Abstract
Tuberculosis is second only to COVID-19 as a cause of death from a single infectious agent. In 2020, almost 10 million people were estimated to have developed tuberculosis and it caused 1·5 million deaths. Around a quarter of deaths caused by antimicrobial resistance are due to rifampicin-resistant tuberculosis. Antimicrobial resistance surveillance systems for many bacterial pathogens are still in the early stages of implementation in many countries, and do not yet allow for the estimation of disease burden at the national level. In this Personal View, we present the achievements, challenges, and way forward for the oldest and largest global antimicrobial resistance surveillance system. Hosted by WHO since 1994, the Global Project on Anti-Tuberculosis Drug Resistance Surveillance has served as a platform for the evaluation of the trends in anti-tuberculosis drug resistance for over 25 years at country, regional, and global levels. With an estimated 465 000 incident cases of multidrug-resistant and rifampicin-resistant tuberculosis in 2019, drug-resistant tuberculosis remains a public health crisis. The COVID-19 pandemic has reversed years of progress in providing essential tuberculosis services and reducing disease burden. The number of people diagnosed with drug-resistant tuberculosis has dropped by 22% since before the pandemic, and the number of patients provided with treatment for drug-resistant tuberculosis has dropped by 15%. Now more than ever, closing gaps in the detection of drug-resistant tuberculosis requires investment in research and development of new diagnostic tools and their rollout, expansion of sample transport systems, and the implementation of data connectivity solutions.
Background
Tuberculosis is a preventable, treatable, and curable disease. However, in 2020, 9·9 million people were estimated to have developed tuberculosis, and 1·5 million people were estimated to have died from it. The COVID-19 pandemic has substantially reduced access to services for the diagnosis and treatment of tuberculosis, resulting in an increase in deaths due to tuberculosis, and a reversal in global progress. Tuberculosis is second only to COVID-19 in terms of death from a single infectious agent.1
Nearly half a million individuals who developed tuberculosis in 2019 had resistance to rifampicin, a crucial first-line drug for the treatment of tuberculosis. Rifampicin resistance could be present either at the onset of disease or it might emerge during the course of the disease due to inadequate treatment.2 Globally, around a quarter of deaths caused by antimicrobial resistance are due to rifampicin-resistant tuberculosis.3 Rifampicin-resistant tuberculosis, which includes multidrug-resistant tuberculosis (defined as combined resistance to rifampicin and isoniazid), accounted for an estimated 180 000 deaths in 2019. Although only 3·3% of patients with new, untreated tuberculosis in 2019 had rifampicin resistance, the costs of providing appropriate diagnosis, treatment, and care for people with drug-resistant tuberculosis are disproportionately high. The epidemic of drug-resistant tuberculosis varies substantially between regions and remains particularly concerning in some countries of eastern Europe and central Asia, including Belarus, Kazakhstan, Kyrgyzstan, Moldova, Russia, Tajikistan, Turkmenistan, and Ukraine. In these countries, the proportion of new, untreated patients with rifampicin-resistant tuberculosis