Abstract
Stress-related disorders display differences at multiple levels according to sex. While most studies have been conducted in male rodents, less is known about comparable outcomes in females. In this study, we found that the chronic restraint stress model (2.5 h/day for 14 days) triggers different somatic responses in male and female adult rats. Chronic restraint produced a loss in sucrose preference and novel location preference in male rats. However, chronic restraint failed to produce loss of sucrose preference in females, while it improved spatial performance. We then characterized the molecular responses associated with these behaviors in the hippocampus, comparing the dorsal and ventral poles. Notably, sex- and hippocampal pole-specific transcriptional signatures were observed, along with a significant concordance between the female ventral and male dorsal profiles. Functional enrichment analysis revealed both shared and specific terms associated with each pole and sex. By looking into signaling pathways that were associated with these terms, we found an ample array of sex differences in the dorsal and, to a lesser extent, in the ventral hippocampus. These differences were mainly present in synaptic TrkB signaling, Akt pathway, and glutamatergic receptors. Unexpectedly, the effects of stress on these pathways were rather minimal and mostly dissociated from the sex-specific behavioral outcomes. Our study suggests that female rats are resilient and males susceptible to the restraint stress exposure in the sucrose preference and object location tests, while the activity of canonical signaling pathways is primarily determined by sex rather than stress in the dorsal and ventral hippocampus.
Keywords: Chronic restraint stress, Neuroplasticity, Hippocampus, Sex, Transcriptomics
Abbreviations: NOR, novel object recognition task; OLT, object recognition test; CRS, chronic restraint stress; LTP, long-term potentiation; CUMS, chronic unpredictable mild stress; CORT, corticosterone; OFT, open field test; FC, female control; MC, male control; FS, female chronic stress; MS, male chronic stress; IPA, Ingenuity Pathway Analysis; RRHO, Rank-rank hypergeometric overlap; CaMKIIα, calcium/calmodulin-dependent protein kinase type II subunit α
1. Introduction
Several diseases display sex differences in their prevalence, onset, severity, and effectiveness of pharmacological treatments. For example, depressive and anxiety disorders are nearly twice as prevalent in females, and are characterized by their association with stress, especially with psychosocial stress (Kessler et al., 1993; McCarthy, 2016). Despite the documented sex differences in the incidence of stress-related neuropsychiatric disorders, there is not much basic research in animals that compares the effects of stress between male and female brains.
Some reports have indicated that female rats and mice show less anxiety-like behavior than males under non-stressful behavioral tasks (Donner and Lowry, 2013). However, upon chronic stress exposure, anxiety-like behaviors consistently increase in male rats (Ulloa et al., 2010); whereas the responses in female rats are more diverse, probably depending on the type of task employed, the stressor applied, and estrous cycle (ter Horst et al., 2012). Normally, male rats and mice learn faster in radial arm and Morris water mazes, but show impairments in spatial learning and memory after chronic stress; in contrast, chronically stressed females display improved spatial performance (Bowman et al., 2001; Luine et al., 2017). Some reports using chronic stress models in male rodents have show altered behavior associated with dendritic retraction and reduction of spine density, both in glutamatergic neurons of the hippocampal CA region (Pittenger and Duman, 2008; Castaneda et al., 2015; Garcia-Rojo et al., 2017) (reviewed in (Qiao et al., 2016)). In contrast, chronically stressed female rats fail to show dendritic retraction at any estrous phase, and insted display an increase in mature spines in CA1 neurons (McLaughlin et al., 2010). Furthermore, long-term potentiation (LTP) –one of the main models of synaptic plasticity underlying learning and memory– is more difficult to induce in females than males (Woodburn et al., 2021), but is favored during proestrus (Warren et al., 1995).
The differences in dendritic spine density and LTP detected between female and male sexes –both under basal conditions and chronic stress– may be associated with variations in the expression of different subtypes of glutamate receptors. A study in hippocampal extracts indicated that female rats have lower levels of metabotropic glutamate receptors (mGluR1 and mGluR2), but higher levels of the NMDA receptor (NMDAR) subunit GluN2B, compared with males (Ning et al., 2017). Furthemore, another study in whole hippocampal extracts found that chronic unpredictable mild stress (CUMS) induced a reduction in PSD95 (a neuronal PDZ protein), and the NMDAR subunits GluN2A and GluN2B in female rats; while CUMS reduced the levels of GluN1 (the mandatory subunit of NMDARs) and increased GluN2B levels in males (Ning et al., 2017). Importantly, one confounding variable rarely considered in molecular studies is the molecular and functional heterogeneity of the hippocampus along its longitudinal axis. Lesion studies in rats have indicated that the dorsal hippocampus (septal portion) is responsible for cognitive functions (learning, memory and spatial navigation); while the ventral hippocampus (temporal portion) participates in the control of hedonic and anxious behaviors, and negative regulation of the neuroendocrine stress axis (Fanselow and Dong, 2010; Strange et al., 2014). However, most of the biochemical studies have mainly considered either whole hippocampus or DH and, subsequently, have not allowed the comparison between the two poles of the hippocampus. Furthermore, the effects of stress on signaling pathways relevant for neuroplasticity across the brain have been extensively described for males, but not for female rodents (Lin et al., 2009).
Using the chronic restraint stress (CRS) model, we explored the interaction between sex as a biological variable and CRS on behavior, transcriptomic signatures by RNA sequencing (RNA-seq), and synaptic regulatory molecular pathways in the dorsal and ventral hippocampus, separately. We hypothesized that sex differences in the transcriptional profiles of the dorsal and ventral hippocampus, along with specific changes in signaling pathways, may correlate with the differences triggered by CRS in novel location and sucrose preference. Our findings unveiled that sex differences in CRS-induced behavior are related to both specific transcriptional signatures and neurotrophic signaling pathways in DH and VH.
2. Material and methods
2.1. Experimental design and animals
Adult Sprague-Dawley rats were obtained from the animal facility of the Faculty of Chemical and Pharmaceutical Sciences of Universidad de Chile. Efforts were made to minimize the number of animals and their suffering. All procedures were performed according to the protocols established and approved by the Institutional Committee for Animal Care and Use (CICUA) of Universidad de Chile (CBE approval codes: CBE2018-04, CBE2019-01, 19309-CYQ-UCH, 20397-CYQ-UCH) and the Agencia Nacional de Investigación y Desarrollo (ANID), in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication, 8th Edition, 2011). Newborn rats were assigned to female or male sex according to external genitalia. Adult male and female Sprague-Dawley rats weighing 300–350 g and 240–280 g at the beginning of the procedures, respectively, were housed in groups of 3–4 per cage (all assigned to the same experimental group). The rats were maintained in separate rooms by both sex and stress, with controlled temperature (22–23 °C), humidity (45%), and standard light:dark cycle (12 h:12 h). We employed a 2 × 2 between-subject factors design to explore the effects of sex (female or male) and stress (control or restraint). Three different cohort of rats were employed: one cohort was exclusively used for behavioral analysis (females: control = 10, stress = 10; males: control = 10, stress = 10); a second cohort for RNA-seq studies (females: control = 5, stress = 5; males: control = 5, stress = 5); and a third cohort for synaptoneurosome preparation and western blotting (females: control = 7, stress = 6; males: control = 7, stress = 9).
Food (Prolab® RMH 3000, LabDiet, St. Louis, MO, USA) and filtered tap water were freely provided, except during restraint stress sessions. Food consumption was daily monitored per cage during all experimental procedures by placing 200 g of rat chow onto the food cage each morning and weighting the remaining food the next morning. The cage bedding was routinely changed every 4 days. All rats were weighed once per day for 15 days prior to and during the experimental procedures. In the case of female rats, vaginal smears were daily collected (8–10 a.m.) to determine the estrous cycle stage according to published guidelines (Cora et al., 2015). The procedure consisted in a gentle and quick restraint of the rat, followed by applying 0.3 mL of filtered tap water into the vaginal canal using a plastic Pasteur pipette, and finally 2–3 flushing to collect cells. Then, a small amount of the cell suspension was added onto a microscope slide and immediately visualized under a light microscope with 10X magnification. The estrous cycle stage was assigned by the abundance and types of cells present across several fields of the slide (Fig. S1). Female rats displaying regular 3–5 days estrous cycles only were used for subsequent experiments.
At the end of experimental procedures, animals not assayed in behavioral tests were euthanized by decapitation for brain dissection and a sample of trunk blood was immediately collected for analyzing serum corticosterone (CORT) levels. CORT levels were determined by ELISA (Enzo Life Sciences, NY, USA; Cat. ADI-900-097) following the manufacturer's instructions. Additionally, bilateral adrenal glands were dissected, weighted, and the bilateral weight average was normalized to the animal weight at the endpoint.
2.2. Experimental and restraint stress procedures
In order to use samples for other studies that could include administration of drugs, both control and stressed animals were daily injected subcutaneously with propylenglycol (1 μl/g of weight), prior to every stress session. During stress sessions, both female and male controls were left undisturbed in their homecages. The animals assigned to the stress groups were positioned into ventilated size-matched acrylic tubes to restrict their movement during 2.5 h per day (tube diameter: 80 mm diameter for males and 55 mm for females). Then, the tubes were horizontally placed in pairs inside a clean cage with the two animals orientated back-to-back. Restraint sessions were conducted during the morning (between 9:00 a.m. and 12:00 p.m.) to avoid circadian rhythm influences. During restraint sessions, the animals did not have access to water and food, and were returned to their homecages immediately after the procedure. In the case of males, restraint sessions were performed every day for 14 consecutive days, as previously described (Castaneda et al., 2015). Considering that estrous cycling may influence the outcomes in females, we controlled for this variable by choosing the estrus stage as the endpoint, due to the low levels of circulating estrogens, hence minimizing the acute neuromodulatory effects of estrogens (reviewed in (Taxier et al., 2020)). In order to reproduce the 14 days chronic stress protocol in females –while also considering 4 days estrous cycles lengths in average– the start of the restraint protocol was performed during metestrus or diestrus, and was continued for 13–15 days (depending on individual cycle lengths). This timeline allowed a high probability of finding a proestrus in the day 14 of restraint and, therefore, an estrus on day 15 (24 h after the last stress session).
