Abstract
Performance of the biomaterials used for regenerative medicine largely depends on biocompatibility; however, the biological mechanisms underlying biocompatibility of a biomaterial within the host system is poorly understood. In addition to the classical immune response against non-self-entities, the sterile inflammatory response could limit the compatibility of biological scaffolds. Whereas the immediate to short-term host response to a biomaterial implant have been characterized, the long-term progression of host–biomaterial relationship has not been described. This article explores the novel concept of biomaterials-driven sterile inflammation (BSI) in long-term biodegradable implants and throws light for possible explanation for the onset of BSI and the associated damage-associated molecular patterns. The understanding of BSI would advance the current strategies to improve biomaterial–host tissue integration and open novel translational avenues in biomaterials-based tissue regeneration.
Impact statement
Understanding the novel concept of biomaterials-driven sterile inflammation and associated damage-associated molecular patterns in long-term biodegradable implants would determine their success and improves the tissue engineering and regenerative strategies.
Keywords: biomaterials, sterile inflammation, DAMPs, biocompatibility, implant immunology
Introduction
Biomaterials for regenerative medicine is an emerging field that is essentially encompassed in biocompatibility. The specific biological mechanisms underlying the biocompatibility of a biomaterial within the host system is poorly understood. Indeed, the concept of biocompatibility has often been misinterpreted to be a characteristic of the biomaterial itself instead of a characteristic of the biomaterial–host system. The widely accepted implanted device is thereby commonly characterized by their highly inert chemical and biological properties, including specific grades of polyethylene, polypropylene, polydimethylsiloxane, polyethylene terephthalate, polytetrafluoroethylene, alumina, zirconia, carbon, titanium–aluminum–vanadium, cobalt–chromium, and platinum–iridium.1
This understanding may be acceptable for implantable devices that do not require a dynamic bioactive profile. However, scaffolds used for therapeutic purposes such as gene and drug delivery, tissue engineering, and regenerative medicine necessarily require bioactivity.2 Therefore, proper understanding of immunobiology is required for the development of a biomaterial scaffold aiming to function optimally until the processes of repair and regeneration is completed.3
Williams et al. (2014) suggests mandatory and optional specifications for these biocompatible systems. Among the specifications for the ideal biomaterial, this article explores the inflammatory pathways associated with biomaterial implants, specifically the novel concept of biomaterials-driven sterile inflammation (BSI). Sterile inflammation has been described as an inflammatory pathway that results from trauma or biochemically induced injury, however, devoid of any pathogen/microorganism or foreign infectious agents. During sterile inflammation, damage-associated molecular patterns (DAMPs), indicators of tissue injury, are released into the extracellular matrix (ECM) and recruit cells such as macrophages and neutrophils to further increase the pool of chemokines and cytokines.4
Sterile inflammatory signals from the implant procedure may resolve and lie dormant until the biomaterial degradation begins. Hence, the consideration of BSI associated with a biomaterial, especially one that requires extended residence in its host system, is necessary for improved biological performance. Although the initial inflammatory events facilitate the healing responses, the hyperactivation of inflammatory signals compromises the biocompatibility of the biomaterial. Herein, this article provides insights on the principles and possible biological mechanisms underlying the BSI and its impact on the success of biomaterials with extended residence in the host tissue.
Biomaterials in Tissue Regeneration
Significant progress has been achieved in the field of biomaterials science and versatile biomaterials with improved performance has been introduced.5 However, much consideration needs to be given to the specific biomaterial to be used for a particular biomedical application. For instance, biocompatibility, biomimicry, bioactive features, and biodegradability are only a few qualities to be considered.6 Depending on the specific application, biomaterials with definitive characteristics are preferred. For example, in cases of bone tissue regeneration, the mechanical properties of the biomaterial of interest need to be similar to the bone tissue that differs significantly for cardiovascular tissue engineering. Moreover, in the setting for a myocardial infarction, hydrogels become an ideal choice to address the free radicals, oxygen tension, and inflammatory cytokines while providing the three-dimensional microenvironment for delivered cell to function.7
In general, the biomaterial needs to be porous to allow cell migration and nutrients/waste to traverse, be nontoxic to cells, and be immunocompatible.8 The expectation of a biomaterial implants is to survive in the damaged anatomy, facilitate an instructive healing microenvironment for recruiting stem cells/progenitor cells, and support the cells to differentiate and proliferate for tissue repair.6 Broadly, biomaterials are grouped into natural or synthetic materials. Regardless of their origin, they are expected to be incorporated into the biological host system without any adverse effects.
Natural Biomaterials
Natural biomaterials have been used for their ability to facilitate tissue regeneration by supporting the host tissue function. They are derived from other living systems and can be polysaccharide-based, protein-based, or decellularized tissue-derived biomaterials.9 Natural biomaterials contain inherent availability of specific binding cues and provide the biochemical signals for cell recruitment and communication. However, the residual antigens elicit immunogenic complications. Nonetheless, the natural biomaterials are biocompatible, biodegradable, and possess the ability to remodel damaged structures/tissues in vivo. However, the immunogenic response after implantation, adverse decomposition properties, poor mechanical properties, and the relative difficulty to manipulate its shape and size form hurdles while dealing with natural biomaterials.10,11 Characteristics of commonly used natural biomaterials are given in Table 1.
Table 1.
Characteristics of Natural Biomaterials
| Natural biomaterial | Properties | Limitations | Cytokines | References |
|---|---|---|---|---|
| Collagen | High resistance to tensile forces High conductivity |
Extraction decreases mechanical properties | IFN-γ, IL-13 | 10,12,13 |
| Alginate | Can be made into a gel Structure similar to ECM Minimal immunogenic response Slow degradation |
Requires additional methods for its degradation in mammals | TNF-α, IL-1, IL-6, IL-12, GM-CSF | 10,12,13 |
| Chitosan | Products of degradation can be easily absorbed | Poor mechanical properties | IFN-γ, IL-2, TNF-α, IL-10 | 10,12,13 |
| Silk | Elasticity Mechanical strength Manipulatable degradation rate |
Mild foreign body responses Long-term immune response is unknown |
IFN-γ, IL-2, TNF-α, IL-1β | 10,13 |
| Cellulose | Insoluble in water Strong mechanical properties Anti-inflammatory and anticancer effects |
Requires additional mechanism for degradation | IL-10, IL-6 | 10,14 |
| Bacterial cellulose | High surface-to-mass ratio Strong mechanical properties High crystallinity Low cytotoxicity, immunogenicity |
Limited cell ingrowth capacity due to dense mesh structure Requires additional mechanism for degradation |
No significant proinflammatory cytokines | 10,15 |
| Fibrin | Controllable degradation rate Protective effects against nonbiocompatible materials Facilitates leukocytes, fibroblasts, and endothelial cells functions |
IL-12, IL-1β, TNF-α GM-CSF, IL-1RA, IL-4, IFN-γ, eotaxin, and IL-6 | 10,16 |
ECM, extracellular matrix; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor-necrosis factor-α.
Synthetic Biomaterials
Synthetic biomaterials are easy to reproduce, highly available, and can be chemically modified. Owing to their structural difference from the native tissue, a number of limitations include improper cell adhesion, ability to promote adverse tissue remodeling, and immunogenicity and toxicity. Despite the limitations, synthetic biomaterials are widely used for biomedical applications.10 Polyesters of α-hydroxy acids (polyglycolic acid [PGA], poly-l-lactic acid, poly-lactic-co-glycolic acid [PLGA]) have been approved by the U.S. Food and Drug Administration (FDA) for human clinical use in various applications including sutures.17 They are considered immunocompatible, but there are concerns about the toxic affect from the accumulation of the acidic degradation products.18
Synthetic biomaterials lack cell-recognition signals, giving it a distinct foreign body response that results in fibrous capsule formation around the implant, isolating it from the host environment.19–21 Characteristics of commonly used synthetic biomaterials are given in Table 2.
