Abstract
Objective
Pulse arrival time (PAT) is a potential main feature in cuff-less blood pressure (BP) monitoring. However, the precise relationship between BP parameters and PAT under varying conditions lacks a complete understanding. We hypothesize that simple test protocols fail to demonstrate the complex relationship between PAT and both SBP and DBP. Therefore, this study aimed to investigate the correlation between PAT and BP during two exercise modalities with differing BP responses using an unobtrusive wearable device.
Methods
Seventy-five subjects, of which 43.7% had a prior diagnosis of hypertension, participated in an isometric and dynamic exercise test also including seated periods of rest prior to, in between and after. PAT was measured using a prototype wearable chest belt with a one-channel electrocardiogram and a photo-plethysmography sensor. Reference BP was measured auscultatory.
Results
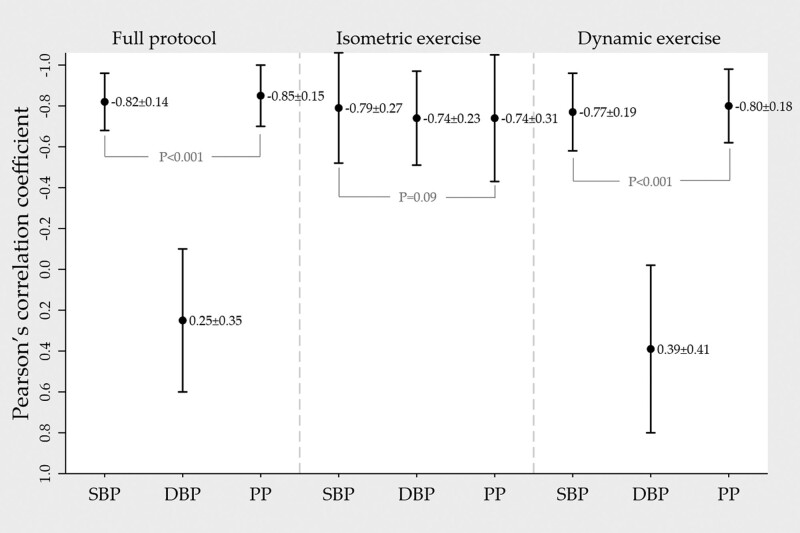
Mean individual correlation between PAT and SBP was −0.82 ± 0.14 in the full protocol, −0.79 ± 0.27 during isometric exercise and −0.77 ± 0.19 during dynamic exercise. Corresponding correlation between PAT and DBP was 0.25 ± 0.35, −0.74 ± 0.23 and 0.39 ± 0.41.
Conclusion
The results confirm PAT as a potential main feature to track changes in SBP. The relationship between DBP and PAT varied between exercise modalities, with the sign of the correlation changing from negative to positive between type of exercise modality. Thus, we hypothesize that simple test protocols fail to demonstrate the complex relationship between PAT and BP with emphasis on DBP.
Keywords: blood pressure monitoring, pulse wave analysis, wearable electronic devices
Introduction
Many studies have confirmed that monitoring blood pressure (BP) during a 24-hour period in ambulatory conditions is superior to office BP in predicting future disease [1]. Still, state-of-the-art 24-hour methods are considered by many as unsatisfactory. Intermittent measurements cannot capture the true hypertensive load, which is also masked by patients being instructed to rest during measurement as motion artifacts and non-steady–state hemodynamic situations easily disrupt the oscillations. Moreover, many find the cuff measurements painful and stressful, especially during night or if BP is elevated, which may affect compliance to monitoring and possibly increase the BP during measurement [2]. Thus, innovation in BP monitoring is motivated by the aim to improve hypertension management.
