Highlights
-
•
Implementation of HIPEC for ovarian cancer is ongoing, aiming to offer this treatment to all eligible patients in the Netherlands.
-
•
Standardization reduces unwanted variation in clinical treatment.
-
•
We intend to standardize patient selection, technical aspects, and perioperative care of CRS and HIPEC.
-
•
This consensus study comprised a two-phase modified Delphi approach.
-
•
Consensus was reached on 82% of items.
Keywords: Ovarian cancer, HIPEC, Consensus, Delphi
Abbreviations: HIPEC, Hyperthermic Intraperitoneal Chemotherapy; CRS, Cytoreductive Surgery; EOC, Epithelial Ovarian Cancer; NACT, Neoadjuvant Chemotherapy
Abstract
Objective
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) is standard of care in the Netherlands in patients with stage III epithelial ovarian cancer following interval cytoreductive surgery (CRS). Differences in patient selection, technical aspects, and perioperative management exist between centers performing HIPEC. Standardization aims to reduce unwanted variation in clinical practice. As part of an implementation process, we aimed to standardize perioperative care for patients treated with CRS and HIPEC using a Delphi-based consensus approach.
Methods
We performed a two-phase modified Delphi method involving a multidisciplinary panel of 40 experts who completed a survey on CRS and HIPEC. During a consensus meeting, survey outcomes and available scientific evidence was discussed. Items without consensus (<75% agreement) were adjusted and evaluated in a second survey.
Results
Consensus was reached in the first round on 51% of items. After two rounds, consensus was reached on the majority of items (82%) including patient selection, preoperative workup, technical aspects of CRS and HIPEC, and postoperative care. No consensus was reached on the role of HIPEC in rare ovarian cancer types, preoperative bowel preparation, timing to create bowel anastomoses, and manipulation of the perfusate.
Conclusions
Dutch experts reached consensus on most items regarding interval CRS and HIPEC for ovarian cancer. This consensus study may help to align treatment protocols and to minimize practice variation. Topics without consensus may be put on the research agenda of HIPEC for ovarian cancer.
1. Introduction
Epithelial Ovarian cancer (EOC) is the 3rd most common gynecologic malignancy among women worldwide (Bray et al., 2018). Due to a lack of specific symptoms, >75% of patients are diagnosed with advanced stage disease (Doubeni et al., 2016). Despite extensive treatment with Cytoreductive Surgery (CRS) and systemic chemotherapy, recurrences occur in>80% of patients highlighting the need for additional treatment (du Bois et al., 2009).
The randomized controlled OVHIPEC-1 trial demonstrated the benefit of the addition of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) with cisplatin to interval CRS in patients with advanced EOC (van Driel et al., 2018). Consequently, in various countries, HIPEC is considered standard treatment in patients with stage III EOC if complete (no visible residual disease) or optimal (<10 mm residual disease) interval CRS is achieved. HIPEC is cost-effective and is fully reimbursed by Dutch health care insurance (Koole et al., 2019). Since March 2019, HIPEC for ovarian cancer is offered in ten Dutch centers. Currently, nationwide implementation is ongoing, aiming to offer this treatment to all eligible patients.
End of 2019, we conducted a survey on practice patterns among the ten Dutch cancer centers performing HIPEC for ovarian cancer (van Stein et al., 2019). Considerable differences were noted regarding patient selection for interval CRS and HIPEC, technical aspects of HIPEC, and perioperative management based on differences in training, personal believes and values. These variation might negatively impact patient outcomes which is supported by multiple examples (Florin et al., 2014, Mahant et al., 2014). Furthermore, differences in patient selection can lead to patients missing out on a beneficial treatment or undergoing a non-beneficial toxic treatment. Standardization aims to reduce unwanted variation in clinical treatment and aims to improve the safety of patient care by reduction of potential errors.
As part of the implementation process, we aimed to standardize CRS and HIPEC in the Netherlands using a Delphi-based consensus approach.
2. Methods
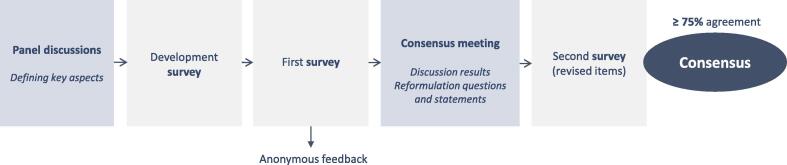
The consensus study was developed by a multidisciplinary scientific committee including an experienced Delphi methodologist. The methodology consisted of two separate rounds in which experts in the field rated statements and answered multiple-choice questions, and a plenary consensus meeting. Fig. 1 shows this process. Our approach combined aspects from the Delphi method and Nominal Group Technique (Jones and Hunter, 1995). Consensus was defined as ≥ 75% agreement amongst panelists. Patient population was defined as patients with primary advanced stage EOC (at least FIGO stage III). If we discuss HIPEC in ovarian cancer it is performed with heated cisplatin 100 mg/m2 for 90 min using the open technique following complete/optimal interval CRS. Essential topics for pre-, intra- and postoperative care for CRS and HIPEC were defined during several sessions of the scientific committee and one gynecological oncologist from each Dutch HIPEC center (Table 1).
Fig. 1.
Overview of modified Delphi process.
Table 1.
pre-, intra-, and postoperative topics.
| Preoperative | Intraoperative | Postoperative |
|---|---|---|
| Patient selection | CRS | Postoperative care |
|
|
|
| Preoperative workup | HIPEC procedure | Follow-up and adjuvant treatment |
|
|
|
The ‘Dutch OVHIPEC Working Group’ panel consists of 12 gynecological oncologists, 8 medical oncologists, 10 surgical oncologists, and 10 anesthesiologists. Selection criteria for panel members included clinical and/or scientific expertise in the field of HIPEC for EOC and availability to participate in the plenary consensus meeting. All Dutch centers performing HIPEC for EOC were represented. These centers perform at least 20 CRS procedures and 10 HIPEC procedures for EOC yearly.
The scientific committee formulated 109 statements and questions (hereinafter referred to as ‘items’). The panelists were asked to complete an online survey with a selection of items related to their discipline. They were encouraged to provide detailed feedback and suggestions for improvement of items. A summary of the first-round results was sent to the panel one week before the consensus meeting. During this meeting (March 26th, 2021), the results of the survey were discussed in light of available scientific evidence. The discussion focused on items with < 75% agreement which were refined and included in a second survey. This survey contained 54 revised items.
