Abstract
Background
Healthy diets have been associated with better cognitive function. Socioeconomic factors including education, poverty, and job complexity may modify the relationship between diet and cognition.
Methods
We used adjusted linear mixed models to examine the association between long-term adherence to the Mediterranean-Dietary Approaches to Stop Hypertension - Intervention for Neurodegenerative Delay (MIND) diet and cognitive function over 8 years of follow-up in Puerto Rican adults residing in the Boston, MA area (aged 45–75 years at baseline). We also examined whether the MIND diet—cognition association was confounded or modified by socioeconomic measures.
Results
In both cross-sectional and longitudinal analyses the highest, versus lowest, MIND quintile was associated with better cognition function (β = 0.093; 95% CI: 0.035, 0.152; p trend = .0019), but not with cognitive trajectory over 8 years. Education <=8th grade (β = −0.339; 95% CI: 0.394, −0.286; p < .0001) and income-to-poverty ratio <120% (β = −0.049; 95% CI: −0.092, −0.007; p = .024) were significantly associated with lower cognitive function, while higher job complexity (β = 0.008; 95% CI: 0.006, 0.011; p < .0001) was associated with better cognition function. These variables acted as confounders, but not effect modifiers of the MIND-diet—cognitive function relationship.
Conclusion
Adherence to the MIND diet was associated with better cognitive function at baseline and over 8 years of follow-up; however, MIND diet was not associated with 8-year cognitive trajectory. More studies are needed to better understand whether the MIND diet is protective against long-term cognitive decline.
Keywords: Global cognitive function, MIND diet, Socioeconomic factors
It is important to identify modifiable risk factors to prevent cognitive decline. The role of dietary pattern in dementia prevention has received increased attention. Higher adherence to the Mediterranean (1,2) or the Dietary Approach to Stop Hypertension (DASH) diets (3,4) has been associated with slower cognitive decline. Recently, Morris et al. (5,6) developed a dietary pattern derived as a combination of the Mediterranean and DASH diets, specifically targeted for prevention of cognitive decline and dementia, named the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet. The MIND diet is based on natural plant foods and limited intakes of animal or high saturated fat foods. It emphasizes berries and green leafy vegetables (5–8), shown to have antioxidant and anti-inflammatory properties, with inhibition of β-amyloid deposition and neurotoxic death (6). Several prospective studies have shown slower cognitive decline among participants with high intake of green leafy vegetables (6). MIND diet components include 10 healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, and wine) and 5 unhealthy food groups (red meat, butter and stick margarine, cheese, pastries, sweets, and fried/fast food).
Mainland Puerto Ricans represent the second largest population of Latinos in the United States (9). Latinos living in the United States have approximately double the risk of AD compared to non-Hispanic whites (10), and Puerto Ricans have the highest risk among Latinos: approximately twice that of Mexican Americans (11), but risk factors for cognitive decline in this population have been understudied. One recent study showed that overall healthier diet quality was associated with better global cognition in older Hispanic/Latinos (12). However, another showed that an association between Mediterranean diet score and cognition was stronger in non-Hispanic whites and men, compared to other racial/ethnic groups and women (13). Higher intake of fruit and vegetables has been inversely associated with cognitive impairment in Mexican Americans (14). Prior work within the Boston Puerto Rican Health Study (BPRHS) has shown positive associations between fruit and vegetable intake and cognition (15), and of the Alternative Healthy Eating Index-2010 (aHEI) and 2-year memory score, among those without type 2 diabetes (2). However, we know of no study that has examined the MIND diet in a large cohort of Puerto Rican adults.
Low socioeconomic status (16) has been associated with increased risk of dementia and cognitive impairment (8,16). Individuals who are socioeconomically disadvantaged tend to have poorer dietary quality (8,17,18) and higher risk of other poor health behaviors (8,19). Low SES may impact cognition due to lower education or occupation complexity (17), and these individuals may be more vulnerable to risk to cognitive health due to poor diet. Low SES has been shown to modify associations between smoking and mortality (20), and between healthy lifestyle and cognitive decline, which was seen only in participants with lower income (8). Understanding whether SES modifies the role of diet on cognitive decline is important for targeting and developing prevention programs among populations with variability in education, job complexity, and income. We, thus, sought to examine whether adherence to the MIND diet was associated with better cognitive function over 8 years of follow-up in a cohort of Boston area Puerto Rican adults, the Boston Puerto Rican Health Study (BPHRS), and whether living in conditions of low SES exacerbates the risk to cognitive health caused by poor diet.
Method
Participants and Data Collection
This analysis was conducted within the Boston Puerto Rican Health Study (BPRHS), which included 1 502 self-identified Puerto Ricans adults in the Boston, MA metro area, aged 45–75 years at baseline (2004–2009). Participants were followed at approximately 2-year (n = 1 258) and 8-year (n = 573) visits and were administered questionnaires analogous to those at baseline (Supplementary Figure 1). As described previously, study participants were recruited through door-to-door enumeration and community approaches (21). Study protocols were approved by the Institutional Review Board at Tufts Medical Center and by the University of Massachusetts Lowell. Trained bilingual interviewers administered questionnaires to participants in their homes, and performed measurements following procedures used in the National Health and Nutrition Examination Survey (NHANES) (22) and the MacArthur Studies of Successful Aging (23). Retraining and review sessions, including checks on scoring of tests and scales, were conducted periodically. Completed interviews were self-and peer-reviewed prior to data entry. Participants provided information on age and education and were asked to self-report whether they had been diagnosed with chronic conditions. Detailed information on prescription and over-the counter medications was collected by asking participants to show medication bottles. Frequency, history and type of alcohol consumption and smoking were assessed. A physical activity score was calculated as the weighted sum of hours spent on typical 24-hour activities, assessed via a modified Paffenbarger questionnaire (24). Diabetes was defined as fasting glucose >= 126 mg/dL or use of diabetes medication. Hypertension was defined as systolic blood pressure >=140, diastolic blood pressure >=90 mm Hg or use of hypertension medication. Depressive symptomatology was estimated using the Center for Epidemiologic Studies Depression scale (CES-D). Genetic polymorphisms, including apoE, were identified with applied Biosystems TaqMan SNP genotyping systems. Dietary intake was assessed with a food frequency questionnaire (FFQ) specifically designed for and previously used in this population (25). The FFQ was based on the National Cancer Institute – Block format, revised to include appropriate foods, portion sizes and recipes for Puerto Ricans (25). Participants self-reported level of education (<=8th grade vs higher), income to poverty ratio (<120% vs higher; based on self-reported family size, total household income and concurrent poverty threshold), and self-reported job title held during most of their working life.
