Abstract
Although parasitic infections do not usually present with disturbance in renal function, glomerular lesions can be seen in most of these infections. The glomerular lesions observed in parasitic infections cover the whole range of glomerular lesions known, but most of them are proliferative. Little is known of the exact pathogenic mechanisms. In this review, we try to explain the glomerular lesions associated with parasitic infections in terms of the specific immunologic events observed during these diseases against the background of recent developments in the general knowledge of the pathogenesis of glomerular disease.
Parasitic infections are a heterogeneous group of diseases that have some common features. Most parasitic infections are chronic, and the host immune response reacts to the different stages of the parasite life cycle involving different parasite antigens. The chronicity of these infections is characterized by fluctuations in antigenemia and therefore in host responses. Although renal disease is not one of the common presenting features, many parasitic infections are associated with glomerular lesions. In this article, we first summarize the terminology used to describe the basic pattern of glomerular lesions pertinent to parasitic infections, then review the glomerulopathies observed and the pathogenic mechanisms thought to be involved in the individual infections, and finally discuss the general mechanisms that can be extracted from these observations.
GLOMERULAR PATHOLOGY
In general, glomerular lesions are described according to (i) the histologic appearance of glomeruli, (ii) the fluorescence pattern reflecting the location and the type of immunoglobulins and complement components in glomeruli, and (iii) the specific ultrastructural changes observed by electron microscopy. The terminology of these descriptive diagnoses refers to the anatomy (Fig. 1) and histology (Fig. 2) of the normal glomerulus; e.g., “mesangioproliferative” suggests the proliferation of mesangial cells, and “subendothelial deposition” refers to deposition of immune complexes on the endothelial side of the glomerular basement membrane.
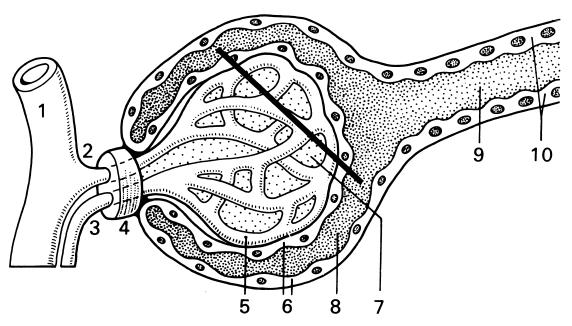
FIG. 1.
Glomerular anatomy. 1, interlobular artery; 2, afferent arteriole; 3, efferent arteriole; 4, juxtaglomerular apparatus; 5, glomerular capillary; 6, epithelial cells and Bowman's capsule; 7, mesangium; 8 and 9 urinary space; 10, proximal tube. Reprinted from reference 36 with permission of the publisher.
FIG. 2.
Glomerular histology. A transverse section through the structure in Fig. 1 is shown, giving details at the electron microscopic level. cap, glomerular capillary; ep, epithelium; bc, Bowman's capsule; ur, urinary space; end, endothelium; mes, mesangium; arrows, glomerular basement membrane. Reprinted from reference 36 with permission of the publisher.
Glomerular lesions observed in parasitic infections cover the whole range of lesions known. Most of these lesions are proliferative and therefore show an accumulation of cells in the glomerular tuft, i.e., a membranoproliferative (synonym of mesangiocapillary) or mesangioproliferative type of glomerulonephritis. Glomerular lesions with little or no proliferation, such as in membranous glomerulopathy, focal segmental glomerulosclerosis, and minimal-change disease, are sometimes seen.
Different clinical syndromes are associated with each type of glomerulopathy. The clinical manifestations range from isolated proteinuria or hematuria to nephrotic syndrome (proteinuria of >3.5 g/day, hypoalbuminemia, generalized edema, and hyperlipidemia), nephritic syndrome (glomerular hematuria, recognized by erythrocyte casts in the urine, and diminished glomerular filtration with some degree of azotemia, oliguria, and hypertension), renal insufficiency, and rapidly progressive glomerulonephritis (nephritic syndrome with doubling of the creatinine level in serum within 3 months as a sign of progressive renal failure). The renal diseases associated with parasitic infections are summarized in Table 1.
TABLE 1.
Renal manifestations associated with parasitic diseasesa
| Parasite (disease) | Host | Clinical manifestations | Renal lesionsb | Pathogenesisb |
|---|---|---|---|---|
| Plasmodium malariae (quartan malaria) | Human | Proteinuria to nephrotic syndrome | Mesangioproliferative GN, membranoproliferative GN | IC, autoimmune component |
| Human | Nephrotic syndrome | Minimal-change disease (rare) | ||
| Human | Nephrotic syndrome | Focal segmental glomerulosclerosis (rare) | ||
| Human | Nephrotic syndrome | Membranous GP (rare) | IC, autoimmune component | |
| Aotus monkey | Nephrotic syndrome | Membranoproliferative GN | IC | |
| Plasmodium falciparum (tertian malaria) | Human | Acute renal failure | Tubulointerstitial damage | Hemolysis and hypoperfusion |
| Human | Proteinuria to nephrotic syndrome (rare) | Mesangioproliferative GN, membranoproliferative GN (rare) | IC, coagulopathy | |
| Aotus monkey | Mesangioproliferative GN | IC, coagulopathy | ||
| Plasmodium berghei | Rat, mouse | Proteinuria | Mesangioproliferative GN | IC, autoimmune component |
| Schistosoma haematobium | Human | Chronic renal failure | Hydronephrosis and pyelonephritis | Vesicoureteral fibrosis and reflux |
| Schistosoma mansoni | Human | Proteinuria | Mesangioproliferative GN, membranoproliferative GN | IC, autoimmune component, portal shunting |
| Human | Nephrotic syndrome | Focal segmental glomerulosclerosis | ||
| Human | Nephrotic syndrome | Membranous GP | ||
| Human | Nephrotic syndrome | Amyloidosis (rare) | ||
| Human | Acute renal failure | Crescentic GN (rare) | ||
| Baboon | Membranoproliferative GN | IC | ||
| Schistosoma japonicum | Primate, rabbit | Mesangioproliferative GN | IC | |
| Membranoproliferative GN | ||||
| Leishmania donovani (kala-azar) | Human | Proteinuria | Mesangioproliferative GN | IC, autoimmune component, coagulopathy |
| Human | Nephrotic syndrome | Amyloidosis | ||
| Hamster | Mesangioproliferative GN | IC | ||
| Trypanosoma brucei rhodesiense, Trypanosoma brucei gambiense | Human | Proteinuria | Mesangioproliferative GN (rare) | IC |
| Trypanosoma brucei brucei | Rhesus monkey, mouse, rat, rabbit | Proteinuria | Mesangioproliferative GN, membranoproliferative GN | IC, autoimmune component |
| Toxoplasma gondii | Human | Proteinuria to nephritic syndrome | Membranoproliferative GN | IC, coagulopathy |
| Human | Nephrotic syndrome | Focal segmental glomerulosclerosis (rare) | Congenital toxoplasmosis | |
| Human | Acute renal failure | Tubulointerstitial nephritis | Sulfadiazine crystal deposition | |
| Trichinella spiralis | Human | Proteinuria | Mesangioproliferative GN, membranoproliferative GN | IC, coagulopathy |
| Human | Mild renal failure | Tubular necrosis | Hypovolemia and myoglobinuria | |
| Opisthorchis viverrini | Hamster | Proteinuria | Mesangioproliferative GN, amyloidosis | IC |
| Babesia bovis, Babesia divergens, Babesia microti | Human | Acute renal failure | Tubular necrosis | Shock and hemolysis |
| Babesia canis | Dog | Acute renal failure | Tubular necrosis | Shock and hemolysis |
| Babesia rhodhaini | Rat | Mild proteinuria | Mesangioproliferative GN | IC |
| Echinococcus granulosus (hydatid disease) | Human | Nephrotic syndrome | Membranous GP (rare) | IC |
| Mass effect | Cyst (generally solitary) | |||
| Wuchereria bancrofti, Brugia malayi (lymphatic filariasis), Onchocerca volvulus (river blindness), Loa loa | Human | Proteinuria to nephritic syndrome | Mesangioproliferative GN, membranoproliferative GN | IC |
| Human | Nephrotic syndrome | Amyloidosis (rare) | ||
| Human | Renal failure | Interstitial nephritis (rare) | ||
| Wuchereria bancrofti | Human | Nephritic syndrome | Acute GN (rare) | |
| Loa loa | Human | Nephrotic syndrome | Membranous GP | |
| Human | Nephrotic syndrome | Focal segmental glomerulosclerosis (rare) |
Membranoproliferative glomerulonephritis is characterized by accentuation of the lobulation of the capillary tuft due to mesangial and endothelial proliferation; this is generally segmental in membranoproliferative glomerulonephritis secondary to infections. The proliferations are seen in association with the typical double contour of the glomerular capillary wall. These lesions are thought to be due to the combination of chronic glomerular showers of antigens, antibodies, and/or immune complexes; activation of the coagulation cascade; and hemodynamic features. All these factors lead, on the one hand, to immune complex depositions in the mesangial and subendothelial areas and, on the other hand, to endothelial-cell damage. The clinical manifestations range from isolated proteinuria or hematuria to the nephrotic syndrome or the nephritic syndrome.
