Abstract
It is estimated that almost one-third of the United States population will be affected by a vocal fold (VF) disorder during their lifespan. Promising therapies to treat VF injury and scarring are mostly centered on VF tissue engineering strategies such as the injection of engineered biomaterials and cell therapy. VF tissue engineering, however, is a challenging field as the biomechanical properties, structure, and composition of the VF tissue change upon exposure to mechanical stimulation. As a result, the development of long-term VF treatment strategies relies on the characterization of engineered tissues under a controlled mechanical environment. In this review, we highlight the importance of bioreactors as a powerful tool for VF tissue engineering with a focus on the current state of the art of bioreactors designed to mimic phonation in vitro. We discuss the influence of the phonatory environment on the development, function, injury, and healing of the VF tissue and its importance for the development of efficient therapeutic strategies. A concise and comprehensive overview of bioreactor designs, principles, operating parameters, and scalability are presented. An in-depth analysis of VF bioreactor data to date reveals that mechanical stimulation significantly influences cell viability and the expression of proinflammatory and profibrotic genes in vitro. Although the precision and accuracy of bioreactors contribute to generating reliable results, diverse gene expression profiles across the literature suggest that future efforts should focus on the standardization of bioreactor parameters to enable direct comparisons between studies.
Impact statement
We present a comprehensive review of bioreactors for vocal fold (VF) tissue engineering with a focus on the influence of the phonatory environment on the development, function, injury, and healing of the VFs and the importance of mimicking phonation on engineered VF tissues in vitro. Furthermore, we put forward a strong argument for the continued development of bioreactors in this area with an emphasis on the standardization of bioreactor designs, principles, operating parameters, and oscillatory regimes to enable comparisons between studies.
Keywords: vocal fold, bioreactor, lamina propria, gene expression, fibrosis, inflammation
Introduction
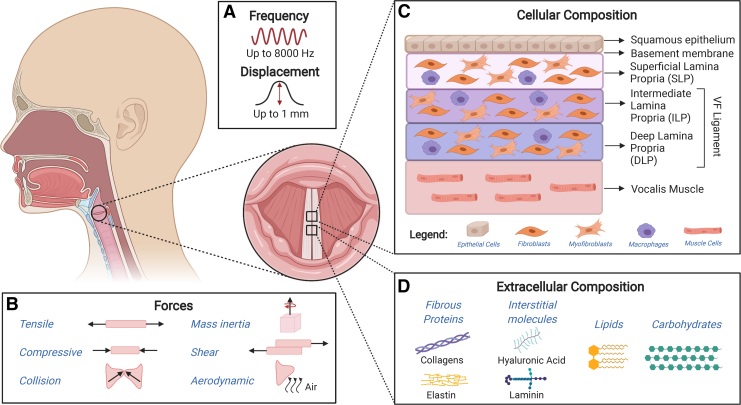
The human vocal folds (VFs), also known as vocal cords, are located in the larynx and have a unique structure and multilayered composition that enable the production of wavelike motion during sound production as depicted in Figure 1. During phonation, the VFs can sustain frequencies up to 8000 Hertz (Hz) and can oscillate at amplitudes of up to 1 mm.1,2 Due to their anatomical location, the VFs are susceptible to various internal and external stimuli that can cause damage leading to irreversible changes in structure and ultimately in vibratory function. Voice disorders represent a significant health care challenge that affects millions of Americans each year. Currently, various treatments for voice disorders, including voice therapy, surgery, or biomaterial injections, have shown promise, but none have fully satisfied the recovery of the VFs.3 While voice therapy may provide temporary relief, in some cases, surgery physically disrupts the native microstructure of the VFs causing scarring.3–6 Although biomaterial injections tend to cause less scarring than surgery, hydrogel formulations to date fail to emulate the complex interstitial microstructure of the VFs.3 As a result, full restoration of the VF microstructure has not yet been achieved at the cellular and interstitial levels.3 Promising tissue engineering efforts focus on restoring the interstitial microenvironment of the VFs using synthetic polymers or naturally-derived biomaterials to enable stratified cellular proliferation with the aid of bioactive factors.7,8
FIG. 1.
Overview of the structure, composition, and forces acting on the vocal folds upon phonation. Schematic illustrating (A) frequency and displacement, (B) forces to which vocal folds undergo during phonation, as well as (C) cellular and (D) extracellular components of the vocal folds. Color images are available online.
One challenge in developing treatments for voice disorders is that their long-term outcomes are complicated by the complex biomechanical stimulation, which occurs during phonation.9 In this context, the use of animal models for preclinical applications is very limited as differences in laryngeal anatomy, phonatory capabilities, tissue structure, and composition largely reduce the scope of studies in vivo.10,11 Although dogs, ferrets, mice, rats, and rabbits have been used in short-term studies, interspecies variability and the inability to simulate the in vivo process of human phonation in these animals preclude direct comparisons between studies. Excised larynges have also been used in short-term VF scarring and replacement studies12–14; however, simulating human phonation in ex vivo models is challenging.15 Therefore, to fully characterize optimized treatments for voice disorders, it is important to develop and utilize bioreactors that reliably simulate the mechanical and cellular environments of the VFs during injury and healing. In this review, we provide a thorough overview of bioreactor designs that mimic the mechanical phonatory conditions of the VFs in vitro.
VF structure and composition
The VFs are composed of three distinct structures: stratified squamous epithelium, lamina propria (LP), and thyroarytenoid (or vocalis) muscle. A thin basement membrane connects the epithelium and the LP.16,17 The epithelium consists of approximately 5–10 layers of closely packed epithelial cells and its role is to protect the deeper layers of tissue by mitigating the impact of internal and external irritants or mechanical stresses that occur during phonation. The layers of epithelial cells are divided into two regions: basal layer, which is connected to the basement membrane, and suprabasal (or luminal) layer. The basement membrane is primarily composed of collagens; however, other proteins such as fibronectin are also present.1,18,19
The LP is a complex trilayered structure subcategorized into the superficial, intermediate, and deep lamina propria. It is heavily involved in sound production and each layer has a distinct structure, molecular composition, and biomechanical properties that determine the vibratory capability of the VFs.18 The LP primarily consists of extracellular matrix (ECM) components such as fibrous proteins (collagen types I, II, and III, elastin), interstitial molecules (glycosaminoglycans: hyaluronic acid [HA]; proteoglycans: decorin, fibromodulin, and versican; glycoproteins: fibronectin and laminin), lipids, and carbohydrates.18 A study by Mora-Navarro et al. detected ∼2000 proteins in a porcine-derived acellular vocal fold lamina proporia extracellular matrix (VFLP-ECM) scaffold.20 It is important to note that the exact distribution of all ECM components is not currently well understood. In addition, the distribution of various ECM components (e.g., elastins) can vary with age and gender.21
Many studies have focused on three important ECM proteins: HA, collagens, and elastins.1 HA is an example of an important glycosaminoglycan that has gained traction in recent studies as its function goes beyond its osmotic and viscoelastic properties. In addition to its important role in water retention and shock absorption, HA interacts with cell surface receptors enabling cell signaling pathways to regulate its degradation, as well as mediate tissue remodeling and inflammation.22 Furthermore, as a biomaterial, its structural versatility permits chemical functionalization into tailored HA derivatives that undergo delayed degradation after injectable delivery.3 Collagens are fibrous proteins that provide tensile strength, maintain LP organization during vibration, and contribute to tissue remodeling during growth and wound healing.1,8 Elastins play an important role in VF biomechanics by acting as stretch and recoil proteins during vibration. In the LP, elastins can be found in three different forms: oxytalan, elaunin, and elastic fibers. By intertwining with inelastic collagens fibrils, elastins regulate stretching to prevent damage during injury, while allowing for prolonged cycles of stretching and recoiling without breaking down.21
The main cell types found in the LP are fibroblasts, myofibroblasts, and macrophages.18,20 Fibroblasts are the most abundant cell type and are found in all layers of the LP. VF fibroblasts are responsible for ECM synthesis and play a key role during normal and diseased conditions. Following injury, fibroblasts can activate and differentiate to myofibroblasts to increase ECM synthesis to promote wound healing and tissue regeneration. However, the prolonged presence of myofibroblasts can lead to abnormal overproduction of ECM components, altering the tissue composition and function resulting in scar formation.20,23 In addition to fibroblasts, macrophages are also heavily involved in orchestrating the complex events involved in VF wound healing and fibrosis.24 Typically, there is a tendency for proinflammatory (or M1 like) macrophages to appear first at the site of injury, followed by a gradual shift toward prohealing (or M2-like) macrophages.25 However, the phenotype and distribution of macrophages in the LP layers have not been well characterized.26
VF disorders and current therapies
Voice disorders are the most common communication disorder in the United States. Approximately 28 million Americans suffer from voice disorders and it is estimated that 29% of the population will develop a voice disorder during their lifespan.3 In addition, the cost associated with health care and lost wages approaches $13 billion dollars and is comparable to conditions such as asthma.27,28 Some examples of voice disorders include contact ulcers, polyps, nodules, cysts, paralysis, cancerous lesions, fibrosis, and so on. Various factors can contribute to voice disorders such as, but not limited to, voice overuse or misuse, gastroesophageal reflux, upper respiratory infections, smoking, radiation, trauma as a result of intubation or surgery, and aging.3,29
Many voice disorders, particularly VF fibrosis, represent a challenging therapeutic scenario since they are associated with significant changes in structure, composition, and mechanical properties of the ECM. The VF scar results in overproduction and random deposition of procollagen, collagen, and fibronectin.20 These changes alter the structure and composition of the VFs, ultimately affecting their vibratory function.
Voice therapy may be used to improve voice quality and reduce vocal fatigue.3,30 Direct voice therapy is the recommended form of treatment for nontraumatic conditions, such as idiopathic VF paralysis and presbylaryngis, in which the VFs undergo changes in their microstructure resulting in loss of bulk and tonicity.31,32 Surgical approaches are being used in the form of LP replacement; however, this approach can result in further complications due to high variability in ECM content and biomechanical properties across individuals.3,30 As an alternative, naturally derived and/or synthetic biomaterial injections [e.g., type I and/or type III collagen, HA, poly(ethylene glycol) (PEG), and porcine urinary bladder matrix] have been used to treat VF injury.3,17
Cell therapy is another promising therapeutic strategy focused on the use of mesenchymal stem cells (MSCs) to reduce scar formation. MSCs have shown remarkable regenerative properties in other tissues of the body and are therefore great therapeutic candidates to aid in VF regeneration.33–37
The importance of bioreactors for VF tissue engineering
Progress in cell therapy research, biomaterials, and tissue engineering has driven major advances in the field of regenerative medicine. Many of these advancements, however, have been accompanied by low therapeutic efficacy.38 One way to increase therapeutic efficacy is to simulate the properties of engineered tissues during preclinical trials in vitro. However, recapitulating the structure, composition, and metabolism of the VFs in vitro is particularly challenging as these factors are altered through mechanical stimulation over time.
Many unanswered questions in the field of VF ontogeny and regeneration would benefit from controlled mechanical stimulation in a VF bioreactor. For instance, there is an ongoing debate in the literature on how different structures of the VF develop throughout life. For example, while most studies propose that the vocal ligament develops after birth with the aid of phonation,39–41 a study published in 200942 suggests that the presence of the vocal ligament at birth may be associated with an individual's genetic profile.42 Although there is consensus that the LP is present at birth as a uniform nonstratified monolayer,39,41,43,44 it is not yet known how the newborn is able to cry at near-maximum levels of pitch and loudness without the presence of a fully formed LP.39 One interesting study aimed to initiate debate in this area, suggesting that the abundance of HA in the newborn VF may facilitate the production of fundamental frequencies ranging from 400 to 600 Hz over extended periods of time without resulting in inflammation or cellular lesions on the site.39 Current trends in the literature suggest that the development of the microstructure of the LP throughout life is heavily dependent on maturity and the development of phonation and speech.39,41,45 Studies have shown that the LP starts to differentiate into a bilaminar structure at the age of 2 months and begins to differentiate into a three-layered structure at 11 months, forming three distinct layers by age 7, which undergo interstitial remodeling between ages 11 and 12, and consolidate into a mature, fully formed complex structure through ages 13 and 17.41,46 This process is not hormonally driven as individuals who are nonphonated from birth or unphonated later in life present hypoplastic VFs without defined VF ligaments and atrophied LP layers, and even the absence of Reinke's space at a mature age.43 This implies that VF fibroblasts require constant biomechanical stimulation to fully develop the LP and maintain tissue homeostasis.16
At the cellular level, it has been suggested that oscillatory stresses can disrupt intracellular adhesions and cellular structures activating mechanotransduction signaling pathways in fibroblasts.47,48 In the context of VF engineering, phonatory forces can stimulate proliferation and repair or, depending on the intensity and duration, induce a cell-mediated inflammatory response, which may lead to the formation of a VF lesion.16
Several studies have shown that oscillatory regimes play a major role in the fibrotic response of VF tissue constructs in vitro by altering the secretion of cytokines, ECM protein concentration, and stiffness.37,47,49,50 Significant changes in matrix and matrix-related gene expression can also be seen in engineered VF tissues upon exposure to oscillatory motion at different amplitudes and frequencies.9,34,36,37,47,49–56 ECM remodeling cytokines and HA are also found in greater concentration after phonation.43,57,58
Animal studies have shown that experimentally induced phonation significantly upregulates messenger RNA (mRNA) expression of the proinflammatory cytokines interleukin (IL)-1β57,59 and transforming growth factor (TGF)-β1,57 as well as the enzymes cyclooxygenase (COX)-257 and matrix metalloproteinase (MMP)-1.59 In agreement with these results, a human subject study showed a significant increase in the secretion of proinflammatory cytokines IL-1β, tumor necrosis factor (TNF)-α, and MMP-8 after 1 h of continuous high-amplitude phonation.60 Acute edemas and other non-neoplastic lesions have also been linked to episodes of loud phonation and attributed to vasodilation followed by capillary rupture and blood plasma infiltration in a process accompanied by inflammatory cytokine release.60,61
Conversely, it has been demonstrated that low impact phonation at the “resonant voice” frequency range has an anti-inflammatory and pro-healing effect with significant decrease in levels of the proinflammatory cytokines IL-1β, IL-6, and MMP-8, followed by an increase in the anti-inflammatory cytokine IL-10 after 24 h.62 Resonant voice frequencies are usually produced through prolonged phonation of /m/, /n/, “ng”, and /j/ focusing on the production of anterior oral vibrations toward the frontal portion of the larynx.62 In agreement with other studies, it has been demonstrated that low magnitude cyclic tensile strain suppresses the upregulation of proinflammatory genes in a magnitude-dependent manner, while promoting collagen type I synthesis in the presence of IL-1β.54
Given the significant effects of the oscillatory environment on the development and maintenance of VF tissues, in vitro systems for VF engineering must be equipped to produce accurate mechanical stimuli, while maintaining cell viability. For this purpose, various bioreactors have been developed. In this review, several bioreactors have been categorized based on the source of vibration into loudspeaker-based (Speaker and Actuator-Based Bioreactors section) (Table 1), actuator-based (Speaker and Actuator-Based Bioreactors section) (Table 2), rheometer-based (Rheometer-Based Bioreactors section) (Table 3), and other (Airflow-Based Bioreactors and Vacuum-Based Bioreactors sections) (Table 4) bioreactors for VF tissue engineering.
