Abstract
Objective
To investigate the clinical characteristics and neuroimaging features of childhood presenting with gray matter heterotopia observed in a single tertiary Pediatric Department in Catania and compare the data with those reported in the literature.
Methods
A retrospectively review of the history, clinical findings, electrophysiological features and magnetic resonance images of 22 children presenting with gray matter heterotopia observed from January 2010 to January 2020.
Results
Among the 22 children included in the study, 17 presented with periventricular heterotopia (PVNH), two with Subcortical Band Heterotopia (SBH), and three with other subcortical heterotopia (SUBH). In the affected children, the ages at first diagnosis ranged from 3 months to 16 years with a mean age of 8.2 years (± 5.4); twelve (54.5%) suffered by developmental delay and intellectual deficit; eleven children (50%) complained of epileptic seizures, mostly focal to bilateral tonic–clonic seizure. In addition, in the periventricular heterotopia group (PVNH), cerebral and systemic malformations were reported in twelve (70%) and in ten (58%) children, respectively, out of seventeen. In the SBH plus SUBH group, epileptic seizures were recorded in 3 (60%) out of 5 children, cerebral malformations in one child and systemic malformations in two children.
Conclusions
Heterotopic gray matter malformations include a group of disorders that manifest with a variety of neurological implications, such as cognitive impairment and epilepsy, and often related with epilepsy, other cerebral malformations and systemic anomalies.
Keywords: Heterotopia, Childhood, Clinical, Neuroimaging, Epilepsy
Introduction
Heterotopic gray matter malformations (HET) are clusters of normal neurons in abnormal locations, mainly due to impaired migration from approximately the 6th to 16th weeks of gestation [1–3]. The increasing availability and resolution of MRI technology and molecular genetics has resulted in a classification published by Barkovich et al. in the 2012 describing different types of malformations of cortical development (MCD) [4]. In 2019, Oegema et al. reviewed this classification [5]. In particular, he distinguished five groups and subdivided them into specific entities. Subcortical Heterotopic gray matter heterotopy (SUBH) was included in Groups 1, 2, 4 and 5, differently periventricular nodular heterotopia (PVNH) and Subcortical Band Heterotopia (SBH) in group 3. In particular, Group 1 includes congenital microcephaly with premigrational reduced proliferation and variable additional MCD; group 2 includes multifocal or focal cortical and subcortical dysgenesis; group 3 includes malformations due to abnormal neuronal migration, PVNH and SBH [6–10]; group 4 includes malformations due to abnormal postmigrational development with subcortical or transmantle components; and group 5 includes heterotopic gray matter brain malformations with bilateral complex patterns.
The diagnosis of GMH requires highly specialized and multidisciplinary expertise. Clinically, it has been commonly related to developmental delay and other systemic malformations. The purpose of this study was to evaluate the clinical and neuroimaging features of gray matter heterotopia observed over a 10-year period in a single tertiary pediatric department to improve the clinicians’ and radiologists’ understanding of the disease.
Methods
We reviewed retrospectively the clinical records of the 22 children presenting with epilepsy and/or development delay and diagnosed at Brain MRI with HET, admitted at single tertiary Pediatric Department at the “Policlinico G. Rodolico” University-Hospital, Catania, Italy from January 2010 to January 2020. In this study, children were grouped according to the main types of heterotopia: seventeen had PVNH (77%), three SBH (13%), and two SUBH (9%). The study was approved by the ethic Committee of the University Hospital of Catania University. We collected the data from the medical charts: sex, age at first diagnosis, seizure types, electroencephalograpic (EEG) results, neuropsychiatric evaluation, were reported together with other possible cerebral or systemic malformations presented by the children. Brain MRI studies included T1-weighted, T2-weighted, fluid-attenuated inversion recovery, diffusion-weighted and post-contrast T1 imaging. EEG was carried out for each child using the international 10–20 system.