3. Results
Response rate was 98% for the first survey and 100% for the second survey. Consensus was reached in the first round on 51% of items. Consensus existed for the majority of items regarding CRS and postoperative care. Items concerning patient selection, preoperative workup, and technical aspects of HIPEC required more discussion and adjustment. Eventually, consensus was reached on 82% of all items. Table 2, Table 3, Table 4 contain items with corresponding panel agreement. Items without consensus or with considerable implications for clinical practice are further discussed in the text. Remaining items are described in supplementary materials. Next to providing an overview of results in this section, we will describe the relevant available evidence that was used to formulate and adjust the items. All available evidence concerning these items for ovarian cancer patients undergoing HIPEC is of a low level (The periodic health examination, 1979).
Table 2.
Panel results on items regarding patient selection for interval CRS with HIPEC and preoperative workup.
| Preoperative | Agreement | ||
|---|---|---|---|
| Patient selection for interval CRS with HIPEC | |||
| Multidisciplinary team meeting | |||
| …. should be present during multidisciplinary team meetings by default/if indicated/never. | By default | If indicated | Never |
| Gynecological oncologist | 100% | 0% | 0% |
| Radiologist | 100% | 0% | 0% |
| Medical oncologist | 100% | 0% | 0% |
| Case-manager (nurse) | 93% | 7% | 0% |
| Urologist | 0% | 86% | 14% |
| Oncological/gastrointestinal surgeon | 37% | 63% | 0% |
| Plastic surgeon | 0% | 59% | 41% |
| Timing indication HIPEC | |||
| The intention to add HIPEC to interval CRS should be clear before start of any treatment. | 90% | ||
| Suspected stage IV | |||
| If stage IV is suspected, this should be confirmed by cytology or histology (if not feasible PET/CT) before administration of NACT. In case the results do not confirm presence of stage IV, a patient is considered eligible for HIPEC. | 79% | ||
| If additional investigation is technically impossible or is inconclusive for stage IV, and after NACT the abnormality is unchanged with an otherwise good response to chemotherapy, a patient is eligible for HIPEC. | 96% | ||
| Resectable stage IV metastases | |||
| Patients with a resectable …. are eligible for HIPEC. | |||
| peritoneal metastasis infiltrating the bowel, liver or spleen | 89%, 89%, 86% | ||
| Sister Mary Joseph Nodule | 82% | ||
| iatrogenic abdominal wall metastasis | 89% | ||
| Patients with a resectable…. do not qualify for HIPEC. | |||
| parenchymal lesion in the liver or spleen | 93%, 79% | ||
| metastasis in paracardial, axillary or inguinal lymph node | 96%, 100%, 93% | ||
| Histological subtype | |||
| Patients with…. are eligible for HIPEC. | |||
| high grade serous carcinoma | 100% | ||
| low grade serous carcinoma | 91% | ||
| high grade endometrioid carcinoma | 96% | ||
| low grade endometrioid carcinoma | 81% | ||
| mucinous carcinoma | 100% | ||
| clear cell carcinoma | 100% | ||
| carcinosarcoma | 65% | ||
| Patients with non-epithelial ovarian, fallopian tube or extra-ovarian tumors do not qualify for HIPEC. | 100% | ||
| Contraindications | |||
| Age is no individual selection criterion for HIPEC. | 76% | ||
| WHO performance status > 2 is a relative contraindication for HIPEC. | 82% | ||
| Creatinine clearance < 60 ml/min or ml/min/1.73 m2 is a relative contraindication for HIPEC. | 80% | ||
| Creatinine clearance can be calculated using Cockcroft Gault formula, MDRD formula, or CKD-EPI formula. | 77% | ||
| Preoperative workup | |||
| Imaging * | |||
| Imaging < 8 weeks prior to CRS (regardless of the addition of HIPEC) should be available. | 83% | ||
| Diagnostic laparoscopy * | |||
| Diagnostic laparoscopy to determine resectability may be performed in a separate session for logistical reasons. | 89% | ||
| Outpatient visit | |||
| Patients should visit…. prior to CRS with HIPEC routinely/if indicated/never. | Routinely | If indicated | Never |
| gynecological oncologist | 100% | 0% | 0% |
| anesthesiologist | 100% | 0% | 0% |
| case-manager (nurse) | 97% | 3% | 0% |
| medical oncologist | 60% | 37% | 3% |
| stoma care nurse | 57% | 43% | 0% |
| social worker | 16% | 81% | 3% |
| oncological/gastrointestinal surgeon | 39% | 61% | 0% |
| dietician | 29% | 71% | 0% |
| physiotherapist | 33% | 64% | 3% |
| Nutritional status | |||
| Prior to NACT, nutritional status should be determined routinely using the Short Nutritional Assessment Questionnaire, Malnutrition Universal Screening Tool, or another validated tool. | 97% | ||
| Preoperative bowel preparation | |||
| Patients should undergo preoperative mechanical bowel preparation: …. | |||
| always | 14% | ||
| in case a colectomy is suspected | 0% | ||
| in case a rectosigmoid resection is suspected | 29% | ||
| in case a colectomy and/or rectosigmoid resection is suspected | 14% | ||
| never | 35% | ||
| A local protocol for preoperative bowel preparation should be available (preferably uniform for gastrointestinal and gynecological procedures). | 82% | ||
| Patient information * | |||
| Basic information for patients undergoing CRS with HIPEC should be uniform and used in all centers in the Netherlands. Center-specific adjustments can be added. | 95% | ||
Note: The bold values represent items for which ≥ 75% of the panel members chose the same option (=consensus). Valid answers: “can't judge (unqualified to answer)” excluded.
Table 3.