Study Outcome—Global Cognitive Function Score
Participants completed a battery of neuropsychological tests to assess cognitive function at baseline, 2-, and 8-year follow-up. The testing battery was designed by a Latino neuropsychologist, in Spanish, and normed/standardized using a U.S. Spanish-speaking population (26). The cognitive assessments were administered by trained native Spanish speakers in the participant’s language of choice (98% in Spanish) at the participants’ home, in consultation with a clinical neuropsychologist (TMS). The battery included the Mini-Mental State Examination (MMSE) as a measure of overall cognitive function (27); a 16-word list learning task for verbal memory; Digit Span forward and backward for attention and working memory (26); the Stroop test for processing speed, cognitive flexibility, and response to inhibition (26); clock drawing (28) and figure copying (29) for visual-spatial organization; and verbal fluency to assess verbal ability and executive function (26). Individual test scores were transformed to Z-scores and a global cognitive function composite score (GCS) was computed as the arithmetic mean of the individual Z-scores. The GCS was used as the outcome variable in this study, as done in prior work (30,31). If a participant did not complete an individual test, the given score was imputed using the minimum Z-score for the same individual test in the rest of the cohort, unless the missing values were due to illiteracy, hearing impairment or poor vision. In these cases, the existing test values for that individual were averaged. Overall, 176 (12.5%), 186 (15.6%), and 54 (10.7%) were missing at least one cognitive score at baseline, 2-, and 8-year follow-ups, respectively.
Exposure—MIND diet
We used FFQ data at baseline, 2-, and 5-year follow-ups to estimate average daily energy, nutrient, and food group intakes, and to construct the MIND diet score at each time point, following methodology described by Morris et al. (6). Nutrient intakes were calculated from the Nutrition Data System for Research software (NDS-R, Nutrition Coordinating Center, University of Minnesota, version (2007–2016). Participants reporting energy intakes < 600 or > 4800 kcal/d, or who had >= 12 questions blank on the FFQ, were excluded as implausible intakes (n = 72) baseline, (n = 21) at 2 years, and (n = 43) at 5 years.
The MIND diet score is based on 10 brain-healthy foods groups: green leafy vegetables (spinach, lettuce, kale), other vegetables, berries, nuts, whole grains, fish, beans, poultry, limited intake of wine (red and white wine), and the use of olive oil as a primary source of fat; and 5 unhealthy foods: butter and margarine, cheese, red meat, and pastries and sweets (6). Each component was assigned a score of 0, 0.5, or 1, and then summed. Total MIND scores were divided into quintiles for analysis, as done previously (6).
Study Covariates
Our cross sectional and longitudinal analyses included the following covariates, based on those commonly used in the literature: age in years, sex (male, premenopausal female or postmenopausal with estrogen use, postmenopausal without estrogen), BMI (kg/m2), physical-activity score, diabetes (y/n), hypertension (y/n), smoking (current vs no), alcohol use (current vs no), APOE e4 carrier (y/n), energy intake (kcal).
We considered several measures of SES as covariates, including income to poverty ratio, education level, and job complexity score, to examine whether these variables acted as confounders (separately or jointly) and/or effect modifiers of the MIND diet—cognition relationship. Income to poverty ratio was calculated based on total household size, total household income, and year-specific poverty cutoff (32). Education (<=8 grade vs higher) and income to poverty ratio (<120% vs higher) were treated as binary variables). A job complexity score was computed based on self-reported job titles using standardized importance scores (0–100) for complex problem-solving skills from O*NET (33), an online data source of national occupational information.
Statistical Methods
All statistical analyses were performed in SAS version 9.4. We computed descriptive statistics (means and proportions) by quintile of MIND diet adherence. In cross-sectional analyses at baseline, we excluded 96 participants due to missing data on MIND diet. Additionally, 76 participants were excluded due to missing covariate data. We assessed the association of adherence to the MIND diet, in quintiles and as a continuous exposure and global cognition cross sectionally, at baseline using general linear regression, adjusted for age, sex/estrogen status (premenopausal female, postmenopausal with no estrogen use, postmenopausal with estrogen use, or male), BMI (kg/m2), physical activity score, APOE e4 allele carrier (yes/no), diabetes status (yes/no), hypertension status (yes/no), depressive symptoms (continuous CES-D score), smoking status (current, yes/no), alcohol intake (current vs no), energy intake (kcal), job complexity score, and income-to-poverty ratio index (yes/no). Global cognitive function was treated as a continuous variable in all analyses.
We subsequently used random-slope repeated measures with time in linear mixed effect models (LMM) (SAS proc mixed), with participant as a random effect and updated exposure and covariate variables to examine the relation between MIND diet, in quintiles and as a continuous exposure, and global cognitive function at baseline, 2- and 8-year follow-up. At the 2 follow-ups, 106 and 120 participants were missing MIND diet, respectively. The covariance structure using compound symmetry for the models was chosen based on the best model fit, where AIC and BIC had the lowest values. The LMM included terms for: age (years), sex (male, premenopausal female or postmenopausal with estrogen use, postmenopausal female not using estrogen), BMI (kg/m2), physical-activity score, diabetes (yes/no), hypertension (yes/no) education level (<= 8th grade vs higher), smoking (current vs no), alcohol use (current vs no), APOE e4 carrier (yes/no), energy intake (kcal), job complexity score, and income to poverty ratio index (yes/no) and time (years). The MIND diet and all covariates were modeled as time varying covariates, based on data collected at the time of each cognitive evaluation.
