Abstract
Background
Delirium is a distressing neurocognitive disorder recently linked to sleep disturbances. However, the longitudinal relationship between sleep and delirium remains unclear. This study assessed the associations of poor sleep burden, and its trajectory, with delirium risk during hospitalization.
Methods
About 321 818 participants from the UK Biobank (mean age 58 ± 8 years [SD]; range 37–74 years) reported (2006–2010) sleep traits (sleep duration, excessive daytime sleepiness, insomnia-type complaints, napping, and chronotype—a closely related circadian measure for sleep timing), aggregated into a sleep burden score (0–9). New-onset delirium (n = 4 775) was obtained from hospitalization records during a 12-year median follow-up. About 42 291 (mean age 64 ± 8 years; range 44–83 years) had repeat sleep assessment on average 8 years after their first.
Results
In the baseline cohort, Cox proportional hazards models showed that moderate (aggregate scores = 4–5) and severe (scores = 6–9) poor sleep burden groups were 18% (hazard ratio = 1.18 [95% confidence interval: 1.08–1.28], p < .001) and 57% (1.57 [1.38–1.80], p < .001), more likely to develop delirium, respectively. The latter risk magnitude is equivalent to 2 additional cardiovascular risks. These findings appeared robust when restricted to postoperative delirium and after exclusion of underlying dementia. Higher sleep burden was also associated with delirium in the follow-up cohort. Worsening sleep burden (score increase ≥2 vs no change) further increased the risk for delirium (1.79 [1.23–2.62], p = .002) independent of their baseline sleep score and time lag. The risk was highest in those younger than 65 years at baseline (p for interaction <.001).
Conclusion
Poor sleep burden and worsening trajectory were associated with increased risk for delirium; promotion of sleep health may be important for those at higher risk.
Keywords: Chronotype, Circadian rhythms, Napping, Perioperative neurocognitive disorders, Sleep health
Delirium is an acute decline in attention and cognition; unfortunately, the prevalence increases dramatically with aging (1). Poor sleep health, which is composed of multidimensional sleep traits (short/long sleep duration, excessive daytime sleepiness, insomnia-like complaints, napping as well as morning/evening chronotype, a closely related circadian measure of preference for sleep timing), has emerged as a potentially modifiable risk factor in conditions implicated in delirium (2–4). For example, many dimensions of sleep health deteriorate with age, after critical illness (5), or prior to Alzheimer’s disease (AD) (6–8). These groups are also the most vulnerable to delirium (9).
Much of the focus on delirium has been on sleep disturbances/disorders around the time of critical illness (10,11) or in those older than 65 years (12). Yet, sleep disorders are often underdiagnosed (13), leading to imprecise associations. Sleep traits are present in all and can be more readily assessed as a widespread gauge of health; this makes any links potentially impactful. Others have shown downstream effects of poor sleep (14,15) to include neuroinflammation, autonomic nervous system (ANS) dysfunction, and cardiometabolic disease. These are also suspected in the etiology of delirium (16,17). Given that sleep is modifiable, our primary objective was to establish prospectively whether earlier life poor sleep is a risk factor for delirium during hospitalization.
In this study, we assessed poor sleep burden, derived from an aggregate sleep score, and new-onset delirium during a median 12 years of follow-up, within a large community sample from the UK Biobank. We also explored these relationships in subsets (postoperative delirium only and after exclusion of underlying dementia). Finally, we tested the trajectory of poor sleep burden and delirium in a smaller follow-up cohort a median 4 years after the first assessment.
Method
Study Participants
Between 2006 and 2010, over 500 000 community-based participants aged between 37 and 70 (mean age 57 ± 8 years, 54% female) from across the United Kingdom were recruited to participate in the UK Biobank (18). Participants completed extensive questionnaires on demographics, lifestyle choices, and medical conditions. Blood/serum samples were also obtained for biochemical analysis. Specific to this study, a total of 321 818 participants (mean age 58 ± 8 years; range 37–74 years; female: 54%) and 42 291 (mean age 64 ± 8 years; range 44–83 years; female: 54%) who had available sleep assessment and at least one subsequent hospitalization episode after baseline and repeat assessments, respectively (Supplementary Figure 1).
A subset of the baseline cohort (n = 61 063; mean age 64 ± 8 years; range 44–83 years) was invited back for reassessment between 2012 and 2020. Participants were followed for up to 15 years from baseline, and up to 9 years after the follow-up visit, until February 2021 (see Supplementary Figure 1 for a flowchart of participant selection). The UK Biobank validation efforts for data collected from participants (eg, sample handling and storage procedures for blood samples or characterization of health-related outcomes) have been described in detail elsewhere (19).
Standard Protocol Approvals, Registrations, and Patient Consents
The UK Biobank received National Research Ethics Approval and participants gave written informed consent. This study was conducted under the terms of UK Biobank access number 40556 and Mass General Brigham institutional review board approval (#2020P002097).
Assessment of Sleep Traits
Sleep health traits were recorded during baseline recruitment for the cohort and at a follow-up visit for a smaller group. For sleep duration, participants were asked “About how many hours sleep do you get in every 24 hours (include naps).” We categorized sleep duration into short (<6 hours/day), normal (6–9 hours), and long (>9 hours) based on prior evidence for U-shape associations with disease (3,15,20). Subjective excessive daytime sleepiness, insomnia-like complaints, and napping were assessed by the answers to the questions: “How likely are you to doze off or fall asleep during the daytime when you don’t mean to? (eg, when working, reading, or driving)” (never/rarely, sometimes, often/all the time), “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” (never/rarely, sometimes, usually), and “Do you have a nap during the day?” (never/rarely, sometimes, usually), respectively. Finally, for chronotype preference, participants were asked “Do you consider yourself to be (1) definitely a ‘morning’ person, (2) more a ‘morning’ than ‘evening’ person, (3) more an ‘evening’ than ‘morning’ person, or (4) definitely an ‘evening’ person.” It is a circadian measure related to preference for the timing of sleep, but is often closely tied to sleep traits and adaptions to typical work schedules. There is, however, a significant proportion (12%) who responded: “Do not know.” In line with other investigators reporting UK Biobank data (21,22), we have classified this group as an intermediate or “neither a morning person nor an evening person” group. Based on the prior association of increasing mortality risk and comorbidities in those who are evening types within this cohort (21), we categorized chronotype into “early/intermediate” (1–3) and “late” (4) groups.
