Supplemental Digital Content is available in the text.
Keywords: ambulatory blood pressure monitoring, hypertension, overactive bladder, urinary incontinence
Abstract
Objectives
To characterize the blood pressure (BP) profile of the new β3-adrenergic receptor agonist, vibegron, in patients with overactive bladder.
Methods
Patients were randomized to once-daily vibegron 75 mg or placebo for 28 days and underwent ambulatory BP monitoring. The primary endpoint was change from baseline (CFB) to day 28 in mean daytime ambulatory systolic BP (SBP). Secondary endpoints were CFB in mean 24-h SBP and in mean daytime and mean 24-h ambulatory diastolic BP (DBP) and heart rate (HR). Safety was assessed through adverse event reporting.
Results
Of 214 patients randomized, 96 receiving vibegron and 101 receiving placebo had evaluable baseline and day 28 measurements. Overall, 39.6 and 30.7% of patients receiving vibegron and placebo, respectively, had preexisting hypertension. The least squares mean difference (LSMD; 90% confidence interval) between vibegron and placebo in CFB in mean daytime SBP was 0.8 (–0.9, 2.5) mmHg. LSMD in CFB in mean daytime DBP and HR was 0.0 mmHg and 0.9 bpm, respectively. No significant differences between treatments were seen in CFB in mean 24-h SBP (LSMD, 0.6 mmHg), DBP (–0.2 mmHg) or HR (1.0 bpm). The most common treatment-emergent adverse event was hypertension, with rates comparable between groups [vibegron: n = 5 (4.7%); placebo: n = 4 (3.7%)]. One patient receiving vibegron took a prohibited medication (phentermine) known to increase BP.
Conclusions
Once-daily vibegron had no statistically significant or clinically relevant effects on BP or HR.
Introduction
Pharmacologic treatment for overactive bladder (OAB), which is characterized by bothersome symptoms of urgency with or without urinary incontinence [1,2], includes anticholinergics and β3-adrenergic receptor agonists [2]. However, treatment with anticholinergics has been associated with QT prolongation and increased heart rate (HR) [3,4], and treatment with a β3-adrenergic agonist may be associated with increases in blood pressure (BP) and HR [5]. Thus, there is an unmet need for safe and effective treatments for OAB that do not have off-target cardiovascular effects. Vibegron is a new β3-adrenergic receptor agonist that showed a favorable efficacy and safety profile in patients with OAB in the EMPOWUR trial with a once-daily dose of 75 mg [6,7]. Vibegron 75 mg was recently approved in the USA for the treatment of adults with OAB.
β3-adrenergic receptors are the most prevalent β-adrenergic receptor subtype expressed on detrusor smooth muscle [8,9]. In-vitro studies have shown that vibegron has high specificity at β3-adrenergic receptors [10]. However, in addition to their expression in the urinary bladder, β-adrenergic receptors are expressed in cardiovascular tissue [11], hence the concern for potential off-target cardiovascular effects.
Adults with OAB have a higher prevalence of cardiovascular comorbidities, such as hypertension, relative to those without OAB [12,13]. Hypertension is associated with an increased risk of cardiovascular events, including congestive heart failure, myocardial infarction and stroke [14,15]. As a result, regulatory guidelines recommend characterization of the pressor effects of new drug compounds, particularly those intended for chronic use [16], with respect to the potential for off-target effects. In the phase 3 EMPOWUR trial to evaluate the efficacy and safety of once-daily vibegron 75 mg in patients with OAB, vibegron was associated with rates of hypertension that were low and similar to placebo [6]. Because cardiovascular risk can be associated with even small increases in BP, ambulatory BP monitoring (ABPM) is a useful tool to detect changes in BP, as low as 2–3 mmHg, and HR over a 24-h period [16]. To characterize the BP and HR profile of vibegron, we performed a dedicated ABPM study of once-daily vibegron 75 mg vs. placebo in patients with OAB.
Methods
Study design and participants
This was a randomized, double-blind, placebo-controlled, parallel-group study in patients with OAB conducted at 10 centers in the USA from December 2018 to June 2019. The study consisted of an up to 45-day screening period; a 28-day treatment period and a 7–10-day follow-up period. Patients were randomly assigned 1:1 to receive once-daily vibegron 75 mg or placebo during the treatment period and did not take any other medication for the treatment of OAB throughout the course of the trial. Randomization was stratified based on age (≤55, >55 years), sex (male/female) and preexisting hypertension (yes/no) and was carried out using an interactive voice or web response system.
