Abstract
We aim to assess the long-term impact of acute kidney injury (AKI) on progression of diabetic kidney disease (DKD) and all-cause mortality and investigate determinants of AKI in Chinese patients with type 2 diabetes (T2D). A consecutive cohort of 9,096 Chinese patients with T2D from the Hong Kong Diabetes Register was followed for 12 years (mean ± SD age 57 ± 13.2 years; 46.9% men; median duration of diabetes 5 years). AKI was defined based on the Kidney Disease: Improving Global Outcomes (KDIGO) criteria using serum creatinine. Estimated glomerular filtration rate measurements were used to identify the first episode with chronic kidney disease (CKD) and end-stage renal disease (ESRD). Polygenic risk score (PRS) composed of 27 single nucleotide polymorphisms (SNPs) known to be associated with serum uric acid (SUA) in European populations was used to examine the role of SUA in pathogenesis of AKI, CKD, and ESRD. Validation was sought in an independent cohort including 6,007 patients (age 61.2 ± 10.9 years; 59.5% men; median duration of diabetes 10 years). Patients with AKI had a higher risk for developing incident CKD (hazard ratio 14.3 [95% CI 12.69–16.11]), for developing ESRD (12.1 [10.74–13.62]), and for all-cause death (7.99 [7.31–8.74]) compared with those without AKI. Incidence rate for ESRD among patients with no episodes of AKI and one, two, and three or more episodes of AKI was 7.1, 24.4, 32.4, and 37.3 per 1,000 person-years, respectively. Baseline SUA was a strong independent predictor for AKI. A PRS composed of 27 SUA-related SNPs was associated with AKI and CKD in both discovery and replication cohorts but not ESRD. Elevated SUA may increase the risk of DKD through increasing AKI. The identification of SUA as a modifiable risk factor and PRS as a nonmodifiable risk factor may facilitate the identification of individuals at high risk to prevent AKI and its long-term impact in T2D.
Introduction
Diabetic kidney disease (DKD), especially type 2 diabetes (T2D), is the most common cause of end-stage renal disease (ESRD) and is associated with increased risk of cardiovascular disease and premature death (1,2). In Asia, it is estimated that >60% of patients with diabetes will develop kidney complications, compared with 30–40% in Europeans despite having similar duration of diabetes (3), which impose a substantial economic burden on health care systems (4,5). Given the continuous increase in the number of people with diabetes, it is particularly important to identify those patients at high risk of progression to DKD and develop effective strategies to prevent and treat the disease. However, since DKD is a complex multifactorial disease and the pathogenesis of the disease remains unclear, investigation of the determinants of progression to DKD is a key research priority.
Acute kidney injury (AKI) is a common problem worldwide, especially among hospitalized patients, affecting ∼13–18% of hospitalized patients and up to 40% of critically ill patients (6,7). Numerous studies have highlighted that AKI is a determinant of renal failure events in selected populations (8,9). For example, in a prospective cohort study in Asia, AKI was associated with more than fivefold higher risk of ESRD after cardiac surgery (10). Although it is well documented that diabetes is the most frequent cause of ESRD, the relationship between AKI and long-term renal complications in patients with diabetes has not been well studied. Recently, Monseu et al. (8) found that AKI was a powerful predictor of major adverse cardiovascular events, myocardial infarction, and other adverse outcomes in a cohort of 1,371 patients with T2D. However, there is a paucity of data on the impact of AKI and its risk factors on long-term renal complications in patients with T2D.
Different clinical factors are known to be associated with AKI, including hypovolemia, diuretic use, and CKD (7). Serum uric acid (SUA) has been identified as a potentially modifiable risk factor for AKI (11,12) and has been associated with DKD in T2D (13). In recent genome-wide association studies (GWAS) a number of genetic variants associated with the concentration of SUA have been identified (14–16), highlighting the presence of genetic factors that can modify SUA concentrations.
In this study, we hypothesized that AKI may have important impact on progression to chronic kidney disease (CKD) and ESRD in patients with T2D. We further sought to identify the clinical predictors of AKI and their biological significance. Having identified SUA as an important determinant of AKI in our cohort, we further explored the role of SUA for AKI and DKD by examining the association between a polygenic risk score (PRS) for SUA and risk of AKI, as well as different DKD outcomes, in our prospective cohort of Chinese with T2D. Better understanding of the main predictors of AKI will enable physicians to identify high-risk patients for early intensification and individualization of treatment to prevent AKI, as well as its potential impact on long-term renal complications.
Research Design and Methods
Study Population
In this study we used the data from the Hong Kong Diabetes Register (HKDR), which was established in 1995 at the Prince of Wales Hospital, the teaching hospital of The Chinese University of Hong Kong (17). The register included consecutively enrolled patients with diabetes who were referred to the Diabetes Mellitus and Endocrine Centre for comprehensive assessment of diabetes complications and metabolic control. Referral sources included hospital-based specialty clinics, community clinics, and general practitioners. Once registered, patients were observed to the time of death. Ethics approval was obtained from the Clinical Research Ethics Committees of The Chinese University of Hong Kong. Written informed consent was obtained from all patients at the time of enrollment for collection of clinical information and biosamples for archival and research purposes. The recruitment methods, definitions, and biochemical investigations have previously been described in detail (18,19).
Since the start of the register in 1995 until 31 December 2007, a consecutive cohort consisting of 10,129 patients with diabetes was assessed. After sequentially excluding 61 patients with non-Chinese or unknown ethnicity, 400 with type 1 diabetes or missing data on type of diabetes, 147 with history of ESRD, and 425 with history of AKI (see definition below), we obtained a prospective cohort of 9,096 patients with detailed information on risk factors, complications, drug use, and clinical outcomes for further analysis (Supplementary Fig. 1).
For validation of the genetic association, we used an independent prospective cohort from the Hong Kong Diabetes Biobank (HKDB), where patients were recruited from 11 diabetes centers at major public hospitals across Hong Kong beginning in 2014 (20). Recruitment and assessment methods were similar to those of HKDR. A total of 6,462 unrelated Chinese patients with T2D from HKDB were genotyped for this analysis. After removing 455 patients who overlapped with HKDR, we included 6,007 patients with T2D for this replication study. All patients gave written informed consent for DNA collection and data analysis for research purposes.