To examine whether baseline MIND diet adherence impacted 8 years cognitive trajectory, we fit linear mixed models, fixing MIND diet and covariates at baseline, allowing only the cognitive outcome to vary over time, and added an interaction term between time and MIND diet to the fully-adjusted linear mixed models. In separate models, we also examined associations between level of education, income to poverty ratio, and job complexity score, and global cognitive function, both cross-sectionally and longitudinally, adjusting for covariates listed above.
We assessed effect modification of the relationship between MIND diet and global cognitive function by education, income to poverty ratio and job complexity score, by adding an interaction between each of these variables and MIND diet to the corresponding regression model and assessing the significance of the interaction term using the Likelihood Ratio Test. Finally, we investigated possible confounding of the association between MIND diet and cognitive function, in both cross-sectional and longitudinal models, by adding education, income to poverty ratio and job complexity score to the models, sequentially, and assessing the impact of adjusting for these SES variables on the beta coefficient for MIND diet. We retained model covariates that resulted in a >10% change in the effect estimates, as suggested by Rothman (34), We also noted improvement in model fit (AIC) associated with adding these covariates to the models.
We conducted several sensitivity analyses. While our primary analyses utilized the complete-case approach, we also conducted sensitivity analyses using missing indicators for all covariates and examined whether the results of this analysis were different from our primary analysis. Also, while our primary results are based on GCS with imputation, we also conducted additional sensitivity analyses restricted to participants who did not have missing data for any of the individual cognitive tests and thus did not require imputation. Furthermore, because of the substantial loss to follow-up in the BPHRS cohort over 8 years, we conducted sensitivity analyses among 470 participants with complete data on MIND diet and GCS, who participated in all 3 waves of follow-up.
Results
The mean age of study participants at baseline was 57.2 ± 7.9 years, and women comprised 70% of the sample (Table 1). The mean for the MIND diet score was 7.39 ± 1.5 SD, with a range of 3–13 out of a total possible 15 points. The prevalence of diabetes was 40%, hypertension 69%, depressive symptomatology 60%, and <= 8th grade education 47%. More than 60% of participants were below 120% of the poverty level and job complexity score tended to be low for most. Individuals with <=8th grade education, under 120 % poverty ratio or with lower employment complexity, tended to have lower MIND diet score (Table 1). In the final cross-sectional model at baseline, after adjusting for covariates, high MIND diet adherence was significantly associated with better cognitive function. The highest, versus lowest quintile difference was 0.182 Z-score units, p = .046, and β trend = 0.028; 95%CI 0.007, 0.049) across quintiles of the MIND diet; p trend = .0098; Table 2). Low education was significantly associated with worse cognitive function (β = −0.306; 95%CI −0.366, −0.246; p < .0001), as was low income to poverty ratio (β = −0.125; 95%CI −0.188, −0.062; p = .0001). Higher job complexity score was significantly associated with better cognitive function (β = 0.007; 95%CI 0.004, 0.010; p < .0001).
Table 1.
Baseline Characteristics of 1,332 BPRHS Study Participants With Global Cognitive Score at Baseline, by Quintile of MIND Diet
| MIND Diet Quintile | ||||||
|---|---|---|---|---|---|---|
| Q1 (n = 315) |
Q2 (n = 162) |
Q3 (n = 321) |
Q4 (n = 309) |
Q5 (n = 225) |
pb | |
| MIND diet score, median ± SD | 5.50 ± 0.56 | 6.50 ± 0.00 | 7.00 ± 0.25 | 8.00 ± 0.25 | 9.50 ± 0.80 | |
| Age, y, mean ± SD | 55.7 ± 7.8 | 56.6 ± 7.2 | 57.7 ± 7.7 | 58.01 ± 7.8 | 57.9 ± 6.7 | .0006 |
| Male n (%) | 99 (31.4) | 49 (30.2) | 85 (26.5) | 91 (29.4) | 48 (21.3) | .019 |
| Female, premenopausal or estrogen use n (%) | 53 (16.8) | 19 (11.7) | 40 (12.5) | 42 (13.6) | 22 (9.78) | |
| Female, postmenopausal no estrogen use n (%) | 163 (51.7) | 94 (58.0) | 196 (61.1) | 176 (56.9) | 155 (68.9) | |
| BMI, mean ± SD | 31.7 ± 7.4 | 31.6 ± 6.0 | 32.2 ± 6.0 | 32.0 ± 6.6 | 32.3 ± 6.5 | .72 |
| Physical activitya mean ± SD | 30.8 ± 4.0 | 31.5 ± 4.8 | 31.1 ± 4.6 | 31.4 ± 4.6 | 32.6 ± 4.8 | .0003 |
| Current smoking n (%) | 98 (31.1) | 47 (29.0) | 81 (25.2) | 66 (21.4) | 25 (11.1) | <.0001 |
| Current alcohol use n (%) | 209 (66.3) | 115 (70.9) | 222 (69.2) | 222 (71.8) | 166 (73.8) | .37 |
| Diabetes n (%) | 114 (36.2) | 56 (34.6) | 145 (45.2) | 122 (39.5) | 96 (42.7) | .085 |
| Hypertension n (%) | 190 (60.3) | 111 (68.5) | 238 (74.1) | 224 (72.5) | 158 (70.2) | .019 |
| <=8th grade education n (%) | 152 (48.2) | 77 (47.5) | 168 (52.3) | 143 (46.3) | 90 (40.0) | .08 |
| APOE E4 n (%) | 51 (16.2) | 28 (17.3) | 55 (17.1) | 62 (20.1) | 48 (21.3) | .27 |
