Abstract
The population of adult survivors of childhood cancer continues to grow as survival rates improve. While it is well-established that these survivors experience a variety of complications and comorbidities related to their malignancy and treatment, this risk is modified by many factors that are not directly linked to their cancer history. Research evaluating the influence of patient-specific demographic and genetic factors, premorbid and comorbid conditions, health behaviors and aging has identified additional risk factors influencing cancer treatment-related toxicity and possible targets for intervention in this population. Furthermore, although current long-term follow-up guidelines comprehensively address specific therapy-related risks and provide screening recommendations, the risk profile of the population continues to evolve with ongoing modification of treatment strategies and emergence of novel therapeutics. To address the multifactorial modifiers of cancer treatment-related health risk and evolving treatment approaches, a patient-centered and risk-adapted approach to care that often requires a multidisciplinary team approach including medical and behavioral providers is necessary for this population.
Keywords: Adolescent, Antineoplastic Agents, Cancer, Child, Delivery of Health Care, Neoplasms, Risk, Survivors, Treatment Outcome
Introduction
With therapeutic advances over the past decades, 5- and 10-year survival rates for childhood cancer now exceed 80%.1,2 This progress permits increased focus on the importance of reducing morbidity and mortality related to the prior childhood cancer experience while continuing research aimed at further increasing survival rates. In 2010 there were an estimated 379,100 survivors of childhood cancer in the United States, an increase of over 50,000 from estimates five years prior, with numbers anticipated to reach 500,000 by 2020 based on current incidence and long-term survival rates.3 Childhood cancer survivors experience a substantial burden of chronic disease that increases in prevalence with advancing time from completion of therapy. Given the unique health risks associated with childhood cancer and its therapy, a risk-based and patient-centered care approach for individual survivors focusing on both therapeutic exposure related risks and patient-specific factors outside of treatment is recommended to anticipate and comprehensively address the complications and comorbidities affecting this growing population.4,5
Pediatric oncologists have pioneered risk-adapted therapy based on cancer and host characteristics in an effort to optimize disease outcomes and reduce late effects. Emerging research into primary and secondary prevention strategies is further informing risk-based care of this population. Despite this, we are only beginning to understand the complex interplay of the multiple factors contributing to morbidity in a given childhood cancer survivor. Similar to the heterogeneity of chronic and comorbid conditions in the general population, factors such as genetics, health behaviors, aging and health care access and utilization modify outcomes within the survivor population. Understanding these modifiers and how we may influence the impact of late effects through tailored primary cancer therapies and early diagnosis and intervention for late effects are key to improving the care of childhood cancer survivors into adulthood.
In this review, we will briefly discuss prior research regarding disease- and therapy-related toxicities and then explore emerging areas of survivor research that consider the complex nature of late effects and possible targets for mitigation. We will specifically address not only cancer- and therapy-related risk factors, but also genetic and patient-specific factors as they relate to risk for toxicity. Additionally, we will explore how comorbid conditions, aging, health-related behaviors, as well as health care access and utilization, influence outcomes and should be considered in a risk-based approach to care of the childhood cancer survivor (Figure 1).
Figure 1.
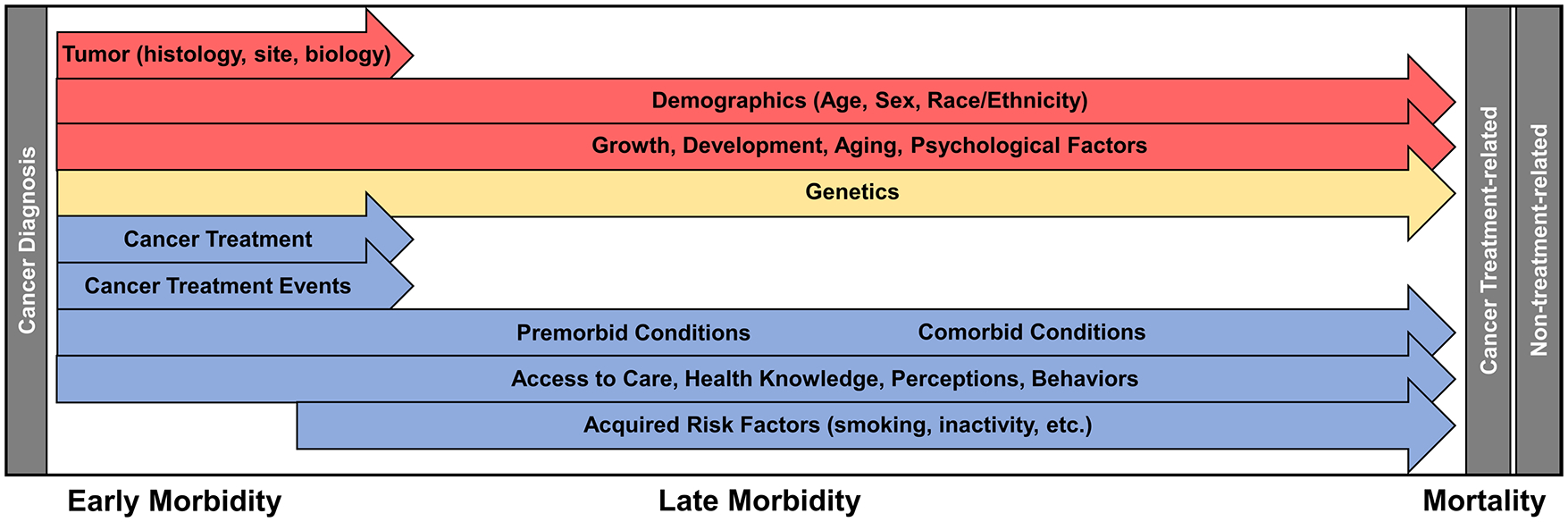
Factors influencing morbidity and mortality of the childhood cancer survivor. Each arrow demonstrates a different factor affecting morbidity and mortality which exert their effect along a continuum of care. Note that all effectors can begin exerting influence on morbidity during the period of cancer-directed therapy. Factors are separated into those that cannot be modified (red), those for which future interventions are plausible (yellow) and those for which there are known targets for interventions or areas where therapy and surveillance have already been modified (blue).
Disease and Therapy Related Toxicities
Childhood cancer encompasses a heterogeneous group of malignant conditions, arising from histologically distinct tissues. Overall, the most common types of childhood cancer include leukemia, specifically acute lymphoblastic leukemia (ALL), central nervous system (CNS) tumors and lymphomas.1 A number of disease-specific factors impact the type and intensity of cancer therapy received, and thus risk of treatment-related toxicities. Treatment, including chemotherapeutic agents and doses, varies widely and typically involves multi-modal therapy in children with biologically aggressive or advanced malignancies. Previous research has investigated treatment-related toxicities by diagnosis and individual chemotherapeutic agent (Table 1). For example, among survivors of ALL and CNS tumors, patterns of executive functioning differ despite sharing common exposures, such as CNS-directed chemotherapy and cranial irradiation.6 Additionally, cancer histology differs within diagnostic groups, which has implications for risk stratification and outcome. For instance, in rhabdomyosarcoma, prior studies have established that the alveolar subtype confers poor prognosis for survival, which has led to the use of more intensive treatment approaches for children with this histology compared to those with the more favorable embryonal subtype.7
Table 1.
Organ System Dysfunction Associated with Pediatric Cancer Therapy
| Organ System | Specific Late Effect | Therapeutic Exposure | Modifying Factors |
|---|---|---|---|
| Auditory | Sensorineural hearing loss Tinnitus Vertigo |
Cisplatin Carboplatin Radiation impacting the ear |
Younger age (< 4 years) at treatment at higher risk |
| Tympanosclerosis Otosclerosis Eustachian tube dysfunction Conductive hearing loss |
Radiation impacting the ear | Younger age at treatment at higher risk | |
| Cardiac | Cardiomyopathy Arrhythmias Subclinical left ventricular dysfunction Valvular Disease Pericardial Disease |
Anthracyclines (daunorubicin, doxorubicin, idarubicin, epirubicin) Radiation impacting the heart |
Younger age (< 5 years) at treatment at higher risk for all except pericardial disease Females at higher risk of cardiomyopathy and valvular disease Family history Genetic factors (variants effecting anthracycline metabolism) Health behaviors (diet, exercise, alcohol, smoking) Cardiovascular risk factors (hypertension, obesity, diabetes) Non-Hispanic Black race increases risk |
| Vascular | Coronary artery disease including myocardial infarction Carotid or subclavian artery disease Stroke Moyamoya Occlusive cerebral vasculopathy |
Radiation impacting cardiovascular or cerebrovascular structures | History of Down syndrome, sickle cell disease or neurofibromatosis Female gender protective for myocardial infarction Non-Hispanic Black race increases risk Family history Health behaviors (diet, exercise, alcohol, smoking) Cardiovascular risk factors (hypertension, obesity, diabetes) |
| Organ System | Specific Late Effect | Predisposing Therapy | Modifying Factors |
| Endocrine/ Metabolic | Overweight/obesity | Radiation impacting the brain (≥18 Gy) or TBI Abdominal radiation Hypothalamic injury from tumor growth or surgery |
Younger age (<10 years) at treatment Girls at higher risk Health behaviors (diet, exercise) Mental health (certain antidepressant use) |
| Metabolic syndrome Diabetes mellitus |
Radiation impacting the brain (≥18 Gy) or TBI Abdominal radiation Cisplatin (with CRT) Glucocorticoids HSCT |
Younger age (< 4 years) at treatment Minority populations at higher risk Health behaviors (diet, exercise) |
|
| Primary Hypothyroidism | Radiation impacting thyroid gland HSCT (due to antibody transfer) |
Younger age at irradiation at higher risk Girls at higher risk |
|
| Hypothalamic/pituitary dysfunction (growth hormone deficiency, central precocious puberty, LH/FSH deficiency, TSH deficiency, ACTH deficiency) |
Tumors located within or near the hypothalamus Radiation impacting hypothalamic-pituitary axis including TBI (dose dependent response with growth hormone being most sensitive Tyrosine Kinase Inhibitors |
Radiation-related deficiencies may appear at lower doses with longer follow-up | |
| Decreased bone mineral density | Methotrexate Glucocorticoids TBI |
Health behaviors (weight bearing exercise, smoking) | |
| Secondary Thyroid Cancer | Irradiation of the neck | Background risk increased in patients with genetic predisposition (DICER 1, PTEN) | |
| Hepatic | Chronic hepatitis C | Blood product transfusion prior to donor screening implemented in 1992 | Health behaviors (IV drug abuse) |
| Hepatic dysfunction Hepatic steatosis/cirrhosis |
Thioguanine Methotrexate Mercaptopurine Busulfan History of treatment related veno-occlusive disease |
History of chronic hepatitis Health behaviors (alcohol use) Obesity Diabetes mellitus Concurrent statin therapy |
|
| Immune/spleen | Asplenia Hyposplenia |
Radiation (≥ 40 Gy) impacting spleen Splenectomy |
History of chronic graft versus host disease |
| Musculoskeletal | Low lean muscle mass/weakness Decreased exercise capacity Frailty or accelerated aging |
Possible effects of: Corticosteroids Doxorubicin Radiation therapy impacting brain, abdomen or pelvis Lower extremity amputation |
Female sex Comorbid conditions (endocrine dysfunction) Lifestyle (physical activity) Health Behaviors (smoking) |
| Scoliosis | Radiation therapy impacting chest wall or thoracic spine Chest wall resection |
||
| Nervous system | Peripheral sensory or motor Neuropathy |
Cisplatin Vinca alkaloids |
History of Charcot-Marie-Tooth disease |
| Clinical leukoencephalopathy (spasticity, ataxia, dysarthria, dysphagia) Hemiparesis Seizures |
Methotrexate (high dose intravenous or intrathecal) Cytarabine (high dose intravenous or intrathecal) Radiation impacting the brain |
Younger age at treatment at higher risk | |
| Neurocognitive deficits | Methotrexate (high dose intravenous or intrathecal) Radiation impacting the brain Corticosteroids in patients not receiving radiation |
Younger age at treatment at higher risk Female sex at higher risk Psychologic and socioeconomic factors |
|
| Pulmonary | Interstitial pneumonitis Pulmonary fibrosis Restrictive lung disease Obstructive lung disease |
Bleomycin Busulfan Carmustine (BCNU) Lomustine (CCNU) Radiation impacting the lungs |
Younger age at treatment at higher risk Health behaviors (smoking) Occupational exposures |
| Renal | Glomerular toxicity Tubular toxicity (renal tubular acidosis, Fanconi’s syndrome, hypophosphatemic rickets) |
Cisplatin Carboplatin Ifosfamide Radiation impacting the kidney Surgical resection of kidney |
Mononephric Comorbid conditions (HTN, diabetes) |
| Urinary Tract - Bladder | Hemorrhagic cystitis Bladder fibrosis Dysfunctional voiding Vesicoureteral reflux Hydronephrosis |
Cyclophosphamide Ifosfamide Radiation impacting the bladder Surgery impacting the bladder/bladder outlet |
No predisposing host factors |
| Reproductive system | Infertility Reduced ovarian reserve |
Cyclophosphamide or other alkylating therapy Heavy Metals Radiation therapy impacting gonads |
Risk increases with age at exposure in females. |
| Sexual Dysfunction | Pelvic floor surgery – female Bladder/prostate surgery – males Surgery affecting spinal cord |
Health behaviors (alcohol, illicit drug use) Comorbid endocrine dysfunction Mental health disorders |
|
| Vision | Cataracts | Busulfan Radiation impacting the eye |
CRT = cranial radiation therapy; HSCT = Hematopoietic stem cell transplant
