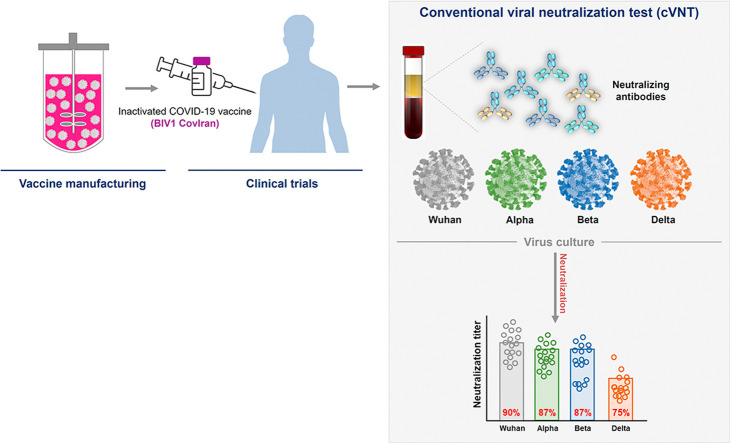
Abstract
Objectives
The BIV1-CovIran vaccine is highly effective against COVID-19. The neutralizing potency of all SARS-CoV-2 vaccines seems to be decreased against variants of concern. We assessed the sensitivity of the Alpha (B.1.1.7), Beta (B.1.351), and Delta (B.1.617.2) variants to neutralizing antibodies (NAbs) present in sera from individuals who had received the BIV1-CovIran candidate vaccine compared with an original Wuhan-related strain.
Methods
The ability of vaccine serum to neutralize the variants was measured using the conventional virus neutralization test. The correlation of spike (S) protein antibody and anti-receptor binding domain with neutralizing activity was investigated.
Results
The current study demonstrated that 29 of 32 (90.6%; 95% CI: 75.0–98.0) of the vaccinees developed NAbs against a Wuhan-related strain. It is noteworthy that 28 (87.50%) and 24 of 32 (75%) of the recipients were able to produce NAbs against Alpha, Beta, and Delta variants, respectively. Serum virus-neutralizing titres for different SARS-CoV-2 strains were weakly correlated with anti–receptor binding domain antibodies (Spearman r = 36-42, p < 0.05), but not S-binding antibodies (p > 0.05).
Discussion
Although there was a reduction in neutralization titres against the Alpha, Beta, and Delta variants compared with the Wuhan strain, BIV1-CovIran still exhibited potent neutralizing activity against the SARS-CoV-2 variants of concern.
Keywords: BIV1-CovIran vaccine, COVID-19 vaccine, SARS-CoV-2 variants, Virus neutralization test
Graphical abstract
Introduction
SARS-CoV-2 continues to mutate with the passing of time, leading to variants of concern (VOCs) in the circulating viral strains during the ongoing COVID-19 pandemic [1,2]. The emergence of VOCs has posed an increased risk of affecting global epidemiology owing to their high transmissibility, as they become the most dominant strain in affected countries. VOCs can lead to waves of infection and potentially escape vaccine-induced immune responses due to their immune evasion abilities.
The most important emerging VOCs isolated from different parts of the world are Alpha (variant 20I/501Y.V1, lineage B.1.1.7) in the United Kingdom (September 2020), Beta (variant 20H/501Y.V2, lineage B.1.351) in South Africa (May 2020), Gamma (variant B.1.1.248, lineage P.1) in Brazil (November 2020), and Delta VOC (variant B.1.617.2, lineage AY) in India (October 2020) [3,4]. Omicron (B.1.1.529), the fifth of these VOCs, is characterized by 37 amino acid substitutions in the S protein, 15 occurring in the receptor-binding domain, which made Omicron able to evade neutralizing antibody (NAb) stimulated via past infection or vaccination [5,6]. Omicron replicated more rapidly in the upper respiratory system than all other SARS-CoV-2 variants but displayed less efficient replication and fusion activity in the lower respiratory system [7]. It is unclear whether Omicron's high rate of transmissibility is due to intrinsic virological characteristic or immune evasion [8].