2.3. Fecal pellet output
To assess the stress response to restraint sessions, the number of fecal pellets produced by each animal during the 2.5 h restraint interval was measured every stress session, and an average of all sessions was calculated per animal. In the case of control animals, and considering that individual housing may produce stress, the average number of pellets produced by grouped animals per cage was obtained at the end of a 2.5 h interval, everytime the bedding was changed (ie. every 4 days). Of note, the timing of these measurments in control animals was coincident with the 2.5 h of restraint sessions. Each time the measurement was performed was counted as a single datapoint; therefore, replicas represent distinct measurements.
2.4. Behavioral studies
To explore the effects of sex and chronic restraint on different behaviors, we employed a battery of tests that allowed repeated testing in a single cohort of animals. The behavioral tests used were, in chronological order: open field test (OFT), sucrose preference test (SPT), and object location/novel object recognition tests (OLT/NORT). The timeline of testing is depicted in Fig. 2A. All tests were performed in the morning during the light phase of the photoperiod (9–14 h), 24 h after a stress session and prior to any restraint session, to avoid acute effects of stress. In males, OFT was performed on day 13, 24 h after the last stress session. On day 15, 24 h after the last restrain, males were probed for SPT, followed by OLT/NORT. In females, the day of testing depended on individual cycles lengths. Since estrus was selected as the endpoint, OFT was performed always in diestrus on day 12–14, and SPT followed by OLT/NORT were performed always in estrus on day 14–16.
Fig. 2.
Anhedonic-, anxiety-like behaviors and memory performance following chronic stress exposure. (A) The timeline shows the day in which the behavioral tests were performed. (B) Sucrose consumption test. Bar graphs indicate the preference for 1% sucrose instead of drinking water, as a percentage of the total fluid intake in control and stressed animals. A decrease in sucrose preference was observed only in stressed males compared to controls. n = 9–10 rats per group. (C) Object location and (D) Object recognition tests are related to novelty discrimination. The data are represented as a discrimination index. Chronic stress decreased the discrimination index of object location in males, and increased it in females. n = 9–10 rats per group. (E) The open field test was conducted to evaluate anxiety-like behaviors. Representation of animal trajectory of controls and stressed animals in an arena. (F) Quantitative analysis of animal behavior in the arena, represented as time spent at center, (G) number of crossings and (H) path length (n = 9–12 rats per group). Basally, females exhibited fewer crosses and a shorter path length than males. All graphs show individual datapoints, and bars represent the mean ± SEM. Data were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Full statistical details are shown in Table S2.
2.4.1. Open field test (OFT)
The first habituation session to the OLT/NORT arena was considered as an open field test. Each rat was placed inside a squared arena (60 × 60 cm) facing one corner. The trajectory of the rat in the arena was recorded during 10 min and analyzed using the free software Tracker (https://physlets.org/tracker/). For quantification, the arena was divided in 9 equal squares of 20 × 20 cm: the central square was defined as the center, the 4 corner squares as the corners, and the remaining 4 wall squares as the walls.
2.4.2. Sucrose preference test
To evaluate anhedonic-like behavior, we performed the sucrose preference test, which evaluates the preference for sweet-flavored water over tap water. Briefly, the rats were introduced to the sucrose solution by placing each rat in individual cages with two identical bottles containing 1% sucrose. All animals were trained for 3 h each day for 7 consecutive days prior to the first stress session. In male rats, the test was performed at day 15, 24 h after the last stress session. In female rats, the test was performed in estrus on day 14–16, depending on cycle lengths. During the test, rats were given a free choice between two bottles, one filled with 1% sucrose and the other filled with tap water. The mass of the bottles was measured before and after the test, and the position of the bottles was randomly switched to prevent side preference in drinking behavior. Sucrose preference was calculated as a percentage of the total amount of liquid ingested.
2.4.3. Object location task (OLT) and novel object recognition task (NORT)
To assess the effect of stress on hippocampal-dependent memory, we performed the object location task (OLT), followed by the novel object recognition task (NORT) (Barker and Warburton, 2011; Vogel-Ciernia and Wood, 2014). Prior to the test day, animals were habituated to the arena (60 × 60 cm square) once daily for two consecutive days. The habituation sessions consisted in placing the rat in the arena facing the same corner each time, and allowing the animal to explore the arena for 15 min. On the test day, rats were submitted to the training phase of OLT, in which two identical objects were located at adjacent corners of one side of the arena. Then, the rat was placed facing the same corner of this arena and was allowed to explore both objects for 5 min. After a delay of 5 min, the testing phase began by placing one of the two objects in the original position and the other one in a diagonal corner, allowing the rats to explore for 5 min. After a second delay of 5 min, the NORT phase began by placing the same object in the novel location of the OLT and a new different object in the OLT familiar location, allowing the rats to explore for 5 min. The position of the moved object and the entry corner were counterbalanced across rats. Each phase was videorecorded and then analyzed by two different observers blinded to the experimental groups. In male rats, the habituations were performed on days 13 and 14, and the OLT/NORT test on day 15, 24 h after the last stress session. In female rats, the habituation sessions were conducted on diestrus on day 12–14 and day 13–15, followed by the OLT/NORT testing in estrus on day 14–16, depending on cycle lengths. Data were expressed as a discrimination index (DI) determined with the formula: DI = (time spent exploring the object in a novel location − time spent in exploring object in a familiar location)/(total exploration time) for OLT; and DI = (time spent in exploring the novel object − time spent in exploring the familiar object)/(total exploration time) for NORT. The DI values range between −1 and 1; where a negative value indicates a preference for familiar location or object, and a positive value represents a preference for exploring either the object in a novel location or the new object.
2.5. RNA sequencing (RNA-seq) and bioinformatic analyses
For RNA sequencing studies, an independent cohort of animals was used. Twenty-four hours after the last stress session or procedure, bilateral dorsal and ventral hippocampi were dissected from rats quickly after decapitation using RNase-free implements at 4 °C. The dorsal and ventral portions of the hippocampus were defined by their position relative to dorsal-ventral and rostral-caudal axes. Whole hippocampi were quickly placed over an ice-cold Petri dish and dissected the top 2/6 as dorsal hippocampus, the bottom 3/6 as ventral hippocampus, and the middle portion was discarded. The dissected hippocampi were flash-frozen in liquid nitrogen and kept at −80 °C until processing. Then, RNAs were isolated from dorsal and ventral hippocampi as we have previously described (Castaneda et al., 2015), using the RNeasy Mini Kit (Qiagen, California, USA). Five biological replicates were obtained from each group: female control (FC), female chronic stress (FS), male control (MC), male chronic stress (MS). Only the fraction of RNAs longer than 200 nucleotides was used for subsequent sequencing studies. The concentration of the samples was assayed by Qubit™ RNA BR Assay Kit (Invitrogen). The samples held RNA Integrity Numbers (RIN) between 8 and 10, as assessed by the Bioanalyzer system (Agilent Technologies). Ribosomal RNA depletion, cDNA library generation and 150-bp paired end sequencing procedures were performed in the Beijing Genomics Institute (BGI) using the DNBseq platform. At least 40 million clean reads of sequencing depth were obtained for each sample. RNA-seq data was preprocessed using Trim Galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) to remove low-quality bases, and high-quality reads were aligned against the rat reference genome (UCSC/rn6) using Hisat2 (Kim et al., 2019). Then, reads assignment and counting were performed using FeatureCounts from the Subread package (Liao et al., 2014). The resulting count matrices were processed using DESeq2 (Love et al., 2014) to obtain the differentially expressed genes by sex (female control vs male control; female stress vs male stress) and stress (females stress vs control; males stress vs control). Differentially expressed genes were defined as those whose p-values were less than 0.05.
Differentially expressed genes in each dataset were subsequently uploaded to the Ingenuity Pathway Analysis (IPA) software (QIAGEN Digital Insights). The IPA software was used for its ability to analyze mRNA data in the context of known biological response and regulatory networks. IPA returns a score for each network using a p-value based on Fisher's exact test. The software determines the probability that each biological function assigned to that data set was due to chance alone.
2.6. Rank-rank hypergeometric overlap (RRHO)
We compared the stress-induced transcriptional signatures of the dorsal and ventral hippocampus within- and between-sexes using the RRHO2 package (Plaisier et al., 2010; Cahill et al., 2018). We used. the threshold-free expression datasets obtained from DEseq2, contrasting stress vs control for each sex and pole. The RRHO pipeline allows the visualization of overlap heatmaps, where pixels are plotted in one of four quadrants in order to determine the concordance and directional overlap between datasets. Heatmaps generated using RRHO2 have top-right (both decreasing) and bottom-left (both increasing) quadrants, representing the concordant mRNA changes, while the top-left and bottom-right quadrants represent discordant overlaps (opposite directional overlap between datasets). For each comparison, the stratified RRHO method was used on -log (p-values) with the default step size for each quadrant, and adjusted Benjamini-Yekuteli p-values were calculated to determine significant concordant and discordant overlaps.
