Abstract
The efficient manipulation of their host cell is an essential feature of intracellular parasites. Most molecular mechanisms governing the subversion of host cell by protozoan parasites involve the release of parasite-derived molecules into the host cell cytoplasm and direct interaction with host proteins. Among these released proteins, kinases are particularly important as they govern the subversion of important host pathways, such as signalling or metabolic pathways. These enzymes, which catalyse the transfer of a phosphate group from ATP onto serine, threonine, tyrosine or histidine residues to covalently modify proteins, are involved in numerous essential biological processes such as cell cycle or transport. Although little is known about the role of most of the released parasite-derived kinases in the host cell, they are examples of kinases hijacking host cellular pathways such as signal transduction or apoptosis, which are essential for immune response evasion as well as parasite survival and development. Here we present the current knowledge on released protozoan kinases and their involvement in host-pathogen interactions. We also highlight the knowledge gaps remaining before considering those kinases - involved in host signalling subversion - as antiparasitic drug targets.
Keywords: excreted kinase, eukaryote, protozoa, signal transduction, antimicrobial therapy
Introduction
The efficient manipulation of their host cell is an essential feature of intracellular parasites, which they achieve by secreting effectors to maintain their replicative niche within the host cell and to hijack important host pathways. Among those effectors, kinases have been shown to regulate a wide range of pathways, such as signalling or metabolic pathways. The potential key role of secreted kinases in the subversion of host cell signalling pathways, make them candidates of choice for the development of new antiparasitic treatments, particularly with the growing concern of drug resistance. Targeting secreted effectors may reduce the risk of drug resistance, as any mutation to bypass the drug effect may prevent their extra-parasite role and thus result in high fitness costs for the parasite. Despite their importance, only few parasite-secreted kinases have been studied and their functions in the host cell characterised. We focused on two phylums:
-The Apicomplexa (Alveolata) with Plasmodium spp., Toxoplasma gondii and Eimeria tenella , the causative agents of malaria - transmitted by female anopheles mosquitoes -, toxoplasmosis and coccidiosis, respectively;
-The kinetoplastids (Euglenozoa) with Leishmania spp., the causative agent of leishmaniasis, transmitted by the bite of a female sand fly and Trypanosoma cruzi, the causative agent of Chagas disease, spread by Triatominae.
In the present mini review, we describe the known mechanisms of parasite effector secretion and compare the secreted kinome from phylogenetically distant intracellular protozoan parasites. We give examples of the host functions of the few studied secreted kinases and highlight the scientific gaps remaining to fully understand host signalling subversion by parasites.
Mechanisms Of Parasite Effector Release/Secretion
Parasites release virulence factors either as soluble molecules or inside extracellular vesicles (EVs), leading to the modification of the biological and immune functions of their host cell to ensure their survival (Silverman et al., 2010b; Regev-Rudzki et al., 2013). Apicomplexa and kinetoplastids parasites display different mode of host-parasite interactions due to the specificity of their host cell and their mode of cell entry, which might be partly reflected in the mechanism of parasite protein secretion.
Common to Most Apicomplexa and Kinetoplastids
Exocytosis, driven by the active transport of secretory vesicles is the eukaryotic conventional secretion system for proteins containing a hydrophobic domain in N-terminal position [Signal Peptide, SP, see (Beer and Wehman, 2017) for an illustrated review]. These protein-containing vesicles traffic from the Golgi apparatus to the plasma membrane, fuse with the plasma membrane to release the secreted proteins in the extracellular environment (Colombo et al., 2014). This secretion system does not account for all the molecules exported by parasites. In Leishmania, 98% of the secreted proteins lack SP, suggesting the presence of other secretion pathways (Silverman et al., 2008). The second mechanism is unconventional protein secretion (UPS) and refers to proteins either exposed on the cell surface or in the extracellular medium (Balmer and Faso, 2021). The third mechanism is through extracellular vesicles (EVs), lipid-bound vesicles which either bud from the plasma membrane (microvesicles) or are derived from multivesicular bodies that fuse with the plasma membrane (exosomes) (Dlugonska and Gatkowska, 2016; Mathieu et al., 2019; Babatunde et al., 2020) and for more details in mechanisms of secretion see (Teng and Fussenegger, 2021).
Specific to Apicomplexa
Apicomplexa have developed specific strategies to release proteins into the host cell, which might be the consequence of cell entry by invasion, contrary to phagocytosis or endocytosis for kinetoplastids. Cell invasion requires the fast discharge of microneme and rhoptry proteins (perforins, lipases, proteases, adhesins and kinases) implicated in gliding motility, parasite attachment, formation of the moving junction and the hijacking of host cell pathways, which might not be compatible with the slower release of proteins by the secretory or exosomal pathways (Tomavo et al., 2013; Bisio and Soldati-Favre, 2019). Proteins are targeted to those compartments by conventional SP and specific motifs. Microneme secretion is triggered by signalling events, involving intracellular cyclic nucleotides, calcium level and phosphatidic acid (Dubois and Soldati-Favre, 2019) and is followed by rhoptry secretion (Aquilini et al., 2021). In addition to micronemes and rhoptries, Plasmodium species create, in the host cell cytoplasm, a network of membranous structures of parasite origin, called Maurer’s clefts. These structures are attached to the host cytoskeleton and act as extracellular secretory and trafficking organelles for the parasite (Lanzer et al., 2006) but little is known about their biogenesis and functions. Finally, some proteins contain PEXEL motifs in Plasmodium (Marti et al., 2004) and TEXEL motifs in Toxoplasma (Coffey et al., 2015), which are required for their release via exporters located on the parasitophorous vacuole membrane (PVM). The PVM derives from the host cell membrane and is modified by incorporation of parasite proteins, to avoid phagolysosome fusion (de Koning-Ward et al., 2009; Marino et al., 2018). This pathway corresponds to the default constitutive secretion pathway (Venugopal et al., 2020).
Exoproteomes of Apicomplexa and Trypanosomatids
There is a growing body of data on the exo-proteome, whatever the mechanism of secretion used by parasites. It contains a range of protein classes including proteases, kinases, membrane proteins, heat shock proteins or nucleic acids, which induce specific modifications in the host cell (Montaner et al., 2014). Only little is known about the mechanisms involved in cargo selection of these EVs. Leishmania HSP100 has a strong impact on protein cargo composition: its deletion affects the immune status of the host cell and parasite survival (Silverman et al., 2011). The EV composition is sensitive to environmental cues (Hassani et al., 2011) and might contribute to the spread of drug resistance (Douanne et al., 2020). EVs have an essential role during infection (Torrecilhas et al., 2020); co-egestion of Leishmania and its EVs by the sand fly induces the inflammatory recruitment of neutrophils and macrophages (Atayde et al., 2015). EVs are involved in immune evasion; T. cruzi exosomes aggravate the infection due to severe inflammatory response and increase the parasite burden (Trocoli Torrecilhas et al., 2009). Several vesicular virulence factors from T. cruzi have been involved in host invasion, intracellular parasite proliferation or immune evasion (Costa et al., 2016). For Apicomplexa, T. gondii exosomes has been shown to activate a pro-inflammatory immune response (Li et al., 2018), and small non-coding RNAs and genomic DNA contained in EVs released from RBC infected by P. falciparum are detected by the STING pathway, favouring parasite survival (Sisquella et al., 2017). However, accessing parasite-derived EVs is challenging, as apicomplexans parasites cannot be cultured without their host cell, thus most of the data available on their exo-proteome is in fact from host-derived EVs.
Secreted/Excreted Parasite Kinases
Phosphorylation, an essential reversible post-translational modification, affects every cellular process (Ardito et al., 2017). It acts as a molecular switch for many biological processes, including signal transduction networks in response to extracellular stimuli. Phosphorylation is catalysed by kinases, which transfers phosphate from ATP onto proteins, sugars or lipids. Upon phosphorylation, the chemical properties, conformation, localisation and/or activity of the molecule change, inducing rapid downstream effects in the cell (Hunter and Sibley, 2012). To survive, intracellular pathogens need to exploit the host pathways either to fulfil their needs for proliferation or to inhibit the host defence responses. Targeting the phospho-proteome of the host is the fastest way to subvert a large repertoire of biological and immune processes (Regev-Rudzki et al., 2013; Carrera-Bravo et al., 2021).
