Abstract
Aims:
To determine whether sleep blood pressure (BP) is associated with increased cardiovascular disease (CVD) risk in youth with type 1 diabetes (T1DM).
Methods:
We enrolled youth with T1DM, 12–21 years old. Carotid-femoral Pulse Wave Velocity (PWVcf) assessed arterial stiffness, a CVD marker. Sleep systolic and diastolic BP variables were obtained from 24-hour BP Monitoring. Linear regression models analyzed the relationship of each BP variable with PWVcf, adjusted for HbA1c. Correlation of sleep BP with urine microalbumin-to-creatinine ratio (UAC) was examined.
Results:
Nocturnal hypertension was found in 36% and abnormal dipping in 48% of the 25 participants, aged 17.7 ± 2.2 years old. Sleep systolic BP [beta = 0.039, 95% Confidence Interval (CI; 0.006–0.073)], diastolic BP [beta = 0.058, 95% CI (0.003–0.114)], Mean Arterial Pressure (MAP) [beta = 0.075, 95% CI (0.018–0.131)] and MAP index [beta = 3.547, 95% CI (0.867–6.227)] were significantly associated with PWVcf. Sleep diastolic BP, load, MAP correlated with UAC.
Conclusions:
Blood pressure alterations during sleep are common in youth with T1DM and they are associated with arterial stiffness and UAC. Larger studies are needed to confirm our results and examine whether interventions that target sleep and night-time BP could decrease CVD risk.
Keywords: Ambulatory blood pressure monitoring, Type 1 diabetes, Arterial stiffness, Cardiovascular, Children, Youth
1. Introduction
Children with type 1 diabetes (T1DM) are exposed early in life to factors that elevate their cardiovascular disease (CVD) risk compared to their healthy peers.1 Some of these CVD risk factors include poor glycemic control, high blood pressure (BP), obesity, hyperlipidemia.1 In fact, children with T1DM are considered to be in the highest tier of CVD risk by the American Heart Association.2 Children in this tier are at greatest risk for accelerated atherosclerosis. The American Academy of Pediatrics’ Clinical Practice Guideline (AAP CPG) for Screening and Management of High Blood Pressure in Children and Adolescents recommends screening these youth for hypertension at every health care encounter as a result.2,3
Studies in adults have shown that both high nocturnal BP and lack of dipping at night are strong predictors of increased CVD morbidity and mortality.4,5 Patients with isolated nocturnal hypertension are often underdiagnosed and undertreated, as the only way to identify these patients is via 24-hour ambulatory blood pressure monitoring (ABPM). This is particularly notable in that nocturnal hypertension is considered a stronger predictor of CVD mortality than day-time hypertension.5,6
In pediatric patients, CVD events are rare and therefore surrogate markers of subclinical cardiovascular disease are used to evaluate the burden of CVD in youth.7 Several studies have shown that youth with T1DM have evidence of subclinical atherosclerosis, manifested as high arterial stiffness and increased carotid intima media thickness.7
There are limited studies investigating the role of abnormal BP patterns during sleep with arterial stiffness in youth with T1DM. We therefore sought to investigate the association of nocturnal blood pressure, blood pressure dipping (% drop in sleep BP compared to wake), blood pressure load (% readings in hypertensive range), and mean arterial pressure (MAP) with arterial stiffness as assessed by carotid-femoral pulse wave velocity (PWVcf) in youth with T1DM. As a secondary objective, we also examined the correlation of the same sleep BP variables with urine microalbumin to creatinine ratio (UAC), given the known relationship of diabetic kidney disease with CVD.8
2. Methods
2.1. Subjects
This project is a secondary analysis of data collected in the clinical study, “Identifying Children with type 1 diabetes at high risk for CVD” (Clinical Trials Number: NCT02275091) which was designed to investigate lipoprotein dysfunction in youth with T1DM.9 Children between the ages of 12–21 years with a history of T1DM for at least one year were eligible; those treated with statins were ineligible and excluded from participation. Recruitment letters were sent to patients with T1DM who are followed at the Pediatric Endocrine Divisions of Georgetown University (GU) and Children’s National Health System (CNHS) in Washington, DC. This study was approved by the Georgetown-Howard Universities’ Center for Clinical and Translational Science (GHUCCTS) Institutional Review Board (IRB). All adult patients and all parents of pediatric patients with T1DM provided written informed consent and all children provided written assent.
A research nurse conducted the anthropometric measurements. Waist circumference was measured at the perimeter between the iliac crest and the rib cage in centimeters. Height was obtained in centimeters with participants in their bare/stocking feet using a wall mounted stadiometer. Height and waist were measured in triplicate and averaged. Fasting LDL-C cholesterol was measured with NMR spectroscopy as previously described.9 HbA1c and urine albumin to creatinine ratio (UAC) were measured at the core lab at Georgetown University. The HbA1c was measured using a turbidimetric inhibition immunoassay. The urine microalbumin to creatinine ratio was measured with Siemens Vista chemistry platform with normal values reported as <30 μg albumin/g creatinine.