Eligible participants were adults 40–75 years of age with OAB with or without urge urinary incontinence. Key exclusion criteria were the use of any prohibited medications (without suitable washout period when indicated); uncontrolled hypertension [systolic BP (SBP) >160 mmHg, diastolic BP (DPB) >95 mmHg, or both] or resting HR >100 bpm at screening; cerebrovascular, cardiovascular or other neurovascular events or interventions within 6 months before screening; and QT interval corrected using Fridericia’s formula >450 milliseconds for men or >470 milliseconds for women or other arrhythmias. Preexisting hypertension was not exclusionary as long as it was managed; up to 55% of patients could have a medical history of hypertension, hypertension at baseline, or both history of and baseline hypertension.
Ambulatory blood pressure methods and outcomes
Patients were fitted with an ABPM device at baseline and on day 28 of the study and returned to the clinic on days 1 and 29 after completion of 24-h assessments. Ambulatory BP recordings were considered invalid if the patient had ≥6 consecutive or ≥8 individual readings missed between 8:00 a.m. and 9:59 p.m. or 20 individual readings missed overall.
The primary endpoint was change from baseline to day 28 in mean daytime (defined as the time interval in which the patient was awake) ambulatory SBP. Key secondary endpoints were change from baseline to day 28 in mean daytime DBP and HR and in mean 24-h SBP, DBP and HR. Additional exploratory endpoints included change from baseline to day 28 in maximum SBP, DBP and HR from 0.5 to 6.5 h, corresponding to the time to the maximum concentration of vibegron, after the time of cuff fitting at baseline and day 28.
Safety
Adverse events were collected from the time of informed consent until follow-up was completed. The adverse events were coded using the Medical Dictionary for Regulatory Activities version 21.1. Treatment-emergent adverse events (TEAEs) were defined as adverse events occurring during the period from the first dose of double-blind study treatment through 10 days after the last dose. Hypertension was considered an adverse event of clinical interest if the following criteria were met: for patients without hypertension at baseline, SBP ≥140 mmHg or DBP ≥90 mmHg (at two consecutive visits for the average of three measurements); for patients with baseline hypertension, SBP increase ≥20 mmHg or DBP increase ≥10 mmHg (at two consecutive visits for the average of three measurements); or the initiation of, or increase in the dose of, medication for the treatment of hypertension. This definition is consistent with the hypertension criteria from the pivotal phase 3 EMPOWUR trial for vibegron 75 mg in patients with OAB [6]. Safety was also evaluated through clinical laboratory assessments and vital signs.
Statistical analyses
The primary hypothesis was to test if the difference in change from baseline to day 28 in mean daytime ambulatory SBP between vibegron and placebo was ≥3.5 mmHg. A sample size of 90 evaluable patients per treatment group would have 75% power to detect a treatment effect of 3.5 mmHg in daytime SBP, assuming an SD of 10 mmHg and a 2-sided significance level of 0.10, which is equivalent to the power needed to rule out a treatment difference of ≥3.5 mmHg. Therefore, an upper bound of the 90% confidence interval (CI) for the treatment differences of <3.5 mmHg would result in the conclusion that the true treatment difference is <3.5 mmHg. Given an estimated 10% dropout rate or unevaluable ambulatory BP data, the planned enrollment was 200 patients.
Ambulatory BP parameters were analyzed for the full analysis set, which included all participants who were randomized, took ≥1 dose of double-blind study treatment and had evaluable baseline and day 28 ABPM. Safety outcomes were analyzed for the safety set, which included all participants who received ≥1 dose of double-blind study treatment. All endpoints were analyzed using linear models and included terms for treatment, age group (≤55, >55 years), sex (male/female), preexisting hypertension (yes/no) and baseline ambulatory BP value. Mean ambulatory BP parameter values for each treatment group and the treatment differences were calculated with 2-sided 90% CIs. CIs not overlapping 0 were considered statistically significant. There were no adjustments for multiplicity.
Percentages of patients meeting prespecified categorical thresholds for change from baseline in ambulatory SBP (≥15 mmHg), DBP (≥10 mmHg) and HR (≥10 bpm) are presented descriptively. Additional analyses were performed in predefined subgroups of patients by age (≤75th, >75th percentile; 75th percentile defined as a median age of 66 years) and by preexisting hypertension (yes/no).
Ethics statement
This study was conducted in accordance with the International Conference on Harmonisation for Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice. The investigators obtained approval from an institutional review board or independent ethics committee before study initiation. All patients provided written informed consent at the screening visit before participating in any study procedures.