Cuff-less BP assessment has received increasing research attention in the past decade [3–5]. Pulse wave propagation times such as pulse arrival time (PAT) and pulse transit time (PTT) are commonly used surrogate measurements. The theoretical basis behind PAT as a BP surrogate marker is described in the arterial wall and pulse wave propagation models [6]. In short, if the pressure within a vessel increases, the pulse waves travel faster. This is detectable as a decrease in the measured pulse wave propagation time. PAT, defined as the time interval from an R-wave in an electrocardiogram (ECG) signal to a fiducial point in a peripheral photo-plethysmography (PPG) waveform, is particularly popular due to measurement simplicity, only requiring a simple ECG signal as a proximal timing reference and a second continuous bio-signal such as PPG as a distal timing reference. However, it includes the pre-ejection period (PEP), defined as the time delay from the electrical onset of systole to the mechanical onset of the pulse wave transit time initiated by aortic valve opening. Whether or not exclusion of PEP is necessary for satisfactory accuracy in estimation of BP remains unknown. While some studies argue that simple PAT measurements are inaccurate due to PEP variability [7] others have demonstrated better accuracy of PAT compared to PTT [8]. Extensive research on PAT has demonstrated its relative dependency on BP changes [4,5,9,10]. Still, a key challenge is the transformation of PAT as a single parameter to both SBP and DBP. Most studies investigated PAT and its ability to predict or track both SBP and DBP in experimental protocols where both BP parameters change in the same directions [4]. Thus, test protocols with simple BP altering methods have potential pitfalls. BP regulation and its variations are complex. SBP and DBP may not always covary, for example during different exercise states [11] or in individuals with increased arterial stiffness where pulse pressure (PP) amplification more easily causes isolated rises in systolic pressure [12]. Current evidence indicates a strong correlation between SBP changes and PAT [4]. On the contrary, there is insufficient knowledge on the association between PAT and DBP and on how PAT is affected when SBP and DBP do not change in the same direction [7,13–15].
A differing BP response in isometric compared to dynamic exercise is well known [11]. Isometric exercise generally produces a ‘pressor effect’ causing both SBP and DBP to increase. Dynamic exercise generally introduces a large PP, where SBP increases markedly while DBP is less affected.
Thus, as a step to enable continuous, cuff-less SBP and DBP measurements, the aim of the present study was to utilize the differential BP response in isometric versus dynamic exercise to investigate the effects of differing BP alterations on PAT on an individual level.
Methods
Subjects and recruitment
This study included subjects reflecting the general adult population with a broad range of age as well as inclusion BPs. Subjects with atrial fibrillation, pregnancy or any contraindication to standard cardiac stress testing were excluded [16]. From December 2019 to September 2020, 80 subjects 18–79 years of age were recruited among volunteers and from a local hypertension registry after approval from its steering committee. Five subjects were excluded from the test protocol for the following reasons; inaudible or difficult to auscultate Korotkoff sounds during exercise (n = 2), poor signal quality (n = 1), baseline SBP >180 mmHg (n = 1) and vasovagal reaction (n = 1).
Test device and estimation of pulse arrival time
The test device is a fully wearable and easy-to-use chest belt with three standard electrodes for ECG and a PPG sensor with potential for seamless integration with clinical applications. Technical details on the device have been published previously [17,18], and an upgraded version (new casing, a higher sampling rate of 1 kHz, new PPG sensor) was used in the present study. PAT was calculated for each cardiac cycle from the R-peak in the ECG to the foot in the PPG waveform. Corresponding PAT measurements for each reference SBP and DBP measurement were calculated by finding the median PAT value from 10 valid cardiac cycles before and after. The PAT values were filtered using a gliding filter with a window size of 30 cycles, only keeping the cycles where the PAT value was within a 20% difference from the median value within the window. Subjects were fitted with the test device and an appropriately sized cuff on the non-dominant arm for reference auscultatory BP measurements.
Study protocol
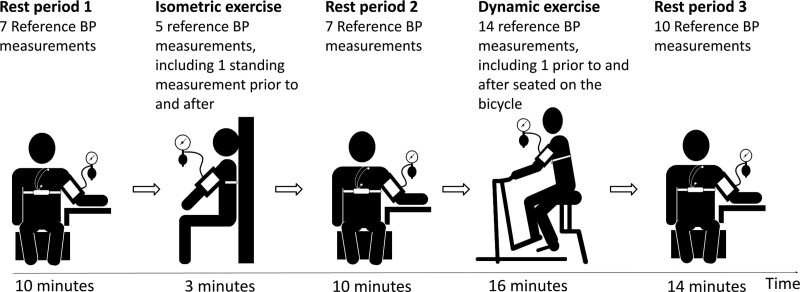
The test protocol (Fig. 1) consisted of an isometric leg exercise, an incremental cycle ergometer test and seated periods of rest before, in-between and after. Prior to the isometric exercise, subjects were instructed to adjust the ankle, knee and hip angle to endure for 3 minutes. The cycle ergometer test was performed on a standard cardiac stress test ergometer cycle (Ergoline Ergoselect 200, GmbH, Bitz, Germany) and consisted of four increments lasting 4 minutes each. The three first increments had stepwise increasing workload and the fourth was a recovery period with equal workload to the first increment. The cycle ergometer test aimed for submaximal exertion during the third increment. Standardization of cycle workload was achieved by each subject determining their fitness level by the rating of perceived capacity tool, which rates maximum exercise capacity for 30 minutes based on metabolic equivalents [19]. Subsequently, the maximum workload during the third increment was calculated to two to three metabolic equivalents below the rating of perceived capacity.