Table 2.
Characteristics of Synthetic Biomaterials
| Synthetic biomaterials | Properties | Limitations | Cytokines | References |
|---|---|---|---|---|
| Polyglycolic acid | High melting point Very high tensile strength |
Rapid degradation compromises mechanical strength Susceptible to inflammatory response from increase in glycolic acid Hydrolytic instability |
IL-1β, IL-6, GM-CSF, TNF-α | 10,13 |
| Polylactic acid | Thermoplastic Manipulatable degradation rate |
Long-term implants | IL-6, IL-12/23, IL-10 | 10,13,22 |
| Poly-lactic-co-glycolic acid | Modifiable qualities to meet specific requirements Degrades into metabolic monomers: lactic acid and glycolic acid |
Degradation products may be a source of inflammation | TNF-α, IL-6, TGF-β1 | 13 |
| Polycaprolactone | Able to mimic ECM structure Slow degradation rate |
Not effective on its own | TNF-α, IL-1β, IL-6 | 10,13 |
| Polyetheretherketone | Thermoplastic Chemically stable Elastic Radiolucent |
High stiffness may be unsuitable for many applications | TNF-α, IL-1β, IL-6, IL-4, IFN-γ | 23 |
| Polyethylene glycol | Water soluble Organic solvents soluble Nontoxic Nonimmunogenic Nonantigenic |
Requires cross-linked groups to create insoluble structure | 10 | |
| Polymethyl methacrylate | Lightweight Good mechanical properties Low toxicity Inert Slow degradation Nonbiodegradable |
Degradation products has been a concern for aseptic loosening Thermal necrosis |
GM-CSF, IL-6, TNF-α, and MMPs | 24,25 |
MMP, matrix metalloproteinase; TGF-β1, transforming growth factor-β1.
With advancement in material science, the distinction between natural and synthetic biomaterials has been blurred for several biomedical applications. For example, biologic or synthetic coating has been shown to improve biocompatibility and biological performance of implants.26,27 Moreover, the hybrid systems comprising natural and synthetic components has been reported to be superior than their individual applications.11 For instance, Fukunishi et al. developed a hybrid nanofiber system using polycaprolactone and chitosan blend for vascular tissue engineering, which was successfully tested in a sheep model.28 As discussed previously, the specification for the biomaterial of choice differs and depends significantly on the type of tissue and application. Hence, any generalization made across organ systems should be taken with a grain of salt.
Biomaterial Implants and Inflammation
A series of cellular events rapidly unfold once the biomaterial is implanted into the host system. The process begins with the adsorption of blood proteins such as albumin, immunoglobulin G, and fibrinogen to the biomaterial surface forming a protein coat before host cells interact with the biomaterial. It is believed that adsorbed host fibrinogen triggers histamine release from mast cells to promote the recruitment of phagocytes to the implant surface and subsequently activates the acute inflammatory response.29 In addition, the newly formed protein corona activates the complement system, platelets, coagulation proteins resulting in a short-lived matrix/thrombus at the tissue–material interface.30,31 This temporary matrix composed of mitogens, chemoattractants, cytokines, and growth factors mediates the further recruitment and activation of macrophages and other immune cells to facilitate inflammatory responses.30 Meanwhile, DAMPs are released by dying cells into the environment as a result of the surgical/implantation procedure. These DAMPs are recognized by pattern recognition receptors (PRRs) on macrophages and dendritic cells (DCs) to further promote inflammation.32
Upon the release of DAMPs and bioactive signals, the acute inflammatory responses are elicited, which is characterized by the increased recruitment of polymorphonuclear leukocytes followed by the macrophages. Neutrophils act as first line of defense against the implantation injury; however, because of their short life span macrophages are recruited within 48 h. Hence, these acute inflammatory episodes are resolved within a week in the absence of infection.33 In addition, in the presence of persisting inflammatory stimuli, the macrophages are recruited to implantation site by complement factors, platelet-derived growth factor (PDGF), macrophage chemoattractant protein 1–4, macrophage inflammatory protein-1α (MIP-1α), MIP-1b, and transforming growth factor (TGF)-β.34 Because of their plasticity, macrophages orchestrate the inflammatory response by changing their phenotypes in response to the tissue microenvironment. These macrophages are proinflammatory phenotypes and secrete additional proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8, MCP-1, and MIP-1β.2
Tissue macrophages are among the first responders to foreign invasion, including implants. As first responders their function is to phagocytose the cellular debris and foreign particles, present antigens to adaptive immune cells, and produce cytokines. Superior plasticity upgrades the macrophages to be the excellent effector cell; however, challenges do exist to characterize the multiple coexisting phenotypes under pathological conditions. The term “classically activated” macrophage is used to refer to effector macrophages in cell-mediated immune responses. Classically activated macrophages (M1) require the combination of interferon-γ (IFN-γ) and TNF signaling to develop microbicidal or tumoricidal abilities and to secrete proinflammatory signals.35 M1 macrophages are short lived because of the transient nature of IFN-γ production by natural killer (NK) cells; however, they can undergo transition to adaptive immunity by utilizing the sustained production of IFN-γ by the T helper 1 cell that maintains their phenotype.35 Classically activated (M1) macrophages are further characterized by the increased levels of inflammatory cytokines such as IL-1, IL-6, and TNF-α.36
Moreover, the macrophages utilize IL-4 and IL-13 for their differentiation to the wound healing phenotype (M2). The IL-4 from mast cells, basophils, and neutrophils promotes the differentiation of naive T cells into Th2 cells, which are the major source of IL-4, forming a strong positive feedback loop.35,37 In addition, IL-4 and IL-13-treated macrophages do not present antigens to T cells, produce significantly less proinflammatory cytokines than their M1 counterpart, and are less effective at removing intracellular pathogens with oxygen and nitrogen radicals.35 Instead, the wound healing macrophages downregulate proinflammatory pool to facilitate tissue healing through activation of fibroblasts to promote tissue remodeling.35,38,39
A third major category of macrophages has been described as the regulatory macrophage (Mreg). The predominant function of Mreg is the production of the inflammatory signal attenuating IL-10 activity. A battery of differentiation signal for Mreg including PGE2, adenosine, dopamine, histamine, melanocortin, and vasoactive intestinal peptide has been identified. Furthermore, there has been evidence of bacterial and viral pathogens exploiting regulatory macrophages to limit proinflammatory signals to permit intracellular growth.35,40–42 Hence, the understanding regarding the macrophage phenotype switch and underlying regulatory signals highlight the pathological consequence resulting in impaired regenerative responses after biomaterial implantation.
Certainly, the plasticity of macrophages paves the way to immunomodulation and the predominance of a certain phenotype could be a key determinant of the course of BSI.38,43 This presentation of macrophages is an oversimplification when in reality their phenotype is most adequately characterized on a spectrum; however, this topic remains controversial in the literature and requires further study. Nonetheless, after macrophage activity, the formation of granulation tissue displays multiple functions in the wound healing process.32,44 Several cell types are part of the granulation tissue; endothelial cells to form new blood vessels for nutrient and leukocyte transport, leukocytes to clear away contamination, and fibroblasts that make up the majority of the cells. Fibroblasts secretes neo-ECM at the damaged site to support the activity of healing elements including growth factors and cells.44 An overview of macrophage profiles is given in Table 3, the relative concentration of each will determine the host response.
