Abstract
Transoral robotic surgery (TORS) using the da Vinci Surgical system was approved by the US Food and Drug Administration in 2009. Currently, most available safety information on TORS procedures describes adverse events occurring in the context of clinical trials or series at high-volume academic centers. The goal of this study was to catalog reported adverse events associated with the da Vinci device in head and neck procedures by querying an FDA database. A search was performed on the MAUDE database inspecting for TORS safety incident reports generated from January 2009 through May 2020 using key words “da Vinci” and “Intuitive Surgical”. A total of 3312 medical device records were produced. Of these 36 head and neck adverse events, reports were identified through manual screening of the data by the authors. Death was found to be the most common adverse event reported overall, manifesting in 44% of all reported incidents. The most frequent source of mortality was found to be hemorrhaging in the perioperative period rather than incidents of device malfunction or structural damage from surgery. This was found to be similar to the results of other published series for transoral ablative surgery. This study suggests that the small number of reported adverse events related to TORS with the da Vinci system seems to mirror what would be expected from the same procedures using other methods for transoral surgery.
Keywords: Robotic surgery, MAUDE, Da Vinci, Intuitive surgical, TORS
Introduction
In 2009, the US Food and Drug Administration (FDA) approved the use of transoral robotic surgery (TORS) using the da Vinci system (Intuitive Surgical Inc., Sunnyvale, CA) for the treatment of early-stage benign and malignant tumors of the upper aerodigestive tract [1–3]. This approval augmented the growing list of surgical procedures performed using the da Vinci system which includes gynecologic, urologic, cardiothoracic, and general surgery applications [1, 4, 5].
To improve device safety, the FDA requires mandatory reporting of all adverse events by submitting a narrative summary to the MAUDE (Manufacturer and User Facility Device Experience) database [2, 4]. This post-marketing surveillance provides a passive means to understand adverse events associated with a device’s use and the influence those events may have on patients [4].
Several studies have previously analyzed various features of the adverse event data recorded for the da Vinci system on the FDA MAUDE database with most attention paid to mechanical failures, software failures, or device-related injuries [6–9]. Others have looked more in depth at adverse events specific to individual fields like urology [10] and gynecology [11] or a combination of the two [12]. One group has also performed a global assessment of adverse event risk for the subspecialties (gynecology, urology, cardiovascular, head and neck) that utilize the da Vinci surgical robot [4].
These publications suggest that underreporting of adverse events remains highly characteristic of records searched within the MAUDE database [4, 5, 9, 12]. Underreporting may limit an adequate understanding of the true risks and benefits associated with use of the da Vinci device [9]. Given the increasing use of robotic platforms in surgery, providers need to be informed of known adverse events to be able to counsel the patients about risks inherent to these procedures [5, 9, 12].
Currently, most available safety information on head and neck TORS procedures describes perioperative adverse events through prospective or retrospective studies [2, 3, 5, 13–15]. There exists little to no available information in terms of studies evaluating intraoperative adverse events. Additionally, the study results available on safety and feasibility tend to represent the experiences from trials performed at larger academic facilities [2, 3, 13] which may be different than results from smaller institutions and community hospitals.
The goal of this study was to catalog reported adverse events associated with the da Vinci device in head and neck procedures by querying an FDA database. A manual, longitudinal assessment of medical device records on the MAUDE database was performed to describe the types, distribution, and trends of adverse events being reported in robotic head and neck surgery since initial FDA approval of TORS procedures.
Materials and methods
MAUDE
The MAUDE database provides access to medical device reports (MDR) submitted to the FDA by mandatory reporters (manufacturers, importers, and device user facilities) [4]. Voluntary reporters who include patients and physicians may also contribute narratives. The database is updated on a weekly basis and can be searched for up to 10 years of prior reports for adverse events involving medical devices.
Primary uses of MAUDE are to monitor a medical product’s performance, identify device-related safety issues, and evaluate the benefits and risks for procedures that a device is used for. The benefit of MAUDE is that data generated on death, injury, and device malfunctions are provided in a contextual narrative that can help to potentially appreciate the underlying cause of an incident [4].