exceeds 20%. Only 86% of patients diagnosed with rifampicin-resistant tuberculosis in 2019 started treatment. In the cohort of patients enrolled in treatment for rifampicin-resistant tuberculosis in 2017, only 57% had successful treatment outcomes, which was defined as either having completed treatment with no evidence of treatment failure, or showing evidence of cured disease. The absence of decentralised and ambulatory models for the provision of treatment and care for patients with drug-resistant tuberculosis in many countries probably contributes to this low proportion.2
Impressive achievements across 25 years
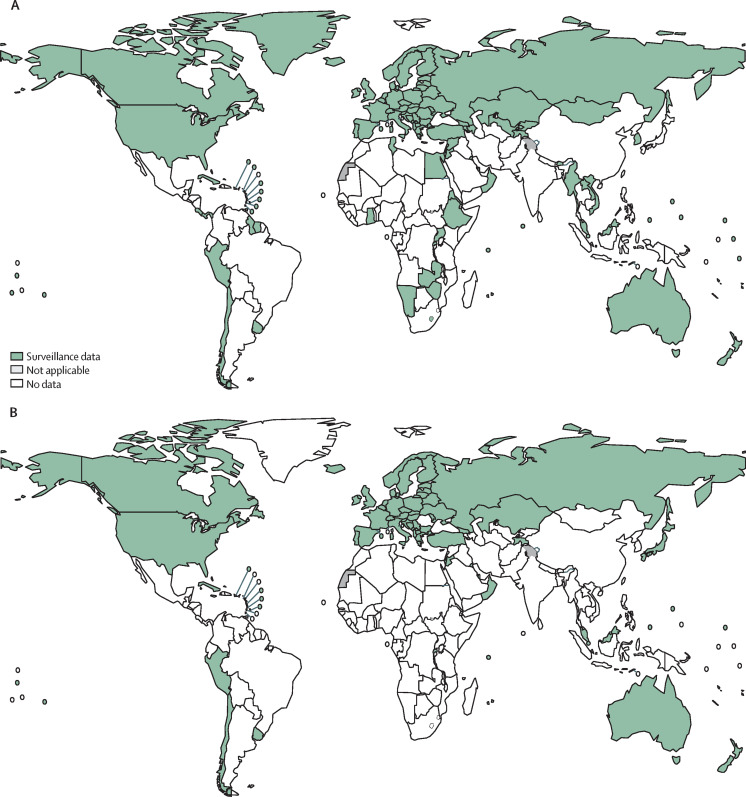
The Global Project on Anti-Tuberculosis Drug Resistance Surveillance is the oldest and largest global surveillance system for antimicrobial resistance in the world.4 Hosted by WHO since 1994, the Global Project has served as a common platform for the evaluation of the magnitude and trends surrounding drug-resistant tuberculosis over 25 years, across country, regional, and global scales. Supported by a WHO global network of supranational reference laboratories for tuberculosis, the data collected are derived from two main sources: (1) routine drug susceptibility testing (DST) using molecular or phenotypic methods for at least rifampicin in the majority (≥80%) of patients with bacteriologically confirmed pulmonary tuberculosis, thus constituting a continuous surveillance system; and (2) periodic epidemiological surveys in settings where routine testing capacity has not yet been established. Implementation of these approaches is detailed in the sixth edition of WHO's global guidance for the surveillance of drug-resistant tuberculosis, published in April, 2021.5 By May, 2021, 125 countries had data on rifampicin-resistant tuberculosis from continuous surveillance, and 53 had data from surveys for at least 1 year since 1994. Collectively, these data accounted for more than 99% of the world's population and estimated number of rifampicin-resistant tuberculosis cases. Some of these data could be outdated or only available for individual years, preventing an assessment of trends over time. Nonetheless, there have been continued improvements in surveillance systems over the 5 year period from 2014 to 2019, with the number of WHO member states with data from routine surveillance increasing from 86 to 125 (figure 1 ).2 The nationally representative data collected by the Global Project can be used to inform timely public health actions, which can be integrated into national strategic plans for tuberculosis. These include guiding resource allocation, assessing the appropriateness of treatment regimens, planning future drug procurement needs, informing national tuberculosis diagnostic algorithms, and prioritising research and development needs. By contrast, antimicrobial resistance surveillance systems for other bacterial pathogens are still in the early stages of implementation in many countries and do not yet allow an estimation of disease burden at the national level. Infrastructure for clinical microbiological diagnostics, including obtaining appropriate samples from a representative group of patients and performing antimicrobial susceptibility testing, is weak.6
Figure 1.
WHO member states with high-quality data on rifampicin resistance among new bacteriologically confirmed pulmonary tuberculosis cases from routine continuous surveillance systems
Data are for 2019 (A) and 2014 (B).
