1. Introduction
Patients with Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) are at higher risk of acquiring the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and have a poor outcome after infection, with a mortality rate of about 30%, increasing to 48% in patients with Myelofibrosis (MF) [1], [2].
COVID-19 vaccines have been readily and highly recommended in hematological patients [3], [4], [5]. In Italy, the BNT162b2 (Pfizer-BioNTech) mRNA vaccine was particularly recommended in frail individuals.
MPN patients may have lower responses to vaccines due to reduced immunological competence that is related to both the hematological disease and the immunosuppressive and/or myelotoxic effects of the treatments [6], [7]. Table 1 summarizes previous reports on serological response in MPN patients [8], [9], [10], [11], [12], [13]. Also, in a recent systematic review, the rate of seroconversion after SARS-CoV-2 vaccine was 78% in the MPN cohort [14].
Table 1.
Selected studies on humoral response against SARS-CoV-2 vaccination in MPN patients.
| study | Patients | MPN type | Methods of Ab detection and type of Ab quantified | Type of vaccine for MPN patients | Therapy at the time of Ab detection | Timing of Ab evaluation | seroconversion rate |
|---|---|---|---|---|---|---|---|
| Pimpinelli et al. 2021[8] | n. 30 Ph-negative MPNs n. 42 Multiple Myeloma n. 20 Chronic Myeloid Leukemia n. 36 Healthy volunteers |
n. 11 ET n. 11 PV n. 8 MF |
chemiluminescent immunoassay for the quantitative determination of anti-S1- and anti-S2-specific IgG Ab titer | 100% BNT162b2 mRNA (Pfizer-BioNTech) | n. 6 ruxolitinib n. 20 hydroxyurea n. 2 anagrelide n. 2 interferon |
Day 1 Day 21 Day 35 |
Not applicable 52.0% (including CML) 52.8% control 88% (including CML) 100% controls |
| Pimpinelli et al. 2021[9] | n. 42 Ph-negative MPNs | n. 17 ET n. 15 PV n. 10 MF |
chemiluminescent immunoassay for the quantitative determination of anti-S1- and anti-S2-specific IgG Ab titer | 100% BNT162b2 mRNA (Pfizer-BioNTech) | n. 8 ruxolitinib n. 29 hydroxyurea n. 3 anagrelide n. 2 interferon |
Day 1 Day 21 Day 35 |
Not applicable 10% MF 68.8% ET/PV 60.0% MF 93.8% ET/PV |
| Guglielmelli et al. 2021[10] | n. 30 Ph-negative MPNs n. 14 Healthy volunteers |
n. 7 ET n. 10 PV n. 13 MF |
method not specified. Quantitative determination of anti S-protein IgG Ab titer, anti-receptor binding domain Ab titer and neutralizing Ab index |
83% Spikevax mRNA (Moderna) 17% BNT162b2 mRNA (Pfizer-BioNTech) |
n. 18 ruxolitinib n. 5 hydroxyurea n. 1 anagrelide n. 1 interferon n. 5 no therapy |
Day 1 Day 21 (Pfizer) or Day 28 (Moderna) |
Not applicable Neutralizing Ab in 33.3% of ruxolitinib patients 58.3% of no-ruxolitinib patients 100% of control |