Kinases
Most studies on kinases refers to protein kinases. Protozoan parasite kinomes contain orthologues for 6 of the 8 groups of conventional eukaryotic PKs (ePK): AGC, CAMK, CK1, CMGC, STE and TKL) and some “others” that share ePK folding but cannot be assigned to any major ePK group from humans (Peixoto et al., 2010; Talevich et al., 2011). One additional group Kinetoplastids, NEK family, is involved in cell cycle and cytoskeletal functions. Apicomplexa contains also specific ePK (FIKK, ROPK and WNG), differentially conserved and/or duplicated within Apicomplexa parasite phylum (Ward et al., 2004; Beraki et al., 2019). While only one FIKK gene was identified in coccidia (Toxoplasma, Eimeria) and in most Plasmodium species, this group is expanded in P. falciparum with 20 kinases and in several Plasmodium sp. infecting apes (Adderley et al., 2021). Most of the rhoptry proteins are kinases (ROPK), either active, inactive (lacking a complete catalytic triad) or non-canonical (active with differences in conserved residues) (Bradley et al., 2005). Finally, very recently, a new group of four kinases specific to coccidia, and missing the typical glycine loop was identified (WNG1-3 and BPK1) (Beraki et al., 2019). BPK1 is associated with bradyzoite cyst wall, with a crucial role in in vivo cyst infectivity (Boothroyd, 2013; Buchholz et al., 2013). In Eimeria, two WNGs are predicted, but their functions remain unknown. To date, only TgWNG1 has been functionally characterized: it is involved in the phosphorylation of GRA, a family of effectors stored in dense granule vesicles and secreted to develop the intra-vacuolar network, implicated in survival of parasite. Although as important as protein kinases, there are no comprehensive studies available on carbohydrate, lipid, nucleoside or other kinases, but only individual publications (Pereira et al., 2011).
Host Functions of Secreted Kinases
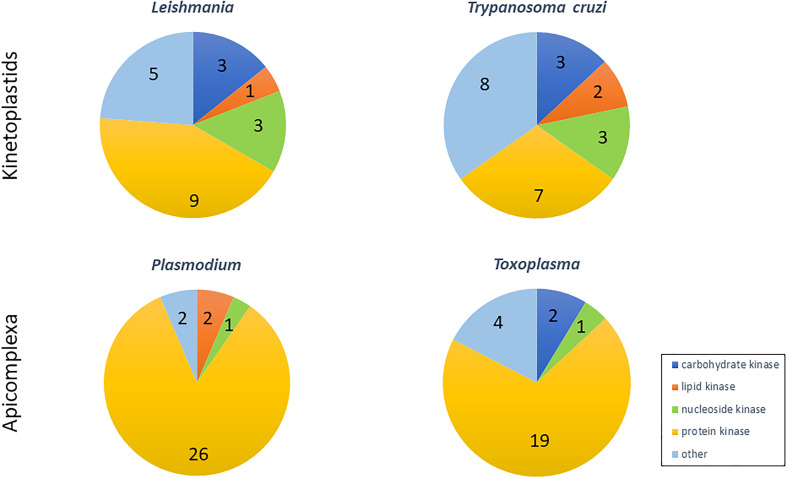
Proteomic characterisation of parasite exo-proteomes revealed the presence of only few kinases, mostly involved in pathways such as glycolysis, cellular energy homeostasis or cell signalling ( Table 1 ). While in Apicomplexa most secreted kinases target proteins, in Leishmania and T. cruzi, more than half of the kinases target nucleosides, carbohydrates or lipids ( Figure 1 ). Eight kinases are released by both kinetoplastids and Apicomplexa ( Table 1 , bold), suggesting that host subversion mediated by those kinases might be conserved between parasites. Five kinases might be kinetoplastid-specific ( Table 1 , italic) and only one might be Apicomplexa-specific (CDPKs, Table 1 , underlined). This low number of specific kinases may be due to the scarce proteomic data available for these parasites.
Table 1.
Kinases secreted by protozoan parasites.
| Organism | Kinase Name | Kinase Class | References | Stages |
|---|---|---|---|---|
| Leishmania | 6-phospho-1-fructokinase, putative | carbohydrate | Silverman et al., 2008; Silverman et al., 2010a; Douanne et al., 2020 | Stationary-phase promastigotes, logarithmic promastigotes |
| adenosine kinase, putative | nucleoside | Silverman et al., 2008 | Stationary-phase promastigotes | |
| adenylate kinase, putative | nucleoside | Silverman et al., 2008; Silverman et al., 2010a; Hassani et al., 2011; Douanne et al., 2020 | Stationary-phase promastigotes, logarithmic promastigotes and axenic amastigotes | |
| casein kinase I, putative CK1.2 | protein | Silverman et al., 2008; Silverman et al., 2010a | Stationary-phase promastigotes | |
| casein kinase II, alpha chain, Putative LmjF.02.0360 | protein | Douanne et al., 2020 | Logarithmic promastigotes | |
| mevalonate kinase | lipid | Bamra et al., 2021 | Promastigotes and amastigotes | |
| cdc2-related kinase 9 | protein | Silverman et al., 2008 | Stationary-phase promastigotes | |
| glycogen synthase kinase3-putative | protein | Douanne et al., 2020 | Logarithmic promastigotes | |
| glycosomal phosphoenolpyruvate carboxykinase, putative | other | Silverman et al., 2008; Hassani et al., 2011; Douanne et al., 2020 | Stationary-phase promastigotes and logarithmic promastigotes | |
| hexokinase, putative | carbohydrate | Silverman et al., 2008; Hassani et al., 2011 | Stationary-phase promastigotes | |
| mitogen activated protein kinase, putative,map kinase, putative | protein | Silverman et al., 2008 | Stationary-phase promastigotes | |
| mitogen-activated protein kinase 3, putative,map kinase 3, putative | protein | Silverman et al., 2008 | Stationary-phase promastigotes | |
| nucleoside diphosphate kinase b | nucleoside | Silverman et al., 2008; Silverman et al., 2010a; Hassani et al., 2011; Douanne et al., 2020 | Stationary-phase promastigotes, logarithmic promastigotes and axenic amastigotes. | |
| phosphoglycerate kinase | other | Douanne et al., 2020; Mandacaru et al., 2019; Ribeiro et al., 2018; Brossas et al., 2017; Queiroz et al., 2016; Bayer-Santos et al., 2013; Hassani et al., 2011 | Logarithmic promastigotes, stationary-phase promastigotes and Epimastigotes, Vero cells infected with trypomastigotes, Trypomastigotes, Tissue culture-derived trypomastigotes and trypomastigotes. | |
| protein kinase, putative LmjF34.0030 | protein | Silverman et al., 2008 | Stationary-phase promastigotes | |
| pyruvate dehydrogenase lipoamide kinase, putative | other | Silverman et al., 2008 | Stationary-phase promastigotes | |
| pyruvate kinase, putative | other | Silverman et al., 2008; Silverman et al., 2010a; Douanne et al., 2020; Ribeiro et al., 2018 | Stationary-phase promastigotes and logarithmic promastigotes, Tissue culture-derived trypomastigotes. | |
| pyruvate phosphate dikinase, putative | other | Silverman et al., 2008; Silverman et al., 2010a; Douanne et al., 2020 | Stationary-phase promastigotes and logarithmic promastigotes | |
| serine/threonine-protein kinase, putative LINF_290033500/LmjF29.2570 identity to human Nek1 | protein | Douanne et al., 2020 | Logarithmic promastigotes | |
| serine/threonine-protein kinase, putative LmjF25.2340 identity to human AKT1 | protein | Silverman et al., 2010a | Stationary-phase promastigotes | |
| tagatose-6-phosphate kinase-like protein | carbohydrate | Silverman et al., 2008 | Stationary-phase promastigotes | |
| Trypanosoma cruzi | adenosine kinase, putative | nucleoside | Brossas et al., 2017 | Trypomastigotes |
| mevalonate kinase | lipid | Ferreira et al., 2016 | Metacyclic trypomastigote and extracellular amastigote cultures | |
| adenylate kinase, putative | nucleoside | Queiroz et al., 2016; Ribeiro et al., 2018 | Trypomastigotes, Tissue culture-derived trypomastigotes | |
| arginine kinase | other | Bayer-Santos et al., 2013; Queiroz et al., 2016; Brossas et al., 2017; Ribeiro et al., 2018; Mandacaru et al., 2019 | Epimastigotes, Vero cells infected with trypomastigotes, Trypomastigotes, Tissue culture-derived trypomastigotes and trypomastigotes. | |
| fucose kinase | carbohydrate | Ribeiro et al., 2018 | Tissue culture-derived trypomastigotes | |
| galactokinase | carbohydrate | Ribeiro et al., 2018 | Tissue culture-derived trypomastigotes | |
| glycosomal phosphoenolpyruvate carboxykinase, putative | other | Queiroz et al., 2016; Ribeiro et al., 2018; Mandacaru et al., 2019 | Trypomastigotes, Tissue culture-derived trypomastigotes and trypomastigotes. | |
| hexokinase | carbohydrate | Bayer-Santos et al., 2013, Mandacaru et al., 2019 | Epimastigotes and trypomastigotes. | |
| mitogen-activated protein kinase, putative | protein | Bayer-Santos et al., 2013; Brossas et al., 2017; Ribeiro et al., 2018 | Epimastigotes, Vero cells infected with trypomastigotes, Tissue culture-derived trypomastigotes | |
| NIMA-related kinase, putative | protein | Queiroz et al., 2016 | Trypomastigotes | |
| nucleoside diphosphate kinase B | nucleoside | Bayer-Santos et al., 2013; Brossas et al., 2017; Queiroz et al., 2016; Ribeiro et al., 2018; Mandacaru et al., 2019 | Epimastigotes, Vero cells infected with trypomastigotes, Trypomastigotes, Tissue culture-derived trypomastigotes and trypomastigotes. | |
| phosphatidylinositol-3-Kinase | lipid | Bayer-Santos et al., 2013 | Epimastigotes | |
| Protein kinase Tc00.1047053506211.210 MAPKKK identity to human PAK1/PAK3 | protein | Ribeiro et al., 2018 | Tissue culture-derived trypomastigotes | |
| Protein kinase, putative TcCLB.508641.170 identity to human PKC theta | protein | Brossas et al., 2017 | Vero cells infected with trypomastigotes | |
| protein kinase-A catalytic subunit | protein | Queiroz et al., 2016 | Trypomastigotes | |
| pyridoxal kinase, putative | other | Queiroz et al., 2016; Ribeiro et al., 2018 | Trypomastigotes, Tissue culture-derived trypomastigotes | |
