Abstract
Background
To investigate changes in gait performance over time and how these changes are associated with the decline in structural network efficiency and cognition in older patients with cerebral small vessel disease (SVD).
Methods
In a prospective, single-center cohort with 217 older participants with SVD, we performed 1.5T MRI scans, cognitive tests, and gait assessments evaluated by Timed UP and Go (TUG) test twice over 4 years. We reconstructed the white matter network for each subject based on diffusion tensor imaging tractography, followed by graph-theoretical analyses to compute the global efficiency. Conventional MRI markers for SVD, that is, white matter hyperintensity (WMH) volume, number of lacunes, and microbleeds, were assessed.
Results
Baseline global efficiency was not related to changes in gait performance, while decline in global efficiency over time was significantly associated with gait decline (ie, increase in TUG time), independent of conventional MRI markers for SVD. Neither baseline cognitive performance nor cognitive decline was associated with gait decline.
Conclusions
We found that disruption of the white matter structural network was associated with gait decline over time, while the effect of cognitive decline was not. This suggests that structural network disruption has an important role in explaining the pathophysiology of gait decline in older patients with SVD, independent of cognitive decline.
Keywords: Cognition, Gait, Network efficiency, Small vessel disease
Cerebral small vessel disease (SVD) is prevalent in the older population and is associated with cognitive decline and gait impairment (1). Gait impairment is clinically important, as it predicts adverse clinical events, including falls, functional dependence, and death (2–4). Despite its importance in these clinical outcomes, the underlying causes of gait impairment in SVD patients are incompletely understood.
A potential mechanism is that SVD affects the microstructural integrity of white matter tracts, and therefore disrupting structural network efficiency, required for optimal information processing and integration in motor tasks (1,5,6). Diffusion tensor imaging (DTI) followed by tractography can be applied to construct structural networks (6,7). Using this approach, some studies have shown that lower structural network efficiency is related to worse gait performance in patients with SVD (8,9). Also, structural network disruption is associated with reduced cognitive performance and decline (5,10). The strong relation between cognitive performance and gait may be another explanation for gait impairment in patients with SVD (11–13). We previously demonstrated that the relation between structural network efficiency and gait performance was indeed mediated by cognitive functioning in patients with sporadic SVD at cross-sectional level (14). However, it is still not clear whether structural network efficiency can predict gait decline, and whether this relation is mediated by cognitive decline over time. Longitudinal data are needed to provide stronger support for the causal mechanisms of cognition and structural network efficiency in explaining gait decline in SVD.
We, therefore, investigated in this study whether baseline structural network efficiency and cognitive performance predicts gait decline. In addition, we examined whether decline in structural network connectivity is associated with gait worsening in patients with SVD over 4 years and whether this is mediated by cognitive decline. Based on our cross-sectional findings, we hypothesized that change in cognition over time would mediate the relation between change in structural network efficiency and gait decline in patients with SVD.
Materials and Methods
Study Population
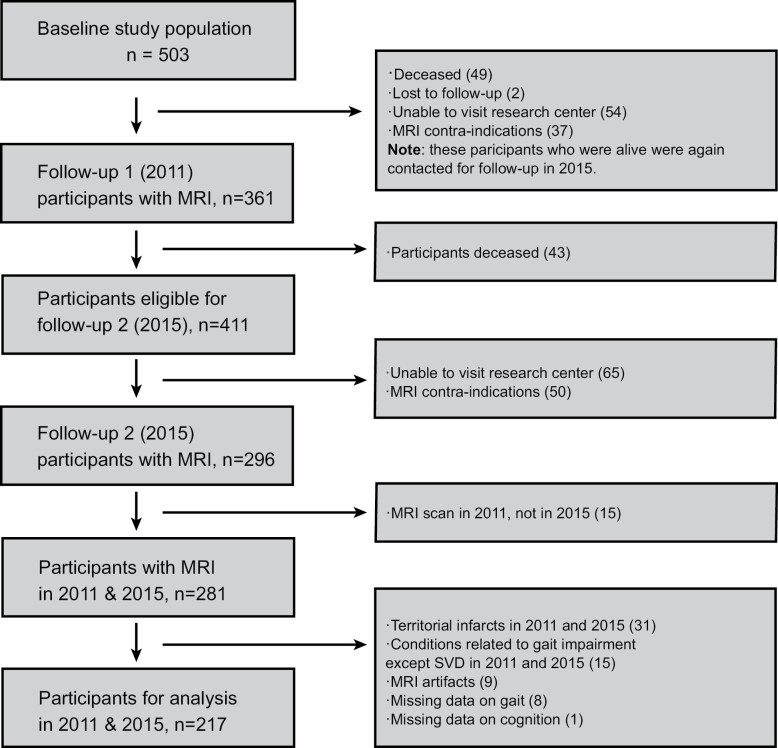
