Abstract
Background
Coronavirus disease-19 (COVID-19) is implicated by active endotheliitis, and cardiovascular morbidity. The long-COVID-19 syndrome implications in atherosclerosis have not been elucidated yet. We assessed the immediate, intermediate, and long-term effects of COVID-19 on endothelial function.
Methods
In this prospective cohort study, patients hospitalized for COVID-19 at the medical ward or Intensive Care Unit (ICU) were enrolled and followed up to 6 months post-hospital discharge. Medical history and laboratory examinations were performed while the endothelial function was assessed by brachial artery flow-mediated dilation (FMD). Comparison with propensity score-matched cohort (control group) was performed at the acute (upon hospital admission) and follow-up (1 and 6 months) stages.
Results
Seventy-three patients diagnosed with COVID-19 (37% admitted in ICU) were recruited. FMD was significantly (p < 0.001) impaired in the COVID-19 group (1.65 ± 2.31%) compared to the control (6.51 ± 2.91%). ICU-treated subjects presented significantly impaired (p = 0.001) FMD (0.48 ± 1.01%) compared to those treated in the medical ward (2.33 ± 2.57%). During hospitalization, FMD was inversely associated with Interleukin-6 and Troponin I (p < 0.05 for all). Although, a significant improvement in FMD was noted during the follow-up (acute: 1.75 ± 2.19% vs. 1 month: 4.23 ± 2.02%, vs. 6 months: 5.24 ± 1.62%; p = 0.001), FMD remained impaired compared to control (6.48 ± 3.08%) at 1 month (p < 0.001) and 6 months (p = 0.01) post-hospital discharge.
Conclusion
COVID-19 patients develop a notable endothelial dysfunction, which is progressively improved over a 6-month follow-up but remains impaired compared to healthy controls subjects. Whether chronic dysregulation of endothelial function following COVID-19 could be accompanied by a residual risk for cardiovascular and thrombotic events merits further research.
Keywords: COVID-19, Flow-mediated dilation, Endothelial function, Endothelium
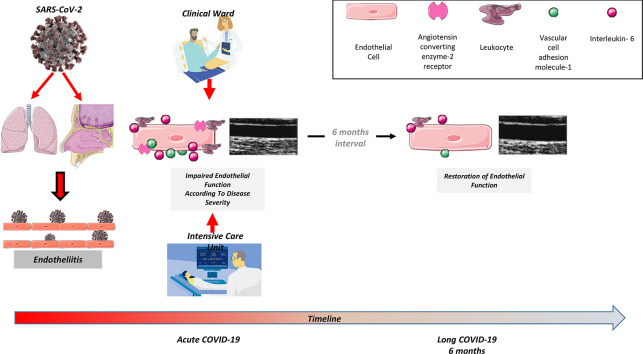
Graphical abstract
1. Introduction
Coronavirus Disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and initially reported in Wuhan, China, constitutes a global health threat. Other than the direct catastrophic pulmonary complications of the SARS-CoV-2, the emergence of the COVID-19 pandemic has especially affected subjects with cardiovascular risk factors or documented cardiovascular disease [[1], [2], [3], [4]]. Existing evidence shows that COVID-19 affects the cardiovascular system in an adverse manner and confirms the increased incidence and worse prognosis of patients presented with pulmonary embolism, thromboembolic complications, acute coronary syndrome, and myocarditis in the course of the disease caused by SARS-CoV-2 [5].
Based on the available pathophysiological data, SARS-CoV-2 primarily targets epithelial cells of nasal, bronchial, and pulmonary epithelium through the viral structural spike (S) protein that binds to the angiotensin-converting enzyme 2 (ACE2) receptor [6]. This receptor is expressed in the outer membrane of several other cell types - especially on vascular endothelium cells [6] - causing active SARS-CoV-2-related endotheliitis documented by viral elements within the endothelial cells, leading to disruption of their membranes and pyroptosis [[7], [8], [9], [10]]. In this sense, the regulatory role of ACE2 receptors and vascular endothelium on inflammatory leucocyte recruitment, cytokines release, and intravascular coagulation may control - among other factors - disease severity and complications [11].
Indeed, many histopathological studies have highlighted the involvement of vascular endothelium in COVID-19 since patients with severe pneumonia had impaired endothelial function [7,12,13], a finding further documented by experimental models [14]. Flow-mediated dilation (FMD) of the brachial artery represents a non-invasive method used to test the systemic vascular endothelial function and is a valuable tool in studying the pathophysiology and the prognosis of cardiovascular disease (CVD) [15,16]. Moreover, FMD is an independent predictor of CV events and mortality [17].
Therefore, the primary aim of this study was to evaluate the immediate effect of COVID-19 on endothelial function integrity – as assessed by FMD - with a secondary aim to test the intermediate and long-term effects on FMD, as well as the association between FMD and disease severity.
2. Material and methods
2.1. Study population
This is a prospective cohort study performed in the “Sotiria” General Hospital for Chest Diseases, Athens, Greece. We recruited 73 patients admitted to the medical ward (3rd Department of Medicine, Athens Medical School, “Sotiria” General Hospital for Chest Diseases) or the Intensive Care Unit (ICU) (Respiratory Failure Center, Intensive Care Unit, “Sotiria” General Hospital for Chest Diseases) with SARS-CoV-2-related infection. The study was carried out between November 20, 2020, and March 31, 2021. Inclusion criteria were adult patients (age ≥ 18 years) admitted to hospital with SARS-CoV-2 infection confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay of nasopharyngeal or bronchial swabs, in at least one biological sample.
Cases were compared to a control group from the historical records of our research group after applying appropriate propensity score matching described in detail in the statistical analysis section.
