Abstract
Background
Concerns over the neurotoxic potential of retained gadolinium in brain tissues after intravenous gadolinium-based contrast agent (GBCA) administration have led to pronounced worldwide use changes, yet the clinical sequelae of gadolinium retention remain undefined.
Purpose
To assess clinical and neurologic effects and potential neurotoxicity of gadolinium retention in rats after administration of various GBCAs.
Materials and Methods
From March 2017 through July 2018, 183 male Wistar rats received 20 intravenous injections of 2.5 mmol per kilogram of body weight (80 human equivalent doses) of various GBCAs (gadodiamide, gadobenate, gadopentetate, gadoxetate, gadobutrol, gadoterate, and gadoteridol) or saline over 4 weeks. Rats were evaluated 6 and 34 weeks after injection with five behavioral tests, and inductively coupled plasma mass spectrometry, transmission electron microscopy, and histopathology were performed on urine, serum, cerebrospinal fluid (CSF), basal ganglia, dentate nucleus, and kidney samples. Dunnett post hoc test and Wilcoxon rank sum test were used to compare differences between treatment groups.
Results
No evidence of differences in any behavioral test was observed between GBCA-exposed rats and control animals at either 6 or 34 weeks (P = .08 to P = .99). Gadolinium concentrations in both neuroanatomic locations were higher in linear GBCA-exposed rats than macrocyclic GBCA-exposed rats at 6 and 34 weeks (P < .001). Gadolinium clearance over time varied among GBCAs, with gadobutrol having the largest clearance (median: 62% for basal ganglia, 70% for dentate) and gadodiamide having no substantial clearance. At 34 weeks, gadolinium was largely cleared from the CSF and serum of gadodiamide-, gadobenate-, gadoterate-, and gadobutrol-exposed rats, especially for the macrocyclic agents (range: 70%–98% removal for CSF, 34%–94% removal for serum), and was nearly completely removed from urine (range: 96%–99% removal). Transmission electron microscopy was used to detect gadolinium foci in linear GBCA-exposed brain tissue, but no histopathologic differences were observed for any GBCA.
Conclusion
In this rat model, no clinical evidence of neurotoxicity was observed after exposure to linear and macrocyclic gadolinium-based contrast agents at supradiagnostic doses.
© RSNA, 2022
Summary
There was no evidence of differences in clinical or histopathologic neurotoxicity parameters between rats exposed to supradiagnostic human dose equivalents of commercially available gadolinium-based contrast agents and saline-exposed rats.
Key Results
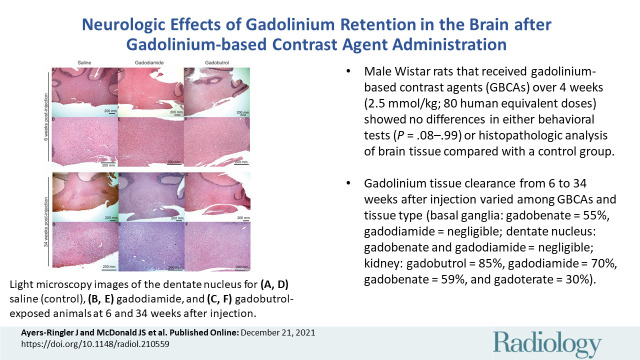
■ Male Wistar rats that received intravenous gadolinium-based contrast agents (GBCAs) over 4 weeks (2.5 mmol/kg; 80 human equivalent doses) showed no evidence of differences in either behavioral tests (P = .08 to P = .99) or histopathologic analysis of brain tissue compared with a control group.
■ Gadolinium tissue clearance from 6 to 34 weeks after injection varied among GBCAs and tissue type (basal ganglia: gadobenate = 55%, gadodiamide = negligible; dentate nucleus: gadobenate and gadodiamide = negligible; kidney: gadobutrol = 85%, gadodiamide = 70%, gadobenate = 59%, and gadoterate = 30%).
Introduction
MRI plays an invaluable clinical role in diagnostic clinical medicine. MRI examinations provide superior delineation of soft tissues without exposure to ionizing radiation inherent to other diagnostic imaging modalities. Gadolinium-based contrast agents (GBCAs) further expand the utility of MRI in the detection of a wide variety of disease processes that would otherwise be undetectable with unenhanced MRI or other imaging modalities.
Recent studies have confirmed the retention of gadolinium in tissues after GBCA exposure in patients and preclinical models (1–8). Higher concentrations of gadolinium and slower washout of gadolinium over time have been observed among linear contrast agents compared with macrocyclic contrast agents but with intraclass differences (1,2,5,8). The acute toxicities of free gadolinium are well known and are often due to the ability of the element to disrupt calcium-mediated cellular processes (9–14). Fortunately, acute toxicity from GBCA exposure is exceedingly rare and can be avoided through adherence to standard clinical dosing and administration routes. However, the chronic toxicity of gadolinium and GBCA exposure remain undefined. Long-term retention of GBCAs within tissues provides an opportunity for dechelation, with the potential to form more biologically active forms of gadolinium. Studies of the potential clinical effects of gadolinium retention have focused on neurologic and cognitive effects (15,16), as (a) gadolinium is known to cross or circumvent the blood brain barrier and deposit in brain tissue, particularly in the dentate nucleus and basal ganglia (5,6,17), and (b) free gadolinium is a documented neurotoxin (18).
The purpose of the current study was to assess the clinical and neurologic effects and potential neurotoxicity of gadolinium retention in rats after administration of various GBCAs. Effects were assessed by behavioral studies, histologic characteristics, transmission electron microscopy, and inductively coupled plasma mass spectrometry.
Materials and Methods
Research reported in our study was financially supported by an investigator-initiated research grant from GE Healthcare. None of the authors are or have been employed by GE Healthcare. The authors maintained control of the study data and the submitted manuscript at all times.
The design and execution of this single-center study were subject to institutional animal care and use committee oversight.
