Abstract
In November 2017, two groups of P. conspicillatus pups from separate locations in Far North Queensland presented with neurological signs consistent with Australian bat lyssavirus (ABLV) infection. These pups (n = 11) died over an 11-day period and were submitted to a government laboratory for testing where ABLV infection was confirmed. Over the next several weeks, additional ABLV cases in flying foxes in Queensland were also detected. Brain tissue from ABLV-infected flying foxes during this period, as well as archived brain tissue, was selected for next-generation sequencing. Phylogenetic analysis suggests that the two groups of pups were each infected from single sources. They were likely exposed while in crèche at night as their dams foraged. This study identifies crèche-age pups at a potentially heightened risk for mass ABLV infection.
Keywords: lyssavirus, public health, whole genome sequencing, zoonoses
1 |. INTRODUCTION
Australian bat lyssavirus (ABLV) is the only lyssavirus endemic to Australia and causes both human and animal fatalities in the state of Queensland (Annand & Reid, 2014; Francis et al., 2014; Hanna et al., 2000). There are two genetic variants of ABLV: a flying fox variant that has been detected in the four most common Australian flying fox species (Pteropus sp.), and an additional variant, to date detected only in the yellow-bellied sheathtail bat (Saccolaimus flaviventris). The prevalence of ABLV antigen—indicative of current infection—in wild bats is less than 1%; however, prevalence is higher in sick or injured bats which are more likely to be found grounded or ‘hanging low’ (Barrett, 2004; Field, 2018). Between 2013 and 2016, ABLV was detected in 8 to 14 bats per year in Queensland, Australia (Wildlife Health Australia, 2017). Because of its zoonotic risk and high case fatality rate, ABLV in bats is of concern to public health authorities and to wildlife carers who oversee the welfare, treatment and rehabilitation of sick and injured bats in Australia.
There is an extensive network of volunteer wildlife carers in Australia that rescue sick, injured and orphaned native animals for rehabilitation and release. In Queensland, wildlife, including bats, is protected under the Nature Conservation Act 1992. The state Department of Environment and Science licenses people to rescue and keep wildlife in accordance with the conditions of their licence and legislation regarding the care of sick, injured or orphaned protected animals (QLD DES, 2013). In addition to being responsible for the rescue, acute care and rehabilitation of bat species, wildlife carers also provide a crucial service to their communities by responding to incidents where bats are found in public spaces and private homes. Due to the low likelihood but potentially fatal consequences of exposure to ABLV, members of the public are urged to avoid contact with bats whenever possible. Health and biosecurity authorities recommend contacting a vaccinated, licensed wildlife carer if a bat is found in need of help (https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/livestock/animal-welfare/pests-diseases-disorders/australian-bat-lyssavirus).
On 31 October 2017, a group of spectacled flying fox pups (Pteropus conspicillatus; n = 9; Group 1) were found hanging from a fence near an established flying fox colony in Far North Queensland (FNQ). The pups ranged in age between 4 and 7 weeks of age based on forearm measurements taken by wildlife carers. One pup had bite wounds to the head severe enough to necessitate euthanasia. Three of the eight remaining rescued pups had wounds consistent with recent bites from another bat. Within a day of rescue, the pups had been distributed to several wildlife carers to be hand-raised. As early as 13 days after the rescue, rescued pups began presenting with clinical signs consistent with ABLV infection; over a 9-day period, 6 of the 8 pups found together had died or were euthanized. The pups presented with clinical signs including but not limited to paresis, aggression and cranial nerve defects (Table 1). Cranial nerve defects impacting tongue and mouth movements can be observed in one of the Group 1 pups at https://animalhealthaustralia.com.au/incidence-of-australian-bat-lyssavirus-in-queensland. The carers reported their suspicion of ABLV infection to Biosecurity Queensland.
TABLE 1.
Clinical signs of ABLV in rescued pups (Group 1)
| Sample ID number | Bat carer | Weight loss/nil weight gain | ‘Wobbly’, uncoordinated | Paresis | Aggression | Laboured breathing | Dysphonic | Cranial nerve defects | ‘Irritable/distressed/agitated’ | Days post-rescue |
|---|---|---|---|---|---|---|---|---|---|---|
| QA018 | 1 | X | nr | nr | nr | nr | nr | nr | nr | 10–13 |
| QA019 | 1 | nr | X | X | X | X | nr | nr | X | 13–15 |
| QA020 | 2 | X | nr | X | nr | nr | nr | X | nr | 13–19 |
| QA021 | 3 | nr | nr | X | X | X | X | nr | X | 16–17 |
| QA022 | 3 | X | X | nr | X | nr | X | nr | X | 19–21 |
| QA023 | 1 | X | nr | X | X | nr | nr | X | X | 19–21 |
Note: X, clinical sign reported; nr, not reported. Paresis (of varying severity), aggression, ‘agitation’ and weight loss/failure to gain weight were the most common clinical signs exhibited amongst pups in Group 1. In each instance, clinical signs were limited to duration of 2–3 days until euthanasia or natural death. Clinical signs specific to individual bats were not recorded for Group 2.
