Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread throughout the world, which becomes a global public health emergency. Undernourishment prolongs its convalescence and has an adverse effect on its prognosis, especially in diabetic patients. The purpose of this study was to evaluate the prevalence and characteristics of undernourishment and to determine how it is related to the prognostic outcomes in the diabetic patients with coronavirus disease 2019 (COVID-19). A retrospective, multicenter study was conducted in 85 diabetic COVID-19 patients from three hospitals in Hubei Province. All patients were assessed using the European Nutritional Risk Screening 2002 (NRS-2002) and other nutritional assessments when admitted. Of them, 35 (41.18%) were at risk of malnutrition (NRS score ≥3). Severe COVID-19 patients had a significantly lower level of serum albumin and prealbumin and higher NRS score than non-severe patients. Multivariate logistic regression analysis showed that serum prealbumin and NRS score increased the likelihood of progression into severe status (P<0.05). Meanwhile, single factor and multivariate analysis determined that grade of illness severity was an independent predictor for malnutrition. Furthermore, prealbumin and NRS score could well predict severe status for COVID-19 patients. The malnutrition group (NRS score ≥3) had more severe illness than the normal nutritional (NRS score <3) group (P<0.001), and had a longer length of in-hospital stay and higher mortality. Malnutrition is highly prevalent among COVID-19 patients with diabetes. It is associated with severely ill status and poor prognosis. Evaluation of nutritional status should be strengthened, especially the indicators of NRS-2002 and the level of serum prealbumin.
Keywords: NRS-2002, malnutrition, COVID-19, diabetes
Introduction
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections was first reported in December 2019. The World Health Organization declared a global pandemic of 2019 coronavirus disease (COVID-19) in March 2020. Now it is affecting more than 180 countries and leading to high mortality globally[1]. As the number of confirmed cases increased, the massive information of patients with COVID-19 has been widely reported. But the knowledge concerning nutritional status of the patients with COVID-19 is contemporarily limited, especially in diabetic patients. Malnutritional status can have a significant adverse impact on the health, leading to major complications of hospitalized patients, especially those suffering from infection. Also, malnutrition is one of the key factors for poor prognosis and high mortality rate in critically ill patients. Previous studies have shown a relationship between nutritional status and virus infection[2–3]. Host nutritional status can impact the reaction to the pathogen and likewise impact the genetic modification of viral genome[4]. Considering the prevalence and importance of malnutrition, it is necessary to screen for malnutrition in COVID-19 patients[5]. One of the recommended tools is Nutritional Risk Screening-2002 (NRS-2002)[6]. In many studies on COVID-19, diabetes has been reported to be associated with higher morbidity and mortality[7]. Also, diabetes is one of the most important and general risk factors for malnutrition. But for COVID-19 patients with diabetes, their nutritional status has less been reported. Given the above mentioned, our study aimed to evaluate the prevalence of risk for malnutrition (as assessed by the NRS-2002 and other nutritional indicators) in the diabetic COVID-19 patients, and to assess the relationship between nutritional status and disease severity.
Materials and methods
Study population
Type 2 diabetes mellitus (T2DM) patients aged 18 years or older who were admitted to the Huangshi Central Hospital, Huangshi Hospital of Traditional Chinese Medicine, and Huangshi Youse Hospital from February 2020 to April 2020 for treating COVID-19 were enrolled in this study. The exclusion criteria for the study were as follows: (1) history of malignant tumor; (2) severe gastrointestinal disorders (such as stomach or bowel excision, ulcerative colitis, and Crohn's disease); and (3) other conditions that can cause severe undernourishment before inclusion. All patients were diagnosed with SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction (RT-PCR) assay. The study was approved by the Ethics Committee of the Affiliated Sir Run Run Hospital of Nanjing Medical University (2020-SR-008).
Data collection
Baseline demographic data (age, gender, height, and weight), underlying diseases (hypertension, coronary heart disease, and chronic pulmonary obstructive disease), nutritional indicators (body mass index [BMI], serum albumin, serum prealbumin, triceps skinfold thickness (TSF), arm medium circumference [AMC], lymphocyte count, and NRS-2002 score) and laboratory data (leukocytes count, hemoglobin, platelets, urea, creatinine, C-reactive protein [CRP], interleukin-6, and PaO2/FiO2) were obtained on admission. All biochemical analyses were performed using routine methods at the local laboratory. Clinical outcomes included severity of disease, mortality, and length of hospital stay. Mortality was defined as death before discharge. Length of stay was defined as duration of hospitalization before discharge or death. On Admission, all enrolled patients were divided into two groups according to the severity of illness: non-severe group and severe group. Patients with any of the following features were categorized as having severe status: respiratory rate of at least 30 breaths per minute; oxygen saturation of 93% or less at a rest state; arterial partial pressure of oxygen to fractional inspired oxygen ratio of 300 mm Hg or less; and more than 50% progression of lesions on lung imaging within 24 to 48 hours. The risk for malnutrition was evaluated by the NRS-2002, which was applied by a trained clinical examiner. NRS-2002 consists of two parts. The first part is about the assessment of the patient's nutritional status and food intake problems, and the second contains information related to the impact of disease severity on nutritional status. Each part is scored from 0 to 3 points. Patients received an extra point for age above 70 years. With 7 points in total, the higher the number of points, the greater the risk of malnutrition[6].
