Abstract
The family Flaviviridae contains three genera: Hepacivirus, Flavivirus, and Pestivirus. Worldwide, more than 170 million people are chronically infected with Hepatitis C virus and are at risk of developing cirrhosis and/or liver cancer. In addition, infections with arthropod-borne flaviviruses (such as dengue fever, Japanese encephalitis, tick-borne encephalitis, St. Louis encephalitis, Murray Valley encephalitis, West Nile, and yellow fever viruses) are emerging throughout the world. The pestiviruses have a serious impact on livestock. Unfortunately, no specific antiviral therapy is available for the treatment or the prevention of infections with members of the Flaviviridae. Ongoing research has identified possible targets for inhibition, including binding of the virus to the cell, uptake of the virus into the cell, the internal ribosome entry site of hepaciviruses and pestiviruses, the capping mechanism of flaviviruses, the viral proteases, the viral RNA-dependent RNA polymerase, and the viral helicase. In light of recent developments, the prevalence of infections caused by these viruses, the disease spectrum, and the impact of infections, different strategies that could be pursued to specifically inhibit viral targets and animal models that are available to study the pathogenesis and antiviral strategies are reviewed.
SPECTRUM OF DISEASE
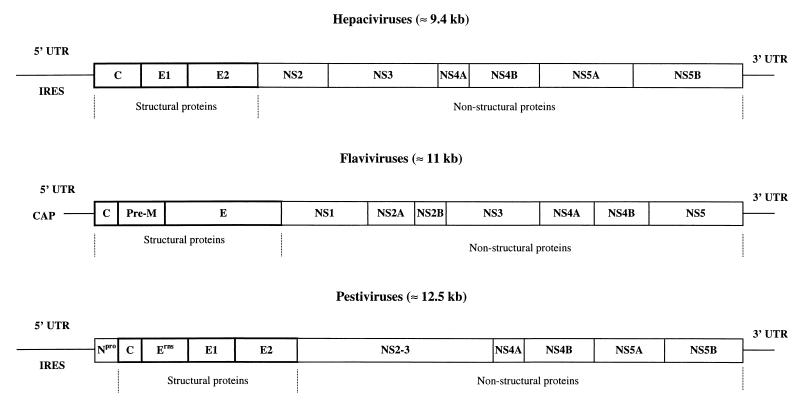
Flaviviridae are enveloped, positive single-stranded RNA viruses. This virus family contains three genera: Hepacivirus (hepatitis C virus [HCV]), Flavivirus (e.g., Yellow fever virus [YFV], Dengue fever virus [DENV], Japanese encephalitis virus [JEV], Tick-borne encephalitis virus [TBEV]) and Pestivirus (Bovine viral diarrhea virus [BVDV], Classical swine fever virus [CSFV], and Border disease virus [BDV]). Hepatitis G virus/GB-virus C (HGV/GBV-C) is classified within the family Flaviviridae but has not been assigned to a genus. Although viruses belonging to different genera have different biological properties and do not show serological cross-reactivity, great similarity in terms of virion morphology, genome organization, and presumed replication strategy have been noted (33, 195, 246). The organization of the genome and its nomenclature, as well as the replication strategy, are presented in Fig. 1 and 2 and Table 1.
FIG. 1.
Genomic organization of members of the Flaviviridae. The viral genome consists of a single-stranded RNA molecule of positive polarity which is capped in flaviviruses and contains an IRES in hepaciviruses and pestiviruses. UTR are present at the 5′ and 3′ ends of the genome. Boxes indicate mature proteins generated by proteolytic processing.
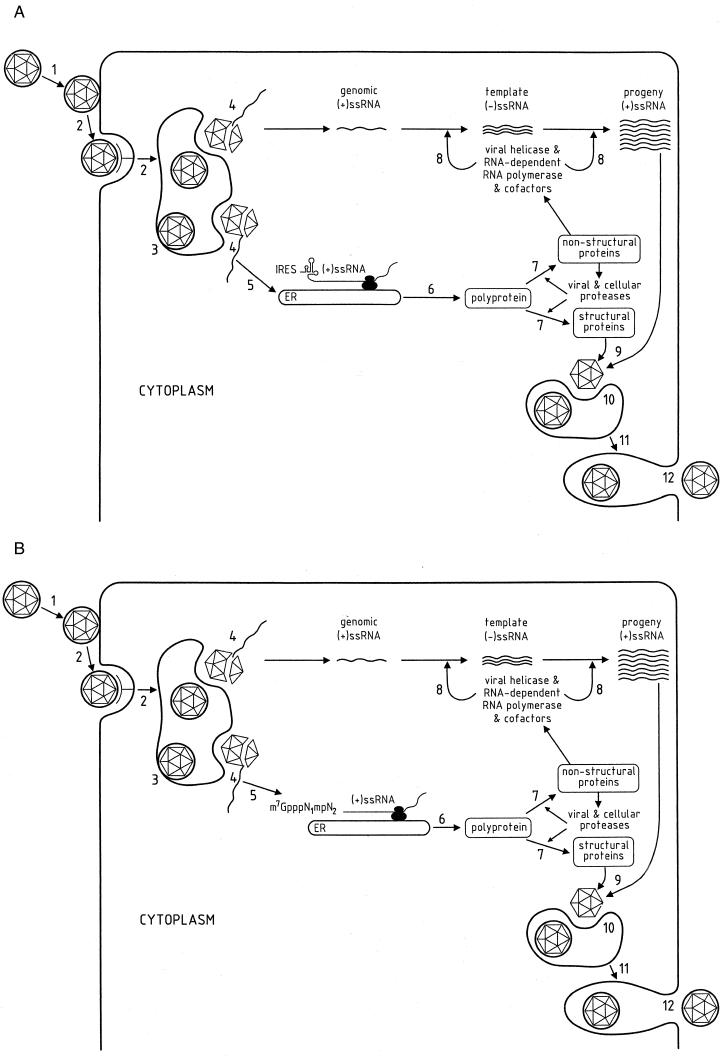
FIG. 2.
Replicative cycle of members of the Flaviviridae. The presumed replication cycles of the hepaciviruses and pestiviruses (A) and of the flaviviruses (B) are shown. 1, adsorption; 2, receptor-mediated endocytosis; 3, low-pH fusion in lysosomes; 4, uncoating; 5, IRES-mediated initiation of translation (A) or cap-mediated initiation of translation (B); 6, translation of the viral RNA into viral precursor polyprotein; 7, co- and posttranslational proteolytic processing of the viral polyprotein by cellular and viral proteases; 8, membrane-associated synthesis of template minus-strand RNA and progeny plus-strand RNA; 9, assembly of the nucleocapside; 10, budding of virions in the endoplasmatic reticulum; 11, transport and maturation of virions in the endoplasmatic reticulum and the Golgi complex; 12, vesicle fusion and release of mature virions. ss, single stranded.
TABLE 1.
5′ and 3′ UTR, structural proteins, and nonstructural proteins of members of the Flaviviridae and their function
| Protein | Hepacivirus | Flavivirus | Pestivirus |
|---|---|---|---|
| 5′ UTR | 342 bases (80) | 67–132 bases (33) | 385 bases (37) |
| Structural proteins | |||
| CAP | —a | Type I m7GpppN1mpN2 (245) | — |
| IRES | HCV (135, 232) | — | BVDV (188), CSFV (196, 207) |
| Npro | — | — | Autoprotease (p20) (248) |
| C | Nucleocapsid protein (12) | Nucleocapsid protein (33) | Nucleocapsid protein (p14) |
| Pre-M | — | Precursor membrane glycoprotein (244) | — |
| E | — | Envelope glycoprotein (33) | — |
| Erns | — | — | Envelope glycoprotein with RNase activity (gp48) (25) |
| E1 | Envelope glycoprotein (12) | — | Envelope glycoprotein (gp25) (200) |
| E2 | Envelope glycoprotein (12) | — | Envelope glycoprotein (gp53) (200) |
| Nonstructural proteins | |||
| NS1 | — | Role in early RNA replication (144), secreted after glycosylation (33) | — |
| NS2 | Constituent of the protease (12) | — | Function unknown (p54) (17) |
| NS2A | — | Function unknown; binds strongly to the 3′UTR, NS3, and NS5 (152) | — |
| NS2B | — | Cofactor of the NS3 (33, 152) | — |
| NS3 | Constituent of the protease; N terminus: serine-type protease; C terminus: NTPase-helicase (12) | Serine protease; NTPase-helicase (33) | N terminus: serine-type protease, C terminus: NTPase-helicase (p80) (252) |
| Expression characteristic for cytopathic biotypes (223) | |||
| NS2-3 | — | — | Expression characteristic for noncytopathic biotypes (223) |
| NS4A | NS3 protease factor (12) | Function unknown; binds strongly to most of the other nonstructural proteins (152) | NS3 protease cofactor (p10) (252) |
| NS4B | Function unknown | Function unknown | Function unknown (p30) (236) |
| NS5 | — | RdRp (33) | — |
| NS5A | Induction of interferon resistance by repression of the cellular PKR protein kinase (68) | — | Function unknown (p58) |
| NS5B | RdRp (12) | — | RdRp (p75) |
| 3′UTR | Variable length (120) | 114–585 bases (33) | 225 bases (37) |
—, does not apply to this genus.
Hepaciviruses
Infections with HCV were referred to as non-A, non-B hepatitis until the causative agent was identified in 1989 (38). HCV infections occur worldwide. The World Health Organization (WHO) recently estimated that 3% of the world's population has been infected with HCV, which means that more than 170 million chronic carriers are at risk of developing cirrhosis and/or liver cancer (133). Many HCV carriers are intravenous-drug users or blood product recipients. With the introduction of blood donor screening for HCV, the frequency of HCV-associated posttransfusion hepatitis has been declining in recent years. Other possible routes of transmission are needle stick accidents, procedures such as body piercing and tattooing, and traditional scarifications and circumcision in developing countries (118, 260). Sexual and perinatal transmission (181, 194, 228) may also occur, although less frequently (the risk is probably <5%) (133). In many cases, no risk factor(s) could be identified. After infection with HCV, an acute infection develops, mostly with a subclinical (anicteric) course. Three weeks after exposure, HCV RNA becomes detectable, and shortly before the onset of clinical signs, levels of alanine aminotransferase (ALT) in serum start to rise. Only one-third of patients develop jaundice or other signs of acute hepatitis, which may last for 2 to 12 weeks; however, the majority of cases remain asymptomatic. At least 85% of acutely infected patients develop chronic HCV infection (3). Although the symptoms of acute hepatitis may resolve, the ALT levels generally remain elevated and HCV RNA persists. About one-third of these patients have persistently normal ALT levels, although most of them have histological evidence of chronic hepatitis (133). In general, the higher the ALT levels, the more severe the symptoms of chronic hepatitis (133). Chronic hepatitis C, whether associated with symptoms or asymptomatic, can lead to cirrhosis and end-stage liver disease. In studies with a 10- to 20-year follow-up, cirrhosis developed in 20 to 30% of the patients, 1 to 5% of whom may develop liver cancer during the next 10 years (56, 109, 187). Together with alcoholic liver disease, hepatitis C is the most common cause of cirrhosis and the major indication for liver transplantation. After liver transplantation, recurrence of HCV infection is common, but in many cases the recurrence is mild and the long-term survival averages 65% after 5 years (references 90, 93, 112, 175, and 197 and references therein). At the current rate of infection, the pool of chronic hepatitis C patients would increase by 4% in the next decade but remain stable thereafter. The number of deaths caused by HCV in the United States is currently estimated at 8,800 per year; by the year 2008 this may be as high as 35,000 (44).
So far, little is known about HGV/GBV-C infections. GB viruses (GBV-A, GBV-B, and GBV-C) are phylogenetically related to HCV (108, 145, 253). GBV-A and GBV-B are naturally occurring viruses in tamarins; humans are the natural host for GBV-C. GBV-C is found mostly as a coinfection associated with HCV and is transmitted in the same way (59, 60).
Flaviviruses
Currently, more than 70 flaviviruses have been reported, and many of them cause important human diseases. All human flaviviruses are transmitted by vectors such as ticks and mosquitoes, making the disease very difficult to eradicate (169). Based on phylogenetic analysis, 72 species of flaviviruses have been grouped into 14 clades, which in turn can be grouped in three clusters: the mosquito-borne cluster, the tick-borne cluster, and the no-vector cluster. All flaviviruses of human importance belong to the first two clusters; the last cluster holds a few viruses which have been isolated from mice or bats; however, no arthropod vector or natural route of transmission has yet been demonstrated (125).
Although an efficient vaccine exists against YFV, this virus is still a leading cause of hemorrhagic fever and related mortality (up to 50%) worldwide (167). Another flavivirus, DENV, threatens up to 2.5 billion people and is still emerging throughout the world. Over 100 million cases of DENV and at least 500,000 cases of dengue hemorrhagic fever (DHF), including in about 25,000 fatal cases, occur annually worldwide (77, 195, 250). All the DENV strains that have ever been isolated can be divided into four serotypes, which cause similar disease in humans (77, 249). Although the primary infection is (often) subclinical (especially in children) and induces lifelong immunity for that particular serotype, a second infection with a different serotype may lead to the development of DHF or dengue shock syndrome (combined mortality, up to 5%) (126, 193). DHF has been classified into four grades according to the severity of shock and bleeding (249). It is generally assumed that antibody-dependent enhancement of infection plays an important role in the complex pathogenesis of DENV infections, but this needs further study (21, 154, 171). International travel and uncontrolled urbanization have resulted in an increased spread of the mosquito vector (Aedes aegypti and Aedes albopictus). Infections in most tropical and subtropical regions now are hyperendemic (prevalence of two or more serotypes), which enhances the occurrence of DHF and dengue shock syndrome (77).
A third important member of the Flavivirus genus is JEV. This mosquito-borne virus is the leading cause of viral encephalitis worldwide. Approximately 50,000 cases occur annually in Asia and result in high mortality (30%) or in long-lasting neurological sequelae (30%) (105, 166).