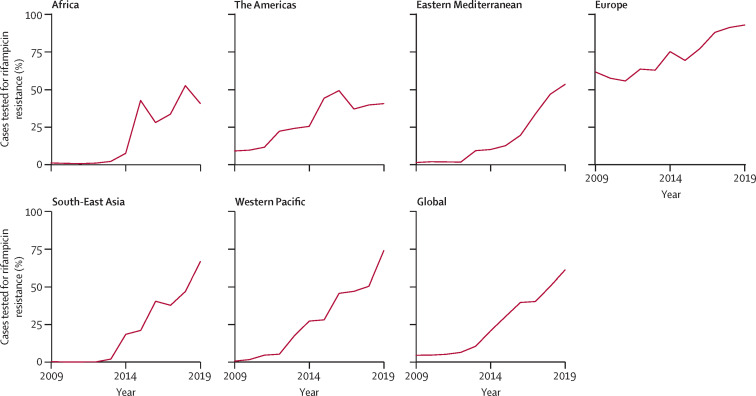
Impressive achievements have been made in the detection of drug-resistant tuberculosis, contributing to the strengthening of surveillance systems. The diagnosis of rifampicin resistance no longer relies solely on culture methods and phenotypic DST, which require months to obtain results. Since 2011, rapid molecular tests have been crucial for improving access to DST by enabling decentralised testing that can be done directly using sputum samples. A variety of different tests with a rapid turnaround time are now available,7 ensuring earlier detection and initiation of appropriate treatment. In 2019, 61% of people with bacteriologically confirmed tuberculosis were tested for rifampicin resistance, a notable increase from 51% in 2017, and only 7% in 2012 (figure 2 ).
Figure 2.
Percentage of bacteriologically confirmed pulmonary tuberculosis cases tested for rifampicin resistance, globally and for WHO regions, 2009–19
Reproduced from WHO's Global TB Report 2020.2
In some high-income countries, next-generation sequencing has become routine practice due to scientific innovation and continued reductions in costs. Sequencing is a valuable public health tool that allows the detection and tracing of multi-country outbreaks of drug-resistant tuberculosis, as shown by the pilot of the EUSeqMyTB consortium across the EU and European Economic Area.8, 9 The integration of sequencing into national anti-tuberculosis drug resistance surveys in Djibouti, Democratic Republic of the Congo, Eritrea, and Eswatini has shown its value.5, 10, 11, 12 The benefits of sequencing include comprehensive results for a greater range of drugs, identification of mutations missed by commercial assays, and epidemiological insights into transmission. In settings where culture methods cannot yet be reliably implemented, targeted sequencing of specific genes can be done directly on sputum samples.11
In combination with improved access to diagnostics leading to better case detection, new treatment options could greatly improve the quality of life for people with drug-resistant tuberculosis. These improvements should also facilitate the transition towards case-based surveillance and more accurate monitoring of treatment outcomes. Since 2020, the regimen recommended by WHO for the treatment of rifampicin-resistant tuberculosis no longer relies on injectable agents administered for a period of up to 24 months. Instead, it consists entirely of oral medications administered for 9–11 months, excluding patients with additional resistance to some other drugs or with extensive tuberculosis disease. 90 countries reported using all-oral medication regimens during 2020.1 For the treatment of rifampicin-resistant tuberculosis with additional fluoroquinolone resistance, WHO has approved the use of an all-oral regimen, BPaL (ie, combined treatment with bedaquiline, pretomanid, and linezolid), administered for 6–9 months under operational research conditions for particular patient groups.13 The results of ongoing clinical trials of new combinations of drugs should become available in 2022 for both drug-susceptible tuberculosis and drug-resistant tuberculosis, including universal regimens that can be used for both.2
Ongoing challenges remain
The COVID-19 pandemic has reversed years of progress in providing essential tuberculosis services and reducing the disease burden. The most obvious impact is an 18% drop in the number of people diagnosed and reported to have tuberculosis globally, falling from 7·1 million in 2019 to 5·8 million in 2020, which is below the 9·9 million people who were estimated to have developed tuberculosis in 2020. Similarly, there was a 22% drop in the number of people diagnosed with drug-resistant tuberculosis, falling from 201 997 in 2019, to 157 903 in 2020. The number of people provided with treatment for drug-resistant tuberculosis dropped by 15% from 177 100 in 2019 to 150 359 in 2020, only reaching around one of three in need.1
