Abstract
Rationale: High-flow nasal therapy (HFNT) has beneficial effects in patients hospitalized with acute hypoxemic respiratory failure. HFNT has not been extensively studied following hospitalization for an acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Objective: We explored the feasibility of conducting a multicentered trial to evaluate the use of HFNT to increase the time to next moderate/ severe exacerbation in patients recently hospitalized for a COPD exacerbation. In this pilot study we measured the hours of home daily HFNT use, maximally tolerated flow rates and temperature, and side effects for a period of 90 days.
Methods: Patients were enrolled in a 90-day, open-labeled pilot study of HFNT to determine the safety and feasibility of home use for daily outpatient COPD management. Patients ≥ 40 years of age with prior hospitalization within the past 12 weeks for an AECOPD were enrolled. COPD was the primary diagnosis in all patients.
Results: Thirty patients presented for HFNT titration. Two dropped out; 1 after receiving a lung transplant and the other was lost to follow-up. The remaining 28 patients completed 90 days of HFNT. None withdrew from HFNT due to intolerance. Use of HFNT averaged 6.8 (2.1) hours daily.
Conclusions: Daily home HFNT for up to 3 months is feasible in COPD patients following hospitalization for AECOPD. Improvements observed in disease-specific quality of life, respiratory symptoms, and 6-minute walk distance suggest the need for a prospective multicenter controlled clinical trial.
Keywords: copd, acute exacerbation of COPD, high-flow nasal therapy, home care
Introduction
Acute exacerbations account for 68% of chronic obstructive pulmonary disease (COPD) total care costs in the United States; hospitalizations account for the majority of costs.1 COPD is the 3rd leading cause of rehospitalization; re-hospitalization is associated with significant morbidity and mortality and also increases costs.2 The Agency for Health Care Policy Research reported, in 2008, that 21% of U.S. COPD patients were readmitted within 30 days and in 85% of readmissions, COPD was the primary or secondary diagnosis.3 Although many drug therapies prevent COPD exacerbations, as of yet, none primarily reduce mortality rates.2,4 Therapies that reduce ventilatory workload, are well tolerated, and easy to implement are needed as treatments for patients with COPD, especially in patients at risk for repeated hospitalization.
High flow nasal therapy (HFNT) is an approach that may help manage patients with COPD at risk for repeated exacerbations, especially those at risk for repeated hospitalization. HFNT delivers warmed, humidified air-oxygen blends with flow rates up to 60L/min with fraction of inspired oxygen (FiO2) from 21%–100%. HFNT enhances oxygenation by closely matching the patient’s inspiratory flow rate and minimizing ambient air entrainment during spontaneous breathing.5 Because of its higher flow rate, HFNT washes out nasopharyngeal dead space,6 improves ventilation efficiency,7 and provides a small but incremental flow-based increase in positive airway pressure.8 These are associated with an increase in end-inspiratory lung volume, reduced airway resistance, and increased functional residual capacity.9,10 HFNT has been reported to be better tolerated than standard nasal oxygen therapy because the delivered warmed, humidified gases reduces airway cooling and desiccation and may, thereby, moisten secretions and facilitate mucociliary clearance.11,12
Clinical trials using HFNT in acute hypoxemic respiratory failure have shown HFNT to be superior to oxygen (O2) or noninvasive ventilation (NIV) in ventilator free days and 90-day mortality13 and non-inferior to NIV in preventing reintubation after cardiothoracic surgery.14 HFNT has been reported superior to O2 in decreasing reintubation in critically ill patients at risk for reintubation,15 and reducing hospitalization stays following thoracic resection.16 HFNT improves oxygenation for the same level of FiO2 compared to a Venturi mask but with better patient comfort, fewer desaturations, less interface displacements, and a lower reintubation rate.17,18 In contrast to data about HFNT use in the above patient populations, data about its use in patients with COPD in the outpatient setting is limited.19-20-21-22 The mechanisms of potential benefit in this population have been described,23 but home use of HFNT is not available. The success of any clinical trial is largely driven by site investigators’ resources and capabilities, and the willingness of study participants to complete all study-related procedures and adhere to the proposed intervention. With that in mind, we performed a single-center study of HFNT use that incorporated many of the procedures and interventions that might be used in a multicentered trial to determine study feasibility. In this 90-day pilot study we measured the hours of daily HFNT use, the ranges of maximally tolerated flow rates and temperature, as well as any side effects that resulted in poor tolerance or termination of HFNT use.