| cPoverty index under 120% n (%) | 211 (73.2) | 110 (70.5) | 219 (71.3) | 208 (71.5) | 133 (61.0) | .034 |
| Job complexity score mean ± SD | 40.3 ± 9.6 | 40.7 ± 9.0 | 41.3 ± 10.1 | 41.6 ± 9.8 | 43.8 ± 11.1 | .0037 |
| Energy intakes, kcal/day mean ± SD | 2 109 ± 894 | 2 042 ± 952 | 2 090 ± 902 | 2 096 ± 860 | 2 245 ± 812 | .17 |
| CES-D >=16 n (%) | 184 (58.4) | 110 (67.9) | 203 (63.2) | 178 (57.6) | 116 (51.6) | .011 |
| Global cognitive z-score mean ± SD | −0.063 ± 0.570 | −0.129 ± 0.630 | −0.082 ± 0.556 | 0.019 ± 0.526 | 0.119± 0.521 | <.0001 |
| MIND components servings/d (mean ± SD) | ||||||
| Green leafy vegetablesd | 0.193 ± 0.217 | 0.299 ± 0.326 | 0.374 ± 0.418 | 0.524 ± 0.447 | 0.836 ± 0.589 | <0.0001 |
| Other vegetables | 0.254 ± 0.188 | 0.288 ± 0.213 | 0.352 ± 0.269 | 0.428 ± 0.324 | 0.776 ± 0.594 | <0.0001 |
| Berries | 0.025 ± 0.043 | 0.028 ± 0.040 | 0.027 ± 0.049 | 0.035 ± 0.050 | 0.116 ± 0.189 | <0.0001 |
| Nuts | 0.086 ± 0.206 | 0.113 ± 0.278 | 0.156 ± 0.347 | 0.183 ± 0.363 | 0.324 ± 0.576 | <0.0001 |
| Olive oil | 0.029 ± 0.090 | 0.055 ± 0.133 | 0.083 ± 0.347 | 0.138 ± 0.197 | 0.251 ± 0.383 | <0.0001 |
| Butter -Margarine | 0.085 ± 0.159 | 0.062 ± 0.136 | 0.085 ± 0.150 | 0.078 ± 0.137 | 0.093 ± 0.150 | 0.30 |
| Cheese | 0.408 ± 0.502 | 0.272 ± 0.308 | 0.303 ± 0.377 | 0.273 ± 0.341 | 0.285 ± 0.318 | 0.0001 |
| Whole grains | 0.642 ± 0.656 | 0.693 ± 0.758 | 0.836 ± 0.825 | 1.11 ± 0.879 | 1.635 ± 1.14 | <0.0001 |
| Fish | 0.165 ± 0.200 | 0.214 ± 0.185 | 0.266 ± 0.272 | 0.313 ± 0.284 | 0.362 ± 0.329 | <0.0001 |
| Beans | 0.257 ± 0.279 | 0.307± 0.276 | 0.334 ± 0.325 | 0.347 ± 0.304 | 0.457 ± 0.380 | <0.0001 |
| Poultry | 0.187 ± 0.190 | 0.212 ± 0.199 | 0.292 ± 0.286 | 0.389 ± 0.320 | 0.475 ± 0.351 | <0.0001 |
| Red meat | 0.513 ± 0.487 | 0.527 ± 0.549 | 0.427 ± 0.443 | 0.373 ± 0.384 | 0.345 ± 0.404 | <0.0001 |
| Fast-fried-food | 0.179 ± 0.201 | 0.163 ± 0.174 | 0.151 ± 0.200 | 0.118 ± 0.143 | 0.100 ± 0.152 | <0.0001 |
| Pastries sweets | 0.859 ± 0.940 | 0.607 ± 0.853 | 0.614 ± 0.684 | 0.592 ± 0.755 | 0.486 ± 0.550 | <0.0001 |
| Wine | 0.015 ± 0.112 | 0.012 ± 0.066 | 0.045 ± 0.271 | 0.044 ± 0.177 | 0.092 ± 0.325 | 0.0005 |
n = number of participants; Q = quintile; SD = standard deviation.
aPhysical activity score (range 24.3–62.6), weighted for total time sleeping and lying down, sitting, light, moderate, and vigorous activity.
b p value based on Chi square test for categorical or F statistics for ANOVA for continuous variables.
cPhysical activity score was calculated as the weighted sum of hours spent on typical 24-hour activities, assessed via a modified Paffenbarger questionnaire.
dSpinach, lettuce, kale.
Table 2.
Cross-sectional Association Between MIND Diet Adherence (quintiles) and Global Cognitive Score Among BPRHS Study Participants
| MIND Diet Quintile | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p trend | |
| Model 1a | Ref | −0.057 | 0.030 | 0.093 | 0.158 | <.0001 |
| n = 1 332 | (−0.148, 0.034) | (−0.045, 0.105) | (0.017, 0.169) | (0.073, 0.243) | ||
| Model 2b | Ref | −0.030 | 0.014 | 0.072 | 0.116 | .002 |
| n = 1 138 | (0.127, 0.067) | (−0.065, 0.093) | (−0.008, 0.152) | (0.027, 0.205) | ||
| Model 3c | Ref | −0.094 | −0.003 | 0.057 | 0.118 | .0005 |
| n = 1 260 | (−0.186, −0.002) | (−0.079, 0.074) | (−0.021, 0.134) | (0.032, 0.205) | ||
| Model 4d | Ref | −0.065 | −0.005 | 0.047 | 0.092 | .0098 |
| n = 1 081 | (−0.162, 0.033) | (−0.085, 0.075) | (−0.035, 0.129) | (0.002, 0.182) | ||
| MIND Diet as a Continuous Exposure | ||||||
| β | 95% CI | p | ||||
| Model 5e n = 1 081 |
0.027 | (0.008, 0.046) | .0062 | |||
Notes:
aModel 1 = adjusted for age (y), sex (male, premenopausal female or postmenopausal with estrogen use, postmenopausal without estrogen), BMI (kg/m2), physical-activity score, diabetes (y/n), hypertension (y/n) education level (<=8th grade vs higher), smoking (current vs no), alcohol use (current vs no), APOE e4 carrier (y/n), energy intake (kcal).
bModel 2= Model 1 + job complexity score
cModel 3= Model 1 + poverty index
dModel 4= Model 1 + job complexity score + poverty index
eModel 5= adjusted for Model 1 + job complexity score + poverty index n = 1 081
Similar results were observed in linear mixed models over 8 years of follow-up, after adjusting for time varying covariates. Greater adherence to the MIND diet score was significantly associated with 3 repeated measures of global cognition score over time. After adjustment for the covariates listed above, participants in the highest, versus lowest, quintile of MIND diet had significantly better cognitive function (β = 0.098; 95%CI 0.045, 0.150; p = .00030, β trend = 0.024; 95%CI 0.012, 0.037; p trend = .0002; Table 3, Model 1). As in cross-sectional analyses, <= 8th grade, versus higher, education (β = −0.339; 95%CI −0.394, −0.286; p < .0001), low versus higher income-to-poverty-ratio (β = −0.049; 95%CI −0.092, −0.007; p = .024), and higher job complexity score, (β = 0.008; 95CI% 0.006, 0.011; p < .0001) were each associated with cognitive function in linear mixed models over 8 years of follow-up.