However, even with such benefit, there are important limitations to consider in interpreting study results derived from MAUDE data. As a passive surveillance system, information from the MAUDE database cannot be used to assess the incidence or prevalence of any issue identified within the narrative reports. Further, some reports rendered may be incomplete, inaccurate, or distorted based on the reporting source [4]. It is also important to note that disclosures made on MAUDE do not necessarily attribute cause of an adverse event to be the result of improper performance by the device itself, merely that the device was involved during the process of or leading up to an observed adverse event.
Data collection and processing
A search was performed on the MAUDE database (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm) inspecting for TORS safety incident reports generated from January 2009 through May 2020 using key words “da Vinci” and “Intuitive Surgical”. Inclusion criteria for TORS cases included reported surgical event logs describing the use of a da Vinci robot system to perform either a “transoral robotic surgery” or other head and neck surgical case (“thyroidectomy”, “laryngectomy”, ect.) using a TORS approach.
Currently, there are four generations of the da Vinci Surgical System approved for use in certain head and neck procedures by the U.S. FDA which includes the da Vinci Xi, da Vinci Si, da Vinci S, and the Standard da Vinci. The newest of these systems is the da Vinci Xi which received FDA approval in 2014. A separate search was also conducted specifically searching for head and neck surgery adverse events on the newer da Vinci Xi system from April 2014 through May 2020 using key words “da Vinci Xi”, “Xi”, and “Intuitive Surgical”.
Adverse events collected were further screened for record repeats which were consolidated when identified. Adverse event reports were grouped initially based on the general outcomes which included death, injury, and device malfunction. Categorized records were then subdivided by year and procedure type to better understand reporting tendencies. Descriptive statistical methods were utilized to process the data which included the calculation of means, standard deviation, and confidence intervals.
Results
