Abstract
The mammalian liver's regenerative ability has led researchers to engineer animals as incubators for expansion of human hepatocytes. The expansion properties of human hepatocytes in immunodeficient mice are well known. However, little has been reported about larger animals that are more scalable and practical for clinical purposes. Therefore, we engineered immunodeficient swine to support expansion of human hepatocytes and identify barriers to their clinical application. Immunodeficient swine were engineered by knockout of the recombinase-activating gene 2 (RAG2) and fumarylacetoacetate hydrolase (FAH). Immature human hepatocytes (ihHCs) were injected into fetal swine by intrauterine cell transplantation (IUCT) at day 40 of gestation. Human albumin was measured as a marker of engraftment. Cytotoxicity against ihHCs was measured in transplanted piglets and control swine. We initially detected higher levels of human albumin in cord blood of newborn FAH/RAG2-deficient (FR) pigs compared with immunocompetent controls (196.26 ng/dL vs. 39.29 ng/dL, p = 0.008), indicating successful engraftment of ihHCs after IUCT and adaptive immunity in the fetus. Although rare hepatocytes staining positive for human albumin were observed, levels of human albumin did not rise after birth, but declined, suggesting rejection of xenografted ihHCs. Cytotoxicity against ihHCs increased after birth by 3.8% (95% CI: [2.1%–5.4%], p < 0.001) and inversely correlated with declining levels of human albumin (p = 2.1 × 10−5, R2 = 0.17). Circulating numbers of T cells and B cells were negligible in FR pigs. However, circulating natural killer (NK) cells exerted cytotoxicity against ihHCs. NK cell activity was lower in immunodeficient piglets after IUCT than in naive controls (30.4% vs. 40.1%, p = 0.011, 95% CI for difference [2.7%–16.7%]). In conclusion, ihHCs were successfully engrafted in FR swine after IUCT. NK cells were a significant barrier to expansion of hepatocytes. New approaches are needed to overcome this hurdle and allow large-scale expansion of human hepatocytes in immunodeficient swine.
Impact statement
There is currently a need for robust expansion of human hepatocytes. We describe an immunodeficient swine model into which we engrafted immature human hepatocytes (ihHCs). We identified the mechanism of the eventual graft rejection by the intact NK cell population, which has not been previously shown to have a significant role in xenograft rejection. By both improving engraftment and reducing NK cell-mediated cytotoxicity toward the graft through intrauterine cell transfer, we confirmed the presence of residual adaptive immunity in this model of immunodeficiency and the ability to induce hyposensitization in the NK cell population by taking advantage of the fetal microenvironment.
Keywords: hepatocyte transplant, hereditary tyrosinemia, genetic immunodeficiency, animal model, natural killer cell
Introduction
No reliable means of engrafting and expanding human hepatocytes within a large animal host exist. Recent advances in embryo complementation have permitted generation of xenogeneic whole organs within swine, which in turn has proven the feasibility of deriving both simple tissues and more complex structures in cross-species hosts.1,2 What this work has demonstrated on a large scale has also been shown on a cellular level. Namely, human hematopoietic stem cells survive and contribute to the body plan of swine after in utero cell transplantation (IUCT), both as pure human cells and as human–pig hybrid cells.3,4 These experiments suggest that no absolute hurdle exists to establishing human–swine chimeras.
The possibility of xenogeneic chimeras is well established in immunodeficient mice also deficient in fumarylacetoacetate hydrolase (FAH).5 The FAH defect leads to apoptosis of native hepatocytes due to abnormal tyrosine metabolism, while wild-type (WT) donor hepatocytes engraft and expand robustly after transplantation. While other xenogeneic chimerism models have been proposed, FAH deficiency is uniquely devoid of significant off-target systemic effects and can be manipulated through treatment with nitisinone (NTBC) to exert or remove selective pressure favoring WT donor hepatocyte expansion.6,7 The most successful immunodeficiency for expansion of human hepatocytes in mice results from combined inactivation of the recombinase-activating gene 2 (RAG2) and interleukin-2 receptor gamma (IL2rg) with the FAH deficiency to produce FRG mice.8 Although FRG mice support the expansion of human hepatocytes, the use of mice is suboptimal due to their diminutive size and potential transmission of murine retroviruses.
In contrast, swine hosts are more appropriate as either a preclinical model or source of cells for clinical application due to their similarity in size, longevity, anatomy, and physiology to humans.9 Furthermore, pigs reproduce human diseases with a higher fidelity than mice; and mice, unlike pigs, have diverged significantly from humans in their innate and adaptive immunity and expression of inflammatory markers.10–12
To circumvent the limitations of FRG mice, we sought to produce a less severe model of immunodeficiency in swine, which would still possess the selective advantage of FAH deficiency favoring expansion of human hepatocytes. RAG2-deficient swine have an excellent health record and were previously shown to readily form mature human teratomas when injected with human induced pluripotent stem cells.13 We sought to enhance RAG2 immunodeficiency with IUCT. We have previously shown the induction of hyposensitization through IUCT by injecting human cells into fetal pigs before maturation of pre-T cells in the fetal thymus (before day 50 of gestation).14 IUCT also provides a favorable fetal microenvironment for maturation of immature donor cells.15
Our primary aim was to study engraftment, expansion, and maturation of immature human hepatocytes (ihHCs) in FAH/RAG2-deficient (FR) pigs. We aimed to address three major barriers to engraftment: (1) the effect of the fetal microenvironment on engraftment, (2) swine adaptive immunity targeting human antigens, and (3) NK cell-mediated cytotoxicity against engrafted cells in swine. In doing so, we sought to investigate which immunologic process would limit engraftment in gestation before development of the host immune system. The current report provides a mechanistic explanation of our findings and plan for future studies.
Methods
All pigs were domestic white swine. Use of swine was approved by the Institutional Animal Care and Use Committees of both the University of Missouri and Mayo Clinic School of Graduate Medical Education and conformed with the U.S. National Research Council and U.S. Public Health Service policies for the humane care and use of laboratory animals. Recombinant DNA usage was approved by biosafety committees at both the University of Missouri and Mayo Clinic School of Graduate Medical Education.