2.7. Western blot of synaptoneurosome-enriched and whole homogenate fractions from rat hippocampus
Animals of a third independent cohort not tested for behavioral analyses were euthanized 24 h after the last stress session or procedure and bilateral dorsal and ventral hippocampi were obtained as described above for RNA-seq studies. The hippocampi were processed to obtain protein extracts from hippocampal whole homogenates and synaptoneurosome-enriched fractions (Fig. S2), as we have reported (Aguayo et al., 2018a, 2018b; Roman-Albasini et al., 2020). Western blot was performed as we have previously described (Aguayo et al., 2018a, 2018b; Roman-Albasini et al., 2020). Briefly, 30 μg or 15 μg of protein of whole hippocampal homogenates or synaptoneurosomes, respectively, were resolved in 10% SDS-polyacrylamide gels. Proteins were then electroblotted onto 0.2 μm nitrocellulose membranes. Membranes were finally processed for Western blot, according to the conditions depicted in Table S1. Blots were then incubated with the appropriate HRP-conjugated secondary antibody: anti-rabbit IgG (Invitrogen, cat #31460) or anti-mouse IgG (Invitrogen, cat #31460). Membranes were developed by incubation with enhanced chemiluminescent substrate (Clarity Western ECL Substrate, Bio-rad, USA) and imaged with Syngene (Cambridge, UK). To determine the phosphorylation status of target proteins, blots incubated and developed with phospho-specific antibodies were stripped and reprobed for total target levels. Bands were quantified with the ImageJ software (https://imagej.nih.gov/ij), and normalized to β-actin immunoreactivity as a loading control, or to total target immunoreactivity in the case of phosphoproteins. To maximize the yield of protein samples, gels were often cut at different molecular weights and each blot fragment was probed individually with an antibody. Therefore, β-actin blots were often shared between different protein targets. To provide full transparency and avoid repetitive actin blots in the main figures, we included representative actin blots for each SDS-PAGE run in the supplement, where we indicate which targets in the main text figures were utilized as their respective loading controls (Fig. S3).
2.8. Statistical analysis
For most physiological, behavioral and Western blot data, two-way ANOVA (2 × 2) was utilized to explore the effects of sex, stress, and their interaction (sex × stress). Planned comparisons (female control vs male control; female control vs female stress; male control vs male stress; and female stress vs male stress) were performed with Fisher's test, whenever a significant main effect or interaction was detected. All the computed F- and p-values of two-way ANOVA are reported in the supplementary material (Table S2), while key significant effects are also reported in the main text and figures. For each determination, outliers were defined as those exceeding the mean ± two standard deviations. To determine the effect of restraint stress in the length of the estrous cycle, the non-parametric Mann-Whitney test was used. The statistical software GraphPad Prism (GraphPad Software Incorporated, V. 8.0.2) was used for all analyses.
3. Results
3.1. Females and males are sensitive to chronic stress according to physiological parameters
First, we validated the stress exposure in both female and male rats by assessing physiological parameters. During the stress response, centrally produced corticotropin-releasing hormone triggers an activation of colonic motility, increasing the fecal pellet output (Tache and Million, 2015). Accordingly, we determined the mean value of feces evacuated in 2.5 h during the 14 days of stress treatment. In comparison to control animals, stressed animals displayed increased fecal pellet output (stress: F(1,73) = 52.78, p < 0.0001; Fig. 1A). Additionally, stressed females defecated more pellets that males (sex: F(1,73) = 4.499, p = 0.0373). Chronic stress exposure is known to diminish weight gain in male rodents. To get a global effect of the stress response, we compared the body weight changes at the treatment endpoint, expressed as the percentage of baseline for each rat. We noted that males gained more weight than females (sex: F(1,79) = 50.45, p < 0.0001; Fig. 1B). Furthermore, we observed that chronic stress reduced weight gain in males, but not in females (sex × stress: F(1,79) = 9.789, p = 0.0025; Fig. 1B). Next, we evaluated the cumulative 24 h food consumption during the 14 days of procedures. Food consumption during treatments was significantly higher in male than female rats (sex: F(1,28) = 49.55, p < 0.0001; Fig. 1C). Moreover, only stressed females displayed a reduction in food consumption, compared to sex-matched controls (stress: F(1,28) = 9.065, p = 0.0055; Fig. 1C). Interestingly, the reduced weight gain observed in stressed males compared to controls was not related to decreased food consumption, and probably responds to a stress-induced metabolic abnormality (triggered, for instance, by corticosterone). Additionally, we did not observe stress-induced variations in estrous cycling (Mann-Whitney U = 215, two-tailed p = 0.3951; Fig. 1D). To evaluate HPA axis changes produced by chronic stress, we determined both adrenal gland weights and unstimulated corticosterone levels. We found that the normalized adrenal weight was higher in females than males (sex: F(1,41) = 115.7, p < 0.0001; Fig. 1E), and was increased by chronic stress only in females (stress: F(1,41) = 4.479, p = 0.0404; Fig. 1E). Finally, the corticosterone serum levels were not affected neither by stress nor sex (Fig. 1F). Altogether, these results show that the fecal pellet output is increased by chronic stress in both females and males, but only stressed males show a body weight gain variation under stress. Moreover, adrenal gland weight and food consumption are basally determined by sex and only stressed females displayed variations in these parameters.
Fig. 1.
Female and male rats are sensitive to chronic restraint stress. (A) Fecal output was determined in control cages (2.5 h) and stressed animals during the stress session. Restraint stress increased fecal output in both males and females (n = 16–23 rats per group). (B) Variation of body weight at the end of the 14-day treatment. Chronic stress decreases weight gain only in males (n = 19–23 rats per group). (C) Cumulative food consumption. Males exhibited higher food intake than females regardless of the experimental condition, while stress decreased food intake only in females (n = 7–9 cages per group). (D) Effect of chronic restraint stress on the estrous cycle. Vaginal smears were taken to determine the progression of the estrous cycle during the treatments (n = 21–24 rats per group). (E) Adrenal gland weight of control and stressed animals were normalized to body weight. This ratio was higher in females than males, despite the experimental condition. Stress decreased this ratio in females (n = 10–12 rats per group). (F) Serum corticosterone levels (8 a.m.) in control and stressed animals (n = 10–12 rats per group). All graphs show individual datapoints, and bars represent the mean ± SEM. Data in A, B, C, E and F were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Full statistical details are shown in Table S2. In D, conditions were compared by the Mann-Whitney test (ns).
3.2. Sex-dependent changes in sucrose preference, memory and anxiety-like behaviors following two weeks of restraint stress
We have previously reported that CRS produces depressive- and anxiety-like behaviors in male rats (Bravo et al., 2006; Ulloa et al., 2010; Castaneda et al., 2015; Garcia-Rojo et al., 2017), accompanied by a loss in novel location preference in the OLT (Roman-Albasini et al., 2020). To determine whether female rats displayed the same behavioral susceptibility to this stress paradigm, we conducted a battery of behavioral test in both female and male rats (Fig. 2A). The sucrose preference test was used as a readout of anhedonic-like behavior at the treatment endpoint (Fig. 2A and B). We observed a strong sex-dependent effect of stress on sucrose preference 24 h after the last stress session (sex × stress: F(1,33) = 15.85, p = 0.0004; Fig. 2B). Notably, while both female and male controls showed sucrose preference (∼80%), chronic stress ablated sucrose preference in male rats (∼30%), while sucrose preference was unaffected by CRS in female rats (Fig. 2B). These results show that chronic restraint stress induces loss of sucrose preference only in male rats.
Next, to evaluate the effects of chronic stress on contextual memory, we submitted rats to the object location task (OLT), which strongly depends on the dorsal hippocampus (Barker and Warburton, 2011); followed by the novel object recognition task (NORT), which relies on the perirhinal cortex (Barker and Warburton, 2011). In the OLT, female controls displayed a lower DI than male controls (Fig. 2C). Furthermore, we observed a sex-divergent effect of stress on the OLT discrimination index (sex × stress: F(1,33) = 14.48, p = 0.0006; Fig. 2C). Indeed, while stress increased the discrimination index in females, it decreased the discrimination index in males (Fig. 2C). Additionally, the total exploration time was comparable between groups. In the NORT, we observed that female and male controls could identify a novel object similarly, without any effect of stress (Fig. 2D). These results indicate that, while female controls do not discriminate in the OLT, stressed females display preferred exploration of the novel location over the familiar one. Conversely, CRS triggers a loss of this spatial ability in males. These observations suggest that chronic stress triggers an enhancement and detriment of spatial memory in female and male rats, respectively. In contrast, novel object recognition is insensitive to restraint stress and both female and male rats perform similarly.
Since a first introduction to the OLT/NORT arena is required prior to these tests, we considered this instance as an opportunity to measure open field test parameters. Representative trajectories from each group are shown in Fig. 2E. Time spent at the center of the arena did not display significant differences (Fig. 2F). On the other hand, the number of crossings through the center of the arena was larger in female than male controls (sex: F(1,36) = 9.746, p = 0.0035; Fig. 2G). Additionally, the path length was also larger in female than male controls (sex: F(1,37) = 9.011, p = 0.0048; Fig. 2H). However, no effect of stress was observed in both parameters (Fig. 2G and H). These open field parameters suggest that neither stress nor sex influence anxiety-like behavior. Of note, further tests aimed at assessing anxiety-like behaviors should help establish a more robust conclusion.
Overall, these results suggest that female rats are resilient against chronic restraint stress in terms of sucrose preference and spatial memory; whereas male rats display a susceptible endophenotype.
3.3. Chronic restraint stress triggers sex-dependent transcriptional profiles in the dorsal and ventral hippocampus
Considering that chronic restraint stress produced rather distinct behavioral outcomes in female and male rats, we next sought to unveil molecular correlates that could explain these differences. Moreover, these behavioral tests may involve either directly or indirectly the dorsal (spatial memory) and ventral (anhedonic-like behavior) hippocampal formation (Fanselow and Dong, 2010; Strange et al., 2014). Thus, we utilized RNA-seq to determine the transcriptomic profiles that result from chronic restraint in the dorsal and ventral hippocampus, separately, in both female and male rats (Fig. 3A). An independent cohort of animals not submitted to behavioral test was used (Fig. 3A). We observed a notable sex-specific transcriptional profiles of stress-responsive genes in both the dorsal and ventral hippocampus (Fig. 3B and C, respectively). In the dorsal hippocampus, we detected 1315 and 442 unique stress-sensitive genes in female and male rats, respectively, with only 37 (∼2%) differentially expressed genes shared between female and male rats (Fig. 3B, right). When stress-sensitive genes were ordered by fold change for either sex, rather discordant distributions of the gene sets were observed (Fig. 3B, left). When considering fold change direction, females displayed a larger set of upregulated (689) than downregulated (663) stress-sensitive genes in the dorsal hippocampus. Similarly, males had a greater number of upregulated (254) than downregulated (225) genes. Notably, of the 37 shared stress-sensitive genes between the female and male dorsal hippocampus, 24 were in the same change direction (8 downregulated and 18 upregulated).