If the SARS-CoV-2 pandemic is not effectively controlled, the emerging VOCs, which diverge sharply from their ancestral strain counterparts, can challenge global health system [9]. Due to the emergence of antigenic distance variants, resistance to NAbs provoked by vaccination is the main concern [10]. The current evidence shows that the level of protection against each SARS-CoV-2 variant may be different [11]. In COVID-19, an indispensable component of acquired immunity against this virus is the induction of NAbs that attach to the SARS-CoV-2 spike (S) protein and prevent the virus from attaching to cell receptors [12]. NAbs mostly inhibit the binding of the virus ligand to cellular receptors, although in some cases, they may block conformational changes essential for fusion of the virus with the host cell membrane or proteolytic cleavage [13]. NAbs evaluations are valuable to determine vaccine potency against VOCs and to predict breakthrough infections [14].
As an inactivated vaccine, the BIV1-CovIran vaccine (Shifa Pharmed Industrial Group, Tehran, Iran) induced high levels of NAbs against the Wuhan strain of SARS-CoV-2 in preclinical studies and phase I, II, and III clinical trials [15]. However, it remains unclear whether formulated inactivated–SARS-CoV-2 vaccines developed against the original Wuhan strain of the virus will still be effective against VOCs. To clarify this, we examined and reported the neutralizing activity of sera from BIV1-CovIran vaccinees against the newly emerged variants of SARS-CoV-2.
Methods
In this study, we assessed the effect of the original strain of Wuhan SARS-CoV-2 and VOCs such as Alpha, Beta, and Delta on humoral immunity elicited by the inactivated vaccine. To this end, we studied one cohort of individuals vaccinated with two doses of the CovIran vaccine. Subsequently, we examined the neutralizing activity of vaccine sera. The sera of individuals vaccinated with the CovIran vaccine were collected 4–6 weeks after the second injection. Afterwards, the conventional virus neutralization test (cVNT) was separately conducted by preincubating the vaccine sera with different types of SARS-CoV-2 on the culture system of Vero cells. In the next step, we compared the Wuhan strain–neutralizing titres of each vaccinee to titres for each of the VOCs. Receptor binding domain (RBD)- and S-binding antibody levels in adults who had received two doses of the CovIran vaccine were measured via ELISA and compared to that of the placebo cohort.
Study population and serum specimen
Serum samples were obtained from 32 fully vaccinated adult individuals who participated in the first and second phases of the BIV1-CovIran candidate vaccine clinical trials. The studies were approved by the National Research Ethics Committee under reference codes IR.NREC.1399.003, IR.NREC.1399.007, and IR.NREC.1399.008. The sera samples were taken 4–6 weeks after the injection of the second dose (5 μg inactivated vaccine).
SARS-CoV-2 strains
The clinical isolates of SARS-CoV-2, including Alpha (lineage B.1.1.7), Beta (lineage B.1.351), Delta (lineage B.1.617.2), and the original Wuhan strain, were isolated from nasopharyngeal swab samples, determined using the SARS-CoV-2 RT-PCR test (GA SARS-CoV-2 OneStep RT-PCR Kit), and confirmed via sequencing. Virus isolation and initial passage were carried out in Vero cells. SARS-CoV-2 was inoculated and titrated on Vero cells. Viral infectivity was calculated as tissue culture infectious dose 50% (TCID50) via the Karber method. All procedures with viruses were conducted in a biosafety level 3 facility (BSL-3), and the approved standard operating procedures were followed when working with live SARS-CoV-2.
cVNT
A cVNT was conducted to verify the presence of NAbs in sera. Sera from vaccinees were assessed against SARS-CoV-2 variants as described earlier [16]. Briefly, sera samples were incubated at 56°C for 30 minutes to inhibit the complement activity. Next, each serum sample was diluted two-fold, serially. Each dilution was exposed to an equal amount of 100 TCID50 of SARS-CoV-2. After 90 minutes incubating at 37°C with 5% CO, the mixture was added into monolayer Vero cells for 1 hour at 37°C. The inoculum was removed at 1 hour post-infection, and infected cells were then replenished with fetal bovine serum (FBS)-free DMEM supplemented with 1% penicillin-streptomycin for 72 hours. Finally, the virus cytopathic effect on the cell monolayer was observed. Neutralization titre was presented as the highest serum dilution that inhibited any virus cytopathic effect in triplicate wells.