This study is a part of the Radboud University Nijmegen Diffusion Tensor and Magnetic resonance Cohort (RUN DMC) study, an ongoing longitudinal prospective single-center study that aims to investigate the risk factors and clinical consequences of sporadic SVD among the older. A detailed description of the patient recruitment and study rationale of the RUN DMC study has been described in the study protocol (15). Baseline data collection was performed in 2006, with 3 follow-ups (2011, 2015, and 2020). Due to the MRI upgrade between 2006 and the first follow-up (2011), we only included participants with data available from 2011 and 2015, who were scanned with identical MR scanners and protocols. Of the 503 baseline patients in 2006, 281 underwent repeated MRI assessment in 2011 and 2015. Additional 64 patients were excluded due to: (1) territorial infarcts present on imaging in 2011 and 2015, as these infarcts are considered potential confounders for gait performance (n = 31); (2) gait-related disorders other than SVD (eg, polyneuropathy, arthrosis in lower extremities, musculoskeletal constraints, lumbar disc herniation, parkinsonism) (n = 15); (3) MRI artifacts (n = 9); (4) missing data on gait (n = 8); (5) missing data on cognition (n = 1). This brought the final sample size for analysis in our study to 217 (Figure 1).
Figure 1.
Flow chart of the study population. Given the scanner upgrade between 2006 and 2011, we only included the study population from 2011 and 2015.
In this study, we will refer to the 2011 assessment as “baseline” and the 2015 assessment as “follow-up.” The Medical Review Ethics Committee Region Arnhem–Nijmegen approved the study and all participants gave written informed consent.
Cardiovascular Risk Factors
We assessed the presence of hypertension, smoking, diabetes mellitus, and hypercholesterolemia by using standardized assessments and structured questionnaires (15).
Gait Assessment
Gait performance was assessed using the Timed Up and Go (TUG) test (16). The mean time (seconds) and the number of steps from the 3 repetitive TUG tests were used for analyses. Gait impairment was defined as TUG time > 12 seconds (17).
Neuropsychological Assessments
For each participant, we calculated an z-score for cognitive index as a measure of global cognitive functioning. Briefly, this was calculated as the mean of the z-scores of the Speed-Accuracy Trade-Off (SAT) score, the mean of the 1-letter subtask of the Paper-Pencil Memory Scanning Task (PPMST) (18), the mean of the Symbol-Digit Substitution Task (SDST) (19), the mean of the SAT score of the reading task of the Stroop test (19), the mean of the added score on the 3 learning trials, and the mean of the delayed recall of the Rey Auditory Verbal Learning Test (RAVLT) (20). We also constructed z-scores specifically for executive function and psychomotor speed, since these two cognitive domains are primarily affected in SVD and were found to have stronger associations with gait than other cognitive domains (14).
Executive function was evaluated as the mean of the z-scores of the verbal fluency task, the Verbal Series Attention Test, and the interference score of the Stroop Test, while the interference score of the Stroop Test was calculated by dividing the SAT scores of color-word task by the mean of the reading and color naming tasks of the Stroop Test. Psychomotor speed was calculated as the mean of the z-scores of the SAT score of the 1-letter subtask of the PPMST, the mean of the SAT score of the reading subtask of the Stroop test, and the mean of the SDST. To account for possible material-specific practice effects, parallel versions of the RAVLT, RCFT and verbal fluency test were used for the follow-up assessment in 2015.
Performance across tests are made comparable by transforming the raw test scores into z-scores (individual test score minus mean test score, divided by the standard deviation). Z-scores of individuals in 2011 and 2015 were calculated using the mean and SD of the baseline (2011) study population. Higher z-scores indicate better cognitive performance. Changes in cognition over time were calculated for each participant individually, by subtracting scores in 2011 from the follow-up scores in 2015.
MRI Protocol
MR images were acquired on a single 1.5 Tesla scanner (Siemens, Magnetom Avanto). The protocol consisted of the following whole-brain scans: T1-weighted 3D Magnetization Prepared RApid Gradient Echo (MPRAGE) sequence (TR/TE/TI: 2250/2.95/850 ms, isotropic voxel size: 1.0 mm3), Fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI: 14240/89/2200 ms, voxel size: 1.2 × 1.0 × 2.5 mm, interslice gap 0.5 mm), T2*-weighted gradient echo sequence (voxel size: 1.3 × 1.0 × 5.0 mm; interslice gap 1.0 mm), and a DTI sequence (TR/TE: 10200/95, isotropic voxel size: 2.5 mm3), 7 unweighted scans, 61 diffusion-weighted scans at b = 900 s/mm2. Full acquisition details have been described previously (21).
