Abstract
Background and Objectives
To investigate the long-term effect of permanent demyelination on axonal attrition by examining an association between intereye asymmetry of the multifocal visual evoked potential (mfVEP) latency delay and subsequent thinning of retinal ganglion cell axons in patients with a long-standing history of unilateral optic neuritis (ON).
Methods
Only patients with a significant degree of chronic demyelination (intereye latency asymmetry >5 ms) were included in this study. The level of optic nerve demyelination was estimated at baseline by the latency delay of mfVEP, while the degree of axonal loss was assessed by thinning of the retinal nerve fiber layer (RNFL) thickness between baseline and follow-up visits. Low-contrast visual acuity (LCVA) was also evaluated at baseline and follow-up. Patients were examined twice with an average interval of 6.1 ± 1.4 years.
Results
From 85 examined patients with multiple sclerosis, 28 satisfied inclusion criteria. Latency of the mfVEP was delayed, and RNFL thickness was reduced in ON eyes compared with fellow eyes at both visits. There was significant correlation between latency asymmetry and baseline or follow-up intereye RNFL thickness asymmetry. Intereye asymmetry of LCVA at baseline correlated with baseline latency asymmetry of mfVEP and baseline asymmetry of RNFL thickness. Latency of the mfVEP in ON eyes improved slightly during the follow-up period, whereas latency of the fellow eye remained stable. By contrast, RNFL thickness significantly declined in both ON and fellow eyes during the follow-up period. The rate of RNFL thinning in ON eyes, however, was more than 2 times faster compared with the fellow eyes (p < 0.001). Furthermore, baseline latency asymmetry significantly correlated with the rate of RNFL thinning in ON eyes during the follow-up (p < 0.001), explaining almost half of the variability of temporal RNFL progression. For each millisecond of latency delay (i.e., ∼0.5 mm of demyelination along the optic nerve), temporal RNFL thickness was annually reduced by 0.05%.
Discussion
Our study provides clear in vivo evidence that chronic demyelination significantly accelerates axonal loss. However, because this process is slow and its effect is mild, long-term monitoring is required to establish and confidently measure the neurodegenerative consequences of demyelination.
Multiple sclerosis (MS) is the most common cause of neurologic disability in young adults. Dysregulation of the adaptive immune system, a principal pathophysiologic driver of MS, results in inflammatory demyelination and progressive tissue injury.
The persistent clinical (disability) and radiologic (brain atrophy) features of early relapsing-remitting multiple sclerosis (RRMS) are primarily determined by substantial axonal damage within acute inflammatory lesions.1,2 However, although existing treatments significantly diminish the formation of new lesions, they do not fully arrest disease progression,3,4 suggesting the contribution of other, potentially neurodegenerative mechanisms.
The nature of the neurodegenerative mechanisms in MS is largely unknown. Although there are strong experimental data suggesting that ongoing CNS neurodegeneration is linked to the permanent loss of myelin sheaths surrounding surviving axons within MS lesions,5 human data on the role of chronic demyelination in MS progression are limited.6
There are several reasons why the visual system is an ideal model to study the effect of chronic demyelination on axonal loss. First, optic neuritis (ON), which leads to chronic demyelination in the optic nerve, is a frequent event in MS. It is the presenting symptom of MS in approximately 20% of cases and approximately 50% of patients with MS will experience symptomatic ON during the course of the disease.7 In addition, ON in MS is typically unilateral, which provides an internal control and reduces intersubject variability when using asymmetry analysis.8 Furthermore, the degree of demyelination can be objectively measured by the latency of multifocal visual evoked potentials (mfVEP),9 whereas optical coherence tomography (OCT) provides accurate estimation of retinal ganglion cell (RGC) axonal loss.10
Therefore, in this study, we used the visual system to examine the long-term effect of permanent demyelination on axonal attrition by investigating an association between intereye asymmetry of the mfVEP latency delay in patients with a long-standing history of unilateral ON and subsequent thinning of RGC axonal (as measured by retinal nerve fiber layer [RNFL] thickness), determined after an average interval of 6 years.
Methods
Participants
Eighty-five consecutive patients with relapsing-remitting MS (diagnosed based on the 2010 revised McDonald criteria11) were enrolled in this study. Patients with a history of other ocular or neurologic diseases were excluded.
All patients underwent low-contrast visual acuity (LCVA), mfVEP, and OCT testing. Patients with a history of unilateral ON at least 12 months before enrollment who reached at least 5 years of follow-up and had intereye latency asymmetry of more than 5 ms12 at the study baseline (indicating a significant degree of chronic optic nerve demyelination) were selected for further analysis. A numerical value of 5 ms was predefined based on the 95th percentile of mfVEP latency asymmetry in normal population.13
Time since the onset of ON was obtained from patient's records. The diagnosis of ON was based on clinical findings, which included an appropriate history and objective examination findings (decreased visual acuity, a visual field defect, color vision loss, relative afferent pupil defect, and a compatible fundus examination). The long-term data analyzed in this study were collected between July 2010 and June 2021.
Standard Protocol Approvals, Registrations, and Patient Consents
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of the University of Sydney. Written consent was signed by all participants.
OCT Scans
All patients had a peripapillary ring scan at baseline and follow-up using the Heidelberg Spectralis OCT as described previously.6 Data were reported following the APOSTEL recommendations.14 The same operator performed all scans. Both global and temporal RNFL thicknesses were analyzed. OSCAR-IB criteria15 were used to check image quality.
