Abstract
Iron is an important element for life; however, intracellular labile iron overload can lead to the generation of reactive oxygen species and cellular damage. Although iron is mainly utilized for heme synthesis and is incorporated into hemoglobin, body iron status is often implicated in the pathogenesis of cardiovascular diseases. In a cell, iron is used for basic processes such as cell growth, maintenance, and repair. Thus, iron is considered to be involved in the pathogenesis of arteriosclerosis. In fact, clinical and experimental studies have shown an association between iron and arteriosclerosis. These data suggest the crosstalk between iron and arteriosclerosis. However, iron metabolism in arteriosclerosis is often complicated, and the systemic and cellular mechanisms of iron homeostasis in arteriosclerosis remain completely unsolved. Thus, in this review, we aimed to examine the role of iron in arteriosclerosis.
Keywords: Iron, Arteriosclerosis, Atherosclerosis, Transferrin receptor 1
Introduction
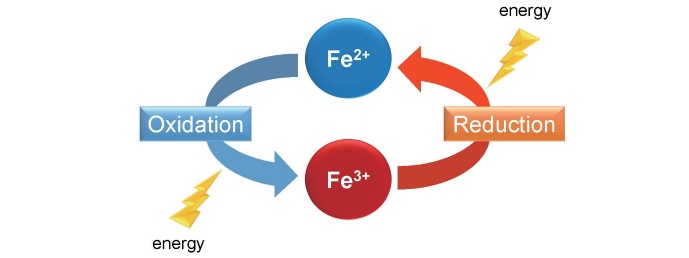
Iron is an important element for life and is known to be involved in oxidation-reduction reactions, changing between oxidation states its ferrous form (Fe 2+ ) and ferric form (Fe 3+ ). It has been determined to be involved in oxygen transport and storage and energy metabolism ( Fig.1 ) ; however, intracellular labile iron overload can lead to the generation of reactive oxygen species. In fact, iron overload has been found to induce adverse health problems and is associated in the pathogenesis of several metabolic diseases 1 , 2) . On the other hand, iron deficiency is also detrimental to health besides erythropoiesis such as cognitive development or birth defects 3) . Thus, cellular and tissue iron levels must be exquisitely governed to maintain systemic and cellular iron homeostasis. Iron is mainly utilized for heme synthesis and is incorporated into hemoglobin; however, body iron status is also implicated in the pathogenesis of cardiovascular diseases.
Fig.1. Ferrous iron (Fe 2+ ) and ferric iron (Fe 3+ ) .
Iron is involved in the oxidation-reduction reactions, changing between oxidation states Fe 2+ and Fe 3+ . Further, iron plays a role for oxygen transport and storage and energy metabolism.
As regard to aortic diseases, both iron deficiency and iron overload can contribute to the pathogenesis of arteriosclerosis and atherosclerosis, respectively 4 - 7) . Iron metabolism in arteriosclerosis and atherosclerosis is often complicated, and the systemic and cellular mechanisms of iron homeostasis in arteriosclerosis and atherosclerosis remain to be largely unknown. Thus, in this review, we focus on the role of iron in arteriosclerosis and atherosclerosis.
Systemic Iron Transport
More than 70% of body iron exists as heme within hemoglobin. Further, 20% of body iron is stored in the liver and 5% in macrophages. Most of the iron is recycled as erythrocytes in the body. Macrophages phagocytize senescent erythrocytes, degrade hemoglobin-derived heme, and export to the circulation. The released iron is mainly used in hematopoiesis in the bone marrow, while excess body iron is stored in the liver 8) .
Systemic iron homeostasis is tightly regulated by the peptide hormone hepcidin in the liver and maintained through dietary absorption in the duodenum. In the duodenum, dietary iron (Fe 3+ ) is transported into enterocyte via apical membrane iron transporter, divalent metal transporter 1 (DMT1) after reduction to iron (Fe 2+ ) by the ferrireductase, that is, duodenal cytochrome b (Dcyt-b). Iron is then stored or exported into the circulation by the basolateral membrane transporter, that is, ferroportin 9) .