With an estimated 465 000 incident cases of multidrug-resistant tuberculosis and rifampicin-resistant tuberculosis in 2019 before the COVID-19 pandemic, the number of people living with the disease (prevalent cases) is even higher. Despite steady increases in the coverage of rifampicin DST, less than half (44%) of the estimated global incident cases of multidrug-resistant tuberculosis and rifampicin-resistant tuberculosis were detected and notified in 2019, and only slightly more than a third (38%) were enrolled on second-line treatment.2 There are particularly large gaps in the detection, recording, and reporting of rifampicin resistance among children with tuberculosis, due to challenges in obtaining suitable samples for bacteriological confirmation and DST.14 Between 2018 and 2019, 8986 children were enrolled on treatment for rifampicin-resistant tuberculosis, but this number was only 7·8% of the 5 year (2018–22) target of 115 000 that was set in the political declaration of the UN high-level meeting on tuberculosis in 2018.2
Together with rifampicin, isoniazid is an essential component of the first-line treatment regimen for tuberculosis recommended by WHO. It is also the most commonly used preventive drug for tuberculosis. However, the increased DST capacity that has been established for rifampicin is not yet available for isoniazid. In many countries, diagnostic algorithms are driven by initial screening for rifampicin resistance, followed by further DST in patients for whom a rifampicin-resistant treatment regimen is indicated.15 With an estimated 1 million cases of tuberculosis emerging in 2019 estimated to have resistance to isoniazid without concurrent rifampicin resistance, there is a much higher burden of this form of drug-resistant tuberculosis than with the 465 000 incident cases of rifampicin-resistant tuberculosis.2 A modified, fluoroquinolone-containing regimen is recommended for the treatment of isoniazid-resistant, rifampicin-susceptible tuberculosis, but many eligible patients might not be detected, thus receiving inappropriate treatment and care.16
With susceptibility to fluoroquinolones being crucial to the success of the all-oral treatment regimen for rifampicin-resistant tuberculosis, the definitions of extensively drug-resistant (XDR)-tuberculosis and preXDR tuberculosis have been updated. From 2021 onwards, preXDR tuberculosis is defined as rifampicin-resistant tuberculosis with concurrent fluoroquinolone resistance.17 With new and repurposed drugs now core components of the all-oral regimen for rifampicin-resistant tuberculosis, the definition of XDR tuberculosis from 2021 onwards is as follows: preXDR plus resistance to any group A drug recommended for the treatment of rifampicin-resistant tuberculosis (currently, either bedaquiline or linezolid). Of the rifampicin-resistant tuberculosis cases detected in 2019, 71% were tested for resistance to fluoroquinolones. This proportion is greater than the amount tested in 2018 (65%), yet gaps remain. In 2019, only 69 countries provided high-quality data through routine continuous surveillance systems, defined as implementing routine DST for rifampicin for 80% or more of patients with bacteriologically confirmed pulmonary tuberculosis, as well as routine DST for fluoroquinolones for 80% or more of patients with pulmonary rifampicin-resistant tuberculosis. A challenge for the surveillance of XDR tuberculosis is that molecular markers of resistance are not yet well understood, and therefore phenotypic testing done on culture isolates is currently the only reliable way to test for resistance to bedaquiline and linezolid. This knowledge gap is a barrier to the expansion of DST for monitoring the emergence of resistance. In the case of bedaquiline, resistance-conferring mutations are scattered without a defined genetic hotspot region, meaning that the development of a rapid molecular assay might not be feasible.18
For laboratory results to be meaningful and actionable, they need to be correctly linked to individual patients and their clinical data in a timely manner. Many diagnostic tests, including molecular assays and liquid culture, can produce results in a digital format, and diagnostic connectivity solutions to allow rapid analysis and communication of results are available.19 However, implementation can be constrained by the absence of unique patient identification codes, multiple samples being tested from the same patient, recurrent episodes of tuberculosis for the same patient, or referral to other health facilities for testing.