Other important flaviviruses that cause encephalitis are also responsible for high mortality rates or neurological sequelae. Two important subtypes of TBEV exist, i.e., the European and Eastern subtypes. The mortality rate associated with infection by the Eastern subtype (also referred to as Russian spring-summer encephalitis virus [RSSEV]) is ∼20%; for infection by the Western subtype (also referred to as Central European encephalitis virus [CEEV]) this value is 1 to 2% (85). Although the last large epidemic caused by Murray Valley encephalitis virus (MVEV) occurred in 1974, new cases of MVEV infection are reported regularly, especially in Western Australia (153). West Nile virus (WNV) is endemic mainly in Africa and the Middle East and around the Mediterranean Sea. In 1996 an outbreak of WNV infection with 373 cases and 17 deaths was reported in Romania (81, 231). Recently (late August and September 1999), an outbreak of encephalitis caused 77 cases in the New York City area, with six deaths. Although the cause was first identified serologically as St. Louis encephalitis virus, partial sequence analysis showed the virus to have higher similarity to the West Nile virus (T. Briese, X.-Y. Jia, C. Huang, L. J. Grady, and W. I. Lipkin, Research Letter, Lancet 354:1261–1262, 1999). Although there are no recent reports of outbreaks or epidemics of St. Louis encephalitis virus (SLEV), the virus causing this disease is endemic in the western United States and is responsible for severe disease (123). Omsk hemorrhagic fever virus (OHFV) is responsible for a number of infections annually in rural areas in the Omsk region in Russia. The case fatality rate is 0.5 to 3% (169). Annually, 400 to 500 virologically diagnosed cases of Kyasanur forest disease virus (KFDV) infections are reported in India (169). Louping ill virus (LIV) mainly infects sheep, but up to 1991 at least 37 documented cases of infection in humans have been reported, most of them acquired during laboratory experiments or diagnosis (43).
Pestiviruses
The genus Pestivirus contains three important animal viruses: CSFV, BVDV, and BDV. Two main routes of infection have been described: the oronasal route and the transplacental route. The latter is responsible for the development of persistently infected animals, which are a threat to the rest of the livestock. Although infection of their respective host causes severe disease that is usually followed by death, all viruses are able to cross the interspecies barrier with great ease, usually causing a milder disease in other hosts (57, 226, 235).
BVDV infections are associated with severe mucosal disease in cattle, although swine and other ruminants are also susceptible to the virus (163, 226). Field isolates can be categorized into noncytopathic and cytopathic biotypes depending on their behavior in cell culture (8, 176). Acute postnatal infection of cattle with BVDV is characterized by high morbidity and low mortality rates (182). This mild disease is marked by ulceration of the nose, mouth, and gastrointestinal mucosa, which causes the virus to spread quickly because of continuous salivation, nasal discharge, coughing, or diarrhea (227). Severe disease resembling CSFV infection or OHF has been recorded in calves, although this (acute) hemorrhagic condition is rare (58). The main target organs for viral replication are lymphoid tissues and epithelial and all major lymphocytic cells, although cells of the gastrointestinal tract, glands, and neurons also support viral replication (26, 58). Depending on the time of gestation, transplacental infection with a noncytopathic strain may cause calves to be stillborn, to become persistently infected, or to experience growth retardation or severe neurological malformations (86). At 6 months to 2 years old, these persistently infected calves develop mucosal disease, which is characterized by severe ulceration of the gastrointestinal tract, and they die about 2 weeks after onset of the disease (140, 141, 224). The mucosal disease is associated with the conversion of the primary infecting noncytopathic strain (or biotype) into a cytopathic strain, which results either from insertion of a cleavage site in the NS2-3 protein or duplication of the NS2-3 gene (thus causing the expression of the NS3 protein instead of the NS2-3 protein) (54, 124, 164, 165, 224). Estimates of economic losses due to BVDV vary and depend mainly on the pathogenicity of the strain. At an estimated annual incidence of acute infections of 34%, annual losses due to low-virulence BVDV strains were estimated at $20 million per 106 calvings and the losses due to highly virulent strains were $57 million per million calvings (92).
Two other pestiviruses cause infection and disease in livestock. CSFV, also known as hog cholera virus, is an important, highly contagious pathogen of swine that is easily transmitted by aerosol, contaminated clothing, or direct contact (127, 129). Wild boar are responsible for introducing or reintroducing CSFV into many domestic swine herds (111). The virus has an almost worldwide distribution and leads to severe economic losses (238). Although severe acute infections with mortality rates varying from 30% up to 90–100% were previously described, chronic CSFV infections are more common today. After infection, pigs suffer from milder symptoms than those associated with the acute form, and the severity of disease depends on whether the infecting strain is of the cytopathic or noncytopathic biotype. Viremia is less pronounced, and a neutralizing-antibody response develops (122). After 2 weeks to several months, the condition of the infected animals deteriorates to a severe leukopenia, which is particularly notable with the lymphocytes (183, 215), and they finally die of neurologic and hemorrhagic symptoms. Infection before day 41 of gestation is lethal. Fetuses that become infected after day 41 and before day 85 may appear normal, but persistent viremia is detected and all of them will die within 1 year of the onset of disease, which is referred to as late-onset CSFV infection. Because of the structure of the porcine placenta, some piglets in a given litter may become infected whereas others may not. After day 85 of gestation until birth, piglets are no longer susceptible to infection (227).
BDV of sheep and goats causes a mild clinical disease following acute postnatal infection (177). However, congenital infection acquired between days 16 and 64 of gestation may cause fetal death, abortion, or endocrine, nervous, skeletal, integumentary, and immune system abnormalities in lambs (30, 177). As with BVDV and CSFV, BDV isolates can, depending on their behavior in cell culture, be divided in noncytopathic and cytopathic biotypes, of which the latter are also characterized by the production of the NS3 nonstructural protein instead of the NS2-3 protein (16).
Therapy for pestivirus infections is not believed to be an option. Instead, when an outbreak or epidemic of CSFV occurs, populations of infected pigs are slaughtered to prevent further virus transmission. The overall strategy for BVDV is (i) vaccination or (ii) identification and removal of persistently infected animals (143). Efficient vaccination may lead to the situation where no more persistently infected calves are born (22, 40, 41).
CURRENT STRATEGIES FOR THE TREATMENT OF INFECTIONS
Hepaciviruses
Currently, chronic or early-diagnosed acute hepatitis C is treated with α-2 interferon alone (131) or in combination with ribavirin (45, 47). The interferons approved for HCV are α-2b interferon (Intron-A), α-2a interferon (Roferon-A), consensus interferon (r-metIFN-Con1), and α-1n interferon (Welferon); all appear to be clinically equivalent (72). Interferon therapy, which is expensive, is associated with many side effects (especially after prolonged therapy) and is effective in only a subset of patients (159). Forty percent of patients with chronic HCV have an initial response to interferon therapy but may subsequently relapse. Only 15 to 20% of patients have a sustained virological response (46, 162). Interferon treatment of patients who had already developed cirrhosis resulted in clinical improvement and reduced the progression to hepatocellular carcinoma (HCC) (19). Therapies (i) with higher interferon dosages (107), (ii) initiated during the acute phase of the infection (192), (iii) with other types of interferon (including pegylated interferon, which results in a more sustained drug concentration) (29, 73, 84), (iv) with other treatment schedules (66), or (v) for longer duration (230) are currently being evaluated. Clinically relevant factors favoring the outcome of treatment with interferon are low pretreatment levels of HCV RNA (less than 2 × 106 copies/ml); patient younger than 40 years, presence of minimal fibrosis, female gender, and the absence of genotype 1b virus (184). Viral factors that may promote the progression of the liver disease include high HCV levels in serum, viral genotype 1b, and a high degree of viral genetic diversity (73, 149). Negative host factors may include immune deficiency, alcohol consumption, and coinfection with Human immunodeficiency virus (HIV) or HBV (73). The National Institutes of Health have recommended against the use of interferon for chronic HCV patients with normal levels of liver enzymes (156). Patients with normal or minimally elevated levels of transaminases in serum have a more benign course of infection with minimal risk of progression to cirrhosis. Evidence has suggested that interferon therapy is not usually beneficial and may even be harmful in this cohort of patients, perhaps as the result of an immunomodulatory action of interferon that may alter the balance of host immune reactions and viral replication (156). Antiviral therapy with an interferon-based regimen is also not recommended in decompensated patients with cirrhosis, because of an increased adverse-effect profile (73).
Data obtained from multicenter studies showed that ribavirin as monotherapy was not more effective than placebo in reducing or eliminating HCV RNA levels (136, 263). Because ribavirin lowered the ALT levels and improved liver histology in most patients without being antivirally active, an immunomodulatory role has been attributed to this drug (98). When therapy with ribavirin was stopped, ALT levels in serum rose to pretreatment levels. A major side effect of ribavirin after prolonged treatment may be reversible hemolytic anemia (91). Close monitoring of hemoglobin is therefore required. Ribavirin in combination with α-2b interferon (marketed as Rebetron) has been approved in the European Union for the treatment of both naive and relapsing hepatitis C. The product has received marketing authorization throughout the 15 member states of the European Union. It is already being marketed in the United States and approved in Canada. Rebetron is approved for the treatment of chronic hepatitis C infection in patients who have relapsed after interferon therapy and for previously untreated patients with chronic hepatitis C, elevated ALT levels, high inflammatory activity or fibrosis, or HCV RNA-positive serum but without liver decompensation. In a large international study, a significantly greater sustained virological response was found in patients treated with the combination of interferon plus ribavirin than in patients treated with interferon alone (190). Improved sustained response rates with combined treatment were also recently reported in a large multicenter trial in the United States (162). In a study with 345 patients with chronic HCV who relapsed after interferon treatment, combination treatment resulted in a higher rate of virological, biochemical, and histological response than did treatment with interferon alone (45). In another study with 912 patients, interferon alone or in combination with ribavirin was given as initial treatment for chronic hepatitis C. The rate of sustained virological response and histological improvement was better in those receiving the combination therapy (162). Overall, viral clearance in about 40% of the patients was obtained with these combination regimens. Thus, although combination therapy results in a marked improvement in the ability to treat chronic HCV infection, ∼60% of the patients still do not respond to treatment. There is therefore an obvious need for more potent therapies. The effect of the combination of interferon and ribavirin has also been evaluated in patients dually infected with HCV and HGV and in HGV-positive patients. However, no significant reduction of the HGV RNA levels in serum was observed (132, 199, 257).
Flaviviruses
At present, no vaccine or effective antiviral treatment exists for the prevention or treatment of infections with DENV. Supportive care and symptomatic treatment through hydration or aggressive fluid management, if hypotention develops during the course of DHV, are the most important aids to improve survival. Treatment with the corticosteroids methylprednisolone, hydrocortisone hemisuccinate (214, 221), or carbazochrome sodium sulfonate (AC-17) (which decreases capillary permeability) (222) did not reduce mortality in children with severe dengue shock syndrome.
Ribavirin has significant in vivo activity against a number of RNA viruses and has proven to be effective in the treatment of Influenza virus (71), Respiratory syncytial virus (216), Lassa fever virus (161; E. L. Stephen and P. B. Jahrling, Letter, Lancet i:268–269, 1979), and Hanta virus infections (95). The in vitro and in vivo activity of ribavirin against flaviviruses (such as YFV and DENV) is, however, very weak (Table 2) (32, 67, 94, 96, 178; our unpublished data). In a blinded, placebo-controlled study, prophylactic ribavirin treatment of rhesus monkeys infected with DENV had little or no effect on viremia (155). In mice, intraperitoneal administration of ribavirin had no effect on survival following intracerebral inoculation with DENV. However, treatment with ribavirin-2′,3′,5′-triacetate, a prodrug of ribavirin, resulted in a significant increased survival time and rate. This may be explained by the higher capability of this molecule to cross the blood-brain barrier (119). Ribavirin had no beneficial effect on the course of YFV infections in rhesus monkeys, as assessed by measuring viremia and liver dysfunction and scoring for encephalitis and mortality (94). Rhesus monkeys treated with the nuclease-resistant complex of polyriboinosinic-polyribocytidylic acid (an interferon inducer) were protected against virus-induced mortality after challenge with a virulent wild-type YFV strain (211). Treatment of weanling mice and baby hamsters with the interferon inducer 10-carboxymethyl-9-acridanone prevented death caused by peripheral JEV infection (225). One case study described the beneficial effect of recombinant human alpha interferon on the course of JEV infection (82). Dexamethasone did not prevent death caused by edema-induced increases in intracranial pressure in patients with severe JEV (88).
TABLE 2.
Susceptibility of YFV and DENV to a selection of antiviral agents
| Antiviral agent | EC50 (μg/ml)a for:
|
|
|---|---|---|
| YFV | DENV | |
| Ribavirin | 28 ± 18 | 49 ± 13 |
| EICAR | 0.8 ± 0.6 | 2.4 ± 0.8 |
| Mycophenolic acid | 0.08 ± 0.05 | 0.4 ± 0.3 |
| Tiazofurin | 25 ± 14 | 98 ± 13 |
| Selenazofurin | 3.6 ± 1.5 | 10 ± 5.7 |
Concentration required to reduce the virus-induced cytopathic effect in Vero cells by 50%.
VIRAL REPLICATION CYCLE
To discuss potential targets for antiviral therapy, it is necessary that the viral life cycle first be reviewed. The initial site of interaction of DENV with the host cell is heparan sulfate (34, 35), which allows the virus to concentrate on the surface of the cell (Fig. 2). After this interaction, the virus is assumed to bind with high affinity and specificity to a less common receptor or coreceptor, which in turn triggers receptor-mediated endocytosis. Endosomatic pH changes trigger the fusion between the viral envelope and the endosomal membrane, expelling the nucleocapsid in the cytosol, whereupon the positive single-stranded RNA genome is uncoated. Recently, it was demonstrated that the HCV E2 envelope protein binds to CD81, a tetraspanin that is expressed on various cell types including hepatocytes and B lymphocytes (186). After release of the viral genome into the cytoplasm, the 5′ untranslated region (5′UTR) directs the RNA to the ribosomes, where the translation of the single open reading frame into a precursor polyprotein occurs. Viruses belonging to the genus Flavivirus have a short 5′UTR containing a type I m7GpppN1mpN2 cap structure (245). The 5′UTR of the genera Pestivirus (188) and Hepacivirus (HCV [232] and HGV [185]), however, contains an internal ribosomal entry site (IRES), which directs the ribosome to the first triplet coding for the polyprotein. Recently, it was also shown that the 3′ end of the HCV genome enhances the efficiency of translation of the viral RNA (102). The viral polyprotein is processed co- and posttranslationally into individual and functional viral proteins. This processing is carried out by cellular proteases (signalases [191]) and viral proteases, which therefore represent an interesting antiviral target. Indeed, inhibition of the protease activity should prevent the viral proteins from maturing and thus prevent viral replication. The RNA-dependent RNA polymerase, associated with cofactors, produces minus-strand single-stranded RNA, which in turn serves as a template for the production of new plus-strand single-stranded RNA genomes (18). Subcellular localization of viral proteins presumed to be involved in replication of the virus and ultrastructural analysis of infected cells indicate that viral replication is closely associated with membranes. After replication, the viral genome is encapsidated in the nucleocapsid proteins and directed to the endoplasmic reticulum or other membranous structures induced by viral infection, where the immature virus, surrounded by a lipid envelope containing viral proteins, buds off into the lumen. Passing through the secretory pathway, the envelope proteins become glycosylated. Finally, mature viruses are released into the extracellular space (33, 246).