On 6 November 2017, a large group of P. conspicillatus pups (n = 18; Group 2) were found on the ground under a tree near an established flying fox colony approximately 80 kilometres south-west from where the first group of P. conspicillatus pups were rescued. Forearm measurements indicated that these pups ranged from 4 to 9 weeks of age. Two weeks later, the carer reported to Biosecurity Queensland that several pups (n = 5) had developed clinical signs consistent with ABLV. Clinical signs specific to individual bats were not recorded. Within two days, all 5 affected pups died or were euthanized. These pups were each isolated as soon as abnormal clinical signs were recognized, minimizing the opportunity for further transmission to other bats in care.
Whole carcasses from the two clusters of affected pups (n = 11) were submitted to the Queensland Government Biosecurity Sciences Laboratory, Coopers Plains, QLD. Real-time PCR testing of fresh brain detected the flying fox variant of ABLV in all 11 pups. Concurrent real-time PCR tests for the S. flaviventris ABLV variant were negative. Nine of the 11 pups were also tested by fluorescent antibody test, corroborating the diagnosis of ABLV infection. These cases were initially reported by wildlife and animal health authorities in Wildlife Health Australia’s ‘ABLV Bat Stats’ and in Animal Health Australia’s Animal Health Surveillance Quarterly (AHA, 2017; WHA, 2017).
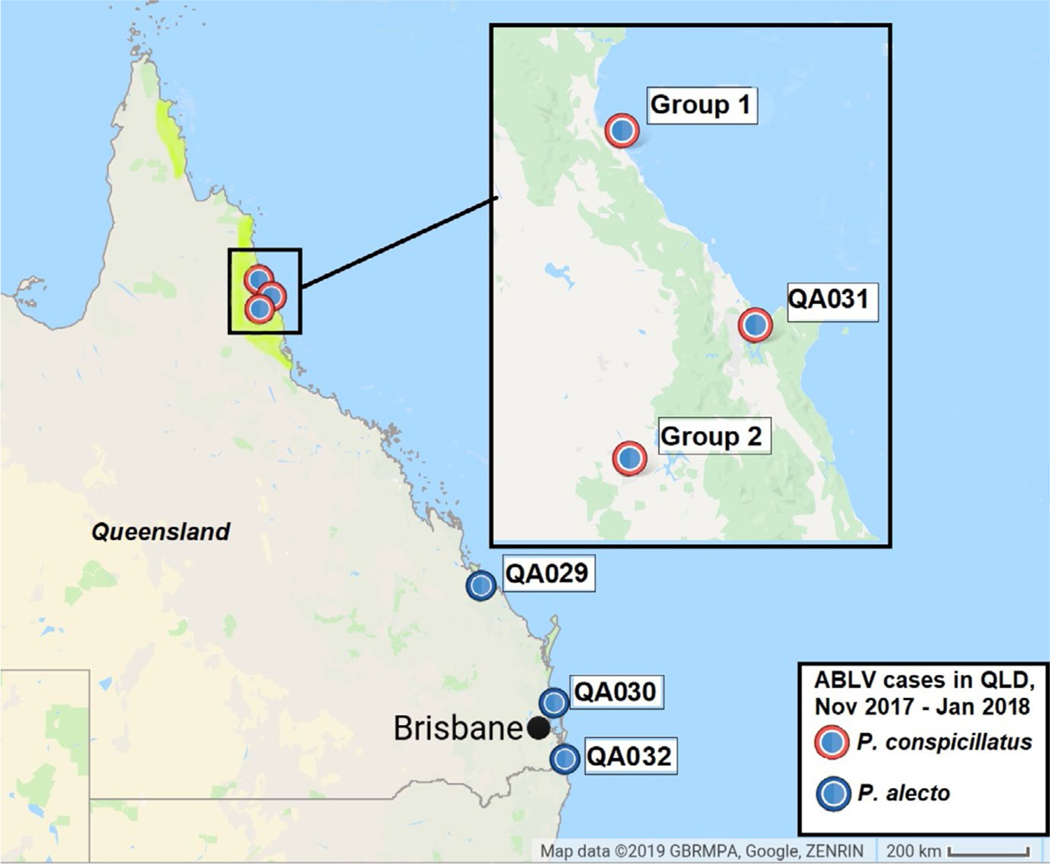
In the weeks following this temporal cluster of ABLV infections in FNQ, ABLV was detected in a further 3 Pteropus alecto from South East and Central Queensland, and an additional P. conspicillatus pup from FNQ (Figure 1). Due to the high number of ABLV infections detected during this 8-week period, as well as the unprecedented clustering and suspected mass infection amongst rescued pups (where mass infection is defined as more than 4 individuals infected as part of an incident), the molecular epidemiology of these cases was investigated using next-generation sequencing (NGS).