Fig. 3.
Female and male rats express different transcriptional responses in the dorsal and ventral hippocampus after chronic stress. (A) Timeline representing the endpoint for RNA-seq studies and the relationship between the hippocampal poles with behavioral analyses. (B) Heatmap and Venn diagram of genes differentially regulated by chronic stress in the dorsal female hippocampus, compared with genes differentially expressed in males. (C) Heatmap and Venn diagram of genes differentially regulated by chronic stress in the ventral male hippocampus, compared with genes differentially expressed in males. RNA-seq was performed on n = 5 rats of each sex and group. Differentially expressed RNAs were defined as those with p < 0.05.
Similar sex asymmetries were observed for the stress-responsive genes in the ventral hippocampus (Fig. 3C). Indeed, 300 and 480 unique genes were differentially expressed in the female and male ventral hippocampus, respectively, and only 19 (∼2.4%) were overlapped between females and males (Fig. 3C, Venn diagram). The comparison by fold change also indicated striking sex-specific transcriptional profiles (Fig. 3C, heatmaps). Moreover, the transcriptional profile of females was shifted toward upregulation vs downregulation (176 vs. 143). Conversely, the stress-sensitive genes of the male ventral hippocampus were trended toward downregulation vs upregulation (252 vs. 247). Lastly, of the 19 genes sensitive to chronic stress in both the female and male ventral hippocampus, 12 exhibited concordant change directions (7 upregulated and 5 downregulated). Overall, these results evidence a rather specific transcriptional response to chronic restraint stress according to sex in both the dorsal and ventral hippocampus.
3.4. Identification of statistically significant overlaps between gene expression signatures of the dorsal and ventral hippocampus
To identify putative significant overlaps of the transcriptional signatures produced by chronic restraint stress between the dorsal and ventral hippocampus for each sex, we conducted a rank-rank hypergeometric overlap (RRHO) test. In females, we detected a significant coherence of co-upregulated and co-downregulated genes between the hippocampal poles (Fig. 4A, left). In contrast, in males, a significant overlap was detected in downregulated genes between the dorsal and ventral hippocampus (Fig. 4A, right). These within-sex overlaps suggest that only genes that are concordant between the dorsal and ventral hippocampus display significant overlap. Next, we used RRHO to compare the gene expression patterns produced by chronic stress, according to both sex and hippocampal region (Fig. 4B). Notably, only a major overlap was observed between upregulated genes in the female ventral and male dorsal hippocampus (Fig. 4B, bottom right), while the other comparisons revealed little or no overlap. These between-sexes analyses evidence that significant overlaps only occur between co-expressed genes of the female ventral and male dorsal hippocampus, while the rest of the stress-induced transcriptional profiles are specific for each sex and hippocampal pole.
Fig. 4.
Rank-rank hypergeometric overlap (RRHO) of the dorsal and ventral hippocampal transcriptional signatures induced by chronic restraint. The transcriptional profiles induced by chronic restraint were compared (A) within each sex and (B) between hippocampal poles and sexes, to identify concordant and discordant significant overlaps of gene expression in a threshold-free manner. Each pixel represents the degree of overlap according to a hypergeometric test, where the adjusted p-value is determined by the color (warmer colors indicate increased significance). Higher overlaps were observed for concordant changes between the female dorsal and ventral hippocampus, and co-downregulated signatures between the male dorsal and ventral hippocampus. The between-sexes comparisons revealed a significant overlap between the co-upregulated profiles of the female ventral and male dorsal hippocampus. RRHO analysis was performed on RNA-seq samples (n = 5 rats of each sex and group). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. The sex-specific transcriptional signatures produced by chronic stress in the rat dorsal and ventral hippocampus are related to distinct physiological functions
In order to discover putative functional roles of the chronic stress-induced sex-specific transcriptional signatures in both the dorsal and ventral hippocampus, we analyzed differentially expressed genes by means of the Ingenuity Pathway Analysis (IPA, Fig. 5). Enrichment analyses of DEGs revealed important signaling pathways that may be significantly influenced by chronic stress, mostly in a sex- and hippocampal pole-specific manner.
Fig. 5.
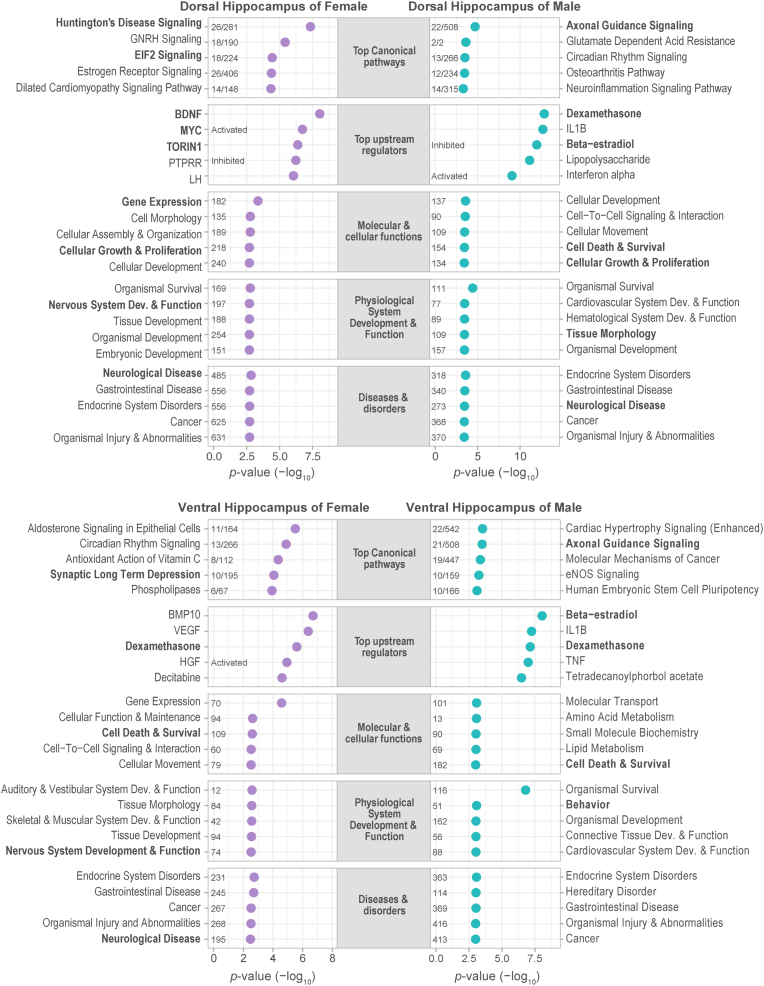
Summary of canonical pathways, upstream regulators, related diseases, and physiological function of the sex-specific transcriptional signatures of chronic stress. The functional enrichment analysis was conducted using the Ingenuity Pathway Analysis™ software. p-values for the overlap between differentially expressed genes and each term geneset was calculated by the Fisher's exact test. Where applicable, the numbers along each bubble represent the number of overlaps.
In the dorsal hippocampus, the female top canonical pathways included Huntington's disease and the initiation of translation by eIF2 signaling. On the other hand, the top pathways of the male dataset were related to axonal guidance, neuroinflammation and circadian rhythm. Furthermore, among the top predicted upstream regulators, BDNF and the mTOR inhibitor Torin-1 (Audet-Walsh et al., 2017) were found for the stress-responsive genes of the female dorsal hippocampus; whereas dexamethasone (a glucocorticoid receptor agonist) and beta-estradiol were enriched for the male dataset. When gene sets were categorized according to various ontologies, the female set contained genes enriched for gene expression, nervous system development and function, and neurological diseases (Fig. 5). In contrast, the stress-sensitive genes of the male dorsal hippocampus were related to cell growth and proliferation, cell death and survival, tissue morphology, and neurological diseases (Fig. 5).
Furthermore, the IPA analysis conducted with the stress-responsive genes of the ventral hippocampus also revealed interesting sex differences and similarities. Within the top canonical pathways, the female dataset was enriched for aldosterone signaling, circadian rhythm and synaptic long-term depression; whereas axonal guidance signaling was enriched in males (Fig. 5). Notably, glucocorticoids may be putative upstream regulators for both female and male stress-induced genes in the ventral hippocampus, given the enrichment of the dexamethasone geneset in both female and male datasets. Furthermore, among the enriched functional ontologies, cell death and survival was detected in both females and males. In contrast, other enriched ontologies were specific for each sex. For instance, nervous system development and function, and neurological diseases were enriched for the stress-responsive genes in the female ventral hippocampus, while the behavior geneset was enriched in males.
When contrasting enriched terms between the dorsal and ventral poles for each sex, some terms were overlapped. For example, development and function of the nervous system, along with neurological disease, were shared between the female dorsal and ventral enriched terms. On the other hand, a larger number of shared terms between the dorsal and ventral hippocampus was found for males (e.g., axonal guidance, dexamethasone, beta-estradiol, and cell death and survival). Despite these similarities, our results globally indicate specific functional enrichments according to sex and the hippocampal pole.
