Abstract
Molecular chaperones are a family of proteins that maintain cellular protein homeostasis through non-covalent peptide folding and quality control mechanisms. The chaperone proteins found within mitochondria play significant protective roles in mitochondrial biogenesis, quality control, and stress response mechanisms. Defective mitochondrial chaperones have been implicated in aging, neurodegeneration, and cancer. In this review, we focus on the two most prominent mitochondrial chaperones: mtHsp60 and mtHsp70. These proteins demonstrate different cellular localization patterns, interact with different targets, and have different functional activities. We discuss the structure and function of these prominent mitochondrial chaperone proteins and give an update on newly discovered regulatory mechanisms and disease implications.
1. Introduction
I (YB) would like to start with an acknowledgment of Dr. Bruce Ames from a personal perspective. Although I haven’t worked under Dr. Ames, I can certainly say that I was his student. Not only have I read many of his papers, dating back to my graduate school years, but I also spent two weeks sitting in a classroom at the Buck Institute for Aging Research where I enjoyed lectures by Dr. Ames and other established scholars at the 2003 11th Annual Summer Training Course in Experimental Aging Research organized by the National Institute on Aging. I can also relate to Dr. Ames as we both spent significant academic time at Caltech. I remember Dr. Ames mentioned the French connection he and my postdoc mentor Dr. Giuseppe Attardi had in a restaurant overseeing the beautiful Golden Gate bridge. I attended Dr. Ames’ seminars both in Caltech and here in San Antonio, and I witnessed one of his research styles of exploring new research fields every 10 years or so. Thanks to his influence, I exhibit in this review my undertaking to explore a new research territory: mitochondrial homeostasis maintained by the mitochondrial chaperone system.
Mitochondria are ubiquitous organelles in eukaryotic cells whose primary function is to generate energy through oxidative phosphorylation [1]. Mitochondria also play a central role in apoptosis [2], calcium signaling [3], and cell proliferation [4]. Reactive oxygen species are by-products of oxidative phosphorylation thought to be pathogenic agents for many diseases and also implicated in aging [5]. Intracellular ROS also mediate normal and pathological processes [6]. Mitochondrial homeostasis, or mitostasis, is a term used to describe the overall mechanisms implicated in the maintenance of functional mitochondria. It encompasses maintaining the genome, proteome, and functional integrity of the mitochondrion. As mitochondrial function is altered by cell stress, mitochondrial homeostasis is an essential component of cellular homeostasis.
Disruption of mitochondrial homeostasis, or mitochondrial dysfunction, is strongly implicated in various human diseases [6–8]. Mitochondrial disorders are the most common group of inborn errors of metabolism, with an estimated prevalence of 1 in 5,000 live births [9]. In addition, mitochondrial dysfunction is implicated in aging, metabolic disorders, neurodegenerative diseases and cancer[9].
Molecular chaperones are a family of proteins that facilitate protein folding and unfolding, as well as assembly and disassembly of protein complexes [10]. Heat shock proteins are a family of proteins that are generated in response to stressful conditions initially discovered during heat shock studies [11]. The terms ‘molecular chaperone’ and ‘heat shock protein’ are used interchangeably. Chaperones have been classified into groups according to their molecular weight into the families of Hsp40 (DNAJ), mtHsp60 (chaperonin), mtHsp70, Hsp90, Hsp100, and the small heat shock proteins [12]. These proteins fold nascent peptides and refold damaged ones [13,14]. Mitochondrial chaperones are also necessary for the import of newly synthesized peptides from the cytosol into mitochondria. They may also regulate organelle biogenesis[15].
Molecular chaperones are present in all cell compartments, typically have long half-lives, and are tightly regulated. Different chaperone proteins assemble into different complexes and cooperate to carry out different cellular functions [16,17]. In mitochondria, the chaperones mtHsp60 and mtHsp70are the two most important mediators of protein homeostasis [18]. These two proteins have different localization patterns, interact with different partners, and have different structural and functional mechanisms [18].
2. Stressors, stress and mitochondrial chaperones
The expression of biological traits is largely the result of interactions between the environment and genes. Environmental stressors include physical stress: heat and irradiation, oxygen stressors, pH, infection and inflammation, nutritional requirements, , toxins, and mechanical stressors such as compression and shearing [19].
As a response, stress is characterized by an organized program which attempts to counteract the damaging effects of the stressors and restore cell homeostasis. The key effect of stressors is protein denaturation, and thus the central stress response consists of mechanisms that prevent protein damage, restore misfolded proteins to their native or functional configuration, and degrade irreversibly denatured proteins. Molecular chaperones are critical for these tasks as they aid other proteins to fold correctly, to refold when reversibly denatured, to assemble into complexes, and to move to different cellular compartments. Finally, as a quality control mechanism, chaperones also participate in the degradation of proteins which are nonfunctional due to mutations or excessive damage.
Mitochondrial stress response restores mitochondrial homeostasis which is important for overall cellular homeostasis. Disruption of mitochondrial homeostasis associated with defective mitochondrial chaperone proteins have been shown to cause human diseases. Mitochondrial homeostasis is largely maintained by mitochondrial chaperones mtHsp60 and mtHsp70 which regulate key aspects of mitochondrial biogenesis and mitochondrial quality control.
In this review, we focus on two of the most prominent molecular chaperones, mtHsp70 and mtHsp60; their structure and function (Fig. 1) are summarized in the following sections. Normal function of these proteins protects cells against stressors and restores mitochondrial and cellular homeostasis (Fig. 2A); on the other hand, insufficient function leads to degenerative disease, and inappropriate gain-of-function is associated with carcinogenesis (Fig. 2B,C). The detailed physiologic functions of mtHsp60 and mtHsp70 are outlined in Figure 3.
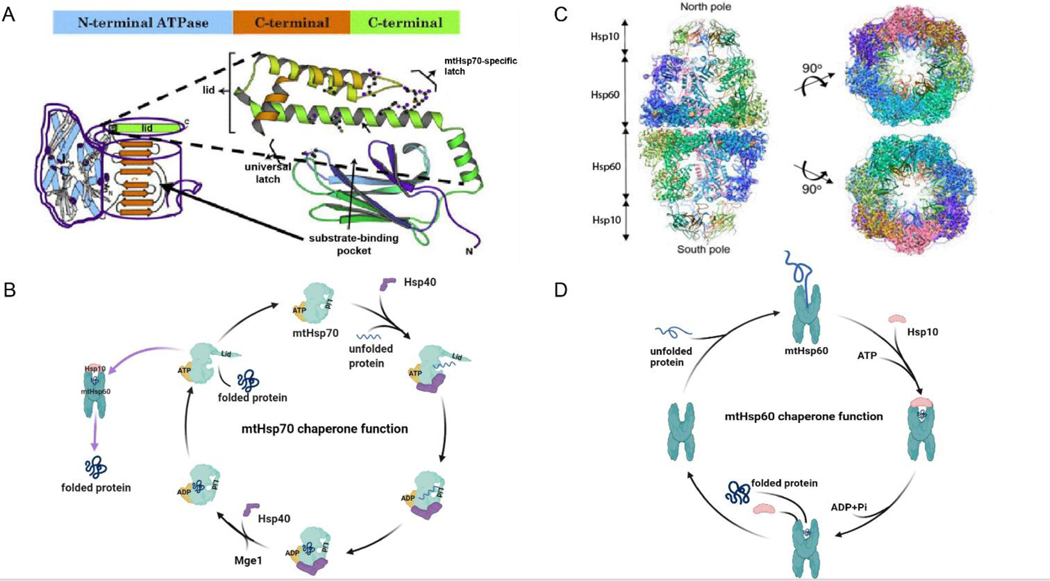
Figure 1: mtHsp70 and mtHsp60 structure and basic function.
(A) Molecular structure of mtHsp70 with three domains: N-terminal domain (ATP domain), substrate binding domain, and C-terminal domain. (B) Working model of mtHsp70 chaperone system on protein folding. (C) MtHsp60 complexed with cochaperone Hsp10. (D) Working model of mtHsp60 chaperone system on protein folding. Fig.1A and 1C are adapted from prior figures by Kaul et al [128] and Nismeblat et al [43].
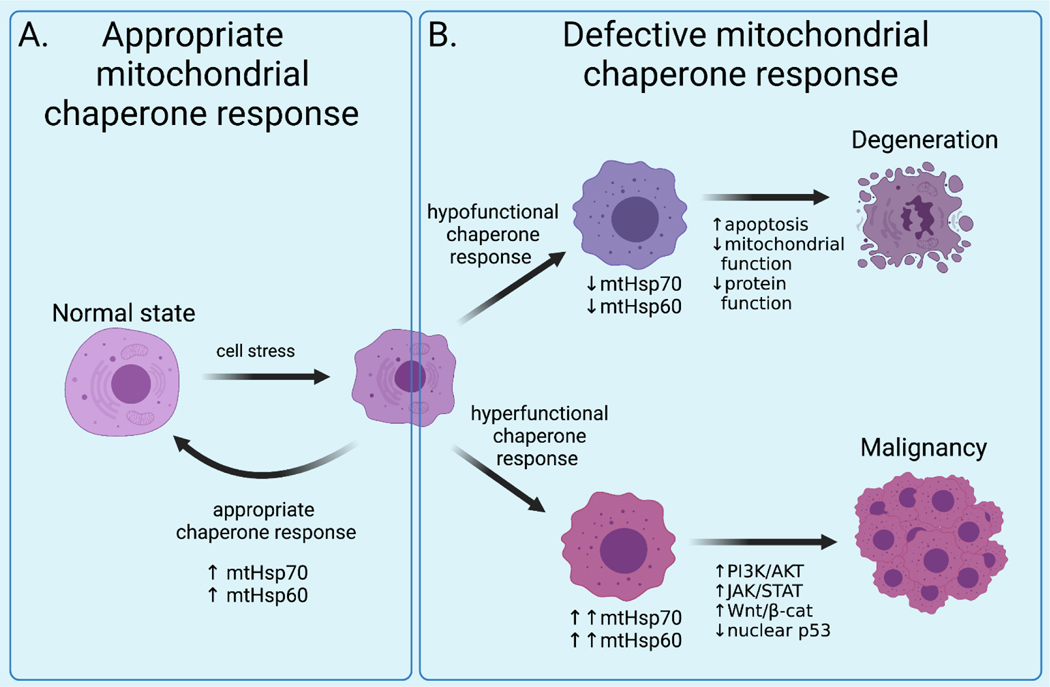
Figure 2: Overview of physiologic or pathologic roles of mitochondrial chaperones in human health and disease.
