Abstract
Aims: Hemodialysis vintage and serum phosphorus levels adversely affect outcomes in patients on hemodialysis. Whether these factors have a similar prognostic impact on patients who are on hemodialysis and have chronic limb-threatening ischemia (CLTI) has not been systematically studied. We aimed to explore the risk factors, including hemodialysis vintage and serum phosphorus levels, on clinical outcomes after endovascular therapy (EVT) in hemodialysis patients with CLTI.
Methods: The current study rerospectively analyzed 374 hemodialysis patients with CLTI presenting with ischemic tissue loss (age: 72.3±9.0 years, male: 73.3%, diabetes mellitus: 68.2%, Rutherford 5: 75.9%, 6: 24.1%, WIfI stage 4: 50.0%) primarily treated with EVT between April 2007 and December 2016. The primary outcome measure was 1-year amputation-free survival (AFS), while the secondary outcome measure was 1-year wound healing. Predictors for each outcome were evaluated by Cox proportional hazards model.
Results: Multivariate analysis significantly associated longer hemodialysis vintages with higher serum phosphorus levels (hazard ratio [HR], 0.599; 95% confidence interval [CI], 0.394-0.910; p =0.016) with 1-year AFS. Longer vintages for hemodialysis with higher serum phosphorus levels were marginally, but not significantly, associated with 1-year wound healing. (HR, 0.684; 95% CI, 0.467–1.000; p =0.050).
Conclusion: Longer hemodialysis vintages with higher serum phosphorus levels adversely affect outcomes after EVT for hemodialysis patients with CLTI presenting with ischemic tissue loss.
Keywords: Amputation-free survival (AFS), Chronic limb-threatening ischemia (CLTI), Hemodialysis vintage, Serum phosphorus level, Wound healing
Introduction
The number of patients with chronic kidney disease (CKD), including hemodialysis, has increased over the years, presumably due to the aging society as well as the diabetes mellitus (DM) and hypertension (HTN) pandemics 1) . CKD itself is a well-known risk factor in peripheral arterial disease (PAD) etiology since it is a part of cardiovascular disease. A previous study reported that patients with more advanced CKD stages had more severe clinical presentations of PAD at initial diagnosis 2) . The management and prevention of patients with CLTI are pressing issues in terms of both medical costs and public health. They have poor prognoses with high mortality and amputation rates unless they undergo appropriate revascularization 3 - 6) . Currently, because of recently-developed dedicated devices and accumulated experience, endovascular therapy (EVT) outcomes have improved considerably. EVT is now becoming an attractive alternative first-line treatment for CLTI 3 , 4) . Despite this major paradigm shift and improved outcomes, CKD, especially hemodialysis, is still a major prognostic factor in clinical outcomes after revascularization. The prediction of outcomes in hemodialysis patients with CLTI is clinically important for risk stratification 7) .
Aim
Although hemodialysis vintage and serum phosphorus levels adversely impact outcomes in the general population on hemodialysis, whether these have a similarly prognostic impact on outcomes in the hemodialysis patients with CLTI presenting tissue loss has not been systematically studied. Therefore, we sought to explore the risk factors, including hemodialysis vintage and serum phosphorus levels, for clinical outcomes after revascularization in hemodialysis patients with CLTI.
Methods
Participants
We used a retrospective database of 508 consecutive hemodialysis patients with CLTI presetting with ischemic tissue loss who primarily underwent EVT between January 2007 and December 2016. We excluded patients who had missing baseline data ( n =98) or had not been evaluated for hemodialysis vintage as well as serum phosphorus levels ( n =36). We analyzed the remaining 374 patients ( Fig.1 ) . The current study was performed under the Declaration of Helsinki and approved by the hospital’s ethics committee.
Fig.1.