Results
Participants
Of 455 patients screened, 214 were randomized, and all received ≥1 dose of vibegron 75 mg (n = 106) or placebo (n = 108) (Supplementary Figure 1, Supplemental digital content 1, http://links.lww.com/BPMJ/A151). Of those randomized, 197 were included in the full analysis set. Overall, 97.2% of patients completed the study. One patient in each group took a prohibited concomitant medication during the study: one patient in the placebo group took a nasal sympathomimetic for cold-like symptoms on the morning of day 29 (the actual date of the day 28 visit) and one patient in the vibegron group took phentermine for weight loss for approximately 5 months before screening and twice daily during the study. The patient randomized to the vibegron group taking the prohibited concomitant medication phentermine was later identified as an extreme outlier for ABPM parameters.
Demographic characteristics were well balanced between groups (Table 1). Overall mean age was 59.3 years, and most patients (74.6%) were female. Of the study population, 69 patients (35%) had preexisting hypertension, which was present in more patients in the vibegron group (39.6%) than in the placebo group (30.7%). At baseline, mean SBP, DBP and HR were generally similar between groups (Table 1).
Table 1.
Patient baseline demographics and clinical characteristics (full analysis seta)
| Characteristic | Placebo (N = 101) | Vibegron (N = 96) | Overall (N = 197) |
|---|---|---|---|
| Mean (SD) age, years | 59.2 (8.2) | 59.4 (8.6) | 59.3 (8.4) |
| Age group, n (%) | |||
| ≥55 years | 66 (65.3) | 66 (68.8) | 132 (67.0) |
| ≥65–≤75 years | 33 (32.7) | 31 (32.3) | 64 (32.5) |
| Women, n (%) | 74 (73.3) | 73 (76.0) | 147 (74.6) |
| Mean (SD) SBP, mmHgb | 121.5 (13.5) | 119.8 (15.5) | 120.7 (14.5) |
| Mean (SD) DBP, mmHgb | 76.3 (8.2) | 75.0 (8.7) | 75.7 (8.4) |
| Mean (SD) HR, bpmb | 70.3 (8.9) | 71.7 (9.7) | 71.0 (9.3) |
| Comorbid disorders of clinical relevance, n (%)c | |||
| Preexisting hypertensiond | 31 (30.7) | 38 (39.6) | 69 (35.0) |
| Type 2 diabetes | 6 (5.9) | 16 (16.7) | 22 (11.2) |
| Hyperlipidemia | 12 (11.9) | 11 (11.5) | 23 (11.7) |
| Hypercholesterolemia | 9 (8.9) | 12 (12.5) | 21 (10.7) |
| Obesity | 10 (9.9) | 8 (8.3) | 18 (9.1) |
| Type 1 diabetes | 1 (1.0) | 2 (2.1) | 3 (1.5) |
| Chronic kidney disease | 0 (0) | 2 (2.1) | 2 (1.0) |
| Antihypertensive use, n (%) | |||
| ACE inhibitor or angiotensin II antagonist | 15 (14.9) | 24 (25.0) | 39 (19.8) |
| Calcium channel blocker | 12 (11.9) | 9 (9.4) | 21 (10.7) |
| Diuretic | 9 (8.9) | 3 (3.1) | 12 (6.1) |
| Beta blocking agent | 6 (5.9) | 3 (3.1) | 9 (4.6) |
ACE, angiotensin-converting enzyme; BP, blood pressure; DBP, diastolic BP; HR, heart rate; SBP, systolic BP.
All participants who were randomized, took ≥1 dose of double-blind study treatment, and had evaluable baseline and day 28 ambulatory BP measurements.
In-clinic baseline measurement.
Based on medical history.
Preexisting hypertension was defined as baseline SBP ≥140 mmHg and/or DBP ≥90 mmHg (based on clinical visit measurements) or a medical history of hypertension.
Ambulatory blood pressure findings
There were no statistically significant differences (90% CIs include 0) in mean daytime SBP, DBP or HR after 28 days of treatment with vibegron compared with placebo (Table 2). At day 28, least squares mean (90% CI) change from baseline in mean daytime SBP was 0.8 (–0.6 to 2.2) mmHg for vibegron and 0.0 (–1.4 to 1.4) mmHg for placebo [least squares mean difference (90% CI), 0.8 (–0.9 to 2.5) mmHg; Table 2]. Least squares mean difference (90% CI) between vibegron and placebo in change from baseline in mean daytime DBP was 0.0 (–1.2 to 1.1) mmHg and in mean daytime HR was 0.9 (–0.3 to 2.0) bpm (Table 2).