A: In the appropriate chaperone response, stress such as reactive oxygen species induce accumulation of damaged protein and mitochondrial dysfunction and other cellular pathologies which the cell responds to by upregulating expression of mitochondrial chaperones mtHSP60 and mtHSP70, in turn decreasing ROS production, enhances protein repair, and restores mitochondrial function. B: In hypofunction of the chaperone system, cell stress is not met with a sufficient chaperone response, leading to overwhelming oxidative damage, irreversible protein damage, and irreversible mitochondrial dysfunction which results in cell degeneration or death. In cancer, DNA damage, epigenetic rewiring (i.e., HSPA9 promoter methylation, histone actylation) and also post-translational modifications (such as HSP70 protein lysine methylation) lead to hyperfunction of chaperone elements. This is often accompanied by loss of network redundancy. The resulting overexpression of mtHSP60 and/or mtHSP70 drives malignant transformations via various pathways including modification of PI3k, Jak/STAT, Wnt, and p53 with phenotypes of decreased apoptosis as well as increased immortalization, proliferation, and stemness.
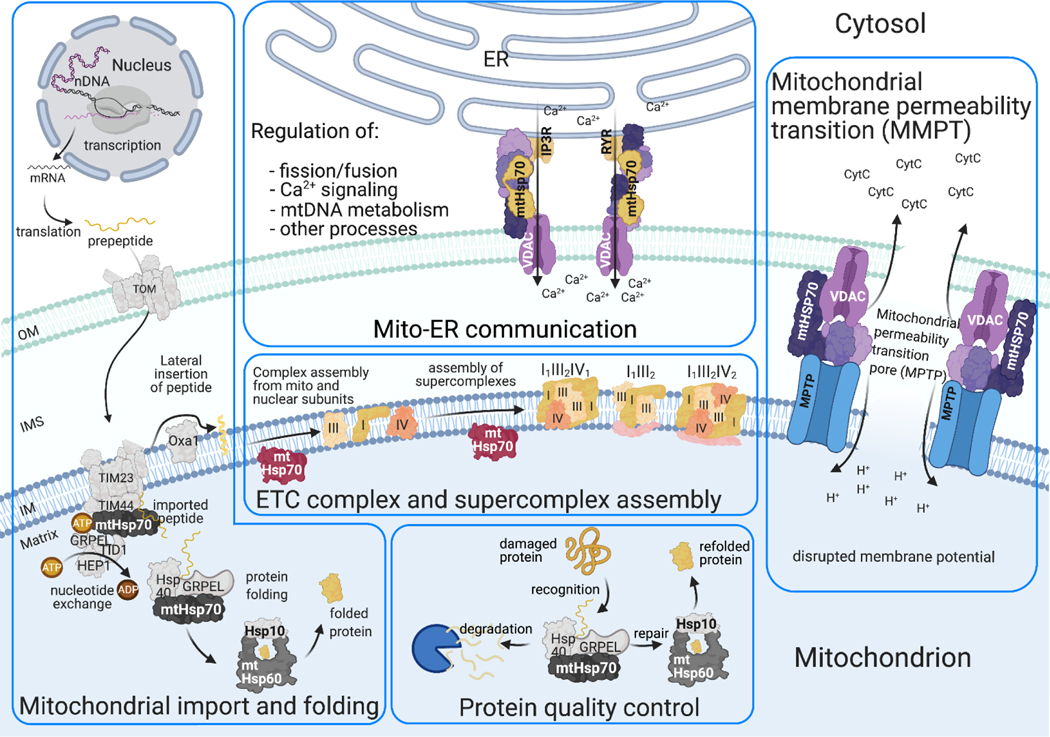
Figure 3:
Working model of physiologic functions of mtHsp60 and mtHsp70
2.1. mtHsp70
mtHsp70 is a 74 kDa mitochondrial protein also known as mortalin, PBP74, or Grp75 [20] that belongs to the heat shock protein 70 (Hsp70) family. Human mtHsp70 is encoded by the gene HspA9B (GeneID: 3313) with a 2.8 kb transcript from an 18 kb region on human chromosome 5q31.1 and consists of 17 exons [21]. To date, separate crystal structures of isolated functional domains have been elucidated for mtHsp70’s 3 main functional regions: an N-terminal ATPase with a nucleotide-binding domain (NBD), a surface groove domain for peptide substrates, and the C-terminal domain which acts as a lid that covers the substrate binding domain when substrate is bound [22] (Fig. 1A). Due to challenges with protein solubility, a crystal structure of mtHsp70 is lacking from the literature, although an ab initio model of the whole protein has been generated [23].
Members of the Hsp70 family provide cells with protection against potentially lethal damage from cell stress, assist in folding and transport of newly synthesized polypeptides, and aid in assembling multi-protein complexes [24]. This protein family also has “unfoldase” activity which involves the recognition of misfolded or aggregated proteins followed by their unfolding and spontaneous refolding to the natively refoldable species [25]. mtHsp70 proteins work in conjunction with various other proteins to form quality control complexes [26].
mtHsp70 is known to interact with a diverse variety of proteins implicated in many different functions apart from protein folding and quality control (Fig. 1B). Specifically, mtHsp70 is involved in cell cycle regulation, inflammation, mitochondrial import, mitochondrial protein biogenesis, electron transport chain assembly, ER-mitochondria communication, and Fe/S cluster biogenesis [27]. (Table 1, Fig. 3).
Table 1:
Protein interactions with mtHsp70 under recent investigation
| Protein | Subcellular location | Function | Reference |
|---|---|---|---|
| IP3R - VDAC1 complex | Mitochondria Associated Membrane | Calcium transport from endoplasmic reticulum to mitochondria | [119] |
| HEP1 | Matrix side of Inner mitochondrial membrane | Co-chaperone, stimulates Hsp70 ATPase activity | [120] |
| GRPEL1/2 | Mitochondrial matrix, | Co-chaperone, nucleotide exchange factor for Hsp70 | [120] |
| TID-1 | Mitochondrial matrix (TID-1S), cytosol (TID-1L) | Co-chaperone, stimulates Hsp70 ATPase activity | [120] |
| PERK / IRE1/ ATF6 | ER lumen | Unfolded protein response | [121] |
| Lon protease | Mitochondrial matrix | Inhibits apoptosis by stabilizing mtHsp60, stabilizes Hsp70-Hsp60 complex. | [122] |
| TLR2 | Plasma membrane | Extracellular Hsp70 and mtHsp60 recognized as DAMP and trigger pro-inflammatory cascade. | [123] |
2.1.1. mtHsp70 in mitochondrial import and folding of nDNA-encoded pre-peptides
The mitochondrial endosymbiotic theory holds that an early ancestor of the eukaryotic cell engulfed a prokaryotic organism with its own distinct genome, the progenitor of what is now the mitochondrion. Over millions of years, genes encoding over 1000 distinct mitochondrial proteins were transferred to chromosomes within the nucleus. Accordingly, a cellular mechanism evolved for transport of newly transcribed mitochondrial proteins from the cytosol into the mitochondria. The key component of this system is the mitochondrial import motor which recognizes nascent mitochondrial peptides found in the cytosol and translocates them across the mitochondrial outer and inner membranes. About 70% of all mitochondrial proteins synthesized in the nucleus are labeled with a cleavable N-terminal mitochondrial localization presequence [28]. The mitochondrial Translocase of the Outer Membrane (TOM) complex recognizes these peptides and facilitates their translocation from the cytosol to the intermembrane space where they are delivered to the translocase of the inner membrane (TIM) complex, which drives import into the mitochondrial matrix via ATP hydrolysis. mtHsp70 plays important roles in both phases of transport, functioning as a partner with both TOM and TIM complexes. It is the essential mediator of the two biochemical steps involved in both stages of import, namely unfolding of the preprotein for extrusion through the translocase, and powering translocation. In fact, mtHsp70 is the only ATPase involved in the mitochondrial import process.
For mitochondrial import to occur, TOM and TIM23 form a supercomplex which facilitates transport across both mitochondrial membranes, but the formation of this supercomplex requires an active presequence translocase-associated motor (PAM) [24], a functional complex found on the matrix side of the inner membrane. The PAM maintains a close association with the TIM23 complex due to direct interaction of TIM44 with mtHsp70 which tethers mtHsp70 to the inner membrane near the import pore. ATP hydrolysis by mtHsp70 in the PAM complex drives the import of precursor peptides into the mitochondrial matrix. The ATPase reaction takes place within mtHsp70’s nucleotide binding domain and the rate is controlled by nucleotide exchange factors GRPEL1 (homolog of yeast Mge1) and GRPEL2 which are found within the matrix [29]. The precise mechanism by which this ATP hydrolysis reaction is converted into kinetic energy remains unknown; however, it is thought to involve a conformational change that creates a tractional force which is coupled to the prepeptide via the substrate binding region of mtHsp70, resulting in mtHsp70 pulling the peptide across the translocase complex [22].
After translocation, mtHsp70 is the first chaperone that contacts the peptide within the mitochondrial matrix and thus is the first chaperone involved in post-translocational folding. In the matrix, mtHsp70 cooperates closely with mtHsp60 to perform folding functions. Together with the help of co-chaperones, mtHsp70 recognizes exposed hydrophobic amino acid residues on misfolded or unfolded peptide chains and stabilizes them through non-covalent interactions, resolulbilizing them and facilitating the refolding of the peptide. For damaged proteins, if the insult is irreparable the chaperone complex may facilitate the peptide’s degradation by ATP-dependent proteases. mtHsp70 chaperones work closely with DNAJs and nucleotide exchange factors (NEFs) [29]. The identity of the specific Hsp40(DNAJ) cochaperone determines the substrate specificity of the chaperone complex. Upon recognition of a damaged peptide, the chaperone protein hydrolyzes ATP to further stabilize the interaction. The rate of ATP hydrolysis, and therefore the rate of the folding reaction, is controlled by these specific NEFs (for example Mge1 as in Fig. 1B). Of note, whether mtHsp70 participates in membrane import or folding within the matrix is determined by which of the J-family co-chaperones it associates with. For example, J-domain protein mdj-1 mediates mtHsp70 folding activity together with mtHsp60 in the matrix [15]; on the other hand, Pam18 and Pam16 are J-domain proteins that regulate mtHsp70 import activity and are necessary for peptide translocation [30,31].
2.1.2. mtHsp70 in synthesis and stabilization of native mitochondrial proteins and electron transport chain (ETC) complexes
Apart from its role in import and folding of imported pre-peptides from the cytosol, mtHsp70 is also directly involved in expression of mtDNA-encoded peptides as well as stabilization of mitochondrial proteins and functional complexes.
mtHsp70 facilitates the functionality of ETC subunits and complexes, and also entire ETC supercomplexes. mtHsp70 maintains functionality of ribosomal protein VAR1, the only mtDNA-encoded subunit of the mitoribosome (especially under heat stress) and a vital member of the mitochondrial protein translation complex [32]. mtHsp70 also facilitates the assembly of mtDNA-encoded subunits 6, and 9 of the ATP synthase complex (Complex V) and also protects these subunits from proteolytic degradation [32]. mtHsp70 was also shown to interact strongly with Complex IV in association with Mge1 [26].
