Abstract
The only natural reservoir of Neisseria meningitidis is the human nasopharyngeal mucosa. Depending on age, climate, country, socioeconomic status, and other factors, approximately 10% of the human population harbors meningococci in the nose. However, invasive disease is relatively rare, as it occurs only when the following conditions are fulfilled: (i) contact with a virulent strain, (ii) colonization by that strain, (iii) penetration of the bacterium through the mucosa, and (iv) survival and eventually outgrowth of the meningococcus in the bloodstream. When the meningococcus has reached the bloodstream and specific antibodies are absent, as is the case for young children or after introduction of a new strain in a population, the ultimate outgrowth depends on the efficacy of the innate immune response. Massive outgrowth leads within 12 h to fulminant meningococcal sepsis (FMS), characterized by high intravascular concentrations of endotoxin that set free high concentrations of proinflammatory mediators. These mediators belonging to the complement system, the contact system, the fibrinolytic system, and the cytokine system induce shock and diffuse intravascular coagulation. FMS can be fatal within 24 h, often before signs of meningitis have developed. In spite of the increasing possibilities for treatment in intensive care units, the mortality rate of FMS is still 30%. When the outgrowth of meningococci in the bloodstream is impeded, seeding of bacteria in the subarachnoidal compartment may lead to overt meningitis within 24 to 36 h. With appropriate antibiotics and good clinical surveillance, the mortality rate of this form of invasive disease is 1 to 2%. The overall mortality rate of meningococcal disease can only be reduced when patients without meningitis, i.e., those who may develop FMS, are recognized early. This means that the fundamental nature of the disease as a meningococcus septicemia deserves more attention.
In 1919, long before the era of antibiotics and intensive care, Herrick stated with respect to meningococcal infections, “no other infection so quickly slays” (217). More than 80 years later, this still holds true. Because healthy young children are primarily the victims of this disease, its incidence continues to increase, and the mortality is still 10% (204, 346, 348, 376); social, medical and scientific vigilance is still required (126).
Neisseria meningitidis is an exclusively human, gram-negative, bean-shaped pathogenic diplococcus that, similar to other gram-negative bacteria, is surrounded by an outer membrane composed of lipids, outer membrane proteins (OMPs), and lipopolysaccharides. Moreover, pathogenic meningococci are enveloped by a polysaccharide capsule attached to this outer membrane.
Meningococci reveal more genetic diversity than most other pathogenic human bacteria. This is explained partly by horizontal intraspecies recombination and incorporation from closely related Neisseria species (80). Traditionally, strains were characterized by using antibodies that recognize surface-exposed epitopes on the capsule or the outer membrane. By this technique, 13 serogroups (identifying capsule antigens), 20 serotypes (identifying class 2/3 OMP antigens), and 10 subtypes (identifying class 1 OMP antigens) have been defined (151). An example of this serological typing is B:4:P1.4, indicating serogroup B, serotype 4, and subtype P1.4. By using the antigenic properties of lipopolysaccharide, renamed lipooligosaccharide (LOS) because of its relatively short sugar chain, another 13 immunotypes, designated by the letter L, can be distinguished (298, 413). Further additional typing is possible by using the antigenic properties of immunoglobulin A1 (IgA1) proteases and pili (452).
Serotyping is of great importance for the development of vaccination strategies. However, although phenotypic characterization may reveal close genetic relatedness, serotyping is not suitable for modern epidemiologic purposes (80). Typing schemes based on variation of a few genes which are probably under selection pressure will not identify the overall relatedness of the chromosomal genome of N. meningitidis (80). By using genetic approaches, in particular multilocus enzyme electrophoresis, which identifies naturally occurring allelic variation in multiple chromosomal housekeeping genes, a better insight into the epidemiology and clonal expansion of disease-causing N. meningitidis can be gained. Other techniques used to this end are DNA fingerprinting and PCR (36, 148, 424, 541).
EPIDEMIOLOGICAL TRENDS
Meningococcal disease occurs worldwide as endemic infections (2, 80, 242, 346, 383, 421). Strains of serogroups B and C cause the majority of infections in industrialized countries. Strains of serogroups A and, to a lesser extent, C dominate in third-world countries (2, 80, 242, 315, 346, 383, 421). The incidence of meningococcal disease during the last 30 years varies from 1–3/100,000 in most industrialized nations to 10–25/100,000 in some third-world countries. These different attack rates reflect the different pathogenic properties of N. meningitidis strains and different socioeconomic, environmental, and climatological conditions.
Sub-Saharan Africa has a special epidemiological pattern. This region, designated the meningitis belt, was first described by Lapeyssonnie in 1963 and comprised 10 countries i.e., Burkina Faso, Ghana, Togo, Benin, Niger, Nigeria, Chad, Cameroon, Central African Republic, and The Sudan (271). Later, Ethiopia, Mali, Guinea, Senegal, and the Gambia were added, to form what is presently denoted the expanded meningitis belt (183, 383). In this region, meningococcal disease caused by serogroup A occurs in yearly recurrent waves. The disease attack rate rises at the end of the dry season and declines rapidly after the beginning of the rainy season (2, 271, 315, 346, 383, 421). During epidemic peaks, the disease incidence may approach 1,000/100,000 inhabitants (383). Initially, a cyclic pattern with epidemics every 8 to 12 years was reported, but this has not been confirmed in later studies for most of the countries (271, 315, 383).
Since the end of the 1960s, widespread epidemics due to genetically closely related strains of N. meningitidis belonging to seven clonal complexes have occurred (80). The largest outbreaks, which originated in northern China and spread to the south and later globally, were caused by two clones of serogroup A (subgroups I and III) (2, 80, 553). The subgroup III clone spread to the Indian subcontinent in 1983 to 1987. In 1987, this clone reached the Middle East and caused a massive epidemic among pilgrims during the Haj in Mecca (2, 80, 383, 421). From here the organism was transported with the Hajis (318), causing epidemics in 1988 in The Sudan and Chad and in the following years in Ethiopia, Kenya, and Uganda (315). In the 1990s, the epidemic moved to countries south of the traditional meningitis belt, reaching Nigeria and South Africa in 1996 (305). In that year, more than 150,000 cases and at least 16,000 deaths were reported in Africa (2, 80, 189). Interestingly, transfer of strains from the same clonal complex by Hajis to the United States and Europe did not elicit epidemics in these parts of the world (316, 318).
In most industrialized countries, serogroup B strains have prevailed the last 30 years. Most of these strains belong to a few clonal complexes, identified as ET-5, lineage III, cluster A4, and ET-37 (80). In northwestern Europe (Norway, Iceland, England, and The Netherlands), hyperendemic infections with an attack rate of 4 to 50/100,000 have prevailed since the mid-1970s (2, 80, 242, 315, 346, 383, 414, 421). This persistent relatively high attack rate is caused mainly by serogroup B strains belonging to the ET-5 complex or lineage III. This strain circulates slowly through the population with a low transmissibility but a high degree of virulence (2, 80, 242, 346, 383, 415, 421).
Group B isolates with ET-5 characteristics were discovered in China in 1974 and in China, Japan, Thailand, Spain, Cuba, Chile, and Brazil in the 1980s. During the 1990s, ET-5 strains have also spread to North Africa, Israel, and Australia (80). In the United States, cases were reported among Cuban immigrants, but in contrast to northwestern Europe, no large epidemics developed. However, in the 1990s an epidemic outbreak caused by the ET-5 clonal complex occurred in the U.S. Pacific Northwest (Oregon) (85). Although in a few cases capsule switching from serogroup B to C was observed, the genetic relatedness and the disproportionate number of cases among young adults demonstrated clearly the transition from an endemic to an epidemic situation (86, 347, 461).
At the same time as the ET-5 clonal complex spread around the world, strains belonging to another serogroup B clonal complex designated lineage III (ET-24 and ET-25) emerged in Europe. First discovered in the Netherlands in 1980, this clone became the most prevalent clone, consisting almost exclusively of B:4:P1.4 strains by 1990 (415). Later, this organism spread to other European countries, including Finland, Norway, and Iceland, although only a few cases occurred in these countries (80). From the beginning of the 1990s, New Zealand experienced a sharp rise in cases caused by lineage III strains (302). In the second half of the 1990s, an increasing number of cases were observed in the United Kingdom, Belgium, and Chile (80, 503).
Strains belonging to the ET-37 clonal complex, which often express serogroup C capsule polysaccharide but also may express serogroup B, W-135, and Y, are found worldwide (80). An isolate belonging to this clonal complex was traced back to at least 1917. In the 1960s, ET-37 strains caused outbreaks among military personnel in the United States (80). In the 1970s, these strains probably spread to Brazil, causing large serogroup C epidemics (80). At the same time, ET-37 strains that expressed the serogroup B capsule were isolated in China, and in the late 1970s serogroup B ET-37 strains were recovered in South Africa (80). In other African countries, serogroup C as well as serogroups W135 and Y strains belonging to the ET-37 complex emerged. In the 1980s, most pathogenic serogroup C strains isolated in Europe and the United States belonged to this complex (80, 357).
When ET-37 variant strains designated ET-15 appeared at the end of the 1980s in North America, the disease attack rate increased (15, 234, 386). In addition, an increasing number of local outbreaks, such as school-based clusters or epidemics originating in a jail, occurred (465, 549). Nevertheless, 90% of the serogroup C cases in the United States are sporadic (378). In the 1990s, ET-15 strains caused outbreaks in Israel, the Czech Republic, Australia, and England (80). Recently, isolates belonging to the ET-15 complex with a serogroup B capsule were found in Canada (256).
In parts of the United States, serogroup Y strains belonging to the ET-508 and related clones emerged in the mid-1990s as an important cause of endemic case clusters. Approximately one-third of the cases in certain areas of the United States are due to this serogroup Y strain (375, 444). Serogroup C strains are responsible for another one-third of cases in the United States, with the remainder of cases being caused by serogroup B and uncommon serogroups (375). The relatively high incidence of serogroup Y cases in the United States is of particular interest since in Europe this serogroup is found almost exclusively in patients with terminal complement deficiencies (140).
Epidemiological studies by modern molecular methods have disclosed a complex picture of a few pathogenic meningococcal clones spreading worldwide. However, the mechanism by which potential pathogenic strains cause large-scale epidemics in some regions while other regions remain unaffected is largely unknown (318). It appears that the occurrence of invasive meningococcal disease is not determined solely by the introduction of a new virulent bacterial strain but also by other factors that enhance transmission and by the susceptibility of the population (444).
CONDITIONS FOR INVASIVE DISEASE
At least four conditions have to be met before invasive disease can occur (421). These conditions are (i) exposure to a pathogenic strain, (ii) colonization of the naso-oropharyngeal mucosa, (iii) passage through that mucosa, and (iv) survival of the meningococcus in the bloodstream. These processes are influenced by bacterial properties, climatological and social conditions, preceding or concomitant viral infections, and the immune status of the patient.
Exposure to Meningococci
The human naso-oropharyngeal mucosa is the only natural reservoir of N. meningitidis. Meningococci are transferred from one person to another by direct contact or via droplets for a distance up to 1 m (331). No exact figures are available, but it seems plausible that the survival of bacteria in these droplets is influenced by climatological conditions such as temperature and humidity. During periods of endemic infection, approximately 10% of the population harbors meningococci in the nose (77, 82, 83, 182). However, 9 of 10 strains isolated from carriers are considered nonpathogenic because they are not associated with the clones cultured from patients with invasive meningococcal disease (33). In three different cross-sectional studies in Norway and the United Kingdom, the carriage rate in children younger than 4 years was <3%. The carriage rate increases with age to a maximum of 24 to 37% at 15 to 24 years, and decreases to <10% at older ages (33, 77, 82, 83). Most adults and children harbor the nonpathogenic Neisseria lactamica (77, 173). The carriage rate of meningococci is higher in lower socioeconomic classes, probably because of crowding, and under conditions where people from different regions are brought together, as for military recruits, pilgrims, boarding-school students, or prisoners (175, 176, 318, 351, 418, 465).
Predicting disease from carriage rates during periods of endemic infection is impossible (S. Kellerman, K. McCombs, P. Pathela, C. Drenzek, A. Margolis, L. Gilbert, J. Berschling, M. Ray, L. Cobb, W. Cheek, J. Koehler, O. Blake, N. Rosenstein, W. Baughman, M. Farley, and D. Stephens, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L32, 1998). The transmission rate of virulent clones is higher, and invasive disease often occurs within the first week after acquisition (130, 300), whereas some persons may carry pathogenic meningococci for many months or years without becoming ill (72). Calculations in Norway suggest that during the endemic situation, acquisition of the pathogenic clones ET-5 and ET-37 induced illness in only 1% of persons harboring these clones (82).
Colonization of the Naso-Oropharyngeal Mucosa
Why certain strains colonize the naso-oropharyngeal mucosa and others do not is the subject of extensive research (310, 329, 364). Colonization takes place both at the exterior surface of the mucosal cell and intra- or subepithelially (446, 449). Damage to the nasopharyngeal ciliated epithelium may be the first step in colonization (379, 451). Physical damage by active or passive smoking increases the risk for carriage and invasive disease (144, 199, 442, 458), as do stressful events and preceding viral infections which either alter the integrity of the mucosal surface or influence local or systemic immunity (78, 199, 317, 380).
Pili are the major adhesins that contribute to the attachment to mucosal cells. These filamentous glycosylated protein appendages emanate from the bacterial surface, traverse the capsular polysaccharide, and bind to receptors on nasopharyngeal cells, i.e., the membrane cofactor protein or CD46 (251, 511). Binding to this receptor transduces a signal to the host cell (250).
After primary binding, further contact with the host cell is established via the class 5 OMPs, Opa and Opc. Opa binds to carcinoembryonic antigen CD66 receptors (513). Interaction with these receptors on phagocytic and endothelial cells mediates phagocytosis and cytokine production (310). Opc binds to heparan sulfate proteoglycan receptors (118). Binding to both types of receptors stimulates the engulfment of meningococci by epithelial cells and transcellular traversal (108). Interestingly, these processes are hindered by the presence of a capsule or sialylated LOS (450, 512), factors that are indispensable for survival of meningococci in the bloodstream (see “Survival of the meningococcus in the bloodstream” below).
During carriage and invasion, the expression of pili, class 5 OMPs, capsule, and LOS is highly variable and subject to phase switching (on/off) and antigenic variation (3, 119, 245, 329). These mechanisms can be used by the bacterium to circumvent host immunity (329, 364).
Invasion or Penetration of the Naso-Oropharyngeal Mucosa
Meningococci pass through the mucosal epithelium via phagocytic vacuoles as a result of endocytosis (306, 329, 381, 446, 448, 450). During invasion, several bacterial factors modulate the metabolism of the mucosal cell (510). As mentioned above, binding of pili and class 5 OMPs to their receptors transduces a signal to the host cell. PorB, a class 2/3 OMP, may translocate into target cell membranes and affect the maturation of phagosomes (401). IgA1 proteases are OMPs that inactivate specific IgA1 (323, 514). By stimulation of the degradation of a membrane glycoprotein in endosomes and lysosomes, they also promote the survival of meningococci in epithelial cells (18).
Frequently, invasive disease is preceded by Mycoplasma pneumoniae or viral (influenza A virus) upper respiratory tract infections; it is assumed that this preceding infection promotes invasion (289, 317, 547). However, it should be noted that these epidemiological studies are prone to confounding bias, since respiratory infections increase mechanical transmission of meningococci by sneezing and coughing and since meningococci themselves may cause signs of upper airway infection (339; J. V. Pether, R. J. Scott, and P. Hancock, Letter, Lancet 344:1636, 1994).
Survival of the Meningococcus in the Bloodstream
Meningococci can survive and proliferate in the bloodstream by virtue of particular bacterial virulence factors or incompleteness of the host defense.
Members of the Neisseriaceae have developed a mechanism for acquiring iron from human transferrin by using transferrin binding proteins (353, 417). However, the most essential bacterial virulence factor for survival in the bloodstream is the polysaccharide capsule, which protects against complement-mediated bacteriolysis and phagocytosis by neutrophils, Kupffer cells, and spleen macrophages (260). In brief, sialic acid residues in the group B and C capsule and possibly LOS immunotype L3 decrease the serum bactericidal activity by enhancing the affinity of the alternative-pathway inhibitor factor H to C3b and thus inhibiting complement activation (112, 136, 137, 239, 245, 248, 260, 398, 515). In addition, some class 1 OMPs impede ingestion of the meningococcus by neutrophils via downregulation of the Fcγ receptor and the C1 and C3 receptor (34), and IgA1 proteases break IgA1 in the hinge region and liberate monomeric Fabα fragments, which can block the access for intact IgG or IgM (323).
Host defense after meningococcal invasion is determined by humoral and cellular responses belonging to the innate and adaptive immune systems. Specific antibodies provide full protectivity. However, because the production of antibodies takes at least 1 week after colonization, the initial defense is dependent primarily on elements of the innate response.
Cornerstones of the early innate defense are complement-mediated bacteriolysis and opsonophagocytosis. Early complement activation occurs via the mannose binding lectin and the alternative pathway. Some genetically determined variants of mannose binding lectin predispose to invasive disease (219). Alternative-pathway defects such as X-linked properdin deficiency may lead to overwhelming invasive disease (138, 435). Deficiency of one of the terminal complement factors increases the chance for invasive disease, mainly by uncommon serogroups, up to 6,000-fold (112, 139, 140, 397). However, due to the rarity of these deficiencies, only a few cases can be explained by these defects. In a Norwegian survey of 98 individuals who survived meningococcal disease, no patient with a complete deficiency was found, and a Dutch study of 29 children surviving fulminant shock revealed only 1 case (115, 223). Deficiency of protein C, a regulator involved in the coagulation, anticoagulation, and fibrinolytic systems, leads to extensive diffuse intravascular coagulation (DIC) and necrosis (138).
In normal individuals, the incidence of meningococcal disease is reciprocally related to the titer of specific antibodies, with the highest incidence occurring from 6 to 24 months of age, when maternal antibodies have disappeared (175, 176). Throughout life, specific antibodies are induced by the continuously repeated and intermittent carriage of different meningococci and N. lactamica (173, 175, 176, 247). Certain enteric bacteria have a capsule that is structurally and immunologically identical to the capsular polysaccharide of meningococci. This is the case for Bacillus pumilus and serogroup A meningococci, and for Escherichia coli K1 and serogroup B strains (178, 253, 504). It has been suggested that these bacteria contribute to the defense against meningococci by the induction of cross-reacting antibodies (253, 504). On the other hand, it has been suggested that IgA antibodies, which do not activate complement, may adhere to important epitopes and block these epitopes for the bactericidal effects of complement-activating IgG and IgM antibodies (185, 186). The importance of blocking IgA antibodies is still being debated.
Although specific antibodies provide full protection against invasive disease, only a few cases have occurred in patients with hypogammaglobulinemia (216, 281); J. L. Bass, R. Nuss, K. A. Mehta, P. Morganelli, and L. Bennett, Letter, N. Engl. J. Med. 309:430, 1983). Interestingly, a genetic polymorphism of the Fcγ receptor [Fcγ-RIIa(CD32)] that binds IgG2 poorly and leads to diminished opsonophagocytosis predisposes individuals to meningococcal disease (62).
The relevance of cellular immune defects is less well established. There is no proven relation between meningococcal disease and HLA phenotype (266). Splenectomy is a well-defined risk factor for overwhelming infections with encapsulated bacteria; however, invasive meningococcal disease is only rarely observed in splenectomized individuals (227, 294). Immunosuppressive drugs or autoimmune diseases such as lupus erythematosus are risk factors (292, 312). Human immunodeficiency virus seropositivity is not a defined risk factor (63, 334), although cases in human immunodeficiency virus-seropositive individuals have been reported (447).
In conclusion, growth of the meningococcus in the bloodstream can occur when intravascular killing is impaired, either because of special properties of the meningococcus itself or because of a naive or defective immune system of the host.
CLINICAL PRESENTATION OF INVASIVE MENINGOCOCCAL DISEASE
Once viable meningococci have reached the bloodstream, different disease manifestations can develop. In some patients, probably those with low degrees of bacteremia, meningococci are cleared spontaneously, leaving behind a so-called transient meningococcemia characterized by a short febrile flu-like episode (104, 162, 427, 459; S. P. Taubkin, Letter, Pediatr. Infect. Dis. J. 1:374). When the bacteremia is not cleared, clinically overt disease develops. In these cases, the ultimate clinical presentation is determined by bacterial properties such as endotoxin release and by host characteristics such as the host immune status (see “Survival of the Meningococcus in the Bloodstream” above) and possibly endotoxin responsiveness.
Endotoxin release is a strain-specific virulence factor. During growth and lysis of meningococci, endotoxin is released in the form of vesicular outer membrane structures (blebs) consisting of up to 50% of LOS, and OMPs, lipids, and capsular polysaccharides (117, 479). During invasive disease, these structures can be visualized in plasma or cerebrospinal fluid (CSF) by electron microscopy (48, 445). Strains isolated from patients with meningococcal septic shock liberate more endotoxin than do strains isolated from patients with chronic benign meningococcemia (368).
Endotoxin-induced cytokine production differs among individuals (64). This genetically determined trait is referred to as endotoxin responsiveness. Originally it was suggested that patients who develop overwhelming sepsis are highly endotoxin responsive, i.e., genetically inclined to produce larger amounts of proinflammatory cytokines such as tumor necrosis factor (TNF) (326, 533). Later studies suggested that a shortage in TNF production and an increased production of the anti-inflammatory interleukin-10 (IL-10) correlate with a poor prognosis (531). However, this latter view conflicts with observational studies by Waage et al., who found a striking association between massively elevated levels of bioactive TNF in serum and death (517, 518). Since shock and high cytokine concentrations are encountered particularly in patients with low titers of bactericidal antibodies and high titers of capsular antigen or endotoxin, unimpeded and massive outgrowth of meningococci is probably much more important than the phenomenon of endotoxin responsiveness (54, 55, 57, 129, 194, 495, 535).
In nearly all patients who develop shock and in most patients with meningitis, the beginning of the bacteremic phase is marked by the onset of chills, acute fever, low-back pain, thigh pain, or generalized muscle aches (296). Within a few hours, fulminant meningococcal sepsis (FMS) may develop without signs of meningitis; this condition is characterized by high concentrations of endotoxin and cytokines in plasma (Fig. 1). Because one of the striking features of meningococci is their propensity to invade the meninges (217, 327, 329), patients with less marked bacterial proliferation in the bloodstream and less cytokinemia present after 18 to 36 h with meningitis. In these patients, blood cultures are often negative at time of hospitalization. Due to the limited growth of bacteria in the bloodstream and the seeding of meningococci in the subarachnoid space, patients with meningitis have compartmentalized high concentrations of endotoxin and cytokines in the CSF (50, 495).
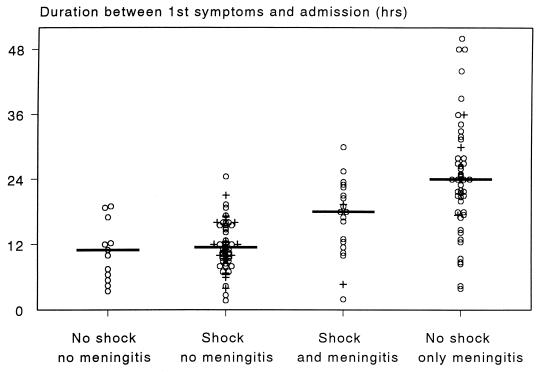
FIG. 1.
The time elapsed between the first symptoms of disease (noted by the patient, parents, or relatives) and the moment of hospital admission of 140 patients with invasive meningococcal disease admitted from 1984 to 1998 to the ICU of the University Hospital, Nijmegen. Patients are classified into four groups: (i) no shock and no meningitis (n = 13), (ii) shock and no meningitis (n = 57), (iii) shock and meningitis (n = 20), and (iv) meningitis without shock (n = 50). Shock is defined as the presence of systolic hypotension, refractory to a fluid bolus and requiring vasopressors, of <100 mm Hg in adults, <85 mm Hg in children 4 to 14 years old, and <75 mm Hg in children younger than 4 years. Meningitis is defined as >108 leukocytes/liter or CSF or, when no spinal tap is performed, the presence of nuchal rigidity (163, 475); J. F. Sinclair, C. H. Sheoch, and D. Hallworth, Letter, Lancet ii:38, 1987). Horizontal lines indicate median values. Crosses refer to fatalities.
Based on the sequence of pathophysiological events, patients with invasive meningococcal disease can be classified into four groups (163): (i) patients with bacteremia without shock, (ii) patients with bacteremia with shock but no meningitis (i.e., FMS), (iii) patients with shock and meningitis, and (iv) patients with meningitis alone. This classification correlates with the duration of disease before hospitalization (Fig. 1); the site, severity, and pattern of mediator activation; and the prognosis. Classification of patients into one of these clinically easily recognizable groups is a great help for clinical decision-making, particularly for the installation of immediate and maximal intensive-care support (54, 55, 192, 193, 495, 498, 517; M. Van Deuren, J. van der Ven-Jongekrijg, and J. W. M. van der Meer, Proc. 3rd Int. Symp. Chemotactic Cytokines, abstr. P-28, 1992).
