Abstract
Colorectal cancer (CRC) incidence among individuals age younger than 50 years continues to rise with etiologies unknown. Synchronously, early-onset CRC disparities have grown more pronounced. As translational research across diverse populations has been limited, we advocate for mechanistic studies that address this challenge to mitigate CRC disparities among young adults.
Despite reductions in the overall colorectal cancer (CRC) burden, the incidence of CRC among individuals younger than age 50 (early-onset CRC; EOCRC) has increased over the past two decades worldwide. Long-term projections suggest that by 2030, approximately one in ten colon cancer and one in four rectal cancer diagnoses in the US will be EOCRC1. Yet across diverse populations, the CRC — and EOCRC — burden sharply varies. By sex, CRC incidence rates are nearly one-third higher among men, but women have a higher incidence of right-sided colon cancers. Among patients with EOCRC, men harbor a 12–18% increased hazard of disease-specific death versus women2. The proportion of individuals with EOCRC is also nearly twofold higher among people of colour versus white people. The disproportionate burden of CRCs in young African Americans (AAs) may reflect larger shares of this adult population that are <37 years of age. However, as clinical features of EOCRC differ across racial or ethnic groups, and survival after disease diagnosis is significantly worse among AAs versus white people, these data point to unique molecular underpinnings of EOCRC.
Evidence is accumulating that suggests the biology of EOCRC is unique compared with CRCs diagnosed among individuals age 50+ years. Young patients often experience more aggressive disease when compared to cases diagnosed at older ages. Differences in genomic landscapes have emerged between EOCRC versus late-onset CRC. Integrated, multi-omics study of EOCRC has recently implicated deregulated redox homeostasis — via perturbation of NRF2-mediated oxidative stress response, glutathione metabolism and the CXCL12–CXCR4 chemokine pathway — as a distinct molecular phenotype of EOCRC among European patients3. It is very likely that future studies will also reveal key pathways unique to EOCRC. However, in the absence of studies that examine the biology of EOCRC and molecular mechanisms underpinning disease development and progression across diverse populations, there remains a significant barrier in translating these findings and reducing the disproportionate CRC burden among young individuals worldwide.
EOCRC disparities
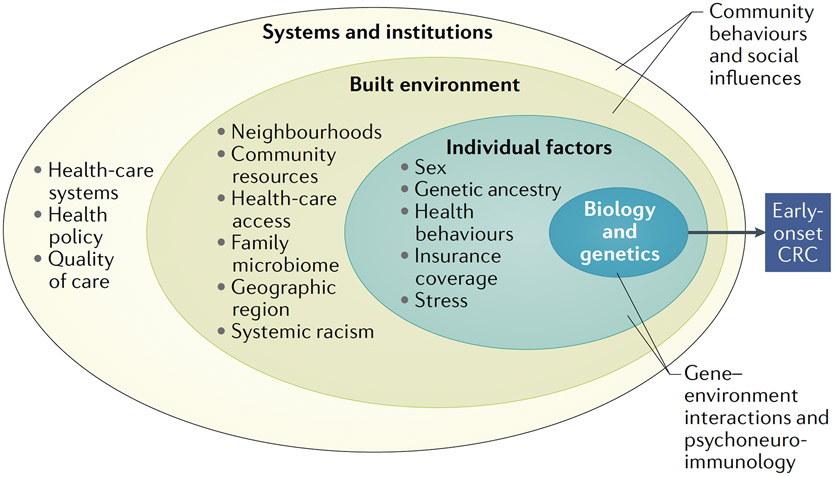
Distinct EOCRC patterns across diverse population subgroups reflect a complex interplay of biology, genetics, behaviour and social determinants of health (FIG. 1). Yet among these factors that contribute to EOCRC disparities, biology is the least understood. Consequently, advancement in our understanding of the molecular mechanisms that drive EOCRC disparities is an indispensable step in a multifaceted approach toward reducing and mitigating inequities.
Fig. 1 ∣. Multi-level factors and domains contributing to early-onset CrC disparities.
CRC, colorectal cancer.
Biological clues
Initial insights into potential molecular mechanisms contributing to the pronounced racial and ethnic disparities in EOCRC posit roles for epigenetics and immune responses. Epigenome-wide analysis of DNA methylation in normal colons from AA and white individuals of all ages identified differences in epigenetic ageing of normal colon by race4. Among AA individuals, accelerated epigenetic ageing was observed in the right colon versus individual-matched left colon. Conversely, age deceleration was observed in the right colon among white individuals. As DNA methylation is largely influenced by the environment, questions remain as to how these exposures accumulate and how they may contribute to different epigenetic profiles across populations, as well as how these patterns might associate with EOCRC risk. Interestingly, immune response profiles may also contribute to biologic differences in EOCRC by race or ethnicity. For example, colon tumours from AA patients may have impaired anti-tumour immunity as compared with tumours from white patients5. In light of the high prevalence of the microsatellite instability (MSI)-immune subtype — defined in part by MSI, immune infiltration and activation, and hypermutation — previously reported among patients with EOCRC6, immune-specific patterns may present potential molecular targets for mechanistic study across diverse EOCRC groups.