Derivation of Poor Sleep Burden and Sleep Trajectory
For each sleep trait (sleep duration, excessive daytime sleepiness, insomnia, napping, and chronotype), a score was assigned: 0 for “normal” 6–9 hours sleep duration, “never/rarely” excessive daytime sleepiness, insomnia-like complaints, or napping, and “early/intermediate” chronotype; 1 for “sometimes” experience excessive daytime sleepiness, insomnia, or napping, and “late” chronotype; and 2 for generally extremes of sleep duration (“short” or “long”), “often/all the time/usually” experience excessive daytime sleepiness, insomnia, or napping. All component scores were summed for each participant to obtain a sleep score ranging from 0 to 9, where higher scores are indicative of more tendencies toward a poorer cumulative sleep burden. We used the score to classify poor sleep burden in a way that keeps group power as balanced as possible with increments of 2 points (representing one significant or 2 minor poor sleep traits) as follows: “minimal” (0–1), “mild” (2, 3), “moderate” (4, 5), and “severe” (6–9; see Supplementary Figure 2A for further details). Sleep burden trajectory was defined for the repeat assessment group as the difference between the sleep scores at follow-up and at baseline. The distribution of the sleep score and change at repeat assessment are shown in Supplementary Figure 2B.
Assessment of Delirium Diagnosis
The UK Biobank has released hospitalization records linked to study participants during the follow-up period from the National Health Service of the United Kingdom. These comprise of hospitalization dates and corresponding International Classification of Disease (ICD)-10 coded diagnoses. In keeping with similar studies using this data (23,24), we identified incident delirium diagnosis as the first date of occurrence for the code F05, included in hospital admissions health records. These cases formed the basis of our main analysis. The earliest delirium date was compared to (a) date of baseline assessment and (b) date of follow-up assessment, to derive the time-to-event. Based on this, we excluded 61 and 27 cases where delirium predated the baseline and follow-up assessment, respectively.
Assessment of Covariates
We assessed participant medical history through a combination of self-report during nurse-led interviews or health records and medication use at baseline time of assessment. Covariates were grouped into (a) demographics, (b) presence of any sleep disorders, (c) body mass index (BMI)/lifestyle factors, and (d) significant cardiovascular risk/disease (CVD)/comorbidities and mean reaction time. Demographics included age, sex, ethnicity, education, and deprivation level. Age at sleep assessment was calculated in years based on their dates of birth. Sex (male/female) and ethnicity were self-reported. Because the majority of participants self-identified as of British or “White” European descent (94%), we included ethnicity as European and non-European. Education was college-level (yes/no). Townsend deprivation index (TDI), a median score based on national geographic census data, was used to classify high/low deprivation. Existing sleep disorders (any from sleep apnea, insomnia, and other disorders including hypersomnia, sleep-wake disorders, and narcolepsy-catalepsy) were derived from ICD-10, within the group G47, and included as a covariate in models. For BMI/lifestyle factors, we included physical activity (summed metabolic equivalent minutes [MET-min] per week for all activities) and alcohol use (<4 drinks/≥4 drinks per week), in addition to BMI (calculated as weight [kg] divided by height squared [m2]). Significant CVD/comorbidities factors included a risk score (0–5) based on the presence of hypertension, high cholesterol, current smoking, diabetes and ischemic heart disease (from self-report and ICD-10), cancer (yes/no, in response to “has a doctor ever told you that you have had cancer?”), respiratory diseases (chronic obstructive pulmonary disease, asthma, or pulmonary fibrosis), dementia/Parkinson’s disease, gastrointestinal disorders (liver disease, inflammatory bowel disease), and renal disorders (kidney failure, dialysis, nephropathies, or pyelonephritis). We also included serum 25-hydroxyvitamin D (25[OH]D, a proxy for vitamin D levels categorized as sufficient >50 nmol/L, low 25–50 nmol/L, and deficient <25 nmol/L), given its recent links to delirium within the same cohort (23,24). Sunlight exposure is also a major source of vitamin D via the skin. Although sunlight regulates the circadian timing system, it may also be affected by one’s sleep traits/behaviors. We also included hypnotic/sedative use self-reported at the time of sleep assessment (yes/no). Finally, as a proxy for cognition, reaction time tests (average timed tests of symbol matching) were completed through a touchscreen tool and recorded in milliseconds.
Statistical Analysis
The characteristics of those who developed delirium compared to those who were hospitalized but remained delirium-free during follow-up were compared using t-tests for continuous variables (eg, age, BMI, deprivation, physical activity, reaction time, CVD risk score) or chi-squared tests for categorical variables (eg, sex, ethnicity, presence/absence of comorbidities). Cox proportional hazards models were used to assess the predictive value of the continuous sleep score and the poor sleep burden groups on incident delirium and reported as hazards ratios (HRs) and corresponding 95% confidence intervals (CIs). Model A: the core model controlled for demographics (age, sex, college education, ethnicity, and deprivation); Model B: further controlled for the presence of any sleep disorder; Model C: accounted for BMI, alcohol use, and physical activity; Model D: final adjustment was made for CVD risk, significant comorbidities, and reaction time. In the smaller follow-up cohort, we used a modified core model adjusting for demographics and sleep disorders to avoid overfitting. When testing sleep burden trajectory, we also included the baseline sleep score and time lag between sleep assessments.
For sensitivity analysis, we further excluded a small subset of the delirium group who had “delirium superimposed on dementia” (F05.1), given the overlapping link between sleep and dementia. We labeled these cases as “nondementia-related delirium.” At the same time, we analyzed operation/procedure coding from the UK Biobank and matched dates of operations within 3 days (25) prior to incident delirium—these were then considered as a subset cohort of postoperative delirium and tested separately. We then tested by subgroups of interest (age, sex, BMI, CVD risk, vitamin D status, and preexisting sleep disorder). Models were also repeated after exclusion of the “Do not know” group.
Time-to-event was calculated as the time interval in years between date of sleep assessments and date of delirium. For those who remained delirium-free, we censored follow-up at February 2021, the latest date of available records. The proportional hazards assumption was assessed using the global χ 2 test in R-package cox.zph (survival) incorporating methods described by Grambsch and Therneau (26). Efron’s method was used to handle ties. All other statistical analyses were performed using JMP Pro (version 14; SAS Institute, Cary, NC). A p value of less than .05 was used for statistical significance.
Results
In total, 4 775 (14.8 per 1 000) from 321 818 UK Biobank participants developed incident delirium (median time: 12.0 years [range 2 months to 15 years; SD 1.8 years]). Compared to those with no incident delirium (Table 1), participants with incident delirium were more likely to be older (64.0 years vs 57.8), male (57.3% vs 45.7%), have lower rates of college attendance (20.7% vs 30.0%), from areas of greater deprivation (TDI −0.62 vs −1.25), and be slightly less active (2 586 vs 2 651 MET-min/week). Ethnic background and alcohol consumption were similar between the 2 groups. The delirium group also had more CVD risks (score 1.24 vs 0.70), higher BMI (28.7 vs 27.7 kg/m2), higher hypnotic/sedative use (5.3% vs 1.5%), more comorbidities such as cancer (12.1% vs 8.8%), and higher rates of vitamin D deficiency (17.1% vs 13.3%).