Table 2.
Change from baseline to day 28 in mean daytime ambulatory SBP, DBP and HR
| Ambulatory BP parameter | Placebo (N = 101) | Vibegron (N = 96) |
|---|---|---|
| SBP, mmHg | ||
| Mean (SD) baseline | 126.6 (10.5) | 125.8 (11.1) |
| Least squares mean change at day 28 (90% CI) | 0.0 (–1.4 to 1.4) | 0.8 (–0.6 to 2.2) |
| Least squares mean difference vs. placebo (90% CI) | 0.8 (–0.9 to 2.5) | |
| DBP, mmHg | ||
| Mean (SD) baseline | 75.7 (8.3) | 75.9 (8.0) |
| Least squares mean change at day 28 (90% CI) | 0.6 (–0.4 to 1.5) | 0.5 (–0.5 to 1.5) |
| Least squares mean difference vs. placebo (90% CI) | 0.0 (–1.2 to 1.1) | |
| HR, bpm | ||
| Mean (SD) baseline | 76.3 (8.7) | 76.9 (8.3) |
| Least squares mean change at day 28 (90% CI) | 0.2 (–0.7 to 1.1) | 1.1 (0.1 to 2.0) |
| Least squares mean difference vs. placebo (90% CI) | 0.9 (–0.3 to 2.0) | |
BP, blood pressure; DBP, diastolic BP; HR, heart rate; SBP, systolic BP.
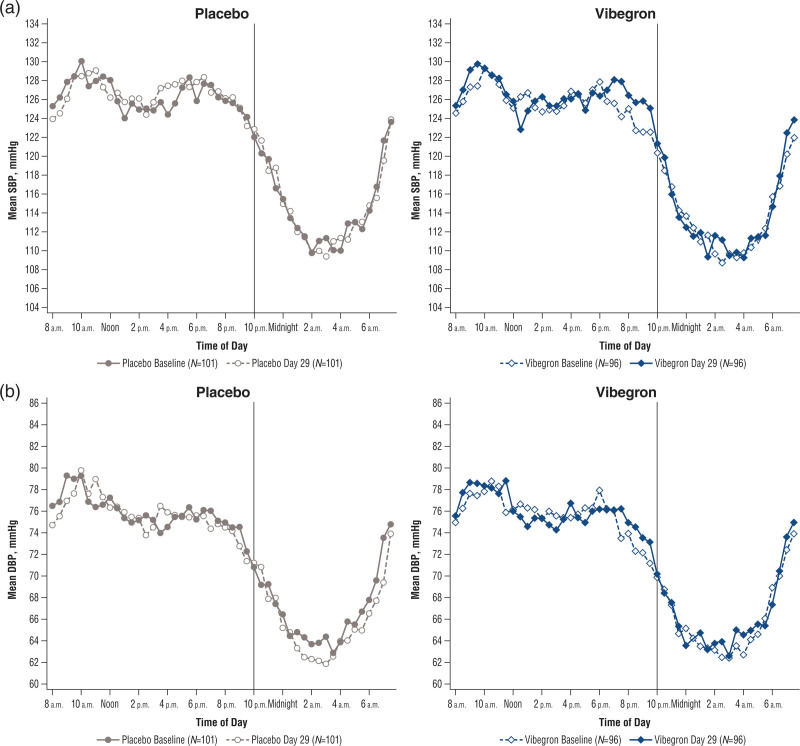
There were no statistically significant differences between vibegron and placebo for the mean changes from baseline in 24-h SBP [least squares mean difference (90% CI), 0.6 (–1.0 to 2.1) mmHg], DBP [–0.2 (–1.3 to 0.9) mmHg] or HR [1.0 (–0.1 to 2.0) bpm] (Table 3; Fig. 1; Supplementary Figure 2, Supplemental digital content 1, http://links.lww.com/BPMJ/A151).
Table 3.