Methods
Study participants were recruited from the pulmonary care unit of the Ambulatory Care Center of Temple University Hospital. Eligibility requirements were age ≥40 years and hospital admission for an acute exacerbation of COPD (AECOPD) within the past 12 weeks. All patients had to have recovered from their COPD exacerbation and be clinically stable. Patients must have had COPD as the primary diagnosis and have smoked ≥10 pack years. The main exclusion criteria were upper airway or nasal complications that prohibited the use of HFNT. Patients using any positive airway pressure (PAP) therapy (e.g., continuous-PAP or non-invasive positive-pressure ventilation) ≤4 weeks before study entry were excluded. Women of childbearing potential had a urine pregnancy test to ensure that they were not pregnant. Patients provided informed consent and expressed a willingness to participate in all study-related activities.
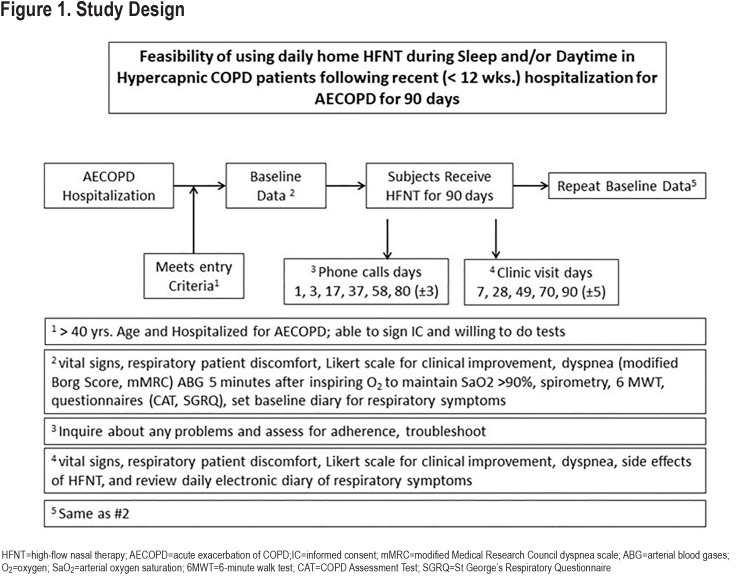
Study Design
The overall trial design is presented in Figure 1. Patients who met eligibility criteria and provided informed consent were scheduled for an outpatient visit. Prior to any other study-related procedures, patients were screened for a high likelihood of sleep apnea:
Snoring-Tiredness-Observed apnea-high blood Pressure-Body mass index (BMI)-age-neck circumference-gender questionnaire (STOPBang)24scores ≥5 or STOPBang score ≥ 2 plus BMI > 35 kg/m2
Berlin questionnaire scores suggesting a high likelihood of sleep apnea with increased risk of sleep-related accidents25
excessive daytime sleepiness (i.e., either a high [>15] score on the Epworth Sleepiness Scale or a“fall asleep” accident or “near miss” accident in the prior 12 months).26
Intervention
All study participants were asked to use the myAirvo™ 2 device (Fisher & Paykel Healthcare) for a minimum of 4 hours per night. The myAirvo™ 2 device is a humidifier with an integrated flow generator that delivers high-flow, warmed, and humidified respiratory gases to spontaneously breathing patients. The flow rate was initially set at 25 L/min and titrated upward to maintain a blood oxygen saturation determined by pulse oximetry (SpO2)of 90% or more at a temperature of 34°-37° C. Airflow was delivered through the OptiflowTM+ nasal cannula (Fisher & Paykel Healthcare) sized for patient comfort.
Measurements
Demographic data including age, gender, race, current medications, and comorbid conditions was collected at enrollment. At the baseline visit, vital signs were obtained as were dyspnea scores (modified Borg score,27,28 modified Medical Research Council [mMRC] dyspnea score29). Arterial blood gases (ABG) after 5 minutes of inspiring supplemental oxygen to keep arterial oxygen saturation (SaO2)> 90% were collected as well as the use of accessory muscles of ventilation. Pre-and post-bronchodilator spirometry was performed. Patients completed the COPD Assessment Test (CAT),30 and the St George’s Respiratory Questionnaire (SGRQ).31 Patients performed a 6-minute walk test (6MWT).32 The patient’s Global initiative for chronic Obstructive Lung Disease (GOLD) grade33 was determined and Body mass index-airway Obstruction-Dyspnea-Exercise (BODE) scores were calculated.34 For the primary outcome of patient comfort, a 100mm visual analog scale was used and anchored at 0 (no discomfort) and 100 (maximal imaginable discomfort). A 5-point Likert scale was used for patients to report their sensation of shortness of breath from “marked deterioration” to “marked improvement.” Patient comfort and sensation of shortness of breath were recorded at the baseline visit and at each of 5 subsequent clinic visits. Based on these reports, changes were made to flow rates and temperatures as needed.