Table 1.
Participants Characteristics and Sleep Health Traits in Delirium and Nondelirium Groups
| New-Onset Delirium Participants (n = 4 775) | Nondelirium Participants (n = 321 818) | ||
|---|---|---|---|
| Mean (SD), or % | Mean (SD), or % | p | |
| Demographics | |||
| Age at baseline | 64.0 (5.4) | 57.8 (7.9) | <.001 |
| Male | 57.3% | 45.7% | <.001 |
| College attendance | 20.7% | 30.0% | <.001 |
| Ethnic background (European) | 95.5% | 94.2% | .078 |
| Townsend deprivation index* | −0.62 (3.4) | −1.25 (3.1) | <.001 |
| BMI/lifestyle | |||
| Body mass index (kg/m2) | 28.7 (5.5) | 27.7 (4.9) | <.001 |
| Physical activity (MET-min)† | 2586 (2835) | 2651 (2752) | .036 |
| Alcohol (≥4 drinks/week) | 47.5% | 46.5% | .15 |
| Cardiovascular risk/comorbidities/cognition | |||
| CVD risk score‡ | 1.24 (1.1) | 0.70 (0.9) | <.001 |
| Dementia/Parkinson’s disease | 2.5% | 0.2% | <.001 |
| Respiratory disease | 14.2% | 14.0% | .59 |
| Liver/GI disease | 9.1% | 8.9% | .66 |
| Renal disease | 1.6% | 1.5% | .84 |
| Cancer diagnosed | 12.1% | 8.8% | <.001 |
| Vitamin D (deficient)§ | 17.2% | 13.3% | <.001 |
| Hypnotic/sedative use | 5.3% | 1.5% | <.001 |
| Cognition (reaction time)‖ | 613 (145) | 563 (119) | <.001 |
| Sleep traits and disorders | |||
| Sleep disorders¶ | 1.5% | 0.9% | <.001 |
| Sleep duration (h/day) | <.001 | ||
| Short (<6) | 8.1% | 6.1% | |
| Normal (6–9) | 87.4% | 92.7% | |
| Long (>9) | 4.4% | 2.1% | |
| Excessive daytime sleepiness | <.001 | ||
| Never/rarely | 64.1% | 74.5% | |
| Sometimes | 30.0% | 22.3% | |
| Often/all the time | 5.8% | 3.1% | |
| Insomnia-like complaints | <.001 | ||
| Never/rarely | 21.8% | 22.5% | |
| Sometimes | 43.8% | 47.3% | |
| Usually | 34.4% | 30.2% | |
| Napping | <.001 | ||
| Never/rarely | 41.2% | 54.3% | |
| Sometimes | 47.6% | 39.9% | |
| Usually | 11.2% | 5.8% | |
| Chronotype | .02 | ||
| Early/intermediate | 91.5% | 92.1% | |
| Late | 8.5% | 7.9% | |
| Poor sleep behavior burden | <.001 | ||
| Minimal (0–1) | 27.7% | 37.8% | |
| Mild (2, 3) | 46.5% | 46.2% | |
| Moderate (4, 5) | 20.1% | 13.5% | |
| Severe (≥6) | 5.8% | 2.5% |
Notes: SD = standard deviation; CVD = cardiovascular disease; MET = metabolic equivalent; BMI = body mass index; GI = gastrointestinal. UK Biobank participant characteristics at baseline expressed as mean (SD) for continuous variables or number (percentage) for categorical variables. Participants were compared based on delirium status (new-onset delirium vs delirium-free participants). Categorical data presented as a percentage of participants present. p values from one-way analysis of variance tests for continuous measures and Pearson’s chi-squared tests for categorical data.
*Higher value indicated worse deprivation.
†METS-min/week increase.
‡CVD risk score: summed hypertension, cholesterol, diabetes mellitus, smoking status, and ischemic heart disease.
§Vitamin D levels: sufficient >50 nmol/L, low 25–50 nmol/L, and deficient <25 nmol/L.
‖Cognition reaction time in milliseconds: average timed tests of symbol matching.
¶Sleep disorders: any from sleep apnea, insomnia, and other disorders such as hypersomnia, sleep-wake disorders, and narcolepsy-catalepsy.
Participants with incident delirium were more likely to be short (8.1% vs 6.1%) or long (4.4% vs 2.1%) sleepers and to report “often/all the time” excessive daytime sleepiness (5.8% vs 3.1%), “usually” napping (11.2% vs 5.8%), and slightly more late chronotypes (8.5% vs 7.9%). While a higher proportion reported “usually” having insomnia in the delirium group (34.4% vs 30.2%), this was reversed in those reporting “sometimes” having insomnia (43.8% vs 47.3%). A higher proportion of delirium participants also had sleep disorders (1.5% vs 0.9%). The overall incidence of a clinical sleep disorder diagnosis was 1.0% and increased in line with poor sleep burden groups (there was a 6-fold increase from minimal to severe poor sleep burden groups; 0.6%–3.8%, p for trend <0.001; Supplementary Figure 3A).
Poor Sleep Burden Increases Risk for Incident Delirium Over Time
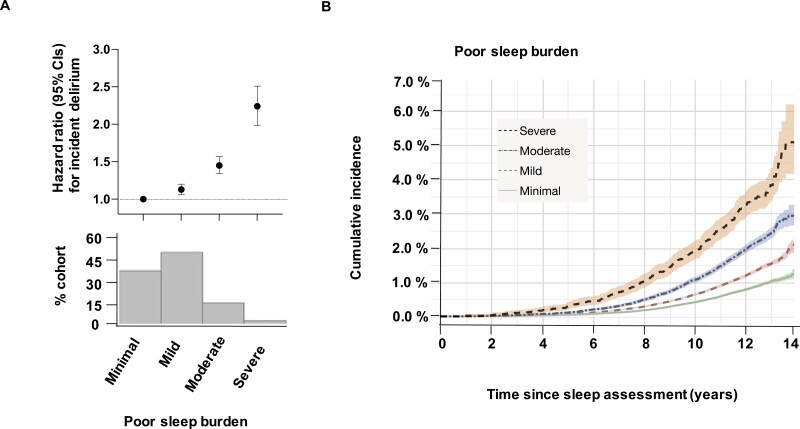
After categorizing into increasing extents of poor sleep burden (none–minimal/mild/moderate/severe), there were stepwise increases in the risk for incident delirium (Figure 1A). This also translated into progressively higher cumulative incidence for delirium across the follow-up period (Figure 1B). For all delirium cases, each 1-point increase in sleep score predicted a 12% increased risk (HR = 1.12, 95% CI: 1.10–1.14 p < .001; Table 2, Model A). Moderate (HR = 1.18, 95% CI: 1.08–1.28, p = .002) and severe (HR = 1.57, 95% CI: 1.38–1.80, p < .001) burden groups remained significantly predictive in the fully adjusted Model D (Table 2). Mild burden participants showed slightly elevated risks in Models A–C, but were no longer significant in the fully adjusted Model D (HR = 1.05, 95% CI: 0.98–1.12, p = .18). These results were similar in postoperative delirium (attenuated for the mild/moderate burden groups) and in nondementia-related delirium cases. Using coefficients (ratio of the natural log of HRs) from the full model, the risks of moderate and severe poor sleep burdens were equivalent to an additional 1.3 and 3.4 years of aging or 0.7 and 1.8 increased CVD risk score, respectively (Supplementary Table 1).