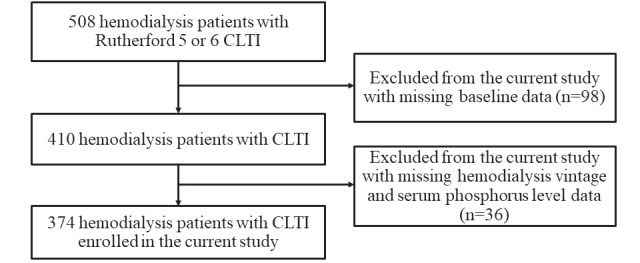
Flow chart of the study population
EVT Strategy
Laboratory data and prescribed medications were generally checked upon admission before revascularization. The severity of ischemia in the lower limbs was assessed hemodynamically by the ankle-brachial index (ABI) and skin perfusion pressure (SPP). Lower limb arteries were evaluated routinely by duplex ultrasound, and the morphology of arterial lesions was assessed by diagnostic angiogram before revascularization. EVT was indicated when the lesion showed 75% stenosis or greater of the vessel diameter on diagnostic angiography with hemodynamic significance. EVT was done according to the generally accepted principles. In the aortoiliac lesions, a primary stenting strategy was conducted. In the femoropopliteal lesions, a stent was allowed in angioplasty failure cases, which included severe dissection, residual stenosis, or a significant pressure gradient of 10 mmHg or greater as a provisional stenting strategy. Below-the-knee (BTK) lesions were generally treated with plain angioplasty under a health insurance system. Drug-coated balloon and atherectomy devices were not used because they were not approved in Japan during the study period.
Follow-Up Protocol
The follow-up interval and modality for ischemic changing were basically at the physician’s direction, with the typical practice being every 2–4 weeks until the wound healed and every 3 months after that for as long as possible, using ABI, arterial duplex ultrasound, and SPP, if needed. Re-intervention was generally indicated for limbs with recurrent symptoms or delayed wound healing accompanied by re-occlusion or restenosis with hemodynamic significance measured by ABI, duplex ultrasound, or SPP.
Study Outcomes
The current study’s primary outcome measure was 1-year amputation-free survival (AFS), while the second outcome measure was a 1-year wound healing rate.
Definitions
CLI was defined under the TASC II guidelines 8) . A non-ambulatory status was defined as wheelchair dependence or bedridden status, as assessed upon admission. Hypertension was defined as systolic blood pressure >130 mmHg, diastolic blood pressure >80 mmHg, or hypertension treatment history. DM was defined as fasting plasma glucose levels ≥ 126 mg/dl, casual plasma glucose levels were ≥ 200 mg/dl, hemoglobin A1c levels ≥ 6.5%, plasma glucose levels ≥ 200 mg/dl 2 hours after a 75-g oral glucose tolerance test, or patients treated for diabetes 9) . Coronary artery disease was defined as the presence of symptoms, a history of infarction, or a history of any cardiac revascularization. Major amputation was defined as the surgical excision of the limb above the ankle. Any amputation at or distal to the Lisfranc ligament was not considered a limb salvage failure. Amputation data were obtained via outpatient clinic follow-up contact. AFS was defined as freedom from major amputation or death.
Serum phosphorus levels were obtained as part of the basic metabolic panel on a non-dialysis day as part of admitted preoperative vascular patients’ routine care. The serum phosphorus level was not normally distributed; therefore, the serum phosphorus level’s median value was used for analysis. The median value of the vintage of hemodialysis was used as the cutoff value for analysis.
Statistical Analysis
Unless mentioned otherwise, data are presented as the median (interquartile range) for continuous variables and as percentages for discrete variables. We performed chi-square test for discrete variables and Mann–Whitney U tests for ordinal variables to assess baseline characteristic differences between groups. Prognostic outcomes were assessed with the Kaplan–Meier method, and the differences between groups were assessed with the log-rank test when necessary. Cox proportional hazards regression analysis was used to determine the association of clinical characteristics with 1-year AFS and wound healing rate.
Multivariate Cox regression analysis determined predictors for 1-year AFS or 1-year wound healing. Clinically specified predictors achieving p <0.05 in univariate analysis were entered into the multivariate Cox regression analysis. The hazard ratio (HR) and 95% confidence interval (CI) were reported. A p -value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Version 24.0J (SPSS Inc., Chicago, IL, USA).