ELISA
Venous blood was taken through venipuncture within evacuated blood tubes with gel and clot activator. Sera were separated via centrifugation at 2000 × g for 10 minutes at room temperature and divided into two similar aliquots of roughly 1.5 mL, which were preserved at –70°C until all measurements were carried out. The measure of anti-spike IgG and anti-RBD IgG was carried out according to the manufacturer's instructions (Pishtaz Teb Zaman Diagnostics, Tehran, Iran).
Statistical analysis
Statistical analysis of results was performed by using GraphPad Prism software, version 9.0. For cVNT experiments, a Friedman test with Dunn's multiple comparison was conducted between each of the SARS-CoV-2 strains. A linear regression was applied to assess whether there was a statistically significant relationship between age and neutralization activity. To compare neutralization activities in 4-week and 6-week vaccine groups, a multiple Mann-Whitney test was performed between the samples obtained from each of the groups for each strain. Comparison of NAb activity against the Wuhan strain and the VOCs between individuals aged >50 and < 50 years was conducted via Mann-Whitney test. To determine the correlation between neutralizing capacity against Alpha (g), Beta (h), and Delta (i) variants and the wildtype Wuhan strain, the Spearman test was used. A comparison of anti-RBD and anti-S antibodies levels in vaccinated and nonvaccinated cohorts was conducted via Mann-Whitney test. Significant differences between 4-week and 6-week RBD- and S-binding activities were assessed via a Wilcoxon test. Spearman correlation coefficients were carried out to evaluate correlation between cVNT and ELISA tests.
Results
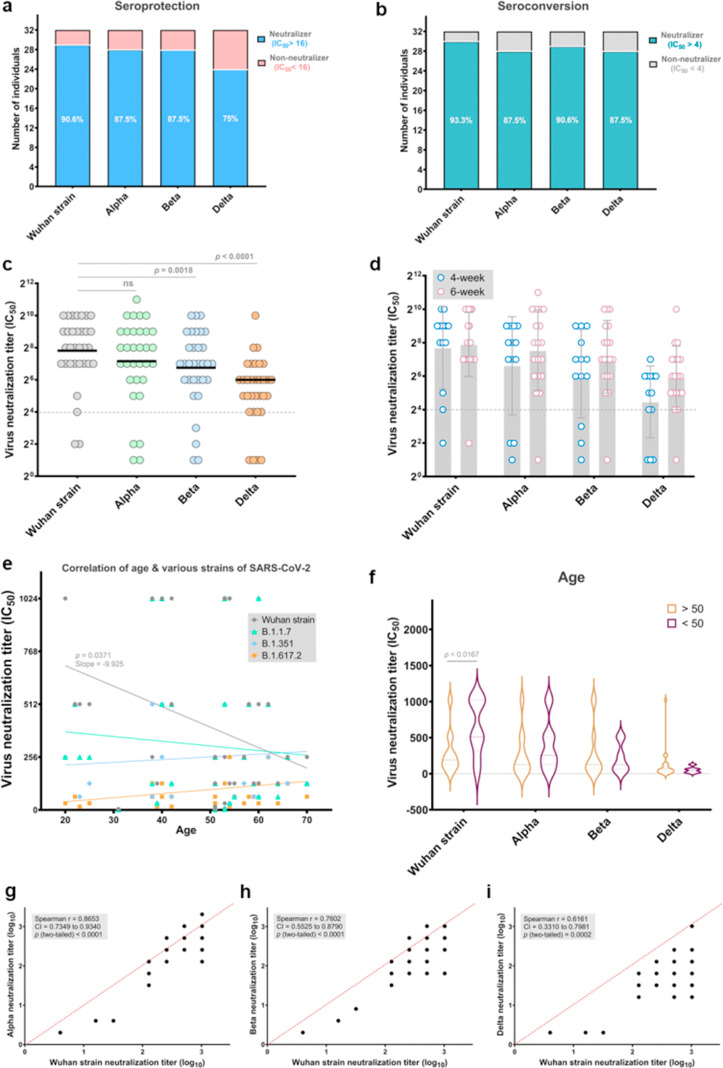
We detected remarkable neutralizing activity against the Wuhan strain of SARS-CoV-2 in 29 of 32 (90.6%; 95% CI: 75.0–98.0) vaccinees (half-maximal neutralization titre, IC50 > 16 as a detection cut-off). Eight of 32 vaccinated persons (25%) in the present study lost all neutralizing activity against the Delta variant, and 12.5% (4 of 32) were unable to neutralize Alpha and Beta variants at detectable levels (Fig. 1 (a)). Of the vaccines, 12.5% (4/32) had no detectable neutralizing activity (IC50, 16 after vaccination (Fig. 1(b)). Geometrical mean titres (GMTs) for Beta and Delta variants were reduced 2- and 5.6-fold relative to the Wuhan strain (p < 0.0018 and p < 0.0001, respectively), whereas neutralizing activity against the Alpha variant was not considerably changed in sera (Fig. 1(c)). In serum samples from participants who had received a second dose of CovIran 28 or 42 days earlier, we found no significant difference in neutralizing activity. This indicated that there is no change in neutralizing capacity against SARS-CoV-2 at 4 to 6 weeks after vaccination (Fig. 1(d)).