Ambulatory BP monitoring was conducted for 24 h (OnTrak, Spacelabs). An appropriate size BP cuff was applied to the non-dominant arm of each participant. BP measurements were taken every 30 min during waking hours and every hour during sleep and the 24-hour monitoring period was initiated in the daytime. Participants were encouraged to avoid strenuous activity during the period of ambulatory BP monitoring. Actigraphy data from the ABPM device determined the sleep time of each participant. Sleep MAP, systolic and diastolic BP, systolic and diastolic dipping (dip = (1 − [wake BP/sleep BP]) * 100), systolic and diastolic BP load were used to assess BP patterns during sleep. Because the hypertensive range varies by sex and height, to compare values across all pediatric participants, we calculated an index BP value for each parameter. The sleep systolic, diastolic and MAP indices were calculated as measured BP value/age-height specific 95th percentile for each participant.10
The prevalence of nocturnal hypertension was determined according to published pediatric guidelines for participants <18 years and according to adult ABPM guidelines (>120/70 for sleep SBP and DBP respectively) for those 18 years of age and older. Abnormal diastolic and systolic dipping was defined as less than 10% decrease during sleep. Abnormal sleep SBP and DBP load was defined as >25%.
To assess arterial stiffness, we measured PWVcf using the SphygmoCor Cardiovascular System (AtCor Medical) device. Triplicate measurements were taken after 15 min rest, in a quiet room with stable temperature for each participant. The average result from these three measurements was used in the analysis. Higher PWVcf indicates greater arterial stiffness.
2.2. Statistical analysis
Statistical analyses were conducted in Stata 15. Categorical variables were summarized using frequencies and percentages and continuous variables with means and standard deviations (SD). Linear regression analyses were performed to examine the association of each of the ABPM sleep parameters (nocturnal MAP index, Systolic BP index, Diastolic BP index, systolic and diastolic BP load and systolic and diastolic dipping) separately with PWVcf. These models were repeated by adjusting for HbA1c. Correlations between sleep BP variables, UAC and HbA1c were examined using Pearson correlation coefficient. Statistical significance was defined as P < 0.05.
3. Results
A total of 26 participants underwent 24-hour ABPM from November 2017 to August 2019. One participant did not wear the ABPM during sleep and was excluded from the analysis. The mean (SD) age of participants was 17.7 ± 2.2 years, the mean (SD) HbA1c was 8.21 ± 1.37% and the mean (SD) duration of diabetes was 10.2 ± 5.0 years. The demographics of the participants are shown in Table 1. None of these participants were on treatment with anti-hypertensive medications.
Table 1.
Basic characteristics of 25 youth with type 1 diabetes mellitus.
| Age (years) | 17.7 ± 2.2 |
| Female sex | 10 (40%) |
| Race | White: 17 (68%) |
| Black: 6 (24%) | |
| Other: 2 (8%) | |
| Duration of diabetes (years) | 10.17 ± 5.02 |
| LDL-cholesterol mg/dL (n = 22) | 88.14 ± 26.12 |
| Waist to height ratio | 0.44 ± 0.04 |
| HbA1c % | 8.21 ± 1.37 |
| Urine microalbumin to creatinine ratio (mcg/mg) | 6.4 [3.09–12.57] |
| Pulse Wave Velocity (carotid-femoral) m/s | 4.42 ± 0.93 |
Data are presented as mean ± SD or median IQR [25th–75th percentile] for continuous variables and N (%) for categorical variables.
HbA1c – hemoglobin A1c; LDL – low density lipoprotein.
Overall, the average nocturnal systolic and diastolic BP for the cohort was within the normal range, as indicated by the index <1 (Table 2). However, 36% of our cohort had nocturnal HTN defined as sleep SBP or DBP >95th percentile plus systolic or diastolic BP load >25%. Also, 48% of our cohort had overall abnormal dipping, defined as systolic or diastolic dipping <10%.
Table 2.
Sleep ambulatory blood pressure monitoring parameters in 25 youth with T1DM.
| Absolute value (mmHg) | Index valuea | Abnormalb (%) | |
|---|---|---|---|
| Nocturnal SBP (mmHg) | 110 ± 11 | 0.97 ± 0.94 | 32% |
| Nocturnal DBP (mmHg) | 61 ± 7 | 0.92 ± 0.10 | 16% |
| Nocturnal MAP | 79 ± 7.1 | 1.01 ± 0.14 | 36% |
| Nocturnal SBP load | 28 ± 26 | 44% | |
| Nocturnal DBP load | 25 ± 22 | 52% | |
| Systolic dipping | 10.54 ± 6.07 | 44% | |
| Diastolic dipping | 15.19 ± 7.40 | 24% |
Values are reported as mean ± SD.
Index = measured value/95th percentile value.
Abnormal values are defined as >sex-height specific 95th percentile for nocturnal SBP, DBP, MAP; ≥25% for SBP and DBP load; and <10% for systolic and diastolic dipping.