Owing to the fact that substantial thinning of the RNFL was present in ON eyes at baseline, the relative change of RNFL was used for analysis, as described previously.6
Multifocal VEP Recordings and Analysis
The level of demyelination in the visual pathways was assessed by the latency delay of mfVEP recorded using the Vision Search system (VisionSearch, Sydney, Australia) with standard stimulus conditions as described previously.16 In brief, 4 gold disk electrodes (Grass, West Warwick, RI) were used for bipolar recording with 2 electrodes positioned 4 cm on either side of the inion, 1 electrode 2.5 cm above, and another 4.5 cm below the inion in the midline. Electrical signals were recorded along 2 channels, measured as the difference between superior and inferior and between the left and right electrodes. The quality of the VEP signal was assessed by AI-assisted in-house software, which was also used to estimate the latency of individual segments. Latency was calculated as the mean of 56 sectors. Because postchiasmal/optic radiation lesions are believed to affect latency of the mfVEP in a similar way, we used between-eye difference (asymmetry) to estimate the degree of demyelination in ON eyes.
Low-Contrast Visual Acuity Recording and Analysis
LCVA was tested unilaterally, using Sloan letter logarithmic translucent contrast charts at 2.5% and 1.25% contrast levels (Precision Vision, La Salle). Testing was performed on a retro-illuminated background at the distance of 4 m. The charts were scored based on the number of letters identified correctly (maximum of 70 letters per chart).17
Statistics
Statistical analysis was performed using SPSS (version 24.0, IBM). The Pearson correlation coefficient was used to measure statistical dependence between 2 numerical variables. p < 0.05 was considered statistically significant. Partial correlation was adjusted for age, sex, duration of the follow-up, and time since the onset of ON. Comparisons between groups were made using the Student t test. Longitudinal changes and difference between ON and fellow eyes were assessed using paired two-sample t tests. The Shapiro-Wilk test was used to test the normal distribution.
Data Availability
Data can be made available on the request of other investigators.
Results
Of 85 patients with RRMS enrolled in this study, 49 had a clinical history of ON at least 12 months before the study. Of those, 28 patients satisfied the inclusion criteria and were selected for the analysis, presented in this study (see flowchart in Figure 1). Twenty-one patients (out of 85 patients) were excluded because of the following: 4 patients had extremely low mfVEP amplitude (presumably caused by the severe damage of optic nerve fibers during acute ON), 3 patients had binocular ON, 12 patients had mfVEP latency asymmetry <5 ms, and in 2 cases, the quality of OCT scans was low.
Figure 1. Flowchart of Patient Selection.
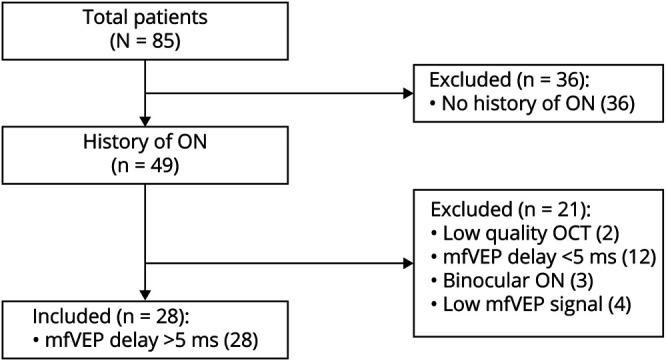
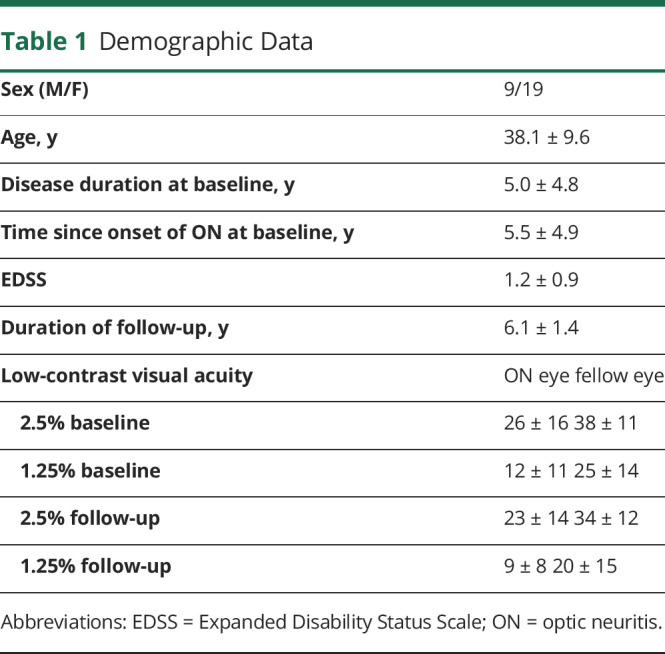
Patients selected for the analysis were examined twice with an average interval of 6.1 ± 1.4 years. Demographic data are provided in Table 1.
Table 1.
Demographic Data
At study baseline, 4 patients were receiving interferon-based therapy, 6-copaxone, 8-gilenya, 3-tysabri, 2-aubagio, 2-tecfidera, and 1-ocrelizumab and 2 patients received no therapy. This has changed at the follow-up to the following: 1 interferon-based therapy, 3-copaxone, 6-gilenya, 2-tysabri, 1-aubagio, 4-tecfidera, 1-ocrelizumab, 5-ocrevus, 2-lemtrada, and 1-plegridy and 2 patients received no therapy.
