Abstract
Recent observations have begun to support a role for Bartonella spp. as animal as well as human pathogens. Bartonella spp. are vector-transmitted, blood-borne, intracellular, gram-negative bacteria that can induce prolonged infection in the host. Persistent infections in domestic and wild animals result in a substantial reservoir of Bartonella organisms in nature that can serve as a source for inadvertent human infection. The prevalence of bacteremia can range from 50 to 95% in selected rodent, cat, deer, and cattle populations. Dogs infected with Bartonella spp. can develop lameness, endocarditis, granulomatous lymphadenitis, and peliosis hepatis, lesions that have also been reported in association with human infection. Understanding the role of Bartonella spp. as pathogens in cats and other wild or domestic animals awaits the results of additional studies. Considering the extensive animal reservoirs and the large number of insects that have been implicated in the transmission of Bartonella spp., both animal and human exposure to these organisms may be more substantial than is currently believed.
When historians examine the events that have contributed to the many rapid advances in knowledge related to “emerging infectious diseases” during the latter portion of the 20th century, it is probable that research contributions related to Bartonella infections will prove to be of substantial comparative medical importance. Knowledge derived from studies of the biologic behavior of Bartonella spp. in animals, as compared to behavior in the human host, should provide important insights into the biological behavior and immunopathogenesis of these diseases. It is becoming increasingly obvious that Bartonella organisms are highly adapted to facilitate intracellular persistence in a wide variety of animals, including human beings. For example, Bartonella bacteremia can be found in up to 50% of the domestic and feral cat populations in regions where fleas are endemic (42). In addition, cats can be coinfected with more than one Bartonella sp. (41). In the southeastern United States and the United Kingdom, Bartonella spp. have been isolated from the blood of 42 and 62% of rodents, respectively (11, 64). Strikingly, nearly 100% of deer sampled at a single point in time in either California or France were found to have bacteremia (20; B. B. Chomel, R. W. Kasten, K. Yamamoto, C. Chang, T. E. Honadel, and Y. Kikuchi, Abstr. First Int. Conf. Bartonella Emerg. Pathogens, Main Speaker, p. 31).
In recent years, there have been several excellent reviews (2, 25, 73, 75, 87, 88, 100) and a text (98) detailing the clinical, diagnostic, pathophysiologic, and microbiologic aspects of Bartonella infection, with particular emphasis on human infection. However, collective recent observations have begun to support a role for Bartonella spp. as animal as well as human pathogens. This review will focus on Bartonella infection in animals, particularly as it relates to carriership, reservoir potential, pathogenicity, and the zoonotic potential for human infection. As information related to these concepts is in a rapid state of development, substantial advancement in our understanding of Bartonella infection in animals is anticipated in the future.
SPECTRUM OF ANIMAL INFECTIONS
Bartonella Species
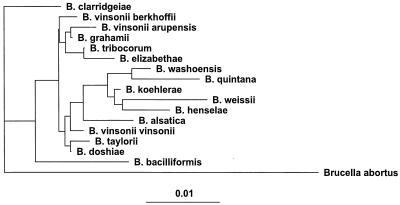
Although continuing to expand rapidly, the genus Bartonella is currently composed of 16 species. In 1993, Brenner and colleagues (17) proposed that previously designated Rochalimaea species be united with the genus Bartonella and renamed Bartonella quintana, B. vinsonii, B. henselae, and B. elizabethae. This reclassification also resulted in the transfer of these organisms from the family Rickettsiaceae to the family Bartonellaceae, which included Bartonella bacilliformis, and removed the family Bartonellaceae from the order Rickettsiales. In 1995, Birtles and colleagues (12) proposed the unification of the genus Grahamella with the genus Bartonella, which resulted in five additional Bartonella species: Bartonella talpae, B. peromysci, B. grahamii, B. taylorii, and B. doshiae. In effect, these reclassifications eliminated the previous genera Rochalimaea and Grahamella. Historically, Grahamella species were characterized as arthropod-transmitted hemotropic gram-negative bacteria of small mammals, fish, and birds, which remain of no known pathogenic consequence for higher mammals. In 1996, Bartonella vinsonii subsp. berkhoffii, isolated from dogs, was designated a new subspecies (57), and Bartonella clarridgeiae, isolated from a cat, was characterized as a new species (70). Bartonella tribocorum was isolated from the blood of wild rats and characterized in 1998 (43). Bartonella washoensis (GenBank accession no. AF070463) was isolated from a patient with cardiac disease, and a rodent reservoir was implicated. During the first 11 months of 1999, Bartonella koehlerae, isolated from the blood of domestic cats (32); Bartonella alsatica, isolated from the blood of wild rabbits (44); Oryctolagus caniculus and Bartonella vinsonii subsp. arupensis, isolated from the blood of a cattle rancher (106); and Bartonella weissi, isolated from a cat (GenBank accession no. AF199502), were added to the growing list of Bartonella species and subspecies. The phylogenetic relationship of these Bartonella species, based upon the 16S rRNA gene, is depicted in Fig. 1.
FIG. 1.
Phylogenetic dendrogram prepared from 1,272 nucleotides of Bartonella 16S DNAs. The dendrogram was based on the maximum-likelihood method, with Brucella abortus included as an outgroup. Scale bar, 1% nucleotide difference.
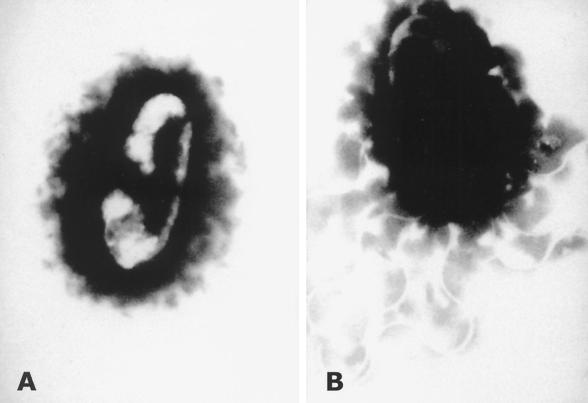
Microscopically, all Bartonella spp. are gram-negative bacilli or coccobacilli. The isolation and characterization of Bartonella spp. have been reviewed in substantial detail elsewhere (96, 97). Bartonella bacilliformis and B. clarridgeiae have flagella, which in the case of B. bacilliformis facilitates erythrocyte invasion. Polar structures resembling fimbriae have been observed on B. tribocorum. Other Bartonella species, such as Bartonella henselae, appear to lack flagella (Fig. 2). Based upon in vitro studies using human umbilical vein endothelial cells, B. henselae is internalized by an actin-dependent invasome-mediated mechanism of cellular invasion (30).
FIG. 2.
B. henselae (A) and B. clarridgeiae (B) stained with 2% phosphotungstic acid (pH 7.2). Magnification, ×31,000. (Reproduced from reference 58 with permission of the publisher.)
As a genus, Bartonella are catalase, oxidase, urease, and nitrate reductase negative. Those members formerly classified as Grahamella and B. quintana (Fuller strain) are uniquely positive in the Voges-Proskauer test. Biochemical profiles of Bartonella spp. are fairly neutral except for the production of peptidases, which varies slightly among species (97). Using DNA fingerprinting, B. henselae isolates can be differentiated into two types (type I and type II), based upon differences in the 16S rRNA gene sequence (9), or four variants (variants I to IV) on the basis of several alternative DNA fingerprinting methods (94, 96). Although the same B. henselae subtype can be identified in cat scratch disease (CSD) patients and the blood of their cats, definitive correlations between subtypes and specific disease manifestations have not yet been reported (95).
Role of Insect Vectors
In general, knowledge related to vector transmission of Bartonella organisms is very incomplete. Several insects have been implicated in Bartonella transmission, including sand flies (36), the human body louse (93), the cat flea (23, 34, 45), the vole ear mite (3), and ticks (81, 106) (Table 1). Fleas (Xenopsylla cheopis) collected from rats in downtown Los Angeles were commonly infected (61%) with Bartonella strains, including B. elizabethae (L. Beati, B. A. Ellis, M. Rood, S. Eldaieef, and R. L. Regnery, First Int. Conf. Bartonella Emerg. Pathogens, 1999, abstr. 13, p. 34). Certain Bartonella spp. appear to be more highly adapted to cause persistent infection in rodents (28, 106; Beati et al., abstr. 13). Infection with rodent Bartonella spp. such as B. elizabethae and B. vinsonii subsp. arupensis in aberrant hosts such as human beings can result in endocarditis and febrile illness, respectively (28, 106).
TABLE 1.