Incidentally, other compartmentalized metastatic infections, such as arthritis or pericarditis, can develop; the latter is caused mainly by serogroup C (38, 404, 529; T. W. Austin and K. R. Gurr, Letter, Clin. Infect. Dis. 20:473, 1995; D. S. Damary, D. A. Sherlock, and J. Croall, Letter, J. Infect. 35:320–321, 1997). Cellulitis and endophthalmitis have been found occasionally (292, 338; T. Sleep and M. Graham, Letter, Br. J. Ophthalmol. 81:1016–1017, 1997). Primary pyogenic arthritis or pericarditis should not be confused with the reactive, immune complex-mediated arthritis or pericarditis, which is often combined with a rash and recrudescence of fever and occurs in 10 to 20% of the patients on days 4 to 7 during the convalescent phase of meningococcal disease (184). A small group of patients, probably less than 1% and consisting mainly of adults, can present with one or more episodes of spiking fever, arthralgia, or arthritis and a recurrent rash; this syndrome is designated chronic benign meningococcemia (360; R. F. Wynn, R. B. S. Laing, C. L. S. Leen, P. Stratham, and F. X. Emmanuel, Letter, Clin. Infect. Dis. 18:829–830, 1994). How these patients tolerate the potentially lethal bacterium for several weeks in their bloodstream is not understood.
In addition to these blood-borne infections, other meningococcal infections such as primary meningococcal conjunctivitis, pneumonia, sialadenitis, adnexitis, or pelvic inflammatory disease have been reported (9, 20, 24, 164, 402; J. D. Cher, W. J. Maxwell, N. Frustajer, M. Marin, and L. D. Wiviott, Letter, Clin. Infect. Dis. 17:134–135, 1993). Meningococcal pneumonia occurs principally in immunocompromised or elderly patients (447). The diagnosis is easily overlooked because clinically there is nothing to differentiate it from pneumonia of other nosocomial causes (246), meningococci are easily missed in cultures of respiratory secretions, and the disease responds to standard antibiotic therapy (105). Meningococcal infections in the upper or lower airways, genitals, and anus differ clearly from invasive disease, since they develop without preceding bacteremia. However, vigilance is still needed since these infections may precede invasion and may cause secondary cases (246, 337, 395; G. Holdsworth, H. Jackson, and E. Kaczmarski, Letter, Lancet 348:1443, 1996).
PATHOPHYSIOLOGY OF INVASIVE DISEASE
Pathophysiology of Fulminant Meningococcal Sepsis
FMS is characterized by shock and DIC, two interrelated processes. Shock and DIC have common causal mechanisms and reinforce each other. For instance, microvascular thrombosis leads to hypoperfusion (i.e., shock) and shock induces endothelial damage and DIC. To quote Hardaway, “Shock is both cause and effect of DIC” (200).
Pathophysiology of shock.
Shock is caused by capillary leakage, inappropriate vascular tone, intravascular microthrombi, and myocardial dysfunction. The central activator that elicits these derangements is meningococcal endotoxin (6, 69, 212), and the severity of shock correlates with the degree of endotoxinemia (54, 55, 57, 495).
From the early onset of disease, meningococcal endotoxin activates zymogens belonging to the complement system, contact system, and kallikrein-bradykinin system. Since activation of these zymogens requires only proteolytic cleavage by a serine protease, the activated factors of these systems appear immediately. Similarly, neutrophils release elastase and other lysosomal proteinases instantaneously from their storage pools (88, 440). Endotoxin also induces the production, expression and release of mediators such as tissue factor (TF), tissue plasminogen activator (TPA), and the pro- and anti-inflammatory cytokines. Since these latter mediators are proteins that have to be synthesized, they appear approximately 1 to 2 h later than the activated zymogens and proteins released from storage pools (88). Nearly all these mediators can induce shock, either alone or in synergy.
In the early stage of invasive disease, complement is activated mainly by the alternative pathway and partly by the classical or mannose-binding lectin pathway (51, 55, 209). The degree of complement activation correlates with the severity of shock (55, 209, 224). Because complement activation takes place on blebs, no effective complement-mediated bacteriolysis occurs. The relative deficiency of the regulatory proteins C4 binding protein (C4Bp) and C1 inhibitor (C1-INH), due to consumption and downregulated production, contributes to the unimpeded activation of complement via the classical pathway (209). The resulting increase of the anaphylatoxins C3a and C5a causes, in concert with bradykinin and the elastase-induced endothelial damage, vasodilatation and capillary leakage.
High cytokine concentrations correlate with the severity of shock. After the first observation in 1987 by Waage et al. that TNF levels in serum correlated with mortality (518), similar patterns were reported for other cytokines such as IL-1β, IL-6, IL-8, leukemia inhibitory factor, gamma interferon, granulocyte colony-stimulating factor, monocyte colony-stimulating factor, and granulocyte-monocyte colony-stimulating factor (168, 192, 263, 495, 498, 517, 526, 527; Van Deuren et al., Abstr. Proc. 3rd Int. Symp. Chemotactic Cytokines). Likewise, the levels of the anti-inflammatory cytokines IL-10, IL-12, and IL-1 receptor antagonist (IL-1Ra) and both TNF soluble receptors (TNFsRs) are increased (113, 169, 210, 283, 489, 495, 496, 498). The exact role of these cytokines in the genesis of shock is difficult to define, since their activity is modulated by the simultaneous presence of soluble cytokine receptors. For instance, IL-6 activity is upregulated by the diminished levels of IL-6 soluble receptor (IL-6sR) in plasma (14, 154) while TNF activity is downregulated by the increased TNFsR levels (169).
Because the ratio of TNF to TNFsR is higher in severe shock than in moderate disease, the original concept was that the proinflammatory cytokine activity outweighs the anti-inflammatory activity in severe shock (169). However, more recent studies underline the importance of proinflammatory cytokines in the initial defense against bacteria. The production of proinflammatory cytokines by peripheral blood cells is downregulated in the early stages of invasive disease (493, 496). Relatives of persons who died of their meningococcal infection produced less TNF and more anti-inflammatory IL-10 than did relatives of survivors (531). In addition, it was shown that the plasma of patients with shock inhibits the cytokine-inducing and procoagulatory activities of endotoxin (56, 206) and that patients with fatal sepsis of various etiologies have lower levels of TNF and higher levels of IL-10 (500). Together, these observations suggest that in severe shock, in spite of a strong proinflammatory state, anti-inflammatory responses that are regulatory in nature are highly upregulated.
Regardless of the clinical course, the increased concentrations in plasma of all cytokines and other mediators involved in the genesis of shock and DIC (see the next section) decline rapidly after the start of antibiotic therapy, suggesting an efficient clearing mechanism and downregulated production (158, 488, 493, 496, 517, 526, 527). Antibiotic-induced increases in the levels of endotoxin or cytokines have never been demonstrated in clinical studies (54, 158, 487, 517). In the Intensive Care Unit (ICU) of the University Hospital Nijmegen, we monitored the course of TNF and IL-1 from admission to recovery or death in 37 FMS patients; TNF and IL-1 concentrations increased after admission in only 1 patient (487). Since this patient was the only patient in the study who did not receive prompt antibiotic treatment because the diagnosis was missed initially, these data advocate strongly against postponement of antibiotic therapy.
The decrease in the level of endotoxin has a half-life of approximately 3 h (54, 494). The half-lives for TNF and IL-1 are 1 to 3 h and 2 h, respectively (158, 488, 517). The half-life for IL-6 is 2 to 6 h (154, 517); R. G. I. Westendorp, A. Brand, J. Haanen, V. W. M. van Hinsbergh, J. Thompson, R. van Furth, and E. A. Meinders, Letter, Am. J. Med. 92:577–578, 1992), similar to that of IL-8 and IL-10 (283); Van Deuren, et al., Abstr. Proc. 3rd Int. Symp. Chemotactic cytokines). In contrast, the half-life of TNFsRs is 3 to 5 days (489). IL-1Ra and plasminogen activator inhibitor 1 (PAI-1) levels may peak shortly after admission and decline afterward, with a half-life of approximately 6 to 12 h (52, 498). Interestingly, the complement system is the only mediator system that shows ongoing activation during the first 12 to 24 h in spite of the initiation of antibiotic therapy (51, 55, 209), and it is tempting to hypothesize that the clinical deterioration that is sometimes observed after the start of therapy (the Jarisch-Herxheimer reaction) (30) is caused by this continuing activation of complement.
DIC and myocardial depression further aggravate the shock state (31, 45, 314). One of the typical features of FMS is that myocardial depression caused by endovascular thrombosis, vasculitis, and a circulating myocardial depressant factor (possibly TNF or IL-1) reaches its maximum within a few hours after admission (39, 202, 208, 268, 309). Echocardiography performed at this time shows an increased end-diastolic volume and decreased left-ventricular shortening fraction. The cardiodepressive state may last for 7 to 10 days. Involvement of the conductive system may cause life-threatening bradyarrhythmias (392, 426). Cardiac tamponade due to an immune-complex-mediated sterile pericarditis may lead to hemodynamic deterioration during the recovery phase of any invasive meningococcal infection (321, 356).
Pathophysiology of DIC.
Skin hemorrhages are the hallmark of invasive meningococcal disease. Microscopically, these lesions are characterized by endothelial damage and hemorrhages around and microthrombi in small vessels, consistent with a generalized Sanarelli-Shwartzman reaction. The lesions are a reflection of the endotoxin- and cytokine-primed vasculitis that is mediated by the upregulation of adhesion molecules on endothelium and degranulating activated neutrophils (13, 220, 322, 439). Clinically, they are the visual manifestations of DIC and consumption coagulopathy. Although DIC is a generalized phenomenon affecting all organs, the adrenals are particularly vulnerable. Adrenal hemorrhages, diagnosed postmortem as Waterhouse-Friderichsen syndrome, may lead to transitory adrenal insufficiency (39, 43, 201, 522). It is of interest that the intracerebral vessels remain spared during FMS.
Most of our knowledge of sepsis-induced DIC originates from infusion experiments with endotoxin, TNF, or other cytokines such as IL-1 and IL-6 and in vitro experiments with monocytes or endothelial cells exposed to these compounds (287, 288, 412, 486). One of the initial procoagulant processes elicited was the expression of TF, the initial step in the activation of the extrinsic coagulation pathway (218) that ultimately results in thrombin formation. In these experimental studies, thrombin inactivation is impaired by the downregulation of thrombomodulin on endothelial cells. This downregulation also impairs protein C function, which results in less inactivation of FVa and FVIIIa and reduced activation of fibrinolysis. In these infusion experiments, activation of fibrinolysis by TPA occurs only during the first 3 h; after this time the fibrinolysis subsides because of the increasing concentrations of PAI-1 (287, 288).
Although endotoxin is a well-known activator of Hageman factor (FXII) (320), the intrinsic coagulation pathway or contact system is not activated in the low-dosage endotoxin infusion models. However, in patients with septic shock or in baboons with lethal experimental bacteremia, FXII is clearly activated (252, 336, 359), whereas the levels of C1-INH and α2-macroglobulin, the natural inhibitors of FXIIa, are decreased (106, 107). FXII activation during sepsis has multiple effects. FXIIa is the initiating protease of the contact system and activator of fibrinolysis, as well as an activator of complement. FXIIa further induces the conversion of prekallikrein to kallikrein, the enzyme that cleaves high-molecular-weight kininogen into the potent vasodilator nonapeptide bradykinin. Both complement activation and bradykinin formation induce hypotension. In addition, FXIIa stimulates the release of elastase by neutrophils, which probably plays an important role in the genesis of acute respiratory distress syndrome (ARDS) (79, 124).
Contact activation has been demonstrated in meningococcal infections. In patients with severe FMS, low levels of prekallikrein (60) and high levels of the elastase-antiprotease complex that reflects neutrophilic activation by endotoxin, TNF, FXIIa, or complement (53, 440) are found.
The significance of contact activation in lethal-sepsis models is demonstrated by the inhibition of FXIIa with monoclonal antibodies. FXIIa inhibition reverses hypotension and attenuates complement and elastase release but does not inhibit DIC (238, 358). However, in view of the myriad interactions between the various mediator networks, the close relationship between shock and DIC, and the fact that activation of the intrinsic coagulation pathway in FMS is well established (543), further study to elucidate the role of FXII activation in the genesis of DIC in patients with FMS is warranted.
In meningococcal infections, severe DIC is associated with a very poor prognosis (211, 507). Østerud and Flaegstad showed that an increased activity of TF on monocytes is associated with a high fatality rate (341). Other poor prognostic signs are a low concentration of FVII, FX, FV, FVIII, and fibrinogen in plasma, reflecting severe consumption-coagulopathy, and increased fibrinopeptide A levels, mirroring fibrin formation (58, 101, 141, 170, 272, 307). Also, low concentrations of the anticoagulant factors antithrombin III (AT-III), protein C, and protein S are associated with a poor prognosis (58, 142, 272, 278, 365; D. R. Powars, Z. R. Rogers, M. J. Patch, W. G. McGehee, and R. B. Francis, Jr., Letter, N. Engl. J. Med. 317:571–572, 1987). Similarly, low plasminogen levels in the very early stage and high PAI-1 concentrations, showing inhibition of fibrinolysis, are associated with a severe course (52, 53, 134, 263; R. G. J. Westendorp, A. Brand, J. Haanen, V. W. M. van Hinsbergh, J. Thompson, R. van Furth, and E. A. Meinders, Letter, Am. J. Med. 92:577–578, 1992). This latter factor is of special importance, since it was recently shown that persons who are inclined to produce more PAI-1 due to polymorphism in the PAI-1 promoter gene have a greater chance of developing severe shock (215, 261, 530).
Outcome of Fulminant Meningococcal Sepsis
The mortality rate of FMS is high and varies from 20 to 80% in different studies. This wide range can be explained partly by selection bias. Tertiary-care centers see more severely ill patients but may document better survival rates because a portion of the patients who die will do so before arrival at the ICU (Fig. 2). Variation in the reported mortality rates can be explained further by the diversity in the natural course of the disease and quality of medical treatment in the hours before arrival at the ICU. Finally, the difference can be explained by different disease definitions. Clinical studies include mainly patients with well-defined shock criteria, whereas many epidemiological studies define sepsis simply by the presence of a purpuric rash or positive blood culture. Clearly, unequivocal definitions are needed for reliable comparison of patients, therapies, and outcomes (H. T. Sorensen, F. H. Steffersen, G. L. Schonheyder, G. L. Nielsen, and J. Olsen, Letter, Br. Med. J. 316:1016–1017, 1998; M. J. Tarlow and A. M. Geddes, Letter, Lancet 340:1481, 1992).
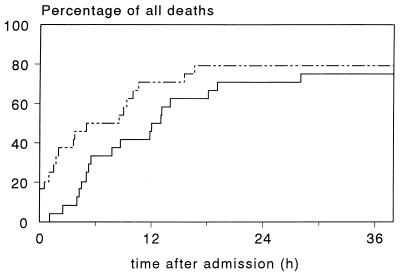
FIG. 2.
Time of death for 24 patients who died of meningococcal septic shock, with respect to the time of hospital admission (continuous line) or ICU admission (dotted line).
Clinical deterioration is overwhelming, and approximately half of the patients who die will do so within 24 h after the first symptoms occur. As estimated from 24 patients who died in the ICU of the Nijmegen University Hospital (Fig. 2) and data reported in the literature (66, 133, 290, 299, 307, 309, 333), one-third of the patients with fatal disease die within 6 h after hospital admission and one-third die between 6 and 18 h. Death at a later stage is still determined by the course in the early hours, as shown by the fact that the principal cause of death after 24 h is withdrawal of treatment because of poor neurological prognosis after prolonged cerebral hypoperfusion in the early hours (313).
Recovery may be complicated by hemorrhages, ARDS, anuria, and multiple-organ failure. In some cases ARDS develops within a few hours after admission (537). Skin and limb necrosis requiring amputation or plastic surgery is seen in 10 to 20% of patients. Of the 55 survivors of FMS in the ICU of the Nijmegen University Hospital from 1984 to 1998, 6 (11%) needed amputation of an extremity (4 patients) or digits (2 patients) while another 6 (11%) needed reconstructive skin surgery. Muscle necrosis results in myoglobinuria, which can lead to renal failure after 2 to 3 days (47, 490). Bone ischemia may lead to osteonecrosis (67).
After 4 to 10 days, 10 to 20% of patients with meningococcal infections have a recrudescence of fever that generally is accompanied by a rash and sometimes by a sterile arthritis or pericarditis (131, 321, 404, 535). As these immunecomplex-mediated manifestations subside spontaneously, only symptomatic therapy is needed.
Pathophysiology of Meningococcal Meningitis
A great deal of our knowledge of the pathophysiology of bacterial meningitis is based on experiments in which bacteria are inoculated intracisternally (372, 480). Consequently, these models do not incorporate the immunological and endocrinological effects of a preceding bacteremia. The relatively rare studies that use an intranasal bacterial challenge mimic the human situation more closely (408).
The mechanisms behind the propensity of meningococci to invade the meninges and their passage across the blood-brain barrier are poorly understood (327, 329). Once in the subarachnoid space, where the principal humoral and cellular host defense mechanisms are absent (432, 555), meningococci proliferate uncontrolled (57). The evolving endotoxin liberation elicits compartmentalized (i.e., confined to the subarachnoid space) activation of proinflammatory cytokines such as TNF, IL-1, IL-6, IL-8, nitric oxide, monocyte colony-stimulating factor, and platelet-activating factor and anti-inflammatory cytokines such as IL-1Ra, IL-10, IL-12, TNFsR-p55, TNFsR-p75, and IL-1sR type II (IL-1sRII) (12, 57, 192, 264, 295, 429, 498, 502, 517, 519–521). Among these, TNF and IL-1 enhance the permeability of the blood-brain barrier and promote the influx of neutrophils by upregulation of adherence molecules (258, 374, 377, 428). The subsequent release of neutrophil products contributes to the development of clinically overt meningitis.
Antibiotics do not halt the meningeal inflammatory process immediately (11, 467, 469). Some studies show even a transitory worsening of the inflammation after antibiotic administration, possibly due to the enhancement of endotoxin release (153, 324). This finding contrasts with studies of sepsis, where antibiotic-induced endotoxin release has never been observed (see “Pathophysiology of shock,” above, and “Antibiotic treatment” below). A possible explanation for this discrepancy is that the clearance of endotoxin and/or the regulation of the production of cytokines in the CSF differs from that in the blood compartment.
Analogous to the high concentrations of endotoxin, TNF, IL-1, and IL-6 in the plasma of patients with FMS, the concentration of these mediators in CSF of patients with meningitis is increased. However, cytokine activation in CSF is not a mere copy of that in blood during FMS. For instance, the ratio in CSF of TNFsR-p75 to TNFsR-p55 and the pattern of IL-1sRII in patients with meningococcal meningitis differs from that in plasma of patients with FMS (495, 498). This dissimilarity can be explained by a different kinetic behavior of these mediators, a different cellular source, or a different interplay with other mediator systems because zymogens, the starting players in the immune response in the blood compartment, are absent in native CSF.
The differences between FMS and meningitis are also evident clinically and histopathologically. The major difference between sepsis and meningitis is that in meningitis the inflammatory response is localized in an extravascular compartment devoid of zymogens belonging to the complement and coagulation systems. While meningococcal sepsis is the most devastating form of sepsis, with a high rate of mortality and sequelae caused by endovascular inflammation and thrombosis, meningococcal meningitis has a relatively low rate of mortality and neurological sequelae compared to other types of bacterial meningitis (21, 172, 419).
Outcome of Meningococcal Meningitis
Since the skull cannot expand, cerebral edema will result in increased intracranial pressure and impeded cerebral perfusion (16). Sometimes fatal brain stem herniation occurs. The 1 to 5% mortality rate associated with meningococcal meningitis is caused almost exclusively by this nearly intractable rapidly fatal complication (335; W. T. Conner and J. A. Minielly, Letter, Lancet ii:967–969, 1980; T. Stephenson, Letter, Br. Med. J. 316:1015, 1998). In most of these cases, autopsy shows not only meningitis but also encephalitis in the regions adjacent to the meninges. In the 58 patients with meningococcal meningitis without shock, referred from 1984 to 1998 to the ICU of the University Hospital Nijmegen, brain stem coning occurred in five patients: three times before arrival at the emergency room and twice within 1 h before arrival at the ICU.
Reportedly, 8 to 20% of survivors suffer from neurological sequelae, varying from sensorineural deafness, mental retardation, spasticity, and/or seizures to concentration disturbances (21, 123, 131, 135, 363, 410). The incidence of neurological sequelae after meningococcal meningitis is lower than after pneumococcal meningitis (21), perhaps because in meningococcal meningitis the subarachnoidal inflammation is extinguished faster (393). Cerebral abscesses do not occur after meningococcal meningitis.
DIAGNOSIS AND RECOGNITION OF PATIENTS AT RISK
Acute meningococcal disease, in particular FMS, can be fatal within a few hours. Therefore, early diagnosis and immediate recognition of (imminent) deterioration is pivotal.
Early diagnosis of meningococcemia is extremely difficult and requires a high degree of suspicion (179, 180, 388). Typically, a completely healthy child complains of myalgia of sudden onset, chills, and fever (296). After 4 to 6 h, there may be a transient clinical improvement that conceals the ongoing deterioration. At this early stage, symptoms and signs are absent or confusing (97, 524). The initial skin manifestations resemble a viral rash (303, 388, 478; P. Baxter and B. Priestly, Letter, Lancet i:1166–1167, 1988); the neck is supple and examination of CSF, including a Gram stain, is inconclusive (93; T. Stephenson, Letter, Br. Med. J. 316:1015, 1998). Anecdotic reports of patients who are sent home from the emergency room at this stage illustrate the diagnostic pitfalls dramatically (97). During the first hours, parental concern is probably the best guide. In a retrospective study of ICU patients at the University Hospital Nijmegen, 60% of the parents or relatives mentioned great concern such that medical attention was sought twice or more before admission (491). In the later stages, when the characteristic hemorrhagic skin lesions become apparent, it is easier to recognize the disease. In patients with FMS, these lesions appear 6 to 12 h after onset of disease.
The early stages of meningococcal meningitis resemble that of FMS, since the early symptoms are determined by the sudden entry of meningococci in the bloodstream. However, in general the course is more insidious. The characteristic hemorrhagic skin lesions become apparent only 12 to 18 h after first disease symptoms, and in 20% of the patients these lesions never develop (49, 400, 477). When a patient presents with fever, headache, photophobia, irritability, vomiting, loss of consciousness, neck stiffness, and skin lesions, the diagnosis will not be missed. However, when focal neurology or behavioral disturbances dominate and skin lesions are absent, the diagnosis can still be overlooked (19).
Prompt bacteriological diagnosis in patients with FMS is possible with a Gram stain of a skin lesion biopsy specimen, buffy coat, or CSF (349, 499). In patients with meningococcal meningitis, skin lesions seldom reveal meningococci and only CSF samples are positive (499). Cultures become positive after 12 to 24 h. Prior antibiotic therapy jeopardizes the recovery of bacteria from cultures of blood and CSF but not from skin biopsy specimens (499). Other methods not affected by prior antibiotic administration are antigen detection or PCR on meningococcal DNA in blood or CSF (40, 41, 81, 332, 484).
Early diagnosis of FMS and recognition of patients at risk are crucial for the timely start of life-saving antibiotic and antishock therapy. In 1966, Stiehm and Damrosch published the first prognostic scoring system for the recognition of patients at risk (454). Meanwhile, multiple scoring systems have been published, all based on quickly available clinical and laboratory parameters (5, 8, 23, 26, 42, 116, 133, 149, 156, 160, 163, 170, 193, 211, 249, 262, 274–277, 293, 333, 340, 473, 475, 483, 507, 539; F. Leclerc, V. Hue, A. Martinot, and F. Delepoulle, Letter, Am. J. Dis. Child. 145:1090–1091, 1991; J. Sinclair, C. H. Skeoch, and D. Hallworth, Letter, Lancet ii:38, 1987). In summary, indicators of FMS and a poor prognosis are the extremes of age; a short period between onset of disease and admission; the absence of meningitis; progressive or widespread skin lesions; shock as shown by a slow capillary refill, cold acra, hypotension, or metabolic acidosis; a moderately elevated or normal C-reactive protein concentration in serum; the absence of leukocytosis; and the presence of thrombocytopenia, DIC, and hypofibrinogenemia. As discussed above (see “Pathophysiology of shock”), high concentrations of cytokines in plasma are also associated with a poor outcome (213). Although the levels of these mediators are a direct reflection of the inflammatory process, long laboratory turnaround times make them unsuitable for prognostic evaluation in daily practice.