| pyruvate kinase 2, putative | other | Queiroz et al., 2016 | Trypomastigotes | |
| pyruvate phosphate dikinase 2 | other | Queiroz et al., 2016 | Trypomastigotes | |
| pyruvate phosphate dikinase 1 | other | Bayer-Santos et al., 2013; Ribeiro et al., 2018 | Epimastigotes, Tissue culture-derived trypomastigotes | |
| serine/threonine protein kinase TcCLB.508153.400/TCSYLVIO_004423 identity to human Nek1 | protein | Bayer-Santos et al., 2013; Ribeiro et al., 2018 | Epimastigotes, Tissue culture-derived trypomastigotes | |
| serine/threonine-protein kinase 10, putative TcCLB.506401.110 identity to human STK10 | protein | Queiroz et al., 2016 | Trypomastigotes | |
| Plasmodium | Camk2d calcium/calmodulin_dependent protein kinase II_ delta isoform 1 | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte |
| Camk2d Isoform 1 of Calcium/calmodulin_dependent protein kinase type II delta chain | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte | |
| Camk2d Isoform 2 of Calcium/calmodulin_dependent protein kinase type II delta chain | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte | |
| Camk2d Isoform 3 of Calcium/calmodulin_dependent protein kinase type II delta chain | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte | |
| Camk2d Isoform 4 of Calcium/calmodulin_dependent protein kinase type II delta chain | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte | |
| casein kinase 2, alpha subunit | protein | Abdi et al., 2017 | Trophozoite P. falciparum infected erythrocyte | |
| diacyl glycerol kinase | lipid | Gualdron-Lopez et al., 2018 | Trophozoite P. vivax infected erythrocyte | |
| FIKK10.1 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK13 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK14 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK1 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK4.1 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK4.2 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK7.1 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK9.1 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK9.3 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK9.6 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK10.2 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK11 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| FIKK12 | protein | Hiller et al., 2004 | Trophozoite P. falciparum infected erythrocyte | |
| pacsin2 Protein kinase C and casein kinase substrate in neurons protein 2 | protein | Martin-Jaular et al., 2011 | Trophozoite P. yoelii infected reticulocyte | |
| phosphatidylinositol 4-kinase, putative | lipid | Abdi et al., 2017 | Trophozoite P. falciparum infected erythrocyte | |
| phosphoglycerate kinase | other | Mantel et al., 2013; Abdi et al., 2017 | Trophozoite P. falciparum infected erythrocyte | |
| pseudo protein kinase 1, putative PF3D7_0321400 | protein | Abdi et al., 2017 | Trophozoite P. falciparum infected erythrocyte | |
| pyruvate kinase | other | Vincensini et al., 2005; Mantel et al., 2013 | Trophozoite P. falciparum infected erythrocyte | |
| serine/threonine protein kinase, putative PF3D7_1441300 | protein | Abdi et al., 2017 | Trophozoite P. falciparum infected erythrocyte | |
| calcium-dependent protein kinase CDPK1 | protein | Lal et al., 2009 | Purified microneme organelle | |
| calcium-dependent protein kinase CDPK4 | protein | Lal et al., 2009 | Purified microneme organelle | |
| adenylate kinase | nucleoside | Vincensini et al., 2005 | Trophozoite P. falciparum infected erythrocyte | |
| Toxoplasma | calcium-dependent protein kinase CDPK1 | protein | Wowk et al., 2017; Ramirez-Flores et al., 2019 | Tachyzoite T. gondii infected human foreskin fibroblast, Acellular tachyzoites |
| calcium-dependent protein kinase CDPK2A | protein | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| calcium-dependent protein kinase CDPK3 | protein | Wowk et al., 2017; Ramirez-Flores et al., 2019 | Tachyzoite T. gondii infected human foreskin fibroblast, Acellular tachyzoites | |
| casein kinase I | protein | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| CMGC kinase, CK2 family | protein | Wowk et al., 2017; Ramirez-Flores et al., 2019 | Tachyzoite T. gondii infected human foreskin fibroblast, Acellular tachyzoites | |
| hexokinase | carbohydrate | Wowk et al., 2017; Ramirez-Flores et al., 2019 | Tachyzoite T. gondii infected human foreskin fibroblast, Acellular tachyzoites | |
| nucleoside diphosphate kinase | nucleoside | Lee et al., 2014 | Acellular tachyzoites | |
| phosphoenolpyruvate-carboxykinase I | other | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| phosphofructokinase PFKII | carbohydrate | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| phosphoglycerate kinase | other | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| pyruvate kinase | other | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| rhoptry kinase family protein ROP39 | protein | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| selenide, water dikinase | other | Wowk et al., 2017 | Tachyzoite T. gondii infected human foreskin fibroblast | |
| ROP2 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP4 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP5 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP8 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP11 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP16 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP17 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP18 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| ROP38 - PK-like | protein | Bradley et al., 2005 | Purified rhoptry organelle | |
| WNG1 (With-No-Gly-Loop) | protein | Beraki et al., 2019 | Bradyzoite | |
| WNG2 | protein | Beraki et al., 2019 | Bradyzoite | |
| WNG3 | protein | Beraki et al., 2019 | Bradyzoite | |
| BPK1 bradyzoite pseudokinase 1 | protein | Buchholz et al., 2013 | Bradyzoite | |
| Eimeria a | pyruvate kinase | other | Labbé et al., 2006 | Schizont from in vitro infected cells |
| hexokinase | carbohydrate | Sun et al., 2016 | Sporozoite from in vitro infected cells | |
| calcium-dependent protein kinase CDPK1 | protein | Dunn et al., 1996 | Sporozoite from in vitro infected cells | |
| calcium-dependent protein kinase CDPK2 | protein | Dunn et al., 1996 | Sporozoite from in vitro infected cells | |
| calcium-dependent protein kinase CDPK3 | protein | Han et al., 2013 | Sporozoite and schizont from in vitro infected cells | |
| calcium-dependent protein kinase CDPK4 | protein | Wang et al., 2016a | Sporozoite and merozoite from in vitro infected cells | |
| ETH_00000075 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00005190 - EtROP1 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00005400 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00005840 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00005905 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00020620 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00026495 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00027695 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00027700 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00027855 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| ETH_00028765 | protein | Oakes et al., 2013 | Purified rhoptry organelle | |
| WNG1, predicted | protein | Beraki et al., 2019 | ND b | |
| WNG4, predicted | protein | Beraki et al., 2019 | ND b |
Secreted kinases listed for Eimeria are underestimated, due to a lack of datasets. Eimeria pyruvate kinase (Labbé et al., 2006), hexokinase (Sun et al., 2016) and CDPK (Dunn et al., 1996; Han et al., 2013; Wang et al., 2016a) are secreted by an unknown mechanism.
Not determined.
Kinase class refers to the kinase substrate. Kinases common to kinetoplastids and Apicomplexa are indicated in bold, kinases specific to kinetoplastids or to Apicomplexa are indicated in italic or underlined, respectively. Based on experimental procedures of cited references for Apicomplexa, kinases from organelles are secreted in the host cell cytoplasm, not in the extracellular medium. To the author knowledge, only P. falciparum TKL2 and PfCK1 were detected in extracellular medium and at the erythrocyte membrane (Abdi et al., 2013) and maybe associated to immunomodulatory functions. Most of the secreted proteins of Kinetoplastids are released inside the host cell. Ndk and AK seem to be secreted in the extracellular environment of the host cell due to their role. Limitations concerning secretome preparation and characterization have been reviewed (Severino et al., 2013).