3.6. Sex and stress determine TrkB signaling in the dorsal, but not ventral hippocampus of rats
Considering that one of the upstream regulators suggested by IPA was BDNF, we evaluated changes in TrkB signaling in synaptoneurosomes isolated from the dorsal and ventral hippocampus (Fig. 6). In the dorsal hippocampus, phosphorylation of the full-length (FL) TrkB receptor at Tyr817 was increased by stress only in males (sex: F(1,18) = 13.74, p = 0.0016; Fig. 6B). Notably, the levels of FL-TrkB were significantly higher in the dorsal hippocampus of females compared to males, irrespective of stress (sex: F(1,19) = 38.04, p < 0.0001; Fig. 6C). Moreover, the levels of a truncated form of TrkB (T-TrkB) were reduced by stress in the male but not female dorsal hippocampus (sex × stress: F(1,18) = 5.302, p = 0.0334; Fig. 6D). We also evaluated the phosphorylation status of calcium/calmodulin-dependent protein kinase type II subunit α (CaMKIIα), a downstream effector of TrkB. Phosphorylation of CaMKIIα at Thr286 (an autophosphorylation site, which renders a constitutively active kinase) was increased in the dorsal region only in stressed males compared to male controls (stress: F(1,18) = 8.009, p = 0.0111; Fig. 6E); whereas CaMKIIα total levels were unaffected (Fig. 6F). These results suggest an overall reduced activity of TrkB-CaMKIIα signaling in the male dorsal hippocampus compared to females, independently of stress. However, this pathway seems to be activated by chronic stress in the male dorsal hippocampus, perhaps caused by reduced levels of the signaling-incompetent truncated form of TrkB.
Fig. 6.
Sex and stress determine TrkB signaling in the dorsal, but not ventral hippocampus. Representative Western blots of targets evaluated in synaptoneurosomes from the (A) dorsal and (G) ventral poles. pTyr817-TrKB relative to full-length (FL) TrkB in (B) dorsal and (H) ventral hippocampus. Restraint stress increased TrKB phosphorylation only in the male dorsal hippocampus. (C, I) Males exhibited lower FL-TrkB levels than females in the dorsal hippocampus, regardless of the experimental condition. (D, J) A decrease in truncated (T)-TrkB was observed in both dorsal and ventral hippocampal poles of stressed males, compared to controls. (E, K) CaMKIIα phosphorylation at Thr286 increased in the dorsal hippocampus of stressed males, compared to controls. (F, L) CaMKIIα levels decreased in the ventral hippocampus of stressed males, compared to controls. In females, both CaMKIIα phosphorylation and total levels were insensitive to stress. Total protein levels were normalized using β-Actin (TrkB: run 1; CaMKIIα: run2; Fig. S3). All graphs show individual datapoints, and bars represent the mean ± SEM. Data were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ****p < 0.0001). Full statistical details are shown in Table S2n = 5–6 per group.
Similar determinations were conducted in synaptoneurosomes from the ventral hippocampus (Fig. 6G-L). While neither phosphorylation nor the total levels of the FL-TrkB receptor experienced variations (Fig. 6H and I), the levels of T-TrkB were reduced by chronic stress in the male but not female ventral hippocampus (sex × stress: F(1,19) = 4.687, p = 0.0433; Fig. 6J). On the other hand, CaMKIIα levels –but not its phosphorylation– were reduced by stress in the ventral hippocampus only in males (sex: F(1,18) = 6.505, p = 0.0201; Fig. 6L). These observations suggest that TrkB-CaMKIIα signaling may be more sensitive to sex and stress effects in the dorsal but not ventral hippocampus.
3.7. Akt signaling axis differs between the ventral and dorsal hippocampus of female and male rats
TrkB may also signal through the PI3K-Akt pathway. Members of this pathway also participate in numerous enriched pathways and processes according to IPA (for instance; eIF2 signaling, cell growth, proliferation, survival and death; Fig. 5). Thus, we next evaluated whether sex and stress modulate the Akt pathway in synaptoneurosomes of the dorsal and ventral hippocampus (Fig. 7). Upstream of Akt, the protein and lipid phosphatase PTEN acts as a negative regulator of the pathway, and its phosphorylation at Ser380 leads to an inhibition of its phosphatase activity. In the dorsal hippocampus, PTEN phosphorylation and total levels, along with Akt full-activity phosphorylation (Ser473) and total levels, were unaffected by sex and stress (Fig. 7B–E). Nonetheless, the Akt-mediated phosphorylation of GSK3β at Ser9 was higher in the dorsal hippocampus of females vs male controls (sex: F(1,18) = 10.00, p = 0.0054; Fig. 7F). On the other hand, GSK3β total levels were significantly higher in stressed males compared to stressed females (F(1,19) = 5.063, p = 0.0365; Fig. 7G). Another important downstream effector of the Akt pathway is the mTOR complex 1 (mTORC1), which is activated by phosphorylation of mTOR at Ser2448. The phosphorylation levels of mTOR variated according to sex and stress (sex × stress: F(1,17) = 10.26, p = 0.0052; Fig. 7H); whereby control females showed higher phosphorylation levels than control males, while stress triggered a reduction only in females (Fig. 7H). Moreover, mTOR total levels were higher in the dorsal hippocampus of stressed females than stressed males (sex × stress: F(1,19) = 7.894, p = 0.0112; Fig. 7I). However, mTORC1-mediated phosphorylation of the eukaryotic translation initiation factor 4E-binding protein (4E-BP1) at Thr37/46 and its total protein levels were insensitive to sex and stress (Fig. 7J–K). These results suggest that the Akt pathway may be more active in female than male controls, whereas chronic restraint effects seem negligible in the dorsal hippocampus.
Fig. 7.
The Akt signaling axis differs between the ventral and dorsal hippocampus of female and male rats. Western blot analyses from hippocampal synaptoneurosomes. Representative Western blots of (A) dorsal and (L) ventral hippocampal poles. (B, M) PTEN phosphorylation at Ser380. (C, N) Total PTEN levels. Basally, males exhibited higher levels of total PTEN than females in the ventral hippocampus. (D, O) AKT phosphorylation at Ser473. (E, P) Total Akt levels. (F, Q) GSK-3β Ser9 phosphorylation. Basally, females showed lower p-GSK-3β levels than males in the dorsal hippocampus. In the ventral pole, stress increased p-GSK-3β levels only in females. (G, R) Total GSK-3β. (H, S) mTOR phosphorylation at Ser2448. Basally, females showed higher p-mTOR levels than males in the dorsal hippocampus; however, restraint stress reduced this ratio only in females. (I, T) Total mTOR levels. (J, U) 4E-BP1 Thr37/46 phosphorylation. (K, V) Total 4E-BP1 levels. Basally, females showed lower 4E-BP1 levels than males in the ventral hippocampus; however, chronic stress increased 4E-BP1 levels only in females. Total protein levels were normalized using β-Actin (PTEN: run 3; Akt: run 4; mTOR: run 2; GSK and 4E-BP1: run 5; Fig. S3). All graphs show individual datapoints, and bars represent the mean ± SEM. Data were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ****p < 0.0001). Full statistical details are shown in Table S2n = 5–6 per group.
In the ventral hippocampus (Fig. 7L–V), phosphorylation of PTEN was not affected by sex nor stress (Fig. 7M). However, total PTEN levels were higher in male than female controls (sex: F(1,20) = 10.73, p = 0.0038; Fig. 7N). Neither Akt phosphorylation at Ser473 nor its total levels were influenced by sex or stress in synaptoneurosomes of the ventral hippocampus (Fig. 7O and P), although GSK3β phosphorylation was increased in females by chronic stress (stress: F(1,20) = 4.546, p = 0.0456; Fig. 7Q). Furthermore, total GSK3β levels were higher in stressed males compared to females (sex: F(1,20) = 5.812, p = 0.0257; Fig. 7R). Conversely, mTOR phosphorylation and its total levels, along with 4E-BP1 phosphorylation, were insensitive to sex and stress (Fig. 7S-U). Nonetheless, total 4E-BP1 levels were higher in control males than females (sex: F(1,19) = 5.860, p = 0.0257; Fig. 7V), and stress triggered a rise in its levels only in females (stress: F(1,19) = 7.023, p = 0.0158; Fig. 7V). Altogether, these data suggest that –in contrast to the dorsal hippocampus– the Akt pathway seems similarly active between female and male controls. However, the increase in GSK3β inhibitory phosphorylation is suggestive of a neuroprotective response to chronic stress in the female ventral hippocampus.
3.8. ERK-CREB signaling differs by sex in the dorsal and ventral hippocampus
Besides the PI3K-Akt pathway, the TrkB receptor also signals downstream via the ERK-CREB axis. Thus, we evaluated the phosphorylation status of ERK and CREB in whole hippocampal homogenates. In the dorsal hippocampus, the activating phosphorylations of ERK1-2 in Thr185/Tyr187 were higher in female controls than males (sex: F(1,20) = 13.54, p = 0.0015; Fig. 8B). On the other hand, total ERK1-2 levels were not affected by sex nor stress (Fig. 8C). Regarding CREB, we observed the same profile as ERKs; where CREB phosphorylation at Ser133 was higher in females than males, irrespective of stress (sex: F(1,19) = 12.89, p = 0.0019; Fig. 8D), and its total levels were similar across groups (Fig. 8E). In the ventral hippocampus, ERKs phosphorylation was insensitive to sex and stress, but its total levels were higher in female control than in males (sex: F(1,20) = 6.912, p = 0.0161; Fig. 8H). Similarly to the dorsal region, CREB phosphorylation was consistently higher in females than males in the ventral hippocampus (sex: F(1,20) = 32.68, p < 0.0001; Fig. 8I); however, this CREB phosphorylation profile was discordant with ERKs phosphorylation in the ventral hippocampus. These differences may be related to the lower total CREB levels in female than male controls (sex: F(1,20) = 4.573, p = 0.0450; Fig. 8J). As a whole, these data indicate that females express higher levels of pCREB in both hippocampal poles, suggesting a neuroprotective effect that was insensitive to stress.
Fig. 8.
ERK-CREB signaling in dorsal and ventral female and male hippocampus evaluated in whole homogenates. Representative Western blots of phosphorylated and total ERK1-2 and CREB levels in (A) dorsal and (F) ventral hippocampus. (B, G) ERK1-2 phosphorylation at Thr185/Tyr187. Females exhibited higher basal p-ERK1-2 levels than males in the dorsal hippocampus, and this ratio was insensitive to stress. (C, H) Total ERK1-2 levels. Females showed higher total ERKs levels in the ventral hippocampus than male controls. (D, I) p-Ser133-CREB. Females showed a higher p-CREB/total CREB ratio than males in the dorsal and ventral hippocampus, irrespective of the experimental condition. (E, J) Total CREB levels. Females exhibited higher basal CREB levels than males in the ventral hippocampus, irrespective of stress. Total protein levels were normalized using β-Actin (PTEN: run 3; Akt: run 4; mTOR: run 2; GSK and 4E-BP1: run 5; Fig. S3). All graphs show individual datapoints, and bars represent the mean ± SEM. Data were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ****p < 0.0001). Full statistical details are shown in Table S2n = 5–6 per group.