Production of genetically engineered swine recipients
Our method for gene editing by CRISPR/Cas9 and zygote injection in pigs has been published.16 Additional information can be found in Supplementary Data S1. For in vitro fertilization (IVF), ovaries from prepubertal gilts were obtained from an abattoir (Smithfield Farmland, Milan, MO). Oocytes were matured, prepared, and washed with semen, as described.17 Fah guides 1 and 2 (Supplementary Fig. S1A, B, Supplementary Table S1) and Cas9 RNA (Sigma, St. Louis, MO) were coinjected. Zygotes were cultured to the blastocyst stage. On day 6, blastocysts were collected and surgically transferred into surrogate gilts in estrus.18 Gilts were given 25 mg of NTBC daily by mouth throughout the pregnancy. Fetuses generated from Fah CRISPR-injected zygotes were collected on day 35; two fetuses were biallelically modified in exon 5 of Fah and one was found to be Fah−/−. The Fah−/− cell line was established as described, and the presence of insertions and deletions (INDELs) was confirmed (Supplementary Table S1).17 RAG2 CRISPR/Cas9 plasmids for both RAG2 guides (Supplementary Fig. S1B, C, Supplementary Table S1) were transfected into the Fah−/− fetal fibroblast cell line, as previously reported.19 INDELs were confirmed (Supplementary Table S1). A biallelically modified fibroblast cell colony was found to have a one-base insertion at the cut site of RAG2 guide 1 binding on one allele, while the other allele had a 43-bp deletion that disrupted the RAG2 guide 2 binding site. To produce somatic cell nuclear transfer (SCNT) embryos, oocytes were matured as previously described for IVF. SCNT was performed using the double knockout (Fah−/−/RAG2−/−) cell line, as previously reported.17 Embryos were transferred into surrogates who were given NTBC throughout the pregnancy. A day-35 fetal fibroblast cell line was created and used for further SCNT and embryo transfer to produce litters taken to birth.
Donor cells and care of recipient swine
NTBC (25 mg) was given daily by mouth to pregnant gilts from the time of embryo transfer through the time of IUCT on gestational day 40. Gilts were placed under general anesthesia and a lower midline laparotomy was performed. Under ultrasound guidance, all viable fetuses received a direct intrahepatic injection of 107 freshly thawed immature human hepatocytes (ihHCs, iCell Hepatocytes 2.0; Cellular Dynamics International, Madison, WI). All gilts were then given 25 mg of NTBC by mouth, cycling 7 days on to 7 days off. On day 117 of gestation, gilts were placed under general anesthesia and a cesarean section was performed under strict sterile conditions in a peroxide-fogged operating theater. Gilts were then euthanized through barbiturate overdose.
Piglets were reared by hand in a peroxide-fogged, positive pressure clean room with an HEPA filtered air supply (bioBUBBLE, Inc., Fort Collins, CO). Piglets were fed sterile bovine colostrum (Huvepharma, Inc., Peachtree City, GA) for the first 24 h of life and ultrapasteurized bovine milk until weaned to sterilized chow and water. Piglets were given 1 mg/kg NTBC by mouth daily, cycling 7 days on to 7 days off. At day 43 of life, a single piglet underwent ultrasound-guided portal vein injection of 108 freshly thawed ihHCs. A group of four piglets was not given any NTBC and observed throughout life to confirm that the chronic phenotype of HT1 was consistent with previous findings.20 Group sizes were dictated by litter size.
Amino acid analysis and human albumin ELISA
Cord blood was obtained at cesarean section. For later samples, the femoral vein was accessed percutaneously through ultrasound guidance. Serum and plasma were separated using standard protocols. For amino acid analysis, blood was dried on 903 Protein Saver Cards (GE Healthcare, Pittsburgh, PA). Amino acid and succinylacetone levels were measured by tandem mass spectrometry, as described.21 Cord blood human albumin was measured in triplicate with an enzyme-linked immunosorbent assay (ELISA) quantification kit (Bethyl Laboratories, Montgomery, TX) as per manufacturer's instructions. Plates were read on an EPOCH 2 plate reader with GEN5 (version 2.09) software (BioTek, Winooski, VT).
Histopathology
Tissue samples were formalin fixed and paraffin embedded. For hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and trichrome staining, slides were prepared using standard protocols. FAH immunohistochemistry (IHC) was performed using a rabbit polyclonal anti-FAH antibody22 and a Bond III automatic stainer (Leica, Buffalo Grove, IL) with a 20-min antigen retrieval step using Bond Epitope Retrieval Solution 2 (Leica) and stained with diaminobenzidine (Leica). Slides were interpreted by four pathologists (T.M., M.S., M.B., and A.W.). Human albumin IHC was performed using a rabbit polyclonal anti-human albumin antibody (ab2406; Abcam, Cambridge, MA) in the same manner.
Flow cytometry
Piglet peripheral blood leukocytes (PBLs) were isolated from cord blood as per manufacturer's protocol with SepMate isolation tubes (Stemcell Technologies, Vancouver, BC). After isolation, PBLs were passed through a 70-μm filter to achieve a single-cell suspension and plated. Antibodies against CD45 (FITC conjugated, Clone K252.1E4; Bio-Rad, Hercules, CA), CD8 (PE conjugated, Clone 76-2-11; BD Biosciences, Franklin Lakes, NJ), CD3 (PE-cy7 conjugated, Clone BB23-8E6-8C8; BD Biosciences), CD4 (PerCP-Cy5.5, Clone 74-12-4; BD Biosciences), and CD335 (APC conjugated, Clone VIV-KM1; Bio-Rad) were used for staining at a dilution of 1:100. Viability was determined with Ghost Dye Red 780 (Tonbo Biosciences, San Diego, CA) at a dilution of 1:1000. BD LSRII and Cytek flow cytometers were used to run the samples. Data were analyzed using FlowJo, v10, software (FlowJo LLC, Ashland, OR).
