Abstract
Background:
Surgical brain injury (SBI) which occurs due to the inadvertent injury inflicted to surrounding brain tissue during neurosurgical procedures can potentiate blood brain barrier (BBB) permeability, brain edema and neurological deficits. This study investigated the role of neurotrophin 3 (NT-3) and tropomyosin related kinase receptor C (TrkC) against brain edema and neurological deficits in a rat SBI model.
Methods:
SBI was induced in male Sprague Dawley rats by partial right frontal lobe resection. Temporal expression of endogenous NT-3 and TrkC was evaluated at 6, 12, 24 and 72 h after SBI. SBI rats received recombinant NT-3 which was directly applied to the brain surgical injury site using gelfoam. Brain edema and neurological function was evaluated at 24 and 72 h after SBI. Small interfering RNA (siRNA) for TrkC and Rapl was administered via intracerebroventricular injection 24 h before SBI. BBB permeability assay and western blot was performed at 24 h after SBI.
Results:
Endogenous NT-3 was decreased and TrkC expression increased after SBI. Topical administration of recombinant NT-3 reduced brain edema, BBB permeability and improved neurological function after SBI. Recombinant NT-3 administration increased the expression of phosphorylated Rapl and Erk5. The protective effect of NT-3 was reversed with TrkC siRNA but not Rapl siRNA.
Conclusions:
Topical application of NT-3 reduced brain edema, BBB permeability and improved neurological function after SBI. The protective effect of NT-3 was possibly mediated via TrkC dependent activation of Erk5.
Keywords: Surgical brain injury, Brain edema, Neurotrophin 3, Tropomyosin related kinase receptor C, Extracellular signal related kinase 5
1. Introduction
Neurosurgery is one of the most practiced surgical procedures in daily hospital care either in emergency or elective situations. Neurosurgical procedures can cause inevitable injury to surrounding neural tissue due to trauma inflicted by the surgical technique itself. Additionally, the proximity of vulnerable anatomic regions in the central nervous system to the surgical site poses a risk of injury to the normal surrounding tissue during surgeries (Jadhav and Zhang, 2008). Especially after brain tumor surgeries, neural tissue close to the surgical removal area can have tissue edema which aggravates postoperative neurologic deficits depending on both the surgical location and severity of surgery. In a pre-clinical setting, the surgical brain injury (SBI) rat model, which involves a right partial frontal lobe resection has been established to demonstrate brain injury associated with neurosurgical procedures (Jadhav et al., 2007; Sulejczak et al., 2008).
Endogenous growth factors in recombinant form have been used as neuroprotective molecules in various brain injury and stroke models (Ren and Finklestein, 2005). Neurotrophins are endogenous polypeptide growth factors that play crucial roles during neuronal growth and differentiation primarily by acting on their specific tyrosine kinase receptors. Mitogen activated protein kinase (MAPK) cascade is the essential downstream pathway for neurotrophins functioning inside the cell (Cai et al., 2014). Neurotrophin-3 (NT-3) is one of the members of the neurotrophin family which shows a higher expression profile throughout embryogenesis period and thereafter declines during adulthood. Topical application of NT-3 has previously been shown to have neuroprotective properties in cerebral ischemia and spinal cord injury animal models (Zhang et al., 1999; Sharma, 2007).
Neurotrophin-3 binds to tropomyosin related kinase receptor C (TrkC), a receptor with tyrosine kinase activity, to exert its effects. Rapl is a small G protein that mediates neurotrophin signaling involved in neuronal growth and polarization, and it has been shown to function as an upstream of MAPK cascade with the binding of NT-3 to its receptor TrkC (York et al., 1998; Je et al., 2006). Extracellular signal-regulated kinase 5 (ERK5) is a member of MAPKs family with slight structural differences from other classical MAPKs and has a role in cell survival and neuronal differentiation (Nishimoto et al., 2005; Nishimoto and Nishida, 2006). Previous study indicated the involvement of endothelial Rap1-Erk5 interaction during angiogenesis (Doebele et al., 2009) and brain derived neurotrophic factor (BDNF) activated Erk5 via RAPI mediated signaling in cortical neurons (Wang et al., 2006). However, there is no research on the association of NT-3 and Rap1-Erk5 in brain injury models including SBI.
The objective of the current study was to evaluate the blood brain barrier (BBB) protective effects of NT-3 and to evaluate the contribution of its receptor TrkC and Rap1-Erk5 pathway in the protective effects of NT-3 in a SBI rat model.
2. Materials and methods
2.1. Animals and surgical brain injury model
All procedures in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University and followed instructions of NIH Guide for Care and Use of Laboratory Animals. Adult male Sprague Dawley rats (weight 280—300 g) were housed in the animal facility for a minimum of 3 days before surgery with a 12 h light/dark cycle and ad libitum access to food and water. A total of 162 male Sprague Dawley rats were used. Animal groups and numbers used per group/per outcome are shown in Table 1.
Table 1.
Animal groups and numbers used per outcome in the study.
| Experimental groups | Animals used | Mortality | Total | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BWC (24 h) | BWC (72 h) | Evans blue assay | Western blot | IHC | |||
|
| |||||||
| Experiment 1 | |||||||
| Sham | 3 | 3 | |||||
| SBI | 16 | 1 | 17 | ||||
| Experiment 2 | |||||||
| Sham | |||||||
| SBI | 2 | 2 | |||||
| Experiment 3 | |||||||
| Sham | 8 | 6 | 6 | 20 | |||
| SBI + Vehicle | 8 | 7 | 7 | 2 | 24 | ||
| SBI + NT-3 (5 μg) | 8 | 1 | 9 | ||||
| SBI + NT-3 (10 μg) | 8 | 6 | 7 | 2 | 23 | ||
| Experiment 4 | |||||||
| Sham | 6 | 6 | |||||
| SBI + Vehicle | 8 | 2 | 10 | ||||
| SBI + NT-3 (10 μg) | 10 | 10 | |||||
| SBI + NT-3 + Scramble siRNA | 9 | 1 | 10 | ||||
| SBI + NT-3 + Rapl siRNA | 10 | 10 | |||||
| SBI + NT-3 + TrkC siRNA | 8 | 2 | 10 | ||||
| SBI + Scramble siRNA | 4 | 4 | |||||
| SBI + TrkC siRNA | 4 | 4 | |||||
| Total | 32 | 19 | 20 | 78 | 2 | 11 | 162 |
SBI, Surgical Brain Injury; NT-3, Neurotrophin 3;TrkC, Tropomyosin related kinase receptor C; BWC, Brain Water Content; IHC, Immunohistochemistry.