Change from baseline to day 28 in mean 24-h SBP, DBP and HR
| Ambulatory BP parameter | Placebo (N = 101) | Vibegron (N = 96) |
|---|---|---|
| SBP, mmHg | ||
| Mean (SD) baseline | 123.0 (10.0) | 122.1 (11.0) |
| Least squares mean change at day 28 (90% CI) | 0.0 (–1.2 to 1.3) | 0.6 (–0.7 to 1.9) |
| Least squares mean difference vs. placebo (90% CI) | 0.6 (–1.0 to 2.1) | |
| DBP, mmHg | ||
| Mean (SD) baseline | 72.6 (7.9) | 72.8 (7.6) |
| Least squares mean change at day 28 (90% CI) | 0.7 (–0.2 to 1.6) | 0.5 (–0.4 to 1.4) |
| Least squares mean difference vs. placebo (90% CI) | –0.2 (–1.3 to 0.9) | |
| HR, bpm | ||
| Mean (SD) baseline | 74.1 (8.3) | 74.4 (7.6) |
| Least squares mean change at day 28 (90% CI) | –0.2 (–1.0 to 0.7) | 0.8 (–0.1 to 1.7) |
| Least squares mean difference vs. placebo (90% CI) | 1.0 (–0.1 to 2.0) | |
BP, blood pressure; DBP, diastolic BP; HR, heart rate; SBP, systolic BP.
Fig. 1.
Mean 24-h ambulatory (a) SBP and (b) DBP for placebo (left) and vibegron (right). BP, blood pressure; DBP, diastolic BP; SBP, systolic BP.
Similarly, there were no statistically significant differences between vibegron and placebo observed for maximum changes from baseline in SBP [least squares mean difference (90% CI), 1.7 (–0.8 to 4.2) mmHg], DBP [1.2 (–0.4 to 2.8) mmHg] or HR [1.5 (–1.1 to 4.0) bpm] from 0.5 to 6.5 h post dose (Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/BPMJ/A151).
Overall, few patients (≤4 patients in any group) met predefined categorical thresholds (exploratory endpoints) for mean daytime and mean 24-h SBP (≥15 mmHg), DBP (≥10 mmHg) and HR (≥10 bpm); furthermore, percentages of patients meeting such thresholds were similar between vibegron and placebo (Table 4). Slightly higher numbers of patients receiving vibegron relative to placebo met predefined categorical thresholds for maximum (0.5–6.5 h) SBP (6/92 patients vs. 4/96 patients, respectively), DBP (9/92 vs. 5/96) and HR (20/92 vs. 17/96).
Table 4.
Percentage of patients meeting predefined categorical thresholds for change from baseline to day 28 in SBP, DBP and HR
| Ambulatory BP parameter, n (%) | Placebo (N = 101) | Vibegron (N = 96) |
|---|---|---|
| SBP, ≥15 mmHg | ||
| Mean daytime | 2 (2.0) | 2 (2.1) |
| Mean 24-h | 0 (0) | 2 (2.1) |
| Maximum (0.5–6.5 h)a,b | 4 (4.2) | 6 (6.5) |
| DBP, ≥10 mmHg | ||
| Mean daytime | 2 (2.0) | 2 (2.1) |
| Mean 24-h | 0 (0) | 1 (1.0) |
| Maximum (0.5–6.5 h)a,b | 5 (5.2) | 9 (9.8) |
| HR, ≥10 bpm | ||
| Mean daytime | 4 (4.0) | 3 (3.1) |
| Mean 24-h | 3 (3.0) | 3 (3.1) |
| Maximum (0.5–6.5 h)a,b | 17 (17.7) | 20 (21.7) |
BP, blood pressure; DBP, diastolic BP; HR, heart rate; SBP, systolic BP.
Defined as the maximum of the hourly means between 0.5 and 6.5 h post cuff fitting, corresponding to the time to maximum concentration of vibegron.
Placebo, N = 96; vibegron, N = 92.
Mean changes from baseline at day 28 were comparable between the age subgroups (≤75th percentile and >75th percentile; Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/BPMJ/A151) and patients with and without preexisting hypertension (Table 5) for all ambulatory BP endpoints. The percentages of patients in each age subgroup meeting predefined categorical thresholds for mean daytime and 24-h SBP, DBP and HR were also similar. Among patients in the >75th age percentile, slightly higher numbers receiving vibegron vs. placebo met predefined thresholds for maximum (0.5–6.5 h) SBP (3/20 patients vs. 0/26 patients, respectively) and HR (4/20 vs. 3/26); among patients in the ≤75th age percentile, a slightly higher number receiving vibegron vs. placebo met predefined threshold for maximum DBP (7/72 vs. 2/70) (Supplementary Table 3, Supplemental digital content 1, http://links.lww.com/BPMJ/A151). A higher percentage of patients with preexisting hypertension receiving vibegron vs. placebo met predefined thresholds for maximum (0.5–6.5 h) SBP, DBP and HR (Supplementary Table 4, Supplemental digital content 1, http://links.lww.com/BPMJ/A151).