| Tzarfati et al. 2021[11] | n. 51 Aggressive NHL n. 40 Indolent NHL n. 16 Hodgkin Lymphoma n. 53 Multiple Myeloma n. 34 Chronic Lymphocytic Leukemia n. 15 Acute Leukemia n. 16 Myelodysplastic syndromes n. 68 Ph-negative MPNs n. 22 Chronic Myeloid Leukemia |
Not specified | chemiluminescence immunoassay for the quantitative determination of anti-S1- and anti-S2-specific IgG Ab titer | 100% BNT162b2 mRNA (Pfizer-BioNTech) | Not specified for MPN (n.12 patients treated with ruxolitinib) | 30–60 days after second dose | 84% (MPNs overall) |
| Chowdhury et al. 2021[12] | n. 35 Ph-negative MPNs n. 12 Chronic Myeloid Leukemia n. 13 Myelodysplastic Syndrome n. 232 Healthy volunteers |
n. 17 ET n. 11 PV n. 7 MF |
chemiluminescent microparticle immunoassay for the determination of quantitative anti S-protein IgG Ab titer and qualitative determination of N-protein Ab | 37% BNT162b2 mRNA (Pfizer-BioNTech) 63% ChAdOx1 viral vector (Oxford-AstraZeneca) |
n. 4 ruxolitinib n. 1 ruxolitinib + hydroxyurea n. 12 hydroxyurea n. 1 anagrelide n. 8 interferon n. 8 no therapy n. 1 investigational BET-inhibitor |
> 14 days after the first dose of vaccine | 63% ET 46% PV 50% MF 97% control |
| Fiorino et al. 2021[13] | n. 42 Myelofibrosis n. 40 Healthy volunteers |
n. 42 MF | ELISA for the quantitative determination of anti S-protein IgG Ab titer and ACE2/RBD binding inhibition assay to evaluate the seroconversion with inhibition activity | 78.6% Spikevax mRNA (Moderna) 21.4% BNT162b2 mRNA (Pfizer-BioNTech) |
n. 16 ruxolitinib n. 26 no ruxolitinib |
Pre-vaccination Day 7 after 2nd dose Day 30 after 2nd dose |
Not applicable 98% Control 18% Ruxo patients 54% No-ruxo patients 98% Control 40% Ruxo patients 54% No-ruxo patients |
| Auteri et al., 2022 | n. 52 Ph-negative MPNs | n. 13 ET n. 23 PV n. 16 MF |
electro-chemiluminescent immunoassay for the quantitative determination of receptor binding domain IgG Ab titer and qualitative determination of N-protein IgG and IgM Ab | 100% BNT162b2 mRNA (Pfizer-BioNTech) | n. 22 ruxolitinib n. 15 hydroxyurea n. 2 anagrelide n. 6 interferon n. 1 busulfan n. 6 no therapy |
> 5 weeks after second dose | 100% ET 100% PV 93.7% MF No/poorer response* 0 ET 21.7% PV 50% MF |
Ab = antibody, MPN = Myeloproliferative Neoplasm, ET = Essential Thrombocythemia, PV = Polycythemia Vera, MF = Myelofibrosis, CML = Chronic Myeloid Leukemia, NHL: non-Hodgkin lymphoma. *no/poorer response was defined as Ab titer below first quartile
2. Methods
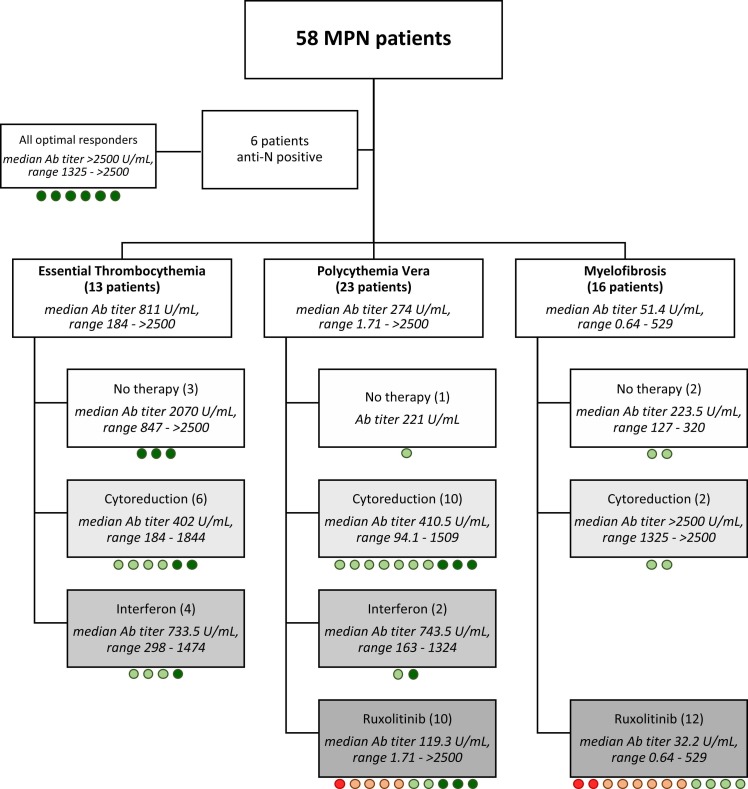
To capture the humoral response over time, serologic tests for SARS-CoV-2 antibodies (Ab) were performed according to routine practice in 58 consecutive MPN patients after at least 5 weeks from the second dose of the BNT162b2 vaccine referred for hematologic examination during the period from April to May 2021 ( Fig. 1). Concurrently, qualitative anti-N antibody analysis was performed to exclude patients who had asymptomatic infection before serologic evaluation.