Fig. 1.
Illustration of the test protocol with isometric exercise, dynamic exercise and rest periods. The dynamic exercise consisted of four 4-minute increments with increasing workload from the first through the third and a fourth recovery increment.
A trained physician measured reference auscultatory BP every 1 to 1½ minute throughout the protocol with an aneroid sphygmomanometer (Maxi-Stabil 3; Welch Allyn, Skaneateles Falls, New York, USA). Korotkoff I determined systolic pressure and Korotkoff V diastolic pressure. In case of inaudible Korotkoff V during exercise, Korotkoff IV was used. Reference BP was measured 43 times in each subject; seven measurements during the first rest period, three measurements during the isometric exercise, seven reference measurements during the second rest period, 12 measurements during the dynamic exercise and 10 measurements during the third rest period. In addition, standing measurements were taken prior to and after the isometric exercise, and seated on the cycle prior to and after the dynamic exercise. PAT measurements from the test device were obtained continuously throughout the test protocol. Because SBP and DBP from the reference BP measurements were separated in time and not from the same cardiac cycle, each SBP and DBP was noted to the nearest second to allow for PAT calculations from 10 cardiac cycles before and after the exact time when SBP or DBP was measured. Five subjects with corrupted test-device signals detected in the offline analysis were invited back for re-test to differentiate between subtypes of observable waveforms in the PPG and ECG signals and noise and were included in the analysis with data from the second attempt.
Data and statistical analyses
All analyses were performed offline using the Python programming language using the following packages: NumPy (1.18.2), SciPy (1.4.1), NeuroKit2 (0.0.40), Pandas (1.0.3) and Plotly (4.7.1) [20–24]. Continuous variables were evaluated for normality by visual inspection of histograms and the Shapiro-Wilk test. The strength of the association between BP variables and PAT was investigated in each subject using Pearson’s correlation. Since it was not possible to measure more than three reference BP measurements during the isometric exercise, two measurement pairs taken standing prior to and after exercise were included for increased statistical power. However, a control analysis including only the three measurements during active isometric exercise was performed, and showed a non-significant reduction in the correlation coefficients and still a significant difference between the regression coefficients for PAT and SBP between the two exercise modalities (data not shown). Correlation coefficients were classified in strength in the following way; r = 0–0.19 was considered very weak, 0.2–0.39 as weak, 0.4–0.59 as moderate, 0.6–0.79 as strong and 0.8–1 as very strong [25]. Further analysis of the relationship between PAT and BP parameters was performed with simple linear regression per individual with PAT as the dependent variable and BP parameters as independent variables. Mean of both individual Pearson’s correlation coefficients and regression coefficients were compared between exercise modalities with Wilcoxon Sign Rank Test after assessing for test assumptions. Unless otherwise specified, all continuous variables are presented as mean ± SD, while categorical values are presented as absolute numbers with percentage in parentheses and P < 0.05 was chosen as significance level.
Results
General characteristics and group average change in measured variables during exercise
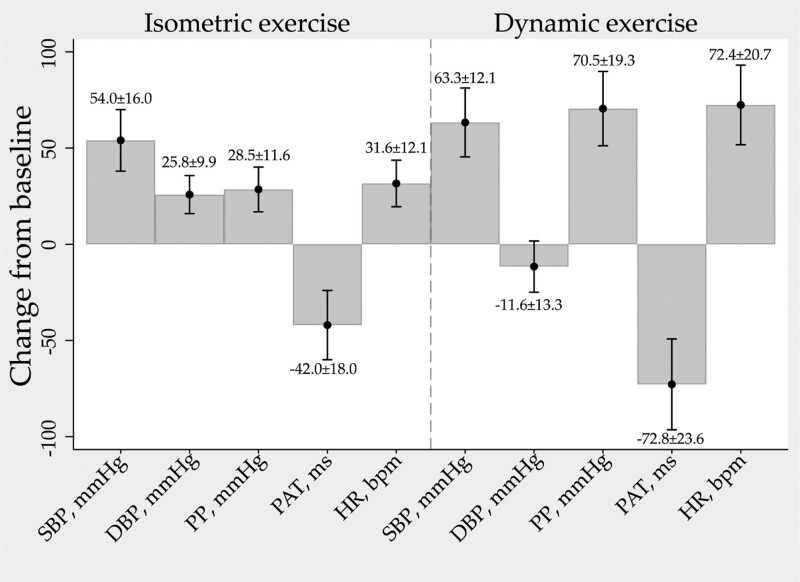
General characteristics of the test subjects are presented in Table 1. Group average change from baseline (defined as the average of the two last measurements during rest period 1) to maximum or minimum for all parameters during the two exercise modalities are presented in Fig. 2. SBP, DBP, HR and PP increased while PAT decreased during isometric exercise. During dynamic exercise SBP, HR and PP increased while DBP decreased slightly and PAT decreased.