Table 5.
Change from baseline to day 28 in ambulatory SBP, DBP and HR by preexisting hypertension status
| Ambulatory BP parameter, mean (SD) | With preexisting hypertensiona | Without preexisting hypertension | ||
|---|---|---|---|---|
| Placebo (N = 31) | Vibegron (N = 38) | Placebo (N = 70) | Vibegron (N = 58) | |
| Daytime SBP, mmHg | ||||
| Baseline | 132.8 (10.5) | 131.2 (10.2) | 123.8 (9.3) | 122.3 (10.3) |
| Change from baseline at day 28 | –0.9 (8.0) | –0.1 (10.6) | 0.0 (6.0) | 1.1 (5.8) |
| Daytime DBP, mmHg | ||||
| Baseline | 78.5 (8.0) | 77.2 (9.7) | 74.4 (8.2) | 75.1 (6.5) |
| Change from baseline at day 28 | –0.4 (5.3) | 0.0 (7.2) | 0.5 (4.3) | 0.2 (3.7) |
| Daytime HR, bpm | ||||
| Baseline | 75.3 (9.5) | 76.1 (9.5) | 76.7 (8.4) | 77.5 (7.5) |
| Change from baseline at day 28 | 0.2 (4.7) | 1.2 (5.2) | 0.4 (5.0) | 1.3 (4.8) |
| 24-h SBP, mmHg | ||||
| Baseline | 129.1 (9.6) | 127.6 (10.2) | 120.3 (9.0) | 118.5 (10.1) |
| Change from baseline at day 28 | –0.8 (7.5) | –0.6 (9.5) | 0.1 (5.3) | 1.3 (5.6) |
| 24-h DBP, mmHg | ||||
| Baseline | 75.4 (7.4) | 74.4 (9.2) | 71.4 (7.9) | 71.8 (6.2) |
| Change from baseline at day 28 | –0.1 (4.9) | –0.2 (6.8) | 0.8 (3.5) | 0.5 (3.4) |
| 24-h HR, bpm | ||||
| Baseline | 73.8 (9.0) | 73.9 (8.5) | 74.2 (8.0) | 74.8 (7.1) |
| Change from baseline at day 28 | –0.1 (4.5) | 0.9 (4.7) | 0.1 (4.7) | 1.1 (4.3) |
| Maximum (0.5–6.5 h) SBP, mmHgb | ||||
| Baseline | 142.4 (13.0) | 141.5 (12.8) | 133.3 (11.6) | 130.6 (12.9) |
| Change from baseline at day 28c | –1.1 (10.7) | 1.8 (15.3) | 0.0 (9.6) | 1.7 (8.8) |
| Maximum (0.5–6.5 h) DBP, mmHgb | ||||
| Baseline | 86.7 (10.2) | 84.3 (11.4) | 83.1 (9.1) | 82.4 (7.4) |
| Change from baseline at day 28c | –1.7 (7.6) | 1.5 (8.5) | –0.9 (6.6) | –0.4 (6.2) |
| Maximum (0.5–6.5 h) HR, bpmb | ||||
| Baseline | 83.1 (12.7) | 85.3 (15.0) | 86.2 (13.7) | 86.8 (10.8) |
| Change from baseline at day 28c | –0.2 (9.7) | 2.1 (10.4) | 0.5 (12.0) | 0.9 (12.5) |
BP, blood pressure; DBP, diastolic BP; HR, heart rate; SBP, systolic BP.
Defined as baseline SBP ≥140 mmHg and/or DBP ≥90 mmHg (based on clinical visit measurements) or as SBP <140 mmHg and/or DBP <90 mmHg in patients taking antihypertensives.
Defined as the maximum of the hourly means between 0.5 and 6.5 h post cuff fitting, corresponding to the time to maximum concentration of vibegron.
With preexisting hypertension: placebo, N = 30; vibegron, N = 36; without preexisting hypertension: placebo, N = 66; vibegron, N = 56.