Association Between Cross-sectional Measures of Demyelination and Axonal Loss
As expected, latency of the ON eyes was significantly delayed compared with fellow eyes at both baseline and follow-up visits (p < 0.001 for both time points, Table 2). Similarly, thickness of RNFL in ON eyes was significantly reduced compared with fellow eyes at both time points (p < 0.001 for all, Table 2).
Table 2.
Baseline and Follow-up mfVEP, RNFL, and LCVA Data
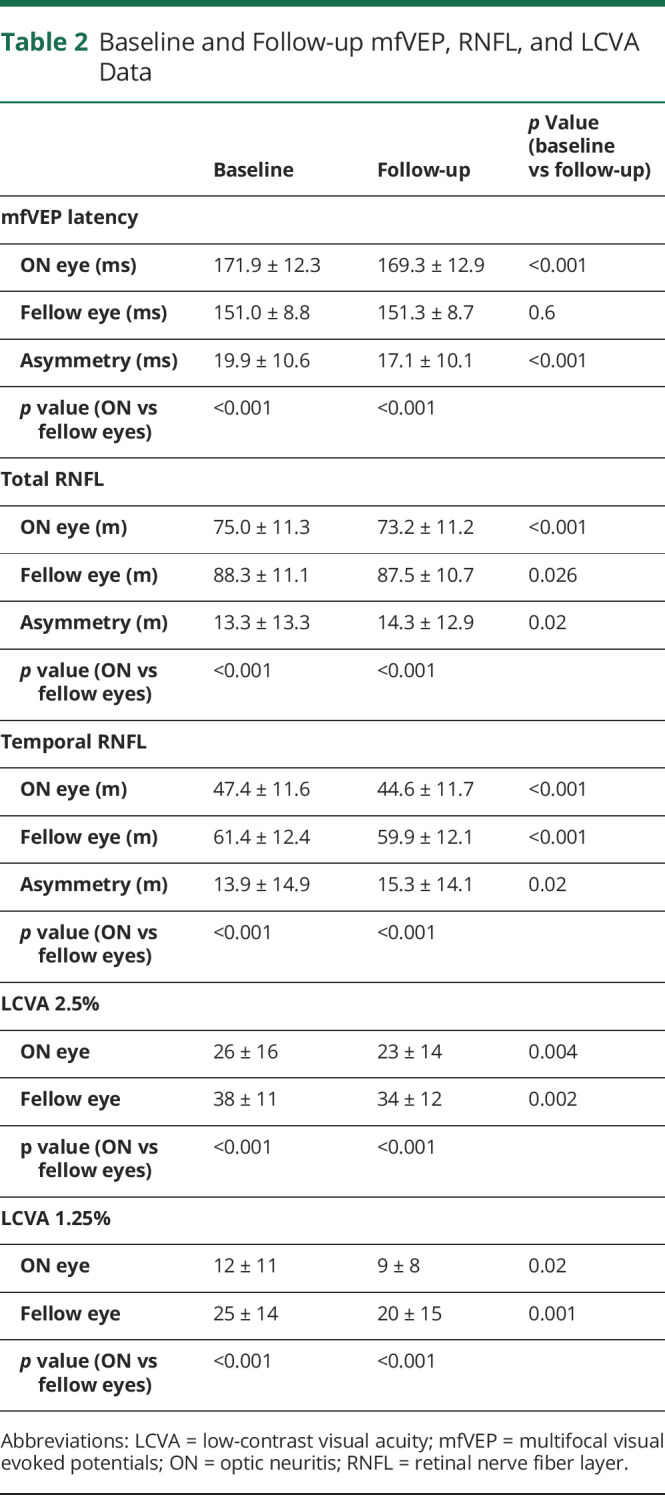
There was significant correlation between baseline intereye latency asymmetry and baseline intereye RNFL thickness asymmetry (both total and temporal RNFL thickness) (r = 0.57, p = 0.004 and r = 0.63, p = 0.001 for total and temporal RNFL thickness, respectively) (Figure 2, A and B), which remained significant after adjusting for age, sex, duration of the follow-up, and time since the onset of ON (partial correlation, r = 0.49, p = 0.016 and r = 0.55, p = 0.005 for total and temporal RNFL thickness, respectively). This relationship remained unchanged (if slightly higher) at the follow-up visit (r = 0.63, p = 0.001 and r = 0.68, p < 0.001 for total and temporal RNFL thickness, respectively, or r = 0.60, p = 0.002 and r = 0.62, p = 0.001 for partial correlation, Figure 2, C and D).
Figure 2. Correlation Between Intereye Latency Asymmetry and Intereye RNFL Thickness Asymmetry.
(A) Baseline intereye latency asymmetry vs baseline intereye total RNFL thickness asymmetry. (B) Baseline intereye latency asymmetry vs baseline intereye temporal RNFL thickness asymmetry. (C) Baseline intereye latency asymmetry vs follow-up intereye total RNFL thickness asymmetry. (D) Baseline intereye latency asymmetry vs follow-up intereye temporal RNFL thickness asymmetry.