FIGURE 1.
ABLV sequences derived from infected Pteropus conspicillatus and Pteropus alecto bats submitted to Biosecurity Queensland between November 2017 and January 2018. The distribution of P. conspicillatus, restricted to the northern part of the state, is marked in yellow
2 |. MATERIALS AND METHODS
2.1 |. Samples
We sequenced brain tissue from 15 flying foxes from Queensland infected with ABLV during the 8 weeks of November 2017 and January 2018. These were compared with samples from 16 ABLV-infected Queensland bats submitted previously over a much longer time course between January 2013 and May 2017 that had been stored at −80°C at the Biosecurity Sciences Laboratory (Table 2).
TABLE 2.
ABLV sequences from selected flying foxes submitted to the Biosecurity Sciences Laboratory, Biosecurity Queensland
| Sample ID number | Species | Date of submission (month/year) | Location (within QLD) | GenBank accession No. | Notes |
|---|---|---|---|---|---|
| QA001 | P. scapulatus | 12/2012 | South East | MK944082 | |
| QA002 | P. scapulatus | 01/2013 | South East | MK944083 | |
| QA003 | P. alecto | 03/2013 | South East | MK944084 | |
| QA004 | P. alecto | 04/2013 | South East | MK944085 | |
| QA006 | P. scapulatus | 09/2013 | Central | MK944086 | |
| QA007 | P. scapulatus | 10/2013 | South East | MK944087 | |
| QA008 | P. scapulatus | 12/2013 | South East | MK944088 | |
| QA009 | P. alecto | 02/2014 | South East | MK944089 | |
| QA010 | P. scapulatus | 04/2014 | South East | MK492318 | |
| QA011 | P. scapulatus | 04/2014 | South East | MK944090 | |
| QA012 | P. scapulatus | 05/2014 | South East | MK944091 | |
| QA013 | P. alecto | 05/2014 | South East | MK944092 | |
| QA014 | P. alecto | 01/2015 | South East | MK944093 | |
| QA015 | P. alecto | 05/2015 | North | MK944094 | |
| QA016 | P. alecto | 11/2016 | South East | MK492319 | |
| QA017 | P. poliocephalus | 05/2017 | South East | MK944095 | |
| QA018 | P. conspicillatus | 11/2017 | Far North | MK492309 | Group 1 |
| QA019 | P. conspicillatus | 11/2017 | Far North | MK92310 | Group 1 |
| QA020 | P. conspicillatus | 11/2017 | Far North | MK492311 | Group 1 |
| QA021 | P. conspicillatus | 11/2017 | Far North | MK492312 | Group 1 |
| QA022 | P. conspicillatus | 11/2017 | Far North | - | Group 1 |
| QA023 | P. conspicillatus | 11/2017 | Far North | - | Group 1 |
| QA024 | P. conspicillatus | 11/2017 | Far North | MK492313 | Group 2 |
| QA025 | P. conspicillatus | 11/2017 | Far North | - | Group 2 |
| QA026 | P. conspicillatus | 11/2017 | Far North | - | Group 2 |
| QA027 | P. conspicillatus | 11/2017 | Far North | - | Group 2 |
| QA028 | P. conspicillatus | 11/2017 | Far North | - | Group 2 |
| QA029 | P. alecto | 12/2017 | Central | MK492314 | |
| QA030 | P. alecto | 12/2017 | South East | MK492315 | |
| QA031 | P. conspicillatus | 01/2018 | Far North | MK492316 | |
| QA032 | P. alecto | 01/2018 | South East | MK492317 |
Note: ABLV sequences from 15 flying foxes submitted between November 2017 and January 2018 and archived brain tissue from 16 flying foxes submitted between January 2013 and May 2017. Two sequences from Group 1 and all but one from Group 2 were not submitted to GenBank as they were identical.