Study Design and Animals
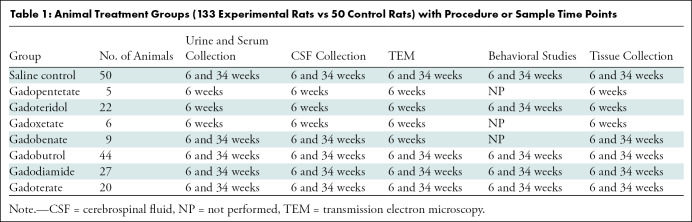
Healthy male Wistar rats (Charles River Laboratories) (mean weight, 200 g; age range, 5–7 weeks at first injection) received intravenous injections of GBCA (n = 133) or saline (n = 50) from March 2017 through July 2018. Animals that died over the course of the study were excluded from final analyses. Behavioral studies were performed, and urine, serum, and cerebrospinal fluid (CSF) were collected at 6 and 34 weeks after the final injection (Table 1) to simulate 3 and 20 years after chronic GBCA exposure in humans, respectively (19). Animals were then humanely euthanized and perfused with ice-cold saline followed by fixative, and the brain and kidneys were harvested.
Table 1:
Animal Treatment Groups (133 Experimental Rats vs 50 Control Rats) with Procedure or Sample Time Points
GBCA Administration
Animals in each treatment group were given tail vein injections of their respective GBCA once a day for 5 days for 4 consecutive weeks (20 injections total) or twice per day for 5 days for 4 consecutive weeks (40 injections total) for gadoxetate, equivalent to a total dose of 50 mmol/kg (80 human equivalent doses), or saline (Baxter) (control group) (C.R.F., 1 year of experience; S.H., 3 years of experience; D.R.J., 5 years of experience; B.S., 1 year of experience; and G.T., 1 year of experience). Seven GBCAs were administered: gadodiamide (Omniscan, GE Healthcare), gadobenate dimeglumine (MultiHance, Bracco), gadopentetate dimeglumine (Magnevist, Bayer), gadoxetate disodium (Eovist, Bayer), gadoterate meglumine (Clariscan, GE Healthcare), gadobutrol (Gadavist, Bayer), and gadoteridol (ProHance, Bracco). Percutaneous tail vein injections were performed using vaporized isoflurane (1%–3%, inhalation).
Behavioral Studies
Rats underwent behavioral testing at 6 and 34 weeks after final injection at our institutional rodent behavioral facility. Behavioral testing was designed to assess neurologic and cognitive function, particularly of the dentate nucleus and basal ganglia. Tests included open field (assessing locomotor function and anxiety), novel object recognition (memory, cognitive function), Y maze (spatial working memory, short-term memory, locomotor), social interaction (social anxiety), and horizontal ladder rung walking (motor coordination, balance, planning). All testing was performed by experienced personnel (J.A., 6 years of animal experience; M.A.C., 1 year of animal experience; C.R.F., 1 year of animal experience; D.R.J., 5 years of animal experience; B.S., 1 year of animal experience; and K.M.W., 5 years of animal experience), who were blinded to contrast agent exposure. Detailed study protocols are provided in Figures E1–E6 (online) and Table E1 (online).
Biologic Fluid Collection
Urine and blood samples were collected from rats at baseline (pre-GBCA injection), 6 weeks, and 34 weeks after final injection. Rats undergoing urine sample collection were housed individually in metabolic cages. Uncontaminated urine was collected over a 14-hour period. Blood was drawn via jugular venipuncture from sedated rats, allowed to clot, centrifuged for serum isolation, and stored at –80°C until analysis.
CSF was collected at 6 or 34 weeks after injection immediately before sacrifice for tissue collection (D.D., 22 years of experience). After anesthetization, a needle was inserted through the dura mater and into the cisterna magna, and CSF was collected, as previously described (20). The sample was stored at –80°C until testing.
Tissue Processing and Histopathologic Analysis
Tissue processing was performed as described in Appendix E1 (online). All slides were independently reviewed by a veterinary pathologist (D.D., 22 years of experience) and a board-certified pathologist blinded to contrast agent exposure.
Mass Spectrometry
Gadolinium levels were quantified by blinded technologists using a fully validated inductively coupled plasma mass spectrometry assay, as previously described (5,6). The limits of detection of this assay for biologic fluid samples and tissue samples are 0.1 ng/mL and 0.1 μg/g, respectively.
Transmission Electron Microscopy
Transmission electron microscopy with energy dispersive x-ray spectroscopy (Tecnai 12 transmission electron microscopy from FEI [Thermo Fisher] equipped with an Oxford X-mat EDX detector) was performed by blinded technologists to characterize the distribution of gadolinium deposits in tissues, as previously described (5,6).
Statistical Analyses
All statistical analyses were performed using JMP, version 14 (SAS Institute) (21). Continuous variables were presented as medians with interquartile ranges due to nonnormal data distributions, unless otherwise noted. Dunnett post hoc test (behavioral test results) and Wilcoxon rank sum test with post hoc analysis (tissue and biologic fluid gadolinium results) were used to compare differences between treatment groups. Details of these analyses are described in Appendix E1 (online). Significance was assigned when P ≤ .05.
Results
Animal Population
A total of 183 healthy rats were included in this study (Table 1). Six rats per group were used for biologic fluids and tissue samples (6 weeks after injection for all contrast agents and saline; 34 weeks after injection for gadodiamide, gadoterate meglumine, gadobutrol, gadobenate dimeglumine, and saline). Six rats per group underwent urine and serum analysis at both time points (6 and 34 weeks after injection of gadodiamide, gadoterate meglumine, gadobutrol, gadobenate dimeglumine, and saline). Sixteen GBCA-exposed rats per group underwent behavior testing (gadodiamide, gadoterate meglumine, gadobutrol, and gadoteridol). Forty saline-exposed rats were used to provide a control for each behavior group.
Gadolinium Quantification in Tissues
All GBCA-exposed groups had higher median gadolinium concentrations in the dentate nucleus than did the control group at 6 weeks (P = .005) and 34 weeks (P = .007 to P = .004) (Table 2; Table E2, Fig E8 [online]), with higher concentrations observed when comparing linear and macrocyclic GBCAs (P < .001). Both GBCA class and ionicity were associated with gadolinium concentrations (Table E3 [online]). Similar results were observed within the basal ganglia (6 weeks: P = .005 to P = .02; 34 weeks: P = .008 to P = .03; linear vs macrocyclic contrast agent, P < .001) and in renal tissues (6 weeks: P = .005; 34 weeks P = .008 to P = .01; linear vs macrocyclic contrast agent, P < .001).