Results
The age at first diagnosis ranged from 3 months to 16 years with a mean age of 8.2 years (± 5.4). Twelve children were males (54.6%) and ten (45.4%) were females. The results obtained are distinguished on the basis of the type of HET and beneath reported:
Periventricular nodular heterotopia (PVNH)
This group of children includes nine females (52.9%) and eight males (47.1%), with mean age of establishing a diagnosis of 8.1 years (± 6.1). This group of children includes nine females (52%) and eight males (47%), with mean age of the first diagnosis of 8 years (± 6).
Table 1 shows the clinical features. Figure 1 shows the main characteristics and intensity of periventricular nodules on MRI.
Table 1.
Data of 17 patients with periventricular heterotopia
| ID patient | Patient sex age at the diagnosis | Seizure | Seizure types | EEG | Heterotopic localisation | Outcome | Other CNS malformations | Systemic malformations/manifestations |
|---|---|---|---|---|---|---|---|---|
| ID-101 |
Female 10.75 y |
Yes | FS | Left FE | Unilateral | Delayed | No | No |
| ID-102 |
Male 13.3 y |
Yes | F to Bil | Right FE | Unilateral | Normal | No | No |
| ID-103 |
Female 8.3 y |
No | Normal | Bilateral | Delayed |
VM Hemispheric cyst AVM PVL Polymicrogyria CVH |
Skeletal Dysplasia Ostium secundum atrial septal defect |
|
| ID-104 |
Female 0.25 y |
Yes | IS | Multifocal FE | Unilateral | Normal |
ACC Aicardi Syndrome |
No |
| ID-105 |
Female 0.75 y |
Yes | IS | GE | Bilateral | Normal | VM | Congenital Hypertrophic cardiomyopathy |
| ID-106 |
Male 4.25 y |
Yes | GS | Multifocal FE | Bilateral | Delayed |
VM Microcephaly Polymicrogyria |
No |
| ID-107 |
Male 3 y |
Yes | GS | Normal | Unilateral | Delayed | No | Classical Ehlerse–Danlos syndrome (COL5A1) |
| ID-108 |
Female 1.6 y |
No | Normal | Bilateral | Normal |
VM ACC CVH Dandye-Walker cyst |
Strabismus | |
| ID-109 |
Male 2 y |
No | Normal | Bilateral | Delayed | VM |
Skeletal dysplasia Strabismus |
|
| ID-110 |
Female 0.8 y |
No | Normal | Unilateral | Delayed | No | No | |
| ID-111 |
Female 15 y |
Yes | F to bil | Right FE | Bilateral | Normal | No | GH deficiency |
| ID-112 |
Male 12.3 y |
No | Normal | Unilateral | Delayed |
VM ACC |
Hypertelorism Nystagmus Conjunctival hypertrophy |
|
| ID-113 |
Male 14 y |
No | Normal | Bilateral | Delayed | VM | Papillary edema | |
| ID-114 |
Male 2 y |