Subjects with a history of: a) end-stage renal failure, b) active malignancy, c) previous or current autoimmune diseases, and d) subjects with COVID-19 who did not agree to participate in the study and/or did not sign the Informed Consent Form, were excluded.
All study parameters were evaluated on: i) the acute phase of SARS-CoV-2 infection (between 24 and 72 h after hospital admission), ii) the mid-term phase (28 days after hospital discharge), and iii) the long-term phase (6 months after hospital discharge).
The study was approved by the hospital's Ethics Committee (protocol number: 29542/6-11-20) with the collaboration of the Athens Medical School of the National and Kapodistrian University of Athens, Greece, and was carried out following the Declaration of Helsinki (1989). All individuals were informed about the study's aims and provided written informed consent.
2.2. Clinical and laboratory measurements
Demographics and medical history data of COVID-19 subjects were collected at the Emergency Department during the acute phase by trained physicians. Clinical data were collected for all patients: i) in the acute phase evaluation (between 24 and 72 h after hospital admission) and through-out hospitalization; ii) in the mid-term phase (28 days after hospital discharge) and iii) in the long-term phase (6 months after hospital discharge). Patients were classified with worsening clinical severity according to the World Health Organization-Ordinal Scale for Clinical Improvement WHO-OSCI ordinal clinical scale [18]. According to study inclusion criteria, we included COVID-19 patients with a score from 3 to 7.
All subjects were evaluated for the presence of myocardial injury according to standard definitions [19] and based on clinical evaluation of biomarkers (Troponin I, D Dimers), clinical presentation, and diagnostic test (i.e. echocardiography, cardiac magnetic resonance imaging, computed tomography pulmonary angiography).
Subjects were characterized as having long COVID-19 syndrome based on the presence of at least one of the following (fatigue, shortness of breath, anxiety, chest pain, altered mental status, cough, depression, tachycardia/palpitation, myalgia, joint pain, mobility problems) lasting at least for 2 months, 3 months after COVID-19 diagnosis, not justified by alternative conditions and with significant impact in everyday activities [20].
Several laboratory parameters and circulating cytokines were measured as described in detail in the Supplementary Material, S1.
2.3. Flow-mediated dilation assessment
Endothelial function was evaluated by estimating the FMD in the brachial artery [16,21], and is described in more detail in the Supplementary Material, S1.
2.4. Statistical analysis
All statistical calculations were performed using IBM SPSS software (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) and G*Power (version 3.0.10). Statistical analysis is described in detail in the Supplementary Material, S1.
3. Results
3.1. Baseline characteristics of the study population
In this analysis we included 73 patients hospitalized for SARS-CoV-2 after application of the exclusion criteria (Supplementary Fig. 1). Patients hospitalized with COVID-19 were predominantly male (63%), with a mean age of 60 (SD: 12) years. The median duration of hospital stay was 14 days (IQR: 8, 26). When compared to their propensity score-matched controls, no major differences were noted in the prevalence of arterial hypertension (COVID-19: 46.6% vs. Control: 56.5%; p = 0.24), diabetes mellitus (COVID-19: 27.4% vs. Control: 19.7%; p = 0.28), coronary artery disease (COVID-19: 9.6% vs. Control: 13.4%; p = 0.48), and Dyslipidemia (COVID-19: 31.5% vs. Control: 38.4%; p = 0.39) (Supplementary Table 1). Approximately 37% of the study population consisted of patients admitted to the ICU, with a median ICU stay of 17 days (IQR: 9, 31). Patients hospitalized in the ICU were older with a higher frequency of active smoking, arterial hypertension, dyslipidemia, and coronary artery disease in their medical history (Table 1 ).
Table 1.
Differences in clinical characteristics, laboratory tests, and endothelial function between patients with COVID-19 hospitalized in a medical ward or in an ICU.
| Medical ward (N = 46) | ICU (N = 27) | p | |
|---|---|---|---|
| Mean age in years (SD) | 56.4 (12.3) | 68.0 (10.4) | <0.001 |
| Male sex, n (%) | 26 (56.5) | 20 (74.1) | 0.13 |
| Smoking, n (%) | 3 (6.5) | 8 (29.6) | 0.010 |
| Systolic BP in mmHg (SD) | 132 (15) | 137 (10) | 0.16 |
| Diastolic BP in mmHg (SD) | 81 (13) | 77 (8) | 0.16 |
| MAP in mmHg (SD) | 98 (12) | 97 (7) | 0.68 |
| History of hypertension, n (%) | 15 (32.6) | 19 (70.4) | 0.002 |
| History of dyslipidemia, n (%) | 10 (21.7) | 13 (48.1) | 0.02 |
| History of DM, n (%) | 11 (23.9) | 9 (33.3) | 0.38 |
| History of CAD, n (%) | 2 (4.3) | 5 (18.5) | 0.05 |
| Lab tests | |||
| Median CRP in mg/dl (IQR) | 4.2 (1.7, 8.8) | 9.6 (5.5, 14.7) | 0.001 |
| Median IL-6 in pg/ml (IQR) | 3.30 (1.73, 8.22) | 69.2 (18.99, 90.30) | <0.001 |
| Median VCAM-1 in ng/ml (IQR) | 125 (847, 1553) | 1545 (1234, 2285) | 0.020 |
| Median Ferritin in ng/ml (IQR) | 354 (187, 507) | 697 (364, 1229) | <0.001 |
| Median LDH in IU/l (IQR) | 304 (242, 395) | 449 (347, 546) | <0.001 |
| Median D-Dimers in μg/ml (IQR) | 0.58 (0.40, 1.00) | 2.16 (0.95, 4.80) | <0.001 |
| Median hsTnI in pg/ml (IQR) | 5 (3, 8) | 35 (14, 85) | <0.001 |
| Endothelial function | |||
| Mean FMD in % (SD) | 2.33 (2.57) | 0.48 (1.01) | 0.001 |
COVID-19: coronavirus disease-19, ICU: intensive care unit, BP: blood pressure, MAP: mean arterial pressure, DM: diabetes mellitus, CAD: coronary artery disease, CRP: C reactive protein, IL-6: interleukin-6, IQR: interquartile range, LDH: lactate dehydrogenase, hsTnI: high sensitivity troponin I, FMD: flow-mediated dilation; VCAM-1: Vascular cell adhesion molecule 1.