Result
Table 1 summarizes the study population’s limb characteristics. Two hundred and seventy-four (73.3%) patients were male, and the mean age was 72.3±9.0 years old. One hundred and seventy-two (50.0%) patients had a non-ambulatory status on admission. Notable comorbidities included hypertension (60.4%; 226/374), diabetes mellitus (68.2%; 255/374) and coronary artery disease (50.3%; 188/374). The median level of serum phosphate was 4.5 (3.7–5.4) mg/dl, and the median hemodialysis vintage was 6.1 (2.6–10.8) years. Ninety-two patients had longer vintages for hemodialysis (>6.1years) and higher serum phosphorus levels (>4.5 mg/dl). Regarding the Rutherford classification, 284 (75.9%) patients were classified in stage 5 and 90 (24.1%) in stage 6. WIfI stage IV, which was the highest risk for major amputation, was observed in 187 (50.0%) of the population. One hundred and seventy-one (45.7%) patients were CLTI due to isolated BTK lesions. The mean ABI and SPP at the dorsalis pedis and plantar surface before EVT were 0.65 (0.53–0.77), 28.0 (17.0–40.5) mmHg, and 31.0 (20.0–43.0) mmHg, respectively ( Table 2 ) .
Table 1. Baseline characteristics of study population.
| Variables | |
|---|---|
| Patients status ( n = 374) | |
| Age (years old) | 72.3±9.0 |
| Male gender, n (%) | 274 (73.3) |
| Body mass index (kg/m ² ) | 21.4±3.6 |
| Non-ambulatory status, n (%) | 172 (46.0) |
| Albumin, g/dL | 3.2±0.6 |
| 3.2 (2.8-3.6) | |
| Risk factors | |
| Hypertension, n (%) | 226 (60.4) |
| Dyslipidemia, n (%) | 98 (26.2) |
| Diabetes mellitus, n (%) | 255 (68.2) |
| Coronary artery disease, n (%) | 188 (50.3) |
| Cerebrovascular disease, n (%) | 36 (9.6) |
| Median levels of serum phosphate (mg/dl) | 4.5 (3.7-5.4) |
| Median levels of serum calcium (mg/dl) | 8.8 (8.3-9.3) |
| Median dialysis vintage (years) | 6.1 (2.6-10.8) |
Data are shown as mean±standard deviation or median with the first and third quartile as continuous data as appropriate. Categorical data are expressed as number with percentages.
Table 2. Baseline lower limb and lesion characteristics.
| Number of patients | 374 |
|---|---|
| Rutherford classification | |
| 5, n (%) | 284 (75.9) |
| 6, n (%) | 90 (24.1) |
| CRP, mg/dL | 1.30±5.64 |
| WIfI classification | |
| 1 (Very low risk) | 27 (7.2) |
| 2 (Low risk) | 64 (17.1) |
| 3 (Moderate risk) | 96 (25.7) |
| 4 (High risk) | 187 (50.0) |
| Ankle-brachial index on admission | 0.65±0.20 |
| 0.65 (0.53-0.77) | |
| Skin perfusion pressure at dorsalis pedis side | 29.8±18.2 |
| 28.0 (17.0-40.5) | |
| plantar side, mmHg | 32.3±18.1 |
| 31.0 (20.0-43.0) | |
| Aorto-iliac lesion, n (%) | 46 (12.3) |
| Femoro-popliteal lesion, n (%) | 190 (50.8) |
| BTK lesion, n (%) | 333 (89.0) |
| Isolated BTK lesion, n (%) | 171 (45.7) |
Data are shown as mean±standard deviation or median with the first and third quartile as continuous data as appropriate. Categorical data are expressed as number with percentages.