In bivariate linear regression models (see Table 3), both sleep MAP index and diastolic dipping were significantly associated with PWVcf (see Figs. 1 and 2). Specifically, for every one-unit increase in the sleep MAP index the PWV increased by 3.103 m/s [95% Confidence Interval (CI) 0.415–5.791, P = 0.026] and for each percentage point increase in diastolic dipping, the PWVcf decreased by 0.051 m/s [(95% CI −0.100 to −0.002), P = 0.044]. After adjusting for HbA1c, sleep MAP index remained significantly associated with PWVcf [beta = 3.547, (95% CI 0.867–6.227), P = 0.012], and the continuous variables of sleep systolic BP, diastolic BP and MAP (mmHg) all became significantly associated with PWVcf (see Table 3). After adjustment for HbA1c, PWVcf was not significantly associated with any of the other sleep related ABPM variables. There was no significant difference in PWVcf between the group of participants with BP dipping <10% and those with BP dipping ≥10% either (data not shown).
Table 3.
Association of nocturnal blood pressure parameters with carotid-femoral Pulse Wave Velocity (PWVcf) among 25 youth with type 1 diabetes mellitus.
| PWVcf (unadjusted) | PWVcf (adjusted)a | |||
|---|---|---|---|---|
| Beta [95% CI] | P-value | Beta [95% CI] | P-value | |
| Nocturnal SBP (mmHg) | 0.031 [−0.002–0.064] | 0.065 | 0.039 [0.006–0.073] | 0.024 |
| Nocturnal SBP index | 2.754 [−1.325–6.833] | 0.176 | 3.635 [−0.572–7.843] | 0.087 |
| Nocturnal DBP (mmHg) | 0.045 [−0.009–0.099] | 0.096 | 0.058 [0.003–0.114] | 0.039 |
| Nocturnal DBP index | 2.368 [−1.342–6.077] | 0.200 | 3.171 [−0.667–7.009] | 0.101 |
| Nocturnal MAP (mmHg) | 0.045 [−0.008–0.098] | 0.093 | 0.075 [0.018–0.131] | 0.012 |
| Nocturnal MAP index | 3.103 [0.415–5.791] | 0.026 | 3.547 [0.867–6.227] | 0.012 |
| Nocturnal SBP load | 0.004 [−0.011–0.020] | 0.551 | 0.006 [−0.010–0.021] | 0.463 |
| Nocturnal DBP load | 0.004 [−0.014–0.023] | 0.623 | 0.006 [−0.0127–0.025] | 0.504 |
| Systolic dipping | −0.045 [−0.108–0.018] | 0.152 | −0.045 [−0.114–0.0316] | 0.252 |
| Diastolic dipping | −0.051 [−0.100 to −0.002] | 0.044 | −0.051 [−0.108–0.005] | 0.074 |
Adjusted for HbA1c.
Fig. 1.
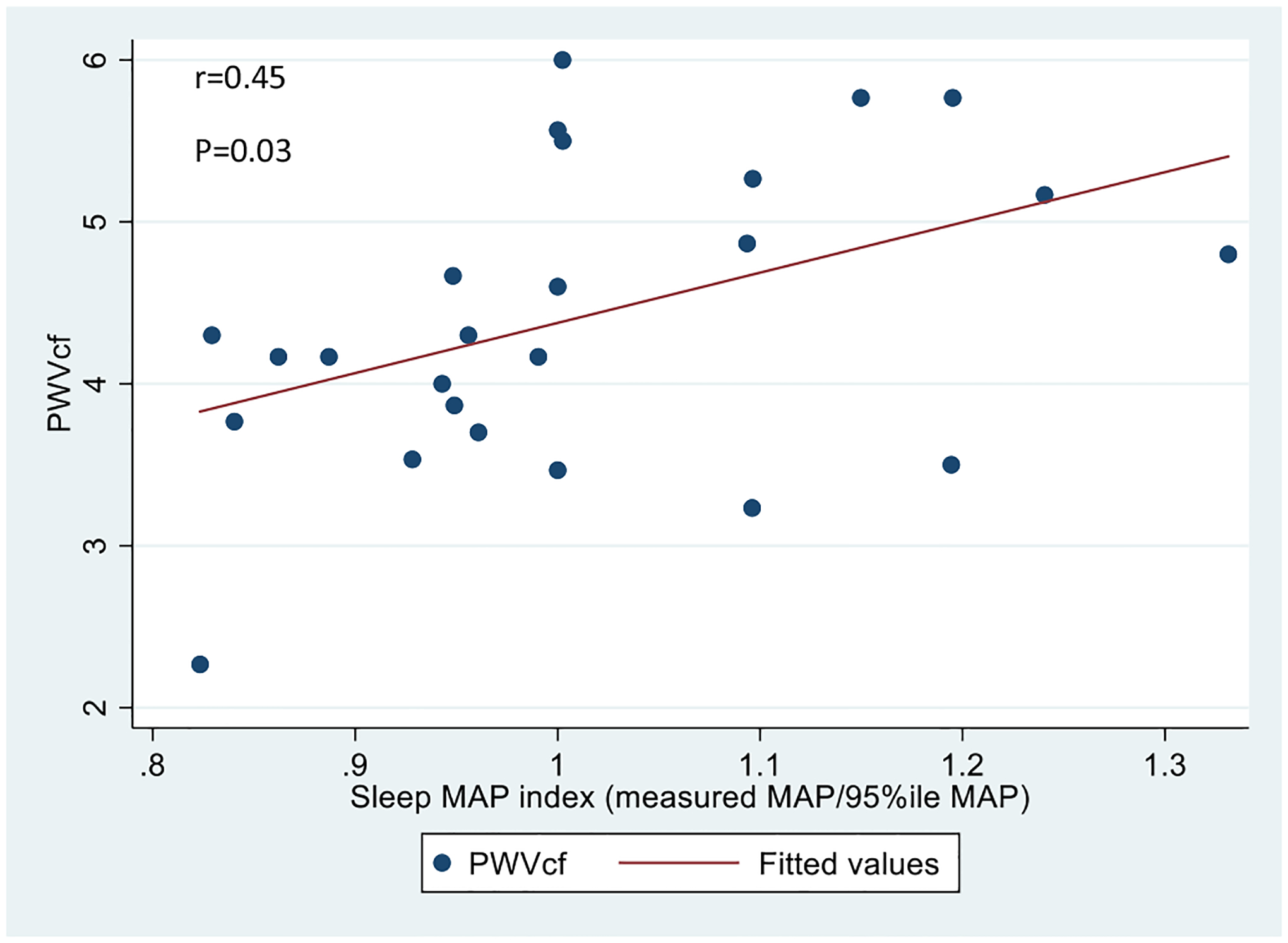
Association of carotid-femoral PWV with sleep MAP index in youth with T1DM.
Fig. 2.
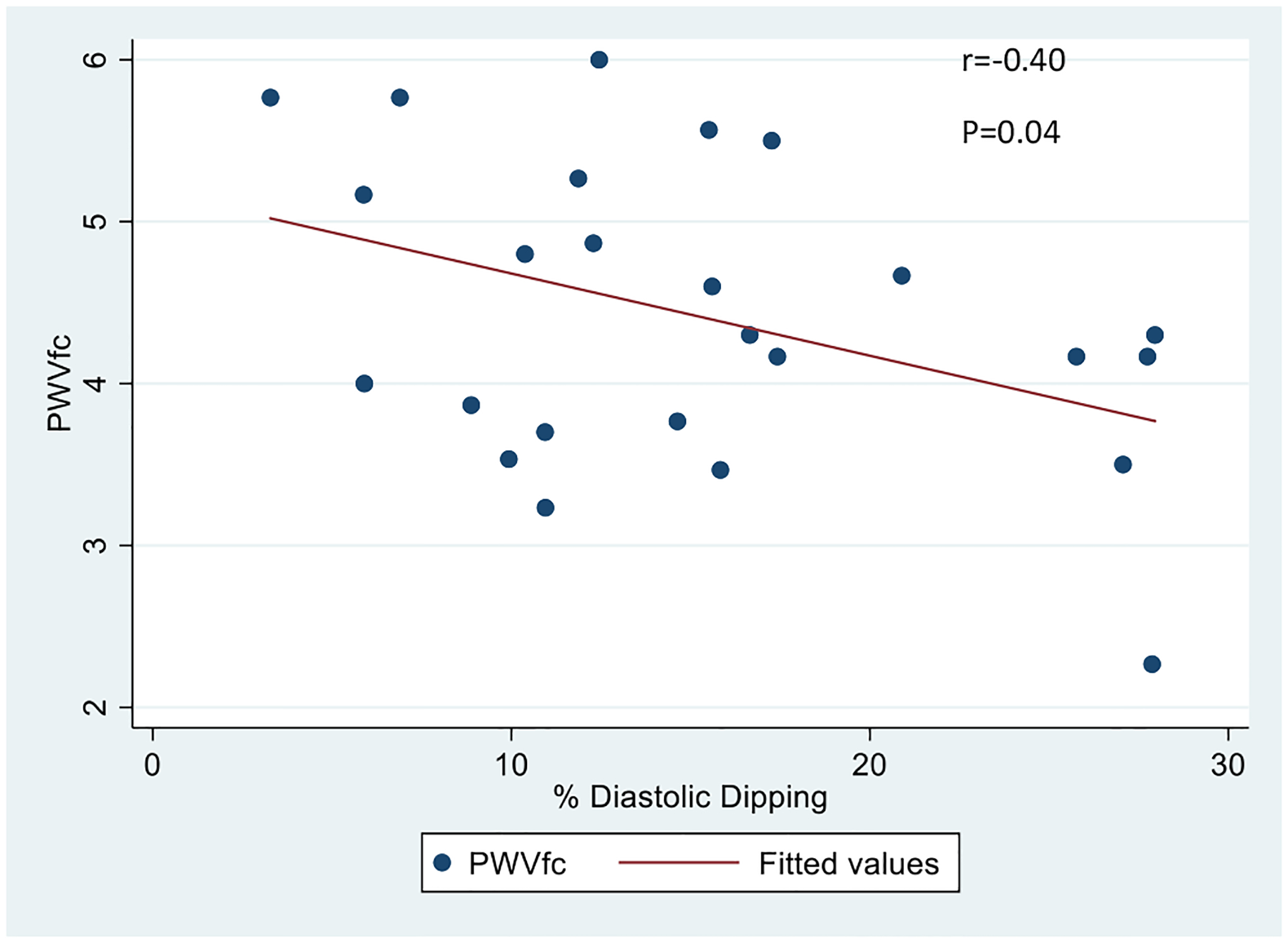
Association of carotid-femoral PWV with % diastolic dipping in youth with T1DM.