Patients were provided with an electronic daily COPD symptom diary to record breathlessness, cough, consistency and quantity of sputum production, and the presence of wheezing, sore throat, or nasal congestion as well as daily peak flow readings.35 Patients were provided with a PersonalBest® (Philips Respironics) peak flow meter and asked to make 3 peak flow maneuvers. The best of the 3 maneuvers was recorded on the electronic symptom diary.
After a 2-week run-in period for symptom reporting, the patient returned to initiate HFNT therapy. Follow-up clinic visits and telephone contacts are shown in Figure 1.
At the conclusion of the study patients were offered the opportunity to continue to use HFNT therapy if they wished to do so.
Sample Size
This pilot study was powered to detect an unacceptably high rate of safety issues or unforeseen problems. The sample size was estimated based on the method described by Viechtbauer et al for detecting problems that arise in pilot studies. Using a 95% confidence threshold and 10% probability that a safety event occurred (defined as the need to discontinue HFNT due to intolerance) the required sample size was 29 patients.
Statistical Analysis
Descriptive statistics (mean [standard deviation (SD)] or median [interquartile range (IQR)])are reported for continuous variables to characterize the patient population in terms of demographic factors and severity of airflow obstruction. Changes in arterial blood gases, 6MWT, CAT and SGRQ scores, and spirometry values were analyzed using paired t-tests. All tests were performed using JMP® Pro 14.2.0, © 2018 SAS Institute Inc.
Study Conduct, Approval, and Registration
This trial was conducted in accordance with the Declaration of Helsinki principles. The study protocol was approved by the Western Institutional Review Board (Approval Number: 20170689), and written informed consent was obtained from all study participants before they entered the trial. This trial was registered with ClinicalTrials.gov (NCT03221387) prior to the enrollment of the first study participant.
Funding Source
Fisher & Paykel Healthcare contributed towards the funding for this study.
Results
Forty-one patients who were recently hospitalized for a COPD exacerbation were consented for the study (Figure 2). Two of these were subsequently excluded because of a high risk of having sleep apnea (by questionnaire) and 1 by a sleep study that revealed sleep apnea. Eight patients did not return for the high-flow titration visit and did not receive study intervention. Two patients who did start high-flow therapy subsequently dropped out before the end of the 90-day evaluation visit (1 received a lung transplant and another was lost to follow-up).
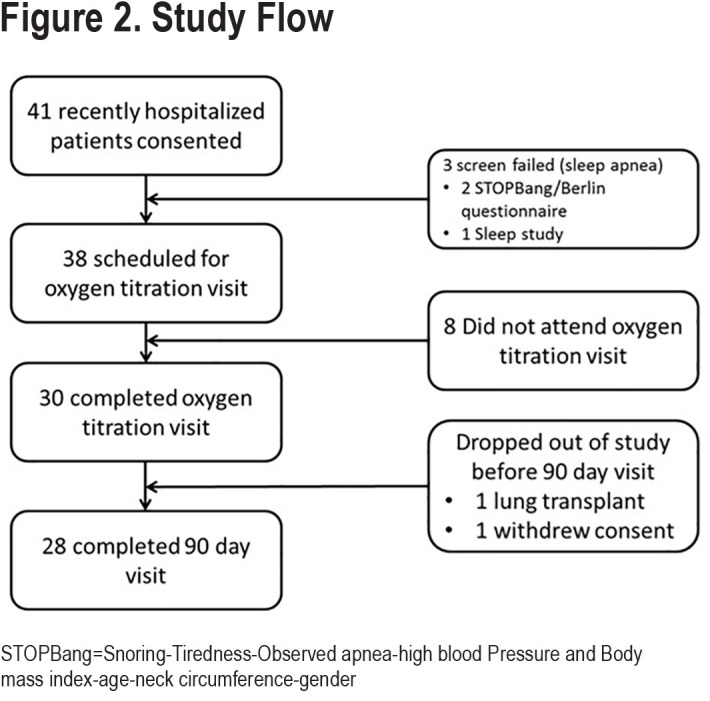
This report reflects the findings of the 28 patients who completed the 90-day visit. Baseline characteristics of the study participants are presented in Table 1.
Nasal High Flow parameters
HFNT was delivered at 35 LPM in 24 patients and 30 LPM in 4 patients. Prior to the application of HFNT, 16patients (57%) maintained adequate oxygen saturations with an FiO2 of 21% (room air). The remaining 12 patients required supplemental oxygen with FiO2 ranging between 26% and 32%. No changes were made to the oxygen prescription after application of the device. All patients left the oxygen titration visit with heated humidified high flow at 37° C. One patient subsequently asked that the temperature be reduced to 34° C at a follow-up visit.
Primary Outcomes
Of the 30 patients initially enrolled in the study, 28 continued to use HFNT throughout the 90-day observation period.