Iron Transport in the Cells
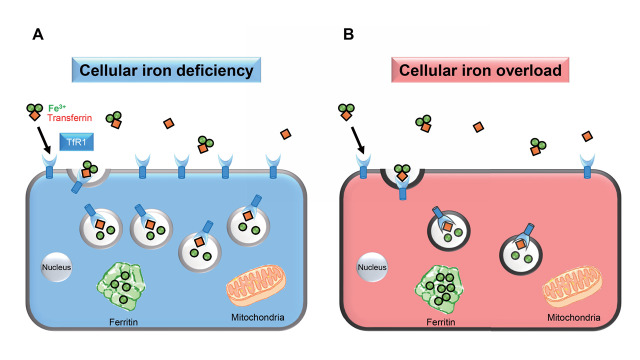
On a cellular level, iron is used for basic processes such as cell growth, maintenance, and repair. Thus, local iron must be tightly controlled for cellular homeostasis. Most cells regulate iron uptake via a cellular iron transporter, that is, transferrin receptor 1 (TfR1). TfR1 is ubiquitously expressed on the cell surface and has been found to be involved in cellular iron uptake through its interaction with transferrin. Transferrin has two iron-binding sites, the extracellular di-Fe 3+ binds to transferrin in the circulation. Transferrin-bound di-Fe 3+ is then transported into the cells by TfR1. Then, TfR1-transferrin di-Fe 3+ complex is internalized; iron is then released into the cells by endocytosis. Transferrin is recycled back to the circulation, and TfR1 is back to cell membrane 10) . In cellular iron deficiency, TfR1 is upregulated; then, cellular iron uptake is accelerated. Meanwhile, in cellular iron overload, TfR1 is downregulated, and cellular iron uptake is suppressed ( Fig.2 ) . Iron is then stored in ferritin in the cells.
Fig.2. A cellular iron transporter, transferrin receptor 1.
TfR1-transferrin di-Fe 3+ complex is transported into the cells by endocytosis after binding to transferrin receptor 1 (TfR1). (A) If cellular iron is deficient, TfR1 is upregulated, and cellular iron uptake is then accelerated. (B) If cellular iron is in excess, TfR1 is downregulated, and cellular iron uptake is inhibited.
Until now, the role of TfR1 in cardiovascular diseases remains under investigation. For instance, cardiomyocyte-specific TfR1 deleted mice were created, and the role of TfR1 in the heart was examined. Cardiomyocyte-specific TfR1 deleted mice died by post-natal day 11 due to cardiac hypertrophy and dysfunction, caused by iron deficiency and associated with mitochondrial failure 11) . Previously, we have examined the role of TfR1 in the mechanism of limb ischemia and renal fibrosis, wherein in limb ischemia models study, blood flow recovery after limb ischemia was attenuated in TfR1 heterozygous deleted mice, along with decreased expression of ferritin and mitochondrial complex I in ischemic adductor muscles 12) . In renal fibrosis models study, we assessed the effects of heterozygous TfR1 deletion in the pathogenesis of renal fibrosis using obstructive nephropathy and diabetic kidney disease models. As per our findings, TfR1 heterozygous deleted mice exhibited attenuated renal fibrosis, along with reduced renal expression of ferritin and 4-hydroxynonenal in both models 13) . These results suggest that TfR1 is involved in the pathogenesis of cardiovascular diseases.
Cellular Iron Transport in Arteriosclerosis
Iron is reported to contribute to the mechanism of both arteriosclerosis and atherosclerosis. Impaired endothelial function and increased intima-media thickness of the carotid artery have been determined to be associated with iron overload in patients with hereditary hemochromatosis 6) . In a 64-slice coronary computed tomography scanning study, an association between increased serum ferritin levels and the presence of coronary artery calcium score, which is a marker of early coronary artery atherosclerosis, has been reported in 12,033 men 14) . In addition, a study using venous occlusion plethysmography has shown that deferoxamine, an iron chelator, improved nitric oxide-mediated, endothelium-dependent vasodilation in patients with coronary artery diseases 15) . Although several investigations on iron and aortic diseases have been performed, the causal relationship between iron and aortic diseases is yet to be fully understood. Also, it is almost unknown whether cellular iron regulating system is involved in the mechanisms of aortic diseases.