Figure 1.
Proportion and number of kinases classes according to their substrates, identified in secretome of Kinetoplastids (Leishmania and Trypanozoma) and Apicomplexa (Toxoplasma and Plasmodium).
Glycolytic Kinases
Glycolytic kinases are located in the glycosomes of kinetoplastids and in the cytosol and the apicoplast of Apicomplexa (Saito et al., 2002; Fleige et al., 2007). They regulate glycolysis but have additional biological functions, as moonlighting proteins. For instance, Leishmania hexokinase, a glycolytic enzyme, also acts as a haemoglobin (Hb) receptor, allowing Hb internalisation (Krishnamurthy et al., 2005). L. donovani aldolase, another glycolytic enzyme, interacts with and activates the host SHP-1 (protein tyrosine phosphatase). SHP-1 inhibits M1 macrophage polarization, creating a more favourable environment for Leishmania (Nandan et al., 2007; Garg et al., 2020). Although important in number, nothing is known about the host functions of glycolytic kinases, but their release by most the parasites suggest important roles in the host cell.
Nucleoside Diphosphate Kinase (Ndk)
Ndk catalyses the transfer of phosphate from nucleoside triphosphate (NTP) to nucleoside diphosphate (NDP) to maintain ATP cellular homeostasis (Kolli et al., 2008). This kinase also plays roles in the regulation of gene transcription, DNA repair, differentiation and apoptosis (Yu et al., 2017). Ndk seems important for drug resistance in T. cruzi and in Leishmania, its overexpression leads to a decrease sensitivity to antimony (SbIII) (Moreira and Murta, 2016); for DNA damage responses in T. cruzi (Miranda et al., 2008); and for parasite replication in T. gondii (Lykins et al., 2018). In these parasites, Ndk is released in EVs (Silverman et al., 2008; Silverman et al., 2010a; Bayer-Santos et al., 2013; Lee et al., 2014; Brossas et al., 2017). In Leishmania, the release of ndk prevents extracellular ATP (eATP)-mediated cytolysis of infected macrophages (Kolli et al., 2008). eATP, a signal nucleotide, binds to and activates the P2X7 receptor, which is responsible for the pore formation in the membrane of macrophages, resulting in cell death (Kolli et al., 2008; Kulkarni et al., 2019). By transferring phosphate from ATP to NDP, Ndk decreases eATP, thus prevents ATP-induced changes in mitochondrial permeability of macrophages. Furthermore, Ndk participates in the host purine salvage by protozoan parasites by utilizing eATP to produce other NTPs such as GTP (Kolli et al., 2008) These functions might be conserved in T. cruzi and T. gondii, which also release Ndk.
Casein Kinase 1
Casein kinase 1 (CK1) is a serine/threonine protein kinase that regulates a wide range of biological processes (Xu et al., 2019; Rachidi et al., 2021). In Leishmania, three paralogs are released: L-CK1.4 through the classical secretory pathway, L-CK1.5 and L-CK1.2 via exosomes ( Table 1 ). Nothing is known about the role of these paralogs in the host cell, except for L-CK1.2. This kinase phosphorylates human IFNAR1 receptor, physiological target of human CK1α, to promote its ubiquitination and subsequent degradation, leading to the attenuation of the cellular response to interferon α/β (Liu et al., 2009). Recently additional host proteins phosphorylated by L-CK1.2 were identified (Smirlis et al., 2022). Several pathways, such as apoptosis, actin skeleton organisation or RNA processing were shown to be potentially regulated by L-CK1.2, which corresponds to pathways altered during Leishmania infection (Smirlis et al., 2020). These findings suggest that L-CK1.2 might replace human CK1 and phosphorylate host proteins to modify the immune status of the host cell. Among the three CK1 isoforms encoded by T. gondii, only CK1α is secreted in EVs (Donald et al., 2005; Wowk et al., 2017; Rachidi et al., 2021). In contrast to its kinetoplastid orthologs, it is still unclear whether TgCK1α is essential for T. gondii survival or what are its functions in the host cell. However, TgCK1α is not a candidate drug target, as its deletion increases T. gondii virulence (Wang et al., 2016b). Finally, P. falciparum expresses only one CK1, which is secreted by potentially hijacking the trafficking system of the host cell (Dorin-Semblat et al., 2015). Ten PfCK1-interacting host proteins were identified and are involved in various pathways, such as post-translational modifications, translation and protein trafficking/export (Batty et al., 2020).
Adenylate Kinase (AK)
AK catalyses the transfer of a phosphate group from ATP to AMP to generate two ADPs. It regulates homeostasis of adenine nucleotides and plays an important role in the regulation of the energy metabolism. AK has been detected in the exo-proteome of Leishmania and T. cruzi ( Table 1 ). Recent data from L. donovani, suggests that AK2a prevents ATP-mediated cytolysis of macrophages, similarly to Ndk (Kulkarni et al., 2019).
Mevalonate Kinase
L. donovani Mevalonate kinase (MVK) is a glycosomal enzyme, secreted by the parasite via a non-classical secretion pathway (Bamra et al., 2021). MVK catalyses the phosphorylation of mevalonic acid into mevalonate-5-phosphate, which is part of the cholesterol biosynthesis pathway. Macrophage infection with L. donovani over-expressing MVK leads to an increase in parasite internalisation. During extracellular amastigotes invasion, T. cruzi MVK induces the phosphorylation of host Src/FAK, involved in cytoskeleton remodelling of the host (Ferreira et al., 2016), and the phosphorylation of the host P38 and ERK leading to cytoskeleton and microfilament remodelling, which favour parasite internalisation. Moreover, LdMVK is an immuno-suppressor, which favours anti-inflammatory cytokines through ERK1/2, increasing parasite survival (Bamra et al., 2021).
FIKKs
The 18 FIKKs secreted by P. falciparum, display an important non-redundant role in cytoskeletal connections, nutrients permeability and ubiquitination of RBC proteins, as shown by the phosphoproteomic profile of their systematic invalidated mutants (Davies et al., 2020). For instance, FIKK4.1 and FIKK4.2 are involved in cytoadhesion of the RBC to the vascular endothelium, by regulating the number/size of knobs formed on the RBC membrane (Kats et al., 2014). FIKK4.1, FIKK7.1 and FIKK12 phosphorylate host cell cytoskeleton proteins, thus modifying RBC rigidity (Nunes et al., 2010; Brandt and Bailey, 2013). Finally, FIKK9.1, FIKK10.1 and FIKK10.2, exported via Maurer’s clefts, are essential for parasite survival (Siddiqui et al., 2020).
ROPKs
ROPK, secreted from the rhoptries, are involved in host-pathogen interaction. Although not all ROPK are functionally characterized, a systematic and targeted T. gondii ROPK knockout screen (Fox et al., 2016) highlighted the role of 20 ROPKs in the establishment of a chronic infection. After their secretion in the host cell cytoplasm, TgROP5, TgROP17 and TgROP18 form a complex on the cytosolic side of the PVM (Etheridge et al., 2014). TgROP5 binds immune-related GTPases (IRG) to decrease their polymerisation rate (Behnke et al., 2012). IRG are then phosphorylated by TgROP18, to prevent their recruitment to the PVM and preserve it (Fleckenstein et al., 2012). Additionally, TgROP17 is also involved in GRA translocation through the PVM, in association with the MYR complex (Panas et al., 2019). TgROP16 (Saeij et al., 2006), TgROP17 (Drewry et al., 2019), TgROP18 (Fentress and Sibley, 2011) and TgROP38 (Peixoto et al., 2010) are known to interfere with and regulate host pathways such as immune response and apoptosis. TgROP16 is localized to the host cell nucleus after invasion (Ong et al., 2010), phosphorylates signal transducer and activator of transcription STAT6 and STAT3 (Yamamoto et al., 2009; Butcher et al., 2011) to bypass the protective immune-response of the host cell. In E. tenella, among 28 ROPKs differentially expressed during the life-cycle (Ribeiro E Silva et al., 2021), only EtROP1 has been functionally characterised (Diallo et al., 2019). It interacts with host p53, to inhibit host cell apoptosis and induce G0/G1 cell cycle arrest. Interestingly, EtROP1 kinase activity is only required for cell cycle arrest, supporting the hypothesis of an additional kinase that would be responsible for p53 phosphorylation. ROPK inhibitors may offer new therapeutic treatments to control coccidiosis (Simpson et al., 2016).
