Supplemental Digital Content is available in the text.
Keywords: cardiac output, cardiography, sitting position, systolic blood pressure, total peripheral resistance
Abstract
Objective
The sit-up test is used to assess orthostatic hypotension, without the use of a tilt table, in populations who are unable to stand. The primary objective of this study was to determine the differences in blood pressure and hemodynamic responses between the sit-up and head-up tilt tests. The secondary objective was to determine the hemodynamic responses related to changes in blood pressure during each test.
Methods
Nineteen healthy volunteers (nine males, aged 24.3 ± 2.4 years) underwent the sit-up and head-up tilt tests. Systolic and diastolic blood pressure, heart rate, stroke volume, cardiac output, and total peripheral resistance were measured.
Results
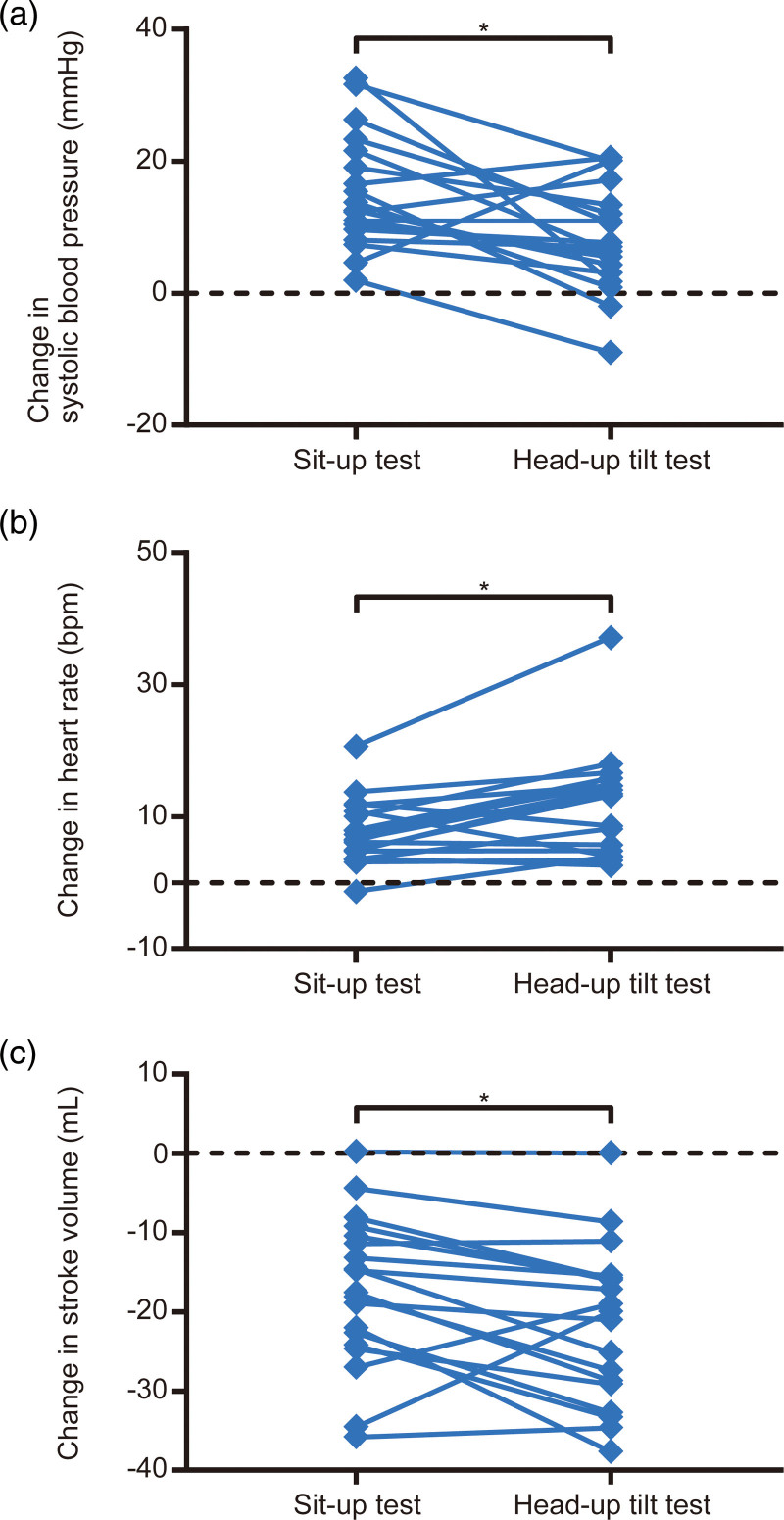
The increase in systolic blood pressure (15 ± 9 vs. 8 ± 8 mmHg) was greater, while the increase in heart rate (8 ± 5 vs. 12 ± 8 bpm) and reduction in stroke volume (−17 ± 10 vs. −21 ± 10 ml) were smaller during the sit-up test than during the head-up tilt test (P < 0.05). Additionally, the increases in blood pressure variables were significantly associated with the increase in total peripheral resistance (P < 0.05), but not with changes in other hemodynamic variables in both tests.
Conclusion
Although the magnitudes of changes in systolic blood pressure, heart rate, and stroke volume differed between the tests, the hemodynamic variable related to changes in blood pressure was the same for both tests. These results may contribute to the clinical application of the sit-up test for identifying the presence and hemodynamic mechanisms of orthostatic hypotension.