Conventional MRI Markers of SVD
The rating of SVD-markers was based on the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria (22). WMH was segmented semiautomatically using FLAIR and T1 sequences as described previously (23). All segmentations were visually inspected for segmentation errors by 1 trained rater, who was blinded for clinical data. WMH volumes were normalized to intracranial volume. Lacunes were manually rated on T1-weighted and FLAIR images, and microbleeds on the T2*-weighted images. These markers (WMH, lacune, microbleeds) were rated by 2 trained raters, followed by a consensus meeting. Inter and intra-rater reliability were excellent (24). Gray matter volume (GMV) and white matter volume (WMV) were calculated employing Statistical Parametric Mapping 12 (SPM, https://www.fil.ion.ucl.ac.uk/spm). SPM12 unified segmentation on T1 sequences and was computed by summing all voxels belonging to tissue class multiplied by voxel volume (mL). Total brain volume was calculated by summing GMV and WMV.
DTI Analysis
Local principal component analysis filter was used to denoise the raw diffusion weighted data (25). We corrected for cardiac and head motion as well as eddy currents by using the PATCH (Patching ArTefacts from Cardiac and Head motion) algorithm as reported previously (26). This well-established method was shown to be robust and sensitive to detect and correct the most frequently occurring DWI artifacts with excellent performance. Susceptibility distortions were unwrapped by normalizing the images to the T1 images in the phase-encoding direction via SPM12. We then used FMRIB Software Library (FSL) to extract brain tissue and calculate the diffusion tensor (27) and an in-house software to conduct whole-brain deterministic tractography by seeding from a 0.5 mm3 grid, with streamlines terminated when the angle between principal eigenvectors > 40° or fractional anisotropy (FA) < 0.2 (5).
Structural Network Construction
We parcellated each brain into 45 regions per hemisphere using the Automatic Anatomical Label (AAL) template (28). Cerebellar regions were excluded since the tractography technique employed in this study is unsuitable for tracing cerebellar connections. T1-weighted images were linearly registered to the b0-image by FMRIB’s Linear Image Registration Tool (FLIRT, part of FSL) (29) and nonlinearly registered to the Montreal Neurological Institute (MNI) 152 template using Advanced Normalization Tools (ANTs) (30). These transformations were combined to register the AAL template to each subject’s diffusion space.
Connectivity weights were ascribed to edges to capture information about connection strength. Edge weights () were computed for each edge based on the lengths (in mm), l of the set of N streamlines with endpoints terminating in each pair of nodes i and j, , modified from Hagmann et al. (31). This includes a scaling factor to correct for the number of seeds per millimeter.
This weighting technique has the benefit of simple interpretation, that is, represents the seeding-corrected number of unique streamlines passing between i and j. Edges were thresholded at to minimize noise-related false positives (10). This resulted in a weighted 90×90 connectivity matrix for each individual.
Network Measures
We used the brain connectivity toolbox (http://www.brain-connectivity-toolbox.net) to compute graph-theoretical measures. Efficiency between two regions (nodes) is defined as the inverse length of the shortest path between them, reflecting the ease with which pairs of regions communicate. Global efficiency reflects integration over the entire network and is estimated by averaging efficiency for all node pairs (32).
Statistical Analyses
Clinical and imaging characteristics of participants are presented as mean ± SD for normally distributed data, median, and interquartile ranges (IQR) for the skewed distributed parameters. Normalized WMH volume was log-transformed to obtain normal distribution. Changes in gait, cognition, and neuroimaging characteristics were compared by using a paired t-test, Wilcoxon signed-rank test, or McNemar test when appropriate.
To examine the longitudinal relation between structural brain metrics (ie, total brain volume and WMH volume) and change in global efficiency, we used multiple linear regression, adjusted age, sex, follow-up duration (time between baseline and follow-up assessment), and baseline global efficiency.
First, we performed multivariable linear regression to investigate whether baseline global efficiency and cognitive index were associated with changes in gait, respectively. Second, we performed multivariable linear regression to investigate the relation between change in global efficiency and change in gait. Third, we examined whether change in cognition predicted change in gait also via multivariable linear regression. Adjustments were made for the following potential confounders: age, gender, height, follow-up duration, baseline TUG test variables (to account for baseline gait performance) (Model 1), and additionally with baseline conventional SVD markers, that is, WMH, lacunes, and microbleeds (Model 2).