3.2. Laboratory findings in patients with COVID-19
Increased levels of inflammatory markers [median CRP 6.7 mg/dl (3.1–11.4) versus reference range < 0.7 mg/dl; p < 0.001], acute phase reactants [median ferritin 430 ng/ml (256–745), reference range 5-204 ng/ml; p < 0.001], markers of tissue damage [median LDH 360 IU/l (268–455), reference range 135-248 IU/l; p < 0.001], myocardial injury [median hsTnI 7.9 pg/ml (4.0–35.2), reference range < 15.6 pg/ml; p = 0.05], and thrombosis [median D-dimers 0.88 μg/ml (0.49–1.94), reference range < 0.5 μg/ml; p = 0.001] were observed in patients hospitalized for COVID-19. We further stratified patients according to ICU admission and noted a significantly higher expression of the examined markers in those hospitalized in an ICU setting (Table 1). As far as IL-6 is concerned, it was found that it is significantly elevated in COVID-19 patients treated in the ICU compared to those hospitalized in the medical ward [69.2 pg/ml (18.99, 90.30) vs. 3.30 pg/ml (1.73, 8.22); p < 0.001, respectively]. IL-6 was also found significantly elevated in deceased COVID-19 patients compared to survivors [54.09 pg/ml (18.11, 89.05) vs. 4.97 pg/ml (2.16, 26.4); p < 0.001; respectively]. VCAM-1 was also significantly elevated in COVID-19 patients hospitalized in the ICU compared to the medical ward [1545 ng/ml (1234, 2285) vs. 1215 ng/ml (847, 1553); p = 0.02, respectively].
During the follow-up, the values of all the examined markers mentioned above were significantly improved and were found to range within normal limits for surviving patients hospitalized either in the ICU or in medical wards (Supplementary Table 2).
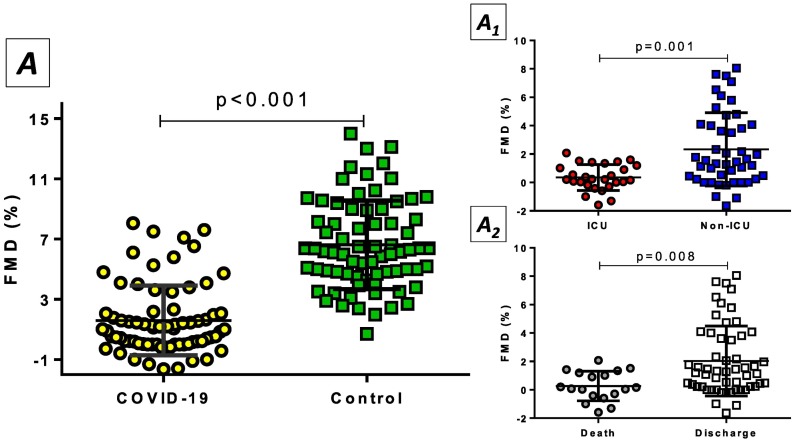
3.3. Endothelial function during the acute COVID-19 phase
Endothelial function - being assessed by FMD - was significantly impaired in patients hospitalized for COVID-19 when compared to propensity score-matched controls [1.65% (SD: 2.31) vs. 6.51% (SD: 2.91); p < 0.001, respectively] (Fig. 1 , Panel A1). Moreover, the higher the score on the WHO-OSCI ordinal clinical scale the more impaired (lower) FMD values [level 3: 2.27% (SD: 1.28), level 4: 2.15% (SD: 2.72), level 5: 1.99% (SD: 1.84), level 6: 1.77% (SD: 2.50), level 7: 0.33% (SD: 1.00); p = 0.05]. Subgroup analysis according to sex demonstrated similar FMD values between female and male subjects [female: 1.69% (SD: 2.48) vs. male: 1.62% (SD: 2.22); p = 0.91] (Supplementary Table 3).
Fig. 1.
The impact of acute COVID-19 on endothelial function. A) Individuals hospitalized for COVID-19 had significantly worsened endothelial function as estimated by flow-mediated dilation (FMD) of brachial artery compared to a propensity score-matched control group. A1) Admission to the intensive care unit (ICU) resulted in severely decreased FMD values compared to patients hospitalized at the medical ward. A2) Lower FMD was detected in patients who died compared to COVID-19 patients who were discharged from the hospital.