In pure mesangioproliferative glomerulonephritis, glomeruli exhibit extension of the mesangial matrix, proliferation of mesangial cells, and immunoglobulin deposits in these mesangial areas. The clinical manifestations in this context vary from minimal to mild proteinuria.
Membranous glomerulopathy is characterized by the presence of electron-dense deposits containing immunoglobulins along the epithelial side of the basement membrane. By light microscopy, glomeruli either appear normal or exhibit diffuse thickening of the glomerular capillary wall with formation of spikes. Patients with these lesions present with nephrotic syndrome.
Another cause of nephrotic syndrome is focal segmental glomerulosclerosis; this rarely occurs in parasitic diseases. This lesion is characterized by segmental sclerosis and collapse of the capillary tuft of some but not all glomeruli.
Minimal-change disease is the last entity associated with nephrotic syndrome in parasitic diseases. Diffuse effacement of epithelial foot processes (podocytes) as seen by electron microscopy and the absence of lesions by light microscopy and immunofluorescence are the hallmark of this entity.
Only occasionally, crescentic glomerulonephritis is associated with parasitic infections. The crescents are the consequence of accumulation of inflammatory and epithelial cells in Bowman's (urinary) space and occur in all patients in whom the glomerular basement membrane is severely damaged. In these cases, glomerular filtration is deteriorating rapidly and the patients present clinically with rapidly progressive glomerulonephritis.
MALARIA
Malaria is one of the most prevalent infectious diseases in the world. It is the first parasitic infection that was clearly shown to be associated with nephrotic syndrome in tropical areas (29, 43). Moreover, areas of the world with a high incidence of nephrotic syndrome overlap with those where Plasmodium malariae occurs (65). Four species of plasmodia—Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium falciparum—are responsible for malaria. All four species of Plasmodium have complex sexual cycles in their insect vectors; the sexual cycle ends with the production of sporozoites, which are inoculated into the mammalian host by the bite of the female anopheline mosquito. After a brief passage in the peripheral blood, the sporozoites invade hepatocytes. In the liver, merozoites are produced by a process of asexual multiplication; they rupture and return to the circulation, where they are ingested by erythrocytes. In the erythrocytes, a second cycle takes place (merozoite-trophozoite-schizont-merozoite).
Only two of the malaria parasites, namely, P. malariae (quartan malaria) and P. falciparum (falciparum malaria), are clearly associated with renal disease, and this occurs only in a small percentage of patients.
Plasmodium malariae
Renal involvement in the course of quartan malaria is generally characterized by nephrotic syndrome. A membranoproliferative type of glomerulonephritis with relatively sparse proliferation of endothelial and mesangial cells is the most common type of glomerular lesion encountered in quartan malaria (Fig. 3). As expected from this type of lesion, granular deposits of immunoglobulin M (IgM), IgG, and C3 are observed in mesangial and subendothelial areas by immunofluorescence. Focal and segmental glomerulosclerosis can be seen as well. Focal and segmental glomerulosclerosis are generally superimposed on or a result of the above-mentioned lesions and sometimes lead to global glomerulosclerosis (67). Since chronic glomerular disease associated with P. malariae is usually not reversible by treating the infection, a role for genetic and environmental factors is suspected (38).
FIG. 3.
P. malariae-associated glomerulonephritis characterized by a membranoproliferative pattern with diffuse mesangial proliferation, irregular thickening of the capillary walls, swelling of endothelial cells, and influx of inflammatory cells. Periodic acid-Schiff staining. Original magnification, ×400. Courtesy of D. Droz, Hopital Necker, Paris, France.
Plasmodium falciparum
In contrast to infection with P. malariae, there is little to suggest that glomerulonephritis is commonly a dominant and sole lesion in patients infected with P. falciparum. Nephrotic syndrome or proteinuria is rare and, if present, is usually associated with the same type of glomerular lesions as in quartan malaria. However, renal involvement in falciparum malaria is usually transient and disappears when the infection is brought under control. A striking feature of falciparum malaria is the occurrence of acute renal failure with or without overt hemoglobinuria. The pathology in this context consists of tubulointerstitial damage such as tubular necrosis, hemoglobin and cellular casts in tubuli, and interstitial edema (67). These lesions seem to be due to impaired blood flow in the microcirculation as a consequence of increased rigidity and adhesiveness of erythrocytes, hypovolemia, intravascular coagulation, and hemolysis.
Pathogenesis of Glomerular Disease
Malarial nephritides are thought to result from the combination of endothelial damage and immune-complex deposition. The local release of inflammatory mediators and the disturbance of the microvasculature by intravascular hemolysis and coagulation may lead to glomerular endothelium activation and damage. Humoral immunity is thought to play a central role in malarial nephritis since immunoglobulins are deposited in glomeruli and circulating immune complexes are detected (52, 78). However, immune complexes containing malarial antigens represent only a fraction of the immune deposits in glomeruli. Hyperimmunoglobulinemia and autoantibodies are frequently encountered in patients infected with Plasmodium spp. This suggests that polyclonal B-cell activation may participate in malarial nephritis. Moreover, malaria induces a generalized T-cell immunosuppression secondary to the production of immunosuppressive cytokines by macrophages and/or T cells. This could in turn play a role in polyclonal B-cell activation.