CRP, C-reactive protein; WIfI, Wound, Ischemia, and foot Infection classification system; BTK, Below-the-knee
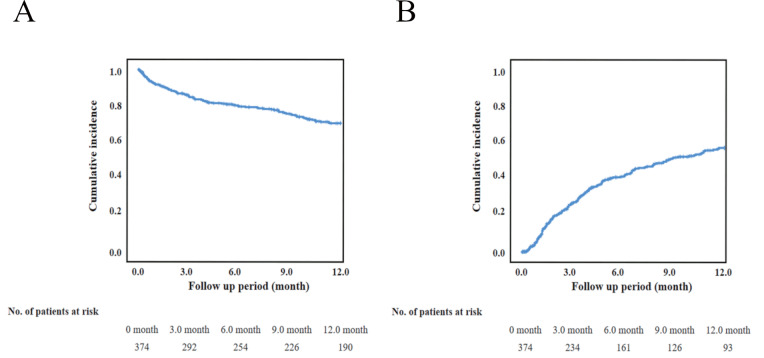
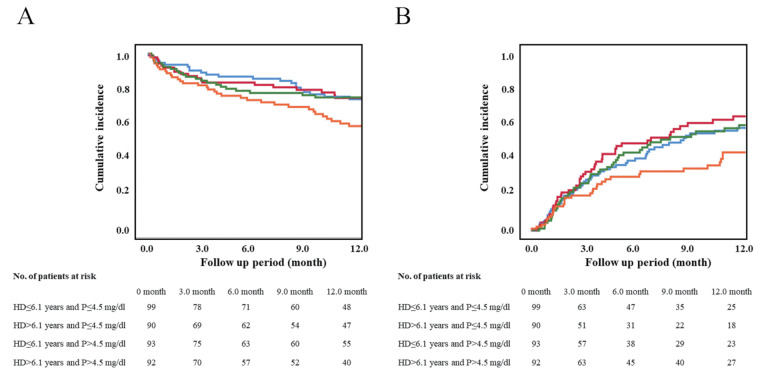
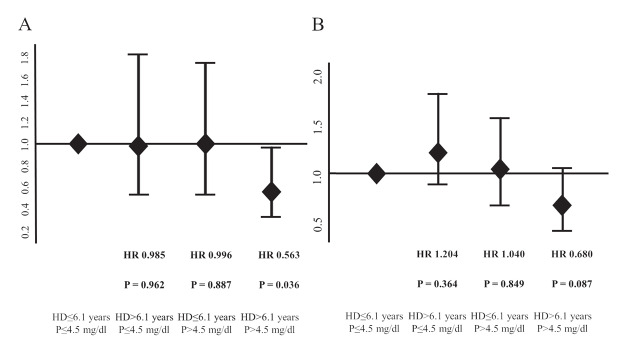
As shown in Fig.2 , the 1-year AFS and 1-year wound healing rates were 70.5%±2.5% and 57.1%±3.0%, respectively. The study population was categorized into four subgroups according to serum phosphorus levels (>4.5 mg/dl or ≤ 4.5 mg/dl) and hemodialysis vintages (>6.1 years or ≤ 6.1 years). The 1-year AFS was significantly lower in the group with longer hemodialysis vintages and higher serum phosphorus levels than in the group with others ( p =0.009) (Fig.3A) . The 1-year wound healing rate was significantly lower in the group with longer hemodialysis vintages and higher serum phosphorus levels ( p =0.019) (Fig.3B) . The subgroup with phosphorus levels >4.5 mg/dl and hemodialysis vintages >6.1 years had a significantly lower 1-year AFS than those with levels ≤ 4.5 mg/dl and hemodialysis vintages ≤ 6.1 years. The HR was 0,563 (95% CI, 0.329–0.962; p =0.036], while the subgroup with >4.5 mg/dl and with ≤ 6.1 years and the subgroup with ≤ 4.5 mg/dl and with >6.1 years had an HR of 0.996 (95% CI, 0.531–1.730; p =0.887) and 0.985 (95% CI, 0.538–1.805; p =0.962), respectively, relative to those with phosphorus levels ≤ 4.5 mg/dl and hemodialysis vintages ≤ 6.1 years. The association of hemodialysis vintage and serum phosphorus level with 1-year AFS is shown in Fig.4A . The subgroup with phosphorus levels >4.5 mg/dl and hemodialysis vintages >6.1 years did not have a significantly lower 1-year wound healing compared to those with phosphorus levels <4.5 mg/dl and hemodialysis vintages <6.1 years ( Fig.4B ) . The HR was 0.680 (95% CI, 0.437–1.057; p =0.087], while the subgroup with >4.5 mg/dl and with ≤ 6.1 years and the subgroup with ≤ 4.5 mg/dl and with >6.1 years had an HR of 1.040 (95% CI, 0.695–1.556; p =0.849) and 1.204 (95% CI, 0.806–1.798; p =0.364), respectively.
Fig.2. Kaplan-Meier analysis for amputation-free survival (AFS) and wound healing.
The 1-year AFS (2-A) and wound healing (2-B) rate were 70.5±2.5% and 57.1±3.0, respectively.
Fig.3. Kaplan-Meier analysis for amputation-free survival (AFS) and wound healing by serum phosphorus level and vintages of hemodialysis.
A: 1-year AFS was significantly lower in the group with longer hemodialysis vintages and higher serum phosphorus levels than in the group with others ( p =0.009).
B: 1-year wound healing was significantly lower in the group with longer hemodialysis vintages and higher serum phosphorus levels than in the group with others ( p =0.019).