Table 2:
Gadolinium Levels in Tissue at 6 and 34 Weeks after Injection
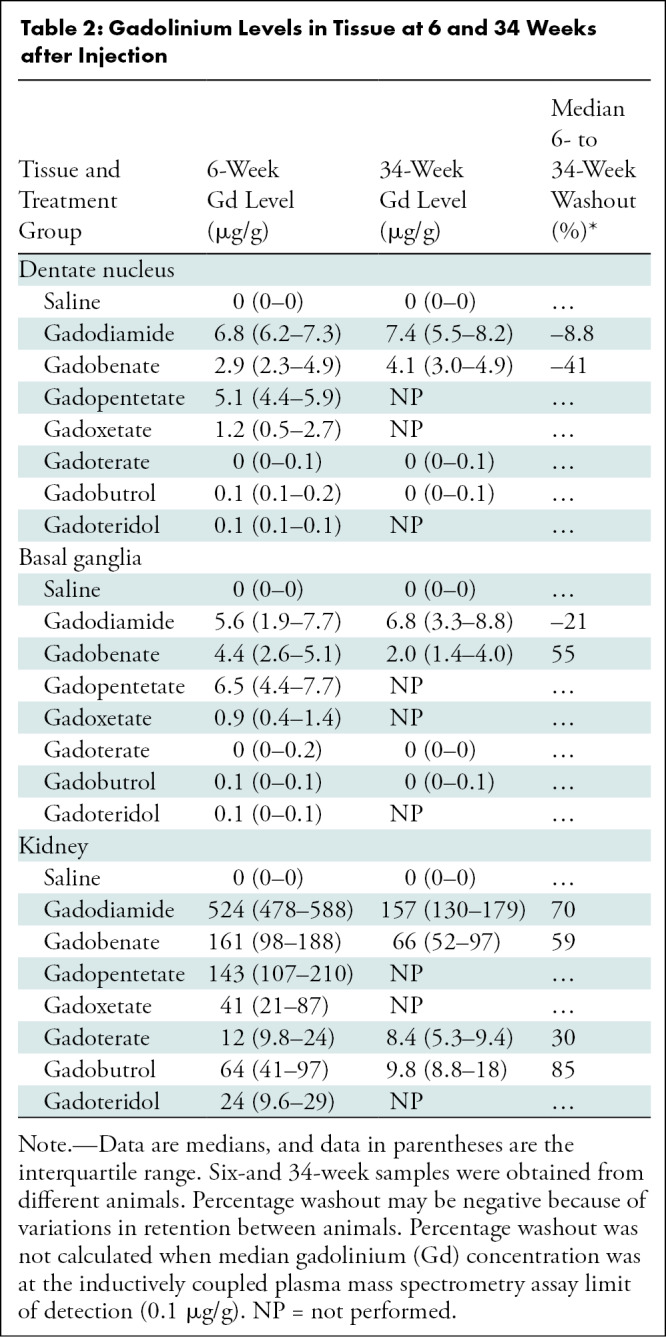
Differences in median gadolinium tissue washout between 6 and 34 weeks after injection were observed between some GBCAs (Table 2). Gadobenate showed a 55% median decrease in gadolinium in the basal ganglia at 34 weeks and no decrease in the dentate nucleus, whereas gadodiamide showed no decrease in either region. In renal tissue, gadolinium washout between 6 and 34 weeks was 85% for gadobutrol, 70% for gadodiamide, 59% for gadobenate, and 30% for gadoterate.
Gadolinium Quantification in Biologic fluids
In urine samples, all GBCA-exposed groups had higher median urine gadolinium concentrations compared with the control group at 6 weeks after exposure (P < .001) and persisted at 34 weeks (P = .03 to P = .003) (Table 3, Fig E9 [online]). However, gadolinium was almost completely eliminated (range, 95%–99%) from urine by 34 weeks for the four GBCAs examined (gadodiamide, gadobenate, gadoterate, gadobutrol).
Table 3:
Gadolinium Levels in Biologic Fluid at 6 and 34 Weeks after Injection
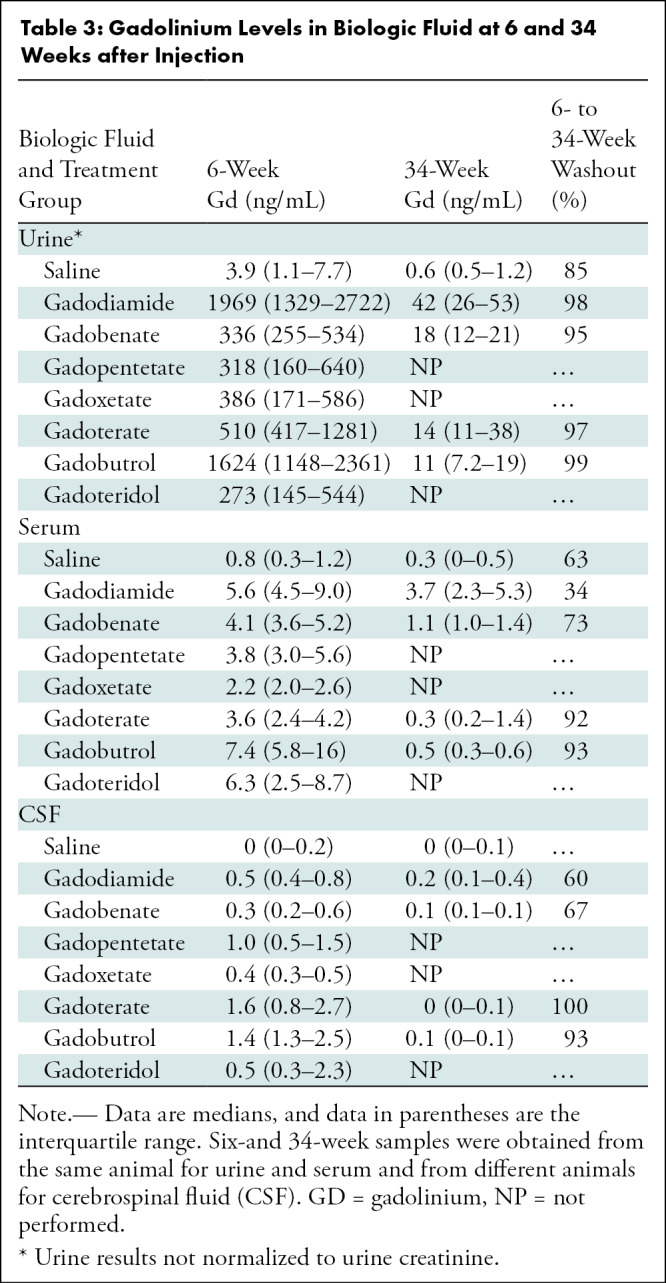
In serum samples, at 6 weeks, all GBCA-exposed groups had a higher median gadolinium concentration than the saline concentration in the control group (P < .001 to P = .01). At 34 weeks, gadodiamide (P = .003) and gadobenate (P = .01) had a higher median gadolinium concentration than saline but not gadobutrol (P = .24) or gadoterate (P = .47). Gadolinium was largely cleared from serum by 34 weeks after macrocyclic exposure (92%–93% removal), with less removal after linear GBCA exposure (34%–73% removal).