Baseline Clinical and Laboratory Measurements
Details of assessment methods and definitions of end points have been described for our previous study (21). In brief, at enrollment and regularly thereafter, patients underwent clinical assessments and laboratory investigations after fasting for 8 h overnight. Aside from documentation of demographic data and clinical assessment of complications, fasting blood was sampled for measurement of plasma glucose, HbA1c, lipid profile (total cholesterol, HDL cholesterol, triglycerides, and calculated LDL cholesterol), and renal function. Random spot urinary sample was used to assess albumin-to-creatinine ratio (ACR). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used for estimated glomerular filtration rate (eGFR) (22). The precision performance of all assays was within the manufacturer’s specifications.
Definition of Clinical Outcomes
Clinical outcomes were defined with use of hospital discharge diagnoses based on the ICD-9 and mortality as censored on or before 30 June 2017. In the Hong Kong Hospital Authority Central Computer System admissions to all public hospitals are recorded; public hospitals provide ∼95% of inpatient bed-days in Hong Kong (23). Details of all medical admissions of the cohort were retrieved from this system with a unique identifier number. In addition, all subsequent outpatient and inpatient blood tests for renal function were retrieved for assessment of longitudinal changes in renal function.
AKI was diagnosed and staged in line with the criteria used in previous studies (24). Briefly, the criteria were derived from the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group guidelines (25). Serum creatinine (SCr) criteria were used to define AKI episode. Baseline creatinine values were determined by a modification of the National Health Service (NHS) England AKI “e-alert” algorithm, in which a hierarchy of criteria was used for changes of creatinine from the previous 48 h and 7, 90, and 365 days. Longitudinal measurements of serum creatinine were almost all exclusively measured in the same chemical pathology laboratory at the Prince of Wales Hospital, which has been accredited by the Royal College of Pathologists of Australasia Quality Assurance Programs. In detail, AKI was defined on the basis of one of three criteria: 1) SCr level ≥1.5 times higher than the median of all SCr values 8–90 days prior, or 91–365 days prior if no tests were done between 8 and 90 days prior; 2) SCr ≥1.5 times higher than the lowest SCr within 7 days; or 3) SCr >26 μmol/l higher than the lowest SCr within 48 h. AKI was further staged according to the ratio of index to baseline SCr values (index/baseline SCr ≥1.5–2.0, ≥2.0–3.0, and ≥3.0 for stages 1, 2, and 3, respectively). Stage 3 was also defined as SCr increase of ≥353.6 μmol/L (≥4.0 mg/dL). A 7-day AKI recovery status was defined according to the ratio of last SCr within 7 days of AKI to baseline SCr at diagnosis: ratio <1.2, ≥1.2–1.5, and ≥1.5 for complete recovery, partial recovery, and nonrecovery. The first episode of AKI was used as clinical end point for Cox regression analyses in this study. Follow-up time was defined as the period from the baseline visit to the date of the first clinical end point or the censored dates—whichever came first. In addition, we also evaluate the number of AKI episodes each individual experienced during the follow-up period. Each individual is categorized as having experienced no episodes of AKI or one, two, or three or more episodes of AKI.
Hospital discharge summaries (ICD-9 codes) were used to identify the first hospitalization with a renal event. CKD was defined as 1) fatal and nonfatal diabetes with renal manifestations (code 250.4), CKD (code 585), or unspecified renal failure (code 586); 2) dialysis (ICD-9 procedure code 39.95) or peritoneal dialysis (ICD-9 procedure code 54.98); or 3) eGFR <60 mL/min/1.73 m2 during follow-up period. ESRD was defined as the presence of a dialysis code (procedure code 39.95 or 54.98), a code of kidney transplant (procedure code 55.6 or diagnosis codes 996.81 or V42.0), or eGFR <15 mL/min/1.73 m2 during the follow-up period.
Due to the short follow-up period of patients from the HKDB, there were few individuals with incident events for each clinical outcome. To increase the sample sizes, we performed a case-control study for each clinical outcome by classifying both those with prevalent and those with incident events as case subjects and those without clinical outcome over at least 10 years’ duration of diabetes as control subjects in the replication cohort of HKDB.
PRS
In a subset of 5,736 patients from the primary cohort with available GWAS data, we developed a PRS using 28 independent genetic variants known to be associated with SUA from previous published GWAS in European populations (14). We then explored the associations between the PRS and clinical outcomes in this subcohort.
We extracted these single nucleotide polymorphisms (SNPs) from imputed genome-wide genotyping data from our cohort using the Illumina 2.5-8 Exome v1.0 BeadChip array (Illumina, San Diego, CA). Standard quality control and imputation of genotyping data have previously been described (26). We selected independent common SNPs (linkage disequilibrium coefficient r2 < 0.5; minor allele frequency >0.01) available in our data set with good genotyping quality (call rate >97%; P > 0.001 in Hardy-Weinberg equilibrium) to construct the final PRS. After excluding one SNP with minor allele frequency <0.01 and poor imputation quality (R2 < 0.5), we obtained 27 independent common SNPs to develop the PRS (Supplementary Table 1).
We developed the weighted PRS by summing the score of reported risk alleles for each SNP based on an additive genetic model, weighted by the effect size of the SUA-related SNP as reported in the literature. The PRS was rescaled to a score to express the SD using the following formula: (individual PRS value – population mean PRS)/population SD of PRS. The final analysis is 1) based on the genetic risk per SD of the standardized PRS and 2) tertile analysis of the PRS for clinical outcomes (tertile 1: low; tertile 2, intermediate; and tertile 3, high). In the replication cohort, we performed genotyping using the Illumina Infinium Global Screening Array and conducted quality control and imputation using criteria similar to those used in the discovery cohort of HKDR. The same SNPs and methods were used to construct the PRS for replication in HKDB. As sensitivity analyses, we repeated analyses with the PRS by removing the top one or top four SNPs most strongly associated with SUA to examine the impact on the association between the PRS and outcomes.