Safety
Study drug discontinuation due to TEAEs occurred in three patients receiving vibegron (one patient each: nausea/vomiting/dizziness; back pain and feeling hot/anxiety) and one patient receiving placebo (BP increase). Overall, 46.2% of patients in the vibegron group and 25% in the placebo group experienced ≥1 TEAE (Supplementary Table 5, Supplemental digital content 1, http://links.lww.com/BPMJ/A151). There was no clustering of TEAEs in any one category for either treatment group. There were no deaths in the study. Serious TEAEs occurred in one patient (0.9%) in each group (vibegron: postoperative pain; placebo: hypoglycemia); no serious TEAEs were considered to be related to study treatment by the investigator. Hypertension was reported as a TEAE in five patients [4.7%, (95% CI, 1.6–10.7%)] receiving vibegron and four patients [3.7% (95% CI, 1.0–9.2%)] receiving placebo; no event of hypertension with vibegron was considered related to study treatment by the investigator. Of the five reported events of hypertension with vibegron, one occurred in a patient taking a prohibited medication (phentermine) known to increase BP. In the subgroup analyses of patients with or without preexisting hypertension, there were no differences in the incidence of hypertension between the vibegron and placebo groups. No differences in clinical laboratory parameters or in-clinic vital signs (Supplementary Table 6, Supplemental digital content 1, http://links.lww.com/BPMJ/A151) were observed in patients receiving vibegron compared with placebo.
Discussion
This double-blind, randomized, placebo-controlled trial evaluated the effects of the once-daily β3-adrenergic receptor agonist vibegron 75 mg on BP and HR in patients with OAB and conformed to the regulatory guidance on the assessment of pressor effects during drug development [16]. Treatment with vibegron was not associated with statistically significant or clinically meaningful increases in mean daytime, mean 24-h or maximum ambulatory BP or HR compared with placebo. The upper bound of the 90% CI for the treatment difference of the primary endpoint of change from baseline in mean daytime ambulatory SBP was <2.5 mmHg, which was in fact lower than the prespecified threshold of 3.5 mmHg, providing strong evidence that vibegron does not induce a pressor effect. While a slightly higher number of patients receiving vibegron vs. placebo met predefined categorical thresholds for maximum SBP, DBP and HR up to 6.5 h post dose, these results are of minor clinical importance to the overall daytime and 24-h data that support no differences in BP between vibegron and placebo. Treatment with vibegron was found to be safe and well tolerated over the 28-day treatment period and up to 10 days of follow-up, consistent with results seen in the 12-week EMPOWUR and 40-week EMPOWUR extension trials [6,7].
The subgroup analyses, although not powered to detect differences between groups in this study, showed comparable results among patients in the two age subgroups (≤75th percentile and >75th percentile) and in patients with and without preexisting hypertension for SBP, DBP and HR. The prevalence of OAB increases with age [1,17], and older adults with OAB are more likely to have cardiovascular comorbidities and use more concomitant medications compared with those without OAB [12,13,18]. A previous subpopulation analysis of the EMPOWUR trial showed similar safety of vibegron in adults ≥65 and ≥75 years of age compared with the overall population, with low rates of hypertension that were comparable with placebo [19]. The lack of increases in ambulatory BP among older adults or those with preexisting hypertension in the current trial suggests that treatment with vibegron remains safe from a BP perspective in these populations.
The incidence of adverse events was comparable to the larger phase 3 EMPOWUR trial, with the exception of hypertension [6]. In EMPOWUR, hypertension was reported in 1.7% of patients treated with vibegron (placebo, 1.7%). In the present study, adverse events of hypertension were reported in 5 of 106 patients (4.7%) receiving vibegron and 4 of 108 patients (3.7%) receiving placebo. One patient with hypertension in the vibegron group was taking a prohibited medication known to be associated with hypertension, and thus the reported adverse event of hypertension in this patient was not surprising. This patient was also identified as an extreme outlier for BP and HR outcomes. Only two serious TEAEs were reported in this ABPM study, and neither was considered by the investigator to be related to study treatment. Similarly, no TEAEs of hypertension with vibegron were considered to be related to study treatment.
Our study is limited by its short duration (28 days); however, vibegron has been shown to reach steady-state concentrations within 7 days of once-daily dosing [20]. Furthermore, the high rates of baseline hypertension and use of concomitant cardiovascular medications are strengths of the study because these are common in the OAB patient population [18] and may enhance the risk of destabilized hypertension during a trial, a phenomenon that was not observed. We had less exclusionary criteria for our study than one would expect for an ambulatory BP study; hence these results may be more generalizable to real-world practice of patients with OAB.
Conclusion
In patients with OAB, once-daily vibegron 75 mg was not associated with statistically significant or clinically meaningful effects on BP or HR overall and in patients with or without preexisting (controlled) hypertension.
Acknowledgements
Medical writing and editorial support was provided by Wendy Kandell, PhD, and Krystina Neuman, PhD, CMPP, of The Curry Rockefeller Group, LLC (Tarrytown, New York, USA) and was funded by Urovant Sciences (Irvine, California, USA).