In CSF samples at 6 weeks, all GBCA-exposed groups had higher median concentrations compared with the control group (P = .002 to P = .006). At 34 weeks, only gadodiamide was higher than in the control group (P = .02) but not gadobutrol (P = .41), gadobenate (P = .14), or gadoterate (P = .65). gadolinium was largely cleared from CSF by 34 weeks after macrocyclic exposure (93%–100% removal), with less clearance after linear exposure (60%–67% removal).
Gadolinium-based contrast agent ionicity, but not class, was typically associated with gadolinium concentrations in biologic fluids (Table E3 [online]). Some macrocyclic GBCAs demonstrated higher urine, serum, and CSF gadolinium concentrations at 6 weeks than some linear GBCAs (ie, gadobutrol- and gadoterate-exposed rats had higher urine gadolinium concentration than did gadobenate-exposed rats (median, 1624 and 510 ng/mL, respectively vs 336 ng/mL). However, at 34 weeks, linear GBCAs demonstrated consistently higher biologic fluid gadolinium concentrations than macrocyclic GBCAs.
Behavioral Tests
Comprehensively, no GBCA-exposed group performed better or worse than the saline control group in any of the five behavioral tests at either 6 or 34 weeks (P = .08 to P = .99; Tables E4–E8, Figs E9–E13 [online]). Details of the results of these tests are in Appendix E1 (online). We found no evidence of differences between the GBCA-exposed groups and the control group for the open field test (overall locomotion or percentage distance traveled in the center zone), Y maze test (number of arm entries or percentage alternation), novel object recognition test (percentage time exploring the novel objects), social interaction test (percentage time with stranger rat), or horizontal ladder rung walking test (average foot fault score on test ladder).
Transmission Electron Microscopy
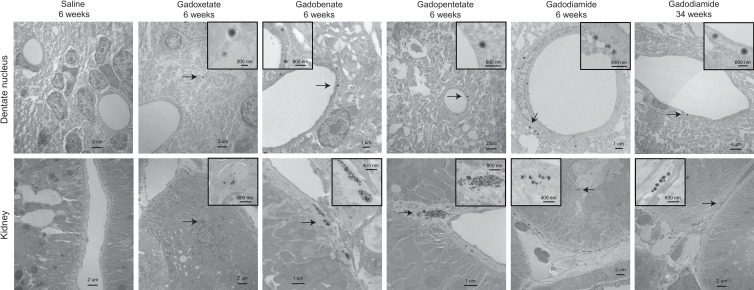
At 6 weeks, all samples exposed to gadodiamide, gadobenate, and gadopentetate and six of nine samples exposed to gadoxetate (two dentate nucleus, one basal ganglia, three kidney) demonstrated foci of gadolinium (Table 4, representative spectra, Fig E14 [online]). More foci were observed in samples exposed to gadodiamide and gadobenate compared with samples exposed to gadopentetate and gadoxetate. None of the samples exposed to macrocyclic GBCAs demonstrated gadolinium foci at 6 weeks. At 34 weeks, seven of nine samples exposed to gadodiamide (all dentate nucleus and basal ganglia, one kidney) and one of nine samples exposed to gadobutrol (basal ganglia) were positive for gadolinium.
Table 4:
Number and Type of Tissues with Gadolinium Foci Confirmed with Transmission Electron Microscopy with Energy Dispersive X-ray Spectroscopy
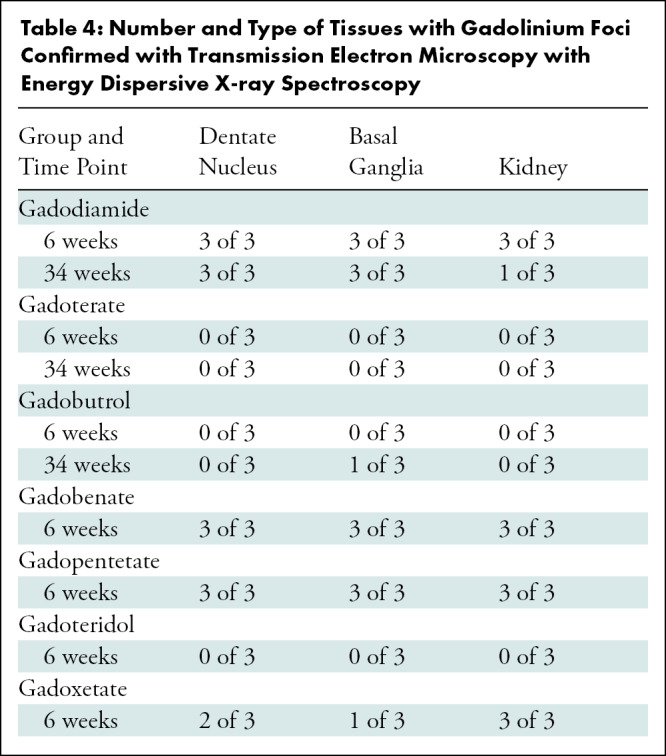
In brain tissues, gadolinium was predominantly endothelial in distribution in the dentate nucleus and basal ganglia, but a smaller amount was also observed to be within the neuropil (Fig 1). In renal tissues, foci were predominantly localized to the endothelial and subendothelial regions (Fig 1). Foci were rarely observed in the glomerulus.