Anticipated late effects of therapy are frequently related to age at exposure. Thus, it is relevant to note that incidence rates for diagnostic groups vary with age; Hodgkin lymphoma and bone tumors become more common as age increases, while renal and eye/orbital tumors follow the inverse pattern.1 Genetic factors may also impact risk for late effects. Some malignancies, such as Wilms tumor, hepatoblastoma and neuroblastoma, may be associated with genetic or developmental anomalies. As a specific example, Wilms tumor and hepatoblastoma occur at increased rates in the first decade of life in children with Beckwith-Wiedemann Syndrome.8 Other genetic anomalies or associated cancer predisposition syndromes (e.g. Li-Fraumeni Syndrome) may confer an increased lifetime cancer risk and require additional considerations during treatment planning to avoid certain exposures, such as radiation therapy.9 Genetic and genomic testing can help assess the personal risk of cancer development based on a germline mutation, and also can be informative in predicting tumor behavior. The current molecular classification of medulloblastoma, a CNS tumor, has four distinct subgroups, WNT, SHH, group 3 and group 4, which characteristically associate with mean age of diagnosis, tumor behavior, and treatment outcomes.10 This has motivated the development of recent clinical trials evaluating therapy reductions in the favorable risk WNT sub-group, including significantly decreased craniospinal irradiation (CSI) doses.11
The extent of disease and response to therapy are additional factors that affect outcomes. Greater tumor burden at diagnosis may correlate to higher stage or higher risk disease requiring more intensified therapy as compared to the patient with minimal disease. In ALL, recent studies have focused on stratification of CNS-directed therapy based on historical associations between CNS status at diagnosis with CNS relapse risk and overall survival. The Children’s Oncology Group (COG) and other groups have focused on restricting the use of CNS irradiation to the highest risk patients and at minimal doses.12 Others have demonstrated comparable event-free survival with elimination of cranial irradiation in first-line therapy by intensifying CNS-directed systemic and intrathecal chemotherapy.13 Likewise, the significance of minimal residual disease (MRD) in ALL as an important prognostic indicator during induction therapy has been elucidated. The use of this technology has refined risk-directed therapy by placing increased numbers of patients in provisional low risk groups and reserving therapy intensification for poor responders.14–16 With response-guided therapeutic modifications, we expect decreased neurocognitive deficits, as well as increased numbers of patients treated for high-risk ALL in the next generations of ALL survivors.
Late effects research has provided strong evidence for associations between therapeutic exposures and risk of specific outcomes, and in doing so has guided risk-adapted treatment, to limit exposures for those with favorable presentations, as well as surveillance strategies (Table 1). Specific toxicities for many chemotherapeutic agents are well known, such as the risk of infertility with the use of alkylating agents, cardiomyopathy associated with anthracycline use and ototoxicity related to platinum-based therapy.17 Research has established dose-response relationships for the adverse effects from many of these agents, helping to guide therapeutic decisions, as well as targeted screening of childhood cancer survivors.3 However, there is still much to learn regarding the interplay of patient specific factors, combined modality therapies and future lifestyle influences on chemotherapy related late effects.
Similarly, radiotherapy for children has evolved during the past two decades and advancements in delivery technologies have significant implications for late effects.18 A rise in the utilization of intensity-modulated radiation therapy (IMRT), a type of photon therapy allowing improved dose conformation around the tumor and organs at risk, has led to concerns of increased secondary malignancy risk. These concerns relate to low-dose radiation exposure to a larger volume of uninvolved tissues with the increase in number of fields needed to deliver IMRT.19 These exposures can be reduced through the use of proton radiotherapy, and with respect to adverse toxicities, early data suggest a potential benefit over photon radiotherapy,20 when radiation is planned for structures with well-defined tolerances (cochlea, salivary glands, lung, bowel, kidneys, etc.).21 For example, the shift to proton radiotherapy is anticipated to substantially reduce specific toxicities, such as hearing loss via reduced cochlear dose with conformal boost methods,22 primary hypothyroidism and cardiac dysfunction due to the elimination of an exit dose from posteriorly directed CSI in both leukemia and brain tumor patients,23,24 and neurocognitive decline by reducing exposure to healthy brain regions.25 Organs known to be at high risk for morbidity from the combined effects of chemotherapy and radiation, such as the heart, may be more successfully spared with these novel techniques.26,27 Future therapeutic trials should incorporate organ sparing and radiation dose reduction strategies in a disease and site specific manner.28
Like radiotherapy, surgery plays an important role in local control of many pediatric solid tumors, with long-term effects better appreciated as overall survival for childhood cancer has improved. Adverse effects of surgical local-control procedures on organ function, functional status and quality of life have prompted evolution from radical resections to organ/tissue sparing surgeries and increased utilization of non-invasive strategies that leverage advances in diagnostic imaging technologies. For example, treating Wilms tumor in non-syndromic children with unilateral radical nephrectomy, without nephrotoxic chemotherapy or ionizing radiation, appears to result in low risk for significant long-term contralateral renal dysfunction.29 In contrast, survivors with bilateral Wilms tumor or those with syndromic Wilms tumor (WAGR: Wilms tumor + aniridia + genitourinary malformation + mental retardation, and Denys-Drash syndrome) are at a substantially higher risk for long-term renal dysfunction.30 Thus, the recommendation for nephron-sparing surgery for bilateral Wilms tumor balances dual goals of achieving local control and long-term survival while decreasing the risk of renal dysfunction. Likewise, surgical resection represents a common strategy for achieving local control of CNS tumors that often confers high risk for morbidity and premature mortality.31 In patients with craniopharyngioma who are often treated with surgery alone, the location of the tumor inherently results in organ dysfunction (pan-hypopituitarism), functional impairment (visual deficits, strabismus and facial nerve palsy), and other chronic health conditions (obesity).32 Recognition of late-effects associated with hypothalamic injury has resulted in recommendations for an experienced, multidisciplinary treatment approach including hypothalamus-sparing surgery with or without the addition of irradiation, preferably proton therapy, for unfavorably located tumors.32
Long-term effects of surgery on functional status are well appreciated, such as in survivors requiring chest wall resection in whom scoliosis is common and may lead to impaired pulmonary function and decreased exercise tolerance.33,34 In survivors of extremity sarcomas, low scores on physical performance measures are reported, but not on mental or emotional measures.35 In this population, limb-salvage surgery seems to offer better gait efficiency and return to normal living compared with above-knee amputation, but neither influence quality of life nor subjective well-being.36
Health outcomes research is continually expanding with the discovery and integration of novel therapies including new chemotherapeutic agents, targeted therapies, immunotherapy and new or improved hematopoietic stem cell transplant (HSCT) techniques. As an example, the use of tyrosine kinase inhibitors (TKIs) is increasing in pediatric leukemia management and we are beginning to learn the associated long-term effects, primarily from patients previously treated for chronic myelogenous leukemia (CML). Endocrinopathies associated with off-target action of TKIs include abnormal growth, impaired thyroid function and bone remodeling, as well as concerns for changes in pubertal development and glucose metabolism.37 Additionally, as individuals are living longer after intensified multimodal therapy (e.g. survivors of high-risk neuroblastoma or those who received allogeneic HSCT as part of their therapy), continued follow-up to establish long-term outcomes is needed. As with all such research in pediatric cancer patients, challenges in determining therapeutic exposure risks and appropriate surveillance exist due to the variable nature of therapy and the latency to presentation of many late effects.
Patient-Specific Demographic Factors
Risk of adverse late health outcomes among childhood cancer survivors not only differs by primary cancer diagnosis and treatment exposure, but may vary according to patient-specific factors such as sex, race/ethnicity and age at diagnosis. Male survivors have higher absolute rates of late mortality (death ≥5 years from diagnosis), which is attributable to the lower life expectancy of males in the general population. Conversely, standardized mortality ratios, which compare the observed number of deaths in survivors to expected numbers based on general population rates and thus account for the sex difference in the general population, are higher in female survivors than males, suggesting that the long-term impact of cancer and its treatment may disproportionally increase risk for death in females.38–40 In the Childhood Cancer Survivor Study, which examined 34,033 five year survivors of childhood cancer in North America, female survivors had higher standardized mortality ratios (SMRs) for all-cause mortality (females, SMR 21.2; males, SMR 10.0) as well as for mortality due to health-related causes (females, SMR 16.4; males, SMR 10.9), subsequent neoplasms (females, SMR 28.4; males, SMR 22.8), and cardiac (females, SMR 16.1; males, SMR 9.8) or pulmonary (females, SMR 20.4; males, SMR 15.9) causes.38 Standardized all cause (females, SMR 11.7; males, SMR 7.9) and non-neoplastic cause (females, SMR 4.2; males, SMR 2.4) mortality was also higher for females in an analysis of 34,489 five year survivors in the British Childhood Cancer Survivor Study, although the same was not true for mortality due to subsequent neoplasms (females, SMR 5.7; males, SMR 6.8).39 Female sex has also been associated with overall increased risk of chronic health conditions and impaired health status.41–44 Certain studies have identified that females are particularly susceptible to anthracycline-mediated cardiomyopathy compared to males.27,45,46 Additionally, female survivors are at a higher risk for subsequent malignant neoplasms, due largely to their increased risk of breast cancer.47–50 Breast cancer risk is driven primarily by radiation exposure, but female survivors in CCSS not exposed to chest radiation also had increased risk compared to the general population, and alkylating agents and anthracyclines were associated with breast cancer risk in this group.4,51 Despite varying results across studies, evidence supports sex-specific differences with higher risk among females compared to males for cognitive dysfunction after cranial radiation, obesity, hypothyroidism, and radiation-induced changes in pubertal timing.52 In general, epidemiologic studies of late effects have not specifically focused on examining sex differences, but the higher risk among females has emerged from multivariable models that typically include factors such as cancer diagnosis and/or treatment exposures. The underlying mechanisms responsible for differences in risk of some late effects by sex are not well understood. Hormonal differences may play a role, but many children undergo treatment prior to puberty, when such differences would be expected to be minimal.