Fig.4. Forest plots for 1-year amputation-free survival and wound healing categorized by phosphorus level and hemodialysis vintage.
A: The subgroup with phosphorus level >4.5 mg/dl and hemodialysis vintage >6.1 years had a significantly lower 1-year AFS compared to those with level ≤ 4.5 mg/dl and hemodialysis vintage ≤ 6.1 years. * p <0.05
B: The subgroup with phosphorus level >4.5 mg/dl and hemodialysis vintage >6.1 years had not a significantly lower 1-year wound healing compared to those without level >4.5 mg/dl and hemodialysis vintage >6.1 years.
Table 3 shows predictors of 1-year AFS after EVT. After multivariate analysis, body mass index (HR, 1.090; 95% CI, 1.020–1.164; p =0.012), non-ambulatory status (HR, 0.530; 95% CI, 0.343–0.818; p =0.004), low serum albumin level (HR, 1.692; 95% CI, 1.184–2.415; p =0.004), WIfI stage 4 (HR, 0.561; 95% CI, 0.364–0.865; p =0.009) and longer vintages for hemodialysis with higher serum phosphorus levels (HR, 0.599; 95% CI, 0.394–0.910; p =0.016) were significantly associated with 1-year AFS.
Table 3. Cox-regression analysis for 1-year amputation-free survival (AFS).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Male gender | 1.506 (0.607-2.188) | P = 0.868 | ||
| Body mass index | 1.145 (1.071-1.225) | P <0.001 | 1.090 (1.020-1.164) | P = 0.012 |
| Non-ambulatory status | 0.373 (0.246-0.564) | P <0.001 | 0.530 (0.343-0.818) | P = 0.004 |
| Hypertension | 0.775 (0.519-1.154) | P = 0.210 | ||
| Diabetes mellitus | 0.697 (0.464-1.049) | P = 0.084 | ||
| Coronary artery disease | 0.880 (0.591-1.307) | P = 1.137 | ||
| Cerebrovascular disease | 0.909 (0.497-1.664) | P = 0.757 | ||
| Serum calcium level | 1.110 (0.862-1.429) | P = 0.419 | ||
| Serum phosphorus level | 0.909 (0.810-1.020) | P = 0.106 | ||
| Serum albumin level | 2.232 (1.631-3.049) | P <0.001 | 1.692 (1.184-2.415) | P = 0.004 |
| WIfI stage 4 | 0.438 (0.289-0.664) | P <0.001 | 0.561 (0.364-0.865) | P = 0.009 |
| HD> 6.1 years and P> 4.5 mg/dl | 0.577 (0.380-0.874) | P = 0.009 | 0.599 (0.394-0.910) | P = 0.016 |
The model included HD> 6.1 years and P> 4.5 mg/dl, body mass index, non-ambulatory status, serum albumin level, WIfI stage 4. WIfI, Wound, Ischemia, and foot Infection classification system; HD, hemodialysis; P, phosphorus
Table 4 shows the predictors of 1-year wound healing after EVT. After multivariate analysis, WIfI stage 4 (HR, 0.713; 95% CI, 0.519–0.979; p =0.037) was significantly associated with 1-year wound healing. Longer vintages for hemodialysis with higher serum phosphorus levels (HR, 0.684; 95% CI, 0.467–1.000; p =0.050) were marginally but not significantly associated with 1-year wound healing.
