Abstract
Background
Exercise training may affect the blood levels of brain-derived neurotrophic factor (BDNF), but meta-analyses have not yet been performed comparing pre- and post-intervention BDNF concentrations in patients with multiple sclerosis (PwMS).
Objective
To perform a meta-analysis to study the influence of exercise on BDNF levels and define components that modulate them across clinical trials of exercise training in adults living with multiple sclerosis (MS).
Method
Five databases (PubMed, EMBASE, Cochrane Library, PEDro database, CINAHL) were searched up to June 2021. According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, we included 13 articles in the meta-analysis, including 271 subjects. To investigate sources of heterogeneity, subgroup analysis, meta-regression, and sensitivity analysis were conducted. We performed the meta-analysis to compare pre- and post-exercise peripheral levels of BDNF in PwMS.
Results
Post-exercise concentrations of serum BDNF were significantly higher than pre-intervention levels (Standardized Mean Difference (SMD): 0.33, 95% CI: [0.04; 0.61], p-value = 0.02). Meta-regression indicated that the quality of the included studies based on the PEDro assessment tool might be a source of heterogeneity, while no significant effect was found for chronological age and disease severity according to the expanded disability status scale.
Conclusion
This systematic review and meta-analysis shows that physical activity increases peripheral levels of BDNF in PwMS. More research on the effect of different modes of exercise on BDNF levels in PwMS is warranted.
1. Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, which plays an essential role in neuroregeneration and neuroprotection [1]. More specifically, BDNF participates in various fundamental domains as follows: (1) neuronal and oligodendroglial survival and growth, (2) neurotransmitter modulation, and (3) neural plasticity. BDNF modulates several brain functions, such as memory and learning, by having a major role in the development of brain circuits [2]. Besides the central nervous system, BDNF has been found in different organs, such as the brain, lungs, heart, spleen, gastrointestinal tract, and liver. Actually, several cell types, including neurons, glia cells, fibroblasts, vascular smooth muscle cells, and thymic stroma release BDNF [2].
Reports on the alterations of the BDNF levels in patients with multiple sclerosis (PwMS) are controversial, but overall, BDNF is usually increased during relapse, with normal levels during remission phases [3–5]. When the demyelination process is actively evolving, BDNF levels tend to increase, probably as a compensatory mechanism against neuronal and glial damage [6]. In addition to microglia, astrocytes and neurons, central nervous system (CNS) infiltrating Immune cells (e.g., T cells and phagocyte cells) can secrete BDNF in demyelinating MS lesions [6].
Supervised exercise or physical activity has been a promising nonpharmacological therapeutic approach for PwMS to help them with their functional capacity and mental health [7]. A systematic review of the literature showed that five out of eight studies demonstrated positive impact of exercise on cognitive status of PwMS [8]. Furthermore, several studies have reported a lower annual relapse rate in PwMS who do exercise compared to patients without regular physical practice [9]. Conversely, several studies have shown significant effects of exercise on increasing peripheral BDNF concentrations in healthy populations in parallel with improved quality of life and well-being [10–13], which indicates a potential neuroprotective effect for exercise. Furthermore, several studies have reported a lower annual relapse rate in MS patients who underwent exercise compared to patients without regular physical practice [9].
Considering that axonal loss and cerebral atrophy occur in MS, exercise prescription could promote neuroprotection, neuroregeneration, and neuroplasticity and reduce long-term disability by increasing BDNF levels. In this context, exercise-induced BDNF increase has been regarded as one of the underlying mechanisms supporting the positive effects of exercise in PwMS [14]. Physical activity increases DNA demethylation in the BDNF promoter region, resulting in higher production of the neurogenesis-promoting signaling molecule [15]. Increased TrkB receptor (a BDNF receptor in the astrocytes) sensitivity may be linked with increased BDNF sensitivity, necessitating the reduction of BDNF synthesis and release following exercise [16]. Notably, prolonged physical activity raised BDNF mRNA levels in the hippocampal dentate gyrus, which was associated with substantial changes in the amounts of TrkB [17].
As exercise training is becoming a vital component in the therapeutic toolbox of MS patients, to date, international guidelines are available [18]. Rehabilitation groups, e.g., International Progressive MS Alliance, have suggested that future research should focus on progressive MS rehabilitation and investigate the effect of exercise on the pattern of disease progression in these patients [19]. It is worth mentioning that pioneers in the field developed a framework to strengthen the standardization, quality and scope of MS rehabilitation studies that would help PwMS with their quality of life (MoXFo initiative) [20].
Herein, we aimed to conduct an up-to-date meta-analysis to determine whether exercise training significantly affects peripheral BDNF concentrations in PwMS. We also discuss the association of these markers with chronological age, EDSS score, and the quality of the existing literature.
2. Materials and methods
This review was registered (#CRD42021256621) in the International Prospective Register of Systematic Reviews (PROSPERO). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [21] were followed for this meta-analysis.
2.1. Search strategy
We performed an online search in PubMed, EMBASE, PEDro database, CINAHL, and Cochrane Central Register of Controlled Trials (Cochrane Library) databases up to June 1st, 2021, to detect original studies with focus on BDNF changes after exercise in MS patients. Medical Subject Headings (MeSH) and Emtree were used to retrieve PubMed and Embase results, respectively. Our search strategy is presented in the supplementary material. In addition, reference lists of retrieved studies were searched for additional relevant reports.
2.2. Selection criteria
Studies were included if (1) they were peer-reviewed clinical trial articles, (2) BDNF blood levels were measured quantitatively using enzyme-linked immunoassays (ELISA) or other assays, (3) BDNF measured before and after an exercise intervention, and (4) the exact values of the BDNF marker were either given within the manuscript or provided by the authors of the original study for performing the meta-analysis. Exclusion criteria were as follows: (1) pediatric MS, (2) case reports, case series, letters, commentaries, abstracts, protocols, review articles, and animal and in vitro studies. Two authors (P.S and A.K) independently performed the screening and eligibility assessment. In case of discrepancy, the two authors discussed and consulted with the third author (S.M) and resolved the conflict.
2.3. Data extraction
Two reviewers independently extracted (1) bibliographic information (study title, year of publication, first author, study type, and country), (2) demographic and clinical features of the sample (number of patients and controls, age, sex, disease duration, mean expanded disability status scale [EDSS] score), (3) methodological details (diagnostic criteria, characteristics of the ELISA or other assay), and (4) levels of the BDNF before and after the intervention. We communicated with the studies’ corresponding authors for additional information if the absolute values of the levels of BDNF were not given in the published manuscript. The inter-rater reliability between reviewers was calculated using the kappa coefficient [22].
2.4. Quality assessment
The methodological quality of the included studies was assessed by two reviewers (P.S. and S.M.) independently, based on the PEDro scale [23]. PEDro is a reliable and valid checklist consisting of 11 items as follows: (1) eligibility criteria, (2) random allocation, (3) concealed allocation, (4) baseline comparability, (5) masked participants, (6) masked therapists, (7) masked assessors, (8) adequate follow up, (9) intention to treat analysis, (10) between-group comparison, and (11) point estimates and variability [23–25]. Note that the eligibility criteria item does not contribute to the total score. Thus, each study gains a score from 0 to 10. We categorized studies based on their PEDro score; below 4 as “poor” quality, a score between 4 and 5 indicating “fair” quality, a score of 6 to 8 considered to be of “good” quality, and a score of 9 to 10 indicating “excellent” quality [26]. Any disagreements were resolved by discussion between two reviewers.