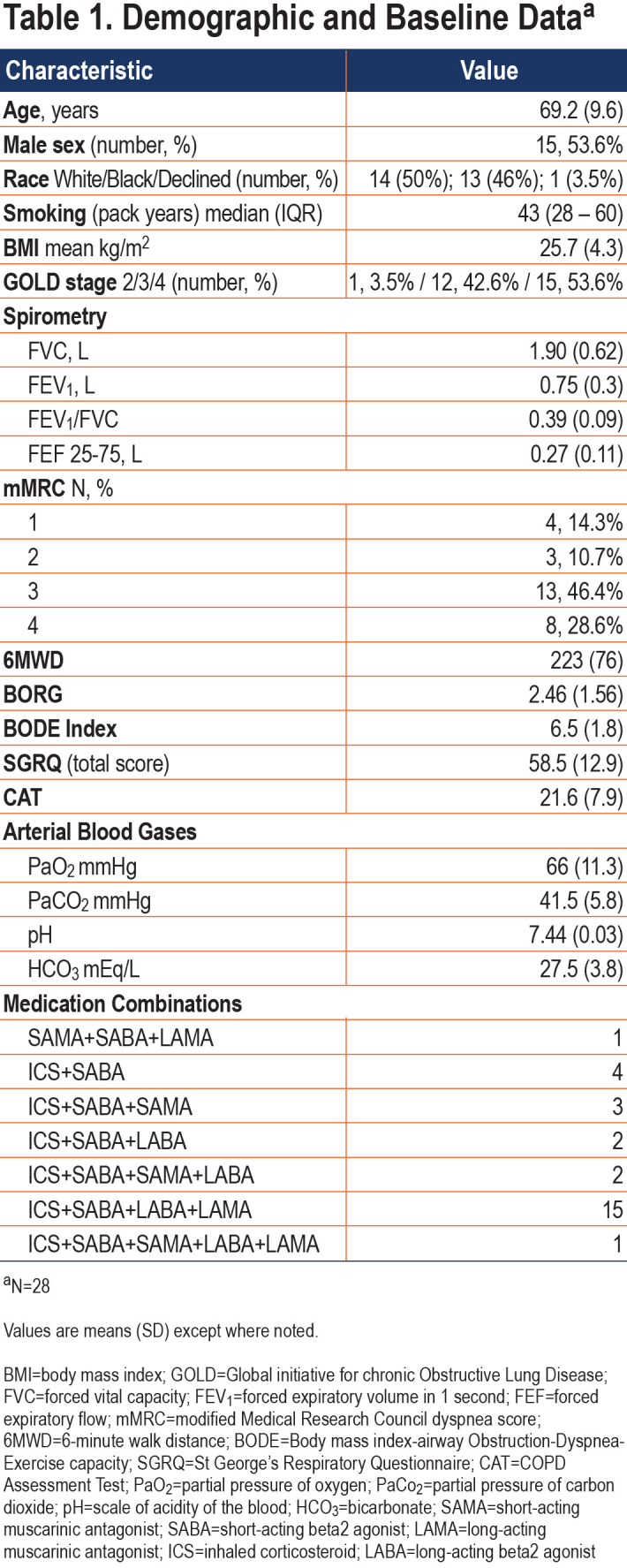
Patients used HFNT for an average of 6.8 (SD 2.1) hours daily. Visual analog scores for patient comfort are shown in Figure 3. The scores among all patients averaged between 20mm and 30mm for each of the clinic visits although, there was substantial variability in scores, hence, they are reported as mean score ± standard error of the mean (SEM).