Few studies have comprehensively examined the role of race/ethnicity in late health outcomes among long-term survivors of childhood cancer. In the CCSS, non-Hispanic black survivors had higher all-cause mortality than non-Hispanic white survivors, although this disparity was abrogated after adjusting for socioeconomic status (SES).53 No differences by race/ethnicity were observed in the overall cumulative incidence of severe, disabling, life-threatening or fatal chronic health conditions, but risk of cardiac conditions and stroke was higher in non-Hispanic black survivors, and both non-Hispanic black and Hispanic survivors were more likely to report having diabetes compared to non-Hispanic white survivors.53,54 These differences were largely attributable to underlying disparities in SES and cardiovascular risk factors. Importantly, studies have not identified a race/ethnicity-specific impact of treatment exposures on risk of late health effects in survivors, but future examinations of genetic susceptibility may reveal racial/ethnic differences in specific genetic variants that modify risk of late effects.
Because childhood is a period of critical growth and development, the age of cancer diagnosis and treatment can impact risk of subsequent treatment-related late effects. Risk of anthracycline-induced cardiotoxicity appears to be highest among children exposed in the first few years of life,27,46,55 although further research is needed to definitively establish the independent contribution of age to cardiotoxicity risk.56 Similarly, the adverse impact of cranial irradiation on cognitive function is particularly apparent among younger children in earlier stages of brain development.57 Conversely, the adverse impact of cancer treatments on female reproductive function increases with older age at exposure due to the natural decline in oocyte number with age, leading to sterility at lower doses or premature ovarian failure at shorter intervals post-therapy.58–61 Additionally, recent evidence suggests that the association between chest radiation and increased breast cancer risk is highest when exposure occurs near the time of menarche.62,63 Although these patient-specific factors are not modifiable, understanding their impact informs the selection of frontline treatments for diseases in which more than one equally efficacious approach is available, as well as surveillance and prevention strategies among survivors. The evolution of therapy for Hodgkin lymphoma gives a prime example. Specifically, use of response-adapted therapy approaches, which allow for elimination of radiation therapy based on disease response without negatively impacting overall survival, limit therapeutic exposures and potential for late-effects, such as breast cancer risk in females.64,65 Recent regimens have included lower cumulative doses of anthracyclines, alkylating agents and bleomycin compared to previous regimens in an attempt to reduce cardiovascular, pulmonary and fertility complications in addition to second malignant neoplasm rates.64
Genetics
Genetic variation can contribute to chronic disease risk among childhood cancer survivors in two ways. First, genetic predispositions known to confer moderate to high risk in the general population may contribute similarly to disease risk among survivors of childhood cancer.66,67 For instance, a germline mutation in a cancer predisposition gene such as TP53, BRCA1, or BRCA2, which is known to greatly increase an individual’s lifetime risk of developing cancer in the general population 68–70, is also a significant factor in determining subsequent cancer risk among childhood cancer survivors, independent of cancer therapy-related exposures.71 Second, inherited genetic variation in pathways influencing treatment efficacy and toxicity may alter risk among survivors receiving certain treatment exposures.72 For example, ovarian tumors with BRCA mutations have shown sensitivity to poly(ADP-ribosyl) polymperase (PARP) inhibitors related to impairment of DNA repair pathways, resulting in improved survival over those with BRCA negative tumors.73 Genetic factors may also enhance risks for late onset toxicity as seen in patients with hereditary retinoblastoma and germline Rb-1 mutations, in whom radiotherapy confers a 3.1 fold increased risk of subsequent neoplasms over nonhereditary patients.74
One of the most well-studied chronic health conditions following cancer therapy among survivors is subsequent cancer.49,75–77 Survivors of childhood cancer have a substantially higher risk of developing additional cancers than do individuals who have not previously had cancer during childhood. In addition, their risk of cancer is influenced by treatments received during childhood and by genetics.78,79 Historically, an increased risk of hereditary cancer has been characterized by early age of cancer onset in the survivor or family members, a higher than expected number of cancers among family members and a personal history of multiple primary cancers or unusual clustering and presentations of cancer.80 However, with advances in genetic sequencing technologies, we are now able to assess the impact of many germline variants on subsequent cancer risk by directly identifying these variants in an individual’s genome. In a recent study, whole-genome sequencing of 2,988 childhood cancer survivors identified that 5.7% carried an underlying germline mutation in a known cancer predisposition gene that affected their risk of developing certain types of subsequent neoplasms in a complex manner depending on prior radiotherapy exposures.71 Specifically, among survivors not treated with irradiation, mutation carriers had a significantly 5.6-fold increased risk of developing a subsequent neoplasm, the most common being basal cell carcinoma, meningioma, thyroid and breast cancer. Among mutation carriers, radiation therapy was significantly associated with a 2.3-fold increased risk of developing ≥2 subsequent neoplasms. This emerging data suggests that genetic risk in the childhood cancer survivor population as a whole may be significantly higher than previously understood and support the consideration of genetic counseling referral for all survivors to discuss the potential benefits and implications of testing after consultation and risk-assessment.71
Several studies have investigated the contribution of genetic variation to the risk of developing non-cancer chronic diseases among childhood cancer survivors. Many of these studies have adopted a candidate gene approach, selecting candidates based on known drug metabolism pathways or the underlying pathobiology of the chronic disease. Among the most extensively studied long-term outcomes to date is anthracycline-induced cardiotoxicity. Genes in which polymorphisms have been associated with cardiomyopathy and/or congestive heart failure include, but are not limited to SLC2A3, ABCB1, ABCB4, and ABCC1, which are involved in drug transport81; CBR3, which is associated with the two-electron reduction of anthracyclines, a main route of anthracycline metabolism82,83; CELF4, a regulator of alternate protein splicing, including cardiac troponin84; and HAS3 and NCF4, both of which are implicated in defense against reactive oxygen species.85–87 Similarly, studies among ALL survivors have identified that polymorphisms in genes likely to affect the action of anti-leukemia medications, such as VDR and TYMS, are associated with osteonecrosis,88 while CRHR1 polymorphisms alter the risk of bone density deficits.89 Although profiling of relevant genetic variation may characterize the risk of a specific disease and identify sub-populations of survivors at high risk, most reported gene-disease associations among childhood cancer survivors have not been extensively replicated, and consequently, their utility has not yet been established.
Interaction of Cancer Therapy with Premorbid and Comorbid Conditions
The implications of childhood cancer therapy for adult health are substantial. Investigators have reported a high prevalence of chronic illness among childhood cancer survivors and, by applying a modification of the National Cancer Institute’s (NCI’s) Common Terminology Criteria for Adverse Events (CTCAE), have described the severity of these late outcomes.90 Age-related health conditions appear earlier and with greater severity than might otherwise be expected. Approximately two-thirds of survivors in the CCSS reported at least one chronic health condition at a mean age of 26.6 years (range: 18.0–48.0), and the adjusted relative risk of a chronic condition in survivors was 3.3 (95% CI, 3.0–3.5) compared with siblings.43 In a large Nordic cohort, childhood cancer survivors were found to have a 1.8-fold risk of hospitalization compared to population based controls, with younger survivors having a higher relative risk than those in later decades of life.91 Subclinical disease can be significant, but is only identified if childhood cancer survivors are systematically screened. In a single-center study of 1,362 five-year survivors, 1,015 (75%) survivors had at least one chronic health condition.41 Participants in the St. Jude Lifetime (SJLIFE) cohort were systematically screened per the COG Long-Term Follow-Up Guidelines, and were found to have a particularly high prevalence of pulmonary, auditory, endocrine, cardiac, and neurocognitive abnormalities. The estimated cumulative prevalence by age 45 years was 95.5% (95% CI 94.8–98.6%) for any chronic condition and 80.5% (95% CI 73.0–86.6%) for a serious/disabling or life-threatening condition.5 However, childhood cancer survivors typically experience more than one chronic health condition. Among CCSS participants, 24-year-old survivors experienced the same cumulative incidence of severe, disabling, life-threatening or fatal health conditions as 50-year-old siblings (19.6%). At age 50 or older, 22.5% of survivors reported two or more CTCAE grade 3–5 conditions compared to 4.3% of siblings.4 The burden of disease among this young adult population can be significant. Using the cumulative burden, a novel measurement of disease burden that incorporates multiple health conditions and recurrent events into a single metric, Bhakta et al. estimated that by 50 years of age, survivors in the SJLIFE cohort would have, on average, 17.1 chronic health conditions, more than four of which are expected to be severe/disabling or life-threatening, representing a two-fold greater health burden compared to an age- and sex-matched community control population.28
Traditional risks to health, such as obesity, diabetes mellitus, and hypertension, which may occur on a background of advancing age, genetic predisposition, or suboptimal lifestyle behaviors, may have more than additive effects on cancer-related complications and contribute to worse long-term health outcomes. In a CCSS report, the risk of major cardiac events among survivors who had been exposed to anthracycline and/or chest-directed radiation was potentiated in a more than additive way by modifiable cardiovascular risk factors, most notably hypertension.92 Smoking and alcohol consumption were independently associated with severe bone mineral density deficits and frailty,93 a phenotype shown to be predictive of mortality in childhood cancer survivors.94 Neurocognitive and emotional problems may also influence health behaviors and adherence to screening.95 Poor health-related quality of life and the frequent need for psychotropic drugs (antidepressants, analgesics) may further complicate care.96 Consideration of chronic conditions, patient specific factors, health behaviors and early interventions could modify the trajectory of anticipated morbidity and mortality experienced by childhood cancer survivors, as demonstrated in this example of cardiomyopathy (Figure 2).
Figure 2.
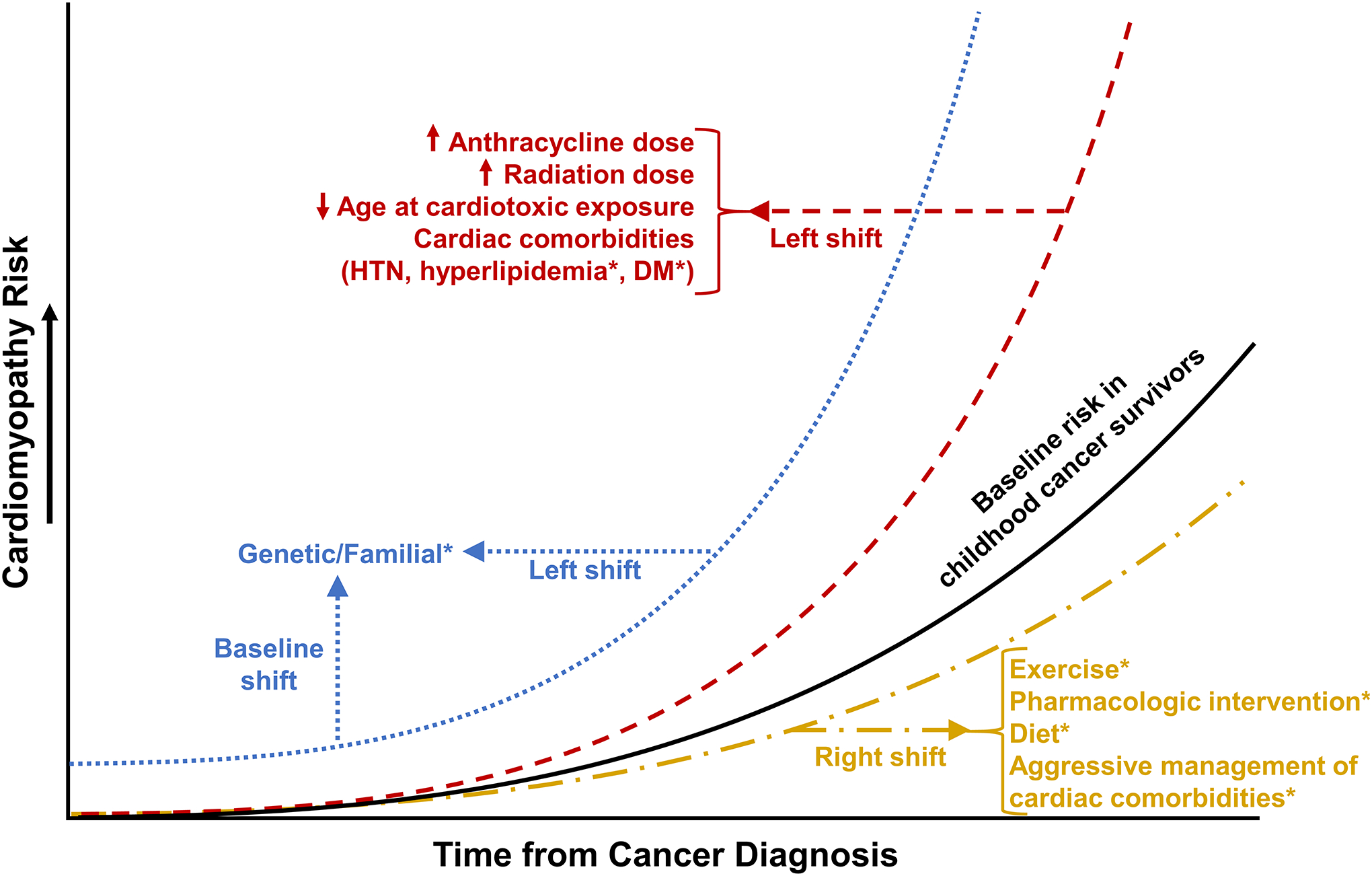
Conceptual schematic of cardiomyopathy risk and modifiers in childhood cancer survivors. Factors designated by an asterisk are under active investigation (knowledge gaps). HTN hypertension, DM diabetes mellitus. Reproduced with permission from Springer Publishing Company. Ehrhardt, MJ, Fulbright JM, Armenian SH. Cardiomyopathy in childhood cancer survivors: lessons from the past and challenges for the future. Curr Oncol Rep, 2016. 18(4): 22.