2.5. Statistical methods
We estimated a standardized mean difference (SMD) (Hedges’ g) and 95% confidence interval (CI) for each between-group comparison as the included studies were conducted in a 16-year period and were susceptible to having different ELISA assays. The SMD of ≤0.2, 0.2–0.8, and ≥0.8 represented small, moderate, and large effect sizes, respectively. Meta-analyses were performed for comparisons for which results from at least three individual datasets were available.
If the values reported in the manuscript were given as a median and interquartile range (IQR) or median and range, and we were not able to retrieve the mean ± standard deviation (SD) from the authors, we used statistical methods suggested by Luo et al. [27] and Wan et al. [28] to convert these values.
To assess heterogeneity between studies in the between-group meta-analyses, we used Cochrane’s Q-test and the I2-index. The I2-indices of ≤25%, 26–75%, and 75%≤ represented low, moderate, and high heterogeneity degrees, respectively [29]. We utilized random effect models according to the DerSimonian and Laird method [30]. Random-effects models are preferred if significant heterogeneity is expected, as they account for variable underlying effects in estimates of uncertainty, including both within- and between-study variance. We visualized the results of the meta-analysis as forest plots and the drapery plot. The drapery plot is a supporting figure to forest plot and was proposed to demonstrate confidence intervals assuming a fixed significance threshold and prevent researchers from exclusively relying on the p-value < 0.05 significance threshold [31,32].
To further assess the causes of heterogeneity, we conducted a sensitivity analysis to identify influential cases for meta-analyses with significant heterogeneity and including ten or more studies. Each time we omitted one study and recalculated the effect size (Leave-One-Out Analyses). To reduce the heterogeneity among individual studies, we conducted a subgroup analysis based on the type of intervention used in each study.
Publication bias was initially assessed by visual observation of the degree of funnel plot asymmetry. Then, we used Egger’s bias test [33] and Begg-Mazumdar Kendall’s [34] to objectively confirm the visual perception from the funnel plot. A p-value < 0.1 was considered as evidence of publication bias. Funnel plots and Egger’s plots are available. When there was evidence of publication bias, we adjusted the effect sizes using the trim-and-fill method [35].
All computations and visualizations were carried out using R version 4.0.4 (R Core Team [2020]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria), and STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) for metaregression and Egger’s plots. We used following packages: “meta” (version 4.17–0), “metafor” (version 2.4–0), “dmetar” (version 0.0–9), and “tidyverse” (version 1.3.0). All forest plots and the drapery plot were designed using R. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Selection of studies
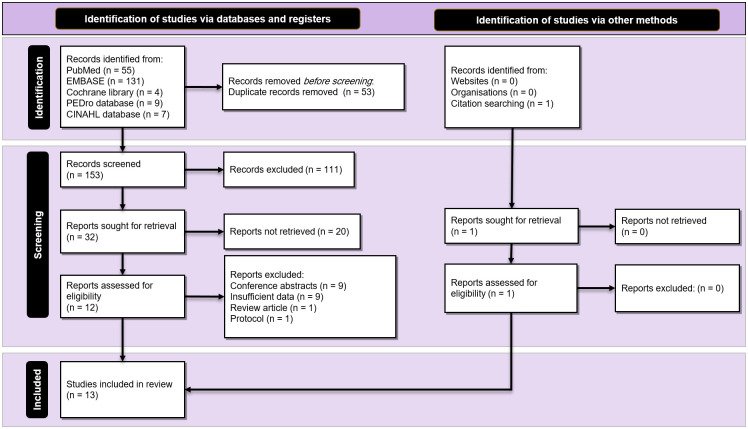
The search strategy retrieved a total yield of 199 studies. After the removal of duplicates, 153 studies remained. Title/abstracts screening identified 32 potentially eligible studies, and 13 original clinical trials met the criteria to be included in the meta-analysis [36–48]. No further studies that were appropriate for inclusion were identified via hand searching and checking references. Fig 1 illustrates the process of study selection according to the PRISMA guideline.
Fig 1. PRISMA diagram.
Study selection process according to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guideline.
The agreement between the two independent reviewers for study selection was excellent for both titles/abstracts (kappa = 1.00; percentage agreement = 99.98%) and full-text (kappa = 1.00; percentage agreement = 100%).
3.2. Study characteristics
Studies were published between 2004 and 2021, and a total of 271 patients with MS participated in the 13 studies included in the current analysis [36–39,41–48]. Outcome measures in these studies were serum BDNF [36–42,44–48], plasma BDNF [43], IL-6 [36,37,46–48], nerve growth factor (NGF) [36,37,40], matrix metalloproteinase-2,-9 (MMP-2, MMP-9) [42], neurotrophin-3,-4 (NT-3, NT-4) [45], glial cell line-derived neurotrophic factor (GDNF) and ciliary neurotrophic factor (CNTF) [45], insulin-like Growth Factor (IGF) [44], sphingosine-1-phosphate (S1P) [43], suppressor of cytokine signaling-1,-3 (SOCS1, SOCS3) [41], tumor necrosis factor α (TNF- α) [37], vitamin D-binding protein (VDBP) [40], and serotonin [42]. Given the type of exercise in each study, we categorized them into three main groups; (1) six studies used aerobic programs [36,37,42,46–48], (2) five studies applied combined training [38,40,41,44,45], and (3) two studies stated anaerobic training [39,43]. In addition, the frequency of exercise of included studies varied from two to three sessions per week, and intervention varied from 6 to 60 min per session over a period of three to 24 weeks.
All but two studies measured BDNF through enzyme-linked immunosorbent assay (ELISA). Bansi et al. [37] and Wens et al. [38] used flow cytometry and Meso Scale, respectively, for BDNF assessment. The characteristics of included studies are detailed in Table 1.
Table 1. Baseline and exercise intervention characteristics of included studies.