Introduction
Postural change from the supine to an upright position is accompanied by a redistribution of the intravascular volume, in which gravity causes approximately 500 ml of blood to pool in the lower extremities and splanchnic veins. This results in a rapid reduction in the central blood volume and subsequent reduction in the stroke volume (SV), cardiac output (CO), and mean blood pressure [1]. Subsequently, baroreflex activation stimulates the sympathetic nervous system and diminishes the activity of the parasympathetic nervous system to increase the heart rate (HR), cardiac contractility, total peripheral resistance (TPR), and venous return [2]. Impairments in one or more of these compensatory responses to restore blood pressure result in orthostatic hypotension [1,2]. Conversely, excessive peripheral vasoconstriction due to sympathetic hyperactivity from overcompensation related to venous pooling in the legs in the upright position may lead to orthostatic hypertension [3–5]. Orthostatic hypotension and orthostatic hypertension are reportedly associated with an increased risk of cardiovascular disease, stroke, cognitive decline, and all-cause mortality [1,3–8]. Therefore, the assessment of postural changes in blood pressure is important to provide appropriate interventions to minimize the occurrence of orthostatic hypotension and orthostatic hypertension.
Active standing and head-up tilt tests are standard orthostatic stress tests to assess postural changes in blood pressure in both research and clinical practice [9,10]. Although the active standing test does not require specialized equipment, such as a tilt table, it may be difficult for individuals who present with balance impairment or difficulty transferring to a standing position to complete the test. The head-up tilt test is a passive orthostatic stress test that can be performed on individuals who have difficulty standing up without aid. However, the use of a tilt table makes the head-up tilt test impractical for clinical settings. To overcome the limitations of the head-up tilt test, the sit-up test was developed and was used to identify the presence of orthostatic hypotension in individuals with spinal cord injury and stroke [11–14]. In the sit-up test, participants are passively moved from the supine to sitting position with the assistance of an assessor. Changes in SBP and DBP during the sit-up test have demonstrated substantial day-to-day reliability [12]. Additionally, the same cut-off points for blood pressure reduction that are recommended for the standard orthostatic stress tests for orthostatic hypotension [10] have been applied to the sit-up test [12–14]. Nevertheless, the hydrostatic effect of gravity, which causes blood pooling in the lower body, may be smaller in the sitting position than in the standing position [15].
Although blood pressure and hemodynamic variables significantly change during the sit-up and head-up tilt tests, even in healthy adults [11,16–20], no direct comparison has been made between the tests for blood pressure and hemodynamic responses. Therefore, from an ethical and scientific perspective, we believe that understanding the difference in blood pressure and hemodynamic responses between the sit-up and head-up tilt tests in healthy young individuals is essential before conducting studies to compare the responses between the tests in patients with orthostatic hypotension. In addition, assessment of the hemodynamic mechanisms underlying postural blood pressure changes can provide useful information for selecting treatment approaches for orthostatic hypotension [21]. HR, SV, CO, and TPR are related to baroreflex-mediated blood pressure control [2]. The increase in TPR is thought to be a major contributor to blood pressure restoration during a postural change from the supine to standing position [8]. However, it remains unclear whether the hemodynamic variables related to the changes in blood pressure are the same for the sit-up and head-up tilt tests.
Therefore, the primary objective of this study was to compare the blood pressure and hemodynamic responses between the sit-up and head-up tilt tests in healthy young individuals. Studies of healthy individuals reported that blood pressure increased from supine to upright position despite the reduction in SV during the postural change [11,16–20]. We hypothesized that the reduction in SV during the sit-up test would be smaller than that during the head-up tilt test due to differences in the hydrostatic effect between the sitting and standing positions [15,22,23], thus, resulting in a larger increase in blood pressure during the sit-up test than during the head-up tilt test. The secondary objective was to identify hemodynamic variables related to postural blood pressure changes during each test. We hypothesized that the changes in blood pressure variables would be more strongly associated with the change in TPR than the changes in HR, SV, and CO during the sit-up test as well as the head-up tilt test.
Materials and methods
Study design
This study used a cross-sectional observational design. Experimental procedures were approved by the appropriate ethics committees of the Tokyo Bay Rehabilitation Hospital (approval number: 239-2) and Shinshu University (approval number: 4615). Participants provided written informed consent prior to participation. The study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013.
Participants
Twenty-one healthy adult volunteers participated in this study. The inclusion criteria were as follows: (1) 20–40 years of age, (2) no underlying diseases, and (3) no history of syncope. Participants were excluded if they had limited range of motion and/or pain that could affect the sit-up and head-up tilt tests.
Orthostatic stress testing
The experimental protocol consisted of the sit-up and head-up tilt tests, with a 10-min rest period between each test. The order of the tests was randomized for each participant. The tests were performed by two trained assessors in a quiet room, at a comfortable temperature. Participants were instructed to refrain from eating and consuming caffeinated products for at least 2 h and to avoid intense physical activity for at least 12 h prior to each test [9]. The tests were performed between 6:00 and 7:00 p.m.