HbA1c was significantly correlated with average sleep MAP (r = 0.495, P = 0.012) (Fig. 3), overall systolic dipping (r = 0.473, P = 0.017) and overall diastolic dipping (r = 0.459, P = 0.021). No significant correlation was found between HbA1c and sleep MAP index, sleep DBP index, sleep SBP index.
Fig. 3.
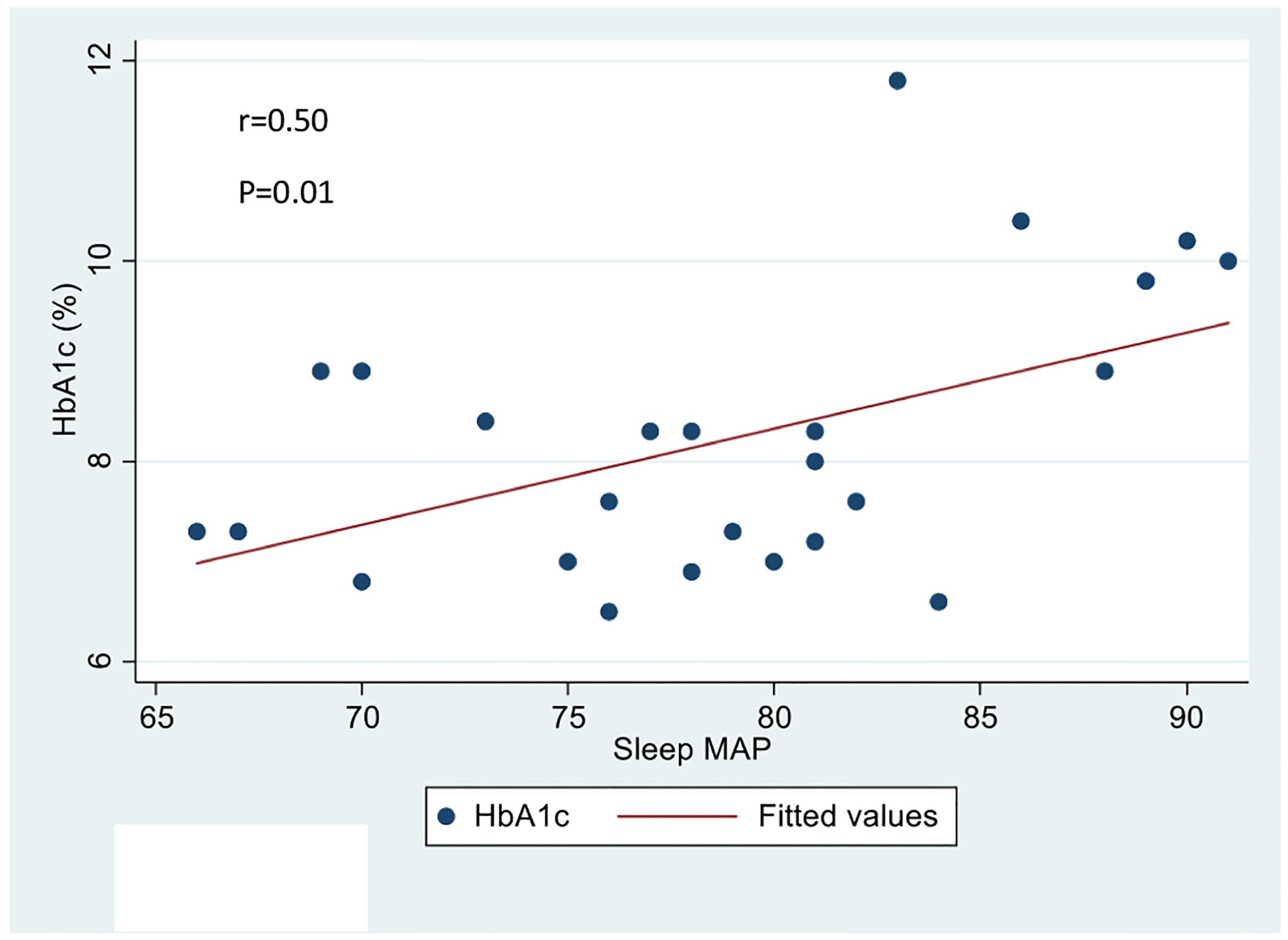
Association of HbA1c with sleep MAP in youth with T1DM.