In this regard, we have investigated the iron regulating system using experimental model of arteriosclerosis and shown the impaired expression of aortic iron transporters in hypertensive model rats. In that study, we first confirmed iron deficiency and upregulated aortic TfR1 expression in rats given an iron-deficient diet. As per our immunohistochemical analysis, it was determined that TfR1 was expressed in the media layer of the aorta. The rats given an iron-deficient diet exhibited upregulated aortic TfR1 expression and downregulated ferritin heavy subunits (ferritin H) expression because of systemic iron deficiency. Of interest, we found that both aortic TfR1 and ferritin H expression were upregulated in Dahl salt-sensitive hypertensive rats with hypertension 16) . Dahl salt-sensitive hypertensive rats with hypertension showed anemia and decreased serum iron levels compared with rats given a normal diet 17) . These results suggested that dysregulated iron regulating system occurred in the aorta of hypertensive model rats. In addition, dietary iron restriction has attenuated the development of arteriosclerosis in these hypertensive rats, suggesting that cellular iron intake has contributed to the development of arteriosclerosis through upregulated aortic TfR1 expression 16) . Iron is required for cell growth, maintenance, and repair. Thus, augmented aortic TfR1 may have contributed to the mechanism of arteriosclerosis. Based on these data, we further investigated TfR1 expression in 5/6 nephrectomized chronic kidney disease (CKD) model rats. The CKD model rats showed hypertension and hypertensive arteriosclerosis. Of interest, aortic TfR1 expression was also upregulated in the media layer of aorta in CKD model rats, and dietary iron restriction suppressed the development of arteriosclerosis in CKD model rats 18) . Taken together, these results suggest that cellular iron intake contributes to arteriosclerosis through upregulated aortic TfR1 expression ( Fig.3 ) . Although further studies on cellular iron intake in the mechanism of arteriosclerosis are necessary, understanding the iron regulating system in arteriosclerosis may lead to a new therapeutic target in arteriosclerosis.
Fig.3. Aortic transferrin receptor 1 in arteriosclerosis.
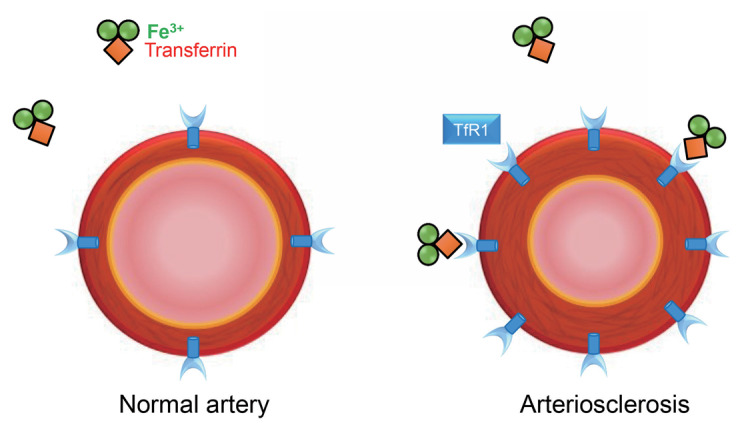
Cellular iron intake contributes to the pathogenesis of arteriosclerosis through upregulated aortic transferrin receptor 1 (TfR1) expression.