Insects associated with the transmission of Bartonella spp.
| Species | Insect vector |
|---|---|
| B. bacilliformis | Sand flies (Lutzomyias sp.) |
| B. quintana | Human body louse (Pedicular humanis) |
| B. henselae | Cat flea (Ctenocephalides felis) |
| B. vinsonii subsp. vinsonii | Vole ear mite (Trombicula miroti) |
| B. vinsonii subsp. berkhoffii | Ticks (species unknown) |
| B. vinsonii subsp. arupensis | Deer tick (Ixodes scapularis) |
Experimentally, B. henselae was transmitted by transferring fleas from bacteremic cattery cats to specific-pathogen-free (SPF) cats (23). In this study, it was not established whether Ctenocephalides felis served as both a mechanical and biologic vector; however, cat fleas can become infected and support the replication of B. henselae following ingestion of a blood meal from an infected cat (45). Bartonellae are visible in dissected flea guts and can be cultured from flea feces up to 9 days after fleas were fed infected blood (45). More recently, cats have been experimentally infected with B. henselae by intradermal inoculation of feces derived from infected fleas (34). In the same study, oral transmission, using fleas or flea feces, was not accomplished. Cats fed fleas that had fed upon B. henselae-infected cats and 45 mg of fresh flea feces from B. henselae-exposed fleas did not seroconvert or become bacteremic. Although as yet unproven, it is now generally accepted that B. henselae transmission from cats to humans is similar to transmission of B. quintana to humans. Mechanical transmission occurs when louse feces containing B. quintana are inoculated by scarification of the skin or through contact with conjunctival membranes. Although the exact mode of transmission of B. henselae, the most frequent cause of CSD, remains unclear, contamination of the claws or teeth with infected flea feces may be required for transmission. When uninfected SPF cats were housed with B. henselae-infected bacteremic cats in an ectoparasite-free environment, there was no evidence of Bartonella transmission (23). Elimination of flea and tick infestation, as potentially demonstrated in a household containing three pets, one each persistently infected with B. henselae, B. clarridgeiae, and B. vinsonii subsp. berkhoffii, may interrupt the transmission cycle (11). Although tick transmission has seldom been proposed as a means of Bartonella transmission to humans, substantial evidence is mounting to support tick transmission of these agents among animals (46, 81, 99; Chomel et al., Main Speaker, p. 31). Ixodes scapularis ticks in the United States and Ixodes ricinus ticks in The Netherlands appear to be frequently infected with Bartonella spp. (46, 99).
Host Specificity
Because all Bartonella spp. are believed to be vector-transmitted, blood-borne, intracellular organisms, vector preference for certain hosts could influence the transmission of these organisms. Presumably, this vector preference is partly responsible for the more frequent association of a given Bartonella sp. with a specific host, i.e., B. henselae, B. clarridgeiae, and B. koehlerae in cats, B. vinsonii subsp. berkhoffii in dogs and coyotes (Canis latrans), B. alsatica in wild rabbits, and B. quintana in human beings. However, preferential infectivity in specific hosts also appears to play a role in determining which animals will become infected with a particular Bartonella sp. For example, Bartonella isolates obtained from three Peromyscus species were all in the same phylogenetic cluster, whereas isolates from cotton rats (Sigmodon hispidus) from the same area or from more distant locations were contained within three clusters (64). In the United Kingdom, the same Bartonella sp. was isolated from five different species of small woodland mammals (11). Following experimental inoculation, dogs do not become bacteremic with B. henselae and cats do not become bacteremic with B. vinsonii subsp. berkhoffii (B. B. Chomel, R. W. Ermel, R. W. Kasten, K. Yamamoto, C.-C. Chang, R. Heller, D. Weber, A. Poland, Y. Piemont, H. J. Boulouis, and N. C. Pedersen, Int. Conf. Rickettsiae Rickettsial Dis. Am. Soc. Rickettsiol. 14th Sesquiannual Joint Meet., 1999, abstr. 41). At least in cats (41, 42, 62) and rodents (64), simultaneous infection with more than one Bartonella sp. (B. henselae and B. clarridgeiae in cats) has been documented.
Other as yet undetermined factors are presumably involved in determining host specificity. For example, although the cat flea, C. felis, frequently infests dogs within the same household that contains cats infected with B. henselae or B. clarridgeiae, to date these organisms have not been isolated from the blood of a dog. However, cats infected with a B. clarridgeiae-like strain obtained from a coyote subsequently developed bacteremia (Chomel et al., abstr. 41). Although host specificity may be responsible for the association of B. henselae with the feline reservoir, an alternative explanation may be a failure to isolate B. henselae from dog blood due to a low concentration of bacteria in the blood. Recently, B. henselae DNA was amplified and sequenced from the liver of a dog with peliosis hepatis (53), a lesion associated with B. henselae or B. quintana infection in human patients, particularly in those who are immunocompromised (54, 88). Unfortunately, blood was not available from this dog for culture or PCR analysis. Isolation of Bartonella spp. from embryos of naturally infected rodents suggests the possibility of transplacental transmission (65). Congenital transmission could also contribute to a predilection for a given Bartonella sp. to more frequently infect a specific host species. As with other infectious agents, differences in placentation among different animal species could serve as a rate-limiting barrier for transplacental transmission.
Seroepidemiologic Studies
In veterinary medicine, the most extensive Bartonella seroepidemiologic studies have involved domestic and feral cats (21, 22, 24, 35, 37, 49, 104). Based upon more recent observations, there may be at least three limitations to the accurate interpretation of seroprevalence data derived from these cat studies (21, 22, 24, 35, 37, 49, 104). First, the patterns of cross- reactivity among the four Bartonella spp. isolated from cats have not been delineated. Second, the extent to which other organisms such as Chlamydia spp. and Coxiella burnetti, which have been associated with serological cross-reactions in people (69, 74), falsely influence seroprevalence data derived from cats is not known. Third, an unknown number of cats will not have detectable antibodies despite being bacteremic at the time of sample collection (24, 59). Despite these limitations, seroprevalence surveys indicate that a remarkable number of cats throughout the world appear to have been exposed to Bartonella spp. Thus, cats have the potential to act as a substantial reservoir for human infection. Seroprevalence to B. henselae antigens is much higher (40 to 70%) in cats that live in warm, humid regions of the world in which severe flea infestation is expected. In the United States, regional seroprevalence varied from 3.7 to 54.6%; only the northern Rocky Mountain-Great Plains region had a seroprevalence rate of less than 10% (49). Of potential public health concern, feral cats are more likely to be seroreactive or bacteremic than pet cats from the same region (21–24, 104). However, seroprevalence in bobcats, mountain lions, and large cats maintained at zoological parks can be comparable to the prevalence found in the domestic cat population in the same geographic area (92; Chomel et al., abstr.). As the nocturnal opossum is frequently infested with C. felis, this animal as well as others in nature may be responsible for transporting infected fleas to repopulate different sites within a community.
Seroprevalence in cattery cats is generally bimodal. Foley et al. (35) found either that most cats within a given cattery had serologic evidence of exposure or that very few or none were exposed. Of note, flea infestation was the most important risk factor associated with high Bartonella seroprevalence in cattery cats. Seroprevalence in cats was 47% in Hawaii (31), 15% in Japan (104), 54% in Indonesia (71), 40% in Israel (4), 11% in Egypt (22), 7% in Portugal (22), 48% in Singapore (77), 21% in South Africa (51), and 24% in Zimbabwe (51). When sera from cats in North Carolina and Israel were tested by indirect fluorescent antibody (IFA) to both B. henselae and B. quintana antigens, antibodies to B. quintana were significantly higher in cats from Israel (4). However, absorption of sera with B. henselae or B. quintana antigens supported exposure to B. henselae rather than B. quintana. These results suggest that in Israel, cats may be exposed to one or more antigenically different Bartonella species, subspecies, or strains that, immunologically, more readily recognize B. quintana antigens.