Statistical Analysis
Analyses were performed using R (version 3.1.0) (https://www.R-project.org). We used Cox proportional hazards regression model for longitudinal association analysis. Since AKI and its status of severity and recovery changed over time during the follow-up period, these variables were used as time-dependent covariates in the Cox model to examine their effects on different clinical outcomes (27). A procedure of backward stepwise model selection was used to determine the predictors for incident AKI. Linear regression was used to examine the association between PRS and SUA. Association between PRS and clinical outcomes was examined, with adjustment of clinical risk factors, with or without consideration of SUA. Individuals with missing values were excluded in regression analysis. As sensitivity analyses, we also performed a Mendelian randomization (MR)-Egger intercept test to compare the strength of association of each SNP with SUA with the strength of its association with AKI (28). The MR-Egger method permits a pleiotropic effect of one or more genetic variants, as long as the size of such pleiotropic effects is independent of the size of the effect of the genetic variant on the risk factor (29). The intercept can be viewed as an estimate of the (horizontal) average pleiotropic effect of genetic variants (28). All data are expressed as percentages, means and SDs, or medians and interquartile ranges (IQRs) as appropriate. All statistical tests were two sided, and P value <0.05 was considered statistically significant.
Data and Resource Availability
Data cannot be shared publicly because of ethics, as individuals did not consent to sharing of individual-level genetic data on a public platform. Data are available for analysis by qualified researchers who write to contact us requesting the data and meet the criteria to access and analyze the data. Readers and colleagues who are interested in obtaining further information about the study can contact the Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, at hkido@cuhk.edu.hk.
Results
Cohort Description
Of 9,096 patients with T2D included in the analysis (mean ± SD age 57 ± 13.2 years; 46.9% men; median duration of diabetes 5 years [IQR 1–10]), 3,209 (35.3%) developed at least one AKI episode during a median follow-up period of 12.3 y ears (IQR 7.7–17) (1,114 [12.2%], 642 [7.1%], and 1,453 [16%] had one, two, and three or more episodes of AKI, respectively). The overall incidence rate of AKI was 29.2 (95% CI 28.2–30.2) per 1,000 person-years. The median number of AKI episodes was 2 (IQR 2–4). With respect to the severity of the first AKI episode, 2,367 (73.8%), 579 (18%), and 263 (8.2%) of 3,209 had AKI stage 1, 2, and 3. Within 7 days, 1,086 (33.8%) patients had complete recovery and 521 (16.2%) and 440 (13.7%) had partial recovery and nonrecovery. The remaining patients (1,162 [36.2%]) had no repeated measurements of SCr within 7 days. Compared with patients without AKI episodes, patients who developed AKI were older; had longer duration of diabetes; had higher HbA1c, urinary ACR, and SUA; had lower eGFR; and were more likely to have multiple comorbidities and to use multiple medications at baseline (Table 1).
Table 1.
Baseline characteristics of T2D patients with or without an AKI event during follow-up period
| AKI event | No AKI events | P | |
|---|---|---|---|
| N | 3,209 | 5,887 | |
| Age (years) | 61.4 ± 12.5 | 54.7 ± 12.9 | <0.001 |
| Male sex | 45.4 (1,457) | 47.6 (2,805) | 0.04 |
| Diabetes duration (years) | 7 (2–12) | 4 (1–10) | <0.001 |
| Smoking status | |||
| Former | 19.2 (615) | 14.6 (858) | <0.001 |
| Current | 12.7 (407) | 13.1 (769) | <0.001 |
| BMI (kg/m2) | 25.4 ± 4.1 | 25.1 ± 4.0 | 0.002 |
| HbA1c (%) | 7.9 ± 1.8 | 7.5 ± 1.7 | <0.001 |
| HbA1c (mmol/mol) | 63 ± 20.2 | 58 ± 18.9 | <0.001 |
| Triglyceride (mmol/L) | 1.5 (1–2.2) | 1.4 (1–2) | <0.001 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.766 |
| LDL cholesterol (mmol/L) | 3.1 ± 1 | 3.1 ± 1 | 0.869 |
| Systolic BP (mmHg) | 138.7 ± 20.7 | 132.4 ± 20 | <0.001 |
| Diastolic BP (mmHg) | 75.3 ± 11.4 | 75.6 ± 10.7 | 0.161 |
| ACR (mg/mmol) | 3.6 (1.1–18.1) | 1.5 (0.7–6.3) | <0.001 |
| eGFR (mL/min/1.73 m2) | 76.8 (59.2–93) | 88.4 (70.6–102) | <0.001 |
| SUA (mmol/L) | 0.4 ± 0.1 | 0.36 ± 0.1 | <0.001 |
| Sensory neuropathy | 27 (865) | 18.5 (1,091) | <0.001 |
| Retinopathy | 33.3 (1,070) | 22 (1,293) | <0.001 |
| Diagnosed comorbidity | |||
| CKD | 26.1 (839) | 15.3 (903) | <0.001 |
| CHF | 2.7 (88) | 1.3 (76) | <0.001 |
| Coronary heart disease | 10.2 (328) | 5.7 (338) | <0.001 |
| Stroke | 3.9 (126) | 2 (119) | <0.001 |
| Peripheral vascular disease | 7 (226) | 4.8 (280) | <0.001 |
| Baseline drug treatment | |||
| Lipid-lowering drugs | 20.4 (656) | 16.4 (965) | <0.001 |
| Antihypertensive drugs | 56.9 (1,827) | 39.3 (2,313) | <0.001 |
| RAS inhibitors (ACEIs or ARBs) | 28.4 (912) | 18.1 (1,064) | <0.001 |
| Oral antihyperglycemic drugs | 70.1 (2,251) | 65.2 (3,838) | <0.001 |
| Insulin | 21.1 (677) | 12.4 (732) | <0.001 |
Data are means ± SD, percentage (number), or median (IQR) unless otherwise indicated. t test or Mann-Whitney rank sum test was used for the continuous variables, and χ2 test was used for the categorical variables. ACEIs, ACE inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure.
In the replication HKDB cohort including 6,007 patients (mean ± SD age 61.2 ± 10.9 years; 59.5% men; median duration of diabetes 10 years [IQR 4–16], SUA 0.38 ± 0.1 mmol/L), 1,233 (20.5%) had experienced at least one AKI episode before date of assessment or during follow-up. Patients with AKI had similar age and HbA1c but higher BMI, ACR, and SUA (Supplementary Table 2).