Animal models developed in different species such as monkeys, chickens, mice, and rats have confirmed that plasmodia may induce immune complex-mediated glomerulonephritis (13, 26). Mice and rats infected with Plasmodium berghei develop only mild proteinuria associated with a transient pure mesangial glomerulonephritis. In later studies, proliferative glomerular changes with overt proteinuria, more similar to malaria-associated glomerular disease in humans, were obtained (49, 79). Since malarial antigens are detected in glomeruli before immunoglobulin deposits can be seen, the in situ formation of immune complexes is suggested (13, 26). Others describe a role for DNA-binding antibodies and cell-mediated immunity in the development of malarial nephritis (49, 79).
SCHISTOSOMIASIS
Although schistosomiasis is one of the oldest and most widespread parasitic infections, its association with glomerular disease was established only in the 1970s (4, 10). In some areas of endemic infection, more than 90% of adults are infected with schistosomes. Infection occurs through contact with water containing the infective free-swimming cercariae. These burrow through the epidermis and/or mucous membranes and enter the bloodstream. The male carries the female to the submucosal venules of the bladder (Schistosoma haematobium) or the intestines (Schistosoma mansoni), where the female deposits her eggs. These eggs secrete an enzymatic substance which destroys surrounding tissue, and the eggs are discharged in the lumen of the gut or bladder, from where they are shed with urine and faeces.
Schistosoma haematobium
Schistosoma haematobium gives rise to renal problems as a result of direct invasion of the urinary tract. Egg deposition may induce chronic bladder infection, calcification and fibrosis with extension to the ureters, and carcinoma of the bladder, especially squamous cell carcinoma. The fibrosis may lead to irregular ureteral stenoses and eventually to dilatation, resulting in either hydronephrosis or reflux nephropathy. In the rare reported cases of glomerular diseases associated with S. haematobium infection, concomitant chronic salmonellosis seems to be involved (10).
Schistosoma mansoni
Patients with chronic S. mansoni infection involving portal hypertension and an enlarged spleen (hepatosplenic schistosomiasis) have an increased frequency (15%) of renal disease; this infection is clinically characterized by variable proteinuria ranging from asymptomatic to nephrotic syndrome (4, 10, 61). The pathological changes vary.
Membranoproliferative glomerulonephritis is one of the most common manifestations of this infection, together with mesangioproliferative glomerulonephritis (Fig. 4). Focal and segmental glomerulosclerosis frequently appears later in the course of the disease. Occasionally, membranous glomerulopathy and crescentic glomerulonephritis are seen as well. Immunofluorescence and electron microscopy reveal the presence of immune complexes containing IgM, IgG, IgA, IgE, complement components, and schistosomal antigens in the mesangium and along the endothelial side of the capillary wall. Sometimes renal histological changes are found to precede clinical manifestations (5, 9, 69).
FIG. 4.
Mesangioproliferative glomerulonephritis secondary to S. mansoni infection, showing diffuse mesangial widening. Periodic acid-Schiff staining. Original magnification, ×250. Courtesy of D. Droz, Hopital Necker, Paris, France.
Pathogenesis of Glomerular Disease
The insights into the pathogenesis of glomerular disease associated with schistosomiasis share many features with malarial nephritis. The presence of schistosomal worm antigens in the glomerular deposits and the detection of circulating immune complexes containing schistosomal antigens were described first (39, 72). This strongly supports the hypothesis of an immune-complex glomerulonephritis. Moreover, Hillyer and Lewert (35) found precipitating antibodies to DNA in sera from hamsters infected with Schistosoma japonicum, suggesting a role for anti-DNA antibodies in a context of polyclonal B-cell activation. On the other hand, Fujiwara et al. (28) showed that polyclonal B-cell activation alone was not sufficient to induce glomerular damage in infected mice. This suggests that additional mechanisms such as genetic and environmental factors (chronic salmonellosis) participate in the development of full-blown nephritis in this context. The absence of amelioration of the glomerular disease with the treatment of the infection and the lack of correlation between the severity of the nephritis and the intensity of the parasite infection support the hypothetical role of autoimmunity in schistosomal nephritis. Moreover, since glomerular changes are particularly prevalent in hepatosplenic schistosomiasis, portal-systemic shunting might be involved in the development of glomerular lesions. Accordingly, ligation of the portal vein in mice enhances immune-complex deposits in the kidneys (72). Glomerular lesions, however, are more severe and more common in hepatosplenic schistosomiasis than in hepatic cirrhosis, confirming the role of (auto)immune mechanisms in schistosomal nephritis (5). In addition, the severity of the glomerular lesions and proteinuria is correlated with the impairment of hepatic macrophage function (11). This macrophage function may involve the clearance of circulating immune complexes and eventually the clearance of other nephritogenic factors.
LEISHMANIASIS
Leishmania donovani
Glomerular lesions are observed with visceral leishmaniasis (kala-azar) caused by Leishmania donovani. Cutaneous or mucocutaneous leishmaniasis caused by other Leishmania species (Leishmania tropica, Leishmania mexicana, etc.) are not associated with renal disease. Leishmanial parasites are transmitted by the bite of sand flies of the genera Phlebotomus and Lutzomyia. L. donovani are found intracellularly in monocytes and endothelial cells.
A prospective study has shown that 60% of patients with kala-azar have mild proteinuria with benign changes in the urinary sediment (microscopic hematuria and leukocyturia) (25). The pathological picture is a glomerulonephritis ranging from purely mesangioproliferative to membraneoproliferative, sometimes associated with focal and segmental collapse of capillary loops. Moreover, tubulointerstitial damage is generally present and consists of tubular degeneration and inflammatory infiltration. Amyloidosis can be a complication of kala-azar. Using immunofluorescence, IgG, IgM, IgA, and C3 are seen in the mesangium with some extensions along the capillary loop. On the ultrastructural level, irregular thickening of the glomerular basement membrane is seen together with subendothelial and subepithelial dense deposits (22). Renal involvement seems to revert with the cure of the leishmanial infection.
Pathogenesis of Glomerular Disease
Kala-azar is usually associated with hyperimmunoglobulinemia with high IgG levels, circulating immune complexes, and high titers of rheumatoid factor and cryoglobulin. Together with the presence of immunoglobulins in the glomeruli, this suggests the pivotal role of polyclonal B-cell activation and “classical” B-cell activation in the pathogenesis of leishmanial nephritis. This is also supported by data obtained with hamsters infected with L. donovani (63). These animals developed glomerular lesions mimicking the human leishmanial glomerulonephritis counterpart. In this model, L. donovani antigens are detected in glomeruli, suggesting their pathogenetic role (63).
TRYPANOSOMIASIS
Trypanosoma brucei
African trypanosomiasis is a protozoal disease caused by motile hemoflagellates of the genus Trypanosoma. Trypanosoma brucei rhodesiense (tropical east Africa) and Trypanosoma brucei gambiense (tropical west and central Africa) are transmitted by the bite of tsetse flies (genus Glossina). T. b. rhodesiense is the more acute of the two forms, often causing death within 1 year if not treated. The Gambian form is usually characterized by several bouts of clinical activity alternating with latent periods that persist for a number of years. The clinical stages of trypanosomiasis range from the trypanosomal chancre that appears at the site of inoculation to febrile attacks, diffuse intravascular coagulation (T. b. rhodesiense), lymphadenopathy (T. b. gambiense), and eventually progressive brain dysfunction leading to sleeping sickness, cachexia, and death. Although glomerular disease associated with T. brucei infection has been described in several species, human glomerulonephritis associated with African trypanosomiasis is limited to an occasional report (8), as is the association of Trypanosoma cruzi infection (Chagas' disease or American trypanosomiasis) with nephritis (19).