Concluding Remarks
Released divergent kinases that alter host signalling pathways are interesting as they co-evolve with their host targets to insure their proper function within the host and are thus less prone to mutations that would lead to drug resistance. Some compounds that target those secreted kinases have already been identified. P. falciparum Pyruvate Kinase is efficiently targeted by antimalarial drugs, such as LZ1 (Fang et al., 2019) or suramin, which also targets trypanosomatids Pyruvate Kinase (Zhong et al., 2020). L-CK1.2 has been validated as a drug target and several compounds with anti-leishmanial activity have been identified, for review see (Rachidi et al., 2021). The similarity between PfCK1 and L-CK1.2 suggests that it might also be a good antimalarial drug target. Given the expanse of their effects on the host cell, understanding the roles that kinases secreted by parasites play in the subversion of host cell signalling will help uncover crucial drug targets.
Author Contributions
AS and NR wrote the first draft of the manuscript. SS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was funded by the French Government (Agence Nationale de la Recherche) Investissement d’Avenir programme, Laboratoire d’Excellence (LabEx) “French Parasitology Alliance For Health Care” (ANR-11-LABX-0024-PARAFRAP) and by the French government, ANR TEXLEISH (ANR-21-CE18-0026).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdi A. I., Carvalho T. G., Wilkes J. M., Doerig C. (2013). A Secreted Plasmodium Falciparum Kinase Reveals a Signature Motif for Classification of Tyrosine Kinase-Like Kinases. Microbiology-Sgm 159, 2533–2547. doi: 10.1099/mic.0.070409-0 [DOI] [PubMed] [Google Scholar]
- Abdi A. I., Yu L., Goulding D., Rono M. K., Bejon P., Choudhary J. (2017). Proteomic Analysis of Extracellular Vesicles From a Plasmodium Falciparum Kenyan Clinical Isolate Defines a Core Parasite Secretome. Wellcome Open Res. 2, 50. doi: 10.12688/wellcomeopenres.11910.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adderley J., Williamson T., Doerig C. (2021). Parasite and Host Erythrocyte Kinomics of Plasmodium Infection. Trends Parasitol. 37 (6), 508–524. doi: 10.1016/j.pt.2021.01.002 [DOI] [PubMed] [Google Scholar]
- Aquilini E., Cova M. M., Mageswaran S. K., Dos Santos Pacheco N., Sparvoli D., Penarete-Vargas D. M., et al. (2021). An Alveolata Secretory Machinery Adapted to Parasite Host Cell Invasion. Nat. Microbiol. 6 (4), 425–442. doi: 10.1038/s41564-020-00854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardito F., Giuliani M., Perrone D., Troiano G., Lo Muzio L. (2017). The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy. Int. J. Mol. Med. 40 (2), 271–280. doi: 10.3892/ijmm.2017.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayde V. D., Aslan H., Townsend S., Hassani K., Kamhawi S., Olivier M. (2015). Exosome Secretion by the Parasitic Protozoan Leishmania Within the Sand Fly Midgut. Cell Rep. 13 (5), 957–967. doi: 10.1016/j.celrep.2015.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde K. A., Subramanian B. Y., Ahouidi A. D., Murillo P. M., Walch M., Mantel P.-Y. (2020). Role of Extracellular Vesicles in Cellular Cross Talk in Malaria. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer E. A., Faso C. (2021). The Road Less Traveled? Unconventional Protein Secretion at Parasite-Host Interfaces. Front. Cell Dev. Biol. 9, 662711. doi: 10.3389/fcell.2021.662711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamra T., Shafi T., Das S., Kumar M., Dikhit M. R., Kumar A., et al. (2021). Leishmania Donovani Secretory Mevalonate Kinase Regulates Host Immune Response and Facilitates Phagocytosis. Front. Cell. Infect. Microbiol. 11, 641985. doi: 10.3389/fcimb.2021.641985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty M. B., Schittenhelm R. B., Dorin-Semblat D., Doerig C., Garcia-Bustos J. F. (2020). Interaction of Plasmodium Falciparum Casein Kinase 1 With Components of Host Cell Protein Trafficking Machinery. IUBMB Life 72 (6), 1243–1249. doi: 10.1002/iub.2294 [DOI] [PubMed] [Google Scholar]
- Bayer-Santos E., Aguilar-Bonavides C., Rodrigues S. P., Cordero E. M., Marques A. F., Varela-Ramirez A., et al. (2013). Proteomic Analysis of Trypanosoma Cruzi Secretome: Characterization of Two Populations of Extracellular Vesicles and Soluble Proteins. J. Prot. Res. 12 (2), 883–897. doi: 10.1021/pr300947g [DOI] [PubMed] [Google Scholar]
- Beer K. B., Wehman A. M. (2017). Mechanisms and Functions of Extracellular Vesicle Release In Vivo-What We Can Learn From Flies and Worms. Cell Adhes. Migr. 11 (2), 135–150. doi: 10.1080/19336918.2016.1236899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M. S., Fentress S. J., Mashayekhi M., Li L. X., Taylor G. A., Sibley L. D. (2012). The Polymorphic Pseudokinase ROP5 Controls Virulence in Toxoplasma Gondii by Regulating the Active Kinase Rop18. PloS Pathog. 8 (11), e1002992. doi: 10.1371/journal.ppat.1002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraki T., Hu X., Broncel M., Young J. C., O'Shaughnessy W. J., Borek D., et al. (2019). Divergent Kinase Regulates Membrane Ultrastructure of the Toxoplasma Parasitophorous Vacuole. P. Ntl. Acad. Sci. U. S. A. 116 (13), 6361–6370. doi: 10.1073/pnas.1816161116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio H., Soldati-Favre D. (2019). Signaling Cascades Governing Entry Into and Exit From Host Cells by Toxoplasma Gondii. Annu. Rev. Microbiol. Gottesman 73, 579–599. doi: 10.1146/annurev-micro-020518-120235 [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C. (2013). Have It Your Way: How Polymorphic, Injected Kinases and Pseudokinases Enable Toxoplasma to Subvert Host Defenses. PloS Pathog. 9 (4), e1003296. doi: 10.1371/journal.ppat.1003296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. J., Ward C., Cheng S. J., Alexander D. L., Coller S., Coombs G. H., et al. (2005). Proteomic Analysis of Rhoptry Organelles Reveals Many Novel Constituents for Host-Parasite Interactions in Toxoplasma Gondii. J. Biol. Chem. 280 (40), 34245–34258. doi: 10.1074/jbc.M504158200 [DOI] [PubMed] [Google Scholar]
- Brandt G. S., Bailey S. (2013). Dematin, a Human Erythrocyte Cytoskeletal Protein, Is a Substrate for a Recombinant FIKK Kinase From Plasmodium Falciparum. Mol. Biochem. Parasitol. 191 (1), 20–23. doi: 10.1016/j.molbiopara.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Brossas J.-Y., Nicolas Gulin J. E., Catalina Bisio M. M., Chapelle M., Marinach-Patrice C., Bordessoules M., et al. (2017). Secretome Analysis of Trypanosoma Cruzi by Proteomics Studies. PloS One 12 (10), e0185504. doi: 10.1371/journal.pone.0185504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz K. R., Bowyer P. W., Boothroyd J. C. (2013). Bradyzoite Pseudokinase 1 Is Crucial for Efficient Oral Infectivity of the Toxoplasma Gondii Tissue Cyst. Eukaryotic Cell 12 (3), 399–410. doi: 10.1128/ec.00343-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher B. A., Fox B. A., Rommereim L. M., Kim S. G., Maurer K. J., Yarovinsky F., et al. (2011). Toxoplasma Gondii Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control. PloS Pathog. 7 (9), e1002236. doi: 10.1371/journal.ppat.1002236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera-Bravo C., Koh E. Y., Tan K. S. W. (2021). The Roles of Parasite-Derived Extracellular Vesicles in Disease and Host-Parasite Communication. Parasitol. Int. 83, e102373. doi: 10.1016/j.parint.2021.102373 [DOI] [PubMed] [Google Scholar]