3.9. AMPA and NMDA glutamate receptors subunits are modulated by sex mainly in the dorsal hippocampus of rats
Until now, we have described that sex, stress and the hippocampal pole act in concert upon the activity of signaling pathways relevant to synaptic plasticity. In an effort to evaluate changes that could impact glutamatergic neurotransmission, we determined the levels of glutamate AMPA and NMDA receptor subunits in synaptoneurosomes. The AMPA receptor subunit GluA1 may be phosphorylated at Ser831 by CaMKII and PKC, increasing its conductance. In the dorsal hippocampus, pSer831-GluA1 displayed a strong interaction between sex and stress (sex × stress: F(1,18) = 16.75, p = 0.0007; Fig. 9B); where females experienced a reduction in GluA1 phosphorylation by stress, but the opposite effect was observed in males (Fig. 9B). Regarding total GluA1 levels, male controls displayed lower levels than females, which were further reduced by chronic stress only in males (sex × stress: F(1,17) = 5.105, p = 0.0373; Fig. 9C). This difference in GluA1 levels may explain the increased phosphorylation status in males, but the lower phosphorylation levels in females, and may be attributed to diminished upstream kinase activity. Additionally, synaptic GluA2 levels were significantly higher in females compared to males in the dorsal hippocampus (sex: F(1,17) = 61.25, p < 0.0001; Fig. 9D). Furthermore, the levels of the mandatory NMDA receptor subunit –GluN1– were also higher in females than males (sex: F(1,19) = 98.97, p < 0.0001; Fig. 9E), and were slightly but significantly increased by stress only in females. The levels of the auxiliary NMDA receptor subunit GluN2A were also higher in the female than male dorsal hippocampus (sex: F(1,20) = 34.34, p < 0.0001; Fig. 9F); whereas GluN2B levels were similar across groups (Fig. 9G). These results suggest that higher levels of GluA1-and GluA2-containig AMPA receptors, along with GluN2A-containing NMDA receptors, are present in synaptoneurosomes of the dorsal hippocampus of females compared to males.
Fig. 9.
Sex and stress determine the levels of both AMPA and NMDA receptor subunits in the hippocampal synaptoneurosome fraction. Representative Western blots of synaptoneurosomal fractions of (A) dorsal and (H) ventral hippocampal poles. (B, I) p-Ser831 GluA1. In the dorsal hippocampus, restraint stress decreased p-GluA1/GluA1 ratio in females and increased it in males. In the ventral hippocampus, p-GluA1/GluA1 ratio was insensitive to stress in both females and males. (C, J) Total GluA1 levels. In the dorsal hippocampus, females showed higher GluA1 basal levels, and restraint stress decreased GluA1 levels only in males. In the ventral hippocampus, GluA1 levels were insensitive to stress in both females and males. (D, K) GluA2 levels. Females exhibited higher GluA2 levels than males in both the dorsal and ventral hippocampus, regardless of stress. (E, L) GluN1 levels. In the dorsal hippocampus, females showed higher GluN1 levels than males, and chronic stress increased GluN1 levels only in females. In the ventral hippocampus, chronic stress decreased GluN1 levels only in males. (F, M) GluN2A levels. In the dorsal hippocampus, females exhibited higher levels of GluN2A than males, despite chronic restraint. (G, N) GluN2B levels. Total protein levels were normalized using β-Actin (GluA1: run 5; GluA2 and GluN1: run 8; GluN2A: run 2; GluN2B: run 4; Fig. S3). All graphs show individual datapoints, and bars represent the mean ± SEM. Data were analyzed by two-way ANOVA, followed by Fischer's test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Full statistical details are presented in Table S2n = 5–6 per group.
In the ventral hippocampus, on the other hand, neither GluA1 phosphorylation nor its total levels were affected by sex and stress (Fig. 9I and J). However, similarly to the dorsal region, GluA2 and GluN1 were higher in females than males (sex: F(1,17) = 29.40, p < 0.0001; sex: F(1,19) = 17.80, p = 0.0005; Fig. 9K and L). Notably, there was a trend toward significance in a reduction of GluA2 levels (Fig. 9K), and a significant reduction in GluN1 levels, both in stressed males compared to sex-matched controls (Fig. 9L). Conversely, both GluN2A and GluN2B were insensitive to stress irrespective of sex (Fig. 9M and N). These data support the notion that similar effects of sex are observed for GluA2 and GluN1 in both hippocampal regions, although males display more sensitivity to stress in the ventral, but not the dorsal hippocampus.
4. Discussion
Our study demonstrates that female and male rats exposed to chronic restraint stress show different behavioral responses, particular transcriptional signatures, and differential patterns in signaling pathways relevant for neuroplasticity in both the dorsal and ventral hippocampus.
In our study, chronic restraint produced effective stress responses in both female and male rats according to fecal pellets output. However, females decreased their food consumption with no variations in body weight; but males decreased their body weight gain, independently of food consumption. Interestingly, chronic restraint plus cold stress reduced food intake in both female and male rats; however, this effect was lost after five days of stress in males, while females showed a more delayed recovery (Pare et al., 1999). Similarly, the reduction in body weight gain triggered by subchronic restraint was lesser in female than male rats (Vieira et al., 2018). Together, these observations suggest that both specific and shared somatic alterations are produced by chronic restraint in both female and male adult rats.
4.1. Sex-specific effects of chronic restraint stress in sucrose preference and spatial memory
Sucrose preference behavior is the most frequently used test to evaluate pleasure experience and seems to depend on sex and stress exposure (Willner et al., 1987; Figueroa et al., 2015; Willner, 2017). Some evidence indicate that the estrous cycle may influence the behavioral outcome in this test (Liang et al., 2008). For instance, naïve females in estrus show an increase in sucrose preference compared to rats in metestrus or diestrus (Liang et al., 2008). Although the homotypic stress exposure produced changes in somatic parameters in both female and male rats, we observed a loss in sucrose preference only in males. In agreement with our results, several chronic stress models consistently decrease sucrose preference in males (Ma et al., 2019), but not in females under chronic restraint stress for 4 weeks (Zhu et al., 2014). In contrast, unpredictable chronic mild stress (Zhu et al., 2014) and subchronic variable stress (SCVS) produces a reduced sucrose preference in females (Hodes et al., 2015; Williams et al., 2020). However, while we conducted the SPT in estrus, it is important to note that these studies did not mention if the behavioral studies were evaluated in a particular estrous cycle stage.
Importantly, the ventral hippocampus integrates the cortico-mesolimbic circuitry of reward and heightened ventral hippocampal-nucleus accumbens activity mediates SCVS-induced reduction in sucrose preference in females (Williams et al., 2020) which can be linked to anhedonia-like behavior (Willner et al., 1987; Figueroa et al., 2015; Willner, 2017). Therefore, chronic restraint may trigger abnormal hyperactivity of the male ventral hippocampus, while females may be more resistant to show variations in neuronal activity related to this pole. Other behavioral test should be used in the future to demonstrate that females are resilient to stress-induced anhedonic behavior.
In addition, the ventral hippocampus is a component of the anxiety circuit (Parfitt et al., 2017). In the elevated plus maze, chronic restraint increases anxiety-like behavior in males (Ulloa et al., 2010). However, in our study, the time spent at the center of an open field did not differ neither by sex nor stress. Hence, anxiety-like behavior may depend on the evaluating task. Besides, female rodents show less anxiety-like behavior than males under non-stressful behavioral tasks (Donner and Lowry, 2013). Moreover, these behavioral responses are more diverse in females, probably depending on the type of task, stressor, and estrous cycle (ter Horst et al., 2012).
In contrast to the ventral region, the dorsal hippocampus integrates critical networks for spatial learning and memory (Strange et al., 2014). Some reports have described the effects of sex and estrous cycle over different spatial memory tasks. For instance, there were not any sex differences in latencies to reach a hidden platform in a Morris water maze but, females in estrus exhibited a slower learning time (Healy et al., 1999). In contrast, both in a modified version of Morris water maze (in which the learning to locate a hidden platform was conducted in a single session) (Berry et al., 1997) and in the radial arm maze test (Stackman et al., 1997), there were not any significant influence of estrous cycle.
Certain studies indicated no influences of the estrous cycle in the performance of rats (Liang et al., 2008) or mice (Spencer et al., 2008) in the OLT (a dorsal hippocampus-dependent task). In contrast, other studies have described that rats in either estrus or proestrus have larger exploration times for novel features –compared to rats in diestrus– in both the OLT (Frye et al., 2007; Paris and Frye, 2008) and NORT (perirhinal cortex-dependent contextual memory) (Paris and Frye, 2008). Furthermore, another report found that chronic restraint stress enhanced the performance of females rats in the radial arm maze compared to controls when performed on diestrus; but both control and stressed rats had impaired acquisition during proestrus (Bowman et al., 2001). Nonetheless, there is not much information about the effects of stress, male and female sex, and estrous cycle on OLT and NORT. We described that chronic restraint differentially impacts males and females in the OLT; but not in the novel object recognition test, which is hippocampus-independent at short retention times (Cohen and Stackman, 2015). In the OLT, males lost their preference for the novel location after chronic restraint. Similarly, chronic stress impairs spatial memory in males by various tasks: radial arm maze (Luine et al., 1994), Y-maze (Conrad et al., 2003), water maze (Kitraki et al., 2004) and object placement (Beck and Luine, 2002; Luine et al., 2017) Thus, stress impairs the ability of males to perform spatial memory tasks, but females have greater difficulty in performing spatial memory tasks at baseline (Luine, 2016). Females show greater cognitive resilience against chronic stress, outperforming naïve females at the object placement task (Beck and Luine, 2002; Bisagno et al., 2003); nonetheless, these studies did not consider the estrous cycle as a variable. These findings agree with our observations conducted in estrus; i.e., stressed females exhibit a similar profile to control males, but different from control females and stressed males.