Table 3.
Longitudinal Association, Over 8 Years of Follow-up, Between MIND Diet Adherence (quintiles) and Global Cognitive Score Among BPRHS Study Participants
| MIND Diet Quintile | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p-trend | |
| Model 1a | Ref | 0.004 (−0.05, 0.056) | 0.033 (−0.010, 0.077) | 0.053 (0.006, 0.100) | 0.098 (0.045, 0.150) | .00020 |
| Model 2b | Ref | 0.013 (−0.044, 0.071) | 0.032 (−0.016,0.079) | 0.045 (−0.006, 0.096) | 0.080 (0.024, 0.137) | .0047 |
| Model 3c | Ref | −0.013 (−0.067, 0.041) | 0.012 (−0.033, 0.057) | 0.053 (0.004, 0.102) | 0.110 (0.056, 0.165) | <.0001 |
| Model 4d | Ref | 0.005 (−0.053, 0.064) | 0.006 (−0.043, 0.055) | 0.047 (−0.006, 0.099) | 0.093 (0.035, 0.152) | .0019 |
| MIND Diet as a Continuous Exposure | ||||||
| β | 95% CI | p | ||||
| Model 5e | 0.0213 | (0.008, 0.034) | .0013 | |||
aModel 1= adjusted for age (y), sex (male, premenopausal female or postmenopausal with estrogen use, postmenopausal without estrogen), BMI (kg/m2), physical activity score, diabetes (y/n), hypertension (y/n) education level (<=8th grade vs higher), smoking (current vs no), alcohol use (current vs no), APOE e4 carrier (y/n), energy intake (kcal); AIC:2652.
bModel 2= adjusted for Model 1 + job complexity score; AIC: 2626
cModel 3= adjusted for Model 1 + poverty index; AIC: 2371
dModel 4= adjusted for Model 1 + job complexity score + poverty index; AIC: 2350 (best
model fit)
eModel 5= Model 5= adjusted for Model 1 + job complexity score + poverty index
Job complexity and income-to-poverty ratio appeared to act as confounders of the association between MIND diet and cognitive function, in both cross-sectional and longitudinal analyses, changing the effect estimate of the MIND diet-cognition association by greater than 10%. In model 4 (fully adjusted for all covariates as well as income-to-poverty-ratio, education, and job complexity), MIND diet remained significantly associated with global cognition function over 8 years, with a mean difference of 0.22 Z-score units between the highest and lowest quintiles (p = .002, β trend = 0.023; 95%CI 0.008, 0.037; p trend = .0019; Table 3). This model also had the best fit, based on AIC. We did not observe effect modification of the MIND diet on cognitive function by education, income-poverty ratio, or job complexity in either cross-sectional or longitudinal analyses (cross-sectional P interaction = 0.69, 0.95, and 0.66, respectively).
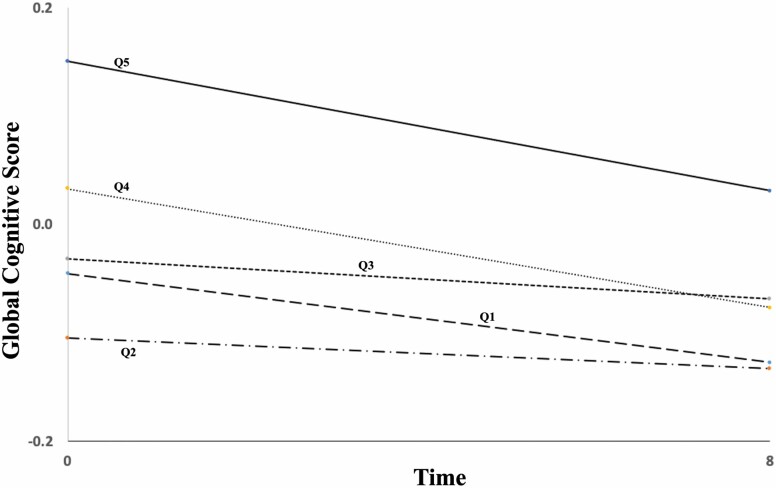
In linear mixed models examining the impact of baseline MIND diet status on 8-year cognitive trajectory, we did not observe interaction between baseline MIND diet and time. Further, we did not observe interaction between education, income to poverty ratio, or job complexity and time on cognition (p interaction = .14, .85, and .70, respectively). Thus, while higher MIND diet adherence was associated with better cognitive function throughout follow-up, baseline MIND diet did not appear to alter the 8-year trajectory in cognitive function; there was no important difference in the rate of change of cognition over time by level of MIND diet (Figure 1).
Figure 1.
Global cognitive function trajectory over 8 years of follow-up, by baseline MIND diet quintile among BPRHS participants. Global cognitive function from linear mixed models adjusted for: age (y), sex (male, premenopausal female or postmenopausal with estrogen use, postmenopausal without estrogen), BMI (kg/m2), physical activity score, diabetes (y/n), hypertension (y/n) education level (<=8th grade vs higher), smoking (current vs no), alcohol use (current vs no), APOE e4 carrier (y/n), energy intake (kcal), job complexity score, and income-to-poverty ratio index.