Table 1.
General characteristics
| Characteristic | Quantity |
|---|---|
| Sex, male (%) | 35 (46.7) |
| Age, years (range) | 47.9 ± 15.5 (18–79) |
| BMI (kg/m2) | 25.6 ± 5.2 |
| Hypertension diagnosis | 32 (43.7) |
| Antihypertensive medication | 31 (41.3) |
| Baseline SBP (range) (mmHg) | 124.4 ± 15.5 (92.5–168) |
| Baseline DBP (range) (mmHg) | 75.9 ± 9.6 (55–104) |
| Baseline PP (mmHg) | 50.0 ± 11.8 |
| Baseline PAT (ms) | 180.8 ± 23.2 |
| SBP distribution at baseline (%) | |
| ≤100 mmHg | 3 (4.0) |
| ≥160 mmHg | 1 (1.3) |
| ≥140 mmHg | 17 (22.7) |
| DBP distribution at baseline (%) | |
| ≤60 mmHg | 4 (5.3) |
| ≥100 mmHg | 2 (2.7) |
| ≥85 mmHg | 12 (16.0) |
Values are presented as absolute numbers with percentages in parentheses or mean ± SD. Baseline values were defined by averaging the two last measurements during the first rest period.
PAT, pulse arrival time (ms); PP, pulse pressure (mmHg).
Fig. 2.
Group average change from baseline in measured physiological variables during the two exercise modalities. Values are presented as mean ± SD. HR, heart rate (bpm); PAT, pulse arrival time (ms); PP, pulse pressure (mmHg).
Correlation between pulse arrival time and blood pressure
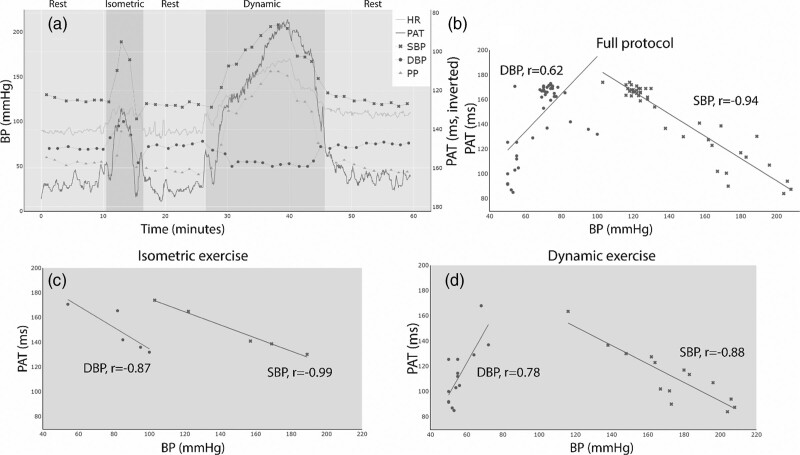
Based on one typical subject, Fig. 3 illustrates how the measured physiological variables varied throughout the experimental protocol (Fig. 3a) and visualizes the correlation analysis for the full protocol (Fig. 3b), isometric exercise (Fig. 3c) and during dynamic exercise (Fig. 3d). The correlation analyses and univariate linear regression were performed separately for the full protocol, the isometric exercise period and the dynamic exercise period. The results of the correlation analyses are presented in Fig. 4.
Fig. 3.
Measurements during the experimental protocol and correlation analysis for one typical subject. (a) All measured physiological variables throughout the test protocol. PAT is inverted on the Y-axis for illustrative purposes. Darker blue background indicates the isometric exercise period and green background indicates the dynamic exercise period. (b) Scatter plot and Pearson’s correlation coefficients of PAT and BP during the full protocol in the same subject as in (a). (c) Scatter plot and Pearson’s correlation coefficients of PAT and BP during the isometric exercise in the same subject as in (a). (d) Scatter plot and Pearson’s correlation coefficients of PAT and BP during dynamic exercise in the same subject as in (a). HR, heart rate (beats per minute); PAT, pulse arrival time (ms); PP, pulse pressure (mmHg).