Table 3.
Macrophage Profiles
| Macrophage classification | Surface marker | Cytokine production | Activation signal | Functions | References |
|---|---|---|---|---|---|
| Classically activated (M1) | CD80 CCR7 CD68 |
IL-1, IL-6, TNF, IL-12, IL-1β | IFN-γ TNF LPS |
Proinflammatory cytokine production Nitrogen and oxygen intermediates production Microbicidal or tumoricidal activity |
43,45–47 |
| Wound healing (M2) | CD163 CD206 (mannose receptor) |
IL-1ra Arginase |
IL-4 IL-13 TGF-β VEGF |
Tissue architecture remodeling Immunoregulation Limit parasitic activity Tumor progression |
36,46 |
| Regulatory (Mreg) | CD80 CD86 |
IL-10 TGF-β |
Dopamine Histamine Adenosine Sphingosine 1-phosphate Melanocortin Vasoactive intestinal peptide |
Immunoregulation | 35 |
Mreg, regulatory macrophage; VEGF, vascular endothelial growth factor.
The foreign body reaction is a unique process that occurs in response to biomaterial implants that is associated with foreign body giant cells (FBGCs).33 Major observation regarding the formation of FBGC are the multinucleated fusion products of macrophages after “frustrated phagocytosis.”32,48 The adhesion receptors (αM-, β1-, and β2-integrins on chemically supportive surfaces) for unfused monocyte attachment and IL-4 and IL-13 signaling facilitate the formation of FBGC.38,49–51 Because implanted biomaterials are coated with host proteins upon implantation, the adsorbed proteins on the biomaterial surface is what signals macrophage fusion. In addition, surface topography of the biomaterial plays a very significant role in the subsequent development of inflammation by FBGC formation.34
Veiseh et al. demonstrated that spherical biomaterial that are 1.5 mm in diameter were more biocompatible than smaller sized or different-shaped materials, independent of materials tested and total surface area.52 Among the adsorbed proteins, vitronectin was superior to encourage the macrophage adhesion and fusion to form FBGC.34,53 Because the FBGC is associated with entire lifespan of the biomaterial, it is logical that they confer an evolutionary advantage (that is not yet well understood) either for their biodegradation or for the macrophages to escape apoptosis.34 Regardless, the presence of FBGC poses a challenge for biocompatibility for implants and additional strategies are warranting to address FBGC formation associated with implant rejection.
As a result of the preceding inflammatory reactions, a fibrous and collagenous capsule forms around the biomaterial by PDGF, vascular endothelial growth factor, TGF-β1 signaling from M2 macrophages, FBGCs, fibroblasts, endothelial cells, and adipocytes.34,54 Matrix metalloproteinases (MMPs) were reported to be active in restructuring the ECM around the implant, attracting fibroblasts and endothelial cells to secrete collagen resulting in fibrous capsule.34 A fibrous capsule encasing the biomaterial implant prevents any interaction with the surrounding tissue and rendering it incapacitated for its purpose.55 In the normal wound healing process, apoptosis and senescence of myoblasts and fibroblasts along with collagen decreasing activity of fibrinolytic macrophage resolves the fibrous capsule.56,57 However, this response is absent in the biological response because of the persisting presence of the biomaterial along with proinflammatory and profibrotic signals.34 Hence, the possibilities of operating the sterile inflammatory events in the vicinity of implant site are higher; however, the concept of BSI is very novel and warrants further understanding.
Biomaterials-Driven Sterile Inflammation
Biodegradable implants are advantageous as they eliminate the need for additional surgical intervention and the associated risks in removing the engineered scaffolds once healing has completed.58 With the goal that the implanted degradable biomaterial scaffold would facilitate host tissue healing and eventually be replaced by the host cells, consideration regarding the interaction between host and the degraded scaffold products are essential. In general, multiple adverse inflammatory phenomenon has been reported in the literature under the terms such as “non-specific foreign body response,” “sterile sinus formation,” and “particle disease.”59–62 These reports refer to an inflammatory response induced by a biomaterial implant as a result of degradation products of biomaterials. The concept of BSI is novel and the scientific literature regarding the molecular immunology underlying BSI is largely unavailable.
The major focus of section aims to give insights into the basic understanding regarding the biology of BSI by presenting commonly used polymers as representative biomaterials. However, the chemistry of biomaterials and the interface biology plays a significant role in the induction of BSI. We propose that BSI opens a novel research avenue dealing with the immunobiology of biomaterials implant.
Sterile inflammation has mostly been characterized as a result of ischemia reperfusion injuries, crystal deposition diseases, and chronic particle-induced diseases like asbestosis and silicosis.63 The sterile inflammatory responses are characterized by the absence of microorganisms/pathogens to trigger an inflammatory response; however, it is instead initiated by endogenous DAMPs.64 Therefore, the inflammation induced by the long-term implant can be a sterile inflammation that is considered to be BSI. Of importance, the inflammation cascades into immune pathology in sterile inflammation with an imbalance of proinflammatory and resolution signaling. The major concern with biomaterial implants has been attributed to their extended residence in the host system and the persistent release of degradation products that may be harmful to healthy host cells and/or act as danger signals to immune cells. Such adverse events not only create a hostile environment for the damaged tissue but also sustain chronic inflammation.
The DAMPs that signal sterile inflammation are classified into two types: (1) signals that are normally contained within living cells and are released into the microenvironment upon cell death and (2) hidden molecular fragments that are exposed after ECM fragmentation.65 Three possible and nonmutually exclusive pathways have been reviewed for sterile danger signals to trigger inflammation: (1) recognition by PRR such as TLRs that sense both endogenous and exogenous signals, (2) through release of intracellular cytokines such as IL-1 and relevant inflammasome activation, and (3) through direct activation of receptors that are normally unrelated to microbial recognition by intracellular content or ECM structures.66 The mechanisms of BSI is in accordance with the known sterile inflammatory disease such as chronic inhalation of asbestos, Alzheimer's disease, and atherosclerosis.67 Hence, these existing frameworks has been under consideration to understand biology of the novel BSI paradigm. Table 4 provides an overview of the common DAMPs and their known stimulus of release. However, the specific DAMPs that are released by biomaterial degradation products are still unknown that warrants further research.
Table 4.
Sterile Inflammation Signals
| Molecular pattern | Receptor | Stimulus of release | References |
|---|---|---|---|
| IL-1 | IL-1R | Necrotic cells Microbial stimuli |
65,68 |
| Uric acid | CD14 | Necrotic cells via xanthine oxidase | 69–71 |
| HMGB1 | RAGE TLR2 TLR4 TLR9 CD24 |
Cell necrosis Macrophage Monocytes Dendritic cells |
66,72,73 |
| Mitochondrial contents | TLR9 FPR1 RAGE NLRP3 |
Intracellular mediators | 74–76 |
| ATP/ADP/adenosine | P2X and P2Y receptors | 77 | |
| S100 | RAGE TLR4 |
Cell necrosis | 78,79 |
In general, integration of biomaterial implants depends on the activation and signaling by immune cells and cytokines released at the site of implantation and much attention has been given to attenuate the postimplant immune response. Several strategies have been developed to modify the biomaterial surface using biological cues to improve the immunocompatibility and biological performance. For instance, the improved regenerative performance was observed in bioceramics coated with bone ECM calcium-binding proteins exhibiting reduced proinflammatory cytokines promoting accelerated regeneration within a 2-week period.27 Herein, we delineate the onset of BSI by a different mechanism centered around the protein corona.