During the first few hours, patients should be monitored closely because shock may develop after the start of antibiotic treatment (236). It should be borne in mind that monitoring of the systolic blood pressure in children is insufficient to trace the development of shock. Better indicators are low diastolic blood pressure, delayed capillary refill, cold extremities, and tachycardia. Nothing surpasses good clinical surveillance supported by frequent laboratory monitoring. For instance, the progression of DIC can be monitored easily by observing an increase in the number and size of skin hemorrhages and a decrease in the platelet count (492).
THERAPY
In spite of the increasing capabilities of ICUs, survival of patients with invasive meningococcal disease has hardly improved during the last few decades (21, 207). The speed and severity of clinical deterioration in patients with FMS often mandates maximal intensive therapy. Due to the lack of good clinical trials, which for a variety of reasons probably never will be carried out, much of this therapy is controversial. General agreement exists only on two aspects: therapy should never be delayed by diagnostic procedures, and antibiotics are the cornerstone of treatment. However, treatment of shock and the use of glucocorticoids, fresh-frozen plasma, plasma exchange, and other immunomodulating or adjuvant therapies all are subject to debate (65, 222, 255, 259, 313, 361, 430).
Based on good clinical practice and insights into the pathophysiology of the disease, any therapeutic regimen should aim at least to provide (i) early recognition, (ii) prompt start of parenteral antibiotic therapy, and (iii) appropriate and frequently repeated prognostic evaluations. In patients with poor prognostic signs or (imminent) shock, the therapy should be extended to include immediate fluid resuscitation, prompt start of mechanical ventilation, and transfer to an adequately equipped ICU.
Antibiotic Treatment
Antibiotics are the cornerstone of treatment. Serum therapy with serum from immunized horses, introduced at the beginning of this century by Jochmann in Germany and Flexner in the United States, has reduced mortality from nearly 100 to 30% (145, 241). Since their introduction in 1937, sulfonamides decreased mortality to 10% (423). In the 1950s and 1960s, sulfonamide resistance necessitated a switch to penicillin or chloramphenicol. Since the 1980s, decreased penicillin susceptibility (MIC, ≥0.25 mg/liter) has been reported in several countries (Spain, Greece, Switzerland, Romania, France, Belgium, United Kingdom, Malawi, South Africa, Canada, and the United States (235, 501; P. Botha, Letter, Lancet i:54, 1988; P. Bray, F. Lomprez, M. Guibourdenche, and J. Y. Riou, Letter, Press Med. 24:1910, 1995; G. Riley, S. Brown, and C. Krishnan, Letter, N. Engl. J. Med. 324:997, 1991; A. Round and W. Hamilton, Letter, Lancet i:702, 1988; E. M. Sutcliffe, D. M. Jones, S. El-Sheikh, and A. Percival, Letter, Lancet i:657–658, 1988). This decreased sensitivity is caused by a reduced affinity to penicillin binding protein type 2 (403). Occasionally, penicillin resistance due to plasmid-related β-lactamase production occurs (122). Chloramphenicol resistance has also been reported (155). For patients infected with penicillin-resistant strains, broad-spectrum cephalosporins (e.g., ceftriaxone) are recommended.
Antibiotic therapy should be started as early as possible. Early antibiotic administration does not hinder microbiological diagnosis when a skin biopsy specimen or PCR is used (73). The concern that early antibiotic administration aggravates the clinical condition by causing (β-lactam) antibiotic-induced endotoxin release (30, 230, 370) has never been confirmed clinically (54, 158, 161, 195, 369). In contrast, postponement of antibiotic therapy will result in an increase of bacterial biomass and a more harmful inflammatory response (161). When contacts of patients with meningococcal disease receive parenteral antibiotics at the time they develop fever, no disease develops (22, 161, 225; R. A. Wall, M. Hasson-King, H. Thomas, and B. M. Greenwood, Letter, Lancet ii:624, 1986). When antibiotic therapy is started later in the course of the disease, i.e., when ischemic lesions have progressed, more bacteria can escape the effect of antibiotics, since meningococci remain viable in the nonperfused center of these lesions for up to 13 h after the start of antibiotic therapy (499). Sometimes the early administration of antibiotics is disputed because the clinical benefit has not been demonstrated in clinical prospective or retrospective studies. However, since the current evidence about the beneficial effects of antibiotics seems convincing (75, 162, 456, 464; K. Cartwright, J. Strang, S. Gossain, and N. Begg, Letter, Br. Med. J. 305:774, 1992), prospective studies are considered unethical. In addition, most retrospective studies will fail to show an effect of early antibiotic therapy because the individual course of the disease is highly variable and the study population will be formed by a case mix of patients with a fulminant course (FMS) and patients with an insidious course (meningitis) (257).
Based on these considerations and the intuitive assumption that treatment will be more effective when it is started before damage has occurred (373), in several countries guidelines are promulgated to allow the referring practioner to start parenteral antibiotic therapy (28; D. Isaacs and P. McIntyre, Letter, Med. J. Aust. 168:195, 1998). Unfortunately, compliance with these guidelines is limited due to a lack of clinical alertness (343, 536, 542; D. Irwin, J. M. Miller, and S. J. Cornell, Letter, Br. Med. J. 312:1538, 1996). A more serious lack of vigilance may occur in the hospital, when diagnostic procedures such as a lumbar tap in FMS patients or CT scanning in meningitis patients delay the start of antibiotic therapy (373, 463, 536, 540).
Treatment of Shock
Shock treatment requires cannulation of a large blood vessel. However, this procedure can be troublesome in hypotensive children with coagulopathy, and fatal bleeding may occur when the vessel is ruptured (494). Therefore, it is highly recommended to puncture a compressible vessel, e.g., the femoral vein. When this procedure fails, prompt surgical section is recommended. In emergency situations, fluid can be infused via an intraosseous needle (221).
Imperative to the management of shock is early fluid resuscitation and the immediate start of mechanical ventilation. Fluid resuscitation in patients with FMS should be done stepwise with steps of 20 ml/kg. Generally, up to 60 ml/kg in the first 1 h and 120 ml/kg in the following 4 to 6 h are required (70). Sometimes 200 ml/kg during the first 24 h is necessary. The presence of concomitant meningitis does not justify limited fluid therapy (367, 434; W. T. Conner and J. A. Minielly, Letter, Lancet ii:967–969, 1980). Fresh-frozen plasma (FFP) is the fluid of choice (see also “Treatment of diffuse intravascular coagulation” and “Plasma or whole-blood exchange” below). Although it has been suggested that FFP aggravates shock by increasing complement-mediated bacteriolysis in patients with a terminal complement deficiency (284), this risk is considered minimal, since patients with these deficiencies rarely, if ever, present with shock (112, 397).
In addition to the extensive capillary leakage, the early stage of FMS is characterized by severe cardiac depression (45, 314). Consequently, pulmonary congestion may develop early, and this limits the amount of fluid that can be administered. In general, inotropic and vasopressive support is needed from an early stage (222, 255, 259, 309). Dobutamine is preferred for its beneficial effects on cardiac function and peripheral oxygenation (509). However, to maintain blood pressure, high dosages of norepinephrine or other α-adrenergics often have to be added, with the risk of aggravating peripheral ischemia. Ionized hypocalcemia, possibly caused by increased IL-1 concentrations in plasma, is found in severe cases and contributes to the decreased cardiac performance and vasoplegia (46, 71). Calcium infusion in these patients increases blood pressure temporarily (90). Hypoglycemia may occur in young infants and should be corrected immediately (394).
In the setting of the fulminant and refractory course of FMS, several alternative approaches have been tried. For instance, naloxone has been used in an effort to counteract the cardiodepressive role of endorphins (P. Cocchi, M. Silenzi, G. Calabri, and G. Salvi, Letter, Pediatr. Infect. Dis. J. 3:187, 1984; M. Tiengo, Letter, Lancet ii:690, 1980). Although later studies did not confirm the originally reported successes, a transitory rise in blood pressure can be observed (191). Recently, extracorporeal membrane oxygenation (ECMO) was claimed to improve survival (174; M. P. Champion, I. A. Murdoch, T. Sajjanhar, and M. J. Marsh, Letter, Lancet 347:201–202, 1996), but the study received much criticism (C. De Munter, S. Nadel, J. Britto, P. Habibi, and M. Levin, Letter, Lancet 349:1398, 1997; F. Leclerc, A. Martinot, R. Cremer, and C. Fourier, Letter, Lancet 349:1397, 1997). Since it took a median of 1 day before the ECMO procedure was started, only patients who survived the early hours of their disease were studied. Therefore, it remains unclear whether ECMO is of help in the more decisive early stage of the disease.
Other experimental extracorporeal therapies such as hemofiltration (HF) and plasma or whole-blood exchange are discussed below.
In the acute stage, FMS may be complicated by anuria due to acute tubular necrosis or cortical necrosis (301). After 2 to 4 days, renal failure may develop because of myoglobinuria (490). Complete anuria at that time is a serious threat and may result in pulmonary congestion, since the cardiodepressive state continues for 10 days whereas from day 3 the extravascular fluid is being mobilized by the resolution of the capillary leak. Therefore, in patients with severe rhabdomyolysis (creatine phosphokinase activity, >20,000 U/liter), alkalinization is recommended to prevent myoglobin-induced renal failure. When anuria develops, filtration or dialysis should be started early to prevent ventilatory problems.
Treatment of DIC
The only successful anti-DIC therapy is antishock therapy. However, endovascular thrombosis, ischemia, and imminent autoamputation may lead to additional treatment. For this purpose, a wide variety of strategies have been suggested. Heparin has been used for several years (100, 437). Although some studies suggest that it leads to less severe distal necrosis, no effect on survival was shown in the few limited trials that have been conducted (159, 198, 270, 299).
When extensive skin or peripheral limb necrosis has progressed, plastic surgery or amputation may be needed (165, 203, 228, 229, 406, 425). However, since the healing of ischemic lesions may be surprisingly good in children, conservative treatment should be continued for as long as possible (203, 228). There are no controlled data on the value of decompressive fasciotomy, but in general the results have been disappointing (127, 228).
New insights into the molecular pathophysiology of DIC may open the way to novel therapies. Thus far, most of these therapies have been tested only in experimental animals, with only a few having been tried in various types of human sepsis or FMS.
The modulating effect of blocking the extrinsic or intrinsic pathways of coagulation has been evaluated in E. coli-infused baboons. Monoclonal antibodies against TF attenuated shock and coagulopathy, whereas monoclonal antibodies against FXIIa abrogated only shock (238, 358, 472). No human studies have been reported.
Nonspecific inhibition of serine proteases, the key enzymes in the activation of the contact, complement, kallikrein-kinin, and fibrinolytic systems, was tried in the 1960s with aprotinin (389). Recently, more sophisticated therapies have been reported. One of them involves replenishment of the coagulation inhibitor protein C currently being explored in a phase III trial (166, 387, 390, 391, 437, 471). Stimulation of fibrinolytic activity by streptokinase or TPA has also been reported, but due to the high PAI-1 levels the effects are limited and the risk for bleeding complications is not negligible (4, 269, 552; S. Nadel, C. De Munter, J. Britto, P. Habibi, and M. Levin, Letter, Crit. Care Med. 26:971–972; 1998; M. Peters and S. Kerr, Letter, Crit. Care Med. 26:972–973, 1998; W. Zenz, Z. Bodó, G. Zobel, S. Fanconi, and A. Rettenbacher, Letter, Crit. Care Med. 26:969–971, 1998). Extensive zymogen activation can also be modulated by replenishment of serine protease inhibitors (serpins) such as AT-III or C1-INH. AT-III, which inhibits thrombin, FXa, FIXa, FXIa, and the FXIIa, seems to be promising in the treatment of sepsis of various etiologies (132) and has been used incidentally in FMS (147, 387; S. Nadel, C. De Munter, J. Britto, P. Habibi, and M. Levin, Letter, Crit. Care Med. 26:971–972, 1998; M. Peters and S. Kerr, Letter, Crit. Care Med. 26:972–973, 1998). C1-INH has been tried out in a human sepsis-like syndrome but not in FMS (190). Based on its potent regulating activity of contact, complement, and kallikrein-kinin system activation, its application seems promising (209). Transfusion of platelets is contraindicated because it increases platelet-derived PAI-1 concentrations and aggravates peripheral ischemia and necrosis (52).
So far, none of the above-mentioned single therapies has been shown to be the “magic bullet” (127). In view of the myriad interactions of mediator activation, this may not be surprising. For the time being, FFP, containing various serpins and anticoagulant factors in physiologically balanced amounts, may be the best alternative for fluid resuscitation as well as immunomodulation.
Immunomodulating Therapies
In experimental models involving infusion of gram-negative bacteria or endotoxin, several antiendotoxin and anticytokine strategies were shown to be protective. Since the pattern of cytokine activation in these models mimics that of FMS (516), it was expected that these strategies would be protective in patients with FMS (328, 508, 534). However, after euphoric preliminary anecdotal reports (196; S. A. Syed, R. H. Taylor, P. M. Crean, and R. J. Stewart, Letter, Lancet 339:496, 1992), controlled trials with human E. coli J5 antibodies and the humanized anti-lipid A IgM antibody HA-1A (Centoxin) failed to show protection (114, 233). To date, the negative results of the antiendotoxin strategies can be explained by the poor neutralizing potency of the antibodies or by the timing of administration. Blocking proximally the action of endotoxin with antiendotoxin drugs will be too late when a patient is already in shock. Recently, a phase III trial and a phase II trial with two novel antiendotoxin drugs, i.e., recombinant amino-terminal fragment of bactericidal/permeability increasing protein (rBPI21) and reconstituted high-density lipoprotein (rHDL), respectively, were started (171, 342). The results are still pending.
Anticytokine strategies, aimed at modulating the mediator cascade more distally, were tried in patients with sepsis of various etiologies. Anti-TNF antibodies, TNFsR constructs, and IL-1Ra all appeared to offer no protection. In fact, some of these strategies had harmful effects (550). Anticytokine therapies have not (yet) been used in patients with meningococcal disease. However, since comparative studies showed that at admission the activation of the mediator network differs among patients in its degree and in its stage, it can be assumed that one uniform immunomodulating monotherapy will not be sufficient. Instead, combination therapies aimed at different time points in the pathological process will be needed (297).
Hemofiltration
HF by the continuous arteriovenous mode was not beneficial in an experimental canine model of E. coli shock (152). Nevertheless, it has been claimed to improve survival by removing proinflammatory cytokines such as TNF and IL-1 or the myocardial depressant factor (188, 409). Some case reports describe its application in patients with FMS (267, 437; C. Best, J. Walsh, J. Sinclair, and J. Beattie, Letter, Lancet 347:202, 1996; G. Connett, M. Waldron, and T. Woodcock, Letter, Lancet 347:611, 1996; S. Morley, A. D'Amore, and R. I. Ross Russell, Letter, Lancet 347:614, 1996).
However, the rationale of the technique should be questioned (488). It is a misconception that detection of TNF or IL-1 in the ultrafiltrate is compatible with a significant removal of these cytokines (29). Significant removal by any procedure will occur only when the clearance achieved by this procedure outweighs the endogenous clearance. Recently, we estimated that for patients with FMS the endogenous clearance of TNF and IL-1 is at least 30 ml/kg/h (487). Since the maximal clearance that can be achieved by HF is equal to the ultrafiltration rate (approximately 10 to 20 ml/kg/min), HF will not be able to contribute substantially to the removal of TNF or IL-1.
Plasma or Whole-Blood Exchange
Exchange transfusions have been used for several years in the treatment of neonatal sepsis (482). In 1979, Scharfman et al. described the first plasmapheresis in meningococcal sepsis (W. B. Scharfman, J. R. Tillotson, E. G. Taft, and E. Wright, Letter, N. Engl. J. Med. 300:1277–1278, 1979). Although plasmapheresis appeared to be detrimental in a later trial with dogs with E. coli sepsis (330), at least 16 clinical case reports or clinical series have suggested beneficial effects of plasma exchange or whole-blood exchange with or without leukapheresis (35, 59, 67, 91, 125, 157, 158, 206, 237, 267, 313, 341, 416, 494; M. A. Lewis, Letter, Lancet 347:612–613, 1996; R. G. J. Westendorp, A. Brand, J. Haaren, V. W. M. van Hinsbergh, J. Thompson, R. van Furth, and E. A. Meinders, Letter, Am. J. Med. 92:577–578, 1992). However, since good clinical trials are lacking, the technique is still controversial (361).
Measuring the course of TNF in plasma before and during exchange sessions showed that the natural decrease in the level of TNF in plasma is accelerated during the exchange procedure (158, 487). However, because the exchange sessions are performed intermittently (59, 158, 494), the overall effect of the procedure on the decline in the level of TNF in the first 24 h is limited (487). The effect of exchange techniques on the concentration in plasma of mediators that have a lower endogenous clearance, such as C-reactive protein and the soluble TNF, IL-1, and IL-6 receptors, is clearer (154, 487, 489, 498), but to date it is unresolved whether this effect has any clinical significance.
It should be noted that plasma or whole-blood exchange not only removes plasma but also replenishes various immunomodulating compounds such as the serpins AT-III and C1-INH and the anticoagulant factors protein C and protein S (443). Since the amounts of these compounds infused during the exchange procedures approximate the quantities used in studies with one of the single products (387, 437), it may well be that the beneficial effect of plasma exchange is explained by the infusion of these large amounts of immunomodulating compounds. Because of the failure of immunomodulating strategies with one single product, plasma exchange or whole-blood exchange may be more than a “heroic action in desperately ill patients” (68). Definitely, only comparative randomized trials will be able to determine the true benefits.
Glucocorticoid Treatment
The use of glucocorticoids in the treatment of FMS is controversial. Until the early 1980s, it was generally accepted that glucocorticoids decreased early mortality in the setting of sepsis (441). However, more recent studies signaled a higher mortality caused partly by a higher secondary infection rate (102, 282). None of these studies included patients with FMS.
One reason why some still advocate the use of glucocorticoids in patients with FMS is the possible occurrence of adrenal hemorrhagic necrosis, an incidental finding visualized echographically or by CT scanning during life but a condition that is observed in 70% of autopsies (8, 39, 66, 156, 201, 307, 333). Although preterminal cortisol levels do not correlate with mortality (290, 548), individual patients may indeed suffer from adrenal insufficiency (43, 205, 354, 522). A Synacthen test performed on day 3 in patients with extensive DIC and shock at the ICU of the Nijmegen University Hospital revealed adrenal insufficiency in 3 of the 23 patients (cortisol increase, <0.10 μmol/liter after 250 μg of Synacthen) and an insufficient response in 4 other patients (cortisol increase, <0.20 μmol/liter). Given these facts, glucocorticoids belong to the standard treatment protocol of FMS in this center. However, in view of its side effects, its use remains debated.
Treatment of Meningitis
In the absence of imminent cerebral herniation or shock, treatment of meningococcal meningitis is relatively simple and demands only parenteral antibiotics and close monitoring. Fluid restriction is not indicated (367, 434, 468, 481).
The primary goal of therapy is to achieve a rapid bactericidal effect in the CSF (373). Diagnostic procedures such as a CT scan should never delay the start of antibiotic administration (128, 463). Some investigators have suggested that antibiotics exacerbate meningeal inflammation by stimulating endotoxin release (11, 324), and have therefore suggested postponing antibiotic treatment until after dexamethasone was given (405). However, these recommendations are dangerous, since proliferating bacteria shed more endotoxin than those exposed to antibiotics and delay in sterilization of CSF worsens the prognosis (153, 273).
Glucocorticoids, in a recommended dexamethasone dose of four administrations of 0.15 mg/kg/day for 3 days, mitigate cerebral inflammation and, according to some studies, reduce the risk of neurological sequelae in Haemophilus influenza meningitis (372, 523). Since a beneficial effect in other types of meningitis has not been demonstrated, the use of this therapy in meningococcal infections is controversial (373, 405). Other immunomodulating therapies have not yet been tried in human bacterial meningitis.
Lumbar puncture risks brain stem coning and is therefore contraindicated when signs of imminent cerebral herniation are present (382, 544; T. Stephenson, Letter, Br. Med. J. 316:1015, 1998; A. P. Winrow, Letter, Br. Med. J. 316:1015, 1998). These patients should receive antibiotics and ICU treatment immediately (mechanical ventilation, eventually mannitol or high-dose barbiturates). This treatment should not be delayed by CT scanning or other diagnostic procedures (128, 373, 463).
In general, performance of a second lumbar puncture or CT scan during recovery is not indicated. Meningococcal meningitis responds to appropriate antibiotics, and cerebral abscesses have never been observed.
PRIMARY PREVENTION
Invasive disease occurs only in patients devoid of specific bactericidal or opsonizing antibodies. Therefore, the best way to prevent disease is to induce these antibodies by vaccination. Ideally, vaccination should not influence naso-oropharyngeal colonization, the natural reservoir of meningococci, since selection pressure may lead to further immunogenic variability of meningococci.
In the 1960s, polysaccharide vaccines based on group A and C capsule were developed (177). At present, a quadrivalent vaccine containing serogroups A, C, W-135, and Y is available (286). These unconjugated polysaccharide vaccines confer protection to persons older than 2 years even when they are complement deficient (411). Vaccination has been highly effective in the control of community outbreaks and epidemics in military centers (285, 304, 396). Similarly, these polysaccharide vaccines are able to control large epidemics in African countries in the meningitis belt, provided that surveillance is adequate and the vaccination is started before the epidemic threshold is passed (505). Vaccination does not reduce the transfer of bacteria to nonvaccinated persons, and carrier status is unaffected (304, 316).
Nevertheless, the value of unconjugated polysaccharide vaccines is limited because these “thymus-independent” vaccines do not induce immunological memory and the response in children younger than 2 years is poor (84, 348). Induction of immunological memory and a higher efficacy in infants can be realized by conjugating the polysaccharide vaccine to a protein (150, 181, 364). The value of these recently developed conjugated vaccines in mass vaccination is currently under investigation.
The major drawback of the presently available vaccines in Western European countries is the absence of activity against group B meningococci. Since group B polysaccharide is a 200-residue α(2→8) homopolymer of N-acetylneuraminic acid (polyα2-8NeuNAc) that mimics the human neuronal cell adhesion molecule (143), the use of group B capsule in a vaccine risks the induction of autoimmunity. Since the antibody response to the group B capsule is limited after natural infections, group B capsular polysaccharide is a poor candidate for vaccine development (187). A novel approach is to use a conjugated chemically modified group B capsule where the N-acetyl group has been replaced by the N-propionyl group (240). Alternative and promising candidates are the surface-exposed OMPs class 1 (PorA) and class 2/3 (PorB) or LOS (121, 364, 506). Large-scale trials with some of these OMP vaccines in Chile, Cuba, Norway, and Brazil yielded different success rates (37, 110, 311, 431, 554). A recently conducted phase II trial in the United Kingdom and the Netherlands with a Dutch hexavalent outer membrane vesicle vaccine derived from two recombinant multivalent OMP strains and based on six class 1 OMPs present in 80% of the group B isolates in the United Kingdom and The Netherlands (485) gave promising results (74, 109, 214).
Administration of meningococcal (group B) vaccine intranasally induces effective bactericidal antibody titers (197). However, since the concomitant induction of secretory antibodies may influence the natural reservoir of meningococci, which may lead to higher immunogenic variability, further epidemiological research is needed before this technique can be introduced.
SECONDARY PROPHYLAXIS
Disease can be prevented by the eradication of the carrier status in subjects likely to harbor virulent meningococci, e.g., housemates of an index patient (422).
Household members and roommates of an index patient have a 1,000-fold-higher chance of acquiring invasive disease than do members of the general population; for pre-primary school contacts, the risk is increased 50-fold (96, 120, 308, 339; R. A. Wall, M. Hasson-King, H. Thomas, and B. M. Greenwood, Letter, Lancet ii:624, 1986). One reason for this increase may be that the conditions needed for invasive disease, in particular the genetic constellation and the occurrence of precedent viral infections, are shared among household members. Since this is not the case for hospital or laboratory personnel, hospital-acquired secondary cases are rare (10, 344, 384, 395; G. Holdsworth, et al., Letter, Lancet 348:1443, 1996).
Chemoprophylaxis for household, day care, and kissing contacts and for medical personnel experiencing intensive contact with oral secretions is recommended by the health authorities of the United States and several European countries. Rifampin is the drug of choice; ciprofloxacin, ofloxacin, minocycline (not in children), and ceftriaxone are good alternatives (167, 243, 371, 420). To prevent reentry of the pathogenic strain into the household after discharge of a patient treated with penicillin, amoxicillin, or chloramphenicol, drugs that do not sterilize the nasopharyngeal mucosa (1), additional prophylaxis before discharge has been suggested (308). However, detailed studies found pathogenic meningococci in only a very small percentage (ca. 1%) of patients after discharge, and therefore the efficacy of sterilizing the naso-oropharynx before discharge is controversial (7, 528).