Undiagnosed or untreated chronic conditions may contribute to worse long-term health outcomes in an already vulnerable population.5 For example, only 31% of childhood cancer survivors with premature ovarian insufficiency (POI) reported receiving hormone replacement therapy in SJLIFE while POI was found to be independently associated with poor bone mineral density and frailty in the same study.98 Furthermore, data remain limited regarding the safety and long-term benefit of certain interventions such as growth hormone replacement in adults99 or sex hormone replacement in women experiencing premature menopause.63,98 While initiation of hormone replacement therapy (HRT) for females in the general population with POI is recommended, with guidance to continue until the age of natural menopause,100 there is not a clear consensus for HRT in childhood cancer survivors. Evidence based guidelines provide direction on surveillance for POI in high risk female childhood cancer survivors,101 but decisions to initiate or continue HRT are considered on an individual basis. The modification of breast cancer risk by hypogonadism and HRT, particularly in those with a history of chest irradiation, has been of particular interest. Recent studies suggest that in hypogonadal individuals, HRT with combined estrogen and progesterone has no, or only moderately, increased risk of breast cancer compared to those not receiving HRT, and nevertheless, remains lower than the risk in non-hypogonadal female survivors who also received chest irradiation.63,102 Further study is needed to investigate the risks and benefits of HRT in this population, in particular, regarding breast cancer risk, as well as cardiovascular and bone mineral density outcomes. Improving general health outcomes by intervening on modifiable risk factors remains an area of active research.
Aging and Frailty
A proportion of childhood cancer survivors are at risk for pre-frailty or frailty, states of reduced physiologic reserve, characterized, respectively, by the presence of two and three or more of the following impairments: 1) low energy expenditure, 2) low lean muscle mass, 3) fatigue, 4) weakness and 5) slow walking speed.103 This phenotype is most often described among older adults, suggesting that childhood cancer survivors may be at risk for accelerated aging. In the SJLIFE cohort, at a mean age of 33.6±8.1 years, pre-frailty was present among 22.2% and frailty among 7.9% of adult survivors of childhood cancer, and was associated with a 2.2-fold (95% CI 1.2–4.2) increased risk for new onset chronic disease and a 2.6-fold (95% CI 1.5–2.8) increased risk for death.94 These data are similar to data from the Cardiovascular Health Study cohort of older adults, ranging in age from 65–101 years of age, where pre-frailty was reported among 15.0%, and frailty among 7.2% of participants.103 This phenotype appears early during the course of childhood cancer therapy. Children with newly diagnosed cancer present with diminished exercise capacity, weakness and low lean muscle mass.105 After cancer therapy, sub-optimal recovery is apparent, as adolescent survivors also have been shown to have lower exercise capacity and reduced muscle strength compared to peers.106,107 This early impairment may perpetuate a sedentary lifestyle that contributes to failed recovery of normal physiologic reserve. Studies in large cohorts of childhood cancer survivors indicate that they are less physically active than their siblings or age and sex-matched controls.108–110
The prevalence of frailty among childhood cancer survivors increases with age and is higher among females. Exposure to cranial, abdominal or pelvic radiation, the development of endocrine dysfunction, and smoking are associated with frailty among adult survivors of childhood cancer.93,99,94 However, those without these exposures are also at risk. It is possible that the disease itself, or exposure to DNA damaging agents, results in loss of existing tissue integrity and underlying cellular function, subsequently impairing the abilities of the cardiopulmonary, musculoskeletal and neurosensory systems to support and respond to movement. Preliminary research among small groups of survivors suggests that telomere attrition, chronic inflammation, and early cellular senescence are potential mechanisms to explain these findings. Arittfin et al. reported shorter leukocyte telomere length, and increased plasma levels of C-reactive protein and interleukins 2, 10 and 17a among adult (age range 18–35 years) survivors of childhood ALL who were five or more years from diagnosis when compared to age- and sex-matched controls.111 Marcoux et al. reported increased expression of p16INK4a, a marker of cellular senescence, in scalp when compared to buttocks biopsies of ten ALL survivors previously exposed to cranial irradiation, suggesting a differential effect in cells exposed to radiation.112 Research also suggests that mitochondrial dysfunction is associated with chemotherapy exposure, and may explain the persistent loss of muscle mass observed in childhood cancer survivors.
Interventions specifically designed to prevent or remediate frail health among childhood cancer survivors have not been tested. Additional research to elucidate the pathobiology of the frailty phenotype should provide biological or molecular targets for intervention. While this work is ongoing, exercise interventions that work in other populations to improve low muscle mass, muscle strength and exercise capacity may benefit those childhood cancer survivors with reduced physiologic reserve.
Health Behaviors
Health behaviors contribute to the expression of late effects in survivors of childhood cancer. Reducing risky and promoting protective health behaviors in this population may help ameliorate adverse health outcomes such as secondary cancers and both cancer and non-cancer related morbidity and mortality. Health behaviors that contribute to late effects of cancer therapy include those related to tobacco, alcohol and illicit drug use, physical inactivity and poor diet, excess sun exposure and risky sexual behaviors (e.g. exposure to HPV). Table 2 describes recent studies that indicate the prevalence of these health behaviors as well as associated risk factors.95,109,110,113–125 Overall, survivors tend to exhibit risky behaviors at rates similar to or just less than their siblings, non-cancer controls, and the general population. However, due to vulnerability as a function of disease and/or treatment, survivors may be more sensitive to these risky behaviors and they should be limited as much as possible.
Table 2.
Selected studies examining prevalence and risk factors regarding health behaviors in childhood cancer survivors.
| Health Behavior | Source | Survivor Population | Comparison Group | Findings | Risk Factors Identified in Survivors* |
|---|---|---|---|---|---|
| Tobacco Use | Gibson et al, 2015102 | N=9397 53% male Age at diagnosis: 0–20 years Age at evaluation: Mean 28 years |
N=4023 siblings and general population from the NHIS | 19% CCS were smokers compared to 24% of siblings and 29% in the general population at baseline; smoking in CCS dropped to 16% at follow-up | Psychological distress and heavy drinking=more likely to smoke; cranial radiation and psychological distress=less likely to quit smoking |
| Emmons et al, 2002103 | N=9709 54% male Age at diagnosis: <21 years Age at evaluation: Median 26 years |
None | 28% were ever smokers; 17% were current smokers | Lower household income; lower educational attainment; older age at cancer diagnosis | |
| Alcohol Use | Rebholz et al, 201294 | N=1049 53.3% male Age at diagnosis: 0 to 10+ years Age at evaluation: 15–40 years |
N=5207 population controls in the Swiss Health Survey | CCS consumed alcohol more frequently than controls (OR=1.7; 95% CI=1.3–2.1) and engaged in binge drinking more frequently (OR 2.9; 95% CI=2.3–3.8) | Male sex; higher educational attainment; geographic location |
| Lown et al, 2008105 | N=10398 54% male Age at diagnosis:<21 years Age at evaluation: 18–48 years |
N=3034 siblings; N=4774 participants National Alcohol Survey | Survivors slightly more likely to be current drinker compared to siblings; less likely to be risky or heavy drinkers | Current age 18–21 years old; male; lower educational attainment; young age at drinking initiation; distress | |
| Illicit Drug Use | Milam et al, 2015106 | N=193 50.3% male Age at diagnosis: Median 12.1 years Age at evaluation: Median 19.9 years |
None | 14% reported current marijuana use | Male sex; depressive symptoms; higher socioeconomic status |
| Klosky et al, 2012107 | N=307 40% male Age at diagnosis: 0–3 years Age at evaluation: Mean 18.1 years |
N=97 siblings | Cocaine/crack rates for current use were 0.6% in survivors vs 1.0% in siblings and 1.6% in survivors vs. 3.1% in siblings for past use | Poor mental health score | |
| Diet | Zhang et al, 2016108 | N=2570 51.6% male Age at diagnosis: Mean 8.3 years Age at evaluation: Mean 32.3 years |
None | Low mean Healthy Eating Index 2010 score of 57.9 out of 100; 15%, 8%, 31% and 34% (respectively) met the recommended intake for fiber, potassium, magnesium and calcium | Sarcoma diagnosis; <5 years at diagnosis; cumulative glucocorticoid dose ≥9000 mg/m2 |
| Smith et al, 2014109 | N=1598 49.2% male Age at diagnosis: Median 7.9 years Age at evaluation: Median 32.7 years |
None; compared to AICR guidelines | 74% and 53% did not consume recommended fruit and vegetables, and complex carbohydrates; 90% and 70% consumed over red meat and sodium recommendation | Survivors with metabolic syndrome | |
| Belle et al, 2017110 | N=1864 52% male Age at diagnosis: Mean 8.8 years Age at evaluation: Mean of 17.2 years since diagnosis |
N=698 siblings N=8258 population controls in the Swiss Health Survey | 93%, 57% and >80% CCS did not meet national dietary guidelines for fruit and vegetable consumption, meat, and dairy intake. Control adherence was equally poor | Female sex, higher education, higher parental education and sport participation were associated with improved adherence | |
| Physical Activity | Wilson et al, 201499 | N=7287 49% male Age at diagnosis: <21 years Age at evaluation: Median 36.1 years |
N=2107 Siblings | 47.5% of survivors vs 41.5% of siblings did not meet CDC physical activity guidelines; 19.0% of survivors reported decline in physical activity vs. 17.6% in siblings | Female sex; older age at survey; black race; lower educational attainment |
| Hocking et al, 2013111 | N=117 36% male Age at diagnosis: <21 years Age at evaluation: Mean 21.6 years |
N=148 Controls | CCS had lower exercise scores than comparison group members at baseline (43.57 vs. 51.68) and 2-month follow-up (42.73 vs. 60.16) | Survivor reported health problems; low-self efficacy | |
| Ness et al, 200998 | N=9301 50.7% male Age at diagnosis: <21 years Age at evaluation: |
N=2886 siblings | 52.1% survivors vs 47% of siblings did not meet CDC guidelines for physical activity; 22.7% survivors vs 14.0% siblings were inactive | Female sex; black race; older age; unable to work; underweight or obese; smoking | |
| Sun Exposure | Levy-Shraga et al, 2015112 | N=143 46.9% male Age at diagnosis: <21 years Age at evaluation: Mean 11.2 years |
N=150 non-cancer patients of primary care pediatric clinics | CCS reported 94 minutes of sun exposure/day; controls reported 81 minutes/day; 39.4% CCS reported wearing sunscreen vs. 38% in controls | Ultra-orthodox religion; older age associated with less sunscreen use |
| Krull et al, 201186 | N= 6440 49% male Age at diagnosis: <21 years Age at evaluation: Mean 32.0 years |
None | 44.4% CCS reported wearing sunscreen; 33.2% of those recommended to get skin exam, received an exam | Older survivors and diagnosis of brain tumor more likely to wear sunscreen; older survivors and income ≥20,000 more likely to get skin exam | |
| Risky Sexual Behavior / HPV | Klosky et al, 2017113 | N=982 54.6% male Age at diagnosis: Median 12.2 years Age at evaluation: Median 16.3 years |
Compared to two population cohorts: NHIS and NIS-Teen | 23.8% CCS vs. 40.5% in general population initiated HPV-vaccination; 13.5% CCS vs 20.8% general population completed vaccination | No provider recommendation; health insurance coverage knowledge; male sex; younger age; perceived barriers |
| Klosky et al, 2014114 | N=307 40% male Age at diagnosis: 0–3.76 years Age at evaluation: Mean 18.1 years |
None | 5% of survivors reported no protection against pregnancy at last intercourse; 10% reported no protection against STDs | Cancer type, time since diagnosis, alcohol use and peer influences |
Unless otherwise specified, listed risk factors all increased the likelihood of the discussed health behavior.