| Study ID | Patients | Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Country | Exercise protocol | Session’s settings | Outcome measures | BDNF measurement protocol | N (Type of MS) | Diagnostic criteria | Age (mean ± SD), years | EDSS Score (mean ± SD) | Disease duration (mean ± SD), years | Female/Male | Pre-exercise BDNF levels (mean ± SD), pg/ml | Post-exercise BDNF levels (mean ± SD), pg/ml |
| Schulz et al., 2004 | Germany | An 8-week (2x/week) bicycle ergometry training program tailored to their individual capabilities (aerobic training) | 30 min at 75% of maximum power in watts | BDNF, IL-6, sIL-6R, NGF | ELISA (Promega, Madison, WI, USA) Serum | 13 | Poser Criteria | 39 ± 9 | 2 ± 1.4 | NA | 9/4 | 4353 ± 3217 | 5930 ± 5178 |
| Bansi et al., 2013 | Switzerland | A 3-week (2x/week) cardiopulmonary exercise test (aerobic training) | 3 minutes at rest (no pedalling) on the cycle ergometer; 3 minutes of unloaded pedalling as a warm up; testing phase until the participant reached a symptom limited maximum | BDNF, IL-6, sIL-6R, NGF, TNF-α | Flow cytometry (Zug, Switzerland) Serum | 52 | revised McDonald criteria | 50.84 ± 7.84 | 4.65 ± 0.93 | NA | 35/18 | 17154.2615 ± 8431.0554 | 18815.7538 ± 7956.1332 |
| Wens et al., 2016 | Belgium | A 24-week (five sessions per 2 weeks) progressive combined training program (Combined training) | Continuous aerobic exercise, 1x6min (start)- 3x10min (final); intensity 12–14 RPE + 35 min resistance exercise; volume: 1x 10 rep—4x15 rep; intensity 12–14 RPE. | BDNF | Meso Scale (Discovery, Rockville, MD, USA) Serum | 15 (RRMS) | McDonald criteria | 42 ± 3 | 2.7 ± 0.3 | NA | 9/6 | 11092 ± 1005 | 12020 ± 942 |
| Ozkul et al., 2018 | Turkey | An 8-week (2x/week) combined exercise training consisting of aerobic training and Pilates training (Combined training) | 25 min aerobic exercise; intensity 60–70% HRmax (week 0–4), 70–80% HRmax (week 5–8). + 50 min resistance exercise with elastic bands; gradual intensity; 10 reps (week 0–4), 20 reps (week 5–8). | BDNF, SOCS1, SOCS3 | ELISA (Shanghai Sunred Biological technology, Shanghai, China) Serum | 18 (RRMS) | McDonald criteria | 34.59 ± 13.88 | 1.36 ± 1.01 | 6.17 ± 6.84 | 14/4 | 1663.2309 ± 2480.1192 | 1947.6375 ± 2553.4374 |
| Eftekhari et al., 2018 | Iran | An 8-week Mat Pilates training | 40–50 min of pilates training; Sets: 1–2 Reps: 3–10; Rest: 60 sec between sets. | BDNF, IL-10 | ELISA (Boster Biological Technology Ltd) Serum | 13 | McDonald criteria | 34.46 ± 7.29 | NA | NA | NA | 10678850 ± 2260170 | 11550140 ± 2619600 |
| Khademosharie et al., 2018 | Iran | A 12 week (3x/week) (combined training) | (2 resistance/1 aerobic) Resistance: Sets: 2–4, Intensity: 60–80% RM; Reps: 8–14; Rest between series: 2–3 min Endurance: 2–4 sets with 4–13 reps at 40–55% HRR; Rest between sets (3–4 min). | BDNF, NGF, VDBP | ELISA (Boster Biollogical Technology, Pleasanton, CA, USA) Serum | 24 (PPMS/SPMS) | revised McDonald criteria | 36.76 ± 6.87 | 3.1 ± 0.5 | NA | 24 | 4223 ± 2084 | 4707 ± 1918 |
| Zimmer et al., 2018 | Germany | A 3-week cardiopulmonary exercise test (aerobic training) | 30 minutes of training, with warm up and cool-down for the first and last 2 minutes. | BDNF, Serotonin, MMP-2, MMP-9 | ELISA (R&D Systems, Inc., Minneapolis, MN, USA) Serum | 27 (14 RRMS, 13 SPMS) | revised McDonald criteria | 51 ± 9.9 | 4.37 ± 1.39 | 11.98 ± 11.34 | 20/7 | 7526.9 ± 10606 | 24663 ± 13019 |
| Jørgensen et al., 2019 | Denmark | A 24-week (2x/week) resistance training (resistance training) | Sets: 3–5, Intensity: 10 repetitions at 15-RM and 6 repetitions at 6-RM; Rest between sets: 2–3 min; Lower limbs: 4 series of 10 repetitions at 10-RM | BDNF, S1P | ELISA (Promega, Madison, WI, USA) Plasma | 16 (RRMS) | NA | 45.09 ± 8.94 | 3 ± 0.81 | NA | 12/4 | 168838.1 ± 154842.5 | 153309.9 ± 109674 |
| Abbaspoor et al., 2020 | Iran | An 8-week (3x/week) aerobic and resistance training (Combined training) | 15–20 min continuous aerobic exercise, intensity at 55–70% HRmáx + 35 min resistance exercise; volume: 1 set (week 1–4)—2 sets (week 5–8); intensity 10–13 (week 1–4), 13–16 (week 5–8) RPE. | BDNF, IGF-1 | ELISA (Shanghai crystal day biotech, China) Serum | 10 | NA | 33.5 ± 6.37 | 3.06 ± 1.2 | 10.25 ± 3.37 | NA | 1960 ± 650 | 1790 ± 550 |
| Devasahayam et al., 2020 | Canada | A 10-week (3x/week) Bodyweight supported treadmill (BWST) (aerobic training) | 40 min BWST, including 5 min of warm-up and cool-down. | BDNF, IL-6 | ELISA (R&D Systems, Inc., Minneapolis, MN, USA) Serum | 10 (3 PPMS, 7 SPMS) | NA | 53.2 ± 15.6 | NA | NA | 9/1 | 67620 ± 20430 | 63460 ± 19970 |
| Banitalebi et al., 2020 | Iran | A 12-week (3x/week) (Combined training) | (IG1, IG2, IG3) 1º Resistance exercise: 3 sets of 12 reps at 40–70% RM. 2º Aerobic exercise: Cycling or running. 20 min at 50–70% HRmax 3º Balance training 4º Stretching exercises | BDNF, GDNF, CNTF, NT-3, NT-4 | ELISA Serum | 45 (RRMS) | NA | 40.83 ± 8.17 | NA | NA | NA | 1822700 ± 925700 | 2552506.7 ± 1121929.5 |
| Devasahayam et al., 2021 | Canada | Graded exercise training (GXT) (aerobic training) | 80 steps per minute during GXT and the workload was increased in ~20-watt increments every 2 min, starting from load level 3 (21 watts) until exhaustion | BDNF, IL-6 | ELISA (R&D Systems, Inc., Minneapolis, MN, USA) Serum | 14 (3 PPMS, 11 SPMS) | McDonald criteria | 54.07 ± 8.46 | NA | NA | 10/4 | 56560 ± 25120 | 56470 ± 44110 |
| Savšek et al., 2021 | Slovenia | A 12-weeks (2x/week) aerobics (aerobic training) | 60 min, consisting of a 6–10 min warm-up, 30–40 min performed at prescribed intensity, and a 6–10 min cool-down | BDNF, IL-6 | ELISA (R&D Systems, Inc., Minneapolis, MN, USA) Serum | 14 (RRMS) | NA | 39.7 ± 6.7 | 2.94 ± 1.61 | 8.4 ± 6.1 | 11/3 | 1923.52 ± 1260.9 | 2011.94 ± 672.48 |
Abbreviations: EDSS: The Expanded Disability Status Scale, BDNF: Brain-derived neurotrophic factor, IL-6: Interleukin-6, IL-10: Interleukin-10, sIL-6R: Soluble IL-6, NGF: Nerve growth factor, TNF- α: Tumor necrosis factor α, SOCS: Suppressor of cytokine signaling, MMP: Matrix metalloproteinase, S1P: Sphingosine-1-phosphate, IGF: Insulin-like Growth Factor, GDNF: Glial cell line-derived neurotrophic factor, CNTF: Ciliary neurotrophic factor, NT: Neurotrophin, RRMS: Relapsing-remitting multiple sclerosis, SPMS: Secondary-progressive multiple sclerosis, PPMS: Primary-progressive multiple sclerosis, VDBP: Vitamin D-binding protein.
3.3. Participants’ characteristics
The combined mean ± SD of the age of participants was 43.72 ± 8.45 years for 271 reported patients. The cumulative number of female and male participants was 153 and 51, respectively, reported by ten studies [36–38,40–43,46–48]. Nine studies reported the EDSS score of the patients [36–38,40–44,48], and the combined EDSS mean ± SD was 3.40 ± 1.5 for 189 reported patients. The combined mean ± SD of the disease duration time of the participants was 9.5 ± 8.7 years, reported by four studies [41,42,44,48]. Not all studies reported the type of MS, but there were 122 RRMS [38,41–43,45,48], 31 SPMS (secondary-progressive multiple sclerosis) [42,46,47], and 6 PPMS (primary-progressive multiple sclerosis) [46,47] patients. The study by Khademosharie et al. [40] did not indicate the number of patients in each MS group (SPMS or PPMS).