Interestingly, mitochondria with mutant mtHsp70 lacking the ability to bind Complex IV demonstrated selective impairment of the assembly of complex IV into respiratory chain supercomplexes. Thus, the association of mtHsp70-Mge1-Complex IV seems to be essential for mature supercomplex formation [26]. Cristae shape, a factor thought to play an important role in supercomplex assembly [33], also seems to be under regulation by mtHsp70 in a manner co-dependent on mtHsp40 via modulation of Opa1 cleavage [34].
2.1.3. mtHsp70 in protein quality control
Damaged peptides often have structural similarity to unfolded nascent peptides, therefore mtHsp70 can also perform protein quality control (PQC) functions. Indeed, mtHsp70s is upregulated during conditions of cell stress. PQC can be broken down into three processes: first, substrate recognition, which is then followed by either refolding or degradation. The substrate specificity of Hsp70s in general is determined by specific J-domain family co-chaperones. Specific proteases can also associate with the mtHsp70 chaperone complex with the aid of reporter proteins. These reporter proteins serve as a tag for degradation and can bind to the misfolded substrate. When repair is not possible, mtHsp70 stabilizes the substrate-bound state of reporter proteins in order to keep the substrate tagged until the proteolytic system can dispose of it. Of note, some proteases may associate with mtHsp70 and perform chaperone functions rather than proteolysis. For example, LONP1 is an AAA+ protease found in the mtHsp70 chaperone complex with DNAJA3, but it also shows intrinsic chaperone activity when working together with mtHsp70 for the folding of Oxa1 [35].
2.1.4. mtHsp70 in mitochondrial quality control, apoptosis, and ER-mitochondria communication
mtHsp70 is a key regulator of life-or-death decision-making in the cell, as well as in the quality control of mitochondria via multiple mechanisms. Mitochondrial membrane potential, calcium dynamics, and mitochondrial permeability are important for mitochondrial quality control and also cell death. mtHsp70 is implicated in each of these processes. To induce mitochondrial calcium overload in conditions of cell stress, calcium microdomains are often transferred from the ER to the mitochondria by a multiprotein complex composed of voltage-dependent anion channel 1 (VDAC1) at the outer mitochondrial membrane which associates with the inositol-1,4,5-trisphosphate receptor (IP3R) on the ER membrane. Facilitation of the interaction between VDAC1 and IP3R by mtHsp70 links the ER with the mitochondrion and results in the transfer of Ca2+ into the mitochondrial matrix.[36] mtHsp70 modulates mitochondrial membrane potential via its interaction with mitochondrial p66Shc. It is thought that mtHsp70 stabilizes p66Shc but that stress conditions induce the release of p66Shc from an inhibitory complex, freeing it to cause damage within the inner mitochondrial membrane [37]. Another well-known role of mtHsp70 in apoptosis regulation is its sequestration of p53, preventing its translocation to the nucleus where p53 would otherwise drive the expression of anti-apoptotic genes [38]. Finally, mtHsp70 works together with J-domain protein JC15 to increase mitochondrial permeability.
2.2. mtHsp60
Mitochondrial heat shock protein 60 (mtHsp60), also known as HLD4 or CPN60, is a 60 kDa molecular chaperone that plays a key role in protein folding. Human mtHsp60 is encoded by HspD1 (Gene ID: 3329), a 13 kb region of human chromosome 2q33.1 that forms a 2.2 kb RNA transcript [40]. Unlike the prokaryotic homolog, GroEL, which exists only as a tetradecamer of two heptameric rings, mtHsp60 can also exist as a single heptameric ring [41]. Each ring serves as an enclosed environment for protein folding (Fig 1C). Some of the interactions with mtHsp60 of recent interest which are discussed in the following sections are summarized in Table 2.
Table 2:
Protein interactions with mtHsp60 under recent investigation
| Protein | Subcellular location | Function | Reference |
|---|---|---|---|
| Survivin | Mitochondrial matrix and cytosol | Inhibitor of apoptosis, stabilized by mtHsp60 | [124] |
| Cyclophilin D | Inner mitochondrial membrane | Component of mitochondrial permeability transition pore that promotes apoptosis, inhibited by mtHsp60. | [124] |
| IKKα/β | Cytosol | Activates IKK/NF-κB survival pathway | [124] |
| apoA-II | Extracellular | Plasma membrane expressed mtHsp60 functions as a cell surface receptor for high density lipoproteins via apoA-II binding | [125] |
| Bax | Cytosol, mitochondrial outer membrane | Induces apoptosis. mtHsp60-Bax complex can sequester Bax, inhibiting apoptosis. However, yeast models have shown that mtHsp60 can stabilize Bax and promote its pro-apoptotic functions. |
[126], [124] |
| TLR4 | Plasma membrane | Induces TNFα and IL-6 release via NFκB and JNK activation. Promotes cell migration in vascular smooth muscle cells via activation of ERK |
[126] |
| TLR2 | Plasma membrane | Extracellular Hsp70 and mtHsp60 recognized as DAMP and trigger pro-inflammatory cascade. | [123] |
| Clusterin | Extracellular, Nucleus, Cytoplasm | Extracellularly, functions as an inhibitor of apoptosis; Intracellularly, can function as a promoter of apoptosis |
[127] |
| P53 | Cytosol | Tumor suppressor, transcription factor | [48] |
| Lon Protease | Mitochondrial matrix | Inhibits apoptosis by stabilizing mtHsp60, stabilizes Hsp70-Hsp60 complex. | [122] |
| Beta-catenin | Plasma membrane (adherens junctions,), cytoplasm/nucleus | Cell-cell adhesion, proto-oncogene, transcription factor | [73] |
2.2.1. mtHsp60 in mitochondrial protein folding
The best characterized representative of mtHsp60 is the prokaryotic homolog, GroEL. GroEL is a tetradecamer formed by two heptameric rings arranged back-to-back, with each ring forming a separate central cavity. Each GroEL subunit has three regions known as the apical, intermediate and equatorial domains. Protein folding occurs in the following manner and is depicted in Figure 1D. First, the polypeptide interacts with hydrophobic residues in the apical domains of the cis ring [42]. Next, ATP binds to the equatorial domains, causing a conformational change in the ring that brings the polypeptide further into the central cavity [42]. Following this step, the co-chaperonin GroES, which also forms a heptameric ring, binds to the apical domains of the GroEL ring, creating an enclosed environment for folding. GroEL’s intrinsic ATPase ability provides the energy for polypeptide release from the apical domains and into the central cavity, where it can be appropriately folded [42]. Release of GroES, ADP, and the folded protein from the cis ring is accomplished by ATP binding to the trans ring. In this model, the GroEL-GroES complex appears as an asymmetric “bullet”, since the GroES heptamer can only bind to one ring at a time [35]. However, recent studies have shown the existence of a symmetrical “football” shaped double ring complex, with GroES heptamers on each end [43]. In contrast, mtHsp60 exists as a range of double and single heptameric ring structures and there is evidence that single ring structures are able to successfully mediate folding [44], even without the help of Hsp10, the eukaryotic homolog to GroES [45].
2.2.2. mtHsp60 in apoptosis
mtHsp60 impacts apoptosis in a variety of ways. Along with Hsp10, mtHsp60 exists as a pre-apoptotic complex with pro-caspase-3 in the intermembrane space of the mitochondria[46]. This complex promotes pro-caspase-3 activation and thus apoptosis in an energy dependent manner, possibly by maintaining it in a protease-sensitive conformation [46].
In contrast, mtHsp60 can also inhibit apoptosis by downregulating caspase activities and upregulating IL-8 and TGF-β [47]. In tumor cells, mtHsp60 stabilizes the mitochondrial pool of survivin, an inhibitor of apoptosis, and forms complexes with p53 and Bax, preventing their pro-apoptotic functions [48]. mtHsp60 is a key component of the mitochondrial unfolded protein response (mtUPR), which maintains protein homeostasis and promotes survival through the involvement of heat shock proteins Hsp10, mtHsp60, and mtHsp70 and proteases ClpP and LONP1[49]. In addition to its role within the mitochondria, the accumulation of cytosolic mtHsp60, which can occur with or without mitochondrial release, has been shown to promote apoptosis in multiple pathways [50]. However, cytosolic accumulation of mtHsp60 has also been shown to promote survival in a Bax-independent manner [50] through the activation of IKK [49]. Ultimately, mtHsp60 can both promote and inhibit apoptosis.
Overall, mtHsp70 and mtHsp60 are two key and essential mitochondrial chaperones that modulate cellular responses to stress through regulation of protein folding, protein quality control, and apoptosis.
3. Roles of mitochondrial chaperones in aging
Aging is the process of gradual decline in organ function and decreased ability to maintain homeostasis under stress conditions [51]. This involves the loss of regeneration potential, disabling of repair functions, and accumulation of genetic and protein damage leading to irreversible dysfunction and eventual cell death.
One of the major theories of aging is the oxidative stress model, which implicates reactive oxygen species as the cause of DNA damage leading to genome instability. The accumulation of mtDNA mutations over time leads to respiratory dysfunction and increased oxidative stress. Another theory of aging involves proteostasis collapse, with cell demise occurring when the rate of inactivation or dysfunction of vital protein exceeds the rates of synthesis and repair.[52]. Mitochondrial chaperones are strongly implicated in both theories of aging, since they regulate the oxidative stress response in mitochondria and also protein homeostasis. With age, chaperone proteins themselves can become damaged; it has been suggested that the combination of sick chaperones and stress is a major contributor to the process of aging.[53] Sick chaperones increase vulnerability of the organism to stressors since chaperones are so important in the anti-stress response of the cell.[53] Therefore, decreased de novo expression rate of chaperones as well as inactivation (or decreased activity) of existing chaperones are important contributors to the aging process in the cell (Fig. 2B).
mtHsp70 together with Tid1 is thought to be the major chaperone complex responsible for scavenging toxic protein aggregates in stressed, diseased, or aging human mitochondria.[54]. Decreased levels of mtHsp70 have been shown to increase vulnerability to stress and accelerate aging. By the same token, progression of the dysfunction associated with aging has been linked with insufficient function and/or expression of mtHsp70. The level of mtHsp70 protein was found to decrease with senescence in human foreskin fibroblasts [55] and knockdown of mtHsp70 caused a progeria-like phenotype in C. elegans worms.[56] Meanwhile, overexpression led to enhance cell survival in human cells and extended lifespan in worms [55,56] In vitro cells with aging phenotypes induced by applying chemical stress were accompanied by decreased levels of mtHsp70, and this change was thought to also involve disruption of the mtHsp70-p53 interaction.[57] As discussed above, mtHsp70 sequesters cytosolic p53 in order to prevent transcription of pro-apoptotic genes. This data supports the idea that the cell must maintain sufficiently high mtHsp70 protein levels to combat the damages due to aging, ultimately with the goal of delaying cell death. In fact, mtHsp70 levels (and thus, its functions) have been noted to be a key determinant which predicts a fixed proliferation potential in normal and aging cells [55]. Interestingly, damaged mtHsp70 can form semi-dysfunctional self-aggregations which are associated with failure of mitochondrial biogenesis [58]. Hsp70-escort protein 1 (Hep1), is an essential co-chaperone to mtHsp70 which helps it resist self-aggregation [59].