Results of sensitivity analyses restricted to 470 participants who completed all 3 waves of follow-up with no missing data were similar to the main results (Supplementary Table 1). Those who were lost to follow up were somewhat older, more likely to be male, to be current smokers, and were less likely to have CES-D >=16% than participants who remained for all 3 waves of follow-up. The 2 groups were similar on other characteristics (Supplementary Table 2). Likewise, restricting analyses to participants who did not have imputed cognitive scores did not change our findings (Supplementary Table 3). Finally, using the missing indicator method instead of the complete case approach to analyses, did not alter out findings, Supplementary Table 4).
Discussion
In this population of older Puerto Rican adults living in the Boston, MA metro area, we investigated the relationship of diet with cognitive function, using a specifically defined diet score, the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, based on foods and nutrients previously shown to prevent dementia. Higher adherence to the MIND diet score was significantly associated with better cognitive function, both at baseline and over 8 years of follow-up. However, baseline MIND diet score did not appear to impact the 8-year cognitive trajectory. These results are consistent with another prospective population-based cohort study, which found that greater long-term adherence to the MIND diet was associated with better verbal memory score in later life (7), but differ from findings by Morris et al., where higher MIND diet score was associated with lower decline in global cognitive score over time (6).
We further investigated whether the association of the MIND diet score with cognitive function varied across social economic factors such as education, income-to-poverty ratio index or occupation complexity score. It is well known that education status is associated with cognition. In our study, individuals with >8th grade education, with income above 120% of the poverty level or with higher job complexity, had significantly better cognition function than those with lower measures. Similarly, another study also found that those with education >= 12 y, versus lower, annual household income of B 33,333 USD, versus lower, or with high versus low demanding jobs had less cognitive decline (8). An analysis of the Framingham Offspring Cohort also showed that high job strain and low control were associated with greater declines in verbal learning and memory (16).
We did not find interaction effects between MIND diet and SES factors, as previously observed in a study where older individuals who were financially disadvantaged had the most cognitive benefit from high intake of vegetables (8). This may have been due to the limited range of SES in this cohort. Still, within the range available, individuals with <=8th grade education, under 120% of the poverty ratio, and with lower employment complexity tended to have lower MIND diet score. Other studies have suggested that the effects of SES on cognition are, in part, due to differences in health behaviors, as those socioeconomically disadvantaged tend to have poorer dietary quality (18) lower physical activity and to participate in other negative health behaviors (17,19).
The MIND diet was based on dietary components of the Mediterranean and DASH diets, with emphasis on natural plant-based foods and limited intake of animal and high saturated fat foods. It highlights consumption of berries and green leafy vegetables, but does not include overall fruit or dairy consumption, foods previously shown to be associated with dementia prevention (35). Several prospective cohort studies have found that higher intake of vegetables, particularly green leafy vegetables, was associated with slower cognitive decline (4,36,37). Several biological mechanisms may apply to the MIND diet in relation to cognition function. For example, low intake of butter and margarine, pastries, and sweets limits exposure to saturated and trans fats, which may improve blood-brain barrier function and decrease AB aggregation (35). Green leafy vegetables are excellent sources of folate, carotenoids, and flavonoids, which have been related to lower risk of dementia and cognitive decline (6). Vitamin E, which is found in vegetable oils, nuts and whole grains, has been shown to be protective of cognition (38). Berries have been demonstrated to improve memory and learning in animal models (39) and to slow cognitive decline in the Nurses’ Health Study (7,40). These dietary components appear to protect the brain through their antioxidant and anti-inflammatory properties (41) or in inhibiting β-amyloid deposition (7,41,42). Fish consumption has also been associated with lower risk of dementia (43). Fish is a rich source of long-chain n-3 fatty acids, which have been shown to reduce oxidative damage and AB formation and increase synaptic proteins and dendritic spine density (7). A recent randomized controlled trial with 37 participants suggested that a MIND diet intervention may reverse the destructive effects of obesity on cognition and brain structure (44). This evidence supports the importance of a healthy diet to prevent cognitive decline.
We did not observe an interaction effect between long-term adherence to the MIND diet and time on cognition function, suggesting that participants with more/less adherence to the MIND diet did not have different trajectories of cognitive function over the time observed. There are several possible reasons why we did not observe an association with trajectory of cognitive decline, which was observed in a prior study by Morris et al. (6). First, nondifferential misclassification in assessment of MIND diet could have biased our results toward the null. However, MIND diet was associated with overall better cognition at each time point, while not associated with cognitive trajectory in our study. Second, Morris et al. performed annual cognitive evaluations over an average of 4.7 years, while our study conducted a total of 3 cognitive assessments, at baseline, 2, and 8 years. This difference in the frequency (lower in our study), and overall duration of cognitive evaluation (longer in our study) may contribute to observed differences in results. A third, and most likely, reason for inconsistent results is the difference between the population in Morris et al. (mainly older and non-Hispanic white) versus our cohort of Puerto Ricans (mainly low SES). Future studies with more populations are needed to further elucidate the relationship between MIND diet adherence and cognitive trajectory.
Our study adds to a growing literature on risk factors for cognitive decline and dementia in Latinos. Dietary pattern has been examined in relation to cognitive decline with mixed findings. A recent study reported that overall healthier diet quality, including adequate consumption of vegetables and whole fruit, was associated with better cognitive function in middle aged and older Latinos (45). Another study showed beneficial associations between the MIND diet, as well as Alternative Healthy Eating index AHEI-2010 pattern in relation to cognitive function in Spain (46). Previous work in the BPRHS showed that adherence to the Mediterranean diet was associated with better memory function only in those without type 2 diabetes (2).
Low education and socioeconomic status are important risk factors for cognitive decline in Latinos (47) and may contribute to the increased incidence rates among Hispanics compared to non-Hispanic whites (47,48). For example, higher language proficiency and bilingualism was associated with higher performance on all cognitive indices (49) among first-generation Americans of Hispanic/Latino origin residing in the United States. Among participants living in low-income neighborhoods, greater area of neighborhood greenness was associated with even greater mental health benefits compared to residents of medium and high-income neighborhoods (50).