The rodent model of SBI was made as established previously (Hyong et al., 2008). Isoflurane 4% was used as for anesthesia induction in an induction chamber and isoflurane 2.5% delivered via nasal mask was used for anesthetic maintenance. Rats were placed prone in a stereotactic frame under surgical microscope. A midline skin incision was made and the periosteum was reflected to expose the skull and bregma was identified. A craniotomy 5 × 5 mm square was made using a microdrill on the right frontal skull. The dura was incised after bone removal and the underlying right frontal lobe was exposed and a partial right frontal lobe resection was performed at a distance of 2 mm lateral to the sagittal suture and 1 mm proximal to the coronal suture. The resection cavity was packed and normal saline irrigation was performed to obtain hemostasis. The incision was closed with sutures and subcutaneous injection of 0.03 mg/kg buprenorphine was administered for postoperative pain. Sham animal underwent identical surgical procedure with craniotomy removal of the bone flap but without partial right frontal lobe resection. Vital parameters were monitored during surgery and recovery. Animals were sacrificed at different time points as indicated in the experiments.
2.2. Animal treatments and experimental groups
2.2.1. Experiment 1
The time course expression of endogenous NT-3 and TrkC expression was evaluated in whole brain samples at 6, 12, 24 and 72 h after SBI. Rats were randomly divided into five groups sham, SBI-6 h, SBI12 h, SBI-24 h and SBI-72 h and whole brain samples were used for western blot. The time course expression of TrkC expression in the right frontal lobe was characterized at 6, 12, 24 and 72 h after SBI. Rats were randomly divided into five groups sham, SBI-6 h, SBI-12 h, SBI-24 h and SBI and right frontal lobe samples were used for western blot.
2.2.2. Experiment 2
The cell types expressing TrkC was identified at 24 h after SBI. The TrkC receptor was co-localized with astrocytes, vascular endothelial cell and neurons at the right frontal lobe perisurgical site using immunofluorescence staining.
2.2.3. Experiment 3
The effect of topical application of recombinant NT-3 in SBI outcomes was evaluated. Recombinant NT-3 was directly delivered to the surgical resection site using gelfoam. Sterile gelfoam was cut into 8 mm3 size piece to fit in the surgical cavity. The gelfoam was soaked in either recombinant NT-3 or saline and then directly applied into the surgical cavity at the end of resection. Two doses of recombinant NT-3 (5 μg and 10 μg) were tested. The dose and delivery of NT-3 was chosen based on previous publication (Zhang et al., 1999; Sharma, 2007). Rats were randomly divided into 4 groups sham, SBI + Vehicle (12 μl saline), SBI + NT-3 (5 μg), SBI + NT-3 (10 μg). Neurological function was evaluated 24 h after SBI after which animals were sacrificed and brain samples were collected for brain water content evaluation.
Next, the effective dose of NT-3 (10 μg) was used to evaluate outcomes 72 h after SBI. Rats were randomly divided into 3 groups sham, SBI + Vehicle (12 μl saline), SBI + NT-3 (10 μg). Neurological function was evaluated at 24 and 72 h after SBI. Animals were sacrificed at 72 h to collect brain samples for brain water content evaluation.
Lastly, BBB permeability was evaluated using Evans blue assay at 24 h after SBI. Rats were randomly divided into 3 groups sham, SBI + Vehicle (12 μl saline), SBI + NT-3 (10 μg). Evans blue dye extravasation assay was performed at 24 h after SBI.
2.2.4. Experiment 4
The role of TrkC and Rapl in pErk5 induction with recombinant NT-3 mediated protection after SBI was evaluated. Rats were randomly divided into 6 groups sham, SBI + Vehicle (12 μl saline), SBI + NT-3 (10 μg), SBI + NT-3 (10 μg) + Scramble siRNA, SBI + NT-3 + Rap1 siRNA, SBI + NT-3 (10 μg) + TrkC siRNA. Neurological function and western blot assay was performed to measure Rapl, pErk5 and Erk5 expression at 24 h after SBI.
The expression of TrkC was evaluated to verify the knockdown of TrkC using siRNA. Rats were randomly divided into 2 groups SBI + Scramble siRNA and SBI + TrkC siRNA. TrkC siRNA or Scramble siRNA 500 pmol was administered by ICV injection 24 h before SBI and brain samples were collected 24 h after SBI for western blot to measure TrkC expression.
2.2.5. Intracerebroventricular injection
The siRNAs were given by intracerebroventicular (ICV) injection 24 h before SBI as previously described (Chen et al., 2013). The rats were anesthetized and placed prone on a stereotactic frame. A burr hole was made using the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral and 3.2 mm below the horizontal plane to inject siRNAs into the ipsilateral ventricle. Using a 10 μl Hamilton syringe, TrkC siRNA (500 pmol), Rapl siRNA (500 pmol) or Scramble siRNA (500 pmol) (all from Origene, MD) in 2 μl sterile saline was injected at a rate of 0.5 μl/min through the burr hole. At the end of injection, the needle was kept in situ for an additional 10 min to prevent leakage and withdrawn slowly over 5 min. The needle was removed, the burr hole was sealed with bone wax and skin incision was sutured.
2.3. Brain water content measurement
The animals were sacrificed under deep isoflurane anesthesia at 24 and 72 h after SBI. Rat brains were immediately removed and dissected into six parts right frontal, left frontal, right parietal, left parietal, cerebellum and brainstem in a petri dish over ice. The wet weights of each brain parts were measured immediately and dry weights were measured after drying in an oven at 104°C for 48 h. The percentage of water content in each region was calculated as [(wet weight-dry weight)/wet weight] × 100% (Yamaguchi et al., 2007).
2.4. Neurological assessment
Neurological assessment was performed by examiner blinded to the groups using modified Garcia test as previously described (Yamaguchi et al., 2007). For the 24 h outcome group, neurological evaluation was performed using Garcia test at 24 h after SBI. For the 72 h outcome group, Garcia test was performed at 24 and 72 h after SBI. The 24 h behavior data of animals from both the 24 and 72 h outcome groups were combined during analysis. The test consisted of 7 parameters which included spontaneous activity, side stroking, vibrissae touch, limb symmetry, climbing, lateral turning, and forelimb walking. Each parameter received a score ranging from O to 3, with a maximum total score of 21.
2.5. Evans blue extravasation assay
The permeability of the BBB was quantified using Evans blue dye extravasation assay as previously described (Suzuki et al., 2010; Sherchan et al., 2017). Evans blue dye 2% at 5 ml/kg was administered by intraperitoneal injection and allowed to circulate for 4 h after which rats were sacrificed. Brain samples were removed after transcardial phosphate buffered saline (PBS) perfusion and dissected into right and left frontal, right and left parietal, brainstem and cerebellum. The right frontal lobe samples were homogenized in PBS 1 ml/300 g and the supernatant was collected after centrifuge was performed at 14,000 rpm for 30 min. The supernatant 500 μl was added to equal amount of trichloroacetic acid (50%) and incubated at 4 °C overnight, followed by centrifuge the next day. Extravasation of Evans blue dye was measured by spectrophotometer at 620 nm and quantified according to the standard curve. The results were expressed as a fold compared to sham.