Associations of AKI With Clinical Outcomes
We examined the associations between AKI and incident CKD, ESRD and all-cause mortality by excluding those prevalent patients for each clinical outcome correspondingly. After adjustment for potential confounding factors including SUA, patients with AKI had a higher risk for developing incident CKD (hazard ratio [HR] 14.3 [95% CI 12.69–16.11]), for developing ESRD (12.1 [10.74–13.62]), and for all-cause death (7.99 [7.31–8.74]) (Table 2). Furthermore, patients who experienced more severe AKI or had poorer recovery of AKI during the first episode had a higher risk of renal complications and all-cause mortality (Table 2). We found that the number of episodes of AKI was also an important factor affecting long-term prognosis. Incidence rates for ESRD among patients with no episodes of AKI and of one, one, and three or more episodes of AKI were 7.1 (95% CI 6.5–7.7), 24.4 (21.9–27.1), 32.4 (28.4–36.7), and 37.3 (34.4–40.4) per 1,000 person-years, respectively. For the CKD end point, the incident rates were 42.2 (40.6–43.9), 132.7 (124.4–141.3), 159.3 (146.7–172.7), and 183.6 (174.1–193.6) per 1,000 person-years.
Table 2.
Associations of AKI (yes/no), the status of AKI severity, and AKI recovery with clinical outcomes
| No. of cases | CKD | ESRD | All-cause death | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| AKI (yes/no) | 3,209 | 14.3 (12.69–16.11) | <0.001 | 12.1 (10.74–13.62) | <0.001 | 7.99 (7.31–8.74) | <0.001 |
| AKI severity | |||||||
| No AKI | 5,887 | Ref. | Ref. | Ref. | |||
| Stage 1 | 2,367 | 10.14 (8.71–11.81) | <0.001 | 7.07 (6.17–8.09) | <0.001 | 6.39 (5.72–7.13) | <0.001 |
| Stage 2 | 579 | 35.04 (26.72–45.96) | <0.001 | 14.47 (11.85–17.68) | <0.001 | 10.57 (9.07–12.31) | <0.001 |
| Stage 3 | 263 | 45.72 (32.21–64.91) | <0.001 | 34.54 (27.3–43.7) | <0.001 | 9.24 (7.48–11.42) | <0.001 |
| AKI recovery | |||||||
| No AKI | 5,887 | Ref. | Ref. | Ref. | |||
| Complete recovery | 1,086 | 17.6 (14.23–21.76) | <0.001 | 4.82 (4.06–5.71) | <0.001 | 5.5 (4.82–6.28) | <0.001 |
| Partial recovery | 521 | 10.01 (7.42–13.51) | <0.001 | 6.16 (4.97–7.63) | <0.001 | 7.46 (6.33–8.79) | <0.001 |
| Nonrecovery | 440 | 21.8 (16.07–29.59) | <0.001 | 18.97 (15.41–23.35) | <0.001 | 11.45 (9.65–13.58) | <0.001 |
AKI, AKI severity, and AKI recovery were used as time-dependent covariates in the Cox models. HRs and P values were adjusted for conventional risk factors at baseline, including age; sex; duration of diabetes; smoking; SUA; BMI; HbA1c; HDL cholesterol; LDL cholesterol; systolic blood pressure; diastolic blood pressure; log-transformed ACR; eGFR; retinopathy; use of lipid-lowering drugs, antihypertensive drugs, RAS inhibitors, and antihyperglycemic drugs; and history of stroke, CHD, or CKD. Ref., referent.
Identification of Clinical Factors Associated With AKI
To identify clinical factors associated with the risk of AKI during follow-up, we conducted multivariable regression analysis for AKI. Using backward stepwise variable selection, we identified SUA as the strongest clinical predictor (HR 3.58 [95% CI 2.39–5.36]) (Table 3) for incident AKI. Other significant independent predictors included age, smoking status, BMI, HbA1c level, hypertension, urinary ACR, retinopathy, and history of stroke, CKD, and history of congestive heart failure (CHF). Moreover, we also examined the associations between SUA at baseline and clinical outcomes of renal complications and mortality. We found that SUA was independently associated with incident CKD (HR 4.13 [2.9–5.89]), ESRD (12.96 [8.11–20.7]), and all-cause mortality (2.48 [1.6–3.82]) during follow up, after adjustment for the conventional baseline confounders (Supplementary Table 3). With further adjustment for AKI, SUA remained significantly associated with CKD and ESRD, but the effect sizes were attenuated (3.34 [2.05–5.43] and 7.30 [4.49–11.86], respectively) (Supplementary Table 3).
Table 3.
Predictors for the first episode of AKI by Cox proportional hazards regression
| HR (95% CI) | P | |
|---|---|---|
| Age (per 1 year) | 1.04 (1.03–1.04) | <0.001 |
| Smoking | ||
| Ex-smoker | 1.15 (1.04–1.28) | 0.008 |
| Current smoker | 1.35 (1.19–1.53) | <0.001 |
| BMI, per kg/m2 | 1.02 (1.01–1.03) | 0.001 |
| HbA1c, per 1% | 1.09 (1.06–1.11) | <0.001 |
| HDL cholesterol, per 1 mmol/L | 1.14 (1.02–1.28) | 0.022 |
| LDL cholesterol, per 1 mmol/L | 0.9 (0.86–0.94) | <0.001 |
| Systolic BP, per 1 mmHg | 1.004 (1.001–1.01) | 0.004 |
| Diastolic BP, per 1 mmHg | 0.99 (0.98–0.99) | <0.001 |
| log (urinary ACR) | 1.24 (1.2–1.28) | <0.001 |
| Retinopathy | 1.2 (1.1–1.32) | <0.001 |
| Stroke history | 1.3 (1.05–1.62) | 0.019 |
| CKD history | 1.29 (1.16–1.43) | <0.001 |
| CHF history | 1.56 (1.23–1.98) | <0.001 |
| Use of antihypertensive drugs | 1.36 (1.24–1.49) | <0.001 |
| SUA | 3.58 (2.39–5.36) | <0.001 |
We identified predictors by performing backward stepwise variable selection. BP, blood pressure.