INHIBITION OF SPECIFIC ANTIVIRAL TARGETS AND ASSAYS TO ASSESS THIS INHIBITION
Cell Culture Systems
An efficient cell culture system for the replication of HCV has not yet been established. Attempts have been made to use human cells of hepatocytic and lymphocytic origin, but low and variable levels of replication and virus-induced cytotoxicity pose important problems. Primary hepatocytes (derived from a human donor) can be infected with HCV isolated from serum of viremic patients, and the virus can, although with much variation, be detected in the supernatant for several weeks after infection. HCV replication has been demonstrated by detection of minus-strand RNA in primary hepatocytes derived from an HCV-negative donor after infection with sera from HCV-positive patients with different viral loads in a strand-specific reverse transcription-PCR (65). However, the availability of primary hepatocytes is limited, and their isolation is time-consuming and labor-intensive, which makes these systems unsuitable for intensive large-scale antiviral studies. Clones of the nonneoplastic hepatocyte line PH5CH were shown to sustain the replication of HCV; this replication was maintained for 70 to 100 days. Alpha interferon was shown to have an antiviral effect in these cell lines (99).
Another example of progress in this domain has been the generation of a continuous human cell line that inducibly expresses the entire HCV open reading frame (170). To this end, the HCV genome has been cloned in a tetracycline-regulated gene expression system. Withdrawal of tetracycline from the cell culture medium results in the generation of an unspliced ∼9-kb transcript. The viral proteins produced are faithfully processed, which indicates that the cellular and viral proteolytic machinery, as well as the posttranslational modifications, is functional. One of the most recent and important examples is the construction of subgenomic selective replicons cloned from a full-length HCV consensus genome from an infected liver. Following transfection of this RNA in human hepatoma cells, these RNAs were found to replicate to high levels, allowing detailed molecular studies of HCV and the study of antiviral drugs (148).
Both pestiviruses and flaviviruses can be readily propagated in cell culture. In vitro cell culture systems with the vaccine strain 17D of YFV has been used to identify new inhibitors of flavivirus replication. These experiments confirmed the weak inhibitory effect of ribavirin on YFV replication (178). The most potent inhibitor of YFV replication identified was the IMP dehydrogenase inhibitor mycophenolic acid and the ribavirin analog EICAR (5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide) (9) (Table 2). Also, several inhibitors of orotidine monophosphate decarboxylase and CTP synthetase resulted in a selective inhibition of YFV replication (178). Recently, some furanonaphthoquinone derivatives were shown to inhibit the replication of JEV in cell culture (217).
Inhibition of Viral Entry
Molecules that specifically interfere with the initial steps (binding and penetration) of viral interaction with the host cell may represent the first barrier against infection. This may be achieved by interference with the viral molecules that bind to their cognate receptor or with the receptor itself. However, at present, for most members of the Flaviviridae, relatively little is known about these initial steps. A better understanding of these processes should be helpful.
Studies with many other viruses have shown that several molecules may act as potent inhibitors of virus binding and/or penetration. These include (i) polyanions (such as sulfated, sulfonated, or carboxylated polymers and polyoxymetalates [100, 179] that block the binding to the host cell of enveloped viruses, including herpesviruses such as cytomegalovirus [CMV] and HIV), and (ii) plant lectins (either mannose, N-galactosamine, or N-acetylglucosamine specific) that are inhibitory to HIV and CMV (10, 11), bicyclams (e.g., AM3100) that hinder the interaction of HIV with its CXCR-4 coreceptor (55, 204), and molecules, such as pirodavir, that prevent the binding of rhinovirus particles with the host cell (4, 83, 198). So far, only the polysulfate PAVAS (a copolymer of acrylic acid and vinyl alcohol sulfate) has been shown to block the infection of primary hepatocytes with HCV (39). It is mandatory, therefore, to examine various other polyanionic (sulfate, sulfonate, carboxylate, oxometalate, etc.) substances for their inhibitory effects on HCV infection. A possible exciting application of compounds that are specifically targeted at the initial interaction of HCV with its host cell would be the addition of such compounds to blood products that have not been screened for the presence of HCV, as is often the case in Third World countries.
Inhibition of the IRES
An example of a possible strategy to discover inhibitors of IRES has been described (31). Initial screening for active compounds was performed with an HCV-IRES-firefly luciferase (Fluci) construct in the Huh7 cell line. To verify selectivity, additional tests were performed with a dual-reporter cell line by using a Renilla luciferase construct (Rluci-IRES-Fluci). Compounds suppressing both signals were discarded as nonselective inhibitors of translation or transcription, whereas compounds that inhibited solely the Fluci signal were selected. These compounds were further studied for their potential inhibitory effect on the replication of an IRES-dependent virus by using chimeric poliovirus whose IRES was replaced by the HCV IRES. The IRES site of poliovirus can indeed be functionally replaced by the related genetic element from HCV. Using a similar chimeric poliovirus, a small (60-nucleotide) RNA (called IRNA, isolated from yeast) was found to inhibit viral replication by competing for critical cellular polypeptides that are required for viral IRES-mediated translation (150, 261).
Inhibition of Capping
In flaviviruses, prevention of capping should elicit an antiviral effect by “disabling” the RNA of the progeny virus. Caps are synthesized by an RNA triphosphatase, a guanylyltransferase, and a methyltransferase. Flaviviruses replicate in the cytoplasm, it can therefore be assumed that these functions are performed by virus-encoded proteins (245). However, much research is still needed to unravel the pathways involved in the capping of flavivirus RNA. Evidence was provided that the flavivirus NS3 protein encodes a triphosphatase in its carboxy-terminal domain (245). A putative methyltransferase domain was identified in the NS5 gene of flaviviruses (121). S-Adenosylmethionine (SAM) acts as a methyl donor in transmethylation reactions. Viral (and cellular) methyltransferases are sensitive to inhibition by S-adenosylhomocysteine (SAH). Specific inhibitors of SAH hydrolase inhibit the replication of RNA viruses such as the rhabdoviruses, reoviruses, and arenaviruses (50). Recently, the SAH hydrolase inhibitor 3-deazaneplanocin A was shown to protect mice against a lethal infection with a mouse-adapted Ebola virus (97). Thus, both viral enzymes (i.e., RNA triphosphatase and methyl transferase) involved in capping, as well as the cellular SAH hydrolase, may be targets for chemotherapeutic intervention in the replicative cycle of flaviviruses.
Inhibition of Protease
The HCV genome encodes two different protease activities: the NS2/3 (putative metalloproteinase) and the NS3 (serine protease). The HCV serine protease is encoded by the NS3 gene and is believed to be one of the main targets for antiviral therapy for members of the Flaviviridae. The proteases of HCV (172, 206, 239) and DENV (234) have been cloned and expressed. The presence of the NS4A protein (or at least part of it) is essential for optimal functioning of the HCV and pestivirus NS3 serine protease (61, 229, 252). The flavivirus NS2b has been proposed to be a cofactor for the flavivirus NS3 (33, 152). Coexpression of the HCV NS3-NS4A complex indeed results in enhanced proteolytic activity compared to that of the NS3 protease alone (203, 220, 247). Site-directed mutagenesis of the HCV protease (209, 233, 254) and the DENV protease (234) and ultrastructural analysis of the HCV protease with and without cofactors (117, 256), has been used to study the properties of amino acid residues in or near the catalytic site. By using protease inhibitors, clues about the structural requirements for achieving effective enzymatic inhibition were determined (53). Because of the low efficiency of the NS3 protease, an assay to determine the activity of the protease based on the natural substrate is not very sensitive. To improve the quality of the assays, Bianchi and colleagues developed synthetic depsipeptide substrates that were shown to have a 100-fold higher turnover rate than the natural substrate did (20, 218).
Several major pharmaceutical groups have set up high-throughput screening systems to identify protease inhibitors. Various methods to discover inhibitors of the enzyme have been developed. Sudo et al. (212) engineered a maltose-binding protein–NS3–NS4A fusion protein and a synthetic peptide that mimics the NS5A-NS5B junction, which is cleaved by the NS3 protease. Degradation of the substrate or inhibition of cleavage could be demonstrated by high-performance liquid chromatography (212). By using poliovirus, a chimeric virus containing the HCV NS3 protease and an NS3-specific cleavage site was generated. Production of infectious virus depended on the activity of the HCV protease (79).
A similar approach to assess the activity of the NS3 protease in cell culture is to use chimeric Sindbis viruses carrying sequences coding for NS3 and its cofactor NS4A. The NS3 cleavage site was introduced between the structural proteins of the Sindbis virus. Production of infectious Sindbis virus particles was found to rely on the activity of the HCV NS3 protease. Disabling the protease by mutagenesis inhibited the production of new Sindbis virus particles (62).
Cho et al. (36) constructed a chimeric gene allowing the expression of the NS3/NS4A-secreted alkaline phosphatase (SEAP) polyprotein. Normal activity of the NS3 protease would result in cleavage of the polyprotein and secretion of the SEAP into the extracellular medium of both transfected or stably transformed cell lines. Inhibition of the protease activity should lead to a reduced secretion of SEAP, which can be conveniently quantified by a chemiluminescence detection method (36). A similar type of assay has been described by Lamarre (130). The amino acid sequence targeted by the HCV NS3 protease was hooked onto the peroxidase-antiperoxidase complex; the complex was then attached to the bottom of 96-well plates. Compounds inhibiting the HCV protease could be readily detected by using the peroxidase-antiperoxidase color reaction after incubation with purified NS3 protease (130). So far, several either nonpeptidic (104, 213) or peptidic (101, 209) inhibitors of the HCV protease have been identified. As for HIV (52, 63), peptidomimetics could be designed. Inhibitors of the HCV protease could also be designed based on studies of the active loop of mutant eglin c, a well-known protease inhibitor (158), and by studying the inhibitory properties of modified natural peptide substrates (101).
Inhibition of RNA-Dependent RNA Polymerase
Another main target is the RNA-dependent RNA polymerase (RdRp). The RNA polymerases of HCV (1, 147, 255, 259) (18), DENV (219), and BVDV (262) have been cloned and expressed. The kinetic properties of the HCV RdRp have been studied in detail (147). An assay in which the RdRp activity is measured in crude extracts of cells infected with a flavivirus has been used for antiviral drug-screening purposes (13, 14). The 5′-triphosphate metabolite of ribavirin is believed to act as an inhibitor of the viral RdRp (94). Besides inhibition of the viral RdRp, two other, non-mutually exclusive mechanisms of antiviral actions of ribavirin have been proposed: (i) inhibition of the IMP dehydrogenase after phosphorylation of ribavirin to ribavirin-5′-monophosphate (RMP), causing a depletion of the intracellular GTP pools, and (ii) inhibition of the guanylyltransferase by ribavirin-5′-triphosphate (RTP), which could interfere with the capping of viral and cellular RNA (74). The precise mechanism of action, i.e., the relative importance of RTP as inhibitor of the viral RdRp in the antiviral activity of ribavirin against flaviviruses, remains to be elucidated.
Several derivatives of ribavirin that are endowed with broad-spectrum antiviral activity have been described. The most potent in this series is EICAR (51). EICAR is 20- to 60-fold more active than ribavirin against YFV (178) and DENV Table 2). The 5′-monophosphate form of EICAR (EICAR-MP) is, like RMP, also an inhibitor of IMP dehydrogenase (9). EICAR 5′-triphosphate (EICAR-TP) is formed intracellularly. This metabolite may, by analogy to RTP, have the potential to inhibit the viral RdRp. Further investigation of the mechanism of the anti-flavivirus action of ribavirin and its analogues may lead to the development of (ribavirin) analogues that are selective and strong inhibitors of the replication of members of the Flaviviridae through a specific interaction with the RdRp.
Inhibition of Helicase
As well as the serine protease activity located at the N terminus of the NS3 protein, helicase and NTPase activities are located in the C terminus of this protein. Helicases are enzymes which unwind double-stranded DNA-DNA, RNA-DNA, or RNA-RNA regions in an ATP-dependent reaction. The function of the helicase of the Flaviviridae is assumed to be the unwinding of the plus and minus RNA strands of the genome after the polymerase reaction.
It is presumed that the helicase-NTPase is essential for viral replication and may thus represent an excellent target for drug development. Inhibitors of the helicase of Herpes simplex virus have been identified (208). The HCV (69, 189) and HGV (134), helicases have been cloned, expressed, and characterized. The helicase of the Flaviviridae belongs to the DEAD (Asp-Glu-Ala-Asp) box family of RNA helicases (115). The functional domain of the HCV NTPase-RNA helicase is about 400 amino acids long (113, 114). The crystal structure of the helicase domain of the HCV NS3 (either with or without a bound single-stranded oligonucleotide) has been reported (116, 258). There may be several potential sites for the interaction of small-molecule inhibitors, including the binding site for ATP and for the single- and double-stranded polynucleotide. These sites are believed to exist in both “open” and “closed” conformations. Trapping the enzymes in either of these conformations may be assumed to block its activity (115).
Ribozymes and Gene Therapy
Ribozymes are RNA molecules composed of a catalytic site that will cleave the target RNA at a specific site and a sequence complementary to a designated site on the target RNA. Ribozymes targeted to highly conserved regions of the HCV genome were shown to cleave the viral RNA and to reduce in vitro translation (180). The ribozymes reduced or eliminated HCV RNA expressed in cultured cells and in primary human hepatocytes that had been isolated from patients with advanced HCV-associated liver disease (138). A luciferase assay to determine the effect of HCV 5′UTR-specific targeted ribozymes on the protein translation process has been reported (202). Due to the RNA structure of ribozymes and hence their high biodegradability, there is a serious delivery problem. A gene therapy approach, using adenovirus-mediated expression of ribozymes, may help to solve this problem (139, 243).
Antisense Oligonucleotide Therapy
Antisense oligonucleotides are able to target and disable viral replication by interfering with the translation process by hybrid arrest of the translational machinery or by the induction of RNase that results in the cleavage of the double-stranded RNA portion of the hybrid (7). Antisense (phosphorothioate) oligonucleotides were shown to inhibit HCV translation in an in vitro model (2, 240). The only antisense oligonucleotide that has been formally approved as an antiviral drug is ISIS 2922 (formiversen). This molecule shows protection against CMV retinitis in AIDS patients when injected intravitreally (64, 160). Although antisense oligonucleotides are designed to act in a very selective way by sequence-dependent binding, side effects are not uncommon, since interaction with DNA (triplex formation) and proteins (through simple charge interactions or even sequence-dependent binding) may also occur (7, 23). Major problems such as rapid digestion by intracellular nucleases and intracellular delivery of the antisense oligonucleotide may be alleviated by modifying the phosphodiester backbone to a phosphorothioate and/or loading the drug in liposomes or DNA-protein complexes which can be targeted to specific cells or tissues by antibodies (7, 23, 76).