Cytotoxicity assays
Whole blood was collected from piglets and washed in lysis buffer (Thermo Fisher Scientific, Waltham, MA) until no heme staining remained. ihHCs were plated and cultured according to the manufacturer protocol for 24–96 h, then incubated for 30 min with pig serum diluted 1:8 in manufacturer-recommended media. Cells were washed and incubated for 1 h with rabbit complement (Pel-Freez, Rogers, AR) diluted 1:16 in media. FlouroQuench (Thermo Fisher Scientific) was applied to each well. For comparisons of complement cytotoxicity between different assays, the percentage of cell death observed in complement-only wells was used as the baseline for each assay. This assay was repeated with the sterile bovine colostrum administered to newborn piglets (Huvepharma, Inc., Peachtree City, GA) to confirm lack of anti-human activity.
For NK cell-mediated cytotoxicity analysis, we first confirmed major histocompatibility complex-1 (MHC-1) expression on ihHCs undergoing in vitro maturation as per the manufacturer protocol. Maturing ihHCs were tested every 24 h to quantify the level of human MHC-I expression from day 0 to day 5 of in vitro culture using a human MHC-I ELISA kit (Proteintech, Rosemont, IL). Whole blood was collected from FR piglets, both having and not having undergone IUCT with human ihHCs, and washed in lysis buffer (Thermo Fisher Scientific) until no heme staining remained. PBL populations of interest (CD3+, CD3−CD8+, and CD3−CD8+CD335+) were sequentially depleted and selected from whole precipitate using MACS anti-mouse IgG microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and the CD3, CD8, and CD335 antibodies listed previously. ihHCs were cocultured with CD3+, CD3−CD8+, and CD3−CD8+CD335+ effector cells in 0.5:1, 1:1, and 2:1 ratios; the 2:1 ratio was selected for further use based on this calibration. Cytotoxicity of ihHCs by effector cells was measured by LDH release using the CyQUANT LDH cytotoxicity assay kit (Invitrogen, Carlsbad, CA).
Statistics
Data normality was assessed with Q-Q plots. Comparisons between two groups of non-normally distributed data were made with Wilcoxon signed-rank and Mann–Whitney U tests. Normally distributed data were assessed with t-tests. Linear regression coefficients were assessed with t-tests and regression correlation with R2 values; p-values were considered significant at the level of α < 0.05.
Experiment
FR swine were engineered and their immunodeficiency and hereditary tyrosinemia phenotypes were confirmed. At day 40 of gestation, 107 ihHCs were injected into each fetal liver. The same procedure was performed on immunocompetent, FAH-deficient control piglets.23 The level of ihHC engraftment was compared through the surrogate marker of serum human albumin concentration between immunodeficient and immunocompetent FAH-deficient piglets.
Rejection of ihHCs, both after engraftment in utero and later through portal vein injection, was monitored through serial measurements of serum human albumin. Cytotoxicity against ihHCs was studied in two ways. First, humoral cytotoxicity was assessed through an in vitro direct cytotoxicity assay against ihHCs using serum of immunodeficient piglets. Change in cytotoxicity was compared within individual piglets over serial measurements and compared among piglets with varying ihHC engraftment. Second, cell-mediated cytotoxicity was evaluated by isolating PBLs from FR swine and coculturing with ihHCs. The level of cytotoxicity against ihHCs was compared between increasingly pure isolates of NK cells. A second comparison was made between NK cells isolated from swine previously exposed to ihHCs through IUCT and NK cells from a control pig not previously exposed.
Results
FAH/RAG2-deficient (FR) pigs
Donor ihHCs were injected by IUCT into nine litters (48 FR fetuses) at gestational day 40. Following IUCT, 18 fetuses were lost before birth consistent with our previous experience.14 Subsequently, 30 piglets were born, of which eight survived to day 14. Thirteen piglets were lost due to perinatal complications. Nine piglets displayed failure to thrive within 14 days of birth and were euthanized. Four of the eight piglets surviving to 14 days received no NTBC to confirm the FAH-deficient phenotype. The remaining four pigs underwent NTBC cycling (7 days on and 7 days off) and ihHC engraftment was monitored. Infections requiring antibiotic therapy were common. The longest-living piglet underwent elective euthanasia after 106 days.
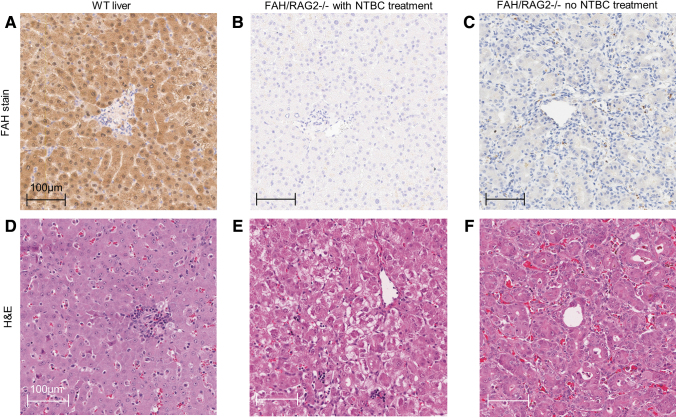
We first sought to confirm the presence of the expected FAH-deficient and immunodeficient phenotypes. Cord blood from FR piglets contained elevated levels of tyrosine (mean 246 μM, SD 74.8 μM) and succinylacetone (mean 1.66 μM, SD 0.473 μM), consistent with hereditary tyrosinemia. FAH enzyme activity of FR piglets was decreased significantly compared with WT pigs and similar to activity previously observed in immunocompetent FAH-deficient pigs (Supplementary Data S2 and Supplementary Fig. S2.1).24,25 Livers from FR pigs revealed no FAH-positive cells on IHC, while all hepatocytes of WT pigs were positive for FAH (Fig. 1A–C). WT livers and livers of FR pigs treated with NTBC were morphologically similar, while livers of FR piglets not receiving NTBC displayed dysplastic changes and abnormal architecture (Fig. 1D–F).