We calculated the variance inflation factor (VIF) in multiple linear regression models to evaluate the degree of multicollinearity. VIF was low (< 3) in all multiple regression models. Given VIF >5 is considered as high multicollinearity, we, therefore, concluded that the multicollinearity was not present (33).
To ensure that the regression results were robust and not driven by outliers, they were identified with the Bonferroni outlier test (“outlierTest” from “car” package in R) and excluded from regression analyses (10 subjects were found) (34). The Bonferroni outlier tests use a t distribution to examine whether the model’s largest studentized residual value’s outlier status is statistically different from the other observations in the model.
Two-tailed p-values <.05 were considered statistically significant. Correction for multiple testing was performed using the Bonferroni method. All statistical analyses were carried out in R, version 3.5.1 (https://www.rproject.org/).
Results
Progression of Gait, Cognition, and Neuroimaging Measures
Baseline characteristics for the study population (n = 217) were shown in Table 1. The mean age of total population was 66.9 years (SD 7.4); 56.6% were men. Both TUG time and the number of steps significantly increased over time. The number of participants with impaired gait significantly increased at follow-up (12.4%) compared to the baseline (4.6%). Cognitive index and psychomotor speed significantly decreased at follow-up compared with baseline, while executive function did not change. We found a nonsignificant decrease in global efficiency during the follow-up, while there was a significant increase in WMH volume, presence of lacunes, and a significant decrease of total brain volume over time (Table 2). Of note, change in WMH volume and brain volume did not predict change in network efficiency (Supplementary Table 1).
Table 1.
Baseline (2011) Characteristics of the Study Population (n = 217)
| Demographics | Baseline |
|---|---|
| Age, years (mean [SD]) | 66.90 (7.4) |
| Sex, male (%) | 123 (56.6) |
| Education, years (mean [SD]) | 5.11 (1.19) |
| Vascular risk factors | |
| Hypertension, n (%) | 163 (75.1) |
| Diabetes, n (%) | 20 (9.2) |
| Hypercholesterolemia, n (%) | 92 (42.4) |
| Smoking ever, n (%) | 35 (16.1) |
| BMI, kg/m2 (mean [SD]) | 27.5 (4.1) |
Notes: Data represent number of participants (%), mean ± SD. BMI = body mass index.
Table 2.
Comparison of Gait, Cognitive, and Neuroimaging Measures at Baseline (2011) and Follow-up (n = 217)
| Gait and Cognitive Measures | Baseline (2011) | Follow-up (2015) | p-value |
|---|---|---|---|
| TUG time, seconds (mean [SD]) | 9.14 (1.60) | 10.07 (1.76) | <.001 |
| TUG steps, n (mean [SD]) | 12.42 (1.70) | 13.66 (2.09) | <.001 |
| Gait impairment, n (%) | 10 (4.6) | 27 (12.4) | <.001 |
| Cognitive index (mean [SD]) | 0.28 (0.65) | 0.23 (0.68) | .015 |
| Executive function (mean [SD]) | 0.24 (0.70) | 0.22 (0.69) | .445 |
| Psychomotor speed (mean [SD]) | 0.32 (0.74) | 0.27 (0.76) | .022 |
| Neuroimaging measures | |||
| Global efficiency (mean [SD]) | 10.68 (2.26) | 10.60 (2.39) | .166 |
| WMH, mL (median [IQR]) | 2.50 [1.14, 6.53] | 3.85 [1.76, 9.20] | <.001 |
| Lacunes presence, n (%) | 26 (12.0) | 48 (22.1) | <.001 |
| Microbleeds presence, n (%) | 38 (17.5) | 48 (23.0) | .067 |
| TBV, mL (mean [SD]) | 1 096.38 (123.09) | 1 074.54 (125.78) | <.001 |
Notes: Data represent number of participants (%), mean ± SD or median (IQR). TBV = total brain volume; WMH = white matter hyperintensity.