No | Normal | Bilateral | Normal | Hemispheric cyst | Cortical cysts in the right kidney | |
| ID-115 |
Female 12 y |
No | Normal | Unilateral |
Enuresis Encopresis |
Spinal Dysraphism with meningocele and tethered cord | No | |
| ID-116 |
Female 16.4 y |
Yes | FS | GE | Unilateral | Normal | VM |
Bicuspid aortic valve GH deficiency |
| ID-117 |
Male 15 y |
No | Normal | Bilateral | Delayed | Ectopic neurohypophysis | No |
ACC agenesis of corpus callosum, AVM arteriovenous malformation, CHD congenital heart disease, CVH cerebellar vermis hypoplasia, FE focal epileptiform, FS focal seizure, F to Bil Focal to Bilateral tonic–clonic seizures, Y years, PVL periventricular leukomalacia, VM ventriculomegaly, GE generalized epileptiform, GH growth hormone, GS generalized seizure, IBD inflammatory Bowel Disease, IS infantile spasms
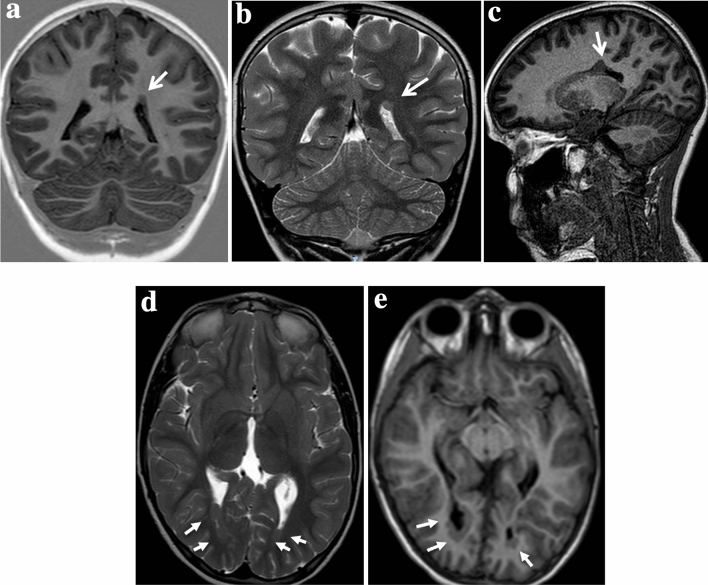
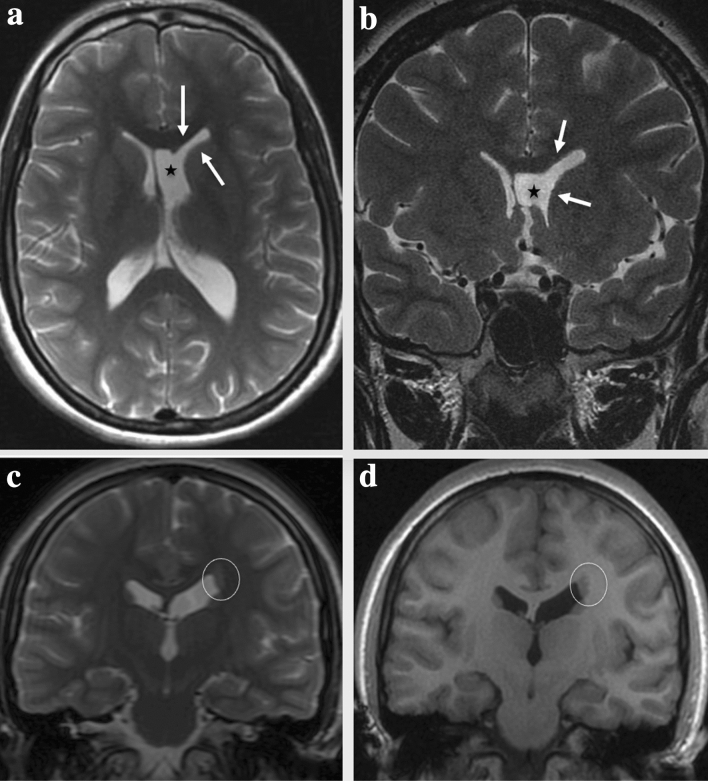
Fig. 1.
Periventricular nodular heterotopia (PVNH). a, b, c Unilateral PVNH (ID 101): Unenhanced magnetic resonance imaging coronal a and mid sagittal c T1-weighted and coronal T2-weighted b shows a small unilateral periventricular nodule, isointense to the gray matter, along the left lateral ventricular wall (arrows) with indentation of the ventricular profile (arrow). d, e Bilateral PVNH (ID103) Unenhanced T2-weighted d and T1-weighted e magnetic resonance axial images shows a few nodules that are lining ventricular occipital horns bilaterally (arrows)
Subcortical band heterotopia (SBH)
This group includes two children, one female and one male, with mean age of establishing a diagnosis of 7.5 years (± 5.5). Table 2 shows clinical features.
Table 2.
Data of two children with SBH
| ID patient | Patient sex age at the diagnosis | Seizure | Seizure types | EEG | Heterotopic Localisation | Psychomotor development | Other CNS malformations | Systemic malformations/manifestations |
|---|---|---|---|---|---|---|---|---|
| ID-201 |
Female 11.3 y |
No | Normal | Bilateral | Delayed | Hemispheric cyst | No | |
| ID-202 |
Male 3.8 y |
No | Multifocal S-W | Unilateral | Delayed | No |
Bicuspid aortic valve Nystagmus |
S-W Spikes-Wave
Subcortical heterotopia (SUBH)
This group includes three children, all males, with mean age of establishing a diagnosis of 9 years (± 1.8). Table 3 shows the clinical features. Figure 2 shows the main characteristics and intensity of periventricular nodules on MRI.