Strengths of our study include a comprehensive battery of culturally appropriate cognitive assessments and a detailed assessment of relevant covariates. Dietary intake was assessed with a validated FFQ designed specifically for this population and linked to a nutrient database that allowed identifying traditional Puerto Rican food components within the MIND diet score. Long-term follow-up allowed us to assess associations between the MIND diet score and cognitive function over time.
A limitation of this study is loss during follow-up. To account of this, we conducted additional sensitivity analyses among participants who remained in the cohort from baseline through year 8 of follow-up, and the results of these analyses did not differ from our main findings. Assessment of MIND diet via FFQ may be subject to recall bias. Further, MIND diet components, characterized by olive oil, nuts, whole grains, green leafy vegetables, and berries, may not reflect Puerto Rican food choices. However, we identified food components specific to traditional Puerto Rican cuisine that fit within the MIND diet categories. MIND diet scores ranged from 3 to 13, in our cohort, providing evidence of sufficient variability in scores, to allow us to successfully examine this exposure in the BPRHS. Our cross-sectional analyses at baseline are subject to potential reverse causation and other biases implicit in cross-sectional design. However, those analyses were supplemented by the longitudinal component, with similar findings. Finally, measurement error in dietary assessment cannot be ruled out, despite the use of a validated FFQ. This cohort study includes Puerto Rican older adults, with lower education than that of the general population, limiting generalizability to other ethnicities and socioeconomic backgrounds.
In conclusion, adherence to the MIND diet was associated with better cognitive function both at baseline and over 8 years of follow-up but did not alter the 8-year cognitive trajectory in older Puerto Rican adults in Massachusetts. Socioeconomic factors, including education, occupation complexity score, and poverty ratio were also associated with cognition. Although individually important, SES factors did not modify the diet-cognition association. These findings have public health implications. Programs to encourage healthy dietary habits may be cost-effective in targeted disadvantaged groups and may help to reduce dementia and other comorbidities. Additional prospective and interventional studies are needed to better understand whether the effects of high adherence to MIND diet varies across social economic factors such as education, poverty ratio index, and occupation complexity score on cognition.
Supplementary Material
Acknowledgments
Author Contribution: T.B. conducted the statistical analysis, wrote and revised the manuscript; T.M.S. led cognitive data acquisition and revised the manuscript; J.-S.L. assisted with statistical analysis and revised the manuscript; X.Z. assisted with statistical analysis and revised the manuscript; K.L.T. is the PI of the BPRHS study, led the data collection, contributed to study conceptualization, and revised the manuscript; N.P. led study conceptualization, assisted with statistical analysis and revised the manuscript.
Funding
This work was supported by the National Institutes of Health (P01 AG023394, P50 HL105185, and R01 AG055948).
Conflict of Interest
None declared.
References
- 1. van de Rest O, Berendsen AAM, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Advances in Nutrition. 2015;6:154–168. doi:10.3945/an.114.007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattei J, Bigornia SJ, Sotos-Prieto M, Scott T, Gao X, Tucker KL. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care. 2019;42(8):1372–1379. doi:10.2337/dc19-0130. PMID: 31123154; PMCID: PMC6647047. [DOI] [PMC free article] [PubMed]
- 3. Smith PJ, Blumenthal JA, Babyak MA, et al. . Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331–1338. doi:10.1161/HYPERTENSIONAHA.109.146795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wengreen H, Munger RG, Cutler A, et al. . Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the cache county study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263–1271. doi:10.3945/ajcn.112.051276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. doi:10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimer’s & Dementia 2015;11:1015–1022. doi:10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berendsen AM, Kang JH, Feskens EJM, de Groot C, Grodstein F, van de Rest O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. 2018;22:222–229. doi:10.1016/j.jamda.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 8. Weng PH, Chen JH, Chiou JM, et al. . The effect of lifestyle on late-life cognitive change under different socioeconomic status. PLoS One. 2018;13(6):e0197676. doi:10.1371/journal.pone.0197676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrión-Baralt JR, Suárez-Pérez E, del Rio R, Moore K, Silverman. Prevalence of dementia in Puerto Rican veterans is higher than in mainland US veterans. J Am Geriatr Soc. 2010;58:798–799. doi:10.1111/j.1532-5415.2010.02789.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang MX, Stern Y, Marder K, et al. . The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi:10.1001/jama.279.10.751 [DOI] [PubMed] [Google Scholar]
- 11. González HM, Tarraf W, Gouskova N, et al. . Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30(1):68–77. doi:10.1093/arclin/acu066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Estrella ML, Durazo-Arvizu RA, Mattei J, et al. . Alternate healthy eating index is positively associated with cognitive function among middle-aged and Older Hispanics/Latinos in the HCHS/SOL. J Nutr. 2020;150(6):1478–1487. doi:10.1093/jn/nxaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu Y, Guo J, Moshfegh AJ. Race/ethnicity and gender modify the association between diet and cognition in U.S. older adults: National Health and Nutrition Examination Survey 2011-2014. Alzheimers Dement (N Y). 2021;7(1):e12128. doi:10.1002/trc2.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Fisher-Hoch SP, Reininger BM, McCormick JB. Association between fruit and vegetable intake and symptoms of mental health conditions in Mexican Americans. Health Psychol. 2018;37(11):1059–1066. doi:10.1037/hea0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye X, Bhupathiraju SN, Tucker KL. Variety in fruit and vegetable intake and cognitive function in middle-aged and older Puerto Rican adults. Br J Nutr. 2013;109(3):503–510. doi:10.1017/S0007114512001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agbenyikey W, Karasek R, Cifuentes M, et al. . Job strain and cognitive decline: a prospective study of the Framingham offspring cohort. Int J Occup Environ Med. 2015;6(2):79–94. doi:10.15171/ijoem.2015.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapko D, McCormack R, Black C, Staff R, Murray A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia - a systematic literature review. Aging Ment Health. 2018;22(8):915–926. doi:10.1080/13607863.2017.1348471. PMID: 28703027. [DOI] [PubMed]