- Coffey M. J., Sleebs B. E., Uboldi A. D., Garnham A., Franco M., Marino N. D., et al. (2015). An Aspartyl Protease Defines a Novel Pathway for Export of Toxoplasma Proteins Into the Host Cell. Elife 4, e10809. doi: 10.7554/eLife.10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Raposo G., Thery C. (2014). Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Ann. Rev. Cell Dev. Biol. 30, 255–289. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Costa R. W., da Silveira J. F., Bahia D. (2016). Interactions Between Trypanosoma Cruzi Secreted Proteins and Host Cell Signaling Pathways. Front. Microbiol. 7, 388. doi: 10.3389/fmicb.2016.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H., Belda H., Broncel M., Ye X., Bisson C., Introini V., et al. (2020). An Exported Kinase Family Mediates Species-Specific Erythrocyte Remodelling and Virulence in Human Malaria. Nat. Microbiol. 5 (6), 848–884. doi: 10.1038/s41564-020-0702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning-Ward T. F., Gilson P. R., Boddey J. A., Rug M., Smith B. J., Papenfuss A. T., et al. (2009). A Newly Discovered Protein Export Machine in Malaria Parasites. Nature 459 (7249), 945–U966. doi: 10.1038/nature08104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo M. A., Sausset A., Gnahoui-David A., Silva A. R. E., Brionne A., Le Vern Y., et al. (2019). Eimeria Tenella ROP Kinase EtROP1 Induces G0/G1 Cell Cycle Arrest and Inhibits Host Cell Apoptosis. Cell. Microbiol. 21 (7), e13027. doi: 10.1111/cmi.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugonska H., Gatkowska J. (2016). Exosomes in the Context of Toxoplasma Gondii - Host Communication. Ann. Parasitol. 62 (3), 169–174. doi: 10.17420/ap6203.50 [DOI] [PubMed] [Google Scholar]
- Donald R. G. K., Zhong T., Meijer L., Liberator P. A. (2005). Characterization of Two T. Gondii CK1 Isoforms. Mol. Biochem. Parasitol. 141 (1), 15–27. doi: 10.1016/j.molbiopara.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Dorin-Semblat D., Demarta-Gatsi C., Hamelin R., Armand F., Carvalho T. G., Moniatte M., et al. (2015). Malaria Parasite-Infected Erythrocytes Secrete PfCK1, the Plasmodium Homologue of the Pleiotropic Protein Kinase Casein Kinase 1. PloS One 10 (12), e0139591. doi: 10.1371/journal.pone.0139591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douanne N., Dong G., Douanne M., Olivier M., Fernandez-Prada C. (2020). Unravelling the Proteomic Signature of Extracellular Vesicles Released by Drug-Resistant Leishmania Infantum Parasites. PloS Neg. Trop. Dis. 14 (7), e0008439. doi: 10.1371/journal.pntd.0008439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry L. L., Jones N. G., Wang Q., Onken M. D., Miller M. J., Sibley L. D. (2019). The Secreted Kinase ROP17 Promotes Toxoplasma Gondii Dissemination by Hijacking Monocyte Tissue Migration. Nat. Microbiol. 4 (11), 1951–1963. doi: 10.1038/s41564-019-0504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois D. J., Soldati-Favre D. (2019). Biogenesis and Secretion of Micronemes in Toxoplasma Gondii. Cell. Microbiol. 21 (10), e13018. doi: 10.1111/cmi.13105 [DOI] [PubMed] [Google Scholar]
- Dunn P. P. J., Bumstead J. M., Tomley F. M. (1996). Sequence, Expression and Localization of Calmodulin-Domain Protein Kinases in Eimeria Tenella and Eimeria Maxima. Parasitology 113, 439–448. doi: 10.1017/s0031182000081506 [DOI] [PubMed] [Google Scholar]
- Etheridge R. D., Alaganan A., Tang K., Lou H. J., Turk B. E., Sibley L. D. (2014). The Toxoplasma Pseudokinase ROP5 Forms Complexes With ROP18 and ROP17 Kinases That Synergize to Control Acute Virulence in Mice. Cell Host Microbe 15 (5), 537–550. doi: 10.1016/j.chom.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., He X., Zhang P., Shen C., Mwangi J., Xu C., et al. (2019). In Vitro and In Vivo Antimalarial Activity of LZ1, A Peptide Derived From Snake Cathelicidin. Toxins 11 (7), 379. doi: 10.3390/toxins11070379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress S. J., Sibley L. D. (2011). The Secreted Kinase ROP18 Defends Toxoplasma's Border. Bioessays 33 (9), 693–700. doi: 10.1002/bies.201100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira E. R., Horjales E., Bonfim-Melo A., Cortez C., da Silva C. V., De Groote M., et al. (2016). Unique Behavior of Trypanosoma Cruzi Mevalonate Kinase: A Conserved Glycosomal Enzyme Involved in Host Cell Invasion and Signaling. Sci. Rep. 6, 24610. doi: 10.1038/srep24610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein M. C., Reese M. L., Konen-Waisman S., Boothroyd J. C., Howard J. C., Steinfeldt T. (2012). A Toxoplasma Gondii Pseudokinase Inhibits Host IRG Resistance Proteins. PloS Biol. 10 (7), e1001358. doi: 10.1371/journal.pbio.1001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige T., Fischer K., Ferguson D. J. P., Gross U., Bohne W. (2007). Carbohydrate Metabolism in the Toxoplasma Gondii Apicoplast: Localization of Three Glycolytic Isoenzymes, the Single Pyruvate Dehydrogenase Complex, and a Plastid Phosphate Translocator. Eukaryotic Cell 6 (6), 984–996. doi: 10.1128/ec.00061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. A., Rommereim L. M., Guevara R. B., Falla A., Triana M. A. H., Sun Y., et al. (2016). The Toxoplasma Gondii Rhoptry Kinome Is Essential for Chronic Infection. Mbio 7 (3). doi: 10.1128/mBio.00193-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Wahid M., Khan F. (2020). Regulation of Peripheral and Central Immunity: Understanding the Role of Src Homology 2 Domain-Containing Tyrosine Phosphatases, SHP-1 & SHP-2. Immunobiology 225 (1), 151847. doi: 10.1016/j.imbio.2019.09.006 [DOI] [PubMed] [Google Scholar]
- Gualdron-Lopez M., Flannery E. L., Kangwanrangsan N., Chuenchob V., Fernandez-Orth D., Segui-Barber J., et al. (2018). Characterization of Plasmodium vivax Proteins in Plasma-Derived Exosomes From Malaria-Infected Liver-Chimeric Humanized Mice. Front. Microbiol. 9, 1–15. doi: 10.3389/fmicb.2018.01271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H. Y., Zhu S. H., Jiang L. L., Li Y., Dong H., Zhao Q. P., et al. (2013). Molecular Characterization and Analysis of a Novel Calcium-Dependent Protein Kinase From Eimeria Tenella. Parasitology 140 (6), 746–755. doi: 10.1017/s0031182012002107 [DOI] [PubMed] [Google Scholar]
- Hassani K., Antoniak E., Jardim A., Olivier M. (2011). Temperature-Induced Protein Secretion by Leishmania Mexicana Modulates Macrophage Signalling and Function. PloS One 6 (5), e18724. doi: 10.1371/journal.pone.0018724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller N. L., Bhattacharjee S., van Ooij C., Liolios K., Harrison T., Lopez-Estrano C., et al. (2004). A Host-Targeting Signal in Virulence Proteins Reveals a Secretome in Malarial Infection. Science 306 (5703), 1934–1937. doi: 10.1126/science.1102737 [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Sibley L. D. (2012). Modulation of Innate Immunity by Toxoplasma Gondii Virulence Effectors. Nat. Rev. Microbiol. 10 (11), 766–778. doi: 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats L. M., Fernandez K. M., Glenister F. K., Herrmann S., Buckingham D. W., Siddiqui G., et al. (2014). An Exported Kinase (FIKK4.2) That Mediates Virulence-Associated Changes in Plasmodium Falciparum-Infected Red Blood Cells. Int. J. Parasitol. 44 (5), 319–328. doi: 10.1016/j.ijpara.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Kolli B. K., Kostal J., Zaborina O., Chakrabarty A. A., Chang K.-P. (2008). Leishmania-Released Nucleoside Diphosphate Kinase Prevents ATP-Mediated Cytolysis of Macrophages. Mol. Biochem. Parasitol. 158 (2), 163–175. doi: 10.1016/j.molbiopara.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G., Vikram R., Singh S. B., Patel N., Agarwal S., Mukhopadhyay G., et al. (2005). Hemoglobin Receptor in Leishmania Is a Hexokinase Located in the Flagellar Pocket. J. Biol. Chem. 280 (7), 5884–5891. doi: 10.1074/jbc.M411845200 [DOI] [PubMed] [Google Scholar]
- Kulkarni P. G., Shah N., Waghela B. N., Pathak C. M., Pappachan A. (2019). Leishmania Donovani Adenylate Kinase 2a Prevents ATP-Mediated Cell Cytolysis in Macrophages. Parasitol. Int. 72, 101929. doi: 10.1016/j.parint.2019.101929 [DOI] [PubMed] [Google Scholar]