Association Between Global Efficiency and Gait Decline
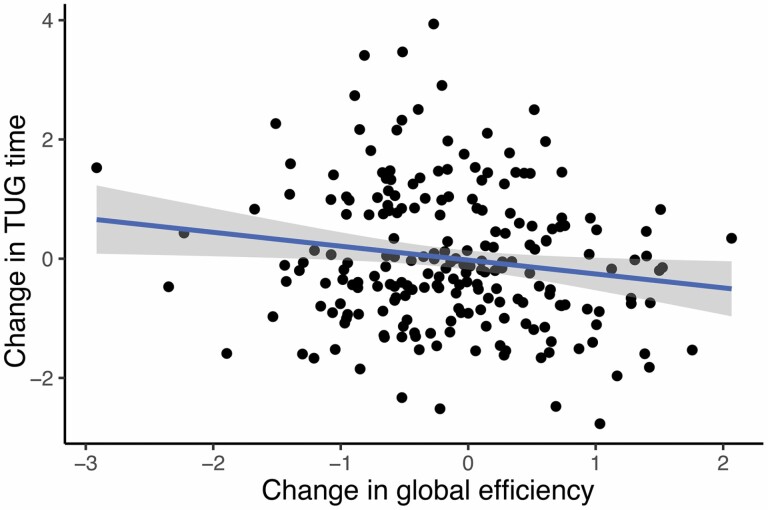
Baseline global efficiency did not predict changes in gait over time as operationalized by TUG time and step numbers, adjusted for the potential confounders. A decline in global efficiency over time was associated with longitudinal changes in TUG time (gait worsening), independent of the potential confounders, but not with change in the number of steps (Figure 2, Table 3). Change in global efficiency explained 3% of variance in TUG time change, on top of SVD markers (Supplementary Table 2). In the post-hoc analysis, we applied fixed network density thresholded at 5%–10%, the results remained similar (data not shown).
Figure 2.
Longitudinal associations between global efficiency and TUG time (β= -0.22; p-value=0.017). Values on x- and y-axis denote the change between baseline and follow-up in global efficiency and TUG time respectively. Beta and p-values were obtained from the linear regression model adjusted for age, sex, height, follow-up duration, baseline TUG time, number of lacunes and microbleeds, white matter hyperintensity volume and total brain volume.
Table 3.
Associations Between Global Efficiency and Change in Timed Up and Go Test Parameters
| Change in TUG Parameters | Δ TUG Time | Δ TUG Step | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| Baseline GE | β [95% CI] | 0.10 [−0.05, 0.24] | 0.28 [0.09, 0.46] | −0.05 [−0.21, 0.10] | 0.05 [−0.15, 0.25] |
| p-value | .201 | .302 | .504 | .606 | |
| ΔGE | β [95% CI] | −0.15 [−0.28, −0.02] | −0.15 [−0.29, −0.02] | −0.06 [−0.20, 0.08] | −0.07 [−0.21, 0.07] |
| p-value | .026 | .021 | .405 | .348 |
Notes: GE = global efficiency; Data present standardized estimates [95% confidence interval] with corresponding p-values after Bonferroni correction for multiple comparisons (ie, two).
Model 1: adjustment for age, sex, height, follow-up duration, baseline TUG test parameters (i.e., time or number of steps).
Model 2: additional adjustment for number of lacunes and microbleeds, white matter hyperintensity volume and total brain volume.
Association Between Cognition and Gait Decline
We found that baseline cognitive index was not associated with change in TUG time and number of steps, adjusted for confounding variables. Furthermore, decline in cognitive index as well as in executive function and psychomotor speed was not associated with change in TUG time and number of steps (Supplementary Table 3).
The lack of the association between decline in global efficiency (predictor) and decline in cognitive function (mediator) prevented us from investigating a possible mediating effect of cognitive decline in the relation between change in global efficiency and gait decline.
Discussion
In this prospective study, we showed that decline in structural network efficiency independently predicted decline in gait performance over a 4-year follow-up in older individuals with SVD. These findings suggest that structural network disruption may have an important role in the pathophysiology of gait decline over time, whereas we did not find an effect of baseline cognitive performance and decline on gait decline.
Our findings showing associations between decline in global efficiency and decline in gait performance complement previous cross-sectional studies (14,35), and they further corroborate the hypothesis that gait decline may be attributable to the disruption of the structural network over time. A potential explanation could be that the cumulative effect of the spatially distributed SVD lesions (ie, SVD MRI markers) and loss of microstructural integrity in the normal-appearing white matter (34,35,36) predispose the brain to the disruption of white matter structural network. Given structural network efficiency was an integrated and sensitive marker to capture MRI visible and invisible lesions/pathologies and gait performance is highly dependent on widespread inter-connected cerebral networks (1,37), disrupted structural network could contribute to gait worsening over time. Besides, we found that volumetric changes, that is, WMH and total brain volume, did not predict change in network efficiency, thereby providing stronger evidence that network efficiency is related to gait longitudinally, independent of volumetric changes. Furthermore, other studies in older population, although cross-sectionally, demonstrated that network efficiency was associated with sensorimotor performance (9,38,39). Taken together, these findings validate the role of network efficiency in motor performance.