3.4. Quality assessment
The median total PEDro score was 6 (IQR = 2.5; mean ± SD = 6.3 ± 1.3; range: 4 to 9) of 10, which indicated an overall good quality of the included studies (Table 2). All studies met the following criteria: baseline comparability, between-group statistical comparison. None of the included studies met the criteria of clinician rater and participant blinding. The agreement between the two reviewers for assessing study quality was excellent (kappa = 1.00; percentage agreement = 100%). Due to the nature of the interventions, in none of the included studies were the participants or therapists blinded.
Table 2. Result of quality assessment of the included studies in the meta-analysis according to the PEDro scale.
| Author, Year | Schulz et al., 2004 | Bansi et al., 2013 | Wens et al., 2016 | Ozkul et al., 2018 | Eftekhari et al., 2018 | Khademosharie et al., 2018 | Zimmer et al., 2018 | Jørgensen et al., 2019 | Abbaspour et al., 2020 | Devasahayam et al., 2020 | Banitalebi et al., 2020 | Devasahayam et al., 2021 | Savšek et al., 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEDro Items | |||||||||||||
| Eligibility criteria | * | * | * | * | * | * | * | * | * | * | * | ||
| Random allocation | * | * | * | * | * | * | * | * | * | * | |||
| Concealed allocation | * | * | * | * | |||||||||
| Baseline comparability | * | * | * | * | * | * | * | * | * | * | * | * | |
| Masked participants | |||||||||||||
| Masked therapists | |||||||||||||
| Masked assessors | * | * | * | * | * | * | |||||||
| Adequate follow-up | * | * | * | * | * | * | * | * | * | * | * | * | |
| Intention to treat analysis | * | * | |||||||||||
| Between-group statistical comparison | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Point estimates and variability | * | * | * | * | * | * | * | * | * | * | * | * | |
| score | 5 | 8 | 6 | 6 | 6 | 4 | 9 | 7 | 6 | 5 | 7 | 5 | 8 |
Astriks* means Yes. Blank means No.
3.5. Comparison of pre- and post-intervention BDNF concentration
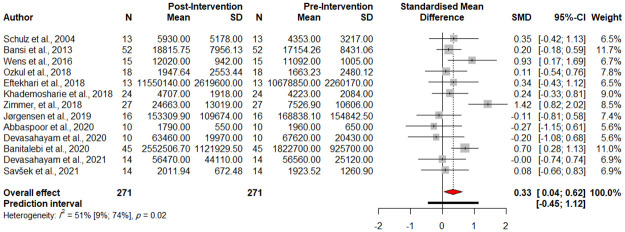
Baseline serum levels of BDNF were significantly higher after exercise intervention (SMD 0.3309, 95% CI [0.0434; 0.6148], p-value = 0.0275, test of heterogeneity: I2 = 51.5%, p-value = 0.0161, Fig 2). A drapery plot is shown to visualize the meta-analysis results based on p-value functions of each study (p-value on the y-axis and the effect size on the x-axis) (S1 Fig).
Fig 2. Forest plot of meta-analysis of pre- and post-intervention levels of BDNF.
The Egger’s test (p-value = 0.38) (S2 Fig), the Begg’s test (p-value = 0.36), and funnel plots (S3 Fig) showed no evidence of publication bias. The Eggers’ test does not indicate the presence of substantial funnel plot asymmetry. A Q value of 24.74 and an I2 index of 51.5% indicate significant heterogeneity and inconsistency, respectively, among included studies.
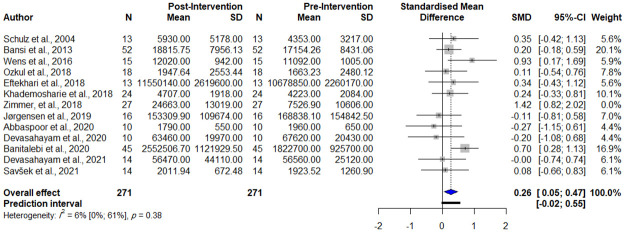
Using the ‘find.outliers’ command in R software, the study by Zimmer et al. [42] was detected to be an outlier; thus, we repeated the meta-analysis and the result is as follows: (SMD 0.26, 95% CI [0.05; 0.47], p-value = 0.0189, test of heterogeneity: I2 = 6.4%, Q = 11.75, p-value = 0.4, Fig 3).
Fig 3. Forest plot of meta-analysis of pre- and post-intervention levels of BDNF removing outliers.
Sensitivity analysis (leave-one-out analysis) showed that the effect size remained significant after omitting each study, and the heterogeneity did significantly reduce (S4–S6 Figs).
Additionally, to find the sources of heterogeneity, metaregression was conducted. The heterogeneity between studies could partially be explained by the PEDro score (R2 = 15.32%). No correlation was found between the effect size and the mean age of the EDSS score. The results of metaregression analysis are shown as bubble plots (Fig 4).
Fig 4. Results of meta-regression.
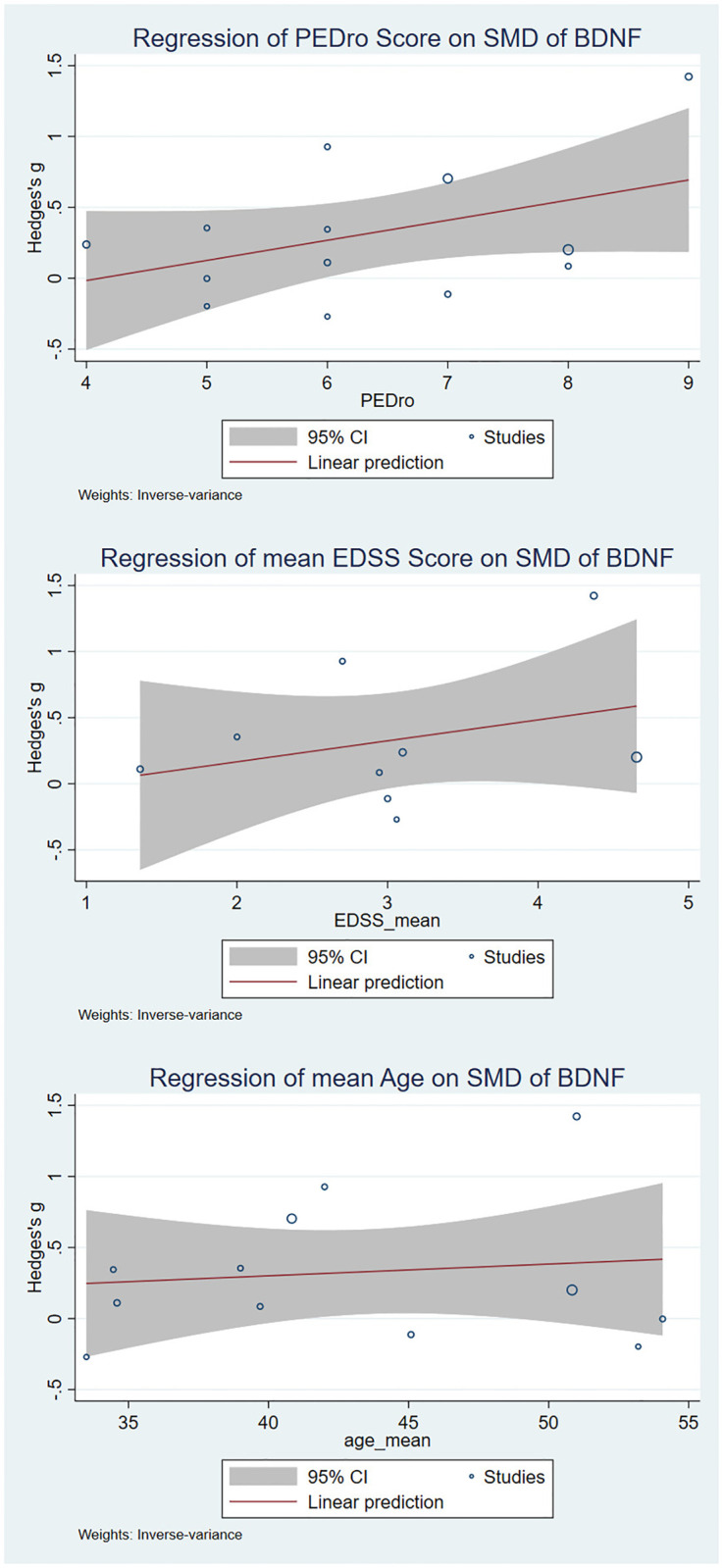
Effects of age, PEDro score, and mean expanded disability status scale (EDSS) score on the effect size of the comparisons of pre-and post-intervention levels of BDNF were assessed wherever for 10 or more original studies data was available.