2.2 |. RNA extraction and next-generation sequencing
Total nucleic acid was extracted from 31 flying fox brain samples using the RNeasy Mini Kit (Qiagen) following manufacturer’s instructions but without the addition of RNAse free DNAse to Buffer RLT. Final elution volume was in 30 μl of Buffer EB. RNA concentration was measured using the Qubit Fluorometer and the Qubit RNA HS Assay Kit (Thermo Fisher Scientific). The sequence library was prepared using the TruSeq Stranded mRNA Library Preparation Kit substituting the Oligo-dT capture beads with those from the RiboZero™ rRNA Removal Kit (Human/Mouse/Rat, Illumina). cDNA was prepared using Superscript™ II Reverse Transcriptase (ThermoFisher Scientific), and all purification steps utilized AMPure XP kit paramagnetic beads (Beckman Coulter). The size and purity of the pooled sequence library were quantified using the 2200 TapeStation (Agilent), with the final equimolar concentration quantified using the Qubit DNA dsDNA HS Assay Kit (ThermoFisher Scientific). The library was sequenced on a NextSeq 500 Sequencing Platform using a NextSeqMid Output Kit v2 300 (Illumina). Indexing quality control and FASTQ file generation were performed using the online server BaseSpace Sequence Hub (Illumina), with additional trimming performed using the Geneious® 11.0.3 (Biomatters) plugin BBDuk (Brian Bushnell). Continuing to use Geneious®, individual reads were paired and mapped to a reference (GenBank accession AF418014).
Consensus sequences were submitted to NCBI GenBank (accession numbers: MK492309–MK492319, MK944082–MK944095).
2.3 |. Phylogenetic and selection analysis
Phylogenetic analysis was performed using whole genome sequence data derived from the NGS and additional whole genome ABLV sequences from flying foxes available on GenBank. Full-length was analysed using the GTR nucleotide substitution model with gammadistributed rate variation as identified using IQtree ModelFinder (Kalyaanamoorthy, Minh, Wong, Haeseler, & Jermiin, 2017). Maximum-likelihood trees were constructed using PhyML with 1000x bootstrap support (Guindon et al., 2010). Phylogenetic tree was formatted using FigTree (version 1.4.4, http://tree.bio.ed.ac.uk/software/figtree/).
3 |. RESULTS
Average coverage across sequences was 4,345. Sequences from the majority of individuals covered 100% of the genome, while remaining sequences covered 99.6%–99.9% of the genome; areas not covered were limited to genomic termini, known to be highly conserved amongst lyssaviruses (Marston et al., 2007) and unlikely to impact downstream analyses.
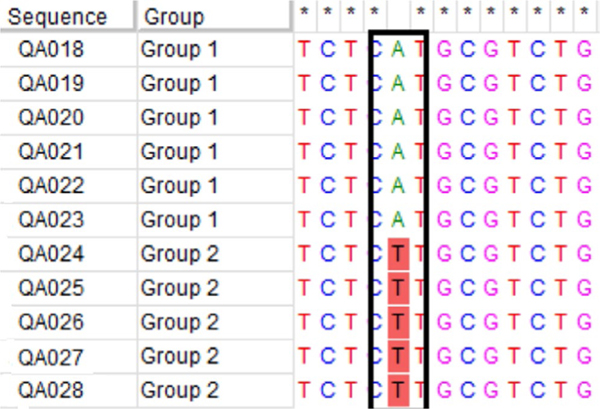
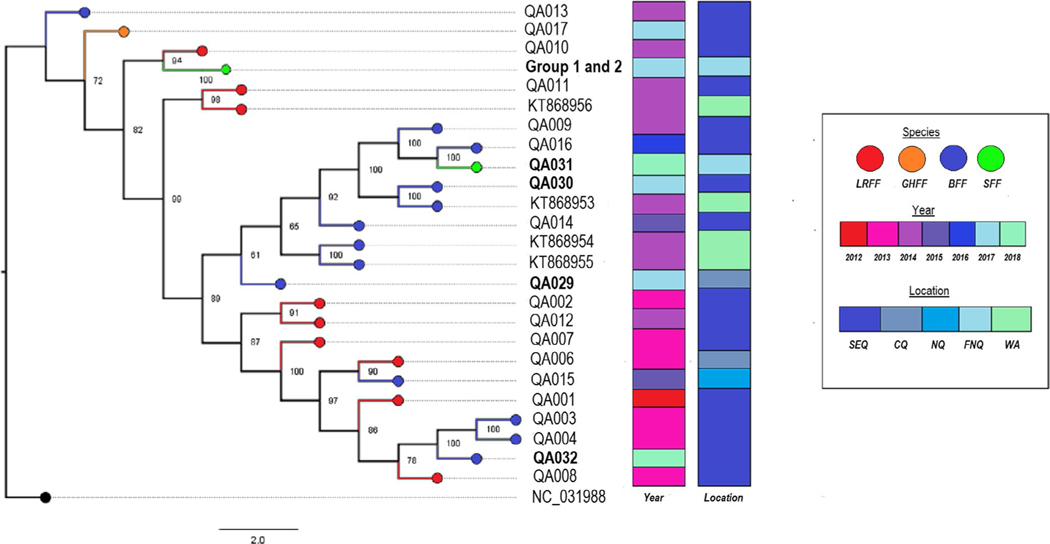
ABLV whole genome sequences from pups in Group 1 (n = 6) and Group 2 (n = 5) were identical or highly similar. A single nucleotide synonymous substitution within the RNA polymerase coding region differentiates Group 1 from Group 2 (Figure 2). Within Group 1, nucleotide sequences were 100% homologous, whereas while slight variations (1–2 nucleotide substitutions) exist in 3 of 6 sequences within Group 1. Whole genome sequences from pups in Groups 1 and 2 (QA0018–QA028) were distinct from sequences from all other ABLV cases submitted to Biosecurity Queensland between November 2017 and January 2018 (QA029–QA032). Comparison with sequences derived from archived ABLV-infected flying fox brain tissue (QA001–QA017) shows that ABLV sequences from Groups 1 and 2 pups are most similar to QA010, extracted from a Pteropus scapulatus in South East Queensland in 2014. The additional ABLV-infected P. conspicillatus pup from Far North Queensland (QA031) was most similar to QA016, a sequence obtained from a P. alecto found in a Brisbane suburb in 2016. The other sequences obtained from ABLV-infected P. alecto between November 2017 and January 2018 show similar diversity and are more homologous to sequences derived from archived flying foxes than to one another (Figure 3).