Fig. 4.
Mean ± SD of individual Pearson’s correlation coefficients between PAT/SBP, PAT/DBP and PAT/PP. Analyses were performed for the full protocol and then separately for the isometric exercise period and dynamic exercise period. PAT, pulse arrival time (ms); PP, pulse pressure (mmHg).
Differences in the pulse arrival time/blood pressure relationship between exercise modalities
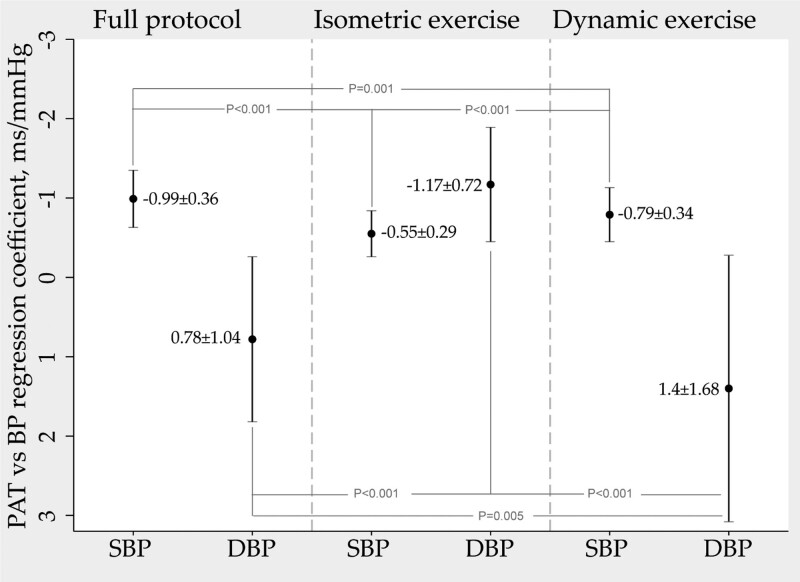
Simple linear regression was performed to determine the equation with the best fit between PAT and BP parameters for each subject for the full protocol and in the isometric and dynamic exercise periods. The results are presented in Fig. 5 as the mean of the individual regression coefficients to allow for a visual representation of the change in PAT per one-unit change in BP as well as comparisons of the regression coefficients between exercise modalities. The mean of individual regression coefficients between PAT and SBP were significantly different when comparing the isometric and dynamic exercise periods (−0.55 ± 0.29 ms/mmHg versus −0.79 ± 0.34 ms/mmHg, P < 0.001).
Fig. 5.
Mean ± SD of the individual regression coefficients between PAT as the dependent variable and SBP and DBP as the independent variable. The analysis was performed for the full protocol and then separately for the isometric exercise period and dynamic exercise period. The presented numerical data in the graph represents change in PAT per one-unit change in BP (ms/mmHg). PAT, pulse arrival time (ms).
Discussion
As a step to enable continuous, cuff-less SBP and DBP measurements, the present study investigated the effects on PAT from distinctly different BP changes during isometric and dynamic exercise. Included subjects represented the general population with broad ranges of age and baseline BPs. The study presents two main findings. First, the lack of a clear association between PAT and DBP was demonstrated by the inconsistent correlation between the parameters in the two exercise modalities. Second, the PAT/SBP slope differed significantly between exercise modalities. A secondary finding was the confirmation of previously known very strong individual correlation between PAT and SBP. To our knowledge, this is the first study to clearly demonstrate the uncertainty of using PAT alone as a surrogate DBP measurement in the same cohort.
Our results demonstrated a strong negative correlation between PAT and DBP in isometric exercise, a weak positive correlation in dynamic exercise and consequently a weak positive overall correlation. A clear demonstration of this discrepancy in a comparable cohort, is previously unreported. A weak association between PAT and DBP has previously been reported [7,14,15], but stand in contrast to the strong correlations reported by the majority of research [4]. In Wibmer et al. [14], a weak association between PAT and DBP was found in patients with an indication of cardiopulmonary exercise testing. Only dynamic exercise was investigated and similarly to us they observed small fluctuations in DBP during dynamic exercise and still a very strong correlation between PAT and SBP. In Marie et al. [15], isometric and dynamic exercise-induced BP changes were studied in the same protocol in five healthy young male subjects with an invasive BP reference. A strong correlation between PAT and DBP was observed during isometric exercise and a moderate correlation during dynamic exercise. Similar to our findings, PAT correlated strongly with SBP changes across all interventions. The results are not directly comparable because exercise intensities and BP changes were of much lower magnitude in Marie et al. [15], and isometric handgrip exercise was performed during the last minute of cycling. Thus, we hypothesize that simple test protocols fail to capture the complex relationship between PAT and BP. This finding is important for ongoing and future research on new methods for BP measurements based on PAT.