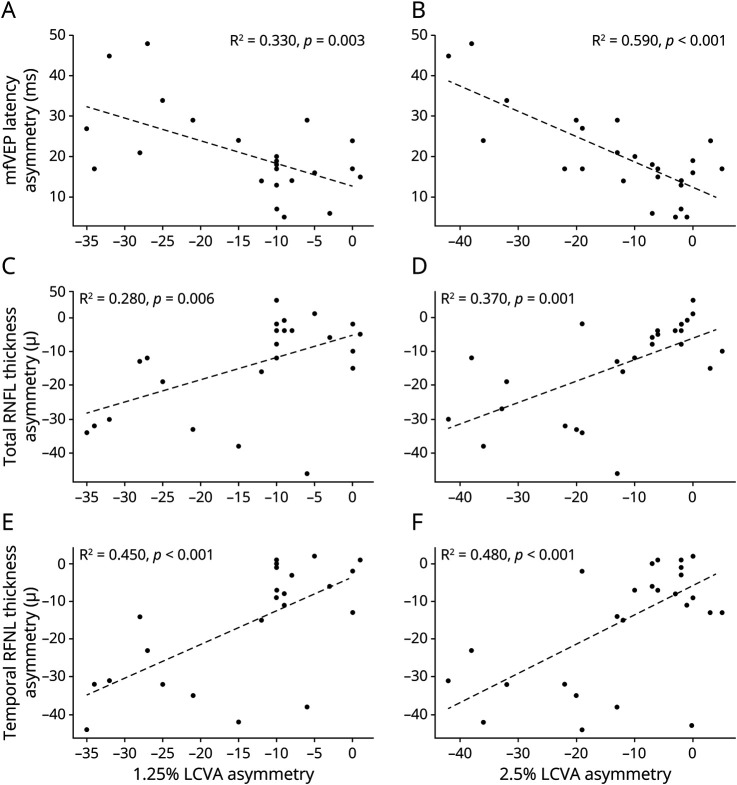
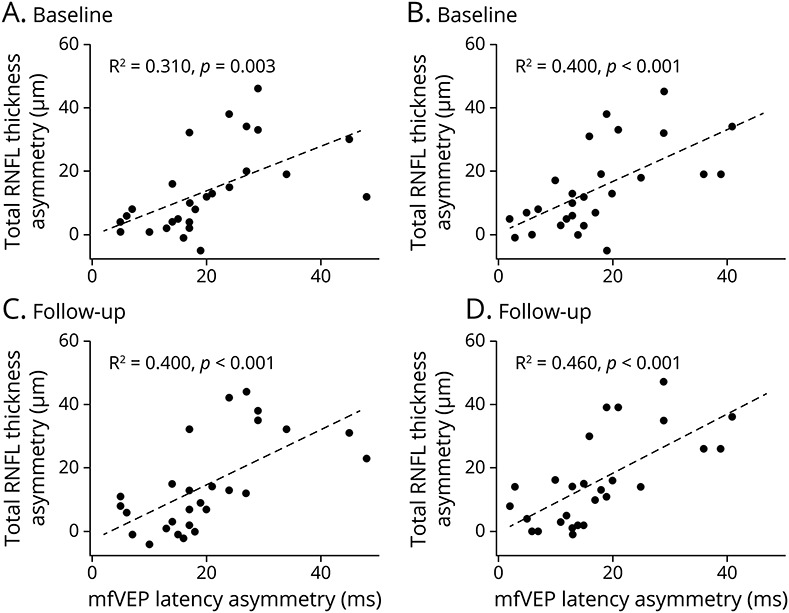
LCVA was also significantly reduced in ON eyes compared with fellow eyes (p < 0.001 for both 2.5% and 1.25% contrasts). In addition, intereye asymmetry of LCVA at baseline correlated with the baseline latency asymmetry of mfVEP and baseline asymmetry of RNFL thickness, both total and temporal RNFL thickness (Table 3 and Figure 3).
Table 3.
Correlation Coefficient of Intereye Asymmetry of LCVA at Baseline With Baseline Latency Asymmetry of mfVEP and Baseline Asymmetry of RNFL Thickness
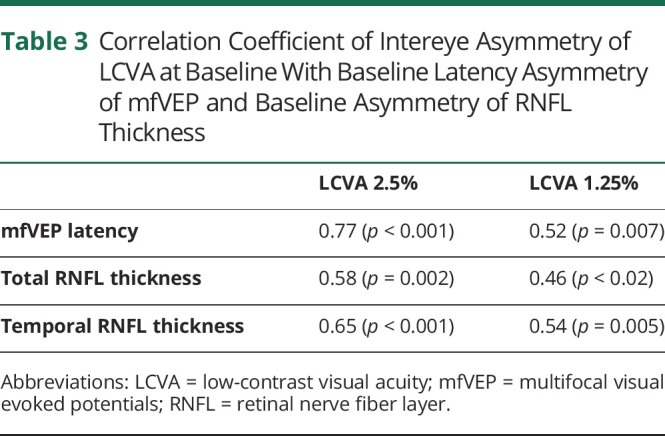
Figure 3. Correlation Between Intereye Asymmetry of LCVA (1.25% and 2.5% Contrast) at Baseline With Baseline Latency Asymmetry of mfVEP (A, B) and Baseline Asymmetry of Total (C, D) and Temporal (E, F) RNFL Thickness.