One of the T. brucei subspecies (T. b. brucei) is not infective in humans. Many vertebrates infected with T. b. brucei do develop disease associated with glomerular involvement. The relative ease in inducing and reproducing experimental disease allows the study of the mechanisms involved in this glomerulopathy, which is seen by some as a model for infection related glomerulopathy. Trypanosomal nephritis has been described in rhesus monkeys (54), rabbits (27, 53), rats (17, 48, 59), and mice (71, 74) and varies from purely mesangioproliferative to membranoproliferative glomerulonephritis. By immunofluorescence, IgM, IgG, and complement components are seen in the mesangium, along with some extensions along the glomerular capillary walls. On the ultrastructural level, large electron-dense deposits are observed in mesangial, subendothelial, and subepithelial areas (Fig. 5).
FIG. 5.
Glomerulopathy of experimental trypanosomiasis. Electron micrographs of a capillary loop of a control mouse, showing normal glomerular histology (A), and of a mouse 8 weeks after inoculation with T. b. brucei, showing swollen endothelial cells and large-electron dense deposits in the mesangium and subendothelially (∗) (B) are presented. cap, glomerular capillary; ep, epithelium; ur, urinary space; end, endothelium; mes, mesangium; arrows, glomerular basement membrane. Magnification, ×1,551.
Pathogenesis of Glomerular Disease
Several hypotheses about the pathogenesis of the glomerular lesions exist. Most studies postulate a preponderant pathogenic role for immune complexes, as with the other parasite-related glomerulopathies. The glomerular immune complexes have been shown in some studies to consist of trypanosomal antigens and antitrypanosomal antibodies (27, 71) and in others to consist of autoantigen-autoantibody complexes (47, 59), especially DNA–anti-DNA complexes (53). Moreover, specific autoantibodies directed against glomerular antigens such as laminin and glomerular glycoproteins have been detected not only in the serum of experimental animals but also in glomerular eluates (17, 74). Therefore, both specific B-cell activation (“classical” pathway) and polyclonal B-cell activation seem to be involved in trypanosomal nephritis. However, humoral immunity alone cannot account for the development of albuminuria, since experiments with different mouse strains show that there is no direct relationship between the glomerular fluorescence pattern, proteinuria, and autoantibody production (76). Moreover, these experiments demonstrate that host-related factors, determined by non-major histocompatibility complex genes, are important for the development of glomerulonephritis associated with African trypanosomiasis. To delineate which parts of the defense system (in addition to the B-cell response) are involved in the development of glomerular disease, the participation of the thymus, spleen, and mononuclear phagocyte system was investigated (75). First, glomerular disease occurs in the absence of thymic tissue, since nude mice infected with T. b. brucei parasites developed albuminuria and some glomerular immunoglobulin deposits in the absence of polyclonal B-cell activation. Thus, the presence of autoantibodies or immune complexes in the context of polyclonal B-cell activation alone could again not explain the full glomerular disease, i.e., histological glomerular changes and albuminuria. Second, these studies have revealed that the spleen is a crucial organ since albuminuria could be prevented or significantly lowered by splenectomy, depending on the timing of the procedure. Third, macrophage depletion leads to a significantly higher albuminuria in infected mice for up to 2 weeks after depletion. Thus, trypanosomal glomerulopathy is independent of T cells, while macrophages have an inhibitory rather than an inducing effect. The spleen enlargement during T. b. brucei infection is not fully understood. It is due in part to an increase in extramedullary hematopoiesis and in part to a proportional increase in the number of CD4+ T cells, CD8+ T cells, and B cells. Moreover, a relative increase in the number of null cells (CD4−, CD8−, Ig−) is observed (51). In this respect, the massive increase in the number of splenic γδ T cells is a particularly interesting finding (B. de Geus, M.-L. F. van Velthuysen, and E. De Heer, unpublished data), especially since these γδ T cells seem to be involved in resistance to T. b. brucei infection. Their role in the pathogenesis of this glomerulopathy should be investigated further.
HYDATID DISEASE
Echinococcus granulosus
Hydatid disease is caused by Echinococcus granulosus. Sheep, cattle, and camels are the common intermediate hosts for this worm, whose eggs are passed in stool and are often transmitted to humans by dogs, which act as intermediate hosts. Therefore, this disease has its highest incidence in countries where sheep and cattle raising is carried out with the help of dogs, i.e., the Middle East, Australia, New Zealand, South Africa, South America, and the Mediterranean area. The embryos escape from the eggs, penetrate the intestinal mucosa of the human host, and enter the portal circulation. Although most larvae are filtered out by the liver and lungs, some organisms escape to the general circulation to involve other sites such as the kidneys (2%). The larvae that are not destroyed develop into hydatid cysts, which are unilocular, in contrast to those of Echinococcus multilocularis, which are multilocular. These cysts can be discovered by accident or can be detected because of pain or accidental rupture, leading to allergic reactions and hypotension. Most reports of hydatid disease and renal involvement concern renal cysts, but a few cases of glomerular lesions associated with hydatid disease have been described. The histological changes in these cases range from the membranous pattern to the membranoproliferative pattern (2, 20; B. Rincon, C. Bernis, A. Garcia, and J. A. Traver, Letter, Nephrol. Dial. Transplant. 8:783–784, 1993). Renal involvement in E. multilocularis infection has not been described.
Pathogenesis of Glomerular Disease
Most cases of glomerular lesions associated with hydatid disease are reported to be reversible by treating the infection (20, 77). Moreover, Viatel et al. (77) eluted echinococcal antigens and antibodies to these antigens from renal tissue of their patient, supporting an immune-complex-mediated pathogenesis. The naturally existing model of E. granulosus-associated membranoproliferative glomerulonephritis in sheep might help to unravel the mechanisms involved in this disease (2), but follow-up of these studies has not been reported.
FILARIASIS
Filarioidea
The parasites causing filariasis belong to the superfamily of Filarioidea. The viviparous female worms discharge microfilariae that migrate to human blood or subcutaneous tissues, where they can wait for weeks or months until they are ingested by hematophagous arthropods. In these vectors, they become filariform larvae, which can mature into adult worms in a new host. Four different parasites, each transmitted by its own specific vector, are responsible for three clinical diseases. The different parasites are identified by their location, periodicity, morphological characteristics, and clinical presentation of the disease they cause. Wuchereria bancrofti and Brugia malayi cause lymphatic filariasis, which is characterized by lymphatic blockade and elephantiasis but is asymptomatic in many cases (24). These infections are endemic in Africa, Southeast Asia, and the coastal planes of South America. River blindness is caused by Onchocerca volvulus, which is endemic throughout tropical Africa and Central and South America and is characterized by blindness and a pruritic skin rash. It is transmitted by blackflies of the genus Simulium, which breed along fast-moving streams. The third disease is loiasis, which is caused by Loa loa. Transient subcutaneous swellings (Calabar) are the hallmark of this disease.
Several studies have recently shown a clear association of filariasis and glomerular disease (24, 46, 55, 57). This association is often difficult to establish due to frequent coinfections (hepatitis B and malaria). Except for an occasional report of acute glomerulonephritis (21), most case reports as well as the studies mentioned describe a mesangioproliferative or membranoproliferative glomerulonephritis (55). Among patients with loiasis, several cases of membranous glomerulopathy and a single case of focal and segmental glomerulosclerosis have been reported (57). During O. volvulus infection, an occasional case of minimal-change nephropathy is found (55).