On the other hand, aging-related stress can drive compensatory increase in the activity of existing mtHsp70 chaperones independent of expression level. For example, oxidative stress drives increased expression of Mge1 (human for GRPEL1), the NEF for mtHsp70 [60], suggesting that the rate of the chaperone reactions catalyzed by mtHsp70 is increased in response to oxidative stress. Magmas is another regulator of mtHsp70’s activity with a facilitative function on mtHsp70 at the import channel that is driven by ROS concentration [61].
The role of mtHsp60 in aging seems to be closely linked to mtHsp70 function. Immunoprecipitation experiments showed that mtHsp60 and mtHsp70 were found to associate in complexes in vivo and that their interaction occurred via mtHsp70’s N-terminal domain [62]. Of note, mtHsp60 has a distinct function from mtHsp70 in aging pathways; overexpression of mtHsp70 extended the in vitro lifespan of normal human fibroblasts, whereas mtHsp60 did not [62]. However, knockdown of either mtHsp70 or mtHsp60 caused growth arrest in osteosarcoma cultures [62]. Experiments in Snell mice, a long-lived mouse model with alterations in the growth hormone pathway, reveal increased resistance to mitochondrial stress due to elevated levels of mtHsp60 and Lon protease, which are key components of the mitochondrial unfolded protein response (mtUPR) [63]. Activation of the mtUPR, along with mitophagy, is shown to reduce oxidative damage and extend the viability of cells exposed to oxidative stress [64].
Altogether, mtHsp70 and mtHsp60 impact cellular and organismal aging by limiting stress which is thought to be a key component of aging. Regulation of mtHsp70 and mtHSP60 activity can have direct consequence on aging phenotypes.
4. Roles of mitochondrial chaperones in cancer
The process of carcinogenesis involves the step-wise development of cellular aberrations resulting in cellular proliferation and immortalization. Since chaperones act as guardians against age-related dysfunction, it is not surprising that the re-wiring of chaperone networks is strongly implicated in cancer (Fig. 2C). The “chaperome”, a term coined by Yan et al recently [65], refers to the large family of chaperone proteins and their cofactors (co-chaperones) as well as many other factors which form functional networks in the cell. In normal cells, the chaperome contains many redundancies; deficiencies in the activity of a particular chaperone are often remedied by another chaperone which may substitute itself into the network in place of the deficient one. Thus, there are two general cellular states of the chaperome, defined by either the presence or the overwhelming absence of chaperone network redundancies. In many cancers, redundancies have been depleted leading to the overwhelming prominence of a single interconnected chaperone pathway contributing to the immortalization phenotype. In such cancers, targeted knockdown or inhibition of a particular chaperone might prove to be an effective chemotherapeutic strategy. In other cancers, the chaperome may behave more like normal cells and retain redundancies, making these cancers poor candidates for chaperone-targeted treatment [65].
Chaperome rewiring defined by overexpression of mtHsp70 has been detected in many epithelial cell malignancies including breast, ovarian, hepatocellular, and cholangiocarcinoma; mtHsp70 contributes to the epithelial-mesenchymal transition via upregulation of PI3/Akt and JAK/STAT pathways, creating a more invasive and metastatic phenotype and also increasing cell proliferation and survival [66]. mtHsp70’s interaction with p53 in the cytosol is responsible for curbing its anti-apoptotic function, its overexpression reducing p53 activity and leaving the cell cycle unchecked. Another important function of mtHsp70 in cancer is its promotion of stem-cell-like behavior, which is associated with chemotherapy resistance and a more aggressive phenotype [67]. Importantly, knockdown of mtHsp70 increased the sensitivity of stem-like cancers to chemotherapy [67].
Epigenetic alterations of mtHsp70 have been implicated in cancer as well. For the Hsp70 family in general, epigenetic aberrations such as DNA hypermethylation of the HspA1A promoter, loss of certain microRNAs which normally inhibit mtHsp70 translation, and changes in histone methylation and acetylation have been documented. For mtHsp70 specifically, post-translational lysine methylation has been associated with a tumor phenotype [68].
Changes in the expression of mtHsp70 co-chaperones are also responsible for altering the sensitivity to chemotherapeutic drugs. For instance, one recent report demonstrated modulation of cell death pathways by DNAJC15 through regulation of mitochondrial permeability transition [29,69,70]. Intriguingly, disruption of the ETC supercomplex has emerged as a potential chemotherapeutic strategy, with promising results in Her2-high breast cancer [71]. Given that mtHsp70 possesses activity as a chaperone in the formation of ETC supercomplexes, this suggests that impairment of supercomplex formation might be a reasonable expectation for chemotherapies targeting mtHsp70.
mtHsp60 expression is increased in a variety of cancer types, including astroglioma, acute myeloid leukemia, Hodgkin’s lymphoma, large bowel adenocarcinoma, ovarian carcinoma, and osteosarcoma [72]. However, in other types of tumors, such as glioblastoma, bladder transitional cell carcinoma, and bronchial adenocarcinoma, mtHsp60 levels are decreased [72]. This differential expression likely reflects mtHsp60s variable impact on apoptosis. Beyond considerations of cell survival, mtHsp60 can also impact progression to metastasis. Overexpression of mtHsp60 is noted to induce metastasis by activating β-catenin via interactions with its apical domain [63]. Aberrant expression of mtHsp60 in tumor cells is regulated by epigenetic factors such as microRNA sequences, including miR-1, miR-206, miR-382, and miR-29, as well as through mtHsp60 acetylation [68]. mtHsp60 can serve as a suitable target for therapy, given its differences in expression in normal vs tumor tissues. For instance, mtHsp60 was highly expressed in adenocarcinoma of the breast, colon, and lung, but undetectable or at very low levels in normal epithelium of the respective tissue [48]. Several existing drugs are thought to have inhibitory effects on mtHsp60 by binding to the equatorial domain at cysteine residues (epolactaene) or by preventing ATPase activity (mizoribine and EC3016, a pyrazolopyrimidine derivative) [74].
Upregulation of mitochondrial chaperones like mtHsp70 and mtHsp60 have been in identified in various types of cancers. Mitochondrial chaperones have also emerged as promising targets for future cancer therapy development.
5. Role of mitochondrial chaperones in neurodegeneration
5.1. Introduction
Disruption of mitochondrial homeostasis and associated mitochondrial dysfunction have long been associated with various neurological diseases and neurodegenerative diseases [75–77]. While gain-of-function changes in mitochondrial chaperone systems are linked with cancer, the opposite is true for neurodegeneration. Deficiencies in mitochondrial chaperone systems are responsible for cell energy deficiencies, neuronal dropout by apoptosis, and the accumulation of toxic protein aggregations which are the hallmark of a class of neurodegenerative diseases known as proteinopathies. The molecular chaperone mtHsp70 has also been shown to have a protective function as it permits cells to survive to otherwise lethal conditions and modulates lifespan. Proteomic analysis of in vitro cultures of adult dorsal root ganglion (DRG) neurons found that mtHsp70 protein was synthesized abundantly in the axon [78], supporting the likely role of mtHsp70 in neurodegeneration due to proteinopathy. Overexpression of mtHsp70 increased sensitivity to cell death stimuli, whereas knockdown of mtHsp70 increased resistance to such insults in studies of hippocampal neurons [79]. mtHsp60 is also implicated in a variety of neurodegenerative processes, and mutations that lead to deficiency are known to cause neurodegenerative disease [80]. The role of mtHsp60 and mtHsp70 in neurodegeneration are summarized in Figure 4.
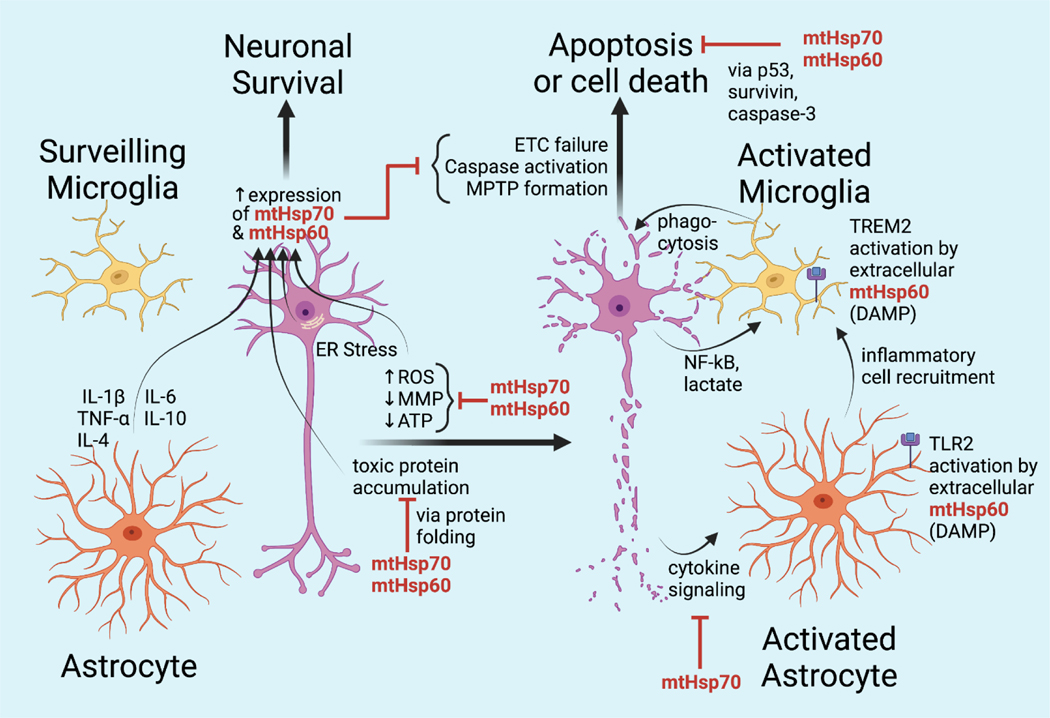
Figure 4:
Current knowledge regarding the roles of mtHsp60 and mtHsp70 in neurodegeneration
mtHsp60 and mtHsp70 have been demonstrated to either co-localize or directly interact with toxic proteins & aggregations, including the more well-known amyloid beta and alpha-synuclein but also HIV-1 Tat protein [81], corpora amylacea [82] and 14–3-3 protein [83]. Such protein-chaperone interactions have been shown to take place in the vicinity of the mitochondria in both neurons and glia, supporting the concept that the mitochondrial dysfunction associated with proteinopathies is a consequence of toxic intracellular proteins directly damaging the mitochondria. Signs of mitochondrial dysfunction may include increased reactive oxygen species levels, decreased mitochondrial membrane potential, decreased ETC complex function, decreased ATP production, or apoptosis. In both neurons and glia, mitochondrial chaperones are able to address these insults either directly, by disposing of toxic proteins, or indirectly by promoting mitochondrial function. Extracellular protein aggregations can also cause mitochondrial dysfunction in proteinopathies via ischemia associated with small vessel occlusion, such as in amyloid angiopathy. Mitochondrial chaperones are important protectors against ischemic damage in neurons [84]. Mitochondrial function of astrocytes is also an important target in the pathogenesis of brain ischemic injury [85] and is also protected by chaperones.