- 18. Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. . Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi:10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 19. Galobardes B, Costanza MC, Bernstein MS, Delhumeau C, Morabia A. Trends in risk factors for lifestyle-related diseases by socioeconomic position in Geneva, Switzerland, 1993-2000: health inequalities persist. Am J Pub Health. 2003;93:1302–1309. doi:10.2105/ajph.93.8.1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pampel FC, Rogers RG. Socioeconomic status, smoking, and health: a test of competing theories of cumulative advantage. J Health Soc Behav. 2004;45(3):306–321. doi:10.1177/002214650404500305 [DOI] [PubMed] [Google Scholar]
- 21. Tucker KL, Mattei J, Noel SE, et al. . The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chumlea WC, Guo SS, Wholihan K, Cockram D, Kuczmarski RJ, Johnson CL. Stature prediction equations for elderly non-Hispanic white, non-Hispanic black, and Mexican-American persons developed from NHANES III data. J Am Diet Assoc. 1998;98(2):137–142. doi:10.1016/S0002-8223(98)00036-4 [DOI] [PubMed] [Google Scholar]
- 23. Seeman TE, Charpentier PA, Berkman LF, et al. . Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49(3):M97–108. doi:10.1093/geronj/49.3.m97 [DOI] [PubMed] [Google Scholar]
- 24. Washburn RA, Smith KW, Goldfield SR, McKinlay JB. Reliability and physiologic correlates of the Harvard Alumni Activity Survey in a general population. J Clin Epidemiol. 1991;44(12):1319–1326. doi:10.1016/0895-4356(91)90093-o [DOI] [PubMed] [Google Scholar]
- 25. Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148(5):507–518. doi:10.1093/oxfordjournals.aje.a009676 [DOI] [PubMed] [Google Scholar]
- 26. Artiola I.Fortuny L, Romo DH, Heaton RK, Pardee Iii RE.. Manual de Normas y Procedimientos Para la Bateria Neuropsicologia. Psychology Press; 2000. doi:10.1207/S15324826AN0803_10 [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 28. Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc. 1989;37(8):730–734. doi:10.1111/j.1532-5415.1989.tb02234.x [DOI] [PubMed] [Google Scholar]
- 29. Beery K. The Development Test of Visual-Motor Integration Manual, revised. Cleveland: Modern Curriculum Press; 1989. doi:10.1007/springerreference_184223 [Google Scholar]
- 30. Mattei J, Bigornia SJ, Sotos-Prieto M, Scott T, Gao X, Tucker KL. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care. 2019;42(8):1372–1379. doi:10.2337/dc19-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palacios N, Scott T, Sahasrabudhe N, Gao X, Tucker KL. Lower plasma vitamin B-6 is associated with 2-year cognitive decline in the Boston Puerto Rican Health Study. J Nutr. 2019;149(4):635–641. doi:10.1093/jn/nxy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://aspe.hhs.gov/prior-hhs-poverty-guidelines-and-federal-register-references Office of the assistant secretary for planning and evaluation.
- 33. The U.S. Department of Labor, Employment & Training Administration, Development. adbtNCfON. O*Net 2021.
- 34. Rothman KJ. Epidemiology: An Introduction. Oxford University Press; 2012. doi:10.1093/occmed/kqh066 [Google Scholar]
- 35. Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol Aging. 2014;35Suppl 2:S59–S64. doi:10.1016/j.neurobiolaging.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–6. doi:10.1016/j.neurobiolaging.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen X, Huang Y, Cheng HG. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: a 3-year cohort study. J Nutr Health Aging. 2012;16:549–552. doi:10.1007/s12603-012-0023-2 [DOI] [PubMed] [Google Scholar]
- 38. Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71(1):1–13. doi:10.1017/S0029665111003296 [DOI] [PubMed] [Google Scholar]
- 39. Willis LM, Shukitt-Hale B, Joseph JA. Recent advances in berry supplementation and age-related cognitive decline. Curr Opin Clin Nutr Metab Care. 2009;12(1):91–94. doi:10.1097/MCO.0b013e32831b9c6e [DOI] [PubMed] [Google Scholar]
- 40. Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135–143. doi:10.1002/ana.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17(8):816–822. doi:10.1096/fj.02-0877com [DOI] [PubMed] [Google Scholar]
- 42. Chan A, Shea TB. Folate deprivation increases presenilin expression, gamma-secretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. J Neurochem. 2007;102(3):753–760. doi:10.1111/j.1471-4159.2007.04589.x [DOI] [PubMed] [Google Scholar]
- 43. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62(12):1849–1853. doi:10.1001/archneur.62.12.noc50161 [DOI] [PubMed] [Google Scholar]
- 44. Arjmand G, Abbas-Zadeh M, fardaei M, Tabatabaee SH, Eftekhari MH. Effect of MIND diet intervention on cognitive performance and brain structure in healthy obese women: a randomized controlled trial. Preprint. bioRxiv. doi:10.1101/2020.04.28.065813 [DOI] [PMC free article] [PubMed]
- 45. Hogervorst E. Vegetable, fruit, and low to moderate alcohol intakes are associated with better cognition in middle-aged and older hispanics/latinos. J Nutr. 2020;150(6):1352–1353. doi:10.1093/jn/nxaa110 [DOI] [PubMed] [Google Scholar]
- 46. Munoz-Garcia MI, Toledo E, Razquin C, et al. . “A priori” dietary patterns and cognitive function in the SUN project. Neuroepidemiology. 2020;54(1):45–57. doi:10.1159/000502608 [DOI] [PubMed] [Google Scholar]
- 47. Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72–83. doi:10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- 48. Díaz-Venegas C, Downer B, Langa KM, Wong R. Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry. 2016;31(9):1004–1012. doi:10.1002/gps.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamar M, León A, Romo K, et al. . The independent and interactive associations of bilingualism and sex on cognitive performance in hispanics/latinos of the hispanic community health study/study of latinos. J Alzheimers Dis. 2019;71(4):1271–1283. doi: 10.3233/JAD-190019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown SC, Perrino T, Lombard J, et al. . Health disparities in the relationship of neighborhood greenness to mental health outcomes in 249,405 U.S. Medicare beneficiaries. Int J Environ Res Public Health. 2018;15(3):430. doi:10.3390/ijerph15030430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.