Association Between Chronic Demyelination in ON and Subsequent Axonal Loss
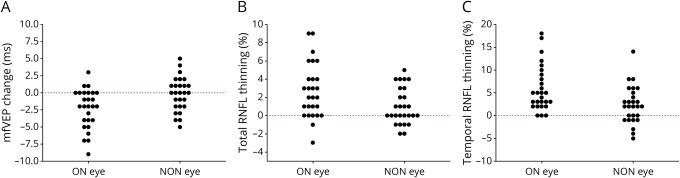
Latency of the mfVEP in ON eyes improved slightly during the follow-up period (averaged shortening: −2.6 ms, p < 0.001) (Table 2 and Figure 4A), whereas latency of the fellow eye remained stable (p = 0.6). This resulted in statistically significant reduction of latency intereye asymmetry between baseline and follow-up visits from 19.9 to 17.2 ms (p < 0.001).
Figure 4. Change mfVEP Latency (A), Total RNFL Thickness (B), and Temporal RNFL Thickness (C) During the Follow-up Period in ON and NON Eyes.
By contrast, we observed a significant reduction of total and temporal RNFL thickness in both ON and fellow eyes during the follow-up period (Table 2 and Figure 4, B and C). The rate of RNFL thinning in ON eyes, however, was significantly larger compared with the fellow eye (2.5% vs 1.0% and 5.8% vs 2.2%, p = 0.002 and 0.001 for total and temporal RNFL thickness, respectively) (Figure 4, B and C). Accordingly, intereye asymmetry of RNFL thickness significantly increased during the follow-up period (p = 0.02 for both total and temporal RNFL thickness, Table 2).
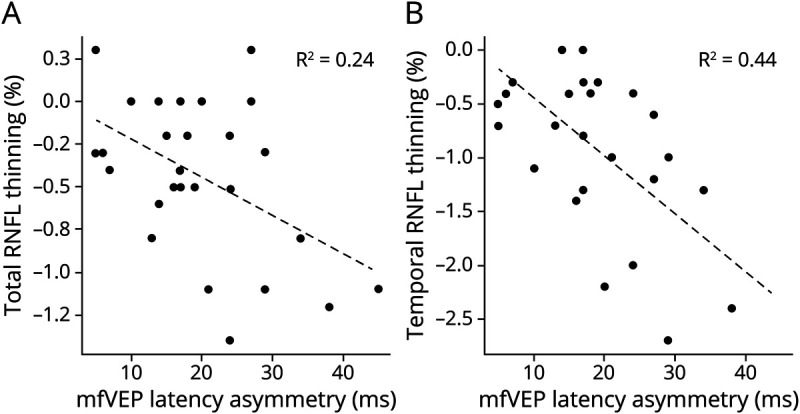
Furthermore, the degree of chronic optic nerve demyelination (latency asymmetry at the baseline) was significantly associated with the annual rate of both total and temporal RNFL thinning in ON eyes (but not in fellow eyes) during the follow-up period (r = 0.47, p = 0.02 and r = 0.67, p < 0.001 for ON eye total and temporal RNFL thinning, respectively) (Figure 5, A and B). This correlation remained unchanged after adjustment for age, sex, and time since the onset of ON (r = 0.52, p = 0.028 and r = 0.68, p < 0.001 for total and temporal RNFL thickness, respectively). For each millisecond of latency delay at baseline (which is believed to be an equivalent of ∼0.5 mm of demyelination along the optic nerve18-20), temporal RNFL thickness was annually reduced by 0.05%.
Figure 5. Correlation Between Baseline mfVP Latency Asymmetry and the Rate of Total (A) and Temporal (B) RNFL Thinning.
There was significant reduction of visual acuity in both ON and fellow eyes (Table 1). However, no correlation was observed between latency asymmetry at baseline and progressive change of LCVA in ON eyes. There was also no significant correlation in ON eyes between the reduction of LCVA and rate of RNFL thinning.
Discussion
The main finding of this study was the observation of an increased rate of RNFL thinning in ON eyes with a substantial degree of chronic demyelination in comparison with the fellow eyes in patients with RRMS. Thus, although RNFL thickness was significantly reduced in both eyes during the follow-up period, progressive thinning of total and temporal RNFL was more than 2 times faster in ON eyes compared with the fellow eyes. Furthermore, by examining the relationship between baseline intereye latency asymmetry and progressive change of RNFL thickness, we found a highly significant association between the degree of chronic optic nerve demyelination and subsequent loss of RGC axons in ON eyes. Chronic demyelination explained almost half of temporal RNFL progression variability. Of note, only patients with clearly measurable chronic demyelination, as evidenced by substantial latency delay in the ON eyes, were included in this analysis.
These findings lend strong support to the notion that chronic demyelination does promote axonal loss. This view until now was mainly based on experimental data. It has been proposed that permanent demyelination may cause axonal damage by rendering axons vulnerable to physiologic stress21-23 by the way of increased energy demands on axonal conduction along demyelinated axons, leading to compromised axoplasmic adenosine triphosphate production,24 ionic imbalance, and Ca2+-mediated axonal degeneration.25 In addition, lack of trophic support from myelin or oligodendrocytes and the disruption of normal axon-myelin interactions were also implicated in the accelerated degeneration of chronically demyelinated axons.22 Furthermore, residual inflammation caused by infiltration of chronic lesions by macrophages and T cells and the activation and proliferation of astrocytes may also contribute to the ongoing damage of demyelinated axons.23,26
However, although we did confirm the detrimental effect of chronic demyelination on axonal survival, suggested by the above experimental studies, the magnitude of the axonal loss caused by permanent demyelination seems to be very modest and is only detectable using a relatively long observation period (annual relative rate of RNFL thinning in ON eyes compared with fellow eyes was +0.25% and +0.6% for total and temporal RNFL thinning, respectively). This may explain why our previous investigations of the relationship between chronic demyelination and axonal damage produced less convincing results.6,27
Our initial short-term study (mean follow-up: 2 years)27 demonstrated a marginally (but not significantly) faster rate of RNFL thinning in ON eyes compared with fellow eyes (1.5 vs 1.3 μ) and did not show any association between RGC axonal loss and latency delay, with the caveat that a first generation temporal domain OCT was used in this study.