ANIMAL MODELS
Experimental HCV Infections
As well as humans, chimpanzees can also be infected with HCV (15, 27, 205) (Table 3) or HGV (28). Although the chimpanzee model has contributed significantly to the understanding of HCV infection, the high cost and availability of these animals limit the extent to which antiviral-drug (and other) studies can be carried out with this model. Small laboratory animals, including mice, are not susceptible to infection with HCV. Alternative models such as the “trimera mouse model” have been established (42). In this model, immunocompetent mice are lethally irradiated and subsequently given bone marrow cells from SCID mice. Thereafter, liver fragments from viremic patients with HCV are transplanted under the kidney capsule. Replication of the virus, as assessed by means of reverse transcription-PCR, was detected for more than 15 days in this model. Another mouse model of HCV involves the highly tumorigenic Huh-7 HCC cells (174). After in vitro infection of these Huh-7 HCC cells with serum isolated from HCV-positive patients, cells are implanted directly into the livers of nude mice. As reported, HCV RNA remained detectable in the serum and liver for more than 49 days postinfection (174). The similarities in genetic organization, replication strategy, and polyprotein processing between HCV and the tamarin virus GBV suggest that it may be possible to construct chimeric HCV-GBV viruses that can be used to infect tamarins. These monkeys are smaller and easier to handle than chimpanzees (73). A murine model that may help elucidate the mechanism of hepatocarcinogenesis and its therapy has recently been published (173). Mice transgenic for the HCV core gene were found to develop steatosis, a histological feature characteristic of chronic hepatitis C, early in life. After the age of 16 months, adenomas appeared, in which, in a nodule-in-nodule like manner, a less differentiated neoplasia resembling human HCC developed.
TABLE 3.
Animal models for infections with members of the Flaviviridae
| Virus | Model | Remarks | Reference(s) |
|---|---|---|---|
| HCV | Chimpanzees | Viremia after peripheral infection | 15, 27, 205 |
| Trimera mouse model | Viral replication after implantation of HCV-positive liver fragments | 42 | |
| Huh-7 HCC mouse model | Viral replication after implantation of HCV-infected HCC cells in nude mice | 174 | |
| Hepatocarcinogenesis | Cirrhosis and liver cancer in mice transgenic for the HCV core protein | 173 | |
| YFV | Monkeys | Morbidity and mortality after peripheral infection | 6, 137, 146, 157, 168 |
| Suckling mice | Encephalitis and death after intracerebral injection with a mouse-adapted YFV strain | 201 | |
| DFV | Monkeys | Viremia after peripheral inoculation; encephalitis after direct intracerebral infection | 5, 155 |
| Suckling mice | Encephalitis after intracerebral but not peripheral inoculation with strain adapted through serial passage in the brain | 24, 110 | |
| Hu-PBL-SCID model | Some (but variable) viral replication | 251 | |
| K562-SCID model | Encephalitis and death after inoculation of the virus in an abdominal erythroleukemia tumor mass | 142 | |
| AG129 mice (IFN-αr/βr-γr) | Encephalitis and death after intraperitoneal inoculation | 103 | |
| JEV | Bonnet macaques | Encephalitis after intracerebral or peripheral inoculation | 70, 75 |
| Immunocompetent mice | Encephalitis and death after peripheral or intracerebral inoculation with some strains | 87 | |
| TBEV | Dogs | Encephalitis and death after bite of an infected tick | 242 |
| Immunocompetent mice | Encephalitis and death after peripheral inoculation with a neurovirulent strain | 237 | |
| Suckling mice | Encephalitis and death after intracerebral or peripheral infection | 89, 106 |
Experimental Flavivirus Infections
In Table 3, the different animal model systems for flavivirus infections are listed. Monkeys have been used to study the neurovirulence of YFV vaccines (137, 157). Experimental infection of rhesus, cynomolgus, or squirrel monkeys with a wild-type YFV strain results in liver damage followed by death, as seen in humans (6, 146, 168). Mouse-adapted strains that were obtained by serial passage of the virus in the brains of suckling mice induce encephalitis and death but only after direct intracerebral inoculation (201). No reports have been published about clinical signs in mice upon peripheral inoculation with mouse-adapted or nonadapted strains of YFV. We have found that intraperitoneal infection of 2-day-old (but not 5-day-old) SCID mice with the vaccine strain Stamaril of YFV results in encephalitis and its associated mortality; in contrast, 2- to 3-day-old NMRI (immunocompetent) mice appeared to be resistant to the infection when not injected directly into the brain (our unpublished data).
Several species of monkeys, such as rhesus monkeys, can be infected experimentally with DENV (155). Although these animals develop viremia, clinical illness or death, as observed in humans, was observed only after intracerebral inoculation with a neurovirulent strain (5). Experimental intracerebral infection of mice with DENV strains that have been passaged in mouse brain may cause encephalitis, especially when newborn or suckling mice are used (24, 110). These mouse-neurotropic DENV strains appear to be attenuated in humans and may be potentially important in the development of a vaccine. Encephalitis caused by DENV in humans is, however, rather rare (151). Peripheral inoculation of DENV in immunocompetent or SCID mice does not cause disease (142, 251; our unpublished data).
A first attempt to establish a murine model for dengue infection by peripheral inoculation of the virus was reported by Wu et al. (251). SCID mice were reconstituted with human peripheral blood lymphocytes and subsequently infected intraperitoneally with DENV (type 1 strain). However, virus production was highly variable in this model (251). A second attempt was recently reported by Lin et al. (142). SCID mice were engrafted intraperitoneally with human erythroleukemia cells (K562). Five weeks after the inoculation of the cells, the resulting abdominal tumor mass was infected with DENV (different strains). Encephalitis and death due to DENV replication was recorded, and viral antigens were detected in the serum, the brain, and the tumor mass (142). Recently, a mouse model that does not make use of human xenografts was reported. Mice that lack the alpha/beta and gamma interferon and receptor genes (AG129 mice) develop disease and die 10 to 12 days after intraperitoneal inoculation with a mouse-adapted type 2 DENV. In contrast to wild-type mice or mice deficient in either the alpha/beta interferon receptor or gamma interferon ligand genes, replication of the virus could be demonstrated in the sera, spleens, and brains of the infected AG129 mice (103).
Intracerebral or subcutaneous inoculation of Bonnet macaques with a neurovirulent strain of JEV resulted in encephalitis (70, 75). The JEV vaccine that is currently used consists of purified inactivated mouse-adapted neurovirulent strains (78, 237). Infection of mice (younger than 3 weeks) with JEV by the intracerebral or peripheral route resulted, depending on the strain used, in encephalitis and death (87). Natural lethal infections with TBEV in dogs bitten by infected ticks have been documented (242). As with JEV, a purified preparation of inactivated TBEV adapted to become neurovirulent in mice can be used as a vaccine (237). Intracerebral inoculation of such virus in suckling or young adult mice results in the development of encephalitis and death (89, 106).
Because of their high pathogenicity for humans, most flaviviruses require special research facilities (BSL3 for YFV [wild-type strains], JEV, LIV, WNV, MVEV, SLEV, and Powassan encephalitis virus and BSL4 for OHFV and RSSEV), which may hinder research in this field. Therefore, an animal model for flavivirus infections that allows researchers to work under BSL2 conditions would help in studying the pathogenesis of and possibly new therapies for flavivirus infections. Ideally, the virus used in such an animal model should (i) be of low pathogenicity for humans, thus reducing the costs of the research and avoiding the need for special facilities for manipulation; (ii) be easy to propagate in cell culture; and (iii) cause morbidity and mortality in small adult laboratory animals following systemic infection. The Modoc virus (48, 49) may fulfill these requirements.
PROSPECTS FOR TREATMENT
Although 170 million people are chronically infected with HCV and are thus at risk of developing cirrhosis, liver failure, and liver cancer, options for therapy are currently limited. Treatment with interferon confers a sustained loss of virus in the blood only in 15 to 20% of the patients and is associated with serious side effects; when interferon is combined with ribavirin, viral clearance may be achieved in up to 40% of patients. Because of the genetic and serological heterogeneity of HCV, the development of effective vaccines will be difficult and is not expected to occur soon. Thus, there is an urgent need for a more (cost-)effective treatment and/or immunoprophylaxis. Because of the lack of a complete cell culture system for HCV replication that can be used for (high-throughput) antiviral drug-screening purposes, several assays have now been elaborated that allow drug screening against specific viral targets (such as the IRES, the viral protease and helicase [which are both encoded by the NS3 gene], and the RdRp [encoded by NS5b]). One may reasonably assume that the major efforts generated by many pharmaceutical groups may in the next coming years result in the discovery of selective inhibitors of HCV replication (241). Such newly discovered inhibitors will first have to be evaluated in the chimpanzee HCV infection model. Despite the major clinical impact of flaviviruses such as DENV, JEV, and TBEV, there is as yet no drug available for the chemoprophylaxis or chemotherapy of infections with these viruses. The intense search for inhibitors of HCV replication will probably result in the discovery of compounds that inhibit the replication of Flaviviridae in general.
ACKNOWLEDGMENTS
We thank Inge Aerts and Christiane Callebaut for dedicated editorial assistance.
P. Leyssen is a Research Assistant from the “Instituut voor Wetenschap and Technologie” (IWT), and J. Neyts is a Post-doctoral Research Assistant from the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (FWO).
REFERENCES
- 1.Al R H, Xie Y, Wang Y, Hagedorn C H. Expression of recombinant hepatitis C virus non-structural protein 5B in Escherichia coli. Virus Res. 1998;53:141–149. doi: 10.1016/s0168-1702(97)00147-0. [DOI] [PubMed] [Google Scholar]
- 2.Alt M, Renz R, Hofschneider P H, Caselmann W H. Core specific antisense phosphorothioate oligodeoxynucleotides as potent and specific inhibitors of hepatitis C viral translation. Arch Virol. 1997;142:589–599. doi: 10.1007/s007050050105. [DOI] [PubMed] [Google Scholar]
- 3.Alter M J, Mast E E, Moyer L A, Margolis H S. Hepatitis C. Infect Dis Clin North Am. 1998;12:13–26. doi: 10.1016/s0891-5520(05)70405-0. [DOI] [PubMed] [Google Scholar]
- 4.Andries K, Dewindt B, Snoeks J, Willebrords R, van Eemeren K, Stokbroekx R, Janssen P A. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob Agents Chemother. 1992;36:100–107. doi: 10.1128/aac.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angsubhakorn S, Moe J B, Marchette N J, Latendresse J R, Palumbo N E, Yoksan S, Bhamarapravati N. Neurovirulence detection of dengue virus using rhesus and cynomolgus monkeys. J Virol Methods. 1987;18:13–24. doi: 10.1016/0166-0934(87)90106-6. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo J I, Apperson S A, Cropp C B, Marafino B J, Jr, Monath T P, Tesh R B, Shope R E, Garcia Blanco M A. Effect of human gamma interferon on yellow fever virus infection. Am J Trop Med Hyg. 1988;38:647–650. [PubMed] [Google Scholar]
- 7.Askari F K, McDonnell W M. Antisense-oligonucleotide therapy. N Engl J Med. 1996;334:316–318. doi: 10.1056/NEJM199602013340508. [DOI] [PubMed] [Google Scholar]
- 8.Baker J C. Bovine viral diarrhea virus: a review. J Am Vet Med Assoc. 1987;190:1449–1458. [PubMed] [Google Scholar]
- 9.Balzarini J, Karlsson A, Wang L, Bohman C, Horska K, Votruba I, Fridland A, Van Aerschot A, Herdewijn P, De Clercq E. Eicar (5-ethynyl-1-beta-d-ribofuranosylimidazole-4-carboxamide). A novel potent inhibitor of inosinate dehydrogenase activity and guanylate biosynthesis. J Biol Chem. 1993;268:24591–24598. [PubMed] [Google Scholar]
- 10.Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W, De Clercq E. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 11.Balzarini J, Schols D, Neyts J, Van Aerschot A, Peumans W, De Clercq E. Alpha-(1-3)- and alpha-(1-6)-d-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991;35:410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartenschlager R. Molecular targets in inhibition of hepatitis C virus replication. Antiviral Chem Chemother. 1997;8:281–301. [Google Scholar]