CCS=Childhood cancer survivor; CCSS=Childhood Cancer Survivor Study; NHIS=National Health Interview Survey; SJLIFE=St. Jude Lifetime Cohort Study; OR=odds ratio; 95% CI=95% Confidence interval; AICR=American Institute for Cancer Research; STDs=Sexually transmitted diseases; NIS-Teen=National Immunization Survey-Teen
As is seen in the general population, engaging in risky or suboptimal health behaviors has implications for the overall health of survivors. For instance, survivors with better diet quality have lower adiposity,126,127 waist circumference127 and BMI127; whereas those with poor diet have an excess risk of conditions associated with the metabolic syndrome.120,128 Similarly, compared to inactive survivors, those with higher rates of physical activity are more likely to have lower percent fat mass, abdominal visceral fat,129 and waist circumferences.130 Survivors with declining physical activity report more chronic musculoskeletal conditions.110 Regarding use of illicit substances in the survivor population, marijuana and tobacco use have been linked to risk of pulmonary complications while cocaine and methamphetamine abuse have been associated with cardiac problems.131 While further research regarding marijuana use is needed, particularly as both recreational and legalized use of various forms have evolved, inhaled marijuana places survivors at risk for respiratory problems and can exacerbate pre-existing pulmonary late effects. In the general population, marijuana smoking is associated with increased forced vital capacity (FVC), in contrast to the reduction seen in tobacco smokers, and has been associated with symptoms of COPD and chronic bronchitis. Additionally, some studies suggest increases in bullous lung disease and hyperinflation, while evidence regarding lung cancer risk remain inconclusive.132,133 Overall, the findings of these studies are limited, in part due to potential confounding from frequent concurrent tobacco use, but demonstrate a need for further research in both the general and survivor population.
Low educational attainment (i.e., less than high school education) and lower household income among survivors have been associated with risky health behaviors, including physical inactivity, smoking, and drinking. As seen in the general population, low educational attainment and annual household income <$20,000 were associated with an increased risk of non-cancer related mortality among survivors.134 These behaviors subsequently place survivors at risk for the development of chronic disease135 and death.134
Several emerging health behaviors need more investigation, such as opioid and medical marijuana use, to understand the prevalence and risk factors in this population. Moreover, there is a need for interventions targeting health behavior change. Future work should draw upon previous findings that have shown efficacy in this population including use of biochemical verification in tobacco interventions,136 assisting with decision-making related to substance use,137 and using websites,138 videogames and other electronic formats to encourage healthy eating. Designing physical activity interventions that utilize group exercise or increase self-efficacy,139 as well as promoting ecological/environmental interventions such as active transportation140 and adventure-based training141,142 are areas to explore. Interventions that target alcohol use, sunscreen behavior and risky sexual behavior are limited and require further investigation.
Socioeconomic and Psychosocial Factors
Socioeconomic Factors and Access to Care
Numerous studies have documented that adult survivors of childhood cancer are at-risk for reduced educational attainment,143–146 increased financial burden,147 underemployment,148,149 dependent living status,150 and limited health insurance coverage.151 Consistent with observations from the general population, SES, specifically low educational attainment and household income, is directly associated with an increased risk of non-cancer related mortality among survivors.134
Lack of health insurance is a significant barrier to accessing medical care in the United States for adults with chronic conditions.152 Adult survivors of childhood cancer potentially face additional barriers to obtaining adequate health insurance coverage compared to the general population.148,153 These factors challenge the delivery of adequate care to survivors and may delay the diagnosis of chronic health conditions.5 Among childhood cancer survivors, decreased access to health care has been associated with reduced utilization of survivor-focused and general preventative health care.154 In a report from the CCSS cohort, nearly 30% of uninsured survivors had received no medical care in the prior two years compared with 10% of insured survivors.155 Importantly, general and survivor-focused health care is critical for prevention and treatment of chronic diseases, a major contributor to late mortality in adult survivors of childhood cancer.156 Survivors of childhood cancer in other countries may face different health insurance challenges. A study of the Norwegian population demonstrated that childhood cancer survivors had a significant increase in utilization of national insurance benefits, specifically for added expenses from disability, compared to the general population.157
Psychological Distress
Adult survivors of childhood cancer are at-risk for the development of psychological symptoms (i.e., depression and anxiety) compared with sibling controls.158 In long-term survivors of childhood cancer, symptoms of psychological distress, particularly suicidal ideation, have been associated with increased risk of all-cause mortality.159 Research has shown that factors such as worry, concern, and the extent to which survivors believe they have control over their health (i.e. locus of control) predict pediatric cancer survivors’ self-reported participation in medical surveillance160 – potentially modifying the likelihood and severity of late effects. Recent research has demonstrated that survivors who develop cardiovascular, endocrine, and/or pulmonary conditions resulting from cancer-directed therapies in childhood are at risk for developing emotional distress (e.g., symptoms of depression and anxiety).161 Further investigations are needed to establish the temporal order between chronic health conditions and psychological distress among survivors as well as to determine the effect of interventions designed to target these modifiable risk factors.
Parental distress is predictive of child distress, during both active cancer treatment162 and survivorship.163 Similarly, family factors, such as cohesion and expressiveness, have been shown to predict child distress during treatment.162 Research from the general population indicates that adverse childhood experiences, including family dysfunction and parental mental illness, significantly increase the risk of chronic health conditions in adulthood.164,165 The potential impact of similar factors such as parental distress and family environment on the morbidity and mortality of aging survivors has not yet been studied.
Chronic Pain, Sleep and Neurocognition
Over half of survivors of childhood cancer report the presence of pain in adulthood. Pain has been shown to impair survivors’ quality of life,166 and to predict persistent psychological distress in survivors over time.167 Although the presence of chronic pain (i.e. persistent or recurrent pain lasting >3 months) has not been examined among adult survivors of childhood cancer, in the general population chronic pain affects 10–11% of adults,168 is associated with depression,169 anxiety,170 decreased activity,171 and increased disability.172 Pain has also been associated with sleep problems and subsequent fatigue in the general population.173 During adulthood, long-term survivors of childhood cancer frequently report poor sleep quality and fatigue, though at levels that are not substantially different than siblings.174 Sleep disturbance and fatigue in survivors has been independently associated with increased risk for neurocognitive impairment, beyond the risk associated with cranial irradiation and neurotoxic chemotherapies.175 Fatigue has also been strongly associated with reduced quality of life.166 Recent research in adolescent survivors of childhood cancer has demonstrated that sleep disturbance is also associated with increased biomarkers of systemic inflammation,176 which may impact future risk for chronic health conditions.
Survivors who receive cranial irradiation,177,178 are exposed to high dose systemic or intrathecal methotrexate,179 or experience treatment-related cardiopulmonary and/or endocrine disease are at increased risk for neurocognitive impairment.180 Social attainment and future health behaviors are negatively impacted by this impairment. Survivors who develop neurocognitive problems during adolescence are more likely to engage in risky health behaviors and less likely to engage in health care utilization as adults.181 Long-term survivors of childhood cancer who develop neurocognitive problems are also less likely to graduate college,182 work full-time,148 and live independently.150
Screening Guidelines and Delivery of Care
Survivorship Guidelines
The many potential late effects of cancer therapy have necessitated a systematic approach to early detection and intervention. To address this need, guidelines have been developed by several leading childhood cancer organizations.183 As guidelines were developed independently by multiple international groups, variations exist in their recommendations regarding patient risk groups and surveillance testing and intervals, due to variation in capacity to implement guidelines. Currently, the International Late Effects of Childhood Cancer Guideline Harmonization Group is working to establish integrated clinical practice guidelines for surveillance of late effects in childhood cancer survivors.183
In North America, the Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers represent the primary survivorship resource for healthcare providers.184,185 The COG guidelines pair evidence linking therapeutic exposures to observed late effects and provide consensus-based screening and management recommendations. Although this strategy can improve the detection of health conditions,5,186 the cost-effectiveness of screening and management and the ultimate impact on clinical outcomes remains uncertain. Two studies on the cost-effectiveness of the COG’s cardiomyopathy screening recommendations provided evidence that although existing screening strategies for cardiomyopathy were cost-effective in survivors at higher risk, optimal cost-efficacy could be achieved through reduced screening frequency from every 1 to 5 years to every 2 to 10 years depending on the risk group which maintained 80% of the health benefits of screening.187,188 However, these studies highlight a need for future research seeking to optimize cost-effective implementation of survivorship guidelines and their impact on clinical outcomes.
In 2010, the International Late Effects of Childhood Cancer Guidelines Harmonization Group was established to address the significant variation among existing guidelines.183 Applying rigorous guideline development methodology, this collaboration has resulted in the completion of four uniform surveillance guidelines (cardiomyopathy, breast cancer, and both male and female gonadal dysfunction), with several others in progress.56,101,189,190 This undertaking will establish an acceptable and generalizable set of surveillance recommendations directing optimal strategies for global delivery of survivorship care.
Models of Care and the Survivorship Care Plans
Different survivorship care models have been utilized across many clinical settings191; however, little evidence exists to prioritize the use of any one approach.192,193 All care models essentially seek to: 1) monitor for treatment-related late effects, 2) educate and engage patients and providers, and 3) provide appropriate management of cancer-related medical issues (Figure 3).194,195 The success of a given approach is largely dependent upon resource availability; therefore, the preferred model is one that can be effectively supported in a given clinical setting.
Figure 3.