A total of 3312 MDR logs were produced using the search methods described. These records consisted of a mix of field testing reports, preventative maintenance logs, and safety event reports for cases in gynecology, urology, general surgery, cardiothoracic, and head and neck procedures. Of these, 43 TORS cases were identified through manual screening of the data by the authors. All adverse events reported on head and neck procedures used the da Vinci Si or earlier systems and no narrative records were discovered that described the Xi system. Of the 43 cases identified, further efforts were undertaken to screen the reported events for duplications. Repeats of cases were identified in 7 instances from the data collected, thereby condensing the total number of actual TORS MDR events to 36.
The number of events reported was found to vary annually over the study period. Figure 1 provides a look at the distribution of annual adverse event reports. The number of reported adverse events for TORS robotics procedures remained small across the 11 years examined and appeared to diminish in recent years.
Fig. 1.
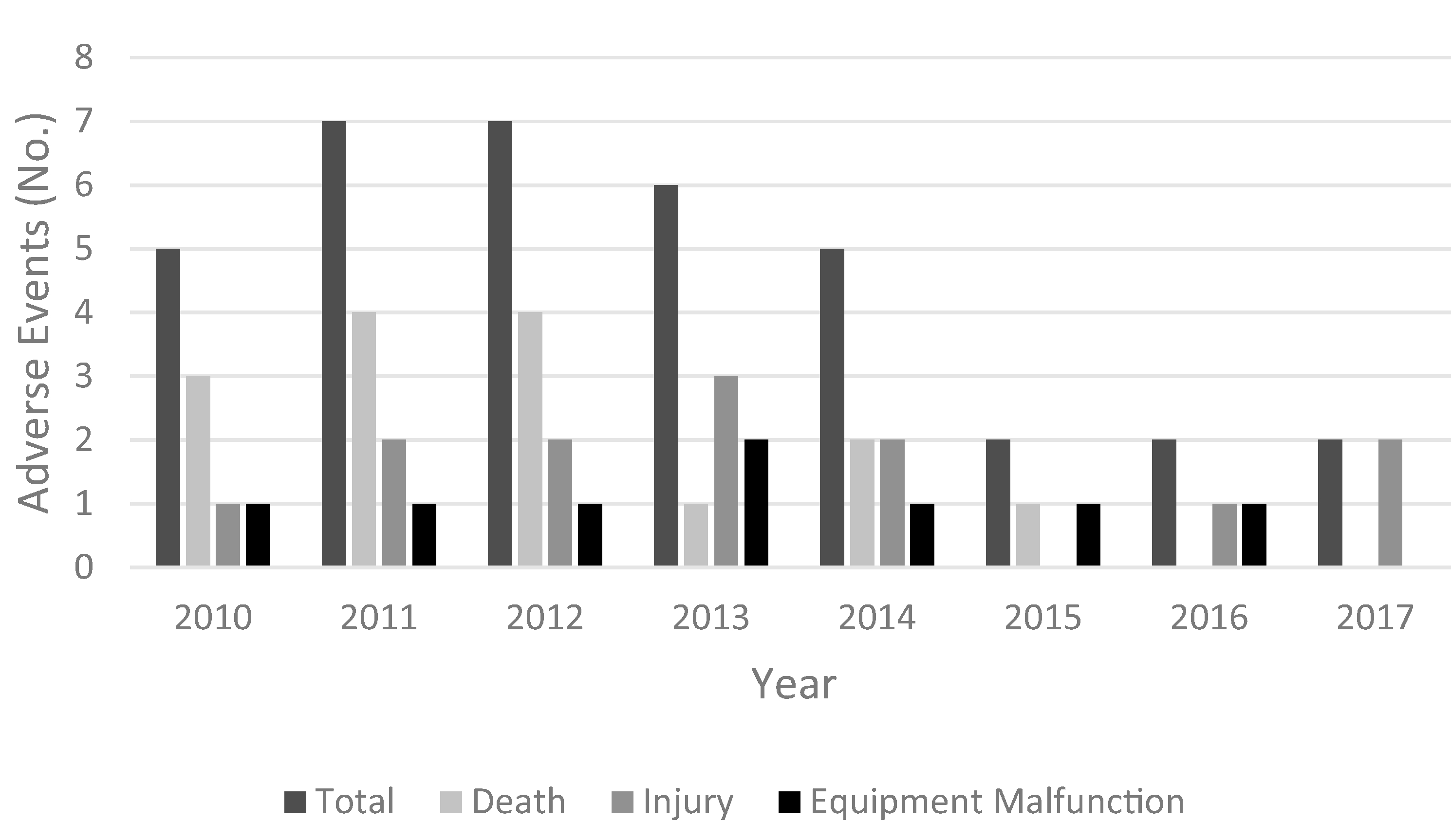
Results from the head and neck MDRs collected from the MAUDE database by year demonstrated a slight annual variation in event reporting. In 2009 and 2018–2020, no adverse events were reported. Overall the total number of events reported for robotic procedures within the head and neck specialty remained small. A greater number of death associated adverse events composing the total of those annually reported can be observed from MDR reports made prior to 2013. Injury and equipment failures appeared to be more commonly reported beyond 2013
The average lag time for all events was 127 days with a range of 4 to 1050 days. Out of the 36 MDRs examined, 5 were incomplete and did not have an event date indicated in the record. Adverse event categories that did not have dates recorded consisted of 2 injuries and 3 deaths. As a result, the injury calculations for lag time were made from 9 events and those for death were from 12 events. A longer average delay in reporting was found for adverse events resulting in patient death. Device malfunction had the lowest lag in reporting time on average. High variability in reporting time was identified within each adverse event category and no differences in average lag times between categories were found to be significant based on the 95% confidence interval. Average lag times for each adverse event category are presented in Table 1.
Table 1.
Adverse event reporting lag time
| Adverse event | Average days (± S.D.) | 95% confidence interval |
|---|---|---|
|
| ||
| Death | 151 (309) | ± 196 |
| Injury | 143 (194) | ± 149 |
| Malfunction | 78 (111) | ± 86 |
Head and neck MDR records were further analyzed according to the type of procedure performed and associated adverse event experienced (death, injury, device malfunction). The results of this analysis are presented in Table 2.
Table 2.