- 13.Bartholomeusz A, Tomlinson E, Wright P J, Birch C, Locarnini S, Weigold H, Marcuccio S, Holan G. Use of a flavivirus RNA-dependent RNA polymerase assay to investigate the antiviral activity of selected compounds. Antiviral Res. 1994;24:341–350. doi: 10.1016/0166-3542(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 14.Bartholomeusz A I, Wright P J. Synthesis of dengue virus RNA in vitro: initiation and the involvement of proteins NS3 and NS5. Arch Virol. 1993;128:111–121. doi: 10.1007/BF01309792. [DOI] [PubMed] [Google Scholar]
- 15.Bassett S E, Brasky K M, Lanford R E. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becher P, Meyers G, Shannon A D, Thiel H J. Cytopathogenicity of border disease virus is correlated with integration of cellular sequences into the viral genome. J Virol. 1996;70:2992–2998. doi: 10.1128/jvi.70.5.2992-2998.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrens S E, Grassmann C W, Thiel H J, Meyers G, Tautz N. Characterization of an autonomous subgenomic pestivirus RNA replicon. J Virol. 1998;72:2364–2372. doi: 10.1128/jvi.72.3.2364-2372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens S E, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Benvegnu L, Chemello L, Noventa F, Fattovich G, Pontisso P, Alberti A. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer. 1998;83:901–909. doi: 10.1002/(sici)1097-0142(19980901)83:5<901::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi E, Steinkuhler C, Taliani M, Urbani A, Francesco R D, Pessi A. Synthetic depsipeptide substrates for the assay of human hepatitis C virus protease. Anal Biochem. 1996;237:239–244. doi: 10.1006/abio.1996.0235. [DOI] [PubMed] [Google Scholar]
- 21.Bielefeldt-Ohmann H. Pathogenesis of dengue virus diseases: missing pieces in the jigsaw. Trends Microbiol. 1997;5:409–413. doi: 10.1016/S0966-842X(97)01126-8. [DOI] [PubMed] [Google Scholar]
- 22.Bolin S R. Control of bovine viral diarrhea infection by use of vaccination. Vet Clin North Am Food Anim Pract. 1995;11:615–625. doi: 10.1016/s0749-0720(15)30470-9. [DOI] [PubMed] [Google Scholar]
- 23.Bonn D. Prospects for antisense therapy are looking brighter. Lancet. 1996;347:820. doi: 10.1016/s0140-6736(96)90886-7. [DOI] [PubMed] [Google Scholar]
- 24.Bray M, Men R, Tokimatsu I, Lai C J. Genetic determinants responsible for acquisition of dengue type 2 virus mouse neurovirulence. J Virol. 1998;72:1647–1651. doi: 10.1128/jvi.72.2.1647-1651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruschke C J, Hulst M M, Moormann R J, Van Rijn P A, Van Oirschot J T. Glycoprotein Erns of pestiviruses induces apoptosis in lymphocytes of several species. J Virol. 1997;71:6692–6696. doi: 10.1128/jvi.71.9.6692-6696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruschke C J, Weerdmeester K, Van Oirschot J T, Van Rijn P A. Distribution of bovine virus diarrhoea virus in tissues and white blood cells of cattle during acute infection. Vet Microbiol. 1998;64:23–32. doi: 10.1016/s0378-1135(98)00249-1. [DOI] [PubMed] [Google Scholar]
- 27.Bukh J, Apgar C L, Engle R, Govindarajan S, Hegerich P A, Tellier R, Wong D C, Elkins R, Kew M C. Experimental infection of chimpanzees with hepatitis C virus of genotype 5a: genetic analysis of the virus and generation of a standardized challenge pool. J Infect Dis. 1998;178:1193–1197. doi: 10.1086/515683. [DOI] [PubMed] [Google Scholar]
- 28.Bukh J, Kim J P, Govindarajan S, Apgar C L, Foung S K, Wages J, Yun A J, Shapiro M, Emerson S U, Purcell R H. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis. 1998;177:855–862. doi: 10.1086/515255. [DOI] [PubMed] [Google Scholar]
- 29.Cacopardo B, Benanti F, Brancati G, Romano F, Nunnari A. Leucocyte interferon-alpha retreatment for chronic hepatitis C patients previously intolerant to other interferons. J Viral Hepat. 1998;5:333–339. doi: 10.1046/j.1365-2893.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 30.Caffrey J F, Dudgeon A M, Donnelly W J, Sheahan B J, Atkins G J. Morphometric analysis of growth retardation in fetal lambs following experimental infection of pregnant ewes with border disease virus. Res Vet Sci. 1997;62:245–248. doi: 10.1016/s0034-5288(97)90198-3. [DOI] [PubMed] [Google Scholar]
- 31.Cai W, Maier E, Morin N, Ilan E, Schwedesl J, Cai P, Hubermanl K, Dagan S, Rondo R, Heguy A, Mounir S. Symposium on Emerging Therapies for Chronic Viral Hepatitis. 1998. Discovery of anti-HCV IRES compound, general session I, presentation 5. [Google Scholar]
- 32.Canonico P G, Kende M, Luscri B J, Huggins J W. In-vivo activity of antivirals against exotic RNA viral infections. J Antimicrob Chemother. 1984;14(Suppl. A):27–41. doi: 10.1093/jac/14.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- 33.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Maguire T, Marks R M. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho Y G, Yang S H, Sung Y C. In vivo assay for hepatitis C viral serine protease activity using a secreted protein. J Virol Methods. 1998;72:109–115. doi: 10.1016/s0166-0934(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 37.Chon S K, Perez D R, Donis R O. Genetic analysis of the internal ribosome entry segment of bovine viral diarrhea virus. Virology. 1998;251:370–382. doi: 10.1006/viro.1998.9425. [DOI] [PubMed] [Google Scholar]
- 38.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 39.Clarysse C, Neyts J, De Clercq E, Ammack N, Yap S H. Congress of the American Association for the Study of Liver Diseases and the International Association for the Study of the Liver. 1998. Sulfated polymers but not ribavirin or interferon inhibit the infection of immortalized human hepatocytes by HCV. [Google Scholar]
- 40.Cortese V S, Grooms D L, Ellis J, Bolin S R, Ridpath J F, Brock K V. Protection of pregnant cattle and their fetuses against infection with bovine viral diarrhea virus type 1 by use of a modified-live virus vaccine. Am J Vet Res. 1998;59:1409–1413. [PubMed] [Google Scholar]
- 41.Cortese V S, Whittaker R, Ellis J, Ridpath J F, Bolin S R. Specificity and duration of neutralizing antibodies induced in healthy cattle after administration of a modified-live virus vaccine against bovine viral diarrhea. Am J Vet Res. 1998;59:848–850. [PubMed] [Google Scholar]
- 42.Dagan S, Nussbaum O, Eren R, Ben-Moshe O, Arazi Y, Berre S, Lubin I, Shouval D, Galun E, Reisner Y, Ilan E. Symposium on Emerging Therapies for Chronic Viral Hepatitis. 1998. The trimera pouse: an HBV and HCV infection model for evaluation of antiviral therapeutic agents, general session II, presentation 3. [Google Scholar]
- 43.Davidson M M, Williams H, Macleod J A. Louping ill in man: a forgotten disease. J Infect. 1991;23:241–249. doi: 10.1016/0163-4453(91)92756-u. [DOI] [PubMed] [Google Scholar]
- 44.Davis G L. Anticipated mortality of hepatitis C and impact of treatment. Hepatology. 1998;28:390A. [Google Scholar]
- 45.Davis G L. Combination therapy with interferon alfa and ribavirin as retreatment of interferon relapse in chronic hepatitis C. Semin Liver Dis. 1999;19(Suppl. 1):49–55. [PubMed] [Google Scholar]
- 46.Davis G L, Balart L A, Schiff E R, Lindsay K, Bodenheimer H C, Jr, Perrillo R P, Carey W, Jacobson I M, Payne J, Dienstag J L, et al. Treatment of chronic hepatitis C with recombinant alpha-interferon. A multicentre randomized, controlled trial. The Hepatitis Interventional Therapy Group. J Hepatol. 1990;11(Suppl. 1):S31–S35. doi: 10.1016/0168-8278(90)90160-s. [DOI] [PubMed] [Google Scholar]
- 47.Davis G L, Esteban Mur R, Rustgi V, Hoefs J, Gordon S C, Trepo C, Shiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 48.Davis J W, Hardy J L. In vitro studies with Modoc virus in Vero cells: plaque assay and kinetics of growth, neutralization, and thermal inactivation. Appl Microbiol. 1973;26:344–348. doi: 10.1128/am.26.3.344-348.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis J W, Hardy J L, Reeves W C. Modoc viral infections in the deer mouse Peromyscus maniculatus. Infect Immun. 1974;10:1362–1369. doi: 10.1128/iai.10.6.1362-1369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Clercq E. Antiviral agents: characteristic activity spectrum depending on the molecular target with which they interact. Adv Virus Res. 1993;42:1–55. doi: 10.1016/s0065-3527(08)60082-2. [DOI] [PubMed] [Google Scholar]
- 51.De Clercq E, Cools M, Balzarini J, Snoeck R, Andrei G, Hosoya M, Shigeta S, Ueda T, Minakawa N, Matsuda A. Antiviral activities of 5-ethynyl-1-beta-d-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob Agents Chemother. 1991;35:679–684. doi: 10.1128/aac.35.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 53.Dimasi N, Martin F, Volpari C, Brunetti M, Biasiol G, Altamura S, Cortese R, De Francesco R, Steinkuhler C, Sollazzo M. Characterization of engineered hepatitis C virus NS3 protease inhibitors affinity selected from human pancreatic secretory trypsin inhibitor and minibody repertoires. J Virol. 1997;71:7461–7469. doi: 10.1128/jvi.71.10.7461-7469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donis R O, Dubovi E J. Differences in virus-induced polypeptides in cells infected by cytopathic and noncytopathic biotypes of bovine virus diarrhea-mucosal disease virus. Virology. 1987;158:168–173. doi: 10.1016/0042-6822(87)90250-9. [DOI] [PubMed] [Google Scholar]
- 55.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 56.Dutta U, Kench J, Byth K, Khan M H, Lin R, Liddle C, Farrell G C. Hepatocellular proliferation and development of hepatocellular carcinoma: a case-control study in chronic hepatitis C. Hum Pathol. 1998;29:1279–1284. doi: 10.1016/s0046-8177(98)90257-x. [DOI] [PubMed] [Google Scholar]
- 57.Edwards S, Roehe P M, Ibata G. Comparative studies of border disease and closely related virus infections in experimental pigs and sheep. Br Vet J. 1995;151:181–187. doi: 10.1016/s0007-1935(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 58.Ellis J A, West K H, Cortese V S, Myers S L, Carman S, Martin K M, Haines D M. Lesions and distribution of viral antigen following an experimental infection of young seronegative calves with virulent bovine virus diarrhea virus-type II. Can J Vet Res. 1998;62:161–169. [PMC free article] [PubMed] [Google Scholar]
- 59.Fabris P, Biasin M R, Infantolino D, Romano L, Benedetti P, Tositti G, Pellizzer G P, Zanetti A R, Stecca C, Marchelle G, de Lalla F. HGV/GBV-C in liver tissue and in sera from patients with chronic hepatitis C. Infection. 1998;26:283–287. doi: 10.1007/BF02962248. [DOI] [PubMed] [Google Scholar]
- 60.Fabris P, Infantolino D, Biasin M R, Benedetti P, Tositti G, Bettini C, Marchelle G, de Lalla F. HGV/GBV-C infection in patients with acute hepatitis of different etiology and in patients with chronic hepatitis C. J Gastroenterol. 1998;33:57–61. doi: 10.1007/pl00009967. [DOI] [PubMed] [Google Scholar]
- 61.Failla C, Tomei L, De Francesco R. Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J Virol. 1994;68:3753–3760. doi: 10.1128/jvi.68.6.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filocamo G, Pacini L, Nardi C, Bartholomew L, Scaturro M, Delmastro P, Tramontano A, De Francesco R, Migliaccio G. Selection of functional variants of the NS3-NS4A protease of hepatitis C virus by using chimeric Sindbis viruses. J Virol. 1999;73:561–575. doi: 10.1128/jvi.73.1.561-575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 64.Flores-Aguilar M, Besen G, Vuong C, Tatebayashi M, Munguia D, Gangan P, Wiley C A, Freeman W R. Evaluation of retinal toxicity and efficacy of anti-cytomegalovirus and anti-herpes simplex virus antiviral phosphorothioate oligonucleotides ISIS 2922 and ISIS 4015. J Infect Dis. 1997;175:1308–1316. doi: 10.1086/516461. [DOI] [PubMed] [Google Scholar]
- 65.Fournier C, Sureau C, Coste J, Ducos J, Pageaux G, Larrey D, Domergue J, Maurel P. In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J Gen Virol. 1998;79:2367–2374. doi: 10.1099/0022-1317-79-10-2367. [DOI] [PubMed] [Google Scholar]
- 66.Fujiwara K, Mochida S, Matsuo S, Ogata I, Hayashi S, Sato Y. Randomized control trial of interferon-beta injections at 12-h intervals as a therapy for chronic hepatitis C. Hepatol Res. 1998;12:240–251. [Google Scholar]
- 67.Gabrielsen B, Phelan M J, Barthel Rosa L, See C, Huggins J W, Kefauver D F, Monath T P, Ussery M A, Chmurny G N, Schubert E M, et al. Synthesis and antiviral evaluation of N-carboxamidine-substituted analogues of 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamidine hydrochloride. J Med Chem. 1992;35:3231–3238. doi: 10.1021/jm00095a020. [DOI] [PubMed] [Google Scholar]
- 68.Gale M, Blakely C M, Kwieciszewski B, Tan S L, Dossett M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallinari P, Brennan D, Nardi C, Brunetti M, Tomei L, Steinkuhler C, De Francesco R. Multiple enzymatic activities associated with recombinant NS3 protein of hepatitis C virus. J Virol. 1998;72:6758–6769. doi: 10.1128/jvi.72.8.6758-6769.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh S N, Goverdhan M K, Sathe P S, Chelliah S C, Naik S V, Godbole P V, Banerjee K. Protective effect of 6-MFA, a fungal interferon inducer against Japanese encephalitis virus in bonnet macaques. Indian J Med Res. 1990;91:408–413. [PubMed] [Google Scholar]