Panel results on items regarding HIPEC.
| Intraoperative | Agreement |
|---|---|
| HIPEC | |
| Chest tube | |
| After diaphragmatic surgery, routine prophylactic chest tube placement can be omitted. | 79% |
| Thoracic chemoperfusion | |
| Any defect in the diaphragm should be closed before start of HIPEC preventing the pleural cavity will be rinsed simultaneously. | 88% |
| Prevention of nephrotoxicity | |
| Administration of sodium thiosulfate is started with a bolus administered…. | |
| simultaneous with heating/filling the abdominal cavity (approximately 20–30 min before first dose of cisplatin) | 50% |
| 5–10 min before first dose of cisplatin | 46% |
| simultaneous with first dose of cisplatin | 4% |
| Urine output of at least 0.5 ml/kg/h should be aimed for during and after HIPEC. | 58% |
| Adequate perfusion of the kidneys should be aimed for during and after HIPEC. | 96% |
| Hemodynamics | |
| Goal-directed fluid therapy with a balanced fluid solution should be administered. | 78% |
| If a vasopressor is indicated, the use of norepinephrine is recommended. | 88% |
| Routine administration of corticosteroid should be omitted. | 85% |
| Drains | |
| Three inflow catheters and two draining catheters should be used. | 65% |
| Inflow and outflow drains should not be placed through the fascia. | 82% |
| Routine use of postoperative abdominal drain(s) should preferably be avoided. | 81% |
| Intra-abdominal temperature | |
| Intra-abdominal temperature should be monitored continuously throughout the perfusion by sensors placed in at least 3 abdominal quadrants. | 95% |
| Temperature of the perfusate should be > 40.5 °C < 42.5 °C. | 88% |
| Stirring | |
| The HIPEC perfusate should be manipulated manually throughout the perfusion. | 63% |
| Plastic sheet * | |
| The wound and retractor should be covered by a plastic hood. | 85% |
| Core temperature * | |
| What measures can be taken to anticipate for a rise in core temperature? | |
| Switch off hotlines | 100% |
| Switch off bear hugger | 100% |
| Switch off warming mattress | 97% |
| Lower room temperature | 55% |
| Ice bags in the neck of a patient | 13% |
| Bowel anastomoses | |
| .… is preferably created after HIPEC. | |
| Low rectal anastomosis | 80% |
| Colon anastomosis | 75% |
| Small bowel anastomosis | 68% |
| Closing skin * | |
| The choice to use skin sutures or staples to close the skin is not affected by the addition of HIPEC to CRS. | 100% |
Note: The bold values represent items for which ≥ 75% of the panel members chose the same option (=consensus). Valid answers: “can't judge (unqualified to answer)” excluded.
Table 4.
Panel results on items regarding postoperative care following CRS and HIPEC, follow-up and adjuvant treatment.
| Postoperative | Agreement |
|---|---|
| Postoperative care following CRS and HIPEC | |
| Timing of extubation | |
| Early extubation should preferably be performed. | 63% |
| Postoperative monitoring | |
| Patients should be routinely admitted to an intensive, medium or post anesthesia care unit. | 88% |
| Postoperative nausea and vomiting * | |
| The Dutch guideline (‘postoperative pain’) should be followed to prevent for and treat postoperative nausea and vomiting. | 92% |
| Nasogastric tube | |
| Routine postoperative use of a nasogastric tube can be avoided in the absence of risk factors for delayed gastric emptying. | 68% |
| Postoperative nutrition | |
| Early postoperative oral intake resumption is recommended. | 79% |
| Analgesia | |
| Preferred intra- and postoperative analgesia is comparable with other major abdominal surgeries. | 87% |
| Thoracic epidural analgesia should be performed routinely intra- and postoperatively. | 97% |
| The aim should be to stop epidural analgesia 72 h postoperatively. | 79% |
| Thrombosis prophylaxis | |
| Extended (at least 28 days) thrombosis prophylaxis with LMWH should be administered. | 80% |
| LMWH should be started postoperative, but not within six hours after surgery. | 97% |
| Laboratory tests | |
| CRP assessment at day 3 postoperative should be performed routinely. | 92% |
| Excreta * | |
| Body fluids of a patient should be considered contaminated for the first 7 days after HIPEC with cisplatin. | 92% |
| Follow-up and adjuvant treatment | |
| Outpatient visit * | |
| There should be at least one postoperative follow-up check (by phone) in the HIPEC center. | 81% |
| Start adjuvant chemotherapy | |
| Chemotherapy should preferably be re-started within 4 weeks after surgery regardless of the addition of HIPEC to CRS.If a patient needs more time to recover, chemotherapy should preferably be started within 6 weeks. | 90% |
Note: The bold values represent items for which ≥ 75% of the panel members chose the same option (=consensus). Valid answers: “can't judge (unqualified to answer)” excluded.
3.1. Preoperative
3.1.1. Patient selection for interval CRS with HIPEC
3.1.1.1. Multidisciplinary team meeting
The panel agreed that a gynecological oncologist, radiologist, medical oncologist, and case-manager should always be present during multidisciplinary team (MDT) meetings and that other medical specialists should participate if needed. MDTs are Dutch standard of care in oncology. Eligibility for either primary or interval CRS is determined in a preoperative MDT meeting. Following 2 cycles of neoadjuvant chemotherapy (NACT), tumor response is evaluated again by the MDT.
3.1.1.2. Timing indication HIPEC and suspicion of stage IV disease
Consensus was reached that the intention to add HIPEC to interval CRS should be clear before start of NACT, and that HIPEC should be scheduled after evaluation of NACT. The final decision to perform HIPEC however, should be made during surgery after achieving complete or optimal CRS.
If FIGO stage IV is suspected, consensus was reached that this should be confirmed by cytology or histology (if not feasible positron emission tomography (PET)/CT) before administration of NACT. In case the results do not confirm presence of stage IV, a patient is considered eligible for HIPEC after interval CRS. If additional investigations to diagnose stage IV are either technically impossible or inconclusive, and the abnormality is unchanged following NACT with an otherwise good response to chemotherapy, a patient is regarded eligible for HIPEC. Imaging plays a significant role in staging EOC. PET/CT may be useful in the presence of lesions outside the abdomen and pelvis or indeterminate lymph node appearance, with a good specificity but a relatively poor sensitivity to detect metastases (Khiewvan et al., 2017). The diagnostic efficiency of PET/CT to detect malignant pleural effusion is moderate, precluding its use for discriminating malignant from benign pleural effusion (Porcel et al., 2015).
3.1.1.3. Resectable stage IV metastases
The panel agreed that patients with parenchymal lesions in the liver or spleen, or metastases in paracardial, axillary, or inguinal lymph nodes do not qualify for HIPEC, even if complete resection of these lesions is accomplished. The value of adding HIPEC to CRS after hematogenous or extra-abdominal lymphogenous metastatic spread is not established.