2.6. Western blot analyses
Western blotting was performed at 24 h after SBI as described previously (Sherchan et al., 2016). Rats were transcardially perfused with ice-cold PBS after which the brain was removed and dissected into 6 regions as in brain water measurement process. The brain samples were put in liquid nitrogen and stored at −80°C until use. The ipsilateral residual right frontal samples were homogenized in RIPA lysis buffer for protein extraction (Santa Cruz Biotechnology, TX) and centrifuged at 14,000 g at 4°C for 30 min. The supernatant was obtained and protein concentration in the samples was measured using a detergent-compatible assay (Bio-Rad, CA). Equal amounts (50 μg) of protein were loaded on SDS-PAGE gel and allowed to run using electrophoresis. Next steps included transfer to a nitrocellulose membrane, blocking and incubation with primary antibodies overnight at 4°C. The primary antibodies used were rabbit polyclonal anti-Neurotrophin-3 (1:200), rabbit polyclonal TrkC antibody (1 :500), goat polyclonal anti-Erk5 and anti-pErk5 (1:500), mouse monoclonal anti-Rapl antibody (1:500) (all from Abcam, MA). For loading control, the same membranes were blotted with primary antibody goat anti-Actin (1:5000; Santa Cruz Biotechnology, TX). The following day, the nitrocellulose membranes were incubated with appropriate secondary antibodies (1:2000; Santa Cruz Biotechnology, TX) for 2 h at room temperature. Immunoblot bands were further probed with a chemiluminescence reagent kit ECL Plus (GE Healthcare, IL). The bands were analyzed by Image J software.
2.7. Immunohistochemistry
Immunohistochemistry staining was performed 24 h after SBI as described previously (Sherchan et al., 2016). During sacrifice rats were transcardially perfused with ice-cold PBS followed by 10% formalin. The brain samples were kept in 10% formalin at 4°C for 24 h and then in 30% sucrose with PBS until the brain samples sank to the bottom. The right frontal brain samples were cut into 10 μm thick coronal sections in a cryostat (CM3050S; LeicaMicrosystems). The brain sections were incubated overnight at 4°C with the following primary antibodies; rabbit polyclonal anti-TrkC (1:100) (Abcam, MA) co-stained with mouse monoclonal anti-GFAP (1:100), mouse monoclonal anti-NeuN (1:100), and goat anti-Von Willebrand factor (1:100) (all from Santa Cruz Biotechnology, TX). The sections were then incubated with appropriate FITC- or Texas Red-conjugated secondary antibodies for 2 h at room temperature. The sections were visualized with a fluorescence microscope (Olympus BX51).
2.8. Statistical analysis
Statistical analysis was performed using the Sigma Plot 10.0 and Sigma Stat version 3.5 (Systat Software, San Jose, CA, USA). Data were expressed as mean ± SD. One-way ANOVA followed by Student Neuman Keuls test was used to analyze statistical differences between groups. P values < 0.05 was considered statistically significant.
3. Results
3.1. Temporal expression of endogenous neurotrophin-3 (NT-3) after SBI
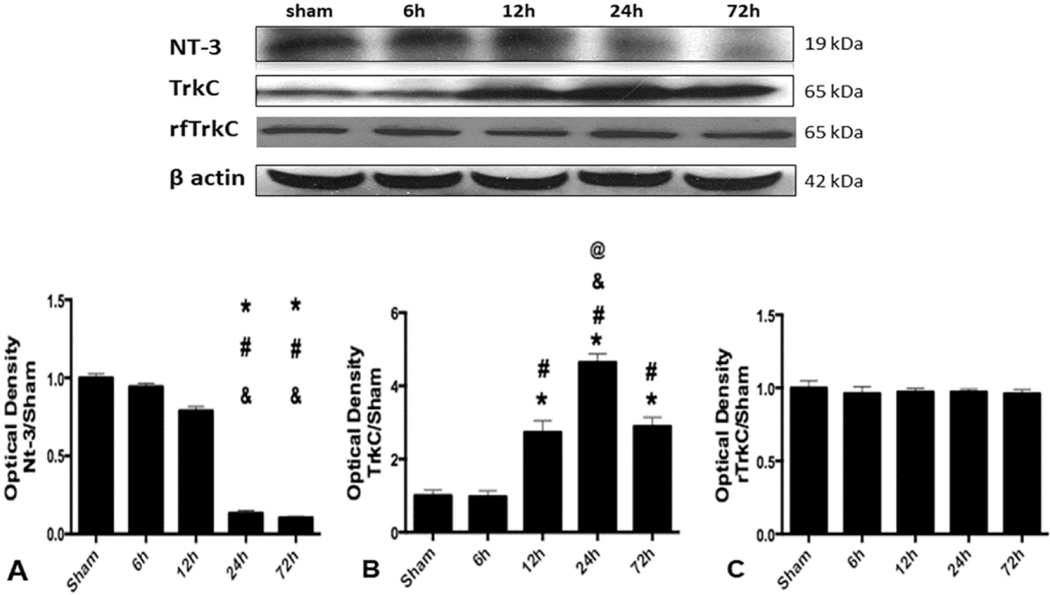
The expression of endogenous NT-3 in whole brain samples was evaluated at 6, 12, 24 and 72 h after SBI. The expression of NT-3 significantly declined at 24 and 72 h after SBI compared to sham operated animals and SBI animals at 6 and 12 h after injury (Fig. 1A).
Fig. 1.
Endogenous expression of NT-3 and TrkC after SBI. Representative western blot bands for NT-3, TrkC in whole brain sample and right frontal region TrkC (rfTrkC) in sham and SBI rats at 6, 12, 24 and 72 h following SBI. Quantitative analysis of (A) NT-3 (B) TrkC and (C) rfTrkC expression in the western blot bands. Data were expressed asrnean ± SD. *p < 0.05 versus sham, #p < 0.05 versus SBI-6h, &p < 0.05 versus SBI-12h, %p < 0.05 versus SBI-24h, @p < 0.05 versus SBI-72 h.
3.2. Temporal expression of endogenous TrkC after SBI
The expression of endogenous TrkC in whole brain samples was evaluated at 6, 12, 24 and 72 h after SBI. The expression of TrkC significantly increased at 12, 24 and 72 h after SBI compared to sham operated animals and SBI animals at 6 h after injury (Fig. 1B). The expression of TrkC was highest at 24 h after SBI. The expression of TrkC in the right frontal region only was not different between the groups (Fig. 1C).