Figure 1.
Poor sleep burden and incident delirium. (A) Hazard ratios (±95% CI) for incident delirium using Cox proportional hazards regression models adjusted for age, sex, education, ethnicity, and deprivation level; percentage of the cohort by sleep disturbance burden group in the panel below. (B) Unadjusted cumulative incidence plot showing the percentage of cohort with the first diagnosis of delirium over time, in the 4 sleep burden groups (minimal = 0–1, mild = 2–3, moderate = 4–5, and severe = ≥6, based on the sleep score). CI = confidence interval.
Table 2.
Effects of Poor Sleep Burden on Incident Delirium
| Poor Sleep Burden | All Delirium (n = 4 775) | Postoperative Delirium (n = 1 613) | Nondementia-Related Delirium (n = 3 943) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Model A | ||||||
| Sleep score* | 1.12 (1.10–1.14) | <.001 | 1.10 (1.07–1.13) | <.001 | 1.14 (1.12–1.17) | <.001 |
| Mild | 1.13 (1.06–1.20) | <.001 | 1.23 (1.01–1.26) | .02 | 1.14 (1.06–1.22) | .002 |
| Moderate | 1.44 (1.34–1.57) | <.001 | 1.33 (1.17–1.53) | <.001 | 1.51 (1.40–1.65) | <.001 |
| Severe | 2.23 (1.98–2.51) | <.001 | 2.03 (1.64–2.51) | <.001 | 2.47 (2.17–2.80) | <.001 |
| Model B | ||||||
| Sleep score* | 1.10 (1.08–1.13) | <.001 | 1.09 (1.06–1.13) | <.001 | 1.14 (1.11–1.16) | <.001 |
| Mild | 1.12 (1.05–1.20) | .003 | 1.12 (1.00–1.25) | .04 | 1.13 (1.06–1.21 | <.001 |
| Moderate | 1.43 (1.33–1.55) | <.001 | 1.31 (1.14–1.50) | <.001 | 1.50 (1.37–1.63) | <.001 |
| Severe | 2.18 (1.93–2.24) | <.001 | 1.99 (1.60–2.48) | <.001 | 2.41 (2.11–2.74) | <.001 |
| Model C | ||||||
| Sleep score* | 1.08 (1.06–1.10) | <.001 | 1.08 (1.05–1.11) | <.001 | 1.12 (1.10–1.14) | <.001 |
| Mild | 1.10 (1.03–1.17) | .003 | 1.10 (0.98–1.22) | .09 | 1.11 (1.03–1.19) | .005 |
| Moderate | 1.37 (1.27–1.48) | <.001 | 1.25 (1.09–1.44) | .001 | 1.42 (1.30–1.55) | <.001 |
| Severe | 2.01 (1.78–2.27) | <.001 | 1.84 (1.48–2.29) | <.001 | 2.17 (1.90–2.48) | <.001 |
| Model D | ||||||
| Sleep score* | 1.07 (1.06–1.09) | <.001 | 1.05 (1.01–1.08) | .005 | 1.07 (1.05–1.09) | <.001 |
| Mild | 1.05 (0.98–1.12) | .18 | 1.06 (0.94–1.19) | .33 | 1.05 (0.97–1.13) | .22 |
| Moderate | 1.18 (1.08–1.28) | .002 | 1.09 (0.94–1.27) | .24 | 1.20 (1.09–1.32) | .002 |
| Severe | 1.57 (1.38–1.80) | <.001 | 1.45 (1.14–1.85) | .0025 | 1.72 (1.49–1.98) | <.001 |
Notes: HR = hazard ratio; 95% CI = 95% confidence interval. Cox proportional hazards models for the continuous sleep score (
*each 1-point increase), and comparing poor sleep burden groups against reference group “none/minimal,” for all delirium cases, postoperative delirium, and nondementia-related delirium subgroups. Model A is our core model adjusting for demographics (age, sex, education, ethnic background, and deprivation). Model B includes Model A plus sleep disorders. Model C includes Model B with physical activity, alcohol consumption, and body mass index. Model D adds on Model C with cardiovascular risk score, reaction time, comorbidities: dementia/Parkinson’s, respiratory disease, gastrointestinal diseases, renal diseases, cancer diagnosis, vitamin D status, and hypnotics. Two-sided p value for HR in comparison with the reference category, without adjustment for multiple comparisons.
Follow-Up Sleep Score and Worsening Sleep Trajectory Associated With Increased Risk for Delirium
In our smaller follow-up cohort, 240 (5.7 per 1 000) from 42 105 participants developed incident delirium (median follow-up time: 4.0 years [range 2 months to 11.2 years; SD 2.7 years]). The median time lag from baseline sleep assessment was 7.6 years (range 2.5–13.8 years; SD 2.7 years). There was a 0.21 (SD = 1.26) score increase overall. After adjusting for demographics and sleep disorders, each point increase in the follow-up sleep score was associated with a 12% increased risk for delirium (Table 3; HR = 1.12, 95% CI: 1.03–1.22, p = .01) and 61% increased risk when categorized into the moderate/severe burden cohort (Table 3; HR = 1.61, 95% CI: 1.12–2.23, p = .01). After further controlling for a participant’s baseline sleep score and time lag, an increased sleep burden score of 2 or more during repeat assessment was associated with an additional 79% risk (Table 3; HR = 1.79, 95% CI: 1.23–2.62, p = .002). Improvement in the sleep score was not significantly associated with reduced risk (Table 3; HR = 0.83, 95% CI: 0.59–1.16, p = .28).
Table 3.