After this, a longer follow-up study (mean observation period: 3.5 years)6 also demonstrated faster tRNFL thinning in ON eyes compared with the fellow eyes (1.3% vs 1.0% tRNFL thinning per year), although was still not statistically significant for both total and temporal RNFL thinning. The survival analysis, however, demonstrated a significant difference between the 2 groups. Furthermore, in cases of more severe optic nerve demyelination, progressive loss of temporal (but not total) RNFL was significantly faster in the ON eyes than in the fellow eyes. In addition, faster loss of tRNFL significantly correlated with the degree of ON-related demyelination.
This study benefitted from an even longer (6 years) follow-up period by clearly demonstrating the difference in the rate of RGC axonal attrition between ON and fellow eyes and a significant correlation between the degree of chronic demyelination and RNFL thinning for both temporal and total RNFL measures. Applying asymmetry analysis and careful patient selection by verifying the presence and degree of chronic demyelination in ON eyes using latency of the mfVEP also provided an additional advantage for this study.
Limited (up to 2 years) observation periods,28,29 mixed patient's cohorts,29-31 or lack of validation of the degree of chronic demyelination29-31 may explain why some of the previously reported studies failed to detect accelerated axonal loss in the eyes of patients with RRMS with a history of ON. The most recent study,32 which followed mixed cohort of patients with MS for 5 years, showed a significant relationship between RNFL thinning and previous history of ON only in the progressive, but not in patients with relapsing-remitting MS. However, contrary to patients with progressive MS, the baseline degree of optic nerve damage caused by acute ON (and, therefore, degree of chronic demyelination) in ON eyes of patients with RRMS was minimal, if any. We believe that the patient selection (using the VEP or OCT criteria33,34) is extremely important if one wants to study the effects of chronic demyelination because at the time of the enrollment, almost half of the clinically diagnosed patients with ON in our cohort did not show significant residual chronic demyelination, indicating either potential over-diagnosis or ‟complete” spontaneous remyelination of optic nerve lesions in the early postacute period.9
Our study demonstrated that the process of axonal attrition caused by the chronic loss of myelin, while measurable, is slow and, when combined with the limited sensitivity of modern OCT technology in eyes that have already lost a substantial amount of RNFL, requires a lengthy observation period to be reliably detected. This is contrary to substantial axonal transection occurring during acute ON,35 corroborated by significant correlation between baseline intereye latency asymmetry (which reflects the length of the acute optic nerve inflammation18) and baseline intereye RNFL thickness asymmetry.
Although the correlation between mfVEP latency and RGC axonal thinning was statistically significant for both total and temporal RNFL, thinning of the temporal RNFL was more evident and more strongly associated with the degree of chronic demyelination. Better correlations of mfVEP latency with temporal RNFL (both cross-sectionally and longitudinally) are likely to reflect better topographical correspondence between the 2 because the central area of the visual field, which represents most (80%) of the mfVEP segments,36 is subserved by the temporal RNFL. In addition, ON more frequently affects the fibers traveling in the center of the nerve,37,38 as manifested by the longest latency delay observed in central mfVEP segments.9 This is possibly due to the proximity of those fibers to the central retinal vessels that travel in the anterior nerve head.
LCVA, used in this study as a functional measure of vision,39,40 was also significantly diminished in ON eyes compared with fellow eyes. It demonstrated a significant association with the level of chronic demyelination in optic nerve and baseline asymmetry of RNFL thickness, confirming our previous observation.16 The correlation was stronger for 2.5% compared with 1.25%, which is likely to be due to a higher variability of the 1.25% LCVA measure. However, although there was a significant reduction of LCVA in both ON and fellow eyes, no correlation was observed between progressive LCVA change in ON eyes and mfVEP latency asymmetry at baseline or the rate of RNFL thinning, implying lower sensitivity of this functional measure in monitoring subtle axonal damage caused by chronic loss of myelin.41
There are several limitations in this study. First, there is potentially an alternative explanation for accelerated axonal loss observed in ON eyes, namely, that slow-burning inflammation at the rim of chronic active MS lesions can potentially cause axonal degeneration and accompanying lesion expansion.42,43 Recent studies have demonstrated that this process is frequent even in the relapsing-remitting stage of the disease.44,45 This is, however, less likely because there was no electrophysiologic evidence of lesion growth (i.e., no increase of latency delay in the ON eye or increase of intereye latency asymmetry, which would complement lesion expansion). On the contrary, latency in the ON eye slightly, but significantly, shortened during the follow-up period, suggesting continuous spontaneous remyelination,9 cortical plasticity,46 beneficial treatment effects, or preferential loss of heavily demyelinated optic nerve axons. The limited effect of slow-burning rim inflammation on axonal loss within lesional tissue may be related to the anatomy of the optic nerve. Thus, geometrically, the optic nerve represents a cylindrical shape with a radius of 1.6–2 mm and a length of approximatey 45–50 mm.47 An average lesion in the optic nerve, as detected by magnetic resonance imaging, is approximatey 20–25 mm in length and often occupies the full cross section of the nerve.48 As a result, the area of potential expansion is limited to the proximal and distal ends of the lesion along the optic nerve,9 contrary to most brain lesions, which are typically fully surrounded by normal white matter.49 These anatomical characteristics of optic nerve lesions, therefore, may provide a unique opportunity for investigating the effect of chronic demyelination in isolation.