Table 3.
Data of three probands with subcortical heterotopia (SUBH)
| ID patient | Patient sex and age at the diagnosis | Seizure | Seizure tupes types | EEG | Heterotopic Localisation | Psychomotor development | Other CNS manlformations | Systemic malformations |
|---|---|---|---|---|---|---|---|---|
| ID-301 |
Male 8.5 y |
Yes | FS | F. S-W Right | Nodular | Normal | No | Strabismus |
| ID-302 |
Male 7.5 y |
Yes | F to Bil | F to Bil | Nodular | Normal | No | No |
| ID-303 |
Male 11 y |
Yes | FS | Normal | Nodular | Normal | No | No |
FS focal seizures, F to Bil focal to bilateral tonic–clonic seizures, F. S-W frontal spike and wave frontal
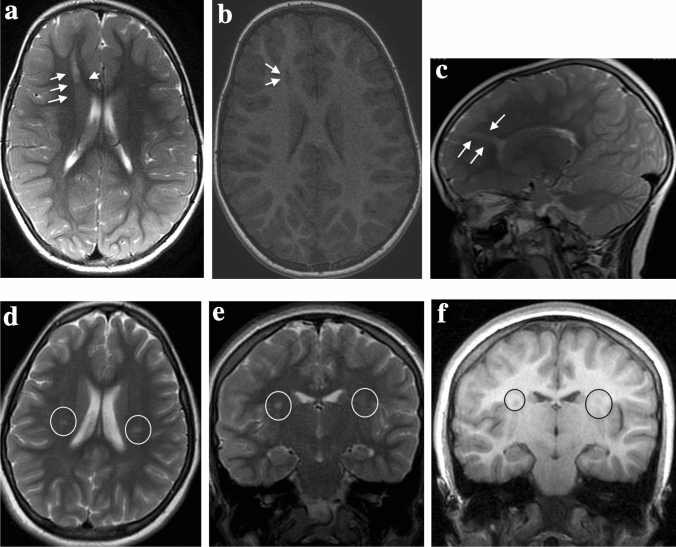
Fig. 2.
Subcortical heterotopia (SUBH). a–c (ID 302) Non-contrast enhancement magnetic resonance imaging, Axial T2- weighted image (a) and T1-weighted image (b) and mid-sagittal T2-weighted image show mantle stripe, isointense to the cerebral cortex with a serpiginous pattern, which stretches in oblique cranio-caudal direction from the right frontal cortico-subcortical region to the anterior horn of the homolateral lateral ventricle (arrows), compatible with sub-cortical heterotopia. d–f (ID 303) Non-contrast enhancement magnetic resonance imaging axial d and coronal e T2-weighted image and coronal T1-weighted image f shows a bilateral nodule of SNH (circles), isointense to gray matter
Discussion
In this retrospective review, we selected 22 children affected by HET: 17 presenting with PVNH 2 with SBH, and 3 with SUBH. HET manifests with variable clinical expression, mainly presenting with DD/ID, epilepsy and other brain and systemic malformations [3, 9–12]. In this series, 12 out of 22 (54%) subjects showed delayed cognitive function, and 11 out of 22 subjects (50%) showed epilepsy with a lower frequency than the results from other studies that reported cognitive disability and epilepsy in a greater number of cases [11, 12].