Fig. 1.
Patient flow diagram, Cytoreduction included hydroxyurea, anagrelide, and busulfan. Ab: antibody. Circles represent Ab response. Dark green: better response (4th quartile); light green: intermediate quartiles; orange: poorer response (Ab titer ≥5–60 BAU/mL); red: negative/inconclusive.
The test quantified the IgG Ab titers against the anti-S receptor binding domain (RBD) by electro-chemiluminescence. The Elecsys® Anti-SARS-CoV-2 ECLIA assay (Roche Diagnostics AG, Rotkreuz, Switzerland) performed on the cobas e 801 analyzer (Roche Diagnostics) was used.
The assay uses recombinant proteins representing the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) in a double-antigen sandwich assay format, which favors detection of late, mature and high affinity antibodies against SARS-CoV-2.
The dosage units (U/mL) of the Elecsys assay are considered equal to the units of the WHO international standard (BAU/mL) for anti-SARS-CoV-2 S (RBD) antibodies. The conversion factor is 1.
Anti-S (RBD) were negative if < 0.8 BAU/mL, inconclusive if ≥ 0.8 to < 5 BAU/mL and positive if ≥ 5 BAU/mL. Patients above this upper cut-off level were considered responders. To further characterize this cohort, patients with Ab titers within the first quartile (<60 BAU/mL) were defined “no/poorer -responders” while patients with Ab titer values within the last quartile (>830 BAU/mL) were defined as “better responders”.
Comparisons of quantitative variables between groups of patients were carried out by Wilcoxon-Mann-Whitney rank-sum test or by Kruskal-Wallis and Dann test, as appropriate, while associations between categorical variables were tested by the χ2 test. Variables significantly associated to antibody titers in univariate analysis were considered in the multivariable analysis (MVA), carried out using a logistic regression model.
3. Results
Notably, 46% of patients experienced localized inflammation and 13.8% of patients had transitory fatigue/headache/malaise after vaccine inoculation. After a median of 15.4 weeks after serological evaluation (range, 9.6 −17), no SARS-CoV-2 infection, no thrombotic events and no long-term vaccine-related adverse events have been documented among vaccinated patients.
Clinical and laboratory characteristics of the study cohort are summarized in Supplemental Table 1.
Median time from second vaccine inoculation and serological evaluation was 10.6 weeks (range, 5.8–15). Median Ab titers in the cohorts that were evaluated after 5–10 or 10–11 or 11–15 weeks were comparable (519, 140, 214, respectively, p = 0.06). Median time from vaccine to serological test was comparable across hematological neoplasms (10.1, 10 and 11 weeks in ET, PV, and MF patients, respectively, p = 0.11) and between RUX-treated (10.2 weeks) and RUX-untreated (10.6 weeks) patients (p = 0.49).
In 6 patients, Ab anti-N were identified, indicating a previous asymptomatic contact with SARS-CoV-2. Among the remaining 52 patients, 13 had ET, 23 PV and 16 MF (5 Primary). Median Ab titer was 279.5 BAU/mL (range 0.6 - >2500).
Median antibody titer was significantly associated to the type of MPN (Supplemental Fig. 1). It was lower in MF (median antibody titer: 51.4 BAU/mL, range 0.6–529) compared to PV (median, 274 BAU/mL, range 1.7 - >2500, p < 0.001) and ET (811 BAU/mL, range 184 - >2500, p = 0.001) patients. This difference was significant also comparing ET and PV (p = 0.03). Also, a poorer response (Ab <60 BAU/mL) was observed in zero, 21.7% and 50% of ET, PV, and MF patients, respectively (p = 0.007). Accordingly, no MF patient achieved a better response (compared to 46.1% and 30.5% in ET and PV, respectively, p = 0.012).
Analyzing the three diseases singularly, no clinical-laboratory characteristic, including disease duration, was associated with median Ab titers. However, among MF patients, a higher frequency of lower response was observed in patients with spleen palpable > 5 cm below costal margin (85.7% versus 22.2% in patients with smaller spleen, p = 0.012). This was confirmed also evaluating only ruxolitinib-treated MF patients (p = 0.01). In MF, Dynamic International Prognostic Score system (DIPSS) risk category [15] was not associated with the Ab response (p = 0,48).