The evidence suggests that the major roles of mitochondrial molecular chaperones in the context of neurodegenerative disease are neuroprotection, regulation of glial cell function, as well as glioprotection. Since the neuroprotective effects of mitochondrial chaperones have been explored in detail with disease-specific models, we will therefore discuss them later in separate sections. Because experiments in glial cells are scarce, we will discuss their implications broadly in the following section using findings from various neurodegenerative disease models.
5.2. Mitochondrial chaperones in glioprotection and neuroglial signalling
mtHsp70 has important functions involving both astrocytes and microglia. Overexpression of mtHsp70 in neurons attenuated lipopolysaccharide-induced oxidative and metabolic stress, and suppressed proinflammatory activation of microglial cells via modulation of neuronal cytokine release [86]. In astrocyte-derived cells treated with amyloid beta, a transient increase in expression of mtHsp70 triggered inhibition of the caspase-3 pathway, promoting the expression of mtHsp60 and Hsp90 and the survival of astrocyte-derived cells [87]. In experiments with HIV-1 Tat protein, mtHsp70 seemed to protect against the indirect neuron death induced by toxin-mediated astrocyte dysfunction; downregulation of mtHsp70 by HIV-1 Tat protein was associated with findings of astrocytic dysfunction and neuron death, while on the other hand, overexpression of mtHsp70 in astrocytes reduced inflammation and also rescued neurons from astrocyte-mediated death [81]. Increased immunoreactivity of mtHsp70 was observed in MS lesions, particularly in astrocytes and axons coinciding with regions of enhanced mitochondrial oxidative stress [88]. Overexpression of mtHsp70 in rat brain was associated with improved mitochondrial function in terms of protection of complex IV activity, reduction of free radicals, decreased lipid peroxidation, and maintenance of sufficient ATP levels [84]. Overexpression of mtHsp70 in astrocytes was shown to protect against ischemic damage and was associated with decreased ROS production, preservation of mitochondrial membrane potential, and preserved ATP levels and cell viability [89]. ER stress was also shown to induce expression of mtHsp70 and its co-chaperone Lon protease in a PERK-dependent manner, and the overexpression of these proteins promoted assembly of ETC complex I and II and protected against mitochondrial dysfunction caused by the toxin brefeldin as well as hypoxic damage [90].
mtHsp60 also plays a protective role in neurons and in glial cells. In astrocytes, increased ROS levels were found to increase expression of mtHsp60 and antioxidant enzymes, especially in the presence of ATP [91]. As discussed above, toxic protein aggregations in astrocytes have been shown to co-localize with mtHsp60, suggesting its deployment for chaperone functions. mtHsp60 may also serve as a cell damage signal and in some contexts, mtHsp60 may drive cell death rather than cell survival pathways. Excess secretion of mtHsp60 by neurons appears to be a damage-associated molecular pattern which stimulates cell surface receptors on nearby astrocytes and microglia. Neurons releasing mtHsp60 exosomes were targeted for phagocytosis by microglia via TREM2 receptor activation [92]. Extracellular mtHsp60 was also shown to activate astrocyte-dependent inflammation pathways via TLR2 receptor activation [93]. The overproduction of mtHsp60 triggers inflammatory cascades; on the other hand, its expression is induced by diverse cytokines including IL-1beta, TNF-alpha, IL-4, IL-6 and IL-10 [94]. Potentially an amplification cascade, this configuration suggests that a tipping point may exist where mtHsp60 levels activate cell death rather than cell survival pathways. Lastly, cell type-specific pathways are important in the regulation of mtHsp60 as a response to cell stress. For example, in optic nerve head astrocytes, TGF-beta2 and hydrogen peroxide treatment did not have any effect on mtHsp60 and −70 expression.[95].
5.3. Mitochondrial chaperones in Alzheimer’s Disease
Alzheimer’s disease (AD) is a debilitating illness with short term memory loss as a significant early symptom. At the molecular level, the disease is associated with extracellular senile plaques composed of amyloid β (Aβ) aggregates, one of the major pathological hallmarks of AD. Defective mitochondrial chaperones have been implicated in AD, and there is evidence implicating mitochondrial chaperone mtHsp70 specifically. There is also a longstanding hypothesis that amyloid toxicity mediates mitochondrial dysfunction in AD. In spite of this, the underlying mechanisms of how mitochondrial chaperones play a role in AD remain largely unknown.
Mitochondrial alterations have been observed in pyramidal neurons from the brains of AD patients [96]. These mitochondria exhibited reduced size and broken cristae structures [97]. Such mitochondrial dysfunction is one of the earliest features of AD [98,99]. Furthermore, amyloid β associates with mitochondria and more importantly, its levels correlate with both the extent of mitochondrial dysfunction as well as the degree of cognitive impairment in AD mouse models [98]. Amyloid β was shown to impair respiratory chain function directly [100] and induce oxidative stress [101,102]. A more careful survey on mitochondrial structural and functional alterations in AD mouse models revealed that mitochondrial damage was severely augmented in the vicinity of amyloid β plaques [103], suggesting that amyloid β mediates mitochondrial toxicity in AD.
Many studies demonstrate a role for mtHsp70 specifically as a protective agent against Alzheimer’s disease. Overexpression of mtHsp70 reduced damage, apoptosis, and cytotoxicity from amyloid beta and also reversed amyloid beta-induced mitochondrial dysfunction. Protection of mitochondrial function against amyloid beta toxicity was associated with inhibition of the mitochondrial permeability transition pore, decrease in caspase activation, and reduced oxidative stress and cytosolic calcium release. Recently, insult from Aβ treatment was shown to increase the expression and interaction of IP3R, Grp75, and vdac1 and led to an increased endoplasmic reticulum (ER)-mitochondria association. From these data, it would seem that therapies aimed at potentiating the activity or increasing the expression of mtHsp70 might be good candidates for neuroprotective agents in the treatment of Alzheimer’s disease.
The role of mtHsp60 in Alzheimer’s disease is uncertain. mtHsp60 may have a neuroprotective effect, as mtHsp60 overexpression mitigates the toxic effects of Aβ on complex IV activity [104]. mtHsp60 in combination with mtHsp70 and Hsp90 limited the production of free radicals and prevented caspase-9 activation which are important mediators of β-amyloid induced neuronal dysfunction and death [104]. Importantly, mtHsp60 inhibits Aβ aggregation by selective action on peptides that act as seeds for fibrillogenesis; this blocks the initiation of Aβ fibril formation [105]. mtHsp60 may also have a damaging effect, as shown by its ability to associate with amyloid precursor protein (APP) and mediate its movement to the mitochondria, leading to mitochondrial dysfunction[106]. It is also thought to trigger neuronal cell death in Alzheimer’s via extracellular activation of TLR4 [107]. However, several authors have suggested that contaminants with affinity for TLRs could be responsible for many of the reported in vitro effects of Hsp proteins [108].
5.4. Mitochondrial chaperones in Parkinson’s disease and Lewy Body dementia
Parkinson’s disease (PD) is characterized by dropout of dopaminergic cells in the substantia nigra of the basal ganglia causing parkinsonian symptoms such as resting tremor and shuffling gait, whereas Lewy body dementia is characterized by first the onset of hallucinations and REM sleep behavior disorders but then eventually parkinsonian features. Both diseases share significant overlap in terms of symptoms and pathology. Importantly for this review, both diseases involve impairment of mitochondria and proteasomes and are associated with increased toxic damage to cells from protein aggregations composed of alpha-synuclein.
In a proteomics study of mtHsp70, its levels were significantly lower in Parkinson’s brains as well as in a cell model of Parkinson’s disease [109]. The expression of mtHsp70 in astrocytes was found to be lower in the midbrain of PD patients compared to controls [110]. Interestingly, serum levels of mtHsp70 showed negative correlation with α-Synuclein levels in PD patients [111]. Knockdown of mtHsp70 induced loss of synaptic mitochondria in a Drosophila Parkinson’s disease model, suggesting a role of mtHps70 in PD-associated cognitive decline [112].
Evidence of the mechanisms by which mtHsp70 is involved in Parkinson’s disease is scant at the present. Pathologic variants in the genes encoding the mtHsp70 chaperone system may be important in the pathogenesis of Parkinson’s disease, although this is under debate [113,114]; however, mutations in the HspA9 gene have been found in Parkinson’s disease patients [115]. Parkin, an E3 ubiquitin ligase, the PTEN induced putative kinase 1 (PINK1), and DJ-1, a mtHsp70 cochaperone, are the major genes associated with familial early onset Parkinson’s disease [116]. Evidence suggests that PINK1 acts upstream of parkin and that DJ-1 stabilizes PINK1 [116]. It has been shown that DJ-1 regulates the integrity and function of ER-mitochondria association through the mtHsp70/IP3R3/VDAC1 interaction [117].
mtHsp60’s role in Parkinson’s disease is similar to its role in Alzheimer’s. mtHsp60 has been shown to prevent fibril formation of alpha-synuclein, with the apical domain alone being sufficient for suppression of fibrils [118]. Additionally, mtHsp60 may play a positive role in Parkinson’s disease by maintaining the function of complex IV of the electron transport chain, which is implicated in the pathogenesis of Parkinson’s disease [116]. Both of these mechanisms have also been demonstrated as anti-amyloid mechanisms in Alzheimer’s disease.
Generally speaking, mitochondrial chaperones such as mtHsp70 and mtHsp60 have a protective role in various neuronal cells including neurons, astrocytes and microglial cells. Mitochondrial dysfunction associated with mitochondrial chaperone deficiency has been implicated in neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease.