Pathogenesis of Glomerular Disease
Glomerular disease associated with filariasis is thought by most authors to be immune complex mediated. Parasitic antigens were demonstrated in glomeruli from 9 of 18 patients with proliferative glomerular disease due to onchocerciasis, supporting this hypothesis about the pathogenesis in these 9 patients (55). Treatment of patients with bancroftian and onchocerciasis infection was shown to increase the incidence of hematuria and proteinuria, which remained after treatment (24, 33, 56). This is an argument in favor of the pathogenicity of immune complexes, since it is presumed that there is an increase in the number of circulating immune complexes during treatment due to the disintergration of parasites. These complexes are cleared after effective treatment. In L. loa and B. malayi infection, however, treatment did not have any effect on glomerular lesions (46; V. K. G. Pillay, E. Kirch, and N. A. Kurtzman, Letter, JAMA 225:179, 1973). Therefore, other factors are thought to be involved. To date, rheumatoid factor is the sole mediator which has been investigated for its role in glomerular disease. Although elevated levels of rheumatoid factor were found in loiasis patients, no correlation with the presence of glomerular disease has been established (1).
BABESIOSIS
Babesia is a tick-borne intraerythrocytic parasite. In Europe, Babesia bovis and Babesia divergens are transmitted from cattle to humans. Asplenic persons are particularly susceptible to this disease. In the United States, most infections are caused by a rodent parasite, Babesia microti, usually in patients with intact spleens. In severe cases involving massive hemolysis, renal involvement, resulting in acute renal failure is seen (16, 70). In South Africa, canine babesiosis is observed and is caused by Babesia canis; infections result in similar symptoms (50). Glomerular involvement has been described only in a rat model (6). In this model, a transient proteinuria is seen, with proliferative glomerular changes and mesangial IgG and complement.
TOXOPLASMOSIS
Toxoplasmosis is caused by Toxoplasma gondii; it is a ubiquitous infection and less common in hot, cold, and arid areas. Infection occurs by the ingestion of cysts or oocyts or by the transplacental or hematogeneous (transfusion, transplantation) route. Most recent reports about renal disease associated with T. gondii infection deal with the problem of acute renal failure due to sulfadiazine crystal deposition in the urinary tract after treatment of Toxoplasma encephalitis with this drug. In the early 1970s, Huldt (40) described a mesangioproliferative glomerulonephritis in mice infected with T. gondii. Glomerular immune complexes were shown to contain Toxoplasma antigen. In a subsequent retrospective study of 150 cases of membranoproliferative or mesangioproliferative glomerulonephritis, 1 was shown to be related to Toxoplasma infection (30). Apart from this, an occasional case of congenital glomerulosclerosis due to congenital toxoplasmosis is reported (66).
TRICHINOSIS
Trichinosis is an infection of humans and other mammals by Trichinella spiralis. This disease is characterized by diarrhea, myositis, fever, prostration, periorbital edema, eosinophilia, and occasionally myocarditis, pneumonitis, or encephalitis. People are infected by ingestion of infected pork or bear meat, particularly in Europe and North America. The majority of infections are asymptomatic. A few cases of membranoproliferative glomerulonephritis associated with trichinosis have been described (44, 64, 68). Severe cases were fatal and were accompanied by acute tubular necrosis and myoglobinuria (44); in other cases, urinary sediment abnormalities disappeared after the infection was brought under control (68). Immune complexes were seen in glomeruli, but Trichinella antigen was not looked for.
OPISTHORCHIASIS
Opisthorchiasis is caused by Opisthorchis felineus in eastern and central Europe and by Opisthorchis viverrini in Thailand and Laos. The infection is transmitted by eating uncooked fish and is characterized by hepatic lesions produced by the adult worm in the large bile ducts. Fifty percent of infected patients have a bile duct carcinoma at autopsy. Even though polyclonal B-cell activation is seen in a few patients with opisthorchiasis (15), glomerular lesions have not yet been found and probably have not been looked for. Syrian hamsters infected with O. viverrini experience a mesangioproliferative glomerulonephritis characterized by deposition of immune complexes (consisting of IgG, C3, and O. viverrini antigen) in the mesangium with some extensions along the capillary wall. These immune complexes are gradually replaced by amyloid (14).
GENERAL CONSIDERATIONS AND CONCLUSIONS
Parasitic protozoa account for more morbidity and mortality than any other class of infectious organisms. However, only recently have the tools of immunology and molecular biology been applied to protozoal diseases and opened new research horizons. These investigations have taught us new immunologic pathways involved in response to parasite infestations. Although few of these new technologies and developments have been applied to the field of the nephropathology, they may be useful tools to highlight the mechanisms of infection-related glomerular diseases. These results may in turn give new insights into the pathogenesis of primary (“idiopathic”) glomerulopathies. It is beyond the scope of this paper to review all mechanisms underlying immunologically mediated glomerulonephritides. Instead, we focus on relevant pathogenic factors leading to glomerular injuries in the context of parasitic infections. These mechanisms are schematically summarized in Fig. 6.
FIG. 6.
Interacting closely with one another, the innate, cellular, and humoral compartments of the immune system play a role in fighting parasitic infections and in turn are involved in the pathogenesis of associated glomerulopathies. During infection, several inflammatory mediators are released. These factors may, on the one hand, directly damage the different glomerular cell types or may, on the other hand, participate in the activation of specific subsets of T and B cells, resulting in different levels of antigen, antibody, and immune complexes. Depending on the site of the immune-complex deposition and on the type of primary damaged glomerular cells, different glomerular lesions may develop. Ag, antigen; Ab, antibody; GN, glomerulonephritis; GP, glomerulopathy; IC, immune complex; NK, natural killer cell.
A fundamental feature of most parasitic infections is their chronicity, characterized by fluctuations in antigenemia and therefore in host immune responses. There are many reasons for this, including weak natural immunity and the ability of parasites to escape the immune system of their host. The respective roles of natural immunity, cellular immunity, and humoral immunity in fighting parasitic diseases are still a matter of debate. Results obtained with gene knockout mice have convincingly shown that acquisition of resistance to intracellular parasites is generally a function of CD4+ αβ T cells, which produce gamma interferon (IFN-γ) and thus are of the TH1 type (41). Extracellular parasites are best counteracted by a combination of TH2- and TH1-type cells, e.g., TH0 cells (62). However, this useful rule suffers exceptions and should not be considered a dogma (3). Indeed, depending on the parasite strain and the life cycle stage, a given cytokine can exercise the opposite effect on the course of the disease. To escape the protective response of T-helper cells, different parasites manipulate the immune system of a susceptible host. Striking examples are African trypanosomiasis (caused by an extracellular parasite) and leishmaniasis (caused by an intracellular parasite). Defense against T. b. brucei depends on the production of interleukin-4, a TH2-type cytokine (7). However, the parasite itself produces a T-lymphocyte triggering factor, which triggers CD8+ T lymphocytes to proliferate and secrete IFN-γ, which in turn stimulates parasite growth. In contrast, to counteract the protective response of TH1 cells, Leishmania spp. induce transforming growth factor β (a cytokine with macrophage inhibiting or deactivating capabilities), enhance the expression of interleukin-10 (a TH2-type cytokine with immunosuppressive properties), and interfere with the IFN-γ signalling pathway (12, 45). On the one hand, this leads to a general T-cell immunosuppression, while on the other hand, it leads to a TH2-mediated response. This type of response is associated with polyclonal B-cell activation (31). Polyclonal B-cell activation is implicated in the onset of glomerular disease associated with systemic autoimmunity in numerous experimental models (32). Despite these data, the direct role of the TH2-type cytokines in parasitic glomerulopathies has not been investigated.