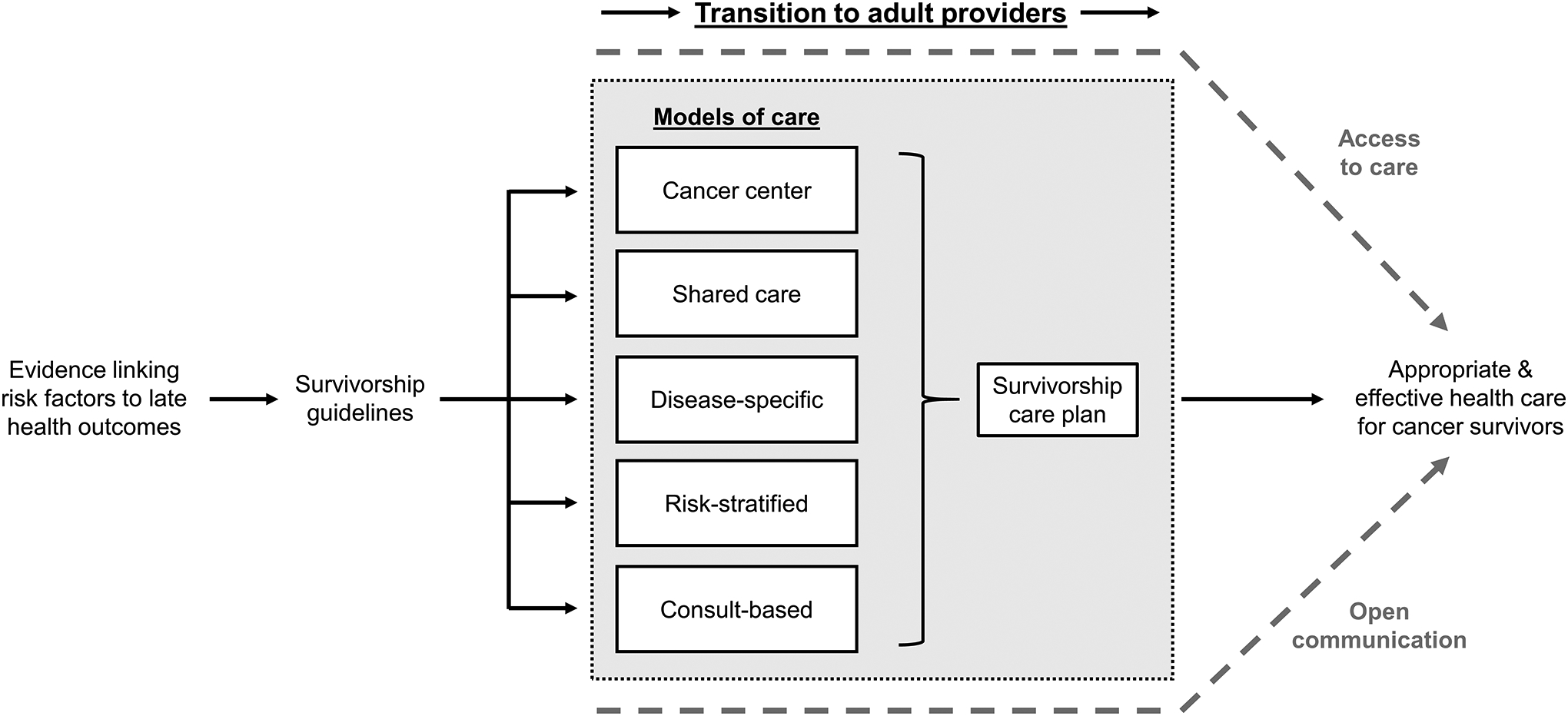
The continuum of childhood cancer survivor care delivery.
“Cancer center” model: care provided within the cancer center, either by the primary oncology or dedicated survivorship teams. “Shared-care” model: care initially provided in the cancer center, with later transfer to community providers with ongoing communication and specialty support from the cancer center. “Disease-specific” model: cancer center-based survivorship clinics designed to specifically address the needs of a particular at-risk population (e.g. central nervous system tumor survivors). “Risk-stratified” model: stratified care is provided based on risk categorization, with survivors at higher risk of late effects being seen at more comprehensive centers and those with lower risk cared for in the community. “Consult-based” model: care administered in the community care provider’s office (i.e. the general pediatric or adult medicine provider) with consultation for specific late effects obtained as needed without ongoing specialty involvement unless clinically indicated.
In high-income countries, many children are diagnosed with and treated for cancer at comprehensive cancer centers, where institutional commitments may be leveraged to support resource-intense multidisciplinary care models, typically involving a pediatric oncologist, internist, appropriate sub-specialty providers for individual patient needs and behavioral clinicians such as a social worker and psychologist. This approach capitalizes on availability of specialty services to provide centrally coordinated and administered comprehensive care. While some authors suggest that multidisciplinary clinics such as these are optimal,191 these centers are often specialized to care for children and may not be best suited to provide quality, cost-effective care to adult survivors of childhood cancer.196 Conversely, regions with fewer resources may choose to utilize consult-based approaches, in which assessments are performed by specialists and care subsequently transferred to the primary care team.197 Many have advocated for a more intermediate approach, such as the shared care model, which involves initial coordination of care by the cancer center, with carefully planned transition of care to primary providers who then have access to ongoing support from the cancer center.198 This approach requires excellent communication systems, which can be challenging in the current healthcare structure. An alternative approach, the patient-centered medical home, has not been widely utilized in cancer survivors but is often implemented to provide community-based care for children with other medically complex conditions (e.g. diabetes) with the goal of accessible, coordinated and patient-centered care. This strategy could facilitate collaborative care between primary providers and survivorship specialists through routine specialist visits to the patient-centered medical home, providing an alternative shared care approach to community-based care for childhood cancer survivors.199,200
For aging survivors, delivery of care is complicated by the need to transition not only from treatment to survivorship and/or primary care, but also from pediatric to adult providers. The importance of effective transition of care has been recognized and is under active investigation in other pediatric chronic conditions, such as sickle cell and congenital heart disease.201,202 Within general pediatrics, some have suggested that beginning the process early, engaging the child in medical decision-making, and maintaining health insurance are important factors in this transition.203 Unique to childhood cancer survivors is that this transition often occurs during the hiatus between active cancer treatment and the onset of late-effects, raising concern that survivors may fail to recognize the importance of ongoing follow-up care. While data to address these concerns are lacking, they underscore a belief in the importance of empowering survivors through ongoing cancer treatment-related history and associated health risk education, emphasizing the value of continued follow-up care.195 Additionally, guidelines in existence tend to emphasize medical outcomes rather than psychosocial or socioeconomic factors impacting survivor care and transition. The COG Long-Term Follow-Up Guidelines briefly address psychosocial outcomes,184 such as education and employment deficits, social withdrawal and dependent living, mental health disorders, and risky health behaviors, however, similar to guidelines from international groups,204 there are challenges in meeting these needs, as well as medical needs, including specific system (e.g. health insurance, access to mental health and rehabilitation services), provider (e.g. setting of practice, training, perceptions of care) and survivor (e.g. self-efficacy, health literacy and knowledge) barriers (Figure 4).
Figure 4.
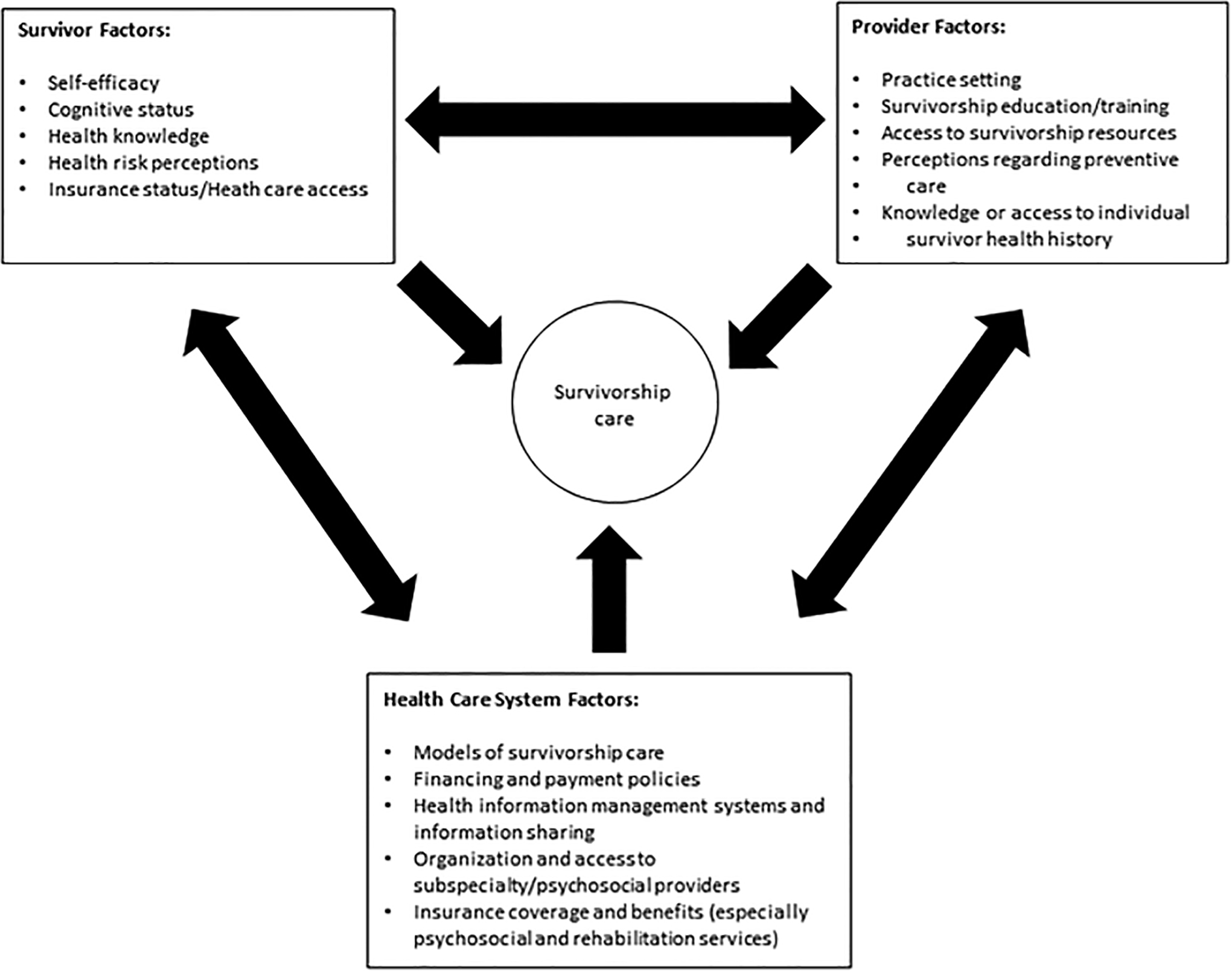
Survivor, provider and health care system factors that may act as barriers to survivorship care. The arrows demonstrate that factors in one domain may influence other domains. Directed interventions should be developed within the setting and context of care system.
Regardless of the chosen model of survivorship and transition care delivery, leading organizations have recognized the growing need to optimize the systematic delivery of evidence-based recommendations to providers and cancer survivors of all ages. The use of personalized treatment summaries and survivorship care plans, which document possible complications of cancer-treatment, signs of recurrence, and recommended follow-up, is supported by many organizations.192 These documents are intended to enhance adherence, improve communication between oncology and primary care providers, and ultimately improve delivery of care. However, their effectiveness remains only anecdotal, and existing studies raise concerns about: 1) the feasibility of providing these documents to both patients and providers and 2) which components of the document are essential for inclusion.155,197,205,206
Conclusions
Comprehensive, risk-based care of the childhood cancer survivor is a complex and multi-faceted undertaking. It requires the cooperation of clinicians and patients as well as the care network responsible for these patients. Although we have identified many contributors to morbidity and mortality in this population, ongoing research is needed to assess the efficacy of interventions, to identify possible targets for mitigation of late effects, and to develop effective screening strategies to assist with early diagnosis and prevention. The heterogeneity of the childhood cancer survivor population, not only in cancer type and treatment factors, but also demographics, genetics, health behaviors and socioeconomic factors highlights the importance of a patient-specific, risk-based approach to care. New challenges and opportunities for research will emerge as the treatment of pediatric cancer continues to introduce novel agents, therapeutic reductions and alternate treatment approaches whose effects will be realized only in the next generation of childhood cancer survivors.