Compared to COVID-19 patients hospitalized in a medical ward, critically ill COVID-19 patients admitted to ICU were found to have considerably lower FMD values [2.33% (SD: 2.57) vs. 0.48% (SD: 1.01); p = 0.001; respectively] (Fig. 1, Panel A1). Even after adjustment for confounders (age, smoking, hypertension, dyslipidemia, CAD), subjects admitted in the ICU had impaired FMD (by 1.79%, p = 0.007) compared to subjects admitted to the medical ward (Supplementary Table 4). FMD was also lower (impaired) in subjects who died than subjects discharged from the hospital [0.45% (SD: 1.14) vs. 2.07% (SD: 2.47); p = 0.008, respectively]. (Fig. 1, Panel A2), though this association faded after multivariate adjustment (results not shown). Additionally, the cumulative mortality was not significantly different when the study population was stratified according to median FMD (Supplementary Fig. 2). Following ROC curve analysis (Supplementary Fig. 3), FMD had a moderate discriminative ability concerning mortality (AUROC: 0.69, p = 0.01), with values equal or <1.54% having a sensitivity of 89.5% and a specificity and 44.4%. Moreover, FMD levels were adversely correlated with IL-6 (r = −0.38, p = 0.005), D-Dimers (r = −0.47, p < 0.001), and hsTnI (r = −0.34, p = 0.003), as well as with the length of hospital stay (r = −0.28, p = 0.02).
Concerning cardiovascular complications, 12 patients (9 ICU, 3 non-ICU) were characterized as having myocardial injury (hsTnI increases above the 99th percentile of upper reference limit). Of those cases, two were attributed to pulmonary embolism, two were diagnosed with myocarditis, and the rest were considered as type 2 myocardial infarction. Interestingly, FMD was significantly impaired in subjects who developed myocardial injury 0.41% (SD: 0.87) compared to the rest of the study group 1.91% (SD: 2.44), p = 0.03].
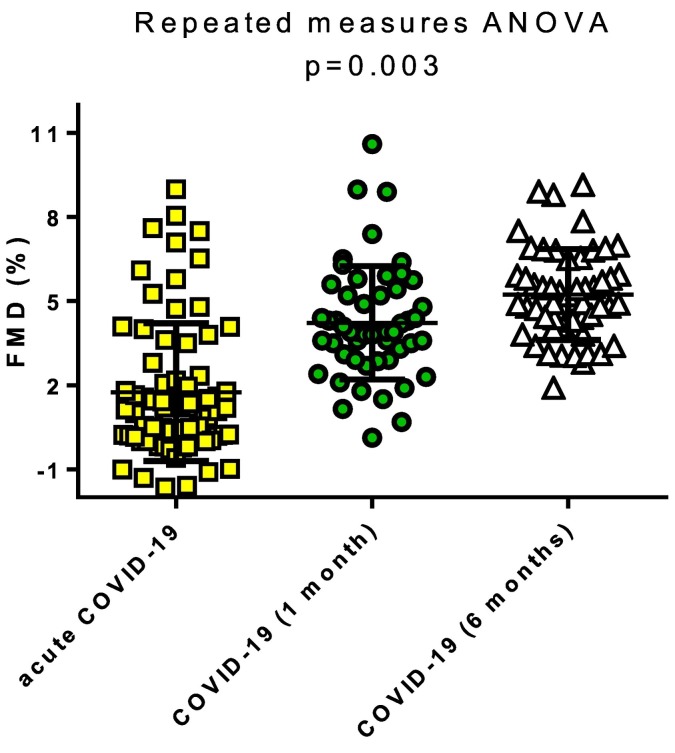
3.4. Endothelial function during the follow-up period
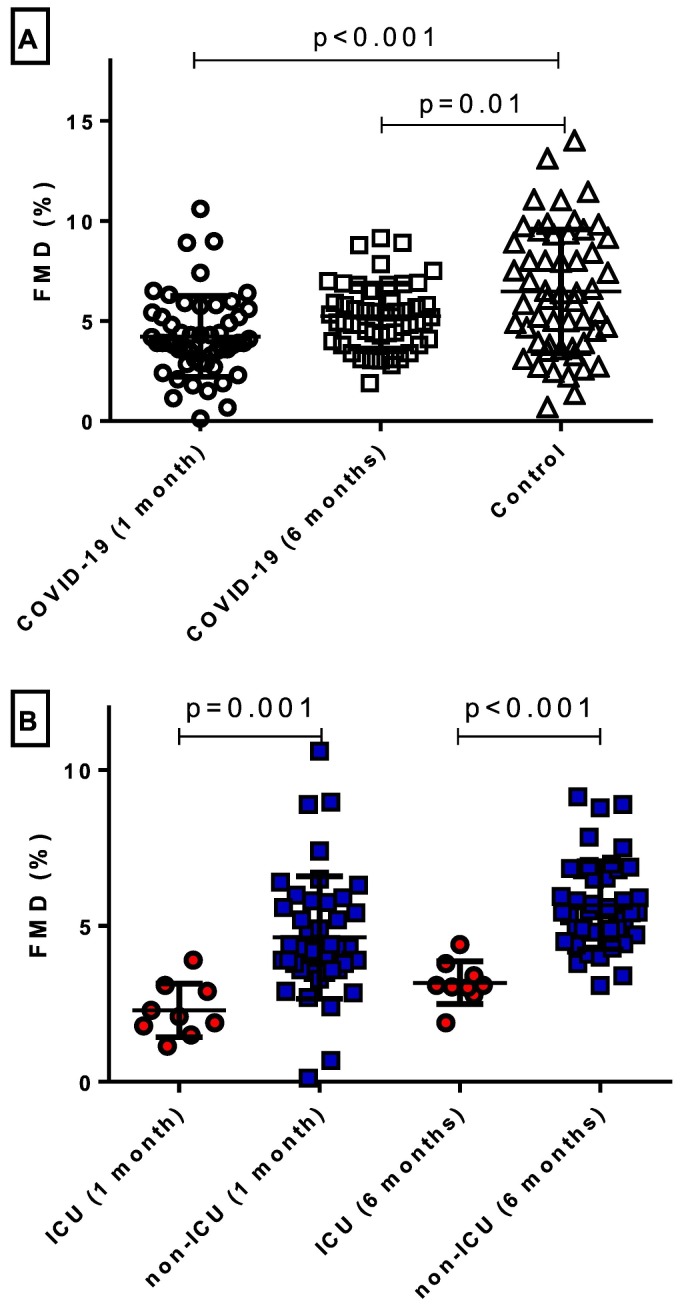
Endothelial function was reassessed at 1 and 6 months after the acute phase of COVID-19. A significant improvement in FMD from the acute hospital phase to 1 and 6 months of follow-up was observed [Acute: 1.75% (SD: 2.19) vs. 1 month: 4.23% (SD: 2.02), vs. 6 months: 5.24% (SD: 1.62); p = 0.001] (Fig. 2 ). Due to the deaths of patients (n = 18) in the initial COVID-19 cohort and the loss of participants to follow-up (n = 3), a new propensity score-matched cohort was determined as the comparison control group. A similar prevalence of cardiovascular risk factors was noted between the two groups (Supplementary Table 5). Nevertheless, endothelial function remained impaired one-month post-COVID-19 hospitalization [FMD in COVID-19 patients: 4.23% (SD: 2.02) vs. Control: 6.48% (SD: 3.08); p < 0.001]. Interestingly, a significant difference in FMD also persisted during the 6-month follow-up values of patients initially hospitalized for COVID-19 and the propensity score-matched control group [COVID−19: 5.24% (SD: 1.62) vs. Control: 6.48% (3.08); p = 0.01] (Fig. 3 , Panel A), after adjustment for multiple comparisons. No sex-related differences were observed during the follow-up period (Supplementary Fig. 4).