4.2. Sex and chronic restraint shape the molecular signature of the dorsal and ventral hippocampus
Considering the heterogeneous role of the hippocampal longitudinal axis in object location memory and reward, our results support the hypothesis that chronic restraint produce impairments in the male dorsal and ventral hippocampus, while females show remarkable resilience against the loss of sucrose preference, and improved performance on the OLT. Several studies have addressed the heterogeneity of the rodent hippocampus along its dorsal-ventral axis by microarray (Leonardo et al., 2006) and RNA-seq (Cembrowski et al., 2016). Notably, acute stress in male mice induces distinct transcriptional and proteomic changes between the hippocampal poles, with the ventral hippocampus being more sensitive (Floriou-Servou et al., 2018). By considering both the sex and hippocampal poles, our study revealed sex-specific transcriptional signatures of the rat dorsal and ventral hippocampus in response to chronic restraint, which agrees with vast findings from both depressed patients and animal models of chronic stress across the brain (Bagot et al., 2015; Labonte et al., 2017; Seney et al., 2018; Rowson et al., 2019; Brivio et al., 2020; Paden et al., 2020). We also noted an overlap in co-upregulated genes by chronic restraint between the female ventral and male dorsal hippocampus, suggesting a molecular convergence between the opposite poles and sex under stress. In the IPA analysis, several terms were overlapped between the female ventral and male dorsal hippocampus. Thus, it is plausible that part of the stress transcriptional signature in the male dorsal hippocampus impairs spatial performance, while promotes resilience in the female ventral hippocampus. Such convergent effects require further investigation.
Few reports have explored differences in signaling pathways between the female and male brains under baseline or stress conditions. We detected a large number of sex differences in the dorsal hippocampus, irrespective of stress. These molecular differences correlated with the stinkingly different performance of unstressed females and males in the OLT. We detected that the FL-TrkB receptor is more abundant in the female dorsal hippocampus, in contrast to males, suggesting a heightened availability of the receptor to signal at the synapse. Similarly, the activity of the Akt pathway –assessed by its downstream targets GSK3β and mTOR– seems larger in synaptic fractions of the female dorsal hippocampus, in contrast to males. Likewise, a recent study showed that Akt, GSK3β and p70S6K phosphoprotein levels were higher in the female dorsal dentate gyrus compared to males, and these differences do not depend on estradiol (Sheppard et al., 2021). Additionally, we detected that phosphorylations of ERK and CREB were also higher in the female dorsal hippocampus relative to males. However, considering the hippocampal subareas, it was reported that ERK phosphorylation is higher in the male dorsal dentate gyrus, while no differences were observed in CA1 area (Sheppard et al., 2021). Considering the known role of these pathways, our observations support the notion that the female dorsal hippocampus exhibits a molecular phenotype that may be associated with neuroprotection. Interestingly, the activity of these pathways may regulate glutamatergic neurotransmission and impact behavioral outcomes in a sex-biased manner (Wickens et al., 2018). We detected higher synaptic GluA1 and GluA2 levels in the dorsal hippocampus of females in contrast to males, in agreement with a previous report (Monfort et al., 2015). Accordingly, CA1 AMPA excitatory potentials were reported to be larger in females than males (Monfort et al., 2015). Moreover, we detected higher GluN1 and GluN2A subunit levels in synaptoneurosomes of the female dorsal hippocampus, in contrast to males. This was similar to a previous report, which showed that females have more levels of GluN1 and GluN2B in the whole hippocampus (Wang et al., 2015); however, we detected that synaptic GluN2B levels were similar between female and male controls. These results suggest a higher glutamatergic excitability in the female dorsal hippocampus that is insensitive to chronic restraint and dissociated from OLT performance.
We noted that the ventral hippocampus showed more similarities between the sexes in signaling pathways. However, the transcriptional signatures between the female and male ventral hippocampus were highly dissociated; hence, they may converge in functional pathways by different mechanisms. Among the differences, we noted higher PTEN levels in the male ventral hippocampus, compared to females. PTEN levels have been reported to increase upon chronic stress exposure in the prefrontal cortex of male mice (Wang et al., 2021). Thus, higher PTEN basal levels may be associated with stress susceptibility. As in the dorsal region, CREB phosphorylation was also higher in the ventral hippocampus of females, relative to males. Of note, this effect was dissociated from ERK phosphorylation, suggesting the participation of other upstream regulators (Ehrlich and Josselyn, 2016). Overall, at baseline, the dorsal pole seems less comparable between the sexes than the ventral hippocampus.
Unexpectedly, we detected few effects of stress on the activity of canonical pathways in the dorsal and ventral hippocampus of both sexes. For instance, the truncated TrkB receptor was reduced by stress in the dorsal hippocampus of males, which was accompanied by an increase in the phosphorylations of both the full-length receptor and its downstream effector, CaMKIIα. Furthermore, the phosphorylation of GluA1 at Ser831 –mediated by CaMKIIα or PKC– was also increased in the male dorsal hippocampus by stress. Considering the impairment of stressed males on spatial tasks, further studies should address whether the TrkB-CaMKIIα-GluA1 axis activation in the male dorsal hippocampus plays a compensatory or facilitating role on this cognitive decline. On the other hand, stress reduced the phosphorylation of Ser831-GluA1 in the female dorsal hippocampus; suggesting that this synaptic phenomenon may be related to enhanced spatial performance, although probably more dependent on PKC than CaMKIIα activity.
5. Conclusions
Overall, our findings highlight the importance of evaluating stress responses not only by sex, but also by segregating the dorsal and ventral hippocampus. The nature and extent of these differences may provide relevant insights for understanding the molecular events that may define a pro-resilient or pro-susceptible phenotype to stress-related diseases.
Data availability disclosure statement
The authors declare that all supporting data and method descriptions are available within the article, or from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Felipe A. Olave: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft. Felipe I. Aguayo: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Funding acquisition. Luciano Román-Albasini: Conceptualization, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Wladimir A. Corrales: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Visualization. Juan P. Silva: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Visualization. Pablo I. González: Validation, Formal analysis. Sara Lagos: Validation, Formal analysis. María A. García: Investigation, Writing – original draft. Matías Alarcón-Mardones: Validation, Formal analysis. Paulina S. Rojas: Validation, Formal analysis. Xiaojiang Xu: Formal analysis, Investigation. John A. Cidlowski: Resources, Writing – review & editing. Esteban Aliaga: Conceptualization, Writing – review & editing. Jenny Fiedler: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None.
Acknowledgements
This work was supported by Fondo Nacional de Ciencia y Tecnología (FONDECYT), Agencia Nacional de Investigación y Desarrollo (ANID) de Chile, grant Nº 119-0899 to JLF and N° 21180695 to FA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100440.
Contributor Information
Esteban Aliaga, Email: ealiaga@ucm.cl.
Jenny Fiedler, Email: jfiedler@ciq.uchile.cl.
Appendix ASupplementary data
The following is the Supplementary data to this article:
A supplementary file containing Figs. S1–3 and Tables S1–2 accompanies this article.