Figure 1:
Tissue localization of gadolinium deposits. Cellular localization of gadolinium deposits (arrows) using transmission electron microscopy are shown for dentate nuclei (top row) and kidney (bottom row) tissues of control and gadolinium-based contrast agent–exposed rats harvested at indicated postinjection time points.
Histologic Characteristics
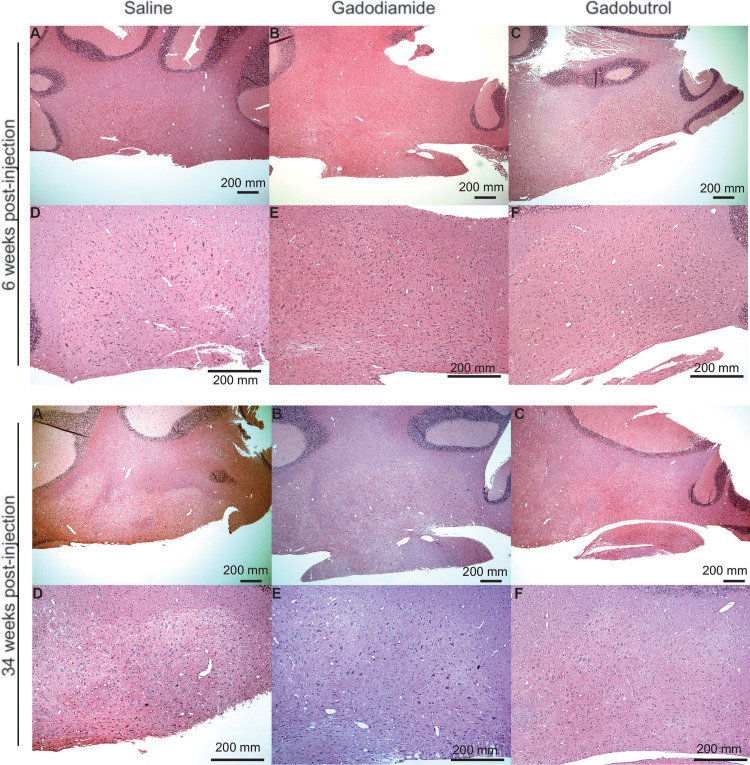
We found no evidence of histopathologic abnormality in the cerebellar roof nuclei (including the dentate nucleus) or basal ganglia at either 6 or 34 weeks in any GBCA-exposed sample (Fig 2).
Figure 2:
Histopathologic analysis. Representative light microscopy images of the dentate nucleus are shown for (A, D) saline (control), (B, E) gadodiamide, and (C, F) gadobutrol-exposed animals at 6 and 34 weeks after injection. (Originial magnification, ×40 in A-C and ×100 in D-F; hematoxylin-eosin stain.)
Discussion
Despite concerns about gadolinium retention in brain tissue after exposure to gadolinium-based contrast agents (GBCAs), the potential neurologic effects of retained gadolinium have not been fully assessed. Our study found no evidence of clinical or histopathologic neurotoxicity due to chronic gadolinium retention within a well-established rat model exposed to supradiagnostic human dose equivalents of various commercially available GBCAs when compared with control rats given saline. General mobility, spatial memory, short-term memory, social interactions, and balance and coordination all appeared to be unaffected by the GBCAs in our study (P = .08 to P = .99). Linear GBCA exposure was associated with higher gadolinium concentrations in the dentate nucleus and basal ganglia compared with rats exposed to macrocyclic GBCAs (dentate nucleus median gadolinium concentrations were 1.2–6.8 μg/g gadolinium for linear GBCAs and 0–0.1 μg/g gadolinium for macrocyclic GBCAs at 6 weeks, 4.1–7.4 μg/g gadolinium for linear GBCAs and 0 μg/g gadolinium for macrocyclic GBCAs at 34 weeks; basal ganglia median gadolinium concentrations were 0.9–6.5 μg/g gadolinium for linear GBCAs and 0–0.1 μg/g gadolinium for macrocyclic GBCAs at 6 weeks, 2.0–6.8 μg/G gadolinium for linear GBCAs and 0 μg/g gadolinium for macrocyclic GBCAs at 34 weeks; P < .001 for all comparisons). Gadolinium clearance from tissues varied among GBCAs and between brain and renal tissues (median percentage decrease in gadolinium from 6 weeks to 34 weeks after injection—basal ganglia: gadobenate = 55%, gadodiamide = negligible; dentate nucleus: gadobenate and gadodiamide = negligible; kidney: gadobutrol = 85%, gadodiamide = 70%, gadobenate = 59%, and gadoterate = 30%).
Our findings correlate with previous preclinical and clinical studies (2,5,6,8,17,22–24). However, our study was able to better compare the washout kinetics of various linear and macrocyclic GBCAs in various tissues and biologic fluids and demonstrate that macrocyclic GBCAs appear to have more complete washout versus linear agents. Intraclass differences in these washout property biodistributions were also observed between GBCAs, as higher gadolinium biologic fluid concentrations at 6 weeks after injection were observed among some macrocyclic agents compared with linear agents (ie, gadobutrol- and gadoteridol-exposed rats had higher serum gadolinium concentrations than gadodiamide and gadobenate-exposed rats [median 7.4 ng/mL and 6.3 ng/mL vs 5.6 ng/mL and 4.1 ng/mL]). Such findings underscore the complex biodistribution of retained GBCAs that demand a more holistic understanding of retained Gd in animals and humans before GBCA class alone is used as a differentiator of potential GBCA safety as it relates to retained forms of gadolinium.
To date, gadolinium retention within brain tissue has yet to be correlated with histopathologic findings of injury or toxicity. Behavior tests can detect perturbations due to toxicologic sequalae before or in the absence of any histologic changes. Thus, we used a battery of behavioral tests to evaluate the clinical neurotoxic potential of chronic exposure to supradiagnostic concentrations of GBCAs. The behavior tests we performed were chosen to target the dentate nucleus and basal ganglia—the brain regions showing the highest levels of MRI enhancement (6,17). The dentate nucleus, although important in skeletal motor functions, is also involved in spatial learning, exploration, and cognition (25–29). The basal ganglia are involved in many different functions, including motivation, memory, volitional fine motor control, impulse control, and anxiety-related disorders (30–38). The behavior tests we performed revealed no significant differences between treatment groups and the control group at 6 or 34 weeks after the final GBCA injection, consistent with findings from other studies (39,40).