FIGURE 2.
A single nucleotide substitution (T --> A) on the RNA polymerase coding region differentiates Group 1 and Group 2
FIGURE 3.
Maximum-likelihood tree of whole genome ABLV sequences with 1,000× bootstrap support. Four ABLV whole genomes from bats in Western Australia are included for comparison, and NC_031998.1 (Gannoruwa bat lyssavirus) is used as an outgroup. The 11 sequences comprising Group 1 and Group 2 are clustered together, while the additional sequence from a P. conspicillatus pup in FNQ during the same time period as the pup cluster (QA031) is notably distant. Sequences from flying foxes submitted between November 2017 and January 2018 are in bold. aSpecies names are condensed to LRFF (little red flying fox, P. scapulatus), GHFF (grey-headed flying fox, P. poliocephalus), BFF (black flying fox, P. alecto) and SFF (spectacled flying fox, P. conspicillatus). bLocations are condensed as SEQ (South East Queensland), CQ (Central Queensland), NQ (North Queensland), FNQ (Far North Queensland) and WA (state of Western Australia)
Apart from the highly similar sequences of Groups 1 and 2, two sequences from archived tissue (QA003, QA004) within a small spatiotemporal window of 7 weeks and less than 5 km appeared to be closely related, with only three nucleotide substitutions. This is in contrast to all other sequences derived from archived flying fox samples which, despite a substantial sampling bias towards bats from South East Queensland, do not indicate any pattern of transmission specific to location or a single species based on the similarity of viral sequences, consistent with previous molecular work on the flying fox ABLV variant (Barrett, 2004; Foord et al., 2006; Guyatt et al., 2003).
4 |. DISCUSSION
With the exception of the P. conspicillatus pup clusters, all other ABLV cases between November 2017 and January 2018 were sufficiently genetically distinct to eliminate the possibility of a propagating epidemic along the Queensland coast in the summer of 2017 (Figure 3). Despite close geographic proximity, an additional ABLV case in a P. conspicillatus pup (QA031), which occurred approximately two months after the ABLV pup clusters, was not closely related. The endangered P. conspicillatus is largely confined to the northern Wet Tropics region of FNQ (Department of the Environment, 2019), yet ABLV sequences from infected P. conspicillatus (groups 1 and 2, QA031) cluster with sequences from archived brain tissue collected from P. scapulatus (QA010) and P. alecto (QA016) submitted from South East Queensland. These more nomadic flying fox species presumably play a role in the geographic dissemination of ABLV (Field, 2004).
There appear to be a number of distinct ABLV lineages that circulate across temporal, geographic and Pteropus species in the state of Queensland. Notably, sequences available on GenBank from infected bats from Western Australia are embedded within these lineages despite enormous geographic distances (Figure 3). This is consistent with previous molecular studies into ABLV epidemiology which indicates that flying fox species form one continuous host population when it comes to ABLV infection, including the geographically limited P. conspicillatus (Barrett, 2004; Foord et al., 2006). ABLV is, to our knowledge, unique in this respect considering that most bat lyssaviruses are markedly species-specific (Marston et al., 2018).