- 71.Gilbert B E, Wilson S Z, Knight V, Couch R B, Quarles J M, Dure L, Hayes N, Willis G. Ribavirin small-particle aerosol treatment of infections caused by influenza virus strains A/Victoria/7/83 (H1N1) and B/Texas/1/84. Antimicrob Agents Chemother. 1985;27:309–313. doi: 10.1128/aac.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gish R G. Standards of treatment in chronic hepatitis C. Semin Liver Dis. 1999;19(Suppl. 1):35–47. [PubMed] [Google Scholar]
- 73.Gonzalez-Peralta R P, Galasso G J, Poynard T, Schalm S, Thomas H C, Wright T L. Summary of the first international symposium on viral hepatitis. Antiviral Res. 1999;42:77–96. doi: 10.1016/s0166-3542(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 74.Goswami B B, Borek E, Sharma O K, Fujitaki J, Smith R A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979;89:830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- 75.Goverdhan M K, Kulkarni A B, Gupta A K, Tupe C D, Rodrigues J J. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992;36:277–283. [PubMed] [Google Scholar]
- 76.Grimaldi S, Lisi A, Pozzi D, Santoro N. Attempts to use liposomes and RBC ghosts as vectors in drug and antisense therapy of virus infection. Res Virol. 1997;148:177–180. doi: 10.1016/s0923-2516(97)89906-2. [DOI] [PubMed] [Google Scholar]
- 77.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta R K, Misra C N, Gupta V K, Saxena S N. An efficient method for production of purified inactivated Japanese encephalitis vaccine from mouse brains. Vaccine. 1991;9:865–867. doi: 10.1016/0264-410x(91)90004-p. [DOI] [PubMed] [Google Scholar]
- 79.Hahm B, Back S H, Lee T G, Wimmer E, Jang S K. Generation of a novel poliovirus with a requirement of hepatitis C virus protease NS3 activity. Virology. 1996;226:318–326. doi: 10.1006/viro.1996.0659. [DOI] [PubMed] [Google Scholar]
- 80.Han J H, Shyamala V, Richman K H, Brauer M J, Irvine B, Urdea M S, Tekamp O P, Kuo G, Choo Q L, Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5′ untranslated region and poly(A) tails at the 3′ end. Proc Natl Acad Sci USA. 1991;88:1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han L L, Popovici F, Alexander J P, Laurentia V, Tengelsen L A, Cernescu C, Gary H E, Ion Nedelcu N, Campbell G L, Tsai T F. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J Infect Dis. 1999;179:230–233. doi: 10.1086/314566. [DOI] [PubMed] [Google Scholar]
- 82.Harinasuta C, Nimmanitya S, Titsyakorn U. The effect of interferon-alpha A on two cases of Japanese encephalitis in Thailand. Southeast Asian J Trop Med Public Health. 1985;16:332–336. [PubMed] [Google Scholar]
- 83.Hayden F G, Hipskind G J, Woerner D H, Eisen G F, Janssens M, Janssen P A, Andries K. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob Agents Chemother. 1995;39:290–294. doi: 10.1128/aac.39.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heathcote J. Consensus interferon: a novel interferon for the treatment of hepatitis C. J Viral Hepat. 1998;5(Suppl. 1):13–18. doi: 10.1046/j.1365-2893.1998.0050s1013.x. [DOI] [PubMed] [Google Scholar]
- 85.Heinz F X, Mandl C W. The molecular biology of tick-borne encephalitis virus. APMIS. 1993;101:735–745. doi: 10.1111/j.1699-0463.1993.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 86.Hewicker T M, Liess B, Trautwein G. Brain lesions in calves following transplacental infection with bovine-virus diarrhoea virus. Zentralbl Veterinarmed Reihe B. 1995;42:65–77. doi: 10.1111/j.1439-0450.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 87.Higgs S, Gould E A. Differences in fusogenicity and mouse neurovirulence of Japanese encephalitis viruses. Arch Virol. 1991;119:119–133. doi: 10.1007/BF01314328. [DOI] [PubMed] [Google Scholar]
- 88.Hoke C H, Jr, Vaughn D W, Nisalak A, Intralawan P, Poolsuppasit S, Jongsawas V, Titsyakorn U, Johnson R T. Effect of high-dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis. 1992;165:631–637. doi: 10.1093/infdis/165.4.631. [DOI] [PubMed] [Google Scholar]
- 89.Holzmann H, Heinz F X, Mandl C W, Guirakhoo F, Kunz C. A single amino acid substitution in envelope protein E of tick-borne encephalitis virus leads to attenuation in the mouse model. J Virol. 1990;64:5156–5159. doi: 10.1128/jvi.64.10.5156-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoofnagle J H. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 91.Hoofnagle J H, Lau D, Conjeevaram H, Kleiner D, Di Bisceglie A M. Prolonged therapy of chronic hepatitis C with ribavirin. J Viral Hepat. 1996;3:247–252. doi: 10.1111/j.1365-2893.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 92.Houe H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet Microbiol. 1999;64:89–107. doi: 10.1016/s0378-1135(98)00262-4. [DOI] [PubMed] [Google Scholar]
- 93.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1035–1058. [Google Scholar]
- 94.Huggins J W. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11(Suppl. 4):S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 95.Huggins J W, Kim G R, Brand O M, McKee K T., Jr Ribavirin therapy for Hantaan virus infection in suckling mice. J Infect Dis. 1986;153:489–497. doi: 10.1093/infdis/153.3.489. [DOI] [PubMed] [Google Scholar]
- 96.Huggins J W, Robins R K, Canonico P G. Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses. Antimicrob Agents Chemother. 1984;26:476–480. doi: 10.1128/aac.26.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huggins J W, Zhang Z X, Bray M. Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J Infect Dis. 1999;179(Suppl. 1):S240–S247. doi: 10.1086/514316. [DOI] [PubMed] [Google Scholar]
- 98.Hultgren C, Milich D R, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79:2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 99.Ikeda M, Sugiyama K, Mizutani T, Tanaka T, Tanaka K, Shimotohno K, Kato N. Hepatitis G virus replication in human cultured cells displaying susceptibility to hepatitis C virus infection. Biochem Biophys Res Commun. 1997;235:505–508. doi: 10.1006/bbrc.1997.6818. [DOI] [PubMed] [Google Scholar]
- 100.Ikeda S, Neyts J, Verma S, Wickramasinghe A, Mohan P, De Clercq E. In vitro and in vivo inhibition of ortho- and paramyxovirus infections by a new class of sulfonic acid polymers interacting with virus-cell binding and/or fusion. Antimicrob Agents Chemother. 1994;38:256–259. doi: 10.1128/aac.38.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ingallinella P, Altamura S, Bianchi E, Taliani M, Ingenito R, Cortese R, De Francesco R, Steinkuhler C, Pessi A. Potent peptide inhibitors of human hepatitis C virus NS3 protease are obtained by optimizing the cleavage products. Biochemistry. 1998;37:8906–8914. doi: 10.1021/bi980314n. [DOI] [PubMed] [Google Scholar]
- 102.Ito T, Tahara S M, Lai M M. The 3′-untranslated region of hepatitis C virus RNA enhances translation from an internal ribosomal entry site. J Virol. 1998;72:8789–8796. doi: 10.1128/jvi.72.11.8789-8796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson A J, Roehrig J T. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kakiuchi N, Komoda Y, Komoda K, Takeshita N, Okada S, Tani T, Shimotohno K. Non-peptide inhibitors of HCV serine proteinase. FEBS Lett. 1998;421:217–220. doi: 10.1016/s0014-5793(97)01566-4. [DOI] [PubMed] [Google Scholar]
- 105.Kalita J, Misra U K. EEG in Japanese encephalitis: a clinico-radiological correlation. Electroencephalogr Clin Neurophysiol. 1998;106:238–243. doi: 10.1016/s0013-4694(97)00123-5. [DOI] [PubMed] [Google Scholar]
- 106.Kaluzova M, Eleckova E, Zuffova E, Pastorek J, Kaluz S, Kozuch O, Labuda M. Reverted virulence of attenuated tick-borne encephalitis virus mutant is not accompanied with the changes in deduced viral envelope protein amino acid sequence. Acta Virol. 1994;38:133–140. [PubMed] [Google Scholar]
- 107.Kanai K, Kako M, Kumada T, Tsubouchi H, Aikawa T, Kojima M, Harada H, Kawasaki T, Nakashima M, Okamoto H, Mishiro S. High-dose (9 MU) long-term (60 weeks) alfa-interferon therapy for chronic hepatitis patients infected with HCV genotype 1b. Arch Virol. 1998;143:1545–1554. doi: 10.1007/s007050050397. [DOI] [PubMed] [Google Scholar]
- 108.Karayiannis P, Pickering J, Zampino R, Thomas H C. Natural history and molecular biology of hepatitis G virus GB virus C. Clin Diagn Virol. 1998;10:103–111. doi: 10.1016/s0928-0197(98)00033-6. [DOI] [PubMed] [Google Scholar]
- 109.Kasai Y, Takeda S, Takagi H. Pathogenesis of hepatocellular carcinoma: a review from the viewpoint of molecular analysis. Semin Surg Oncol. 1996;12:155–159. doi: 10.1002/(SICI)1098-2388(199605/06)12:3<155::AID-SSU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 110.Kawano H, Rostapshov V, Rosen L, Lai C J. Genetic determinants of dengue type 4 virus neurovirulence for mice. J Virol. 1993;67:6567–6575. doi: 10.1128/jvi.67.11.6567-6575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kern B, Depner K R, Letz W, Rott M, Thalheim S, Nitschke B, Plagemann R, Liess B. Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF virus persistence as a mechanism for virus perpetuation. Zentralbl Veterinarmed Reihe B. 1999;46:63–67. doi: 10.1046/j.1439-0450.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 112.Khettry U, Robiou C, Jenkins R L, Loda M, Lewis W D. Recurrent hepatitis C in liver allografts: early histologic indicators. Int J Surg Pathol. 1998;6:197–204. [Google Scholar]
- 113.Kim D W, Gwack Y, Han J H, Choe J. Towards defining a minimal functional domain for NTPase and RNA helicase activities of the hepatitis C virus NS3 protein. Virus Res. 1997;49:17–25. doi: 10.1016/s0168-1702(97)01452-4. [DOI] [PubMed] [Google Scholar]
- 114.Kim D W, Kim J, Gwack Y, Han J H, Choe J. Mutational analysis of the hepatitis C virus RNA helicase. J Virol. 1997;71:9400–9409. doi: 10.1128/jvi.71.12.9400-9409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim J L, Caron P R. Crystal structure of hepatitis C NS3 RNA helicase reveals a possible enzyme mechanism and suggests multiple drug binding sites. Int Antiviral News. 1998;6:26–28. [Google Scholar]
- 116.Kim J L, Morgenstern K A, Griffith J P, Dwyer M D, Thomson J A, Murcko M A, Lin C, Caron P R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 117.Kim J L, Morgenstern K A, Lin C, Fox T, Dwyer M D, Landro J A, Chambers S P, Markland W, Lepre C A, O'Malley E T, Harbeson S L, Rice C M, Murcko M A, Caron P R, Thomson J A. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87:343–355. doi: 10.1016/s0092-8674(00)81351-3. . (Erratum 89:159, 1997.) [DOI] [PubMed] [Google Scholar]
- 118.Kleter B, Brouwer J T, Nevens F, van Doorn L J, Elewaut A, Versieck J, Michielsen P P, Hautekeete M L, Chamuleau R A, Brenard R, Bourgeois N, Adler M, Quint W G, Bronkhorst C M, Heijtink R A, Hop W J, Fevery J, Schalm S W. Hepatitis C virus genotypes: epidemiological and clinical associations. Benelux Study Group on Treatment of Chronic Hepatitis C. Liver. 1998;18:32–38. [PubMed] [Google Scholar]
- 119.Koff W C, Pratt R D, Elm J L, Jr, Venkateshan C N, Halstead S B. Treatment of intracranial dengue virus infections in mice with a lipophilic derivative of ribavirin. Antimicrob Agents Chemother. 1983;24:134–136. doi: 10.1128/aac.24.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koonin E V. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. J Gen Virol. 1993;74:733–740. doi: 10.1099/0022-1317-74-4-733. [DOI] [PubMed] [Google Scholar]
- 122.Kosmidou A, Buttner M, Meyers G. Isolation and characterization of cytopathogenic classical swine fever virus (CSFV) Arch Virol. 1998;143:1295–1309. doi: 10.1007/s007050050376. [DOI] [PubMed] [Google Scholar]
- 123.Kramer L D, Presser S B, Hardy J L, Jackson A O. Genotypic and phenotypic variation of selected Saint Louis encephalitis viral strains isolated in California. Am J Trop Med Hyg. 1997;57:222–229. doi: 10.4269/ajtmh.1997.57.222. [DOI] [PubMed] [Google Scholar]
- 124.Kummerer B M, Stoll D, Meyers G. Bovine viral diarrhea virus strain Oregon: a novel mechanism for processing of NS2-3 based on point mutations. J Virol. 1998;72:4127–4138. doi: 10.1128/jvi.72.5.4127-4138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuno G, Chang G J, Tsuchiya K R, Karabatsos N, Cropp C B. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kurane I, Rothman A L, Livingston P G, Green S, Gagnon S J, Janus J, Innis B L, Nimmannitya S, Nisalak A, Ennis F A. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch Virol Suppl. 1994;9:59–64. doi: 10.1007/978-3-7091-9326-6_7. [DOI] [PubMed] [Google Scholar]
- 127.Laevens H, Deluyker H, Koenen F, Van Caenegem G, Vermeersch J P, de Kruif A. An experimental infection with a classical swine fever virus in weaner pigs. II. The use of serological data to estimate the day of virus introduction in natural outbreaks. Vet Q. 1998;20:46–49. doi: 10.1080/01652176.1998.9694837. [DOI] [PubMed] [Google Scholar]
- 128.Reference deleted.