Our results showed that age was not correlated with PWVcf (r = 0.015, p = 0.94), with systolic dipping (r = −0.08, p = 0.69) or with diastolic dipping (r = 0.01, p = 0.96). There was no difference by sex in PWVcf (4.4 (SD = 1.1) vs 4.4 (SD = 0.75); p = 0.90), in systolic dipping (10.4 (SD = 6.8) vs 10.7 (SD = 5.1); p = 0.92) or in diastolic dipping (14.2 (SD = 7.8) vs 16.6 (SD = 6.9); p = 0.44).
To address potential confounding effect of age and gender, we further adjusted our regression models by sex and age despite the risk of overfitting. The relationship between PWVcf and sleep MAP index did not change in this extended model (Beta estimate for sleep MAP index = 3.9, SE = 1.4, p = 0.01); however, the model was not significant overall due to high number of covariates with a small sample size (Prob>F = 0.12).
With regards to our secondary aim, UAC was significantly correlated with sleep diastolic load (r = 0.49, P = 0.020), sleep MAP (r = 0.43, P = 0.046) and sleep diastolic BP (r = 0.43, P = 0.047). Urine UAC was not significantly correlated with the other sleep BP parameters (systolic or diastolic dipping, diastolic BP load, sleep systolic BP, BP indices; data not shown).
4. Discussion
In a cohort of 25 youth aged 12 to 21 years of age with T1DM duration for an average of 10 years, we demonstrate a high prevalence of abnormal nocturnal blood pressure findings. In addition, several of these sleep parameters – MAP, SBP, DBP - were associated with increasing PWVcf, a marker of arterial stiffness, even after adjusting for hemoglobin A1c, a marker of glycemic control. Sleep diastolic load, sleep MAP and sleep diastolic BP were also found to correlate with UAC, a marker for glomerular hyperfiltration and sclerosis. These novel findings highlight the increased CVD risk that is present among children and young adults with T1DM and suggest the presence of subclinical cardiovascular disease in these youth.
The SEARCH for diabetes in youth study, a multi-center epidemiologic study, showed that the age-adjusted prevalence of high arterial stiffness was 11.6% among 1746 participants with T1DM (mean age, 17.9 ± 4.1 years).11 Furthermore, CVD risk factors such as metabolic syndrome, obesity, large waist, elevated lipids and high BP are associated with worsening arterial stiffness over time in youth with T1DM.12,13 In addition to these traditional CVD risk factors, children with T1DM are at greater risk for arterial stiffness based on their disease duration and severity. In a more recent analysis of the SEARCH cohort,13 arterial stiffness was higher in participants with glycated hemoglobin ≥9% and those with lower insulin sensitivity or longer duration of diabetes mellitus.13
Very limited studies have examined the relationship of ambulatory BP with arterial stiffness in pediatric patients with T1DM. Sulakova et al. explored this using ABPM data from 84 pediatric patients with T1DM and 27 healthy controls, using the ABPM-derived ambulatory arterial stiffness index (AASI) to estimate arterial stiffness.14 The AASI is calculated based on a formula that considers the relationship between the systolic and diastolic BP, with higher AASI indicating greater arterial stiffness. These authors found that children with T1DM and either ambulatory hypertension or white coat hypertension had higher AASI compared to healthy controls.14 While this is compelling, AASI does not directly measure arterial stiffness and the reproducibility of the AASI method has been questioned by some investigators in patients with T1DM.15,16 In another pediatric study, the relationship of ABPM with carotid intima media thickness (cIMT), another surrogate marker of atherosclerosis, was examined. Among 82 pediatric patients with T1DM, children with T1DM and night-time hypertension had higher cIMT than children with T1DM who were normotensive.17
Our study extends the findings described in the above pediatric studies in that we show a relatively high prevalence of abnormal nocturnal BP and a strong relationship of sleep BP parameters with arterial stiffness, a measure of subclinical CVD, in youth with T1DM. We also found that diastolic dipping had a negative association with arterial stiffness; in other words, greater diastolic BP dipping is associated with more favorable arterial stiffness and, conversely, less extreme dipping is associated with increased arterial stiffness.
Our findings have important clinical implications for both prevention of CVD and treatment of nocturnal HTN. Adult studies that have shown dipping status to be cardioprotective18,19 and lack of nocturnal dipping to be associated with CV morbidity and mortality.4,20,21 Also, as discussed earlier, nocturnal HTN is a strong predictor of increased CVD morbidity and mortality in adults.4,5 Therefore, in youth with T1DM, there might be a window of opportunity to intervene early in the development of CVD and halt the progression of arterial stiffness and permanent HTN. Our findings suggest that improving BP patterns during sleep may be an impactful future therapeutic target. Studies in normal adults have shown that the quality of sleep is associated with BP dipping.22 Well-designed clinical trials that investigate the effect of interventions to lower nocturnal BP and improve quality of sleep such as earlier bedtime, limiting caffeine, avoidance of electronic devices and dinner close to bedtime could improve arterial stiffness and other measures of CVD risk in youth with T1DM. Furthermore, our group has reported a case of a lean child with T1DM with obstructive sleep apnea and there are also a few other studies indicating obstructive sleep apnea can be present even in lean adult patients with T1DM.23,24 Sleep distorted breathing has also been described in young children with T1DM.25,26 More research is also needed to uncover the role of sleep in promoting abnormal sleep BP patterns in youth with T1DM.