Table 1.
Speaker-Based Bioreactors
| References | Frequency | Displacement | Characterization methods | Forces acting in the system | Cell culture conditions |
Exposure time | Cell viability | ||
|---|---|---|---|---|---|---|---|---|---|
| 2D or 3D | Cell types | Biomaterials | |||||||
| T.1.A (Zerdoum et al.49) | 200 Hz | 74 ± 2 μm (mid-membrane); 47 ± 3.6 μm (within gel) | LDV; Digital Image Correlation (Correlated Solutions); FEA (ANSYS software) | Tensile by vibration | 3D | Bone marrow-derived hMSCs | HPC gels (HA, PEG, and collagen) | Pilot studies of 5, 15, 30, 60, and 120 min; static 7-day preculture; 1 h on/1 h off for 12 h/day for 3 days | 81.0% ± 2.1% |
| T.1.B (Kirsch et al.56) | Linear chirp; 50-250-50 Hz | Not reported | LDV | Tensile by vibration | 2D | Immortalized hVFFs | Pronectin-coated silicone base (BioFlex) | Static 1-day preculture; 8 h off, 16 h of 1 min on/1 min off for total of 2 days | No difference compared to static controls |
| T.1.C (Tong et al.34) | 200 Hz | 40 μm | Not reported | Tensile | 3D | Bone marrow-derived hMSCs | PCL scaffolds | 3-Day static preculture; 1 h on/1 h off for 12 h/day for 3 days, followed by 3 days off | No significant difference compared to static controls |
| T.1.D (Zerdoum et al.85) | 100–300 Hz | 40 μm (mid-membrane) | LDV | Tensile | 3D | Bone marrow-derived hMSCs | PCL scaffolds and fibronectin | 3-Day static culture; 1 h on/1 h off vibration at 200 Hz for 12 h/day for up to 7 days | No loss in viability |
| T.1.E (Tong et al.36) | 100–300 Hz | 40 μm (mid-membrane) | LDV | Tensile | 3D | Bone marrow-derived hMSCs | Fibronectin-soaked PCL scaffolds inserted into silicone disk | 3-Day static preculture; CT culture: 12 h/day for 7 days; OF culture: 1 h on/1 h off for 12 h for 7 days | No significant difference compared to static controls |
| T.1.F (Farran et al.55) | 60–300 Hz | 1–30 μm | LDV; tensile measurements of silicone membranes using a Rheometrics mechanical analyzer | Tensile; compressive | 2D | Primary human NFFs | Collagen-coated silicone membranes | 1-Day static preculture; 1 h followed by 6 h rest | Not reported |
CT, continuous; 2D, two dimensional; 3D, three dimensional; FEA, finite element analysis; HA, hyaluronic acid; hMSCs, human mesenchymal stem cells; hVFF, human vocal fold fibroblasts; LDV, laser doppler vibrometry; NFFs, neonatal foreskin fibroblasts; OF, on-off; PCL, poly(ɛ-caprolactone); PEG, poly(ethylene glycol).
Table 2.
Actuator-Based Bioreactors
| References | Frequency | Displacement | Characterization methods | Forces acting in the system | Cell culture conditions |
Exposure time | Cell viability | ||
|---|---|---|---|---|---|---|---|---|---|
| 2D or 3D | Cell types | Biomaterials | |||||||
| T.2.A (Bartlett et al.35) | 200 Hz | 20% Longitudinal tensile strain | Not reported | Tensile by vibration; strain and compression through mechanical actuation | 3D | Primary hVFFs (monoculture); human BM-MSC (monoculture); human AT-MSC (monoculture) | Fibronectin-soaked polyether polyurethane scaffolds | 2-Day static preculture; 24 h: 12 h at 200 Hz and 20% tensile stress every third minute, 12-h rest | Not reported |
| T.2.B (Kim et al.52) | 205 Hz | 19.14 μm at the center; 47.1 μm at 9 mm from center (from Kim et al.53) | Bioreactor characterized in Kim et al.53 | Tensile | 2D | hVFFs (monoculture); hMFSCs (monoculture) | BioFlex culture plates | Precultured until confluence (static); 2 s on/2 s off for 4 h | hVFFs: no significant difference; hMFSCs: increased viability (p < 0.05) |
| T.2.C (Kim et al.53) | 205 Hz | 19.14 μm at the center; 47.1 μm at 9 mm from center | LDV | Tensile | 2D | hVFFs | Type I collagen-coated BioFlex culture plates | Precultured until confluence (static); 2, 6, or 10 h; 6-h rest | Increased proliferation at 10 h compared to 2 and 6 h of vibration |
| T.2.D (Bartlett et al.33) | 200 Hz | 20% Longitudinal tensile strain | Not reported | Tensile by vibration; strain and compression by mechanical actuation | 3D | Primary hVFFs (monoculture); human BM-MSC (monoculture); human AT-MSC (monoculture) | Fibronectin-soaked polyether polyurethane scaffolds | 2-Day static preculture; 24 h: 12 h at 200 Hz and 20% tensile stress every third minute, 12-h rest | Proliferation or apoptosis did not differ by cell type or mechanical condition |
| T.2.E (Gaston et al.37) | 200 Hz | 20% Strain | Not reported | Tensile; impact | 3D | Immortalized hVFFs (monoculture); BM-MSC (monoculture) | Fibronectin-soaked Tecoflex strips | Precultured statically until cells attached to the scaffold; 8-h exposure | 96% Viability for both hVFFs and BM-MSC |
| T.2.F (Kutty and Webb51) | 100 Hz | 1 mm | Digital stroboscope | Tensile by vibration | 3D | NHDF | Methacrylated hyaluronic acid (GMHA) hydrogels cross-linked to Tecoflex films mixed with acrylate-PEG-GRGDS (fibronectin-derived cell adhesion peptide) | Overnight static preculture; 2 s on/2 s off for 4 h/day for 1, 3, 5, and 10 days | <60% Viability |
| T.2.G (Wolchok et al.47) | 100–200 Hz | 0.9 mm at 100 Hz for single well; <0.25 mm for six-well plate | Fluorescent microspheres attached to substrates (displacement calculated using an image analysis software) | Tensile by vibration | 3D | Human laryngeal fibroblasts | Fibronectin-soaked Tecoflex substrates | 2-Day static preculture; 14 min over 6-h period (15-s vibration followed by 30-s rest) followed by 18-h rest; 1-s vibration followed by 2-s rest for 6 h followed by 18-h rest; exposure time varied from 1 to 21 days | Not reported |
| T.2.H (Titze et al.50) | 20–200 Hz | 1 mm | Stroboscopic measurements at 1 Hz offset frequency | Tensile | 3D | Human laryngeal fibroblasts | Porous substrates (Tecoflex and fibronectin) | 3-Day static culture; 6 h of continuous 100 Hz vibration at 20% strain; 100 Hz at 20% strain for 6 h/day for 7 days | >95% Viable; second condition: regions of cell death present |
AT-MSC, adipose-derived mesenchymal stromal cells; BM-MSC, bone marrow-derived mesenchymal stromal cells; hMFSCs, human macula flava stellate cells; NHDF, normal human dermal fibroblasts.
Table 3.
Rheometer-Based Bioreactors
| References | Frequency | Displacement | Characterization methods | Forces acting in the system | Cell culture conditions |
Exposure time | Cell viability | ||
|---|---|---|---|---|---|---|---|---|---|
| 2D or 3D | Cell types | Biomaterials | |||||||
| T.3.A (Titze et al.83) | 100 Hz | 1 kPa vibrational stress; 2 kPa oscillatory stress | Real-time feedback by rheometer software (Malvern Instruments) | Oscillatory stresses; shear stresses | 2D | hVFF | Fibronectin-treated glass coverslips | Static overnight incubation postseeding; stress regimes: 10 s on/10 s off for 2 h | No quantitative data reported; qualitative observations report low adhesion for substrates seeded with 1000 cells/mm2, medium adhesion for ∼2500 cells/mm2, and patchy adhesion for 5000 cells/mm2 |
| T.3.B (Klemuk et al.78) | Up to 150 Hz; tested: 0.1, 1, 10, 35, 43, 67, and 70 Hz | Not reported | Real-time feedback by rheometer software (Malvern Instruments) | Oscillatory stresses; shear stresses | 3D | Human tracheal scar fibroblasts (T31) | Fibronectin-soaked Tecoflex substrates | 2-Week static culture until cells were elongated across the scaffold pores; 25% duty ratio (45 s on and 135 s off) for 2 h; 75% duty ratio (45 s off and 135 s on) for 2 h | Viability was not affected by vibration conditions |
Table 4.
Other Bioreactors for Vocal Fold Tissue Engineering
| References | Frequency | Displacement | Characterization methods | Forces acting in the system | Cell culture conditions |
Exposure time | Cell viability | ||
|---|---|---|---|---|---|---|---|---|---|
| 2D or 3D | Cell types | Biomaterials | |||||||
| T.4.A (Latifi et al.9)a | ∼100 Hz | Subglottal pressure of 14.9 cmH2O | Computational models (SolidWorks and ADINA software); mechanical longevity test; fatigue test; biochemical stability test; rheometry; static subglottal pressure, dynamic subglottal and supraglottal pressure measurements | Tensile; compressive; aerodynamic; impact; inertial; shear | 3D | hVFF | VF replicas: silicone rubber (Dragon Skin and Ecoflex) containing hVFF, HA, gelatin, and PEG cross-linker | 3-Day static preculture; 2 h/day for 4 days | 91.3% ± 2.4% |
| T.4.B (Branski et al.54)b | 0.005, 0.05, and 0.5 Hz | 3%, 6%, 9%, and 18% cyclic tensile strain | Not reported | Tensile | 2D | rVFF | Collagen type I-coated Bioflex II plates | 4–5 Days static preculture; 4, 24, 36, and 48 h | >99% Cell viability |
Airflow-based bioreactor.
Computer-assisted cyclic vacuum controller-based bioreactor.
rVFF, rabbit vocal fold fibroblasts; VF, vocal fold.
Mimicking the Mechanical Environment of the VFs
VF biomechanics
In 1988, Titze63 demonstrated that the self-sustained oscillation of the VFs was made possible through the propagation of mucosal waves along the LP. More specifically, the interaction between the distinct biomechanical properties of each of the LP layers allows the freedom of motion necessary for self-sustained oscillation.64 Therefore, the protein composition of each LP layer serves a specific purpose in the propagation of the mucosal wave; for instance, fibrous proteins such as collagen and elastin are responsible for maintaining the shape of the VFs under stress and strain during phonation, whereas interstitial proteins control water content, viscosity, and tissue size, as well as the size and density of the collagen fibers.64
Although the biomechanical properties of the VFs are largely determined by the ECM composition of the LP, properties such as viscosity, elasticity, pliability, rigidity, and stiffness can be altered upon changes in VF tension and length during phonation.18 The anisotropic behavior of the VFs has been verified through stress-strain experiments in which VFs show a nonlinear response during phonation; this nonlinear behavior can be attributed to the mix of collagen and elastin fibers gradually engaging in motion in the anteroposterior and transverse directions of the VFs in a process that promotes changes in VF stiffness and tension.65
As a result, therapeutic agents for VF regeneration must incorporate similar properties that sustain this anisotropic, nonlinear behavior, while resisting the dynamic oscillatory environment of the VFs.
Phonation
To date, the most widely accepted theory of phonation is the myoelastic-aerodynamic theory proposed by van den Berg66 in 1958, in which he postulated that the VFs are actuated by a stream of air delivered by the lungs and trachea.
Physiologically, phonation is the result of highly specialized neuromuscular coordination between the respiratory and laryngeal muscles67 in a concerted effort to convert the kinetic energy of the subglottic Bernoulli pressure68 into self-sustained VF oscillation, thereby modulating glottal airflow into acoustic resonance. As a result, the mechano-environment of the VFs is subject to complex fluid-structure-acoustic interactions between the VFs, the glottis, laryngeal muscles, and air being expelled out of the lungs into the upper airways and the vocal tract.65 More specifically, the VFs are subject to four major mechanical stressors: laryngeal shortening/lengthening, self-sustained oscillation, lateral collision, and air pressure forces in the form of subglottal pressure.