Follow-Up Poor Sleep Burden, Sleep Burden Trajectory, and Risk for Delirium
| N (%) | HR (95% CI) | p | |
|---|---|---|---|
| Poor sleep burden | |||
| Sleep score* | 42 105 (100%) | 1.12 (1.03–1.22) | .01 |
| Minimal | 15 345 (36.5%) | REF | REF |
| Mild | 20 063 (47.7%) | 1.28 (0.90–1.76) | .17 |
| Moderate/severe | 6 695 (15.9%) | 1.61 (1.12–2.23) | .01 |
| Sleep burden trajectory | |||
| Improved (score change −1 or more) | 10 000 (23.8%) | 0.83 (0.59–1.16) | .28 |
| No change (0) | 16 652 (39.6%) | REF | REF |
| Mild worsening (+1) | 10 105 (24.0%) | 1.06 (0.73–1.52) | .76 |
| Significant worsening (+2 or more) | 5 348 (12.7%) | 1.79 (1.23–2.62) | .002 |
Notes: HR = hazard ratio; CI = confidence interval; REF = reference group. Cox proportional hazards models: results presented as hazard ratio (95% confidence interval) and p value. Our poor sleep burden model used our aggregate sleep score as a continuous measure and categorized into burden groups with non/minimal as reference, and included age at follow-up date, sex, college education, deprivation, sleep disorders, and ethnic background. Sleep score trajectory model calculated the change in sleep score from baseline to follow-up and categorized as improved (score change ≤−1), no change (0; reference group), mild worsening (+1), significant worsening (≥+2) and calculated additional risk after inclusion of demographics, sleep disorders, baseline sleep score, and the time lag from baseline to follow-up.
*Risk per 1-point increase.
Incident Delirium Risk by Subgroups
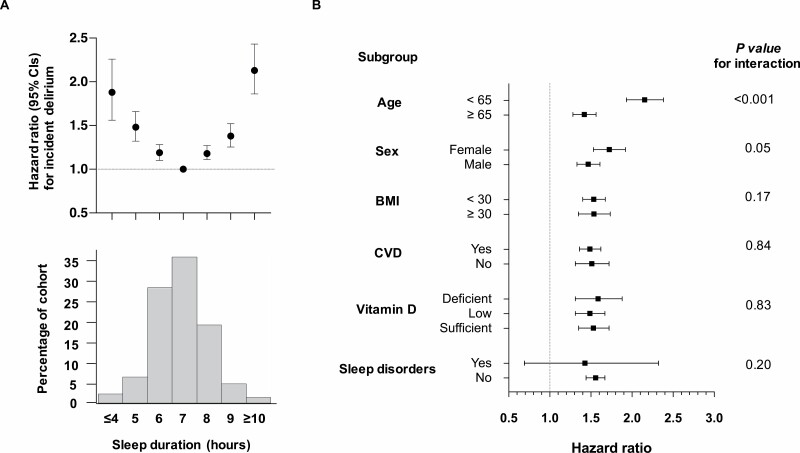
We further examined risk for delirium by hourly sleep duration, as well as by age, sex, obesity, CVD risk, vitamin D status, and presence/absence of any sleep disorders (Figure 2). There was a U-shaped risk profile (adjusted for age, sex, education, and ethnicity) where more extreme durations away from 7 hours (reference group) were associated with rising risk for incident delirium (Figure 2A). We also found a significant interaction between the age of reporting sleep and the extent of poor sleep burden (moderate/severe vs none/minimal) on the risk for incident delirium. Those aged younger than 65 years (cases 2 177/249 949 [8.7 per 1 000]) with moderate/severe poor sleep burden were at more than double the risk (HR = 2.15, 95% CI: 1.93–2.38, p < .001) compared to those aged 65 years or older (cases 2 589/71 835 [36.0 per 1 000]; HR = 1.41, 95% CI: 1.28–1.56, p < .001) at the time of sleep assessment (Figure 2B). However, poor sleep burden was equally predictive in males and females, in obese (BMI ≥30)/nonobese (BMI <30), presence/absence of CVD, differing levels of vitamin D, and when sleep disorders are excluded (Figure 2B).
Figure 2.
Subgroup analysis of delirium risk. (A) Delirium risk by hourly sleep duration shows a U-shaped profile (adjusted for age, sex, education, ethnicity, and deprivation). (B) Forest plot of hazard ratios and 95% confidence intervals for moderate/severe sleep burden (vs none/minimal), predicting incident delirium based on subgroups of participants by age, sex, BMI, CVD risk, vitamin D levels, and presence of any sleep disorder. BMI = body mass index; CVD = cardiovascular disease.
Individual Sleep Health Traits and Incident Delirium
The effects of sleep traits on incident delirium were also estimated using Cox proportional hazards models (Supplementary Table 2). After adjusting for age, sex, education, ethnicity, and deprivation, short (HR = 1.39, 95% CI: 1.27–1.53, p < .001) and long sleepers (HR = 1.89, 95% CI: 1.67–2.15, p < .001) were at increased risk for incident delirium compared to normal duration sleepers. Those who reported “sometimes” (HR = 1.21, 95% CI: 1.14–1.28, p < .001) and “often” (HR = 1.71, 95% CI: 1.53–1.92, p < .001) excessive daytime sleepiness or “sometimes” (HR = 1.19, 95% CI: 1.12–1.26, p < .001) and “often” (HR = 1.52, 95% CI: 1.39–1.66, p < .001) napping were similarly at increased risk compared to the “never/rarely” groups, respectively. However, participants reporting insomnia symptoms were not found to be at higher risk. Finally, late chronotype participants (HR = 1.28, 95% CI: 1.17–1.41, p < .001) were also at increased risk compared to early/intermediate chronotype. These results remained significant although effect sizes were moderately attenuated in our fully adjusted model (Model D, Supplementary Table 2). Results remained the same for all models when those reporting “Do not know” for chronotype were excluded (data not shown). Interestingly, we found an interaction between sleep duration and napping such that the risk from short or long sleep was highest within those who reported napping in a 24-hour period (p for interaction = .007; Supplementary Figure 3B). Someone reporting sleeping less than 6 hours per day with any napping had a higher risk (1.85 [1.48–2.31], p < .001) than someone without napping (1.36 [1.23–1.50], p < .001), compared to normal sleepers with no naps as reference and after adjusting for demographics. No interactions were found for sleep duration with the other sleep traits.
Discussion
In this cohort of 321 818 community-based middle-aged to older men and women, we found that those with moderate and severe poor sleep burden were 18% and 57% more likely to experience hospital-diagnosed delirium during follow-up, when compared with those with none/minimal burden. The findings remained when including only postoperative delirium and after exclusion of underlying dementia. More recent repeated sleep burden scores in a smaller cohort of 42 291 confirmed the association with delirium risk, and more importantly, participants who experienced worsened sleep burden trajectory (score increase of ≥2 vs no change from baseline) were at 79% increased risk regardless of their baseline sleep score or time lag between sleep assessments. Although delirium diagnosis was more prevalent at an older age, the additional risk from poor sleep appeared to be highest when reported before age of 65 years.