In literature HET is associated with epilepsy [13–15]; about this, in our experience 11/22 patients (50%) developed epilepsy. Several studies reported that periventricular heterotopia is the type of GMH more associated with the epilepsy (80–90%) [12, 16], which was more common focal seizure than general [12, 13], and more detected in females than males [4, 15]. Differently, in our periventricular group we detected epilepsy in 8/17 patients (47%), with a female predominance (female to male ratio 5:3). About the type of epilepsy, we found generalized seizures in 4/8 patients, followed by two patients with infantile spasms and two with focal seizure. In the report of Srour et al., all patients with infantile spasms showed bilateral lesions [16]: in our case series, only 1/2 with infantile spasms showed bilateral lesions in accordance with the study of Hung et al. [11]. Consistent with the literature, the most common EEG abnormality was focal epileptiform.
About the subcortical group, we collected three patients affected by epilepsy, two focal type and one generalized seizures, and no other anomalies. Only one patient had strabismus. Raza et al. report a study with ten patients affected by subcortical heterotopia [17]: they describe an association with subcortical heterotopia, central nervous system (CNS) anomalies and neurological dysfunction. Probably due to the low number of patients, we did not detect these findings.
About the band group, we collected two patients with psychomotor delay, without seizures. One of the two patients had hemispheric cystic. Hung et al. describe 10 patients with band heterotopia [11], associating it with brain anomalies and congenital malformations. As the precedent group, due to the low number of patients, it is difficult compare our results.
HET can be associated with other CNS anomalies: in our case series, especially in periventricular group, we noted the recurrence of ventriculomegaly (8/17) in accordance with the literature (Fig. 3). In contrast to the paper Hung et al. and Srour et al. [11, 16], we found that bilateral heterotopia are more likely have ventriculomegaly instead of unilateral type. Ventriculomegaly is followed by agenesis of corpus callosum (3/17), cerebellar vermis hypoplasia (Fig. 4a) (2/17) and polymicrogyria (2/17) (Fig. 4b, c). In addition, we found in 2/17 hemispheric cyst (Fig. 5a, b).
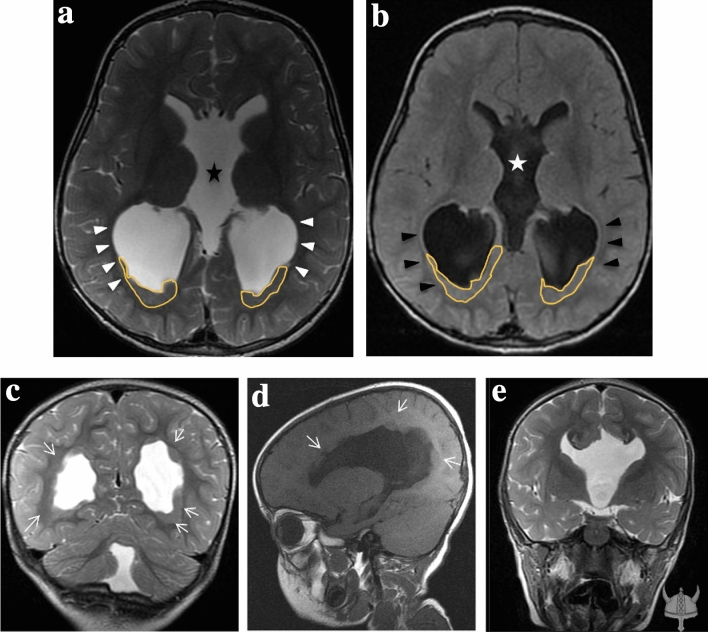
Fig. 3.