Anatomically, the VFs are located directly above the cricoid cartilage and, within the larynx, are anteriorly attached to the thyroid cartilage and posteriorly attached to the anterolateral surface of the arytenoid cartilages.65 The laryngeal muscles engaged in phonation interact with the cricothyroid and cricoarytenoid joints, as well as with the cricoid cartilage, changing the geometry, position, and mechanical properties of the VFs.65 More specifically, the thyroarytenoid and the cricothyroid muscles regulate VF tension by shortening or lengthening the VFs in a process that alters their tension and stiffness.69 In this context, tension and stiffness are not to be confused as tension refers to the state of physical elongation of the VFs, whereas stiffness is a parameter derived from the VF's biomechanical structure. Even though stiffness is often referred to as an intrinsic property of the VFs, when under mechanical stress, the nonlinear structure of the VF promotes changes to the VF's biomechanical structure,18 thereby changing its stiffness through anisotropic and viscoelastic behaviors.65
As the velocity of the subglottal air stream increases as it passes through the glottis, pressure decreases in the vicinity of the LPs, resulting in net energy transfer from the airflow to the VFs.70 Self-sustained oscillation of the VFs is achieved as they alternate between convergent and divergent conformations in the absence of damping. Damping occurs at the end of a vocal emission as the VFs abduct and cease sound production.71
Another important factor in phonation is VF size. VFs vary in size depending on age and gender, typically measuring between 11 and 15 mm lengthwise in adult women and 17 and 21 mm in adult men.65 As a result, the VFs can be flexible enough to permit oscillatory movements ranging from 50 to 8000 Hz2 at amplitudes of 0.1–1 mm50 for varying periods of time. More commonly, however, the VFs vibrate at frequencies ranging from 100 to 1000 Hz.72
Bioreactors for VF Tissue Engineering
Bioreactors are designed to act as a controlled environment for cells and tissues to develop in vitro by providing favorable conditions, such as optimal temperature, pressure, substrate or scaffold support, regulatory biochemical signals, and physicochemical stimulation.73 Reliable in vitro models or bioreactors for VF tissue engineering must incorporate precise control of four main variables: frequency, amplitude, programmable oscillatory regimes, as well as a suitable environment for prolonged cell viability.50 In addition to being biocompatible, the materials used in these systems must resist on-off stress regimes and frictional energy dissipation.50
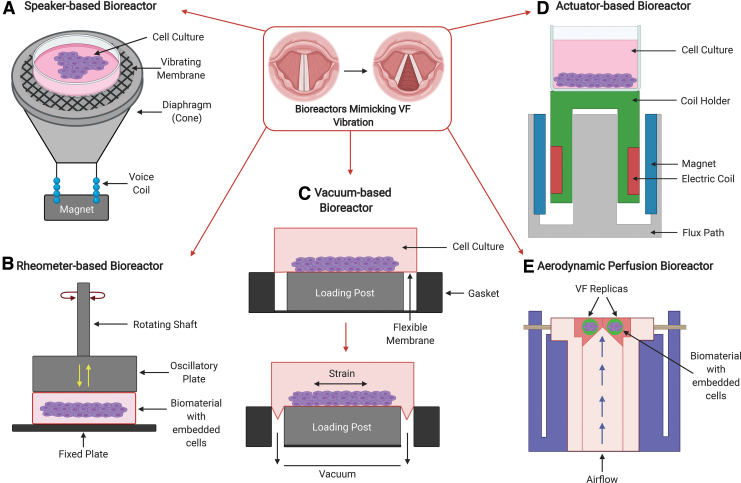
In this review, we grouped bioreactors according to working principles described by the original authors. Table 1 lists bioreactors that were described as speaker-based bioreactors, even though, in principle, these are equipped with voice coil actuators to generate oscillatory motion. Table 2 lists actuator-based bioreactors, as they were referred to in the original articles. Table 3 presents a compilation of rheometer-based bioreactors that explore in-plane and out-of-plane shear forces through mechanical and oscillatory stretching, as shown in Figure 2. Finally, Table 4 lists two very distinct principles that rely on air flow perfusion9 and on vacuum generated cyclic tensile strain,54 respectively. Figure 2 presents a schematic of the bioreactor principles listed on Tables 1–4 with an emphasis on the types of forces exerted on the scaffolds or substrates in each bioreactor.
FIG. 2.
Schematic of the working principle and forces acting in each type of bioreactor. (A) Speaker-based bioreactor principle in which tensile forces are transmitted to the substrate or scaffold by sound waves travelling through the air (Table 1). (B) Rheometer-based bioreactor principle in which in-plane stress and strain are applied through rotation around the axis of a piston, which also applies out-of-plane oscillatory forces (Table 3). (C) Vacuum-based or cyclical tensile strain bioreactor principle (adapted from Branski et al.54 and www.flexcellint.com/product/transwell-holder) in which a flexible substrate is stretched in all directions with the aid of a vacuum applied beneath the flexible substrate (T.4.B in Table 4). (D) Actuator-based bioreactor principle in which the tensile forces are transmitted directly onto the substrate or scaffold through direct contact (Table 2). (E) Aerodynamic perfusion bioreactor principle (adapted from Latifi et al. 2016) in which the pressurized airflow displaces the substrate or scaffold in the direction of the airflow following the same principle of the myoelastic-aerodynamic theory in which the VFs are actuated by the subglottal airflow (T.4.A in Table 4). VFs, vocal folds. Color images are available online.
Commonly used biomaterials in VF bioreactors
Poly(ɛ-caprolactone) (PCL) is a Food and Drug Administration (FDA)-approved polymer that has been widely used in the biomedical field due to its biocompatibility, biodegradability, and superior mechanical properties. In the context of VF tissue engineering, PCL has been used as a porous/fibrous scaffold in bioreactors to recapitulate the structure of the VF ligament, as well as an injectable material in the form of microspheres in solution for in vivo therapies. One limitation is that PCL is relatively hydrophobic and, as a result, a coating with collagen, fibronectin, fibrin, gelatin, or growth factors is often needed to promote better cell adhesion and proliferation.3,36,74
Another commonly used substrate for VF bioreactors is the commercially available BioFlex Culture Plate, a flexible-bottom cell culture plate made of a clear, rubber-like membrane. The plates can be further coated with ECM proteins such as collagen, laminin, elastin, and fibronectin.75 Tecoflex SG-80A, a commercially available aliphatic polyether-based thermoplastic polyurethane, is also used as a scaffold for VF bioreactors.76 This nonbiodegradable, medical grade polymer is biocompatible and has been formulated for solution processing. As a polyurethane, Tecoflex has a copolymer backbone comprising of hard and soft segments, imparting elastomeric properties that typically have high tensile and tear strengths with long elongation capabilities.77 Tecoflex can be solvent casted into a film or porous scaffold with similar properties to the VF ligament and has been used in actuator and rheometer type bioreactors.37,47,50,51,77,78 Similar to PCL, Tecoflex is not amenable to cell attachment; however, it is slightly hydrophobic allowing for adsorption of proteins like fibronectin.77
Fibronectin is one of the most popular and well-characterized biomaterials used to coat scaffolds and substrates in VF bioreactors. Fibronectin's important role in wound healing79 and ability to bind to other ECM proteins, such as collagen, fibrin, and glycosaminoglycans such as heparin sulfate and HA, makes it the ideal candidate for experimentation in vitro.80 For further information, comprehensive reviews of biomaterials for VF tissue engineering can be found in Li et al.3 and Wrona et al.17
Speaker- and actuator-based bioreactors
The most popular type of VF bioreactor in the scientific literature is the speaker- or actuator-based bioreactor. Commonly used in cone and flat panel loudspeakers, actuators convert electrical signals into mechanical motion.81 In a speaker, an electronic circuit digitally controls the current passing through a metallic coil that is connected to a vibrating membrane.81 The current passing through the metallic coil (also known as a voice coil) generates a magnetic field that interacts with a fixed magnet; the amount of current passing through the coil determines the strength of the magnetic field and its physical position relative to the magnet following the principle of an electric motor and, as a result, the membrane attached to the voice coil oscillates and compresses the air generating sound waves that are proportional to the electrical signal.82
This level of digital control allows researchers to accurately program oscillatory routines through hardware-software integration for defined periods of time. In addition, actuator-based bioreactors can be engineered to accommodate modular experimental setups onto which customized cell culture plates can be fitted for multiple replicates. As depicted in Figure 2, most bioreactors described to date use electromagnetic voice coil actuators to mechanically drive oscillation through oscillating air pressure in the form of sound waves (Fig. 2A) or through hard contact (Fig. 2D).55 Speaker-based bioreactors shown on Table 1 are trending in the literature as these are commercially available, modular, and oftentimes waterproof, and can be run in parallel. Most importantly, they offer programmable routines featuring a wide range of frequencies and amplitudes that can be remotely controlled by Bluetooth or other forms of electronic communication. The main limitation of using speaker-based bioreactors lies in the fact that, depending on the bioreactor design, there may be energy losses in the form of sound waves to the environment. In this regard, actuator-based bioreactors offer an advantage through hard contact with the bioreactor scaffold or substrate. Overall, these two types of bioreactor offer a versatile and easy-to-use option for VF tissue engineering.
Rheometer-based bioreactors
Rheometer-based bioreactors capable of applying lateral sheer and out-of-plane oscillatory forces (Fig. 2B) are also featured in the literature; however, these are less common. Table 3 lists two relatively recent studies that use rheometer-based bioreactors78,83; however, these cover a narrower frequency range (<150 Hz) when compared to speaker- and actuator-based bioreactors (<300 Hz). Rheometer platforms tend to be more complex than other types of bioreactors as they rely on heavy instrumentation to guarantee instrumental precision during rotation. This imposes limitations in terms of modularity and size when compared to speaker- and actuator-based bioreactors.
Airflow-based bioreactors
One of the most complex bioreactors incorporating aerodynamic perfusion was proposed by Latifi et al. in 201484 and more extensively characterized in 2016.9 This unique study, listed on T.4.A in Table 4, shows that it is possible to incorporate airflow into cell culture to simulate the subglottic pressure and sustain self-oscillation of VF replicas (Fig. 2E). The verisimilitude of this bioreactor with the anatomy of the larynx is highly attractive as a larger number of variables can be incorporated into this design. Such a sophisticated bioreactor design has theoretical advantages over other bioreactor types in terms of types of forces applied to the VF replicas; however, the complexity of its design, fabrication, operation, and maintenance may result in high variability across experimental units.
Vacuum-based bioreactors
Vacuum-based bioreactors are another option for VF engineering as they offer a stretchable and bendable membrane that can be precisely controlled (Fig. 2C). As listed on T.4.B in Table 4, vacuum-based bioreactor studies are rare and tend to operate at lower frequencies (<1 Hz). Although these bioreactors may be available commercially, their operation relies on metered vacuum for precision, which may translate into higher cost and size when compared with other bioreactors.
Parameter considerations
Figure 2 outlines how different forces are exerted on the substrate or scaffold through each method. In effect, the different principles illustrated in Figure 2 can produce very different overall force propagation on the substrate or scaffold as they act through different propagation media and vectors. This has significant implications on the biomechanics of the cells and engineered tissues tested in each bioreactor as the anisotropic behavior of the VF tissue is highly dependent on the magnitude and direction of forces applied to the cells and the ECM. It can be argued that none of the described bioreactors are able to fully recreate all the forces observed in vivo as the oscillatory motion of the LP is the result of a multifactorial set of variables that include the nature and direction of the applied forces, propagation media, structure, anatomy, size, thickness, and stretch of the VFs. As a result, bioreactors that incorporate a maximum number of controlled variables are the most promising to study VF disease, healing, and injury in vitro.
However, depending on the parameter being investigated, it is possible that certain types of bioreactors may suffice to provide clues on how cells and synthetic biomaterials behave under different regimes of vibration. In this sense, VF bioreactors provide a valuable approximation of the VF mechano-environment, permitting the evaluation of a series of biomaterials, and the viability of cellular cultures and co-cultures. At the very least, the structural integrity of novel biomaterials must be tested to survive the vibratory environment of the VFs before clinical translation. The very nature of the VFs calls for dynamic VF tissue engineering methods, regardless of their ability to simulate all forces exerted on the VFs in vivo. In addition, the ability to test many experimental units in parallel in a bioreactor is vastly advantageous to evaluate multiple variables at the same time.
In all bioreactors, frequency and displacement were controlled remotely either through an electric switch or computer software. The great majority of bioreactors were characterized using a Laser Doppler vibrometer to evaluate the accuracy of the frequency and displacement being transmitted from the bioreactor core to the scaffold or flexible substrate. Other characterization techniques involved stroboscopic photography, fluorescent particle tracking using a high-speed camera and built-in instrument software. Most articles characterized and described the physical forces acting on the scaffold or flexible substrates as tensile, compressive, aerodynamic, impact, inertial, or shear; mechanical forces exerted on single cells were not reported.
When engineering a bioreactor, one of the most important aspects to consider is the biocompatibility of the materials used. In addition, it is important to establish whether the bioreactor will incorporate fluid flow, gas exchange, and a sterile platform for three-dimensional (3D) or two-dimensional (2D) cell culture. Although 2D and 3D studies seem to be equally prevalent in the literature, 3D cell culture models were the most popular models used in actuator- and speaker-based bioreactors (Tables 1 and 2). The most popular cell types investigated in all types of bioreactors were the human bone marrow-derived mesenchymal stem cells (hBM-MSCs) and a variety of fibroblasts. Among all choices of biocompatible substrates for 3D cell culture, fibronectin-coated Tecoflex was the preferred choice featuring in almost all types of bioreactors, followed by the biodegradable polymers PCL and HPC gels (HA, PEG, and collagen) as flexible scaffolds. The most popular choices of substrate for 2D cell culture were the commercially available Bioflex and Bioflex II flexible culture plates.