Fig. 2.
Follow-up evaluation of endothelial function in COVID-19 survivors. A significant improvement was observed over the follow-up duration (1 month and 6 months) in flow-mediated dilation (FMD) in patients initially hospitalized for COVID-19.
Fig. 3.
Intermediate and long-term effect of COVID-19 on endothelial function. A) Follow-up evaluation of endothelial function at 1 and 6 months after hospitalization for COVID-19 demonstrated significantly impaired flow-mediated dilation (FMD) when compared to propensity score-matched controls. B) Patients hospitalized in an intensive care unit (ICU) had remarkably worse FMD compared to individuals hospitalized in a non-ICU setting at 1 and 6 months after the index admission.
When taking ICU admission during the hospitalization into account, the magnitude of endothelial dysfunction was stronger in patients admitted to the ICU compared to those hospitalized at the medical ward, both at one month [2.29% (SD: 0.86) vs. 4.63% (1.96); p = 0.001, respectively] and 6 months follow-up [3.18% (SD: 0.69) vs. 5.67% (SD: 1.41); p < 0.001, respectively] (Fig. 3, Panel B). We further proceeded to the stratification of the study population according to the median baseline FMD (1.28%). Although we did not note any major differences in the examined biomarkers at 1- and 6-month follow-up, FMD was significantly more impaired both at 1- and 6-month follow-up in those with baseline FMD values below the median (Supplementary Table 6). Interestingly, a greater increase from baseline was noted in subjects with baseline FMD below the median. Finally, even though none of the patients were vaccinated prior to hospitalization, 11 patients completed their vaccination during the follow-up period. When analyzing those subjects compared to the rest of the study population that completed the follow-up, we found no association between post-infection vaccination status with the degree of endothelial impairment across the follow-up period (Supplementary Fig. 5).
According to symptoms presentation in the 6-month follow-up, the majority of subjects (58%) were found to have long COVID-19. There was no difference in FMD values at the 6-month follow-up according to the presence or not of the long-COVID-19 syndrome [4.98% (SD: 1.90) vs. 5.02% (SD: 1.15); p = 0.42].
4. Discussion
In this study, we documented that during the acute phase of COVID-19, impaired endothelial function correlates with the severity of the disease and the critically ill stage, as well as with IL-6 and VCAM-1. Moreover, cardiovascular complications were associated with impaired FMD. We also demonstrated that following recovery from COVID-19, there is a time progressive restoration of endothelial function at one month and six months following SARS-CoV-2 infection. Despite the improvement of endothelial function following acute COVID-19, even six months after diagnosis of infection, FMD remained impaired compared to control subjects without COVID-19. Interestingly, we demonstrated that non-ICU hospitalized COVID-19 patients have better restoration of endothelial function six months following SARS-CoV-2 infection.
4.1. Acute COVID-19 and endothelial function
Endothelial dysfunction has been characterized as a critical component of COVID-19 with great prognostic significance since it has been associated with clinical complications and adverse outcomes [22,23]. Although the importance of endothelial dysfunction has been reported and discussed in the setting of the acute SARS-CoV-2 infection, it is unclear whether a complete resolution ensues in the mid-term or long-term follow-up, as a chronic dysregulation of endothelial function could be accompanied by a residual risk for cardiovascular and thrombotic events.
To our knowledge, this is the first prospective study to evaluate the course of endothelial dysfunction in COVID-19 in the acute phase of the SARS-COV-2 infection as well as in the mid- and long-term follow-up, paired with laboratory evaluation of common laboratory markers of COVID-19 severity and specific endothelial and inflammatory biomarkers. According to our results, patients hospitalized for SARS-CoV2 had impaired endothelial function, evaluated by brachial artery FMD, compared to propensity score-matched non-COVID-19 controls. When considering FMD in the acute phase of COVID-19, although available studies have found impaired values, there are disparities regarding the extent of endothelial dysfunction or FMD lowering. In the study of Oliveira et al., a mean FMD of <0% was reported [24], while Heubel et al. in a cohort of hospitalized COVID-19 patients at a stable state reported mean FMD values of approximately 5.5% [25]. In the most recently published study by Bianconi et al., the median brachial FMD was reported at 4.4% in the entire COVID-19 population of 408 patients, with non-survivors or those requiring ICU admission exhibiting even lower values [22]. A potential explanation of these differences could be the disease severity (ICU admission, length of stay) and the differences in risk factor profiles (i.e. history of hypertension, diabetes mellitus, coronary artery disease) of the study participants. Moreover, significantly impaired FMD was also associated with in-hospital mortality in our study, confirming previous results [22,24]. However, we found that endothelial impairment has only a modest sensitivity and low specificity for identification of patients at high risk of death.