Footnotes
DISCLOSURES: Wassim Chemaitilly reports personal fees from Pfizer and Novo-Nordisk outside the submitted work. The remaining authors made no disclosures.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32(12):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter AL, Conklin HM, Tyc VL, et al. Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. J Clin Exp Neuropsychol. 2014;36(8):818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crist WM, Garnsey L, Beltangady MS, et al. Prognosis in children with rhabdomyosarcoma: a report of the Intergroup Rhabdomyosarcoma Studies I and II. Intergroup Rhabdomyosarcoma Committee. J Clin Oncol. 1990;8(3):443–452. [DOI] [PubMed] [Google Scholar]
- 8.Maas SM, Vansenne F, Kadouch DJ, et al. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A. 2016;170(9):2248–2260. [DOI] [PubMed] [Google Scholar]
- 9.Schneider K, Zelley K, Nichols KE, Garber J. Li-Fraumeni Syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews(R). Seattle,WA: University of Washington, Seattle: 1993. [Google Scholar]
- 10.Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer TC, Mahoney EL, Pomeroy SL. Medulloblastoma: molecular classification-based personal therapeutics. Neurotherapeutics. 2017;14(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winick N, Devidas M, Chen S, et al. Impact of initial CSF findings on outcome among patients with National Cancer Institute standard- and high-risk B-Cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2017;35(22):2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pui CH, Pei D, Coustan-Smith E, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16(4):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 17.Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. 2004;54(4):208–236. [DOI] [PubMed] [Google Scholar]
- 18.Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013;10(1):52–60. [DOI] [PubMed] [Google Scholar]
- 19.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83–88. [DOI] [PubMed] [Google Scholar]
- 20.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87(1):46–52. [DOI] [PubMed] [Google Scholar]
- 21.Yock TI, Tarbell NJ. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1(2):97–103. [DOI] [PubMed] [Google Scholar]
- 22.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. [DOI] [PubMed] [Google Scholar]
- 23.Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Howell RM, Homann K, et al. Predicted risks of radiogenic cardiac toxicity in two pediatric patients undergoing photon or proton radiotherapy. Radiat Oncol. 2013;8(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodin NP, Munck Af Rosenschold P, Aznar MC, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011;50(6):806–816. [DOI] [PubMed] [Google Scholar]
- 27.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Interiano RB, Delos Santos N, Huang S, et al. Renal function in survivors of nonsyndromic Wilms tumor treated with unilateral radical nephrectomy. Cancer. 2015;121(14):2449–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Interiano RB, McCarville MB, Santos ND, et al. Comprehensive renal function evaluation in patients treated for synchronous bilateral Wilms tumor. J Pediatr Surg. 2017;52(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller HL. Risk-adapted, long-term management in childhood-onset craniopharyngioma. Pituitary. 2017;20(2):267–281. [DOI] [PubMed] [Google Scholar]
- 33.Gawade PL, Hudson MM, Kaste SC, et al. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev. 2014;10(4):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Interiano RB, Kaste SC, Li C, et al. Associations between treatment, scoliosis, pulmonary function, and physical performance in long-term survivors of sarcoma. J Cancer Surviv. 2017;11(5):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Pineda I, Hudson MM, Pappo AS, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. J Cancer Surviv. 2017;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malek F, Somerson JS, Mitchel S, Williams RP. Does limb-salvage surgery offer patients better quality of life and functional capacity than amputation? Clin Orthop Relat Res. 2012;470(7):2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samis J, Lee P, Zimmerman D, Arceci RJ, Suttorp M, Hijiya N. Recognizing endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic myelogenous leukemia. Pediatr Blood Cancer. 2016;63(8):1332–1338. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173–3181. [DOI] [PubMed] [Google Scholar]
- 41.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. [DOI] [PubMed] [Google Scholar]
- 42.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 43.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 44.Reulen RC, Winter DL, Lancashire ER, et al. Health-status of adult survivors of childhood cancer: a large-scale population-based study from the British Childhood Cancer Survivor Study. Int J Cancer. 2007;121(3):633–640. [DOI] [PubMed] [Google Scholar]
- 45.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’ Tumor Study group. J Clin Oncol. 2001;19(7):1926–1934. [DOI] [PubMed] [Google Scholar]
- 46.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–1743. [DOI] [PubMed] [Google Scholar]
- 47.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21(23):4386–4394. [DOI] [PubMed] [Google Scholar]
- 48.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93(8):618–629. [DOI] [PubMed] [Google Scholar]
- 49.Teepen JC, van Leeuwen FE, Tissing WJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study Cohort: role of chemotherapy. J Clin Oncol. 2017;35(20):2288–2298. [DOI] [PubMed] [Google Scholar]
- 50.MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Phillips N, McBride ML. Risk of a second malignant neoplasm among 5-year survivors of cancer in childhood and adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48(4):453–459. [DOI] [PubMed] [Google Scholar]
- 51.Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(9):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25(28):4477–4489. [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Leisenring WM, Ness KK, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(14):1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatia S, Gibson TM, Ness KK, et al. Childhood cancer survivorship research in minority populations: A position paper from the Childhood Cancer Survivor Study. Cancer. 2016;122(15):2426–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324(12):808–815. [DOI] [PubMed] [Google Scholar]
- 56.Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91(5):1723–1728. [DOI] [PubMed] [Google Scholar]
- 59.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(16):2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sklar C Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33(1):2–8. [DOI] [PubMed] [Google Scholar]
- 61.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–744. [DOI] [PubMed] [Google Scholar]
- 62.Cooke R, Jones ME, Cunningham D, et al. Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer. 2013;108(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moskowitz CS, Chou JF, Sklar CA, et al. Radiation-associated breast cancer and gonadal hormone exposure: a report from the Childhood Cancer Survivor Study. Br J Cancer. 2017;117(2):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly KM. Hodgkin lymphoma in children and adolescents: improving the therapeutic index. Blood. 2015;126(22):2452–2458. [DOI] [PubMed] [Google Scholar]
- 65.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006;98(1):15–25. [DOI] [PubMed] [Google Scholar]
- 67.Ripperger T, Bielack SS, Borkhardt A, et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am J Med Genet A. 2017;173(4):1017–1037. [DOI] [PubMed] [Google Scholar]
- 68.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365–1372. [DOI] [PubMed] [Google Scholar]
- 69.Chompret A, Brugieres L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000;82(12):1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metcalfe KA, Lynch HT, Ghadirian P, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005;96(1):222–226. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, L. Wilson C, Easton J, et al. Germline mutations in cancer predisposition genes and risk for subsequent neoplasm among long-term survivors of childhood cancer in the St. Jude Lifetime Cohort [abstract]. Paper presented at: AACR Annual Meeting; April 1–5, 2017, 2017; Washington, District of Columbia. [Google Scholar]
- 72.Bhatia S Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2048–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pothuri B BRCA1- and BRCA2-related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;24 Suppl 8:viii22–viii27. [DOI] [PubMed] [Google Scholar]
- 74.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272–2279. [DOI] [PubMed] [Google Scholar]
- 75.Armstrong GT, Liu W, Leisenring W, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2011;29(22):3056–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017;317(8):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305(22):2311–2319. [DOI] [PubMed] [Google Scholar]
- 78.Inskip PD, Sigurdson AJ, Veiga L, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 2016;94(4):800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhatia S Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer. 2015;121(5):648–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70–87. [DOI] [PubMed] [Google Scholar]
- 81.Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30(13):1422–1428. [DOI] [PubMed] [Google Scholar]
- 82.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112(12):2789–2795. [DOI] [PubMed] [Google Scholar]
- 83.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(13):1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Sun CL, Quinones-Lombrana A, et al. CELF4 variant and anthracycline-related cardiomyopathy: a Children’s Oncology Group genome-wide association study. J Clin Oncol. 2016;34(8):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Liu W, Sun CL, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group. J Clin Oncol. 2014;32(7):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossi D, Rasi S, Franceschetti S, et al. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B-cell lymphoma treated with R-CHOP21. Leukemia. 2009;23(6):1118–1126. [DOI] [PubMed] [Google Scholar]
- 87.Wojnowski L, Kulle B, Schirmer M, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112(24):3754–3762. [DOI] [PubMed] [Google Scholar]
- 88.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22(19):3930–3936. [DOI] [PubMed] [Google Scholar]
- 89.Jones TS, Kaste SC, Liu W, et al. CRHR1 polymorphisms predict bone density in survivors of acute lymphoblastic leukemia. J Clin Oncol. 2008;26(18):3031–3037. [DOI] [PubMed] [Google Scholar]
- 90.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity-grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Fine Licht S, Rugbjerg K, Gudmundsdottir T, et al. Long-term inpatient disease burden in the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study: A cohort study of 21,297 childhood cancer survivors. PLoS Med. 2017;14(5):e1002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson CL, Chemaitilly W, Jones KE, et al. Modifiable factors associated with aging phenotypes among adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(21):2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47(9):1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7(1):104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2015;33(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921. [DOI] [PubMed] [Google Scholar]
- 101.van Dorp W, Mulder RL, Kremer LC, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. J Clin Oncol. 2016;34(28):3440–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krul IM, Opstal-van Winden AWJ, Aleman BMP, et al. Breast Cancer Risk After Radiation Therapy for Hodgkin Lymphoma: Influence of Gonadal Hormone Exposure. Int J Radiat Oncol Biol Phys. 2017;99(4):843–853. [DOI] [PubMed] [Google Scholar]
- 103.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 105.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(4):1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Brussel M, Takken T, van der Net J, et al. Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil. 2006;9(3):267–274. [DOI] [PubMed] [Google Scholar]
- 107.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1356–1363. [DOI] [PubMed] [Google Scholar]
- 109.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson CL, Stratton K, Leisenring WL, et al. Decline in physical activity level in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ariffin H, Azanan MS, Abd Ghafar SS, et al. Young adult survivors of childhood acute lymphoblastic leukemia show evidence of chronic inflammation and cellular aging. Cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 112.Marcoux S, Le ON, Langlois-Pelletier C, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol. 2013;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gibson TM, Liu W, Armstrong GT, et al. Longitudinal smoking patterns in survivors of childhood cancer: An update from the Childhood Cancer Survivor Study. Cancer. 2015;121(22):4035–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2002;20(6):1608–1616. [DOI] [PubMed] [Google Scholar]
- 115.Rebholz CE, Kuehni CE, Strippoli MP, et al. Alcohol consumption and binge drinking in young adult childhood cancer survivors. Pediatr Blood Cancer. 2012;58(2):256–264. [DOI] [PubMed] [Google Scholar]
- 116.Lown EA, Goldsby R, Mertens AC, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103(7):1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milam J, Slaughter R, Meeske K, et al. Substance use among adolescent and young adult cancer survivors. Psychooncology. 2016;25(11):1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klosky JL, Howell CR, Li Z, et al. Risky health behavior among adolescents in the Childhood Cancer Survivor Study cohort. J Pediatr Psychol. 2012;37(6):634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang FF, Ojha RP, Krull KR, et al. Adult survivors of childhood cancer have poor adherence to dietary guidelines. J Nutr. 2016;146(12):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120(17):2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belle F, Wengenroth L, Weiss A, et al. Low adherence to dietary recommendations in adult childhood cancer survivors. Clin Nutr. 2017;36(5):1266–1274. [DOI] [PubMed] [Google Scholar]