- 129.Laevens H, Koenen F, Deluyker H, Berkvens D, de Kruif A. An experimental infection with a classical swine fever virus in weaner pigs. I. Transmission of the virus, course of the disease, and antibody response. Vet Q. 1998;20:41–45. doi: 10.1080/01652176.1998.9694836. [DOI] [PubMed] [Google Scholar]
- 130.Lamarre D. Biological characterization and peptide-based inhibition of the hepatitis C virus NS3 serine protease, general session I, presentation 7. Symposium on Emerging Therapies for Chronic Viral Hepatitis. 1998. [Google Scholar]
- 131.Lau D Y, Kleiner D E, Ghany M G, Park Y, Schmid P, Hoofnagle J H. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology. 1998;28:1121–1127. doi: 10.1002/hep.510280430. [DOI] [PubMed] [Google Scholar]
- 132.Lau J Y, Qian K, Detmer J, Collins M L, Orito E, Kolberg J A, Urdea M S, Mizokami M, Davis G L. Effect of interferon-alpha and ribavirin therapy on serum GB virus C/hepatitis G virus (GBV-C/HGV) RNA levels in patients chronically infected with hepatitis C virus and GBV-C/HGV. J Infect Dis. 1997;176:421–426. doi: 10.1086/514059. [DOI] [PubMed] [Google Scholar]
- 133.Lavanchy D, Purcell R, Hollinger F B, Howard C, Alberti A, Kew M, Dusheiko G, Alter M, Ayoola E, Beutels P, Bloomer R, Ferret B, Decker R, Esteban R, Fay O, Fields H, Fuller E C, Grob P, Houghton M, Leung N, Locarnini S A, Margolis H, Meheus A, Miyamura T, Mohamed M K, Tandon B, Thomas D, Head H T, Toukan A U, Van Damme P, Zanetti A, Arthur R, Couper M, D'Amelio R, Emmanuel J C, Esteves K, Gavinio P, Griffiths E, Hallaj Z, Heuck C C, Heymann D L, Holck S E, Kane M, Martinez L J, Meslin F, Mochny I S, Ndikuyeze A, Padilla A M, Rodier G M, Roure C, Savage F, Vercauteren G. Global surveillance and control of hepatitis C. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 134.Laxton C D, McMillan D, Sullivan V, Ackrill A M. Expression and characterization of the hepatitis G virus helicase. J Viral Hepat. 1998;5:21–26. doi: 10.1046/j.1365-2893.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 135.Le S Y, Liu W M, Maizel J V., Jr Phylogenetic evidence for the improved RNA higher-order structure in internal ribosome entry sequences of HCV and pestiviruses. Virus Genes. 1998;17:279–295. doi: 10.1023/a:1008073905920. [DOI] [PubMed] [Google Scholar]
- 136.Lee J H, von Wagner M, Roth W K, Teuber G, Sarrazin C, Zeuzem S. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C virus. J Hepatol. 1998;29:29–35. doi: 10.1016/s0168-8278(98)80175-x. [DOI] [PubMed] [Google Scholar]
- 137.Levenbook I S, Pelleu L J, Elisberg B L. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and histological examination. J Biol Stand. 1987;15:305–313. doi: 10.1016/s0092-1157(87)80003-3. [DOI] [PubMed] [Google Scholar]
- 138.Lieber A, He C Y, Polyak S J, Gretch D R, Barr D, Kay M A. Elimination of hepatitis C virus RNA in infected human hepatocytes by adenovirus-mediated expression of ribozymes. J Virol. 1996;70:8782–8791. doi: 10.1128/jvi.70.12.8782-8791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lieber A, Kay M A. Adenovirus-mediated expression of ribozymes in mice. J Virol. 1996;70:3153–3158. doi: 10.1128/jvi.70.5.3153-3158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liebler Tenorio E M, Greiser W I, Pohlenz J F. Organ and tissue distribution of the antigen of the cytopathogenic bovine virus diarrhea virus in the early and advanced phase of experimental mucosal disease. Arch Virol. 1997;142:1613–1634. doi: 10.1007/s007050050184. [DOI] [PubMed] [Google Scholar]
- 141.Liebler Tenorio E M, Pohlenz J F. Experimental mucosal disease of cattle: changes in cell proliferation in lymphoid tissues and intestinal epithelium. J Comp Pathol. 1997;117:339–350. doi: 10.1016/s0021-9975(97)80081-3. [DOI] [PubMed] [Google Scholar]
- 142.Lin Y L, Liao C L, Chen L K, Yeh C T, Liu C I, Ma S H, Huang Y Y, Huang Y L, Kao C L, King C C. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lindberg A E, Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Vet Microbiol. 1999;64:197–222. doi: 10.1016/s0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- 144.Lindenbach B D, Rice C M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Linnen J, Wages J, Zhang Keck Z Y, Fry K E, Krawczynski K Z, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J W, Young L, Piatak M, Hoover C, Fernandez J, Chen S, Zou J C, Morris T, Hyams K C, Ismay S, Lifson J D, Kim J P, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 146.Liu C T, Griffin M J. Changes in body fluid compartments, tissue water and electrolyte distribution, and lipid concentrations in rhesus macaques with yellow fever. Am J Vet Res. 1982;43:2013–2018. [PubMed] [Google Scholar]
- 147.Lohmann V, Korner F, Herian U, Bartenschlager R. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J Virol. 1997;71:8416–8428. doi: 10.1128/jvi.71.11.8416-8428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 149.Lopez Labrador F X, Ampurdanes S, Gimenez Barcons M, Guilera M, Costa J, de Anta M T J, Sanchez Tapias J M, Rodes J, Saiz J C. Relationship of the genomic complexity of hepatitis C virus with liver disease severity and response to interferon in patients with chronic HCV genotype 1b interferon. Hepatology. 1999;29:897–903. doi: 10.1002/hep.510290306. [DOI] [PubMed] [Google Scholar]
- 150.Lu H H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lum L C, Lam S K, Choy Y S, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 152.Mackenzie J M, Khromykh A A, Jones M K, Westaway E G. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 153.Mackenzie J S, Broom A K. Australian X disease, Murray Valley encephalitis and the French connection. Vet Microbiol. 1995;46:79–90. doi: 10.1016/0378-1135(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 154.Mady B J, Kurane I, Erbe D V, Fanger M W, Ennis F A. Neuraminidase augments Fc gamma receptor II-mediated antibody-dependent enhancement of dengue virus infection. J Gen Virol. 1993;74:839–844. doi: 10.1099/0022-1317-74-5-839. [DOI] [PubMed] [Google Scholar]
- 155.Malinoski F J, Hasty S E, Ussery M A, Dalrymple J M. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antiviral Res. 1990;13:139–149. doi: 10.1016/0166-3542(90)90029-7. [DOI] [PubMed] [Google Scholar]
- 156.Marcellin P, Levy S, Erlinger S. Therapy of hepatitis C: patients with normal aminotransferase levels. Hepatology. 1997;26:133S–136S. doi: 10.1002/hep.510260723. [DOI] [PubMed] [Google Scholar]
- 157.Marchevsky R S, Mariano J, Ferreira V S, Almeida E, Cerqueira M J, Carvalho R, Pissurno J W, da Rosa A P, Simoes M C, Santos C N, et al. Phenotypic analysis of yellow fever virus derived from complementary DNA. Am J Trop Med Hyg. 1995;52:75–80. doi: 10.4269/ajtmh.1995.52.75. [DOI] [PubMed] [Google Scholar]
- 158.Martin F, Dimasi N, Volpari C, Perrera C, Di Marco S, Brunetti M, Steinkuhler C, De Francesco R, Sollazzo M. Design of selective eglin inhibitors of HCV NS3 proteinase. Biochemistry. 1998;37:11459–11468. doi: 10.1021/bi980283w. [DOI] [PubMed] [Google Scholar]
- 159.Martinot Peignoux M, Boyer N, Pouteau M, Castelnau C, Giuily N, Duchatelle V, Auperin A, Degott C, Benhamou J P, Erlinger S, Marcellin P. Predictors of sustained response to alpha interferon therapy in chronic hepatitis C. J Hepatol. 1998;29:214–223. doi: 10.1016/s0168-8278(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 160.Marwick C. First “antisense” drug will treat CMV retinitis. JAMA. 1998;280:871. [PubMed] [Google Scholar]
- 161.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 162.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 163.Meehan J T, Lehmkuhl H D, Cutlip R C, Bolin S R. Acute pulmonary lesions in sheep experimentally infected with bovine viral diarrhoea virus. J Comp Pathol. 1998;119:277–292. doi: 10.1016/s0021-9975(98)80050-9. [DOI] [PubMed] [Google Scholar]
- 164.Mendez E, Ruggli N, Collett M S, Rice C M. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J Virol. 1998;72:4737–4745. doi: 10.1128/jvi.72.6.4737-4745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Meyers G, Thiel H J. Molecular characterization of pestiviruses. Adv Virus Res. 1996;47:53–118. doi: 10.1016/s0065-3527(08)60734-4. [DOI] [PubMed] [Google Scholar]
- 166.Misra U K, Kalita J, Srivastava M. Prognosis of Japanese encephalitis: a multivariate analysis. J Neurol Sci. 1998;161:143–147. doi: 10.1016/s0022-510x(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 167.Monath T P. Yellow fever: a medically neglected disease. Report on a seminar. Rev Infect Dis. 1987;9:165–175. doi: 10.1093/clinids/9.1.165. [DOI] [PubMed] [Google Scholar]
- 168.Monath T P, Brinker K R, Chandler F W, Kemp G E, Cropp C B. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am J Trop Med Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- 169.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 961–1034. [Google Scholar]
- 170.Moradpour D, Wakita T, Wands J R, Blum H E. Tightly regulated expression of the entire hepatitis C virus structural region in continuous human cell lines. Biochem Biophys Res Commun. 1998;246:920–924. doi: 10.1006/bbrc.1998.8727. [DOI] [PubMed] [Google Scholar]
- 171.Morens D M. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994;19:500–512. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 172.Mori A, Yamada K, Kimura J, Koide T, Yuasa S, Yamada E, Miyamura T. Enzymatic characterization of purified NS3 serine proteinase of hepatitis C virus expressed in Escherichia coli. FEBS Lett. 1996;378:37–42. doi: 10.1016/0014-5793(95)01423-3. [DOI] [PubMed] [Google Scholar]
- 173.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 174.Mounir S. Symposium on Emerging Therapies for Chronic Viral Hepatitis. 1998. Hepatitis C virus replication in the nude mouse model, general session II, presentation 4. [Google Scholar]
- 175.Negro F, Giostra E, Rubbia Brandt L, Mentha G, Colucci G, Morel P, Quadri R, Perrin L, Hadengue A. IgM anti-hepatitis C virus core antibodies as marker of recurrent hepatitis C after liver transplantation. J Med Virol. 1998;56:224–229. [PubMed] [Google Scholar]
- 176.Nettleton P F, Entrican G. Ruminant pestiviruses. Br Vet J. 1995;151:615–642. doi: 10.1016/S0007-1935(95)80145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nettleton P F, Gilray J A, Russo P, Dlissi E. Border disease of sheep and goats. Vet Res. 1998;29:327–340. [PubMed] [Google Scholar]
- 178.Neyts J, Meerbach A, McKenna P, De Clercq E. Use of the yellow fever virus vaccine strain 17D for the study of strategies for the treatment of yellow fever virus infections. Antiviral Res. 1996;30:125–132. doi: 10.1016/0166-3542(96)89697-5. [DOI] [PubMed] [Google Scholar]
- 179.Neyts J, Snoeck R, Schols D, Balzarini J, Esko J D, Van Schepdael A, De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 180.Ohkawa K, Yuki N, Kanazawa Y, Ueda K, Mita E, Sasaki Y, Kasahara A, Hayashi N. Cleavage of viral RNA and inhibition of viral translation by hepatitis C virus RNA-specific hammerhead ribozyme in vitro. J Hepatol. 1997;27:78–84. doi: 10.1016/s0168-8278(97)80283-8. [DOI] [PubMed] [Google Scholar]
- 181.Osella A R, Massa M A, Joekes S, Blanch N, Yacci M R, Centonze S, Sileoni S. Hepatitis B and C virus sexual transmission among homosexual men. Am J Gastroenterol. 1998;93:49–52. doi: 10.1111/j.1572-0241.1998.049_c.x. [DOI] [PubMed] [Google Scholar]
- 182.Pastoret P P, Lambot M. Biologie de l'infection des bovins par le Pestivirus responsable de la maladie des muqueuses. Bull Mem Acad R Med Belg. 1995;150:244–250. . (In French.) [PubMed] [Google Scholar]
- 183.Pauly T, Konig M, Thiel H J, Saalmuller A. Infection with classical swine fever virus: effects on phenotype and immune responsiveness of porcine T lymphocytes. J Gen Virol. 1998;79:31–40. doi: 10.1099/0022-1317-79-1-31. [DOI] [PubMed] [Google Scholar]
- 184.Pawlotsky J M, Germanidis G, Neumann A U, Pellerin M, Frainais P O, Dhumeaux D. Interferon resistance of hepatitis C virus genotype 1b: relationship to nonstructural 5A gene quasispecies mutations. J Virol. 1998;72:2795–2805. doi: 10.1128/jvi.72.4.2795-2805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pickering J M, Thomas H C, Karayiannis P. Predicted secondary structure of the hepatitis G virus and GB virus-A 5′untranslated regions consistent with an internal ribosome entry site. J Viral Hepat. 1997;4:175–184. doi: 10.1046/j.1365-2893.1997.00143.x. [DOI] [PubMed] [Google Scholar]
- 186.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 187.Pontisso P, Belluco C, Bertorelle R, De Moliner L, Chieco Bianchi L, Nitti D, Lise M, Alberti A. Hepatitis C virus infection associated with human hepatocellular carcinoma: lack of correlation with p53 abnormalities in Caucasian patients. Cancer. 1998;83:1489–1494. [PubMed] [Google Scholar]
- 188.Poole T L, Wang C, Popp R A, Potgieter L N, Siddiqui A, Collett M S. Pestivirus translation initiation occurs by internal ribosome entry. Virology. 1995;206:750–754. doi: 10.1016/s0042-6822(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 189.Porter D J. Inhibition of the hepatitis C virus helicase-associated ATPase activity by the combination of ADP, NaF, MgCl2, and poly(rU). Two ADP binding sites on the enzyme-nucleic acid complex. J Biol Chem. 1998;273:7390–7396. doi: 10.1074/jbc.273.13.7390. [DOI] [PubMed] [Google Scholar]
- 190.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha 2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha 2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 191.Pryor M J, Gualano R C, Lin B, Davidson A D, Wright P J. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J Gen Virol. 1998;79:2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- 192.Quin J W. Interferon therapy for acute hepatitis C viral infection—a review by meta-analysis. Aust N Z J Med. 1997;27:611–617. doi: 10.1111/j.1445-5994.1997.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 193.Ramirez Ronda C H, Garcia C D. Dengue in the Western Hemisphere. Infect Dis Clin North Am. 1994;8:107–128. [PubMed] [Google Scholar]
- 194.Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, Vierucci A. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. Br Med J. 1998;317:437–441. doi: 10.1136/bmj.317.7156.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 931–960. [Google Scholar]
- 196.Rijnbrand R, van der Straaten T, Van Rijn P A, Spaan W J, Bredenbeek P J. Internal entry of ribosomes is directed by the 5′ noncoding region of classical swine fever virus and is dependent on the presence of an RNA pseudoknot upstream of the initiation codon. J Virol. 1997;71:451–457. doi: 10.1128/jvi.71.1.451-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosen H R, Gretch D R, Oehlke M, Flora K D, Benner K G, Rabkin J M, Corless C L. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation. 1998;65:1178–1182. doi: 10.1097/00007890-199805150-00006. [DOI] [PubMed] [Google Scholar]
- 198.Rosenwirth B, Oren D A, Arnold E, Kis Z L, Eggers H J. SDZ 35-682, a new picornavirus capsid-binding agent with potent antiviral activity. Antiviral Res. 1995;26:65–82. doi: 10.1016/0166-3542(94)00066-h. [DOI] [PubMed] [Google Scholar]
- 199.Rostaing L, Izopet J, Moussion F, Alric L, Verdier D, That H T, Duffaut M, Durand D, Puel J, Suc J M. HCV RNA clearance after treatment with interferon-alpha in chronic hemodialysis patients with or without coinfection by HGV/HGBV-C. Nephrologie. 1997;18:281–286. . (In French.) [PubMed] [Google Scholar]
- 200.Rümenapf T, Unger G, Strauss J H, Thiel H-J. Processing of the envelope glycoproteins of pestiviruses. J Virol. 1993;67:3288–3294. doi: 10.1128/jvi.67.6.3288-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ryman K D, Xie H, Ledger T N, Campbell G A, Barrett A D. Antigenic variants of yellow fever virus with an altered neurovirulence phenotype in mice. Virology. 1997;230:376–380. doi: 10.1006/viro.1997.8496. [DOI] [PubMed] [Google Scholar]
- 202.Sakamoto N, Wu C H, Wu G Y. Intracellular cleavage of hepatitis C virus RNA and inhibition of viral protein translation by hammerhead ribozymes. J Clin Investig. 1996;98:2720–2728. doi: 10.1172/JCI119097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sali D L, Ingram R, Wendel M, Gupta D, McNemar C, Tsarbopoulos A, Chen J W, Hong Z, Chase R, Risano C, Zhang R, Yao N, Kwong A D, Ramanathan L, Le H V, Weber P C. Serine protease of hepatitis C virus expressed in insect cells as the NS3/4A complex. Biochemistry. 1998;37:3392–3401. doi: 10.1021/bi972010r. [DOI] [PubMed] [Google Scholar]
- 204.Schols D, Struyf S, Van Damme J, Este J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shimizu Y K, Igarashi H, Kiyohara T, Shapiro M, Wong D C, Purcell R H, Yoshikura H. Infection of a chimpanzee with hepatitis C virus grown in cell culture. J Gen Virol. 1998;79:1383–1386. doi: 10.1099/0022-1317-79-6-1383. [DOI] [PubMed] [Google Scholar]
- 206.Shoji I, Suzuki T, Chieda S, Sato M, Harada T, Chiba T, Matsuura Y, Miyamura T. Proteolytic activity of NS3 serine proteinase of hepatitis C virus efficiently expressed in Escherichia coli. Hepatology. 1995;22:1648–1655. [PubMed] [Google Scholar]
- 207.Sizova D V, Kolupaeva V G, Pestova T V, Shatsky I N, Hellen C U. Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. J Virol. 1998;72:4775–4782. doi: 10.1128/jvi.72.6.4775-4782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Spector F C, Liang L, Giordano H, Sivaraja M, Peterson M G. Inhibition of herpes simplex virus replication by a 2-amino thiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J Virol. 1998;72:6979–6987. doi: 10.1128/jvi.72.9.6979-6987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Steinkuhler C, Biasiol G, Brunetti M, Urbani A, Koch U, Cortese R, Pessi A, De Francesco R. Product inhibition of the hepatitis C virus NS3 protease. Biochemistry. 1998;37:8899–8905. doi: 10.1021/bi980313v. [DOI] [PubMed] [Google Scholar]
- 210.Reference deleted.