In both tests, the participants remained in a resting supine position on a motorized tilt table (SPR-7001; SAKAI Medical Co., Ltd., Tokyo, Japan) for 10 min before the postural change [9,12,13]. In the sit-up test, participants were passively moved from the supine position to sitting position within 30 s and were then maintained in the sitting position for 3 min with the assistance of an assessor [13]. Participants were instructed not to assist with the maneuver during the test. The height of the tilt table was adjusted such that both of the participants’ feet reached the floor while sitting. In the head-up tilt test, participants were supported by belts at the level of the hips and by a footrest. After the supine rest for 10 min, the tilt table was elevated to an angle of 70° over the course of approximately 30 s and was maintained for 3 min [9].
Measurement of blood pressure and hemodynamic variables
Task Force Monitor 3040i (CNSystems Medizintechnik, Graz, Austria) was used for the noninvasive measurement of blood pressure and hemodynamic variables. A digital cuff was positioned around the second phalanx of the middle finger on the right hand to provide a continuous noninvasive measurement of arterial blood pressure. The right arm was supported by a sling, so that the finger cuff remained at the level of the heart. Continuous measurements of beat-to-beat SBP and DBP were obtained using the vascular unloading technique on the finger [24,25]. These blood pressure values were automatically and continuously corrected to oscillometric blood pressure values obtained in the left arm. HR, SV, CO, and TPR were measured as hemodynamic variables on a beat-to-beat basis. HR was measured using six-lead electrocardiography. SV was derived using an improved transthoracic impedance cardiography method, as previously described [26]. Three short-band electrodes were placed on the participants: one on the neck and two below the thorax. SV was calculated using the following formula:
where Vth is the electrical participating thoracic volume, LVET is the left ventricular ejection time, (dZ/dt)max is the maximal rate of reduction in impedance for a given heartbeat, and Z0 is the base impedance. CO was calculated as the product of HR and SV. TPR was calculated according to Ohm’s law: TPR = mean blood pressure/CO × 80 [27]. Data were filtered using a ±5 s moving average filter to remove signal noise [28]. The averaged values of blood pressure and hemodynamic variables during the supine and upright periods were calculated from data collected during the final 2 min of each period [28].
Statistical analysis
Sample size was calculated using a power analysis with a test family = t-tests, statistical test = means: difference between two dependent means (matched pairs), an alpha value of 0.05, and a statistical power of 0.95. To our knowledge, there were no studies that reported data to calculate the effect size for the difference between changes in blood pressure and hemodynamic variables during sitting up and those during standing up in young healthy individuals. Therefore, based on the results of a study comparing the changes in blood pressure variables, HR, and CO between the active postural changes from the supine to sitting position and from the supine to standing position in elderly patients [29], we used an estimated effect size of 0.80 [30] for the comparative analysis. The power analysis required a minimum sample size of 19.
Data normality was tested using the Shapiro–Wilk test, which determined that the assumption of normality was met for all variables (P > 0.05). Blood pressure and hemodynamic variables were subjected to a two-way repeated-measures analysis of variance (ANOVA) with two positions (supine and upright positions) and two tests (sit-up and head-up tilt tests) as within-subject factors. Post-hoc analyses were performed using a paired t-test with Bonferroni correction. The changes in blood pressure and hemodynamic variables during the tests were calculated by subtracting the values in the supine position from the values in the upright position. Changes in blood pressure and hemodynamic variables were compared between the sit-up and head-up tilt tests using a paired t-test. In addition, the Bland-Altman analysis [31] was used to examine the degree of agreement between the two tests for changes in blood pressure variables. Bias was calculated by subtracting the value of the head-up tilt test from the value of the sit-up test. The limits of agreement were defined as bias ± 1.96 × SD of the difference. Furthermore, we examined the associations between the changes in blood pressure and hemodynamic variables using Pearson’s product-moment correlation coefficient to identify the changes in hemodynamic variables related to changes in blood pressure variables during each test. Statistical analyses were performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, CA, USA). Statistical significance was set at P < 0.05.
Results
Participants
Two of the 21 participants were excluded from the analysis, because hemodynamic data could not be measured due to technical difficulties. Finally, 19 participants (nine males, age 24.3 ± 2.4 years, 20.9 ± 1.8 kg m−2 of BMI) were included in the analysis.