After the exposure/deposition of serum proteins and soluble mediators at the implant surface, the chemical composition of the biomaterial contributes less to the inflammatory response than to the protein corona.80,81 In addition to serum proteins, endogenous DAMPs that are released as a result of the surgical trauma also adhere to the biomaterial surface.82,83 The behavior of the protein coat is predicated on the redistribution of charged proteins on the surface, structural changes made to the protein molecule, polarity changes on the protein surface, and the dehydration status of the biomaterial surface.67,84,85 These variables make it difficult to map out the mechanism of action for any given biomaterial at an implant site.
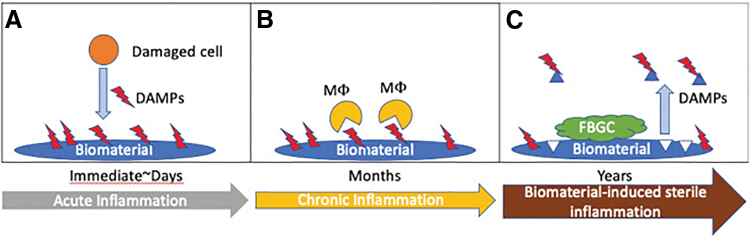
In general, prominent proteins found on among the adhered proteins are plasmin, fibrinogen, and fibronectin that act as ligands for TLR4 pathway in sterile inflammation.83,86–89 After TLR4 activation, the adapter protein MyD88 facilitates activation of nuclear factor kappa B (NF-κB) and MAPKs for the production of inflammatory cytokines such as TNF-α and IL-6.90,91 Although this model of pattern recognition is described for the onset of acute inflammation, to the best of our knowledge, the literature regarding the fate of the adsorbed protein coat upon biomaterial degradation is rare. However, the possibilities of the re-release of the adsorbed DAMPs into the local environment are greater during the course of biodegradation. Hence, it is logical that the injectable biomaterial scaffold elicits minimal BSI warrants extensive research. A hypothesized pathway of DAMP release is given in Figure 1.
FIG. 1.
Proposed BSI pathway: (A) DAMPs released from injured cells from the implant procedure adhere to the biomaterial surface immediately. Some DAMPs are recognized by immune cells and contribute to the acute inflammation. Others remain “dormant” owing to their orientation. (B) Chronic inflammation is characterized by the dominant presence of macrophages that can recognize DAMPs through PRR. Macrophages may also fuse to form FBGC. (C) The degradation of the biomaterial causes adhered DAMPs to be released into the microenvironment. BSI, biomaterials-driven sterile inflammation; DAMP, damage-associated molecular pattern; FBGCs, foreign body giant cells; PRR, pattern recognition receptor. Color images are available online.
The release of DAMPs from the protein corona will lead to an immune response in the surrounding tissue as any viable cell can respond to DAMPs.92 Of more interest in recent years is the involvement of mast cells in the immune response to biomaterials. Mast cells possess an abundance of receptors, including the five main classes of PRRs that are likely to be activated by DAMPs: TLRs and C-type lectin receptors on the membrane, NLRs (nucleotide oligomerization domain-like receptor) and RLRs (retinoic acid-inducible gene-I-like receptor) in the cytoplasm, and DNA sensors.93,94 Tang et al. demonstrated the important role of mast cells and histamine release in the acute inflammatory response and their role in tissue repair such as bone healing.95–97 The critical role of mast cells and mast cell degranulation at the implant surface, and response to DAMP activation and their prohealing/regenerative effects warrant further research.
In addition, aseptic loosening in joint replacements is driven by implant-derived wear debris resulting in progressive osteolysis mediated by macrophage-driven activation of osteoclasts, ultimately leading to loss of implant stability, bone erosion, and joint instability owing to the destruction of soft tissue.98 At present, the mechanism underlying the onset of aseptic loosening is unclear; however, recent/emerging insights into the expanded role of mast cells contributes to further understanding. In aseptic loosening and other particle-induced inflammation such as asbestos and silica, the sterile inflammatory component NLRP3 has been upregulated.99,100 The inflammasome NLRP-3 signals the activation of the proinflammatory cytokine IL-1β that involves the action of caspase-1.73 The activation of NLRP-3 signaling is triggered by reactive oxygen species, lysosomal content leakage, and alterations in ionic influx/efflux.101 Subsequently, IL-1β recruits additional neutrophils and monocytes to the implant zone and upregulates other proinflammatory cytokines.
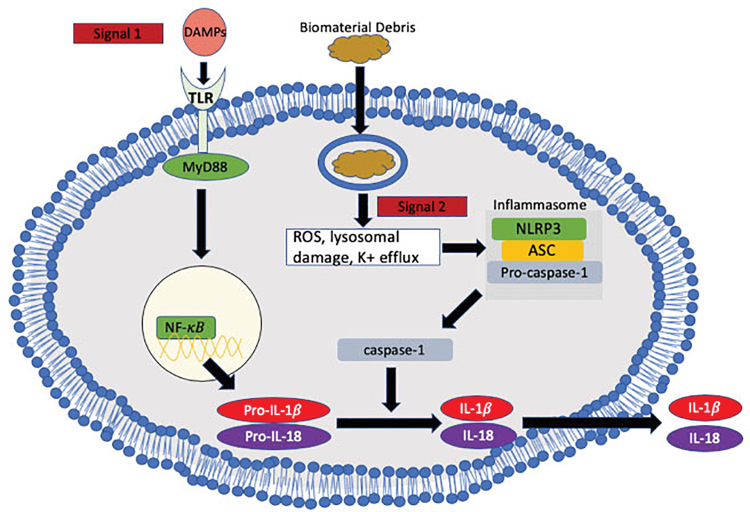
The NLRP-3 activation involves the priming signal that could be biomaterials-driven DAMPs leading to the inflammasome assembly and the proteolytic activation of pro-IL-1β to IL-1β.102 TNF produced by particle-challenged macrophages in the implant degrading environment act as prime signal for NF-κB activation when wear particles were insufficient to activate NLRP-3.102 Figure 2 shows how biomaterial debris can contribute to activation of the NLRP3 inflammasome.
FIG. 2.
Release of IL-1β and IL-18 by NLRP3 inflammasome activation: The production of mature IL-1β and IL-18 requires two signals. Signal 1: biomaterial degradation products cause local cell necrosis leading to the release of DAMPs. Recognition of DAMPs by TLR eventually leads to NF-κB-induced transcription of pro-IL-1β and pro-IL-18. Signal 2: Endocytosis of crystals has been shown to activate NLRP3 inflammasome pathway. We speculate that endocytosis of biomaterial debris can lead to similar activation. NF-κB, nuclear factor kappa B. Color images are available online.
HMGB1 is a well-characterized endogenous DAMP released upon cell damage, shown to be highly expressed and released by mononuclear cells after surgical trauma into the serum, leading to elevated IL-6 secretion.103 Normally, HMGB1 functions in the maintenance of chromatin architecture and modulation of gene expression.104 HMGB1 is released from multiple cell types, including monocytes, macrophages, DCs, NK cells, endothelial cells, and platelets.79 HMGB1 upregulation occur as a result of phagocytosis of apoptotic cells by macrophages, macrophage release after activation of NLRP3, and in response to proinflammatory cytokines.105–107 HMGB1-mediated signaling operates mainly through TLR4 axis and RAGE axis. In general, HMGB1/TLR4 axis induces macrophage activation108 and HMGB1/RAGE activates the ERK MAP kinase pathway for cell migration and expression of MMPs.79
In a seminal study, HMGB1 was detected at higher levels in animals that received a poly lactide-co-glycolide acid (PLGA) scaffold implant compared with naive controls,109 suggesting that HMGB1 is released by necrotic cells in response to the implant. In our recent study on mitochondrial DAMPs, the HMGB1 mitochondria homolog, mitochondrial transcription factor A (mt-TFA), caused a similar immune response as HMGB1 that ultimately lead to vein graft fibrosis and failure.64 Although these agents have been widely acknowledged to be a part of sterile inflammatory pathways, their release and activation upon biomaterials implantation are largely unknown and warrants further investigation.46,110
Molecular fragments of the ECM serve as danger signals in sterile inflammation. MMPs degrade structural components of the ECM to produce changes in cell behavior and are actively secreted by biomaterial adherent macrophages and FBGCs. The profile of MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) is largely determined by the chemistry of the biomaterial, although the mechanisms are still unknown.111 It is possible that in biomaterial degradation, changes in material chemistry overtime leads to a differential MMP/TIMP profile that may be challenging to predict and warrants extensive research.