Associations of PRS for SUA
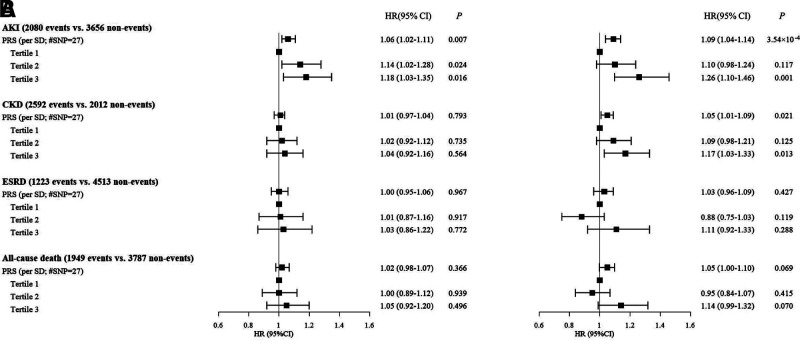
Given the strong association identified between SUA and renal outcomes in our analysis, we undertook additional analyses to explore the mediating effects of SUA on renal outcomes in diabetes, using available genotyping data from our cohort. We explored the associations between a PRS derived from SUA-related SNPs and clinical outcomes, with adjustment for confounding factors. As would be expected, the PRS was significantly associated with SUA at baseline after adjustment (β [SE] 0.014 [0.001] per SD; P = 4.26 × 10−20). With adjustment for conventional confounding factors, the PRS was significantly associated with incident AKI (HR 1.09 [95% CI 1.04–1.14] per SD; P = 3.54 × 10−4) and CKD (1.05 [1.01–1.09] per SD; P = 0.021) (Fig. 1). No significant associations were found between the PRS for SUA and ESRD or all-cause mortality.
Figure 1.
Associations between PRS derived from 27 SNPs identified to be associated with SUA in European populations and clinical outcomes in the discovery cohort of HKDR. A: HRs were derived from the models without adjustment. B: HRs were adjusted for conventional risk factors including age; sex; duration of diabetes; smoking; BMI; HbA1c; HDL cholesterol; LDL cholesterol; systolic blood pressure; diastolic blood pressure; log-transformed ACR; eGFR; retinopathy; use of lipid-lowering drugs (yes/no), antihypertensive drugs (yes/no), RAS inhibitors (yes/no), and antihyperglycemic drugs (yes/no); and history of stroke, CHD, or CKD.
With respect to the replication in HKDB, the PRS derived from 27 SNPs was strongly associated with SUA (β [SE] 0.014 [0.002] per SD; P = 3.15 × 10−13) after adjustment for confounders. Moreover, the PRS was also significantly associated with AKI (odds ratio 1.14 [95% CI 1.04–1.25] per SD; P = 0.007) and CKD (1.15 [1.05–1.27] per SD; P = 0.004) after adjustment for potential confounding factors (Supplementary Table 4).
Sensitivity Analysis
We performed the following sensitivity analyses to assess the robustness of our findings. First, since AKI was not excluded in the definitions of CKD and ESRD, for some patients AKI might be misclassified as CKD or ESRD events, which would lead to overestimation of effect sizes between AKI and clinical outcomes. Therefore, we redefined CKD and ESRD by excluding AKI and using eGFR, which remained low over 3 months (eGFR <60 and <15 mL/min/1.73 m2 for CKD and ESRD, respectively), in addition to using diagnostic codes as described in the primary analysis. Furthermore, we extended the censor date to 30 June 2019 and reexamined the associations between AKI and incident CKD, ESRD, and all-cause mortality. We found that the associations were consistent with that in the primary analysis but the effect sizes were reduced (Supplementary Table 5). Second, we further investigated the associations between uric acid and CKD, ESRD, and all-cause mortality in males and females (Supplementary Table 6). Similar to our primary results derived from the entire cohort, SUA was independently associated with incident AKI, CKD, ESRD, and all-cause mortality after adjustment for the conventional baseline confounders in both the male and female subcohorts. However, the effect sizes for ESRD were markedly higher for males compared with females (HR 21.91 [95% CI 10.66–45.02] for males vs. 7.61 [3.96–14.63] for females). Third, we defined hyperuricemia as SUA >0.42 mmol/L in men and >0.36 mmol/L in women according to the criteria from the National Kidney Foundation (30) and examined the associations between hyperuricemia and clinical outcomes in males, females, and all participants (Supplementary Table 7). Similar to our primary analysis in which SUA was used as a continuous variable, hyperuricemia was significantly associated with all clinical outcomes except all-cause mortality in females. Fourth, as death was a potential competing risk for CKD and ESRD in our study, we examined the associations between the status of AKI and the outcomes of CKD and ESRD, with death considered as a competing risk, using Fine-Gray subdistribution hazards model. In using the subdistribution hazard model we then considered the possibility of someone dying before the opportunity to develop CKD or ESRD. The results were overall similar to those of the original Cox regression analysis and support our main conclusions that AKI remains a strong predictor of CKD or ESRD, even after consideration of death as competing risk (Supplementary Table 8). Fifth, we repeated the analysis, using only the most strongly associated SNP, rs2231142, near ABCG2, which was associated with SUA in our first cohort, HKDR, at genome-wide significance, as the instrumental variable. The results likewise showed association with AKI in HKDR (1.08 [1.01–1.17]; P = 0.037). We further repeated the analysis by removing the top SNP, rs2231442, and found significant association of a PRS containing the remaining 26 SNPs with AKI (1.08 [1.04–1.14] per SD; P = 0.001). A further analysis where we used a trimmed PRS with 23 SNPS, after the top 4 SNPs for SUA were removed, yielded similar results, with significant association with AKI (1.08 [1.03–1.14] per SD; P = 0.003). Finally, we performed an MR-Egger intercept test and compared the strength of association between each SNP and SUA against the association between each SNP and AKI in the discovery data set. The plot is supportive of a direct relationship between SUA and AKI (Supplementary Fig. 2). The intercept estimates from the MR-Egger method did not significantly deviate from zero (P = 0.816), indicating that genetic pleiotropy did not give rise to causal bias. Lookup of the top four SNPs for SUA did not reveal any association with any other traits that may contribute toward AKI or DKD, other than rs1260326 near GCKR being reported to be associated with lipid, glucose, CKD, and T2D in the GWAS catalog.