FIG. 1.
Immunohistochemical and H&E staining of liver tissue of FR pigs demonstrates no visible FAH+ hepatocyte xenograft and no inflammatory infiltrate or vasculopathy indicative of acute rejection. (A) WT tissue with FAH staining showing diffuse cytoplasmic expression. (B) FR pigs on NTBC, with FAH staining. (C) FR pigs on no NTBC treatment, with FAH staining. (B, C) Loss of FAH expression without visible FAH+ cells. (D) WT tissue with H&E staining. (E) FR pig on NTBC, with H&E staining. Hepatocellular ballooning is evident in zone 3. (F) FR pig on no NTBC treatment, with H&E staining, demonstrates dysplastic change with atypical hepatocyte architecture and pseudoacinar forms. Neither (E) nor (F) demonstrates vasculopathy or lymphocytic, neutrophilic, or macrocytic infiltrate, which would be evident with acute xenograft rejection. H&E, hematoxylin and eosin.
Despite detectable levels of human albumin in cord blood and later blood collections, we were not able to identify ihHCs as FAH-expressing hepatocytes by IHC in liver samples, nor were we able to confirm a mechanism of ihHC destruction, as liver tissue appeared void of lymphocytic, macrocytic, or neutrophilic infiltrate, lacked vasculopathy, and did not show visible evidence of immune rejection (Fig. 1E, F).
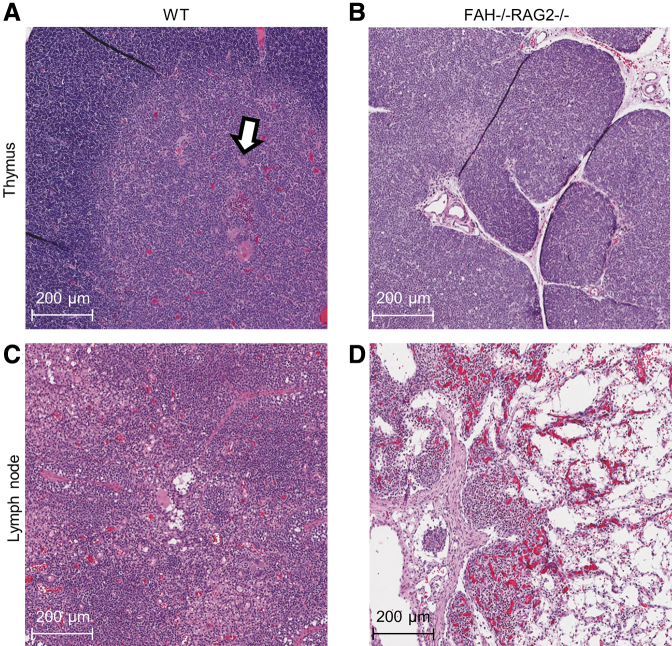
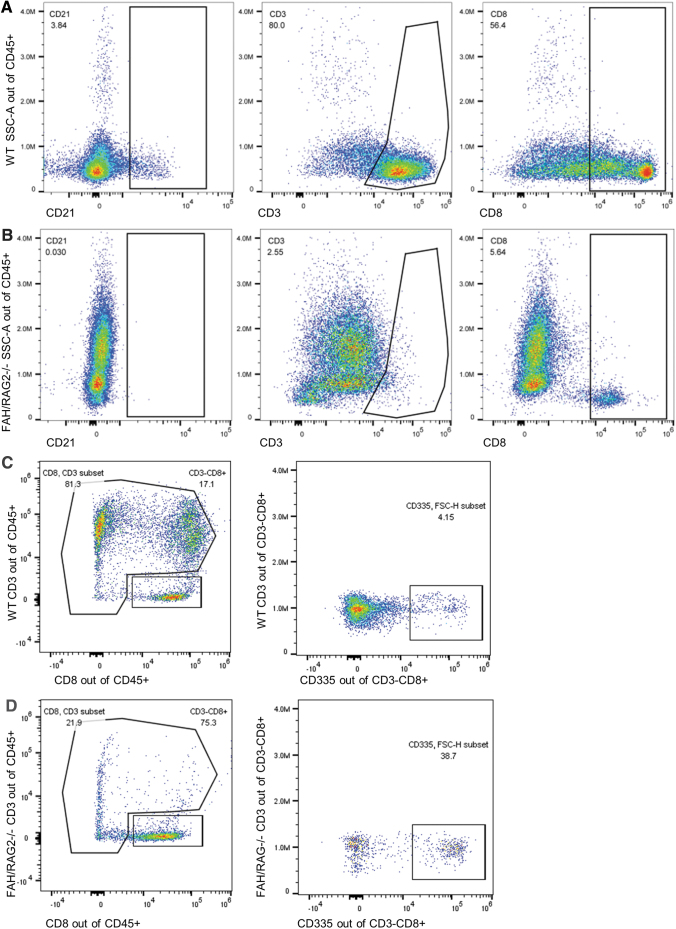
Having confirmed the FAH-deficient phenotype, we investigated the RAG2-immunodeficient phenotype. FR piglets were prone to infection despite being raised in a clean environment. Thymi of FR piglets were uniformly grossly hypoplastic and their thymic tissue displayed abnormal histology with decreased lobule size and lack of corticomedullary differentiation of maturing thymocytes when compared with WT pigs (Fig. 2A, B). Lymph nodes from FR pigs were grossly hypoplastic. On histology, we identified the stromal architecture of lymph nodes, including capsules and medullary sinuses, but the lymphocyte population was depleted compared with WT controls and replaced with fibrofatty tissue (Fig. 2B, C). Flow cytometry of PBLs was performed on cord blood. Compared with WT controls, FR piglets had severely depleted populations of circulating CD21+ B cells and CD3+ T cells (Fig. 3A, B).