Biodegradation and BSI
Understanding of the biomaterial degradation may be helpful to determine the initiation of BSI. The processes of biomaterial degradation includes photodegradation, hydrolytic, thermal, oxidative, mechanical, and biodegradation.58,112 Naturally, biodegradation is complicated by the presence of blood, interstitial fluids, the diverse cell types, and the location of the implant. In addition, the chemical profile of degradation products by hydrolysis or enzymatic digestion are more challenging to predict because their mechanistic interactions are largely unknown.113 This section attempts to create a model for the onset of BSI using the reported findings from the commonly studied homopolymers and copolymers of lactic and glycolic acid (polylactic acid [PLA], PGA, and PLGA) as model biomaterials by reviewing their degradative components and the presence of inflammatory cells and cytokine profile in situ.
PGA is a polyester that has been successful in a versatile of biomedical applications including tissue engineering as scaffolds.114–117 The degradation profile of PGA is similar in vivo and in vitro; however, the rate of degradation varies.118 PGA degrades initially to glycolide molecules, followed by glyoxylic acid, and finally to glycine, which is a standard amino acid.119 Although the temporal and spatial degradation of PGA implants were uniform in an in vivo study in rabbits, it was found that the tissue response was inconsistent.119 For instance, for orthopedic implants of PGA, there is a 5% incidence of a local foreign body reaction during the final stage of in vivo degradation. The clinical symptoms range from a painful erythematous papule to development of extensive osteolytic lesions.61,120 The FBGC were reported to be the dominant cell type from 3 to 6 weeks of implantation that was then overshadowed by macrophages at week 12.121 Furthermore, the giant cells predominantly operated on the surface of the implant, whereas the macrophages were found within the interstices.121
The degradation time of PLA is significantly longer than PGA121 because of its higher resistance to hydrolytic attack, making PLA an ideal choice for biomedical applications.58 Upon degradation, PLA releases lactic acid (LA) into the local environment. The local concentration of LA is minimal initially, which gradually increases to significant amounts as PLA is broken down into small oligomers. The increases in LA concentration results in the drop in pH and can lead to inflammation or tissue necrosis.122 PLGA is derived from the racemic mixture of PLA and PGA. PLGA exhibits a heterogeneous degradation pattern initiated with autocatalysis and the carboxylic acid monomers released from degradation reduces pH and further induces hydrolytic degradation.122 This is seen in the in vivo hydrolysis of PLGA microspheres in drug delivery applications where the lower internal pH leads to denaturation of the encapsulated proteins.123 PLGA erosion is facilitated by the uptake of water resulting in hydrolysis and leads to the formation of acidic oligomeric fragments.124 Zolnik et al. demonstrated that the topographic changes occurring in PLGA-based biomaterials at physiologic pH (7.4) initiates the surface erosion, followed by agglomeration, and finally forming a large block polymer fragment.
At present, limited information is available regarding the immune cell interaction with biomaterial-degraded products. A recent study demonstrated that DCs interact with biomaterials and the degradation products through TLRs (mainly TLR4, TLR2, and TLR6).125 Of interest, the silencing of TLR4 on DCs greatly reduced expression of IL-1β, IL-6, IL-10, IL-12p40, RANTES, and TNF-α.125 In addition, the polydopamine degradation products suppressed inflammation of macrophages by downregulating TLR-4-MyD88-NF-κB pathway.126 These studies suggest the potential of biomaterial degradation products to elicit a chronic immune response. A further understanding is required to advance implant design and possibly serve as a therapeutic target.
In a study of cell viability in response to the acidic degradation products of PLGA and PCL (poly e-caprolactone), mouse aortic smooth muscle cells were observed to respond more poorly in relation to the faster degradation rate of PLGA in vitro.127 Furthermore, the in vivo study showed that PCL exhibited significantly higher levels of inflammation, correlating to its higher degree of angiogenesis observed.127 It is speculated that the acidic environment functions as an impediment to cellular migration, including inflammatory cells. Although establishment of new vasculature is necessary for cell recruitment for tissue healing, inflammatory cells are also able to use these channels to form undesirable inflammation. The detailed mechanism of how pH affects immune cell function and biomaterial–tissue interface remains to be studied.
Translational Significance
We believe that the deciphering the knowledge regarding BSI biology paves the way for novel strategies in the development of compatible biomaterial implants. Understanding the mechanisms underlying DAMP release and inflammasome activation further advances the design of biomaterial implants. A better understanding of BSI first involves characterizing the degradation chemistry of biomaterials and the interface biology in vivo. This requires observation of chemical breakdown of biomaterials in animal models, specifically the identity/composition of degradation products and its effect on the tissue environment. Moreover, those degradation products warrant to be correlated with specific DAMP molecules, whether through cell necrosis, ECM breakdown, and/or direct interaction with cells. Hence, intervention steps such as incorporating sterile inflammation inhibitors with the scaffold, chemical/biological tuning of biomaterials, and altering the implantation procedure need to be adopted for addressing the BSI-driven adverse immune reactions after biomaterials implantation.
This will be a steppingstone in developing treatment for patients suffering from BSI and prevent BSI in future patients, ultimately leading to the design of effective biomaterial-based therapeutics. With its wide array (drug delivery, tissue grafts, implants, and scaffolds) of clinical applications, advancement in the implant design improves the quality of treatment for many patients. Hence, the concepts of BSI provide a novel framework to consider biocompatibility for the safer applications of biomaterial implants.
Summary and Future
This review highlights the need for further studies on the events of long-term implant failure. Current reviews are very rare making the characterization of BSI difficult and speculative. Much of the research available are focused on the initial biocompatibility of the implant and the characterization of the immune response and thus little attention has been given to biodegradable implants requiring extended residence in the host. Numerous potential studies are required for the full characterization of BSI requiring the collaboration of the scientific community. Further research on the degradation products of biomaterials in vivo would reveal novel danger signals for the onset of BSI. The complex environment in a living host necessitates in vivo observations, particularly the fate of the initially adsorbed protein layer containing DAMPs upon biomaterial degradation.
Studies including cytokine profile in BSI would reveal the key mediators triggering the immune responses through immune cells primed by BSI signals. Understanding regarding the molecular signaling underlying the BSI-mediated immune responses and identifying the biomaterials derived/triggered priming signals possesses immense translational potential in next-generation tissue engineering; however, it warrants further investigations. In addition, it is logical that the chemistry of biomaterials and initial interface biological reactions are crucial for immunocompatibility of tissue engineering implants. Hence, the development of tunable and intelligent biomaterials capable of sensing the BSI that subsequently respond by favoring/activating immunocompatible signaling is warranted. Moreover, effective strategies for taming the BSI need to be discovered, which provide opportunity for the design and fabrication of BSI-free/BSI-resistant biomaterials for improving the immunocompatibility. Altogether, unveiling the mystery of BSI opens novel avenues for improving the compatibility and biological performance of tissue engineering biomaterials.