3.6. Aerobic exercise vs. combined exercise
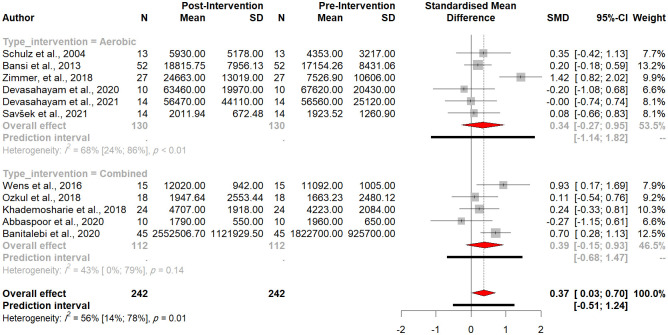
Subgroup analysis revealed no significant difference between aerobic and combined exercise subgroups (Q = 0.03, p-value = 0.86) (Fig 5).
Fig 5. Forest plot of the subgroup analysis (type of intervention).
3.7. Duration of exercise intervention
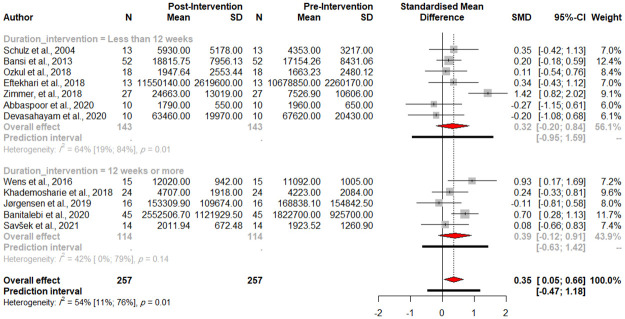
We performed a subgroup analysis to assess the effect of the duration of physical exercise on BDNF levels. Out of 12 studies that reported the duration of their intervention, five scheduled the program for 12 weeks or more, and seven conducted the program for less than 12 weeks. The subgroup analysis did not show a significant difference between the two abovementioned groups (Between groups Q = 0.07, p-value = 0.7914, Fig 6). The results for each group are as follows: Less than 12 weeks: (n = 7, SMD 0.3193, 95%CI [-0.2039; 0.8425] Q = 16.67, I2 = 64.0%), 12 weeks or more: (n = 5, SMD 0.3941, 95%CI [-0.1200; 0.9083], Q = 6.86, I2 = 41.7%).
Fig 6. Forest plot of the subgroup analysis (duration of intervention).
4. Discussion
The main finding of our study is that physical exercise significantly increases baseline serum levels of BDNF in PwMS. Subgroup analysis did not reveal any significant difference due to the type of exercise and the duration of the exercise training program.
Our meta-regression suggested that the existing heterogeneity in the meta-analysis results was not significantly related to sex or chronological age. It is plausible that variations in the quality of included studies could partially explain the existed heterogeneity in the meta-analysis results. Variations in the BDNF measurement procedures and techniques (as pointed out in the study by Hirsch et al. [49]), protocol and settings of physical activity programs, and the ratio of included participants with RRMS or progressive MS in the included investigations might be other contributors to the heterogeneity in the meta-analysis results. We were not able to evaluate the association between exercise program intensity and session time and changes in baseline peripheral BDNF concentrations. As all studies reported serum concentrations of BDNF, it is plausible that resting peripheral BDNF concentration is not a source of heterogeneity in this analysis.
Several pieces of evidence suggest that physical activity contributes to CNS functioning through multiple mechanisms [50–52], including (1) increasing cerebral blood flow, (2) endocannabinoid and neurotransmitter modulation, (3) alterations in neuroendocrine responses, and (4) structural changes in the CNS [50–52]. Given that exercise increases BDNF levels, this has been regarded as one of the potential mechanisms by which exercise affects brain health and functioning. BDNF improves synaptic potentiation, synaptic plasticity, and neuronal activity, also modifying axodendritic morphology [7,53,54]. In addition, BDNF enhances quantal neurotransmitter release by influencing presynaptic terminals, which potentiates synaptic transmission [55].
Exercise-induced increase of BDNF levels can contribute to plastic changes following physical activity [7,56]. Although no previous study has simultaneously investigated BDNF and neuroimaging changes after exercise in PwMS, several studies have demonstrated the effect of exercise in brain structure. Prakash et al. [57] used a cross-sectional design to demonstrate that aerobic exercise affects both grey matter volume and white matter integrity in PwMS. Klaren et al. [58] reported that the volume of several areas of the brain, including the hippocampus, thalamus, caudate, putamen, and pallidum, relates to the level of moderate/vigorous physical activity. Kjølhede et al. [59] showed that the trend of reduced brain atrophy is nonsignificant in PwMS who attended a six months (2 days/week) resistance training program.
Multiple central and peripheral factors, influence the levels of BDNF [10]. In MS, treatment [60,61], sex [62], age [63], body mass index (BMI) [64], and disease status, as assessed by EDSS [65,66], have been associated with the peripheral levels of BDNF. Our results confirm that physical activity can also influence BDNF levels in PwMS. As the increase in serum levels of BDNF may reflect both peripheral and CNS changes [2], it remains to be determined the main source and/or target of these enhanced BDNF levels.
A previous meta-analysis of studies measuring BDNF after exercise in healthy adults revealed that aerobic training can increase BDNF concentrations more than resistance training [12]. Moreover, the duration of exercise was highly associated with BDNF increase [10]. In contrast, our subgroup meta-analysis considering the type of intervention (aerobic vs. resistance training) and duration of exercise (less than 12 weeks vs. 12 weeks or more) showed no significant difference in test for between-groups differences (random-effects model).
Frequency, intensity, duration, and mode of exercise are essential variables in the context of achieving positive rehabilitation outcomes for adults with MS [67,68]. Our subgroup meta-analysis considering the type of intervention (aerobic vs. resistance training) and duration of exercise (less than 12 weeks vs. 12 weeks or more) showed no significant difference in test for between-groups differences (random-effects model). Recent interest in High Intensity Interval Training suggests that alternative exercise modalities may also offer promise in inducing BDNF related neuroplasticity in PwMS [69,70].