Blood pressure and hemodynamic responses in the sit-up and head-up tilt tests
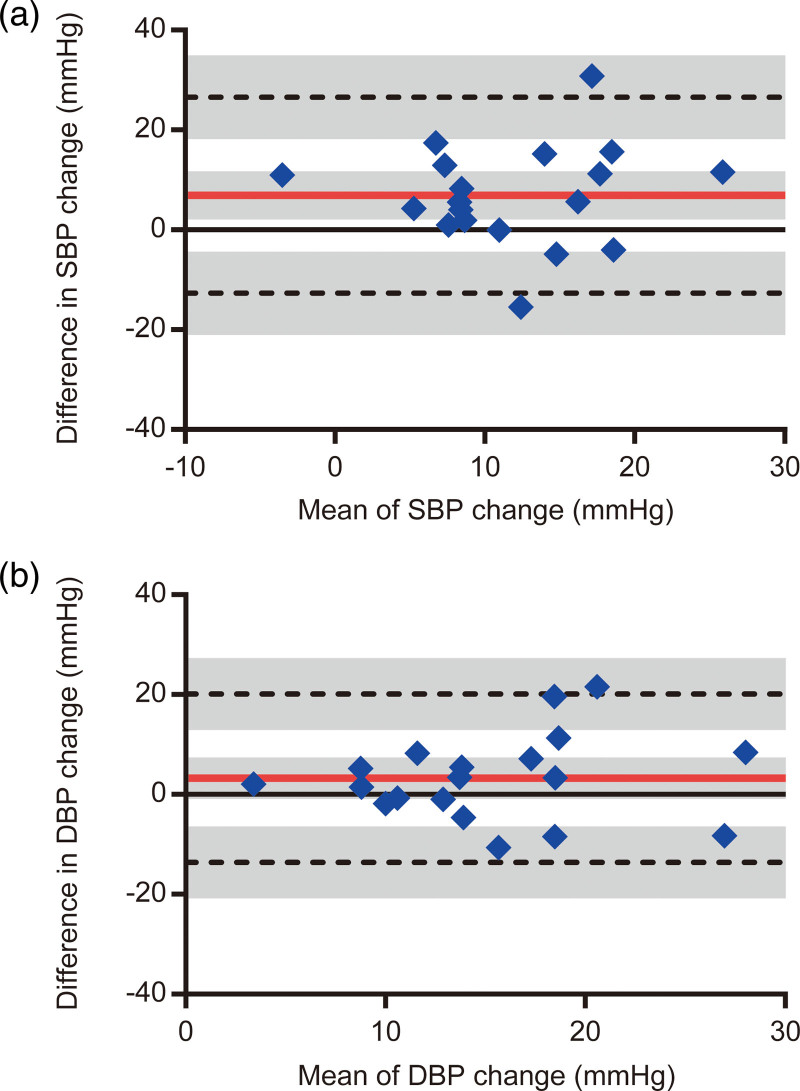
There were no adverse orthostatic symptoms associated with either the sit-up or head-up tilt test. Examples of blood pressure and hemodynamic responses to sit-up and head-up tilt tests are shown in Supplementary Fig. 1, Supplemental digital content 1, http://links.lww.com/BPMJ/A150. The results of the two-way repeated-measures ANOVA are presented in Table 1. For SBP, an interaction between position and test was significant (P = 0.007). Although SBP in the supine position was not significantly different between the sit-up and head-up tilt tests [mean difference = 0, 95% confidence interval (CI) = −5 to 5, P = 0.999], SBP in the upright position was significantly higher in the sit-up test than in the head-up tilt test (mean difference = 7, 95% CI = 2–12, P = 0.003). Additionally, SBP in the upright position was significantly higher than that in the supine position for both the sit-up (mean difference = 15, 95% CI = 10–20, P < 0.001) and head-up tilt tests (mean difference = 8, 95% CI = 3–13, P < 0.001). Participants exhibited an increase in SBP during the sit-up test, while two participants exhibited a reduction in SBP during the head-up tilt test (Fig. 1a). In the two participants who experienced a decrease in SBP of 9 mmHg and 2 mmHg during the head-up tilt test, SBP increased by 2 mmHg and 15 mmHg during the sit-up test, respectively. The increase in SBP during the sit-up test was significantly more than that during the head-up tilt test (mean difference = 7, 95% CI = 2–12, P = 0.007; Fig. 1a). The Bland-Altman analysis for the change in SBP showed that the bias was 7 mmHg and the limits of agreement ranged from −13 to 27 mmHg (Fig. 2a). For DBP, an interaction between position and test (P = 0.116) and a main effect of test (P = 0.096) were significant. No significant difference in DBP between the tests were observed in the supine (mean difference = 1, 95% CI = −3 to 5, P = 0.999) and upright positions (mean difference = 5, 95% CI = 0–8, P = 0.058); however, a main effect of test was significant (P < 0.001). DBP was significantly higher in the upright position than in the supine position for the sit-up (mean difference = 17, 95% CI = 13–21, P < 0.001) and head-up tilt tests (mean difference = 14, 95% CI = 9–18, P < 0.001). The increase in DBP did not significantly differ between the tests (mean difference = 3, 95% CI = −1 to 7, P = 0.116; Supplementary Fig. 2A, Supplemental digital content 1, http://links.lww.com/BPMJ/A150). The Bland-Altman analysis for the change in DBP indicated that the bias was 3 mmHg, and the limits of agreement were from −14 to 20 mmHg (Fig. 2b).
Table 1.
Results of the two-way repeated measures analysis of variance for blood pressure and hemodynamic variables
| Variable | Position | Sit-up test | Head-up tilt test | Main effect | Interaction | |
|---|---|---|---|---|---|---|
| Position | Test | Position × test | ||||
| Systolic blood pressure (mmHg) | Supine | 108 ± 11 | 108 ± 12 | F = 61.12 | F = 4.936 | F = 9.124 |
| Upright | 123 ± 13 | 116 ± 11 | P < 0.001 | P = 0.039 | P = 0.007 | |
| Diastolic blood pressure (mmHg) | Supine | 68 ± 5 | 67 ± 7 | F = 118.1 | F = 3.096 | F = 2.732 |
| Upright | 85 ± 9 | 80 ± 8 | P < 0.001 | P = 0.096 | P = 0.116 | |
| Heart rate (bpm) | Supine | 68 ± 8 | 67 ± 7 | F = 50.48 | F = 3.783 | F = 12.66 |
| Upright | 75 ± 7 | 79 ± 9 | P < 0.001 | P = 0.068 | P = 0.002 | |
| Stroke volume (ml) | Supine | 81 ± 12 | 80 ± 10 | F = 88.30 | F = 10.22 | F = 6.231 |
| Upright | 63 ± 9 | 58 ± 7 | P < 0.001 | P = 0.005 | P = 0.023 | |
| Cardiac output (l min−1) | Supine | 5.40 ± 0.68 | 5.34 ± 0.68 | F = 27.22 | F = 4.335 | F = 0.343 |
| Upright | 4.73 ± 0.57 | 4.59 ± 0.43 | P < 0.001 | P = 0.052 | P = 0.566 | |
| Total peripheral resistance (dyne s cm−5) | Supine | 1176 ± 195 | 1173 ± 199 | F = 99.01 | F = 0.924 | F = 1.030 |
| Upright | 1635 ± 291 | 1577 ± 214 | P < 0.001 | P = 0.349 | P = 0.324 | |
P-values marked in bold indicate a significant main effect and a significant interaction.