Disclosure Statement
There are no potential conflicts of interest or financial disclosures associated with the authors of this article.
Funding Information
FT received start-up funding from WUHS and DA received NIH-R01 funding.
References
- 1. Williams, D.F. Biocompatibility in clinical practice: predictable and unpredictable outcomes. Prog Biomed Eng 1, 013001, 2019. [Google Scholar]
- 2. Williams, D.F. The biomaterials conundrum in tissue engineering. Tissue Eng Part A 20, 1129, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Kaiser, N.J., and Coulombe, K.L.K.. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed Mater 10, 034003, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thankam, F.G., Roesch, Z.K., Dilisio, M.F., et al. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci Rep 8, 8918, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finosh, G.T., and Jayabalan, M.. Regenerative therapy and tissue engineering for the treatment of end-stage cardiac failure: new developments and challenges. Biomatter 2, 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdulghani, S., and Mitchell, G.R.. Biomaterials for in situ tissue regeneration: a review. Biomolecules 9, 750, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camci-Unal, G., Annabi, N., Dokmeci, M.R., Liao, R., and Khademhosseini, A.. Hydrogels for cardiac tissue engineering. NPG Asia Mater 6, e99, 2014. [Google Scholar]
- 8. Turnbull, G., Clarke, J., Picard, F., et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater 3, 278, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ha, T.L.B., Quan, T.M., Vu, D.N., and Si, D.M.. Naturally Derived Biomaterials: Preparation and Application. Regenerative Medicine and Tissue Engineering [Internet]. IntechOpen, 2013. Available at: https://www.intechopen.com/books/regenerative-medicine-and-tissue-engineering/naturally-derived-biomaterials-preparation-and-application (accessed August 2, 2020).
- 10. Manoukian, O.S., Sardashti, N., Stedman, T., et al. Biomaterials for Tissue Engineering and Regenerative Medicine.. Encycl Biomed Eng [Internet]. Elsevier, USA, pp. 462–82, 2019. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9780128012383640989 (accessed May 24, 2020).
- 11. Gnanaprakasam Thankam, F., Muthu, J., Sankar, V., and Kozhiparambil Gopal, R.. Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf B Biointerfaces 107, 137, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Chung, L., Maestas, D.R., Housseau, F., and Elisseeff, J.H.. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev 114, 184, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Sarkar, K., Xue, Y., and Sant, S.. Host Response to Synthetic Versus Natural Biomaterials. In: Corradetti, B., ed. Immune Response Implant Mater Devices [Internet]. Cham: Springer International Publishing, pp. 81–105, 2017. Available at: http://link.springer.com/10.1007/978-3-319-45433-7_5 (accessed July 24, 2020).
- 14. Čolić, M., Mihajlović, D., Mathew, A., Naseri, N., and Kokol, V.. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 22, 763, 2015. [Google Scholar]
- 15. Wang, J., Zhu, Y., and Du, J.. Bacterial cellulose: a natural nanomaterial for biomedical applications. J Mech Med Biol 11, 285, 2011. [Google Scholar]
- 16. Lourenço, E.S., de Almeida Barros Mourão, C.F., Leite, P.E.C., Granjeiro, J.M., Calasans-Maia, M.D., and Alves, G.G.. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J Biomed Mater Res A 106, 1373, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Kim, B.-S., and Mooney, D.J.. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol 16, 224, 1998. [DOI] [PubMed] [Google Scholar]
- 18. Ignatius, A.A., and Claes, L.E.. In vitro biocompatibility of bioresorbable polymers: poly(L, DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials 17, 831, 1996. [DOI] [PubMed] [Google Scholar]
- 19. Pariente, J.-L., Kim, B.-S., and Atala, A.. In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J Biomed Mater Res 55, 33, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Morris, A.H., Stamer, D.K., and Kyriakides, T.R.. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol 29, 72, 2017. [DOI] [PubMed] [Google Scholar]
- 21. Chung, L., Maestas, D.R., Lebid, A., et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Sci Transl Med [Internet]. American Association for the Advancement of Science 12, eaax3799, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulkarni, R.K., Pani, K.C., Neuman, C., and Leonard, F.. Polylactic acid for surgical implants. Arch Surg 93, 839, 1966. [DOI] [PubMed] [Google Scholar]
- 23. Barkarmo, S., Östberg, A.-K., Johansson, C.B., et al. Inflammatory cytokine release from human peripheral blood mononuclear cells exposed to polyetheretherketone and titanium-6 aluminum-4 vanadium in vitro. J Biomater Appl 33, 245, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Trindade, M.C.D., Lind, M., Nakashima, Y., et al. Interleukin-10 inhibits polymethylmethacrylate particle induced interleukin-6 and tumor necrosis factor-α release by human monocyte/macrophages in vitro. Biomaterials 22, 2067, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Webb, J.C.J., and Spencer, R.F.. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br 89-B, 851, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Chen, J., Chen, C., Chen, Z., Chen, J., Li, Q., and Huang, N.. Collagen/heparin coating on titanium surface improves the biocompatibility of titanium applied as a blood-contacting biomaterial. J Biomed Mater Res A 95A, 341, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Mansour, A., Abu-Nada, L., Al-Waeli, H., et al. Bone extracts immunomodulate and enhance the regenerative performance of dicalcium phosphates bioceramics. Acta Biomater 89, 343, 2019. [DOI] [PubMed] [Google Scholar]
- 28. Fukunishi, T., Best, C.A., Sugiura, T., et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 11, e0158555, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zdolsek, J., Eaton, J.W., and Tang, L.. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med 5, 31, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson, J.M., Rodriguez, A., and Chang, D.T.. Foreign body reaction to biomaterials. Semin Immunol 20, 86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang, L., and Eaton, J.W.. Inflammatory responses to biomaterials. Am J Clin Pathol 103, 466, 1995. [DOI] [PubMed] [Google Scholar]
- 32. Vasconcelos, D.P., Águas, A.P., Barbosa, M.A., Pelegrín, P., and Barbosa, J.N.. The inflammasome in host response to biomaterials: bridging inflammation and tissue regeneration. Acta Biomater 83, 1, 2019. [DOI] [PubMed] [Google Scholar]
- 33. Li, J.J., and Zreiqat, H.. Tissue response to biomaterials. In: Narayan, R., ed. Encycl Biomed Eng [Internet]. Oxford: Elsevier, pp. 270–7, 2019. Available at: www.sciencedirect.com/science/article/pii/B9780128012383998805 (accessed June 9, 2020).