While others have shown that certain sleep characteristics just before hospitalization may be associated with delirium (4,11,27), this prospective study provides evidence that patterns of poor sleep may be an indicator of independent risk years before delirium. Single sleep characteristics in isolation are important, but the sleep burden metric integrates multiple dimensions of sleep (28) and is novel in our application to delirium. In aggregate, it may act as a better proxy for sleep regulation (29), and/or circadian misalignment (30,31), mismatches between our body clocks and the timing of external behaviors. For example, it is worth noting that even mild burden participants are at slightly increased risk that was attenuated by comorbidities in the final model, but to put the severe burden group’s risk magnitude into context, it is equivalent to the risk of being nearly 4 years older or having an extra 2 cardiovascular risk factors.
In line with these observations, sleep/circadian regulation has been associated with both cardiometabolic diseases (diabetes (32), ischemic heart disease (15), heart failure (33), or stroke (34)) and neurodegenerative conditions such as mild cognitive impairment (35) and AD (8,36–39). Even though delirium is generally transient after acute illness and hospitalization, it is also associated with increased risk for AD (16). This may reflect preclinical AD vulnerability to delirium, which makes our results consistent with recent links between poor sleep/circadian regulation and AD (8,40). It is thus plausible that common mechanisms such as neuroinflammation, ANS dysfunction, and cardiometabolic risks, implicated in both sleep (14,15) and delirium (16,17), interact with normal aging (36) and contribute to a spectrum of neurodegenerative diseases.
Whether these results point to a causal role, or an unmasking of cognitive vulnerability, is unclear. We hypothesize that the consequences of poor sleep play a causal role in delirium risk, rather than underlying disease linked to sleep disturbances being the culprits, as many of these were controlled for in our final model. In addition, the results were significantly stronger in younger participants less than 65 years when there is a lower likelihood of underlying neurodegenerative diseases, independent of a domain of cognition (reaction time), and robust when underlying dementia was excluded. Given that the UK Biobank was also relatively healthy at recruitment, this makes the relationship between sleep and delirium less likely to be fully accounted for by concomitant underlying disease. Further work is needed to understand the exact timing of risk increases and through which particular mechanisms. Here, the baseline cohort had sleep assessed over a decade prior to incident delirium, but the results were consistent in a smaller repeat assessment cohort only 4 years prior. In addition, a worsening trajectory for the sleep score was associated with additional risk compared to no change, regardless of a participant’s baseline sleep score. However, improvements in sleep did not reach significance for protection, but this warrants further replication as only 240 cases accumulated during the median 4-year follow-up. Altogether, sleep traits may be on a causal chain of multisystemic contributing factors, but it appears to be important in delirium risk across time and age groups.
When we examined individual sleep traits, results suggested that “excess” sleep behaviors were the strongest predictors for delirium (long sleep duration, unexpected excessive daytime sleepiness, and napping outside of the main sleep period). For example, we demonstrate for the first time, a U-shaped relationship between sleep duration and delirium, centered at 7 hours, but the effect sizes were greatest for the longest duration of 10 or more hours (Figure 2A), and consistent with a recent polysomnography study in delirium patients (41). Yet, it is important to note that those sleeping less than 6 hours per night (1 in 16) still have a 20% increased risk, consistent with recent links to AD (3) and myocardial infarction (15). Interestingly, “sometimes” and “often” insomnia-type complaints had no significant effect on incident delirium beyond the core model. This is an important finding because insomnia is the most common sleep complaint in the world, and this was no different in the UK Biobank, where between a third and half of participants reported some form of insomnia-type complaint. One explanation is that these are not true diagnoses of insomnia per se, rather that participants were asked about perceived inability to initiate or maintain sleep. Answers may have been influenced by self-perceptions of sleep rather than true sleep disruption. Insomnia may also have been highly correlated with other risk factors we adjusted for.
We also found that evening chronotypes (those who report optimal alertness in the evening and preference for later bedtimes) were at greater risk for delirium than early/intermediate types. Chronotype is an indication of one’s preference for sleep timing, an important dimension for sleep health. Evening chronotypes can struggle to adapt to societally enforced work/education schedules leading to a mismatch or misalignment with their underlying circadian rhythm. This underlying circadian misalignment is also seen in sleep disorders and is thought to contribute to an increased risk for cardiometabolic disease (42) and mortality (21) relative to early chronotypes. These relationships may account for the observation that evening chronotype was at higher risk in all models until CVD risks and mortality-related commodities were incorporated in Model D. Clearly, follow-up work examining objective measures of sleep and circadian rest/activity patterns are needed to untangle these factors in the context of future delirium risk.
In further sensitivity analysis, poor sleep burden was equally predictive in men and women and across obesity status, CVD risk, vitamin D sufficiency, and when those with diagnosed sleep disorders were removed. A formal sleep disorder diagnosis via ICD-10 or self-report appeared uncommon (0.6% in none/minimal burden group, and 3.8% in the severe group) and was likely an underestimation, but was associated with delirium risk after adjusting demographics (Supplementary Table 1, Model B). However, this effect was no longer significant when overlapping risks factors such as BMI and other lifestyle factors were included. As sleep disorders accumulate with aging, future work in this cohort may be able to address this link further; currently, there is insufficient power to confirm whether baseline sleep disorders independently predict delirium, which is why a flexible sleep burden metric can be potentially more useful in clinical practice (43).
Of note, effect sizes were significantly stronger in those younger than 65 at the time of sleep assessment (Figure 2B; p < .001 for interaction). The UK Biobank is a relatively healthy cohort compared to the general population. And in capturing people during middle age, where AD plaques/tangles are rarer in the brain, it is less likely that poor sleep is a symptom of preclinical dementia, rather that these associations are a true risk factor for delirium risk. Similarly, given that both comorbidities and sleep disturbances are more common in older participants, the extrinsic influences of medication or biological interactions with disease associated with the aging process may have attenuated the signal (44). For individual sleep traits, the interaction with age was confirmed for sleep duration, napping, and insomnia, but not excessive daytime sleepiness nor chronotype (data not shown). Sleep traits and daily activity patterns often change over time with age, and the exact relationship with neurodegenerative disorders remains unclear (36). In addition, the etiology of sleep behaviors is multifactorial, and some traits may be more influenced by personal preference or societal norms than others. For example, napping or “siestas” outside of the main sleep period is widespread and even beneficial among certain Mediterranean countries (45) and mainland China (46), but associated with poor outcome in those where it is not (47,48). However, it appears that from this UK cohort, where siesta is not part of the culture, that frequent napping at younger ages confers more risk for future incident delirium than at older ages. We also observed that the combination of short sleep and napping in a 24-hour period conferred a higher risk than short sleep alone. One interpretation is that when self-reported sleep duration during the 24-hour period is partially occupied by napping, this either limits nighttime sleep duration and together compounds risk, or conversely, napping is a compensatory behavior and a sign of inadequate/poor quality nighttime sleep. Additionally, the interaction between napping and short sleep duration may contribute to misalignment between daily behaviors and underlying circadian rhythms. This chronic stressor is a potential risk factor for delirium that needs to be formally tested (eg, with objective actigraphy measures) in future studies. On the other hand, experiencing excessive daytime sleepiness is unlikely to ever be “normal” and is thus equally detrimental to risk for delirium, regardless of age. Further work is needed to untangle which traits and combinations are most sensitive to delirium risk and at which age groups.