Chemoprophylaxis may fail because it is given too late or given to the wrong person or because of noncompliance (96, 146, 243, 308). Rifampin resistance, fortunately rare, is another cause of failure (96, 98, 418; P. Yagupsky, S. Ashkerazi, and C. Block, Letter, Lancet 341:1152–1153, 1993). The effect of chemoprophylaxis is further limited because it does not prevent reintroduction of the pathogenic strain from a carrier outside the group, and therefore late secondary cases still occur (457).
Based on the observation that approximately half of the secondary cases in families develop within 24 h in children younger than 15 years, the Norwegian health authorities advise treating these possible coprimary cases with phenoxymethylpenicillin for 1 week (161, 225, 265). Although this strategy has dramatically reduced the number of coprimary fatalities in families, no controlled studies are available and the strategy is debated in other countries (D. M. Jones, Letter, Br. Med. J. 312:1537, 1996; A. Pollard, R. Booy, S. Nadel, and M. Levin, Letter, Br. Med. J. 312:1536, 1996; T. Stokes, R. Shukla, and P. Monk, Letter, Br. Med. J. 312:1536–1537, 1996). One of the concerns is the development of penicillin resistance.
EPILOGUE
Because in many cases vaccination is impossible and secondary chemoprophylaxis is of limited value, early diagnosis and prompt appropriate treatment remain the only ways of reducing morbidity and mortality. This requires not only medical acumen and expertise but also permanent vigilance by a well-informed public (179, 180, 388). Unfortunately, the latter is not the case. Media publicity often misinforms and misdirects vigilance by overemphasizing features such as headache, nausea, and stiff neck while neglecting the early and characteristic skin lesions (474). Even in 2000, the old thinking that meningococcal disease consists primarily of meningitis lives firmly in the minds of many people including physicians (345; A. Corke, R. Anderson, M. Darda, J. Bhachu, V. Franklin, S. Chen, J. Taylor, N. Choudbury, and T. Christmas, Letter, Br. Med. J. 312:311–312, 1996). With respect to this, little has changed since the time of Herrick, who began his article in 1919 with the following (217):
The meningeal picture resulting from meningococcus infection has so fixed the attention of clinicians and pathologists that the possibilities of extrameningeal infection have had scant notice. This has resulted in a general failure to recognize the fundamental nature of the disease as a meningococcus septicemia, which has in turn had important consequences in the field of diagnosis and treatment.
ACKNOWLEDGMENTS
We acknowledge the staff of the Intensive Care Unit of the University Hospital Nijmegen, Nijmegen, The Netherlands (head, S. J. H. van Leeuwen), where the clinical part of this study was conducted.
REFERENCES
- 1.Abramson J S, Spika J S. Persistence of Neisseria meningitidis in the upper respiratory tract after intravenous antibiotic therapy for systemic meningococcal disease. J Infect Dis. 1985;151:370–371. doi: 10.1093/infdis/151.2.370. [DOI] [PubMed] [Google Scholar]
- 2.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 159–175. [Google Scholar]
- 3.Achtman M, Wall R A, Bopp M, Kusecek B, Morelli G, Saken E, Hassan King M. Variation in class 5 protein expression by serogroup A meningococci during a meningitis epidemic. J Infect Dis. 1991;164:375–382. doi: 10.1093/infdis/164.2.375. [DOI] [PubMed] [Google Scholar]
- 4.Aiuto L T, Barone S R, Cohen P S, Boxer R A. Recombinant tissue plasminogen activator restores perfusion in meningococcal purpura fulminans. Crit Care Med. 1997;25:1079–1082. doi: 10.1097/00003246-199706000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Algren J T, Lal S, Cutliff S A, Richman B J. Predictors of outcome in acute meningococcal infection in children. Crit Care Med. 1993;21:447–452. doi: 10.1097/00003246-199303000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Alpert G, Baldwin G, Thompson C, Wainwright N, Novitsky T J, Gillis Z, Parsonnet J, Fleisher G R, Siber G R. Limulus antilipopolysaccharide factor protects rabbits from meningococcal endotoxin shock. J Infect Dis. 1992;165:494–500. doi: 10.1093/infdis/165.3.494. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez F, Aguilera A, Garcia-Gago M. Effect of chemoprophylaxis on the meningococcal carrier state after infection. Pediatr Infect Dis J. 1991;10:700. doi: 10.1097/00006454-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Andersen B M. Mortality in meningococcal infections. Scand J Infect Dis. 1978;10:277–282. doi: 10.3109/inf.1978.10.issue-4.04. [DOI] [PubMed] [Google Scholar]
- 9.Andersen J, Lind I. Characterization of Neisseria meningitidis isolates and clinical features of meningococcal conjunctivitis in ten patients. Eur J Clin Microbiol Infect Dis. 1994;13:388–393. doi: 10.1007/BF01971995. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Laboratory-acquired meningococcal infection. Commun Dis Rep Weekly. 1992;2:39. [PubMed] [Google Scholar]
- 11.Arditi M, Ables L, Yogev R. Cerebrospinal fluid endotoxin levels in children with H. influenzae meningitis before and after administration of intravenous ceftriaxone. J Infect Dis. 1989;160:1005–1011. doi: 10.1093/infdis/160.6.1005. [DOI] [PubMed] [Google Scholar]
- 12.Arditi M, Manogue K R, Caplan M, Yogev R. Cerebrospinal fluid cachectin/tumor necrosis factor-α and platelet-activating factor concentrations and severity of bacterial meningitis in children. J Infect Dis. 1990;162:139–147. doi: 10.1093/infdis/162.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Argenbright L W, Barton R W. Interactions of leukocyte integrins with intercellular adhesion molecule 1 in the production of inflammatory vascular injury in vivo. The Shwartzman reaction revisited. J Clin Investig. 1992;89:259–272. doi: 10.1172/JCI115570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arranz E, Blanco-Quiros A, Solis P, Garotte J A. Lack of correlation between soluble CD14 and IL-6 in meningococcal septic shock. Pediatr Allergy Immunol. 1997;8:194–199. doi: 10.1111/j.1399-3038.1997.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 15.Ashton F E, Ryan J A, Borczyk A, Caugant D A, Mancino L, Huang D. Emergence of a virulent clone of Neisseria meningitidis serotype 2a that is associated with meningococcal group C disease in Canada. J Clin Microbiol. 1991;29:2489–2493. doi: 10.1128/jcm.29.11.2489-2493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashwal S, Stringer W, Tomasi L, Schneider S, Thompson J, Perkin R. Cerebral blood flow and carbon dioxide reactivity in children with bacterial meningitis. J Pediatr. 1990;117:523–530. doi: 10.1016/s0022-3476(05)80683-3. [DOI] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Ayala P, Lin L, Hopper S, Fukuda M, So M. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect Immun. 1998;66:5001–5007. doi: 10.1128/iai.66.10.5001-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin L N, Henderson A, Thomas P, Wright M. Acute bacterial meningitis in young adults mistaken for substance abuse. Br Med J. 1993;306:775–776. doi: 10.1136/bmj.306.6880.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball J H, Young D A. Primary meningococcal pneumonia. Am Rev Respir Dis. 1974;109:480–483. doi: 10.1164/arrd.1974.109.4.480. [DOI] [PubMed] [Google Scholar]
- 21.Baraff L J, Lee S I, Schriger D L. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993;12:389–394. doi: 10.1097/00006454-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Baraff L J, Oslund S, Prather M. Effect of antibiotic therapy and etiologic microorganism on the risk of bacterial meningitis in children with occult bacteremia. Pediatrics. 1993;92:140–143. [PubMed] [Google Scholar]
- 23.Barquet N, Domingo P, Caylà J A, Gonz'ález J, Rodrigo C, Fernández-Viladrich P, Moraga-Llop F A, Marco F, Vázquez J, Sáez-Nieto J A, Casal J, Canela J, Foz M for the Barcelona Meningococcal Disease Surveillance Group. Prognostic factors in meningococcal disease. Development of a bedside predictive model and scoring system. JAMA. 1997;278:491–496. doi: 10.1001/jama.278.6.491. [DOI] [PubMed] [Google Scholar]
- 24.Barquet N, Gasser I, Domingo P, Moraga F A, Macaya A, Elcuaz R. Primary meningococcal conjunctivitis: report of 21 patients and review. Rev Infect Dis. 1990;12:838–847. doi: 10.1093/clinids/12.5.838. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Bausher J C, Baker R C. Early prognostic indicators in acute meningococcemia: implications for management. Pediatr Emerg Care. 1986;2:176–179. doi: 10.1097/00006565-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Reference deleted.
- 28.Begg N. Reducing mortality from meningococcal disease. Br Med J. 1992;305:133–134. doi: 10.1136/bmj.305.6846.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellomo R, Tipping P, Boyce N. Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med. 1993;21:522–526. doi: 10.1097/00003246-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz F E, Vallabh P, Altman D I, Diamantes F, Van Wyk H J P, Stroucken J M M. Jarisch-Herxheimer reaction in meningococcal meningitis. Am J Dis Child. 1983;137:599. doi: 10.1001/archpedi.1983.02140320075018. [DOI] [PubMed] [Google Scholar]
- 31.Berthier J C, Hartemann E. L'incompétence myocardique dans le purpura méningococcique de l'enfant. Etude hémodynamique précoce. Presse Med. 1987;16:519–522. [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Bevanger L, Bergh K, Gisnås G, Caugant D A, Frøholm L O. Identification of nasopharyngeal carriage of an outbreak strain of Neisseria meningitidis by pulsed-field gel electrophoresis versus phenotypic methods. J Med Microbiol. 1998;47:993–998. doi: 10.1099/00222615-47-11-993. [DOI] [PubMed] [Google Scholar]
- 34.Bjerknes R, Guttormsen H-K, Solberg C O, Wetzler L M. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect Immun. 1995;63:160–167. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorvatn B, Bjertnaes L, Fadnes H O, Flaegstad T, Gutteberg T J, Kristiansen B-E, Pape J, Rekvig O P, Østerud B, Aanderud L. Meningococcal septicaemia treated with combined plasmapheresis and leucapheresis or with blood exchange. Br Med J Clin Res Ed. 1984;288:439–441. doi: 10.1136/bmj.288.6415.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorvatn B, Hassan-King M, Greenwood B, Haimanot R T, Fekade D, Sperber G. DNA fingerprinting in the epidemiology of African serogroup A Neisseria meningitidis. Scand J Infect Dis. 1992;24:323–332. doi: 10.3109/00365549209061338. [DOI] [PubMed] [Google Scholar]
- 37.Bjune G, Høiby E A, Grønnesby J K, Arnesen Ø, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 38.Blaser M J, Reingold A L, Alsever R N, Hightower A. Primary meningococcal pericarditis: a disease of adults associated with serogroup C Neisseria meningitidis. Rev Infect Dis. 1984;6:625–632. doi: 10.1093/clinids/6.5.625. [DOI] [PubMed] [Google Scholar]
- 39.Böhm N. Adrenal, cutaneous and myocardial lesions in fulminating endotoxinemia (Waterhouse-Friderichsen syndrome) Pathol Res Pract. 1982;174:92–105. doi: 10.1016/S0344-0338(82)80032-0. [DOI] [PubMed] [Google Scholar]
- 40.Borrow R, Claus H, Guiver M, Smart L, Jones D M, Kaczmarski E B, Frosch M, Fox A J. Non-culture diagnosis and serogroup determination of meningococcal B and C infection by a sialyltransferase (siaD) PCR ELISA. Epidemiol Infect. 1997;118:111–117. doi: 10.1017/s0950268896007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borrow R, Guiver M, Sadler F, Kaczmarski E B, Fox A J. False positive diagnosis of meningococcal infection by the IS1106 PCR ELISA. FEMS Microbiol Lett. 1998;162:215–218. doi: 10.1111/j.1574-6968.1998.tb13001.x. [DOI] [PubMed] [Google Scholar]
- 42.Borschsenius F, Bruun J N, Michaelsen T E, Tonjun T. Serum C-reactive protein in systemic infections due to Neisseria meningitidis. NIPH Ann. 1986;9:15–21. [PubMed] [Google Scholar]
- 43.Bosworth D C. Reversible adrenocorticol insufficiency in fulminant meningococcemia. Arch Intern Med. 1979;139:823–824. [PubMed] [Google Scholar]
- 44.Reference deleted.
- 45.Boucek M M, Boerth R C, Artman M, Graham T P, Jr, Boucek R J., Jr Myocardial dysfunction in children with acute meningococcemia. J Pediatr. 1984;105:538–542. doi: 10.1016/s0022-3476(84)80416-3. [DOI] [PubMed] [Google Scholar]
- 46.Boyce B F, Yates A J, Mundy G R. Bolus injections of recombinant human interleukin-1 cause transient hypocalcemia in normal mice. Endocrinology. 1989;125:2780–2783. doi: 10.1210/endo-125-5-2780. [DOI] [PubMed] [Google Scholar]
- 47.Brandtzaeg P. Pathogenesis of meningococcal infections. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 71–114. [Google Scholar]
- 48.Brandtzaeg P, Bryn K, Kierulf P, Øvstebø R, Namork E, Aase B, Jantzen E. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Investig. 1992;89:816–823. doi: 10.1172/JCI115660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandtzaeg P, Dahle J S, Høiby E A. The occurrence and features of hemorrhagic skin lesions in 115 cases of systemic meningococcal disease. NIPH Ann. 1983;6:183–190. , 202–203. [PubMed] [Google Scholar]
- 50.Brandtzaeg P, Halstensen A, Kierulf P, Espevik T, Waage A. Molecular mechanisms in the compartmentalized inflammatory response presenting as meningococcal meningitis or septic shock. Microb Pathog. 1992;13:423–431. doi: 10.1016/0882-4010(92)90010-l. [DOI] [PubMed] [Google Scholar]
- 51.Brandtzaeg P, Høgåsen K, Kierulf P, Mollnes T E. The excessive complement activation in fulminant meningococcal septicemia is predominantly caused by alternative pathway activation. J Infect Dis. 1996;173:647–655. doi: 10.1093/infdis/173.3.647. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P, Joø G B, Brusletto B, Kierulf P. Plasminogen activator inhibitor 1 and 2, alpha-2-antiplasmin, plasminogen, and endotoxin levels in systemic meningococcal disease. Thromb Res. 1990;57:271–278. doi: 10.1016/0049-3848(90)90326-8. [DOI] [PubMed] [Google Scholar]
- 53.Brandtzaeg P, Kierulf P. Endotoxin and meningococcemia: intravascular inflammation induced by native endotoxin in man. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides. II. Immunopharmacology and pathophysiology. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 327–346. [Google Scholar]
- 54.Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun J N, Halvorsen S, Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 55.Brandtzaeg P, Mollnes T E, Kierulf P. Complement activation and endotoxin levels in systemic meningococcal disease. J Infect Dis. 1989;160:58–65. doi: 10.1093/infdis/160.1.58. [DOI] [PubMed] [Google Scholar]
- 56.Brandtzaeg P, Osnes L, Øvstebo R, Joø G B, Westvik Å-B, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184:51–60. doi: 10.1084/jem.184.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandtzaeg P, Øvstebø R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650–652. doi: 10.1093/infdis/166.3.650. [DOI] [PubMed] [Google Scholar]
- 58.Brandtzaeg P, Sandset P M, Joø G B, Øvstebø R, Abildgaard U, Kierulf P. The quantitative association of plasma endotoxin, antithrombin, protein C, extrinsic pathway inhibitor and fibrinopeptide A in systemic meningococcal disease. Thromb Res. 1989;55:459–470. doi: 10.1016/0049-3848(89)90054-6. [DOI] [PubMed] [Google Scholar]
- 59.Brandtzaeg P, Sirnes K, Folsland B, Godal H C, Kierulf P, Bruun J N, Dobloug J. Plasmapheresis in the treatment of severe meningococcal or pneumococcal septicaemia with DIC and fibrinolysis. Preliminary data on eight patients. Scand J Clin Lab Investig. 1985;178(Suppl. 45):53–55. [PubMed] [Google Scholar]
- 60.Brandtzaeg P, Waage A, Mollnes T E, Oktedalen O, Kierulf P. Severe human septic shock involves more than tumor necrosis factor. In: Sturk A, van Deventer S J H, ten Cate J W, Büller H R, Thijs L G, Levin J, editors. Bacterial endotoxins: cytokine mediators and new therapies for sepsis. New York, N.Y: Wiley Liss; 1991. p. 25. [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Bredius R G M, Derkx B H F, Fijen C A P, de Wit T P M, de Haas M, Weening R S, van de Winkel J G J, Out T A. Fc gamma receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J Infect Dis. 1994;170:848–853. doi: 10.1093/infdis/170.4.848. [DOI] [PubMed] [Google Scholar]
- 63.Brindle R, Simani P, Newnham R, Waiyaki P, Gilks C. No association between meningococcal disease and human immunodeficiency virus in adults in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 1991;85:651. doi: 10.1016/0035-9203(91)90380-h. [DOI] [PubMed] [Google Scholar]
- 64.Bruin K F. Ph.D. thesis. Amsterdam, The Netherlands: University of Amsterdam; 1994. [Google Scholar]
- 65.Busund R, Straume B, Revhaug A. Fatal course in severe meningococcemia: clinical predictors and effect of transfusion therapy. Crit Care Med. 1993;21:1699–1705. doi: 10.1097/00003246-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 66.Cahalane S F, Waters M. Fulminant meningococcal septicaemia. A hospital experience. Lancet. 1975;ii:120–121. doi: 10.1016/s0140-6736(75)90017-3. [DOI] [PubMed] [Google Scholar]
- 67.Campbell W N, Joshi M, Sileo D. Osteonecrosis following meningococcemia and disseminated intravascular coagulation in an adult: case report and review. Clin Infect Dis. 1997;24:452–455. doi: 10.1093/clinids/24.3.452. [DOI] [PubMed] [Google Scholar]
- 68.Campion E W. Desperate diseases and plasmapheresis. N Engl J Med. 1992;326:1425–1427. doi: 10.1056/NEJM199205213262109. [DOI] [PubMed] [Google Scholar]
- 69.Caputo G L, Baldwin G, Alpert G, Parsonnet J, Gillis Z A, Siber G, Fleisher G. Effect of meningococcal endotoxin in a rabbit model of shock. Circ Shock. 1992;36:104–112. [PubMed] [Google Scholar]
- 70.Carcillo J A, Davis A L, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–1245. [PubMed] [Google Scholar]
- 71.Cardenas-Rivero N, Chernow B, Stoiko M A, Nussbaum S R, Todres I D. Hypocalcemia in critically ill children. J Pediatr. 1989;114:946–951. doi: 10.1016/s0022-3476(89)80435-4. [DOI] [PubMed] [Google Scholar]
- 72.Cartwright K. Meningococcal carriage and disease. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 115–146. [Google Scholar]
- 73.Cartwright K, Kroll S. Optimising the investigation of meningococcal disease. Early treatment with benzylpenicillin is important and doesn't jeopardise diagnosis. Br Med J. 1997;315:757–758. doi: 10.1136/bmj.315.7111.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cartwright K, Morris R, Rumke H, Fox A, Borrow R, Begg N, Richmond P, Poolman J. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine. 1999;17:2612–2619. doi: 10.1016/s0264-410x(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 75.Cartwright K, Reilly S, White D, Stuart J. Early treatment with parenteral penicillin in meningococcal disease. Br Med J. 1992;305:143–147. doi: 10.1136/bmj.305.6846.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reference deleted.
- 77.Cartwright K A, Stuart J M, Jones D M, Noah N D. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cartwright K A V, Jones D M, Smith A J, Stuart J M, Kaczmarski E B, Palmer S R. Influenza A and meningococcal disease. Lancet. 1991;338:554–557. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 79.Carvalho A C, DeMarinis S, Scott C F, Silver L D, Schmaier A H, Colman R W. Activation of the contact system of plasma proteolysis in the adult respiratory distress syndrome. J Lab Clin Med. 1988;112:270–277. [PubMed] [Google Scholar]
- 80.Caugant D A. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS. 1998;106:505–525. [PubMed] [Google Scholar]
- 81.Caugant D A, Høiby E A, Frøholm L O, Brandtzaeg P. Polymerase chain reaction for case ascertainment of meningococcal meningitis: application to the cerebrospinal fluids collected in the course of the Norwegian meningococcal serogroup B protection trial. Scand J Infect Dis. 1996;28:149–153. doi: 10.3109/00365549609049066. [DOI] [PubMed] [Google Scholar]
- 82.Caugant D A, Høiby E A, Magnus P, Scheel O, Hoel T, Bjune G, Wedege E, Eng J, Frøholm L O. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323–330. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caugant D A, Kristiansen B E, Frøholm L O, Bovre K, Selander R K. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect Immun. 1988;56:2060–2068. doi: 10.1128/iai.56.8.2060-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ceesay S J, Allen S J, Menon A, Todd J E, Cham K, Carlone G M, Turner S H, Gheesling L L, DeWitt W, Plikaytis B D, Greenwood B. Decline in meningococcal antibody levels in African children 5 years after vaccination and the lack of an effect of booster immunization. J Infect Dis. 1993;167:1212–1216. doi: 10.1093/infdis/167.5.1212. [DOI] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention. Serogroup B meningococcal disease—Oregon, 1994. Morbid Mortal Weekly Rep. 1995;44:121–134. [Google Scholar]
- 86.Centers for Disease Control and Prevention. Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:13–21. [PubMed] [Google Scholar]
- 87.Reference deleted.
- 88.Chan B, Kalabalikis P, Klein N, Heyderman R, Levin M. Assessment of the effect of candidate anti-inflammatory treatments on the interaction between meningococci and inflammatory cells in-vitro in a whole blood model. Biotherapy. 1996;9:221–228. doi: 10.1007/BF02620735. [DOI] [PubMed] [Google Scholar]
- 89.Reference deleted.
- 90.Chernow B. Calcium: does it have a therapeutic role in sepsis? Crit Care Med. 1990;18:895–896. [PubMed] [Google Scholar]
- 91.Churchwell K B, McManus M L, Kent P, Gorlin J, Galacki D, Humphreys D, Kevy S V. Intensive blood and plasma exchange for treatment of coagulopathy in meningococcemia. J Clin Apheresis. 1995;10:171–177. doi: 10.1002/jca.2920100403. [DOI] [PubMed] [Google Scholar]
- 92.Reference deleted.
- 93.Coll M-T, Uriz M-S, Pineda V, Fontanals D, Bella F, Nava J M, Deulofeu F, Morera M-A, Martí C, Lite J, Garau J, Font B. Meningococcal meningitis with ‘normal’ cerebrospinal fluid. J Infect. 1994;29:289–294. doi: 10.1016/s0163-4453(94)91197-5. [DOI] [PubMed] [Google Scholar]
- 94.Reference deleted.
- 95.Reference deleted.
- 96.Cooke R P D, Riordan T, Jones D M, Painter M J. Secondary cases of meningococcal infection among close family and household contacts in England and Wales, 1984–7. Br Med J. 1989;298:555–558. doi: 10.1136/bmj.298.6673.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cookson S T, Corrales J L, Lotero J O, Regueira M, Binsztein N, Reeves M W, Ajello G, Jarvis W R. Disco fever: epidemic meningococcal disease in northeastern Argentina associated with disco patronage. J Infect Dis. 1998;178:266–269. doi: 10.1086/517450. [DOI] [PubMed] [Google Scholar]
- 98.Cooper E R, Ellison III R T, Smith G S, Blaser M J, Reller L B, Paisley J W. Rifampicin-resistant meningococcal disease in a contact patient given prophylactic rifampicin. J Pediatr. 1986;108:93–96. doi: 10.1016/s0022-3476(86)80776-4. [DOI] [PubMed] [Google Scholar]
- 99.Reference deleted.
- 100.Corrigan J J, Jr, Jordan C M. Heparin therapy in septicemia with disseminated intravascular coagulation. Effect on mortality and on correction of hemostatic defects. N Engl J Med. 1970;283:778–782. doi: 10.1056/NEJM197010082831502. [DOI] [PubMed] [Google Scholar]
- 101.Cremer R, Leclerc F, Jude B, Sadik A, Leteurtre S, Fourier C, Martinot A, Diependaele J F. Are there specific haemostatic abnormalities in children surviving septic shock with purpura and having skin necrosis or limb ischaemia that need skin grafts or limb amputations? Eur J Pediatr. 1999;158:127–132. doi: 10.1007/s004310051032. [DOI] [PubMed] [Google Scholar]
- 102.Cronin L, Cook D J, Carlet J, Heyland D K, King D, Lansang M A, Fisher C J., Jr Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 103.Reference deleted.