The linear relationship between PAT and SBP differed significantly between exercise modalities, suggesting that PAT is dependent on the characteristic of the BP change or other physiological changes. One previous study also indicated that the PAT/BP slope is altered across different BP changes in the same subject [26].The inclusion of the PEP, a known source of error in PAT measurements [7] shown to decrease more in dynamic exercise compared to isometric exercise [27], is one possible explanation. Furthermore, in our study PP demonstrated significantly stronger correlation with PAT compared to SBP for both the full protocol and dynamic exercise, indicating that the maximum exerted pressure on the arterial wall is more important compared to an increase in both SBP and DBP. However, this contrasts with previous hypotheses stating that PAT is more dependent on the mean arterial pressure [28]. The role of PP changes on PAT is scarcely researched, but showed superior correlation compared to SBP in one study from a large bio-signal database [8]. Lastly, there is evidence of a BP independent effect of HR on pulse wave velocity (PWV), where increasing HR increases PWV [29,30]. The effect of HR on PWV is difficult to investigate because HR and BP often change in the same direction and existing research also show conflicting results [29]. Future research needs to investigate the above-discussed physiological parameters and the implications on PAT accuracy.
Regarding SBP, our results are consistent with established evidence of a very strong negative correlation with PAT with most studies reporting correlation coefficients between −0.8 and −0.9 [4]. These findings indicate that PAT is a potential main feature in surrogate measurement of SBP in ambulatory monitoring. All associations between PAT and BP variables discussed in this article represent individual associations between PAT and BP. The current use of PAT as a BP surrogate requires calibration with a cuff measurement [4] to adjust for individual offsets [4,6].
A strong negative correlation between PAT and DBP shown during isometric exercise is similar to findings in previous studies [4,15,31], as well as studies applying a BP changing method where SBP and DBP change in the same directions, such as the cold pressor test [32], mental arithmetic stress test [32] or the Valsalva maneuver [33]. This suggests that DBP can be predicted from PAT during specific conditions, however, it is unlikely to be able to capture all DBP variations during ambulatory conditions.
The present study measured PAT and BP during active exercise to investigate the effects of BP on PAT during large BP fluctuations. Previous comparable studies have investigated dynamic exercise-induced BP changes, most commonly cycle ergometry or treadmill running. However, BP measurements were mainly registered post-exercise or when exercise was intermittently stopped [34–36]. As BP changes rapidly towards ‘normal’ level immediately after stopping the exercise [11,37], the actual BP during the active exercise may have been masked. This may in part explain why strong negative DBP correlations have been previously reported from dynamic exercise-induced BP changes.
In this study, PAT was measured from a vascular pathway different from the brachial artery reference cuff measurement site. PAT measured at chest level detects pulse waves that propagate from the aorta to the skin vasculature via a mixture of central elastic arteries and the muscular internal thoracic arteries, and it is not known if this PAT reflects central BP rather than brachial BP.
PPG as well as ECG signals are susceptible to corruption by artifacts from noise. After retrospect visual inspection of seven outliers with a correlation between SBP and PAT less than −0.70, it is likely that this is a result of motion artifacts and noise in the PPG and ECG waveforms. Still, we did not omit them from the analysis as algorithms that could identify all artifacts are currently not available. These findings emphasize the importance of signal processing and robust methods to detect corrupt waveforms.
Limitations
The BP measurement method is the major limitation in all studies with protocols involving exercise and is a matter of debate and conflicting evidence regarding accuracy and appropriate noninvasive method [38–40]. Invasive measurements are generally considered as the gold standard during exercise but were not an available alternative in this study due to ethical considerations. Particularly DBP is difficult to measure during exercise and is known to either increase slightly, decrease slightly or remain unchanged during dynamic exercise [11]. The magnitude and direction of DBP change during dynamic exercise differ depending on study population, exercise modality and body position as well as workload intensity [37,39,41]. In one study, auscultatory measurements during dynamic exercise compared to an invasive reference showed a –5 ± 7 mmHg difference [42]. On the contrary, the auscultatory method is considered acceptable during exercise [38]. Although we acknowledge that high precision noninvasive BP measurements during exercise are not possible, it is unlikely that the uncertainty from the BP measurement method would have affected the study conclusions; that PAT is not consistently and strongly correlated to DBP changes across various hemodynamic states. The correlation and regression analyses were performed for each individual subject. With only three measurements during isometric exercise, a standing measurement immediately prior to and after was included to increase statistical power.