Fig. 1.
Neutralization of wild-type Wuhan strain and variants of concern (VOCs) Alpha, Beta, and Delta by vaccine sera. (a) Percentages of vaccines that have seroprotective neutralization titres (neutralizer, IC50 >16) and non-neutralizers (IC50 < 16) are indicated in light blue and coral, respectively. Neutralization titres of sera against the indicated Wuhan strain and VOCs are expressed as IC50 values. The numbers indicate the percentage of neutralizers. (b) Percentages of vaccinated individuals who have no detectable serum neutralizing titres (IC50 < 4) and seroconservative titres (IC50 > 4) against the indicated wild-type Wuhan strain and VOCs. Individuals with an IC50 of neutralization above 4 were considered neutralizers and are showed in teal. Non-neutralizers are in grey. (c) IC50 titres of sera from vaccinated individuals (n = 32) against Wuhan strain and Alpha, Beta, and Delta variants. Each dot represents the IC50 for a single donor. The black lines show geometric mean titres (GMTs). The threshold for neutralizing activity was set at an IC50 of 16 (dashed line). A two-sided Friedman test with Dunn's multiple comparison was conducted between each of the SARS-CoV-2 strains. (d) Neutralizing titre of sera from vaccinees, sampled at week 4 (n = 13) and week 6 after vaccination (n = 19) with CovIran. A multiple Mann-Whitney test was performed between samples from week 4 and week 6 after vaccination; p values were not significant. (e) Correlation of participant age with serum neutralizing antibody titre against the Wuhan strain and VOCs. (f) Comparison of neutralizing antibody activity against the Wuhan strain and VOCs between two age groups (individuals >50 years and <50 years of age). Analysis for comparison of neutralizing activity of age groups was performed via Mann-Whitney test. (g–i) Correlation of neutralizing capacity against Alpha (g), Beta (h), and Delta (i) variants with that of wild-type Wuhan strain. Spearman r was used to determine correlations. Values from statistical analysis are depicted in the grey box.
The reduction in neutralizing capacity against the SARS-CoV-2 Wuhan strain was greater for older than younger adults, whereas no correlation was found between age and GMTs for VOCs after vaccination (Fig. 1(e) and (f)). The Wuhan strain GMT for older persons with age >50 years was reduced 2.4-fold relative to those with age <50 years (p < 0.0167).
We found that Wuhan strain neutralizing titres were highly correlated with the Alpha variant at the individual level (Fig. 1(g)). In contrast, the correlation of the neutralizing titres for the Wuhan strain was weak with Beta (Fig. 1(h)) and Delta variants (Fig. 1(i)).