Consensus was achieved that patients with resectable peritoneal metastases with infiltration into the bowel, liver, or spleen, a resectable Sister Mary Joseph Nodule, or a resectable iatrogenic abdominal wall metastasis after diagnostic laparoscopy or ascites drainage are eligible for CRS plus HIPEC. Because these lesions are not metastasized hematogenously, patients potentially benefit from HIPEC after complete resection of the locally infiltrated lesion. It should be noted that discrimination between parenchymal liver or spleen metastases and peritoneal infiltration into these organs can be challenging based on imaging alone.
3.1.1.4. Histological subtype
There was consensus that HIPEC can be performed in patients with all types of epithelial ovarian, fallopian tube, or extra-ovarian tumors. Patients with non-epithelial tumors are not considered eligible for HIPEC. No consensus was reached about the eligibility for HIPEC in case of a carcinosarcoma.
Post-hoc subgroup analyses for RFS and OS in the OVHIPEC-1 trial showed a homogenous effect of HIPEC across high-grade serous vs. other tumor histology (van Driel et al., 2018). However, numbers are small and efficacy of HIPEC may differ between EOC subtypes warranting further research. The synergistic effect of chemotherapy and hyperthermia in serous and endometrioid cell lines is well investigated (Vos et al., 2022). There are no in-vitro studies in rare EOC subtypes such as carcinosarcoma, mucinous, or clear cell carcinoma.
3.1.1.5. Contra-indications
There was consensus that age is not a separate selection criterion for HIPEC but should be part of a broader clinical assessment including factors like frailty, comorbidity, expected years of life, and physiological age.
The panel acknowledged that World Health Organization (WHO) performance status > 2 is a relative contraindication for HIPEC.
Consensus was achieved that creatinine clearance < 60 ml/min or ml/min/1.73 m2 (using either MDRD, Cockcroft-Gault formula, or CKD-EPI) should be considered a relative contraindication for HIPEC. In patients with creatinine clearance below this threshold, HIPEC could be delivered at the discretion of the medical oncologist dependent on extent, cause, treatability, and comorbidity. This statement is based on the knowledge cisplatin can cause nephrotoxicity. Although plasma concentration of cisplatin is low during intraperitoneal perfusion, adequate renal function is a prerequisite for cisplatin use (Zivanovic et al., 2015). No studies were found to help set the cutoff for kidney function at which intraperitoneal cisplatin administration is contra-indicated. A generally recognized threshold for systemic cisplatin eligibility is creatinine clearance > 60 ml/min.
3.1.2. Preoperative workup
3.1.2.1. Outpatient visit
Consensus was reached on routinely visiting a gynecological oncologist, case-manager, and anesthesiologist in the HIPEC center prior to interval CRS and HIPEC. A social worker should be visited routinely according to only 19% of the panel. The majority of experts agreed a medical oncologist and stoma-care nurse should be visited routinely but no consensus was reached. In case a patient does not visit a stoma-care nurse prior to surgery, another health worker/specialist should inform the patient on the possibility and consequences once a stoma is created. Although no consensus was reached, a large part of the panel agreed on a preoperative visit when indicated to an oncological/gastrointestinal surgeon, dietician, or physiotherapist.
3.1.2.2. Nutritional status
There was consensus that prior to NACT, the patient’s nutritional status should be determined routinely using the Short Nutritional Assessment Questionnaire, Malnutrition Universal Screening Tool, or another validated tool so action can be taken if necessary. Preoperative malnutrition affects postoperative outcomes (Reece et al., 2019). Assessing the preoperative nutritional state is a critical step for nutrition support and postoperative recovery.
3.1.2.3. Preoperative bowel preparation
No consensus existed on administration of preoperative mechanical bowel preparation. According to the panel, every hospital should follow local protocol that is preferably uniform for gynecological and gastrointestinal oncological procedures. Multiple systematic reviews and meta-analyses have shown that the use of mechanical bowel preparation in patients undergoing colorectal surgery is not associated with a decrease of infection, anastomotic leak, re-surgery, or mortality (Nelson et al., 2016, Fotopoulou et al., 2021). Therefore, Enhanced Recovery After Surgery (ERAS) and European Society of Gynaecological Oncology (ESGO) guidelines, do not recommend routine mechanical bowel preparation in EOC patients undergoing CRS (Nelson et al., 2016, Fotopoulou et al., 2021). However, the combination of mechanical bowel preparation with oral antibiotics seems to be associated with a decrease of surgical site infections, intra-abdominal infections, and anastomotic leaks in colorectal surgery (Fotopoulou et al., 2021).
3.2. Intraoperative
3.2.1. CRS
As HIPEC is part of a surgical procedure, also a number of general CRS topics were discussed. On 10/11 items consensus was reached. No consensus was achieved on optimal patient positioning for CRS. Results on CRS items are shown in supplementary materials.
3.2.2. HIPEC
3.2.2.1. Chest tube
According to the panel, routine prophylactic chest tube placement after diaphragmatic surgery can be omitted. A diaphragmatic defect should preferably be closed with positive pulmonary pressure without placing a chest tube. In this case, attention should be paid to total HIPEC perfusate volumes, because the perfusate can accidentally enter the thoracic cavity which would require drainage. Prophylactic use of a chest tube could potentially prevent postoperative pulmonary complications e.g. pleural effusion, pneumonia, and pneumothorax (Fotopoulou et al., 2021, Vilkki and Gunn, 2020). Nevertheless, ESGO guidelines do not recommend routine chest tube placement since the incidence of these complications is low (Fotopoulou et al., 2021).
3.2.2.2. Thoracic chemoperfusion
There was consensus that any defect in the diaphragm should be closed before HIPEC preventing that the pleural cavity will be perfused simultaneously. Hyperthermic intrapleural chemotherapy with cisplatin is investigated in patients with malignant pleural effusion or malignant pleural tumors (Migliore et al., 2015, Zhao et al., 2017). However, patients with FIGO stage IV EOC based on malignant pleural effusion are not eligible to undergo HIPEC in the Netherlands. Tumor involvement of the diaphragm, leading to a diaphragmatic resection might increase the risk of iatrogenic contamination of the pleural cavity. The role of simultaneous intraperitoneal and intrapleural chemoperfusion in controlling thoracic contamination in EOC patients remains unclear. A retrospective study including 102 patients who underwent CRS-HIPEC for colorectal cancer including diaphragm resection, showed that simultaneous intrapleural perfusion was associated with more thoracic recurrences (17% vs. 2.3% (p = 0.04)) (Ahmed et al., 2014).