Adverse event distribution by procedure type
| Case type | No. (%) | Adverse events |
||
|---|---|---|---|---|
| Death | Injury | Device malfunction | ||
|
| ||||
| TORS (unspecified) | 13 (36.1) | 4 | 4 | 5 |
| BOT | 8 (22.2) | 6 | 1 | 1 |
| SAP | 3 (8.3) | 1 | 2 | 0 |
| Thyroidectomy | 3 (8.3) | 0 | 2 | 1 |
| Multiple site resection* | 3 (8.3) | 2 | 1 | 0 |
| Pharyngectomy | 2 (5.5) | 0 | 2 | 0 |
| Partial laryngectomy | 1 (2.7) | 1 | 0 | 0 |
| Supraglottic resection | 1 (2.7) | 1 | 0 | 0 |
| Neck dissection | 1 (2.7) | 0 | 1 | 0 |
| Tonsillectomy | 1 | 0 | 1 | 0 |
| Total | 36 | 15 | 13 | 8 |
BOT base of tongue, SAP sleep apnea procedure, TORS transoral robotic surgery
Surgical resection areas included portions in various combination of the pharynx, tonsils, palate, and base of tongue
Unspecified TORS procedures were most commonly reported to have an adverse event and the most prominent category with narratives of device malfunction and injury. Base of tongue (BOT) was second in the quantity of adverse events reported and had the greatest number of patient deaths.
Death was found to be the most common adverse event reported overall, composing 42% of all recorded incidents. In the vast majority, 93% (n = 14), of reported deaths the cited cause of death in the narrative was from postoperative surgical site hemorrhage. Hemorrhaging was noted as the reason for adverse event reporting in 53% (n = 18) of all cases. For the 18 cases with hemorrhaging, 83% (n = 14) had fatal outcomes and 17% (n = 3) were non-fatal. The average postoperative time to surgical site hemorrhage of any outcome was 5 days after a robotics procedure with a range from 2 to 10 days. In 5 cases, there was no recorded postoperative day in the record for when bleeding occurred. In the only case of patient death not cited to be caused by postoperative bleeding, patient mortality resulted from a pulmonary infection obtained after aspirating during a postoperative hemorrhage.
For cases of injury, cautery lesions were the most commonly recorded composing 46% (n = 6) of events. Cautery burns most often occurred on the lip when a non-insulated segment of the cautery unit contacted the lip during the procedure. In one instance of cautery burn, the incorrect grounding pad was placed on the patient. Remaining causes of injury that were identified during or surrounding the time of a robotic procedure included non-fatal hemorrhage, 31% (n = 4); nerve compression, 9% (n = 1); and unintended laceration, 9% (n = 1). In one case of injury, the attributed source was not identified in the report.
We found no reports of the da Vinci surgical system implicated as the direct cause of any surgical catastrophe. Instead, the deaths and injuries observed were described by both physicians and manufacturer representatives as complications known as risks with the listed procedures.
Attributed causes of device malfunction reported in MDRs included power supply failure (n = 2), endoscope camera manipulator malfunction (n = 2), cautery component malfunction (n = 2), non-intuitive robotic arm movements (n = 1), and persistent error code messages (n = 1). In all but 1 case, the decision was made to convert to an open procedure. In the one instance of device malfunction where the TORS procedure continued, the issue was resolved by exchanging the monopolar cautery unit.
Discussion
Head and neck procedures utilizing the da Vinci system have previously published lower numbers of disclosed reports on the MAUDE database than other specialties (urology, gynecology, cardiothoracic), but the submitted reports contain a higher number of serious adverse events [4, 5]. The other specialty applications represent larger case volumes reflecting the more common incidence of urologic and gynecologic cancers as well as specialty preferences in device utilization. The serious adverse events, including death, identified in the MAUDE system in the head and neck area are known complications of other approaches to transoral cancer resection. This study is not designed to compare the relative incidence of postoperative hemorrhage with transoral surgery utilizing the da Vinci system compared to other devices utilized for transoral resection.
In this study, post-operative hemorrhage represented the most common adverse event reported through MDR logs and the most common cause of death. One solution that has been proposed to address this concern is the prophylactic ligation of branch vessels from the external carotid artery supplying the TORS operational site. Several studies [16–18] have evaluated this technique although its impact on hemorrhage incidence has been mixed. It remains important to emphasize that the postoperative course for the majority of patients undergoing TORS procedures often tends to be simple and uneventful with quick return of alimentary function and brief hospital stays [3]. A number of multicenter reports examining the safety and efficacy of TORS help to better appreciate the prevalence of adverse events which are described most often in the postoperative setting [3, 13–15]. A systemic review of 247 cases performed by de Almeida et al. compiling adverse event reports from seven different prospective TORS procedure studies identified postoperative hemorrhage in 2% of patients with 1 case developing a neck hematoma and all cases resolving without mortality. Multivariate analysis performed by Zevallos et al. identified a mortality rate of < 1% associated with TORS procedures. Overall risk of both operative and perioperative wound site hemorrhage related mortality remains low in TORS but represents a potential danger to be recognized when recommending TORS procedures to patients.