- Labbé M., Peroval M., Bourdieu C., Girard-Misguich F., Pery P. (2006). Eimeria Tenella Enolase and Pyruvate Kinase: A Likely Role in Glycolysis and in Others Functions. Int. J. Parasitol. 36 (14), 1443–1452. doi: 10.1016/j.ijpara.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Lal K., Prieto J. H., Bromley E., Sanderson S. J., Yates J. R., Wastling J. M., et al. (2009). Characterisation of Plasmodium Invasive Organelles; An Ookinete Microneme Proteome. Proteomics 9 (5), 1142–1151. doi: 10.1002/pmic.200800404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., Wickert H., Krohne G., Vincensini L., Breton C. B. (2006). Maurer's Clefts: A Novel Multi-Functional Organelle in the Cytoplasm of Plasmodium Falciparum-Infected Erythrocytes. Int. J. Parasitol. 36 (1), 23–36. doi: 10.1016/j.ijpara.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Lee W.-K., Ahn H.-J., Baek J.-H., Lee C.-H., Yu Y. G., Nam H.-W. (2014). Comprehensive Proteome Analysis of the Excretory/Secretory Proteins of Toxoplasma Gondii. B. Korean Chem. Soc. 35 (10), 3071–3076. doi: 10.5012/bkcs.2014.35.10.3071 [DOI] [Google Scholar]
- Liu L., Tucker S. C., Satir B. H. (2009). Toxoplasma PRP1 Is an Ortholog of Parafusin (PFUS) in Vesicle Scaffold Assembly in Ca2+-Regulated Exocytosis. Eur. J. Cell Biol. 88 (5), 301–313. doi: 10.1016/j.ejcb.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Li Y., Xiu F., Mou Z., Xue Z., Du H., Zhou C., et al. (2018). Exosomes Derived From Toxoplasma Gondii Stimulate an Inflammatory Response Through JNK Signaling Pathway. Nanomedicine 13 (10), 1157–1168. doi: 10.2217/nnm-2018-0035 [DOI] [PubMed] [Google Scholar]
- Lykins J. D., Filippova E. V., Halavaty A. S., Minasov G., Zhou Y., Dubrovska I., et al. (2018). CSGID Solves Structures and Identifies Phenotypes for Five Enzymes in Toxoplasma Gondii. Front. Cell. Infect. Microbiol. 8, 352. doi: 10.3389/fcimb.2018.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandacaru S. C., Queiroz R. M. L., Alborghetti M. R., de Oliveira L. S., de Lima C. M. R., Bastos I. M. D., et al. (2019). Exoproteome Profiling of Trypanosoma cruzi During Amastigogenesis Early Stages. Plos One 14 (11), e0225386. doi: 10.1371/journal.pone.0225386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel P.-Y., Hoang A. N., Goldowitz I., Potashnikova D., Hamza B., Vorobjev I., et al. (2013). Malaria-Infected Erythrocyte-Derived Microvesicles Mediate Cellular Communication Within the Parasite Population and With the Host Immune System. Cell Host Microbe 13 (5), 521–534. doi: 10.1016/j.chom.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino N. D., Panas M. W., Franco M., Theisen T. C., Naor A., Rastogi S., et al. (2018). Identification of a Novel Protein Complex Essential for Effector Translocation Across the Parasitophorous Vacuole Membrane of Toxoplasma Gondii. PloS Pathog. 14 (1), e1006828. doi: 10.1371/journal.ppat.1006828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M., Good R. T., Rug M., Knuepfer E., Cowman A. F. (2004). Targeting Malaria Virulence and Remodeling Proteins to the Host Erythrocyte. Science 306 (5703), 1930–1933. doi: 10.1126/science.1102452 [DOI] [PubMed] [Google Scholar]
- Martin-Jaular L., Nakayasu E. S., Ferrer M., Almeida I. C., del Portillo H. A. (2011). Exosomes From Plasmodium yoelii-Infected Reticulocytes Protect Mice From Lethal Infections. Plos One 6 (10), e26588. doi: 10.1371/journal.pone.0026588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M., Martin-Jaular L., Lavieu G., Thery C. (2019). Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 21 (1), 9–17. doi: 10.1038/s41556-018-0250-9 [DOI] [PubMed] [Google Scholar]
- Miranda M. R., Canepa G. E., Bouvier L. A., Pereira C. A. (2008). Trypanosoma Cruzi Nucleoside Diphosphate Kinase 1 (TcNDPK1) has a Broad Nuclease Activity. Parasitology 135 (14), 1661–1666. doi: 10.1017/s0031182008005106 [DOI] [PubMed] [Google Scholar]
- Montaner S., Galiano A., Trelis M., Martin-Jaular L., del Portillo H. A., Bernal D., et al. (2014). The Role of Extracellular Vesicles in Modulating the Host Immune Response During Parasitic Infections. Front. Immun. 5, 433. doi: 10.3389/fimmu.2014.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D. S., Murta S. M. F. (2016). Involvement of Nucleoside Diphosphate Kinase B and Elongation Factor 2 in Leishmania Braziliensis Antimony Resistance Phenotype. Parasite Vector 9, 641. doi: 10.1186/s13071-016-1930-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan D., Tran T., Trinh E., Silverman J. M., Lopez M. (2007). Identification of Leishmania Fructose-1,6-Bisphosphate Aldolase as a Novel Activator of Host Macrophage Src Homology 2 Domain Containing Protein Tyrosine Phosphatase SHP-1. Biochem. Biophys. Res. Commun. 364 (3), 601–607. doi: 10.1016/j.bbrc.2007.10.065 [DOI] [PubMed] [Google Scholar]
- Nunes M. C., Okada M., Scheidig-Benatar C., Cooke B. M., Scherf A. (2010). Plasmodium Falciparum FIKK Kinase Members Target Distinct Components of the Erythrocyte Membrane. PloS One 5 (7), e11747. doi: 10.1371/journal.pone.0011747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes R. D., Kurian D., Bromley C., Ward C., Lal K., Blake D. P., et al. (2013). The Rhoptry Proteome of Eimeria tenella Sporozoites. Int. J. Parasitol. 43 (2), 181–188. doi: 10.1016/j.ijpara.2012.10.024 [DOI] [PubMed] [Google Scholar]
- Ong Y. C., Reese M. L., Boothroyd J. C. (2010). Toxoplasma Rhoptry Protein 16 (ROP16) Subverts Host Function by Direct Tyrosine Phosphorylation of STAT6. J. Biol. Chem. 285 (37), 28731–28740. doi: 10.1074/jbc.M110.112359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas M. W., Ferrel A., Naor A., Tenborg E., Lorenzi H. A., Boothroyd J. C. (2019). Translocation of Dense Granule Effectors Across the Parasitophorous Vacuole Membrane in Toxoplasma-Infected Cells Requires the Activity of ROP17, a Rhoptry Protein Kinase. mSphere 4 (4), e00276-00219. doi: 10.1128/mSphere.00276-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L., Chen F., Harb O. S., Davis P. H., Beiting D. P., Brownback C. S., et al. (2010). Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases That Regulate Host Responses. Cell Host Microbe 8 (2), 208–218. doi: 10.1016/j.chom.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C. A., Bouvier L. A., Camara M.d. L.M., Miranda M. R. (2011). Singular Features of Trypanosomatids' Phosphotransferases Involved in Cell Energy Management. Enzyme Res. 2011, 576483–576483. doi: 10.4061/2011/576483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz R. M. L., Ricart C. A. O., Machado M. O., Bastos I. M. D., de Santana J. M., de Sousa M. V., et al. (2016). Insight Into the Exoproteome of the Tissue-Derived Trypomastigote Form of Trypanosoma cruzi. Front. Chem. 4, 42. doi: 10.3389/fchem.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi N., Knippschild U., Spath G. F. (2021). Dangerous Duplicity: The Dual Functions of Casein Kinase 1 in Parasite Biology and Host Subversion. Front. Cell. Infect. Microbiol. 11, 655700. doi: 10.3389/fcimb.2021.655700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Flores C. J., Cruz-Miron R., Mondragon-Castelan M. E., Gonzalez-Pozos S., Rios-Castro E., Mondragon-Flores R. (2019). Proteomic and Structural Characterization of Self-Assembled Vesicles From Excretion/Secretion Products of Toxoplasma gondii . J. Proteomics 208, 103490. doi: 10.1016/j.jprot.2019.103490 [DOI] [PubMed] [Google Scholar]
- Regev-Rudzki N., Wilson D. W., Carvalho T. G., Sisquella X., Coleman B. M., Rug M., et al. (2013). Cell-Cell Communication Between Malaria-Infected Red Blood Cells via Exosome-Like Vesicles. Cell 153 (5), 1120–1133. doi: 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- Ribeiro K. S., Vasconcellos C. I., Soares R. P., Mendes M. T., Ellis C. C., Aguilera-Flores M., et al. (2018). Proteomic Analysis Reveals Different Composition of Extracellular Vesicles Released by Two Trypanosoma cruzi Strains Associated With Their Distinct Interaction With Host Cells. J. Extracell. Vesicles 7 (1), 1463779. doi: 10.1080/20013078.2018.1463779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro E Silva A., Sausset A., Bussiere F. I., Laurent F., Lacroix-Lamande S., Silvestre A. (2021). Genome-Wide Expression Patterns of Rhoptry Kinases During the Eimeria Tenella Life-Cycle. Microorganisms 9 (8), 1621. doi: 10.3390/microorganisms9081621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij J. P. J., Boyle J. P., Coller S., Taylor S., Sibley L. D., Brooke-Powell E. T., et al. (2006). Polymorphic Secreted Kinases Are Key Virulence Factors in Toxoplasmosis. Science 314 (5806), 1780–1783. doi: 10.1126/science.1133690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Maeda T., Nakazawa M., Takeuchi T., Nozaki T., Asai T. (2002). Characterisation of Hexokinase in Toxoplasma Gondii Tachyzoites. Int. J. Parasitol. 32 (8), 961–967. doi: 10.1016/s0020-7519(02)00059-0 [DOI] [PubMed] [Google Scholar]