The high percentage of children demonstrating abnormal nocturnal dipping in our study population (44% for systolic BP and 24% for diastolic BP) highlights the concept that children with T1DM are at increased CVD risk. As endorsed by the AAP CPG, children with T1DM should be screened regularly for hypertension and there may be an expanded role for ABPM in these at-risk youth.3 However, there is no recommended schedule for screening normotensive children with T1DM with ABPM and gaps in the use of ABPM among pediatric patients still exist.3 Providers should therefore remember to screen youth with T1DM for hypertension at every health care encounter and have a low threshold for referral for ABPM, particularly in children with T1DM and other CVD risk factors, such as obesity and poor glycemic control.
We also found a significant correlation of sleep diastolic BP load, MAP and diastolic BP with UAC. These results are in agreement with results from a prospective, observational study of 75 adolescents with T1DM that found high nocturnal BP to be associated with the development of microalbuminuria over a 5 year period of time.27 In that study, all subjects had normal urine albumin to creatinine ratio and the increase in nocturnal BP preceded the development of microalbuminuria.27 Similar results were reported in a large multicenter observational study of 2105 children with T1DM.28 In this study, nocturnal diastolic BP and diastolic dipping were associated with urine microalbuminuria.28 In adult patients with T1DM a lack of nocturnal BP dipping is associated with increased albuminuria and a faster decline of glomerular filtration rate in patients with diabetic nephropathy.29
Microalbuminuria is often the first manifestation of diabetic kidney disease. This can later progress to macro-albuminuria and ultimately kidney failure. Kidney failure, or end stage renal disease, is a long-term complication of poorly controlled diabetes and a strong risk factor for CVD in patients with T1DM.8 Identifying clinical targets to delay kidney disease progression and CVD is therefore a high priority. The above studies suggest that future randomized clinical trials aimed at improving nocturnal blood pressure might present novel approaches towards halting the progression of kidney disease, and ultimately CVD, in this vulnerable population. It is worth mentioning that a large randomized controlled study done in pediatric patients with chronic kidney disease showed that targeting 24-hour MAP in the low range of normal (<50th percentile) was superior to targeting a 24-hour MAP in the normal range (50–95th percentile) in terms of slowing the progression of kidney disease.30 Whether similar beneficial results might be true for patients with T1DM who are treated to a lower sleep BP or MAP target to decrease microalbuminuria remains to be examined.
A limitation of our pilot study is the small sample size that did not allow us to adjust for additional CVD risk factors that are known to be associated with arterial stiffness such as LDL-cholesterol, duration of diabetes and waist to height ratio. Future studies are needed to validate our results in a bigger sample of participants which can inform future clinical trials designed to examine the effect of sleep interventions and/or nocturnal administration of antihypertensive medications on CVD risk. Such studies could inform future clinical guidelines. Another potential limitation to our study is that we did not collect data from participants regarding the impact of cuff inflation on sleep quality during the monitoring period. While it is possible that the nocturnal BP measurements may have resulted in sleep disturbance and the obtained measurements, we implemented several approaches to minimize this potential effect. All children started their 24-hour monitoring period in the daytime, giving them several hours to acclimatize to the measurements. We also implemented a longer interval between measurements during sleep (1 h) than done in other studies (30 min) which should have minimized any sleep disturbance. Finally, we interpreted all measurements and classified all blood pressures according to published pediatric normative ABPM values which were presumably subjected to the same risk of sleep disturbance. For these reasons, we feel the risk of altered measurements due to poor sleep was minimized.
To summarize, in our pilot study of 25 youth with T1DM, we found a strong relationship between sleep BP parameters and PWVcf, a measure of arterial stiffness and marker of subclinical atherosclerosis. Furthermore, almost half of the patients with T1DM were exposed to increased BP during their sleep or lacked a physiologic and cardioprotective BP dipping pattern during sleep. Patients and providers might be unaware of these abnormal nocturnal BP patterns unless 24-hour ABPM is obtained. Further studies are needed to validate our findings in a larger group of patients, to determine the characteristics of pediatric patients with T1DM at high risk for sleep BP abnormalities, and evaluate whether early interventions that target sleep BP parameters could improve their future risk for CVD.
Highlights.
Sleep BP parameters are associated with arterial stiffness.
Sleep BP parameters are associated with urine microalbumin to creatinine ratio.
Approximately a third of the patients with T1DM have nocturnal hypertension.
Approximately 50% of patients with T1DM lack a physiologic BP dip during sleep.
Acknowledgements
We would like to thank all youth and their families for participating in this study.