While the biological mechanisms contributing to EOCRC disparities by sex remain unknown to date, variation in life-course exposure to sex-steroid hormones could contribute to sex-specific metabolic phenotypes of CRC. Colonic length also differs between men and women7. As the gut milieu — comprised of metabolites produced from the environment, microbiome and diet — may differ along the length of the colorectum, this may partly account for sex-specific influences in CRC metabolism. This is supported by recent study of normal colon and primary tumour tissues that showed levels of metabolites involved in the pentose phosphate pathway and glycolysis differ by sex8. For women with right-sided colon cancers, enhanced asparagine metabolism may also increase serine and threonine uptake to promote tumour development. However, as these findings derive from a patient cohort age 55+ years, future studies will need to discern whether comparable results are observed among patients with EOCRC.
Social and environmental factors (including chronic exposure to adverse social conditions and systemic racism) that may influence mechanisms of CRC progression remain largely unknown. Chronic stress may impair the immune response and contribute to disease progression. In mice injected with colon cancer cells in the spleen, chronic stress elevated catecholamine levels and promoted liver metastasis that was reversed with β-blocker treatment9. Thus, it will be critical to consider the biological impact of stress and mediators of stress on mechanisms of disease progression within the EOCRC population.
Although initial evidence provides clues to the biology of CRC disparities, there is a dearth of studies specifically focused on dissecting the molecular mechanisms underlying EOCRC disparities. Such work is essential to tailor prevention and intervention strategies within diverse populations, and to close the gaps in health disparities among young patients with CRC.
The path forward
Amid the current absence of large-scale -omics studies across diverse subgroups within the EOCRC population, the advent of EOCRC organoids provides an essential model system for biological studies to characterize molecular alterations and to explore distinct tumour drivers, biomarkers and therapeutic targets. For example, the derivation of organoids using tissues collected from 20 patients with microsatellite-stable EOCRC in Hong Kong revealed novel oncogenic pathway cooperativity intricately linked to signaling processes along the colonic crypt axis among patients with EOCRC carrying a PTPRK–RSPO3 fusion10. While the generalizability of this biobank may be limited across population subgroups, these results illustrate the importance of leveraging patient-derived models, such as organoids and xenografts, to elucidate the biologic mechanisms underpinning EOCRC disparities. Overall, there is an imminent need to integrate mechanistic approaches with human studies to unravel molecular underpinnings of EOCRC disparities.
Conclusion
We advocate for studies on the biology of EOCRC across diverse populations as these should deliver an abundance of knowledge in the race to unravel the underpinnings of this epidemic. We also believe that collaborations will be vital toward achieving this goal worldwide and to bring scientific breakthroughs to all diverse populations diagnosed with EOCRC. Together, these discoveries should translate into personalized EOCRC prevention and detection strategies and therapeutic modalities that should mitigate disparities and reduce the global burden of EOCRC.
Acknowledgements
A.N.H. was supported by the National Institutes of Health (NIH) K12 HD043483 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. This work was also supported by the American Cancer Society (#IRG-19-139-59) to A.N.H., and NIH/National Cancer Institute R01CA229259 to C.H.L.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bailey CE et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 150, 17–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holowatyj AN et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J. Clin. Oncol 34, 2148–2156(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holowatyj AN et al. Distinct molecular phenotype of sporadic colorectal cancers among young patients based on multiomics analysis. Gastroenterology 158, 1155–1158 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devall M et al. Racial disparities in epigenetic aging of the right vs left colon. J. Natl Cancer Inst 10.1093/jnci/djaa207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes J et al. Immune-related gene expression and cytokine secretion is reduced among African American colon cancer patients. Front. Oncol 10, 1498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willauer AN et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 125, 2002–2010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders BP et al. Why is colonoscopy more difficult in women? Gastrointest. Endosc 43, 124–126 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Cai Y et al. Sex differences in colon cancer metabolism reveal a novel subphenotype. Sci. Rep 10, 4905 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L et al. Effect of chronic psychological stress on liver metastasis of colon cancer in mice. PLoS ONE 10, e0139978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HHN et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut 69, 2165–2179 (2020). [DOI] [PubMed] [Google Scholar]