3.3. Cellular localization of TrkC after SBI
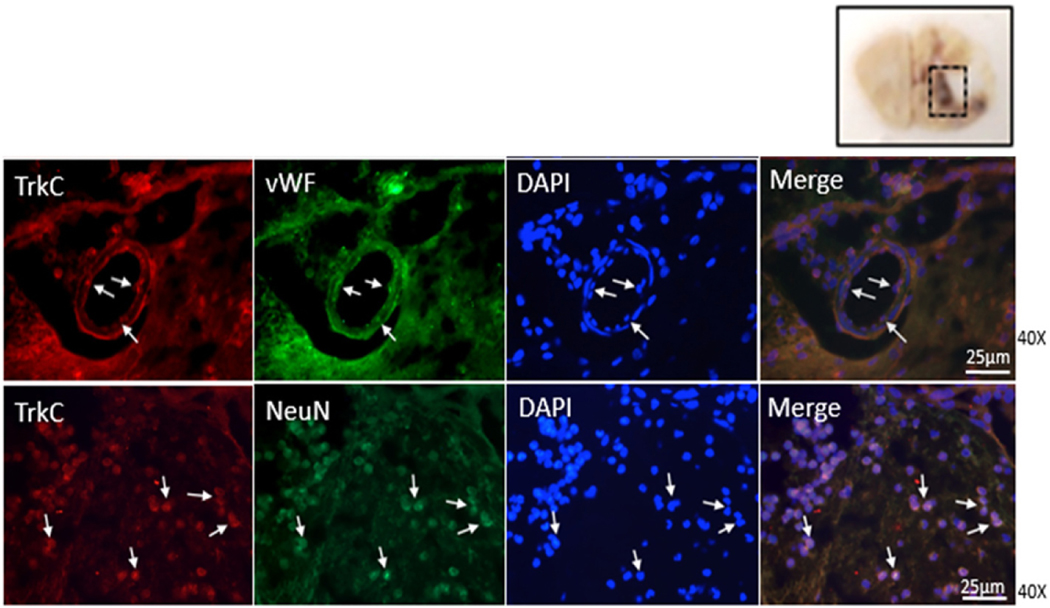
The cell types expressing TrkC receptor at the perisurgical site in right frontal perisurgical site was evaluated 24 h after SBI. Double immunofluorescence staining of brain sections of SBI rats demonstrated that TrkC receptor co-localized with markers of endothelial cells and neurons at the perisurgical site 24 h after SBI (Fig. 2).
Fig. 2.
Representative images of immunofluorescence staining showing co-localization of TrkC (Texas Red/red) with endothelial cell marker vWF and neuronal marker NeuN (both FITC/green) at the right frontal perisurgical site 24 h after SBI. Scale bar: 25 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Effects of topical administration of recombinant NT-3 on brain edema and neurological function 24 and 72 h after SBI
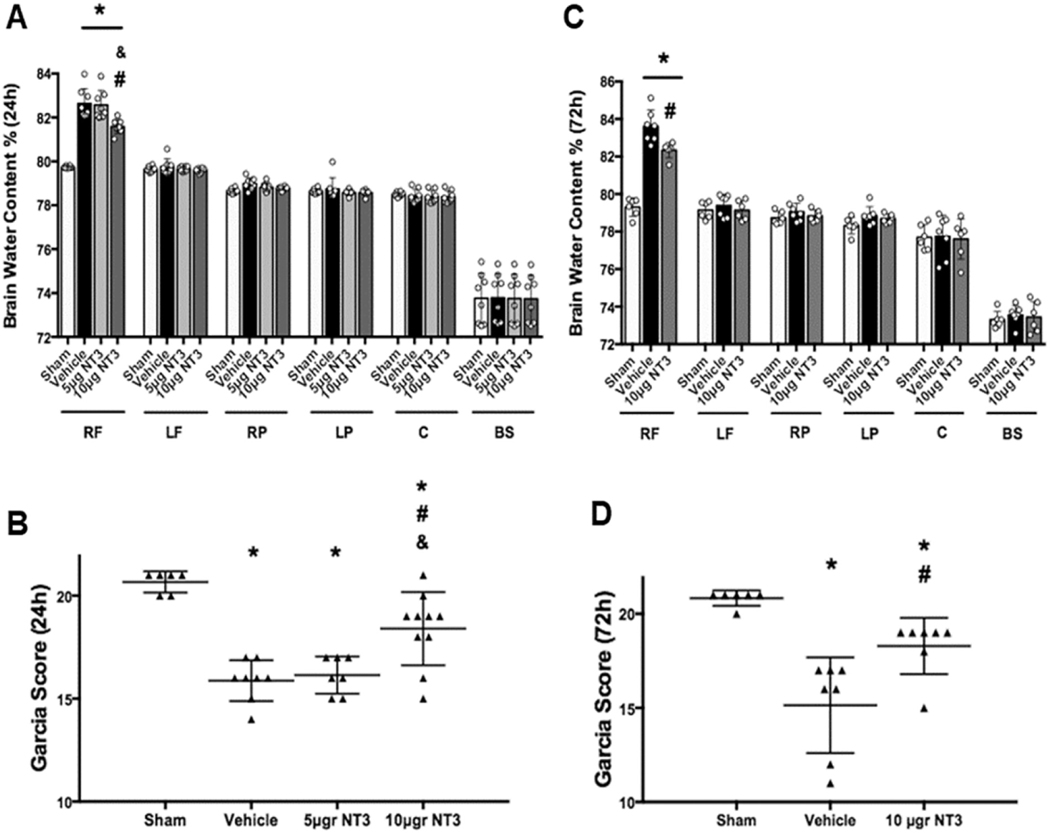
The SBI animals had significantly higher brain water content at the perisurgical site of the right frontal lobe compared to sham operated animals at 24 and 72 h after SBI (Fig. 3A and Fig. 3C, respectively). Recombinant NT-3 (10 μg) embedded in gelfoam significantly reduced brain water content at 24 and 72 h after SBI compared to vehicle group (Fig. 3A and C, respectively). Garcia test showed SBI rats had significantly worse neurological function at 24 and 72 h after injury, and recombinant NT-3 (10 μg) significantly improved neurological function at 24 and 72 h after SBI (Fig. 3B and D, respectively). Recombinant NT-3 (5 μg) did not significantly reduce brain water content and neurological deficits 24 h after SBI (Fig. 3A and B, respectively) and therefore, recombinant NT-3 (5 μg) was not used for further studies.
Fig. 3.
Brain water content and Garcia neurological test scores at 24 and 72 h after SBI. Recombinant NT-3 (10 μg) reduced brain edema and improved neurological function at 24 h after SBI (A and B, respectively) and at 72 h after SBI (C and D, respectively). Brain samples were divided into six parts: right frontal (RF), left frontal (LF), right parietal (RP), left parietal (LF), cerebellum (C) and brainstem (BS) to measured brain edema. Data were expressed as mean ± SD. *p < 0.05 versus sham, #p < 0.05 versus SBI + Vehicle, &p < 0.05 versus SBI + NT-3 (5 μg).