Due to the relatively recent recognition that dogs can be infected with B. vinsonii subsp. berkhoffii and potentially other Bartonella spp., seroprevalence data are limited. Seroprevalence was determined in 1,920 clinically ill dogs from North Carolina and surrounding states that were evaluated at a veterinary teaching hospital (81). Using a cutoff reciprocal titer of ≥64, 3.6% of the dogs had antibodies to B. vinsonii subsp. berkhoffii. Risk factors that could be associated with seroreactivity included heavy tick infestation (odds ratio [OR], 14.2), cattle exposure (OR, 9.3), rural rather than urban environment (OR, 7.1), and heavy flea infestation (OR, 5.6). These data indicated that exposure to B. vinsonii subsp. berkhoffii was more likely in dogs in rural environments that were allowed to roam and likely to have a history of heavy tick and flea infestation. Using sera from dogs experimentally infected with R. rickettsii or Ehrlichia canis, cross-reactivity to Bartonella antigens was not detected; however, 36 and 52% of serum samples derived from dogs naturally infected with Ehrlichia canis or Babesia canis, respectively, were reactive to B. vinsonii antigens (81). As both E. canis and B. canis are transmitted by Rhipicephalus sanguineous, this tick may be involved in the transmission of B. vinsonii, particularly in kennels with severe tick infestation problems. The possibility of tick transmission was further supported by two additional studies (15, 63) involving dogs from the same geographic region infected with one or more Ehrlichia spp. Seroreactivity to B. vinsonii subsp. berkhoffii antigens was detected in 4 of 12 (33%) dogs diagnosed with ehrlichiosis (15) and in 23 of 27 (85%) Walker hounds in a kennel with severe tick infestation (63). Seroprevalence, using B. vinsonii subsp. berkhoffii antigens, was 10% (4 of 40 dogs) in dogs with suspected tick-borne illness from Israel (5). Using an enzyme-linked immunosorbent assay, 35% of 869 serum samples derived from coyotes in California contained antibodies to B. vinsonii subsp. berkhoffii antigens (19). Antibodies to B. vinsonii subsp. berkhoffii were detected in 66 of 483 (14%) sera from military working dogs stationed in southern France or Africa (B. Davoust, M. Drancourt, M. Boni, D. Parzy, J. Seignot, V. Roux, and D. Raoult, Int. Conf. Rickettsiae Rickettsial Dis. Am. Soc. Rickettsiol. 14th Sesquiannu. Joint Meet., 1999, abstr. 232B) and in 163 of 1,873 (9%) sera from U.S. government-owned dogs (Chomel et al., Main Speaker, p. 31).
PREVALENCE, PERSISTENCE, AND PATHOGENICITY
Prevalence of Bacteremia
The high prevalence of antibodies to Bartonella spp. in animals supports the possibilities of frequent exposure, persistent infection, and recurrent infection. In addition to seroprevalence data, a substantial number of recent studies have utilized blood culture to detect Bartonella bacteremia. Bartonella spp. can be isolated from blood more efficiently following lysis of erythrocytes and leukocytes (85, 96). This can be achieved by using a lysis solution (Isolator blood lysis tubes; Wampole, Cranbury, N.J.) or by freezing the EDTA-treated blood samples at −70°C for 24 h prior to inoculation of a blood agar plate. Detection of Bartonella species in conventional automated blood culture systems is less reliable (96). As most Bartonella species are highly fastidious, prolonged incubation periods (4 to 6 weeks) at low CO2 concentrations (5 to 10%) maintained at 35°C can be required for visualization of colonies.
The prevalence of bacteremia within rodent populations can be quite high. In the United Kingdom and in the southeastern United States, 23 of 37 (62.2%) rodents and 119 of 279 (42.2%) rodents, respectively, were bacteremic (11, 64). In the southeastern United States, rodent Bartonella isolates cluster within four phylogenetic groups, based on homology of the citrate synthase gene (11). Despite the high prevalence of bacteremia, most infected rodents have low or undetectable levels of serum antibodies by IFA. Bartonella spp. were visualized in blood smears of shrews (10% of Sorex cinereus, 20% of Sorex fumens, and 14% of Blarina brevicauda) collected from Pennsylvania (66).
Bartonella spp. have also been isolated from the blood of a diverse group of wild animals from the western United States, including 2 of 7 mountain lions, 5 of 13 bobcats, 17 of 54 coyotes, 4 of 6 gray foxes, 17 of 100 elk, and 39 of 42 black-tailed deer (19, 20; Chomel et al., Main Speaker, p. 31, and abstr. 41). Although genetically similar, isolates from pumas and bobcats differ from B. henselae isolates obtained from domestic cats (Chomel et al., abstr. 41). B. alsatica was isolated from 9 of 30 blood samples from wild rabbits in France (44). Recently, Bartonella bacteremia was reported in 47 (89%) of 53 beef cattle from Oklahoma and 11 (17%) of 63 dairy cattle from California (20). Tick, louse, or other vector transmission of Bartonella species in deer or cattle would also seem more likely, as fleas infrequently infest these species.
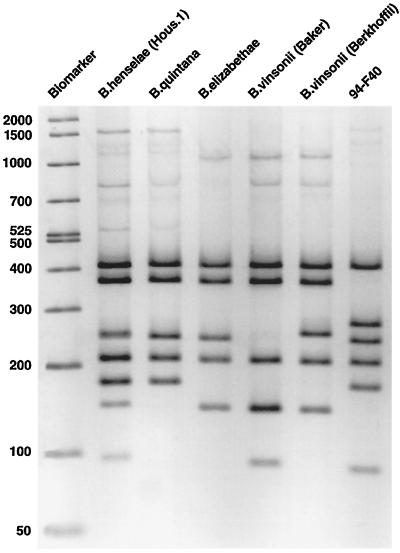
Bartonella spp. have been isolated from the blood of cats from San Francisco (54), North Carolina (52), Hawaii (31), Japan (72), Sydney, Australia (13, 33), New Zealand (50), the Netherlands (10), Nancy, France (42), and Indonesia (71) (Table 2). In Nancy (42), 17 isolates (34%) were B. henselae type I (identical to the Houston I type strain) (85), 19 isolates were B. henselae type II (identical to the BA-TF strain) (90), and 15 isolates (30%) were identical to the type strain (ATCC 51734) (70) of B. clarridgeiae (42). In Indonesia, six B. henselae and three B. clarridgeiae isolates were obtained (71). Bartonella spp. were isolated from six of seven domestic cats belonging to four patients diagnosed with bacillary angiomatosis and from 17 of 19 domestic cats associated with CSD patients (54, 56). B. clarridgeiae, isolated from a kitten, was implicated as the cause of CSD in a veterinarian (58). The isolate, designated 94-F40 (nucleotide sequence accession number U64691), was initially differentiated from other type strains by PCR-restriction fragment length polymorphism (RFLP) (Fig. 3).
TABLE 2.
Isolation of Bartonella spp. from cats
FIG. 3.
PCR-RFLP profiles of selected Bartonella type strains. The isolate 94-F40 was implicated as a cause of CSD in a veterinarian. The 16S rRNA gene was digested with DdeI. Sizes are shown in daltons. (Reproduced from reference 58 with permission of the publisher.)
When using the currently recommended microbiologic techniques, there appears to be considerable variation in the degree of difficulty associated with the isolation of Bartonella spp. from the blood of different animal species. For example, in our laboratory, isolation of B. henselae from the blood of naturally or experimentally infected cats is frequently successful; however, isolation of B. vinsonii from seroreactive dogs or dogs from which B. vinsonii subsp. berkhoffii DNA has been amplified from the same processed EDTA-treated blood sample is rarely successful, even when tissue culture isolation is employed (16). Retrospectively, long-term administration of immunosuppressive doses of corticosteroids for a presumptive diagnosis of systemic lupus erythematosus may have facilitated the isolation of the original type strain of B. vinsonii subsp. berkhoffii from a dog with endocarditis (14). Three other isolates of B. vinsonii subsp. berkhoffii that could be differentiated from the type strain by restriction digestion were cultured from a dog with hyperadrenocorticism, a healthy blood donor, and a healthy pet (57, 61). B. vinsonii subsp. berkhoffii has been isolated from the blood of 17 of 54 (32%) coyotes in California (19). Ease of isolation from the blood of an individual cat may be of particular relevance if, hypothetically, B. henselae proves to be considerably easier to culture than B. clarridgeiae or B. koehlerae. B. koehlerae appears to be more fastidious and requires inoculation on chocolate agar for isolation (32). In cats, accurate isolation data will be important to assess vaccine efficacy, as heterologous challenge with a different Bartonella sp. does not confer protection (107).
Persistence of Bacteremia
Several lines of evidence support the conclusion that Bartonella spp. generally cause persistent infection in the susceptible host. When sampled at a single point in time, the high percentage of bacteremic animals that are detected indirectly supports persistent infection in several animal species for which long-term isolation studies in vector-free environments have not been performed. Prolonged periods of bacteremia have been documented in naturally (sequentially sampled for at least 15 months) (56) and experimentally (sampled for at least 454 days) (59, 60, 62) infected SPF cats. However, given current difficulties in documenting low levels of bacteremia, the maximum period of persistence in most animal species is unknown. Within an experimental group, individual cats can have highly variable patterns of relapsing bacteremia (59, 62). Sequential sera and Bartonella isolates obtained from the same cat at identical time points failed to identify obvious differences in sodium dodecyl sulfate-polyacrylamide gel electrophoresis or Western immunoblot patterns, indicating that alterations in surface antigenic structure, as have been demonstrated for relapsing fever borreliae (91) in people, are not responsible for the relapsing pattern of bacteremia in cats (62).