(ID 108): Agenesis of the corpus callosum and cavum septum pellicidum, ventriculomegaly and cerebellar vermis atrophy in patient with bilateral periventricular nodular heterotopia (PVNH). a, b Non-contrast enhancement axial T2-weighted MR image a and T1-weighted MR image b shows enlargement of the ventricles in particular the images show dilatation of the third ventricle (star) and lateral ventricles with widely spaced parallel bodies (arrowheads) in patient with bilateral periventricular nodular heterotopia (white lines). c Unenhanced T2-weighted MR coronal image show bilateral periventricular nodular heterotopia (PVNH) (arrows), enlarged lateral ventricles and cerebellar vermis atrophy. d Unenhanced T1-weighted MR midsagittal image show dysmorphic ventricular system and periventricular nodular heterotopia (arrows). e T2 -weighted MR coronal image shows the “Viking helmet appearance” refers to the morphology of the lateral ventricles in the coronal plane in patients with dysgenesis of the corpus callosum. The cingulate gyrus is everted into narrowed and elongated frontal horns
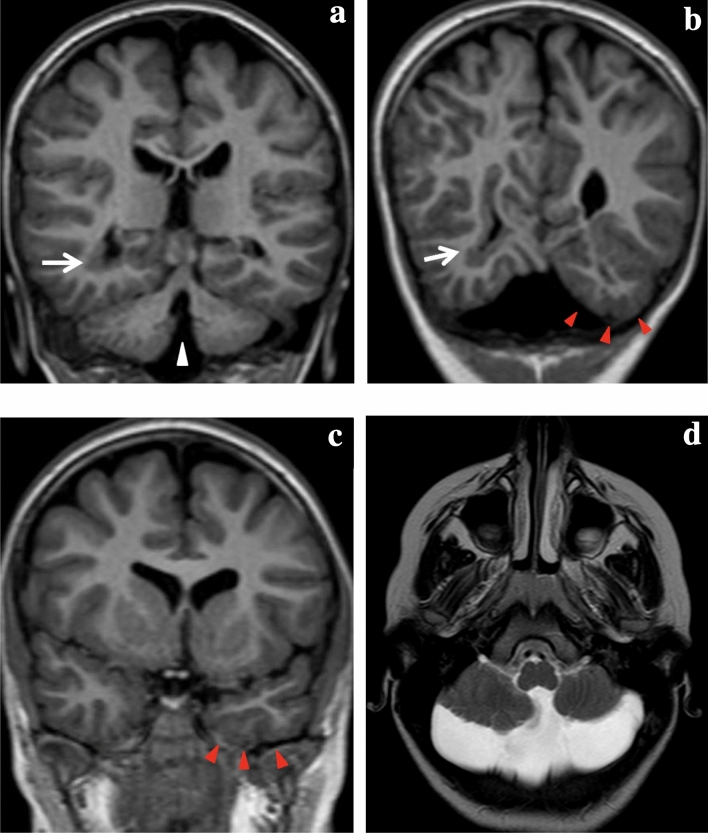
Fig. 4.
(ID 103) Brain malformations associated with monolateral periventricular heterotopia (PVNH) a, b T1-weighted coronal MR images demonstrates nodular periventricular heterotopia (white arrows) in the right lateral ventricle, cerebellar vermis hypoplasia a (white arrowhead) b, c T1-weighted coronal MR images shows pachygyric appearance of the left parahippocampal gyrus with a ‘nubby’ appearance to the cortical surface (white arrows) d T2-weighted assial MR image show cerebellar hemisphere and vermis hypoplasia
Fig. 5.
(ID 109): Dysmorphic left lateral ventricles and periventricular nodular heterotopia (PVNH). a, b Unenhanced axial T2-weighted (T2w) magnetic resonance image a and unenhanced coronal T2-weighted (T2w) magnetic resonance image b shows dysmorphic left lateral ventricles (white arrows) that appear fused with cyst of the cavum septum pellucidum (black star). c, d Coronal T2-weighted (T2w) magnetic resonance image c and T1 3D FSPGR (fast spoiled gradient echo) coronal magnetic resonance image d shows periventricular nodular heterotopia (white circle)
In accordance with the data of Hung et al., we report the case of a patient with Dandy-Walker cyst and the case of a patient with Ehlers-Danlos syndrome. Advances in genetic show a correlation between connective tissue disorder and periventricular heterotopia [18]: our experience confirms this date.
Interestingly, we report the case of a patient with periventricular heterotopia and Aicardi syndrome, showing seizures and agenesis of corpus callosum, typical features described in the syndrome [19].