Antibodies produced during the course of parasitic infection, either by the “classical” pathway or in the context of polyclonal B-cell activation, may accumulate in glomeruli in different ways such as passive trapping of immune complexes, in situ immune-complex formation (binding of antibody to the antigen that was “planted” previously in glomeruli), and direct binding of autoantibodies to glomerular autoantigens. However, different studies have clearly established that these antibodies are neither sufficient nor necessary for the development of proteinuria, one of the parameters of glomerular dysfunction. Moreover, results obtained with Xid mice (B1-deficient mice) infected with Leishmania have even, surprisingly, shown that B cells contribute to the progression of the disease (37). Unfortunately, renal pathology was not investigated in this setting. These observations affirm the importance of other parts of the defense system in the pathogenesis of glomerular diseases.
With the exception of B cells and CD4+ T cells, innate immunity is also implicated in the pathogenesis of glomerular disease. Macrophages, granulocytes, natural killer cells, double-negative γδ T cells, and CD8+ T cells are parts of the host response to parasitic infection and may participate in the glomerular damage. Thus, it is of interest that part of the glomerular hypercellularity in murine malaria is due to an influx of CD8+ T cells (49). These components of the primary immune system act through an intricate network of cytokines and other inflammatory mediators such as complement factors, oxygen radicals, enzymes, and kinines. These mediators are involved in glomerular lesions in other experimental models, either by causing direct cellular damage (42) or by activating the coagulation cascade, and may therefore be responsible for the glomerular injury in parasitic infections as well.
To conclude, although the association of parasitic infections with glomerular injury is clear, the pathogenesis of parasitic glomerulonephritides is not. As in autoimmunity, different mechanisms and host and environmental factors are at play and are interrelated (summarized in Fig. 6).
REFERENCES
- 1.Adebajo A O, Akinsola A, Maizels R M, Cawston T R, Hazleman B L. Rheumatoid factor and rheumatoid isotypes in loiasis with and without accompanying glomerulonephritis. Trans R Soc Trop Med Hyg. 1992;86:667–669. doi: 10.1016/0035-9203(92)90183-d. [DOI] [PubMed] [Google Scholar]
- 2.Albano Edelweiss M I, Lizardo-Daudt H M. Naturally existing model of glomerulonephritis mediated by immune complexes associated with hydatidosis in sheep. Nephron. 1991;57:253–254. doi: 10.1159/000186269. [DOI] [PubMed] [Google Scholar]
- 3.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 4.Andrade Z A, Andrade S G, Sadigursky M. Renal changes in patients with hepatosplenic schistosomiasis. Am J Trop Med Hyg. 1971;20:77–83. doi: 10.4269/ajtmh.1971.20.77. [DOI] [PubMed] [Google Scholar]
- 5.Andrade Z A, Rocha H. Schistosomal glomerulopathy. Kidney Int. 1979;16:23–29. doi: 10.1038/ki.1979.99. [DOI] [PubMed] [Google Scholar]
- 6.Annable C R, Ward P A. Immunopathology of the renal complications of babesiosis. J Immunol. 1974;112:1–8. [PubMed] [Google Scholar]
- 7.Bakhiet M, Jansson L, Buscher P, Holmdahl R, Kristensson K, Olsson T. Control of parasitemia and survival during Trypanosoma brucei brucei infection is related to strain-dependent ability to produce IL-4. J Immunol. 1996;157:3518–3526. [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Ugoretz R G, Braude A L. Disseminated intravascular coagulation in trypanosomiasis. Arch Intern Med. 1973;131:574–577. doi: 10.1001/archinte.131.4.574. [DOI] [PubMed] [Google Scholar]
- 9.Barsoum R S. Schistosomal glomerulopathies. Kidney Int. 1993;44:1–12. doi: 10.1038/ki.1993.205. [DOI] [PubMed] [Google Scholar]
- 10.Barsoum R S, Bassily S, Baligh O K, Elissa M, El-Sheemy N, Affify N, Hassaballa A. Renal disease in hepatosplenic schistosomiasis: a clinicopathological study. Trans R Soc Trop Med Hyg. 1977;71:387–391. doi: 10.1016/0035-9203(77)90035-9. [DOI] [PubMed] [Google Scholar]
- 11.Barsoum R S, Sersawy G, Haddad S, Hashem M B, Kamel M, Wassef N, Francis M, Ghonaimy E, Soliman M, Khashab O. Hepatic macrophage function in schistosomal glomerulopathy. Nephrol Dial Transplant. 1988;3:612–616. doi: 10.1093/oxfordjournals.ndt.a091715. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan C, Gessner A, Solbach W, Rollinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 13.Boonpucknavig S, Boonpucknavig V, Bhamarapravati N. Immunopathological studies of Plasmodium berghei-infected mice. Arch Pathol. 1972;94:322–330. [PubMed] [Google Scholar]
- 14.Boonpucknavig S, Boonpucknavig V, Tanvanich S, Doungchawee G, Thamavit W. Development of immune-complex glomerulonephritis and amyloidosis in Syrian golden hamsters infected with Opisthorchis viverrini. J Med Assoc Thail. 1992;75(Suppl. 1):7–19. [PubMed] [Google Scholar]
- 15.Boonpucknavig S, Kurathong S, Thamavit W. Detection of antibodies in sera from patients with opisthorchiasis. J Clin Lab Immunol. 1986;19:135–137. [PubMed] [Google Scholar]
- 16.Brasseur P, Gorenflot A. Human babesiosis in Europe. Mem Inst Oswaldo Cruz. 1992;87(Suppl. 3):131–132. doi: 10.1590/s0074-02761992000700019. [DOI] [PubMed] [Google Scholar]
- 17.Bruijn J A, Oemar B S, Ehrich J H, Foidart J M, Fleuren G J. Anti-basement membrane glomerulopathy in experimental trypanosomiasis. J Immunol. 1987;139:2482–2488. [PubMed] [Google Scholar]
- 18.Chugh K S, Sakhuja V. Glomerular disease in the tropics. Am J Nephrol. 1990;10:437–450. doi: 10.1159/000168167. [DOI] [PubMed] [Google Scholar]
- 19.Costa R S, Monteiro R C, Lehuen A, Joskowicz M, Noël L-H, Droz D. Immune complex-mediated glomerulopathy in experimental Chagas' disease. Clin Immunol Immunopathol. 1991;58:102–114. doi: 10.1016/0090-1229(91)90152-z. [DOI] [PubMed] [Google Scholar]
- 20.Covic A, Mititiuc I, Caruntu L, Goldsmith D J. Reversible nephrotic syndrome due to mesangiocapillary glomerulonephritis secondary to hepatic hydatid disease. Nephrol Dial Transplant. 1996;11:2074–2076. doi: 10.1093/oxfordjournals.ndt.a027101. [DOI] [PubMed] [Google Scholar]