2D and 3D cell cultures were exposed to varying oscillatory regimes in all types of bioreactors. Exposure regimes ranged from as little as a total of 5 min a day to as much as 12 h a day. The reported length of the studies varied from 1 to 21 days. Most studies evaluated cell viability of oscillatory versus static cultures at the phenotypic and genotypic levels. More specifically, many studies focused on measuring the gene expression of ECM-related genes to evaluate the effects of oscillatory regimes on ECM-related protein production and remodeling.
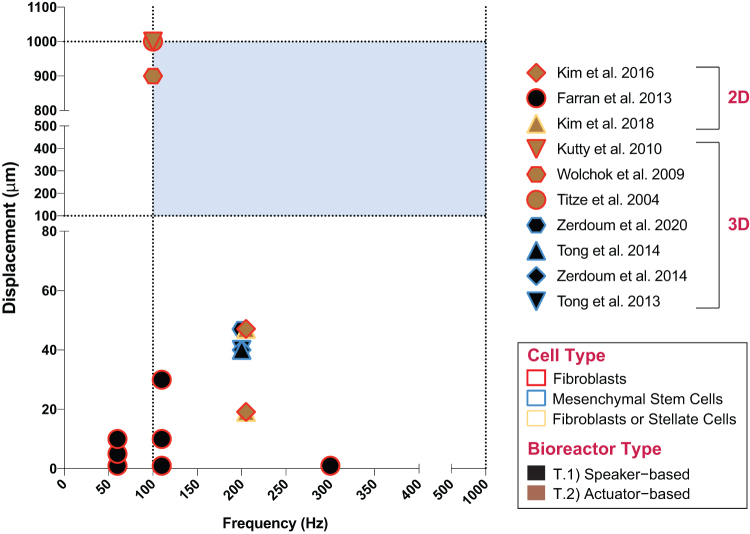
Figure 3 shows that most studies overlap with regard to frequency and displacement as it is advantageous to reproduce previously cited parameters for comparison and validation. However, only three studies (T.2.F–H)47,50,51 portrayed in Figure 3 utilize parameters that are congruent with the common frequency and amplitude range of human phonation (blue region in the graph). Interestingly, although the majority of the studies fall within the most common frequency range of human phonation (100–1000 Hz),72 most of the listed bioreactors operate at amplitudes or out-of-plane displacements below 60 μm (T.1.A, C, F and T.2.B, C),34,49,52,53,55 with the exception of three studies that report displacements around 1000 μm (T.2.F–H).47,50,51 It is important to highlight that most bioreactors described in the literature to date do not reproduce VF tissue stiffness or anysotropy,85 nor do they incorporate more specialized features capable of simulating the bilateral collision84,85 of the VFs during phonation, with the exception of attempts by Latifi et al. (T.4.A in Table 4).9
FIG. 3.
Cell culture bioreactor parameters. The blue region in the graph marks the regions of interest for common human phonation with frequencies ranging between 100 and 1000 Hz at amplitudes or displacements between 100 and 1000 μm. (Graph only includes studies that reported both frequency [Hz] and displacement [μm] values. Studies that reported displacement in different units are not included. Graph only includes values used for cell studies.) Color images are available online.
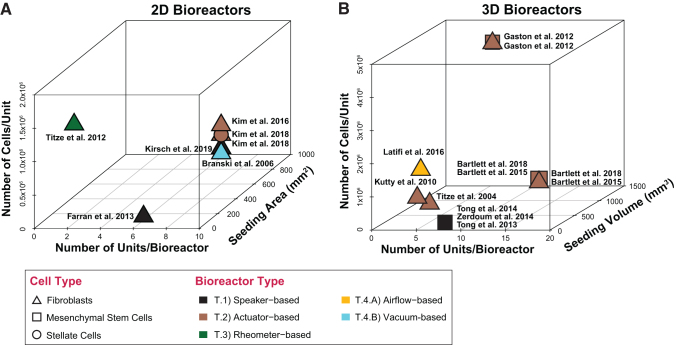
Bioreactor scalability
Bioreactor capacity is a function of sample size and the total number of experimental units. To maximize experimental reproducibility and reduce cost, experimental bioreactors are usually equipped to process small sample sizes and a limited number of experimental units. Depending on the types of cells and biomaterials involved, it is many times cost prohibitive to run large samples during the early phases of research and development.
In the case of the VF bioreactors reported in the literature, we note that most bioreactors use less than two million cells per experimental unit, with an average number of 10 experimental units per bioreactor as shown in Figure 4. One study by Klemuk et al. is an exception to this rule with 16 million cells per experimental unit; however, the authors do not report the total number of units in their bioreactor (T.3.B).78 Conversely, studies that report the use of very low numbers of cells per experimental unit tend to accommodate a statistically significant number of experimental units in their bioreactors.
FIG. 4.
Bioreactor scalability. (A) XYZ scatter plot of two-dimensional bioreactors (X, number of units/bioreactor; Y, seeding area [mm2]; and Z, total number of cells/unit). (B) XYZ scatter plot of three-dimensional bioreactors (X, number of units/bioreactor; Y, seeding volume [mm3]; and Z, total number of cells/unit). (Graph only includes studies that reported values for all parameters on the XYZ scatter plot.) Color images are available online.
The choice of sample size may be important depending on the application of the bioreactor. Larger sample sizes may enable the development of implantable scaffolds or substrates for clinical translation, whereas smaller sample sizes may enable rapid multifactorial screening for the development of new drugs and therapeutics in samples that can be tailored for patient use.
Scalability is an important factor to consider when engineering multiunit VF bioreactors as experimental units may be designed to simultaneously oscillate at precise frequencies and amplitudes. This has important implications for the choice of bioreactor material, design, and engineering, as well as for experimental reproducibility and validity.
Influence on Cellular Behavior
It is a well-known fact that the upregulation of proinflammatory markers correlates with suboptimal healing outcomes at the tissue and organ levels.86–88 Some of the proinflammatory markers known to interfere with the healing process include cytokines such as IL-1β, TNF-α, prostaglandin E2 (PGE2), and MMPs.89,90 In addition to inflammation, the upregulation of profibrotic markers such as collagen type I, α smooth muscle actin, and biglycan also hinders optimal healing outcomes.91
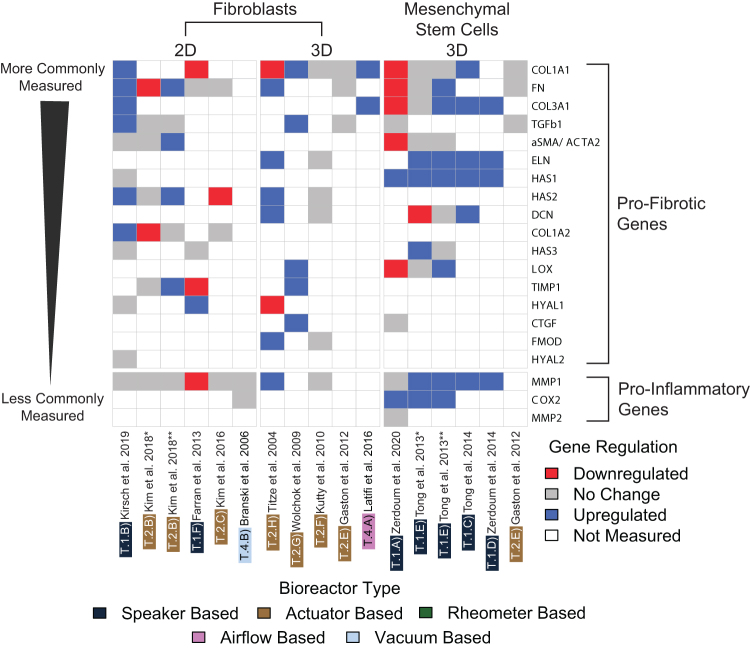
Figure 5 shows the expression profiles of proinflammatory and profibrotic genes upon exposure to different oscillatory regimes and forces in VF bioreactors. Proinflammatory genes reported in the VF bioreactor literature included COX2, MMP-1, and MMP-2.
FIG. 5.
Gene expression profiles of profibrotic and proinflammatory genes involved in vocal fold tissue engineering. Heat map showing the most commonly measured genes categorized into profibrotic or proinflammatory. Studies have been categorized according to cell type (fibroblasts or mesenchymal stem cells) and cell culture format (2D or 3D). (*Kim et al. 2018,52 hVFF; **Kim et al. 2018,52 hMFSCs; *Tong et al. 2013,36 continuous oscillatory regime; **Tong et al. 2013,36 intermittent oscillatory regime). 2D, two dimensional; hMFSCs, human macula flava stellate cells; hVFF, human vocal fold fibroblast. Color images are available online.
COX2 expression was upregulated in studies involving MSCs (T.1.A, E* and **)36,49 in different culture formats; however, no change in expression was observed in rabbit VF fibroblasts in 2D culture (T.4.B).54
MMP-1 expression was predominantly upregulated in MSCs; however, this gene displayed contradictory expression in studies involving fibroblasts showing downregulation (T.1.F)55 and upregulation (T.2.H)50 and remaining unchanged (T.1.B; T.2.B*, C, F; T.4.B).51–54,56 MMP-2 expression remained unchanged in MSCs in 3D culture (T.1.A).49
The expression of the profibrotic gene α smooth muscle actin gene, aSMA/ACTA2, was distinct in fibroblasts and MSCs, showing upregulation in human macula flava stellate cells (hMFSCs) (T.2.B**)52 in 2D culture and downregulation in hBM-MSCs in 3D culture (T.1.A).49 aSMA/ACTA2 is one of the key genes involved in fibrosis and its expression remained unchanged in human vocal fold fibroblasts (hVFFs) in 2D culture (T.1.B; T.2.B*),52,56 and in hBM-MSCs in 3D culture (T.1.E* and **).36
Lysyl oxidase (LOX) gene expression showed a mixture of upregulation and downregulation in MSCs at different oscillatory regimes, with downregulation seen in oscillatory regimes at 200 Hz and an out-of-plane amplitude of 47 μm that involved 1 h on/1 h off cycles over a 3-day period (T.1.A).49 Interestingly, LOX was upregulated in a 3D MSC culture on a similar oscillatory regime consisting of 1 h on/1 h off cycles, also at 200 Hz and an out-of-plane amplitude of 40 μm, over a period of 7 days, 12 h a day (T.1.E**).36 Therefore, it appears that the forces applied to the cell culture, as well as the extent and periodicity of exposure to the oscillatory regime play a key role in the LOX gene expression. LOX upregulation was seen in human laryngeal fibroblasts (hLFs) in 3D culture at intermittent oscillatory regimes at 100 Hz (T.2.G).47
The expression of collagen type I alpha-1 chain (COL1A1) was shown to be very distinct under different oscillatory regimes. COL1A1 expression appears to be inextricably linked to oscillatory regimes and cell culture conditions, as this gene showed downregulation (T.1.A, F; T.2.H)49,50,55 and upregulation (T.1.B, C; T.2.G; T.4.A),9,34,47,56 and remained unchanged in studies involving fibroblasts (T.2.E, F)37,51 and MSCs (T.1.E* and **, T.2.E).36,37
Collagen type I alpha-2 chain (COL1A2) expression showed upregulation (T.1.B)56 in hVFFs in 2D culture. One noteworthy study (T.2.B* and **)52 that compared 2D cultures under the same oscillatory regime showed that COL1A2 expression was downregulated in hVFFs, however, remained unchanged in hMFSCs. Collagen type III alpha-1 chain (COL3A1) was mostly evaluated in 3D cultures involving MSCs and in only two studies involving 2D (T.1.B)56 and 3D (T.4.A)9 fibroblast cultures. COL3A1 expression was mostly upregulated (T.1.C, D, E**)34,36,85 in MSCs in 3D culture, except for two studies with very similar oscillatory regimes in which it was downregulated (T.1.A)49 on an intermittent 1 h on/1 h off regime at 200 Hz with an amplitude of 47 ± 3.6 μm over a period of 3 days and remained unchanged (T.1.E*)36 on both continuous and intermittent 12-h regimes at 200 Hz with an amplitude of 40 μm over a period of 7 days. Once again, it appears that the periodicity and overall length of exposure seem to play a strong role on gene expression.
The expression of the connective tissue growth factor (CTGF) gene that encodes the production of CTGF was upregulated (T.2.G)47 in hLFs in 3D culture; however, it remained unchanged (T.1.A)49 in MSCs in 3D culture. The gene responsible for the production of the proteoglycan decorin, DCN, was upregulated in hVFFs (T.2.H)50 and in hBM-MSCs (T.1.C)34 in 3D culture. In one study (T.1.E* and **),36 DCN expression in hBM-MSCs was downregulated and remained unchanged upon exposure to continuous and intermittent oscillatory regimes, respectively, suggesting that the periodicity of the oscillatory regimes plays a major role on DCN expression.