Values are presented as the mean ± SD.
Fig. 1.
Comparisons of changes in (a) systolic blood pressure, (b) heart rate, and (c) stroke volume between the sit-up and head-up tilt tests. (a) Mean ± SD of the change in systolic blood pressure was 15 ± 9 mmHg for the sit-up test and 8 ± 8 mmHg for the head-up tilt test. (b) Mean ± SD of the change in heart rate was 8 ± 5 bpm for the sit-up test and 12 ± 8 bpm for the head-up tilt test. (c) Mean ± SD of the change in stroke volume was −17 ± 10 ml for the sit-up test and −21 ± 10 ml for the head-up tilt test. “*”, Significant difference between the sit-up and head-up tilt tests (P < 0.05).
Fig. 2.
Bland-Altman plots between the sit-up and head-up tilt tests for (a) systolic blood pressure and (b) diastolic blood pressure changes. The red solid and black dashed horizontal lines represent the bias and limits of agreement, respectively. Bias was calculated by subtracting the value of the head-up tilt test from the value of the sit-up test. The gray shaded areas illustrate the 95% confidence interval for the bias and limits of agreement.
Regarding hemodynamic variables, a significant interaction between position and time was observed for HR (P = 0.002) and SV (P = 0.023). In the supine position, there was no significant difference in HR (mean difference = 0, 95% CI = −2 to 3, P = 0.999) and SV (mean difference = 1, 95% CI = −3 to 4, P = 0.999) between the tests. In the upright position, HR in the sit-up test was significantly lower than that in the head-up tilt test (mean difference = −4, 95% CI = −7 to −1, P = 0.002), while SV was significantly higher in the sit-up test than in the head-up tilt test (mean difference = 5, 95% = 1–8, P = 0.003). HR in the upright position was significantly higher than that in the supine position for the sit-up (mean difference = 7, 95% CI = 5–10, P < 0.001) and head-up tilt tests (mean difference = 12, 95% CI = 9–15, P < 0.001). The increase in HR was significantly smaller during the sit-up test than during the head-up tilt test (mean difference = −4, 95% CI = −7 to −2, P = 0.002; Fig. 1b). Conversely, SV was significantly lower in the upright position than in the supine position for both the sit-up (mean difference = −17, 95% CI = −21 to −14, P < 0.001) and head-up tilt tests (mean difference = −21, 95% CI = −25 to −18, P < 0.001). The reduction in SV during the sit-up test was significantly smaller than that during the head-up tilt test (mean difference = 4, 95% CI = 1–8, P = 0.023; Fig. 1c). For CO and TPR, there was no significant interaction between position and test (CO: P = 0.566; TPR: P = 0.324) and no significant main effect of test (CO: P = 0.052; TPR: P = 0.329). In the supine position, there were no significant differences in CO (mean difference = 0.06, 95% CI = −0.20 to 0.32, P = 0.999) and TPR (mean difference = 2, 95% CI = −112 to 117, P = 0.999) between the tests. In the upright position, there were also no significant differences in CO (mean difference = 0.14, 95% CI = −0.12 to 0.39, P = 0.840) and TPR (mean difference = 58, 95% CI = −56 to 172, P = 0.907) between the tests. The main effect of position was significant for both CO and TPR (P < 0.001). CO in the upright position was significantly lower than that in the supine position for the sit-up (mean difference = −0.68, 95% CI = −0.94 to −0.42, P < 0.001) and head-up tilt tests (mean difference = −0.75, 95% CI = −1.01 to −0.49, P < 0.001). Conversely, TPR was significantly higher in the upright position than in the supine position for both tests (sit-up test: mean difference = 459, 95% CI = 345–574, P < 0.001; head-up tilt test: mean difference = 404, 95% CI = 290–518, P < 0.001). There were no significant differences in the decrease in CO (mean difference = 0.07, 95% CI = −0.91 to 0.33, P = 0.566; Supplementary Fig. 2B, Supplemental digital content 1, http://links.lww.com/BPMJ/A150) and the increase in TPR (mean difference = 55, 95% CI = −59 to 170, P = 0.324; Supplementary Fig. 2C, Supplemental digital content 1, http://links.lww.com/BPMJ/A150) between the tests.