- 104.Dashefsky B, Teele D W, Klein J O. Unsuspected meningococcemia. J Pediatr. 1983;102:69–72. doi: 10.1016/s0022-3476(83)80290-x. [DOI] [PubMed] [Google Scholar]
- 105.Davies B I, Spanjaard L, Dankert J. Meningococcal chest infections in a general hospital. Eur J Clin Microbiol Infect Dis. 1991;10:399–404. doi: 10.1007/BF01968018. [DOI] [PubMed] [Google Scholar]
- 106.De Boer J P, Creasey A A, Chang A, Abbink J J, Roem D, Eerenberg A J, Hack C E, Taylor F B., Jr Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic proteinases in sepsis: studies using a baboon model. Infect Immun. 1993;61:5035–5043. doi: 10.1128/iai.61.12.5035-5043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Boer J P, Creasey A A, Chang A, Roem D, Eerenberg A J M, Hack C E, Taylor F B., Jr Activation of the complement system in baboons challenged with live Escherichia coli: correlation with mortality and evidence for a biphasic activation pattern. Infect Immun. 1993;61:4293–4301. doi: 10.1128/iai.61.10.4293-4301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dehio C, Gray-Owen S D, Meyer T F. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 109.De Kleyn, E., R. de Groot, J. Labadie, R. Lafeber, G. van den Dobbelsteen, L. van Alphen, G. W. van Ommen, M. Wale, R. Jultman, and H. C. Rümke. Immunogenicity and safety of a hexavalent meningococcal outer membrane vaccine in children of 2–3 and 7–8 years of age. Vaccine, in press. [DOI] [PubMed]
- 110.De Moraes J C, Perkins B A, Camargo M C C, Hidalgo N T R, Barbosa H A, Sacchi C T, Landgraf I M, Gattas V L, Vasconcelos H D G, Plikyatis B D, Wenger J D, Broome C V. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 111.Reference deleted.
- 112.Densen P. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin Microbiol Rev. 1989;2(Suppl.):S11–S17. doi: 10.1128/cmr.2.suppl.s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Derkx B, Marchant A, Goldman M, Bijlmer R, van Deventer S. High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis. 1995;171:229–232. doi: 10.1093/infdis/171.1.229. [DOI] [PubMed] [Google Scholar]
- 114.Derkx B, Wittes J, McCloskey R. Randomized placebo-controlled trial of HA-1A, a human monoclonal anti-endotoxin antibody, in children with meningococcal septic shock. Clin Infect Dis. 1999;28:770–777. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 115.Derkx H H, Kuijper E J, Fijen C A, Jak M, Dankert J, van Deventer S J. Inherited complement deficiency in children surviving fulminant meningococcal septic shock. Eur J Pediatr. 1995;154:735–738. doi: 10.1007/BF02276718. [DOI] [PubMed] [Google Scholar]
- 116.Derkx H H F, van den Hoek J, Redekop W K, Bijlmer R P G H, van Deventer S J H, Bossuyt P M M. Meningococcal disease: a comparison of eight severity scores in 125 children. Intensive Care Med. 1996;22:1433–1441. doi: 10.1007/BF01709565. [DOI] [PubMed] [Google Scholar]
- 117.DeVoe I W, Gilchrist J E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973;138:1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Vries F P, Cole R, Dankert J, Frosch M, van Putten J P. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol Microbiol. 1998;27:1203–1212. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 119.De Vries F P, van Der Ende A, van Putten J P, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Wals P, Hertoghe L, Borlée-Grimée I, De Maeyer-Cleempoel S, Reginster Haneuse G, Dachy A, Bouckaert A, Lechat M F. Meningococcal disease in Belgium. Secondary attack rate among household, day-care nursery and pre-elementary school contacts. J Infect. 1981;3(Suppl. 1):53–61. doi: 10.1016/s0163-4453(81)80009-6. [DOI] [PubMed] [Google Scholar]
- 121.Diaz Romero J, Outschoorn I M. Current status of meningococcal group B vaccine candidates: capsular or noncapsular? Clin Microbiol Rev. 1994;7:559–575. doi: 10.1128/cmr.7.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dillon J R, Pauzé M, Yeung K-H. Spread of penicillinase-producing and transfer plasmids from the gonococcus to Neisseria meningitidis. Lancet. 1983;i:779–781. doi: 10.1016/s0140-6736(83)91846-9. [DOI] [PubMed] [Google Scholar]
- 123.Djupesland G, Gedde-Dahl T W. Sequelae of meningococcal disease studied about six weeks after hospital admission. NIPH Ann. 1983;6:85–90. [PubMed] [Google Scholar]
- 124.Donnelly S C, MacGregor I, Zamani A, Gordon M W G, Robertson C E, Steedman D J, Little K, Haslett C. Plasma elastase levels and the development of the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1428–1433. doi: 10.1164/ajrccm.151.5.7735596. [DOI] [PubMed] [Google Scholar]
- 125.Drapkin M S, Wisch J S, Gelfand J A, Cannon J G, Dinarello C A. Plasmapheresis for fulminant meningococcemia. Pediatr Infect Dis J. 1989;8:399–400. doi: 10.1097/00006454-198906000-00015. [DOI] [PubMed] [Google Scholar]
- 126.Duffy T P. Clinical problem-solving. The sooner the better. N Engl J Med. 1993;329:710–713. doi: 10.1056/NEJM199309023291007. [DOI] [PubMed] [Google Scholar]
- 127.Duncan A. New therapies for severe meningococcal disease but better outcomes? Lancet. 1997;350:1565–1566. doi: 10.1016/s0140-6736(05)64007-x. [DOI] [PubMed] [Google Scholar]
- 128.Durand M L, Calderwood S B, Weber D J, Miller S I, Southwick F S, Caviness V S, Jr, Swartz M N. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 129.Edwards E A. Immunological investigations of meningococcal disease. II. Some characteristics of group C antigen of Neisseria meningitidis in the sera of patients with fulminant meningococcemia. J Infect Dis. 1974;129:538–544. doi: 10.1093/infdis/129.5.538. [DOI] [PubMed] [Google Scholar]
- 130.Edwards E A, Devine L F, Sengbusch G H, Ward H W. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis. 1977;9:105–110. doi: 10.3109/inf.1977.9.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 131.Edwards M S, Baker C J. Complications and sequelae of meningococcal infections in children. J Pediatr. 1981;99:540–545. doi: 10.1016/s0022-3476(81)80250-8. [DOI] [PubMed] [Google Scholar]
- 132.Eisele B, Lamy M, Thijs L G, Keinecke H O, Schuster H P, Matthias F R, Fourrier F, Heinrichs H, Delvos U. Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intensive Care Med. 1998;24:663–672. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 133.Emparanza J I, Aldamiz-Echevarria L, Perez-Yarza E G, Larrañaga P, Jiminez J L, Labiano M, Ozcoidi I. Prognostic score in acute meningococcemia. Crit Care Med. 1988;16:168–169. doi: 10.1097/00003246-198802000-00015. [DOI] [PubMed] [Google Scholar]
- 134.Engebretsen L F, Kierulf P, Brandtzaeg P. Extreme plasminogen activator inhibitor and endotoxin values in patients with meningococcal disease. Thromb Res. 1986;42:713–716. doi: 10.1016/0049-3848(86)90351-8. [DOI] [PubMed] [Google Scholar]
- 135.Erickson L, De Wals P. Complications and sequelae of meningococcal disease in Quebec, Canada, 1990–1994. Clin Infect Dis. 1998;26:1159–1164. doi: 10.1086/520303. [DOI] [PubMed] [Google Scholar]
- 136.Estabrook M M, Christopher N C, Griffiss J M, Baker C J, Mandrell R E. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J Infect Dis. 1992;166:1079–1088. doi: 10.1093/infdis/166.5.1079. [DOI] [PubMed] [Google Scholar]
- 137.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fijen C A, Derkx B H, Kuijper E J, Mannens M, Poort S R, Peters M, Daha M R, Dankert J. Fulminant meningococcal septic shock in a boy with combined inherited properdin and protein C deficiency. Clin Exp Immunol. 1995;102:290–296. doi: 10.1111/j.1365-2249.1995.tb03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fijen C A P, Kuijper E J, Hannema A J, Sjöholm A G, van Putten J P M. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet. 1989;iii:585–588. doi: 10.1016/s0140-6736(89)90712-5. [DOI] [PubMed] [Google Scholar]
- 140.Fijen C A P, Kuijper E J, Tjia H G, Daha M R, Dankert J. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin Infect Dis. 1994;18:780–784. doi: 10.1093/clinids/18.5.780. [DOI] [PubMed] [Google Scholar]
- 141.Fijnvandraat K, Derkx B, Peters M, Bijlmer R, Sturk A, Prins M H, van Deventer S J H, ten Cate J W. Coagulation activation and tissue necrosis in meningococcal septic shock: severely reduced protein C levels predict a high mortality. Thromb Haemostasis. 1995;73:15–20. [PubMed] [Google Scholar]
- 142.Fijnvandraat K, Peters M, Derkx B, van Deventer S, ten Cate J W. Endotoxin induced coagulation activation and protein C reduction in meningococcal septic shock. Prog Clin Biol Res. 1994;388:247–254. [PubMed] [Google Scholar]
- 143.Finne J, Leinonen M, Mäkelä P H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 144.Fischer M, Hedberg K, Cardosi P, Plikaytis B D, Hoesly F C, Steingart K R, Bell T A, Fleming D W, Wenger J D, Perkins B A. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16:979–983. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 145.Flexner S. The results of the serum treatment in thirteen hundred cases of epidemic meningitis. J Exp Med. 1913;17:553–576. doi: 10.1084/jem.17.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Foster G, Panigrahi H, Walker M. Failure of chemoprophylaxis to prevent meningococcal disease. Br Med J Clin Res Ed. 1986;292:886–887. doi: 10.1136/bmj.292.6524.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fourrier F, Lestavel P, Chopin C, Marey A, Goudemand J, Rime A, Mangalaboyi J. Meningococcemia and purpura fulminans in adults: acute deficiencies of proteins C and S and early treatment with antithrombin III concentrates. Intensive Care Med. 1990;16:121–124. doi: 10.1007/BF02575306. [DOI] [PubMed] [Google Scholar]
- 148.Fox A J, Jones D M, Gray S J, Caugant D A, Saunders N A. An epidemiologically valuable typing method for Neisseria meningitidis by analysis of restriction fragment length polymorphisms. J Med Microbiol. 1991;34:265–270. doi: 10.1099/00222615-34-5-265. [DOI] [PubMed] [Google Scholar]
- 149.Frappat P. Aspects épidémiologique et prognostique des purpuras fulminans de l'enfant. Enquête regionale. Pediatrie. 1987;42:41–43. [PubMed] [Google Scholar]
- 150.Frasch C E. Vaccines for prevention of meningococcal disease. Clin Microbiol Rev. 1989;2(Suppl.):S134–S138. doi: 10.1128/cmr.2.suppl.s134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 152.Freeman B D, Yatsiv I, Natanson C, Solomon M A, Quezado Z M, Danner R L, Banks S M, Hoffman W D. Continuous arteriovenous hemofiltration does not improve survival in a canine model of septic shock. J Am Coll Surg. 1995;180:286–292. [PubMed] [Google Scholar]
- 153.Friedland I R, Jafari H, Ehrett S, Rinderknecht S, Paris M, Coulthard M, Saxen H, Olsen K, McCracken G H., Jr Comparison of endotoxin release by different antimicrobial agents and the effect on inflammation in experimental Escherichia coli meningitis. J Infect Dis. 1993;168:657–662. doi: 10.1093/infdis/168.3.657. [DOI] [PubMed] [Google Scholar]
- 154.Frieling J T M, van Deuren M, Wijdenes J, van Dalen R, Bartelink A K M, van der Linden C J, Sauerwein R W. Interleukin-6 and its soluble receptor during acute meningococcal infections: effect of plasma or whole blood exchange. Crit Care Med. 1996;24:1801–1805. doi: 10.1097/00003246-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 155.Galimand M, Gerbaud G, Guibourdenche M, Riou J-Y, Courvalin P. High-level chloramphenicol resistance in Neisseria meningitidis. N Engl J Med. 1998;339:868–874. doi: 10.1056/NEJM199809243391302. [DOI] [PubMed] [Google Scholar]
- 156.Gårdlund B. Prognostic evaluation in meningococcal disease. A retrospective study of 115 cases. Intensive Care Med. 1986;12:302–307. doi: 10.1007/BF00261740. [DOI] [PubMed] [Google Scholar]
- 157.Gårdlund B, Sjölin J, Nilsson A, Roll M, Wickerts C-J, Wikstrom B, Wretlind B. Plasmapheresis in the treatment of primary septic shock in humans. Scand J Infect Dis. 1993;25:757–761. doi: 10.3109/00365549309008575. [DOI] [PubMed] [Google Scholar]
- 158.Gårdlund B, Sjölin J, Nilsson A, Roll M, Wickerts C-J, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 159.Gaskins R A, Jr, Dalldorf F G. Experimental meningococcal septicemia. Effect of heparin therapy. Arch Pathol Lab Med. 1976;100:318–324. [PubMed] [Google Scholar]
- 160.Gedde-Dahl T W, Bjark P, Høiby E A, Høst J H, Bruun J N. Severity of meningococcal disease: assessment by factors and scores and implications for patient management. Rev Infect Dis. 1990;12:973–992. doi: 10.1093/clinids/12.6.973. [DOI] [PubMed] [Google Scholar]
- 161.Gedde-Dahl T W, Høiby E A, Brandtzaeg P, Eskerud J R, Bøvre K. Some arguments on early hospital admission and treatment of suspected meningococcal disease cases. NIPH Ann. 1990;13:45–60. [PubMed] [Google Scholar]
- 162.Gedde-Dahl T W, Høiby E A, Eskerud J R. Unbiased evidence on early treatment of suspected meningococcal disease. Rev Infect Dis. 1990;12:359. doi: 10.1093/clinids/12.2.359-a. , 361–354. [DOI] [PubMed] [Google Scholar]
- 163.Gedde-Dahl T W, Hoiby E A, Schillinger A, Lystad A, Bovre K. An epidemiological, clinical and microbiological follow-up study of incident meningococcal disease cases in Norway, winter 1981–1982. Material and epidemiology in the MenOPP project. NIPH Ann. 1983;6:155–168. [PubMed] [Google Scholar]
- 164.Gelfand M S, Cleveland K O, Campagna C, Zolyomi A. Meningococcal cellulitis and sialadenitis. South Med J. 1998;91:287–288. doi: 10.1097/00007611-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 165.Genoff M C, Hoffer M M, Achauer B, Formosa P. Extremity amputations in meningococcemia-induced purpura fulminans. Plast Reconstr Surg. 1992;89:878–881. doi: 10.1097/00006534-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 166.Gerson W T, Dickerman J D, Bovill E G, Golden E. Severe acquired protein C deficiency in purpura fulminans associated with disseminated intravascular coagulation: treatment with protein C concentrate. Pediatrics. 1993;91:418–422. [PubMed] [Google Scholar]
- 167.Gilja O H, Halstensen A, Digranes A, Mylvaganam H, Aksnes A, Høiby E A. Use of single-dose ofloxacin to eradicate tonsillopharyngeal carriage of Neisseria meningitidis. Antimicrob Agents Chemother. 1993;37:2024–2026. doi: 10.1128/aac.37.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Girardin E, Grau G E, Dayer J-M, Roux-Lombard P, Lambert P H The J5 Study Group. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 169.Girardin E, Roux-Lombard P, Grau G E, Suter P, Gallati H, Dayer J-M The J5 Study Group. Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. Immunology. 1992;76:20–23. [PMC free article] [PubMed] [Google Scholar]
- 170.Giraud T, Dhainaut J-F, Schremmer B, Regnier B, Desjars P, Loirat P, Journois D, Lanore J-J. Adult overwhelming meningococcal purpura. A study of 35 cases, 1977–1989. Arch Intern Med. 1991;151:310–316. [PubMed] [Google Scholar]
- 171.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N J, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 172.Gold R. Bacterial meningitis—1982. Am J Med. 1983;75:98–101. doi: 10.1016/0002-9343(83)90079-7. [DOI] [PubMed] [Google Scholar]
- 173.Gold R, Goldschneider I, Lepow M L, Draper T F, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 174.Goldman A P, Kerr S J, Butt W, Marsh M J, Murdoch I A, Paul T, Firmin R K, Tasker R C, Macrae D J. Extracorporeal support for intractable cardiorespiratory failure due to meningococcal disease. Lancet. 1997;349:466–469. doi: 10.1016/s0140-6736(96)12106-1. [DOI] [PubMed] [Google Scholar]
- 175.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grados O, Ewing W H. Antigenic relationship between Escherichia coli and Neisseria meningitidis. J Infect Dis. 1970;122:100–103. doi: 10.1093/infdis/122.1-2.100. [DOI] [PubMed] [Google Scholar]
- 179.Granier S, Owen P, Pill R, Jacobson L. Recognising meningococcal disease in primary care: qualitative study of how general practitioners process clinical and contextual information. Br Med J. 1998;316:276–279. doi: 10.1136/bmj.316.7127.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Granier S, Owen P, Stott N C H. Recognizing meningococcal disease: the case for further research in primary care. Br J Gen Pract. 1998;48:1167–1171. [PMC free article] [PubMed] [Google Scholar]
- 181.Granoff D M, Forrest B, Rappuoli R. Meningococcal polysaccharide-protein conjugate vaccines. Int J Infect Dis. 1997;1:152–157. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 182.Greenfield S, Sheehe P R, Feldman H A. Meningococcal carriage in a population of “normal” families. J Infect Dis. 1971;123:67–73. doi: 10.1093/infdis/123.1.67. [DOI] [PubMed] [Google Scholar]
- 183.Greenwood B M. Bacterial meningitis. London, United Kingdom: Academic Press, Ltd.; 1987. The epidemiology of acute bacterial meningitis in tropical Africa; pp. 61–91. [Google Scholar]
- 184.Greenwood B M, Mohammed I, Whittle H C. Immune complexes and the pathogenesis of meningococcal arthritis. Clin Exp Immunol. 1985;59:513–519. [PMC free article] [PubMed] [Google Scholar]
- 185.Griffis J M. Mechanisms of host immunity. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 35–70. [Google Scholar]
- 186.Griffiss J M. Epidemic meningococcal disease: synthesis of a hypothetical immunoepidemiologic model. Rev Infect Dis. 1982;4:159–172. doi: 10.1093/clinids/4.1.159. [DOI] [PubMed] [Google Scholar]
- 187.Griffiss J M, Yamasaki R, Estabrook M, Kim J J. Meningococcal molecular mimicry and the search for an ideal vaccine. Trans R Soc Trop Med Hyg. 1991;85(Suppl. 1):32–36. doi: 10.1016/0035-9203(91)90338-y. [DOI] [PubMed] [Google Scholar]
- 188.Grootendorst A F, van Bommel E F H, van der Hoven B, van Leengoed L A M G, van Osta A L. High volume hemofiltration improves right ventricular function in endotoxin-induced shock in the pig. Intensive Care Med. 1992;18:235–240. doi: 10.1007/BF01709839. [DOI] [PubMed] [Google Scholar]
- 189.Guibourdenche M, Høiby E A, Riou J Y, Varaine F, Joguet C, Caugant D A. Epidemics of serogroup A Neisseria meningitidis of subgroup III in Africa, 1989–94. Epidemiol Infect. 1996;116:115–120. doi: 10.1017/s095026880005233x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hack C E, Ogilvie A C, Eisele B, Eerenberg A J, Wagstaff J, Thijs L G. C1-inhibitor substitution therapy in septic shock and in the vascular leak syndrome induced by high doses of interleukin-2. Intensive Care Med. 1993;19:S19–S28. doi: 10.1007/BF01738946. [DOI] [PubMed] [Google Scholar]
- 191.Hackshaw K V, Parker G A, Roberts J W. Naloxone in septic shock. Crit Care Med. 1990;18:47–51. doi: 10.1097/00003246-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 192.Halstensen A, Ceska M, Brandtzaeg P, Redl H, Naess A, Waage A. Interleukin-8 in serum and cerebrospinal fluid from patients with meningococcal disease. J Infect Dis. 1993;167:471–475. doi: 10.1093/infdis/167.2.471. [DOI] [PubMed] [Google Scholar]
- 193.Halstensen A, Pedersen S J H, Haneberg B, Bjorvatn B, Solberg C O. Case fatality of meningococcal disease in western Norway. Scand J Infect Dis. 1987;19:35–42. doi: 10.3109/00365548709032375. [DOI] [PubMed] [Google Scholar]
- 194.Halstensen A, Sjursen H, Vollset S E, Frøholm L O, Naess A, Matre R, Solberg C O. Serum opsonins to serogroup B meningococci in meningococcal disease. Scand J Infect Dis. 1989;21:267–276. doi: 10.3109/00365548909035696. [DOI] [PubMed] [Google Scholar]
- 195.Halstensen A, Vollset S E, Haneberg B, Høiby E A, Solberg C O. Antimicrobial therapy and case fatality in meningococcal disease. Scand J Infect Dis. 1987;19:403–407. doi: 10.3109/00365548709021672. [DOI] [PubMed] [Google Scholar]
- 196.Hameter B M, Westra P, van der Zande F L, Versluis D J, van't Wout J W, Maartense E, de Kievit W. Immunotherapy with human monoclonal antibody against endotoxin (HA-1A) in meningococcal sepsis/shock. Clin Intensive Care. 1993;4:116–120. [Google Scholar]
- 197.Haneberg B, Dalseg R, Wedege E, Høiby E A, Haugen I L, Oftung F, Andersen S R, Naess L M, Aase A, Michaelsen T E, Holst J. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haneberg B, Gutteberg T J, Moe P J, Østerud B, Bjorvatn B, Lehmann E H. Heparin for infants and children with meningococcal septicemia. Results of a randomized therapeutic trial. NIPH Ann. 1983;6:43–47. [PubMed] [Google Scholar]
- 199.Haneberg B, Tønjum T, Rodahl K, Gedde Dahl T W. Factors preceding the onset of meningococcal disease, with special emphasis on passive smoking, stressful events, physical fitness and general symptoms of ill health. NIPH Ann. 1983;6:169–173. [PubMed] [Google Scholar]
- 200.Hardaway R M. Pathology and pathophysiology of disseminated intravascular coagulation. In: Cowley R A, Trump B F, editors. Pathophysiology of shock, anoxia and ischaemia. Baltimore, Md: The Williams & Wilkins Co.; 1982. pp. 186–197. [Google Scholar]
- 201.Hardman J M, Earle K M. Meningococcal infections: a review of 200 fatal cases. J Neuropathol Exp Neurol. 1967;26:119. [PubMed] [Google Scholar]
- 202.Hardman J M, Earle K M. Myocarditis in 200 fatal meningococcal infections. Arch Pathol. 1969;87:318–325. [PubMed] [Google Scholar]
- 203.Harris N J, Gosh M. Skin and extremity loss in meningococcal septicaemia treated in a burn unit. Burns. 1994;20:471–472. doi: 10.1016/0305-4179(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 204.Hart C A, Rogers T R F. Meningococcal disease. J Med Microbiol. 1993;39:3–25. doi: 10.1099/00222615-39-1-3. [DOI] [PubMed] [Google Scholar]
- 205.Hatherill M, Tibby S M, Hilliard T, Turner C, Murdoch I A. Adrenal insufficiency in septic shock. Arch Dis Child. 1999;80:51–55. doi: 10.1136/adc.80.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Haupt W, Fritzsche H, Hohenberger W, Zirngibl H. Selective cytokine release induced by serum and separated plasma from septic patients. Eur J Surg. 1996;162:769–776. [PubMed] [Google Scholar]
- 207.Havens P L, Garland J S, Brook M M, Dewitz B A, Stremski E S, Troshynski T J. Trends in mortality in children hospitalized with meningococcal infections, 1957 to 1987. Pediatr Infect Dis J. 1989;8:8–11. doi: 10.1097/00006454-198901000-00003. [DOI] [PubMed] [Google Scholar]
- 208.Hazelzet J A, de Groot R. Sepsis-related problems in pediatric patients. In: Reinhart K, Eyrich K, Sprung C, editors. Sepsis: current perspectives in pathophysiology and therapy. 18th ed. Berlin, Germany: Springer-Verlag KG; 1994. pp. 217–228. [Google Scholar]
- 209.Hazelzet J A, de Groot R, van Mierlo G, Joosten K F M, van der Voort E, Eerenberg A, Suur M H, Hop W C J, Hack C E. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect Immun. 1998;66:5350–5356. doi: 10.1128/iai.66.11.5350-5356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hazelzet J A, Kornelisse R F, van der Pouw Kraan T C T H, Joosten K F M, van der Voort E, van Mierlo G, Suur M H, Hop W C J, de Groot R, Hack C E. Interleukin 12 levels during the initial phase of septic shock with purpura in children: relation to severity of disease. Cytokine. 1997;9:711–716. doi: 10.1006/cyto.1997.0215. [DOI] [PubMed] [Google Scholar]
- 211.Hazelzet J A, Risseeuw-Appel I M, Kornelisse R F, Hop W C J, Dekker I, Joosten K F M, de Groot R, Hack C E. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemostasis. 1996;76:932–938. [PubMed] [Google Scholar]
- 212.Hazelzet J A, Stubenitsky R, Petrov A B, van Wieringen G W, van der Voort E, Hess J, Hop W C, Thijs L G, Duncker D J, Poolman J T, Verdouw P D. Cardiovascular aspects of experimental meningococcal sepsis in young and older awake piglets: age-related differences. Shock. 1999;12:145–154. doi: 10.1097/00024382-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 213.Hazelzet J A, van der Voort E, Lindemans J, ter Heerdt P G J, Neijens H J. Relation between cytokines and routine laboratory data in children with septic shock and purpura. Intensive Care Med. 1994;20:371–374. doi: 10.1007/BF01720912. [DOI] [PubMed] [Google Scholar]
- 214.Herbert M A, Heath P T, Mayon-White R T. Meningococcal vaccines for the United Kingdom. Commun Dis Rep Rev. 1995;5:R130–R135. [PubMed] [Google Scholar]
- 215.Hermans P W, Hibberd M L, Booy R, Daramola O, Hazelzet J A, de Groot R, Levin M. 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet. 1999;354:556–560. doi: 10.1016/s0140-6736(99)02220-5. [DOI] [PubMed] [Google Scholar]
- 216.Hermaszewski R A, Webster A D B. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42. [PubMed] [Google Scholar]
- 217.Herrick W W. Extrameningeal meningococcus infections. Arch Intern Med. 1919;23:409–418. [Google Scholar]
- 218.Heyderman R S, Klein N J, Daramola O A, Hammerschmidt S, Frosch M, Robertson B D, Levin M, Ison C A. Induction of human endothelial tissue factor expression by Neisseria meningitidis: the influence of bacterial killing and adherence to the endothelium. Microb Pathog. 1997;22:265–274. doi: 10.1006/mpat.1996.0112. [DOI] [PubMed] [Google Scholar]
- 219.Hibberd M L, Sumiya M, Summerfield J A, Booy R, Levin M the Meningococcal Research Group. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–1053. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 220.Hill W R, Kinney T D. The cutaneous lesions in acute meningococcemia. A clinical and pathologic study. JAMA. 1947;134:513–518. doi: 10.1001/jama.1947.02880230023006. [DOI] [PubMed] [Google Scholar]
- 221.Hodge D. Intraosseous infusions: a review. Pediatr Emerg Care. 1985;1:215–218. doi: 10.1097/00006565-198512000-00012. [DOI] [PubMed] [Google Scholar]
- 222.Hodgetts T J, Brett A, Castle N. The early management of meningococcal disease. J Accid Emerg Med. 1998;15:72–76. doi: 10.1136/emj.15.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Høgåsen K, Michaelsen T, Mellbye O J, Bjune G. Low prevalence of complement deficiencies among patients with meningococcal disease in Norway. Scand J Immunol. 1993;37:487–489. doi: 10.1111/j.1365-3083.1993.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 224.Høgåsen K, Mollnes T E, Brandtzaeg P. Low levels of vitronectin and clusterin in acute meningococcal disease are closely associated with formation of the terminal-complement complex and the vitronectin-thrombin-antithrombin complex. Infect Immun. 1994;62:4874–4880. doi: 10.1128/iai.62.11.4874-4880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Høiby E A, Moe P J, Lystad A, Frøholm L O, Bøvre K. Phenoxymethyl-penicillin treatment of household contacts of patients with meningococcal disease. Antonie Leeuwenhoek. 1986;52:255–257. [Google Scholar]
- 226.Reference deleted.