Conflicts of interest
M.A.W. has received research and/or consulting fees from AbbVie, Ablative Solutions, Boston Scientific, Bristol-Myers Squibb, Janssen Pharmaceuticals, Johnson & Johnson, Medtronic, Novartis, ReCor Medical, Sanofi, and Urovant Sciences and honoraria from Ablative Solutions, Astellas, Boston Scientific, Johnson & Johnson, Medtronic, ReCor Medical, and Sanofi. W.B.W. is a consultant for AstraZeneca, Bristol-Myers Squibb, Cadence Pharma, Takeda, and Travere; has received research funding from the National Institute on Aging; has received royalties from Wolters Kluwer; and is editor-in-chief emeritus of Blood Pressure Monitoring. A.W. is an employee of Apex Biostatistics and consultant for Urovant Sciences. J.K. and C.H.-M. are employees of Urovant Sciences. P.N.M. was an employee of Urovant Sciences as the time the study was conducted. This study was funded by Urovant Sciences.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.bpmonitoring.com.
References
- 1.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 2011; 77:1081–1087. [DOI] [PubMed] [Google Scholar]
- 2.Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and Treatment of Overactive Bladder (Non-neurogenic) in Adults: AUA/SUFU Guideline. 2019.American Urological Association; [Google Scholar]
- 3.Arana A, Margulis AV, McQuay LJ, Ziemiecki R, Bartsch JL, Rothman KJ, et al. Variation in cardiovascular risk related to individual antimuscarinic drugs used to treat overactive bladder: a UK cohort study. Pharmacotherapy 2018; 38:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosa GM, Bauckneht M, Scala C, Tafi E, Leone Roberti Maggiore U, Ferrero S, Brunelli C. Cardiovascular effects of antimuscarinic agents in overactive bladder. Expert Opin Drug Saf 2013; 12:815–827. [DOI] [PubMed] [Google Scholar]
- 5.MYRBETRIQ® (mirabegron extended-release tablets). Full Prescribing Information. 2018.Astellas Pharma US, Inc.; [Google Scholar]
- 6.Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN., Jr. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol 2020; 204:316–324. [DOI] [PubMed] [Google Scholar]
- 7.Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN., Jr. Once-daily vibegron 75 mg for overactive bladder: long-term safety and efficacy from a double-blind extension study of the international phase 3 trial (EMPOWUR). J Urol 2021; 205:1421–1429. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of β-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol 2008; 377:473–481. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi O. β3-adrenoceptors in human detrusor muscle. Urology 2002; 59:25–29. [DOI] [PubMed] [Google Scholar]
- 10.Brucker BM, McHale K, King J, Mudd PN. Selectivity and maximum response of vibegron and mirabegron for β3-adrenergic receptors. Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction 2021 Winter Meeting; 2021 February 25-27; Virtual Congress. [Google Scholar]
- 11.Wachter SB, Gilbert EM. Beta-adrenergic receptors, from their discovery and characterization through their manipulation to beneficial clinical application. Cardiology 2012; 122:104–112. [DOI] [PubMed] [Google Scholar]
- 12.Andersson KE, Sarawate C, Kahler KH, Stanley EL, Kulkarni AS. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int 2010; 106:268–274. [DOI] [PubMed] [Google Scholar]
- 13.Asche CV, Kim J, Kulkarni AS, Chakravarti P, Andersson KE. Presence of central nervous system, cardiovascular and overall co-morbidity burden in patients with overactive bladder disorder in a real-world setting. BJU Int 2012; 109:572–580. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–753. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med 2001; 161:1183–1192. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Assessment of Pressor Effects of Drugs. 2018.Guidance for Industry; [Google Scholar]
- 17.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003; 20:327–336. [DOI] [PubMed] [Google Scholar]
- 18.Ganz ML, Liu J, Zou KH, Bhagnani T, Luo X. Real-world characteristics of elderly patients with overactive bladder in the United States. Curr Med Res Opin 2016; 32:1997–2005. [DOI] [PubMed] [Google Scholar]
- 19.Varano S, Staskin D, Frankel J, Shortino D, Jankowich R, Mudd PN., Jr. Efficacy and safety of once-daily vibegron for treatment of overactive bladder in patients aged ≥65 and ≥75 years: subpopulation analysis from the EMPOWUR randomized, international, phase III study. Drugs Aging 2021; 38:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GEMTESA® (vibegron). Full Prescribing Information. 2020.Urovant Sciences, Inc.; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.