Discussion
AKI is an important determinant of clinical outcomes in different populations. In this large prospective study of Chinese patients with T2D, we analyzed the relationship between AKI episode and long-term outcomes, including all-cause mortality and renal complications. Our main findings were threefold. First, we revealed that AKI was a common occurrence (affecting more than one-third of patients during follow-up) and a powerful predictor of long-term renal complications and all-cause mortality in patients with T2D, and more severe AKI or poorer recovery from AKI was associated with greater risk of complication outcomes. Second, we identified SUA as a significant predictor of AKI and long-term outcomes. Third, genetic variants associated with SUA were associated with the development of AKI and CKD in patients with T2D, suggesting that SUA is genetically linked to the progression of renal complications in T2D.
AKI as Risk Factor for DKD
This analysis is consistent with and extends previous work. In previous studies investigators reported that AKI predicted the risk of mortality (24), renal diseases (31,32), and cardiovascular diseases (33) in the general population. Despite the body of evidence suggesting that diabetes is a major risk factor for the development of AKI in hospitalized patients (34,35), there is a scarcity of data for investigating the role of AKI in disease progression among patients with T2D. Advani recently reviewed the link between AKI and diabetes and proposed that AKI should be considered a complication of diabetes alongside other complications such as CKD and ESRD (36). In a European cohort of 1,371 patients with T2D, researchers highlighted that AKI was a powerful predictor of major adverse outcomes including cardiovascular events and mortality (8). In our large prospective cohort of Chinese patients with T2D followed for 12 years, we systematically demonstrated that AKI status and the severity and recovery status of the first AKI were significantly associated with risk of long-term renal complication outcomes and mortality.
Link Between Uric Acid and Acute and Chronic Kidney Complications
In recent studies investigators have identified a number of predictors or biomarkers of AKI. For example, in a large-scale community-based cohort from a northern California population, older age, lower eGFR, proteinuria, and comorbidity including heart failure and diabetes were identified as independent predictors of recurrent AKI (37). Besides those common risk factors of AKI, we identified SUA as a powerful predictor for the development of AKI in patients with T2D. Uric acid is the final oxidation product of purine metabolism and is excreted by the kidneys (38). Therefore, elevated SUA concentrations are seen in patients with reduced glomerular filtration rate (12). With respect to the impact of SUA in patients with diabetes, Bjornstad et al. (13) reported that SUA was associated with DKD in obese adolescents with T2D. In our Chinese patients with T2D, we found that each unit increase in SUA was associated with more than threefold higher risk of an AKI event. In previous studies it was proposed that uric acid itself may play a causal role in the pathophysiology of CKD (12); however, recently, investigators of an MR study concluded that there were no causal effects of SUA on eGFR level or CKD risk in a European population (39). In our Chinese patients with T2D, we discovered a genetic link between SUA and renal complications, but further studies are needed to investigate whether the relationship between SUA and renal complications in patients with T2D is causal.
Genetic Determinants of SUA Concentration and Use of a PRS
As a potential modifiable risk factor of many diseases including DKD, SUA is also affected by genetic factors, although the mechanism remains unclear. It is estimated that heritability of SUA ranges from 40 to 70% in different populations (14,40,41). Recent GWAS have identified a number of genetic variants associated with the concentration of SUA (14–16). In this larger cohort of Chinese patients with T2D, we derived a PRS using 27 variants that predicted SUA and kidney function. The PRS was independently associated with AKI and CKD in both discovery and replication cohorts. Further analysis would be required to explore the biological mechanisms of the variants in the development of DKD.
We did not find any association with ESRD, which may be due to the small sample sizes for ESRD cases in both cohorts. Furthermore, most of the published genetic variants were identified in European populations, which may not apply to the Chinese population because of differences in genetic architecture (42), and only 1.5% of variance of SUA was explained by the PRS in our cohort. There are few genetic studies in Asian populations on SUA, and more large-scale GWAS are called for to fill this knowledge gap. Given the small effect size of these common SNPs, the use of genome-wide PRS based on thousands of SNPs may further improve the discriminative performance in predicting DKD (43).
Clinical Relevance of SUA for DKD and Treatment Implications
Our study suggests a potentially important role for SUA in the development of AKI and also for long-term renal complications in T2D. Results of analyses with use of the MR-Egger intercept test support SUA being directly linked with the risk of AKI in T2D. The association between the PRS was considerably stronger for AKI than CKD, suggesting that while SUA was strongly associated with the development of AKI (such as through its causal relationship with acute gout flare-up) (16), it has less direct impact on CKD progression in T2D. Interestingly, despite the well-known epidemiological link between urate and DKD, results of an analysis with use of MR suggested that urate is not causally linked to eGFR or progression of diabetic nephropathy in patients with type 1 diabetes in the Finnish Diabetic Nephropathy (FinnDiane) Study cohort (44). As highlighted earlier, an MR study in the general population suggested no causal link between urate and CKD (39). In a recent report from the National Kidney Foundation it was likewise concluded that there are currently insufficient data to suggest that urate is causally linked to hypertension, CKD, or cardiovascular disease (30) and suggested that routine treatment of hyperuricemia in patients with hypertension, kidney disease, or metabolic syndrome/T2D is not currently supported based on current evidence (30). As far as we are aware, no randomized controlled trials have been conducted to date to examine the effect of urate lowering on DKD progression in the setting of T2D.
Other Contributing Factors to AKI
Another important cause for AKI is hospitalization with heart failure. This is supported by our finding in the regression analysis whereby history of CHF was one of the strongest clinical predictors of AKI. The relationship between CHF and AKI is complex and multifactorial, including cardiogenic shock leading to acute tubular necrosis, as well as other prerenal mechanisms, and the impact of diuretic therapy and use of renin-angiotensin system blockers. Other potential causes of AKI include sepsis, dehydration, or the use of nephrotoxic medications. Interestingly, treatment with sodium–glucose cotransporter 2 inhibitors, which also lower SUA, has consistently been shown to have beneficial effects on reducing hospitalization with heart failure, though the exact mechanisms remain unknown (45). We also cannot exclude the possibility that concomitant medications, such as short-term nonsteroidal anti-inflammatory drugs prescribed during episodes of acute gout, may have contributed to some AKI episodes.