3.5. Effects of topical administration of recombinant NT-3 on BBB permeability 24 h after SBI
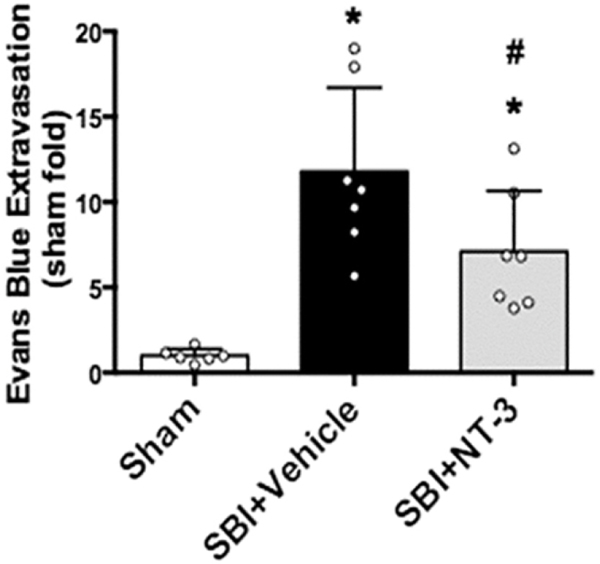
The permeability of BBB was evaluated by measuring Evans blue dye extravasation into the right frontal perisurgical site 24 h after SBI. The extravasation of Evans blue dye at the perisurgical site was significantly increased in SBI rats compared to sham operated animals 24 h after SBI (Fig. 4). Recombinant NT-3 (10 μg) embedded in gelfoam significantly reduced Evans blue dye extravasation 24 h after SBI compared to vehicle group.
Fig. 4.
Evans blue dye extravasation assay at 24 h after SBI. Recombinant NT-3 (10 μg) reduced Evans blue dye extravasation at the right frontal perisurgical site 24 h after SBI. Data were expressed as mean ± SD. *p < 0.05 versus sham, #p < 0.05 versus SBI + Vehicle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Recombinant NT-3 administration increased Rapl and phosphorylated Erk5 (pErk5) expression 24 h after SBI
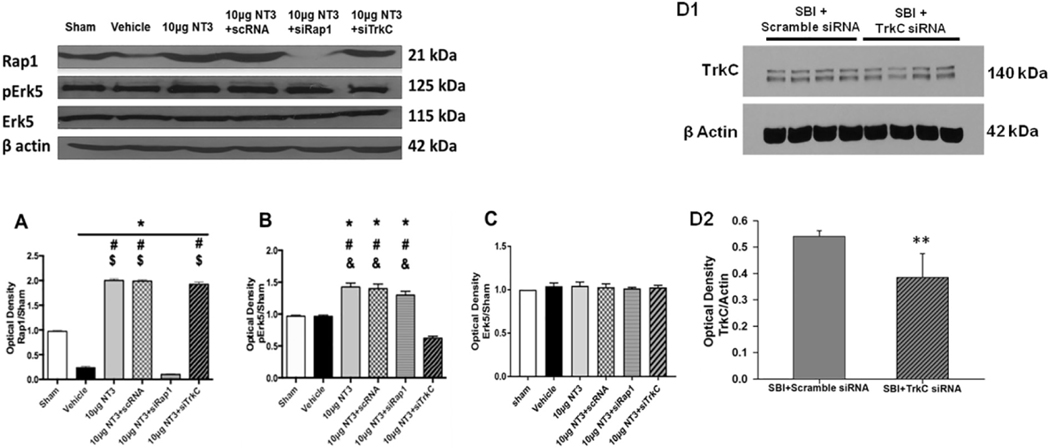
Western blot analysis showed that the expression of Rapl was significantly reduced in the vehicle group 24 h after SBI. Recombinant NT-3 (10 μg) embedded in gelfoam significantly increased Rapl expression compared to vehicle group 24 h after SBI. The administration of Rapl siRNA but not scramble siRNA reversed the effects of recombinant NT-3 (10 μg) on Rapl expression. The administration of TrkC siRNA with recombinant NT-3 (10 μg) did not reverse the effect of recombinant NT-3 (10 μg) on Rapl expression (Fig. 5A).
Fig. 5.
Representative western blot bands and densitometric quantification of Rapl, pErk5/Erk5, ERK5 and TrkC expression at 24 h after SBI. (A) Recombinant NT-3 (10 μg) increased Rapl expression 24 h after SBI which was reversed with Rapl siRNA but not TrkC siRNA. (B) Recombinant NT-3 (10 μg) increased pErk5/Erk5 expression 24 h after SBI which was reversed with TrkC siRNA but not Rapl siRNA. (C) Total Erk5 expression was not different among all the groups. (DI and D2) The expression of TrkC was reduced in the TrkC siRNA group but not with Scramble siRNA. TrkC siRNA or Scramble siRNA was administered 24 h before SBI and brain samples were collected 24 h after SBI. Data were expressed as mean ± SD. *p < 0.05 versus sham, #p < 0.05 versus SBI + Vehicle, $p < 0.05 versus SBI + NT-3 (10μg) + Rapl siRNA, < 0.05 versus SBI + NT-3 (10μg) + TrkC siRNA, < 0.05 versus SBI + Scramble siRNA.
The expression of pErk5/Erk5 was significantly increased 24 h after SBI with recombinant NT-3 (10 μg) embedded in gelfoam compared to vehicle. The expression of pErk5/Erk5 induced by recombinant NT-3 (10 μg) was significantly reduced with TrkC siRNA but not with Rapl siRNA or scramble siRNA administered with recombinant NT-3 (Fig. 5B). Total Erk5 expression was not different among all groups (Fig. 5C).
The administration of TrkC siRNA decreased the expression of TrkC compared to scramble siRNA administration measured 24 h after SBI (Fig. 5 D1 and D2).
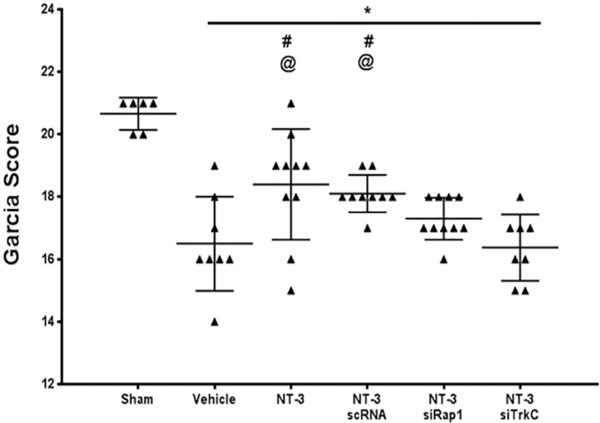
3.7. TrkC knockdown reversed the protective effects of recombinant NT-3 on neurological function 24 h after SBI
Knockdown of TrkC receptor using siRNA reversed the protective effect of recombinant NT-3 (10 μg) on improving neurological function at 24 h after SBI (Fig. 6).