- 34. Klopfleisch, R., and Jung, F.. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A 105, 927, 2017. [DOI] [PubMed] [Google Scholar]
- 35. Mosser, D.M., and Edwards, J.P.. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shrivastava, R., and Shukla, N.. Attributes of alternatively activated (M2) macrophages. Life Sci 224, 222, 2019. [DOI] [PubMed] [Google Scholar]
- 37. Ho, I.-C., and Miaw, S.-C.. Regulation of IL-4 expression in immunity and diseases. Adv Exp Med Biol 941, 31, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Franz, S., Rammelt, S., Scharnweber, D., and Simon, J.C.. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Gratchev, A., Guillot, P., Hakiy, N., et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand J Immunol 53, 386, 2001. [DOI] [PubMed] [Google Scholar]
- 40. Benoit, M., Barbarat, B., Bernard, A., Olive, D., and Mege, J.-L.. Coxiella burnetii, the agent of Q fever, stimulates an atypical M2 activation program in human macrophages. Eur J Immunol 38, 1065, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Kim, C., Wilcox-Adelman, S., Sano, Y., Tang, W.-J., Collier, R.J., and Park, J.M.. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci U S A 105, 6150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miles, S.A., Conrad, S.M., Alves, R.G., Jeronimo, S.M.B., and Mosser, D.M.. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med 201, 747, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ambarus, C.A., Krausz, S., van Eijk, M., et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 375, 196, 2012. [DOI] [PubMed] [Google Scholar]
- 44. Wilmink, J.M., and Van Weeren, P.R.. Treatment of exuberant granulation tissue. Clin Tech Equine Pract 3, 141, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Kzhyshkowska, J., Gudima, A., Riabov, V., Dollinger, C., Lavalle, P., and Vrana, N.E.. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol 98, 953, 2015. [DOI] [PubMed] [Google Scholar]
- 46. Badylak, S.F., Valentin, J.E., Ravindra, A.K., McCabe, G.P., and Stewart-Akers, A.M.. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Jones, J.A., Chang, D.T., Meyerson, H., et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A 83A, 585, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Henson, P.M. The immunologic release of constituents from neutrophil leukocytes: I. the role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol 107, 1535, 1971. [PubMed] [Google Scholar]
- 49. Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—phenotypes, functions and markers. Acta Biomater 43, 3, 2016. [DOI] [PubMed] [Google Scholar]
- 50. McNally, A.K., and Anderson, J.M.. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol 713, 97. [DOI] [PubMed] [Google Scholar]
- 51. Brodbeck, W.G., and Anderson, J.M.. Giant cell formation and function. Curr Opin Hematol 16, 53, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veiseh, O., Doloff, J.C., Ma, M., et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater 14, 643, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McNally, A.K., Jones, J.A., MacEwan, S.R., Colton, E., and Anderson, J.M.. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J Biomed Mater Res A 86A, 535, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhan, W., and Lu, F.. Activated macrophages as key mediators of capsule formation on adipose constructs in tissue engineering chamber models. Cell Biol Int 41, 354, 2017. [DOI] [PubMed] [Google Scholar]
- 55. DiEgidio, P., Friedman, H.I., Gourdie, R.G., Riley, A.E., Yost, M.J., and Goodwin, R.L.. Biomedical implant capsule formation: lessons learned and the road ahead. Ann Plast Surg 73, 451, 2014. [DOI] [PubMed] [Google Scholar]
- 56. Jun, J.-I., and Lau, L.F.. Cellular senescence controls fibrosis in wound healing. Aging 2, 627, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wermuth, P.J., and Jimenez, S.A.. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med 4, 2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anju, S., Prajitha, N., Sukanya, V.S., and Mohanan, P.V.. Complicity of degradable polymers in health-care applications. Mater Today Chem 16, 100236, 2020. [Google Scholar]
- 59. Albrektsson, T., Canullo, L., Cochran, D., and Bruyn, H.D.. “Peri-Implantitis”: a complication of a foreign body or a man-made “Disease.” Facts and Fiction. Clin Implant Dent Relat Res 18, 840, 2016. [DOI] [PubMed] [Google Scholar]
- 60. Werner, J.H., Rosenberg, J.H., Keeley, K.L., and Agrawal, D.K.. Immunobiology of periprosthetic inflammation and pain following ultra-high-molecular-weight-polyethylene wear debris in the lumbar spine. Expert Rev Clin Immunol 14, 695, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weiler, A., Helling, H.-J., Kirch, U., Zirbes, T.K., and Rehm, K.E.. Foreign-body reaction and the course of osteolysis after polyglycolide implants for fracture fixation: experimental study in sheep. J Bone Joint Surg Br 78-B, 369, 1996. [PubMed] [Google Scholar]
- 62. Gallo, J., Goodman, S.B., Konttinen, Y.T., and Raska, M.. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun 19, 213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen, H., Kreisel, D., and Goldstein, D.R.. Processes of sterile inflammation. J Immunol 191, 2857, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thankam, F.G., Ayoub, J.G., Ahmed, M.M.R., et al. Association of hypoxia and mitochondrial damage associated molecular patterns in the pathogenesis of vein graft failure: a pilot study. Transl Res 229, 38, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kono, H., Onda, A., and Yanagida, T.. Molecular determinants of sterile inflammation. Curr Opin Immunol 26, 147, 2014. [DOI] [PubMed] [Google Scholar]
- 66. Chen, G.Y., and Nuñez, G.. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10, 826, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Williams, D.F. Biocompatibility pathways: biomaterials-induced sterile inflammation, mechanotransduction, and principles of biocompatibility control. ACS Biomater Sci Eng 3, 2, 2017. [DOI] [PubMed] [Google Scholar]
- 68. Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27, 519, 2009. [DOI] [PubMed] [Google Scholar]
- 69. Shi, Y., Evans, J.E., and Rock, K.L.. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516, 2003. [DOI] [PubMed] [Google Scholar]
- 70. Kono, H., Chen, C.-J., Ontiveros, F., and Rock, K.L.. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 120, 1939, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scott, P., Ma, H., Viriyakosol, S., Terkeltaub, R., and Liu-Bryan, R.. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol 177, 6370, 2006. [DOI] [PubMed] [Google Scholar]
- 72. Hori, O., Brett, J., Slattery, T., et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270, 25752, 1995. [DOI] [PubMed] [Google Scholar]
- 73. Leso, V., Fontana, L., and Iavicoli, I.. Nanomaterial exposure and sterile inflammatory reactions. Toxicol Appl Pharmacol 355, 80, 2018. [DOI] [PubMed] [Google Scholar]
- 74. Julian, M.W., Shao, G., Bao, S., et al. Mitochondrial transcription factor a serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J Immunol 189, 433, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oka, T., Hikoso, S., Yamaguchi, O., et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang, Q., Raoof, M., Chen, Y., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Romagnoli, R., Baraldi, P.G., Cruz-Lopez, O., et al. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets 12, 647, 2008. [DOI] [PubMed] [Google Scholar]
- 78. Hofmann, M.A., Drury, S., Fu, C., et al. RAGE Mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889, 1999. [DOI] [PubMed] [Google Scholar]
- 79. Tsung, A., Tohme, S., and Billiar, T.R.. High-mobility group box-1 in sterile inflammation. J Intern Med 276, 425, 2014. [DOI] [PubMed] [Google Scholar]
- 80. Anderson, J.M. Biological responses to materials. Annu Rev Mater Res 31, 81, 2001. [Google Scholar]
- 81. Schutte, R.J., Parisi-Amon, A., and Reichert, W.M.. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J Biomed Mater Res A 88A, 128, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lin, T., Tamaki, Y., Pajarinen, J., et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater 10, 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rogers, T.H., and Babensee, J.E.. Altered adherent leukocyte profile on biomaterials in Toll-like receptor 4 deficient mice. Biomaterials 31, 594, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wilson, C.J., Clegg, R.E., Leavesley, D.I., and Pearcy, M.J.. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Eng 11, 1, 2005. [DOI] [PubMed] [Google Scholar]