Funding
This work was supported by funding to Dr. Gourgari from a KL2 Award: Award Numbers KL2TR001432 and UL1TR001409 from the tional Center for Advancing Translational Sciences.
Footnotes
Declarations of competing interest
None.
Author statement
Evgenia Gourgari: Conceptualization, recruitment of participants, writing original draft preparation, acquisition of the financial support for the project leading to this publication.
Mihriye Mete: Data curation, statistical analysis.
Margarita Dimatulac: Recruitment of participants, data collection, reviewing and editing.
Fran Cogen: Recruitment of participants, reviewing and editing.
Tammy Brady: Project design, reviewing and editing.
Uncited reference
References
- 1.Maahs DM, Daniels SR, de Ferranti SD, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2014;130:1532–58. [DOI] [PubMed] [Google Scholar]
- 2.de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 2019;139:e603–34. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Gatteschi C, et al. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 1993;88: 986–92. [DOI] [PubMed] [Google Scholar]
- 5.Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension 2005;45:240–5. [DOI] [PubMed] [Google Scholar]
- 6.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 7.Gourgari E, Dabelea D, Rother K. Modifiable risk factors for cardiovascular disease in children with type 1 diabetes: can early intervention prevent future cardiovascular events? Curr Diab Rep 2017;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 1998;41:784–90. [DOI] [PubMed] [Google Scholar]
- 9.Gourgari E, Playford MP, Campia U, et al. Low cholesterol efflux capacity and abnormal lipoprotein particles in youth with type 1 diabetes: a case control study. Cardiovasc Diabetol 2018;17:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 2014;63:1116–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Talton JW, D’Agostino R Jr, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care 2013;36:3938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbina EM, Isom S, Bell RA, et al. Burden of cardiovascular risk factors over time and arterial stiffness in youth with type 1 diabetes mellitus: the SEARCH for diabetes in youth study. J Am Heart Assoc 2019;8, e010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulakova T, Janda J, Cerna J, Janstova V, Feber J. Assessment of arterial stiffness from ambulatory blood pressure monitoring in children with diabetes mellitus type-1 (DMT1). J Hum Hypertens 2012;26:357–64. [DOI] [PubMed] [Google Scholar]
- 15.Laugesen E, Hansen KW, Knudsen ST, Erlandsen M, Ebbehoj E, Poulsen PL. Reproducibility of the ambulatory arterial stiffness index in patients with type 1 diabetes mellitus. Blood Press Monit 2010;15:18–22. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Wang JG, Dolan E, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension 2006;47:359–64. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Kim JH, Kang MJ, Lee YA, Won Yang S, Shin CH. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care 2011;34: 2180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips RA, Sheinart KF, Godbold JH, Mahboob R, Tuhrim S. The association of blunted nocturnal blood pressure dip and stroke in a multiethnic population. Am J Hypertens 2000;13:1250–5. [DOI] [PubMed] [Google Scholar]
- 19.Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994;24: 793–801. [DOI] [PubMed] [Google Scholar]
- 20.Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res 2012;35:695–701. [DOI] [PubMed] [Google Scholar]
- 21.Tsivgoulis G, Vemmos KN, Zakopoulos N, et al. Association of blunted nocturnal blood pressure dip with intracerebral hemorrhage. Blood Press Monit 2005;10:189–95. [DOI] [PubMed] [Google Scholar]
- 22.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep 2004;27:1097–103. [DOI] [PubMed] [Google Scholar]
- 23.Evgenia Gourgari GP, Konstantinopoulou S. Obstructive sleep apnea in a lean child 427 with type 1 diabetes: is it linked to diabetes? Endocr Pract 2020. (In press). [DOI] [PubMed] [Google Scholar]
- 24.Manin G, Pons A, Baltzinger P, et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabet Med 2015;32:90–6. [DOI] [PubMed] [Google Scholar]
- 25.Perfect MM. Sleep-related disorders in patients with type 1 diabetes mellitus: current insights. Nat Sci Sleep 2020;12:101–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa MP, Multari G, Montesano M, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia 2000;43:696–702. [DOI] [PubMed] [Google Scholar]
- 27.Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- 28.Dost A, Klinkert C, Kapellen T, et al. Arterial hypertension determined by ambulatory blood pressure profiles: contribution to microalbuminuria risk in a multicenter investigation in 2,105 children and adolescents with type 1 diabetes. Diabetes Care 2008;31:720–5. [DOI] [PubMed] [Google Scholar]
- 29.Miller JA, Curtis JR, Sochett EB. Relationship between diurnal blood pressure, renal hemodynamic function, and the renin-angiotensin system in type 1 diabetes. Diabetes 2003;52:1806–11. [DOI] [PubMed] [Google Scholar]
- 30.Group ET, Wuhl E, Trivelli A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med 2009;361:1639–50. [DOI] [PubMed] [Google Scholar]
- 31.Stergiou GS, Alamara CV, Salgami EV, Vaindirlis IN, Dacou-Voutetakis C, Mountokalakis TD. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press Monit 2005;10:143–7. [DOI] [PubMed] [Google Scholar]