In terms of patient injury or equipment failure in TORS, it is notable that such events are far less frequently recorded for head and neck cases than for procedures involving other specialties [4, 5]. This was also experienced to be the case based on manual review of records performed in the present study. Overall, the number and types of reports for patient injury and device malfunction discovered in this study appeared to present more as random, isolated events rather than a hazards sequence highlighting concern with device use. A trend that is somewhat suggestive of inconsistent disclosure of adverse events by mandatory reporting entities.
In this investigation, a low presence of reported adverse events overall for TORS procedures was found which is consistent with other reports in recent literature [3–5, 13–15]. The results also showed an apparent decrease in the number of adverse events reported in recent years for TORS. While the exact cause cannot be explained by the current data set, this was found to contradict findings in a report by Alemzadeh et al. who noted an absolute increase in annual events reported when globally evaluating da Vinci system use in all specialties. Possible explanations exist for why data from their study did not match those of this study. Alemzadeh et al. monitored reporting trends through 2013, a point beyond which our own study found annual reports started to decline. In addition, our analysis specifically evaluated events of head and neck da Vinci system use which may have been offset in prior reports by increased reports from other specialties. Even with such differences, it does seem unlikely for such a trend to be plausible given the persistent growth and influence of da Vinci procedures globally [1, 5, 14]. Further, the notably sporadic disclosure and clear lag times in reporting of events identified in this study present concerns about the efficiency of communication channels for relaying such information and the perceived practicality for doing so by mandatory reporters. Several additional variables that could also have influenced an outcome of lower reporting include technology enhancements, improved surgical techniques, changes in procedure selection, adjustments to disclosure protocols of mandatory reporters, or a refinement of patient selection guidelines [1–3, 5].
Conclusion
This study provides an understanding of reported adverse events specifically related to the use of the da Vinci surgical system for head and neck TORS procedures over the last 11 years as portrayed through MAUDE database narratives. It is recognized that the small data set obtained and limitations of a central database prevents measurement of a true incidence rate of intraoperative and perioperative adverse events associated with TORS procedures. Other key limitations of this study include an inability to detect changes in reporting methods and investigate individual operator proficiency.
Based on the results of this study, reported TORS adverse events appear to be similar in type to known risks of transoral tumor resection. These events are most common in the postoperative setting. Further, the findings of this study appear to be concordant with adverse events identified through the results of prior published studies from academic TORS sites. Although, evident lack of consistency and substance of records by mandatory reporters utilized from the MAUDE database in this study do represent an important area for future focus to ensure the accuracy of such findings.
The presence of incomplete narratives and significant lag times identified in the reporting of adverse events suggests that there exists a lack of incentive to ensure accurate and timely records. We stress the importance of having established channels [5] for reporting adverse event information to better enhance the understanding of adverse events related to surgical devices and thus allow for the continued improvement of technology and surgery technique with the overall goal of better quality of care to patients.
Abbreviations
- BOT
Base of tongue
- FDA
US Food and Drug Administration
- MAUDE
Manufacturer and user facility device experience
- MDR
Medical device reports
- SD
Standard deviation
- SAP
Sleep apnea procedure
- TORS
Transoral robotic surgery
Footnotes
Compliance with ethical standards
Conflict of interest There are no conflicts of interest.