Our study is limited by relatively small sample size of patients who fitted the specific criteria for inclusion, which may hinder the detection of an association between chronic demyelination and LCVA and limit the potential utility of functional measures of axonal loss.
There is also a possibility that a new episode of ON or the development of new lesions in the optic radiations during the follow-up can potentially result in RNFL loss. However, we do not think that the abovementioned factors would have affected our results. As we have previously shown, new OR lesions can affect the RNFL in both eyes through transsynaptic degeneration,50 but this produces similar loss in both eyes and is reflected in a symmetrical delay in the mfVEP. In addition, a new episode of ON is likely to cause substantial unilateral delay on mfVEP, which was not observed in this study.
Another limitation is related to the fact that patients were on various disease-modifying therapies (DMT) that, in some cases, were changed during the follow-up period. Although this potentially affected our results, there is currently no information related to the effect of DMT on the neuroprotection of chronically demyelinated axons available, and this question remains to be addressed in future studies.
The results of this study may have significant implications for the design of clinical trials of remyelinating agents. For example, even highly sensitive structural measures of axonal loss, such as OCT, required >5 years of follow-up to unequivocally establish the effect of chronic demyelination on axonal loss. Therefore, to demonstrate potential neuroprotective effects of treatment-induced remyelination on preventing such a loss, a prohibitively long study duration is likely to be required.
In summary, our study provides clear in vivo evidence that chronic demyelination significantly accelerates axonal loss. However, because this process is slow and its effect is mild, long-term monitoring is required to firmly establish and confidently measure the neurodegenerative consequences of demyelination.
Glossary
- DMT
disease modifying therapies
- LCVA
low contrast visual acuity
- mfVEP
multifocal visual evoked potentials
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- RRMS
relapsing-remitting multiple sclerosis
Appendix. Authors

Contributor Information
Samuel Klistorner, Email: samuel.klistorner@sydney.edu.au.
Yuyi You, Email: yuyi.you@gmail.com.
Stuart L. Graham, Email: stuart.graham@mq.edu.au.
Con Yiannikas, Email: y.con@bigpond.com.
John Parratt, Email: john.parratt@sydney.edu.au.
Michael Barnett, Email: michael@snac.com.au.
Study Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the National Multiple Sclerosis Society (NMSS), Novartis Save Neuron Grant, Sydney Eye Hospital foundation grant, and Sydney Medical School Foundation.
Disclosure
All authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
Publication History
Received by Neurology: Neuroimmunology & Neuroinflammation October 10, 2021. Accepted in final form January 24, 2022. Submitted and externally peer reviewed. The handling editor was Friedemann Paul, MD.
References
- 1.Barkhof F, Jong RD, Sfikas N, Vera AD. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing–remitting multiple sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler. 2014;20(13):1704-1713. [DOI] [PubMed] [Google Scholar]
- 2.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck ES, Reich DS. Brain atrophy in multiple sclerosis: how deep must we go? Ann Neurol. 2018;83(2):208-209. [DOI] [PubMed] [Google Scholar]
- 4.Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler. 2016;22(1):64-72. [DOI] [PubMed] [Google Scholar]
- 5.Mahad DH, Trapp BD, Lassmann H. Progressive multiple sclerosis 1 Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183-193. [DOI] [PubMed] [Google Scholar]
- 6.You Yuyi, Barnett MH, Yiannikas C, et al. . Chronic demyelination exacerbates neuroaxonal loss in MS patients with unilateral optic neuritis. Neurol Neuroimmunol Neuroinflammation. 2020;7(3):e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins T, Ciccarelli O, Toosy A, et al. Dissecting structure-function interactions in acute optic neuritis to investigate neuroplasticity. Hum Brain Mapp. 2010;31(2):276-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham SL, Klistorner AI, Grigg JR, Billson FA. Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma. 2000;9(1):10-19. [DOI] [PubMed] [Google Scholar]
- 9.Klistorner A, Arvind H, Garrick R, Yiannikas C, Paine M, Graham SL. Remyelination of optic nerve lesions: spatial and temporal factors. Mult Scler. 2010;16(7):786-795. [DOI] [PubMed] [Google Scholar]
- 10.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797-812. [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behbehani R, Ali A, Al-Omairah H, Rousseff RT. Optimization of spectral domain optical coherence tomography and visual evoked potentials to identify unilateral optic neuritis. Mult Scler Relat Disord. 2020;41:101988. [DOI] [PubMed] [Google Scholar]
- 13.Klistorner A, Triplett JD, Barnett MH, et al. . Latency of multifocal visual evoked potential in multiple sclerosis: a visual pathway biomarker for clinical trials of remyelinating therapies. J Clin Neurophysiol. 2020;38(3):186-191. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Herranz A, alk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triplett JD, Yiannikas C, Barnett MH, et al. Pathophysiological basis of low contrast visual acuity loss in multiple sclerosis. Ann Clin Transl Neurol. 2018;5(12):1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Der Walt A, Kolbe S, Mitchell P, et al. . Parallel changes in structural and functional measures of optic nerve myelination after optic neuritis. PLoS One. 2015;10(5):e0121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardmeier M, Leocani L, Fuhr P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. MSJ. 2017;23(10):1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald WI. Pathophysiology of conduction in central nerve fibres. In: Visual Evoked Potentials in Man: New Developments. Clarendon Press; 1977:427-437. [Google Scholar]