3.2.2.3. Prevention of nephrotoxicity
No consensus was reached on the optimal timing to start administering sodium thiosulfate. Cisplatin is known to cause nephrotoxicity. A competitive neutralizing agent, such as sodium thiosulfate, is often administered to prevent the occurrence of nephrotoxicity. When sodium thiosulfate is administered concurrently, the dose of cisplatin can be escalated to 270 mg/m2 without causing a significant increase of serum creatinine (Howell et al., 1982). In OVHIPEC-1, sodium thiosulfate was administered as an intravenous bolus (9 g/m2 in 200 ml), followed by continuous infusion (12 g/m2 in 1000 ml) over 6 h (van Driel et al., 2018). Based on historical grounds and for logistical reasons, part of the panel prefers to administer the bolus simultaneous with warming up/filling the abdomen (approximately 20–30 min before first gift of cisplatin). Thiosulfate binds covalently to cisplatin and inactivates it. Both drugs should be present in the systemic circulation simultaneously to make this process happen. The peak level of thiosulfate is reached at the end of the bolus and will then decrease quickly (elimination half-life is approximately 180 min for a dose of 150 mg/kg). The peak of systemic cisplatin is reached approximately 30 min after intraperitoneal perfusion (Cashin et al., 2013). Based on these theoretical grounds, it seems logical to start the bolus simultaneous with or within 10 min before first dose of cisplatin.
There was also no consensus on the statement to aim for a minimum urine output of 0.5 ml/kg/hour during and after HIPEC. During surgery, a rise of plasma antidiuretic hormone occurs which may decrease diuresis. Therefore, urine output may not be the optimal parameter to monitor nephrotoxicity.
Obviously, the panel agreed that adequate perfusion of the kidneys should be aimed for to prevent nephrotoxicity. How to ensure adequate organ perfusion is briefly discussed in the following section.
3.2.2.4. Hemodynamics
There was consensus that goal-directed fluid therapy with a balanced fluid solution should be used routinely with the addition of norepinephrine if a vasopressor is indicated. Extensive surgery such as CRS and HIPEC is associated with blood loss and significant systemic inflammatory response syndrome (SIRS) which leads to vasodilation and capillary leak with fluid and protein loss causing hypotension (Kanakoudis et al., 1996). Also, epidural analgesia-induced sympathetic blockade causes vasodilation which can result in hypotension. Intraoperative maintenance of an adequate intravascular volume by fluid administration maintains tissue perfusion. Goal-directed fluid therapy uses advanced hemodynamic monitoring. Fluid and vasopressor therapy is administered according to hemodynamic parameters like e.g. pulse pressure variation, stroke volume variation, or systolic pressure variation. Adequate intravascular volume is ensured before starting vasopressor therapy, minimizing the risks associated with both hypovolemia (e.g. nephrotoxicity) and hypervolemia (Miller and Myles, 2019). Routine usage of steroids was only supported by a small part of the panel, in spite of some data suggesting better outcome after surgery (Raimondi et al., 2006).
3.2.2.5. Drains
No consensus was reached regarding the number of inflow and outflow drains although the majority of the panel agreed that three inflow catheters and two centrally placed outflow drains should be used, which is similar to the amount used in the OVHIPEC-1 trial (van Driel et al., 2018). No scientific evidence is available regarding the optimal amount of inflow and outflow drains.
There was consensus regarding fixation of inflow and outflow drains. To minimize damage to healthy tissue, the drains should not be placed through the fascia. Alternatively, the drains can be fixated to the retractor.
Consensus was reached that after interval CRS (regardless of whether bowel surgery has been performed) with HIPEC, routine use of abdominal drain(s) should preferably be omitted. Postoperative abdominal drainage is regularly used to prevent accumulation of fluid in the peritoneal cavity, and after bowel surgery it may show anastomotic leakage. However, abdominal drainage does not lead to better outcomes after gynecological oncological surgery, including patients undergoing bowel surgery (Nelson et al., 2016). There are no studies addressing the role of routine abdominal drainage in patients undergoing HIPEC. The Dutch surgical community has good overall experience with avoiding routine use of abdominal drain(s) after CRS and HIPEC for colorectal cancer.
3.2.2.6. Intra-abdominal temperature
The panel acknowledged that intra-abdominal temperature should be monitored continuously throughout perfusion by sensors placed in at least three abdominal quadrants. Consensus was reached that one should aim for an intra-abdominal temperature between 40.5 °C and 42.5 °C. The synergistic effect of chemotherapy and hyperthermia already occurs at moderately elevated temperatures (40–41 °C) (Vos et al., 2022).
3.2.2.7. Stirring
No consensus existed whether the perfusate should be manipulated manually throughout the HIPEC procedure. Manipulation of the abdominal perfusate could potentially allow better exposure of the peritoneal surface to heated chemotherapy. However, no data is available on the effect of stirring on distribution of cisplatin or temperature of the perfusate throughout the peritoneal cavity.
3.2.2.8. Bowel anastomoses
There was consensus to make a low rectal anastomosis or other colon anastomosis after HIPEC. With regards to small bowel anastomoses no consensus was reached. Although not supported by scientific evidence, the possible advantage of creating a bowel anastomosis after HIPEC is that microscopic tumor potentially present in the anastomotic line will be well exposed to HIPEC and thereby reduces anastomotic recurrences (Somashekhar et al., 2022). On the other hand it could technically be more difficult to make an anastomosis after HIPEC due to edema, and suture healing might be negatively affected by heat and chemotherapy, increasing the incidence of anastomotic leaks. Except for one retrospective study suggesting that both strategies are comparable with regards to anastomotic leak/bowel perforation (Somashekhar et al., 2022), there are no comparative studies available investigating the optimal timing.
3.3. Postoperative
3.3.1. Postoperative care following CRS and HIPEC
3.3.1.1. Timing of extubation
No consensus was reached regarding routine early extubation. Available data suggests that immediate postoperative extubation is safe (Cooksley and Haji-Michael, 2011). Early extubation with shorter anesthesia leads to faster recovery. Possibly, no consensus was reached because of unfamiliarity with early extubation after HIPEC and the possible advantages. Education and further discussion with the anesthetic team may allow routine early extubation in the future.