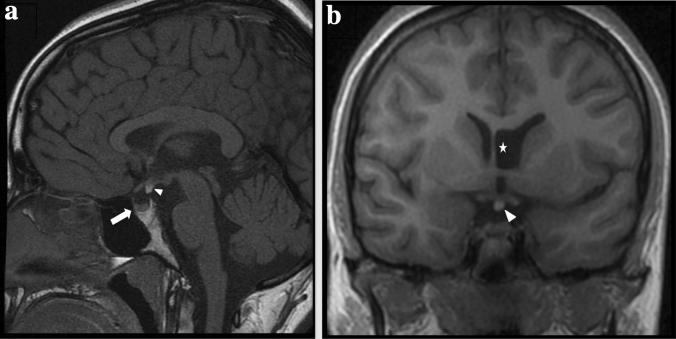
About the other systemic malformations, our experience shows a correlation between heterotopia and cardiac diseases, previously described by other studies [11, 16]. Also, we found ocular abnormalities, especially strabismus, in 6/22 patients. Interestingly, we found two patients with growth hormone deficiency (GH) and heterotopia. We attribute this incidence in our case series, never described before, because our center is a reference department of endocrinology and neurology pediatric. In these patients MRI did not show pituitary lesions, so they received diagnosis of GH deficiency idiopathic. In literature, growth hormone deficiency and heterotopia are rarely correlated, such as Vilboux et al. in a Joubert patient [20]. Mitchell et al. reported a study with a possible correlation between periventricular heterotopia, ectopic posterior pituitary lobe and GH deficiency [21]. In our case series, one patient had ectopic posterior pituitary lobe and periventricular heterotopia (Fig. 6); differently, in two patients with GH deficiency MRI did not show anomalies to the posterior pituitary.
Fig. 6.
(ID 109): Ectopic posterior pituitary lobe. a, b Unenhanced T1-weighted (T1w) midsagittal and coronal magnetic resonance images shows hyperintensity at the median eminence, which corresponds to ectopic posterior pituitary lobe (arrowheads). Anterior pituitary lobe appears normally localized although small and flattened (arrow). b Dysmorphic left lateral ventricles that appear fused with cyst of the cavum septum pellucidum (white star)
Our study had some limitations. The study population was small, especially for the subcortical and band group. All the data were obtained retrospectively. Our patients were only from a single tertiary hospital in Catania. In addition, genetic testing was not performed in our cohort. Our report confirms that heterotopia may be a clue of different and various cerebral and systemic anomalies; for this reason, in case of GMHs for the clinicians it’s important value the cardiac function, ocular abnormalities and eventually skeletal or renal anomalies.
Acknowledgements
The authors also wish to thank American Manuscript Editors for reviewing the manuscript and Springer Editing Sevices (certification number 57FB-A1FD-91C5-F26C-2A7F) for reviewing the manuscript.
Author contributions
All the authors have equally contributed to the writing of the manuscript and critically revised it for intellectual content.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement.
Declarations
Conflict of interest
All authors declare do not have conflict of interests.
Data availability
The data used to support the findings of this study may be released upon application to the corresponding author, who can be contacted at alessandradinora@gmail.com.
Ethical approval
The study was conducted ethically in accordance with the World Medical Association’s Declaration of Helsinki.