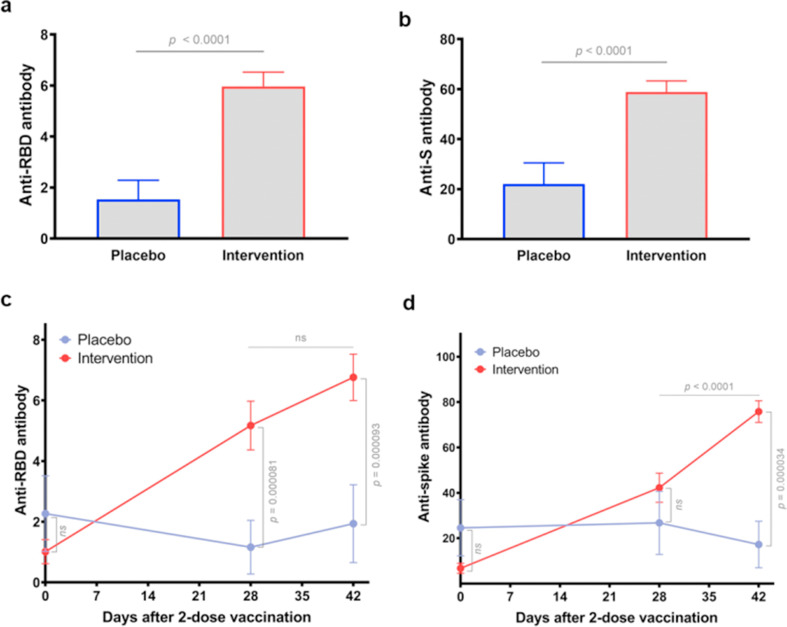
Subsequently, RBD- and S-binding antibody levels in vaccinees were measured through ELISA. Compared to that of the placebo cohort, antibody concentrations in vaccinated individual sera were 2.7-fold higher (p < 0.0001) for RBD (Fig. 2 (a)) and 3.9-fold higher (p < 0.0001) for S (Fig. 2(b)). Moreover, we assessed RBD- and S-binding antibody concentrations on day 0 (pre-dose) and day 28 and day 42 (4 and 6 weeks after the second dose, respectively). In total, there was a considerable increase in RBD- and S-binding antibody levels at 28 and 42 days following the second vaccination (Fig. 2(c) and (d)). Additionally, post-vaccination S-binding antibody levels at day 42 were notably higher than the antibody levels induced by the vaccine at day 28 (p < 0.0001) (Fig. 2(d)). However, there was no further remarkable change in RBD-binding antibody levels between day 28 and 42 (p = 0.1010) (Fig. 2(c)).
Fig. 2.
Levels of receptor binding domain (RBD)- and S-binding antibodies in vaccinees. (a) Comparison of anti-RBD antibodies titres in vaccinees after the second dose of the CovIran vaccine (intervention, n = 36) and nonvaccinated persons (placebo, n = 12). (b) Comparison of S-binding antibodies levels in the total vaccinees (intervention) and nonvaccinated persons (placebo). A Mann-Whitney test was implemented for comparisons. (c) Changes in RBD-binding antibody levels from 4 weeks (day 28) to 6 weeks (day 42) after COVID-19 vaccination. (d) Post-vaccination changes in S-binding antibody levels. Statistical analysis was carried out via a paired, nonparametric Wilcoxon test.