Next-generation sequencing of ABLV from infected bats in Queensland between November 2017 and January 2018 has demonstrated that while viral sequences from each pup within Groups 1 and 2 were significantly different to all other ABLV sequences from other bats during this time period (Figure 3), the sequences within each group were highly similar.
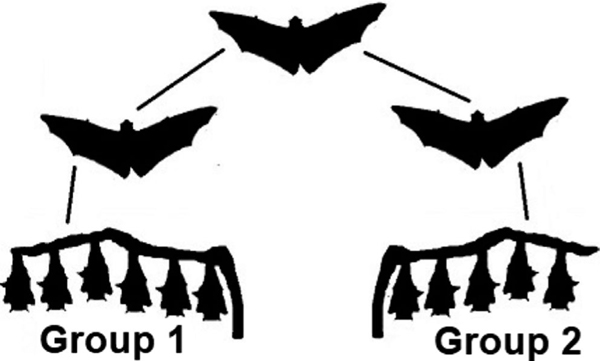
In addition, the degree of homology between Groups 1 and 2 is much higher than anticipated, with only a single nucleotide synonymous substitution within the RNA polymerase coding region clearly delineating Group 1 from 2 (Figure 2). One possibility is that a single bat infected both groups of pups, and a within-host mutation occurred in the period between attacks, differentiating Group 1 and Group 2. However, while point mutations during passage have been documented in lyssaviruses, within-host mutations to our knowledge have not (Bonnaud et al., 2019). It is unlikely that a flying fox, already sufficiently progressed in its disease course to infect a group of pups, could survive long enough to fly approximately 80 kilometres and then infect another group of pups 6 days later. Clinical progression to death in flying foxes rescued with clinical signs due to ABLV is rapid, usually 1–3 days (Barrett, 2004). One clinically ill adult bat that was observed for 8 days prior to death was unable to fly when first observed, suggesting the complex function of flight is lost early in the clinical course (Barrett, 2004). In an experimental study, no ABLV-infected flying foxes exhibiting clinical signs survived >4 days (Barrett, 2004). We speculate that the two clusters were infected by two different bats, but that these two bats had been infected by a single bat (Figure 4). These two bats would have then dispersed during their respective incubation periods prior to infecting pups in Groups 1 and 2.
FIGURE 4.
Hypothesized transmission pathway: pups in Group 1 and Group 2 each infected by a single bat. Based on the nucleotide similarity between ABLV sequences in Group 1 and Group 2, we speculate that two flying foxes were infected by the same source before dispersing at least 80 kilometres to infect the two groups of P. conspicillatus pups
The short period during which observed clinical onset occurred within pups of Group 1 (within nine days) and Group 2 (within 2 days) means it is very unlikely any pups in a group had subsequently infected the others (Table 1). Previous experimental studies of ABLV and RABV in bats have indicated that virus in saliva is only detectable at onset or immediately before onset (<24 hr) prior to recognizable clinical signs (Barrett, 2004; Jackson et al., 2008). While it is possible that pup QA018 (clinical onset Day 10) infected pup QA023 (clinical onset Day 19), the observation that QA023 had bite marks at the time of rescue supports the notion that this pup and the others found on the ground were all exposed prior to coming into care. The onset of clinical signs of ABLV in all 5 pups in Group 2 occurred over only 2 days, precluding the possibility that one infected bat in that group had infected the others.
At 4 to 9 weeks of age flying fox pups cannot fly, but are sufficiently independent to be left in roosting trees in a crèche overnight while dams forage for food (Markus & Blackshaw, 2002). Pups in groups 1 and 2 were found close to the ground on the edge of established colonies; Group 2 was found beneath a tree that may have been used for crèche (J. Mclean personal communication, November 24 2017). Clusters of ABLV infection in pups which have not been reported in adults or neonatal pups suggest pups in crèche may be uniquely susceptible to mass infection due to the combination of their nocturnal communal roosting and their relative inability to avoid bites and scratches from an infected adult. This represents a brief but highly vulnerable period in their development in which they are left alone overnight without the protection of their dams and are too young to fly away.
ABLV infections in Groups 1 and 2 were identified because wildlife carers routinely checked on the welfare of the flying foxes within their local roosting colonies. Similar mass infections of crèche-age flying foxes may have gone undetected. Two additional accounts from Queensland wildlife carers describe attacks towards flying foxes of this age group: a carer observed a group of pre-flight flying foxes being attacked by a highly aggressive, dysphonic adult flying fox who was euthanized shortly thereafter. The pups who were unable to fly away or fend off attack came into care and subsequently declined in condition and died in care from what is suspected ABLV (wildlife carer personal communication, June 25 2018). A previously documented case describes a dam, later confirmed to be infected with ABLV, observed attacking juvenile bats as well as her own pup so severely its skull was punctured (Skerratt, Speare, Berger, & Winsor, 1998). Whether this elevated risk factor extends to other bat species that use crèches is unknown.