FIG. 2.
H&E staining demonstrates a markedly abnormal structure of lymphoid organs in FR pigs consistent with the immunodeficient phenotype. (A) WT thymus with normal architecture and expected corticomedullary differentiation. Hassall's corpuscles (arrow) are seen. (B) FR pig thymus. FR pig sample demonstrates hypoplasia, reduction in lobule size, and lack of corticomedullary differentiation. (C) WT lymph node. (D) FR pig lymph node, with depleted lymphocyte population that has been replaced with fibrofatty tissue. Lymph node architecture is preserved.
FIG. 3.
Peripheral blood leukocytes of FR pigs demonstrate reduced T cell and B cell populations, but normal levels of NK cells. (A) WT pig serum forward and side scatter area distributions of CD21, CD3, and CD8 of CD45+ cells, nonspecific lymphocyte markers. (B) FR pig serum demonstrates significant absence of CD21+, decreased CD3+, and decreased CD8+ cells, consistent with the relative paucity of T cells and B cells in the RAG2−/− phenotype. (C) WT pig serum CD3 and CD8 sorting, then further analysis of the CD335 population of the CD3−CD8+ subset. (D) FR pig serum CD3 and CD8 sorting, demonstrating a CD3−CD8+ population that is not depleted when compared with the WT animal. Further gating of CD335+ and CD3−CD8+ demonstrates persistent populations of both CD335+ and CD335− cells.
Engraftment and rejection of ihHCs
As no engrafted ihHCs were visible on IHC targeting FAH, we investigated human albumin as a surrogate marker of human cell engraftment, as previously reported.14 It was remarkable that newborn FR piglets had higher concentration of human albumin in their cord blood at birth compared with immunocompetent Fah−/− RAG2+/+ control piglets (196.26 vs. 39.29 ng/dL, Mann–Whitney U test p = 0.008, Fig. 4A).
FIG. 4.

Serum human albumin production by transplanted human hepatocytes in FR piglets is increased at birth compared with immunocompetent piglets, while hepatocytes staining positive for human albumin are observed. Human albumin production subsequently shows a downtrend. (A) Serum human albumin at birth, measured in 50 piglets. FR pigs had significantly higher levels of human albumin at birth than control Fah−/− pigs (p = 0.008, Mann–Whitney U test). The addition of RAG2−/− increased engraftment of functional human hepatocytes in the porcine liver. (B) Serum human albumin in four FR piglets measured sequentially after birth. Pig A also received a portal vein injection of ihHCs on day 43 postbirth, indicated by (*) in the image, and serum human albumin concentration was measured on days 2, 10, and 27 after this injection. Human albumin levels declined across all time points in all piglets. (C) Representative anti-human albumin IHC slide of FR pig liver. Rare hepatocytes staining positive for human albumin (white arrows) are scattered throughout the livers of FR pigs who underwent IUCT with ihHCs on gestational day 40. No associated inflammatory cells are present. IUCT, intrauterine cell transplantation; ihHC, immature human hepatocyte.
Four piglets with detectable levels of human albumin at birth lived for more than 14 days; these FR piglets exhibited a steady decline in serum level of human albumin despite repeated cycling of NTBC (Fig. 4B). Additionally, one of these FR piglets underwent ultrasound-guided portal vein injection of 108 ihHCs on day 43 of life and again showed a steady decline in concentration of circulating human albumin despite NTBC cycling (Fig. 4B). Additionally, IHC of experimental livers targeting human albumin revealed rare positively stained hepatocytes consistent with low levels of ihHC engraftment (Fig. 4C).
Humoral cytotoxicity
Humoral cytotoxicity was determined from the presence of anti-ihHC antibodies in pig serum. Serum cytotoxicity against ihHCs was initially low at birth and rose in FR piglet serum collected at subsequent time points: 5.1%, (95% CI: [0.9%–9.2%], p < 0.05, paired t-test). A negative correlation was observed between serum cytotoxicity and circulating levels of human albumin in FR piglets (regression slope = −0.02% cytotoxicity/1 ng/dL albumin, p < 0.05, R2 = 0.17).
NK cell-mediated cytotoxicity
Having confirmed low levels of humoral cytotoxicity, we investigated cytotoxicity derived from the NK cell population of FR pigs as a mechanism for rejection of donor ihHCs. First, expression of the human MHC class 1 antigen was confirmed on ihHCs at all stages of maturation (data not shown). Presence of the MHC class 1 antigen on donor cells suggested a possible target for recognition by porcine NK cells. Next, using flow cytometry, we observed that peripheral blood of FR pigs had an isolated population of CD3−CD8+ cells, a combination of markers seen on NK cells (Fig. 3C, D). Consistent with that possibility, 38.7% of CD3−CD8+ cells were NKp46+ and 61.3% were NKp46−.26,27 We also confirmed that CD335+ NK cells were a subpopulation of the CD3−CD8+ cells (Fig. 3C, D). These results are consistent with T−B−NK+ cells previously described in RAG2-deficient pigs.13 In vitro cytotoxic activity against target ihHCs was determined in viable peripheral blood mononucleated cells collected from three pigs (Fig. 5). As expected, assuming predominantly NK cell-mediated cytotoxicity, increasingly pure subsets of porcine CD335+ cells corresponded to increasing cytotoxicity against ihHCs: the buffy coat was less cytotoxic than the CD3+ subset (p = 3 × 10−7) and the CD3+ subset was less cytotoxic than the CD3−CD8+ and CD335+ subsets (p = 0.0007 and p = 0.013, respectively). In vitro cytotoxic activity of the CD3−8+ subset and CD335+ cells was similar (p = 0.42). Since not all porcine NK cells express CD335, but all should be captured within the CD3−8+ subset, it follows that the majority of NK cell cytotoxicity against ihHCs was from the CD335+ subset.
FIG. 5.