Responsible for regulating the production of the protein elastin, ELN expression was upregulated in all 3D cultures involving MSCs (T.1.C–E),34,36,85 as well as in a 3D culture of hLFs (T.2.H),50 while remaining unchanged in a 3D culture of hDFs (T.2.F).51 Another important modulator of fibrosis regulating the production of the protein fibromodulin, the FMOD gene was upregulated in 3D cultures involving hLFs (T.2.H),50 however, showed no change in expression in 3D cultures with hDFs (T.2.F).51 Fibronectin (FN) expression regulates the production of the high molecular weight glycoprotein fibronectin, a key component of the ECM that binds cells to the ECM through integrin receptors. FN expression in fibroblasts (2D) showed a mixed response of downregulation (T.2.B*),52 upregulation (T.1.B; T.2.B**, H),50,52,56 and unchanged (T.1.F; T.2.C)53,55 expression. Kim et al. 2018 (T.2.B* and **)52 subjected both hVFFs and hMFSCs in 2D culture to the same experimental conditions and observed downregulation and upregulation of the FN gene in these two cell types, respectively. Furthermore, the authors observed that vibrational conditions reduced the stem cell-like properties of hMFSCs, promoting a fibroblast-like phenotype on these cells, concluding that hMFSCs were overall more sensitive to vibration. Tong et al. observed that the periodicity of their oscillatory regime resulted in unchanged and upregulated FN expression on the same cell types (T.1.E* and **).36
The expression of the hyaluronan synthase genes HAS1, HAS2, and HAS3 was also evaluated. HAS1 expression was found to be consistently upregulated in all 3D cultures involving MSCs (T.1.A, C, D, E* and **)34,36,49,85 and remained unchanged in 2D cultures of hVFFs (T.1.B).56 HAS2 expression studies were limited to 2D and 3D fibroblast cultures with very distinct results. Downregulation of HAS2 was only seen in one instance (T.2.C)53 involving hVFFs in 2D culture, with upregulation (T.1.B, T.2.B**, T.2.H)50,52,56 and no change (T.2.B*, T.2.F)52,53 seen in the remaining studies. HAS3 expression followed an interesting trend in a study by Tong et al. in which hBM-MSCs were exposed to the same frequency and amplitude, however, over different oscillatory regimes, resulting in upregulation upon continuous exposure, versus no change during intermittent exposure (T.1.E* and **).36 No change was seen in HAS3 expression in 2D cultures of primary human neonatal foreskin fibroblasts (hNFFs) (T.1.F)55 and hVFFs (T.1.B).56
The HYAL1 and HYAL2 genes that regulate the production of the ECM remodeling enzyme hyaluronidase were also monitored in 2D and 3D fibroblast cultures, with the expression of both genes remaining unchanged in hVFFs (T.1.B).56 HYAL1 was upregulated in hNFFs (T.1.F)55 in 2D culture and in hBM-MSCs (T.1.D)85 in 3D culture, and downregulated in hLFs (T.2.H).50
An important modulator of fibrosis, the gene that regulates the production of the profibrotic cytokine, TGF-β1, was evaluated in all modalities of cell culture. TGF-β1 expression remained unchanged (T.1.A; T.2.E)37,49 in all MSC cell cultures, however, appeared to be either unchanged (T.2.B* and **, E)37,52 or upregulated (T.1.B; T.2.G)47,56 in fibroblast cultures. Gaston et al. (T.2.E)37 compared TGF-β1 expression in hVFFs and hBM-MSCs under the same conditions and both remained unchanged.
Finally, the expression of the TIMP1 gene, which regulates the production of the tissue inhibitor of metallopeptidase 1 glycoprotein, was investigated in fibroblasts in 2D and 3D culture. In the same study (T.2.B** and *),52 TIMP1 expression was both upregulated (hMFSCs) and unchanged (hVFFs) under the same experimental conditions. TIMP1 was downregulated in hNFFs (T.1.F)55 in 2D culture and upregulated in hLFs (T.2.G)47 in 3D culture.
The data shown in Figure 5 suggest that gene expression does not follow a particular trend for any of the genes under the conditions evaluated. Instead, the data suggest that gene expression is the product of multiple variables, including cell type, cell culture format (2D or 3D), and bioreactor size, as well as frequency, amplitude, periodicity, and overall duration of the oscillatory regime. Even though genes such as ELN and HAS1 showed upregulation in most studies involving MSCs, more investigation is needed. As Tong et al. (T.1.E)36 demonstrated, a particular cell type responds differently to different oscillatory regimes. Kim et al. (T.2.B),52 on the other hand, demonstrated that different cell types respond differently to the same oscillatory regime.
While there are no reported ideal gene expression values for VF regeneration in vitro, information on Table 5 may serve as a guide to predict profibrotic or pro-healing outcomes for each gene in the context of VF tissue engineering. Since the VF microenvironment is highly responsive to physicochemical changes, the measurement of gene expression may be a valuable tool to evaluate cell-ECM interactions under static and dynamic conditions and compare tissue response in vitro and in vivo.
Table 5.
Most Studied Proinflammatory and Profibrotic Genes That Play a Significant Role in Vocal Fold Homeostasis
| Marker | Full name | Gene | Function | Expression in healthy tissue | Downregulation | Upregulation |
|---|---|---|---|---|---|---|
| COX2 | Cytochrome c oxidase subunit 2 | MTCO-2 (mitochondrially encoded cytochrome c oxidase II) | Protein coding gene to produce the COX2 enzyme, which converts AA to prostaglandin endoperoxide H293 | Highly restricted94 | Downregulated in the presence of corticosteroids94 | Drastically upregulated during inflammation94 |
| MMP-1 | Matrix metalloproteinase 1 | MMP-1 | Protein coding gene to produce MMP-1 enzyme, which breaks down interstitial collagen type I, II, and III95 | Low expression is required for postnatal development and tissue remodeling96 | Inhibited by TIMPs97 and in the presence of myelodysplastic syndrome98 | Overexpressed during tissue repair95 and considered beneficial in the context of VF regeneration.34 Prolonged overexpression may result in chronic nonhealing wounds.97 Chronologically upregulated on skin (aging)99 |
| MMP-2 | Matrix metalloproteinase 2 | MMP-2 | Protein coding gene to produce gelatinase A, a type IV collagenase that breaks down collagen types IV100 and V,101,102 elastin, and solubilized monomers of collagens I, II, and III,103 and inactivates chemoattractant protein-3104 | Baseline expression is necessary to modulate ECM remodeling, cell growth and migration, inflammation, angiogenesis, and metabolism104 | Inhibited by TIMP2,105 doxycycline,106 statins,104 and oleic acid (18:1 cis-9).107 Proinflammatory if underexpressed as MMP-2 deficiency leads to cardiac and systemic inflammation104 | Highly upregulated in connective tissue diseases.101 Proinflammatory if overexpressed, increasing the deposition of collagen over elastin.108 Chronologically upregulated on skin (aging)99 |
| ACTA2 | Actin alpha 2, smooth muscle | ACTA2 | Gene encoding six distinct globular protein isoforms or actins that assemble into microfilaments. α-smooth muscle actin (α-SMA) is the most studied isoform involved in cellular structural integrity, motility, and intercellular signaling109 | Baseline expression is necessary to maintain vascular health110,111 | Inhibited by (TGF)-β-antagonists.112 Downregulation through antisense mRNA increased cell migration and decreased cell-matrix adhesion113 | Upregulated in the presence of TGF-β1, increasing fibroblast contractile activity,114 and stimulating collagen and ECM production115 |
| LOX | Lysyl oxidase | LOX | Protein coding gene to produce the secretory amine oxidase enzyme LOX, which catalyzes the cross-linking of collagens and elastin116 | Associated with elastin fiber synthesis in infants and collagen fiber synthesis at all ages117 | Downregulated in the presence of β-aminopropionitrile (BAPN)118 | Associated with fibrotic disorders119 and tumor progression120 |
| COL1A1 | Collagen type I alpha 1 chain | COL1A1 | Protein encoding gene for the pro-alpha1 chains of type I collagen, which is a fibril-forming collagen found in most connective tissues and is abundant in bone, cornea, dermis, and tendon121 | Baseline expression is necessary to protect against soft tissue rupture, including tendons and ligaments,122 and to maintain bone density and prevent osteoporosis123 | Downregulation leads to loss of cellular adhesion to ECM components and induces terminal differentiation of keratinocytes.124 Inhibited during mesenchymal stem cell differentiation into chondrocyte-like cells125 | Increase in expression in the presence of TGF-β1.115 Overexpressed in fibrotic tissues126–129 and tumors130,131 |
| COL1A2 | Collagen type I alpha 2 chain | COL1A2 | Protein coding gene for the pro-alpha2 chain of type I collagen found in most connective tissues and is abundant in bone, cornea, dermis, and tendon132 | Baseline expression is necessary to maintain ECM integrity and bone density133 | Downregulated shortly following thermal injury134,135 | Increased expression has been observed during the proliferative phase of wound healing134,136 and linked to tumor suppresion137 |
| COL3A1 | Collagen type III alpha 1 chain | COL3A1 | Protein coding gene for the pro-alpha1 chains of type III collagen, a fibrillar collagen found in stretchy connective tissues frequently in association with type I collagen138 | Baseline expression is essential for collagen fibrilogenesis in many organs139 | Downregulated in the presence of suberoylanilide hydroxamic acid140 and nintedanib141 | Upregulated during the proliferative phase of wound healing,136 profibrotic events,142–144 and tumor proliferation145 |
| CTGF | Cellular communication network factor 2 | CCN2 | Gene that codes the secretion of a matricellular protein that promotes mitosis, also known as connective tissue growth factor, which influences cell adhesion, tissue repair, fibrosis, chondrogenesis, and osteogenesis, and is related to platelet-derived growth factor146,147 | Baseline expression is required for postnatal fibrogenesis,148 ECM organization, cell proliferation, migration, and survival149 | Downregulated during the proliferation and maturation phases of wound healing150 | Upregulated during the inflammatory phase of wound healing150 Overexpression leads to failure to stop tissue repair, leading to pathological scarring in fibrosis and cancer147 |
| DCN | Decorin | DCN | Protein coding gene for the production of decorin, a small leucine-rich proteoglycan that is essential to collagen fibril assembly151 | Baseline expression levels mediate fibrilogenesis, autophagy, inflammation, angiogenesis, and tumorigenesis151 | Downregulated in cancerous tissues152,153 | Upregulation can inhibit fibrosis and scar formation and prevent proliferation and metastasis of tumor cells154 |
| ELN | Elastin | ELN | Protein coding gene to produce elastin, one of the two components of elastic fibers that confers elasticity to the ECM in tissues and organs155 | Baseline expression is required during fetal development and for the maintenance of tissue elasticity156 | Inhibited by basic fibroblast growth factor157 and interleukin-1β.158 Downregulation leads to focal stenosis, hypertension, and vascular stiffness159 and has been linked to aging in mice160, 161 | Upregulated in the tumor microenvironment162 and in fibrotic extensible organs such as in chronic obstructive pulmonary disease163,164 |
| FMOD | Fibromodulin | FMOD | Protein coding gene to produce the small interstitial proteoglycan fibromodulin, which interacts with type I and type II collagen fibrils to inhibit fibrilogenesis165 | Baseline expression levels are essential for muscle development through normal collagen cross-linkage for the maturation of large diameter collagen fibrils166 | Downregulation postpones wound closure by delaying dermal cell migration, impeding angiogenesis, and increasing scar formation167 | Upregulation controls myoblast differentiation168 and enhances angiogenesis during wound healing.167 Overexpression has been associated with scarless wound healing169 and improved tensile strength166 |
| FN | Fibronectin 1 | FN1 | Gene that encodes the production of fibronectin, a glycoprotein that is present on the cell surface and in the ECM and is involved in cell adhesion and migration processes170 | Baseline expression levels are essential for ECM assembly and maintenance171,172 | Downregulation has been associated with the loss of cellular adhesion sites on the ECM173 and reduced vascular remodeling in vivo174 | Upregulation is linked to fibrotic outcomes 175,176 |
| HAS1 | Hyaluronan synthase 1 | HAS1 | Protein coding gene that produces an enzyme that synthesizes hyaluronan or HA found in the ECM. Its functions include joint lubrication, space filling, and enabling cell migration through the ECM177 | Baseline expression is necessary for tissue development and maintenance178 | Downregulation is associated with inflammatory and degenerative arthopathies177 | Upregulation immediately after injury,179 during wound healing and tissue repair to support blood vessels and fibroblasts.177 Associated with scarless healing of the VFs179 |
| HAS2 | Hyaluronan synthase 2 | HAS2 | Protein coding gene that produces an enzyme that synthesizes hyaluronan or HA found in the ECM. Its functions include joint lubrication, space filling, and enabling cell migration through the ECM180 | Baseline expression is essential for embryonic development and necessary for tissue development and maintenance178 with low levels detected in normal VF tissue179 | Downregulation is associated with inflammatory and degenerative arthopathies177 | Highly upregulated immediately after injury179 |
| HAS3 | Hyaluronan synthase 3 | HAS3 | Protein coding gene that produces an enzyme that synthesizes the unbranched hyaluronan or HA found in the ECM and responsible for the regulation of hyaluronan synthesis181 | Baseline expression is necessary for tissue development and maintenance178 | Downregulation immediately after injury179 | Upregulated at chronic/and or later stages of wound healing179 |
| HYAL1 | Hyaluronidase 1 | HYAL1 | Protein coding gene that encodes a lysosomal enzyme, hyaluronidase, which intracellularly degrades HA during cell differentiation, proliferation, and migration182 | Baseline expression is necessary for the development and maintenance of tissue structure182 | Downregulation has been linked with tumor progression183 | Upregulated upon mechanical stimuli184 and in later stages of wound healing179 |
| HYAL2 | Hyaluronidase 2 | HYAL2 | Protein coding gene that encodes a weak acid-active form of the enzyme hyaluronidase, which degrades HA during cell differentiation, proliferation, and migration185 | Baseline expression is necessary for the development and maintenance of tissue structure182 | Downregulation has been linked with tumor progression183 | Upregulated upon mechanical stimuli184 and in later stages of wound healing179 |
| TGF-β1 | Transforming growth factor beta 1 | TGF-β1 | Protein coding gene that encodes a secreted ligand of the TGF-β superfamily of proteins, which bind to SMAD transcription factors to regulate gene expression involved in cell differentiation, growth, proliferation, and activation of other growth factors186 | Low baseline expression in cardiac tissue187 | Inhibited by decorin188 and hyaluronidase,189 both natural TGF-β1 antagonists, as well as antisense oligonucleotides190 and synthetic antagonists.191,192 | Upregulated in fibroproliferative diseases, acute VF injury (acute inflammation), during the neomatrix deposition following injury, as well as in chronic VF lesions193 |
| TIMP1 | Tissue inhibitor of metalloproteinases 1 | TIMP1 | Protein coding gene for the production MMP inhibitors involved in cell proliferation and antiapoptotic function194 | Baseline expression levels are necessary to maintain healthy tissue structure, counter inflammation and apoptosis, and promote cell proliferation195 | Chronologically downregulated during the aging process contributing to matrix degradation and senescence99 | Overexpressed in proliferative cancers196,197 and upregulation has been linked to fibrosis195,198 |
AA, arachidonic acid; COX, cyclooxygenase; CTGF, connective tissue growth factor; ECM, extracellular matrix; LOX, lysyl oxidase; MMP, matrix metalloproteinase; mRNA, messenger RNA; TGF, transforming growth factor.