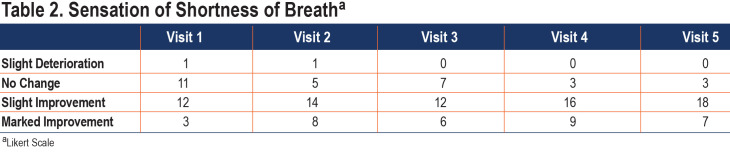
The effect of HFNT on the sensation of breathlessness tended to improve over the course of the study with 25 of 28 patients reporting a slight to marked improvement in the sensation of breathlessness by visits 4 and 5. (Table 2)
Secondary Outcomes
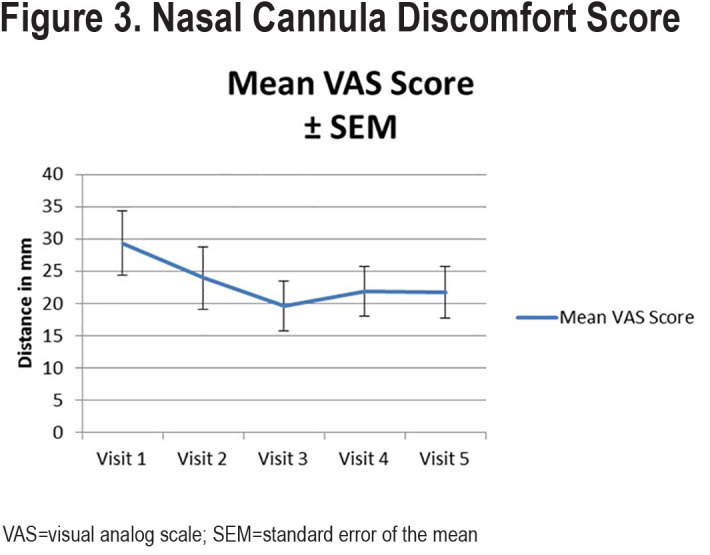
There were no statistically significant changes in any of the secondary outcomes, which included spirometry, arterial blood gases, 6MWT, SGRQ, and the CAT. (Table 3)
COPD Symptom Reports
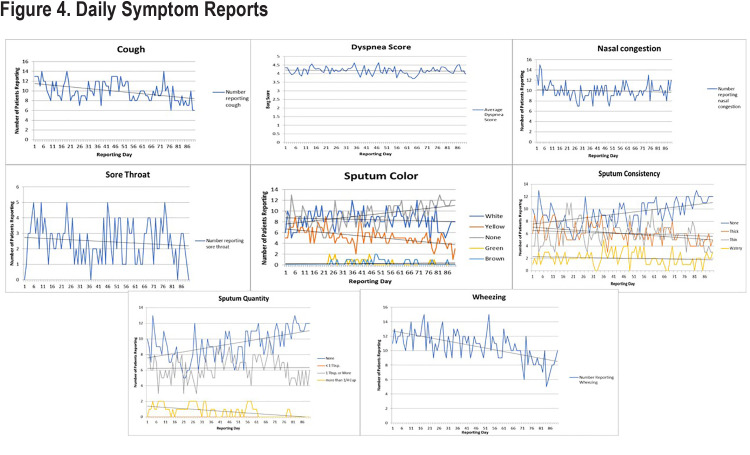
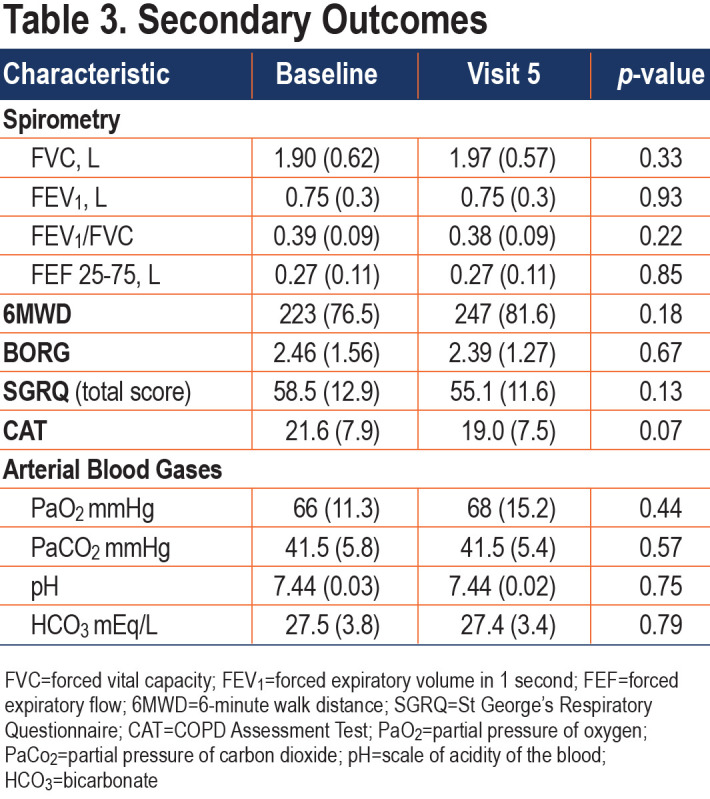
COPD symptom reports showed that approximately 84% of all potential daily symptom reports (Figure 4) were made; median symptom reporting compliance was 85% (IQR 75% –93%). Trends for each of the COPD symptoms and peak flow readings are shown in Figure 5. Over the course of the 90-day study, only 7 reports of increased temperature were made by 6 different patients. Cough and wheezing were the most frequently reported COPD symptoms and tended to decrease over the study period. Nasal congestion and sore throat were less frequently reported, and the number of reports remained stable. Dyspnea scores remained unchanged. Average peak flow readings increased slightly during the reporting period from just over 200L/min to around 235L/min. Several changes were observed in the characteristics of sputum production. The number of patients who reported no sputum production trended upward over the course of the study with patients reporting decreases in thick, thin, and watery sputum consistency. Very few individuals reported production of green or brown sputum. The numbers of patients who produced white or yellow sputum decreased over time with the majority moving towards no sputum production.