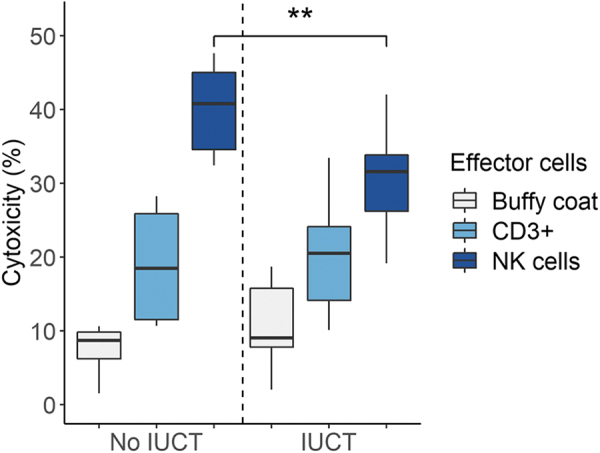
Concentration-dependent NK cell cytotoxicity was reduced after IUCT. PBLs of three FR pigs were tested for cytotoxic activity against ihHCs biweekly from 6 to 14 weeks of age; two pigs were post-IUCT and one pig was not post-IUCT. Cytotoxicity increased with further purification of NK cells. NK cell-mediated cytotoxicity was reduced in two FR pigs after IUCT compared with the non-IUCT FR pig (**p = 0.011, t-test). Cytotoxicity of the buffy coat and CD3+ cells was similar in all FR pigs. Of note, no significant trends in NK cell cytotoxicity were observed over time within an individual FR pig. PBL, peripheral blood leukocyte.
Consistent with the potential importance of NK cells as a barrier to engraftment of ihHCs, piglets that underwent IUCT with engrafted ihHCs from gestation day 40 until birth had lower NK cell cytotoxicity than uninjected RAG-deficient pigs (30.4% compared with 40.1%; p = 0.011, 95% CI for difference [2.7%–16.7%]). Lower cytotoxicity in pigs exposed to human cells was consistent with human chimerism and hypofunctioning adaptive immunity.
Discussion
Our study is the first to report the progress, potential, and remaining challenges of an immunodeficient swine model of FAH deficiency to generate human hepatocytes for clinical use. This study improves upon our previous work with the use of newer, more efficient gene editing technology to generate double knockout FR pigs.25 FAH deficiency was selected to deplete the native hepatocyte population in the setting of the liver's well-described, endogenous regenerative capacity, which we aimed to exploit.8 This model allowed investigation of barriers to large-scale expansion of human hepatocytes in the setting of a preferential metabolic advantage benefiting engrafted FAH+ ihHCs over mutant host hepatocytes.
We have functionally clarified the B−T−NK+ immunodeficient phenotype of RAG2 deficiency in FR pigs. Other reports describe RAG2-deficient animals as SCID and SCID-like animals. We provide data to support that these animals bear a milder immunodeficiency syndrome than previously thought. While RAG2 deficiency yielded significant improvement in fetal engraftment of ihHCs in immunodeficient versus immunocompetent swine, ihHCs present at birth did not undergo robust expansion in FR pigs with cycling of NTBC, as had been expected. Nevertheless, the milder immunodeficiency of FR pigs has advantages over a more severe SCID phenotype produced by adding additional gene knockouts. The potential for disease transmission from pigs to humans (i.e., porcine endogenous retrovirus [PERV]) during production of human hepatocytes in pigs is exceedingly small based on prior reports of humans treated with living pig tissues.28–30 Additionally, the risk of zoonosis may be further reduced both by following published xenotransplantation strategies as done herein and theoretically by permitting some swine host immune functions to allow suppression of host-derived infectious agents.31,32 Further investigation, including directly comparing the zoonotic potential between hosts of varying degrees of immunodeficiency, will be required before approval of immunodeficient swine-derived products.
While we agree with previous reports on severe depletion of mature B cells in RAG2-deficient pigs, we show a small amount of residual functional humoral cytotoxicity, which was not previously observed.13 This difference may be explained by our use of a different (CRISPR/Cas9) gene editing system to inactivate RAG2, leaving the adjacent RAG1 locus intact. Of note, RAG1 has been shown to inefficiently induce V(D)J recombinase activity in the absence of RAG2.33
Like in humans, porcine NK cells express the effector molecule, perforin, from birth and are capable of inducing cell death.26,34 Fetal NK cells have even shown the capability to induce cell death as early as 15 weeks of gestation.35 In our animals, the NK cell population remained physiologically intact and demonstrated significant cytotoxicity in vitro against human ihHC targets. While this is consistent with previous work describing robust NK cell-mediated rejection in xenotransplantation models even in the absence of T and B cell activity, to our knowledge, this is the first study to demonstrate differential NK cell cytotoxicity against xenogeneic targets introduced in utero.36–39 Furthermore, it has been shown that IUCT in the absence of an introduced foreign antigen does not itself induce rejection.40 This NK cell cytotoxicity was sufficient to overcome the selective advantage favoring FAH+ donor cells in the FAH-deficient liver and occurred despite our efforts to induce hyposensitization by IUCT. Preliminary data using mature human hepatocytes, as opposed to ihHCs, appear to show the same little and declining engraftment as presented herein using ihHCs (data not shown).
We conclude that the combined deficiency of FAH and RAG2 contributed to partial hyposensitivity and increased engraftment of ihHCs compared with WT pigs. However, donor ihHCs were recognized as foreign by the intact NK cell population of FR pigs. Therefore, new treatments are needed to eliminate the cytotoxic NK cell population of FR pigs and facilitate robust expansion of human hepatocytes. Both porcine CD335+ and CD335− NK cell populations have been shown to possess cytotoxic activity. These cell populations differ, however, as the CD335+ population has been shown to produce significantly more interferon-γ and CD335 expression can be induced in the CD335− population by interleukins 2, 12, and 18.27 Complex interactions between resting NK cells, activated NK cells, and supporting T cells support the premise that RAG2 deficiency may induce a partial state of tolerance toward donor xenografts through removal of T cell cytokine stimulation.