Our previous cross-sectional study showed that the relation between network efficiency and gait was mediated by cognition (14). Of note, cognition only has a partial mediation role, suggesting that network efficiency has the effect on gait performance not through cognitive decline. Thus, other unobserved/uninvestigated mechanisms may be at play as well. This was further confirmed by the fact that the explained variance of change in network metrics for gait decline is limited (3%). This finding, from another perspective, provides supportive information that other noncognitive contributors may be involved. For instance, age-related brain comorbidities and decline in sensory input is likely to lead to gait decline with advancing age (12,13).
It should be noted that the mediation role of cognitive decline was not found longitudinally. A recent study showed that greater decline in network global efficiency predicted global cognitive decline in SVD population (10), which we did not observe in our study. The lack of association between decline in global efficiency and decline in cognitive function may be explained by the fact that there are potential contributors to cognitive decline in SVD patients that cannot be captured by the change of global efficiency based on diffusion tensor tractography. These contributors could include, for example, disruption of microstructural integrity at strategic location of the white matter, cortical atrophy, decline in functional connectivity, and contribution of neurodegenerative pathologies in SVD patients (37).
In this study, we did not find longitudinal associations between cognitive decline and gait decline, although both gait and cognitive performance worsened over time, suggesting these two are not causally related. Our longitudinal findings are in contrast to other studies that found cognitive decline to predict gait decline over time in an older community-dwelling population (40,41). However, our study was different from theirs in terms of study design, approaches to assess gait parameters and cognition, and in study population (ie, their high-functioning older individuals and non-SVD based compared to our SVD-specific patients). There are several explanations for the lack of a longitudinal association in our study. First, cognition and gait might have different rates of decline over time, therefore their progression might vary considerably across individuals over time (6,42). Second, the presence of brain pathologies (eg, SVD and neurodegeneration) may have a differential impact on gait problems and cognitive deficits. Since the involved brain structures may have different contributions to gait and cognition, changes in cognition may not be related to changes in gait in a time-dependent fashion (43,44). Third, the effect of cognitive functioning on gait performance becomes more prominent in the presence of age-related subclinical comorbidities (ie, sarcopenia and joint problems) with a strategy of cognitive compensation (14). One can speculate that this compensatory effect on gait performance might decrease with more severe cognitive decline and dementia (45). This could explain the reasons that additional cognitive decline is not related to further gait decline.
In our previous cross-sectional study, we found that cognition has a mediation effect between network efficiency and gait performance in SVD cross-sectionally (14). This explanatory mediation framework could help put into perspective the inter-connected relation between structural network efficiency, cognition, and gait performance. However, the lack of association between cognitive decline and gait decline indicates that temporal changes of cognitive function cannot explain the relation between network efficiency and gait decline over time. Therefore, more attention is warranted when explaining the relation between network efficiency, cognition, and gait.
Strengths and Limitations
Major strengths of the study were the large cohort of participants covering a wide range of SVD spectrum. Participants with gait problems caused by other diseases than SVD were excluded. SVD was rated according to standardized procedures to minimize the risk of misclassification. Furthermore, our longitudinal neuroimaging data were consistent, since they were acquired from the same scanner without upgrade or change over the whole follow-up period.
Methodological limitations should be considered regarding structural networks derived from deterministic diffusion tensor tractography, although it has been shown that the reconstruction approach and structural network measures were reliable and reproducible (46). We have acquired relatively low-resolution images and the tractography algorithm might have the limited capacity to detect longer fibers and the inability to resolve crossing/kissing fibers. However, the streamlining algorithm is robust and computationally inexpensive to identify major white matter tracts (47). Another methodological limitation was that DTI acquisition with low b-values and deterministic tractography may be influenced by motion artifacts. Although the PATCH algorithm is accurate and robust to correct motion artifacts, the DTI results may still be driven by residual motion artifacts. High-resolution imaging and more advanced tractography algorithms together with careful examination of motion artifacts and detailed gait assessment are warranted to provide more exhaustive information about white matter networks and gait performance in future studies. Furthermore, in the present study, we used the TUG test to evaluate gait performance longitudinally. We were unable to utilize GAITRite to assess spatial-temporal parameters of the gait, since GAITRite assessment was not performed in 2015. The steady-state gait performance evaluated by the TUG test may not be as precise as GAITRite, given the influence of all TUG components (eg, sit-to-stand, stand-to-sit) on the gait performance. However, previous studies have shown that the TUG test is reliable and valid for quantifying gait functioning in the older population and shorter TUG time is highly correlated with faster gait speed (16,48). In addition, TUG test has been widely used in SVD study, demonstrating that it is a valid approach for SVD population to quantify gait performance (49,50).