Conclusion
The present study demonstrated the lack of a clear association between PAT and DBP, enabled by an experimental protocol that included two different BP-altering exercise interventions. In addition, the change in PAT per unit change in SBP differed significantly between exercise modality. Thus, we raise concern regarding PAT alone as a surrogate BP measurement across various hemodynamic settings and argue that simple test protocols may fail to capture the complex relationship between PAT as a single parameter and both SBP and DBP. Future research should focus on additional parameters to improve the robustness of cuff-less BP estimation and include various BP altering methods. Despite this, our study showed consistent very strong negative correlations on an individual basis between PAT and SBP, suggesting that PAT is a potential main feature in cuff-less BP measurements.
Acknowledgements
This work was supported by the HyperSension project (project number 282039), a research project in the BIA program financed by the Norwegian Research Council.
Conflicts of interest
N.K.M. is with Datek Next AS, a project partner involved in the development of the device prototype. For the remaining authors, there are no conflicts of interest.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 2.van der Steen MS, Lenders JWM, Thien T. Side effects of ambulatory blood pressure monitoring. Blood Pres Monit 2005; 10:151–155. [DOI] [PubMed] [Google Scholar]
- 3.Burnier M, Kjeldsen SE, Narkiewicz K, Oparil S. Cuff-less measurements of blood pressure: are we ready for a change? Blood Press 2021; 30:205–207. [DOI] [PubMed] [Google Scholar]
- 4.Welykholowa K, Hosanee M, Chan G, Cooper R, Kyriacou PA, Zheng D, et al. Multimodal photoplethysmography-based approaches for improved detection of hypertension. J Clin Med 2020; 9:E1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elgendi M, Fletcher R, Liang Y, Howard N, Lovell NH, Abbott D, et al. The use of photoplethysmography for assessing hypertension. NPJ Digit Med 2019; 2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukkamala R, Hahn JO, Inan OT, Mestha LK, Kim CS, Töreyin H, Kyal S. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng 2015; 62:1879–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne RA, Symeonides CN, Webb DJ, Maxwell SR. Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure. J Appl Physiol (1985) 2006; 100:136–141. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Yang S, Lee S, Kim HC. Analysis of pulse arrival time as an indicator of blood pressure in a large surgical biosignal database: recommendations for developing Ubiquitous blood pressure monitoring methods. J Clin Med 2019; 8:1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pielmus AG, Mühlstef J, Bresch E, Glos M, Jungen C, Mieke S, et al. Surrogate based continuous noninvasive blood pressure measurement. Biomed Tech (Berl) 2021; 66:231–245. [DOI] [PubMed] [Google Scholar]
- 10.Pandit JA, Lores E, Batlle D. Cuffless blood pressure monitoring: promises and challenges. Clin J Am Soc Nephrol 2020; 15:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palatini P. Blood pressure behaviour during physical activity. Sports Med 1988; 5:353–374. [DOI] [PubMed] [Google Scholar]
- 12.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness. Hypertension 2018; 72:796–805. [DOI] [PubMed] [Google Scholar]
- 13.Gesche H, Grosskurth D, Küchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol 2012; 112:309–315. [DOI] [PubMed] [Google Scholar]
- 14.Wibmer T, Doering K, Kropf-Sanchen C, Rüdiger S, Blanta I, Stoiber KM, et al. Pulse transit time and blood pressure during cardiopulmonary exercise tests. Physiol Res 2014; 63:287–296. [DOI] [PubMed] [Google Scholar]
- 15.Marie GV, Lo CR, Van Jones J, Johnston DW. The relationship between arterial blood pressure and pulse transit time during dynamic and static exercise. Psychophysiology 1984; 21:521–527. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128:873–934. [DOI] [PubMed] [Google Scholar]
- 17.Austad HOV, JonRøed MHD, Steffen B, Tomas L, Strisland FAE, Seeberg TM. An Unobtrusive Wearable Device for Ambulatory Monitoring of Pulse Transit Time to Estimate Central Blood Pressure. 2016. pp. Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies; 179–186. [Google Scholar]
- 18.Seeberg TM, Orr JG, Opsahl H, Austad HO, Roed MH, Dalgard SH, et al. A novel method for continuous, noninvasive, cuff-less measurement of blood pressure: evaluation in patients with nonalcoholic fatty liver disease. IEEE Trans Biomed Eng 2017; 64:1469–1478. [DOI] [PubMed] [Google Scholar]