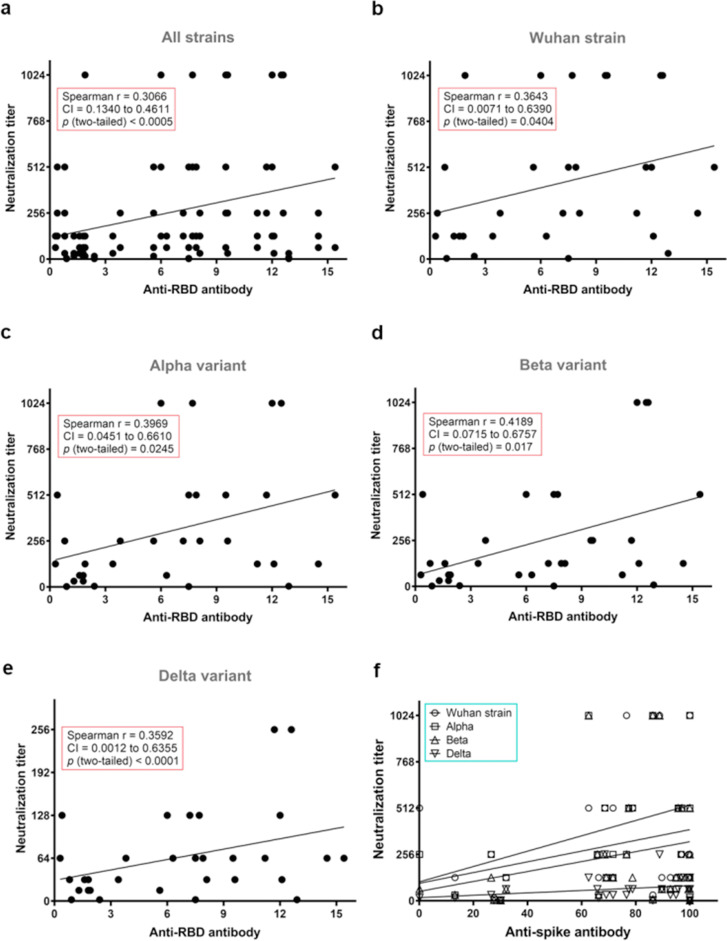
A positive mild correlation between RBD-antibody binding concentrations and virus neutralization titres was observed in this study (Fig. 3 (a)). Moreover, anti-RBD antibodies positively correlated with neutralization titres in each strain in our study (Fig. 3(b–e)). This indicated that the concentration of variant-specific RBD-binding antibody is commensurate with live virus neutralization activity against each strain. It is important to note that high antibody levels could also be observed in the absence of neutralization in some participants. However, no significant correlation was observed between the levels of anti-S antibody and virus neutralization titres for all strains (Fig. 3(f)).
Fig. 3.
Correlation of serological parameters with serum IC50 titres against Wuhan strain and variants of concern (VOCs) Alpha, Beta, and Delta. (a–e) Nonparametric Spearman correlation of receptor binding domain (RBD)-binding antibody levels with IC50 titres from neutralizing sera collected from vaccine recipients (n = 32) against SARS-CoV-2 (a), Wuhan strain (b), Alpha (c), Beta (d), and Delta (e). Spearman r and p values are indicated in the rectangular box in each graph. (f) Correlation of S-binding antibody levels with IC50 titres from neutralizing sera collected from vaccine recipients against each of the SARS-CoV-2 strains.
Discussion
In this study, humoral response cross-reactivity to the original SARS-CoV-2 and new emerging variants was evaluated in sera collected from BIV1-CovIran vaccinees. We report that the neutralizing activity of immune sera against B.1.1.7 and B.1.351 is largely preserved, but it clearly decreased against B.1.617.2 in comparison to the wild type. The delta variant is potentially more challenging because it is less sensitive to sera collected from vaccinees [16]. The lower correlation between neutralizing titre values of the original strain with that of the Delta variant likely demonstrates that some of the epitopes detected via Wuhan strain NAbs are altered in the Delta variant. Our findings for B.1.1.7 and B.1.351 parallel the results of other inactivated vaccines. Wang et al. reported that B.1.1.7 had slight resistance to the neutralizing activity of BBIBP-CorV or CoronaVac vaccine sera, whereas B.1.351 displayed more resistance to the neutralization of vaccine sera (by a factor of 2.5 to 3.3) than the wild-type strain [17]. In line with the current study, the NAb after BNT162b2 vaccination was reduced 5.8-fold against B.1.617.2 compared to wild-type, notably less than against B.1.1.7 (2.6-fold vs. wild-type) and similar to the decrease detected against B.1.351 (4.9-fold vs. wild-type) [18].