The way forward
Drug-resistant tuberculosis remains a public health crisis, and ongoing surveillance of the burden is essential to mounting an effective response. Accurate diagnosis and treatment of tuberculosis, including drug-resistant forms, should be available and accessible to all who need it. All member states of WHO have committed to ending the global tuberculosis epidemic by 2030 through the adoption of WHO's End TB Strategy in 2014, and the UN Sustainable Development Goals in 2015.20, 21 These commitments were reaffirmed at the UN high-level meeting on tuberculosis in 2018. A crucial component required to fulfil these commitments is improved access to diagnostic testing for tuberculosis, including universal DST. Member states have also called for strengthened efforts to combat antimicrobial resistance, including drug-resistant tuberculosis, in a resolution of the World Health Assembly in 2019.20
Now more than ever, closing gaps in the detection of drug-resistant tuberculosis requires investment in laboratory capacity, sample transport systems, and data connectivity solutions. To improve detection requires a multistep process, first requiring improved bacteriological confirmation among presumptive cases of pulmonary tuberculosis. In 2019, globally, only 57% of diagnosed cases of tuberculosis were bacteriologically confirmed, a notable contrast to 84% in high-income countries.2 Improved bacteriological confirmation must be combined with increased DST in cases that are bacteriologically confirmed.
New molecular tools are becoming increasingly available for other drugs. In July, 2021, WHO endorsed the use of additional low-complexity and moderate-complexity nucleic acid amplification tests for isoniazid and fluoroquinolone testing, including rapid cartridge-based assays for both drugs.7 Such tools can be decentralised, thereby reducing gaps in the detection of isoniazid-resistant tuberculosis cases and allowing confirmation of the eligibility for the all-oral regimen for rifampicin-resistant tuberculosis. Furthermore, new molecular tools could play an important role in assessing eligibility for, and monitoring emergence of resistance to, new first-line regimens. After reviewing the evidence of the Study 31/A5349 trial, in June, 2021, WHO endorsed the use of a 4 month rifapentine-containing and fluoroquinolone-containing regimen as a possible alternative to the current 6 month first-line regimen for drug-susceptible pulmonary tuberculosis.22 However, access to rifapentine remains problematic due to limited availability and costs, emphasising the crucial need for more manufacturers.23, 24
Next-generation sequencing has the potential to revolutionise the expansion of comprehensive DST, as it can detect resistance to multiple drugs simultaneously and might also be the only option available when no clear genetic hotspots have been identified for the development of rapid molecular tests. Global uptake has been limited by requirements for advanced bioinformatics and information technology capacity, and by a lack of standardisation for data processing, analysis, and interpretation.25, 26 Portable platforms such as the MinION (developed by Oxford Nanopore Technologies, Oxford, UK), which can be done on raw sputum, and assays done directly on processed sputum, such as Deeplex-MycTB (developed by GenoScreen, Lille, France), could reduce turnaround time and allow live detection of clusters and timely patient management. WHO is collaborating with partners such as the Foundation for Innovative New Diagnostics to address these needs.27 Different sequencing solutions are being piloted in countries to explore the most appropriate approaches in varied settings, with consideration given to priority patient groups for testing, the type of technology to be used (eg, targeted versus whole-genome sequencing, either directly on sputum or on culture isolates), and the model of delivery within the laboratory network. The first WHO catalogue of mutations was published in June, 2021,28 compiling evidence to define the role of different mutations in conferring resistance. This catalogue serves as a global reference for harmonising the analysis and interpretation of sequencing data, and will be reviewed annually.