Hemodynamic variables related to the changes in blood pressure variables during each test
The associations between the changes in blood pressure variables and changes in hemodynamic variables during each test are shown in Table 2. In the sit-up test, the increases in SBP (r = 0.484, P = 0.036) and DBP (r = 0.594, P = 0.007) were significantly associated with an increase in TPR, while no significant associations were observed regarding changes in other hemodynamic variables. In the head-up tilt test, the increases in SBP (r = 0.546, P = 0.016) and DBP (r = 0.619, P = 0.005) were also significantly correlated with only an increase in TPR.
Table 2.
Correlations between the changes in blood pressure variables and hemodynamic variables
| Changes in hemodynamic variables | Changes in blood pressure variables | |||||
|---|---|---|---|---|---|---|
| Systolic blood pressure | Diastolic blood pressure | |||||
| r | 95% CI | P-value | r | 95% CI | P-value | |
| Sit-up test | ||||||
| Heart rate | 0.026 | −0.433 to 0.475 | 0.916 | 0.181 | −0.298 to 0.587 | 0.459 |
| Stroke volume | 0.176 | −0.303 to 0.583 | 0.472 | −0.033 | −0.480 to 0.428 | 0.894 |
| Cardiac output | 0.274 | −0.206 to 0.647 | 0.426 | 0.150 | −0.327 to 0.566 | 0.540 |
| Total peripheral resistance | 0.484 | 0.038–0.769 | 0.036 | 0.594 | 0.191–0.826 | 0.007 |
| Head-up tilt test | ||||||
| Heart rate | −0.324 | −0.678 to 0.153 | 0.177 | −0.168 | −0.578 to 0.310 | 0.491 |
| Stroke volume | 0.085 | −0.384 to 0.519 | 0.731 | −0.047 | −0.491 to 0.416 | 0.848 |
| Cardiac output | −0.109 | −0.537 to 0.363 | 0.657 | −0.146 | −0.563 to 0.330 | 0.551 |
| Total peripheral resistance | 0.546 | 0.122–0.802 | 0.016 | 0.619 | 0.229–0.838 | 0.005 |
P-values marked in bold indicate a significant main effect and a significant interaction.
CI, confidence interval; r, Pearson’s product-moment correlation coefficient.
Discussion
To our knowledge, this study is the first to compare postural changes in blood pressure and hemodynamic variables between the sit-up and head-up tilt tests. The directions of changes in blood pressure and hemodynamic variables during the sit-up test were similar to those during the head-up tilt test. However, the sit-up test elicited a larger increase in SBP, a smaller increase in HR, and a smaller reduction in SV than the head-up tilt test. In addition, in both tests, increases in SBP and DBP were associated with an increase in TPR, but not with changes in other hemodynamic variables. These results may have important implications for the clinical application of the sit-up test, such as identifying the presence and hemodynamic mechanisms of orthostatic hypotension.
Comparisons of blood pressure and hemodynamic responses to the sit-up and head-up tilt tests
As a rest period of at least 5-min is considered sufficient to establish a stable baseline [9,28], the sit-up and head-up tilt tests were performed in random order with a 10-min rest period between each test. These procedures may help us reduce order bias and carryover effects, which resulted in no significant differences in all blood pressure and hemodynamic variables in the supine position between the tests.
We found increases in the mean values of the blood pressure variables during both tests, which support our hypothesis. In addition, SV and CO decreased, while HR and TPR increased during both tests. The reductions in SV and CO may indicate a reduction in venous return to the heart after postural change from the supine to the upright position [1,2,8,32–35]. The increases in HR and TPR suggest baroreflex-mediated compensatory sympathetic activation and reduced parasympathetic activation [1,2,8,36,37]. The increase in blood pressure variables during the tests suggest that the reduction in CO was compensated by the increase in TPR.
We found a higher SV in the upright position during the sit-up test than during the head-up tilt test. Compared with the sitting position, more blood is pooled in the lower extremities as a result of gravitational forces in the standing position, which leads to a smaller venous return in the standing position than in the sitting position [15]. Therefore, SV in the sitting position has been reported to be larger than that in the standing position [22,23]. Moreover, we observed a higher CO and TPR in the upright position during the sit-up test than during the head-up tilt test; however, these differences were not statistically significant. The differences observed in the mean values of these hemodynamic variables between the tests were thought to result in a higher SBP in the upright position during the sit-up test than during the head-up tilt test.
Regarding the changes in blood pressure variables during the orthostatic stress tests, the increase in SBP was significantly greater during the sit-up test than during the head-up tilt test with the bias of 7 mmHg. We also found that all participants exhibited an increase in SBP during the sit-up test, while two participants exhibited a reduction in SBP during the head-up tilt test. Additionally, the limits of agreement indicated that the change in SBP measured during the sit-up test may be between 13 mmHg below and 27 mmHg above the head-up tilt test. For the change in DBP, we found that there was no significant difference between the tests and the bias was 3 mmHg. However, the limits of agreement showed that the change in DBP measured during the sit-up test may be between 14 mmHg below and 20 mmHg above the head-up tilt test. Given that a reduction in SBP of at least 20 mmHg or DBP of 10 mmHg are used as a cut-off for orthostatic hypotension [10], the limits of agreement for the changes in SBP and DBP observed in this study were broad. These results suggest that the same cut-off points for blood pressure reduction that are recommended for the standard orthostatic stress tests of orthostatic hypotension probably should not be applied to the sit-up test. As all the participants did not have orthostatic hypotension, further research is needed to compare the blood pressure and hemodynamic responses between the tests in a clinical population with an increased prevalence of orthostatic hypotension.