Moving forward, we hypothesize that the incomplete hyposensitization we described herein may be overcome by pretreatment with novel anti-NK cell agents such as blocking antibodies and suppressive drugs to eliminate residual immunity.41,42 In addition, we have made significant progress and recently produced our first litters of triple knockout FRG pigs with the addition of IL2rg−/− to the Fah−/−/RAG2−/− genotype (S.L.N. and J.B.L., personal communication). As in FRG mice bearing the same three deficiencies, these pigs are expected to be more severely deficient in their repertoires of T, B, and NK cells compared with our double knockout FR pig counterparts. The FRG pig is most promising as a model to remove the remaining immunological barriers to robust large-scale expansion of human hepatocytes that we have described herein. While other experimental techniques have been proposed for large-scale hepatocyte expansion, such as blastocyst complementation, many experimental and ethical barriers to these techniques remain. For example, evolutionarily divergent interspecies blastocyst complementation has consistently yielded low percentages of chimerism,43–47 and research to restrict the lineage of human pluripotent stem cells to non-neural tissue in developing chimeras is ongoing.48–50
Conclusions
We have produced a porcine model of FAH/RAG2 deficiency into which we transplanted ihHCs in utero. RAG2 immunodeficiency improved ihHC engraftment as demonstrated by increased serum human albumin at birth. The improvement in engraftment with genetic immunodeficiency provides evidence of cellular compatibility between swine and humans. With further refinements, such as anti-NK cell-directed therapies or further immunodeficiency (i.e., FRG pig), it can be realistically predicted that a porcine model could serve as an in vivo bioreactor for large-scale expansion of human hepatocytes. Such expansion will greatly benefit patients in need of supportive therapies and as an individualized approach to pharmaceutical applications of human hepatocytes.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Tom Meier, Jodi Scholz, Amy Andrews, Michael Blanco, and Kathryn LaVallee as well as Nicholas Rindels, Stacy Hall, Heidi Zell, Emily Freiermuth, Cassandra Fjeld, Kasey Strand, Christopher Tweite, Lindsey Grudem, and Duane Meixner for their assistance in the care of the animals in this investigation.
Authors' Contributions
E.N., E.L., A.S., K.W., and S.N. wrote the manuscript.
E.N., D.J.J., S.M., J.G., A.A.R., W.Z., Y.J., T.M., M.S., M.B., A.W., F.J., K.W., A.S., M.S., K.W., A.M., E.W., P.R., B.A., R.H., and S.N. performed the experiments.
E.L. and E.N. performed the statistical analysis.
E.W., R.P., B.A., J.L., A.J., R.H., J.P., and S.N. provided senior supervision.
Disclosure Statement
S.N., R.H., and J.L. are inventors of FAH-deficient swine. The FAH-deficient swine patent is owned by Mayo Clinic and Oregon Health and Science University and licensed to Cytotheryx (Rochester, MN). J.L. is a board member of Cytotheryx.
Funding Information
Funding sources include the National Institutes of Health (grants R01-DK56733 and P30DK084567), J. Willard and Alice S. Marriott Foundation, Mayo Foundation for Medical Education and Research, Wallace H. Coulter Foundation, and Regenerative Medicine Minnesota.
This article utilized resources at the National Swine Resource and Research Center funded by a U42 grant (U42 OD011140) from NIH Office of Research Infrastructure Programs. Genetically modified swine described herein are available using RRID NSRRC: 0085.
Supplementary Material
References
- 1. Matsunari, H., Nagashima, H., Watanabe, M., et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. PNAS 110, 4557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kobayashi, T., Yamaguchi, T., Hamanaka, S., et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Ogle, B.M., Butters, K.A., Plummer, T.B., et al. Spontaneous fusion of cells between species yields transdifferentiation and retroviral transfer in vivo. FASEB J 18, 548, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Ogle, B.M., Cascalho, M., and Platt, J.L.. Biological implications of cell fusion. Nature reviews. Mol Cell Biol 6, 567, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Grompe, M. Fah knockout animals as models for therapeutic liver repopulation. Adv Exp Med Biol 959, 215, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Aravalli, R.N. Generating liver using blastocyst complementation: opportunities and challenges. Xenotransplantation 28, e12668, 2021. [DOI] [PubMed] [Google Scholar]
- 7. Matsunari, H., Watanabe, M., Hasegawa, K., et al. Compensation of disabled organogeneses in genetically modified pig fetuses by blastocyst complementation. Stem Cell Reports 14, 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azuma, H., Paulk, N., Ranade, A., et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol 25, 903, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walters, E.M., and Prather, R.S.. Advancing swine models for human health and diseases. Mo Med 110, 212, 2013. [PMC free article] [PubMed] [Google Scholar]
- 10. Mestas, J., and Hughes, C.C.. Of mice and not men: differences between mouse and human immunology. J Immunol 172, 2731, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Seok, J., Warren, H.S., Cuenca, A.G., et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110, 3507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whyte, J.J., and Prather, R.S.. Genetic modifications of pigs for medicine and agriculture. Mol Reprod Dev 78, 879, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee, K., Kwon, D.N., Ezashi, T., et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A 111, 7260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher, J.E., Lillegard, J.B., McKenzie, T.J., Rodysill, B.R., Wettstein, P.J., and Nyberg, S.L.. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl 19, 328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogle, B., Knudsen, B., Nishitai, R., Ogata, K., and Platt, J.. Toward development and production of human T cells in swine for potential use in adoptive T cell immunotherapy. Tissue Eng Part A 15, 1031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitworth, K.M., Benne, J.A., Spate, L.D., et al. Zygote injection of CRISPR/Cas9 RNA successfully modifies the target gene without delaying blastocyst development or altering the sex ratio in pigs. Transgenic Res 26, 97, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitworth, K.M., Lee, K., Benne, J.A., et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod 91, 78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee, K., Redel, B.K., Spate, L., et al. Piglets produced from cloned blastocysts cultured in vitro with GM-CSF. Mol Reprod Dev 80, 145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross, J.W., Whyte, J.J., Zhao, J., Samuel, M., Wells, K.D., and Prather, R.S.. Optimization of square-wave electroporation for transfection of porcine fetal fibroblasts. Transgenic Res 19, 611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elgilani, F., Mao, S.A., Glorioso, J.M., et al. Chronic phenotype characterization of a large-animal model of hereditary tyrosinemia type 1. Am J Pathol 187, 33, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turgeon, C., Magera, M.J., Allard, P., et al. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin Chem 54, 657, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Wang, X., Montini, E., Al-Dhalimy, M., Lagasse, E., Finegold, M., and Grompe, M.. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol 161, 565, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hickey, R.D., Mao, S.A., Glorioso, J., et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res 13, 144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kvittingen, E.A., Jellum, E., and Stokke, O.. Assay of fumarylacetoacetate fumarylhydrolase in human liver—deficient activity in a case of hereditary tyrosinemia. Clin Chim Acta 115, 311, 1981. [DOI] [PubMed] [Google Scholar]
- 25. Hickey, R.D., Lillegard, J.B., Fisher, J.E., et al. Efficient production of Fah-null heterozygote pigs by chimeric adeno-associated virus-mediated gene knockout and somatic cell nuclear transfer. Hepatology 54, 1351, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Talker, S.C., Käser, T., Reutner, K., et al. Phenotypic maturation of porcine NK- and T-cell subsets. Dev Comp Immunol 40, 51, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Mair, K.H., Essler, S.E., Patzl, M., Storset, A.K., Saalmüller, A., and Gerner, W.. NKp46 expression discriminates porcine NK cells with different functional properties. Eur J Immunol 42, 1261, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Heneine, W., Tibell, A., Switzer, W., et al. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet 352, 695, 1998. [DOI] [PubMed] [Google Scholar]
- 29. Paradis, K., Langjord, G., Long, Z., et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science 285, 1236, 1999. [DOI] [PubMed] [Google Scholar]
- 30. Demetriou, A.A., Brown, R.S.Jr., Busuttil, R.W., et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg 239, 660; Discussion 667–670, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Onions, D.E., and Witt, C.J.. Xenotransplantation: an overview of microbiological risks and potentials for risk management. Rev Sci Tech 19, 289, 2000. [DOI] [PubMed] [Google Scholar]
- 32. Onions, D., Cooper, D.K., Alexander, T.J., et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation 7, 143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oettinger, M., Schatz, D., Gorka, C., and Baltimore, D.. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248, 1517, 1990. [DOI] [PubMed] [Google Scholar]
- 34. Rukavina, D., Laskarin, G., Rubesa, G., et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood 92, 2410, 1998. [PubMed] [Google Scholar]
- 35. Phillips, J.H., Hori, T., Nagler, A., Bhat, N., Spits, H., and Lanier, L.L.. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon, delta proteins. J Exp Med 175, 1055, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cascalho, M., and Platt, J.L.. The immunological barrier to xenotransplantation. Immunity 14, 437, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Xia, G., Ji, P., Rutgeerts, O., and Waer, M.. Natural killer cell- and macrophage mediated discordant guinea pig—>rat xenograft rejection in the absence of complement, xenoantibody and T cell immunity. Transplantation 70, 86, 2000. [PubMed] [Google Scholar]
- 38. Li, S., Waer, M., and Billiau, A.D.. Xenotransplantation: role of natural immunity. Transpl Immunol 21, 70, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Lin, Y., Vandeputte, M., and Waer, M.. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J Immunol 158, 5658, 1997. [PubMed] [Google Scholar]
- 40. Peranteau, W.H., Endo, M., Adibe, O.O., and Flake, A.W.. Evidence for an immune barrier after in utero hematopoietic-cell transplantation. Blood 109, 1331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guerrero, A. A2a adenosine receptor agonists and their potential therapeutic applications. An update. Curr Med Chem 25, 3597, 2018. [DOI] [PubMed] [Google Scholar]
- 42. Kuldova, M., Svoboda, J., Kovaru, F., Vannucci, L., Kovaru, H., and Fiserova, A.. NK cell-mediated cytotoxicity modulation by A2 adenosine receptor agonist in different mammalian species. Folia Microbiol 54, 364, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Tan, T., Wu, J., Si, C., et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell 184, 2020–2032. e2014, 2021. [DOI] [PubMed] [Google Scholar]
- 44. Wu, J., Platero-Luengo, A., Sakurai, M., et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 168, 473–486.e415, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Das, S., Koyano-Nakagawa, N., Gafni, O., et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol 38, 297, 2020. [DOI] [PubMed] [Google Scholar]
- 46. Nowak-Imialek, M., Wunderlich, S., Herrmann, D., et al. In vitro and in vivo interspecies chimera assay using early pig embryos. Cell Reprogram 22, 118, 2020. [DOI] [PubMed] [Google Scholar]
- 47. Fu, R., Yu, D., Ren, J., et al. Domesticated cynomolgus monkey embryonic stem cells allow the generation of neonatal interspecies chimeric pigs. Protein Cell 11, 97, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Masaki, H., Kato-Itoh, M., Takahashi, Y., et al. Inhibition of apoptosis overcomes stage-related compatibility barriers to chimera formation in mouse embryos. Cell Stem Cell 19, 587, 2016. [DOI] [PubMed] [Google Scholar]
- 49. Kobayashi, T., Kato-Itoh, M., and Nakauchi, H.. Targeted organ generation using Mixl1-inducible mouse pluripotent stem cells in blastocyst complementation. Stem Cells Dev 24, 182, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park, C.H., Jeoung, Y.H., Uh, K.J., et al. Extraembryonic Endoderm (XEN) cells capable of contributing to embryonic chimeras established from pig embryos. Stem Cell Reports 16, 212, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.