6. Conclusions and future directions
Mitochondrial molecular chaperones are key players in the stress response which restores mitochondrial homeostasis, an important component of cellular homeostasis. mtHsp60 and mtHsp70 are the most prominent mitochondrial molecular chaperones and are crucial for mitochondrial protein import and folding, formation and maintenance of the oxidative phosphorylation machinery, ER-mitochondrial communication, mitochondrial quality control and regulation of apoptosis. As summarized in Figure 2, failure to maintain mitochondrial homeostasis with proper mitochondrial chaperone function will lead to severe health consequences. On one hand, compromised chaperone activity might lead to degenerative diseases and premature aging; on the other hand, over-reactive chaperones could be markers for cancer development. In the coming years, the precise details and working mechanisms connecting different carcinogenesis and cell death signaling incorporating mitochondrial chaperone system leading to tumorigenesis and degenerative phenotypes will be explored. More importantly, in-depth investigation on mitochondrial chaperones and associated regulation of mitochondrial homeostasis will promise hopes for develop protective regiments for age-related degenerative diseases and therapeutical targets for various cancer types.
Mitochondrial chaperones play an important role in mitochondrial homeostasis.
Mitochondrial chaperones integrate mitochondrial stress responses.
mtHsp60 and mytHsp60 are two key mitochondrial chaperones.
Deficient mitochondrial chaperone system has been implicated in aging and age-related degenerative diseases
Overreaction of mitochondrial chaperone system has been implicated in tumorigenesis and could sever as therapeutical target.
7. Acknowledgements
Work in YB’s lab has been supported by National Institute of Health (R01 GM109434 and GM130129) and William and Ella Owens Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Attardi G, Schatz G, Biogenesis of mitochondria, Annu Rev Cell Biol. 4 (1988) 289–333. 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- [2].Wang X, The expanding role of mitochondria in apoptosis, Genes Dev. 15 (2001) 2922–2933. [PubMed] [Google Scholar]
- [3].Falcke M, Hudson JL, Camacho P, Lechleiter JD, Impact of mitochondrial Ca2+ cycling on pattern formation and stability, Biophys J. 77 (1999) 37–44. 10.1016/S0006-3495(99)76870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rustin P, Mitochondria, from cell death to proliferation, Nat Genet. 30 (2002) 352–353. 10.1038/ng0402-352. [DOI] [PubMed] [Google Scholar]
- [5].Ames BN, Shigenaga MK, Oxidants are a major contributor to aging, Ann N Y Acad Sci. 663 (1992) 85–96. 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- [6].Bahr T, Welburn K, Donnelly J, Bai Y, Emerging model systems and treatment approaches for Leber’s hereditary optic neuropathy: Challenges and opportunities, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1866 (2020) 165743. 10.1016/j.bbadis.2020.165743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou C, Lyu L, Miao H, Bahr T, Zhang Q, Liang T, Zhou H, Chen G, Bai Y, Redox regulation by SOD2 modulates colorectal cancer tumorigenesis through AMPK-mediated energy metabolism, Molecular Carcinogenesis. 59 (2020) 545–556. 10.1002/mc.23178. [DOI] [PubMed] [Google Scholar]
- [8].Jiang Z, Bahr T, Zhou C, Jin T, Chen H, Song S, Ikeno Y, Tian H, Bai Y, Diagnostic value of circulating cell-free mtDNA in patients with suspected thyroid cancer: ND4/ND1 ratio as a new potential plasma marker, Mitochondrion. 55 (2020) 145–153. 10.1016/j.mito.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF, The epidemiology of mitochondrial disorders—past, present and future, Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1659 (2004) 115–120. 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [10].Hartl FU, Molecular chaperones in cellular protein folding, Nature. 381 (1996) 571–579. 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- [11].Craig EA, Gambill BD, Nelson RJ, Heat shock proteins: molecular chaperones of protein biogenesis., Microbiol Rev. 57 (1993) 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Labbadia J, Morimoto RI, The Biology of Proteostasis in Aging and Disease, Annu Rev Biochem. 84 (2015) 435–464. 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Becker J, Craig EA, Heat-shock proteins as molecular chaperones, Eur J Biochem. 219 (1994) 11–23. 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- [14].Feder ME, Hofmann GE, Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology, Annu Rev Physiol. 61 (1999) 243–282. 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- [15].Voos W, Röttgers K, Molecular chaperones as essential mediators of mitochondrial biogenesis, Biochim Biophys Acta. 1592 (2002) 51–62. 10.1016/s0167-4889(02)00264-1. [DOI] [PubMed] [Google Scholar]
- [16].Czarnecka AM, Campanella C, Zummo G, Cappello F, Mitochondrial chaperones in cancer: from molecular biology to clinical diagnostics, Cancer Biol Ther. 5 (2006) 714–720. 10.4161/cbt.5.7.2975. [DOI] [PubMed] [Google Scholar]
- [17].van den IJssel P, Norman DG, Quinlan RA, Molecular chaperones: small heat shock proteins in the limelight, Curr Biol. 9 (1999) R103–105. 10.1016/s0960-9822(99)80061-x. [DOI] [PubMed] [Google Scholar]
- [18].Deocaris CC, Kaul SC, Wadhwa R, On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60, Cell Stress Chaperones. 11 (2006) 116–128. 10.1379/csc-144r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Macario AJL, Conway de Macario E, Heat-shock response, overview., in: The Encyclopedia of Stress, Academic Press, San Diego, California, USA, 2000: p. 429. [Google Scholar]
- [20].Webster TJ, Naylor DJ, Hartman DJ, Høj PB, Hoogenraad NJ, cDNA Cloning and Efficient Mitochondrial Import of Pre-mtHSP70 from Rat Liver, DNA and Cell Biology. 13 (1994) 1213–1220. 10.1089/dna.1994.13.1213. [DOI] [PubMed] [Google Scholar]
- [21].Xie H, Hu Z, Chyna B, Horrigan SK, Westbrook CA, Human mortalin (HSPA9): a candidate for the myeloid leukemia tumor suppressor gene on 5q31, Leukemia. 14 (2000) 2128–2134. 10.1038/sj.leu.2401935. [DOI] [PubMed] [Google Scholar]
- [22].Voos W, Chaperone-protease networks in mitochondrial protein homeostasis, Biochim Biophys Acta. 1833 (2013) 388–399. 10.1016/j.bbamcr.2012.06.005. [DOI] [PubMed] [Google Scholar]
- [23].Dores-Silva PR, Barbosa LRS, Ramos CHI, Borges JC, Human Mitochondrial Hsp70 (Mortalin): Shedding Light on ATPase Activity, Interaction with Adenosine Nucleotides, Solution Structure and Domain Organization, PLOS ONE. 10 (2015) e0117170. 10.1371/journal.pone.0117170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hartl FU, Molecular chaperones in cellular protein folding, Nature. 381 (1996) 571–579. 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- [25].Sharma SK, De los Rios P, Christen P, Lustig A, Goloubinoff P, The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase, Nat Chem Biol. 6 (2010) 914–920. 10.1038/nchembio.455. [DOI] [PubMed] [Google Scholar]
- [26].Böttinger L, Oeljeklaus S, Guiard B, Rospert S, Warscheid B, Becker T, Mitochondrial heat shock protein (Hsp) 70 and Hsp10 cooperate in the formation of Hsp60 complexes, J Biol Chem. 290 (2015) 11611–11622. 10.1074/jbc.M115.642017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eisenberg E, Greene LE, Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis, Traffic. 8 (2007) 640–646. 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- [28].Mokranjac D, How to get to the other side of the mitochondrial inner membrane – the protein import motor, Biological Chemistry. 401 (2020) 723–736. 10.1515/hsz-2020-0106. [DOI] [PubMed] [Google Scholar]
- [29].Srivastava S, Savanur MA, Sinha D, Birje A, R V, Saha PP, D’Silva P, Regulation of mitochondrial protein import by the nucleotide exchange factors GrpEL1 and GrpEL2 in human cells, J Biol Chem. 292 (2017) 18075–18090. 10.1074/jbc.M117.788463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schulz C, Rehling P, Remodelling of the active presequence translocase drives motor-dependent mitochondrial protein translocation, Nat Commun. 5 (2014) 4349. 10.1038/ncomms5349. [DOI] [PubMed] [Google Scholar]
- [31].Tamadaddi C, Sagar V, Verma AK, Afsal F, Sahi C, Expansion of the evolutionarily conserved network of J-domain proteins in the Arabidopsis mitochondrial import complex, Plant Mol Biol. 105 (2021) 385–403. 10.1007/s11103-020-01095-8. [DOI] [PubMed] [Google Scholar]
- [32].Herrmann JM, Stuart RA, Craig EA, Neupert W, Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA, J Cell Biol. 127 (1994) 893–902. 10.1083/jcb.127.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L, Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency, Cell. 155 (2013) 160–171. 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee B, Ahn Y, Kang S-M, Park Y, Jeon Y-J, Rho JM, Kim S-W, Stoichiometric expression of mtHsp40 and mtHsp70 modulates mitochondrial morphology and cristae structure via Opa1L cleavage, Mol Biol Cell. 26 (2015) 2156–2167. 10.1091/mbc.E14-02-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin C-S, Meng S, Garbis SD, Moradian A, Taylor RW, Sweredoski MJ, Lomenick B, Chan DC, LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding, Nat Commun. 12 (2021) 265. 10.1038/s41467-020-20597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Honrath B, Metz I, Bendridi N, Rieusset J, Culmsee C, Dolga AM, Glucose-regulated protein 75 determines ER–mitochondrial coupling and sensitivity to oxidative stress in neuronal cells, Cell Death Discov. 3 (2017) 1–13. 10.1038/cddiscovery.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG, The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential, J Biol Chem. 279 (2004) 25689–25695. 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- [38].Londono C, Osorio C, Gama V, Alzate O, Mortalin, Apoptosis, and Neurodegeneration, Biomolecules. 2 (2012) 143–164. 10.3390/biom2010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sinha D, D’Silva P, Chaperoning mitochondrial permeability transition: regulation of transition pore complex by a J-protein, DnaJC15, Cell Death Dis. 5 (2014) e1101. 10.1038/cddis.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].HSPD1 heat shock protein family D (Hsp60) member 1 [Homo sapiens (human)] - Gene - NCBI, (n.d.). https://www.ncbi.nlm.nih.gov/gene/3329#reference-sequences (accessed August 24, 2021).