At the time of the serological test, 18 patients (34.6%) were receiving cytoreductive drugs (hydroxyurea, anagrelide, busulfan), 6 patients (11.5%) interferon, and 22 patients (42.3%) ruxolitinib. Six patients were off treatment.
Median ruxolitinib duration was 2.6 years (range, 0.4–16.8) and median ruxolitinib dose was 10 mg BID, with 18.2%, 63.6%, 5.6% and 13.6% patients receiving 5, 10, 15 and 20 mg BID, respectively. The use of ruxolitinib was associated with significantly decreased median Ab titers (33.1 BAU/mL versus 396.5 BAU/mL cytoreduction, p = 0.003; versus 733.5 BAU/mL interferon, p = 0.005; versus 583.5 BAU/mL in off-therapy patients, p = 0.007). In MF, this effect was particularly evident (median Ab titer 32.2 BAU/mL versus 233.5 in patients who were not receiving ruxolitinib, p = 0.05). In PV, only a trend for lower median Ab titers was observed (119.3 BAU/mL versus 393 BAU/mL, p = 0.09). Additionally, lower response was mainly observed in patients treated with ruxolitinib, both overall (59.1% vs 0) and singularly in the PV (50% vs 0) and MF cohorts (66.7% vs 0). In multivariable analysis, including MPN diagnosis (PV versus MF), treatment (ruxolitinib versus no ruxolitinib) and palpable spleen at time of serologic evaluation, ruxolitinib therapy (p = 0.02) and splenomegaly (p = 0.04) remained associated with lower Ab titers. Importantly, ruxolitinib dose did not influence the response, with median Ab titers of 40 and 33 BAU/mL in patients receiving doses of 5–10 mg BID or > 10 mg BID, respectively (p = 0.86). Also, time on ruxolitinib (≥ 1 year or ≥ 2 years) was not associated with response (p = 0.23 and 0.69, respectively).
In PV and ET, interferon therapy was associated with a slightly higher median Ab titer compared to cytoreduction/no therapy (733 versus 410 BAU/mL, p = 0.80).
4. Discussion
With the limitation of the small number of subjects included, we observed that median Ab titers, evaluated after more than one month from completion of vaccine program, are significantly greater in ET, intermediate in PV, and lower in MF. This is consistent with serological evaluations performed at earlier time points and it highlights the immunological differences across MPNs [1].
We also showed how ruxolitinib therapy, even more than disease type, may influence a later vaccine response. This finding reinforces the crucial argument of an impaired early humoral response to the SARS-CoV-2 vaccine in patients receiving ruxolitinib, and extends this issue to the later response as well [10]. However, the dose and duration of ruxolitinib did not affect Ab titers. Therefore, it may be advisable to wait for vaccine administration before starting ruxolitinib, if clinically acceptable, but there are no data to support a dose reduction of ruxolitinib in the peri-vaccinal period, nor to avoid or hasten vaccine administration in ruxolitinib-treated patients. Also, palpable splenomegaly was associated with a marked reduction in humoral response. This suggests, once again, how the severity of the hematological disease corresponds to a more critical immunologic disorder and is associated with vaccine humoral response.
Notably, the rate of response in our cohort was high compared to previous observations [14]. Whether this finding is related to methodological issues, or to different timings of evaluation, remains unclear. Our study, being observational, did not investigate cellular response [16]. Recently, a memory T cell response, not affected by ruxolitinib therapy, was observed in 80% MPN patients after the first dose of the BNT162b2 vaccine [17].
Given its observational nature, the present study has several limitations, including the possibility for selection bias, that may affect the greater-than-expected serological response in MF. Also, the evaluation of no-spike IgG does not entirely exclude the possibility of previous infection that has not seroconverted, especially for the patients on ruxolitinib [18].
Overall, these data highlight the correlation between lower Ab titer, ruxolitinib therapy, and MPN severity. A third vaccine dose must be administered early in patients with MF and PV, particularly those on ruxolitinib therapy and/or with uncontrolled splenomegaly. All patients should continue to take the best preventive measures against COVID-19 even after receiving vaccination.