The lack of histopathologic injury in rat brain tissues exposed to GBCAs, where considerable gadolinium deposits are detected via transmission electron microscopy energy dispersive x-ray spectroscopy, reinforces preliminary findings from our group and others (4–6,41–44). We previously observed gadolinium foci in the brain tissue of gadobutrol-exposed rats 1week after injection (5), suggesting that gadolinium foci are present early after GBCA exposure for all agents, but the more favorable washout of macrocyclic GBCAs eliminates these deposits compared with linear GBCAs. Interestingly, gadoxetate-exposed rats had basal ganglia gadolinium levels comparable with macrocyclic GBCA-exposed rats and dentate nucleus gadolinium levels intermediate to levels observed in rats exposed to other linear and macrocyclic GBCAs, a finding also observed in the recent study from Jost et al (45). This may be explained by the difference in dosing (50 mmol/kg total dose of gadoxetate = 333 human equivalent doses, whereas 50 mmol/kg total dose of other GBCAs = 80 human equivalent doses) or by inherent in vivo differences in biodistribution and bioavailability between gadoxetate and other GBCAs.
Our findings of greater washout of gadolinium between 6 and 34 weeks after injection with macrocyclic GBCAs compared with linear GBCAs concur with findings of other studies (2,8,44). Our findings showed kidney and brain tissue had similar gadolinium washout for rats exposed to gadoterate, gadobutrol, and gadobenate, but rats exposed to gadodiamide had a much higher amount of washout in the kidney (median 70%) compared with the brain (zero washout). Biologic fluid (serum, urine, CSF) washout kinetics did not appear to correlate with the type of GBCA administered and mirrored findings from other preclinical studies (23,24,46–49). Although GBCAs have very similar early elimination kinetics in biofluids, there is a growing body of evidence that a smaller amount of gadolinium is sequestered into one or more tissue compartments during this rapid elimination, where it is slowly released again over time, reequilibrating back into these same biologic fluids and tissues. The propensity for GBCAs to sequester into these tissues and the rates of reequilibration appear to differ considerably between agents and classes and appear to be associated with the kinetic lability of each chelate. Furthermore, as our data show that linear GBCAs circulate in the blood and CSF for a longer time than macrocyclic GBCAs, it remains possible that this longer “dwell time” in these biologic fluids may be a result of greater amounts of sequestered GBCA or may even reflect slower clearance of different chemical forms of gadolinium circulating in the blood or CSF after initial dechelation. This latter theory requires further evaluation with speciation analysis.
Our study had several limitations. First, comparative anatomy indicates that rodent brains are more primitive in structure and complexity when compared with the brains of larger mammals. This potentially makes them less susceptible to the neurotoxic effects of gadolinium exposure or less likely to manifest subtle symptoms when compared with humans and other mammals with more highly evolved neuroanatomy. Second, the life span of a rodent is much shorter than the life span of humans, so the chronic effects of toxicity may not have manifested during the life span of this model, despite the simulated 30-plus years of GBCA exposure. Third, as the clinical manifestations of gadolinium-mediated neural cell toxicity remain largely unknown, we assumed that injury is most likely to occur where gadolinium retention is highest, and we tailored our behavioral testing accordingly. Certain regions of the brain that do not substantially accumulate gadolinium may be uniquely susceptible to injury that was not assessed in our testing paradigms. Fourth, we did not include positive controls for our behavioral tests. We attempted to perform focused dentate lesioning on a subgroup of rats to serve as a positive control; however, these attempts were unsuccessful because of a high mortality rate. Fifth, urine gadolinium results were not normalized to urine creatinine because of budgetary constraints. Sixth, the rats were subjected to high doses of GBCAs with multiple administrations over a short time. The effects of these high doses may be attenuated or absent in patients exposed to these agents at clinically relevant doses and schedules. Seventh, because of study budgetary constraints, not all commercially available GBCAs were assessed at all time points. Eighth, only a limited number of organs and regions of the brain were assessed with inductively coupled plasma mass spectrometry, transmission electron microscopy, and histology in this study, and no speciation analyses were performed.
In conclusion, no clinical or histopathologic evidence of neurotoxicity was observed after exposure of rats to various linear and macrocyclic gadolinium-based contrast agent (GBCAs) when compared with saline-exposed rat controls. Additional studies should be performed to address these limitations and further assess the safety of GBCAs.
Acknowledgments
Acknowledgments
The authors would like to thank Trace Christensen for his technical expertise with transmission electron microscopy and Yong Guo, MD, PhD, for his review of brain histologic slides.
J.A. and J.S.M. contributed equally to this work.
Supported by an investigator-initiated research grant from GE Healthcare. R.J.M. supported in part by an RSNA Research Scholar Grant investigator-initiated research grant.
Disclosures of conflicts of interest: J.A. disclosed no relevant relationships. J.S.M. institution received funding from GE Healthcare; consulting fees from GE Healthcare; member of the American College of Radiology Committee on Drugs and Contrast Media. M.A.C. disclosed no relevant relationships. C.R.F. disclosed no relevant relationships. S.H. disclosed no relevant relationships. D.R.J. disclosed no relevant relationships. B.S. disclosed no relevant relationships. G.T. disclosed no relevant relationships. K.M.W. disclosed no relevant relationships. D.D. disclosed no relevant relationships. D.S.C. scientific advisory board member to Peptron. J.L.S. disclosed no relevant relationships. P.J.J. American Association for Clinical Chemistry Secretary. J.A.B. disclosed no relevant relationships. R.K. institution received grants from the National Institutes of Health (NS076491, NS107111, NS110114). D.F.K. grants from Medtronic, Monarch Medical, NeuroGami Medical, Insera Therapeutics, Balt, Cerenovus, MicroVention, and MiVI Neurovascular; royalties from Marblehead Medical; patents submitted and issued by Marblehead Medical and Kypheze Medical; advisory boards of Minnetronix, Vesalio, and NoNO; stock in Marblehead Medical, Superior Medical Experts, Conway Medical, and Nested Knowledge; senior consultant to the editor of Radiology. R.J.M. unrestricted investigator-initiated research grant from GE Healthcare for development of novel MR contrast agent; investigator-initiated research funding from Bracco Diagnostics for Food and Drug Administration–mandated research on gadolinium retention in humans; member of American College of Radiology Committee on Contrast Safety and Drug Use and Practice Standards.