Subclinical persistence of B. vinsonii subsp. berkhoffii has been documented in a healthy dog for 14 months (61). Allowing for potential difficulties associated with the isolation of B. vinsonii from dog blood (16) or any Bartonella sp. from the blood of immunocompetent people (2, 25, 73, 75, 88), culture may represent an insensitive means to confirm bacteremia. In support of persistent infection in people, B. bacilliformis has been inadvertently transmitted by blood transfusion and B. quintana has been repeatedly isolated from people over extended periods of time (18, 36). Brouqui et al. recently described the isolation of B. quintana from the blood of 10 (14%) out of 71 homeless people, 5 of whom had chronic bacteremia for at least several weeks (18). Collectively, these observations support persistent infection with Bartonella spp. in the blood of cats, dogs, and human beings.
Pathogenicity
With respect to experimental infection studies in cats, the source of inoculum may substantially influence several experimental outcomes. For example, when cultured B. henselae organisms are used as the inoculum, clinical manifestations are generally absent, the duration of bacteremia is short (<3 months), seroconversion is followed by a rapid decline in immunoglobulin G (IgG) antibodies, and a relapsing pattern of bacteremia is not reported (1, 8, 38–40, 80, 89, 107). Presumably, culture on artificial medium results in attenuation of B. henselae strains, perhaps due to downregulation of genes that influence virulence (1, 8, 38–40, 80, 89, 107). These observations are in contrast to the results of studies in which cats are infected by intravenous transfusion or intramuscular administration of blood from chronically infected feline donors (59, 62) or by exposure to infected fleas (24). Following intravenous or intramuscular administration of blood obtained from bacteremic donor cats that had induced CSD in their owners, bacteremia in recipient cats was detectable by day 11, was relapsing in nature, and persisted in some cats for the 454-day duration of the study (59, 62). There was substantial variation in both the pattern of bacteremia and the pattern of IgG antibody production among recipient cats that received blood from the same donor cat. Following flea transmission, bacteremia can be detected within 2 to 4 weeks and IgG antibodies can be detected within 4 to 5 weeks (23). Subsequently, bacteremia, which was relapsing in one kitten, was accompanied by stable IgG antibody titers that were detected throughout the 22-week period of observation (23). There are also obvious limitations to using Bartonella-containing blood or fleas to experimentally infect cats: (i) the inoculum cannot be critically standardized, (ii) more than one Bartonella sp. can be unknowingly transmitted, and (iii) there is always the potential for inadvertent transmission of other known or unknown blood-borne or flea-borne pathogens (i.e., Rickettsia felis). From a clinical perspective, transfusion of Bartonella-infected blood to an anemic sick cat may result in side effects that historically have been unknowingly attributed to a transfusion reaction. Although Bartonella spp. are subtle pathogens during chronic infection of a healthy cat, acute infection of a debilitated cat might induce more serious consequences.
EXPERIMENTAL INFECTION STUDIES
Rodents
Although much of the data has not yet been published, several laboratories have been actively pursuing experimental infection studies with rodents. Perhaps not surprisingly, immunocompetent rodent strains appear to be more resistant to infection with nonrodent Bartonella species. When female C57BL/6 mice were infected with 9.1 × 107 CFU of B. henselae (Houston 1 strain), cultivatable organisms were rapidly cleared; however, DNA could be detected by nested PCR throughout the duration of the study (94 days) (84). In additional experiments, mice infected with at least 5 × 107 B. henselae developed a granulomatous hepatitis with a predominance of CD4+ lymphocytes or CD11b+ monocytes. Significant lesions were not detected in other organs. The authors noted that “a key element in establishing this infection model was probably the passage of bacteria through mice prior to experimental infection.” Subcutaneous inoculation into the hind foot pad induced popliteal lymphadenopathy in female C57BL/6 mice (84).
Genetic susceptibility, age-associated immunocompetence, and pregnancy are factors that can influence Bartonella infection. When a Bartonella strain isolated from a field mouse (Apodemus sp.) was inoculated intravenously, BALB/c mice developed a significantly higher mean level of bacteremia than C57BL/6 or Swiss mice (F. Barrat, D. Bermond, C. Bouillin, C. Gandoin, D. Thibault, B. Chomel, R. Heller, Y. Piemont, and H. J. Boulois, First Int. Conf. Bartonella Emerg. Pathogens, 1999, abstr. 22, p. 47). Older BALB/c mice had significantly higher levels of bacteremia than younger mice, and the level of bacteremia was amplified in gravid mice compared to that in virgin mice. In utero transmission to the fetus was documented by the 18th day of gestation (Barrat et al., abstr.). A recombinant strain of B. tribocorum expressing green fluorescent protein has been used to study the kinetics of erythrocyte infection in rats. Following infection, bacteria are rapidly cleared from the blood within hours but subsequently reappear in erythrocytes on day 4 postinfection (R. Schülein, C. Gille, A. Seubert, Y. Hansmann, R. Heller, Y. Piemont, S. Andersson, and C. Lanz, First Int. Conf. Bartonella Emerg. Pathogens, 1999, Main Speaker, p. 51). Within erythrocytes, green fluorescent protein-expressing B. tribocorum reach a limiting cell density of 10 to 15 bacteria per erythrocyte. Similar to results derived from cats (55, 76) and rabbits (43), only 1 in 1,000 to 5,000 rat erythrocytes became infected throughout the entire 10-week bacteremic phase of infection. The factor(s) that limits infection of cat, rabbit, and rodent erythrocytes remains to be determined. In contrast, nearly 100% of human erythrocytes can be infected with B. bacilliformis, potentially leading to a severe hemolytic crisis associated with Oroya fever (36). When CD-1 mice were inoculated with B. henselae or B. quintana, immune sera with a high degree of species specificity were produced (102).
Cats
Findings derived from several experimental infection studies involving cats are difficult to compare as there has been variability in the type and size of inoculum, the route of infection, and the strain of B. henselae. In a comparative study, intradermal inoculation of culture-propagated B. henselae was more likely to induce infection than intravenous inoculation (1). Strain variability among B. henselae isolates may contribute to enhanced pathogenicity in experimentally infected cats (80). In contrast to what occurred with mice, transplacental or perinatal transmission was not found in cats experimentally infected with B. henselae (40). Cats may also serve as a useful species to study the efficacy of antibiotics for treatment of B. henselae or B. clarridgeiae infections (8, 38, 60, 89).
Dogs
Following experimental inoculation of SPF dogs with culture-grown B. vinsonii subsp. berkhoffii, there was sustained suppression of peripheral blood CD8+ lymphocytes, accompanied by an altered cell surface phenotype and an increase in CD4+ lymphocytes in the peripheral lymph nodes during the 149-day period of study (B. L. Pappalardo, T. Brown, M. Tompkins, and E. Breitschwerdt, Int. Conf. Rickettsiae Rickettsial Dis. Am. Soc. Rickettsiol. 14th Sesquiannu. Joint Meet., 1999, abstr. 175). As the clinical manifestations of natural Bartonella infection in dogs are similar to those associated with human disease, the immunopathologic consequences of infection in dogs and people may prove to be similar. Experimentally, dogs inoculated with B. henselae did not become bacteremic. However, inoculation of dogs with a B. clarridgeiae-like strain isolated from a coyote induced bacteremia (Chomel et al., abstr. 41).
Nonhuman Primates
Few contemporary studies involving nonhuman primates have been reported. Historical accounts of experimental infection of nonhuman primates with B. bacilliformis have been reviewed (36). Bartonella vinsonii subsp. vinsonii was not isolated from two monkeys using yolk sac isolation, and there were no signs of illness or increased temperature (3). When simian immunodeficiency virus strain mac (SIVmac)-infected cynomolgus macaques were inoculated intradermally or subcutaneously with in vitro-cultured B. henselae, B. quintana, or Afipia felis, only the B. quintana-inoculated monkeys became bacteremic and seroconverted (8). Clinical abnormalities were not apparent. Two cynomolgus macaques inoculated subcutaneously with B. henselae-infected blood derived from an experimentally infected SPF cat developed fever and subcutaneous purple-red spots at the inoculation site; however, bacteria did not grow from regional lymph node samples obtained 7 to 9 weeks following inoculation and B. henselae antibodies were not detected (8).