Cellular Iron Transport in Pulmonary Arteriosclerosis
Pulmonary hypertension is a progressing vascular disease characterized by pulmonary vascular remodeling, which induces right ventricular failure and sudden death 19) . Iron is also considered to be implicated in the pathogenesis of pulmonary hypertension. Iron deficiency is often prevalent in patients with pulmonary hypertension 20 - 22) . Iron deficiency without overt anemia, as defined by raised levels of soluble transferrin receptor, is common in patients with idiopathic pulmonary arterial hypertension; further, it is associated with disease severity and poor clinical outcome 20) . Another study also shows that iron deficiency is frequent in patients with idiopathic pulmonary arterial hypertension and associated with a lower exercise capacity regardless of the presence of anemia 21) . Also, the prevalence of unexplained iron deficiency is high in patients with idiopathic pulmonary arterial hypertension 22) . In addition, iron has been linked to the control of pulmonary vascular tone in response to acute hypoxic condition 23 , 24) . An intravenous iron infusion reduced both the elevation in baseline pulmonary artery systolic pressure and the enhanced sensitivity of the pulmonary vasculature to acute hypoxia induced by exposure to sustained hypoxia in a study examining 16 healthy volunteers 23) . Also, two randomized controlled trials showed that approximately 40% of the pulmonary hypertensive response to hypoxia was reversed by an intravenous iron infusion, which reduced pulmonary artery systolic pressure in 22 healthy sea-level resident men 24) .
Based on the experimental results of hypertensive arteriosclerosis, we have investigated the involvement of cellular iron intake in the mechanisms of vascular lesions of pulmonary hypertension (pulmonary vascular remodeling). Of note, TfR1 expression was increased in the remodeled pulmonary artery of monocrotaline-injected pulmonary hypertension model rats. In addition, dietary iron restriction has prevented the development of pulmonary vascular remodeling in monocrotaline-injected pulmonary hypertensive model rats 25) . We have also found that TfR1 heterozygous deleted mice attenuated pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension model mice 26) . Moreover, the depletion of TfR1 by RNA interference has attenuated human pulmonary artery smooth muscle cells proliferation induced by platelet-derived growth factor-BB in vitro 26) . Taken together, these results suggest that cellular iron intake via TfR1 in pulmonary artery may be associated with the pathophysiology of pulmonary vascular remodeling.
Chronic severe iron deficiency can lead to pulmonary vascular remodeling in normal Sprague–Dawley rats 27) . Clinical studies have shown that iron deficiency is noted to be frequent in patients with idiopathic pulmonary arterial hypertension 20 - 22) . In cellular iron deficiency, TfR1 is upregulated; then, cellular iron uptake is accelerated. Collectively, TfR1 in pulmonary artery in response to iron deficiency may contribute to the development of pulmonary vascular remodeling in pulmonary hypertension. As iron deficiency is associated with a poor prognosis in patients with idiopathic pulmonary arterial hypertension 20) , iron deficiency is expected as a therapeutic target for pulmonary hypertension. As regards iron repletion for patients with pulmonary hypertension, recent two randomized, double-blind studies of intravenous iron versus placebo showed that iron repletion to pulmonary hypertension patients with iron deficiency without overt anemia provided no significant clinical benefit at 12 weeks after intravenous iron administration 28) . Besides systemic iron repletion, modulation of TfR1 expression in pulmonary artery might contribute to preventing or treating pulmonary vascular remodeling.
Cellular Iron Transport in Atherosclerosis
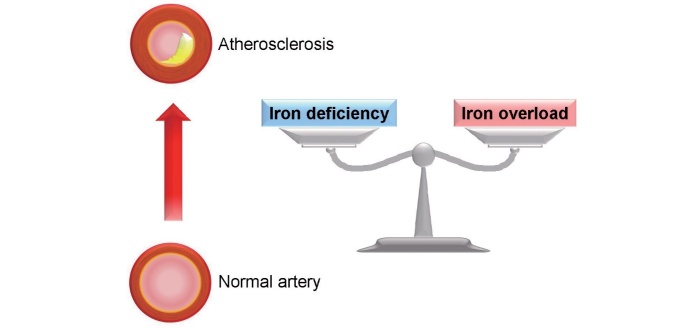