Abbreviations:
- CSF
- cerebrospinal fluid
- GBCA
- gadolinium-based contrast agent
References
- 1. Frenzel T , Apte C , Jost G , Schöckel L , Lohrke J , Pietsch H . Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents: Comparative Study in Rats . Invest Radiol 2017. ; 52 ( 7 ): 396 – 404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jost G , Frenzel T , Boyken J , Lohrke J , Nischwitz V , Pietsch H . Long-term Excretion of Gadolinium-based Contrast Agents: Linear versus Macrocyclic Agents in an Experimental Rat Model . Radiology 2019. ; 290 ( 2 ): 340 – 348 . [DOI] [PubMed] [Google Scholar]
- 3. Kanda T , Fukusato T , Matsuda M , et al . Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy . Radiology 2015. ; 276 ( 1 ): 228 – 232 . [DOI] [PubMed] [Google Scholar]
- 4. Lohrke J , Frisk AL , Frenzel T , et al . Histology and Gadolinium Distribution in the Rodent Brain After the Administration of Cumulative High Doses of Linear and Macrocyclic Gadolinium-Based Contrast Agents . Invest Radiol 2017. ; 52 ( 6 ): 324 – 333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald RJ , McDonald JS , Dai D , et al . Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear versus Macrocyclic Gadolinium Chelates . Radiology 2017. ; 285 ( 2 ): 536 – 545 . [DOI] [PubMed] [Google Scholar]
- 6. McDonald RJ , McDonald JS , Kallmes DF , et al . Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging . Radiology 2015. ; 275 ( 3 ): 772 – 782 . [DOI] [PubMed] [Google Scholar]
- 7. Murata N , Gonzalez-Cuyar LF , Murata K , et al . Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function . Invest Radiol 2016. ; 51 ( 7 ): 447 – 453 . [DOI] [PubMed] [Google Scholar]
- 8. Robert P , Fingerhut S , Factor C , et al . One-year Retention of Gadolinium in the Brain: Comparison of Gadodiamide and Gadoterate Meglumine in a Rodent Model . Radiology 2018. ; 288 ( 2 ): 424 – 433 . [DOI] [PubMed] [Google Scholar]
- 9. Hirano S , Suzuki KT . Exposure, metabolism, and toxicity of rare earths and related compounds . Environ Health Perspect 1996. ; 104 ( Suppl 1 ): 85 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nunn AD , Wedeking P , Marinelli E , et al . Toxicity of gadolinium chelates in rodents . Acad Radiol 1996. ; 3 ( Suppl 2 ): S333 – S335 . [DOI] [PubMed] [Google Scholar]
- 11. Tweedle MF , Wedeking P , Kumar K . Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats . Invest Radiol 1995. ; 30 ( 6 ): 372 – 380 . [DOI] [PubMed] [Google Scholar]
- 12.Bertin A, Michou-Gallani AI, Gallani JL, Felder-Flesch D. In vitro neurotoxicity of magnetic resonance imaging (MRI) contrast agents: influence of the molecular structure and paramagnetic ion. Toxicol In Vitro 2010;24(5):1386–1394. [DOI] [PubMed] [Google Scholar]
- 13. Feng X , Xia Q , Yuan L , Yang X , Wang K . Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity . Neurotoxicology 2010. ; 31 ( 4 ): 391 – 398 . [DOI] [PubMed] [Google Scholar]
- 14. Mallio CA , Rovira À , Parizel PM , Quattrocchi CC . Exposure to gadolinium and neurotoxicity: current status of preclinical and clinical studies . Neuroradiology 2020. ; 62 ( 8 ): 925 – 934 . [DOI] [PubMed] [Google Scholar]
- 15. Habermeyer J , Boyken J , Harrer J , et al . Comprehensive phenotyping revealed transient startle response reduction and histopathological gadolinium localization to perineuronal nets after gadodiamide administration in rats . Sci Rep 2020. ; 10 ( 1 ): 22385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solmaz V , Köse Özlece H , Fatih Bozkurt M , Özkul B , Erbaş O . Repeated gadoteric acid and gadobutrol exposure causes deterioration of behavior and memory functions in rats: MRI, histopathological and biochemical evidence . Brain Res 2021. ; 1754 147256 . [DOI] [PubMed] [Google Scholar]
- 17. Kanda T , Ishii K , Kawaguchi H , Kitajima K , Takenaka D . High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material . Radiology 2014. ; 270 ( 3 ): 834 – 841 . [DOI] [PubMed] [Google Scholar]
- 18. Rogosnitzky M , Branch S . Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms . Biometals 2016. ; 29 ( 3 ): 365 – 376 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andreollo NA , Santos EF , Araújo MR , Lopes LR . Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig 2012. ; 25 ( 1 ): 49 – 51 . [DOI] [PubMed] [Google Scholar]
- 20. Liu L , Duff K . A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse . J Vis Exp 2008. ; 10 ( 21 ): 960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Development Core Team. R : A language and environment for statistical computing . Vienna, Austria: : R Foundation for Statistical Computing; , 2012. . [Google Scholar]
- 22. Murata N , Murata K , Gonzalez-Cuyar LF , Maravilla KR . Gadolinium tissue deposition in brain and bone . Magn Reson Imaging 2016. ; 34 ( 10 ): 1359 – 1365 . [DOI] [PubMed] [Google Scholar]
- 23. Rasschaert M , Emerit A , Fretellier N , et al . Gadolinium Retention, Brain T1 Hyperintensity, and Endogenous Metals: A Comparative Study of Macrocyclic Versus Linear Gadolinium Chelates in Renally Sensitized Rats . Invest Radiol 2018. ; 53 ( 6 ): 328 – 337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robert P , Violas X , Grand S , et al . Linear Gadolinium-Based Contrast Agents Are Associated With Brain Gadolinium Retention in Healthy Rats . Invest Radiol 2016. ; 51 ( 2 ): 73 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bauer DJ , Kerr AL , Swain RA . Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration . Neurobiol Learn Mem 2011. ; 95 ( 2 ): 166 – 175 . [DOI] [PubMed] [Google Scholar]