Efforts are needed to minimise the emergence of resistance to the first new drugs to be made available for the treatment of tuberculosis in more than 50 years. These new drugs include bedaquiline, which is critical for the treatment of rifampicin-resistant tuberculosis, pretomanid, for which clinical trial results of combination therapy for drug-resistant tuberculosis are soon anticipated,29 and the closely related delamanid. Crucial factors in preventing emergence of resistance to new and repurposed drugs include appropriate patient support to ensure treatment adherence, effective combination therapies incorporating several active drug classes, and early detection of resistance to prevent further spread.30, 31 Early detection of resistance relies on investment in research and development of new molecular tools. In August, 2021, WHO released updated target product profiles (TPPs) for DST at peripheral centres to inform research and development priorities. The TPPs provide minimal and optimal requirements for new tools to facilitate affordable and equitable access, including the need for point-of-care solutions.32 For some drugs, portable, targeted next-generation sequencing might be the most promising approach to scaling up testing globally. This upscaling of testing will be reliant on a better understanding of the role of different mutations, which in turn requires paired data from phenotypic DST and whole-genome sequencing. In the interim, in-country capacity for culture and phenotypic testing of these drugs must be strengthened. The expansion of surveillance systems requires more than the strengthening of laboratory capacity and sample transport systems. Countries should strive to transition from paper-based recording and reporting systems to case-based digital surveillance systems, such as those provided by the District Health Information Software 2 (DHIS2). DHIS2 is an open-source, web-based health management information system platform designed in line with WHO standards for service delivery and programme implementation. The DHIS2 case-based module for tuberculosis surveillance provides a relational database with linkage of unique patient identifiers to different laboratory tests and other data. Interactive dashboards allow visualisation of key indicators. DHIS2 can be used for many different diseases, thus consolidating a country's surveillance data into one common platform. It is encouraging that case-based digital surveillance systems covering both drug-susceptible and drug-resistant tuberculosis were available in 136 countries—collectively accounting for 72% of global tuberculosis cases in 2019.2
Conclusion
Impressive progress has been made over the past 25 years in improving the diagnosis of, and access to treatment and care for, drug-resistant tuberculosis. The Global Project on Anti-Tuberculosis Drug Resistance Surveillance serves to guide the development and expansion of the surveillance of antimicrobial resistance among other pathogens. Nonetheless, the global epidemic of drug-resistant tuberculosis remains a public health threat, with the COVID-19 pandemic reversing years of progress. Urgent investment is needed from governments, donors, the commercial sector, academic institutions, non-governmental organisations, and other stakeholders.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank National TB Programmes and WHO supranational tuberculosis reference laboratories for their valuable contributions to the Global Project on Anti-Tuberculosis Drug Resistance Surveillance.
Contributors
The drafting of the manuscript was led by ASD with equal contributions provided by all authors. All authors contributed to the design, implementation, and analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance.
References
- 1.WHO Global tuberculosis report 2021. 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021
- 2.WHO Global tuberculosis report 2020. 2020. https://www.who.int/publications/i/item/9789240013131
- 3.O Neill J. The review on antimicrobial resistance. Tackling drug-resistant infections globally: final report and recommendations. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
- 4.Zignol M, Dean AS, Falzon D, et al. Twenty years of global surveillance of antituberculosis-drug resistance. N Engl J Med. 2016;375:1081–1089. doi: 10.1056/NEJMsr1512438. [DOI] [PubMed] [Google Scholar]
- 5.WHO . 6th edition. World Health Organization; Geneva: 2021. Guidance for the surveillance of drug resistance in tuberculosis. [Google Scholar]
- 6.WHO Global antimicrobial resistance and use surveillance system (GLASS) report 2021. 2021. https://www.who.int/publications/i/item/9789240027336
- 7.WHO WHO consolidated guidelines on tuberculosis, module 3: diagnosis—rapid diagnostics for tuberculosis detection 2021 update. 2021. https://www.who.int/publications/i/item/9789240029415 [PubMed]