CLINICOPATHOLOGIC CORRELATIONS
The Cat
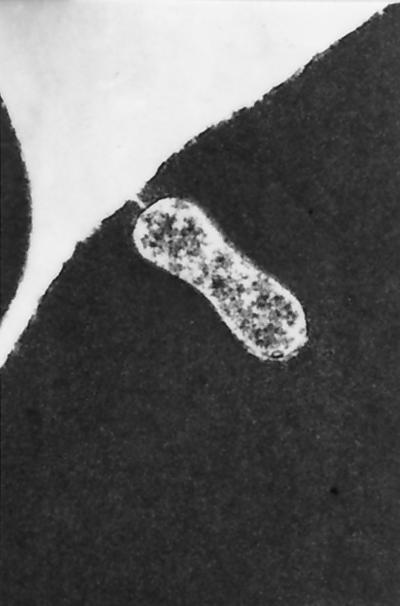
Although B. henselae and B. clarridgeiae can remain a part of the intravascular microbial flora of healthy cats for months to years, recent evidence suggests that cats may pay a biologic price (i.e., development of chronic insidious disease manifestations) for persistent infection. B. henselae has been observed within feline erythrocytes by electron (Fig. 4) and laser confocal (55, 76) microscopy. For reasons that remain unclear, less than 1% of erythrocytes appear to be susceptible to infection (76). In a seroepidemiologic study involving 170 cats from Japan that were tested for antibodies to both feline immunodeficiency virus (FIV) and B. henselae antigens, there was a significant increase in the incidence of lymphadenopathy and gingivitis in cats with serologic evidence of infection with both organisms (105). As FIV causes a progressive decrease in CD4+ lymphocytes, these results suggest that coinfection with B. henselae in an immunodeficient cat can induce specific disease manifestations. Of potentially unique importance to our future understanding of Bartonella spp. as pathogens in cats, a seroepidemiologic survey involving 728 healthy and sick cats from Switzerland identified a statistical correlation between the presence of antibodies to B. henselae antigens and stomatitis, as well as a variety of renal and urinary tract abnormalities (37). It is also of interest that in cats greater than 7 years of age, only clinically ill cats (17 of 110 clinically ill cats) were Bartonella seroreactive. As the seroprevalence in cats from Switzerland is substantially lower (8.3%) than in cats from warmer and more humid climates, detection of statistical associations with renal or urinary tract disease seem more feasible. Since chronic renal failure of undetermined etiology is a major cause of morbidity and mortality in older cats, clarification of the role of Bartonella spp. as a potential cause of chronic renal failure could prove to be of considerable importance. Recently, based upon detection of antibodies in serum and aqueous humor, Bartonella infection was proposed as a cause of uveitis in an immunocompetent cat (67). In a subsequent study, ocular production of Bartonella IgG in aqueous humor was detected in 7 of 49 cats with uveitis, 0 of 49 healthy shelter cats, and 4 of 9 cats experimentally infected with B. henselae (68).
FIG. 4.
Electron photomicrograph of intraerythrocytic B. henselae, illustrating the existence of a pore between the bacterium and the extracellular fluid space. Sample was stained with methanol, uranyl acetate, and lead citrate. Magnification, ×38,000. (Reproduced from reference 55 with permission of the publisher.)
In addition to the above seroepidemiologic and clinical observations, findings derived from experimental infection studies also have begun to support a pathogenic role for Bartonella spp. in cats. Historically, as earlier studies indicated that Bartonella could be isolated from the blood of many naturally infected healthy cats, many experimental infection studies, including those from our laboratory (59, 62), were not designed to critically address the role of B. henselae as a pathogen in cats, and in several earlier studies, histopathologic evaluation was not performed. Detailed histopathologic studies should be a more important consideration in future studies. Abnormalities reported in cats experimentally infected with B. henselae or B. clarridgeiae include fever, mild transient anemia, eosinophilia, lymphadenomegaly, cholangitis, cardiac and renal lesions, neurologic dysfunction, and reproductive failure. Fever, lymphadenomegaly, and anemia inconsistently accompany initial infection (39, 59, 80). Although perinatal transmission was not successful, the same group of investigators reported reproductive failure, characterized by failure to conceive, fetal involution and resorption, and delayed conception in cats inoculated intradermally with B. henselae (40). Transient neurologic dysfunction, characterized by lethargy, disorientation, and lack of response to environmental stimuli, has been reported in cats infected with blood- or culture-derived inocula (40, 59, 80). Focal myocardial inflammation, consisting predominantly of mononuclear cells, has been observed in cats experimentally infected with B. henselae or B. clarridgeiae (39, 59). Although presumably unrelated to Bartonella infection, cataracts developed, without explanation, in SPF cats obtained from a commercial vendor supplying cats without historical problems with cataracts within 1 year following Bartonella infection (59, 62).
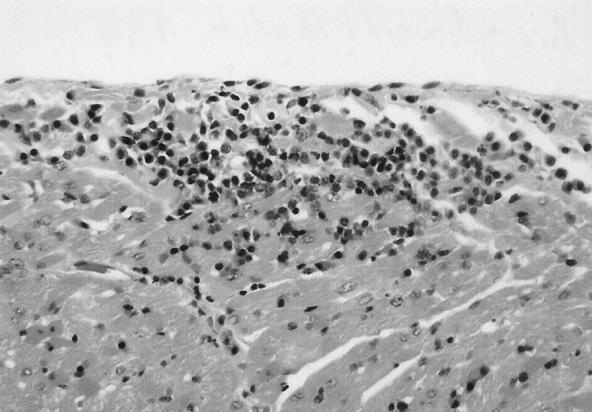
In cats infected with blood inocula containing B. henselae (type II) and/or B. clarridgeiae and monitored for 454 days, a substantial number of unexpected histopathologic lesions, including lymph node hyperplasia, splenic follicular hyperplasia, lymphocytic cholangitis, lymphocytic hepatitis, lymphocytic plasmacytic myocarditis (Fig. 5), and lymphocytic interstitial nephritis were identified (62). Bartonella DNA was amplified from the blood or tissues of individual cats regardless of bacteremia status. An important conclusion from this study, given the endemicity of feline bartonellosis in the natural cat population, is that the definition of normal pathology in healthy or sick cats may require reexamination with regard to the Bartonella infection status. This situation in cats could be analogous to the differences in the interpretations of gastric histopathology in human patients before and after the association of Helicobacter pylori with chronic gastritis and ulcer disease.
FIG. 5.
Feline heart. There is focal accumulation of lymphocytes and plasma cells displacing myocardial fibers with sporadic myocardial fiber. (Reproduced from reference 62 with permission of the publisher.)
The Dog
The spectrum of disease associated with Bartonella infection in dogs is currently unknown. However, from a comparative medical perspective, dogs infected with Bartonella spp. can develop disease manifestations that are similar to lesions reported in human patients, including endocarditis (14, 16), granulomatous lymphadenitis, granulomatous rhinitis (83), and peliosis hepatis (53). With the exception of a single case report of B. henselae-associated peliosis hepatis (53), B. vinsonii subsp. berkhoffii has been implicated on a molecular basis in all other disease processes identified in dogs to date. Endocarditis associated with B. vinsonii occurs in large-breed dogs with a potential predisposition for aortic valve involvement. Intermittent lameness or fever of unknown origin can precede the diagnosis of endocarditis in these dogs for several months. In addition to endocarditis, multifocal areas of severe myocardial inflammation can be found in dogs with B. vinsonii endocarditis. Potentially, myocarditis without endocarditis can result in cardiac arrhythmias, syncope, or sudden death (16). Similarly, myocarditis was identified in a 60-year-old male from Sweden with serologic and molecular evidence of infection with a Bartonella spp. who died suddenly during a running competition (47). Considering these preliminary and incomplete observations derived from experimentally infected cats, naturally infected dogs, and the patient from Sweden, the role of Bartonella spp. as a cause of myocarditis in animals and people deserves future research consideration. On the basis of seroreactivity, visualization of Warthin-Starry, silver-staining bacteria within the lymph node and PCR amplification followed by Southern blot hybridization, granulomatous lymphadenitis due to B. vinsonii subsp. berkhoffii was diagnosed in a dog. The source of infection was believed to be a tick that was removed from the ear 7 days prior to the onset of illness (83). Although the sources of infection differed, the historical course and clinicopathologic findings for this dog were comparable to those of CSD lymphadenitis in human patients. B. henselae was amplified and sequenced on two independent occasions from the liver of a dog with peliosis hepatis (53). Although not identified, extrapolation from the frequent association of peliosis hepatis with HIV-infected individuals supports the possibility of an unidentified cause of immunosuppression in the dog.