- [41].Wang JC-Y, Chen L, Structural basis for the structural dynamics of human mitochondrial chaperonin mHsp60, Sci Rep. 11 (2021) 14809. 10.1038/s41598-021-94236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vilasi S, Bulone D, Caruso Bavisotto C, Campanella C, Marino Gammazza A, San Biagio PL, Cappello F, Conway de Macario E, Macario AJL, Chaperonin of Group I: Oligomeric Spectrum and Biochemical and Biological Implications, Front Mol Biosci. 4 (2018) 99. 10.3389/fmolb.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nisemblat S, Yaniv O, Parnas A, Frolow F, Azem A, Crystal structure of the human mitochondrial chaperonin symmetrical football complex, Proc Natl Acad Sci U S A. 112 (2015) 6044–6049. 10.1073/pnas.1411718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gomez-Llorente Y, Jebara F, Patra M, Malik R, Nisemblat S, Chomsky-Hecht O, Parnas A, Azem A, Hirsch JA, Ubarretxena-Belandia I, Structural basis for active single and double ring complexes in human mitochondrial Hsp60-Hsp10 chaperonin, Nat Commun. 11 (2020) 1916. 10.1038/s41467-020-15698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dubaquié Y, Looser R, Fünfschilling U, Jenö P, Rospert S, Identification of in vivo substrates of the yeast mitochondrial chaperonins reveals overlapping but non-identical requirement for hsp60 and hsp10., EMBO J. 17 (1998) 5868–5876. 10.1093/emboj/17.20.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S, Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells., EMBO J. 18 (1999) 2040–2048. 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kumar S, O’Malley J, Chaudhary AK, Inigo JR, Yadav N, Kumar R, Chandra D, Hsp60 and IL-8 axis promotes apoptosis resistance in cancer, Br J Cancer. 121 (2019) 934–943. 10.1038/s41416-019-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ghosh JC, Dohi T, Kang BH, Altieri DC, Hsp60 Regulation of Tumor Cell Apoptosis *, Journal of Biological Chemistry. 283 (2008) 5188–5194. 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- [49].O’Malley J, Kumar R, Inigo J, Yadava N, Chandra D, Mitochondrial Stress Response and Cancer, Trends Cancer. 6 (2020) 688–701. 10.1016/j.trecan.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chandra D, Choy G, Tang DG, Cytosolic Accumulation of HSP60 during Apoptosis with or without Apparent Mitochondrial Release: EVIDENCE THAT ITS PRO-APOPTOTIC OR PRO-SURVIVAL FUNCTIONS INVOLVE DIFFERENTIAL INTERACTIONS WITH CASPASE-3*, Journal of Biological Chemistry. 282 (2007) 31289–31301. 10.1074/jbc.M702777200. [DOI] [PubMed] [Google Scholar]
- [51].Knapowski J, Wieczorowska-Tobis K, Witowski J, Pathophysiology of ageing, J Physiol Pharmacol. 53 (2002) 135–146. [PubMed] [Google Scholar]
- [52].Santra M, Dill KA, de Graff AMR, Proteostasis collapse is a driver of cell aging and death, PNAS. 116 (2019) 22173–22178. 10.1073/pnas.1906592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Macario AJL, Conway de Macario E, Sick chaperones and ageing: a perspective, Ageing Res Rev. 1 (2002) 295–311. 10.1016/s1568-1637(01)00005-8. [DOI] [PubMed] [Google Scholar]
- [54].Iosefson O, Sharon S, Goloubinoff P, Azem A, Reactivation of protein aggregates by mortalin and Tid1--the human mitochondrial Hsp70 chaperone system, Cell Stress Chaperones. 17 (2012) 57–66. 10.1007/s12192-011-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deocaris CC, Kaul SC, Wadhwa R, From proliferative to neurological role of an hsp70 stress chaperone, mortalin, Biogerontology. 9 (2008) 391–403. 10.1007/s10522-008-9174-2. [DOI] [PubMed] [Google Scholar]
- [56].Kimura K, Tanaka N, Nakamura N, Takano S, Ohkuma S, Knockdown of mitochondrial heat shock protein 70 promotes progeria-like phenotypes in caenorhabditis elegans, J Biol Chem. 282 (2007) 5910–5918. 10.1074/jbc.M609025200. [DOI] [PubMed] [Google Scholar]
- [57].Garg S, Huifu H, Kumari A, Sundar D, Kaul SC, Wadhwa R, Induction of Senescence in Cancer Cells by a Novel Combination of Cucurbitacin B and Withanone: Molecular Mechanism and Therapeutic Potential, J Gerontol A Biol Sci Med Sci. 75 (2020) 1031–1041. 10.1093/gerona/glz077. [DOI] [PubMed] [Google Scholar]
- [58].Kiraly VTR, Dores-Silva PR, Serrão VHB, Cauvi DM, De Maio A, Borges JC, Thermal aggregates of human mortalin and Hsp70–1A behave as supramolecular assemblies, Int J Biol Macromol. 146 (2020) 320–331. 10.1016/j.ijbiomac.2019.12.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dores-Silva PR, Minari K, Ramos CHI, Barbosa LRS, Borges JC, Structural and stability studies of the human mtHsp70-escort protein 1: an essential mortalin co-chaperone, Int J Biol Macromol. 56 (2013) 140–148. 10.1016/j.ijbiomac.2013.02.009. [DOI] [PubMed] [Google Scholar]
- [60].Marada A, Allu PK, Murari A, PullaReddy B, Tammineni P, Thiriveedi VR, Danduprolu J, Sepuri NBV, Mge1, a nucleotide exchange factor of Hsp70, acts as an oxidative sensor to regulate mitochondrial Hsp70 function, Mol Biol Cell. 24 (2013) 692–703. 10.1091/mbc.E12-10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Srivastava S, Sinha D, Saha PP, Marthala H, D’Silva P, Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages, Cell Death Dis. 5 (2014) e1394. 10.1038/cddis.2014.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z, Hirano T, Taira K, Kaul SC, Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60, Biochem J. 391 (2005) 185–190. 10.1042/BJ20050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ozkurede U, Miller RA, Improved mitochondrial stress response in long-lived Snell dwarf mice, Aging Cell. 18 (2019) e13030. 10.1111/acel.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou B, Fang L, Dong Y, Yang J, Chen X, Zhang N, Zhu Y, Huang T, Mitochondrial quality control protects photoreceptors against oxidative stress in the H2O2-induced models of retinal degeneration diseases, Cell Death Dis. 12 (2021) 413. 10.1038/s41419-021-03660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yan P, Wang T, Guzman ML, Peter RI, Chiosis G, Chaperome Networks - Redundancy and Implications for Cancer Treatment, Adv Exp Med Biol. 1243 (2020) 87–99. 10.1007/978-3-030-40204-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kabakov A, Yakimova A, Matchuk O, Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy, Cells. 9 (2020) 892. 10.3390/cells9040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yun C-O, Bhargava P, Na Y, Lee J-S, Ryu J, Kaul SC, Wadhwa R, Relevance of mortalin to cancer cell stemness and cancer therapy, Sci Rep. 7 (2017) 42016. 10.1038/srep42016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ban HS, Han T-S, Hur K, Cho H-S, Epigenetic Alterations of Heat Shock Proteins (HSPs) in Cancer, Int J Mol Sci. 20 (2019) 4758. 10.3390/ijms20194758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sinha D, D’Silva P, Chaperoning mitochondrial permeability transition: regulation of transition pore complex by a J-protein, DnaJC15, Cell Death Dis. 5 (2014) e1101. 10.1038/cddis.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schusdziarra C, Blamowska M, Azem A, Hell K, Methylation-controlled J-protein MCJ acts in the import of proteins into human mitochondria, Hum Mol Genet. 22 (2013) 1348–1357. 10.1093/hmg/dds541. [DOI] [PubMed] [Google Scholar]
- [71].Rohlenova K, Sachaphibulkij K, Stursa J, Bezawork-Geleta A, Blecha J, Endaya B, Werner L, Cerny J, Zobalova R, Goodwin J, Spacek T, Alizadeh Pesdar E, Yan B, Nguyen MN, Vondrusova M, Sobol M, Jezek P, Hozak P, Truksa J, Rohlena J, Dong L-F, Neuzil J, Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2high Breast Cancer, Antioxidants & Redox Signaling. 26 (2017) 84–103. 10.1089/ars.2016.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cappello F, David S, Peri G, Farina F, Macario E, Macario A, Zummo G, Hsp60: Molecular anatomy and role in colorectal cancer diagnosis and treatment, Frontiers in Bioscience (Scholar Edition). 3 (2011) 341–51. [DOI] [PubMed] [Google Scholar]
- [73].Tsai Y-P, Yang M-H, Huang C-H, Chang S-Y, Chen P-M, Liu C-J, Teng S-C, Wu K-J, Interaction between HSP60 and β-catenin promotes metastasis, Carcinogenesis. 30 (2009) 1049–1057. 10.1093/carcin/bgp087. [DOI] [PubMed] [Google Scholar]
- [74].Taldone T, Ochiana SO, Patel PD, Chiosis G, Selective targeting of the stress chaperome as a therapeutic strategy, Trends Pharmacol Sci. 35 (2014) 592–603. 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Onyango IG, Khan SM, Bennett JP, Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases, Front Biosci (Landmark Ed). 22 (2017) 854–872. 10.2741/4521. [DOI] [PubMed] [Google Scholar]
- [76].Area-Gomez E, Guardia-Laguarta C, Schon EA, Przedborski S, Mitochondria, OxPhos, and neurodegeneration: cells are not just running out of gas, J Clin Invest. 129 (2019) 34–45. 10.1172/JCI120848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT, Understanding mitochondrial complex I assembly in health and disease, Biochim Biophys Acta. 1817 (2012) 851–862. 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- [78].Willis D, Li KW, Zheng J-Q, Chang JH, Smit AB, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL, Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons, J Neurosci. 25 (2005) 778–791. 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Voloboueva LA, Lee SW, Emery JF, Palmer TD, Giffard RG, Mitochondrial protection attenuates inflammation-induced impairment of neurogenesis in vitro and in vivo, J Neurosci. 30 (2010) 12242–12251. 10.1523/JNEUROSCI.1752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Magen D, Georgopoulos C, Bross P, Ang D, Segev Y, Goldsher D, Nemirovski A, Shahar E, Ravid S, Luder A, Heno B, Gershoni-Baruch R, Skorecki K, Mandel H, Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy, Am J Hum Genet. 83 (2008) 30–42. 10.1016/j.ajhg.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Priyanka null, Wadhwa R, Chaudhuri R, Nag TC, Seth P, Novel role of mortalin in attenuating HIV-1 Tat-mediated astrogliosis, J Neuroinflammation. 17 (2020) 276. 10.1186/s12974-020-01912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gáti I, Leel-Ossy L, Heat shock protein 60 in corpora amylacea, Pathol Oncol Res. 7 (2001) 140–144. 10.1007/BF03032581. [DOI] [PubMed] [Google Scholar]
- [83].Satoh J, Onoue H, Arima K, Yamamura T, The 14–3-3 protein forms a molecular complex with heat shock protein Hsp60 and cellular prion protein, J Neuropathol Exp Neurol. 64 (2005) 858–868. 10.1097/01.jnen.0000182979.56612.08. [DOI] [PubMed] [Google Scholar]