3.3.1.2. Postoperative monitoring
The panel agreed that patients should be routinely admitted to an intensive care unit (ICU), post anesthesia care unit (PACU) or medium care unit (MCU). MCU, also referred to as intermediate care or high-dependency unit, has a level of care in between that of the ICU and the general ward. Following CRS and HIPEC, physiological changes occur and postoperative challenges, in particular with hemodynamic management, may exist (Cooksley and Haji-Michael, 2011). This may only be manageable on units with high level of care. However, routine admission to this type of unit might not be required for all patients but could hypothetically be based on individual patient characteristics, comorbidities, and/or perioperative risk factors (Mogal et al., 2016).
3.3.1.3. Nasogastric tube
No consensus was reached whether routine postoperative nasogastric intubation can be avoided in the absence of risk factors for delayed gastric emptying (e.g. gastric surgery, resection of omentum minus). Data on elective gastrointestinal surgery does not support routine use of a nasogastric tube (Nelson et al., 2005, Cheatham et al., 1995). It has been associated with adverse effects such as delayed resumption of bowel function and increased postoperative pulmonary complications. Vomiting and abdominal distension might decrease with use of a nasogastric tube, but at the same time discomfort increases (Nelson et al., 2005, Cheatham et al., 1995). Aforementioned results might not completely apply to patients undergoing additional HIPEC because hyperthermia and chemotherapy might delay return of normal gastrointestinal motility.
3.3.1.4. Nutrition
Consensus was reached to follow ERAS recommendations regarding postoperative nutrition including early resumption (<24 h) of oral intake after surgery. For patients with complications preventing oral intake, parenteral nutrition should be considered (Nelson et al., 2016, Hübner et al., 2020). Early postoperative enteral feeding is associated with less mortality, less anastomotic dehiscence, faster resumption of bowel activity, and reduced length of hospital stay (Nelson et al., 2016, Hübner et al., 2020, Raspé et al., 2017).
3.3.1.5. Analgesia
There was consensus that optimal intra- and postoperative analgesia is similar to other major abdominal surgeries and the Dutch guideline can be followed (Postoperatieve pijn Richtenlijnendatabase, 2013). Routine use of thoracic epidural analgesia has proven to be effective in managing severe postoperative pain and is recommended by the consensus panel (Salicath et al., 2018). An attempt should be made to stop epidural analgesia 72 h postoperatively according to the panel. For most patients, after the first 48–72 h a transition to oral pain medication will be possible.
3.3.1.6. Thrombosis prophylaxis
Consensus was reached that Low Molecular Weight Heparin (LMWH) should be started postoperative, but not within six hours after surgery. The panel agreed extended (at least 28 days) thrombosis prophylaxis with LMWH should be administered routinely following CRS and HIPEC. The Dutch multidisciplinary guideline on recommended treatment and prophylaxis for thrombosis includes a risk stratification of perioperative venous thromboembolism. Oncological resections of the abdomen or pelvis are associated with a high risk of thrombosis. The guideline includes the recommendation to consider extended duration of LMWH depending on the extent of the surgical resection (Gould et al., 2012).
3.3.1.7. Laboratory tests
The panel reached consensus that C-reactive protein (CRP) assessment at day 3 postoperative should be performed routinely. In OVHIPEC-1, one of the most common grade 3/4 adverse events was infection in both groups (van Driel et al., 2018). A retrospective analysis of 181 patients undergoing CRS-HIPEC suggested that CRP levels that continue to rise after postoperative day 2 or CRP concentrations above 166 mg/L on postoperative day 3 and 116 mg/L on postoperative day 4, indicate a considerable risk for the presence of high-grade adverse events (van Kooten et al., 2021).
3.3.2. Follow-up and adjuvant treatment
3.3.2.1. Start adjuvant chemotherapy
Consensus was achieved that chemotherapy should preferably be re-initiated within 4 weeks after CRS regardless of the addition of HIPEC. If a patient needs more time to recover, chemotherapy should preferably be started within 6 weeks. In a Dutch cohort, delayed initiation (>37 days) of adjuvant chemotherapy was an independent prognostic factor for worse OS after complete (interval)CRS (Timmermans et al., 2018). OVHIPEC-1 showed no difference in time between surgery and restart of chemotherapy between groups (van Driel et al., 2018).
4. Discussion
This Delphi-based consensus study aimed to reach consensus within a Dutch multidisciplinary expert panel on various perioperative aspects of interval CRS and HIPEC for patients with ovarian cancer. After two rounds including a consensus meeting, consensus was reached on the vast majority of items.
We used a modified Delphi method to determine consensus. This technique ensures anonymity during voting, which allows participants to freely express their opinion. The panel received feedback, which may improve knowledge and encourages the evolution of new ideas. Ideally, this process will lead to consensus. Criteria for a consensus threshold are not clearly defined in literature on Delphi studies, but mostly range from 50 to 97%. We used a 75% threshold, as this is most frequently reported in literature (Diamond et al., 2014).
HIPEC in combination with CRS is a highly complex procedure. Uniform training and proctoring of surgical teams is known to result in improved quality of the operative procedure. The introduction of a standardized protocol for HIPEC for colon cancer in the Netherlands resulted in an improvement of surgical quality and a decrease in complications (Kuijpers et al., 2015). Implementation of HIPEC for patients with EOC should be done in a similar way, enabling a controlled and uniform introduction of this technique while minimizing risks of unnecessary complications. Although the importance of standardization of treatment of ovarian cancer is emphasized by the recently published ESGO guidelines for perioperative management in patients undergoing CRS, standardization of HIPEC was not included and is thus still lacking (Fotopoulou et al., 2021). An initial survey showed that practice variation existed in patient selection criteria, technical aspects, and perioperative management between the ten Dutch centers that perform HIPEC for ovarian cancer. The recommendations following this consensus process aim to standardize CRS with HIPEC and perioperative care. As a result, the performance of routine use of HIPEC during interval CRS is likely to improve and unwanted practice variation will be minimized. The results of this consensus study are also useful for the standardization of the HIPEC procedure in currently ongoing clinical trials such as OVHIPEC-2 (ClinicalTrials.gov: NCT03772028) (Koole et al., 2020).