In the current study, aging was influential in decreasing both NAbs and anti-S-RBD antibodies responses against the wild-type Wuhan strain. Individuals aged >50 years showed significantly reduced levels of antibody against the Wuhan strain, whereas in the VOCs this was not observed. In line with our study, the seroconversion rates of Sinopharm/BBIBP-CorV vaccinated individuals were significantly lower in persons >60 years of age in comparison with those who were 20 to 39 years of age [19]. In the parallel study, age had a statistically substantial impact on both NAbs and anti-S-RBD antibodies. It has become apparent that younger individuals (<50 years) raised higher titres after day 36, and their change rate was strikingly lower than in older individuals (≥50 years) [20]. This finding is in accordance with the influence of age on antibody levels after vaccination with Spikevax (Moderna, Cambridge, MA) [21].
There was an affirmative correlation between RBD-antibody binding levels and virus neutralization titres. Furthermore, anti-RBD antibodies were associated with neutralization titres in each strain in this study. This suggested that the concentration of variant-specific RBD-binding antibodies is related to the neutralization activity of live virus against each strain. In 2020, Salazar et al., concluded that anti-RBD titres could act as a surrogate for virus neutralization titres to identify appropriate plasma donors, although there was no significant correlation between anti-S antibody concentrations and virus neutralization titres for all strains [22]. In contrast to this study, Dolscheid-Pommerich et al., reported that high and low anti-S1 IgG levels were correlated with a positive predictive rate of 72.0% for high-titre NAbs and a negative predictive rate of 90.8% for low-levels NAbs, subsequently [23].
This study has several potential limitations. A relatively small number of individuals in a short time frame after immunization have been evaluated. Moreover, we have not studied the impact of cellular immune response, which may provide broader protection against emerging VOCs than the humoral response, although the inactivated vaccine generally skews the immune response toward a Th2-dominant response [24]. Future investigation is required to adopt new vaccination approaches, such as administering more booster doses, optimal mix and match strategies, and a new formulation based on intranasal administration to stop virus shedding, and to move forward to develop a multivalent candidate vaccine to address emerging variants. Rapidly emerging VOCs may overtake the speed of production of safe novel VOC vaccines.
The results of the present study indicate that the BIV1-CovIran inactivated vaccine is potent against the Alpha, Beta, and Delta variants. Studying the status of vaccinated individuals in different age groups, especially the elderly and immune-compromised patients, and developing new vaccines for emerging variants will be the most important goals of our future studies.
Transparency declaration
HRJ and HJ are the chairman and managing director of the Shifa Pharmed Industrial Group, respectively. MM was the principal investigator and MRS and PT were investigators in the clinical trial. HH was responsible for the contract research organization in the clinical trial, and AA and RA were technology developers. All other authors declare no competing interests. This study was supported by Shifa Pharmed Industrial Group.
Author contributions
Conceptualization: AA and HRJ; methodology: AA, HH, and HK; software: ML, SR, and MM; validation: MS, AA, and RA; formal analysis: ML, HJ, HK, and PT; investigation: MM and HJ; writing—original draft preparation: ML, AA, MRS, and HK. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank all participants from whom the post-immunization human sera were obtained. We sincerely appreciate Sepideh Meidaninikjeh, Maryam Shafaati, and Mehdi Razazian for all fruitful comments, which helped us to improve the quality of this manuscript. Finally, we thank all of the staff of the Shifa Pharmed Industrial Group for manufacturing this vaccine.