- 21.Date A, Kirubakaran M G, Gunasekaran V, Shastry J C M. Acute eosinophilic glomerulonephritis with Bancroftian filariasis. Postgrad Med J. 1979;55:905–907. doi: 10.1136/pgmj.55.650.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Brito T, Hoshino-Shimizu S, Amato Neto V, Duarte I S, Penna D O. Glomerular involvement in human Kala-azar: a light, immunofluorescent and electron microscopic study based on kidney biopsies. Am J Trop Med Hyg. 1975;24:9–18. [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Dreyer G, Ottesen E A, Galdino E, Andrade L, Rocha A, Medeiros Z, Moura I, Casimiro I, Beliz F, Coutinho A. Renal abnormalities in microfilaremic patients with Bancroftian filariasis. Am J Trop Med Hyg. 1992;46:745–751. doi: 10.4269/ajtmh.1992.46.745. [DOI] [PubMed] [Google Scholar]
- 25.Dutra M, Martinelli R, Carvalho E M, Rodrigues L E, Brito E, Rocha H. Renal involvement in visceral leishmaniasis. Am J Kidney Dis. 1985;6:22–27. doi: 10.1016/s0272-6386(85)80034-2. [DOI] [PubMed] [Google Scholar]
- 26.Ehrich J H, Sterzel R B, Deicher H R, Foellmer H G. Rat malarial glomerulonephritis. An experimental model of post-infectious glomerular injury. Virchows Arch B. 1981;37:343–356. [PubMed] [Google Scholar]
- 27.Facer C A, Molland E A, Gray A B, Jenkins G C. Trypanosoma brucei: renal pathology in rabbits. Exp Parasitol. 1978;44:249–261. doi: 10.1016/0014-4894(78)90106-6. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara M, Makino M, Watanabe H. Schistosoma mansoni: induction of severe glomerulonephritis in female BXSB mice following chronic infection. Exp Parasitol. 1988;65:214–221. doi: 10.1016/0014-4894(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 29.Giglioli G. Malaria and renal disease with special reference to British Guiana. Ann Trop Med Parasitol. 1962;56:225–241. doi: 10.1080/00034983.1962.11686115. [DOI] [PubMed] [Google Scholar]
- 30.Ginsburg B E, Wasserman J, Huldt G, Bergstrand A. Case of glomerulonephritis associated with acute toxoplasmosis. Br Med J. 1974;3:664–665. doi: 10.1136/bmj.3.5932.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman M, Baran D, Druet P. Polyclonal activation and experimental nephropathies. Kidney Int. 1988;34:141–150. doi: 10.1038/ki.1988.159. [DOI] [PubMed] [Google Scholar]
- 32.Goldman M, Druet P, Gleichmann E. TH2 cells in systemic autoimmunity: Insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 33.Greene B M, Taylor H R, Humphrey R L. Proteinuria associated with diethylcarbamazine treatment of onchocerciasis. Lancet. 1980;i:254–255. doi: 10.1016/s0140-6736(80)90737-0. [DOI] [PubMed] [Google Scholar]
- 34.Heptinstall R H. Renal manifestations of various infective conditions. In: Heptinstall R H, editor. Pathology of the kidney. Boston, Mass: Little, Brown & Co.; 1992. pp. 1968–1987. [Google Scholar]
- 35.Hillyer G V, Lewert R M. Studies on renal pathology on hamsters infected with Schistosoma mansoni and S. japonicum. Am J Trop Med Hyg. 1974;23:404–411. doi: 10.4269/ajtmh.1974.23.404. [DOI] [PubMed] [Google Scholar]
- 36.Hoedemaeker P J. Nieren en urinewegen. In: Hoedemaeker P J, Bosman F T, Meijer C J L M, Becker A E, editors. Pathologie. Utrecht, The Netherlands: Wetenschappelijke uitgever Bunge; 1995. pp. 345–385. [Google Scholar]
- 37.Hoerauf A, Solbach W, Rollinghoff M, Gessner A. Effect of IL-7 treatment on Leishmania major-infected BALB.Xid mice: enhanced lymphopoiesis with sustained lack of B1 cells and clinical aggravation of disease. Int Immunol. 1995;7:1879–1884. [PubMed] [Google Scholar]
- 38.Houba V. Immunologic aspects of renal lesions associated with malaria. Kidney Int. 1979;16:3–8. doi: 10.1038/ki.1979.96. [DOI] [PubMed] [Google Scholar]
- 39.Houba V. Experimental renal disease due to schistisomiasis. Kidney Int. 1979;16:30–43. doi: 10.1038/ki.1979.100. [DOI] [PubMed] [Google Scholar]
- 40.Huldt G. Studies on experimental toxoplasmosis. Ann N Y Acad Sci. 1971;177:146–155. doi: 10.1111/j.1749-6632.1971.tb35041.x. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann S H. Bacterial and protozoal infections in genetically disrupted mice. Curr Opin Immunol. 1994;6:518–525. doi: 10.1016/0952-7915(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki K, Yaoita E, Yamamoto T, Kihara I. Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int. 1992;41:1517–1526. doi: 10.1038/ki.1992.221. [DOI] [PubMed] [Google Scholar]
- 43.Kibukamusoke J W, Hutt M S R, Wilks N E. The nephrotic syndrome in Uganda and its association with quartan malaria. Q J Med. 1967;36:393–408. [PubMed] [Google Scholar]
- 44.Kociecka W, Gabryel P, Leszyk A, Gustowska L. A case of fatal trichinosis with early renal failure and involvement of the central nervous system. Wiad Parazytol. 1987;33:545–551. [PubMed] [Google Scholar]
- 45.Krishnan L, Guilbert L J, Wegmann T G, Belosevic M, Mosmann T R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- 46.Langhammer J, Birk H W, Zahner H. Renal disease in lymphatic filariasis: evidence for tubular and glomerular disorders at various stages of the infection. Trop Med Int Health. 1997;2:875–884. doi: 10.1046/j.1365-3156.1997.d01-404.x. [DOI] [PubMed] [Google Scholar]
- 47.Lindsley H B, Nagle R B, Stechschulte D J. Proliferative glomerulonephritis, hypocomplementemia, and nucleic acid antibodies in rats infected with Trypanosoma rhodesiense. Am J Trop Med Hyg. 1978;27:865–872. doi: 10.4269/ajtmh.1978.27.864. [DOI] [PubMed] [Google Scholar]
- 48.Lindsley H B, Nagle R B, Werner W A, Stechschulte D J. Variable severity of glomerulonephritis in inbred rats infected with Trypanosoma rhodesiense. Correlation with immunoglobulin class-specific antibody responses to trypanosomal antigens and total IgM levels. Am J Trop Med Hyg. 1980;29:348–357. doi: 10.4269/ajtmh.1980.29.348. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd C M, Wozencraft A O, Williams D G. Cell-mediated pathology during murine malaria-associated nephritis. Clin Exp Immunol. 1993;94:398–402. doi: 10.1111/j.1365-2249.1993.tb08208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobetti R G, Reyers F, Nesbit J W. The comparative role of haemoglobinaemia and hypoxia in the development of canine babesial nephropathy. J S Afr Vet Assoc. 1996;67:188–198. [PubMed] [Google Scholar]