Epidemiologic and experimental studies have shown an association between iron and atherosclerosis. Prospective results from the Bruneck study show the potential association between increased serum ferritin levels and the 5-year progression of carotid atherosclerosis assessed by ultrasonography 4) . From the Kuopio Ischaemic Heart Disease Risk Factor Study in Eastern Finland, high stored iron levels, as assessed by elevated serum ferritin levels, have been identified to be a risk factor for coronary heart disease 5) . Also, associations between increased iron load and endothelium-dependent dilation of the brachial artery and intima-media thickness of the carotid artery are reported in patients with hereditary hemochromatosis 6) . By contrast, a Mendelian randomization study has shown that higher iron status reduces coronary artery disease risk 7) . In experimental studies, iron accumulation is observed in the atherosclerotic lesions of apolipoprotein E-deficient mice 29) . In addition, we have previously reported that iron is involved in the pathophysiology of abdominal aortic aneurysm (AAA) formation in angiotensin II injected apolipoprotein E-deficient mice 30) . Iron deposition was accumulated in AAA walls with the increments of oxidative stress and inflammation. Also, dietary iron restriction has reduced the incidence of AAA formation with attenuation of oxidative stress and inflammation in angiotensin II-induced AAA mice 30) . Iron is known to bind to transferrin in the circulation. When iron exceeds the carrying capacity of transferrin, non-transferrin-bound iron then circulates and promotes organ damage such as vascular endothelial cell dysfunction. Indeed, crossing hereditary haemochromatosis mice with apolipoprotein E-deficient mice was associated with iron accumulation on the aorta and an increase in atherosclerotic lesion compared with control mice 31) . Atherosclerotic plaques contain iron, and this may promote atherosclerotic plaque progression 32) . Iron may also accumulate in atherosclerotic lesions, and the hydroxyl radical is thought to promote the formation of oxidized low-density lipoprotein cholesterol and pro-inflammatory intermediates. In contrast, an earlier study has reported that dietary iron overload decreased atherosclerosis in apolipoprotein E-deficient mice 33) , and iron deficiency is reported to be associated with a risk of coronary artery disease (CAD) 7 , 34) . These are supports to the hypothesis that both iron overload and iron deficiency are associated with the pathogenesis of atherosclerosis ( Fig.4 ) . These findings suggest the importance of appropriate body iron status in the pathogenesis of atherosclerosis. However, the detailed mechanism of this U-shaped association of iron and atherosclerosis remains unknown. In experimental studies reporting a link between iron overload and atherosclerosis, iron deposits appear in atherosclerotic lesions, particularly in the endothelium, intima enriched in foam cells, and smooth muscle cells of the media layer of aorta in apolipoprotein E-deficient mice 29) . Further, iron is mainly present in the media layer of aorta in hereditary haemochromatosis mice crossed with apolipoprotein E-deficient mice 31) . The investigation focusing on cellular iron metabolism may lead to uncover the mechanism of these cellular iron distributions in atherosclerosis and a U-shaped association of iron and atherosclerosis.
Fig.4. Crosstalk between iron and atherosclerosis.
Both iron deficiency and iron overload are associated with the pathogenesis of atherosclerosis. The appropriate body iron status should be considered in patients with atherosclerosis.
In patients with heart failure (HF), iron deficiency has been associated with a poor prognosis 35) . Thus, iron deficiency is expected as a therapeutic target for HF. The European Society of Cardiology guideline 2016 has recommended iron repletion for symptomatic patients with HF with reduced ejection fraction with iron deficiency as class IIa 36) . Also, intravenous iron supplementation has been recommended for symptomatic patients with HF with reduced ejection fraction (NYHA II/III) with iron deficiency as class IIb in American College of Cardiology/American Heart Association/Heart Failure Society of America 2017 guidelines 37) . In fact, iron is necessary to maintain mitochondrial functions in organs with high-energy demands such as the heart. However, iron repletion could be harmful to HF patients complicated with arteriosclerosis. Considering the total iron balance for the body, the risks and benefits of iron repletion should be considered cautiously in patients with arteriosclerosis.
Conclusion
Iron has been determined to contribute to the development of arteriosclerosis and atherosclerosis. Thus, understanding the iron regulating system in these aortic diseases may lead to a new therapeutic target in aortic diseases. However, future studies are needed to address the clinical significance of cellular iron metabolism in aortic diseases.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C) JSPS KAKENHI Grant No. 25460919, 16K09273, and 19K07950 (to Y. Naito) and No. 18K08055 (to T. Tsujino and Y. Naito)
Disclosures
None.