Table 4. Cox-regression analysis for 1-year wound healing.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Male gender | 0.757 (0.555-1.034) | P = 0.080 | ||
| Body mass index | 1.009 (0.971-1.050) | P = 0.639 | ||
| Non-ambulatory status | 0.677 (0.496-0.923) | P = 0.014 | 0.792 (0.572-1.099) | P = 0.163 |
| Hypertension | 0.891 (0.654-1.215) | P = 0.467 | ||
| Diabetes mellitus | 1.038 (0.753-1.431) | P = 0.891 | ||
| Coronary artery disease | 1.135 (0.842-1.528) | P = 0.406 | ||
| Cerebrovascular disease | 0.890 (0.583-1.360) | P = 0.591 | ||
| Serum calcium level | 1.153 (0.961-1.382) | P = 0.125 | ||
| Serum phosphorus level | 0.903 (0.815-1.000) | P = 0.051 | ||
| Serum albumin level | 1.451 (1.118-1.884) | P = 0.005 | 1.249 (0.943-1.654) | P = 0.121 |
| WIfI stage 4 | 0.616 (0.455-0.835) | P = 0.002 | 0.713 (0.519-0.979) | P = 0.037 |
| HD> 6.1 years and P> 4.5 mg/dl | 0.638 (0.437-0.932) | P = 0.020 | 0.684 (0.467-1.000) | P = 0.050 |
The model included HD> 6.1 years and P> 4.5 mg/dl, non-ambulatory status, serum albumin level, WIfI stage 4. WIfI, Wound, Ischemia, and foot Infection classification system; HD, hemodialysis; P, phosphorus
Discussion
Summary of the Current Study
The current study analyzed the impact of hemodialysis vintage and serum phosphorus levels on clinical outcomes. The results revealed longer hemodialysis vintages and higher serum phosphorus levels, poorer comorbidities, and severe wound status were significantly associated with 1-year AFS. On the other hand, WIfI stage 4 was the sole risk factor for 1-year wound healing, while longer hemodialysis vintages with higher serum phosphorus levels were marginally but not significantly associated.
Summary of Previously Reported Studies
CKD, including hemodialysis, is a well-known prognostic factor of mortality and major amputation in CLTI patients after endovascular or surgical revascularization 10 - 12) . In the population limited to CKD patients with CLTI, several comorbidities were prognostic factors after revascularization. The current study’s result supported findings from prior investigations that body mass index, non-ambulatory status, low serum albumin level, and WIfI stage 4 affected 1-year AFS in hemodialysis patients with CLTI presenting with tissue loss 13) . Although these studies analyzed CLTI patients with Rutherford 4 to 6, the current study was limited to CLTI patients with ischemic tissue, leading to a somewhat different result. Another study reported that serum phosphorus level was a predictor of AFS prognosis in CLTI patients 14) . In the current study, the serum phosphorus level was not significantly associated with 1-year AFS among a cohort of hemodialysis patients with ischemic tissue loss, which was different from previous reports 15) . One major possibility for this difference might be the historical background of each study. The previous study enrolled CLTI patients but included Rutherford 4 and non-dialysis CLTI patients. Guidelines for renal replacement therapy have reported that hemodialysis’s vintages are one of the risk factors for vascular calcification and that dialysis vintage significantly increased the risk of cardiac death 16) . Taken together, longer vintages for hemodialysis with higher serum phosphorus levels adversely impact AFS. To the best of our knowledge, the current study was the first to examine the relationship between longer hemodialysis vintages, higher serum phosphorus levels, and AFS.
Another highlight of the current study was to assess the impact of hemodialysis vintage and serum phosphorus level on 1-year wound healing rate. The 1-year wound healing of 57.1% in the current study was consistent with previous studies 12) A multivariate analysis demonstrated that WIfI stage 4 was significantly associated with 1-year wound healing, while longer vintages for hemodialysis with higher serum phosphorus levels were marginally but not significantly associated. Major tissue loss and severe wound infection are both well-known risk factors for delayed wound healing that generally require absolute blood flow 17) . Previous literature had reported that WIfI stage 4 was a strong prognostic factor reflecting wound healing 13 , 18) When the study population was limited to hemodialysis patients with CLTI presenting with ischemic tissue loss, a consistent result was found. Additionally, the current study did not identify the Rutherford classification as an interacting factor like the WIfI classification, presumably because it was not accurate to classify wound severity. At least in performing revascularization in this population, the WIfI classification would be more informative than the Rutherford classification for predicting wound healing. In terms of longer vintages for hemodialysis with higher serum phosphorus levels, we speculated that these would accelerate vessel calcification, leading to the results of suboptimal angioplasties and consequently delayed wound healing. Clinically, serum phosphorus levels, as well as longer vintages for dialysis, have been associated with the presence and extent of arterial calcification among patients on dialysis 19 , 20) . The description regarding the relation between endothelial function, vessel calcification, and serum phosphorus levels has been accordingly added as follows; increasing evidence supports a role for phosphate in the pathogenesis of medial and intimal calcification. Vascular smooth muscle cells respond to increased phosphate levels by undergoing osteochondrogenic differentiation with subsequent mineralization of the extracellular matrix 21 , 22) . When an endovascular approach is clinically applied to hemodialysis patients with CLTI, the presence of calcified arterial lesions is generally opposed to the achievement of technical success, mainly due to the following two clinical issues: 1) difficulty of acquiring acute gain by balloon dilatation, and 2) difficulty of maintaining vessel patency because of early recoil due to calcified arterial walls. Our findings indicate that vessel calcification caused by longer vintages for hemodialysis with higher serum phosphorus levels might reflect such technical difficulties during procedures, and early recoil or restenosis in un-dilatable calcified lesions would result in a lower wound healing rate after endovascular therapy.