- 19.Gjestvang C, Stensrud T, Haakstad LAH. How is rating of perceived capacity related to VO2max and what is VO2max at onset of training? BMJ Open Sport Exerc Med 2017; 3:e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinney W. Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference 2010; 445:56–61. [Google Scholar]
- 21.Inc. PT. Collaborative data science. 2015.Montreal. QC; [Google Scholar]
- 22.Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature 2020; 585:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.team Tpd. pandas-dev/pandas: Pandas. 2020.Zendo; [Google Scholar]
- 24.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0 Contributors. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 2020; 17:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell MJ, Swinscow TDV. Statistics at square one. 2009.11th ed. ed. Wiley-Blackwell/BMJ Books; [Google Scholar]
- 26.Schaanning SG, Skjaervold NK. Rapid declines in systolic blood pressure are associated with an increase in pulse transit time. PLoS One 2020; 15:e0240126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist VA, Spangler RD, Blount SG., Jr. A comparison between the effects of dynamic and isometric exercise as evaluated by the systolic time intervals in normal man. Am Heart J 1973; 85:227–236. [DOI] [PubMed] [Google Scholar]
- 28.Solà J, Proença M, Ferrario D, Porchet JA, Falhi A, Grossenbacher O, et al. Noninvasive and nonocclusive blood pressure estimation via a chest sensor. IEEE Trans Biomed Eng 2013; 60:3505–3513. [DOI] [PubMed] [Google Scholar]
- 29.Tan I, Spronck B, Kiat H, Barin E, Reesink KD, Delhaas T, et al. Heart rate dependency of large artery stiffness. Hypertension 2016; 68:236–242. [DOI] [PubMed] [Google Scholar]
- 30.Spronck B, Tan I, Reesink KD, Georgevsky D, Delhaas T, Avolio AP, Butlin M. Heart rate and blood pressure dependence of aortic distensibility in rats: comparison of measured and calculated pulse wave velocity. J Hypertens 2021; 39:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Sohn J, Park J, Yang S, Lee S, Kim HC. Novel blood pressure and pulse pressure estimation based on pulse transit time and stroke volume approximation. Biomed Eng Online 2018; 17:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block RC, Yavarimanesh M, Natarajan K, Carek A, Mousavi A, Chandrasekhar A, et al. Conventional pulse transit times as markers of blood pressure changes in humans. Sci Rep 2020; 10:16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding X, Yan BP, Zhang YT, Liu J, Zhao N, Tsang HK. Pulse transit time based continuous cuffless blood pressure estimation: a new extension and a comprehensive evaluation. Sci Rep 2017; 7:11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong MY, Pickwell-MacPherson E, Zhang YT. The acute effects of running on blood pressure estimation using pulse transit time in normotensive subjects. Eur J Appl Physiol 2009; 107:169–175. [DOI] [PubMed] [Google Scholar]
- 35.Liu SH, Cheng DC, Su CH. A cuffless blood pressure measurement based on the impedance plethysmography technique. Sensors (Basel) 2017; 17:E1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao J, Shi P, Hu S, Yu H. A revised point-to-point calibration approach with adaptive errors correction to weaken initial sensitivity of cuff-less blood pressure estimation. Sensors (Basel) 2020; 20:E2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund-Johansen P. Blood pressure response during exercise as a prognostic factor. J Hypertens 2002; 20:1473–1475. [DOI] [PubMed] [Google Scholar]
- 38.Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens 2015; 29:351–358. [DOI] [PubMed] [Google Scholar]
- 39.Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159. [DOI] [PubMed] [Google Scholar]
- 40.Myers J, Arena R, Franklin B, Pina I, Kraus WE, McInnis K, Balady GJ; American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention of the Council on Clinical Cardiology, the Council on Nutrition, Physical Activity, and Metabolism, and the Council on Cardiovascular Nursing. Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation 2009; 119:3144–3161. [DOI] [PubMed] [Google Scholar]
- 41.Sheps DS, Ernst JC, Briese FW, Myerburg RJ. Exercise-induced increase in diastolic pressure: indicator of severe coronary artery disease. Am J Cardiol 1979; 43:708–712. [DOI] [PubMed] [Google Scholar]
- 42.White WB, Lund-Johansen P, Omvik P. Assessment of four ambulatory blood pressure monitors and measurements by clinicians versus intraarterial blood pressure at rest and during exercise. Am J Cardiol 1990; 65:60–66. [DOI] [PubMed] [Google Scholar]