Conclusion
In conclusion, decline in structural network efficiency was associated with decline in gait performance in older individuals with cerebral SVD, while decline in cognitive functioning has no predictive effect on gait worsening. Our study highlights that the importance of disrupted white matter network connectivity in explaining gait-related mechanisms in older patients with SVD, independent of cognitive decline.
Supplementary Material
Funding
This work was supported by China Scholarship Council (201706100189 to M.C.), the Dutch Heart Foundation (grant 2016 T044 to A.M.T., grant 2014 T060 to FE.d.L.), and the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 & 2012-06 Heart Brain Connection to A.M.T.), VIDI innovational grant from The Netherlands Organization for Health Research and Development (ZonMw grant 016.126.351 to FE.d.L.).
Author Contributions
M.C. contributed to conceptualization, statistical analysis, and original draft preparation. M.A.J. also contributed to the original draft preparation and revision, and interpretation. D.G.N. revised the manuscript. A.M.T. and FE.d.L. contributed to the design of this study, revision and interpretation of the manuscript.
Conflict of Interest
None declared.
References
- 1. de Laat KF, van Norden AG, Gons RA, et al. . Gait in elderly with cerebral small vessel disease. Stroke. 2010;41(8):1652–1658. doi:10.1161/STROKEAHA.110.583229 [DOI] [PubMed] [Google Scholar]
- 2. Cesari M, Kritchevsky SB, Penninx BW, et al. . Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi:10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 3. Abellan van Kan G, Rolland Y, Andrieu S, et al. . Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi:10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Patel K, et al. . Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83(4):304–311. doi:10.1212/WNL.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuladhar AM, Tay J, van Leijsen E, et al. . Structural network changes in cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2020;91(2):196–203. doi:10.1136/jnnp-2019-321767 [DOI] [PubMed] [Google Scholar]
- 7. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi:10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 8. Reijmer YD, Leemans A, Caeyenberghs K, Heringa SM, Koek HL, Biessels GJ; Utrecht Vascular Cognitive Impairment Study Group. Disruption of cerebral networks and cognitive impairment in Alzheimer disease. Neurology. 2013;80(15):1370–1377. doi:10.1212/WNL.0b013e31828c2ee5 [DOI] [PubMed] [Google Scholar]
- 9. Verwer JH, Reijmer YD, Koek HL, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) Study Group. Physical performance in memory clinic patients: the potential role of the white matter network. J Am Geriatr Soc. 2019;67(9):1880–1887. doi:10.1111/jgs.15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawrence AJ, Zeestraten EA, Benjamin P, et al. . Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology. 2018;90(21):e1898–e1910. doi:10.1212/WNL.0000000000005551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi:10.1037/0894-4105.20.2.215 [DOI] [PubMed] [Google Scholar]
- 12. Takakusaki K. Neurophysiology of gait: From the spinal cord to the frontal lobe: Neurophysiology of gait. Movement Disorders. 2013;28(11):1483–1491. doi:10.1002/mds.25669 [DOI] [PubMed] [Google Scholar]
- 13. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10(1):1–17. doi:10.14802/jmd.16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cai M, Jacob MA, Norris DG, Duering M, de Leeuw FE, Tuladhar AM. Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin. 2021;30:102667. doi:10.1016/j.nicl.2021.102667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Norden AG, de Laat KF, Gons RA, et al. . Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. doi:10.1186/1471-2377-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 17. Bischoff HA, Stähelin HB, Monsch AU, et al. . Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. doi:10.1093/ageing/32.3.315 [DOI] [PubMed] [Google Scholar]
- 18. Elst WVD, Boxtel MPJV, Breukelen GJPV, Jolles J. Assessment of information processing in working memory in applied settings: the paper & pencil memory scanning test. Psychological Medicine. 2007;37(9):1335–1344. doi:10.1017/S0033291707000360 [DOI] [PubMed] [Google Scholar]
- 19. van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. doi:10.1080/13803390591004428 [DOI] [PubMed] [Google Scholar]
- 20. Elst WVD, Boxtel MPJV, Breukelen GJPV, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302. doi:10.1017/S1355617705050344 [DOI] [PubMed] [Google Scholar]
- 21. van Leijsen EMC, van Uden IWM, Ghafoorian M, et al. . Nonlinear temporal dynamics of cerebral small vessel disease: The RUN DMC study. Neurology. 2017;89(15):1569–1577. doi:10.1212/WNL.0000000000004490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wardlaw JM, Smith EE, Biessels GJ, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi:10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghafoorian M, Karssemeijer N, van Uden IW, et al. . Automated detection of white matter hyperintensities of all sizes in cerebral small vessel disease. Med Phys. 2016;43(12):6246. doi:10.1118/1.4966029 [DOI] [PubMed] [Google Scholar]