Coinfection
Recent serologic and molecular evidence indicates that coinfection of dogs with Ehrlichia, Babesia, and Bartonella spp. may be more frequent than previously realized (15, 63, 81). Therefore, the extent to which infection with Bartonella influences the pathophysiology of ehrlichiosis or babesiosis, diseases of much longer historical venue, deserves critical reappraisal. Of similar potential concern in human medicine is the finding of cosegregation of Borrelia burgdorferi, Babesia microti, and a Bartonella sp. in Peromyscus leucopus mice in the north-central United States (46). In The Netherlands, Ixodes ricinus ticks can simultaneously contain B. burgdorferi (sensu lato), Ehrlichia spp., and Bartonella spp. (99). Potentially, Ixodes scapularis ticks, feeding upon infected mice, could subsequently transmit B. burgdorferi, B. microti, Ehrlichia equi (the putative agent of human granulocytic ehrlichiosis), or a rodent Bartonella sp., which are rarely considered pathogenic in human beings (46, 106). These recent observations illustrate the potential difficulty in establishing causation associated with a single pathogen in dogs or people that may be coinfected with multiple tick-transmitted pathogens. As certain Borrelia, Ehrlichia, Babesia, and Bartonella spp. can cause chronic, subclinical infection in dogs, the relative role of each of these organisms to the pathogenesis of specific disease manifestations in a sick, naturally infected dog will remain difficult to establish.
ZOONOTIC POTENTIAL FOR HUMAN INFECTION
Routes of Transmission
Considering the extensive animal reservoirs and the large number of insects that have been implicated in the transmission of Bartonella spp., both animal and human exposure to these organisms may be more substantial than is currently believed. This statement is supported in part by seroprevalence data from healthy human blood donors as well as individuals with more frequent animal contact. Seroprevalence in healthy human blood donors has ranged from 2 to 6% (48, 86, 108) in the United States and 4% in Sweden (47). In Seattle, seroprevalence was 20% among an indigent, inner-city clinic population (48), and in Marseilles, 30% of homeless patients had high antibody titers to B. quintana (18). Two seroprevalence studies involving veterinary professionals from the United States and veterinarians from Europe have reported seroprevalence to B. henselae antigens as 7.1 and 51.1%, respectively (both studies used IFA testing) (78, 79). In addition to exposure to known and putative insect vectors, the high levels of bacteremia currently being documented in numerous domestic and wild animal species indicate that there is a tremendous animal reservoir for these organisms in nature. In this regard, transmission without an insect vector, although perhaps infrequent, seems plausible. For example, as a high percentage of wild rabbits, deer, and beef cattle can be bacteremic, inadvertent blood transmission might occur during the butchering process.
Risk of Transmission
Based upon animal studies as well as recent and historical accounts, the risk of transmission of B. quintana or B. bacilliformis (in regions of South America where bartonellosis is endemic) through human blood transfusion may prove to be a legitimate medical concern. As fleas may be necessary for the transmission of feline Bartonella species to humans, rigorous flea control should be recommended by health care workers, particularly when advising immunocompromised individuals on risks related to pet ownership. Fortunately, several safe and highly effective products have been introduced in recent years that can eliminate or substantially reduce flea infestation in cats and dogs. Although vector competency has not been clearly established for tick species, circumstantial evidence suggests that tick control measures should also be emphasized to avoid the potential of Bartonella transmission to humans. Infrequently, dogs have been implicated in the transmission of Bartonella spp. following a bite or a scratch (52, 103). In future case studies implicating transmission from dogs, definitive documentation of the causative Bartonella sp. in people will be important, as B. vinsonii subsp. berkhoffii is the only species isolated from dogs to date. Children and immunocompromised individuals should be cautioned to avoid behaviors that increase the risk of animal bites or scratches.
CONCLUDING REMARKS
Not too long ago, many were taught during microbiology courses (or medical school training) that blood is generally a sterile medium. Increasingly, this assertion must be qualified with regard to Bartonella spp. as well as other intracellular pathogens that have coevolved with humans and animals to persist in circulating blood cells such as erythrocytes or macrophages for months to years and perhaps longer. As establishing a cause-and-effect relationship in chronic infectious diseases can be extremely difficult, particularly for cats, among which the prevalence of infection is high, substantial effort will be required to clarify the role of Bartonella spp. as a cause of chronic insidious disease in animals and people. On an evolutionary basis, animals with substantial exposure to vector-borne intraerythrocytic Bartonella have presumably developed a highly sophisticated immunologic response to organisms that can persist for long periods within the vasculature. Elucidation of the mechanisms associated with immunologic adaptation to intravascular persistence on a comparative basis may more readily unravel the complexity of this process and thereby benefit humans as well as animals. Despite substantial and rapid progress during the past several years, Bartonella spp. still have many secrets to share with investigators in the future.
ACKNOWLEDGMENTS
We acknowledge the many investigators who have unselfishly shared details of data that are in the process of publication. Numerous sources within the animal health industry provided the financial support to generate much of the data referenced in this review. We gratefully acknowledge the assistance of Kenneth H. Wilson in the preparation of the phylogenetic dendrogram. Our thanks to colleagues and collaborators and, in particular, to Vicki Grantham for editorial assistance.
REFERENCES
- 1.Abbott R C, Chomel B B, Kasten R W, Floyd-Hawkins K A, Kikuchi Y, Koehler J E, Pedersen N C. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/s0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker J A. A rickettsial infection in Canadian voles. J Exp Med. 1946;84:37–51. [PubMed] [Google Scholar]
- 4.Baneth G, Kordick D L, Hegarty B C, Breitschwerdt E B. Comparative seroreactivity to Bartonella henselae and Bartonella quintana among cats from Israel and North Carolina. Vet Microbiol. 1995;50:95–103. doi: 10.1016/0378-1135(96)00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Baneth G, Breitschwerdt E B, Hegarty B C, Pappalardo B, Ryan J. A survey of tick-borne bacteria and protozoa in naturally exposed dogs from Israel. Vet Parasitol. 1998;74:133–142. doi: 10.1016/s0304-4017(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Reference deleted.
- 8.Bergmans A M C, Hulskotte E G J, de Jong C M A, van Amerongen G, Osterhaus A D M E, Schouls L M. Experimental infection of cats and macaques with Bartonella henselae: kinetics of bacteremia and efficacy of antibiotic treatment in cats. Ph.D. thesis. Bilthoven, The Netherlands: Universiteit Utrecht; 1996. pp. 101–119. [Google Scholar]
- 9.Bergmans A M C, Schellekens J F P, van Embden J D A, Schouls L M. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmans A M C, de Jong C M A, van Amerongen G, Schot C S, Schouls L M. Prevalence of Bartonella species in domestic cats in The Netherlands. J Clin Microbiol. 1997;35:2256–2261. doi: 10.1128/jcm.35.9.2256-2261.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birtles R J, Harrison T G, Molyneux D H. Grahamella in small woodland mammals in the U.K.: isolation, prevalence and host specificity. Ann Trop Med Parasitol. 1994;88:317–327. doi: 10.1080/00034983.1994.11812872. [DOI] [PubMed] [Google Scholar]
- 12.Birtles R J, Harrison T G, Saunders N A, Molyneux D H. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995;45:1–8. doi: 10.1099/00207713-45-1-1. [DOI] [PubMed] [Google Scholar]
- 13.Branely J, Wolfson C, Waters P, Gottlieb T, Bradbury R. Prevalence of Bartonella henselae bacteremia, the causative agent of cat scratch disease, in an Australian cat population. Pathology. 1996;28:262–265. doi: 10.1080/00313029600169124. [DOI] [PubMed] [Google Scholar]
- 14.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breitschwerdt E B, Atkins C E, Brown T T, Kordick D L, Snyder P S. Bartonella vinsonii subspecies berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol. 1999;37:3618–3626. doi: 10.1128/jcm.37.11.3618-3626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner D J, O'Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Microbiol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 18.Broqui P, Lascola B, Roux V R, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 19.Chang C-C, Yamamoto K, Chomel B B, Kasten R W, Simpson D, Smith C, Kramer V L. Seroepidemiology of Bartonella vinsonii subsp. berkhoffii infection in California coyotes, 1994–1998. Emerg Infect Dis. 1999;58:711–715. doi: 10.3201/eid0505.990514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang, C.-C., B. B. Chomel, R. W. Kasten, R. Heller, K. M. Kocan, H. Ueno, K. Yamamoto, V. C. Bleich, B. J. Gonzales, P. K. Swift, W. M. Boyce, S. S. Jang, H.-J. Boulouis, and Y. Piemont. Isolation of Bartonella spp. from wild cervids, bovids and domestic cattle in North America. J. Am. Vet. Med. Assoc., in press. [DOI] [PMC free article] [PubMed]
- 21.Childs J E, Rooney J A, Cooper J L, Olson J G, Regnery R L. Epidemiologic observations on infection with Rochalimaea species among cats living in Baltimore, MD. J Am Vet Med Assoc. 1994;204:1775–1778. [PubMed] [Google Scholar]
- 22.Childs J E, Olson J G, Wolf A, Cohen N, Fakile Y, Rooney J A, Bacellar F, Regnery R L. Prevalence of antibodies to Rochalimaea species (cat scratch disease agent) in cats. Vet Rec. 1995;136:519–520. doi: 10.1136/vr.136.20.519. [DOI] [PubMed] [Google Scholar]
- 23.Chomel B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield A N, Abbott R C, Pedersen N C. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chomel B B, Abbott R C, Kasten R W, Floyd-Hawkins K A, Kass P H, Glaser C A, Pederson N C, Koehler J E. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995;33:2445–2450. doi: 10.1128/jcm.33.9.2445-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chomel B B. Cat-scratch disease and bacillary angiomatosis. Rev Sci Tech Off Int Epizoot. 1996;15:1061–1073. doi: 10.20506/rst.15.3.975. [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Reference deleted.