Study Limitations
There were several limitations in the current study. First, this investigation consisted of a retrospective, single-center study and only enrolled patients undergoing EVT and not surgical bypass therapy. Further studies in other groups are required to confirm these results. Second, detailed data on the continuation of medical intervention was not collected. Some literature has reported that these interventions positively affected clinical outcomes 23 - 25) , but such studies are still inconclusive. Third, the WIfI clinical stage was retrospectively evaluated using the photographs of ischemic wounds and medical records, including laboratory examinations at registration. Although efforts were made to obtain consistent and accurate wound evaluations, some amount of error was inevitable. Fourth, we could not collect the baseline characteristics for a substantial amount of the population, leading to unfortunate exclusion. This may impact the study’s outcomes.
Conclusion
Longer hemodialysis vintages with higher serum phosphorus levels adversely impact on outcomes after EVT for hemodialysis patients with CLTI presenting with ischemic tissue loss.
Conflicts of Interest
None.
Financial Disclosure
None.
References
- 1).US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Chapter 10 International comparison. Am J Kidney Dis, 2015; 63: 187-210 [Google Scholar]
- 2).Ann O, Daniel B, Anton S, Michael S, Saunak S, Mary-Margaret C. Renal Insufficiency and Use of Revascularization among a National Cohort of Men with Advanced Lower Extremity Peripheral Arterial Disease. Clin J Am Soc Nephrol, 2006; 1: 297-304 [DOI] [PubMed] [Google Scholar]
- 3).Shikhar A, Karan S, Shishehbor H. Nationwide Trends of Hospital Admission and Outcomes Among Critical Limb Ischemia Patients From 2003–2011. J Am Coll Cardiol, 2016; 67: 1901-1913 [DOI] [PubMed] [Google Scholar]
- 4).Louis N, Gregory M, Michael C, Dennis B, Alexander C, Lynn S, PREVENT III Investigators. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg, 2006; 44: 977-983; discussion: 983-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ann O, Daniel B, Anton S, Michael S, Saunak S, Mary-Margaret C. Renal Insufficiency and Use of Revascularization among a National Cohort of Men with Advanced Lower Extremity Peripheral Arterial Disease. Clin J Am Soc Nephrol, 2006; 1: 297-304 [DOI] [PubMed] [Google Scholar]
- 6).Alap S, Andrew K, Andrew S, John M, Stephen A, Mark N, William H, Ivan C. Clinical outcomes using aggressive approach to anatomic screening and endovascular revascularization in a Veterans Affairs population with critical limb ischemia. Catheter Cardiovasc Interv, 2009; 74: 11-19 [DOI] [PubMed] [Google Scholar]
- 7).Jana O, Brigitta G, Nicolas D, Florian D, Tobias T, Iris B. Survival benefits of revascularization in patients with critical limb ischemia and renal insufficiency. J Vasc Surg, 2012; 56: 737-745 [DOI] [PubMed] [Google Scholar]
- 8).Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, FowkesFG, Kevin B, Joseph C, Isabelle D, Komori K, Lammer J, Liapis C, NovoS, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg, 2007; 33 Suppl 1: S1-75 [DOI] [PubMed] [Google Scholar]
- 9).Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto At, Kondo T, Araki E. Japanese Clinical Practice Guideline for Diabetes 2016. J Diabetes Investig, 2018; 9: 657-697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Theodosios B, Giovanni T, Konstantinos S. The CRITISCH Registry Investigators provide an overview of registry data examining various treatment options for patients with CLI. ENDOVASCULAR TODAY MAY 2019 VOL. 18, NO. 5 [Google Scholar]