- 21.Brück W. Inflammatory demyelination is not central to the pathogenesis of multiple sclerosis. J Neurol. 2005;252(suppl 5):v10-v15. [DOI] [PubMed] [Google Scholar]
- 22.Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999;12(3):295-302. [DOI] [PubMed] [Google Scholar]
- 23.Kornek B, Storch MK, Weissert R, et al. . Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative study of axonal injury in active, inactive and remyelinated lesons. Am J Pathol. 2000;157(1):267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta R, MsDonough J, Yin X, Peterson J, Chang A. Mitochondrial dysfunction cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59(3):478-489. [DOI] [PubMed] [Google Scholar]
- 25.Correale J, Gaitán MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment multiple sclerosis. Brain. 2017;140(3):527-546. [DOI] [PubMed] [Google Scholar]
- 26.Correale J, Farez MF, Cardona AE. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klistorner A, Garrick R, Paine M, et al. . Relationship between chronic demyelination of the optic nerve and short term axonal loss. J Neurol Neurosurg Psychiatry. 2011;83(3):311-314. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Martin E, Pueyo V, Ara JR, et al. . Effect of optic neuritis on progressive axonal damage in multiple sclerosis patients. Mult Scler J. 2011;17(7):830-837. [DOI] [PubMed] [Google Scholar]
- 29.Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263(7):1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abalo-Lojo JM, Treus A, Arias M, Gómez-Ulla F, Gonzalez F. Longitudinal study of retinal nerve fiber layer thickness changes in a multiple sclerosis patients cohort: a long term 5 year follow-up. Mult Scler Relat Disord. 2018;19:124-128. [DOI] [PubMed] [Google Scholar]
- 31.Preiningerova JL, Grishko A, Sobisek L, et al. . Do eyes with and without optic neuritis in multiple sclerosis age equally? Neuropsychiatr Dis Treat. 2018;14:2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakimovski D, Zivadinov R, Vaughn CB, Ozel O, Weinstock-Guttman B. Clinical effects associated with five-year retinal nerve fiber layer thinning in multiple sclerosis. J Neurol Sci. 2021;427:117552. [DOI] [PubMed] [Google Scholar]
- 33.Bsteh G, Hegen H, Altmann P, et al. Validation of inter-eye difference thresholds in optical coherence tomography for identification of optic neuritis in multiple sclerosis. Mult Scler Relat Disord. 2020;45:102403. [DOI] [PubMed] [Google Scholar]
- 34.Nolan-Kenney RC, Liu M, Akhand O, et al. . Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol. 2019;85(5):618-629. [DOI] [PubMed] [Google Scholar]
- 35.Kupersmith MJ, Garvin MK, Wang J, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22(5):641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klistorner A, Arvind H, Nguyen T, et al. Multifocal VEP and OCT in optic neuritis: a topographical study of the structure-function relationship. Doc Ophthalmol. 2009;118(2):129-137. [DOI] [PubMed] [Google Scholar]
- 37.Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7(5):e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebolleda G, Sánchez-Sánchez C, González-López JJ, Contreras I, Muñoz-Negrete FJ. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015;56(2):682-692. [DOI] [PubMed] [Google Scholar]
- 39.Baier ML, Cutter GR, Rudick R. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2015;64(6):992-995. [DOI] [PubMed] [Google Scholar]
- 40.Balcer LJ, Raynowska J, Nolan R, et al. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):734-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klistorner A, Barnett MH. Remyelination trials: are we expecting the unexpected? Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. . Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler. 2019;25(14):1915-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klistorner S, Barnett MH, Yiannikas C, et al. . Expansion of chronic lesions is linked to disease progression in relapsing–remitting multiple sclerosis patients. Mult Scler J. 2021;27(10):1533-1542. [DOI] [PubMed] [Google Scholar]
- 45.Klistorner S, Barnett M, Yiannikas C, et al. . Expansion of chronic MS lesions is associated with an increase of radial diffusivity in periplaque white matter. Mult Scler. 2021. Online ahead of print, doi: 10.1177/13524585211033464. [DOI] [PubMed] [Google Scholar]
- 46.Raz N, Chokron S, Ben-Hur T, Levin N. Temporal reorganization to overcome monocular demyelination. Neurology. 2013;81(8):702-709. [DOI] [PubMed] [Google Scholar]
- 47.Frohman EM, Dwyer MG, Frohman T, et al. . Relationship of optic nerve and brain conventional and non-conventional MRI measures and RNFL, as assessed by OCT and GDx: a pilot study. J Neurol Sci. 2009;282(1-2):96-105. [DOI] [PubMed] [Google Scholar]
- 48.Trip SA, Schlottmann PG, Jones SJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. 2006;31(1):286-293. [DOI] [PubMed] [Google Scholar]
- 49.Fillipi M. MRI technologies in multiple sclerosis. In: Cook SD, ed. Handbook on multiple sclerosis. Taylor & Francis; 2005:179-221. [Google Scholar]
- 50.Klistorner A, Sriram P, Vootakuru N, et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology. 2014;82(24):2165-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available on the request of other investigators.