- 28.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 31.Demers D M, Bass J W, Vincent J M, Person D A, Noyes D K, Staege C M, Samlaska C P, Lockwood N H, Regnery R L, Anderson B E. Cat scratch disease in Hawaii: etiology and seroepidemiology. J Pediatr. 1995;127:23–26. doi: 10.1016/s0022-3476(95)70251-2. [DOI] [PubMed] [Google Scholar]
- 32.Droz A, Chi B, Horn E, Steigerwalt A G, Whitney A M, Brenner D J. Bartonella koehlerae sp. nov., isolated from cats. J Clin Microbiol. 1999;37:1117–1122. doi: 10.1128/jcm.37.4.1117-1122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flexman J P, Lavis N J, Kay I D, Watson M, Metcalf C, Pearman J W. Bartonella henselae is the causative agent of cat scratch disease in Australia. J Infect. 1995;31:341–345. doi: 10.1016/s0163-4453(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 34.Foil L, Andress E, Freeland R L, Roy A F, Rutledge R, Triche P C, O'Reilly K L. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol. 1998;35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 35.Foley J E, Chomel B, Kikuchi Y, Yamamoto K, Pedersen N C. Seroprevalence of Bartonella henselae in cattery cats: association with cattery hygiene and flea infestation. Vet Q. 1998;20:1–5. doi: 10.1080/01652176.1998.9694824. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Caceres U, Garcia F U. Bartonellosis: an immunodepressive disease and the life of Daniel Alcides Carrion. J Clin Pathol. 1991;95(Suppl. 1):S58–S66. [PubMed] [Google Scholar]
- 37.Glaus T, Hofmann-Lehmann R, Greene C, Glaus B, Wolfensberger C, Lutz H. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol. 1997;35:2883–2885. doi: 10.1128/jcm.35.11.2883-2885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene C E, McDermott M, Jameson P H. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682–1685. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guptill L, Slater L, Wu C, Lin T, Glickman L, Welch D, HogenEsch H. Experimental infection of young cats with the zoonotic organism Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 40.Guptill L, Slater L, Wu C-C, Lin T-L, Glickman L T, Welch D F, Tobolski J, Hogenesch H. Evidence of reproductive failure and lack of perinatal transmission of Bartonella henselae in experimentally infected cats. Vet Immunol Immunopathol. 1998;65:177–189. doi: 10.1016/s0165-2427(98)00153-6. [DOI] [PubMed] [Google Scholar]
- 41.Gurfield A N, Boulouis J-J, Chomel B B, Heller R, Kasten R W, Yamamoto K, Piemont Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J Clin Microbiol. 1997;35:2120–2123. doi: 10.1128/jcm.35.8.2120-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller R, Artois M, Xemar V, DeBriel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heller R, Riegel P, Hansmann Y, Delacour G, Bermond D, Dehio C, Lamarque F, Monteil H, Chomel B, Piemont Y. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol. 1998;48:1333–1339. doi: 10.1099/00207713-48-4-1333. [DOI] [PubMed] [Google Scholar]
- 44.Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Lamarque F, Kasten R, Boulouis H-J, Monteil H, Chomel B, Piemont Y. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol. 1999;49:283–288. doi: 10.1099/00207713-49-1-283. [DOI] [PubMed] [Google Scholar]
- 45.Higgins J A, Radulovic S, Jaworski D C, Azad A F. Acquisition of the cat scratch disease agent Bartonella henselae by cat fleas (Siphonaptera: Pulicidae) J Med Entomol. 1996;33:490–495. doi: 10.1093/jmedent/33.3.490. [DOI] [PubMed] [Google Scholar]
- 46.Hofmeister E K, Kolbert C P, Abdulkarim A S, Magera J M H, Hopkins M K, Uhl J R, Ambyaye A, Telford III S R, Cocerill III F R, Persing D H. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177:409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 47.Holmberg M, McGill S, Ehrenborg C, Wesslen L, Hjelm E, Darelid J, Blad L, Engstrand L, Regnery R, Friman G. Evaluation of human seroreactivity to Bartonella species in Sweden. J Clin Microbiol. 1999;37:1381–1384. doi: 10.1128/jcm.37.5.1381-1384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson L A, Spach D H, Kippen D A, Sugg N K, Regnery R L, Sayers M H, Stamm W E. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis. 1996;173:1023–1026. doi: 10.1093/infdis/173.4.1023. [DOI] [PubMed] [Google Scholar]
- 49.Jameson P H, Greene C E, Regnery R L, Dryden M, Marks A, Brown J, Cooper J, Glaus B, Greene R. Seroprevalence of Rochalimaea henselae in pet cats throughout regions of North America. J Infect Dis. 1995;172:1145–1149. doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 50.Joseph A K, Wood C W, Robson J M, Paul S L, Morris A J. Bartonella henselae bacteremia in domestic cats from Auckland. N Z Vet J. 1997;45:185–187. doi: 10.1080/00480169.1997.36023. [DOI] [PubMed] [Google Scholar]
- 51.Kelly P J, Mattheman L A, Hayter D, Downey S, Wray K, Bryson N R, Raoult D. Bartonella (Rochalimaea) henselae in Southern Africa—evidence for infections in domestic cats and implications for veterinarians. Tydskr S-Afr Vet Ver. 1996;67:182–187. [PubMed] [Google Scholar]
- 52.Keret D, Giladi M, Kletter Y, Wientroub S. Cat-scratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br. 1998;80-B:766–767. [PubMed] [Google Scholar]
- 53.Kitchell B E, Fan T M, Walenberg G, Kordick D L, Breitschwerdt E B. Peliosis hepatis in a dog associated with Bartonella henselae. J Am Vet Med Assoc. 2000;216:519–523. doi: 10.2460/javma.2000.216.519. [DOI] [PubMed] [Google Scholar]
- 54.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection: a new zoonosis with the domestic cat as reservoir. J Am Med Assoc. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 55.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kordick D L, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O'Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs: Bartonella vinsonii subsp. vinsonii; an emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 58.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papule, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kordick D L, Breitschwerdt E B. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 60.Kordick D L, Papich M G, Breitschwerdt E B. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997;41:2448–2455. doi: 10.1128/aac.41.11.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kordick D L, Breitschwerdt E B. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4:325–328. doi: 10.3201/eid0402.980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kordick D L, Brown T T, Shin K, Breitschwerdt E B. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol. 1999;37:1536–1547. doi: 10.1128/jcm.37.5.1536-1547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kordick S K, Breitschwerdt E B, Hegarty B C, Southwick K L, Colitz C M, Hancock S I, Bradley J M, Rumbough R, McPherson J T, MacCormack J N. Coinfection with multiple tickborne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosoy M Y, Regnery R L, Tzianabos T, Marston E L, Jones D C, Green D, Maupin G O, Olson J G, Childs J E. Distribution, diversity, and host specificity of Bartonella in rodents from the southeastern United States. Am J Trop Med Hyg. 1997;57:578–588. doi: 10.4269/ajtmh.1997.57.578. [DOI] [PubMed] [Google Scholar]
- 65.Kosoy M Y, Regnery R L, Kosaya O L, Jones D C, Marston E L, Childs J E. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J Wildl Dis. 1998;34:305–309. doi: 10.7589/0090-3558-34.2.305. [DOI] [PubMed] [Google Scholar]
- 66.Laakkonen J, Haukisalmi V, Merritt J F. Blood parasites of shrews from Pennsylvania. J Parasitol. 1998;84:1300–1303. [PubMed] [Google Scholar]
- 67.Lappin M R, Black J C. Bartonella spp. infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc. 1999;214:1205–1207. [PubMed] [Google Scholar]
- 68.Lappin, M. R., D. L. Kordick, K. Karem, and E. B. Breitschwerdt.Bartonella spp. antibodies and DNA in aqueous humor of cats. Am. J. Vet. Res., in press. [DOI] [PMC free article] [PubMed]