- 24. van Leijsen EMC, van Uden IWM, Ghafoorian M, et al. . Nonlinear temporal dynamics of cerebral small vessel disease: The RUN DMC study. Neurology. 2017;89(15):1569–1577. doi:10.1212/WNL.0000000000004490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manjón JV, Coupé P, Concha L, Buades A, Collins DL, Robles M. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8(9):e73021. doi:10.1371/journal.pone.0073021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zwiers MP. Patching cardiac and head motion artefacts in diffusion-weighted images. Neuroimage. 2010;53(2):565–575. doi:10.1016/j.neuroimage.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Jenkinson M, Woolrich MW, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi:10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi:10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 29. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi:10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- 30. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi:10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagmann P, Kurant M, Gigandet X, et al. . Mapping human whole-brain structural networks with diffusion MRI. PLoS One. 2007;2(7):e597. doi:10.1371/journal.pone.0000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3(2):e17. doi:10.1371/journal.pcbi.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheather SJ. Multiple Linear Regression. In: Sheather S, ed. A Modern Approach to Regression with R. Springer Texts in Statistics. New York, NY: Springer; 2009:125–149. doi:10.1007/978-0-387-09608-7_5 [Google Scholar]
- 34. Fox J, Weisberg S.. An {R} Companion to Applied Regression. Second Edition. Thousand Oaks CA: Sage; 2011. https://socialsciences.mcmaster.ca/jfox/Books/Companion/appendices.html. Accessed June 1, 2021. [Google Scholar]
- 35. Reijmer YD, Fotiadis P, Martinez-Ramirez S, et al. . Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138(Pt 1):179–188. doi:10.1093/brain/awu316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83. doi:10.1093/brain/awq343 [DOI] [PubMed] [Google Scholar]
- 37. Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14(7):387–398. doi:10.1038/s41582-018-0014-y [DOI] [PubMed] [Google Scholar]
- 38. Kok JG, Leemans A, Teune LK, et al. . Structural network analysis using diffusion MRI tractography in Parkinson’s disease and correlations with motor impairment. Front Neurol. 2020;11:841. doi:10.3389/fneur.2020.00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlemm E, Schulz R, Bönstrup M, et al. . Structural brain networks and functional motor outcome after stroke-a prospective cohort study. Brain Commun. 2020;2(1):fcaa001. doi:10.1093/braincomms/fcaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2002;57(4):M228–M235. doi:10.1093/gerona/57.4.m228 [DOI] [PubMed] [Google Scholar]
- 41. Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: The Tasmanian Study of Cognition and Gait. J Gerontol A Biol Sci Med Sci. 2015;70(10):1226–1232. doi:10.1093/gerona/glv066 [DOI] [PubMed] [Google Scholar]
- 42. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–113. doi:10.1212/01.wnl.0000316799.86917.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Finsterwalder S, Wuehr M, Gesierich B, et al. . Minor gait impairment despite white matter damage in pure small vessel disease. Ann Clin Transl Neurol. 2019;6(10):2026–2036. doi:10.1002/acn3.50891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dyer AH, Lawlor B, Kennelly SP; NILVAD Study Group. Gait speed, cognition and falls in people living with mild-to-moderate Alzheimer disease: data from NILVAD. BMC Geriatr. 2020;20(1):117. doi:10.1186/s12877-020-01531-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cabeza R, Albert M, Belleville S, et al. . Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701–710. doi:10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lawrence AJ, Tozer DJ, Stamatakis EA, Markus HS. A comparison of functional and tractography based networks in cerebral small vessel disease. Neuroimage Clin. 2018;18:425–432. doi:10.1016/j.nicl.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468–480. doi:10.1002/nbm.781 [DOI] [PubMed] [Google Scholar]
- 48. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96–101. doi:10.1191/026921500675545616 [DOI] [PubMed] [Google Scholar]
- 49. Zhou X, Zhang C, Li L, et al. . Altered brain function in cerebral small vessel disease patients with gait disorders: a resting-state functional MRI study. Front Aging Neurosci. 2020;12:234. doi:10.3389/fnagi.2020.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li P, Wang Y, Jiang Y, et al. . Cerebral small vessel disease is associated with gait disturbance among community-dwelling elderly individuals: the Taizhou imaging study. Aging (Albany NY). 2020;12(3):2814–2824. doi:10.18632/aging.102779 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.