4.1. Ethics approval statement
All patients participated in either of the following retrospective and prospective observational studies: MF-2014-01 study (date of approval: 10/06/2014, approval file number: 068/2014/U), PV-ARC study (date of approval: 12/09/2018, approval file number: 438/2018/Oss/AOUBo) or ET-2014–01 study (date of approval: 10/06/2014, approval file number: 098/2014/O/Oss). All three studies were approved by our local Ethics Committee (Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna (CE-AVEC), address: Via Albertoni 15, 40138, Bologna, Italy, e-mail: cometico@aosp.bo.it).
CRediT authorship contribution statement
Designed research: Palandri F., Auteri G., Di Pietro C., Performed research: Palandri F., Auteri G., Lazzarotto T.Collected data: Palandri F., Auteri G., Di Pietro C., Sutto E. Analyzed and interpreted data: Bartoletti D, Palandri F., Auteri G, Wrote the manuscript: Palandri F., Auteri G, Bartoletti D., Revised and gave final approval: All Authors.
Declaration of Competing interest
The authors declare no competing interests.
Acknowledgments
This work was supported by BolognAIL.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.leukres.2022.106819.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Palandri F., Breccia M., De Stefano V., Passamonti F. Philadelphia-negative chronic myeloproliferative neoplasms during the COVID-19 pandemic: challenges and future scenarios. Cancers. 2021;13:4750. doi: 10.3390/cancers13194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbui T., Vannucchi A.M., Alvarez-Larran A., Iurlo A., Masciulli A., Carobbio A., Ghirardi A., Ferrari A., Rossi G., Elli E., Andrade-Campos M.M., Kabat M.G., Kiladjian J.-J., Palandri F., Benevolo G., Garcia-Gutierrez V., Fox M.L., Foncillas M.A., Morcillo C.M., Rumi E., Osorio S., Papadopoulos P., Bonifacio M., Cervantes K.S.Q., Serrano M.S., Carreno-Tarragona G., Sobas M.A., Lunghi F., Patriarca A., Elorza B.N., Angona A., Mazo E.M., Koschmieder S., Ruggeri M., Cuevas B., Hernandez-Boluda J.C., Abadia E.L., Cirici B.X., Guglielmelli P., Garrote M., Cattaneo D., Daffini R., Cavalca F., Bellosillo B., Benajiba L., Curto-Garcia N., Bellini M., Betti S., De Stefano V., Harrison C., Rambaldi A. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021;35:485–493. doi: 10.1038/s41375-020-01107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.G. SIE, Vaccinazione per COVID-19 nei pazienti con malattie del sangue e sottoposti a trapianto di cellule staminali., 2021.
- 4.COVID-19 resources. COVID-19 and Myeloproliferative Neoplasms: Frequently Asked Questions. Version 6.1; last updated September 3, 2021 〈https://www.hematology.org:443/covid-19/covid-19-and-myeloproliferative-neoplasms〉. American Society of Hematology. Accessed November 8, 2021.
- 5.L. Castelo-Branco, A. Cervantes, G. Curigliano, M.C. Garassino, N. Giesen, P. Grivas, J. Haanen, K. Jordan, U.G. Liebert, F. Lordick, F Lucibello, I. Melero, O. Mir, G. Pentheroudakis, S. Peters, C. Pietsch, F. Scotté, M. von Lilienfeld-Toal. COVID-19 and Cancer. ESMO Statements for vaccination against COVID-19 in patients with cancer (https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination). European Society for Medical Oncology. Accessed November 17, 2021.