Collectively, reported data on the benefits of physical therapy and exercise on cognitive functions, including memory, learning, information processing, attention, and concentration, is noteworthy [71–75]. Numerous research have been conducted to date on the function of BDNF in the acute exercise—cognition connection [76–82]. Although all these studies corroborate the positive effects of acute exercise on cognitive performance, discrepancies exist in the proof that BDNF is responsible for these cognitive advantages [83]. Considering the previously-mentioned studies, exercise-induced alterations in BDNF and its correlation with cognitive performance were specifically tested by Ferris et al. [76], Lee et al. [79], Skriver et al. [80], Tsai et al. [82], and Winter et al. [78]. Additionally, substantial correlations between acute exercise-induced modifications in BDNF concentration and cognitive function have been reported in research measuring memory, but not in studies investigating non-memory aspects of cognition [83]. Regarding the beneficial effects of aerobic exercise, a case study by Leavitt et al. [84] reported a 16.5% increase in hippocampal volume following a 53% improvement in their memory after a 12-week aerobic exercise (30 min, 3 days/week). A systematic review addressing studies on MS and cognition exhibited that out of eight included studies, five studies showed that exercise positively impacts patients’ cognitive status [8]. Additionally, BDNF Val66Met polymorphism has shown protective roles against cognitive impairment in PwMS [85]. Moreover, evidence indicates the neurotrophic role of BDNF on motor-related neurons, which may relieve motor symptoms via modulating neuronal morphology and motility [86].
There is some evidence suggesting that exercise may reduce MS progression. Regarding this association, intense exercise has shown reductions in MS development, excluding known factors and determinants of MS progression [87]. Still, long-term follow-up studies are needed to confirm these findings. In addition, based on pieces of evidence given in a systematic review, physical exercise training may reduce the risk of relapse in PwMS by 27% in the training group versus the control group [9]. However, a lack of papers with standard methodologies assessing the association of exercise training and MS progression exists in the current literature.
Notably, for other neurodegenerative disorders, including Parkinson’s disease (PD) and Alzheimer’s disease (AD), exercise-induced BDNF has been proposed as a protective factor. For example, a meta-analysis of two randomized controlled trials and four pre-experimental studies with a total of 100 patients with PD undergoing physical exercise showed a significant increase in BDNF blood levels in parallel with improvement of motor symptoms (e.g., improvement in Unified Parkinson’s disease rating scale-Part III (MDS-UPDRS-III)) [49]. Another meta-analysis reporting the effects of exercise on neurotrophic factors in cognitively impaired individuals diagnosed with dementia or mild cognitive impairment (MCI) demonstrated positive and significant effects of exercise resulting in higher inflammatory and neurotrophic biomarkers in MCI patients [88].
Future studies should study the association between exercise, neuroplasticity markers and functional outcomes to determine whether the observed neurotrophic effects translate to clinical benefits. Ultimately, based on research findings, exercise may be an invaluable adjunct component to the existing medical treatments. Of note, more human studies are needed to underpin this relationship, as we cannot only rely on animal studies because of humans and animals’ structural and functional brain differences.
The main limitation of this current meta-analysis is that the extant studies on exercise in PwMS involved relatively small number of subjects. Other limitations include short-term interventions (<26 weeks), low levels of disability of most participants (EDSS scores <4), and the inclusion of mainly relapsing-remitting MS or mixed-group patient populations and exclusion of patients with comorbidities that could their relief assessed due to exercise training. Moreover, we only included the intervention group of the included studies and analyzed the pre- and post-intervention levels of BDNF in PwMS who underwent exercise, as another meta-analysis conducted by Ruiz-González et al. [89] compared post-intervention levels of BDNF in both PwMS and controls. It is worth emphasizing that Mackay et al. [90] intended to assess the impact of aerobic exercise on BDNF levels in persons with neurological disorders without segregating neurologic conditions (e.g., MS). Although they performed the meta-analysis considering all of the neurologic disorders, their results align with the current study and indicate that exercise might contribute to increased BDNF concentrations.
5. Conclusion
This systematic review and meta-analysis show that physical activity increases peripheral levels of BDNF in PwMS. Future studies involving more subjects across different forms of the disease undergoing well-designed physical interventions are warranted. These studies should also incorporate multiple measures of BDNF alongside neuroimaging outcomes. These initiatives will ultimately strengthen the quality and scope of the evidence on MS rehabilitation [20].
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We sincerely thank the authors of the included articles in this review due to sharing the relevant data.
Abbreviations
- AD
Alzheimer’s disease
- BDNF
brain-derived neurotrophic factor
- CI
confidence interval
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- EDSS
expanded disability status scale
- ELISA
enzyme-linked immunoassay
- GDNF
glial cell line-derived neurotrophic factor
- IGF
insulin-like Growth Factor
- IQR
interquartile range
- MCI
mild cognitive impairment
- MMP-2, -9
matrix metalloproteinase-2, -9
- NGF
nerve growth factor
- NT-3, -4
neurotrophin-3, -4
- PD
Parkinson’s disease
- PPMS
primary-progressive multiple sclerosis
- PwMS
patients with multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- S1P
sphingosine-1-phosphate
- SD
standard deviation
- SMD
standardized mean difference
- SOCS1,3
suppressor of cytokine signaling-1, -3
- SPMS
secondary-progressive multiple sclerosis
- TNF-α
tumor necrosis factor α
- VDBP
vitamin D-binding protein
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Brigadski T, Leßmann V. The physiology of regulated BDNF release. Cell and Tissue Research. 2020:1–31. doi: 10.1007/s00441-020-03253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Archives of medical science: AMS. 2015;11(6):1164. doi: 10.5114/aoms.2015.56342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. Journal of neuroimmunology. 2005;167(1–2):215–8. doi: 10.1016/j.jneuroim.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Caggiula M, Batocchi A, Frisullo G, Angelucci F, Patanella A, Sancricca C, et al. Neurotrophic factors and clinical recovery in relapsing-remitting multiple sclerosis. Scandinavian journal of immunology. 2005;62(2):176–82. doi: 10.1111/j.1365-3083.2005.01649.x [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Dreyfus CF. The role of growth factors as a therapeutic approach to demyelinating disease. Experimental neurology. 2016;283:531–40. doi: 10.1016/j.expneurol.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nociti V. What is the role of Brain derived neurotrophic factor in Multiple Sclerosis neuroinflammation? Neuroimmunology and Neuroinflammation. 2020;7(3):291–9. [Google Scholar]
- 7.Dalgas U. Exercise therapy in multiple sclerosis and its effects on function and the brain. Neurodegenerative disease management. 2017;7(6s):35–40. doi: 10.2217/nmt-2017-0040 [DOI] [PubMed] [Google Scholar]
- 8.Kalron A, Zeilig G. Efficacy of exercise intervention programs on cognition in people suffering from multiple sclerosis, stroke and Parkinson’s disease: A systematic review and meta-analysis of current evidence. NeuroRehabilitation. 2015;37(2):273–89. doi: 10.3233/NRE-151260 [DOI] [PubMed] [Google Scholar]
- 9.Pilutti LA, Platta ME, Motl RW, Latimer-Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. Journal of the neurological sciences. 2014;343(1–2):3–7. doi: 10.1016/j.jns.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 10.Dinoff A, Herrmann N, Swardfager W, Lanctot KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. European Journal of Neuroscience. 2017;46(1):1635–46. doi: 10.1111/ejn.13603 [DOI] [PubMed] [Google Scholar]