- 11).Michael SC, Andrew B, Philippe K, John VW, Florian D, Robert F, Joseph LM, Jean-Baptiste R, Kalkunte RS, Murad MH, GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg, 2019; 69: 3S-125S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Iida O, Nakamura M, Yamauchi Y, Fukunaga M, Yokoi Y, Yokoi H, Soga Y, Kan Zen, Suematsu N, Inoue N, Suzuki K, Hirano K, Shintani Y, Miyashita Y, Urasawa K, Kitano I, Tsuchiya T, Kawamoto K, Yamaoka T, Uesugi M, Shinke T, Oba Y, Ohura N, Uematsu M, Takahara M, Hamasaki T, Nanto S. A Prospective, Multi-Center, Three-Year Follow-Up Study on Endovascular Treatment for Infra-Inguinal Vessel in Patients With Critical Limb Ischemia OLIVE 3-Year Follow-Up Study. JACC Intv, 2015; 8: 1493-1502 [DOI] [PubMed] [Google Scholar]
- 13).Joseph L, Michael S, David G, Frank B, Andres S, George A. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg, 2014; 59: 220-234 [DOI] [PubMed] [Google Scholar]
- 14).Sara LZ, Peter AS, Klaas HJU, Crystal S, Jinhee O, Kevin M, Marc LS, Raul JG. Elevated serum phosphate levels are associated with decreased amputation-free survival after interventions for critical limb ischemia. J Vasc Surg, 2017; 65: 431-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Hioki H, Miyashita Y, Shiraki T, Iida O, Uematsu M, Miura T, Ebisawa S, Ikeda U. Impact of deteriorated calcium-phosphate homeostasis on amputation-free survival after endovascular revascularization in patients with critical limb ischemia on hemodialysis. Vascular Medicine, 2016; 21: 137-143 [DOI] [PubMed] [Google Scholar]
- 16).Lijie M, Sumei Z. Risk factors for mortality in patients undergoing hemodialysis- A systematic review and meta-analysis. International Journal of Cardiology, 2017; 238: 151-158 [DOI] [PubMed] [Google Scholar]
- 17).Shiraki T, Iida O, Takahara M, Soga Y, Yamauchi Y, Hirano K, Kawasaki D, Fujihara M, Utsunomiya M, Tazaki J, Yamaoka T, Shintani Y, Suematsu N, Suzuki K, Miyashita Y, Tsuchiya T, Uematsu M. Predictors of delayed wound healing after endovascular therapy of isolated infrapopliteal lesions underlying critical limb ischemia in patients with high prevalence of diabetes mellitus and hemodialysis. Eur J Vasc Endovasc Surg, 2015; 49: 565-573 [DOI] [PubMed] [Google Scholar]
- 18).Jeremy DD, John CM, Peter AS, Yifan M, Mark CW, Allen DH, Hence JV, Marc LS. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg, 2016; 64: 616-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Bryan K, Joshua NS, Kyle DR, Donald JP, Stephen LS, Bessie Y, Donald JS, Dennis LA. Serum Phosphate Levels and Mortality Risk among People with Chronic Kidney Disease. J Am Soc Nephrol, 2005; 16: 520-528 [DOI] [PubMed] [Google Scholar]
- 20).Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med, 2000; 342: 1478-1483 [DOI] [PubMed] [Google Scholar]
- 21).Cecilia MG. The emerging role of phosphate in vascular calcification. Kidney Int, 2009 May; 75: 890-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet, 2003; 361: 827-833 [DOI] [PubMed] [Google Scholar]
- 23).Matsukura M, Hoshina K, Shigematsu K, Miyata T, Watanabe T. Paramalleolar Arterial Bollinger Score in the Era of Diabetes and End-Stage Renal Disease - Usefulness for Predicting Operative Outcome of Critical Limb Ischemia. Circ J, 2016; 80: 235-242 [DOI] [PubMed] [Google Scholar]
- 24).Nakama T, Watanabe N, Haraguchi T, Sakamoto H, Kamoi D, Tsubakimoto Y, Ogata K, Satoh K, Urasawa K, Andoh H, Fujita H, Shibata Y. Clinical Outcomes of Pedal Artery Angioplasty for Patients with Ischemic Wounds: Results From the Multicenter RENDEZVOUS Registry. JACC Cardiovasc Interv, 2017; 10: 79-90 [DOI] [PubMed] [Google Scholar]
- 25).Sushant KD, Yi FY, Mao QL. Predictors of delayed wound healing after successful isolated below-the-knee endovascular intervention in patients with ischemic foot ulcers. J Vasc Surg, 2018; 67: 1181-1190 [DOI] [PubMed] [Google Scholar]