Preferably, standardized application of evidence-based care is applied. However, many perioperative topics are not properly investigated. Lack of consensus was generally associated with absence of robust scientific evidence. Because no consensus could be reached for all items, some practice variation will persist. With detailed registration of these items, a comparison between centers will later be possible and help to define best practices. By further investigating the topics on the research agenda (Table 5), an optimal and uniform protocol for HIPEC and perioperative care is pursued.
Table 5.
Research agenda containing topics with lack of consensus.
| Research agenda |
|---|
| Benefit HIPEC in rare ovarian cancer types (e.g. carcinosarcoma) |
| When to use preoperative bowel preparation |
HIPEC technique:
|
| Optimal ways to prevent nephrotoxicity |
| Optimal timing to create bowel anastomoses: before or after HIPEC |
| When to use postoperative gastric decompression |
| Safety and feasibility of direct extubation |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
KWF Dutch Cancer Society provides funding for the implementation project of HIPEC for ovarian cancer in the Netherlands.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.100945.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and management of ovarian cancer. Am. Fam. Physician. 2016;93(11):937–944. [PubMed] [Google Scholar]
- du Bois A., Reuss A., Pujade-Lauraine E., Harter P., Ray-Coquard I., Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- van Driel W.J., Koole S.N., Sikorska K., Schagen van Leeuwen J.H., Schreuder H.W.R., Hermans R.H.M., de Hingh I.H.J.T., van der Velden J., Arts H.J., Massuger L.F.A.G., Aalbers A.G.J., Verwaal V.J., Kieffer J.M., Van de Vijver K.K., van Tinteren H., Aaronson N.K., Sonke G.S. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- Koole S.N., van Lieshout C., van Driel W.J., van Schagen E., Sikorska K., Kieffer J.M., Schagen van Leeuwen J.H., Schreuder H.W.R., Hermans R.H., de Hingh I.H., van der Velden J., Arts H.J., Massuger L.F.A.G., Aalbers A.G., Verwaal V.J., Van de Vijver K.K., Aaronson N.K., van Tinteren H., Sonke G.S., van Harten W.H., Retèl V.P. Cost effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in stage III ovarian cancer on the basis of a randomized phase III trial. J. Clin. Oncol. 2019;37(23):2041–2050. doi: 10.1200/JCO.19.00594. [DOI] [PubMed] [Google Scholar]
- van Stein, R.M., Lok, C.A., van Driel, W.J. Explorative survey OVHIPEC. [Survey]. Unpublished 2019.
- Florin T.A., Byczkowski T., Ruddy R.M., Zorc J.J., Test M., Shah S.S. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American Academy of Pediatrics bronchiolitis guidelines. J. Pediatr. 2014;165(4):786–792.e1. doi: 10.1016/j.jpeds.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahant S., Keren R., Localio R., Luan X., Song L., Shah S.S., Tieder J.S., Wilson K.M., Elden L., Srivastava R. Variation in quality of tonsillectomy perioperative care and revisit rates in children's hospitals. Pediatrics. 2014;133(2):280–288. doi: 10.1542/peds.2013-1884. [DOI] [PubMed] [Google Scholar]
- Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The periodic health examination Canadian Task Force on the Periodic Health Examination. Can. Med. Assoc. J. 1979;121(9):1193–1254. [PMC free article] [PubMed] [Google Scholar]
- Khiewvan B., Torigian D.A., Emamzadehfard S., Paydary K., Salavati A., Houshmand S., Werner T.J., Alavi A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging. 2017;44(6):1079–1091. doi: 10.1007/s00259-017-3638-z. [DOI] [PubMed] [Google Scholar]
- Porcel J.M., Hernández P., Martínez-Alonso M., Bielsa S., Salud A. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest. 2015;147(2):502–512. doi: 10.1378/chest.14-0820. [DOI] [PubMed] [Google Scholar]
- Vos L.M.C., Aronson S.L., van Driel W.J., Huitema A.D.R., Schagen van Leeuwen J.H., Lok C.A.R., Sonke G.S. Translational and pharmacological principles of hyperthermic intraperitoneal chemotherapy for ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;78:86–102. doi: 10.1016/j.bpobgyn.2021.06.004. [DOI] [PubMed] [Google Scholar]
- Zivanovic O., Abramian A., Kullmann M., et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int. J. Cancer. 2015;136(3):699–708. doi: 10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]
- Reece L., Dragicevich H., Lewis C., Rothwell C., Fisher O.M., Carey S., Alzahrani N.A., Liauw W., Morris D.L. Preoperative Nutrition Status and Postoperative Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019;26(8):2622–2630. doi: 10.1245/s10434-019-07415-7. [DOI] [PubMed] [Google Scholar]
- Nelson G., Altman A.D., Nick A., Meyer L.A., Ramirez P.T., Achtari C., Antrobus J., Huang J., Scott M., Wijk L., Acheson N., Ljungqvist O., Dowdy S.C. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations–Part I. Gynecol. Oncol. 2016;140(2):313–322. doi: 10.1016/j.ygyno.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Fotopoulou C., Planchamp F., Aytulu T., Chiva L., Cina A., Ergönül Ö., Fagotti A., Haidopoulos D., Hasenburg A., Hughes C., Knapp P., Morice P., Schneider S., Sehouli J., Stamatakis E., Suria S., Taskiran C., Trappe R.U., Campbell J. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer. 2021;31(9):1199–1206. doi: 10.1136/ijgc-2021-002951. [DOI] [PubMed] [Google Scholar]
- Vilkki V.A., Gunn J.M. Complications related to tube thoracostomy in Southwest Finland hospital district between 2004 and 2014. Scand. J. Surg. 2020;109(4):314–319. doi: 10.1177/1457496919857262. [DOI] [PubMed] [Google Scholar]