Fig. 6.
TrkC siRNA reversed the protective effects of recombinant NT-3 on neurological function 24 h after SBI. Recombinant NT-3 (10 μg) significantly improved Garcia test scores 24 h after SBI which was reversed with TrkC siRNA administration. Data were expressed as mean ± SD. *p < 0.05 versus sham, p < 0.05 versus SBI + Vehicle, @p < 0.05 versus SBI + NT-3 (10 μg) + TrkC siRNA.
4. Discussion
Neurotrophins promote neuroplasticity after acute CNS injury (Bothwell, 2014). Among the members of neurotrophin family, NT-3 plays a major role in neuronal survival, migration and differentiation during embryonic development and its level declines through adulthood (Tabakman et al., 2004). The effects of NT-3 were shown to be concentration and age dependent in neuronal cell culture (Lowenstein and Arsenault, 1996). Recently, the function of NT-3 in mature nervous system was explored in spinal cord injury models (Harvey et al., 2015). In this study, we evaluated the neuroprotective effects of topical application of recombinant NT-3 in a SBI rat model, and we examined the role of its receptor TrkC and downstream mediators Rapl and Erk5 in NT-3 mediated protection.
We first examined the endogenous response of NT-3 and its receptor TrkC to SBI. We observed that the expression of endogenous NT-3 declined at 24 and 72 h after SBI. Previous studies report variable NT-3 expression in response to brain or spinal cord injury. An increase in NT-3 mRNA levels at 4 to 8 h after TBI was reported followed by a decline to control values by 48 h after injury (Felderhoff-Mueser et al., 2002). The levels of NT-3 mRNA and protein expression declined in the hippocampus at 2, 4 and 6 h after TBI in a rat model (Yang et al., 2005). The possible explanation for the variability noted in the expression of NT-3 following injury may be due to a number of factors such as a difference in the injury type, different animal models used in these studies, variability in time points used to measure NT-3 expression and difference in the techniques used to measure NT-3 expression.
Next, we observed that TrkC receptor was expressed by endothelial cells and neurons at the perisurgical site 24 h after SBI. TrkC, the high-affinity tropomyosin-related kinase receptor of NT-3 been shown to mediate the neuroprotective function of NT-3 and has been reported to be located on neurons, astrocytes, microglia and endothelial cells in the central nervous system (Ishitsuka et al., 2012; Lawn et al., 2015). Similar to NT-3, the expression of endogenous TrkC varies in different experimental brain injury models in the literature (Hicks et al., 1999). Previous study showed that the levels of TrkC receptor mRNA was reduced in a spinal cord injury model (Hajebrahimi et al., 2008). In contrast to this study, there was no change in TrkC expression following excitotoxic brain injury (Canals et al., 1999). In a rat model of focal mechanical injury in the hippocampus, the expression of TrkC mRNA increased after the injury reaching a peak at 4 h then tapered off to control levels at 8 h after the injury (Mudó et al., 1993). In the SBI model, we observed an increase in TrkC expression from 12 h up to 72 h after injury in the whole brain samples. However, we did not find a difference in TrkC expression after SBI in the right frontal lobe only samples. It is possible that the right frontal lobe only samples may not be adequate enough to measure the expression of TrkC receptor. Previous study showed that TrkC expression was differentially expressed in various brain regions in the rat central nervous system. A study by Merlio et al. that examined the mRNA expression of different members of Trk family found that cells expressing TrkB and TrkC mRNAs were widely distributed in the rat brain with higher levels of expression in the olfactory formations, neocortex, hippocampus, thalamic and hypothalamic nuclei, brainstem nuclei, cerebellum and spinal cord motoneurons (Merlio et al., 1992). Therefore, the change in TrkC expression is likely to be detected to a greater extent in the whole brain samples than only the right frontal lobe samples.
Exogenous delivery of NT-3 either with recombinant viral vector or gelatin sponge scaffold has been shown to have promising therapeutic effects after ischemic neural injuries. Recombinant NT-3 application with a gelfoam carrier reduced neuronal apoptosis after cerebral ischemia in a rat model (Zhang et al., 1999). In a spinal cord injury model, NT-3/fibroin coated gelatin sponge scaffold allowed controlled release of NT-3 and facilitated regeneration after injury (Li et al., 2016). In this study, we used direct topical application of recombinant NT-3 using a gelfoam which was embedded at the surgical resection site. Gelfoam is a biocompatible, biodegradable and hemostatic gelatin sponge that is widely used in clinical practice as a drug delivery material to maintain sustained release of drugs directly to the injury site (Abada and Golzarian, 2007; Valentine et al., 2009). We first tested two doses of NT-3 (5 μg and 10 μg) and evaluated outcomes at 24 h after SBI. We observed that NT-3 (10μg) dose reduced brain edema and improved neurological function 24 h after SBI. We therefore, use NT-3 (10 μg) dose for rest of the experiments. Our results showed that NT-3 (10 μg) administration improved outcomes up to 72 h after SBI which was evidenced by reduced brain edema and neurological deficits in the NT-3 treatment group. Additionally, BBB permeability 24 h after SBI was reduced with NT-3 (10 μg) administration.
We next assessed whether Rapl and/or Erk5 signaling via TrkC receptor contributed to the protective effects of NT-3. During embryonic neurogenesis, neurotrophin signal transduction involves activation of Trk receptors which has docking sites for adaptor proteins that in turn activate small G proteins and the MAPK pathways (Jossin and Cooper, 2011). Rapl is one of the small Ras-related guanine triphosphatase (GTPase) which functions as a downstream effector of NGF and BDNF signaling during neurite outgrowth and neuronal polarization (Hisata et al., 2007). Previous study reported that NT-3 binding to TrkC activated Rapl throughout long-term structural and functional synaptic changes (Je et al., 2006). Our results showed that expression of Rapl at the perisurgical region decreased in SBI rats and the expression of Rapl increased in SBI rats treated with recombinant NT-3. We therefore knocked down TrkC using siRNA in SBI rats treated with recombinant NT-3 to determine if NT-3 mediated the activation of Rapl via its receptor TrkC. Knockdown of TrkC did not reverse recombinant NT-3 mediated increase in Rapl. Our findings indicate that recombinant NT-3 induced the expression of Rapl, but Rapl may not be the major downstream effector of TrkC activation by NT-3. It is possible that Rapl may be activated through other Trk receptors and neurotrophic factors after SBI.