Editor: L. Kaiser
References
- 1.Rhoads D.D., Plunkett D., Nakitandwe J., Dempsey A., Tu Z.J., Procop G.W., et al. Endemic SARS-CoV-2 polymorphisms can cause a higher diagnostic target failure rate than estimated by aggregate global sequencing data. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00913-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2—what do they mean? JAMA. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 3.Focosi D. SARS-CoV-2 spike protein convergent evolution. Springer; Switzerland: 2021. SARS-CoV-2 variants; pp. 55–71. [Google Scholar]
- 4.Hirabara S.M., Serdan T.D., Gorjao R., Masi L.N., Pithon-Curi T.C., Covas D.T., et al. SARS-COV-2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2022;11:781429. doi: 10.3389/fcimb.2021.781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena S.K., Kumar S., Ansari S., Paweska J.T., Maurya V.K., Tripathi A.K., et al. Characterization of the novel SARS-CoV-2 Omicron (B. 1.1. 529) variant of concern and its global perspective. J Med Virol. 2021;94:1738–1744. doi: 10.1002/jmv.27524. [DOI] [PubMed] [Google Scholar]
- 6.Willett B.J., Grove J., MacLean O., Wilkie C., Logan N., De Lorenzo G., et al. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. MedRxiv. 2022 doi: 10.1101/2022.01.03.21268111. https://www.medrxiv.org/content/10.1101/2022.01.03.21268111v2.full [DOI] [Google Scholar]
- 7.Zhao H., Lu L., Peng Z., Chen L.-L., Meng X., Zhang C., et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with delta variant in TMPRSS2-expressed cells: Omicron variant replication kinetics. Emerg Microbe. Infect. 2022;11:277–283. doi: 10.1080/22221751.2021.2023329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui K.P., Ho J.C., Cheung M.-C., Ng K.-C., Ching R.H., Lai K.-L., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022 doi: 10.1038/s41586-022-04479-6. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Murano K., Guo Y., Siomi H. The emergence of SARS-CoV-2 variants threatens to decrease the efficacy of neutralizing antibodies and vaccines. Biochem Soc Trans. 2021;49:2879–2890. doi: 10.1042/BST20210859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R., Liu J., Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48:102–106. doi: 10.1016/j.jgg.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanne J.H. Covid-19: moderna plans booster doses to counter variants. BMJ. 2021;372:n232. doi: 10.1136/bmj.n232. [DOI] [PubMed] [Google Scholar]
- 12.Xiaojie S., Yu L., Guang Y., Min Q. Neutralizing antibodies targeting SARS-CoV-2 spike protein. Stem Cell Res. 2021;50:102125. doi: 10.1016/j.scr.2020.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann M.F., Mohsen M.O., Zha L., Vogel M., Speiser D.E. SARS-CoV-2 structural features may explain limited neutralizing-antibody responses. NPJ Vaccines. 2021;6:2. doi: 10.1038/s41541-020-00264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdoli A., Aalizadeh R., Aminianfar H., Kianmehr Z., Teimoori A., Azimi E., et al. Safety and potency of BIV1-CovIran inactivated vaccine candidate for SARS-CoV-2: a preclinical study. Rev Med Virol. 2021 doi: 10.1002/rmv.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cevik M., Grubaugh N.D., Iwasaki A., Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G.L., Wang Z.Y., Duan L.J., Meng Q.C., Jiang M.D., Cao J., et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med. 2021;384:2354–2356. doi: 10.1056/NEJMc2103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B. 1.617. 2 and B. 1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeewandara C., Aberathna I.S., Pushpakumara P.D., Kamaladasa A., Guruge D., Jayathilaka D., et al. Antibody and T cell responses to Sinopharm/BBIBP-CorV in naïve and previously infected individuals in Sri Lanka. medRxiv. 2021 doi: 10.1101/2021.07.15.21260621. https://www.medrxiv.org/content/10.1101/2021.07.15.21260621v1 [DOI] [Google Scholar]
- 20.Terpos E., Trougakos I.P., Karalis V., Ntanasis-Stathopoulos I., Gumeni S., Apostolakou F., et al. Kinetics of anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with bnt162b2; a prospective study in 283 health workers. Cells. 2021;10:1942. doi: 10.3390/cells10081942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T.N., Yi X., Zhao P., et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. J Clin Invest. 2020;130(12):6728–6738. doi: 10.1172/JCI141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolscheid-Pommerich R., Bartok E., Renn M., Kümmerer B.M., Schulte B., Schmithausen R.M., et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J Med Virol. 2022;94:388–392. doi: 10.1002/jmv.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsunetsugu-Yokota Y., Ato M., Takahashi Y., Hashimoto S.-I., Kaji T., Kuraoka M., et al. Formalin-treated UV-inactivated SARS coronavirus vaccine retains its immunogenicity and promotes Th2-type immune responses. Jpn J Infect Dis. 2007;60:106–112. [PubMed] [Google Scholar]