- Migliore M., Calvo D., Criscione A., Viola C., Privitera G., Spatola C., Parra H.S., Palmucci S., Ciancio N., Caltabiano R., Maria G.D. Cytoreductive surgery and hyperthermic intrapleural chemotherapy for malignant pleural diseases: preliminary experience. Future Oncol. 2015;11(2s):47–52. doi: 10.2217/fon.14.256. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-Y., Zhao S.-S., Ren M., Liu Z.-L., Li Z., Yang L. Effect of hyperthermic intrathoracic chemotherapy on the malignant pleural mesothelioma: a systematic review and meta-analysis. Oncotarget. 2017;8(59):100640–100647. doi: 10.18632/oncotarget.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Levine E.A., Randle R.W., Swett K.R., Shen P., Stewart J.H., Votanopoulos K.I. Significance of diaphragmatic resections and thoracic chemoperfusion on outcomes of peritoneal surface disease treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann. Surg. Oncol. 2014;21(13):4226–4231. doi: 10.1245/s10434-014-3891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S.B., Pfeifle C.L., Wung W.E., et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann. Intern. Med. 1982;97(6):845–851. doi: 10.7326/0003-4819-97-6-845. [DOI] [PubMed] [Google Scholar]
- Cashin P.H., Ehrsson H., Wallin I., Nygren P., Mahteme H. Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis. Eur. J. Clin. Pharmacol. 2013;69(3):533–540. doi: 10.1007/s00228-012-1405-4. [DOI] [PubMed] [Google Scholar]
- Kanakoudis F., Petrou A., Michaloudis D., Chortaria G., Konstantinidou A. Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. Haemodynamic changes, oxygen consumption and delivery. Anaesthesia. 1996;51(11):1033–1036. doi: 10.1111/j.1365-2044.1996.tb14998.x. [DOI] [PubMed] [Google Scholar]
- Miller T.E., Myles P.S. Perioperative Fluid Therapy for Major Surgery. Anesthesiology. 2019;130(5):825–832. doi: 10.1097/ALN.0000000000002603. [DOI] [PubMed] [Google Scholar]
- Raimondi A.M., Guimarães H.P., Amaral J.L.G.d., Leal P.H.R. Perioperative glucocorticoid administration for prevention of systemic organ failure in patients undergoing esophageal resection for esophageal carcinoma. Sao Paulo Med. J. 2006;124(2):112–115. doi: 10.1590/S1516-31802006000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G., Altman A.D., Nick A., Meyer L.A., Ramirez P.T., Achtari C., Antrobus J., Huang J., Scott M., Wijk L., Acheson N., Ljungqvist O., Dowdy S.C. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations–Part II. Gynecol. Oncol. 2016;140(2):323–332. doi: 10.1016/j.ygyno.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somashekhar S.P., Rohit K.C., Ramya Y., Zaveri S.S., Ahuja V., Namachivayam A.K., Ashwin K.R. Bowel Anastomosis After or Before HIPEC: A Comparative Study in Patients Undergoing CRS+HIPEC for Peritoneal Surface Malignancy. Ann. Surg. Oncol. 2022;29(1):214–223. doi: 10.1245/s10434-021-10661-3. [DOI] [PubMed] [Google Scholar]
- Cooksley T.J., Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) World J. Surg. Oncol. 2011;9:169. doi: 10.1186/1477-7819-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogal H.D., Levine E.A., Fino N.F., Obiora C., Shen P., Stewart J.H., Votanopoulos K.I. Routine Admission to Intensive Care Unit After Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy: Not Always a Requirement. Ann. Surg. Oncol. 2016;23(5):1486–1495. doi: 10.1245/s10434-015-4963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R., Tse B., Edwards S. Systematic review of prophylactic nasogastric decompression after abdominal operations. Br. J. Surg. 2005;92(6):673–680. doi: 10.1002/bjs.5090. [DOI] [PubMed] [Google Scholar]
- Cheatham M.L., Chapman W.C., Key S.P., Sawyers J.L. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann. Surg. 1995;221(5):469–478. doi: 10.1097/00000658-199505000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner M., Kusamura S., Villeneuve L., Al-Niaimi A., Alyami M., Balonov K., Bell J., Bristow R., Guiral D.C., Fagotti A., Falcão L.F.R., Glehen O., Lambert L., Mack L., Muenster T., Piso P., Pocard M., Rau B., Sgarbura O., Somashekhar S.P., Wadhwa A., Altman A., Fawcett W., Veerapong J., Nelson G. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations - Part II: Postoperative management and special considerations. Eur. J. Surg. Oncol. 2020;46(12):2311–2323. doi: 10.1016/j.ejso.2020.08.006. [DOI] [PubMed] [Google Scholar]
- Raspé C., Flöther L., Schneider R., Bucher M., Piso P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur. J. Surg. Oncol. 2017;43(6):1013–1027. doi: 10.1016/j.ejso.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Postoperatieve pijn Richtenlijnendatabase, 2013 [Available from: https://richtlijnendatabase.nl/richtlijn/postoperatieve_pijn/postoperatieve_pijn_-_startpagina.html.
- Salicath J.H., Yeoh E.C., Bennett M.H. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8(8) doi: 10.1002/14651858.CD010434.pub2. Cd010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould M.K., Garcia D.A., Wren S.M., Karanicolas P.J., Arcelus J.I., Heit J.A., Samama C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e227S–e277. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten J.P., Oemrawsingh A., de Boer N.L., Verhoef C., Burger J.W.A., Madsen E.V.E., Brandt-Kerkhof A.R.M. Predictive Ability of C-Reactive Protein in Detecting Short-Term Complications After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Retrospective Cross-Sectional Study. Ann. Surg. Oncol. 2021;28(1):233–243. doi: 10.1245/s10434-020-08619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans M., van der Aa M.A., Lalisang R.I., Witteveen P.O., Van de Vijver K.K., Kruitwagen R.F., Sonke G.S. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol. Oncol. 2018;150(3):446–450. doi: 10.1016/j.ygyno.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Diamond I.R., Grant R.C., Feldman B.M., Pencharz P.B., Ling S.C., Moore A.M., Wales P.W. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Kuijpers A.M.J., Aalbers A.G.J., Nienhuijs S.W., de Hingh I.H.J.T., Wiezer M.J., van Ramshorst B., van Ginkel R.J., Havenga K., Heemsbergen W.D., Hauptmann M., Verwaal V.J. Implementation of a standardized HIPEC protocol improves outcome for peritoneal malignancy. World J. Surg. 2015;39(2):453–460. doi: 10.1007/s00268-014-2801-y. [DOI] [PubMed] [Google Scholar]
- Koole S., van Stein R., Sikorska K., Barton D., Perrin L., Brennan D., Zivanovic O., Mosgaard B.J., Fagotti A., Colombo P.-E., Sonke G., Driel W.J.V. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer. 2020;30(6):888–892. doi: 10.1136/ijgc-2020-001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.