- 227.Holmes F F, Weyandt T, Glazier J, Cuppage F E, Moral L A, Lindsey N J. Fulminant meningococcemia after splenectomy. JAMA. 1981;246:1119–1120. [PubMed] [Google Scholar]
- 228.Huang S, Clarke J A. Severe skin loss after meningococcal septicaemia: complications in treatment. Acta Paediatr. 1997;86:1263–1266. doi: 10.1111/j.1651-2227.1997.tb14859.x. [DOI] [PubMed] [Google Scholar]
- 229.Hudson D A, Goddard E A, Millar K N. The management of skin infarction after meningococcal septicaemia in children. Br J Plast Surg. 1993;46:243–246. doi: 10.1016/0007-1226(93)90176-c. [DOI] [PubMed] [Google Scholar]
- 230.Hurley J C. Antibiotic-induced release of endotoxin: a reappraisal. Clin Infect Dis. 1992;15:840–854. doi: 10.1093/clind/15.5.840. [DOI] [PubMed] [Google Scholar]
- 231.Reference deleted.
- 232.Reference deleted.
- 233.J5 Study Group. Treatment of severe infectious purpura in children with human plasma from donors immunized with Escherichia coli J5: a prospective double-blind study. J Infect Dis. 1992;165:695–701. doi: 10.1093/infdis/165.4.695. [DOI] [PubMed] [Google Scholar]
- 234.Jackson L A, Schuchat A, Reeves M W, Wenger J D. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA. 1995;273:383–389. [PubMed] [Google Scholar]
- 235.Jackson L A, Tenover F C, Baker C, Plikaytis B D, Reeves M W, Stocker S A, Weaver R E, Wenger J D The Meningococcal Disease Study Group. Prevalence of Neisseria meningitidis relatively resistant to penicillin in the United States, 1991. J Infect Dis. 1994;169:438–441. doi: 10.1093/infdis/169.2.438. [DOI] [PubMed] [Google Scholar]
- 236.Jacobs R F, Sowell M K, Moss M M, Fiser D H. Septic shock in children: bacterial etiologies and temporal relationships. Pediatr Infect Dis J. 1990;9:196–200. doi: 10.1097/00006454-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 237.Janbon B, Vuillez J-P, Carpentier F, Barnoud D, André-Poyaud P, Barbe G, Guignier M. Removal of circulating tumor necrosis factor. Its role in septic shock treatment. Ann Med Interne Paris. 1992;143(Suppl. 1):13–16. [PubMed] [Google Scholar]
- 238.Jansen P M, Pixley R A, Brouwer M, de Jong I W, Chang A C, Hack C E, Taylor F B, Jr, Colman R W. Inhibition of factor XII in septic baboons attenuates the activation of complement and fibrinolytic systems and reduces the release of interleukin-6 and neutrophil elastase. Blood. 1996;87:2337–2344. [PubMed] [Google Scholar]
- 239.Jarvis G A, Vedros N A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jennings H J. N-propionylated group B meningococcal polysaccharide glycoconjugate vaccine against group B meningococcal meningitis. Int J Infect Dis. 1997;1:158–164. [Google Scholar]
- 241.Jochmann G. Versuche zur Serodiagnostik un Serotherapie der epidemischen Genickstarre. Dtsch Med Wochenschr. 1906;1:788–793. [Google Scholar]
- 242.Jones D. Epidemiology of meningococcal disease in Europe and the USA. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 147–157. [Google Scholar]
- 243.Jones D M. Control of meningococcal disease. Chemoprophylaxis for some carriers and some contact groups. Br Med J. 1989;298:542–543. doi: 10.1136/bmj.298.6673.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reference deleted.
- 245.Jones D M, Borrow R, Fox A J, Gray S, Cartwright K A, Poolman J T. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992;13:219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 246.Jones E M, Brown N M, Harvey J E, Reeves D S, MacGowan A P. Three cases of meningococcal pneumonia. Thorax. 1997;52:927–929. doi: 10.1136/thx.52.10.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jones G R, Christodoulides M, Brooks J L, Miller A R, Cartwright K A, Heckels J E. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J Infect Dis. 1998;178:451–459. doi: 10.1086/515622. [DOI] [PubMed] [Google Scholar]
- 248.Kahler C M, Martin L E, Shih G C, Rahman M M, Carlson R W, Stephens D S. The (alpha2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kahn A, Blum D. Factors for poor prognosis in fulminating meningococcemia. Conclusions from observations of 67 childhood cases. Clin Pediatr Phila. 1978;17:680–682. doi: 10.1177/000992287801700902. , 687. [DOI] [PubMed] [Google Scholar]
- 250.Kallstrom H, Islam M S, Berggren P O, Jonsson A B. Cell signaling by the type IV pili of pathogenic Neisseria. J Biol Chem. 1998;273:21777–21782. doi: 10.1074/jbc.273.34.21777. [DOI] [PubMed] [Google Scholar]
- 251.Kallstrom H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 252.Kalter E S, Daha M R, ten Cate J W, Verhoef J, Bouma B N. Activation and inhibition of Hageman factor-dependent pathways and the complement system in uncomplicated bacteremia or bacterial shock. J Infect Dis. 1985;151:1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- 253.Kasper D L, Winkelhake J L, Zollinger W D, Brandt B L, Artenstein M S. Immunochemical similarity between polysaccharide antigens of Escherichia coli O7:K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973;110:262–268. [PubMed] [Google Scholar]
- 254.Reference deleted.
- 255.Kennedy N J, Duncan A W. Acute meningococcaemia: recent advances in management (with particular reference to children) Anaesth Intensive Care. 1996;24:197–216. doi: 10.1177/0310057X9602400212. [DOI] [PubMed] [Google Scholar]
- 256.Kertesz D A, Coulthart M B, Ryan J A, Johnson W M, Ashton F E. Serogroup B, electrophoretic type 15 Neisseria meningitidis in Canada. J Infect Dis. 1998;177:1754–1757. doi: 10.1086/517439. [DOI] [PubMed] [Google Scholar]
- 257.Kilpi T, Anttila M, Kallio M J, Peltola H. Severity of childhood bacterial meningitis and duration of illness before diagnosis. Lancet. 1991;338:406–409. doi: 10.1016/0140-6736(91)91032-p. [DOI] [PubMed] [Google Scholar]
- 258.Kim K S, Wass C A, Cross A S, Opal S M. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- 259.Kirsch E A, Barton R P, Kitchen L, Giroir B P. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996;15:967–978. doi: 10.1097/00006454-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 260.Klein N J, Ison C A, Peakman M, Levin M, Hammerschmidt S, Frosch M, Heyderman R S. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J Infect Dis. 1996;173:172–179. doi: 10.1093/infdis/173.1.172. [DOI] [PubMed] [Google Scholar]
- 261.Kornelisse R F. Ph.D. thesis. Rotterdam, The Netherlands: University of Rotterdam; 1996. [Google Scholar]
- 262.Kornelisse R F, Hazelzet J A, Hop W C J, Spanjaard L, Suur M H, van der Voort E, de Groot R. Meningococcal septic shock in children: clinical and laboratory features, outcome, and development of a prognostic score. Clin Infect Dis. 1997;25:640–646. doi: 10.1086/513759. [DOI] [PubMed] [Google Scholar]
- 263.Kornelisse R F, Hazelzet J A, Savelkoul H F J, Hop W C J, Suur M H, Borsboom A N J, Risseeuw-Appel I M, van der Voort E, de Groot R. The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis. 1996;173:1148–1156. doi: 10.1093/infdis/173.5.1148. [DOI] [PubMed] [Google Scholar]
- 264.Kornelisse R F, Hoekman K, Visser J J, Hop W C J, Huijmans J G M, van der Straaten P J C, van der Heijden A J, Sukhai R N, Neijens H J, de Groot R. The role of nitric oxide in bacterial meningitis in children. J Infect Dis. 1996;174:120–126. doi: 10.1093/infdis/174.1.120. [DOI] [PubMed] [Google Scholar]
- 265.Kristiansen B E, Knapskog A B. Secondary prevention of meningococcal disease. High risk contacts should be given chemoprophylaxis and preventive treatment with penicillin. Br Med J. 1996;312:591–592. doi: 10.1136/bmj.312.7031.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kristiansen B E, Thorsby E. HLA histocompatibility antigens and meningococcal disease. NIPH Ann. 1980;3:75–79. [PubMed] [Google Scholar]
- 267.Kumar A, Kanagasundaram N S, Collyns T A, Davison A M. Plasma exchange and haemodiafiltration in fulminant meningococcal sepsis. Nephrol Dial Transplant. 1998;13:484–487. doi: 10.1093/oxfordjournals.ndt.a027853. [DOI] [PubMed] [Google Scholar]
- 268.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo J E. Tumor necrosis factor α and interleukin 1β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kunzer W, Schindera F, Schenck W, Schumacher H. Waterhouse-Friderichsen Syndrom, Abgrenzung, Pathogenese, und Therapie mit Streptokinase. Dtsch Med Wochenschr. 1972;97:270–273. doi: 10.1055/s-0028-1107339. [DOI] [PubMed] [Google Scholar]
- 270.Kuppermann N, Inkelis S H, Saladino R. The role of heparin in the prevention of extremity and digit necrosis in meningococcal purpura fulminans. Pediatr Infect Dis J. 1994;13:867–873. doi: 10.1097/00006454-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 271.Lapeyssonnie L. La méningite cérébro-spinale en Afrique. Bull W H O. 1963;28(Suppl. 1):3–114. [PMC free article] [PubMed] [Google Scholar]
- 272.Laursen B, Faber V, Brock A, Gormsen J, Sørensen H. Disseminated intravascular coagulation, antithrombin III, and complement in meningococcal infections. Acta Med Scand. 1981;209:221–227. doi: 10.1111/j.0954-6820.1981.tb11581.x. [DOI] [PubMed] [Google Scholar]
- 273.Lebel M H, McCracken G H., Jr Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics. 1989;83:161–167. [PubMed] [Google Scholar]
- 274.Leclerc F, Beuscart R, Guillois B, Diependaele J F, Krim G, Devictor D, Bompard Y, van Albada T. Prognostic factors of severe infectious purpura in children. Intensive Care Med. 1985;11:140–143. doi: 10.1007/BF00258539. [DOI] [PubMed] [Google Scholar]
- 275.Leclerc F, Chenaud M, Delepoulle F, Diependaele J F, Martinot A, Hue V. Prognostic value of C-reactive protein level in severe infectious purpura: a comparison with eight other scores. Crit Care Med. 1991;19:430–432. doi: 10.1097/00003246-199103000-00026. [DOI] [PubMed] [Google Scholar]
- 276.Leclerc F, Delepoulle F, Diependaele J F, Martinot A, Hue V, Flurin V, Fourier C, Chenaud M. Severity scores in meningococcal septicemia and severe infectious purpura with shock. Intensive Care Med. 1995;21:264–265. doi: 10.1007/BF01701486. [DOI] [PubMed] [Google Scholar]
- 277.Leclerc F, Giraud T, Delepoulle F, Hue V, Martinot A, Flurin V. Réanimation urgences. Paris, France: Arnette; 1993. Facteurs de gravité des méningococcémies; pp. 105–122. [Google Scholar]
- 278.Leclerc F, Hazelzet J, Jude B, Hofhuis W, Hue V, Martinot A, Van der Voort E. Protein C and S deficiency in severe infectious purpura of children: a collaborative study of 40 cases. Intensive Care Med. 1992;18:202–205. doi: 10.1007/BF01709832. [DOI] [PubMed] [Google Scholar]
- 279.Reference deleted.
- 280.Reference deleted.
- 281.Lederman H M, Winkelstein J A. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985;64:145–156. [PubMed] [Google Scholar]
- 282.Lefering R, Neugebauer E A. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 283.Lehmann A K, Halstensen A, Sørnes S, Røkke O, Waage A. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun. 1995;63:2109–2112. doi: 10.1128/iai.63.6.2109-2112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lehner P J, Davies K A, Walport M J, Cope A P, Würzner R, Orren A, Morgan B P, Cohen J. Meningococcal septicaemia in a C6-deficient patient and effects of plasma transfusion on lipopolysaccharide release. Lancet. 1992;340:1379–1381. doi: 10.1016/0140-6736(92)92561-s. [DOI] [PubMed] [Google Scholar]
- 285.Lennon D, Gellin B, Hood D, Voss L, Heffernan H, Thakur S. Successful intervention in a group A meningococcal outbreak in Auckland, New Zealand. Pediatr Infect Dis J. 1992;11:617–623. [PubMed] [Google Scholar]
- 286.Lepow M L, Beeler J, Randolph M, Samuelson J S, Hankins W A. Reactogenicity and immunogenicity of a quadrivalent combined meningococcal polysaccharide vaccine in children. J Infect Dis. 1986;154:1033–1036. doi: 10.1093/infdis/154.6.1033. [DOI] [PubMed] [Google Scholar]
- 287.Levi M, ten Cate H, van der Poll T, van Deventer S J H. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 1993;270:975–979. [PubMed] [Google Scholar]
- 288.Levi M, van der Poll T, ten Cate H, van Deventer S J H. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Investig. 1997;27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 289.Levitt L P, Bond J O, Hall I E, Jr, Dame G M, Buff E E, Marston C, Prather E C. Meningococcal and ECHO-9 meningitis. Report of an outbreak. Neurology. 1970;20:45–51. doi: 10.1212/wnl.20.1.45. [DOI] [PubMed] [Google Scholar]
- 290.Lewis L S. Prognostic factors in acute meningococcaemia. Arch Dis Child. 1979;54:44–48. doi: 10.1136/adc.54.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Reference deleted.
- 292.Lin V H, Parekh R S, McQuillan M A, Braun D K, Markovitz D M. Meningococcal endocarditis presenting as cellulitis. Clin Infect Dis. 1995;21:1023–1025. doi: 10.1093/clinids/21.4.1023. [DOI] [PubMed] [Google Scholar]
- 293.Lodder M C, Schildkamp R L, Bijlmer H A, Dankert J, Kuik D J, Scholten R J P M. Prognostic indicators of the outcome of meningococcal disease: a study of 562 patients. J Med Microbiol. 1996;45:16–20. doi: 10.1099/00222615-45-1-16. [DOI] [PubMed] [Google Scholar]
- 294.Loggie B W, Hinchey E J. Does splenectomy predispose to meningococcal sepsis? An experimental study and clinical review. J Pediatr Surg. 1986;21:326–330. doi: 10.1016/s0022-3468(86)80195-6. [DOI] [PubMed] [Google Scholar]
- 295.López Cortés L F, Cruz-Ruiz M, Gómez-Mateos J, Jiménez-Hernández D, Palomino J, Jiménez E. Measurement of levels of tumor necrosis factor-α and interleukin-1β in the CSF of patients with meningitis of different etiologies: utility in the differential diagnosis. Clin Infect Dis. 1993;16:534–539. doi: 10.1093/clind/16.4.534. [DOI] [PubMed] [Google Scholar]
- 296.Louria D B, Sen P, Kapila R, Johnson E, Smith L, Roberts R. Anterior thigh pain or tenderness. A diagnostically useful manifestation of bacteremia. Arch Intern Med. 1985;145:657–658. [PubMed] [Google Scholar]
- 297.Lynn W A, Cohen J. Adjunctive therapy for septic shock: a review of experimental approaches. Clin Infect Dis. 1995;20:143–158. doi: 10.1093/clinids/20.1.143. [DOI] [PubMed] [Google Scholar]
- 298.Mandrell R E, Zollinger W D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977;16:471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Manios S G, Kanakoudi F, Maniati E. Fulminant meningococcemia. Heparin therapy and survival rate. Scand J Infect Dis. 1971;3:127–133. doi: 10.3109/inf.1971.3.issue-2.06. [DOI] [PubMed] [Google Scholar]
- 300.Marks M I, Frasch C E, Shapera R M. Meningococcal colonization and infection in children and their household contacts. Am J Epidemiol. 1979;109:563–571. doi: 10.1093/oxfordjournals.aje.a112714. [DOI] [PubMed] [Google Scholar]
- 301.Marotto M S, Marotto P C, Sztajnbok J, Seguro A C. Outcome of acute renal failure in meningococcemia. Renal Failure. 1997;19:807–810. doi: 10.3109/08860229709037221. [DOI] [PubMed] [Google Scholar]
- 302.Martin D R, Walker S J, Baker M G, Lennon D R. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J Infect Dis. 1998;177:497–500. doi: 10.1086/517385. [DOI] [PubMed] [Google Scholar]
- 303.Marzouk O, Thomson A P J, Sills J A, Hart C A, Harris F. Features and outcome in meningococcal disease presenting with maculopapular rash. Arch Dis Child. 1991;66:485–487. doi: 10.1136/adc.66.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Masterton R G, Youngs E R, Wardle J C, Croft K F, Jones D M. Control of an outbreak of group C meningococcal meningitis with a polysaccharide vaccine. J Infect. 1988;17:177–182. doi: 10.1016/s0163-4453(88)91907-x. [DOI] [PubMed] [Google Scholar]
- 305.McGee L, Koornhof H J, Caugant D A. Epidemic spread of subgroup III of Neisseria meningitidis serogroup A to South Africa in 1996. Clin Infect Dis. 1998;27:1214–1220. doi: 10.1086/514971. [DOI] [PubMed] [Google Scholar]
- 306.McGee Z A, Stephens D S, Hoffman L H, Schlech W F d, Horn R G. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev Infect Dis. 1983;5(Suppl. 4):S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- 307.McGehee W G, Rapaport S I, Hjort P F. Intravascular coagulation in fulminant meningococcemia. Ann Intern Med. 1967;67:250–260. doi: 10.7326/0003-4819-67-2-250. [DOI] [PubMed] [Google Scholar]
- 308.Meningococcal Disease Surveillance Group. Meningococcal disease. Secondary attack rate and chemoprophylaxis in the United States, 1974. JAMA. 1976;235:261–265. [PubMed] [Google Scholar]
- 309.Mercier J-C, Beaufils F, Hartmann J-F, Azéma D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16:27–33. doi: 10.1097/00003246-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 310.Meyer T F. Pathogenic neisseriae: complexity of pathogen-host cell interplay. Clin Infect Dis. 1999;28:433–441. doi: 10.1086/515160. [DOI] [PubMed] [Google Scholar]
- 311.Milagres L G, Ramos S R, Sacchi C T, Melles C E A, Vieira V S D, Sato H, Brito G S, Moraes J C, Frasch C E. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–4424. doi: 10.1128/iai.62.10.4419-4424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mitchell S R, Nguyen P Q, Katz P. Increased risk of neisserial infections in systemic lupus erythematosus. Semin Arthritis Rheum. 1990;20:174–184. doi: 10.1016/0049-0172(90)90058-n. [DOI] [PubMed] [Google Scholar]
- 313.Mok Q, Butt W. The outcome of children admitted to intensive care with meningococcal septicaemia. Intensive Care Med. 1996;22:259–263. doi: 10.1007/BF01712247. [DOI] [PubMed] [Google Scholar]
- 314.Monsalve F, Rucabado L, Salvador A, Bonastre J, Cuñat J, Ruano M. Myocardial depression in septic shock caused by meningococcal infection. Crit Care Med. 1984;12:1021–1023. doi: 10.1097/00003246-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 315.Moore P S. Meningococcal meningitis in sub-Saharan Africa: a model for the epidemic process. Clin Infect Dis. 1992;14:515–525. doi: 10.1093/clinids/14.2.515. [DOI] [PubMed] [Google Scholar]
- 316.Moore P S, Harrison L H, Telzak E E, Ajello G W, Broome C V. Group A meningococcal carriage in travelers returning from Saudi Arabia. JAMA. 1988;260:2686–2689. [PubMed] [Google Scholar]
- 317.Moore P S, Hierholzer J, DeWitt W, Gouan K, Djoré D, Lippeveld T, Plikaytis B, Broome C V. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. JAMA. 1990;264:1271–1275. [PubMed] [Google Scholar]
- 318.Moore P S, Reeves M W, Schwartz B, Gellin B G, Broome C V. Intercontinental spread of an epidemic group A Neisseria meningitidis strain. Lancet. 1989;ii:260–263. doi: 10.1016/s0140-6736(89)90439-x. [DOI] [PubMed] [Google Scholar]
- 319.Reference deleted.