We acknowledge several limitations in our study. First, the collection of laboratory data was done according to clinical need during follow-up and hospitalization and not according to a protocol. Ascertainment bias may be introduced, since blood testing was performed at clinical discretion rather than in standardized testing intervals. Nevertheless, given all outpatient as well as inpatient renal function test results were retrieved, it is unlikely that any significant episodes of AKI would have been missed. Second, the definition of AKI was based only on SCr and eGFR and did not include reduced urine output. Third, the recovery status could not be defined for a number of patients due to missing data of repeated measurements of SCr within 7 days, which may induce some biases in the association analysis of AKI recovery. Fourth, although our findings provide evidence that PRS of SUA is associated with the progression of kidney complications in T2D, we cannot establish a causal relationship between them. MR is one of the approaches to investigate whether SUA is causally linked to renal complications in T2D. We are also not able to identify specific causes for each episode of AKI. Lastly, we did not validate our findings in the second cohort using prospective follow-up. However, replication of these findings in a large cross-sectional cohort with retrospective data, together with similar findings in the literature, lent support to our results.
In conclusion, in Chinese patients with T2D, we found that AKI was a common occurrence. SUA was a strong predictor for AKI, which was further associated with all-cause mortality and long-term renal complications in T2D. Moreover, we revealed that SUA was genetically linked to the progression of AKI and CKD in T2D. Young age at diagnosis, tobacco use, BMI, HbA1c level, hypertension, urinary ACR, retinopathy complications, and genetic variants of SUA all independently predicted AKI. The contributions of both modifiable and nonmodifiable risk factors may facilitate the identification of high-risk individuals to prevent AKI and renal complications in patients with T2D.
Article Information
Funding. This work was supported by the NSFC-NHMRC Joint Research Scheme (81561128017), Research Grants Council Theme-based Research Scheme (T12-402/13N), Croucher Foundation Senior Medical Research Fellowship, Focused Innovation Scheme, Vice-Chancellor One-off Discretionary Fund, Postdoctoral Fellowship Scheme of the Chinese University of Hong Kong, and Chinese University of Hong Kong-Shanghai Jiao Tong University Joint Research Fund and by a grant from the Research Grants Council of the Hong Kong Special Administrative Region (CU R4012-18).
The funding sources did not have any role in the design or interpretation of the study or the decision to publish the results.
Duality of Interest. J.C.N.C. serves on advisory boards or steering committees of research and education programs sponsored by AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Servier, and Sanofi. Her affiliated institutions, The Chinese University of Hong Kong and Asia Diabetes Foundation, have received research and educational grants from these companies. R.C.W.M. received research funding from AstraZeneca, Bayer, Boehringer Ingelheim, and Pfizer for carrying out clinical trials, speaker honorarium, or consultancy in advisory boards; all proceeds have been donated to The Chinese University of Hong Kong to support diabetes research. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.J., R.A.O., W.Y.S., J.C.N.C., and R.C.W.M. contributed to study conceptualization. G.J., A.O.L., C.H.T.T., R.O., E.S.L., E.Y.K.C., B.F., K.F.L., S.C.S., G.H., C.C.T., K.P.L., J.Y.L., M.-w.T., G.K., I.T.L., J.K.L., V.T.Y., E.L., S.L., S.F., Y.L.C., C.C.C., N.L.S.T., Y.H., H.-y.L., C.C.S., A.P.S.K., C.K.P.L., W.Y.S., J.C.N.C., and R.C.W.M. contributed to data curation. G.J., A.O.L., W.Y.S., J.C.N.C., and R.C.W.M. contributed to formal analysis. G.J., A.O.L., C.H.T.T., E.S.L., R.A.O., W.Y.S., J.C.N.C., and R.C.W.M. contributed to the investigation process. G.J., A.O.L., C.H.T.T., R.A.O., J.C.NC., and R.C.W.M. contributed to methodology development. G.J., R.A.O., J.C.N.C., and R.C.W.M. contributed to data validation. G.J., R.A.O., J.C.N.C., and R.C.W.M. wrote the original draft of the manuscript. G.J., A.O.L., C.H.T.T., E.S.L., B.F., R.O., E.Y.K.C., K.F.L., S.C.S., G.H., C.C.T., G.K., I.T.L., J.Y.L., M.-w.T., K.P.L., J.K.L., V.T.Y., E.L., S.L., S.F., Y.L.C., C.C.C., N.L.S.T., Y.H., H.-y.L., A.P.S.K., C.K.P.L., R.A.O., C.C.S., W.Y.S., J.C.N.C., and R.C.W.M. wrote, reviewed, and edited the manuscript. R.C.W.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.17268527.
A full list of members of Hong Kong Diabetes Register TRS Study Group and Hong Kong Diabetes Biobank Study Group can be found in the supplementary material online.