Hemodynamic variables related to the changes in blood pressure variables during each test
The results from the correlation analysis support our hypothesis that the changes in blood pressure variables are more strongly associated with the changes in TPR than the changes in other hemodynamic variables during the sit-up test as well as the head-up tilt test. Thus, hemodynamic responses related to the changes in blood pressure may be similar between the tests. Assessment of the hemodynamic mechanisms of orthostatic hypotension can aid treatment selection. van Wijnen et al. [38] demonstrated that a delayed recovery from initial orthostatic hypotension was associated with an impaired increase in TPR, suggesting that the use of vasodilators to treat hypertension has a negative effect on initial orthostatic blood pressure control. If an impaired TPR is the mechanism of orthostatic hypotension, midodrine that is an alpha-1 agonist may be appropriate given that it causes vasoconstriction and would work to increase TPR [21,39]. Conversely, the selection of fludrocortisone and compression therapy may be more appropriate if the driving mechanism of orthostatic hypotension is the reduction in CO due to reduction in SV [21]. Fludrocortisone acts at renal mineralocorticoid receptors to promote sodium and water retention and, thus, increases intravascular volume [39]. Compression therapy reduces venous pooling in the legs and improves venous return to the heart [40]. Therefore, these therapies will help prevent a reduction in SV and maintain CO. Further research is warranted to verify that the sit-up test can be used to determine the hemodynamic mechanisms underlying orthostatic hypotension.
Study limitations
This study had some limitations. First, we evaluated only healthy young participants to demonstrate normal blood pressure and hemodynamic responses to the sit-up test. Thus, extrapolation of the results to other groups, such as older adults and individuals with autonomic impairments that cause orthostatic hypotension, is not possible. Second, as the time required for sitting up during the sit-up test was not strictly controlled in this study, it might have differed between participants. Nevertheless, participants were moved from the supine to sitting position within 30 s. In addition, the measurements in the upright position were defined as the average value obtained during the final 2 min to eliminate the analysis of data obtained during the transient period, which is complete within 20 s in healthy adults [28]. Therefore, the influence of individual differences in the time required to complete the sit-up test on the results of this study may be small. Finally, we used an effect size estimated based on a study of elderly individuals [29] to calculate the sample size. As elderly persons are more prone to changes in blood pressure with a postural challenge [41,42], the sample size was relatively small, which may reduce the power to detect small differences between changes in blood pressure and hemodynamic variables during the sit-up test and those during the head-up tilt test. Nevertheless, we believe the results of the comparative analysis were reasonable.
In conclusion, increases in blood pressure variables, HR, and TPR and reductions in SV and CO were observed during the sit-up and head-up tilt tests. However, the increase in SBP was greater, while the increase in HR and reduction in SV were smaller during the sit-up test than during the head-up tilt test. In addition, increases in the blood pressure variables were associated with an increase in TPR but not with the changes in other hemodynamic variables during both tests. The findings of this study may provide a basis for the clinical application of the sit-up test.
Acknowledgements
This study was supported by a grant from the Funds for Grant-in-Aid for Young Scientists to Kazuaki Oyake (18K17730 and 21K17489).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.bpmonitoring.com.
References
- 1.Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol 2015; 66:848–860. [DOI] [PubMed] [Google Scholar]
- 2.Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic hypotension: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72:1294–1309. [DOI] [PubMed] [Google Scholar]
- 3.Kario K. Orthostatic hypertension-a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol 2013; 9:726–738. [DOI] [PubMed] [Google Scholar]
- 4.Jordan J, Ricci F, Hoffmann F, Hamrefors V, Fedorowski A. Orthostatic hypertension: critical appraisal of an overlooked condition. Hypertension 2020; 75:1151–1158. [DOI] [PubMed] [Google Scholar]
- 5.Magkas N, Tsioufis C, Thomopoulos C, Dilaveris P, Georgiopoulos G, Doumas M, et al. Orthostatic hypertension: from pathophysiology to clinical applications and therapeutic considerations. J Clin Hypertens (Greenwich) 2019; 21:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015; 36:1609–1617. [DOI] [PubMed] [Google Scholar]
- 7.Iseli R, Nguyen VTV, Sharmin S, Reijnierse EM, Lim WK, Maier AB. Orthostatic hypotension and cognition in older adults: a systematic review and meta-analysis. Exp Gerontol 2019; 120:40–49. [DOI] [PubMed] [Google Scholar]
- 8.Magkas N, Tsioufis C, Thomopoulos C, Dilaveris P, Georgiopoulos G, Sanidas E, et al. Orthostatic hypotension: from pathophysiology to clinical applications and therapeutic considerations. J Clin Hypertens (Greenwich) 2019; 21:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheshire WP, Jr, Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res 2019; 29:215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21:69–72. [DOI] [PubMed] [Google Scholar]
- 11.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006; 23:1713–1725. [DOI] [PubMed] [Google Scholar]