References
- 1).Fernandez-Real JM, Manco M: Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol, 2014; 2: 513-526 [DOI] [PubMed] [Google Scholar]
- 2).Jehn M, Clark JM, Guallar E: Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care, 2004; 27: 2422-2428 [DOI] [PubMed] [Google Scholar]
- 3).Musallam KM, Taher AT: Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin, 2018; 34: 81-93 [DOI] [PubMed] [Google Scholar]
- 4).Kiechl S, Wileit J, Egger G, Poewe W, Oberhollenzer F: Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation, 1997; 96: 3300-3307 [DOI] [PubMed] [Google Scholar]
- 5).Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R: High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation, 1992; 86: 803-811 [DOI] [PubMed] [Google Scholar]
- 6).Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, Weiss G: Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol, 2002; 40: 2189-2194 [DOI] [PubMed] [Google Scholar]
- 7).Gill D, Del Greco M F, Walker AP, Srai SKS, Laffan MA, Minelli C: The Effect of Iron Status on Risk of Coronary Artery Disease: A Mendelian Randomization Study-Brief Report. Arterioscler Thromb Vasc Biol, 2017; 37: 1788-1792 [DOI] [PubMed] [Google Scholar]
- 8).Andrews NC: Disorders of iron metabolism. N Engl J Med, 1999; 341: 1986-1995 [DOI] [PubMed] [Google Scholar]
- 9).Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC: The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab, 2005; 1: 191-200 [DOI] [PubMed] [Google Scholar]
- 10).Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML: The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol, 2006; 121: 144-158 [DOI] [PubMed] [Google Scholar]
- 11).Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC: Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep, 2015; 13: 533-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Okuno K, Naito Y, Yasumura S, Sawada H, Asakura M, Masuyama T, Ishihara M: Haploinsufficiency of transferrin receptor 1 impairs angiogenesis with reduced mitochondrial complex I in mice with limb ischemia. Sci Rep, 2019; 9: 13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yasumura S, Naito Y, Okuno K, Sawada H, Asakura M, Masuyama T, Ishihara M: Effects of heterozygous transferrin receptor 1 deletion in pathogenesis of renal fibrosis in mice. Hypertension, 2020; 75: 413-421 [DOI] [PubMed] [Google Scholar]
- 14).Sung KC, Kang SM, Cho EJ, Park JB, Wild SH, Byrne CD: Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler Thromb Vasc Biol, 2012; 32: 2525-2530 [DOI] [PubMed] [Google Scholar]
- 15).Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF Jr, Vita JA: Iron chelation improves endothelial function in patients with coronary artery disease. Circulation, 2001; 103: 2799-2804 [DOI] [PubMed] [Google Scholar]
- 16).Naito Y, Hirotani S, Sawada H, Akahori H, Tsujino T, Masuyama T: Dietary iron restriction prevents hypertensive cardiovascular remodeling in Dahl salt-sensitive rats. Hypertension, 2011; 57: 497-504 [DOI] [PubMed] [Google Scholar]
- 17).Naito Y, Tsujino T, Fujimori Y, Sawada H, Akahori H, Hirotani S, Ohyanagi M, Masuyama T: Impaired expression of duodenal iron transporters in Dahl salt-sensitive heart failure rats. J Hypertens, 2011; 29: 741-748 [DOI] [PubMed] [Google Scholar]
- 18).Naito Y, Fujii A, Sawada H, Hirotani S, Iwasaku T, Okuhara Y, Eguchi A, Ohyanagi M, Tsujino T, Masuyama T: Dietary iron restriction prevents further deterioration of renal damage in a chronic kidney disease rat model. J Hypertens, 2013; 31: 1203-1213 [DOI] [PubMed] [Google Scholar]
- 19).Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB; National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure: Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation, 2006; 114: 1883-1891 [DOI] [PubMed] [Google Scholar]
- 20).Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili, Gibbs JS, Wharton J, Wilkins MR: Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol, 2011; 58: 300-309 [DOI] [PubMed] [Google Scholar]