Contributor Information
Collaborators: Hong Kong Diabetes Register TRS Study Group:, Ronald C.W. Ma, Juliana C.N. Chan, Yu Huang, Hui-yao Lan, Si Lok, Brian Tomlinson, Stephen K.W. Tsui, Weichuan Yu, Kevin Y.L. Yip, Ting Fung Chan, Xiaodan Fan, Wing Yee So, Cheuk Chun Szeto, Nelson L.S. Tang, Andrea O. Luk, Xiaoyu Tian, Guozhi Jiang, Claudia H.T. Tam, Heung Man Lee, Cadmon K.P. Lim, Katie K.H. Chan, Fangying Xie, Alex C.W. Ng, Grace P.Y. Cheung, Ming-wai Yeung, Shi Mai, Fei Xie, Sen Zhang, Pu Yu, Meng Weng, Hong Kong Diabetes Biobank Study Group:, Ronald C.W. Ma, Juliana C.N. Chan, Risa Ozaki, Andrea O. Luk, Wing Yee So, Cadmon King Poo Lim, Ka Fai Lee, Shing Chung Siu, Grace Hui, Chiu Chi Tsang, Kam Piu Lau, Jenny Y.Y. Leung, Man Wo Tsang, Grace Kam, Elaine Cheung, Ip Tim Lau, June Kam-yin Li, Vincent T.F. Yeung, Samuel K.S. Fung, Stanley Lo, Emmy Lau, Yuk Lun Cheng, Stephen Kwok-wing Tsui, Yu Huang, Hui-yao Lan, Weichuan Yu, Brian Tomlinson, Si Lok, Ting Fung Chan, Kevin Yuk-lap Yip, Cheuk Chun Szeto, Xiaodan Fan, Nelson L.S. Tang, Xiaoyu Tian, Claudia H.T. Tam, Guozhi Jiang, Shi Mai, Baoqi Fan, Eric S. Lau, Fei Xie, Sen Zhang, Pu Yu, Meng Wang, Heung Man Lee, Fangying Xie, Alex C.W. Ng, Grace Cheung, Alice P.S. Kong, Elaine Y.K. Chow, Ming Wai Yeung, Chun Chung Chow, Kitty K.T. Cheung, Rebecca Y.M. Wong, So Hon Cheong, Katie K.H. Chan, Chin-san Law, Anthea Ka Yuen Lock, Ingrid Kwok Ying Tsang, Susanna Chi Pun Chan, Yin Wah Chan, Cherry Chiu, Chi Sang Hung, Cheuk Wah Ho, Ivy Hoi Yee Ng, Juliana Mun Chun Fok, Kai Man Lee, Hoi Sze Candy Leung, Ka Wah Lee, Hui Ming Chan, Winnie Wat, Tracy Lau, Rebecca Law, Ryan Chan, Candice Lau, Pearl Tsang, Vince Chan, Lap Ying Ho, Eva Wong, Josephine Chan, Sau Fung Lam, Jessy Pang, and Yee Mui Lee
References
- 1. McKnight AJ, Duffy S, Maxwell AP. Genetics of diabetic nephropathy: a long road of discovery. Curr Diab Rep 2015;15:41. [DOI] [PubMed] [Google Scholar]
- 2. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of Diabetes and kidney disease. Adv Chronic Kidney Dis 2018;25:121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vupputuri S, Kimes TM, Calloway MO, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications 2014;28:10–16 [DOI] [PubMed] [Google Scholar]
- 4. Wu AYT, Kong NCT, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia 2005;48:17–26 [DOI] [PubMed] [Google Scholar]
- 5. Jha V. Current status of chronic kidney disease care in southeast Asia. Semin Nephrol 2009;29:487–496 [DOI] [PubMed] [Google Scholar]
- 6. Ftouh S; Acute Kidney Injury Guideline Development Group convened by the National Clinical Guidelines Centre and commissioned by the National Institute for Health and Care Excellence, in association with The Royal College of Physicians’ Clini . Prevention, detection and management of acute kidney injury: concise guideline. Clin Med (Lond) 2014;14:61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nisula S, Kaukonen KM, Vaara ST, et al.; FINNAKI Study Group . Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med 2013;39:420–428 [DOI] [PubMed] [Google Scholar]
- 8. Monseu M, Gand E, Saulnier PJ, et al.; SURDIAGENE Study Group . Acute kidney injury predicts major adverse outcomes in diabetes: synergic impact with low glomerular filtration rate and albuminuria. Diabetes Care 2015;38:2333–2340 [DOI] [PubMed] [Google Scholar]
- 9. Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015;5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chew ST, Ng RR, Liu W, Chow KY, Ti LK. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol 2017;18:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hahn K, Kanbay M, Lanaspa MA, Johnson RJ, Ejaz AA. Serum uric acid and acute kidney injury: a mini review. J Adv Res 2017;8:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giordano C, Karasik O, King-Morris K, Asmar A. Uric acid as a marker of kidney disease: review of the current literature. Dis Markers 2015;2015:382918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjornstad P, Laffel L, Lynch J, et al.; TODAY Study Group . Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study. Diabetes Care 2019;42:1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Köttgen A, Albrecht E, Teumer A, et al.; LifeLines Cohort Study; CARDIoGRAM Consortium; DIAGRAM Consortium; ICBP Consortium; MAGIC Consortium . Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet 2013;45:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kolz M, Johnson T, Sanna S, et al.; EUROSPAN Consortium; ENGAGE Consortium; PROCARDIS Consortium; KORA Study; WTCCC . Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 2009;5:e1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dehghan A, Köttgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008;372:1953–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan JC, So W, Ma RC, Tong PC, Wong R, Yang X. The complexity of vascular and non-vascular complications of diabetes: the Hong Kong Diabetes Registry. Curr Cardiovasc Risk Rep 2011;5:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Ng MC, Lee SC, et al. Phenotypic heterogeneity and associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care 2003;26:2410–2415 [DOI] [PubMed] [Google Scholar]
- 19. Yang X, So WY, Kong AP, et al. Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 2007;30:65–70 [DOI] [PubMed] [Google Scholar]
- 20. Jiang G, Luk AO, Tam CHT, et al.; Hong Kong Diabetes Register TRS Study Group; Hong Kong Diabetes Biobank Study Group . Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med 2020;17:e1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang XL, So WY, Kong AP, et al. End-stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia 2006;49:2299–2308 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Information Services Department, Hong Kong Special Administrative Region Government . Hong Kong: the facts, 2012. Accessed 1 December 2013. Available from https://www.gov.hk/en/about/abouthk/factsheets/docs/public_health.pdf
- 24. Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [Google Scholar]
- 26. Jiang G, Luk AOY, Tam CHT, et al.; Hong Kong Diabetes Register TRS Study Group . Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int 2019;95:178–187 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 2018;71:851–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heung M, Steffick DE, Zivin K, et al.; Centers for Disease Control and Prevention CKD Surveillance Team . Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis 2016;67:742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018;93:460–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 2014;9:448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 35. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008;3:844–861 [DOI] [PubMed] [Google Scholar]
- 36. Advani A. Acute kidney injury: a bona fide complication of diabetes. Diabetes 2020;69:2229–2237 [DOI] [PubMed] [Google Scholar]
- 37. Liu KD, Yang J, Tan TC, et al. Risk factors for recurrent acute kidney injury in a large population-based cohort. Am J Kidney Dis 2019;73:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin Nephrol 2011;31:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jordan DM, Choi HK, Verbanck M, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019;16:e1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whitfield JB, Martin NG. Inheritance and alcohol as factors influencing plasma uric acid levels. Acta Genet Med Gemellol (Roma) 1983;32:117–126 [DOI] [PubMed] [Google Scholar]
- 41. Nath SD, Voruganti VS, Arar NH, et al. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol 2007;18:3156–3163 [DOI] [PubMed] [Google Scholar]
- 42. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahola AJ, Sandholm N, Forsblom C, Harjutsalo V, Dahlström E; FinnDiane Study Group . The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int 2017;91:1178–1185 [DOI] [PubMed] [Google Scholar]
- 45. McGuire DK, Shih W, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]