Conclusions and Future Directions
Bioreactors are essential to replicate the phonatory conditions of the VFs in vitro. Efforts to date have largely focused on the development of actuator- and loudspeaker-based bioreactors, with a small number of rheometer-based and aerodynamic-based bioreactors. Studies have mostly focused on the application of tensile forces to cell culture through a variety of oscillatory regimes involving frequencies ranging from 50 to 300 Hz and out-of-plane displacements of 0.1–1.0 mm. Very few bioreactors have explored shear, inertial, aerodynamic, or compressive forces. Most bioreactors utilize between 2 and 16 million cells per experimental condition and incorporate a modular design featuring 3–15 experimental units. Cell types, biomaterials, and substrates vary in the literature, with fibroblasts or MSCs in 3D culture being the most popular choice on porous scaffolds. Cell viability and gene expression on static versus dynamic cultures are commonly measured parameters. Even though the native VFs are composed of multiple cell types such as fibroblasts, myofibroblasts, macrophages, epithelial cells, and muscle cells, all bioreactors presented in this review were capable of incorporating monocultures only. Future studies should focus on incorporating multiple cell types simultaneously to better recapitulate the cellular composition of the native VFs, enabling drug response studies, disease modeling, and the preconditioning of implantable scaffolds or engineered tissues before transplantation.
Although there seem to be trends in the literature, the diversity of oscillatory regimes, cell types, biomaterials, and cell culture formats preclude direct comparisons between similar studies. Future investigations should aim to standardize bioreactor test parameters such as cell type, 2D or 3D culture conditions, frequency, and amplitude, as well as the periodicity and length of the oscillatory regimes to yield comparable data. Experiments should focus on using precision tools to map the forces acting on the system and quantify in-plane and out-of-plane displacement, stress, strain, and shear, as well measure cellular responses through proteomics and gene expression.
Another promising approach to optimize VF engineering in the future is to introduce biomaterials that contain anti-inflammatory cytokines and/or growth factors to stimulate a prohealing phenotype.20,91 In the case of VF tissue engineering, this strategy can be used synergistically with oscillatory stimulation to upregulate anti-inflammatory and antifibrotic genes in 2D or 3D culture formats.91,92
Acknowledgment
Images on Figures 1 and 2 were created with BioRender.com.
Authors' Contributions
A.M.G.M. contributed with conceptualization, literature search, data analysis, article writing, and editing. A.B. contributed with conceptualization, literature search, data analysis, article writing, and editing. D.S. contributed with conceptualization and data analysis. D.O.F. contributed with conceptualization and article editing.
Disclosure Statement
No competing financial interests exist.
Funding Information
Financial support for this work was provided by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (R01DC017139, R01DC017743).
References
- 1. Miri, A.K. Mechanical characterization of vocal fold tissue: a review study. J Voice 28, 657, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Svec, J.G., and Granqvist, S.. Guidelines for selecting microphones for human voice production research. Am J Speech Lang Pathol 19, 356, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Li, L., Stiadle, J.M., Lau, H.K., et al. Tissue e engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials 108, 91, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long, J.L. Repairing the vibratory vocal fold. Laryngoscope 128, 153, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benninger, M.S., Alessi, D., Archer, S., et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg 115, 474, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Mattei, A., Magalon, J., Bertrand, B., Philandrianos, C., Veran, J., and Giovanni, A.. Cell therapy and vocal fold scarring. Eur Ann Otorhinolaryngol Head Neck Dis 134, 339, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Ling, C., Li, Q., Brown, M.E., et al. Bioengineered vocal fold mucosa for voice restoration. Sci Transl Med 7, 314ra187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farran, A.J.E., Teller, S.S., Jha, A.K., et al. Effects of matrix composition, microstructure, and viscoelasticity on the behaviors of vocal fold fibroblasts cultured in three-dimensional hydrogel networks. Tissue Eng Part A 16, 1247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latifi, N., Heris, H.K., Thomson, S.L., et al. A flow perfusion bioreactor system for vocal fold tissue engineering applications. Tissue Eng Part C Methods 22, 823, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrett, C.G., Coleman, J.R., and Reinisch, L.. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope 110, 814, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Kutty, J.K., and Webb, K.. Tissue engineering therapies for the vocal fold lamina propria. Tissue Eng Part B Rev 15, 249, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Woodson, G. Developing a porcine model for study of vocal fold scar. J Voice 26, 706, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Long, J.L., Neubauer, J., Zhang, Z., Zuk, P., Berke, G.S., and Chhetri, D.K.. Functional testing of a tissue-engineered vocal fold cover replacement. Otolaryngol Head Neck Surg 142, 438, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Yamashita, M., Kanemaru, S.-I., Hirano, S., et al. Glottal reconstruction with a tissue engineering technique using polypropylene mesh: a canine experiment. Ann Otol Rhinol Laryngol 119, 110, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Birk, V., Döllinger, M., Sutor, A., et al. Automated setup for ex vivo larynx experiments. J Acoust Soc Am 141, 1349, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li, N. Current understanding and future directions for vocal fold mechanobiology. J Cytol Mol Biol 1, 001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wrona, E.A., Peng, R., Amin, M.R., Branski, R.C., and Freytes, D.O.. Extracellular matrix for vocal fold lamina propria replacement: a review. Tissue Eng Part B Rev 22, 421, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Finck, C., and Lejeune, L.. Chapter 10.2 structure and oscillatory function of the vocal folds. Handb Behav Neurosci 19, 427, 2010. [Google Scholar]
- 19. Levendoski, E.E., Leydon, C., and Thibeault, S.L.. Vocal fold epithelial barrier in health and injury: a research review. J Speech Lang Hear Res 57, 1679, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mora-Navarro, C., Badileanu, A., Martins, A.M.G., et al. Porcine vocal fold lamina propria-derived biomaterials modulate TGF-β1-mediated fibroblast activation in vitro. ACS Biomater Sci Eng 6, 1690, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore, J., and Thibeault, S.. Insights into the role of elastin in vocal fold health and disease. J Voice 26, 269, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Entwistle, J., Hall, C.L., and Turley, E.A.. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem 61, 569, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Branco, A., Bartley, S.M., King, S.N., Jetté, M.E., and Thibeault, S.L.. Vocal fold myofibroblast profile of scarring. Laryngoscope 126, E110, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King, S.N., Chen, F., Jetté, M.E., and Thibeault, S.L.. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine 61, 228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiller, K.L., Freytes, D.O., and Vunjak-Novakovic, G.. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann Biomed Eng 43, 616, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaba, S., Nakamura, R., Yamashita, M., et al. Alterations in macrophage polarization in injured murine vocal folds. Laryngoscope 129, E135, 2019. [DOI] [PubMed] [Google Scholar]
- 27. Cohen, S.M., Kim, J., Roy, N., Asche, C., and Courey, M.. Direct health care costs of laryngeal diseases and disorders. Laryngoscope 122, 1582, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Sullivan, P.W., Ghushchyan, V.H., Slejko, J.F., Belozeroff, V., Globe, D.R., and Lin, S.-L.. The burden of adult asthma in the United States: evidence from the Medical Expenditure Panel Survey. J Allergy Clin Immun 127, 363, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Roy, N., Merrill, R.M., Gray, S.D., and Smith, E.M.. Voice disorders in the general population: prevalence, risk factors, and occupational impact. Laryngoscope 115, 1988, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Bartlett, R.S., Thibeault, S.L., and Prestwich, G.D.. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater 7, 024103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller, S. Voice therapy for vocal fold paralysis. Otolaryngol Clin North Am 37, 105, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Mallick, A.S., Garas, G., and McGlashan, J.. Presbylaryngis: a state-of-the-art review. Curr Opin Otolaryngol 27, 168, 2019. [DOI] [PubMed] [Google Scholar]
- 33. Bartlett, R.S., Gaston, J.D., Yen, T.Y., Ye, S., Kendziorski, C., and Thibeault, S.L.. Biomechanical screening of cell therapies for vocal fold scar. Tissue Eng Part A 21, 2437, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tong, Z., Zerdoum, A.B., Duncan, R.L., and Jia, X.. Dynamic vibration cooperates with connective tissue growth factor to modulate stem cell behaviors. Tissue Eng Part A 20, 1922, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bartlett, R.S., Gaston, J.D., Ye, S., Kendziorski, C., and Thibeault, S.L.. Mechanotransduction of vocal fold fibroblasts and mesenchymal stromal cells in the context of the vocal fold mechanome. J Biomech 83, 227, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tong, Z., Duncan, R.L., and Jia, X.. Modulating the behaviors of mesenchymal stem cells via the combination of high-frequency vibratory stimulations and fibrous scaffolds. Tissue Eng Part A 19, 1862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaston, J., Rios, B.Q., Bartlett, R., Berchtold, C., and Thibeault, S.L.. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS One 7, e30965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon, S.G., Kwon, Y.W., Lee, T.W., Park, G.T., and Kim, J.H.. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 22, 36, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schweinfurth, J.M., and Thibeault, S.L.. Does hyaluronic acid distribution in the larynx relate to the newborn's capacity for crying? Laryngoscope 118, 1692, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Sato, K., Hirano, M., and Nakashima, T.. Fine structure of the human newborn and infant vocal fold mucosae. Ann Otol Rhinol Laryngol 110, 417, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Ishii, K., Akita, M., Yamashita, K., and Hirose, H.. Age-related development of the arrangement of connective tissue fibers in the lamina propria of the human vocal fold. Ann Otol Rhinol Laryngol 109, 1055, 2000. [DOI] [PubMed] [Google Scholar]
- 42. Nita, L.M., Battlehner, C.N., Ferreira, M.A., et al. The presence of a vocal ligament in fetuses: a histochemical and ultrastructural study. J Anat 215, 692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sato, K., Umeno, H., Ono, T., and Nakashima, T. Histopathologic study of human vocal fold mucosa unphonated over a decade. 131, 1319, 2011. [DOI] [PubMed]
- 44. Boseley, M.E., and Hartnick, C.J.. Development of the human true vocal fold: depth of cell layers and quantifying cell types within the lamina propria. Ann Otol Rhinol Laryngol 115, 784, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Smith, M.E., and Gray, S.D.. Developmental laryngeal and phonatory anatomy and physiology. Sig 3 Perspect Voice Voice Disord 12, 4, 2002. [Google Scholar]
- 46. Hartnick, C.J., Rehbar, R., and Prasad, V.. Development and maturation of the pediatric human vocal fold lamina propria. Laryngoscope 115, 4, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Wolchok, J.C., Brokopp, C., Underwood, C.J., and Tresco, P.A.. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials 30, 327, 2009. [DOI] [PubMed] [Google Scholar]
- 48. Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB J 20, 811, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Zerdoum, A.B., Saberi, P., Stuffer, A.J., et al. Regulation of stem cell function in an engineered vocal fold-mimetic environment. Regen Eng Transl Med 6, 164, 2020. [PMC free article] [PubMed] [Google Scholar]
- 50. Titze, I.R., Hitchcock, R.W., Broadhead, K., et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech 37, 1521, 2004. [DOI] [PubMed] [Google Scholar]
- 51. Kutty, J.K., and Webb, K.. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J Tissue Eng Regen Med 4, 62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim, D., Lee, S., Lim, J., and Kwon, S.. Characteristics and responses of human vocal fold cells in a vibrational culture model. Laryngoscope 128, E258, 2018. [DOI] [PubMed] [Google Scholar]
- 53. Kim, D., Lim, J.-Y., and Kwon, S.. Development of vibrational culture model mimicking vocal fold tissues. Ann Biomed Eng 44, 3136, 2016. [DOI] [PubMed] [Google Scholar]
- 54. Branski, R.C., Perera, P., Verdolini, K., Rosen, C.A., Hebda, P.A., and Agarwal, S.. Dynamic biomechanical strain inhibits IL-1β–induced inflammation in vocal fold fibroblasts. J Voice 21, 651, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farran, A.J.E., Teller, S.S., Jia, F., Clifton, R.J., Duncan, R.L., and Jia, X.. Design and characterization of a dynamic vibrational culture system. J Tissue Eng Regen Med 7, 213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirsch, A., Hortobagyi, D., Stachl, T., et al. Development and validation of a novel phonomimetic bioreactor. PLoS One 14, e0213788, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Swanson, E.R., Ohno, T., Abdollahian, D., Garrett, C.G., and Rousseau, B.. Effects of raised-intensity phonation on inflammatory mediator gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg 143, 567, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sato, K., Umeno, H., Nakashima, T., Nonaka, S., and Harabuchi, Y.. Expression and distribution of hyaluronic acid and CD44 in unphonated human vocal fold mucosa. Ann Otol Rhinol Laryngol 118, 773, 2009. [PubMed] [Google Scholar]
- 59. Rousseau, B., Ge, P., French, L.C., Zealear, D.L., Thibeault, S.L., and Ossoff, R.H.. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg 138, 62, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Verdolini, K., Branski, R.C., Rosen, C.A., and Hebda, P.A.. Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: a preliminary study. Ann Otol Rhinol Laryngol 112, 1021, 2003. [DOI] [PubMed] [Google Scholar]
- 61. Courey, M.S., Scott, M.A., Shohet, J.A., and Ossoff, R.H.. Immunohistochemical characterization of benign laryngeal lesions. Ann Otol Rhinol Laryngol 105, 525, 1996. [DOI] [PubMed] [Google Scholar]
- 62. Abbott, K.V., Li, N.Y.K., Branski, R.C., et al. Vocal exercise may attenuate acute vocal fold inflammation. J Voice 26, 814.e1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Titze, I.R. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am 83, 1536, 1988. [DOI] [PubMed] [Google Scholar]
- 64. Gray, S.D., Alipour, F., Titze, I.R., and Hammond, T.H.. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol 109, 77, 2000. [DOI] [PubMed] [Google Scholar]
- 65. Zhang, Z. Mechanics of human voice production and control. J Acoust Soc Am 140, 2614, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van den Berg, J. Myoelastic-aerodynamic theory of voice production. J Speech Hear Res 1, 227, 1958. [DOI] [PubMed] [Google Scholar]
- 67. Loucks, T.M.J., Poletto, C.J., Simonyan, K., Reynolds, C.L., and Ludlow, C.L.. Human brain activation during phonation and exhalation: common volitional control for two upper airway functions. Neuroimage 36, 131, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flanagan, J.L. Estimates of intraglottal pressure during phonation. J Speech Hear Res 2, 168, 1959. [DOI] [PubMed] [Google Scholar]
- 69. Yin, J., and Zhang, Z.. The influence of thyroarytenoid and cricothyroid muscle activation on vocal fold stiffness and eigenfrequencies. J Acoust Soc Am 133, 2972, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thomson, S.L., Mongeau, L., and Frankel, S.H.. Aerodynamic transfer of energy to the vocal folds. J Acoust Soc Am 118, 1689, 2005. [DOI] [PubMed] [Google Scholar]
- 71. DeJonckere, P.H., and Lebacq, J.. Damping of vocal fold oscillation at voice offset. Biomed Signal Proces 37, 92, 2017. [Google Scholar]
- 72. Titze, I.R. On the relation between subglottal pressure and fundamental frequency in phonation. J Acoust Soc Am 85, 901, 1989. [DOI] [PubMed] [Google Scholar]
- 73. Plunkett, N., and O'Brien, F.J.. Bioreactors in tissue engineering. Technol Health Care 19, 55, 2011. [DOI] [PubMed] [Google Scholar]
- 74. Dwivedi, R., Kumar, S., Pandey, R., et al. Polycaprolactone as biomaterial for bone scaffolds: review of literature. J Oral Biol Craniofac Res 10, 381, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Flexcell International Corporation. BioFlex® culture plates. https://www.flexcellint.com/product/bioflex-culture-plates (accessed February 18, 2021).