- 51.Makumyaviri A M, Sileghem M R, Ray D, Hamers R, Baetselier P. Systeme lymphocytaire et resistance relative de la souris consanguine a Trypanosoma brucei brucei. Ann Inst Pasteur Immunol. 1988;139:545–556. doi: 10.1016/0769-2625(88)90099-2. [DOI] [PubMed] [Google Scholar]
- 52.McGregor I A, Turner M W, Williams K, Hall P. Soluble antigens in the blood of African patients with severe plasmodium falciparum infection in man. Lancet. 1968;i:881–884. doi: 10.1016/s0140-6736(68)90237-7. [DOI] [PubMed] [Google Scholar]
- 53.Nagle R B, Dong S D, Janacek L L, Guillot J M, Lindsley H B. Glomerular accumulation of monocytes and macrophages in experimental glomerulonephritis associated with Trypanosoma rhodesiense infection. Lab Investig. 1982;46:356–376. [PubMed] [Google Scholar]
- 54.Nagle R B, Ward P A, Lindsley H B, Sadun E H, Johnson A J, Berkaw R E, Hildebrandt P K. Experimental infections with African trypanosomes. VI. Glomerulonephritis involving the alternativepathway of complement activation. Am J Trop Med Hyg. 1974;23:15–26. [PubMed] [Google Scholar]
- 55.Ngu J L, Chatelanat F, Leke R, Ndumbe P, Youmbissi J. Nephropathy in Cameroon: evidence for filarial derived immune-complex pathogenesis in some cases. Clin Nephrol. 1985;24:128–134. [PubMed] [Google Scholar]
- 56.Oremond A D, Hussey J K, Petersen J, Weir J, Edward N. Immune complex glomerulonephritis and chronic anaerobic urinary infection: complications of filariasis. Postgrad Med J. 1983;59:730–733. doi: 10.1136/pgmj.59.697.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pakasa N M, Nseka N M, Nyimi L M. Secondary collapsing glomerulopathy associated with Loa loa filariasis. Am J Kidney Dis. 1997;30:836–839. doi: 10.1016/s0272-6386(97)90090-1. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Rickman W J, Cox H W. Association of autoantibodies with anemia, splenomegaly, and glomerulonephritis in experimental African trypanosomiasis. J Parasitol. 1979;65:65–73. [PubMed] [Google Scholar]
- 60.Reference deleted.
- 61.Rocha H, Cruz T, Brito E, Susin M. Renal involvement in patients with hepatosplenic schistosomiasis mansoni. Am J Trop Med Hyg. 1976;25:108–115. doi: 10.4269/ajtmh.1976.25.108. [DOI] [PubMed] [Google Scholar]
- 62.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 63.Sartori A, Roque-Barreira M C, Coe J, Campos-Neto A. Immune complex glomerulonephritis in experimental kala-azar II. Detection and characterization of parasite antigens and antibodies eluted from kidneys of Leishmania donovani-infected hamsters. Clin Exp Immunol. 1991;87:386–392. doi: 10.1111/j.1365-2249.1992.tb03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoenfeld M R, Edis G T. Trichinoisis and glomerulonephritis. Arch Pathol. 1967;84:625–626. [PubMed] [Google Scholar]
- 65.Seggie J L, Dwomoa A. Nephrotic syndrome in the Tropics. In: Cameron J S, Glassock R J, editors. The nephrotic syndrome. New York, N.Y: Marcel Dekker, Inc.; 1988. pp. 653–695. [Google Scholar]
- 66.Shahin B, Papadopoulou Z L, Jenis E H. Congenital nephrotic syndrome associated with congenital toxoplasmosis. J Pediatr. 1974;85:366–370. doi: 10.1016/s0022-3476(74)80117-4. [DOI] [PubMed] [Google Scholar]
- 67.Sitprija V, Boonpucknavig V. Renal involvement in parasitic diseases. In: Tisher C C, Brenner B M, editors. Renal pathology with clinical and functional correlations. J. P. Philadelphia, Pa: Lippincott Co.; 1994. pp. 626–657. [Google Scholar]
- 68.Sitprija V, Keoplung M, Boonpucknavig V, Boonpucknavig S. Renal involvement in human trichinosis. Arch Intern Med. 1980;140:544–546. [PubMed] [Google Scholar]
- 69.Sobh M A, Moustafa F E, El-Housseini F, Basta M T, Deelder A M, Ghoniem M A. Schistosomal specific nephropathy leading to end-stage renal failure. Kidney Int. 1987;31:1006–1011. doi: 10.1038/ki.1987.99. [DOI] [PubMed] [Google Scholar]
- 70.Uhnoo I, Cars O, Christensson D, Nystrom-Rosander C. First documented case of human babesiosis in Sweden. Scand J Infect Dis. 1992;24:541–547. doi: 10.3109/00365549209052642. [DOI] [PubMed] [Google Scholar]
- 71.van Marck E A E, Beckers A, Deelder A M, Jacob W, Wery M, Gigase P L J. Renal disease in chronic experimental Trypanosoma gambiense infections. Am J Trop Med Hyg. 1981;30:780–789. doi: 10.4269/ajtmh.1981.30.780. [DOI] [PubMed] [Google Scholar]
- 72.van Marck E A E, Deelder A M, Gigase P L J. Effect of portal vein ligation on immune glomerular deposits in Schistosoma mansoni infected mice. Br J Exp Pathol. 1977;58:412–417. [PMC free article] [PubMed] [Google Scholar]
- 73.van Velthuysen M L F. Glomerulopathy associated with parasitic infections. Parasitol Today. 1996;12:102–107. doi: 10.1016/0169-4758(96)80669-7. [DOI] [PubMed] [Google Scholar]
- 74.van Velthuysen M L F, Bruijn J A, van Leer E H G, Fleuren G J. Pathogenesis of trypanosomiasis-induced glomerulonephritis in mice. Nephrol Dial Transplant. 1992;7:507–515. [PubMed] [Google Scholar]
- 75.van Velthuysen M L F, Mayen A E M, Van Rooijen N, Fleuren G J, De Heer E, Bruijn J A. T cells and macrophages in Trypanosoma brucei-related glomerulopathy. Infect Immun. 1994;62:3230–3235. doi: 10.1128/iai.62.8.3230-3235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Velthuysen M L F, Veninga A, Bruijn J A, De Heer E, Fleuren G J. Susceptibility for infection-related glomerulopathy depends on non-MHC genes. Kidney Int. 1993;43:623–629. doi: 10.1038/ki.1993.91. [DOI] [PubMed] [Google Scholar]
- 77.Viatel P, Chenais F, Desgeorges P, Couderc P, Micouin C, Cordonnier D. Membranous nephropathy associated with hydatid disease. N Engl J Med. 1981;304:610–611. doi: 10.1056/NEJM198103053041016. [DOI] [PubMed] [Google Scholar]
- 78.Ward P A, Kibukamusoke J W. Evidence for soluble immune complexes in the pathogenesis of the glomerulonephritis of quartan malaria. Lancet. 1969;i:283–285. doi: 10.1016/s0140-6736(69)91038-1. [DOI] [PubMed] [Google Scholar]
- 79.Wozencraft A O, Lloyd C M, Staines N A, Griffiths V J. Role of DNA-binding antibodies in kidney pathology associated with murine malaria infections. Infect Immun. 1990;58:2156–2164. doi: 10.1128/iai.58.7.2156-2164.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]