- 11.de Azevedo KPM, de Oliveira Segundo VH, de Medeiros GCBS, de Sousa Mata ÁN, García DÁ, de Carvalho Leitão JCG, et al. Effects of exercise on the levels of BDNF and executive function in adolescents: A protocol for systematic review and meta-analysis. Medicine. 2019;98(28). doi: 10.1097/MD.0000000000016445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, et al. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PloS one. 2016;11(9):e0163037. doi: 10.1371/journal.pone.0163037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of psychiatric research. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langeskov-Christensen M, Bisson EJ, Finlayson ML, Dalgas U. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: a scoping review. Journal of the neurological sciences. 2017;373:307–20. doi: 10.1016/j.jns.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. European Journal of Neuroscience. 2011;33(3):383–90. doi: 10.1111/j.1460-9568.2010.07508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:e15092. doi: 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, et al. Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Structure and Function. 2017;222(4):1797–808. doi: 10.1007/s00429-016-1308-8 [DOI] [PubMed] [Google Scholar]
- 18.Latimer-Cheung AE, Ginis KAM, Hicks AL, Motl RW, Pilutti LA, Duggan M, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Archives of physical medicine and rehabilitation. 2013;94(9):1829–36. e7. doi: 10.1016/j.apmr.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 19.Zackowski KM, Freeman J, Brichetto G, Centonze D, Dalgas U, DeLuca J, et al. Prioritizing progressive MS rehabilitation research: A call from the International Progressive MS Alliance. Multiple Sclerosis Journal. 2021:1352458521999970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalgas U, Hvid LG, Kwakkel G, Motl RW, De Groot V, Feys P, et al. Moving exercise research in multiple sclerosis forward (the MoXFo initiative): Developing consensus statements for research. Multiple Sclerosis Journal. 2020;26(11):1303–8. doi: 10.1177/1352458520910360 [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tooth LR, Ottenbacher KJ. The κ statistic in rehabilitation research: An examination. Archives of physical medicine and rehabilitation. 2004;85(8):1371–6. doi: 10.1016/j.apmr.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 23.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical therapy. 2003;83(8):713–21. [PubMed] [Google Scholar]
- 24.Macedo LG, Elkins MR, Maher CG, Moseley AM, Herbert RD, Sherrington C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. Journal of clinical epidemiology. 2010;63(8):920–5. doi: 10.1016/j.jclinepi.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Yamato TP, Maher C, Koes B, Moseley A. The PEDro scale had acceptably high convergent validity, construct validity, and interrater reliability in evaluating methodological quality of pharmaceutical trials. Journal of clinical epidemiology. 2017;86:176–81. doi: 10.1016/j.jclinepi.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 26.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Topics in stroke rehabilitation. 2003;10(1):1–7. [PubMed] [Google Scholar]
- 27.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical methods in medical research. 2018;27(6):1785–805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC medical research methodology. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological methods. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 31.Infanger D, Schmidt-Trucksäss A. P value functions: An underused method to present research results and to promote quantitative reasoning. Statistics in Medicine. 2019;38(21):4189–97. doi: 10.1002/sim.8293 [DOI] [PubMed] [Google Scholar]
- 32.Rücker G, Schwarzer G. Beyond the forest plot: The drapery plot. Research Synthesis Methods. 2021;12(1):13–9. doi: 10.1002/jrsm.1410 [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–101. [PubMed] [Google Scholar]
- 35.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 36.Schulz KH, Gold SM, Witte J, Bartsch K, Lang UE, Hellweg R, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. Journal of the Neurological Sciences. 2004;225(1–2):11–8. doi: 10.1016/j.jns.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 37.Bansi J, Bloch W, Gamper U, Kesselring J. Training in MS: Influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Multiple Sclerosis Journal. 2013;19(5):613–21. doi: 10.1177/1352458512458605 [DOI] [PubMed] [Google Scholar]
- 38.Wens I, Keytsman C, Deckx N, Cools N, Dalgas U, Eijnde BO. Brain derived neurotrophic factor in multiple sclerosis: Effect of 24 weeks endurance and resistance training. European Journal of Neurology. 2016;23(6):1028–35. doi: 10.1111/ene.12976 [DOI] [PubMed] [Google Scholar]
- 39.Eftekhari E, Etemadifar M. Interleukin-10 and brain-derived neurotrophic factor responses to the Mat Pilates training in women with multiple sclerosis. Scientia Medica. 2018;28(4). [Google Scholar]
- 40.Khademosharie M, Tadibi V, Behpoor N, Hamedinia MR. The effect of 12-weeks resistance and endurance training on the serum levels NGF, BDNF, and VDBP in women with multiple sclerosis. International Journal of Applied Exercise Physiology. 2018;7(1):76–86. [Google Scholar]
- 41.Ozkul C, Guclu-Gunduz A, Irkec C, Fidan I, Aydin Y, Ozkan T, et al. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. Journal of Neuroimmunology. 2018;316:121–9. doi: 10.1016/j.jneuroim.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 42.Zimmer P, Bloch W, Schenk A, Oberste M, Riedel S, Kool J, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Multiple Sclerosis Journal. 2018;24(12):1635–44. doi: 10.1177/1352458517728342 [DOI] [PubMed] [Google Scholar]
- 43.Jørgensen MLK, Kjølhede T, Dalgas U, Hvid LG. Plasma brain-derived neurotrophic factor (BDNF) and sphingosine-1-phosphat (S1P) are NOT the main mediators of neuroprotection induced by resistance training in persons with multiple sclerosis—A randomized controlled trial. Multiple Sclerosis and Related Disorders. 2019;31:106–11. doi: 10.1016/j.msard.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 44.Abbaspoor E, Zolfaghari M, Ahmadi B, Khodaei K. The effect of combined functional training on BDNF, IGF-1, and their association with health-related fitness in the multiple sclerosis women. Growth Hormone and IGF Research. 2020;52. [DOI] [PubMed] [Google Scholar]
- 45.Banitalebi E, Ghahfarrokhi MM, Negaresh R, Kazemi A, Faramarzi M, Motl RW, et al. Exercise improves neurotrophins in multiple sclerosis independent of disability status. Multiple Sclerosis and Related Disorders. 2020;43. doi: 10.1016/j.msard.2020.102143 [DOI] [PubMed] [Google Scholar]
- 46.Devasahayam AJ, Chaves AR, Lasisi WO, Curtis ME, Wadden KP, Kelly LP, et al. Vigorous cool room treadmill training to improve walking ability in people with multiple sclerosis who use ambulatory assistive devices: A feasibility study. BMC Neurology. 2020;20(1). doi: 10.1186/s12883-020-1611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devasahayam AJ, Kelly LP, Williams JB, Moore CS, Ploughman M. Fitness Shifts the Balance of BDNF and IL-6 from Inflammation to Repair among People with Progressive Multiple Sclerosis. Biomolecules. 2021;11(4). doi: 10.3390/biom11040504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savšek L, Stergar T, Strojnik V, Ihan A, Koren A, Špiclin Ž, et al. Impact of aerobic exercise on clinical and magnetic resonance imaging biomarkers in persons with multiple sclerosis: An exploratory randomized controlled trial. J Rehabil Med. 2021;53(4):jrm00178. doi: 10.2340/16501977-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch MA, van Wegen EE, Newman MA, Heyn PC. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: a systematic review and meta-analysis. Translational neurodegeneration. 2018;7(1):1–12. doi: 10.1186/s40035-018-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gligoroska JP, Manchevska S. The effect of physical activity on cognition–physiological mechanisms. Materia socio-medica. 2012;24(3):198. doi: 10.5455/msm.2012.24.198-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. Journal of applied physiology. 2006;101(4):1237–42. doi: 10.1152/japplphysiol.00500.2006 [DOI] [PubMed] [Google Scholar]
- 52.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults. Sports medicine. 2002;32(12):741–60. doi: 10.2165/00007256-200232120-00001 [DOI] [PubMed] [Google Scholar]