- 76. The Lubrizol Corporation. Tecoflex™ TPU—Lubrizol. https://www.lubrizol.com/Health/Medical/Polymers/Tecoflex-TPU (accessed February 18, 2021).
- 77. Gaston, J., Bartlett, R.S., Klemuk, S.A., and Thibeault, S.L.. Formulation and characterization of a porous, elastomeric biomaterial for vocal fold tissue engineering research. Ann Otol Rhinol Laryngol 123, 866, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Klemuk, S.A., Jaiswal, S., and Titze, I.R.. Cell viability viscoelastic measurement in a rheometer used to stress and engineer tissues at low sonic frequencies. J Acoust Soc Am 124, 2330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patten, J., and Wang, K. Fibronectin in development and wound healing. Adv Drug Deliver Rev 2020 [Epub ahead of print]; DOI: 10.1016/j.addr.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 80. Lenselink, E.A. Role of fibronectin in normal wound healing. Int Wound J 12, 313, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rashedin, R., and Meydan, T.. Solenoid actuator for loudspeaker application. Sens Actuators Phys 129, 220, 2006. [Google Scholar]
- 82. McBean, J., and Breazeal, C.. Voice coil actuators for human-robot interaction. 2004 IEEE RSJ Int Conf Intelligent Robots Syst IROS IEEE Cat 04ch37566 1, 852, 2004. [Google Scholar]
- 83. Titze, I.R., Klemuk, S.A., and Lu, X.. Adhesion of a monolayer of fibroblast cells to fibronectin under sonic vibrations in a bioreactor. Ann Otol Rhinol Laryngol 121, 364, 2012. [DOI] [PubMed] [Google Scholar]
- 84. Latifi, N., Heris, H.K., Kazemirad, S., and Mongeau, L. Development of a self-oscillating mechanical model to investigate the biological response of human vocal fold fibroblasts to phono-mimetic stimulation. Proceedings of the ASME 2014 International Mechanical Engineering Congress and Exposition. Volume 3: Biomedical and Biotechnology Engineering. Montreal, Quebec, Canada. November 14–20, 2014. V003T03A002. ASME. 10.1115/IMECE2014-38970. [DOI] [Google Scholar]
- 85. Zerdoum, A.B., Tong, Z., Bachman, B., and Jia, X. Construction and characterization of a novel vocal fold bioreactor. J Vis Exp 2014. Aug 1;(90):e51594. doi: 10.3791/51594. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dinarello, C.A. Interleukin-18, a proinflammatory cytokine. Eur Cytokine Netw 11, 483, 2000. [PubMed] [Google Scholar]
- 87. Dinarello, C.A. Interleukin-1. Cytokine Growth F R 8, 253, 1997. [DOI] [PubMed] [Google Scholar]
- 88. Guirao, X., and Lowry, S.F.. Biologic control of injury and inflammation: much more than too little or too late. World J Surg 20, 437, 1996. [DOI] [PubMed] [Google Scholar]
- 89. Grottkau, B.E., Noordin, S., Shortkroff, S., Schaffer, J.L., Thornhill, T.S., and Spector, M.. Effect of mechanical perturbation on the release of PGE2 by macrophages in vitro. J Biomed Mater Res 59, 288, 2002. [DOI] [PubMed] [Google Scholar]
- 90. Long, P., Gassner, R., and Agarwal, S.. Tumor necrosis factor α–dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthritis Rheum 44, 2311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Erndt-Marino, J.D., Jimenez-Vergara, A.C., Diaz-Rodriguez, P., et al. In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment. J Biomed Mater Res Part B Appl Biomater 106, 1258, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen, H., Erndt-Marino, J., Diaz-Rodriguez, P., et al. In vitro evaluation of anti-fibrotic effects of select cytokines for vocal fold scar treatment. J Biomed Mater Res Part B Appl Biomater 107, 1056, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brock, T.G., McNish, R.W., and Peters-Golden, M.. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem 274, 11660, 1999. [DOI] [PubMed] [Google Scholar]
- 94. Crofford, L.J. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl 49, 15, 1997. [PubMed] [Google Scholar]
- 95. Walimbe, T., Calve, S., Panitch, A., and Sivasankar, M.P.. Incorporation of types I and III collagen in tunable hyaluronan hydrogels for vocal fold tissue engineering. Acta Biomater 87, 97, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Page-McCaw, A., Ewald, A.J., and Werb, Z.. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8, 221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Beidler, S.K., Douillet, C.D., Berndt, D.F., Keagy, B.A., Rich, P.B., and Marston, W.A.. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen 16, 642, 2008. [DOI] [PubMed] [Google Scholar]
- 98. Zhao, S., Zhao, Y., Guo, J., et al. Downregulation of MMP1 in MDS-derived mesenchymal stromal cells reduces the capacity to restrict MDS cell proliferation. Sci Rep 7, 43849, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hornebeck, W. Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival. Pathol Biol 51, 569, 2003. [DOI] [PubMed] [Google Scholar]
- 100. Sarker, H., Hardy, E., Haimour, A., Maksymowych, W.P., Botto, L.D., and Fernandez-Patron, C.. Identification of fibrinogen as a natural inhibitor of MMP-2. Sci Rep 9, 4340, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Veidal, S.S., Larsen, D.V., Chen, X., et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin Biochem 45, 541, 2012. [DOI] [PubMed] [Google Scholar]
- 102. Okada, Y., Morodomi, T., Enghild, J.J., et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Eur J Biochem 194, 721, 1990. [DOI] [PubMed] [Google Scholar]
- 103. Doren, S.R.V. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol 44, 224, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fernandez-Patron, C., Kassiri, Z., and Leung, D.. Comprehensive physiology. Compr Physiol 6, 1935, 2016. [DOI] [PubMed] [Google Scholar]
- 105. Corcoran, M.L., Hewitt, R.E., Kleiner, D.E.Jr., and Stetler-Stevenson, W.G.. MMP-2: expression, activation and inhibition. Enzyme Protein 49, 7, 1996. [DOI] [PubMed] [Google Scholar]
- 106. Berry, E., Hernandez-Anzaldo, S., Ghomashchi, F., et al. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc 4, e001868, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Berton, A., Rigot, V., Huet, E., et al. Involvement of fibronectin type II repeats in the efficient inhibition of gelatinases A and B by long-chain unsaturated fatty acids. J Biol Chem 276, 20458, 2001. [DOI] [PubMed] [Google Scholar]
- 108. Shen, M., Lee, J., Basu, R., et al. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol 35, 888, 2015. [DOI] [PubMed] [Google Scholar]
- 109. National Center for Biotechnology Information (NCBI). ACTA2 actin alpha 2, smooth muscle [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene?Db=gene&Cmd=DetailsSearch&Term=59 (accessed December 17, 2020).
- 110. Guo, D.-C., Papke, C.L., Tran-Fadulu, V., et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 84, 617, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cheng, J., Zhou, X., Jiang, X., and Sun, T.. Deletion of ACTA2 in mice promotes angiotensin II induced pathogenesis of thoracic aortic aneurysms and dissections. J Thorac Dis 10, 4733, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yuan, S.-M. α-Smooth muscle actin and ACTA2 gene expressions in vasculopathies. Braz J Cardiovasc Surg 30, 644, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rønnov-Jessen, L., and Petersen, O.W.. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68, 696, 1993. [PubMed] [Google Scholar]
- 114. Hinz, B., Celetta, G., Tomasek, J.J., Gabbiani, G., and Chaponnier, C.. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12, 2730, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Correll, K.A., Edeen, K.E., Redente, E.F., et al. TGF beta inhibits HGF, FGF7, and FGF10 expression in normal and IPF lung fibroblasts. Physiol Rep 6, e13794, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Grimsby, J.L., Lucero, H.A., Trackman, P.C., Ravid, K., and Kagan, H.M.. Role of lysyl oxidase propeptide in secretion and enzyme activity. J Cell Biochem 111, 1231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Szauter, K.M., Cao, T., Boyd, C.D., and Csiszar, K.. Lysyl oxidase in development, aging and pathologies of the skin. Pathol Biol 53, 448, 2005. [DOI] [PubMed] [Google Scholar]
- 118. Lucero, H.A., and Kagan, H.M.Lysyl oxidase: an oxidative enzyme and effector of cell function. 2006. file:///D:/3%20-%20Work/2%20-%20Projects/5%20-%20VF%20Review/Articles/Lucero-Kagan2006_Article_LysylOxidaseAnOxidativeEnzymeA.pdf (accessed December 12, 2020). [DOI] [PMC free article] [PubMed]
- 119. Hämäläinen, E.-R., Jones, T.A., Sheer, D., Taskinen, K., Pihlajaniemi, T., and Kivirikko, K.I.. Molecular cloning of human lysyl oxidase and assignment of the gene to chromosome 5q23.3–31.2. Genomics 11, 508, 1991. [DOI] [PubMed] [Google Scholar]
- 120. Levental, K.R., Yu, H., Kass, L., et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. National Center for Biotechnology Information (NCBI). COL1A1 collagen type I alpha 1 chain [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/1277 (accessed December 13, 2020).
- 122. Collins, M., Posthumus, M., and Schwellnus, M.P.. The COL1A1 gene and acute soft tissue ruptures. Br J Sport Med 44, 1063, 2010. [DOI] [PubMed] [Google Scholar]
- 123. Mann, V., Hobson, E.E., Li, B., et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107, 899, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Adams, J.C., and Watt, F.M.. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell 63, 425, 1990. [DOI] [PubMed] [Google Scholar]
- 125. Chen, G., Liu, D., Tadokoro, M., et al. Chondrogenic differentiation of human mesenchymal stem cells cultured in a cobweb-like biodegradable scaffold. Biochem Biophys Res Commun 322, 50, 2004. [DOI] [PubMed] [Google Scholar]
- 126. Tao, R., Fan, X., Yu, H., et al. MicroRNA-29b-3p prevents Schistosoma japonicum-induced liver fibrosis by targeting COL1A1 and COL3A1. J Cell Biochem 119, 3199, 2018. [DOI] [PubMed] [Google Scholar]
- 127. Giménez, A., Duch, P., Puig, M., Gabasa, M., Xaubet, A., and Alcaraz, J.. Dysregulated collagen homeostasis by matrix stiffening and TGF-β1 in fibroblasts from idiopathic pulmonary fibrosis patients: role of FAK/Akt. Int J Mol Sci 18, 2431, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jiang, B., Zu, W., Xu, J., et al. Botulinum toxin type A relieves sternocleidomastoid muscle fibrosis in congenital muscular torticollis. Int J Biol Macromol 112, 1014, 2018. [DOI] [PubMed] [Google Scholar]
- 129. Felkin, L.E., Lara-Pezzi, E., George, R., Yacoub, M.H., Birks, E.J., and Barton, P.J.R.. Expression of extracellular matrix genes during myocardial recovery from heart failure after left ventricular assist device support. J Heart Lung Transplant 28, 117, 2009. [DOI] [PubMed] [Google Scholar]
- 130. Zhang, Z., Wang, Y., Zhang, J., Zhong, J., and Yang, R.. COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway. Mol Med Rep 17, 5037, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wu, W., Yang, Z., Long, F., et al. COL1A1 and MZB1 as the hub genes influenced the proliferation, invasion, migration and apoptosis of rectum adenocarcinoma cells by weighted correlation network analysis. Bioorg Chem 95, 103457, 2020. [DOI] [PubMed] [Google Scholar]
- 132. National Center for Biotechnology Information (NCBI). COL1A2 collagen type I alpha 2 chain [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/1278 (accessed December 13, 2020).