Cardiovascular complications, including pulmonary embolism, myocarditis, and myocardial injury or acute coronary syndrome are common among patients hospitalized for COVID-19 and adversely affect outcomes [26]. Therefore, endothelial dysfunction may be a common pathophysiologic mechanism, at least concerning myocardial injury of different etiologies.
4.2. Cytokines, adhesion molecules, and endothelial dysfunction
As far as IL-6 is concerned, it has been previously presented as a potent indicator of increased COVID-19 severity [27]. The regulatory role of IL-6 in atherosclerosis initiation and progression has also been extensively reported [28], implying a possible common pathogenetic pathway of COVID-19 with cardiovascular disease.
The association of IL-6 levels with COVID-19 severity was reproduced in our study, with patients hospitalized in the ICU and non-survivors having significantly increased IL-6 compared to patients hospitalized in medical wards and COVID-19 survivors, respectively. Additionally, we found a significant negative correlation of circulating IL-6 with FMD in the acute phase of the disease. The same correlation has been reported in healthy subjects, independently of confounders [29]. Moreover, administration of tocilizumab, an IL-6 inhibitor, may improve FMD, according to the study of Bacchiega et al. in patients with rheumatoid arthritis and increased cardiovascular risk [30]. In the setting of COVID-19, IL-6 inhibition has yielded encouraging results regarding the survival of critically ill patients requiring organ support, the progression to mechanical ventilation, and all-cause mortality [31,32]. Confirmatory to literature data in our study population, improvement of clinical status following hospital discharge was accompanied by a parallel improvement of endothelial function and normalization of IL-6 levels.
VCAM-1 serves as a ligand for integrins on the endothelium. Impaired endothelial function cause leucocyte adhesion, accumulation, and perpetuation of immune responses and inflammation. In the setting of COVID-19, VCAM-1 has been associated with adverse prognosis and ICU hospitalization [33,34]. Interestingly, VCAM-1 levels in COVID-19 patients were found in patients with diffuse endothelial damage in the alveolar epithelium, vascular microthrombi and increased, PaCO2 [35]. Our findings in the acute phase of COVID-19 support the active role of the VCAM-1 in COVID-19 related endothelial impairment. Interestingly, the moderate restoration of VCAM-1 during the follow-up period may explain the lack of FMD values normalization 6 months after acute SARS-CoV-2 infection and may serve as a possible biomarker of disease recovery, which may also be associated with the status of the alveolar epithelium.
4.3. Long COVID-19 and endothelial function
Although endothelial function improved considerably during the follow-up period, it remained significantly lower compared to the propensity score-matched control group at 1 and 6 months after hospitalization. In a recent cross-sectional study of 133 patients following COVID-19 referred for pulmonary rehabilitation, endothelial function remained impaired two months after hospitalization [36]. Importantly the authors noted a sex-related difference since females presented with greater FMD values similar to the control group, a finding which was not observed in our study [36]. The same study group also reported an improvement in FMD following pulmonary rehabilitation [37]. In our study, endothelial function recovered without a specific rehabilitation program, and the additional impact of a structured rehabilitation program in endothelial function improvement following COVID-19 may be further evaluated by a randomized control trial. Moreover, in a cohort of 70 subjects four months after SARS-CoV-2 infection, the reported values of FMD were slightly higher than our findings [38]. However, it should be noted that 34% of their study population consisted of patients with a mild form of the disease not requiring hospitalization, while none of the participants required mechanical ventilation [38]. The only study with a follow-up period (29 ± 3 weeks) comparable to our study setting reported considerably lower FMD values but in a population older than our COVID-19 cohort [39]. Interestingly, long-COVID-19 syndrome 6 months following infection with SARS-CoV-2 was not associated with endothelial function status. Interestingly, there are no data on the impact of post-COVID-19 vaccination in the presentation of the long COVID-19 syndrome. Based on our supplementary analysis in a small population of subjects vaccinated against COVID-19 at least 3 months following recovery of acute COVID-19 we found no difference in endothelial status of post COVID-19 vaccinated and unvaccinated patients.
Another important aspect of our study concerned the association of disease severity at the acute phase with the degree of endothelial function improvement during the follow-up period. Interestingly, hospitalization in the ICU resulted in more impaired FMD values than patients hospitalized in a medical ward but as we have noted the improvement was more potent in subjects with lower baseline values and independent of inflammatory biomarkers levels. This finding contradicts the results presented by Riou et al., who observed similar FMD values regardless of disease severity [40]. It should be noted that their study population comprised considerably fewer participants with ICU hospitalization compared to our cohort, while their FMD at the time of hospitalization was not evaluated.
5. Conclusion
The endothelium is a critical target in patients with COVID-19, implicating not only in the cell infection by SARS-CoV-2 but also in the disease course. Ιn the acute phase of COVID-19, there is significant impairment of the endothelial function proportional to the disease severity and in parallel with circulating inflammatory cytokines. Myocardial injury during the course of COVID-19 is also associated with impaired endothelial function. There is a considerable progressive improvement of endothelial function, following acute SARS-CoV-2 infection, during a 6-month follow-up period but subjects in the post-COVID-19 phase continue to have impaired FMD compared to subjects without a history of COVID-19. These findings emphasize the role of disseminated endotheliitis in COVID-19 and give insights into the mechanisms of cardiac complications following infection with SARS-CoV-2. Whether chronic dysregulation of endothelial function following COVID-19 could be accompanied by a residual risk for cardiovascular and thrombotic events merits further research.