That the pups were found together on the ground, some with fresh bite wounds, supports that Groups 1 and 2 were exposed to ABLV just prior to being found. The apparent incubation periods (n = 11, ranging from 10 to 19 days) in these clusters are shorter than those previously observed in naturally infected flying foxes (30 days and 36–57 days; Field, McCall, & Barrett, 1999; Warrilow et al., 2003). However, the incubation periods are consistent with those observed following the experimental inoculation of flying foxes with a particularly virulent ABLV isolate (Barrett, 2004). Groups 1 and 2 feature 2 amino acid substitutions on the phosphoprotein coding gene and 3 on RNA polymerase coding gene. Although most studies have focused on the role of the glycoprotein for pathogenicity, substitutions on the phosphoprotein and RNA polymerase may impact neuroinvasiveness and the rate of transcription and replication, thereby influencing the incubation period and potentially viral load (Streicker, Altizer, Velasco-Villa, & Rupprecht, 2012; Yamaoka et al., 2013).
This unprecedented cluster of ABLV amongst P. conspicillatus pups, likely exposed while vulnerable in crèche, exhibited a wide array of clinical signs and a brief and unexpectedly consistent incubation period. Wildlife carers and other people who may come into contact with bats in Australia need to be to be alert for bats showing a wide spectrum of potentially subtle neurological signs as more overt clinical signs (e.g., aggression) are not always present. Wildlife carers in Australia should be particularly wary of ABLV clinical signs in the pre-flight age group and can help limit further transmission to other bats in their care by adhering to the Australian Bat Lyssavirus General Biosecurity Obligation guidelines (Queensland Government, 2019).
Investigations into the large number of ABLV-infected bats in Queensland between November 2017 and January 2018 revealed the diversity of ABLV circulating amongst flying fox populations in the state and identified the mass infection of two groups of P. conspicillatus pups. Next-generation sequencing allowed for differentiation of closely related viruses, including nearly identical sequences, which would have been indistinguishable based on N or G gene sequencing alone. This study demonstrates the value of whole genome sequencing for investigations into the transmission of lyssaviruses and other zoonotic pathogens.
Impacts.
Whole genome sequences of Australian bat lyssavirus contribute to an understanding of the diversity of the virus in Pteropus species in Queensland.
A wide range of Australian bat lyssavirus clinical signs in Pteropus conspicillatus pups are documented.
We identify a heightened risk of mass Australian bat lys-savirus infection in crèche-age Pteropus pups.
ACKNOWLEDGEMENTS
We thank Gavin S. Wilkie for his sequencing expertise and assistance, and the staff at Biosecurity Queensland’s Biosecurity Sciences Laboratory. We also want to thank the volunteer wildlife carers of Tolga Bat Hospital, Connie Kerr, Dave Pinson, Jesse Jansen and Alicia Knudsen. This study was funded by Biosecurity Queensland, University of Queensland School of Veterinary Science, and a Wet Tropics Management Authority student grant. Author H. Field was supported in part by the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT (Cooperative Agreement No. AID-OAA-A-14-00102) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Award No. R01AI110964).