Strengths of this study included the large sample size and prospective design over nearly 14 years of follow-up with a repeat assessment in a more recent subset. Sleep disturbances are often underdiagnosed and worsen with age, so the study’s assessment of multiple domains of sleep and overall poor sleep burden is a novel starting point for sleep and delirium fields. Despite these strengths, several limitations must be acknowledged. This is an observational study and should not be interpreted as causal. Sleep measures were based on self-report where poor recall and misclassification are likely present; the caveat being that these would reduce the effect sizes and bias our findings toward the null. While we attempted to categorize poor sleep burden as a starting point for simplicity and maximize power by inclusion of all levels of responses (eg, “never/rarely,” “sometimes,” “often”) (43), it should be acknowledged that this measure has not yet been formally validated, but work in this is ongoing. Future studies should also test the optimal weighting for each level.
There must also be caution interpreting the repeat assessment of sleep and its trajectory from baseline given that the repeat assessment cohort was limited in power. The more recent assessment is informative of the temporal relationship, but relatively few cases of new-onset delirium occurred. It may also have been underpowered to detect the benefits of score improvement. Ceiling and, in particular, floor effects may have biased our results to the null. However, results were unchanged when we excluded the lower (0) or higher scores (8 and 9; data not shown). The large-scale nature of the UK Biobank meant that we did not have access to primary sourced nursing assessment (eg, the Confusion Assessment Method) results or detailed chart review. We relied on electronic health records and ICD-10 coding derived from nursing delirium assessment throughout the United Kingdom. Others have used this approach for delirium (23,24) and a variety of diseases (20,49–51) within this cohort. While this approach is likely highly specific (up to 96%) in a recent study (52), the sensitivity was also reported at 53% for delirium. Thus, we are likely missing a proportion of cases, particularly milder or the hypoactive form. However, these novel findings remain valuable as a starting point for research in sleep health and delirium as long as they are interpreted with the above caveats.
Although we adjusted for major risk factors, there was likely residual confounding given the complex nature of sleep biology and the heterogeneity of delirium. In addition, some of these factors could be on the causal pathway between sleep and delirium rather than being confounders. For example, physical activity, alcohol, or vitamin D levels could be changed because of sleep state and then go on to influence delirium outcomes. This might have implications for the interpretation of the results, particularly the attenuation of risks in those older than 65 years old, where the true effects of sleep are potentially underestimated. Future studies should aim to examine larger samples of repeated sleep assessments and covariates to model the impact of sleep and coexisting risk changes over time on delirium risk, ideally using machine learning techniques. Finally, the UK Biobank is a single population of mostly Caucasian of European descent, limiting the generalizability of these results to other parts of the world.
In conclusion, poor sleep burden is associated with incident delirium a decade later. Poor sleep and delirium are both common problems in older adults. Sleep traits can be relatively simple to assess, but are often unaddressed in practice. These results provide the basis for a better understanding of sleep and delirium, particularly before the age of 65 where there is a potential earlier window for intervention.
Funding
The work was supported by the National Institutes of Health (grant numbers T32GM007592 and R03AG067985 to L.G. and RF1AG059867 and RF1AG064312 to K.H.), the BrightFocus Foundation (A2020886S to P.L.), and the Foundation for Anesthesia Education and Research (GEMSSTAR-04-15-2021 to L.G.).
Conflict of Interest
F.A.J.L.S. has received lecture fees from Bayer HealthCare (2016), Sentara HealthCare (2017), Philips (2017), Vanda Pharmaceuticals (2018), and Pfizer Pharmaceuticals (2018). The other authors declare no conflict.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of the participants and investigators at the UK Biobank.
Contributor Information
Ma Cherrysse Ulsa, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Xi Zheng, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Peng Li, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Division of Sleep Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Arlen Gaba, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Patricia M Wong, Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Richa Saxena, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA; Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
Frank A J L Scheer, Division of Sleep Medicine, Harvard Medical School, Boston, Massachusetts, USA; Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA.
Martin Rutter, Division of Diabetes, Endocrinology & Gastroenterology, The University of Manchester, UK.
Oluwaseun Akeju, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Kun Hu, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Division of Sleep Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Lei Gao, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA.
Data Availability
Data are available from the UK Biobank after submitting an application (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The syntax for conducting the analysis is available upon request. The underlying data are open-access through application to the UK Biobank, and materials and methods will be made freely available through the UK Biobank as part of this project.