- 12.Currie KD, Wong SC, Warburton DE, Krassioukov AV. Reliability of the sit-up test in individuals with spinal cord injury. J Spinal Cord Med 2015; 38:563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang A, Eng JJ, Krassioukov A. Application of the Sit-Up Test for orthostatic hypotension in individuals with stroke. Auton Neurosci 2012; 168:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Wecht JM, Legg Ditterline B, Ugiliweneza B, Maher MT, Lombard AT, et al. Heart rate and blood pressure response improve the prediction of orthostatic cardiovascular dysregulation in persons with chronic spinal cord injury. Physiol Rep 2020; 8:e14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olufsen MS, Ottesen JT, Tran HT, Ellwein LM, Lipsitz LA, Novak V. Blood pressure and blood flow variation during postural change from sitting to standing: model development and validation. J Appl Physiol (1985) 2005; 99:1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley LA, Conway F, Anderson PJ, Tomlinson B, Critchley JA. Non-invasive continuous arterial pressure, heart rate and stroke volume measurements during graded head-up tilt in normal man. Clin Auton Res 1997; 7:97–101. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 2005; 289:R109–R116. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 2003; 88:769–774. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi A, Benitez D, Gaydecki PA, Vohra A, Fitzpatrick AP. Haemodynamic effects of increasing angle of head up tilt. Heart 2000; 83:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onizuka C, Niimi Y, Sato M, Sugenoya J. Arterial blood pressure response to head-up tilt test and orthostatic tolerance in nurses. Environ Health Prev Med 2015; 20:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deegan BM, O’Connor M, Donnelly T, Carew S, Costelloe A, Sheehy T, et al. Orthostatic hypotension: a new classification system. Europace 2007; 9:937–941. [DOI] [PubMed] [Google Scholar]
- 22.Edgell H, Robertson AD, Hughson RL. Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J Appl Physiol (1985) 2012; 112:1482–1493. [DOI] [PubMed] [Google Scholar]
- 23.Middlemiss JE, Cocks A, Paapstel K, Maki-Petaja KM, Sunita , Wilkinson IB, McEniery CM; ACCT Study Investigators. Evaluation of inert gas rebreathing for determination of cardiac output: influence of age, gender and body size. Hypertens Res 2019; 42:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeleazcov C, Krajinovic L, Münster T, Birkholz T, Fried R, Schüttler J, Fechner J. Precision and accuracy of a new device (CNAPTM) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. Br J Anaesth 2010; 105:264–272. [DOI] [PubMed] [Google Scholar]
- 25.Brittain JM, Busk TM, Møller S. Validation of non-invasive haemodynamic methods in patients with liver disease: the Finometer and the Task Force Monitor. Clin Physiol Funct Imaging 2018; 38:384–389. [DOI] [PubMed] [Google Scholar]
- 26.Fortin J, Habenbacher W, Heller A, Hacker A, Grüllenberger R, Innerhofer J, et al. Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med 2006; 36:1185–1203. [DOI] [PubMed] [Google Scholar]
- 27.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart 2003; 89:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finucane C, van Wijnen VK, Fan CW, Soraghan C, Byrne L, Westerhof BE, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res 2019; 29:427–441. [DOI] [PubMed] [Google Scholar]
- 29.Breeuwsma AC, Hartog LC, Kamper AM, Groenier KH, Bilo HJ, Kleefstra N, Van Hateren KJ. Standing orthostatic blood pressure measurements cannot be replaced by sitting measurements. Hypertens Res 2017; 40:765–770. [DOI] [PubMed] [Google Scholar]
- 30.Maher JM, Markey JC, Ebert-May D. The other half of the story: effect size analysis in quantitative research. CBE Life Sci Educ 2013; 12:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310. [PubMed] [Google Scholar]
- 32.Katkov VE, Chestukhin VV. Blood pressure and oxygenation in different cardiovascular compartments of a normal man during postural exposures. Aviat Space Environ Med 1980; 51:1234–1242. [PubMed] [Google Scholar]
- 33.Matzen S, Perko G, Groth S, Friedman DB, Secher NH. Blood volume distribution during head-up tilt induced central hypovolaemia in man. Clin Physiol 1991; 11:411–422. [DOI] [PubMed] [Google Scholar]
- 34.Stead EA, Warren JV, Merrill AJ, Brannon ES. The cardiac output in male subjects as measured by the technique of right atrial catheterization. normal values with observations on the effect of anxiety and tilting. J Clin Invest 1945; 24:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Marshall RJ, Shepherd JT. The effect of changes in posture and of graded exercise on stroke volume in man. J Clin Invest 1960; 39:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp Physiol 2001; 86:677–681. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P, Paudel BH, Singh PN, Limbu P. Heart rate variability: response to graded head up tilt in healthy men. Kathmandu Univ Med J (KUMJ) 2009; 7:252–257. [DOI] [PubMed] [Google Scholar]
- 38.van Wijnen VK, Hove DT, Finucane C, Wieling W, van Roon AM, Ter Maaten JC, Harms MPM. Hemodynamic mechanisms underlying initial orthostatic hypotension, delayed recovery and orthostatic hypotension. J Am Med Dir Assoc 2018; 19:786–792. [DOI] [PubMed] [Google Scholar]
- 39.Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol 2017; 33:1725–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smeenk HE, Koster MJ, Faaij RA, de Geer DB, Hamaker ME. Compression therapy in patients with orthostatic hypotension: a systematic review. Neth J Med 2014; 72:80–85. [PubMed] [Google Scholar]
- 41.Finucane C, O’Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 2014; 130:1780–1789. [DOI] [PubMed] [Google Scholar]
- 42.Wu JS, Yang YC, Lu FH, Wu CH, Chang CJ. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens Res 2008; 31:897–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.