- 122.Hocking MC, Schwartz LA, Hobbie WL, et al. Prospectively examining physical activity in young adult survivors of childhood cancer and healthy controls. Pediatr Blood Cancer. 2013;60(2):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levy-Shraga Y, Cohen R, Ben Ami M, Yeshayahu Y, Temam V, Modan-Moses D. Sun exposure and protection habits in pediatric patients with a history of malignancy. PLoS One. 2015;10(9):e0137453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klosky JL, Hudson MM, Chen Y, et al. Human Papillomavirus Vaccination Rates in Young Cancer Survivors. J Clin Oncol. 2017:JCO2017741843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klosky JL, Foster RH, Li Z, et al. Risky sexual behavior in adolescent survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Health Psychol. 2014;33(8):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Landy DC, Lipsitz SR, Kurtz JM, et al. Dietary quality, caloric intake, and adiposity of childhood cancer survivors and their siblings: an analysis from the cardiac risk factors in Childhood Cancer Survivors Study. Nutr Cancer. 2013;65(4):547–555. [DOI] [PubMed] [Google Scholar]
- 127.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barnea D, Raghunathan N, Friedman DN, Tonorezos ES. Obesity and metabolic disease after childhood cancer. Oncology (Williston Park). 2015;29(11):849–855. [PMC free article] [PubMed] [Google Scholar]
- 129.Slater ME, Ross JA, Kelly AS, et al. Physical activity and cardiovascular risk factors in childhood cancer survivors. Pediatr Blood Cancer. 2015;62(2):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slater ME, Steinberger J, Ross JA, et al. Physical activity, fitness, and cardiometabolic risk factors in adult survivors of childhood cancer with a history of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(7):1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tyc VL, Klosky JL. Lifestyle factors and health risk behaviors. Handbook of Long Term Care of The Childhood Cancer Survivor: Springer; 2015:325–346. [Google Scholar]
- 132.Martinasek MP, McGrogan JB, Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respiratory care. 2016;61(11):1543–1551.133. [DOI] [PubMed] [Google Scholar]
- 133.Ribeiro LI, Ind PW. Effect of cannabis smoking on lung function and respiratory symptoms: a structured literature review. NPJ primary care respiratory medicine. 2016;26:16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cox CL, Nolan VG, Leisenring W, et al. Noncancer-related mortality risks in adult survivors of pediatric malignancies: the Childhood Cancer Survivor Study. J Cancer Surviv. 2014;8(3):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oancea SC, Gurney JG, Ness KK, et al. Cigarette smoking and pulmonary function in adult survivors of childhood cancer exposed to pulmonary-toxic therapy: results from the St. Jude lifetime cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klesges RC, Krukowski RA, Klosky JL, et al. Efficacy of a tobacco quitline among adult survivors of childhood cancer. Nicotine Tob Res. 2015;17(6):710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hollen PJ, Tyc VL, Shannon SV, et al. Factors related to decision making and substance use in adolescent survivors of childhood cancer: a presenting clinical profile. J Cancer Surviv. 2013;7(3):500–510. [DOI] [PubMed] [Google Scholar]
- 138.Li R, Raber M, Chandra J. Developing a healthy web-based cookbook for pediatric cancer patients and survivors: rationale and methods. JMIR Res Protoc. 2015;4(1):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ruble K, Scarvalone S, Gallicchio L, Davis C, Wells D. Group physical activity intervention for childhood cancer survivors: a pilot study. J Phys Act Health. 2016;13(3):352–359. [DOI] [PubMed] [Google Scholar]
- 140.Slater ME, Kelly AS, Sadak KT, Ross JA. Active transportation in adult survivors of childhood cancer and neighborhood controls. J Cancer Surviv. 2016;10(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li HC, Chung OK, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology. 2013;22(11):2601–2610. [DOI] [PubMed] [Google Scholar]
- 142.Chung OK, Li HC, Chiu SY, Ho KY, Lopez V. Sustainability of an integrated adventure-based training and health education program to enhance quality of life among chinese childhood cancer survivors: a randomized controlled trial. Cancer Nurs. 2015;38(5):366–374. [DOI] [PubMed] [Google Scholar]
- 143.Kuehni CE, Strippoli MP, Rueegg CS, et al. Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer. 2012;118(5):1439–1449. [DOI] [PubMed] [Google Scholar]
- 144.Lancashire ER, Frobisher C, Reulen RC, Winter DL, Glaser A, Hawkins MM. Educational attainment among adult survivors of childhood cancer in Great Britain: a population-based cohort study. J Natl Cancer Inst. 2010;102(4):254–270. [DOI] [PubMed] [Google Scholar]
- 145.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97(4):1115–1126. [DOI] [PubMed] [Google Scholar]
- 146.Lorenzi M, McMillan AJ, Siegel LS, et al. Educational outcomes among survivors of childhood cancer in British Columbia, Canada: report of the Childhood/Adolescent/Young Adult Cancer Survivors (CAYACS) Program. Cancer. 2009;115(10):2234–2245.146. [DOI] [PubMed] [Google Scholar]
- 147.Nipp RD, Kirchhoff AC, Fair D, et al. Financial burden in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2017:JCO2016717066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kirchhoff AC, Krull KR, Ness KK, et al. Occupational outcomes of adult childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Cancer. 2011;117(13):3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Med Care. 2010;48(11):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. Predictors of independent living status in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57(7):1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuhlthau KA, Nipp RD, Shui A, et al. Health insurance coverage, care accessibility and affordability for adult survivors of childhood cancer: a cross-sectional study of a nationally representative database. J Cancer Surviv. 2016;10(6):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. A national study of chronic disease prevalence and access to care in uninsured U.S. adults. Ann Intern Med. 2008;149(3):170–176. [DOI] [PubMed] [Google Scholar]
- 153.Kirchhoff AC, Kuhlthau K, Pajolek H, et al. Employer-sponsored health insurance coverage limitations: results from the Childhood Cancer Survivor Study. Support Care Cancer. 2013;21(2):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Casillas J, Castellino SM, Hudson MM, et al. Impact of insurance type on survivor-focused and general preventive health care utilization in adult survivors of childhood cancer: the Childhood Cancer Survivor Study (CCSS). Cancer. 2011;117(9):1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(27):4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johannesen TB, Langmark F, Wesenberg F, Lote K. Prevalence of Norwegian patients diagnosed with childhood cancer, their working ability and need of health insurance benefits. Acta Oncol. 2007;46(1):60–66. [DOI] [PubMed] [Google Scholar]
- 158.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):435–446. [DOI] [PubMed] [Google Scholar]
- 159.Brinkman TM, Zhang N, Recklitis CJ, et al. Suicide ideation and associated mortality in adult survivors of childhood cancer. Cancer. 2014;120(2):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cox CL, Zhu L, Hudson MM, Steen BD, Robison LL, Oeffinger KC. Survivor typologies predict medical surveillance participation: the childhood cancer survivor study. Psychooncology. 2013;22(7):1534–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vuotto SC, Krull KR, Li C, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123(3):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jobe-Shields L, Alderfer MA, Barrera M, Vannatta K, Currier JM, Phipps S. Parental depression and family environment predict distress in children prior to stem-cell transplantation. J Dev Behav Pediatr. 2009;30(2):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bruce M A systematic and conceptual review of posttraumatic stress in childhood cancer survivors and their parents. Clin Psychol Rev. 2006;26(3):233–256. [DOI] [PubMed] [Google Scholar]
- 164.Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34(5):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gilbert LK, Breiding MJ, Merrick MT, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48(3):345–349. [DOI] [PubMed] [Google Scholar]
- 166.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(33):4242–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brinkman TM, Zhu L, Zeltzer LK, et al. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br J Cancer. 2013;109(5):1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. [DOI] [PubMed] [Google Scholar]
- 170.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26(10):888–901. [DOI] [PubMed] [Google Scholar]
- 171.van den Berg-Emons RJ, Schasfoort FC, de Vos LA, Bussmann JB, Stam HJ. Impact of chronic pain on everyday physical activity. Eur J Pain. 2007;11(5):587–593. [DOI] [PubMed] [Google Scholar]
- 172.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C.: The National Academies Press. 2011. [PubMed] [Google Scholar]
- 173.Evans S, Djilas V, Seidman LC, Zeltzer LK, Tsao JCI. Sleep quality, affect, pain, and disability in children with chronic pain: is affect a mediator or moderator? J Pain. 2017;18(9):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). Sleep. 2008;31(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(11):2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheung YT, Brinkman TM, Mulrooney DA, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;123(17):3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brinkman TM, Krasin MJ, Liu W, et al. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: results from the St Jude Lifetime Cohort Study. J Clin Oncol. 2016;34(12):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(35):4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Krull KR, Cheung YT, Liu W, et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(22):2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Krull KR, Huang S, Gurney JG, et al. Adolescent behavior and adult health status in childhood cancer survivors. J Cancer Surviv. 2010;4(3):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kremer LC, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60(4):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. Monrovia, CA: Children’s Oncology Group. October, 2014. www.survivorshipguidelines.org. Accessed Dec. 7, 2017. [Google Scholar]
- 185.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–4990. [DOI] [PubMed] [Google Scholar]
- 186.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30(35):4401–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wong FL, Bhatia S, Landier W, et al. Cost-effectiveness of the children’s oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160(10):672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160(10):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14(13):e621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Skinner R, Mulder RL, Kremer LC, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017;18(2):e75–e90. [DOI] [PubMed] [Google Scholar]
- 191.Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr Blood Cancer. 2006;46(2):159–168. [DOI] [PubMed] [Google Scholar]
- 192.Hewitt M, Greenfield S, Stovall M. From Cancer Patient to Cancer Survivor: Lost in Transition. Washinton, D.C.: The National Academies Press; 2006. [Google Scholar]
- 193.Institute of Medicine and National Research Council. In: Hewitt M, Weiner SL, Simone JV, eds. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, D.C.: National Academies Press; 2003. [PubMed] [Google Scholar]
- 194.Freyer DRV R; Eshelman-Kent D Survivorship Transitions Following Childhood and Adolescent Cancer. In: Schwartz CL, ed. Survivors of Childhood and Adolescent Cancer: A Multidisciplinary Approach. Third ed. New York, NY: Springer International Publishing; 2015:413–424. [Google Scholar]
- 195.Nathan PC, Hayes-Lattin B, Sisler JJ, Hudson MM. Critical issues in transition and survivorship for adolescents and young adults with cancers. Cancer. 2011;117(10 Suppl):2335–2341. [DOI] [PubMed] [Google Scholar]
- 196.World Health Organization. Quality of Care: A Process for Making Strategic Choices in Health Systems. Geneva: WHO, 2006. [Google Scholar]
- 197.Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19–e29. [DOI] [PubMed] [Google Scholar]
- 198.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117–5124. [DOI] [PubMed] [Google Scholar]
- 199.American Academy of Pediatrics Section on Hematology/Oncology CsOG. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123(3):906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hudson SV, Miller SM, Hemler J, et al. Cancer survivors and the patient-centered medical home. Transl Behav Med. 2012;2(3):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Frost JR, Cherry RK, Oyeku SO, et al. Improving sickle cell transitions of care through health information technology. Am J Prev Med. 2016;51(1 Suppl 1):S17–23. [DOI] [PubMed] [Google Scholar]
- 202.Lee A, Bailey B, Cullen-Dean G, Aiello S, Morin J, Oechslin E. Transition of care in congenital heart disease: ensuring the proper handoff. Curr Cardiol Rep. 2017;19(6):55. [DOI] [PubMed] [Google Scholar]
- 203.Reiss J, Gibson R. Health care transition: destinations unknown. Pediatrics. 2002;110(6 Pt 2):1307–1314. [PubMed] [Google Scholar]
- 204.Wallace WH, Thompson L, Anderson RA. Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ. 2013;346:f1190. [DOI] [PubMed] [Google Scholar]
- 205.Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27(3):143–167. [DOI] [PubMed] [Google Scholar]
- 206.Suh E, Daugherty CK, Wroblewski K, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