- 21).Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, van der Laarse WJ, Vonk-Noordegraaf A: Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J, 2011; 37: 1386-1391 [DOI] [PubMed] [Google Scholar]
- 22).Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, Sinclair-McGarvie M, Arnold J, Sheares KK, Morrell NW, Pepke-Zaba J: Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax, 2011; 66: 326-332 [DOI] [PubMed] [Google Scholar]
- 23).Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, Robbins PA: The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol, 2008; 586: 5999-6005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, Dorrington KL, León-Velarde F, Robbins PA: Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA, 2009; 302: 1444-1450 [DOI] [PubMed] [Google Scholar]
- 25).Naito Y, Hosokawa M, Hao H, Sawada H, Hirotani S, Iwasaku T, Okuhara Y, Eguchi A, Hirota S, Ohyanagi M, Tsujino T, Masuyama T: Impact of dietary iron restriction on the development of monocrotaline-induced pulmonary vascular remodeling and right ventricular failure in rats. Biochem Biophys Res Commun, 2013; 436: 145-151 [DOI] [PubMed] [Google Scholar]
- 26).Naito Y, Hosokawa M, Sawada H, Oboshi M, Hirotani S, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Nishimura K, Soyama Y, Fujii K, Mano T, Ishihara M, Tsujino T, Masuyama T: Transferrin receptor 1 in chronic hypoxia-induced pulmonary vascular remodeling. Am J Hypertens, 2016; 29: 713-718 [DOI] [PubMed] [Google Scholar]
- 27).Cotroneo E, Ashek A, Wang L, Wharton J, Dubois O, Bozorgi S, Busbridge M, Alavian KN, Wilkins MR, Zhao L: Iron homeostasis and pulmonary hypertension: Iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res, 2015; 116: 1680-1690 [DOI] [PubMed] [Google Scholar]
- 28).Howard LSGE, He J, Watson GMJ, Huang L, Wharton J, Luo Q, Kiely DG, Condliffe R, Pepke-Zaba J, Morrell NW, Sheares KK, Ulrich A, Quan R, Zhao Z, Jing X, An C, Liu Z, Xiong C, Robbins PA, Dawes T, de Marvao A, Rhodes CJ, Richter MJ, Gall H, Ghofrani HA, Zhao L, Huson L, Wilkins MR: Supplementation with Iron in Pulmonary Arterial Hypertension: Two Randomized Crossover Trials. Ann Am Thorac Soc, 2021; doi: 10.1513/AnnalsATS.202009-1131OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Lee TS, Shiao MS, Pan CC, Chau LY: Iron-deficient diet reduces atherosclerotic lesions in apoe-deficient mice. Circulation, 1999; 99: 1222-1229 [DOI] [PubMed] [Google Scholar]
- 30).Sawada H, Hao H, Naito Y, Oboshi M, Hirotani S, Mitsuno M, Miyamoto Y, Hirota S, Masuyama T: Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol, 2015; 35: 1507-1514 [DOI] [PubMed] [Google Scholar]
- 31).Vinchi F, Porto G, Simmelbauer A, Altamura S, Passos ST, Garbowski M, Silva AMN, Spaich S, Seide SE, Sparla R, Hentze MW, Muckenthaler MU: Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J, 2020; 41: 2681-2695 [DOI] [PubMed] [Google Scholar]
- 32).Sullivan JL: Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp Biol Med (Maywood), 2007; 232: 1014-1020 [DOI] [PubMed] [Google Scholar]
- 33).Kirk EA, Heinecke JW, LeBoeuf RC: Iron overload diminishes atherosclerosis in apoE-deficient mice. J Clin Invest, 2001; 107: 1545-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Das De S, Krishna S, Jethwa A: Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis, 2015; 238: 296-303 [DOI] [PubMed] [Google Scholar]
- 35).Rocha BML, Cunha GJL, Menezes Falcão LF: The Burden of Iron Deficiency in Heart Failure: Therapeutic Approach. J Am Coll Cardiol, 2018; 71: 782-793 [DOI] [PubMed] [Google Scholar]
- 36).Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J, 2016; 37: 2129-2200 [Google Scholar]
- 37).Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C: 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation, 2017; 136: e137-e161 [DOI] [PubMed] [Google Scholar]