The following are the supplementary data related to this article.
Flowchart of patient recruitment with inclusion and exclusion criteria.
Kaplan-Meier plot for the in-hospital survival of the study population categorized according to median flow-mediated dilation (FMD). No differences in survival curves of coronavirus disease-19 hospitalized patients with low and high FMD values as they have been categorized according to median FMD.
Area under receiver operating characteristic curve for flow-mediated dilation regarding in-hospital mortality. Modest sensitivity and low specificity of flow-mediated dilation (FMD) to discriminate coronavirus disease-19 patients at high risk of death.
Follow-up flow-mediated dilation (FMD) evaluation of coronavirus disease-19 survivors according to sex. There was no difference in the trends of FMD improvement in male and female coronavirus disease-19 subjects in the 1- and 6-month follow-up period.
Follow-up flow-mediated dilation (FMD) evaluation of coronavirus disease-19 survivors according to post-hospitalization vaccination status. There was no difference in the trends of FMD improvement in vaccinated and unvaccinated coronavirus disease-19 subjects in the 1- and 6-month follow-up period.
Characteristics of patients hospitalized with COVID-19 and an age- and sex-matched cohort.
Changes in biomarkers of inflammation, tissue damage, thrombosis, and myocardial injury from the acute phase to the 1- and 6-month follow-up period.
Differences in clinical characteristics, laboratory tests, and endothelial function between female and male patients hospitalized with COVID-19.
Regression analysis on the impact of COVID-19 severity on flow-mediated dilation after adjustment for confounders.
Characteristics of patients followed-up after COVID-19 and an age- and sex-matched cohort.
Biomarker profile and endothelial function of patients followed up after hospitalization for COVID-19, stratified according to median FMD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Evangelos Oikonomou: Substantial contributed to the conception and design of the work, to the analysis, or the interpretation of the data and drafted the work; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gerasimos Siasos, Manolis Vavuranakis, Nektarios Souvaliotis, Stamatios Lampsas, Costas Tsioufis and Dimitris Tousoulis: Substantially contributed to the interpretation of data for the work, revised the manuscript for important intellectual, gave final approval of the version to be published and agreed to be accountable for all aspects of the work content.
Garyphallia Poulakou, Panagiotis Thofilis, Theodore G Papaioannou, Anna-Bettina Haidich, Georgia Tsaousi, Ntousopoulos Vasileios, Sakka Vissaria, Georgios Charalambous, Vasiliki Rapti, Asimina Sylvia Raftopoulou and Syrigos Konstantinos: Substantial contributed to design of the work; the acquisition, and interpretation of data for the work; Drafted the work and revised critically the manuscript, gave final approval of the version to be published; And agreed to to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
None.
Acknowledgements
None.
References
- 1.Lazaros G., Oikonomou E., Theofilis P., et al. The impact of COVID-19 pandemic on adult cardiac surgery procedures. Hell. J. Cardiol. 2021;62:231–233. doi: 10.1016/j.hjc.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oikonomou E., Aznaouridis K., Barbetseas J., et al. Hospital attendance and admission trends for cardiac diseases during the COVID-19 outbreak and lockdown in Greece. Public Health. 2020;187:115–119. doi: 10.1016/j.puhe.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassilikos V.P., Pagourelias E.D., Katsos K., et al. Impact of social containment measures on cardiovascular admissions and sudden cardiac death rates during coronavirus disease (COVID-19) outbreak in Greece. Hell. J. Cardiol. 2021;62:318–319. doi: 10.1016/j.hjc.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panagiotakos D., Tsiampalis T. Excess mortality in Greece during 2020: the role of COVID-19 and cardiovascular disease. Hell. J. Cardiol. 2021;62:378–380. doi: 10.1016/j.hjc.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenko E., Badimon L., Bugiardini R., et al. Cardiovascular disease and COVID-19: a consensus paper from the ESC Working Group on coronary pathophysiology & microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA) Cardiovasc. Res. 2021;117:2705–2729. doi: 10.1093/cvr/cvab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsioufis C., Dimitriadis K., Tousoulis D. The interplay of hypertension, ACE-2 and SARS-CoV-2: emerging data as the “Ariadne’s thread” for the “labyrinth” of COVID-19. Hell. J. Cardiol. 2020;61:31–33. doi: 10.1016/j.hjc.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia de Guadiana-Romualdo L., Calvo Nieves M.D., Rodriguez Mulero M.D., et al. MR-proADM as marker of endotheliitis predicts COVID-19 severity. Eur. J. Clin. Investig. 2021;51 doi: 10.1111/eci.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaventura A., Vecchie A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theofilis P., Sagris M., Oikonomou E., et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans P.C., Rainger G.E., Mason J.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmukh V., Motwani R., Kumar A., Kumari C., Raza K. Histopathological observations in COVID-19: a systematic review. J. Clin. Pathol. 2021;74:76–83. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez-Bonilla W.O., Orozco R., Argueta V., et al. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020;105:74–83. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Z., Liu F., Blair R., et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021;11:8076–8091. doi: 10.7150/thno.61810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siasos G., Zografos T., Oikonomou E., Papavassiliou A.G., Stefanadis C., Tousoulis D. Flow-mediated dilation: is it just a research tool or a useful biomarker for cardiovascular prognosis. Int. J. Cardiol. 2015;180:154–157. doi: 10.1016/j.ijcard.2014.11.209. [DOI] [PubMed] [Google Scholar]
- 16.Thijssen D.H.J., Bruno R.M., van Mil A., et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019;40:2534–2547. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Arora R.C., Hiebert B.M., et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging. 2014;15:736–746. doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 18.COVID-19 Therapeutic Trial Synopsis 2020. https://cdn.who.int/media/docs/default-source/blue-print/covid-19-therapeutic-trial-synopsis.pdf?sfvrsn=44b83344_1&download=true [cited 2022 January 2]. Available from:
- 19.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 20.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tousoulis D., Antoniades C., Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart. 2005;91:553–558. doi: 10.1136/hrt.2003.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianconi V., Mannarino M.R., Figorilli F., et al. Low brachial artery flow-mediated dilation predicts worse prognosis in hospitalized patients with COVID-19. J. Clin. Med. 2021;10:5456. doi: 10.3390/jcm10225456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampsas S., Tsaplaris P., Pantelidis P., et al. The role of endothelial related circulating biomarkers in COVID-19. A systematic review and meta-analysis. Curr. Med. Chem. 2021 doi: 10.2174/0929867328666211026124033. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira M.R., Back G.D., da Luz Goulart C., Domingos B.C., Arena R., Borghi-Silva A. Endothelial function provides early prognostic information in patients with COVID-19: a cohort study. Respir. Med. 2021;185:106469. doi: 10.1016/j.rmed.2021.106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heubel A.D., Viana A.A., Linares S.N., et al. Determinants of endothelial dysfunction in non-critically ill hospitalized COVID-19 patients: a cross-sectional study. Obesity (Silver Spring) 2021;30:165–171. doi: 10.1002/oby.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsoularis I., Fonseca-Rodriguez O., Farrington P., Lindmark K., Fors Connolly A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398:599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santa Cruz A., Mendes-Frias A., Oliveira A.I., et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker P.M., Libby P., MacFadyen J.G., et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) Eur. Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310. [DOI] [PubMed] [Google Scholar]
- 29.Esteve E., Castro A., López-Bermejo A., Vendrell J., Ricart W., Fernández-Real J.-M. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 30.Bacchiega B.C., Bacchiega A.B., Usnayo M.J.G., Bedirian R., Singh G., Pinheiro GdRC. Interleukin 6 inhibition and coronary artery disease in a high-risk population: a prospective community-based clinical study. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vela D., Vela-Gaxha Z., Rexhepi M., Olloni R., Hyseni V., Nallbani R. Efficacy and safety of tocilizumab versus standard care/placebo in patients with COVID-19; a systematic review and meta-analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 2021 doi: 10.1111/bcp.15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Investigators R.-C., Gordon A.C., Mouncey P.R., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miwa K., Igawa A., Inoue H. Soluble E-selectin, ICAM-1 and VCAM-1 levels in systemic and coronary circulation in patients with variant angina. Cardiovasc. Res. 1997;36:37–44. doi: 10.1016/s0008-6363(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 34.Muller W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 35.Spadaro S., Fogagnolo A., Campo G., et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care. 2021;25:74. doi: 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrosino P., Calcaterra I., Molino A., et al. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines. 2021;9 doi: 10.3390/biomedicines9080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrosino P., Molino A., Calcaterra I., et al. Clinical assessment of endothelial function in convalescent COVID-19 patients undergoing multidisciplinary pulmonary rehabilitation. Biomedicines. 2021;9 doi: 10.3390/biomedicines9060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambadiari V., Mitrakou A., Kountouri A., et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021;23:1916–1926. doi: 10.1002/ejhf.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jud P., Gressenberger P., Muster V., et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection-a cross-sectional study. Front. Cardiovasc. Med. 2021;8:750887. doi: 10.3389/fcvm.2021.750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riou M., Oulehri W., Momas C., et al. Reduced flow-mediated dilatation is not related to COVID-19 severity three months after hospitalization for SARS-CoV-2 infection. J. Clin. Med. 2021;10 doi: 10.3390/jcm10061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of patient recruitment with inclusion and exclusion criteria.
Kaplan-Meier plot for the in-hospital survival of the study population categorized according to median flow-mediated dilation (FMD). No differences in survival curves of coronavirus disease-19 hospitalized patients with low and high FMD values as they have been categorized according to median FMD.
Area under receiver operating characteristic curve for flow-mediated dilation regarding in-hospital mortality. Modest sensitivity and low specificity of flow-mediated dilation (FMD) to discriminate coronavirus disease-19 patients at high risk of death.
Follow-up flow-mediated dilation (FMD) evaluation of coronavirus disease-19 survivors according to sex. There was no difference in the trends of FMD improvement in male and female coronavirus disease-19 subjects in the 1- and 6-month follow-up period.
Follow-up flow-mediated dilation (FMD) evaluation of coronavirus disease-19 survivors according to post-hospitalization vaccination status. There was no difference in the trends of FMD improvement in vaccinated and unvaccinated coronavirus disease-19 subjects in the 1- and 6-month follow-up period.
Characteristics of patients hospitalized with COVID-19 and an age- and sex-matched cohort.
Changes in biomarkers of inflammation, tissue damage, thrombosis, and myocardial injury from the acute phase to the 1- and 6-month follow-up period.
Differences in clinical characteristics, laboratory tests, and endothelial function between female and male patients hospitalized with COVID-19.
Regression analysis on the impact of COVID-19 severity on flow-mediated dilation after adjustment for confounders.
Characteristics of patients followed-up after COVID-19 and an age- and sex-matched cohort.
Biomarker profile and endothelial function of patients followed up after hospitalization for COVID-19, stratified according to median FMD.