Informed consent
Informed consent was obtained from the parents/or caregivers of the probands.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barkovich AJ, Kuzniecky RI. Gray matter heterotopia. Neurology. 2000;55(11):1603–1608. doi: 10.1212/WNL.55.11.1603. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronica E, Mühlebner A. Neuropathology of epilepsy. Handb Clin Neurol. 2017;145:193–216. doi: 10.1016/B978-0-12-802395-2.00015-8. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Kjos BO. Gray matter heterotopia: MR characteristics and correlation with developmental and neurologic manifestations. Radiology. 1992;182:493–499. doi: 10.1148/radiology.182.2.1732969. [DOI] [PubMed] [Google Scholar]
- 5.Oegema R, Barkovich AJ, Mancini GMS, Guerrini R, Dobyns WB. Subcortical heterotopic gray matter brain malformations: classification study of 107 individuals. Neurology. 2019;93(14):e1360–e1373. doi: 10.1212/WNL.0000000000008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkovich AJ. Morphological characteristics of subcortical heterotopia: MR imaging study. Am J Neuroradiol. 2000;21(2):290–295. [PMC free article] [PubMed] [Google Scholar]
- 7.Donkol RH, Moghazy KM, Abolenin A. Assessment of gray matter heterotopia by magnetic resonance imaging. World J Radiol. 2012;4(3):90–96. doi: 10.4329/wjr.v4.i3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavone L, Corsello G, Pavone P, Iannetti P. Lissencephalic syndromes: brain and beyond. Front Biosci (Sch Ed) 2010;2:85–95. doi: 10.2741/s47. [DOI] [PubMed] [Google Scholar]
- 9.DeMyer W. Megalencephaly: types, clinical syndromes, and management. Pediatr Neurol. 1986;2:321–328. doi: 10.1016/0887-8994(86)90072-X. [DOI] [PubMed] [Google Scholar]
- 10.Jansen PR, Dremmen M, van den Berg A, Dekkers IA, Blanken LME, Muetzel RL, et al. Incidental findings on brain imaging in the general pediatric population. N Engl J Med. 2017;377(16):1593–1595. doi: 10.1056/NEJMc1710724. [DOI] [PubMed] [Google Scholar]
- 11.Hung PC, Wang HS, Chou ML, Lin KL, Hsieh MY, Wong AM. Clinical and neuroimaging findings in children with gray matter heterotopia: a single institution experience of 36 patients. Eur J Paediatr Neurol. 2016;20(5):732–737. doi: 10.1016/j.ejpn.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Dubeau F, Tampieri D, Lee N, Andermann E, Carpenter S, Leblanc R, et al. Periventricular and subcortical nodular heterotopia. A study of 33 patients. Brain. 1995;118(Pt 5):1273–1287. doi: 10.1093/brain/118.5.1273. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia G, Chiapparini L, Franceschetti S, Freri E, Tassi L, Bassanini S, et al. Periventricular nodular heterotopia: classification, epileptic history, and genesis of epileptic discharges. Epilepsia. 2006;47(1):86–97. doi: 10.1111/j.1528-1167.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 14.Barkovich AJ. Subcortical heterotopia: a distinct clinicoradiologic entity. AJNR Am J Neuroradiol. 1996;17(7):1315–1322. [PMC free article] [PubMed] [Google Scholar]
- 15.Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A, et al. The role of periventricular nodular heterotopia in epileptogenesis. Brain. 2005;128(Pt 3):641–651. doi: 10.1093/brain/awh388. [DOI] [PubMed] [Google Scholar]
- 16.Srour M, Rioux MF, Varga C, Lortie A, Major P, Robitaille Y, et al. The clinical spectrum of nodular heterotopia in children: report of 31 patients. Epilepsia. 2011;52(4):728–737. doi: 10.1111/j.1528-1167.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 17.Raza HK, Chen H, Chansysouphanthong T, Zhang Z, Hua F, Ye X, et al. The clinical and imaging features of gray matter heterotopia: a clinical analysis on 15 patients. Neurol Sci. 2019;40(3):489–494. doi: 10.1007/s10072-018-3667-9. [DOI] [PubMed] [Google Scholar]
- 18.Sheen VL, Walsh CA. Periventricular heterotopia: new insights into Ehlers-Danlos syndrome. Clin Med Res. 2005;3(4):229–233. doi: 10.3121/cmr.3.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G. Neuroimaging aspects of Aicardi syndrome. Am J Med Genet A. 2008;146A(22):2871–2878. doi: 10.1002/ajmg.a.32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilboux T, Malicdan MC, Roney JC, Cullinane AR, Stephen J, Yildirimli D, et al. CELSR2, encoding a planar cell polarity protein, is a putative gene in Joubert syndrome with cortical heterotopia, microophthalmia, and growth hormone deficiency. Am J Med Genet A. 2017;173(3):661–666. doi: 10.1002/ajmg.a.38005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell LA, Thomas PQ, Zacharin MR, Scheffer IE. Ectopic posterior pituitary lobe and periventricular heterotopia: cerebral malformations with the same underlying mechanism? AJNR Am J Neuroradiol. 2002;23(9):1475–1481. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study may be released upon application to the corresponding author, who can be contacted at alessandradinora@gmail.com.