Discussion
In our study of HFNT in patients recently discharged from the hospital for a COPD exacerbation, we met our primary objective of assessing the feasibility of daily use over 90 days. In this population of patients with very severe COPD and recent hospitalization, we found acceptable daily usage of HFNT at optimal HFNT settings of flow and humidification and no untoward side effects or technical issues. No patients who were started on HFNT needed to be removed for lack of efficacy or intolerance, although 2 patients dropped out: 1 after receiving a lung transplant and the other was lost to follow-up despite multiple attempted contacts. While we asked patients to use HFNT for a minimum of 4 hours daily, the overall daily use was greater than that and averaged about 6 hours per day. Patients agreed to and were able to complete all study-related assessments.
Pearson has defined comfort as a physical sensation, a psychological state, or a combination of both.36 Measurement of comfort is by definition, subjective. In a comparison study between nasal high-flow therapy and Venturi mask oxygen therapy by Maggiore, patients were asked about discomfort with the nasal interface and symptoms of airway dryness.17 This was done by using a 0 to 10 scale correlating with no discomfort and maximum discomfort respectively. Visual analog scales (VAS) have also been used.36 We utilized a 100mm VAS to assess patient discomfort while using the device. Mean VAS scores averaged near 30mm at the start of the study and decreased over time; however, there was substantial variability in the comfort scores even in the same patient between visits. When used for the assessment of pain, a score of 30mm or less is typically defined as “mild pain.”37 It appears that many patients experienced mild discomfort around the nares using the device at some point over the course of the study, though none discontinued using the device due to this mild discomfort.
Although there were no statistically significant differences in the secondary outcomes, there was a 24-meter increase in 6-minute walk distance, and a 3.4-point drop in the SGRQ. The improvement in 6-minute walk distance parallels the observation in another controlled trial of HFNT versus usual care.21 Their same study showed a stabilization of the SGRQ in the HFNT group compared to a slight worsening in the usual care group. Despite being unpowered to reach this endpoint, our study also showed a 2.6-point drop in the CAT, which just failed to reach statistical significance (p=0.07).
Most of the symptom scores reported in the daily electronic diary remained essentially unchanged over the study including nasal congestion, Borg dyspnea score, presence of sore throat, and peak flow readings. What did trend downward were the number of reports of wheezing and cough, and changes in the color, consistency, and volume of sputum. Patients who produced white or yellow sputum transitioned to none; those producing thick or thin sputum transitioned to watery or no sputum production; and those who were producing a tablespoonful or more of sputum daily transitioned to no or lower levels of sputum production. The reasons for the shift to no or lower levels of sputum production are not known but the application of heated and humidified HFNT has been reported to facilitate secretion removal.38 It may be that HFNT users find it easier to cough and clear secretions, but this was not objectively measured in our pilot study.39 A recent study by Choate and colleagues shows that those who experience more cough and sputum production have worse clinical and quality of life outcomes.40 Even so, there is no evidence to suggest that reducing cough or altering the volume, consistency, or color of sputum results in improvements in clinical or quality of life measures. Our study was not powered to show improvements in these outcomes.
By study’s end, 25 of 28 patients reported a slight to marked improvement in the sensation of breathlessness. This occurred even though there were no measurable changes in the Borg dyspnea scale, daily peak flow measures, arterial blood gases, or spirometry, which suggests that factor(s) other than dyspnea may be represented by this measurement. The finding of improvement in the sensation of breathlessness is consistent with a report of the qualitative experiences of domiciliary HFNT users and their relatives obtained by Storgaard.39 Her structured interviews identified 6 themes that related to therapy adherence. These included: a perceived lower work of breathing, reduced COPD symptoms, improvement in sleep quality, increased activity of daily living, feeling safe, and technology use. Perhaps a reduction in respiratory rate in patients with severe obstruction when using HFNT resulted in a prolongation of expiratory time and decrease in the extent of air-trapping, a known consequence of severe exacerbation in patients hospitalized with a COPD exacerbation.41 Further studies will need to measure the impact of HFNT on air trapping.
Limitations
This study has several limitations. First, as a feasibility study the number of enrolled patients was small and performed at a single clinical center. Second, all patients were assigned to the intervention and served as their own control. Third, the use of the VAS and Likert scale to assess the sensation of shortness of breath have not been validated.