- 133. Stephen, J., Shukla, A., Dalal, A., et al. Mutation spectrum of COL1A1 and COL1A2 genes in Indian patients with osteogenesis imperfecta. Am J Med Genet A 164, 1482, 2014. [DOI] [PubMed] [Google Scholar]
- 134. Zhou, J., Zhang, X., Liang, P., et al. Protective role of microRNA-29a in denatured dermis and skin fibroblast cells after thermal injury. Biol Open 5, 211, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liu, J., Luo, C., Yin, Z., et al. Downregulation of let-7b promotes COL1A1 and COL1A2 expression in dermis and skin fibroblasts during heat wound repair. Mol Med Rep 13, 2683, 2016. [DOI] [PubMed] [Google Scholar]
- 136. Zaitseva, E.L., Tokmakova, A.Y., Petrov, V.M., et al. Genetic parameters of wound healing in patients with neuropatic diabetic foot ulcers. Diabetes Mellitus 20, 344, 2017. [Google Scholar]
- 137. Yu, Y., Liu, D., Liu, Z., et al. The inhibitory effects of COL1A2 on colorectal cancer cell proliferation, migration, and invasion. J Cancer 9, 2953, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. National Center for Biotechnology Information (NCBI). COL3A1 collagen type III alpha 1 chain [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/1281 (accessed December 13, 2020).
- 139. Wang, L., Liu, H., Jiao, Y., et al. Differences between mice and humans in regulation and the molecular network of collagen, type III, alpha-1 at the gene expression level: obstacles that translational research must overcome. Int J Mol Sci 16, 15031, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang, X., Liu, H., Hock, T., Thannickal, V.J., and Sanders, Y.Y.. Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int J Mol Sci 14, 19605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Piñol-Jurado, P., Suárez-Calvet, X., Fernández-Simón, E., et al. Nintedanib decreases muscle fibrosis and improves muscle function in a murine model of dystrophinopathy. Cell Death Dis 9, 776, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stevenson, K., Kucich, U., Whitbeck, C., Levin, R.M., and Howard, P.S.. Functional changes in bladder tissue from type III collagen-deficient mice. Mol Cell Biochem 283, 107, 2006. [DOI] [PubMed] [Google Scholar]
- 143. Hoffman, D.B., Sorensen, J.R., Call, J.A., Corona, B.T., and Greising, S.M.. Temporal changes in pathologic fibrosis following volumetric muscle loss injury. FASEB J 34, 1, 2020. [Google Scholar]
- 144. Hünerwadel, A., Fagagnini, S., Rogler, G., et al. Severity of local inflammation does not impact development of fibrosis in mouse models of intestinal fibrosis. Sci Rep 8, 15182, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang, X.-Q., Tang, Z.-X., Yu, D., et al. Epithelial but not stromal expression of collagen alpha-1(III) is a diagnostic and prognostic indicator of colorectal carcinoma. Oncotarget 7, 8823, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. National Center for Biotechnology Information (NCBI). CCN2 cellular communication network factor 2 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/1490 (accessed December 14, 2020).
- 147. Shi-Wen, X., Leask, A., and Abraham, D.. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth F R 19, 133, 2008. [DOI] [PubMed] [Google Scholar]
- 148. Liu, S., and Leask, A.. CCN2 is not required for skin development. J Cell Commun Signal 5, 179, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hall-Glenn, F., Aivazi, A., Akopyan, L., et al. CCN2/CTGF is required for matrix organization and to protect growth plate chondrocytes from cellular stress. J Cell Commun Signal 7, 219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jun, J.-I., and Lau, L.F.. CCN2 induces cellular senescence in fibroblasts. J Cell Commun Signal 11, 15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. National Center for Biotechnology Information (NCBI). DCN decorin [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/1634 (accessed December 14, 2020).
- 152. Xu, Y., Xia, Q., Rao, Q., et al. DCN deficiency promotes renal cell carcinoma growth and metastasis through downregulation of P21 and E-cadherin. Tumor Biol 37, 5171, 2016. [DOI] [PubMed] [Google Scholar]
- 153. Hu, X., Villodre, E.S., Larson, R., et al. Decorin, a novel negative modulator of E-cadherin in inflammatory breast cancer. bioRxiv 2020.07.07.190496, 2020. [Google Scholar]
- 154. Ma, W., Tan, Y., Cai, S., Chen, H., Du, J., and Cai, S.. The action of decorin in anti-fibrosis and anti-cancer [in Chinese]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 24, 222, 2007. [PubMed] [Google Scholar]
- 155. National Center for Biotechnology Information (NCBI). ELN elastin [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/2006 (accessed December 14, 2020).
- 156. Staiculescu, M.C., Cocciolone, A.J., Procknow, J.D., Kim, J., and Wagenseil, J.E.. Comparative gene array analyses of severe elastic fiber defects in late embryonic and newborn mouse aorta. Physiol Genomics 50, 988, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Carreras, I., Rich, C.B., Panchenko, M.P., and Foster, J.A.. Basic fibroblast growth factor decreases elastin gene transcription in aortic smooth muscle cells. J Cell Biochem 85, 592, 2002. [DOI] [PubMed] [Google Scholar]
- 158. Kuang, P.-P., and Goldstein, R.H.. Regulation of elastin gene transcription by interleukin-1β-induced C/EBPβ isoforms. Am J Physiol Cell Physiol 285, C1349, 2003. [DOI] [PubMed] [Google Scholar]
- 159. Kozel, B.A., Danback, J.R., Waxler, J.L., et al. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension 63, 74, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Misra, V., Lee, H., Singh, A., et al. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics 31, 429, 2007. [DOI] [PubMed] [Google Scholar]
- 161. Walker, A.E., Henson, G.D., Reihl, K.D., et al. Aortic stiffening as a result of reduced elastin content leads to cerebral artery dysfunction. FASEB J 27, 1194.3, 2013. [Google Scholar]
- 162. Li, J., Xu, X., Jiang, Y., et al. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer 20, 217, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Skjøt-Arkil, H., Clausen, R.E., Nguyen, Q.H.T., et al. Measurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm Med 12, 34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Brandsma, C.-A., Berge, M.. van den, Postma, D.S., et al. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax 70, 21, 2015. [DOI] [PubMed] [Google Scholar]
- 165. National Center for Biotechnology Information (NCBI). FMOD fibromodulin [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/2331 (accessed December 14, 2020).
- 166. Al-Qattan, M.M., and Al-Qattan, A.M.. Fibromodulin: structure, physiological functions, and an emphasis on its potential clinical applications in various diseases. J Coll Physicians Surg Pak 28, 783, 2018. [PubMed] [Google Scholar]
- 167. Zheng, Z., Jian, J., Velasco, O., et al. Fibromodulin enhances angiogenesis during cutaneous wound healing. Plastic Reconstr Surg Glob Open 2, e275, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lee, E.J., Nam, J.H., and Choi, I.. Fibromodulin modulates myoblast differentiation by controlling calcium channel. Biochem Biophys Res Commun 503, 580, 2018. [DOI] [PubMed] [Google Scholar]
- 169. Andréasson, K., Gustafsson, R., Rydell-Törmänen, K., Westergren-Thorsson, G., Saxne, T., and Hesselstrand, R.. Limited impact of fibromodulin deficiency on the development of experimental skin fibrosis. Exp Dermatol 25, 558, 2016. [DOI] [PubMed] [Google Scholar]
- 170. National Center for Biotechnology Information (NCBI). FN1 fibronectin 1 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene?term=(fn[gene])%20AND%20(Homo%20sapiens[orgn])%20AND%20alive[prop]%20NOT%20newentry[gene]&sort=weight (accessed December 14, 2020).
- 171. Mosher, D.F. Assembly of fibronectin into extracellular matrix. Curr Opin Struct Biol 3, 214, 1993. [Google Scholar]
- 172. Singh, P., Carraher, C., and Schwarzbauer, J.E.. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26, 397, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sottile, J., and Hocking, D.C.. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 13, 3546, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chiang, H.-Y., Korshunov, V.A., Serour, A., Shi, F., and Sottile, J.. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29, 1074, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu, X.-Y., Liu, R.-X., Hou, F., et al. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep 14, 3669, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Junge, J., Horn, T., and Christoffersen, P.. The occurrence and significance of fibronectin in livers from chronic alcoholics. APMIS 96, 56, 1988. [DOI] [PubMed] [Google Scholar]
- 177. National Center for Biotechnology Information (NCBI). HAS1 hyaluronan synthase 1 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/3036 (accessed December 14, 2020).
- 178. Lee, J.Y., Rountree, R.B., Kingsley, D.M., and Spicer, A.P.. In vivo investigation of hyaluronan and hyaluronan synthase-2 function during cartilage and joint development. In: Hascall, V.C., and Kuettner, K.E., eds. The Many Faces of Osteoarthritis. Basel: Birkhäuser, 2002, pp. 213–218. [Google Scholar]
- 179. Tateya, I., Tateya, T., Watanuki, M., and Bless, D.M.. Homeostasis of hyaluronic acid in normal and scarred vocal folds. J Voice 29, 133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. National Center for Biotechnology Information (NCBI). HAS2 hyaluronan synthase 2 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/3037 (accessed December 14, 2020).
- 181. National Center for Biotechnology Information (NCBI). HAS3 hyaluronan synthase 3 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/3038 (accessed December 14, 2020).
- 182. National Center for Biotechnology Information (NCBI). HYAL1 hyaluronidase 1 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/3373 (accessed December 14, 2020).
- 183. Jin, Z., Zhang, G., Liu, Y., et al. The suppressive role of HYAL1 and HYAL2 in the metastasis of colorectal cancer. J Gastroenterol Hepatol 34, 1766, 2019. [DOI] [PubMed] [Google Scholar]
- 184. Tanimoto, K., Kitamura, R., Tanne, Y., et al. Modulation of hyaluronan catabolism in chondrocytes by mechanical stimuli. J Biomed Mater Res A 93A, 373, 2010. [DOI] [PubMed] [Google Scholar]
- 185. National Center for Biotechnology Information (NCBI). HYAL2 hyaluronidase 2 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/8692 (accessed December 14, 2020).
- 186. National Center for Biotechnology Information (NCBI). TGFB1 transforming growth factor beta 1 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/7040 (accessed December 14, 2020).
- 187. Lijnen, P.J., Petrov, V.V., and Fagard, R.H.. Induction of cardiac fibrosis by transforming growth factor-β1. Mol Genet Metab 71, 418, 2000. [DOI] [PubMed] [Google Scholar]
- 188. Yan, W., Wang, P., Zhao, C.X., Tang, J., Xiao, X., and Wang, D.W.. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-β/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther 20, 1190, 2009. [DOI] [PubMed] [Google Scholar]
- 189. Leng, D., Huang, X., Yi, J., Zhao, H., and Zhang, Y.. HYAL1 is downregulated in idiopathic pulmonary fibrosis and inhibits HFL-1 fibroblast proliferation when upregulated. Biomed Res Int 2020, 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Akagi, Y., Isaka, Y., Arai, M., et al. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 50, 148, 1996. [DOI] [PubMed] [Google Scholar]
- 191. Murakami, S., Takashima, H., Sato-Watanabe, M., et al. Ursolic acid, an antagonist for transforming growth factor (TGF)-β1. FEBS Lett 566, 55, 2004. [DOI] [PubMed] [Google Scholar]
- 192. Huang, S.S., Liu, Q., Johnson, F.E., Konish, Y., and Huang, J.S.. Transforming growth factor β peptide antagonists and their conversion to partial agonists. J Biol Chem 272, 27155, 1997. [DOI] [PubMed] [Google Scholar]
- 193. Branski, R.C., Barbieri, S.S., Weksler, B.B., et al. Effects of transforming growth factor-β1 on human vocal fold fibroblasts. Ann Otol Rhinol Laryngol 118, 218, 2009. [DOI] [PubMed] [Google Scholar]
- 194. National Center for Biotechnology Information (NCBI). TIMP1 TIMP metallopeptidase inhibitor 1 [Homo sapiens (human)]—Gene—NCBI. https://www-ncbi-nlm-nih-gov.libproxy.lib.unc.edu/gene/7076 (accessed December 14, 2020).
- 195. Wang, K., Lin, B., Brems, J.J., and Gamelli, R.L.. Hepatic apoptosis can modulate liver fibrosis through TIMP1 pathway. Apoptosis 18, 566, 2013. [DOI] [PubMed] [Google Scholar]
- 196. Bjerre, C., Vinther, L., Belling, K.C., et al. TIMP1 overexpression mediates resistance of MCF-7 human breast cancer cells to fulvestrant and down-regulates progesterone receptor expression. Tumor Biol 34, 3839, 2013. [DOI] [PubMed] [Google Scholar]
- 197. Omar, O.M., Soutto, M., Bhat, N.S., et al. TFF1 antagonizes TIMP-1 mediated proliferative functions in gastric cancer. Mol Carcinog 57, 1577, 2018. [DOI] [PubMed] [Google Scholar]
- 198. Takawale, A., Zhang, P., Patel, V.B., Wang, X., Oudit, G., and Kassiri, Z.. Tissue inhibitor of matrix metalloproteinase-1 promotes myocardial fibrosis by mediating CD63–integrin β1 interaction. Hypertension 69, 1092, 2018. [DOI] [PubMed] [Google Scholar]