- 26. Gaytán-Tocavén L , Olvera-Cortés ME . Bilateral lesion of the cerebellar-dentate nucleus impairs egocentric sequential learning but not egocentric navigation in the rat . Neurobiol Learn Mem 2004. ; 82 ( 2 ): 120 – 127 . [DOI] [PubMed] [Google Scholar]
- 27. Lalonde R , Strazielle C . The effects of cerebellar damage on maze learning in animals . Cerebellum 2003. ; 2 ( 4 ): 300 – 309 . [DOI] [PubMed] [Google Scholar]
- 28. Noblett KL , Swain RA . Pretraining enhances recovery from visuospatial deficit following cerebellar dentate nucleus lesion . Behav Neurosci 2003. ; 117 ( 4 ): 785 – 798 . [DOI] [PubMed] [Google Scholar]
- 29. Peterson TC , Villatoro L , Arneson T , Ahuja B , Voss S , Swain RA . Behavior modification after inactivation of cerebellar dentate nuclei . Behav Neurosci 2012. ; 126 ( 4 ): 551 – 562 . [DOI] [PubMed] [Google Scholar]
- 30. Aymerich MS , Barroso-Chinea P , Pérez-Manso M , et al . Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats . Eur J Neurosci 2006. ; 23 ( 8 ): 2099 – 2108 . [DOI] [PubMed] [Google Scholar]
- 31. Baunez C , Nieoullon A , Amalric M . In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit . J Neurosci 1995. ; 15 ( 10 ): 6531 – 6541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandes VS , Ribeiro AM , Melo TG , et al . Memory impairment induced by low doses of reserpine in rats: possible relationship with emotional processing deficits in Parkinson disease . Prog Neuropsychopharmacol Biol Psychiatry 2008. ; 32 ( 6 ): 1479 – 1483 . [DOI] [PubMed] [Google Scholar]
- 33. Ito M , Doya K . Validation of decision-making models and analysis of decision variables in the rat basal ganglia . J Neurosci 2009. ; 29 ( 31 ): 9861 – 9874 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koch M , Schnitzler HU . The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation . Behav Brain Res 1997. ; 89 ( 1-2 ): 35 – 49 . [DOI] [PubMed] [Google Scholar]
- 35. Leung BK , Balleine BW . The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions . J Neurosci 2013. ; 33 ( 34 ): 13848 – 13860 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qiu MH , Vetrivelan R , Fuller PM , Lu J . Basal ganglia control of sleep-wake behavior and cortical activation . Eur J Neurosci 2010. ; 31 ( 3 ): 499 – 507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunaydin LA , Kreitzer AC . Cortico-Basal Ganglia Circuit Function in Psychiatric Disease . Annu Rev Physiol 2016. ; 78 ( 1 ): 327 – 350 . [DOI] [PubMed] [Google Scholar]
- 38. Deisseroth K . Circuit dynamics of adaptive and maladaptive behaviour . Nature 2014. ; 505 ( 7483 ): 309 – 317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bussi S , Penard L , Bonafè R , et al . Non-clinical assessment of safety and gadolinium deposition after cumulative administration of gadobenate dimeglumine (MultiHance®) to neonatal and juvenile rats . Regul Toxicol Pharmacol 2018. ; 92 ( 268 ): 277 . [DOI] [PubMed] [Google Scholar]
- 40. Fretellier N , Granottier A , Rasschaert M , et al . Does Age Interfere With Gadolinium Toxicity and Presence in Brain and Bone Tissues?: A Comparative Gadoterate Versus Gadodiamide Study in Juvenile and Adult Rats . Invest Radiol 2019. ; 54 ( 2 ): 61 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith AP , Marino M , Roberts J , et al . Clearance of Gadolinium from the Brain with No Pathologic Effect after Repeated Administration of Gadodiamide in Healthy Rats: An Analytical and Histologic Study . Radiology 2017. ; 282 ( 3 ): 743 – 751 . [DOI] [PubMed] [Google Scholar]
- 42. Gambino JM , James JR , Buchweitz JP , et al . Retention of gadolinium in the brains of healthy dogs after a single intravenous administration of gadodiamide . Am J Vet Res 2018. ; 79 ( 9 ): 949 – 960 . [DOI] [PubMed] [Google Scholar]
- 43. Rasschaert M , Schroeder JA , Wu TD , et al . Multimodal Imaging Study of Gadolinium Presence in Rat Cerebellum: Differences Between Gd Chelates, Presence in the Virchow-Robin Space, Association With Lipofuscin, and Hypotheses About Distribution Pathway . Invest Radiol 2018. ; 53 ( 9 ): 518 – 528 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang ST , Hua ZX , Fan DX , Zhang X , Ren K . Gadolinium Retention and Clearance in the Diabetic Brain after Administrations of Gadodiamide, Gadopentetate Dimeglumine, and Gadoterate Meglumine in a Rat Model . BioMed Res Int 2019. ; 2019 3901907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jost G , Frenzel T , Boyken J , Schoeckel L , Pietsch H . Gadolinium Presence in the Brain After Administration of the Liver-Specific Gadolinium-Based Contrast Agent Gadoxetate: A Systematic Comparison to Multipurpose Agents in Rats . Invest Radiol 2019. ; 54 ( 8 ): 468 – 474 . [DOI] [PubMed] [Google Scholar]
- 46. Bussi S , Coppo A , Botteron C , et al . Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats . J Magn Reson Imaging 2018. ; 47 ( 3 ): 746 – 752 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robert P , Lehericy S , Grand S , et al . T1-Weighted Hypersignal in the Deep Cerebellar Nuclei After Repeated Administrations of Gadolinium-Based Contrast Agents in Healthy Rats: Difference Between Linear and Macrocyclic Agents . Invest Radiol 2015. ; 50 ( 8 ): 473 – 480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rasschaert M , Idée JM , Robert P , et al . Moderate Renal Failure Accentuates T1 Signal Enhancement in the Deep Cerebellar Nuclei of Gadodiamide-Treated Rats . Invest Radiol 2017. ; 52 ( 5 ): 255 – 264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jost G , Frenzel T , Lohrke J , Lenhard DC , Naganawa S , Pietsch H . Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue . Eur Radiol 2017. ; 27 ( 7 ): 2877 – 2885 . [DOI] [PMC free article] [PubMed] [Google Scholar]