Erk5 is a novel MAPK which has been shown to have a supportive role for the cytoskeleton, permeability regulation as well as pro-survival role in cerebral ischemia (Pi et al., 2004; Wang et al., 2009). The phosphorylated (p)-ERK5 signaling pathway has been shown to be involved in neurogenesis and its level declines through postnatal development (Parmar et al., 2015; Wang et al., 2015). Previous study reported the activation of Erk5 in medulloblastoma cell line with NT-3/ TrkC stimulation (Sturla et al., 2005). Likewise, the role of Rap1-Erk5 was reported in angiogenesis (Doebele et al., 2009). Additionally, previous study showed the activation of Erk5 via Rapl signaling cascade after BDNF stimulation in cortical neuronal cell culture (Wang et al., 2006). In this study we proposed that Erk5 is a downstream target of Rapl signaling subsequent to NT-3 binding on TrkC. Our results showed there was no difference in pErk5/Erk5 levels between sham and vehicle SBI groups at 24 h after SBI. Even though endogenous NT-3 was downregulated 24 h after SBI, it is possible that the upregulation of TrkC as a response to injury may offset NT-3 signaling and therefore, does not affect pErk5 expression at 24 h after injury. Additionally, other members of endogenous neurotrophins such as BDNF may stabilize or induce cerebral pErk5 expression as a compensatory neuroprotective mechanism after injury. BDNF is one of the upstream neurotrophic factors for Erk5 activation and its expression was shown to be increased in brain injury models (Mohapel et al., 2005; Bath et al., 2012). Furthermore, we observed that recombinant NT-3 increased the expression of pErk5/Erk5 after SBI which was reversed with TrkC knockdown. However, Rapl knockdown did not reverse NT-3 mediated increase in pErk5/Ekr5 expression after SBI. This suggested that NT-3 regulated the activation of Erk5 dependent on TrkC but Rapl was not the major adaptor protein that regulated Erk5 activation by NT-3. Additionally, the improvement in neurological function observed with recombinant NT-3 was reversed with TrkC knockdown. These observations suggested that Erk5 activation has a possible role in the neuroprotective effects of NT-3 as a downstream mediator of TrkC receptor activation.
There are some limitations in our study. The role of Erk5 in NT-3 induced protection requires further exploration. Previous studies have reported that Erk5 signaling pathway was crucial for cell survival and anti-apoptotic function (Simöes et al., 2016). Erk5 was essential for survival of dopaminergic neurons and inhibition of Erk5 decreased the viability of dopamine neurons in vitro (Parmar et al., 2014). Additionally, phosphorylated Erk5 activated the Ca+ +/cAMP response element binding protein (CREB) which regulates the transcription of survival genes (Watson et al., 2001). Our study focused on the BBB protective effects of NT-3. The role of NT-3 and Erk5 in neuronal surVival needs to be explored in future studies.
In summary, we observed that endogenous NT-3 expression in whole brain sample was downregulated after SBI. Topical administration of recombinant NT-3 using gelfoam directly applied to the surgical injury site reduced brain edema, neurological deficits and BBB permeability after SBI. Recombinant NT-3 increased pErk5/Erk5 expression and improved neurological function which was reversed with the knockdown of TrkC receptor using siRNA but not with Rapl knockdown.
Overall, these results showed that NT-3 was decreased in response to SBI and topical application of recombinant NT-3 improved outcomes after SBI. The protective effect of NT-3 was possibly dependent on TrkC receptor mediated pErk5 activation. As a clinical perspective, topical application of recombinant NT-3 using a gelfoam or other novel delivery techniques may be a potential new therapeutic option particularly after neurosurgical operations.
Acknowledgements
The authors would like to acknowledge Dr. Enkhjargal Budbazar at Loma Lind University for assistance with the revised manuscript.
Funding sources
This study was partially supported by NIH grant NS084921 to JHZ.
Abbreviations:
- SBI
Surgical brain injury
- BBB
Blood brain barrier
- NT-3
Neurotrophin 3
- TrkC
Tropomyosin related kinase receptor C
- MAPK
Mitogen activated protein kinase
- Erk5
Extracellular signal-regulated kinase 5
- BDNF
Brain derived neurotrophic factor
- ICV
Intracerebroventicular
- GFAP
Glial Fibrillary Acidic Protein
- vWF
von Willebrand Factor
- NeuN
Neuronal Nuclei
- TBI
Traumatic brain injury
- GTPase
Guanine triphosphatase
Footnotes
Conflicts of interest
None.
References
- Abada HT, Golzarian J. 2007. Gelatine sponge particles: handling characteristics for endovascular use. Tech. Vasc. Interv. Radiol 10, 257–260. [DOI] [PubMed] [Google Scholar]
- Bath KG, Akins MR, Lee F.s., 2012. BDNF control of adult SVZ neurogenesis. Dev.Psychobiol 54, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M, 2014. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol 220, 3–15. [DOI] [PubMed] [Google Scholar]
- cai J, Hua F, Yuan L, Tang W, Lu J, Yu S, Wang X, Hu Y, 2014. Potential therapeutic effects of neurotrophins for acute and chronic neurological diseases. Biomed. Res. Int. 2014, 601084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals JM, Checa N, Marco S, Michels A, Perez-Navarro E, Alberch J, 1999. The neurotrophin receptors trkA, trkB and trkC are differentially regulated after excitotoxic lesion in rat striatum. Brain Res. Mol. Brain Res 69, 242–248. [DOI] [PubMed] [Google Scholar]
- Chen S, Ma Q, Krafft PR, Hu Q, Rolland II W, Sherchan P, Zhang J, Tang J, Zhang JH, 2013. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol. Dis 58, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebele RC, Schulze-Hoepfner FT, Hong J, Chlenski A, Zeitlin BD, Goel K, Gomes S, Liu Y, Abe MK, Nor JE, Lingen MW, Rosner MR, 2009. A novel interplay between Epac/Rap1 and mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) regulates thrombospondin to control angiogenesis. Blood 114, 4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, Sifringer M, Pesditschek S, Kuckuck H, Moysich A, Bittigau P, Ikonomidou C. 2002. Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol. Dis 11, 231–245. [DOI] [PubMed] [Google Scholar]
- Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M, 2008. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci. Lett 441, 261–266. [DOI] [PubMed] [Google Scholar]
- Harvey AR., Lovett SJ, Majda BT, Yoon JH, Wheeler LP, Hodgetts SI, 2015. Neurotrophic factors for spinal cord repair. which, where, how and when to apply, and for what period of time? Brain Res. 1619, 36–71. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Martin VB, Zhang L, Seroogy KB, 1999. Mild experimental brain injury differentially alters the expression of neurotrophin and neurotrophin receptor mRNAs in the hippocampus. Exp. Neurol 160, 469–478. [DOI] [PubMed] [Google Scholar]
- Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y, 2007. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rapl and ERK and neurite outgrowth. J. Cell Biol 178, 843–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J, 2008. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res. 1215, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, Kuroda J, Kamouchi M, Kitazono T, 2012. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc. Res 83, 352–359. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Zhang JH, 2008. Surgical brain injury: prevention is better than cure. Front. Biosci 13, 3793–3797. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Solaroglu I, Obenaus A, Zhang JH, 2007. Neuroprotection against surgically induced brain injury. Surg. Neurol 67, 15–20 (discussion 20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je HS, Yang F, Zhou J, Lu B. 2006. Neurotrophin 3 induces structural and functional modification of synapses through distinct molecular mechanisms. J. Cell Biol 175, 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Cooper JA, 2011. Reelin, Rapl and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci 14, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S, Krishna N, Pisklakova A, Qu X, Fenstermacher DA, Fournier M, Vrionis FD, Tran N, Chan JA, Kenchappa RS, Forsyth PA, 2015. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J. Biol. Chem 290, 3814–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, Rong LM, Liu S, Ding Y, Shen HY, Long SM, WU JL, Ling EA, zeng Y.s., 2016. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 83, 233–248. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Arsenault L, 1996. The effects of growth factors on the survival and differentiation of cultured dentate gyrus neurons. J. Neurosci 16, 1759–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlio JP, Ernfors P, Jaber M, Persson H, 1992. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience 51, 513–532. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O. Brundin P, 2005. Plateletderived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience 132, 767–776. [DOI] [PubMed] [Google Scholar]
- MudO G, Persson H, Timmusk T, Funakoshi H, Bindoni M, Belluardo N. 1993. Increased expression of trkB and trkC messenger RNAs in the rat forebrain after focal mechanical injury. Neuroscience 57, 901–912. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Nishida E, 2006. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 7, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Kusakabe M, Nishida E. 2005. Requirement of the MEK5-ERK5 pathway for neural differentiation in Xenopus embryonic development. EMBO Rep. 6, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar MS, Jaumotte JD, Wyrostek SL, Zigmond MJ, Cavanaugh JE, 2014. Role of ERKI, 2, and 5 in dopamine neuron survival during aging. Neurobiol. Aging 35, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar MS, Jaumotte JD, Zigmond MJ, Cavanaugh JE, 2015. ERKI, 2, and 5 expression and activation in dopaminergic brain regions during postnatal development. Int. J. Dev. Neurosci 46, 44–50. [DOI] [PubMed] [Google Scholar]
- Pi X, Yan C, Berk BC, 2004. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ. Res 94, 362–369. [DOI] [PubMed] [Google Scholar]
- Ren JM, Finklestein SP, 2005. Growth factor treatrnent of stroke. Curr. Drug Targets CNS Neurol. Disord 4, 121–125. [DOI] [PubMed] [Google Scholar]
- Sharma HS, 2007. A select combination of neurotrophins enhances neuroprotection and functional recovery following spinal cord injury. Ann. N. Y. Acad. Sci 1122, 95–111. [DOI] [PubMed] [Google Scholar]
- Sherchan P, Huang L, Wang Y, Akyol O, Tang J, Zhang JH, 2016. Recombinant Slit2 attenuates neuroinflammation after surgical brain injury by inhibiting peripheral immune cell infiltration via Rob01-srGAP1 pathway in a rat model. Neurobiol. Dis 85, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan P, Huang L, Akyol O, Reis C, Tang J, Zhang JH, 2017. Recombinant Slit2 reduces surgical brain injury induced blood brain barrier disruption via Rob04 dependent Racl activation in a rodent model. Sci. Rep 7, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simöes AE, Rodrigues c.M., Borralho PM, 2016. The MEK5/ERK5 signalling pathway in cancer. a promising novel therapeutic target. Drug Discov. Today 21, 1654–1663. [DOI] [PubMed] [Google Scholar]
- Sturla LM, Cowan CW, Guenther L, Castellino R.c., Kim JY, Pomeroy s.L., 2005. A novel role for extracellular signal-regulated kinase 5 and myocyte enhancer factor 2 in medulloblastoma cell death. Cancer Res. 65, 5683–5689. [DOI] [PubMed] [Google Scholar]
- Sulejczak D, Grieb P, Walski M, Frontczak-Baniewicz M. 2008. Apoptotic death of cortical neurons following surgical brain injury. Folia Neuropathol. 46, 213–219. [PubMed] [Google Scholar]
- Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH, 2010. Mechanisms of osteopontininduced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 41, 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P, 2004. Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog. Brain Res 146, 387–401. [DOI] [PubMed] [Google Scholar]
- Valentine R, Wormald PJ, Sindwani R. 2009. Advances in absorbable biomaterials and nasal packing. Otolaryngol. Clin. N. Am 42, 813–828 (ix). [DOI] [PubMed] [Google Scholar]
- Wang Y, Su B, Xia Z, 2006. Brain-derived neurotrophic factor activates ERK5 in cortical neurons via a Rapl -MEKK2 signaling cascade. J. Biol. Chem 281, 35965–35974. [DOI] [PubMed] [Google Scholar]
- Wang RM, Zhang QG, Li J, Yang L.c., Yang F, Brann D.w., 2009. The ERK5MEF2C transcription factor pathway contributes to anti-apoptotic effect of cerebral ischemia preconditioning in the hippocampal CAI region of rats. Brain Res. 1255, 32–41. [DOI] [PubMed] [Google Scholar]
- Wang W, Lu S, Li T, Pan Y.w., zou J, Abel GM, xu L, Storm DR, Xia Z, 2015. Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function. J. Neurosci 35, 7833–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Kiesse L, Lin M.z., Segal RA, 2001. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci 4, 981–988. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH, 2007. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgicallyinduced brain injury. Neurosurgery 61, 1067–1075 (discussion 1075–1066). [DOI] [PubMed] [Google Scholar]
- Yang JT, Lee TH, weng HH, Chang c.N., Chen WC, Cheng WC, wu JH, 2005. Dexamethasone enhances NT-3 expression in rat hippocampus after traumatic brain injury. Exp. Neurol 192, 437–443. [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, stork PJ, 1998. Rapl mediates sustained MAP kinase activation induced by nerve growth factor.Nature 392, 622–626. [DOI] [PubMed] [Google Scholar]
- Zhang WR, Kitagawa H, Hayashi T, Sasaki C, Sakai K, Warita H, Shiro Y, Suenaga H, Ohmae H, Tsuji S, Itoh T, Nishimura O, Nagasaki H, Abe K. 1999. Topical application of neurotrophin-3 attenuates ischemic brain injury after transient middle cerebral artery occlusion in rats. Brain Res. 842, 211–214. [DOI] [PubMed] [Google Scholar]