- 8.Walker TM, Merker M, Knoblauch AM, et al. A cluster of multidrug-resistant Mycobacterium tuberculosis among patients arriving in Europe from the Horn of Africa: a molecular epidemiological study. Lancet Infect Dis. 2018;18:431–440. doi: 10.1016/S1473-3099(18)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagliani E, Anthony R, Kohl TA, et al. Use of a whole genome sequencing-based approach for Mycobacterium tuberculosis surveillance in Europe in 2017–2019: an ECDC pilot study. Eur Respir J. 2021;57 doi: 10.1183/13993003.02272-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliani E, Hassan MO, Waberi Y, et al. Culture and next-generation sequencing-based drug susceptibility testing unveil high levels of drug-resistant-TB in Djibouti: results from the first national survey. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayomo MK, Mbula VN, Aloni M, et al. Targeted next-generation sequencing of sputum for diagnosis of drug-resistant TB: results of a national survey in Democratic Republic of the Congo. Sci Rep. 2020;10 doi: 10.1038/s41598-020-67479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesfin AB, Araia ZZ, Beyene HN, et al. First molecular-based anti-TB drug resistance survey in Eritrea. Int J Tuberc Lung Dis. 2021;25:43–51. doi: 10.5588/ijtld.20.0558. [DOI] [PubMed] [Google Scholar]
- 13.Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2020;57 doi: 10.1183/13993003.03300-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuaid CF, Cohen T, Dean AS, et al. Ongoing challenges to understanding multidrug- and rifampicin-resistant tuberculosis in children versus adults. Eur Respir J. 2021;57 doi: 10.1183/13993003.02504-2020. [DOI] [PubMed] [Google Scholar]
- 15.Dean AS, Zignol M, Cabibbe AM, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Medicine. 2020;17 doi: 10.1371/journal.pmed.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO WHO consolidated guidelines on tuberculosis, module 4: treatment. Drug-Resistant tuberculosis treatment. 2020. https://www.who.int/publications/i/item/9789240007048 [PubMed]
- 17.WHO Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis. 2021. https://www.who.int/publications/i/item/meeting-report-of-the-who-expert-consultation-on-the-definition-of-extensively-drug-resistant-tuberculosis
- 18.Kadura S, King N, Nakhoul M, et al. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother. 2020;75:2031–2043. doi: 10.1093/jac/dkaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Laboratory Initiative GLI quick guide to TB diagnostics connectivity solutions. 2016. http://www.stoptb.org/wg/gli/assets/documents/gli_connectivity_guide.pdf
- 20.WHO Resolution WHA67.25: antimicrobial resistance. 2014. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R25-en.pdf
- 21.UN Progress towards the achievement of global tuberculosis targets and implementation of the political declaration of the high-level meeting of the General Assembly on the fight against tuberculosis. 2020. https://undocs.org/en/A/75/236
- 22.WHO Treatment of drug-susceptible tuberculosis: rapid communication. 2021. https://www.who.int/publications/i/item/9789240028678
- 23.WHO WHO encourages manufacturers to develop quality assured formulations of the game-changing drug rifapentine. https://www.who.int/news/item/15-07-2021-who-encourages-manufacturers-to-develop-quality-assured-formulations-of-the-game-changing-drug-rifapentine
- 24.WHO Nitrosamine concerns for rifapentine and rifampicin: update and FAQs. 2021. https://extranet.who.int/pqweb/sites/default/files/documents/FAQ_Nitrosamine_18Dec2020.pdf
- 25.Inzaule SC, Tessema SK, Kebede Y, Ouma AEO, Nkengasong JN. Genomic-informed pathogen surveillance in Africa: opportunities and challenges. Lancet Infect Dis. 2021;21:e281–e289. doi: 10.1016/S1473-3099(20)30939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO The use of next-generation sequencing technologies for the detection of mutations associated with drug resistance in Mycobacterium tuberculosis complex: technical guide. 2018. https://www.who.int/publications/i/item/WHO-CDS-TB-2018.19
- 27.GenomeWeb Sequencing-based tuberculosis drug resistance testing gaining momentum. 2020. https://www.360dx.com/clinical-sequencing/sequencing-based-tuberculosis-drug-resistance-testing-gaining-momentum#.YC18qWhKiUk
- 28.WHO Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. 2021. https://www.who.int/publications/i/item/9789240028173
- 29.Médecins Sans Frontières TB PRACTECAL. https://msf.org.uk/tb-practecal
- 30.Ndjeka N, Ismail NA. Bedaquiline and clofazimine: successes and challenges. Lancet Microbe. 2020;1:e139–e140. doi: 10.1016/S2666-5247(20)30097-5. [DOI] [PubMed] [Google Scholar]
- 31.Ismail NA, Omar SV, Joseph L, et al. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine. 2018;28:136–142. doi: 10.1016/j.ebiom.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Target product profile for next-generation tuberculosis drug-susceptibility testing at peripheral centres. 2021. https://www.who.int/publications/i/item/9789240032361 [DOI] [PMC free article] [PubMed]