Funding information
University of Queensland; Biosecurity Queensland; Wet Tropics Management Authority
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- Animal Health Australia (2017). State and Territory Reports – October to December 2017. Animal Health Surveillance Quarterly Report, 22, 17–42. [Google Scholar]
- Annand EJ, & Reid PA (2014). Clinical review of two fatal equine cases of infection with the insectivorous bat strain of Australian bat lyssavirus. Australian Veterinary Journal, 92, 324–332. 10.1111/avj.12227 [DOI] [PubMed] [Google Scholar]
- Barrett J. (2004). Australian bat lyssavirus. PhD thesis, The University of Queensland, Brisbane, Australia. Retrieved from https://espace.library.uq.edu.au/view/UQ:9486
- Bonnaud EM, Troupin C, Dacheux L, Holmes EC, Monchatre-Leroy E, Tanguy M, … Bourhy H. (2019). Comparison of intra- and inter-host genetic diversity in rabies virus during experimental cross-species transmission. PLoS pathogens, 15, e1007799. 10.1371/journal.ppat.1007799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of the Environment (2019). Pteropus conspicillatus – Species profile and threats database. Retrieved from http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=185
- Field H. (2004). The ecology of Hendra virus and Australian bat lyssavirus. PhD thesis, The University of Queensland, Brisbane, Australia. Retrieved from http://espace.library.uq.edu.au/view/UQ:13859 [Google Scholar]
- Field HE (2018). Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa. Zoonoses and Public Health, 65, 742–748. 10.1111/zph.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H, McCall B, & Barrett J. (1999). Australian bat lyssavirus infection in a captive juvenile black flying fox. Emerging Infectious Diseases, 5, 438–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord A, Heine H, Pritchard L, Lunt R, Newberry K, Rootes C, & Boyle DB (2006). Molecular diagnosis of lyssaviruses and sequence comparison of Australian bat lyssavirus samples. Australian Veterinary Journal, 84, 225–230. [DOI] [PubMed] [Google Scholar]
- Francis JR, Nourse C, Vaska VL, Calvert S, Northill JA, McCall B, & Mattke AC (2014). Australian bat lyssavirus in a child: The first reported case. Pediatrics, 133, e1063–e1067. 10.1542/peds.2013-1782 [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, & Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Guyatt KJ, Twin J, Davis P, Holmes EC, Smith GA, Smith IL, … Young PL (2003). A molecular epidemiological study of Australian bat lyssavirus. Journal of General Virology, 84, 485–496. [DOI] [PubMed] [Google Scholar]
- Hanna JN, Carney IK, Deverill JE, Botha JA, Smith GA, Serafin IL, … Searle JW (2000). Australian bat lyssavirus infection: A second human case, with a long incubation period. The Medical Journal of Australia, 172, 597–599. [DOI] [PubMed] [Google Scholar]
- Jackson FR, Turmelle AS, Farino DM, Franka R, McCracken GF, & Rupprecht CE (2008). Experimental rabies virus infection of big brown bats (Eptesicus fuscus). Journal of Wildlife Diseases, 44, 612–621. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, & Jermiin LS (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus N, & Blackshaw JK (2002). Behaviour of the Black Flying Fox Pteropus alecto: 1. An ethogram of behaviour, and preliminary characterisation of mother-infant interactions. Acta Chiropterologica, 4, 137–152. 10.3161/001.004.0203 [DOI] [Google Scholar]
- Marston DA, Banyard AC, McElhinney LM, Freuling CM, Finke S, de Lamballerie X, … Fooks AR (2018). The lyssavirus host-specificity conundrum—rabies virus—the exception not the rule. Current Opinion in Virology, 28, 68–73. 10.1016/j.coviro.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Marston DA, McElhinney LM, Johnson N, Müller T, Conzelmann KK, Tordo N, & Fooks AR (2007). Comparative analysis of the full genome sequence of European bat lyssavirus type 1 and type 2 with other lyssaviruses and evidence for a conserved transcription termination and polyadenylation motif in the G-L 3′ non-translated region. Journal of General Virology, 88, 1302–1314. 10.1099/vir.0.82692-0 [DOI] [PubMed] [Google Scholar]
- QLD Des - Queensland Department of Environment and Science (2013). Code of Practice Care of Sick, Injured or Orphaned Protected Animals in Queensland Nature Conservation Act 1992. Retrieved from: https://environment.des.qld.gov.au/wildlife/caring-for-wildlife/pdfs/cp-wlrehab.pdf
- Queensland Government (2019). Australian bat lyssavirus and your general biosecurity obligation. Retrieved from https://www.qld.gov.au/environment/plantsanimals/wildlife/bats-gbo
- Skerratt LF, Speare R, Berger L, & Winsor H. (1998). Lyssaviral infection and lead poisoning in black flying foxes from Queensland. Journal of Wildlife Diseases, 34, 355–361. [DOI] [PubMed] [Google Scholar]
- Streicker DG, Altizer SM, Velasco-Villa A, & Rupprecht CE (2012). Variable evolutionary routes to host establishment across repeated rabies virus host shifts among bats. Proceedings of the National Academy of Sciences of the United States of America, 109, 19715–19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow D, Harrower B, Smith IL, Field H, Taylor R, Walker GC, & Smith GA (2003). Public health surveillance for Australian bat lyssavirus in Queensland, Australia, 2000–2001. Emerging Infectious Diseases, 9, 262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHA – Wildife Health Australia (2017). ABLV Bat Stats – Australian Bat Lyssavirus Report, December 2017. Retrieved from https://www.wildlifehealthaustralia.com.au/Portals/0/Documents/ProgramProjects/ABLV_Bat_Stats_Dec_2017.pdf
- Yamaoka S, Ito N, Ohka S, Kaneda S, Nakamura H, Agari T, … Sugiyama M. (2013). Involvement of the rabies virus phosphoprotein gene in neuroinvasiveness. Journal of Virology, 87, 12327–12338. 10.1128/JVI.02132-13 [DOI] [PMC free article] [PubMed] [Google Scholar]