- 6.Polverelli N., Breccia M., Benevolo G., Martino B., Tieghi A., Latagliata R., Sabattini E., Riminucci M., Godio L., Catani L., Nicolosi M., Perricone M., Sollazzo D., Colafigli G., Campana A., Merli F., Vitolo U., Alimena G., Martinelli G., Lewis R.E., Vianelli N., Cavo M., Palandri F. Risk factors for infections in myelofibrosis: role of disease status and treatment. A multicenter study of 507 patients. Am. J. Hematol. 2017;92:37–41. doi: 10.1002/ajh.24572. [DOI] [PubMed] [Google Scholar]
- 7.Barosi G. An immune dysregulation in MPN. Curr. Hematol. Malig. Rep. 2014;9:331–339. doi: 10.1007/s11899-014-0227-0. [DOI] [PubMed] [Google Scholar]
- 8.Pimpinelli F., Marchesi F., Piaggio G., Giannarelli D., Papa E., Falcucci P., Pontone M., Di Martino S., Laquintana V., La Malfa A., Di Domenico E.G., Di Bella O., Falzone G., Ensoli F., Vujovic B., Morrone A., Ciliberto G., Mengarelli A. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J. Hematol. Oncol. 2021;14:81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimpinelli F., Marchesi F., Piaggio G., Giannarelli D., Papa E., Falcucci P., Spadea A., Pontone M., Di Martino S., Laquintana V., La Malfa A., Di Domenico E.G., Di Bella O., Falzone G., Ensoli F., Vujovic B., Morrone A., Ciliberto G., Mengarelli A. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J. Hematol. Oncol. 2021;14:119. doi: 10.1186/s13045-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmelli P., Mazzoni A., Maggi L., Kiros S.T., Zammarchi L., Pilerci S., Rocca A., Spinicci M., Borella M., Bartoloni A., Rossolini G.M., Annunziato F., Vannucchi A.M. Impaired response to first SARS-CoV-2 dose vaccination in myeloproliferative neoplasm patients receiving ruxolitinib. Am. J. Hematol. 2021;96:E408–E410. doi: 10.1002/ajh.26305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzog Tzarfati K., Gutwein O., Apel A., Rahimi-Levene N., Sadovnik M., Harel L., Benveniste-Levkovitz P., Bar Chaim A., Koren-Michowitz M. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am. J. Hematol. 2021;96:1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury O., Bruguier H., Mallett G., Sousos N., Crozier K., Allman C., Eyre D., Lumley S., Strickland M., Karali C.S., Murphy L., Sternberg A., Jeffery K., Mead A.J., Peniket A., Psaila B. Impaired antibody response to COVID-19 vaccination in patients with chronic myeloid neoplasms. Br. J. Haematol. 2021;194:1010–1015. doi: 10.1111/bjh.17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorino F., Sicuranza A., Ciabattini A., Santoni A., Pastore G., Simoncelli M., Polvere J., Galimberti S., Auddino S., Baratè C., Montagnani F., Sammartano V., Bocchia M., Medaglini D. The slower antibody response in myelofibrosis patients after two doses of mRNA SARS-CoV-2 vaccine calls for a third dose. Biomedicines. 2021;9:1480. doi: 10.3390/biomedicines9101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N. Gagelmann, F. Passamonti, C. Wolschke, R. Massoud, C. Niederwieser, E. Klyuchnikov, R. Adjallé, B. Mora, F. Ayuk, N. Kröger. Antibody response to COVID-19 vaccination in adults with haematological malignancies: a systematic review and meta-analysis, social science research network, Rochester, NY, 2021. 10.2139/ssrn.3929967. [DOI]
- 15.Passamonti F., Cervantes F., Vannucchi A.M., Morra E., Rumi E., Pereira A., Guglielmelli P., Pungolino E., Caramella M., Maffioli M., Pascutto C., Lazzarino M., Cazzola M., Tefferi A. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 16.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., Markowitz C.E., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington P., de Lavallade H., Doores K.J., O’Reilly A., Seow J., Graham C., Lechmere T., Radia D., Dillon R., Shanmugharaj Y., Espehana A., Woodley C., Saunders J., Curto-Garcia N., O’Sullivan J., Raj K., Kordasti S., Malim M.H., Harrison C.N., McLornan D.P. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35(12):3573–3577. doi: 10.1038/s41375-021-01300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passamonti F., Romano A., Salvini M., Merli F., Porta M.G.D., Bruna R., Coviello E., Romano I., Cairoli R., Lemoli R., Farina F., Venditti A., Busca A., Ladetto M., Massaia M., Pinto A., Arcaini L., Tafuri A., Marchesi F., Fracchiolla N., Bocchia M., Armiento D., Candoni A., Krampera M., Luppi M., Cardinali V., Galimberti S., Cattaneo C., La Barbera E.O., Mina R., Lanza F., Visani G., Marchesi P., Petrucci L., Zaja F., Grossi P.A., Bertù L., Pagano L., Corradini P. ITA-HEMA-COV Investigators*, COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br. J. Haematol. 2021;195(3):371–377. doi: 10.1111/bjh.17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material