- Severino V., Farina A., Chambery A. (2013). Analysis of Secreted Proteins. Method. Mol. Biol. (Clifton N. J.) 1002, 37–60. doi: 10.1007/978-1-62703-360-2_4 [DOI] [PubMed] [Google Scholar]
- Siddiqui G., Proellochs N. I., Cooke B. M. (2020). Identification of Essential Exported Plasmodium Falciparum Protein Kinases in Malaria-Infected Red Blood Cells. Brit. J. Haematol. 188 (5), 774–783. doi: 10.1111/bjh.16219 [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Chan S. K., Robinson D. P., Dwyer D. M., Nandan D., Foster L. J., et al. (2008). Proteomic Analysis of the Secretome of Leishmania Donovani. Genome Biol. 9 (2), R35. doi: 10.1186/gb-2008-9-2-r35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. M., Clos J., de'Oliveira C. C., Shirvani O., Fang Y., Wang C., et al. (2010. a). An Exosome-Based Secretion Pathway Is Responsible for Protein Export From Leishmania and Communication With Macrophages. J. Cell Sci. 123 (6), 842–852. doi: 10.1242/jcs.056465 [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Clos J., Horakova E., Wang A. Y., Wiesgigl M., Kelly I., et al. (2010. b). Leishmania Exosomes Modulate Innate and Adaptive Immune Responses Through Effects on Monocytes and Dendritic Cells. J. Immunol. 185 (9), 5011–5022. doi: 10.4049/jimmunol.1000541 [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Reiner N. E. (2011). Exosomes and Other Microvesicles in Infection Biology: Organelles With Unanticipated Phenotypes. Cell. Microbiol. 13 (1), 1–9. doi: 10.1111/j.1462-5822.2010.01537.x [DOI] [PubMed] [Google Scholar]
- Simpson C., Jones N. G., Hull-Ryde E. A., Kireev D., Stashko M., Tang K., et al. (2016). Identification of Small Molecule Inhibitors That Block the Toxoplasma Gondii Rhoptry Kinase Rop18. ACS Infect. Dis. 2 (3), 194–206. doi: 10.1021/acsinfecdis.5b00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisquella X., Ofir-Birin Y., Pimentel M. A., Cheng L., Abou Karam P., Sampaio N. G., et al. (2017). Malaria Parasite DNA-Harbouring Vesicles Activate Cytosolic Immune Sensors. Nat. Commun. 8, 1985. doi: 10.1038/s41467-017-02083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirlis D., Dingli F., Pescher P., Prina E., Loew D., Rachidi N., et al. (2020). SILAC-Based Quantitative Proteomics Reveals Pleiotropic, Phenotypic Modulation in Primary Murine Macrophages Infected With the Protozoan Pathogen Leishmania Donovani. J. Proteomics 213. doi: 10.1016/j.jprot.2019.103617 [DOI] [PubMed] [Google Scholar]
- Smirlis D., Dingli F., Sabatet V., Roth A., Knippchild U., Loew D., et al. (2022). SILAKin: A Novel High Throughput SILAC and Mass Spectrometry-Based Assay to Identify the Substratome of Kinases Secreted by Pathogens. Front. Cell. Dev. Biol. 9, 800098. doi: 10.3389/fcell.2021.800098. 2005.2005.442720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Liao S., Zhang L., Wu C., Qi N., Lv M., et al. (2016). Molecular and Biochemical Characterization of Eimeria Tenella Hexokinase. Parasitol. Res. 115 (9), 3425–3433. doi: 10.1007/s00436-016-5104-4 [DOI] [PubMed] [Google Scholar]
- Talevich E., Mirza A., Kannan N. (2011). Structural and Evolutionary Divergence of Eukaryotic Protein Kinases in Apicomplexa. BMC Evol. Biol. 11, 321. doi: 10.1186/1471-2148-11-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F., Fussenegger M. (2021). Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 8 (1), 2003505. doi: 10.1002/advs.202003505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomavo S., Slomianny C., Meissner M., Carruthers V. B. (2013). Protein Trafficking Through the Endosomal System Prepares Intracellular Parasites for a Home Invasion. PloS Pathog. 9 (10), e1003629. doi: 10.1371/journal.ppat.1003629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilhas A. C., Soares R. P., Schenkman S., Fernandez-Prada C., Olivier M. (2020). Extracellular Vesicles in Trypanosomatids: Host Cell Communication. Front. Cell. Infect. Microbiol. 10, 602502. doi: 10.3389/fcimb.2020.602502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocoli Torrecilhas A. C., Tonelli R. R., Pavanelli W. R., da Silva J. S., Schumacher R. I., de Souza W., et al. (2009). Trypanosoma Cruzi: Parasite Shed Vesicles Increase Heart Parasitism and Generate an Intense Inflammatory Response. Microbes Infect. 11 (1), 29–39. doi: 10.1016/j.micinf.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Venugopal K., Chehade S., Werkmeister E., Barois N., Periz J., Lafont F., et al. (2020). Rab11A Regulates Dense Granule Transport and Secretion During Toxoplasma Gondii Invasion of Host Cells and Parasite Replication. PloS Pathog. 16 (5), e1008106. doi: 10.1371/journal.ppat.1008106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincensini L., Richert S., Blisnick T., Van Dorsselaer A., Leize-Wagner E., Rabilloud T., et al. (2005). Proteomic Analysis Identifies Novel Proteins of the Maurer’s Clefts, A Secretory Compartment Delivering Plasmodium falciparum Proteins to the Surface of Its Host Cell. Mol. Cell. Proteom. 4 (4), 582–593. doi: 10.1074/mcp.M400176-MCP200 [DOI] [PubMed] [Google Scholar]
- Wang Z., Huang B., Dong H., Zhao Q., Zhu S., Xia W., et al. (2016. a). Molecular Characterization and Functional Analysis of a Novel Calcium-Dependent Protein Kinase 4 From Eimeria Tenella. PloS One 11 (12), e0168132. doi: 10.1371/journal.pone.0168132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang S., Wang W., Gu Y., Liu H., Wei F., et al. (2016. b). Targeted Disruption of CK1 Alpha in Toxoplasma Gondii Increases Acute Virulence in Mice. Eur. J. Protistol. 56, 90–101. doi: 10.1016/j.ejop.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Ward P., Equinet L., Packer J., Doerig C. (2004). Protein Kinases of the Human Malaria Parasite Plasmodium Falciparum: The Kinome of a Divergent Eukaryote. BMC Genomics 5. doi: 10.1186/1471-2164-5-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wowk P. F., Zardo M. L., Miot H. T., Goldenberg S., Carvalho P. C., Moerking P. A. (2017). Proteomic Profiling of Extracellular Vesicles Secreted From Toxoplasma Gondii. Proteomics 17 (15-16), 1600477. doi: 10.1002/pmic.201600477 [DOI] [PubMed] [Google Scholar]
- Xu P., Ianes C., Gaertner F., Liu C., Burster T., Bakulev V., et al. (2019). Structure, Regulation, and (Patho-)Physiological Functions of the Stress-Induced Protein Kinase CK1 Delta (CSNK1D). Gene 715, 144005. doi: 10.1016/j.gene.2019.144005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Standley D. M., Takashima S., Saiga H., Okuyama M., Kayama H., et al. (2009). A Single Polymorphic Amino Acid on Toxoplasma Gondii Kinase ROP16 Determines the Direct and Strain-Specific Activation of Stat3. J. Exp. Med. 206 (12), 2747–2760. doi: 10.1084/jem.20091703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Rao X., Zhang K. (2017). Nucleoside Diphosphate Kinase (Ndk): A Pleiotropic Effector Manipulating Bacterial Virulence and Adaptive Responses. Microbiol. Res. 205, 125–134. doi: 10.1016/j.micres.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Zhong W., Li K., Cai Q., Guo J., Yuan M., Wong Y. H., et al. (2020). Pyruvate Kinase From Plasmodium Falciparum: Structural and Kinetic Insights Into the Allosteric Mechanism. Biochem. Bioph. Res. Commun. 532 (3), 370–376. doi: 10.1016/j.bbrc.2020.08.048 [DOI] [PubMed] [Google Scholar]