- 320.Morrison D C, Cochrane C G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins) J Exp Med. 1974;140:797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Morse J R, Oretsky M I, Hudson J A. Pericarditis as a complication of meningococcal meningitis. Ann Intern Med. 1971;74:212–217. doi: 10.7326/0003-4819-74-2-212. [DOI] [PubMed] [Google Scholar]
- 322.Movat H Z, Burrowes C E. The local Shwartzman reaction: endotoxin-mediated inflammatory and thrombo-hemorrhagic lesions. In: Berry L J, editor. Handbook of endotoxin. 3. Cellular biology of endotoxin. Amsterdam, The Netherlands: Elsevier Science Publisher BV; 1985. pp. 260–302. [Google Scholar]
- 323.Mulks M H, Plaut A G. IgA protease production as a characteristic distinguishing pathogenic from harmless neisseriaceae. N Engl J Med. 1978;299:973–976. doi: 10.1056/NEJM197811022991802. [DOI] [PubMed] [Google Scholar]
- 324.Mustafa M M, Mertsola J, Ramilo O, Sáez-Llorens X, Risser R C, McCracken G H., Jr Increased endotoxin and interleukin-1β concentrations in cerebrospinal fluid of infants with coliform meningitis and ventriculitis associated with intraventricular gentamicin therapy. J Infect Dis. 1989;160:891–895. doi: 10.1093/infdis/160.5.891. [DOI] [PubMed] [Google Scholar]
- 325.Reference deleted.
- 326.Nadel S, Newport M J, Booy R, Levin M. Variation in the tumor necrosis factor-α gene promoter region may be associated with death from meningococcal disease. J Infect Dis. 1996;174:878–880. doi: 10.1093/infdis/174.4.878. [DOI] [PubMed] [Google Scholar]
- 327.Nassif X. Interaction mechanisms of encapsulated meningococci with eucaryotic cells: what does this tell us about the crossing of the blood-brain barrier by Neisseria meningitidis? Curr Opin Microbiol. 1999;2:71–77. doi: 10.1016/s1369-5274(99)80012-5. [DOI] [PubMed] [Google Scholar]
- 328.Nassif X, Mathison J C, Wolfson E, Koziol J A, Ulevitch R J, So M. Tumour necrosis factor alpha antibody protects against lethal meningococcaemia. Mol Microbiol. 1992;6:591–597. doi: 10.1111/j.1365-2958.1992.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 329.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Natanson C, Hoffman W D, Koev L A, Dolan D P, Banks S M, Bacher J, Danner R L, Klein H G, Parrillo J E. Plasma exchange does not improve survival in a canine model of human septic shock. Transfusion. 1993;33:243–248. doi: 10.1046/j.1537-2995.1993.33393174451.x. [DOI] [PubMed] [Google Scholar]
- 331.Nelson J D. Jails, microbes, and the three-foot barrier. N Engl J Med. 1996;335:885–886. doi: 10.1056/NEJM199609193351210. [DOI] [PubMed] [Google Scholar]
- 332.Newcombe J, Dyer S, Blackman L, Cartwright K, Palmer W H, McFadden J, Blackwell L. PCR-single-stranded confirmational polymorphism analysis for non-culture-based subtyping of meningococcal strains in clinical specimens. J Clin Microbiol. 1997;35:1809–1812. doi: 10.1128/jcm.35.7.1809-1812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Niklasson P-M, Lundbergh P, Strandell T. Prognostic factors in meningococcal disease. Scand J Infect Dis. 1971;3:17–25. doi: 10.3109/inf.1971.3.issue-1.03. [DOI] [PubMed] [Google Scholar]
- 334.Nitta A T, Douglas J M, Arakere G, Ebens J B. Disseminated meningococcal infection in HIV-seropositive patients. AIDS. 1993;7:87–90. doi: 10.1097/00002030-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 335.Nugent S K, Bausher J A, Moxon E R, Rogers M C. Raised intracranial pressure: its management in Neisseria meningitidis meningoencephalitis. Am J Dis Child. 1979;133:260–262. [PubMed] [Google Scholar]
- 336.Nuijens J H, Huijbregts C C M, Eerenberg-Belmer A J M, Abbink J J, Strack van Schijndel R J M, Felt-Bersma R J F, Thijs L G, Hack C E. Quantification of plasma factor XIIa-C1-inhibitor and kallikrein-C1-inhibitor complexes in sepsis. Blood. 1988;72:1841–1848. [PubMed] [Google Scholar]
- 337.Ødegaard A. Primary meningococcal conjunctivitis followed by meningitis and septicemia. NIPH Ann. 1983;6:55–57. [PubMed] [Google Scholar]
- 338.Ødegaard A. Unusual manifestations of meningococcal infection. A review. NIPH Ann. 1983;6:59–63. [PubMed] [Google Scholar]
- 339.Olcén P, Kjellander J, Danielsson D, Lindquist B L. Epidemiology of Neisseria meningitidis: prevalence and symptoms from the upper respiratory tract in family members to patients with meningococcal disease. Scand J Infect Dis. 1981;13:105–109. doi: 10.3109/inf.1981.13.issue-2.05. [DOI] [PubMed] [Google Scholar]
- 340.Olivares R, Bouyer J, Hubert B. Risk factors for death in meningococcal disease. Pathol Biol Paris. 1993;41:164–168. [PubMed] [Google Scholar]
- 341.Østerud B, Flaegstad T. Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb Haemostasis. 1983;49:5–7. [PubMed] [Google Scholar]
- 342.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, van der Poll T, ten Cate J W, van Deventer S J H. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Palmer S R, Corson J, Hall R, Payne S, Ludlow J, Deere B, Jones H, Kaul S, Stubbins J, Williams R, Walapu M, Spence A, Jenkins P, Donald D. Meningococcal disease in Wales: clinical features, outcome and public health management. J Infect. 1992;25:321–328. doi: 10.1016/0163-4453(92)91699-c. [DOI] [PubMed] [Google Scholar]
- 344.Paradis J F, Grimard D. Laboratory-acquired invasive meningococcus—Quebec. Can Commun Dis Rep. 1994;20:12–14. [PubMed] [Google Scholar]
- 345.Peltola H. Early meningococcal disease: advising the public and the profession. Lancet. 1993;342:509–510. doi: 10.1016/0140-6736(93)91642-y. [DOI] [PubMed] [Google Scholar]
- 346.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 347.Peltola H, Kataja J M, Makela P H. Shift in the age-distribution of meningococcal disease as predictor of an epidemic? Lancet. 1982;2:595–597. doi: 10.1016/s0140-6736(82)90669-9. [DOI] [PubMed] [Google Scholar]
- 348.Peltola H, Mäkelä H P, Käyhty H, Jousimies H, Herva E, Hällström K, Sivonen A, Renkonen O-V, Pettay O, Karanko V, Ahvonen P, Sarna S. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- 349.Periappuram M, Taylor M R H, Keane C T. Rapid detection of meningococci from petechiae in acute meningococcal infection. J Infect. 1995;31:201–203. doi: 10.1016/s0163-4453(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 350.Reference deleted.
- 351.Pether J V, Lightfoot N F, Scott R J, Morgan J, Steele-Perkins A P, Sheard S C. Carriage of Neisseria meningitidis: investigations in a military establishment. Epidemiol Infect. 1988;101:21–42. doi: 10.1017/s0950268800029198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Reference deleted.
- 353.Pettersson A, Poolman J T, van der Ley P, Tommassen J. Response of Neisseria meningitidis to iron limitation. Antonie Leeuwenhoek. 1997;71:129–136. doi: 10.1023/a:1000179301748. [DOI] [PubMed] [Google Scholar]
- 354.Piccioli A, Chini G, Mannelli M, Serio M. Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endocrinol Investig. 1994;17:821–824. doi: 10.1007/BF03347786. [DOI] [PubMed] [Google Scholar]
- 355.Reference deleted.
- 356.Pierce H I, Cooper E B. Meningococcal pericarditis. Clinical features and therapy in five patients. Arch Intern Med. 1972;129:918–922. doi: 10.1001/archinte.129.6.918. [DOI] [PubMed] [Google Scholar]
- 357.Pinner R W, Gellin B G, Bibb W F, Baker C N, Weaver R, Hunter S B, Waterman S H, Mocca L F, Frasch C E, Broome C V. Meningococcal disease in the United States—1986. Meningococcal Disease Study Group. J Infect Dis. 1991;164:368–374. doi: 10.1093/infdis/164.2.368. [DOI] [PubMed] [Google Scholar]
- 358.Pixley R A, De La Cadena R, Page J D, Kaufman N, Wyshock E G, Chang A, Taylor F B, Jr, Colman R W. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Investig. 1993;91:61–68. doi: 10.1172/JCI116201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pixley R A, DeLa Cadena R A, Page J D, Kaufman N, Wyshock E G, Colman R W, Chang A, Taylor F B., Jr Activation of the contact system in lethal hypotensive bacteremia in a baboon model. Am J Pathol. 1992;140:897–906. [PMC free article] [PubMed] [Google Scholar]
- 360.Ploysangam T, Sheth A P. Chronic meningococcemia in childhood: case report and review of the literature. Pediatr Dermatol. 1996;13:483–487. doi: 10.1111/j.1525-1470.1996.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 361.Pollack M. Blood exchange and plasmapheresis in sepsis and septic shock. Clin Infect Dis. 1992;15:431–433. doi: 10.1093/clind/15.3.431. [DOI] [PubMed] [Google Scholar]
- 362.Reference deleted.
- 363.Pomeroy S L, Holmes S J, Dodge P R, Feigin R D. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323:1651–1657. doi: 10.1056/NEJM199012133232402. [DOI] [PubMed] [Google Scholar]
- 364.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 365.Powars D, Larsen R, Johnson J, Hulbert T, Sun T, Patch M J, Francis R, Chan L. Epidemic meningococcemia and purpura fulminans with induced protein C deficiency. Clin Infect Dis. 1993;17:254–261. doi: 10.1093/clinids/17.2.254. [DOI] [PubMed] [Google Scholar]
- 366.Reference deleted.
- 367.Powell K R, Sugarman L I, Eskenazi A E, Woodin K A, Kays M A, McCormick K L, Miller M E, Sladek C D. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J Pediatr. 1990;117:515–522. doi: 10.1016/s0022-3476(05)80682-1. [DOI] [PubMed] [Google Scholar]
- 368.Prins J M, Lauw F N, Derkx B H F, Speelman P, Kuijper E J, Dankert J, van Deventer S J H. Endotoxin release and cytokine production in acute and chronic meningococcaemia. Clin Exp Immunol. 1998;114:215–219. doi: 10.1046/j.1365-2249.1998.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Prins J M, Speelman P, Kuijper E J, Dankert J, van Deventer S J. No increase in endotoxin release during antibiotic killing of meningococci. J Antimicrob Chemother. 1997;39:13–18. doi: 10.1093/jac/39.1.13. [DOI] [PubMed] [Google Scholar]
- 370.Prins J M, van Deventer S J, Kuijper E J, Speelman P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Chemother. 1994;38:1211–1218. doi: 10.1128/aac.38.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Pugsley M P, Dworzack D L, Horowitz E A, Cuevas T A, Sanders W E, Jr, Sanders C C. Efficacy of ciprofloxacin in the treatment of nasopharyngeal carriers of Neisseria meningitidis. J Infect Dis. 1987;156:211–213. doi: 10.1093/infdis/156.1.211. [DOI] [PubMed] [Google Scholar]
- 372.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 373.Quagliarello V J, Scheld W M. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708–716. doi: 10.1056/NEJM199703063361007. [DOI] [PubMed] [Google Scholar]
- 374.Quagliarello V J, Wispelwey B, Long W J, Jr, Scheld W M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Investig. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Racoosin J A, Whitney C G, Conover C S, Diaz P S. Serogroup Y meningococcal disease in Chicago, 1991–1997. JAMA. 1998;280:2094–2098. doi: 10.1001/jama.280.24.2094. [DOI] [PubMed] [Google Scholar]
- 376.Raman G V. Meningococcal septicaemia and meningitis: a rising tide. Br Med J Clin Res Ed. 1988;296:1141–1142. doi: 10.1136/bmj.296.6630.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ramilo O, Sáez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor α/cachectin and interleukin 1β initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Raymond N J, Reeves M, Ajello G, Baughman W, Gheesling L L, Carlone G M, Wenger J D, Stephens D S. Molecular epidemiology of sporadic (endemic) serogroup C meningococcal disease. J Infect Dis. 1997;176:1277–1284. doi: 10.1086/514123. [DOI] [PubMed] [Google Scholar]
- 379.Rayner C F, Dewar A, Moxon E R, Virji M, Wilson R. The effect of variations in the expression of pili on the interaction of Neisseria meningitidis with human nasopharyngeal epithelium. J Infect Dis. 1995;171:113–121. doi: 10.1093/infdis/171.1.113. [DOI] [PubMed] [Google Scholar]
- 380.Raza M W, El Ahmer O R, Ogilvie M M, Blackwell C C, Saadi A T, Elton R A, Weir D M. Infection with respiratory syncytial virus enhances expression of native receptors for non-pilate Neisseria meningitidis on HEp-2 cells. FEMS Immunol Med Microbiol. 1999;23:115–124. doi: 10.1111/j.1574-695X.1999.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 381.Read R C, Fox A J, Miller K, Gray T, Jones N, Borrows R, Jones D M, Finch R G. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J Med Microbiol. 1995;42:353–361. doi: 10.1099/00222615-42-5-353. [DOI] [PubMed] [Google Scholar]
- 382.Rennick G, Shann F, de Campo J. Cerebral herniation during bacterial meningitis in children. Br Med J. 1993;306:953–955. doi: 10.1136/bmj.306.6883.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Riedo F X, Plikaytis B D, Broome C V. Epidemiology and prevention of meningococcal disease. Pediatr Infect Dis J. 1995;14:643–657. doi: 10.1097/00006454-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 384.Riewerts Eriksen N H, Espersen F, Laursen L, Skinhøj P, Høiby N, Lind I. Nosocomial outbreak of group C meningococcal disease. Br Med J. 1989;298:568–569. doi: 10.1136/bmj.298.6673.568-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Reference deleted.
- 386.Ringuette L, Lorange M, Ryan A, Ashton F. Meningococcal infections in the Province of Quebec, Canada, during the period 1991 to 1992. J Clin Microbiol. 1995;33:53–57. doi: 10.1128/jcm.33.1.53-57.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rintala E, Seppälä O-P, Kotilainen P, Pettilä V, Rasi V. Protein C in the treatment of coagulopathy in meningococcal disease. Crit Care Med. 1998;26:965–968. doi: 10.1097/00003246-199805000-00038. [DOI] [PubMed] [Google Scholar]
- 388.Riordan F A I, Thomson A P J, Sills J A, Hart C A. Who spots the spots? Diagnosis and treatment of early meningococcal disease in children. Br Med J. 1996;313:1255–1256. doi: 10.1136/bmj.313.7067.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Ritter R. Behandlung des Waterhouse-Friderichsen Syndrom (bei Meningokokkensepsis) mit Trasylol. Munch Med Wochenschr. 1967;109:2238–2242. [PubMed] [Google Scholar]
- 390.Rivard G E, David M, Farrell C, Schwarz H P. Treatment of purpura fulminans in meningococcemia with protein C concentrate. J Pediatr. 1995;126:646–652. doi: 10.1016/s0022-3476(95)70369-1. [DOI] [PubMed] [Google Scholar]
- 391.Roback M G, Stack A M, Thompson C, Brugnara C, Schwarz H P, Saladino R A. Activated protein C concentrate for the treatment of meningococcal endotoxin shock in rabbits. Shock. 1998;9:138–142. doi: 10.1097/00024382-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 392.Robboy S J. Atrioventricular-node inflammation—mechanism of sudden death in protracted meningococcemia. N Engl J Med. 1972;286:1091–1093. doi: 10.1056/NEJM197205182862008. [DOI] [PubMed] [Google Scholar]
- 393.Roine I, Foncea L M, Ledermann W, Peltola H. Slow recovery of cerebrospinal fluid glucose and protein concentrations distinguish pneumococcal from Haemophilus influenzae and meningococcal meningitis in children. Pediatr Infect Dis J. 1995;14:905–907. [PubMed] [Google Scholar]
- 394.Romijn J A, Godfried M H, Wortel C, Sauerwein H P. Hypoglycemia, hormones and cytokines in fatal meningococcal septicemia. J Endocrinol Investig. 1990;13:743–747. doi: 10.1007/BF03349613. [DOI] [PubMed] [Google Scholar]
- 395.Rose H D, Lenz I E, Sheth N K. Meningococcal pneumonia. A source of nosocomial infection. Arch Intern Med. 1981;141:575–577. doi: 10.1001/archinte.141.5.575. [DOI] [PubMed] [Google Scholar]
- 396.Rosenstein N, Levine O, Taylor J P, Evans D, Plikaytis B D, Wenger J D, Perkins B A. Efficacy of meningococcal vaccine and barriers to vaccination. JAMA. 1998;279:435–439. doi: 10.1001/jama.279.6.435. [DOI] [PubMed] [Google Scholar]
- 397.Ross S C, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984;63:243–273. [PubMed] [Google Scholar]
- 398.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 399.Reference deleted.
- 400.Rubenstein R, Esterly N B. Meningococcal meningitis with a benign skin rash. Pediatr Dermatol. 1986;3:414–416. doi: 10.1111/j.1525-1470.1986.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 401.Rudel T, Schmid A, Benz R, Kolb H A, Lang F, Meyer T F. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 402.Sacks H S. Meningococcal pneumonia and empyema. Am J Med. 1986;80:290–291. doi: 10.1016/0002-9343(86)90024-0. [DOI] [PubMed] [Google Scholar]
- 403.Sáez-Nieto J A, Lujan R, Berrón S, Campos J, Viñas M, Fusté C, Vazquez J A, Zhang Q-Y, Bowler L D, Martinez Suarez J V, Spratt B G. Epidemiology and molecular basis of penicillin-resistant Neisseria meningitidis in Spain: a 5-year history (1985–1989) Clin Infect Dis. 1992;14:394–402. doi: 10.1093/clinids/14.2.394. [DOI] [PubMed] [Google Scholar]
- 404.Schaad U B. Arthritis in disease due to Neisseria meningitidis. Rev Infect Dis. 1980;2:880–888. doi: 10.1093/clinids/2.6.880. [DOI] [PubMed] [Google Scholar]
- 405.Schaad U B, Kaplan S L, McCracken G H., Jr Steroid therapy for bacterial meningitis. Clin Infect Dis. 1995;20:685–690. doi: 10.1093/clinids/20.3.685. [DOI] [PubMed] [Google Scholar]
- 406.Schaller R T, Jr, Schaller J F. Surgical management of life-threatening and disfiguring sequelae of fulminant meningococcemia. Am J Surg. 1986;151:553–556. doi: 10.1016/0002-9610(86)90542-8. [DOI] [PubMed] [Google Scholar]
- 407.Reference deleted.
- 408.Scheifele D W, Daum R S, Syriopoulou V P, Averill D R, Smith A L. Haemophilus influenzae bacteremia and meningitis in infant primates. J Lab Clin Med. 1980;95:450–462. [PubMed] [Google Scholar]
- 409.Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Removal of pro-inflammatory cytokines with renal replacement therapy: sense or nonsense? Intensive Care Med. 1995;21:169–176. doi: 10.1007/BF01726541. [DOI] [PubMed] [Google Scholar]
- 410.Schildkamp R L, Lodder M C, Bijlmer H A, Dankert J, Scholten R J. Clinical manifestations and course of meningococcal disease in 562 patients. Scand J Infect Dis. 1996;28:47–51. doi: 10.3109/00365549609027149. [DOI] [PubMed] [Google Scholar]
- 411.Schlesinger M, Greenberg R, Levy J, Kayhty H, Levy R. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J Infect Dis. 1994;170:449–453. doi: 10.1093/infdis/170.2.449. [DOI] [PubMed] [Google Scholar]
- 412.Schlichting E, Lyberg T, Solberg O, Andersen B M. Endotoxin liberation from Neisseria meningitidis correlates to their ability to induce procoagulant and fibrinolytic factors in human monocytes. Scand J Infect Dis. 1993;25:585–594. doi: 10.3109/00365549309008547. [DOI] [PubMed] [Google Scholar]
- 413.Scholten R J, Kuipers B, Valkenburg H A, Dankert J, Zollinger W D, Poolman J T. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J Med Microbiol. 1994;41:236–243. doi: 10.1099/00222615-41-4-236. [DOI] [PubMed] [Google Scholar]
- 414.Scholten R J P M, Bijlmer H A, Poolman J T, Kuipers B, Caugant D A, van Alphen L, Dankert J, Valkenburg H A. Meningococcal disease in The Netherlands, 1958–1990: a steady increase in the incidence since 1982 partially caused by new serotypes and subtypes of Neisseria meningitidis. Clin Infect Dis. 1993;16:237–246. doi: 10.1093/clind/16.2.237. [DOI] [PubMed] [Google Scholar]
- 415.Scholten R J P M, Poolman J T, Valkenburg H A, Bijlmer H A, Dankert J, Caugant D A. Phenotypic and genotypic changes in a new clone complex of Neisseria meningitidis causing disease in The Netherlands, 1958–1990. J Infect Dis. 1994;169:673–676. doi: 10.1093/infdis/169.3.673. [DOI] [PubMed] [Google Scholar]
- 416.Schott U, Bjorsell-Ostling E. Sonoclot coagulation analysis and plasma exchange in a case of meningococcal septicaemia. Can J Anaesth. 1995;42:64–68. doi: 10.1007/BF03010573. [DOI] [PubMed] [Google Scholar]
- 417.Schryvers A B, Gonzalez G C. Receptors for transferrin in pathogenic bacteria are specific for the host's protein. Can J Microbiol. 1990;36:145–147. doi: 10.1139/m90-026. [DOI] [PubMed] [Google Scholar]
- 418.Schubiger G, Munzinger J, Dudli C, Wipfli U. Meningokokken-Epidemie in einer Internatsschule: Sekundärerkrankung mit rifampicin-restistentem Erreger unter Chemoprophylaxe. Schweiz Med Wochenschr. 1986;116:1172–1175. [PubMed] [Google Scholar]
- 419.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 420.Schwartz B, Al-Tobaiqi A, Al-Ruwais A, Fontaine R E, A'Ashi J, Hightower A W, Broome C V, Music S I. Comparative efficacy of ceftriaxone and rifampicin in eradicating pharyngeal carriage of group A Neisseria meningitidis. Lancet. 1988;i:1239–1242. doi: 10.1016/s0140-6736(88)92069-7. [DOI] [PubMed] [Google Scholar]
- 421.Schwartz B, Moore P S, Broome C V. Global epidemiology of meningococcal disease. Clin Microbiol Rev. 1989;2(Suppl.):S118–S124. doi: 10.1128/cmr.2.suppl.s118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Schwarz B. Chemoprophylaxis for bacterial infections: principle of and application to meningococcal infections. Rev Infect Dis. 1991;13(Suppl. 2):S170–S173. doi: 10.1093/clinids/13.supplement_2.s170. [DOI] [PubMed] [Google Scholar]
- 423.Schwentker F F, Gelman S, Long P H. The treatment of meningococcic meningitis with sulfanilamide. Preliminary report. JAMA. 1937;108:1407–1408. doi: 10.1001/jama.251.6.788. [DOI] [PubMed] [Google Scholar]
- 424.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Seyfer A E, Kiefer R, Smith A C. The management of dermal necrosis after acute Neisseria infection. Mil Med. 1989;154:598–600. [PubMed] [Google Scholar]
- 426.Shapira M Y, Hirshberg B, Ben-Yehuda A. Asymptomatic temporary atrioventricular dissociation complicating meningococcal meningitis. Int J Cardiol. 1997;62:277–278. doi: 10.1016/s0167-5273(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 427.Shapiro E D, Aaron N H, Wald E R, Chiponis D. Risk factors for development of bacterial meningitis among children with occult bacteremia. J Pediatr. 1986;109:15–19. doi: 10.1016/s0022-3476(86)80564-9. [DOI] [PubMed] [Google Scholar]
- 428.Sharief M K, Ciardi M, Thompson E J. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-α but not interleukin-1β. J Infect Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 429.Shimoda K, Okamura S, Mizuno Y, Harada N, Kubota A, Yamada M, Hara T, Aoki T, Akeda H, Ueda K, Niho Y. Human macrophage colony-stimulating factor levels in cerebrospinal fluid. Cytokine. 1993;5:250–254. doi: 10.1016/1043-4666(93)90012-t. [DOI] [PubMed] [Google Scholar]
- 430.Shneerson J M, Fawcett I W. The complications and management of meningococcal meningitis. Intensive Care Med. 1979;5:5–9. doi: 10.1007/BF01738995. [DOI] [PubMed] [Google Scholar]
- 431.Sierra G V G, Campa H C, Varcacel N M, Garcia I L, Izquierdo P L, Sotolongo P F, Casanueva G V, Rico C O, Rodriguez C R, Terry M H. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. [PubMed] [Google Scholar]
- 432.Simberkoff M S, Moldover N H, Rahal J J., Jr Absence of detectable bactericidal and opsonic activities in normal and infected human cerebrospinal fluids. A regional host defense deficiency. J Lab Clin Med. 1980;95:362–372. [PubMed] [Google Scholar]
- 433.Reference deleted.