- [84].Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG, Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia, J Cereb Blood Flow Metab. 29 (2009) 365–374. 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shih EK, Robinson MB, Role of Astrocytic Mitochondria in Limiting Ischemic Brain Injury?, Physiology (Bethesda). 33 (2018) 99–112. 10.1152/physiol.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Voloboueva LA, Emery JF, Sun X, Giffard RG, Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin, FEBS Lett. 587 (2013) 756–762. 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Calvillo M, Diaz A, Limon DI, Mayoral MA, Chánez-Cárdenas ME, Zenteno E, Montaño LF, Guevara J, Espinosa B, Amyloid-β(25–35) induces a permanent phosphorylation of HSF-1, but a transitory and inflammation-independent overexpression of Hsp-70 in C6 astrocytoma cells, Neuropeptides. 47 (2013) 339–346. 10.1016/j.npep.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [88].Witte ME, Bø L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJG, De Vries HE, van der Valk P, van Horssen J, Enhanced number and activity of mitochondria in multiple sclerosis lesions, J Pathol. 219 (2009) 193–204. 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- [89].Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG, Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro, J Cereb Blood Flow Metab. 28 (2008) 1009–1016. 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, Tohyama M, M Stern D, Ogawa S, Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease, J Cell Biol. 157 (2002) 1151–1160. 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chen HB, Chan Y-T, Hung AC, Tsai Y-C, Sun SH, Elucidation of ATP-stimulated stress protein expression of RBA-2 type-2 astrocytes: ATP potentiate HSP60 and Cu/Zn SOD expression and stimulates pI shift of peroxiredoxin II, J Cell Biochem. 97 (2006) 314–326. 10.1002/jcb.20547. [DOI] [PubMed] [Google Scholar]
- [92].Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J, The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor, J Neurochem. 110 (2009) 284–294. 10.1111/j.1471-4159.2009.06130.x. [DOI] [PubMed] [Google Scholar]
- [93].Jafarzadeh A, Nemati M, Khorramdelazad H, Mirshafiey A, The Toll-like Receptor 2 (TLR2)-related Immunopathological Responses in the Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis, Iran J Allergy Asthma Immunol. 18 (2019) 230–250. 10.18502/ijaai.v18i3.1117. [DOI] [PubMed] [Google Scholar]
- [94].Bajramović JJ, Bsibsi M, Geutskens SB, Hassankhan R, Verhulst KC, Stege GJ, de Groot CJ, van Noort JM, Differential expression of stress proteins in human adult astrocytes in response to cytokines, J Neuroimmunol. 106 (2000) 14–22. 10.1016/s0165-5728(99)00260-x. [DOI] [PubMed] [Google Scholar]
- [95].Yu AL, Moriniere J, Birke M, Neumann C, Fuchshofer R, Kampik A, Bloemendal H, Welge-Lussen U, Reactivation of optic nerve head astrocytes by TGF-beta2 and H2O2 is accompanied by increased Hsp32 and Hsp47 expression, Invest Ophthalmol Vis Sci. 50 (2009) 1707–1717. 10.1167/iovs.08-1961. [DOI] [PubMed] [Google Scholar]
- [96].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA, Mitochondrial abnormalities in Alzheimer’s disease, J Neurosci. 21 (2001) 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Baloyannis SJ, Mitochondrial alterations in Alzheimer’s disease, J Alzheimers Dis. 9 (2006) 119–126. 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- [98].Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC, Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice, J Alzheimers Dis. 20 Suppl 2 (2010) S535–550. 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- [99].Hauptmann S, Scherping I, Dröse S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Müller WE, Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice, Neurobiol Aging. 30 (2009) 1574–1586. 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [100].Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH, Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons, J Alzheimers Dis. 20 Suppl 2 (2010) S609–631. 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP, Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal, J Neurochem. 69 (1997) 273–284. 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- [102].Abramov AY, Canevari L, Duchen MR, Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase, J Neurosci. 24 (2004) 565–575. 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Xie H, Guan J, Borrelli LA, Xu J, Serrano-Pozo A, Bacskai BJ, Mitochondrial Alterations near Amyloid Plaques in an Alzheimer’s Disease Mouse Model, J Neurosci. 33 (2013) 17042–17051. 10.1523/JNEUROSCI.1836-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW, Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis, J Biol Chem. 281 (2006) 29468–29478. 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- [105].Mangione MR, Vilasi S, Marino C, Librizzi F, Canale C, Spigolon D, Bucchieri F, Fucarino A, Passantino R, Cappello F, Bulone D, San Biagio PL, Hsp60, amateur chaperone in amyloid-beta fibrillogenesis, Biochim Biophys Acta. 1860 (2016) 2474–2483. 10.1016/j.bbagen.2016.07.019. [DOI] [PubMed] [Google Scholar]
- [106].Walls KC, Coskun P, Gallegos-Perez JL, Zadourian N, Freude K, Rasool S, Blurton-Jones M, Green KN, LaFerla FM, Swedish Alzheimer Mutation Induces Mitochondrial Dysfunction Mediated by HSP60 Mislocalization of Amyloid Precursor Protein (APP) and Beta-Amyloid, J Biol Chem. 287 (2012) 30317–30327. 10.1074/jbc.M112.365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rodriguez A, Von Salzen D, Holguin BA, Bernal RA, Complex Destabilization in the Mitochondrial Chaperonin Hsp60 Leads to Disease, Front. Mol. Biosci. 0 (2020). 10.3389/fmolb.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Stricher F, Macri C, Ruff M, Muller S, HSPA8/HSC70 chaperone protein, Autophagy. 9 (2013) 1937–1954. 10.4161/auto.26448. [DOI] [PubMed] [Google Scholar]
- [109].Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J, Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease, Mol Cell Proteomics. 5 (2006) 1193–1204. 10.1074/mcp.M500382-MCP200. [DOI] [PubMed] [Google Scholar]
- [110].Cook TJ, Hoekstra JG, Eaton DL, Zhang J, Mortalin is Expressed by Astrocytes and Decreased in the Midbrain of Parkinson’s Disease Patients, Brain Pathol. 26 (2016) 75–81. 10.1111/bpa.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Singh AP, Bajaj T, Gupta D, Singh SB, Chakrawarty A, Goyal V, Dey AB, Dey S, Serum Mortalin Correlated with α-Synuclein as Serum Markers in Parkinson’s Disease: A Pilot Study, Neuromolecular Med. 20 (2018) 83–89. 10.1007/s12017-017-8475-5. [DOI] [PubMed] [Google Scholar]
- [112].Zhu J-Y, Vereshchagina N, Sreekumar V, Burbulla LF, Costa AC, Daub KJ, Woitalla D, Martins LM, Krüger R, Rasse TM, Knockdown of Hsc70–5/mortalin induces loss of synaptic mitochondria in a Drosophila Parkinson’s disease model, PLoS One. 8 (2013) e83714. 10.1371/journal.pone.0083714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chung SJ, Kim M-J, Ryu H-S, Kim J, Kim YJ, Kim K, You S, Kim SY, Lee J-H , Lack of association of mortalin (HSPA9) and other mitochondria-related genes with risk of Parkinson’s and Alzheimer’s diseases, Neurobiol Aging. 49 (2017) 215.e9–215.e10. 10.1016/j.neurobiolaging.2016.09.017. [DOI] [PubMed] [Google Scholar]
- [114].Freimann K, Zschiedrich K, Brüggemann N, Grünewald A, Pawlack H, Hagenah J, Lohmann K, Klein C, Westenberger A, Mortalin mutations are not a frequent cause of early-onset Parkinson disease, Neurobiol Aging. 34 (2013) 2694.e19–20. 10.1016/j.neurobiolaging.2013.05.021. [DOI] [PubMed] [Google Scholar]
- [115].Flachbartová Z, Kovacech B, Mortalin - a multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration, Acta Virol. 57 (2013) 3–15. 10.4149/av_2013_01_3. [DOI] [PubMed] [Google Scholar]
- [116].Rakovic A, Grünewald A, Voges L, Hofmann S, Orolicki S, Lohmann K, Klein C, PINK1-Interacting Proteins: Proteomic Analysis of Overexpressed PINK1, Parkinsons Dis. 2011 (2011) 153979. 10.4061/2011/153979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu Y, Ma X, Fujioka H, Liu J, Chen S, Zhu X, DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1, Proc Natl Acad Sci U S A. 116 (2019) 25322–25328. 10.1073/pnas.1906565116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yamamoto H, Fukui N, Adachi M, Saiki E, Yamasaki A, Matsumura R, Kuroyanagi D, Hongo K, Mizobata T, Kawata Y, Human Molecular Chaperone Hsp60 and Its Apical Domain Suppress Amyloid Fibril Formation of α-Synuclein, Int J Mol Sci. 21 (2019) 47. 10.3390/ijms21010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zhang S-S, Zhou S, Crowley-McHattan ZJ, Wang R-Y, Li J-P, A Review of the Role of Endo/Sarcoplasmic Reticulum-Mitochondria Ca2+ Transport in Diseases and Skeletal Muscle Function, Int J Environ Res Public Health. 18 (2021) 3874. 10.3390/ijerph18083874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Havalová H, Ondrovičová G, Keresztesová B, Bauer JA, Pevala V, Kutejová E, Kunová N, Mitochondrial HSP70 Chaperone System—The Influence of Post-Translational Modifications and Involvement in Human Diseases, Int J Mol Sci. 22 (2021) 8077. 10.3390/ijms22158077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Graner MW, The unfolded protein response in glioblastomas: targetable or trouble?, Future Sci OA. 1 (2015) FSO45. 10.4155/fso.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bota DA, Davies KJA, Mitochondrial Lon protease in human disease and aging: Including an etiologic classification of Lon-related diseases and disorders, Free Radic Biol Med. 100 (2016) 188–198. 10.1016/j.freeradbiomed.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jafarzadeh A, Nemati M, Khorramdelazad H, Mirshafiey A, The Toll-like Receptor 2 (TLR2)-related Immunopathological Responses in the Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis, Iranian Journal of Allergy, Asthma and Immunology. (2019) 230–250. 10.18502/ijaai.v18i3.1117. [DOI] [PubMed] [Google Scholar]
- [124].Huang Y-H, Yeh C-T, Functional Compartmentalization of HSP60-Survivin Interaction between Mitochondria and Cytosol in Cancer Cells, Cells. 9 (2019) 23. 10.3390/cells9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Jeffery CJ, Protein moonlighting: what is it, and why is it important?, Philos Trans R Soc Lond B Biol Sci. 373 (2018) 20160523. 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Duan Y, Tang H, Mitchell-silbaugh K, Fang X, Han Z, Ouyang K, Heat Shock Protein 60 in Cardiovascular Physiology and Diseases, Front Mol Biosci. 7 (2020) 73. 10.3389/fmolb.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chaiwatanasirikul K-A, Sala A, The tumour-suppressive function of CLU is explained by its localisation and interaction with HSP60, Cell Death Dis. 2 (2011) e219–e219. 10.1038/cddis.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kaul SC, Deocaris CC, Wadhwa R, Three faces of mortalin: A housekeeper, guardian and killer, Experimental Gerontology. 42 (2007) 263–274. 10.1016/j.exger.2006.10.020. [DOI] [PubMed] [Google Scholar]