- 69.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetti. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawson P A, Collins M D. Description of Bartonella clarridgeiae sp. nov. isolated from the cat of a patient with Bartonella henselae septicemia. Med Microbiol Lett. 1996;5:64–73. [Google Scholar]
- 71.Marston E L, Finkel B, Regnery R L, Winoto I L, Graham R R, Wignal S, Simanjuntak G, Olson J G. Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin Diagn Lab Immunol. 1999;6:41–44. doi: 10.1128/cdli.6.1.41-44.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maruyama S, Nogami S, Inoue I, Namba S, Asanome K, Katsube Y. Isolation of Bartonella henselae from domestic dogs in Japan. J Vet Med Sci. 1996;58:81–83. doi: 10.1292/jvms.58.81. [DOI] [PubMed] [Google Scholar]
- 73.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 74.Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implication for diagnosis. J Clin Microbiol. 1997;35:2283–2287. doi: 10.1128/jcm.35.9.2283-2287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maurin M, Raoult D. Bartonella infections: diagnostic and management issues. Curr Opin Infect Dis. 1998;11:189–193. [PubMed] [Google Scholar]
- 76.Mehock J R, Greene C E, Gherardini F C, Hahn T-W, Krause D C. Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun. 1998;66:3462–3466. doi: 10.1128/iai.66.7.3462-3466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nasirudeen A M A, Thong M L. Prevalence of Bartonella henselae immunoglobulin G antibodies in Singaporean cats. Pediatr Infect Dis J. 1999;18:276–278. doi: 10.1097/00006454-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Noah D L, Kramer C M, Verbsky M P, Rooney J A, Smith K A, Childs J E. Survey of veterinary professionals and other veterinary conference attendees for antibodies to Bartonella henselae and B. quintana. J Am Vet Med Assoc. 1997;210:342–344. [PubMed] [Google Scholar]
- 79.Nowotny N, Deutz A, Fuchs K, Schuller W, Hinterdorfer F, Auer H, Aspock H. Prevalence of swine influenza and other viral, bacterial and parasitic zoonoses in veterinarians. J Infect Dis. 1997;176:1414–1415. doi: 10.1086/517337. [DOI] [PubMed] [Google Scholar]
- 80.O'Reilly K L, Bauer R W, Freeland R L, Foil L D, Huges K J, Rohde K R, Roy A F, Stout R W, Triche P. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU 16) Infect Immun. 1999;67:3066–3072. doi: 10.1128/iai.67.6.3066-3072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pappalardo B L, Correa M T, York C C, Peat C Y, Breitschwerdt E B. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 82.Reference deleted.
- 83.Pappalardo B L, Brown T, Gookin J L, Morrill C L, Breitschwerdt E B. Granulomatous disease associated with Bartonella infection in two dogs. J Vet Intern Med. 2000;14:37–42. doi: 10.1892/0891-6640(2000)014<0037:gdawii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 84.Regnath T, Mielke M E A, Arvand M, Hahn H. Murine model of Bartonella henselae infection in the immunocompetent host. Infect Immun. 1998;66:5534–5536. doi: 10.1128/iai.66.11.5534-5536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regnery R L, Anderson B E, Clarridge J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Regnery R L, Olson J, Perkins B A, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 87.Regnery R L, Childs J E, Koehler J E. Infections associated with Bartonella species in persons infected with human immunodeficiency virus. J Infect Dis. 1995;21(Suppl. 1):S94–S98. doi: 10.1093/clinids/21.supplement_1.s94. [DOI] [PubMed] [Google Scholar]
- 88.Regnery R, Tappero J. Unraveling mysteries associated with cat-scratch disease, bacillary angiomatosis and related syndromes. Emerg Infect Dis. 1995;1:16–21. doi: 10.3201/eid0101.950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 90.Relman D A, Lepp P W, Sadler K N, Schmidt T M. Phylogenetic relationships among the agent of bacillary angiomatosis, Bartonella bacilliformis, and other alpha proteobacteria. Mol Microbiol. 1992;6:1801–1807. doi: 10.1111/j.1365-2958.1992.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 91.Restrepo B I, Barbour A G. Antigen diversity in the bacterium Borrelia hermsii through “somatic” mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 92.Rotstein D S, Taylor S K, Bradley J, Breitschwerdt E B. Prevalence of Bartonella henselae antibody in free-ranging Florida panthers and translocated Texas cougars from Florida. J Wildl Dis. 2000;36:157–160. doi: 10.7589/0090-3558-36.1.157. [DOI] [PubMed] [Google Scholar]
- 93.Roux V, Raoult D. Body lice as tools for diagnosis and surveillance of reemerging disease. J Clin Microbiol. 1999;37:596–599. doi: 10.1128/jcm.37.3.596-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sander A, Buhler C, Pelz K, von Cramm E, Bredt W. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J Clin Microbiol. 1997;35:584–587. doi: 10.1128/jcm.35.3.584-587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sander A, Ruess M, Deichmann K, Bohn N, Bredt W. Two different genotypes of Bartonella henselae in children with cat scratch disease and their pet cats. Scand J Infect Dis. 1998;30:387–391. doi: 10.1080/00365549850160693. [DOI] [PubMed] [Google Scholar]
- 96.Sander A, Ruess M, Bereswill S, Schuppler M, Steinbrueckner B. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J Clin Microbiol. 1998;36:2973–2981. doi: 10.1128/jcm.36.10.2973-2981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sander A. Microbiological diagnosis of Bartonella species and Afipia felis. In: Schmidt A, editor. Bartonella and Afipia species emphasizing Bartonella henselae. Basel, Switzerland: Karger Publishing Co.; 1998. pp. 98–129. [Google Scholar]
- 98.Schmidt A, editor. Bartonella and Afipia species emphasizing Bartonella henselae (Contributions to microbiology) Vol. 1. Basel, Switzerland: Karger Publishing Co.; 1998. [Google Scholar]
- 99.Schouls L M, Van De Pol I, Rijpkema S G T, Schot C S. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schwartzman W. Bartonella (Rochalimaea) infections: beyond cat scratch. Annu Rev Med. 1996;47:355–364. doi: 10.1146/annurev.med.47.1.355. [DOI] [PubMed] [Google Scholar]
- 101.Reference deleted.
- 102.Slater L N, Coody D W, Woolridge L K, Welch D F. Murine antibody responses distinguish Rochalimaea henselae from Rochalimaea quintana. J Clin Microbiol. 1992;30:1722–1727. doi: 10.1128/jcm.30.7.1722-1727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsukahara M, Tsuneoka H, Lino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998;352:1682. doi: 10.1016/s0140-6736(05)61455-9. [DOI] [PubMed] [Google Scholar]
- 104.Ueno H, Muramatsu Y, Chomel B B, Hohdatsu T, Koyama H, Morita C. Seroepidemiological survey of Bartonella (Rochalimaea) henselae in domestic cats in Japan. Microbiol Immunol. 1995;39:339–341. doi: 10.1111/j.1348-0421.1995.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 105.Ueno H, Hohdatsu T, Muramatsu Y, Koyama H, Morita C. Does coinfection of B. henselae and FIV induce clinical disorders in cats? Microbiol Immunol. 1996;40:617–620. doi: 10.1111/j.1348-0421.1996.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 106.Welch D F, Carroll K C, Hofmeister E K, Persing D H, Robison D A, Steigerwalt A G, Brenner D J. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37:2598–2601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamamoto K, Chomel B B, Kasten R W, Chang C-C, Tseggai T, Decker P R, Mackowiak M, Floyd-Hawkins K A, Pedersen N C. Homologous protection but lack of heterologous protection by various species and types of Bartonella in specific pathogen free cats. Vet Immunol Immunopathol. 1998;65:191–204. doi: 10.1016/s0165-2427(98)00154-8. [DOI] [PubMed] [Google Scholar]
- 108.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Carter M L, Wenger J D. Cat scratch disease in Connecticut: epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]