Availability of data and material Data available within the article or its supplementary materials.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Byrd JK, Ferris RL (2016) Is there a role for robotic surgery in the treatment of head and neck cancer? Curr Treat Opt Oncol 17:29. 10.1007/s11864-016-0405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorincz BB, Jowett N, Knecht R (2016) Decision management in transoral robotic surgery: Indications, individual patient selection, and role in the multidisciplinary treatment for head and neck cancer from a European perspective. Head Neck 38:E2290–E2296 [DOI] [PubMed] [Google Scholar]
- 3.Vergez S, Lallemant B, Ceruse P, Moriniere S, Aubry K, De Mones E, Benlyazid A, Mallet Y (2012) Initial multi-institutional experience with transoral robotic surgery. Head Neck Surg 147(3):475–481 [DOI] [PubMed] [Google Scholar]
- 4.Alemzadeh H, Raman J, Leveson N, Kalbarczyk Z, Iyer RK (2016) Adverse events in robotic surgery: a retrospective study of 14 years of FDA data. PLoS ONE 11(4):e0151470. 10.1371/journal.pone.0151470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA, Ibrahim A, Lyu H, Makary MA (2015) Underreporting of robotic surgery complications. J Healthc Qual 37(2):133–138 [DOI] [PubMed] [Google Scholar]
- 6.Fuller A, Vilos GA, Paulter SE (2012) Electrosurgical injuries during robot assisted surgery: Insights from the FDA MAUDE database. SPIE BiOS 8207:820714 [Google Scholar]
- 7.Andonian S, Okeke Z, Okeke DA, Rastinehad A, Vanderbrink BA, Richstone L, Lee BR (2008) Device failures associated with patient injuries during robot-assisted laparoscopic surgeries: a comprehensive review of FDA MAUDE database. Can J Urol 15(1):3912–3916 [PubMed] [Google Scholar]
- 8.Lucas SM, Pattision EA, Sundaram CP (2012) Global robotic experience and the type of surgical system impact the types of robotic malfunctions and their clinical consequences: an FDA MAUDE review. BJU Int 109(8):1222–1227 [DOI] [PubMed] [Google Scholar]
- 9.Friedman DC, Lendvay TS, Hannaford B (2013) Instrument failures for the da vinci surgical system: a Food and Drug Administration MAUDE database study. Surg Endosc 27(5):1503–1508 [DOI] [PubMed] [Google Scholar]
- 10.Murphy D, Challacombe B, Elhage O, Dasgupta P (2007) Complications in robotic urological surgery. Minerva Urol Nephrol 59(2):191–198 [PubMed] [Google Scholar]
- 11.Manoucheri E, Fuchs-Weizman N, Cohen SL, Wang KC, Einars-son J (2014) MAUDE: analysis of robotic-assisted gynecologic surgery. J Min Invas Gynecol 21(4):592–595 [DOI] [PubMed] [Google Scholar]
- 12.Gupta P, Schomburg J, Lund E, Adejoro O, Konety B (2013) 855 adverse events associated with the davinci surgical system as reported in the FDA MAUDE database. J Urol 189(4):e351. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein GS, O’Malley BW, Magnuson JS, Carroll WR, Olsen KD, Daio L, Moore EJ, Holsinger FC (2012) Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122:1701–1707 [DOI] [PubMed] [Google Scholar]
- 14.de Almeida JR, Byrd JK, Wu R, Stucken CL, Duvvuri U, Goldstein DP, Miles BA, Teng MS, Gupta V, Genden EM (2014) A systemic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systemic review. Laryngoscope 124:2096–2102 [DOI] [PubMed] [Google Scholar]
- 15.Zevallos JP, Mitra N, Swisher-McClure S (2016) Patterns of care an perioperative outcomes in transoral endoscopic surgery for oropharyngeal squamous cell carcinoma. Head Neck 38:402–409 [DOI] [PubMed] [Google Scholar]
- 16.Crawford JA, Bahgat AY, White HN, Magnuson JS (2016) Hemostatic options for transoral robotic surgery of the pharynx and base of tongue. Otolaryngol Clin North Am 49(3):715–725 [DOI] [PubMed] [Google Scholar]
- 17.Pollei TR, Hinni ML, Moore EJ, Hayden RE, Olsen KD, Casler JD, Walter LC (2013) Analysis of postoperative bleeding and risk factors in transoral surgery of the oropharynx. Jama Otolaryngol Head Neck Surg 139(11):1212–1218. 10.1001/jamaoto.2013.5097 [DOI] [PubMed] [Google Scholar]
- 18.Mandal R, Duvvuri U, Ferris RL, Kaffenberger TM, Choby GW, Kim S (2016) Analysis of post-transoral robotic-assisted surgery [DOI] [PubMed] [Google Scholar]