- 211.Stephen E L, Sammons M L, Pannier W L, Baron S, Spertzel R O, Levy H B. Effect of a nuclease-resistant derivative of polyriboinosinic-polyribocytidylic acid complex on yellow fever in rhesus monkeys (Macaca mulatta) J Infect Dis. 1977;136:122–126. doi: 10.1093/infdis/136.1.122. [DOI] [PubMed] [Google Scholar]
- 212.Sudo K, Inoue H, Shimizu Y, Yamaji K, Konno K, Shigeta S, Kaneko T, Yokota T, Shimotohno K. Establishment of an in vitro assay system for screening hepatitis C virus protease inhibitors using high performance liquid chromatography. Antiviral Res. 1996;32:9–18. doi: 10.1016/0166-3542(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 213.Sudo K, Matsumoto Y, Matsushima M, Fujiwara M, Konno K, Shimotohno K, Shigeta S, Yokota T. Novel hepatitis C virus protease inhibitors: thiazolidine derivatives. Biochem Biophys Res Commun. 1997;238:643–647. doi: 10.1006/bbrc.1997.7358. [DOI] [PubMed] [Google Scholar]
- 214.Sumarmo S P, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics. 1982;69:45–49. [PubMed] [Google Scholar]
- 215.Summerfield A, Knotig S M, McCullough K C. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J Virol. 1998;72:1853–1861. doi: 10.1128/jvi.72.3.1853-1861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Taber L H, Knight V, Gilbert B E, McClung H W, Wilson S Z, Norton H J, Thurson J M, Gordon W H, Atmar R L, Schlaudt W R. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics. 1983;72:613–618. [PubMed] [Google Scholar]
- 217.Takegami T, Simamura E, Hirai K, Koyama J. Inhibitory effect of furanonaphthoquinone derivatives on the replication of Japanese encephalitis virus. Antiviral Res. 1998;37:37–45. doi: 10.1016/s0166-3542(97)00058-2. [DOI] [PubMed] [Google Scholar]
- 218.Taliani M, Bianchi E, Narjes F, Fossatelli M, Urbani A, Steinkuhler C, De Francesco R, Pessi A. A continuous assay of hepatitis C virus protease based on resonance energy transfer depsipeptide substrates. Anal Biochem. 1996;240:60–67. doi: 10.1006/abio.1996.0331. [DOI] [PubMed] [Google Scholar]
- 219.Tan B H, Fu J, Sugrue R J, Yap E H, Chan Y C, Tan Y H. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 220.Taremi S S, Beyer B, Maher M, Yao N, Prosise W, Weber P C, Malcolm B A. Construction, expression, and characterization of a novel fully activated recombinant single-chain hepatitis C virus protease. Protein Sci. 1998;7:2143–2149. doi: 10.1002/pro.5560071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics. 1993;92:111–115. [PubMed] [Google Scholar]
- 222.Tassniyom S, Vasanawathana S, Dhiensiri T, Nisalak A, Chirawatkul A. Failure of carbazochrome sodium sulfonate (AC-17) to prevent dengue vascular permeability or shock: a randomized, controlled trial. J Pediatr. 1997;131:525–528. doi: 10.1016/s0022-3476(97)70055-6. [DOI] [PubMed] [Google Scholar]
- 223.Tautz N, Meyers G, Stark R, Dubovi E J, Thiel H-J. Cytopathogenicity of a pestivirus correlates with a 27-nucleotide insertion. J Virol. 1996;70:7851–7858. doi: 10.1128/jvi.70.11.7851-7858.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tautz N, Meyers G, Thiel H-J. Pathogenesis of mucosal disease, a deadly disease of cattle caused by a pestivirus. Clin Diagn Virol. 1998;10:121–127. doi: 10.1016/s0928-0197(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 225.Taylor J L, Schoenherr C, Grossberg S E. Protection against Japanese encephalitis virus in mice and hamsters by treatment with carboxymethylacridanone, a potent interferon inducer. J Infect Dis. 1980;142:394–399. doi: 10.1093/infdis/142.3.394. [DOI] [PubMed] [Google Scholar]
- 226.Terpstra C, Wensvoort G. A congenital persistent infection of bovine virus diarrhoea virus in pigs: clinical, virological and immunological observations. Vet Q. 1997;19:97–101. doi: 10.1080/01652176.1997.9694750. [DOI] [PubMed] [Google Scholar]
- 227.Thiel H-J, Plagemann P G W, Moennig V. Pestiviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1059–1073. [Google Scholar]
- 228.Thomas S L, Newell M L, Peckham C S, Ades A E, Hall A J. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. Int J Epidemiol. 1998;27:108–117. doi: 10.1093/ije/27.1.108. [DOI] [PubMed] [Google Scholar]
- 229.Tomei L, Failla C, Vitale R L, Bianchi E, De Francesco R. A central hydrophobic domain of the hepatitis C virus NS4A protein is necessary and sufficient for the activation of the NS3 protease. J Gen Virol. 1996;77:1065–1070. doi: 10.1099/0022-1317-77-5-1065. [DOI] [PubMed] [Google Scholar]
- 230.Tong M J, Blatt L M, Tong L T, Sayadzadeh K, Conrad A. Long-term retreatment in chronic hepatitis C patients who were non-responders to an initial course of interferon-alpha 2b. J Viral Hepat. 1998;5:323–331. doi: 10.1046/j.1365-2893.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 231.Tsai T F, Popovici F, Cernescu C, Campbell G L, Nedelcu N I. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 232.Tsukiyama K K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Urbani A, Bazzo R, Nardi M C, Cicero D O, De Francesco R, Steinkuhler C, Barbato G. The metal binding site of the hepatitis C virus NS3 protease. A spectroscopic investigation. J Biol Chem. 1998;273:18760–18769. doi: 10.1074/jbc.273.30.18760. [DOI] [PubMed] [Google Scholar]
- 234.Valle R P, Falgout B. Mutagenesis of the NS3 protease of dengue virus type 2. J Virol. 1998;72:624–632. doi: 10.1128/jvi.72.1.624-632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Van Campen H, Williams E S, Edwards J, Cook W, Stout G. Experimental infection of deer with bovine viral diarrhea virus. J Wildl Dis. 1997;33:567–573. doi: 10.7589/0090-3558-33.3.567. [DOI] [PubMed] [Google Scholar]
- 236.van Olphen A L, Donis R O. Identification of bovine viral diarrhea virus nonstructural polypeptide NS4B/P38. Virus Res. 1997;51:197–201. doi: 10.1016/s0168-1702(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 237.Venugopal K, Gould E A. Towards a new generation of flavivirus vaccines. Vaccine. 1994;12:966–975. doi: 10.1016/0264-410x(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 238.Vilcek S, Stadejek T, Ballagi P A, Lowings J P, Paton D J, Belak S. Genetic variability of classical swine fever virus. Virus Res. 1996;43:137–147. doi: 10.1016/0168-1702(96)01326-3. [DOI] [PubMed] [Google Scholar]
- 239.Vishnuvardhan D, Kakiuchi N, Urvil P T, Shimotohno K, Kumar P K, Nishikawa S. Expression of highly active recombinant NS3 protease domain of hepatitis C virus in E. coli. FEBS Lett. 1997;402:209–212. doi: 10.1016/s0014-5793(96)01532-3. [DOI] [PubMed] [Google Scholar]
- 240.Wakita T, Moradpour D, Tokushihge K, Wands J R. Antiviral effects of antisense RNA on hepatitis C virus RNA translation and expression. J Med Virol. 1999;57:217–222. [PubMed] [Google Scholar]
- 241.Walker M A. Hepatitis C virus: an overview of current approaches and progress. Drug Discov Today. 1999;4:518–529. doi: 10.1016/s1359-6446(99)01414-2. [DOI] [PubMed] [Google Scholar]
- 242.Weissenbock H, Suchy A, Holzmann H. Tick-borne encephalitis in dogs: neuropathological findings and distribution of antigen. Acta Neuropathol Berl. 1998;95:361–366. doi: 10.1007/s004010050811. [DOI] [PubMed] [Google Scholar]
- 243.Welch P J, Tritz R, Yei S, Leavitt M, Yu M, Barber J. A potential therapeutic application of hairpin ribozymes: in vitro and in vivo studies of gene therapy for hepatitis C virus infection. Gene Ther. 1996;3:994–1001. [PubMed] [Google Scholar]
- 244.Wengler G, Wengler G. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J Virol. 1989;63:2521–2526. doi: 10.1128/jvi.63.6.2521-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wengler G, Wengler G. The NS 3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology. 1993;197:265–273. doi: 10.1006/viro.1993.1587. [DOI] [PubMed] [Google Scholar]
- 246.Westaway E G. Flavivirus replication strategy. Adv Virus Res. 1987;33:45–90. doi: 10.1016/s0065-3527(08)60316-4. [DOI] [PubMed] [Google Scholar]
- 247.Wilkinson T C, Bunyard P R, Quirk K, Wilkinson C S. Characterisation of an HCV NS3/NS4A proteinase fusion protein expressed in E. coli using synthetic peptide substrates. Biochem Soc Trans. 1997;25:S624. doi: 10.1042/bst025s624. [DOI] [PubMed] [Google Scholar]
- 248.Wiskerchen M, Belzer S K, Collett M S. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J Virol. 1991;65:4508–4514. doi: 10.1128/jvi.65.8.4508-4514.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.World Health Organization. Monograph on dengue/dengue hemorrhagic fever. Regional publication, SEARO no. 22. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 250.World Health Organization. Dengue bulletin. Bull W H O. 1997;21:1–15. [Google Scholar]
- 251.Wu S J, Hayes C G, Dubois D R, Windheuser M G, Kang Y H, Watts D M, Sieckmann D G. Evaluation of the severe combined immunodeficient (SCID) mouse as an animal model for dengue viral infection. Am J Trop Med Hyg. 1995;52:468–476. doi: 10.4269/ajtmh.1995.52.468. [DOI] [PubMed] [Google Scholar]
- 252.Xu J, Mendez E, Caron P R, Lin C, Murcko M A, Collett M S, Rice C M. Bovine viral diarrhea virus NS3 serine proteinase: polyprotein cleavage sites, cofactor requirements, and molecular model of an enzyme essential for pestivirus replication. J Virol. 1997;71:5312–5322. doi: 10.1128/jvi.71.7.5312-5322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yamada Osaki M, Sumazaki R, Kajiwara Y, Miyakawa T, Shirahata A, Matsui A. Natural course of HGV infection in haemophiliacs. Br J Haematol. 1998;102:616–621. doi: 10.1046/j.1365-2141.1998.00793.x. [DOI] [PubMed] [Google Scholar]
- 254.Yamada K, Mori A, Seki M, Kimura J, Yuasa S, Matsuura Y, Miyamura T. Critical point mutations for hepatitis C virus NS3 proteinase. Virology. 1998;246:104–112. doi: 10.1006/viro.1998.9184. [DOI] [PubMed] [Google Scholar]
- 255.Yamashita T, Kaneko S, Shirota Y, Qin W, Nomura T, Kobayashi K, Murakami S. RNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal region. J Biol Chem. 1998;273:15479–15486. doi: 10.1074/jbc.273.25.15479. [DOI] [PubMed] [Google Scholar]
- 256.Yan Y, Li Y, Munshi S, Sardana V, Cole J L, Sardana M, Steinkuehler C, Tomei L, De Francesco R, Kuo L C, Chen Z. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 A resolution structure in a hexagonal crystal form. Protein Sci. 1998;7:837–847. doi: 10.1002/pro.5560070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang G, Caroli Bosc F X, Laffont C, Bianchi D, Dantin S, Lefebvre J C, Doglio A. Hepatitis G and C viruses respond to interferon-alpha with different virologic kinetics. Dig Dis Sci. 1998;43:1307–1310. doi: 10.1023/a:1018824311722. [DOI] [PubMed] [Google Scholar]
- 258.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–467. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]
- 259.Yuan Z H, Kumar U, Thomas H C, Wen Y M, Monjardino J. Expression, purification, and partial characterization of HCV RNA polymerase. Biochem Biophys Res Commun. 1997;232:231–235. doi: 10.1006/bbrc.1997.6249. [DOI] [PubMed] [Google Scholar]
- 260.Zeuzem S, Teuber G, Lee J H, Ruster B, Roth W K. Risk factors for the transmission of hepatitis C. J Hepatol. 1996;24:3–10. [PubMed] [Google Scholar]
- 261.Zhao W D, Wimmer E, Lahser F C. Poliovirus/Hepatitis C virus (internal ribosomal entry site-core) chimeric viruses: improved growth properties through modification of a proteolytic cleavage site and requirement for core RNA sequences but not for core-related polypeptides. J Virol. 1999;73:1546–1554. doi: 10.1128/jvi.73.2.1546-1554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhong W, Gutshall L L, Del Vecchio A M. Identification and characterization of an RNA-dependent RNA polymerase activity within the nonstructural protein 5B region of bovine viral diarrhea virus. J Virol. 1998;72:9365–9369. doi: 10.1128/jvi.72.11.9365-9369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zoulim F, Haem J, Ahmed S S, Chossegros P, Habersetzer F, Chevallier M, Bailly F, Trepo C. Ribavirin monotherapy in patients with chronic hepatitis C: a retrospective study of 95 patients. J Viral Hepat. 1998;5:193–198. doi: 10.1046/j.1365-2893.1998.00099.x. [DOI] [PubMed] [Google Scholar]