- 434.Singhi S C, Singhi P D, Srinivas B, Narakesri H P, Ganguli N K, Sialy R, Walia B N S. Fluid restriction does not improve the outcome of acute meningitis. Pediatr Infect Dis J. 1995;14:495–503. doi: 10.1097/00006454-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 435.Sjöholm A G, Kuijper E J, Tijssen C C, Jansz A, Bol P, Spanjaard L, Zanen H C. Dysfunctional properdin in a Dutch family with meningococcal disease. N Engl J Med. 1988;319:33–37. doi: 10.1056/NEJM198807073190106. [DOI] [PubMed] [Google Scholar]
- 436.Reference deleted.
- 437.Smith O P, White B, Vaughan D, Rafferty M, Claffey L, Lyons B, Casey W. Use of protein-C concentrate, heparin, and haemodiafiltration in meningococcus-induced purpura fulminans. Lancet. 1997;350:1590–1593. doi: 10.1016/s0140-6736(97)06356-3. [DOI] [PubMed] [Google Scholar]
- 438.Reference deleted.
- 439.Sotto M N, Langer B, Hoshino-Shimizu S, de Brito T. Pathogenesis of cutaneous lesions in acute meningococcemia in humans: light, immunofluorescent, and electron microscopic studies of skin biopsy specimens. J Infect Dis. 1976;133:506–514. doi: 10.1093/infdis/133.5.506. [DOI] [PubMed] [Google Scholar]
- 440.Speer C P, Rethwilm M, Gahr M. Elastase-alpha 1-proteinase inhibitor: an early indicator of septicemia and bacterial meningitis in children. J Pediatr. 1987;111:667–671. doi: 10.1016/s0022-3476(87)80240-8. [DOI] [PubMed] [Google Scholar]
- 441.Sprung C L, Caralis P V, Marcial E H, Pierce M, Gelbard M A, Long W M, Duncan R C, Tendler M D, Karpf M. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 442.Stanwell-Smith R E, Stuart J M, Hughes A O, Robinson P, Griffin M B, Cartwright K. Smoking, the environment and meningococcal disease: a case control study. Epidemiol Infect. 1994;112:315–328. doi: 10.1017/s0950268800057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Stegmayr B G. Plasmapheresis in severe sepsis or septic shock. Blood Purif. 1996;14:94–101. doi: 10.1159/000170250. [DOI] [PubMed] [Google Scholar]
- 444.Stephens D S. Uncloacking the meningococcus: dynamics of carriage and disease. Lancet. 1999;353:941–942. doi: 10.1016/S0140-6736(98)00279-7. [DOI] [PubMed] [Google Scholar]
- 445.Stephens D S, Edwards K M, Morris F, McGee Z A. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982;146:568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- 446.Stephens D S, Farley M M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev Infect Dis. 1991;13:22–33. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- 447.Stephens D S, Hajjeh R A, Baughman W S, Harvey R C, Wenger J D, Farley M M. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Intern Med. 1995;123:937–940. doi: 10.7326/0003-4819-123-12-199512150-00007. [DOI] [PubMed] [Google Scholar]
- 448.Stephens D S, Hoffman L H, McGee Z A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983;148:369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- 449.Stephens D S, McGee Z A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981;143:525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- 450.Stephens D S, Spellman P A, Swartley J S. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis. 1993;167:475–479. doi: 10.1093/infdis/167.2.475. [DOI] [PubMed] [Google Scholar]
- 451.Stephens D S, Whitney A M, Melly M A, Hoffman L H, Farley M M, Frasch C E. Analysis of damage to human ciliated nasopharyngeal epithelium by Neisseria meningitidis. Infect Immun. 1986;51:579–585. doi: 10.1128/iai.51.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Stephens D S, Whitney A M, Rothbard J, Schoolnik G K. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J Exp Med. 1985;161:1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Reference deleted.
- 454.Stiehm E R, Damrosch D S. Factors in the prognosis of meningococcal infection. Review of 63 cases with emphasis on recognition and management of the severely ill patient. J Pediatr. 1966;68:457–467. doi: 10.1016/s0022-3476(66)80250-0. [DOI] [PubMed] [Google Scholar]
- 455.Reference deleted.
- 456.Strang J R, Pugh E J. Meningococcal infections: reducing the case fatality rate by giving penicillin before admission to hospital. Br Med J. 1992;305:141–143. doi: 10.1136/bmj.305.6846.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Stuart J M, Cartwright K A, Robinson P M, Noah N D. Does eradication of meningococcal carriage in household contacts prevent secondary cases of meningococcal disease? Br Med J. 1989;298:569–570. doi: 10.1136/bmj.298.6673.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Stuart J M, Cartwright K A V, Robinson P M, Noah N D. Effect of smoking on meningococcal carriage. Lancet. 1989;ii:723–725. doi: 10.1016/s0140-6736(89)90781-2. [DOI] [PubMed] [Google Scholar]
- 459.Sullivan T D, LaScolea L J., Jr Neisseria meningitidis bacteremia in children: quantitation of bacteremia and spontaneous clinical recovery without antibiotic therapy. Pediatrics. 1987;80:63–67. [PubMed] [Google Scholar]
- 460.Reference deleted.
- 461.Swartley J S, Marfin A A, Edupuganti S, Liu L J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Reference deleted.
- 463.Talan D A, Guterman J J, Overturf G D, Singer C, Hoffman J R, Lambert B. Analysis of emergency department management of suspected bacterial meningitis. Ann Emerg Med. 1989;18:856–862. doi: 10.1016/s0196-0644(89)80213-6. [DOI] [PubMed] [Google Scholar]
- 464.Talan D A, Hoffman J R, Yoshikawa T T, Overturf G D. Role of empiric parenteral antibiotics prior to lumbar puncture in suspected bacterial meningitis: state of the art. Rev Infect Dis. 1988;10:365–376. doi: 10.1093/clinids/10.2.365. [DOI] [PubMed] [Google Scholar]
- 465.Tappero J W, Reporter R, Wenger J D, Ward B A, Reeves M W, Missbach T S, Plikaytis B D, Mascola L, Schuchat A. Meningococcal disease in Los Angeles County, California, and among men in the county jails. N Engl J Med. 1996;335:833–840. doi: 10.1056/NEJM199609193351201. [DOI] [PubMed] [Google Scholar]
- 466.Reference deleted.
- 467.Täuber M G, Khayam-Bashi H, Sande M A. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J Infect Dis. 1985;151:528–534. doi: 10.1093/infdis/151.3.528. [DOI] [PubMed] [Google Scholar]
- 468.Täuber M G, Sande E, Fournier M A, Tureen J H, Sande M A. Fluid administration, brain edema, and cerebrospinal fluid lactate and glucose concentrations in experimental Escherichia coli meningitis. J Infect Dis. 1993;168:473–476. doi: 10.1093/infdis/168.2.473. [DOI] [PubMed] [Google Scholar]
- 469.Täuber M G, Shibl A M, Hackbarth C J, Larrick J W, Sande M A. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987;156:456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]
- 470.Reference deleted.
- 471.Taylor F B, Jr, Chang A, Esmon C T, D'Angelo A, Vigano-D'Angelo S, Blick K E. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Investig. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Taylor F B, Jr, Chang A, Ruf W, Morrissey J H, Hinshaw L, Catlett R, Blick K, Edgington T S. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 473.Tesoro L J, Selbst S M. Factors affecting outcome in meningococcal infections. Am J Dis Child. 1991;145:218–220. doi: 10.1001/archpedi.1991.02160020112029. [DOI] [PubMed] [Google Scholar]
- 474.Thomson A P J, Hayhurst G K. Press publicity in meningococcal disease. Arch Dis Child. 1993;69:166–169. doi: 10.1136/adc.69.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Thomson A P J, Sills J A, Hart C A. Validation of the Glasgow Meningococcal Septicemia Prognostic Score: a 10-year retrospective survey. Crit Care Med. 1991;19:26–30. doi: 10.1097/00003246-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 476.Reference deleted.
- 477.Toews W H, Bass J W. Skin manifestations of meningococcal infection; an immediate indicator of prognosis. Am J Dis Child. 1974;127:173–176. doi: 10.1001/archpedi.1974.02110210023003. [DOI] [PubMed] [Google Scholar]
- 478.Tønjum T, Nilsson F, Bruun J N, Haneberg B. The early phase of meningococcal disease. NIPH Ann. 1983;6:175–181. [PubMed] [Google Scholar]
- 479.Troelstra A. Ph.D. thesis. Utrecht, The Netherlands: University of Utrecht; 1998. [Google Scholar]
- 480.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Tureen J H, Täuber M G, Sande M A. Effect of hydration status on cerebral blood flow and cerebrospinal fluid lactic acidosis in rabbits with experimental meningitis. J Clin Investig. 1992;89:947–953. doi: 10.1172/JCI115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Vain N E, Mazlumian J R, Swarner O W, Cha C C. Role of exchange transfusion in the treatment of severe septicemia. Pediatrics. 1980;66:693–697. [PubMed] [Google Scholar]
- 483.Valmari P, Mäkelä M, Kataja M, Peltola H. Multivariate prognostication in bacterial meningitis of childhood. Scand J Infect Dis. 1987;19:29–34. doi: 10.3109/00365548709032374. [DOI] [PubMed] [Google Scholar]
- 484.Van der Ende A, Schuurman I G, Hopman C T, Fijen C A, Dankert J. Comparison of commercial diagnostic tests for identification of serogroup antigens of Neisseria meningitidis. J Clin Microbiol. 1995;33:3326–3327. doi: 10.1128/jcm.33.12.3326-3327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Van der Ley P, Poolman J T. Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect Immun. 1992;60:3156–3161. doi: 10.1128/iai.60.8.3156-3161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Van der Poll T, Lowry S F. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995;3:1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 487.Van Deuren M. Ph.D. thesis. Nijmegen, The Netherlands: University of Nijmegen; 1998. [Google Scholar]
- 488.Van Deuren M. Extracorporal techniques to accelerate the clearance of TNF-α and IL-1β in septic patients. In: Vincent J L, editor. Yearbook of intensive care and emeregency meicine. Berlin, Germany: Springer Verlag KG; 1997. pp. 140–147. [Google Scholar]
- 489.Van Deuren M, Frieling J T M, van der Ven-Jongekrijg J, Neeleman C, Russel F G M, van Lier H J J, Bartelink A K M, van der Meer J W M. Plasma patterns of tumor necrosis factor-α (TNF) and TNF soluble receptors during acute meningococcal infections and the effect of plasma exchange. Clin Infect Dis. 1998;26:918–923. doi: 10.1086/513933. [DOI] [PubMed] [Google Scholar]
- 490.Van Deuren M, Neeleman C, Assmann K J M, Wetzels J F M, van der Meer J W M. Rhabdomyolysis during the subacute stage of meningococcal sepsis. Clin Infect Dis. 1998;26:214–215. doi: 10.1086/517026. [DOI] [PubMed] [Google Scholar]
- 491.Van Deuren M, Neeleman C, van der Meer J W M. Meningokokkenziekte: snel herkennen, snel handelen. Tijdschr Huisartsgeneeskd. 1998;15:397–400. [Google Scholar]
- 492.Van Deuren M, Neeleman C, van't Hek L G F M, van der Meer J W M. A normal platelet count at admission in acute meningococcal disease does not exclude a fulminant course. Intensive Care Med. 1998;24:157–161. doi: 10.1007/s001340050538. [DOI] [PubMed] [Google Scholar]
- 493.Van Deuren M, Netea M G, Hijmans A, Demacker P N M, Neeleman C, Sauerwein R W, Bartelink A K M, van der Meer J W M. Posttranscriptional down-regulation of tumor necrosis factor-α and interleukin-1β production in acute meningococcal infections. J Infect Dis. 1998;177:1401–1405. doi: 10.1086/517824. [DOI] [PubMed] [Google Scholar]
- 494.Van Deuren M, Santman F W, van Dalen R, Sauerwein R W, Span L F, van der Meer J W. Plasma and whole blood exchange in meningococcal sepsis. Clin Infect Dis. 1992;15:424–430. doi: 10.1093/clind/15.3.424. [DOI] [PubMed] [Google Scholar]
- 495.Van Deuren M, van der Ven Jongekrijg J, Bartelink A K M, van Dalen R, Sauerwein R W, van der Meer J W M. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 496.Van Deuren M, van der Ven Jongekrijg J, Demacker P N M, Bartelink A K M, van Dalen R, Sauerwein R W, Gallati H, Vannice J L, van der Meer J W M. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Dis. 1994;169:157–161. doi: 10.1093/infdis/169.1.157. [DOI] [PubMed] [Google Scholar]
- 497.Reference deleted.
- 498.Van Deuren M, van der Ven-Jongekrijg J, Vannier E, van Dalen R, Pesman G, Bartelink A K M, Dinarello C A, van der Meer J W M. The pattern of interleukin-1β (IL-1β) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood. 1997;90:1101–1108. [PubMed] [Google Scholar]
- 499.Van Deuren M, van Dijke B J, Koopman R J J, Horrevorts A M, Meis J F G M, Santman F W, van der Meer J W M. Rapid diagnosis of acute meningococcal infections by needle aspiration or biopsy of skin lesions. Br Med J. 1993;306:1229–1232. doi: 10.1136/bmj.306.6887.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Van Dissel J T, van Langevelde P, Westendorp R G, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 501.Van Esso D, Fontanals D, Uriz S, Morera M A, Juncosa T, Latorre C, Duran M. Neisseria meningitidis strains with decreased susceptibility to penicillin. Pediatr Infect Dis J. 1987;6:438–439. doi: 10.1097/00006454-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 502.Van Furth A M, Seijmonsbergen E M, Langermans J A M, Groeneveld P H P, de Bel C E, van Furth R. High levels of interleukin 10 and tumor necrosis factor-α in cerebrospinal fluid during the onset of bacterial meningitis. Clin Infect Dis. 1995;21:220–222. doi: 10.1093/clinids/21.1.220. [DOI] [PubMed] [Google Scholar]
- 503.Van Looveren M, Vandamme P, Hauchecorne M, Wijdooghe M, Carion F, Caugant D A, Goossens H. Molecular epidemiology of recent Belgian isolates of Neisseria meningitidis serogroup B. J Clin Microbiol. 1998;36:2828–2834. doi: 10.1128/jcm.36.10.2828-2834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Vann W F, Liu T-Y, Robbins J B. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect Immun. 1976;13:1654–1662. doi: 10.1128/iai.13.6.1654-1662.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Veeken H, Ritmeijer K, Hausman B. Priority during a meningitis epidemic: vaccination or treatment? Bull W H O. 1998;76:135–141. [PMC free article] [PubMed] [Google Scholar]
- 506.Verheul A F M, Snippe H, Poolman J T. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol Rev. 1993;57:34–49. doi: 10.1128/mr.57.1.34-49.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Vik Mo H, Lote K, Nordøy A. Disseminated intravascular coagulation in patients with meningococcal infection: laboratory diagnosis and prognostic factors. Scand J Infect Dis. 1978;10:187–191. doi: 10.3109/inf.1978.10.issue-3.06. [DOI] [PubMed] [Google Scholar]
- 508.Villard J, Roux-Lombard P, Hugli A, Dayer J-M. Could natural inhibitors of tumor necrosis factor-α modify the clinical course of fulminant meningococcemia? Crit Care Med. 1993;21:1396–1400. doi: 10.1097/00003246-199309000-00025. [DOI] [PubMed] [Google Scholar]
- 509.Vincent J-L, Roman A, Kahn R J. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med. 1990;18:689–693. doi: 10.1097/00003246-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 510.Virji M. Microbial utilization of human signalling molecules. Microbiology. 1996;142:3319–3336. doi: 10.1099/13500872-142-12-3319. [DOI] [PubMed] [Google Scholar]
- 511.Virji M, Alexandrescu C, Ferguson D J, Saunders J R, Moxon E R. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol. 1992;6:1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 512.Virji M, Makepeace K, Peak I R, Ferguson D J, Jennings M P, Moxon E R. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol Microbiol. 1995;18:741–754. doi: 10.1111/j.1365-2958.1995.mmi_18040741.x. [DOI] [PubMed] [Google Scholar]
- 513.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 514.Vitovski S, Read R C, Sayers J R. Invasive isolates of Neisseria meningitidis possess enhanced immunoglobulin A1 protease activity compared to colonizing strains. FASEB J. 1999;13:331–337. doi: 10.1096/fasebj.13.2.331. [DOI] [PubMed] [Google Scholar]
- 515.Vogel U, Claus H, Heinze G, Frosch M. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect Immun. 1999;67:954–957. doi: 10.1128/iai.67.2.954-957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Waage A, Aasen A O. Different role of cytokine mediators in septic shock related to meningococcal disease and surgery/polytrauma. Immunol Rev. 1992;127:221–230. doi: 10.1111/j.1600-065x.1992.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 517.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;i:355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 519.Waage A, Halstensen A, Espevik T, Brandtzaeg P. Compartmentalization of TNF and IL-6 in meningitis and septic shock. Mediators Inflamm. 1993;2:23–25. doi: 10.1155/S096293519300002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Waage A, Halstensen A, Espevik T, Brandtzaeg P. Cytokines in meningococcal disease. Ballière's. Clin Infect Dis. 1994;1:97–108. [Google Scholar]
- 521.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Wajchenberg B, Leme C E, Tambascia M, Boulos M, Okada H, Cesar F P, Pieroni R R, Mattar E. The adrenal response to exogenous adrenocorticotrophin in patients with infections due to Neisseria meningitidis. J Infect Dis. 1978;138:387–391. doi: 10.1093/infdis/138.3.387. [DOI] [PubMed] [Google Scholar]
- 523.Wald E R, Kaplan S L, Mason E O, Jr, Sabo D, Ross L, Arditi M, Wiedermann B L, Barson W, Kim K S, Yogov R, Hofkosh D for the Meningitis Study Group. Dexamethasone therapy for children with bacterial meningitis. Pediatrics. 1995;95:21–28. [PubMed] [Google Scholar]
- 524.Waldum H L, Fuglesang J E. Fulminant meningococcemia starting as an acute gastroenteritis. Scand J Infect Dis. 1977;9:309–310. doi: 10.3109/inf.1977.9.issue-4.10. [DOI] [PubMed] [Google Scholar]
- 525.Reference deleted.
- 526.Waring P M, Presneill J, Maher D W, Layton J E, Cebon J, Waring L J, Metcalf D. Differential alterations in plasma colony-stimulating factor concentrations in meningococcaemia. Clin Exp Immunol. 1995;102:501–506. doi: 10.1111/j.1365-2249.1995.tb03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Waring P M, Waring L J, Metcalf D. Circulating leukemia inhibitory factor levels correlate with disease severity in meningococcemia. J Infect Dis. 1994;170:1224–1228. doi: 10.1093/infdis/170.5.1224. [DOI] [PubMed] [Google Scholar]
- 528.Weis N, Lind I. Pharyngeal carriage of Neisseria meningitidis before and after treatment of meningococcal disease. J Med Microbiol. 1994;41:339–342. doi: 10.1099/00222615-41-5-339. [DOI] [PubMed] [Google Scholar]
- 529.Wells M, Gibbons R B. Primary meningococcal arthritis: case report and review of the literature. Mil Med. 1997;162:769–772. [PubMed] [Google Scholar]
- 530.Westendorp R G, Hottenga J J, Slagboom P E. Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet. 1999;354:561–563. doi: 10.1016/S0140-6736(98)09376-3. [DOI] [PubMed] [Google Scholar]
- 531.Westendorp R G, Langermans J A, Huizinga T W, Elouali A H, Verweij C L, Boomsma D I, Vandenbroucke J P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 532.Reference deleted.
- 533.Westendorp R G J, Langermans J A M, de Bel C E, Meinders A E, Vandenbroucke J P, van Furth R, van Dissel J T. Release of tumor necrosis factor: an innate host characteristic that may contribute to the outcome of meningococcal disease. J Infect Dis. 1995;171:1057–1060. doi: 10.1093/infdis/171.4.1057. [DOI] [PubMed] [Google Scholar]
- 534.Wester J P, Breumelhof R, Geers A B. Monoclonal antibodies in the treatment of adult fulminant meningococcaemia. Eur J Med. 1992;1:372–373. [PubMed] [Google Scholar]
- 535.Whittle H C, Greenwood B M, Davidson N M, Tomkins A, Tugwell P, Warrell D A, Zalin A, Bryceson A D M, Parry E H J, Brueton M, Duggan M, Oomen J M V, Rajkovic A D. Meningococcal antigen in diagnosis and treatment of group A meningococcal infections. Am J Med. 1975;58:823–828. doi: 10.1016/0002-9343(75)90638-5. [DOI] [PubMed] [Google Scholar]
- 536.Wilks D, Lever A M M. Reasons for delay in administration of antibiotics to patients with meningitis and meningococcaemia. J Infect. 1996;32:49–51. doi: 10.1016/s0163-4453(96)80009-0. [DOI] [PubMed] [Google Scholar]
- 537.Wilson D C, Crean P M, Jenkins J G. Adult respiratory distress syndrome (ARDS) complicating meningococcaemia. Ir J Med Sci. 1991;160:10–11. doi: 10.1007/BF02944724. [DOI] [PubMed] [Google Scholar]
- 538.Reference deleted.
- 539.Wong V K, Hitchcock W, Mason W H. Meningococcal infections in children: a review of 100 cases. Pediatr Infect Dis J. 1989;8:224–227. [PubMed] [Google Scholar]
- 540.Wood A L, O'Brien S J. How long is too long? Determining the early management of meningococcal disease in Birmingham. Public Health. 1996;110:237–239. doi: 10.1016/s0033-3506(96)80109-0. [DOI] [PubMed] [Google Scholar]
- 541.Woods J P, Kersulyte D, Tolan R W, Jr, Berg C M, Berg D E. Use of arbitrarily primed polymerase chain reaction analysis to type disease and carrier strains of Neisseria meningitidis isolated during a university outbreak. J Infect Dis. 1994;169:1384–1389. doi: 10.1093/infdis/169.6.1384. [DOI] [PubMed] [Google Scholar]
- 542.Woodward C M, Jessop E G, Wale M C. Early management of meningococcal disease. Commun Dis Rep Rev. 1995;5:R135–137. [PubMed] [Google Scholar]
- 543.Wuillemin W A, Fijnvandraat K, Derkx B H, Peters M, Vreede W, ten Cate H, Hack C E. Activation of the intrinsic pathway of coagulation in children with meningococcal septic shock. Thromb Haemostasis. 1995;74:1436–1441. [PubMed] [Google Scholar]
- 544.Wylie P A, Stevens D, Drake III W, Stuart J, Cartwright K. Epidemiology and clinical management of meningococcal disease in west Gloucestershire: retrospective, population based study. Br Med J. 1997;315:774–779. doi: 10.1136/bmj.315.7111.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Reference deleted.
- 546.Reference deleted.
- 547.Young L S, LaForce F M, Head J J, Feeley J C, Bennett J V. A simultaneous outbreak of meningococcal and influenza infections. N Engl J Med. 1972;287:5–9. doi: 10.1056/NEJM197207062870102. [DOI] [PubMed] [Google Scholar]
- 548.Zachmann M, Fanconi A, Prader A. Plasma cortisol in children with fulminating meningococcal infection. Helv Paediatr Acta. 1974;29:245–250. [PubMed] [Google Scholar]
- 549.Zangwill K M, Schuchat A, Riedo F X, Pinner R W, Koo D T, Reeves M W, Wenger J D. School-based clusters of meningococcal disease in the United States. Descriptive epidemiology and a case-control analysis. JAMA. 1997;277:389–395. [PubMed] [Google Scholar]
- 550.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 551.Reference deleted.
- 552.Zenz W, Muntean W, Gallistl S, Zobel G, Grubbauer H M. Recombinant tissue plasminogen activator treatment in two infants with fulminant meningococcemia. Pediatrics. 1995;96:144–148. [PubMed] [Google Scholar]
- 553.Zhu P, Hu X, Xu L. Typing Neisseria meningitidis by analysis of restriction fragment length polymorphisms in the gene encoding the class 1 outer membrane protein: application to assessment of epidemics throughout the last 4 decades in China. J Clin Microbiol. 1995;33:458–462. doi: 10.1128/jcm.33.2.458-462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Zollinger W D, Boslego J, Moran E, Garcia J, Cruz C, Ruiz S, Brandt B, Martinez M, Arthur J, Underwood P, Hankins W, Mays J, Gilley J The Chilean National Committee for Meningococcal Disease. Meningococcal serogroup B vaccine protection trial and follow-up studies in Chile. NIPH Ann. 1991;14:211–212. [PubMed] [Google Scholar]
- 555.Zwahlen A, Nydegger U E, Vaudaux P, Lambert P H, Waldvogel F A. Complement-mediated opsonic activity in normal and infected human cerebrospinal fluid: early response during bacterial meningitis. J Infect Dis. 1982;145:635–646. doi: 10.1093/infdis/145.2.635. [DOI] [PubMed] [Google Scholar]