Conclusions
This single center study demonstrates the feasibility of using daily home HFNT in COPD patients recently discharged from the hospital for a COPD exacerbation. The therapy was well tolerated, and no patient needed to be removed from the intervention. The trends for improvements in quality of life measured by SGRQ, the CAT, and 6-minute walk distance suggests that home HFNT may have benefit in patients following a recent COPD hospitalization. These findings require validation by a well-powered, prospective, multicentered, controlled trial.
Abbreviations
Abbreviations: high-flow nasal therapy, HFNT; acute exacerbation of COPD, AECOPD; chronic obstructive pulmonary disease, COPD; fraction of inspired oxygen, FiO2; oxygen, O2; noninvasive ventilation, NIV; positive airway pressure, PAP; informed consent, IC; body mass index, BMI; Snoring-Tiredness-Observed apnea-high blood Pressure-high BMI-age-neck circumference-gender, STOPBang; blood oxygen saturation determined by pulse oximetry, SpO2; modified Medical Research Council, mMRC; arterial oxygen saturation, SaO2; COPD Assessment Test, CAT; St George’s Respiratory Questionnaire, SGRQ; 6-minute walk test, 6MWT; Global initiative for chronic Obstructive Lung Disease, GOLD; BMI-airway Obstruction-Dyspnea-Exercise capacity scale, BODE; standard deviation, SD; interquartile, IQR; partial pressure of oxygen, PaO2; partial pressure of carbon dioxide, PaCO2; scale of acidity of the blood, pH; bicarbonate, HCO3; short-acting muscarinic antagonist, SAMA; short-acting beta2 agonist, SABA; long-acting muscarinic antagonist, LAMA; inhaled corticosteroid, ICS; long-acting beta2-agonist, LABA; standard error of the mean, SEM; visual analog scales, VAS
Funding Statement
Funding, equipment, and supplies were provided by Fisher & Paykel Healthcare
References
- 1.Strassels SA,Smith DH,Sullivan SD,Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344-352. doi: https://doi.org/10.1378/chest.119.2.344 [DOI] [PubMed] [Google Scholar]
- 2.Marchetti N,Criner GJ,Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013;143(5):1444-1454. doi: https://doi.org/10.1378/chest.12-1801 [DOI] [PubMed] [Google Scholar]
- 3.Elixhauser A,Au DH,Podulka J. Readmissions for chronic obstructive pulmonary disease, 2008: statistical brief #121. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Agency for Healthcare Research and Quality (US). 2011.doi: https://pubmed.ncbi.nlm.nih.gov/22049571/ [PubMed] [Google Scholar]
- 4.Wedzicha J,Calverley P,Albert R,et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;50(3):1602265. doi: https://doi.org/10.1183/13993003.02265-2016 [DOI] [PubMed] [Google Scholar]
- 5.Sim MA,Dean P,Kinsella J,Black R,Carter R,Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63(9):938-40. doi: https://doi.org/10.1111/j.1365-2044.2008.05536.x [DOI] [PubMed] [Google Scholar]
- 6.Kumar GK,Nanduri J,Peng YJ,Prabhakar NR. Neuromolecular mechanisms mediating the effects of chronic intermittent hypoxia on adrenal medulla. Respir Physiol Neurobiol. 2015;209:115-119.doi: https://doi.org/10.1016/j.resp.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mündel T,Feng S,Tatkov S,Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol (1985). 2013;114(8):1058-1065. doi: https://doi.org/10.1152/japplphysiol.01308.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parke RL,McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58(10):1621-1624. doi: https://doi.org/10.4187/respcare.02358 [DOI] [PubMed] [Google Scholar]
- 9.Riera J,Pérez P,Cortés J,Roca O,Masclans JR,Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58(4):589-596.doi: https://doi.org/10.4187/respcare.02086 [DOI] [PubMed] [Google Scholar]
- 10.Sztrymf B,Messika J,Bertrand F,et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780-1786. doi: https://doi.org/10.1007/s00134-011-2354-6 [DOI] [PubMed] [Google Scholar]
- 11.Papazian L,Corley A,Hess D,et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42(9):1336-1349.doi: https://doi.org/10.1007/s00134-016-4277-8 [DOI] [PubMed] [Google Scholar]
- 12.Spoletini G,Alotaibi M,Blasi F,Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253-261. doi: https://doi.org/10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 13.Frat JP,Thille AW,Mercat A,et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196.doi: https://doi.org/10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 14.Stephan F,Barrucand B,Petit P,et al. high-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331-2339.doi: https://doi.org/10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 15.Hernández G,Vaquero C,Colinas L,et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565-1574.doi: https://doi.org/10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 16.Ansari BM,Hogan MP,Collier TJ,et al. A randomized controlled trial of high-flow nasal oxygen (optiflow) as part of an enhanced recovery program after lung resection surgery. Ann Thorac Surg. 2016;101(2):459-464. doi: https://doi.org/10.1016/j.athoracsur.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 17.Maggiore SM,Idone FA,Vaschetto R,et al. Nasal High-flow versus venturi mask oxygen therapy after extubation. effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282-288. doi: https://doi.org/10.1164/rccm.201402-0364OC [DOI] [PubMed] [Google Scholar]