References
- 1. Davis DH, Barnes LE, Stephan BC, et al. ; MRC Cognitive Function and Ageing Study . The descriptive epidemiology of delirium symptoms in a large population-based cohort study: results from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). BMC Geriatr. 2014;14:87. doi:10.1186/1471-2318-14-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi:10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. doi:10.1038/s41467-021-22354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Todd O. Poor sleep—an early sign of incipient delirium, or a risk factor in itself? A clinical observational study. Sleep Med. 2013;14:e285. doi:10.1016/j.sleep.2013.11.698 [Google Scholar]
- 5. McKenna H, van der Horst GTJ, Reiss I, Martin D. Clinical chronobiology: a timely consideration in critical care medicine. Crit Care. 2018;22(1):124. doi:10.1186/s13054-018-2041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18(3):307–318. doi:10.1016/S1474-4422(18)30461-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer’s disease. Exp Mol Med. 2015;47:e148. doi:10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao L, Li P, Cui L, et al. Sleep disturbance and incident Alzheimer’s disease: a UK Biobank study of 502,538 middle-aged to older participants. Alzheimer’s & Dementia. 2020;16(S5):e044575. doi:10.1002/alz.044575 [Google Scholar]
- 9. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210–220. doi:10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29(5):707–716. doi:10.1093/sleep/29.5.707 [DOI] [PubMed] [Google Scholar]
- 11. Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi:10.1097/CCM.0000000000003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Westwood AJ, Beiser A, Jain N, et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology. 2017;88(12):1172–1179. doi:10.1212/WNL.0000000000003732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stores G. Clinical diagnosis and misdiagnosis of sleep disorders. J Neurol Neurosurg Psychiatry. 2007;78(12):1293–1297. doi:10.1136/jnnp.2006.111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–1008. doi:10.1126/science.aah4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daghlas I, Dashti HS, Lane J, et al. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019;74(10):1304–1314. doi:10.1016/j.jacc.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fong TG, Vasunilashorn SM, Libermann T, Marcantonio ER, Inouye SK. Delirium and Alzheimer’s disease: a proposed model for shared pathophysiology. Int J Geriatr Psychiatry. 2019;34(6):781–789. doi:10.1002/gps.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ritter C, Tomasi CD, Dal-Pizzol F, et al. Inflammation biomarkers and delirium in critically ill patients. Crit Care. 2014;18(3):R106. doi:10.1186/cc13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi:10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen N, Sudlow C, Downey P, et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1(3):123–126. doi:10.1016/j.hlpt.2012.07.003 [Google Scholar]
- 20. Wyse CA, Celis Morales CA, Graham N, et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK Biobank. Ann Med. 2017;49(5):411–420. doi:10.1080/07853890.2017.1292045 [DOI] [PubMed] [Google Scholar]
- 21. Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 2018;35(8):1–9. doi:10.1080/07420528.2018.1454458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones SE, Lane JM, Wood AR, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi:10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowman K, Jones L, Pilling LC, et al. Vitamin D levels and risk of delirium: a Mendelian randomization study in the UK Biobank. Neurology. 2019;92(12):e1387–e1394. doi:10.1212/WNL.0000000000007136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pilling LC, Jones LC, Masoli JAH, et al. Low vitamin D levels and risk of incident delirium in 351,000 older UK Biobank participants. J Am Geriatr Soc. 2020;69:365–372. doi:10.1111/jgs.16853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77(4):448–456. [PMC free article] [PubMed] [Google Scholar]
- 26. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi:10.1093/biomet/81.3.515 [Google Scholar]
- 27. Todd OM, Gelrich L, MacLullich AM, Driessen M, Thomas C, Kreisel SH. Sleep disruption at home as an independent risk factor for postoperative delirium. J Am Geriatr Soc. 2017;65(5):949–957. doi:10.1111/jgs.14685 [DOI] [PubMed] [Google Scholar]
- 28. Wallace ML, Stone K, Smagula SF, et al. Which sleep health characteristics predict all-cause mortality in older men? An application of flexible multivariable approaches. Sleep. 2018;41:3–9. doi:10.1093/sleep/zsx189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40:2–7. doi:10.1093/sleep/zsw075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi:10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu K, Scheer FA, Ivanov PCh, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149(3):508–517. doi:10.1016/j.neuroscience.2007.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merikanto I, Lahti T, Puolijoki H, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30(4):470–477. doi:10.3109/07420528.2012.741171 [DOI] [PubMed] [Google Scholar]
- 33. Gao L, Lim ASP, Wong PM, et al. Fragmentation of rest/activity patterns in community-based elderly individuals predicts incident heart failure. Nat Sci Sleep. 2020;12:299–307. doi:10.2147/NSS.S253757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koo DL, Nam H, Thomas RJ, Yun CH. Sleep disturbances as a risk factor for stroke. J Stroke. 2018;20(1):12–32. doi:10.5853/jos.2017.02887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wams EJ, Wilcock GK, Foster RG, Wulff K. Sleep-wake patterns and cognition of older adults with amnestic mild cognitive impairment (aMCI): a comparison with cognitively healthy adults and moderate Alzheimer’s disease patients. Curr Alzheimer Res. 2017;14(10):1030–1041. doi:10.2174/1567205014666170523095634 [DOI] [PubMed] [Google Scholar]
- 36. Hu K, Li P, Gao L. Sleep, rest-activity rhythms and aging: a complex web in Alzheimer’s disease? Neurobiol Aging. 2021;104:102–103. doi:10.1016/j.neurobiolaging.2021.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu K, Harper DG, Shea SA, Stopa EG, Scheer FA. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep. 2013;3:2229. doi:10.1038/srep02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: involvement of the circadian pacemaker. Proc Natl Acad Sci U S A. 2009;106(8):2490–2494. doi:10.1073/pnas.0806087106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao L, Li P, Gaba A, Musiek E, Ju YS, Hu K. Fractal motor activity regulation and sex differences in preclinical Alzheimer’s disease pathology. Alzheimers Dement (Amst). 2021;13(1):e12211. doi:10.1002/dad2.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li P, Gao L, Gaba A, et al. Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longev. 2020;1(3):e96–e105. doi:10.1016/s2666-7568(20)30015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibala R, Mekonnen J, Gitlin J, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res. 2021;30(5):e13322. doi:10.1111/jsr.13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu JH, Yun CH, Ahn JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100(4):1494–1502. doi:10.1210/jc.2014-3754 [DOI] [PubMed] [Google Scholar]
- 43. Wallace ML, Yu L, Buysse DJ, et al. Multidimensional sleep health domains in older men and women: an actigraphy factor analysis. Sleep. 2021;44:5–9. doi:10.1093/sleep/zsaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao L, Gaba A, Cui L, et al. Resting heartbeat complexity predicts all-cause and cardiorespiratory mortality in middle- to older-aged adults from the UK Biobank. J Am Heart Assoc. 2021;10(3):e018483. doi:10.1161/JAHA.120.018483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167(3):296–301. doi:10.1001/archinte.167.3.296 [DOI] [PubMed] [Google Scholar]
- 46. Cai H, Su N, Li W, Li X, Xiao S, Sun L. Relationship between afternoon napping and cognitive function in the ageing Chinese population. Gen Psychiatr. 2021;34(1):e100361. doi:10.1136/gpsych-2020-100361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leng Y, Wainwright NW, Cappuccio FP, et al. Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol. 2014;179(9):1115–1124. doi:10.1093/aje/kwu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dashti HS, Daghlas I, Lane JM, et al. ; 23andMe Research Team . Genetic determinants of daytime napping and effects on cardiometabolic health. Nat Commun. 2021;12(1):900. doi:10.1038/s41467-020-20585-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK Biobank participants. Eur Heart J. 2020;41(11):1182–1189. doi:10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–2230. doi:10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li P, Zheng X, Ulsa MC, et al. Poor sleep behavior burden and risk of COVID-19 mortality and hospitalization. Sleep. 2021;44:zsab138. doi:10.1093/sleep/zsab138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sepulveda E, Franco JG, Trzepacz PT, et al. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry. 2016;16:167. doi:10.1186/s12888-016-0878-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the UK Biobank after submitting an application (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The syntax for conducting the analysis is available upon request. The underlying data are open-access through application to the UK Biobank, and materials and methods will be made freely available through the UK Biobank as part of this project.