- 85. Haynes, C.A., and Norde, W.. Globular proteins at solid/liquid interfaces. Colloids Surf B Biointerfaces 2, 517, 1994. [Google Scholar]
- 86. Ward, J.R., Dower, S.K., Whyte, M.K.B., Buttle, D.J., and Sabroe, I.. Potentiation of TLR4 signalling by plasmin activity. Biochem Biophys Res Commun 341, 299, 2006. [DOI] [PubMed] [Google Scholar]
- 87. Mollen, K.P., Anand, R.J., Tsung, A., Prince, J.M., Levy, R.M., and Billiar, T.R.. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26, 430, 2006. [DOI] [PubMed] [Google Scholar]
- 88. Okamura, Y., Watari, M., Jerud, E.S., et al. The extra domain A of fibronectin activates toll-like receptor 4. J Biol Chem 276, 10229, 2001. [DOI] [PubMed] [Google Scholar]
- 89. Love, R.J., and Jones, K.S.. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. J Biomed Mater Res A 101A, 2740, 2013. [DOI] [PubMed] [Google Scholar]
- 90. Laird, M.H.W., Rhee, S.H., Perkins, D.J., et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 85, 966, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Uto, T., Akagi, T., Yoshinaga, K., Toyama, M., Akashi, M., and Baba, M.. The induction of innate and adaptive immunity by biodegradable poly(γ-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 32, 5206, 2011. [DOI] [PubMed] [Google Scholar]
- 92. Zindel, J., and Kubes, P.. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol Mech Dis 15, 493, 2020. [DOI] [PubMed] [Google Scholar]
- 93. Gong, T., Liu, L., Jiang, W., and Zhou, R.. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20, 95, 2020. [DOI] [PubMed] [Google Scholar]
- 94. Redegeld, F.A., Yu, Y., Kumari, S., Charles, N., and Blank, U.. Non-IgE mediated mast cell activation. Immunol Rev 282, 87, 2018. [DOI] [PubMed] [Google Scholar]
- 95. Tang, L., Jennings, T.A., and Eaton, J.W.. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A 95, 8841, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ragipoglu, D., Dudeck, A., Haffner-Luntzer, M., et al. The role of mast cells in bone metabolism and bone disorders. Front Immunol 11, 163, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ramirez-GarciaLuna, J.L., Chan, D., Samberg, R., et al. Defective bone repair in mast cell-deficient Cpa3Cre/+ mice. PLoS One 12, e0174396, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barnsley, L., and Barnsley, L.. Detection of aseptic loosening in total knee replacements: a systematic review and meta-analysis. Skeletal Radiol 48, 1565, 2019. [DOI] [PubMed] [Google Scholar]
- 99. Dostert, C., Pétrilli, V., Van Bruggen, R., Steele, C., Mossman, B.T., and Tschopp, J.. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jo, E.-K., Kim, J.K., Shin, D.-M., and Sasakawa, C.. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. Nature Publishing Group 13, 148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Latz, E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 22, 28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jämsen, E., Pajarinen, J., Kouri, V.-P., et al. Tumor necrosis factor primes and metal particles activate the NLRP3 inflammasome in human primary macrophages. Acta Biomater 108, 347, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Manganelli, V., Signore, M., Pacini, I., et al. Increased HMGB1 expression and release by mononuclear cells following surgical/anesthesia trauma. Crit Care 14, R197, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bertheloot, D., and Latz, E.. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 14, 43, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qin, S., Wang, H., Yuan, R., et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203, 1637, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Willingham, S.B., Allen, I.C., Bergstralh, D.T., et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol 183, 2008, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lu, B., Wang, H., Andersson, U., and Tracey, K.J.. Regulation of HMGB1 release by inflammasomes. Protein Cell 4, 163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Park, J.S., Gamboni-Robertson, F., He, Q., et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290, C917, 2006. [DOI] [PubMed] [Google Scholar]
- 109. Babensee, J.E. Interaction of dendritic cells with biomaterials. Semin Immunol 20, 101, 2008. [DOI] [PubMed] [Google Scholar]
- 110. Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta BBA 1864, 2036, 2017. [DOI] [PubMed] [Google Scholar]
- 111. Jones, J.A., McNally, A.K., Chang, D.T., et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A 84A, 158, 2008. [DOI] [PubMed] [Google Scholar]
- 112. Tamariz, E., and Rios-Ramírez, A.. Biodegradation of medical purpose polymeric materials and their impact on biocompatibility. Biodegrad—Life Sci [Internet]. IntechOpen, 2013. Available at: https://www.intechopen.com/books/biodegradation-life-of-science/biodegradation-of-medical-purpose-polymeric-materials-and-their-impact-on-biocompatibility (accessed February 3, 2021).
- 113. Saltzman, W.M., and Kyriakides, T.R.. Chapter 20—Cell Interactions with Polymers. In: Lanza, R., Langer, R., and Vacanti, J., eds. Princ Tissue Eng Fourth Ed [Internet]. Boston: Academic Press, pp. 385–406, 2014. Available at: www.sciencedirect.com/science/article/pii/B9780123983589000203 (accessed June 16, 2020).
- 114. Mannami, T., Fujiwara, N., Ikeda, G., et al. Gastrointestinal hemorrhage caused by the direct invasion of a hepatocellular carcinoma successfully treated with polyglycolic acid sheet shielding. Endoscopy 51, E20, 2019. [DOI] [PubMed] [Google Scholar]
- 115. Jang, J.-Y., Shin, Y.C., Han, Y., et al. Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg 152, 150, 2017. [DOI] [PubMed] [Google Scholar]
- 116. Otsuki, S., Nakagawa, K., Murakami, T., et al. Evaluation of meniscal regeneration in a mini pig model treated with a novel polyglycolic acid meniscal scaffold. Am J Sports Med 47, 1804, 2019. [DOI] [PubMed] [Google Scholar]
- 117. Hoerstrup Simon P., Ralf S, Sabine D, et al. Functional living trileaflet heart valves grown in vitro. Circulation 102, Iii, 2000. [DOI] [PubMed] [Google Scholar]
- 118. Williams, D.F. Biodegradation of surgical polymers. J Mater Sci 17, 1233, 1982. [Google Scholar]
- 119. Böstman, O., Päivärinta, U., Partio, E., Vasenius, J., Manninen, M., and Rokkanen, P.. Degradation and tissue replacement of an absorbable polyglycolide screw in the fixation of rabbit femoral osteotomies. JBJS 74, 1021, 1992. [PubMed] [Google Scholar]
- 120. Böstman, O., and Pihlajamäki, H.. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials 21, 2615, 2000. [DOI] [PubMed] [Google Scholar]
- 121. Päivärinta, U., Böstman, O., Majola, A., Toivonen, T., Törmälä, P., and Rokkanen, P.. Intraosseous cellular response to biodegradable fracture fixation screws made of polyglycolide or polylactide. Arch Orthop Trauma Surg 112, 71, 1993. [DOI] [PubMed] [Google Scholar]
- 122. Suggs, L.J., Moore, S.A., and Mikos, A.G.. Synthetic Biodegradable Polymers for Medical Applications. Physical Properties of Polymers Handbook. New York, NY: Springer, 2007, p. 939. [Google Scholar]
- 123. Crotts, G., and Park, T.G.. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul 15, 699, 1998. [DOI] [PubMed] [Google Scholar]
- 124. Zolnik, B.S., and Burgess, D.J.. Effect of acidic pH on PLGA microsphere degradation and release. J Controlled Release 122, 338, 2007. [DOI] [PubMed] [Google Scholar]
- 125. Shokouhi, B., Coban, C., Hasirci, V., et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials 31, 5759, 2010. [DOI] [PubMed] [Google Scholar]
- 126. Jin, L., Yuan, F., Chen, C., et al. Degradation products of polydopamine restrained inflammatory response of LPS-stimulated macrophages through mediation TLR-4-MYD88 dependent signaling pathways by antioxidant. Inflammation 42, 658, 2019. [DOI] [PubMed] [Google Scholar]
- 127. Sung, H.-J., Meredith, C., Johnson, C., and Galis, Z.S.. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 25, 5735, 2004. [DOI] [PubMed] [Google Scholar]