- 18.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529-541.doi: https://doi.org/10.4187/respcare.04577 [DOI] [PubMed] [Google Scholar]
- 19.Bräunlich J,Dellweg D,Bastian A,et al. Nasal high-flow versus non-invasive ventilation in patients with chronic hypercapnic COPD. Eur Respir J. 2020;56(suppl 64):4350. doi: https://doi.org/10.1183/13993003.congress-2020.4350 [Google Scholar]
- 20.Bräunlich J,Dellweg D,Bastian A,et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1411-21.doi: https://doi.org/10.2147/COPD.S206111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storgaard LH,Hockey H-U ,Laursen BS,Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2018;13:1195-1205.doi: https://doi.org/10.2147/COPD.S159666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata K,Kikuchi T,Horie T. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. A multicenter randomized crossover trial. Ann Am Thorac Soc. 2018;15(4):432-439.doi: https://doi.org/10.1513/AnnalsATS.201706-425OC [DOI] [PubMed] [Google Scholar]
- 23.Pisani L,Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD. 2017;14(3):346-350.doi: https://doi.org/10.1080/15412555.2017.1315715 [DOI] [PubMed] [Google Scholar]
- 24.Chung F,Abdullah HR,Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638. doi: https://doi.org/10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 25.Netzer NC,Stoohs RA,Netzer CM,Clark K,Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491. doi: https://doi.org/10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.doi: https://doi.org/10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 27.Meek PM. Measurement of dyspnea in chronic obstructive pulmonary disease: what is the tool telling you? Chron Respir Dis. 2004;1(1):29-37. doi: https://doi.org/10.1191/1479972304cd008ra [DOI] [PubMed] [Google Scholar]
- 28.Kendrick KR,Baxi SC,Smith RM. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26(3):216-222.doi: https://doi.org/10.1016/S0099-1767(00)90093-X [DOI] [PubMed] [Google Scholar]
- 29.Bestall JC,Paul EA,Garrod R,Garnham R,Jones PW,Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581-586.doi: https://doi.org/10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones PW,Harding G,Berry P,Wiklund I,Chen WH,Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.doi: https://doi.org/10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 31.Barr JT,Schumacher GE,Freeman S,LeMoine M,Bakst AW,Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22(9):1121-1145.doi: https://doi.org/10.1016/S0149-2918(00)80089-2 [DOI] [PubMed] [Google Scholar]
- 32.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: https://doi.org/10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 33.Global Initiative for Chronic Obstructive Lung Disease (GOLD).Global strategy for the diagnosis, management and prevention of COPD, 2019 report. GOLD website. Published 2019.Accessed September 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf [Google Scholar]
- 34.Celli BR,Cote CG,Marin JM,et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012.doi: https://doi.org/10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 35.Cordova FC,Ciccolella DE,Grabianowski CL,et al. A telemedicine-based intervention reduces the frequency and severity of COPD exacerbation symptoms: a randomized controlled trial. Telemed J E Health. 2016;22(2):114-122.doi: https://doi.org/10.1089/tmj.2015.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson EJ. Comfort and its measurement--a literature review. Disabil Rehabil Assist Technol. 2009;4(5):301-310. doi: https://doi.org/10.1080/17483100902980950 [DOI] [PubMed] [Google Scholar]
- 37.Hawker GA,Mian S,Kendzerska T,French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form Mcgill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(S11):S240-S52. doi: https://doi.org/10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 38.Spoletini G,Alotaibi M,Blasi F,Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253-261. doi: https://doi.org/10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 39.Storgaard LH,Weinreich UM,Laursen BS. COPD patients' experience of long-term domestic oxygen-enriched nasal high flow treatment: a qualitative study. COPD. 2020;17(2):175-183.doi: https://doi.org/10.1080/15412555.2020.1736998 [DOI] [PubMed] [Google Scholar]
- 40.Choate R,Pasquale CB,Parada NA,Prieto-Centurion V,Mularski RA,Yawn BP. The burden of cough and phlegm in people with COPD: a COPD patient-powered research network study. Chronic Obstr Pulm Dis. 2020;7(1):49-59. doi: https://doi.org/10.15326/jcopdf.7.1.2019.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson NJ,Walker PP,Costello RW,Calverley PM. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(12):1510-1516.doi: https://doi.org/10.1164/rccm.200504-595OC [DOI] [PubMed] [Google Scholar]