- 53.Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and molecular neurobiology. 2018;38(3):579–93. doi: 10.1007/s10571-017-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 55.Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. The Neuroscientist. 2002;8(6):524–31. doi: 10.1177/1073858402238511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalgas U. Exercise therapy in MS—An ongoing shift of paradigm. Multiple Sclerosis. 2010;16(10):S30. [Google Scholar]
- 57.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain research. 2010;1341:41–51. doi: 10.1016/j.brainres.2009.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klaren RE, Hubbard EA, Motl RW, Pilutti LA, Wetter NC, Sutton BP. Objectively measured physical activity is associated with brain volumetric measurements in multiple sclerosis. Behavioural Neurology. 2015;2015. doi: 10.1155/2015/482536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kjølhede T, Siemonsen S, Wenzel D, Stellmann J-P, Ringgaard S, Pedersen BG, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Multiple Sclerosis Journal. 2018;24(10):1356–65. doi: 10.1177/1352458517722645 [DOI] [PubMed] [Google Scholar]
- 60.Archer T. Influence of physical exercise on traumatic brain injury deficits: scaffolding effect. Neurotoxicity research. 2012;21(4):418–34. doi: 10.1007/s12640-011-9297-0 [DOI] [PubMed] [Google Scholar]
- 61.Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Gabriela Nielsen M, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. The World Journal of Biological Psychiatry. 2010;11(6):763–73. doi: 10.3109/15622971003611319 [DOI] [PubMed] [Google Scholar]
- 62.Bus B, Molendijk ML, Penninx B, Buitelaar JK, Kenis G, Prickaerts J, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36(2):228–39. doi: 10.1016/j.psyneuen.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 63.Katoh-Semba R, Wakako R, Komori T, Shigemi H, Miyazaki N, Ito H, et al. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. International Journal of Developmental Neuroscience. 2007;25(6):367–72. doi: 10.1016/j.ijdevneu.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 64.El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. The Journal of Clinical Endocrinology & Metabolism. 2006;91(9):3548–52. doi: 10.1210/jc.2006-0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, et al. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(8):1529–31. doi: 10.1016/j.pnpbp.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 66.Molendijk M, Spinhoven P, Polak M, Bus B, Penninx B, Elzinga B. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Molecular psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105 [DOI] [PubMed] [Google Scholar]
- 67.Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E, et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Multiple Sclerosis Journal. 2020;26(12):1459–69. doi: 10.1177/1352458520915629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motl RW. Exercise and multiple sclerosis. Adv Exp Med Biol. 2020;1228:333–43. doi: 10.1007/978-981-15-1792-1_22 [DOI] [PubMed] [Google Scholar]
- 69.Campbell E, Coulter EH, Paul L. High intensity interval training for people with multiple sclerosis: a systematic review. Multiple sclerosis and related disorders. 2018;24:55–63. doi: 10.1016/j.msard.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 70.Gravesteijn A, Beckerman H, De Jong B, Hulst H, De Groot V. Neuroprotective effects of exercise in people with progressive multiple sclerosis (Exercise PRO-MS): study protocol of a phase II trial. BMC neurology. 2020;20:1–11. doi: 10.1186/s12883-019-1585-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velikonja O, Čurić K, Ožura A, Jazbec SŠ. Influence of sports climbing and yoga on spasticity, cognitive function, mood and fatigue in patients with multiple sclerosis. Clinical neurology and neurosurgery. 2010;112(7):597–601. doi: 10.1016/j.clineuro.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 72.Beier M, Bombardier CH, Hartoonian N, Motl RW, Kraft GH. Improved physical fitness correlates with improved cognition in multiple sclerosis. Archives of physical medicine and rehabilitation. 2014;95(7):1328–34. doi: 10.1016/j.apmr.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 73.Briken S, Gold S, Patra S, Vettorazzi E, Harbs D, Tallner A, et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Multiple Sclerosis Journal. 2014;20(3):382–90. doi: 10.1177/1352458513507358 [DOI] [PubMed] [Google Scholar]
- 74.Sandroff BM, Dlugonski D, Pilutti LA, Pula JH, Benedict RH, Motl RW. Physical activity is associated with cognitive processing speed in persons with multiple sclerosis. Multiple Sclerosis and Related Disorders. 2014;3(1):123–8. doi: 10.1016/j.msard.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 75.Sandroff BM, Balto JM, Klaren RE, Sommer SK, DeLuca J, Motl RW. Systematically developed pilot randomized controlled trial of exercise and cognition in persons with multiple sclerosis. Neurocase. 2016;22(5):443–50. doi: 10.1080/13554794.2016.1237658 [DOI] [PubMed] [Google Scholar]
- 76.Ferris LT, Williams JS, Shen C-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and science in sports and exercise. 2007;39(4):728. doi: 10.1249/mss.0b013e31802f04c7 [DOI] [PubMed] [Google Scholar]
- 77.Griffin ÉW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly ÁM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & behavior. 2011;104(5):934–41. doi: 10.1016/j.physbeh.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 78.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of learning and memory. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 79.Lee JK, Koh AC, Koh SX, Liu GJ, Nio AQ, Fan PW. Neck cooling and cognitive performance following exercise-induced hyperthermia. European journal of applied physiology. 2014;114(2):375–84. doi: 10.1007/s00421-013-2774-9 [DOI] [PubMed] [Google Scholar]
- 80.Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, et al. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiology of learning and memory. 2014;116:46–58. doi: 10.1016/j.nlm.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 81.Tonoli C, Heyman E, Buyse L, Roelands B, Piacentini MF, Bailey S, et al. Neurotrophins and cognitive functions in T1D compared with healthy controls: effects of a high-intensity exercise. Applied Physiology, Nutrition, and Metabolism. 2015;40(1):20–7. doi: 10.1139/apnm-2014-0098 [DOI] [PubMed] [Google Scholar]
- 82.Tsai C-L, Chen F-C, Pan C-Y, Wang C-H, Huang T-H, Chen T-C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–31. doi: 10.1016/j.psyneuen.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 83.Piepmeier AT, Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. Journal of Sport and Health Science. 2015;4(1):14–23. [Google Scholar]
- 84.Leavitt V, Cirnigliaro C, Cohen A, Farag A, Brooks M, Wecht J, et al. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase. 2014;20(6):695–7. doi: 10.1080/13554794.2013.841951 [DOI] [PubMed] [Google Scholar]
- 85.Portaccio E, Bellinvia A, Prestipino E, Nacmias B, Bagnoli S, Razzolini L, et al. The Brain-Derived Neurotrophic Factor Val66Met Polymorphism Can Protect Against Cognitive Impairment in Multiple Sclerosis. Frontiers in Neurology. 2021;12(236). doi: 10.3389/fneur.2021.645220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y-Y, Zhang X-Y, Yung W-H, Zhu J-N, Wang J-J. Role of BDNF in central motor structures and motor diseases. Molecular neurobiology. 2013;48(3):783–93. doi: 10.1007/s12035-013-8466-y [DOI] [PubMed] [Google Scholar]
- 87.Wesnes K, Myhr K-M, Riise T, Cortese M, Pugliatti M, Boström I, et al. Physical activity is associated with a decreased multiple sclerosis risk: The EnvIMS study. Multiple Sclerosis Journal. 2018;24(2):150–7. doi: 10.1177/1352458517694088 [DOI] [PubMed] [Google Scholar]
- 88.Stigger FS, Zago Marcolino MA, Portela KM, Plentz RDM. Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: a systematic review and meta-analysis. The Journals of Gerontology: Series A. 2019;74(5):616–24. doi: 10.1093/gerona/gly173 [DOI] [PubMed] [Google Scholar]
- 89.Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neuroscience & Biobehavioral Reviews. 2021. doi: 10.1016/j.neubiorev.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 90.Mackay CP, Kuys SS, Brauer SG. The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural plasticity. 2017;2017. doi: 10.1155/2017/4716197 [DOI] [PMC free article] [PubMed] [Google Scholar]