References
- Aguayo F.I., Pacheco A.A., Garcia-Rojo G.J., Pizarro-Bauerle J.A., Doberti A.V., Tejos M., Garcia-Perez M.A., Rojas P.S., Fiedler J.L. Matrix metalloproteinase 9 displays a particular time response to acute stress: variation in its levels and activity distribution in rat Hippocampus. ACS Chem. Neurosci. 2018;9:945–956. doi: 10.1021/acschemneuro.7b00387. [DOI] [PubMed] [Google Scholar]
- Aguayo F.I., Tejos-Bravo M., Diaz-Veliz G., Pacheco A., Garcia-Rojo G., Corrales W., Olave F.A., Aliaga E., Ulloa J.L., Avalos A.M., Roman-Albasini L., Rojas P.S., Fiedler J.L. Hippocampal memory recovery after acute stress: a behavioral, morphological and molecular study. Front. Mol. Neurosci. 2018;11:283. doi: 10.3389/fnmol.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet-Walsh E., Dufour C.R., Yee T., Zouanat F.Z., Yan M., Kalloghlian G., Vernier M., Caron M., Bourque G., Scarlata E., Hamel L., Brimo F., Aprikian A.G., Lapointe J., Chevalier S., Giguere V. Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer. Gene Dev. 2017;31:1228–1242. doi: 10.1101/gad.299958.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot R.C., Parise E.M., Pena C.J., Zhang H.X., Maze I., Chaudhury D., Persaud B., Cachope R., Bolanos-Guzman C.A., Cheer J.F., Deisseroth K., Han M.H., Nestler E.J. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G.R., Warburton E.C. When is the hippocampus involved in recognition memory? J. Neurosci. : the official journal of the Society for Neuroscience. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K.D., Luine V.N. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol. Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Berry B., McMahan R., Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav. Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bisagno V., Bowman R., Luine V. Functional aspects of estrogen neuroprotection. Endocrine. 2003;21:33–41. doi: 10.1385/endo:21:1:33. [DOI] [PubMed] [Google Scholar]
- Bowman R.E., Zrull M.C., Luine V.N. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Parra C.S., Arancibia S., Andres S., Morales P., Herrera-Marschitz M., Herrera L., Lara H.E., Fiedler J.L. Adrenalectomy promotes a permanent decrease of plasma corticoid levels and a transient increase of apoptosis and the expression of Transforming Growth Factor beta1 (TGF-beta1) in hippocampus: effect of a TGF-beta1 oligo-antisense. BMC Neurosci. 2006;7:40. doi: 10.1186/1471-2202-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivio E., Lopez J.P., Chen A. Sex differences: transcriptional signatures of stress exposure in male and female brains. Gene Brain Behav. 2020;19 doi: 10.1111/gbb.12643. [DOI] [PubMed] [Google Scholar]
- Cahill K.M., Huo Z., Tseng G.C., Logan R.W., Seney M.L. Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci. Rep. 2018;8:9588. doi: 10.1038/s41598-018-27903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda P., Munoz M., Garcia-Rojo G., Ulloa J.L., Bravo J.A., Marquez R., Garcia-Perez M.A., Arancibia D., Araneda K., Rojas P.S., Mondaca-Ruff D., Diaz-Veliz G., Mora S., Aliaga E., Fiedler J.L. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J. Neurosci. Res. 2015;93:1476–1491. doi: 10.1002/jnr.23602. [DOI] [PubMed] [Google Scholar]
- Cembrowski M.S., Wang L., Sugino K., Shields B.C., Spruston N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. Elife. 2016;5 doi: 10.7554/eLife.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.J., Stackman R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., Grote K.A., Hobbs R.J., Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Cora M.C., Kooistra L., Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015;43:776–793. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner N.C., Lowry C.A. Sex differences in anxiety and emotional behavior. Pflueg. Arch. Eur. J. Physiol. 2013;465:601–626. doi: 10.1007/s00424-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D.E., Josselyn S.A. Plasticity-related genes in brain development and amygdala-dependent learning. Gene Brain Behav. 2016;15:125–143. doi: 10.1111/gbb.12255. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J., Sola-Oriol D., Manteca X., Perez J.F., Dwyer D.M. Anhedonia in pigs? Effects of social stress and restraint stress on sucrose preference. Physiol. Behav. 2015;151:509–515. doi: 10.1016/j.physbeh.2015.08.027. [DOI] [PubMed] [Google Scholar]
- Floriou-Servou A., von Ziegler L., Stalder L., Sturman O., Privitera M., Rassi A., Cremonesi A., Thony B., Bohacek J. Distinct proteomic, transcriptomic, and epigenetic stress responses in dorsal and ventral Hippocampus. Biol. Psychiatr. 2018;84:531–541. doi: 10.1016/j.biopsych.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Frye C.A., Duffy C.K., Walf A.A. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rojo G., Fresno C., Vilches N., Diaz-Veliz G., Mora S., Aguayo F., Pacheco A., Parra-Fiedler N., Parra C.S., Rojas P.S., Tejos M., Aliaga E., Fiedler J.L. The ROCK inhibitor fasudil prevents chronic restraint stress-induced depressive-like behaviors and dendritic spine loss in rat Hippocampus. Int. J. Neuropsychopharmacol. 2017;20:336–345. doi: 10.1093/ijnp/pyw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy S.D., Braham S.R., Braithwaite V.A. Spatial working memory in rats: no differences between the sexes. Proc. Biol. Sci. 1999;266:2303–2308. doi: 10.1098/rspb.1999.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E., et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. : the official journal of the Society for Neuroscience. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., McGonagle K.A., Swartz M., Blazer D.G., Nelson C.B. Sex and depression in the national comorbidity survey. I: lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E., Kremmyda O., Youlatos D., Alexis M.N., Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Labonte B., et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo E.D., Richardson-Jones J.W., Sibille E., Kottman A., Hen R. Molecular heterogeneity along the dorsal-ventral axis of the murine hippocampal CA1 field: a microarray analysis of gene expression. Neuroscience. 2006;137:177–186. doi: 10.1016/j.neuroscience.2005.08.082. [DOI] [PubMed] [Google Scholar]
- Liang S., Byers D.M., Irwin L.N. Sex and diet affect the behavioral response of rats to chronic mild stressors. Physiol. Behav. 2008;93:27–36. doi: 10.1016/j.physbeh.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lin Y., Ter Horst G.J., Wichmann R., Bakker P., Liu A., Li X., Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebr. Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents. J. Steroid Biochem. Mol. Biol. 2016;160:189–195. doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V., Villegas M., Martinez C., McEwen B.S. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V., Gomez J., Beck K., Bowman R. Sex differences in chronic stress effects on cognition in rodents. Pharmacol., Biochem. Behav. 2017;152:13–19. doi: 10.1016/j.pbb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Xu Y., Wang G., Li R. What do we know about sex differences in depression: a review of animal models and potential mechanisms. Progress in neuro-psychopharmacology & biological psychiatry. 2019;89:48–56. doi: 10.1016/j.pnpbp.2018.08.026. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M. Multifaceted origins of sex differences in the brain. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016;371:20150106. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.J., Wilson J.O., Harman J., Wright R.L., Wieczorek L., Gomez J., Korol D.L., Conrad C.D. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort P., Gomez-Gimenez B., Llansola M., Felipo V. Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chem. Neurosci. 2015;6:1420–1427. doi: 10.1021/acschemneuro.5b00096. [DOI] [PubMed] [Google Scholar]
- Ning L.N., Zhang T., Chu J., Qu N., Lin L., Fang Y.Y., Shi Y., Zeng P., Cai E.L., Wang X.M., Wang Q., Lu Y.M., Zhou X.W., Zhang Q., Tian Q. Gender-related hippocampal proteomics study from young rats after chronic unpredicted mild stress exposure. Mol. Neurobiol. 2017;55:835–850. doi: 10.1007/s12035-016-0352-y. [DOI] [PubMed] [Google Scholar]
- Paden W., Barko K., Puralewski R., Cahill K.M., Huo Z., Shelton M.A., Tseng G.C., Logan R.W., Seney M.L. Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry. Transl. Psychiatry. 2020;10:59. doi: 10.1038/s41398-020-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare W.P., Blair G.R., Kluczynski J., Tejani-Butt S. Gender differences in acute and chronic stress in Wistar Kyoto (WKY) rats. Integr. Physiol. Behav. Sci. : the official journal of the Pavlovian Society. 1999;34:227–241. doi: 10.1007/BF02688691. [DOI] [PubMed] [Google Scholar]
- Parfitt G.M., Nguyen R., Bang J.Y., Aqrabawi A.J., Tran M.M., Seo D.K., Richards B.A., Kim J.C. Bidirectional control of anxiety-related behaviors in mice: role of inputs arising from the ventral Hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42:1715–1728. doi: 10.1038/npp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.J., Frye C.A. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Plaisier S.B., Taschereau R., Wong J.A., Graeber T.G. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169. doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Li M.X., Xu C., Chen H.B., An S.C., Ma X.M. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Albasini L., Diaz-Veliz G., Olave F.A., Aguayo F.I., Garcia-Rojo G., Corrales W.A., Silva J.P., Avalos A.M., Rojas P.S., Aliaga E., Fiedler J.L. Antidepressant-relevant behavioral and synaptic molecular effects of long-term fasudil treatment in chronically stressed male rats. Neurobiology of stress. 2020;13:100234. doi: 10.1016/j.ynstr.2020.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson S.A., Bekhbat M., Kelly S.D., Binder E.B., Hyer M.M., Shaw G., Bent M.A., Hodes G., Tharp G., Weinshenker D., Qin Z., Neigh G.N. Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2019;44:1207–1215. doi: 10.1038/s41386-019-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M.L., Huo Z., Cahill K., French L., Puralewski R., Zhang J., Logan R.W., Tseng G., Lewis D.A., Sibille E. Opposite molecular signatures of depression in men and women. Biol. Psychiatr. 2018;84:18–27. doi: 10.1016/j.biopsych.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard P.A.S., Puri T.A., Galea L.A.M. Sex differences and estradiol effects in MAPK and Akt cell signalling across subregions of the hippocampus. Neuroendocrinology. 2021 doi: 10.1159/000519072. [DOI] [PubMed] [Google Scholar]
- Spencer J.L., Waters E.M., Milner T.A., McEwen B.S. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman R.W., Blasberg M.E., Langan C.J., Clark A.S. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol. Learn. Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Tache Y., Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxier L.R., Gross K.S., Frick K.M. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci. 2020;21:535–550. doi: 10.1038/s41583-020-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Horst J.P., de Kloet E.R., Schachinger H., Oitzl M.S. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa J.L., Castaneda P., Berrios C., Diaz-Veliz G., Mora S., Bravo J.A., Araneda K., Menares C., Morales P., Fiedler J.L. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol., Biochem. Behav. 2010;97:213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Vieira J.O., Duarte J.O., Costa-Ferreira W., Morais-Silva G., Marin M.T., Crestani C.C. Sex differences in cardiovascular, neuroendocrine and behavioral changes evoked by chronic stressors in rats. Progress in neuro-psychopharmacology & biological psychiatry. 2018;81:426–437. doi: 10.1016/j.pnpbp.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A., Wood M.A. Examining object location and object recognition memory in mice. Current protocols in neuroscience. 2014;69:8 31 31–17. doi: 10.1002/0471142301.ns0831s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Q., Zhang L., Xia Z.Y., Chen J.Y., Fang Y., Ding Y.Q. PTEN in prefrontal cortex is essential in regulating depression-like behaviors in mice. Transl. Psychiatry. 2021;11:185. doi: 10.1038/s41398-021-01312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ma Y., Hu J., Cheng W., Jiang H., Zhang X., Li M., Ren J., Li X. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience. 2015;301:363–374. doi: 10.1016/j.neuroscience.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Warren S.G., Humphreys A.G., Juraska J.M., Greenough W.T. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Wickens M.M., Bangasser D.A., Briand L.A. Sex differences in psychiatric disease: a focus on the glutamate system. Front. Mol. Neurosci. 2018;11:197. doi: 10.3389/fnmol.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E.S., Manning C.E., Eagle A.L., Swift-Gallant A., Duque-Wilckens N., Chinnusamy S., Moeser A., Jordan C., Leinninger G., Robison A.J. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatr. 2020;87:492–501. doi: 10.1016/j.biopsych.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiology of stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Woodburn S.C., Bollinger J.L., Wohleb E.S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiology of stress. 2021;14:100312. doi: 10.1016/j.ynstr.2021.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Shi R., Wang J., Wang J.F., Li X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport. 2014;25:1151–1155. doi: 10.1097/WNR.0000000000000243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data and method descriptions are available within the article, or from the corresponding author upon reasonable request.