Statistical analysis
The results were statistically processed with the statistical software SPSS26.0 (IBM, USA). The data are expressed as means combined with standard deviations (SD), or median depending on normal distribution. Comparisons between the two groups were assessed with a Student's t-test or a Mann-Whitney test, as appropriate. The differences of enumeration data, such as gender, complications, and grade of illness severity, were compared by χ2 test. Associations among variables were assessed with single factor regression analysis and multivariate logistic regression analysis. Receiver operating characteristic (ROC) analysis was used for evaluating the predictive value of variables for the severity of illness. A P-value <0.05 was set to be statistically significant.
Results
Baseline clinical characteristics
During the study period, a total of 85 diabetic patients with COVID-19 were enrolled. The overall mean age was (61.035±14.73) years (range from 25 to 86). According to the NRS-2002, 35 (41.18%) of them were at risk of malnutrition (NRS score ≥3). Their clinical characteristics are shown in Table 1. Age and gender were equally distributed between the severe group and the non-severe group. Height, weight, BMI, and underlying diseases were similar. Patients in the severe group had a significantly lower level of serum albumin, lower level of serum prealbumin, and higher NRS-2002 score than those in the non-severe group. Laboratory variables including leukocytes, lymphocyte count, hemoglobin, urea, CRP, and PaO2/FiO2 differed significantly (P<0.05). There were no significant differences in triceps thickness, upper arm circumference, platelets, creatinine, and interleukin-6 between the two groups.
Table 1. Baseline characteristics and laboratory findings between non-severe and severe groups.
| Characteristics | Severity grade | t/χ2 | P-value | |
| Non-severe group (n=62) | Severe group (n=23) | |||
| aData are presented as mean±SD. P-values were calculated by *chi-square test or #Student's t-test or Mann-Whitney U test. BMI: body mass index; TSF: triceps skinfold thickness; AMC: arm medium circumference; NRS-2002: nutritional risk screening-2002; CRP: C-reactive protein; IL-6: interleukin-6; PaO2/FiO2: arterial partial pressure of oxygen/fraction of inspired oxygen. | ||||
| Demographics | ||||
| Age (years)a | 59.5±14.5 | 65.1±14.7 | −1.558 | 0.123# |
| Male (n [%]) | 26 (41.9) | 14 (60.9) | 2.414 | 0.120* |
| Height (cm)a | 163.8±8.5 | 165.3±8.0 | −0.729 | 0.468# |
| Weight (kg)a | 61.27±13.3 | 62.80±10.2 | −0.497 | 0.620# |
| Underling diseases | ||||
| Hypertension (n [%]) | 21 (33.9) | 9 (39.1) | 0.203 | 0.652* |
| Coronary heart disease (n [%]) | 7 (11.3) | 5 (21.7) | 1.511 | 0.219* |
| Chronic pulmonary obstructive disease (n [%]) | 6 (9.7) | 3 (13.0) | 0.201 | 0.654* |
| Nutritional indicatorsa | ||||
| BMI (kg/m2) | 22.7±4.1 | 22.9±2.8 | −0.227 | 0.821# |
| Serum albumin (g/L) | 39.5±2.9 | 36.2±3.2 | 4.595 | <0.001 # |
| Serum prealbumin (g/L) | 287.9±90.0 | 134.4±60.9 | 7.549 | <0.001 # |
| TSF (% of normal value) | 102.7±36.3 | 100.6±41.1 | 0.230 | 0.819# |
| AMC (% of normal value) | 112.1±14.0 | 103.8±14.7 | 2.351 | 0.024# |
| Lymphocyte count (×109/L) | 1.4±0.5 | 1.0±0.5 | 3.150 | 0.002# |
| NRS-2002 (point) | 1.4±1.3 | 4.3±1.1 | −9.764 | <0.001 # |
| Laboratory indicatorsa | ||||
| Leukocytes count (×109/L) | 6.0±2.7 | 7.9±3.7 | −2.187 | 0.036# |
| Hemoglobin (g/L) | 118.6±14.4 | 98.4±18.2 |
5.335 |
<0.001 # |
| Platelets (×109/L) | 233.7±78.5 | 213.2±109.8 | 0.819 | 0.419# |
| Urea (μmol/L) | 4.5±1.2 | 8.2±6.1 | −2.843 | 0.009# |
| Creatinine (μmol/L) | 58.7±14.3 | 64.2±29.8 | −0.853 | 0.401# |
| CRP (mg/L) | 6.2±10.2 | 44.0±46.3 | −3.743 | 0.001# |
| IL-6 (pg/mL) | 15.7±22.5 | 24.9±23.1 | −1.361 | 0.180# |
| PaO2/FiO2 | 417.4±79.8 | 211.4±98.1 | 6.142 | <0.001 # |
NRS score was an independent predictor for severe status
To explore the risk factor of severe status, single factor regression analysis and multivariate logistic regression analysis were conducted. Increasing odds for developing into severe status were found with NRS-2002, serum albumin, serum prealbumin, leukocytes, lymphocyte count, hemoglobin, urea, CRP, and PaO2/FiO2 (Table 2). Meanwhile, taking AMC, serum albumin, serum prealbumin, score of NRS-2002, lymphocyte count, and P/F ratio as the covariate, multivariate logistic regression analysis showed that serum prealbumin was significantly associated with the presence of severe status (odds ratio [OR], 0.977; 95% confidence interval [CI], 0.960–0.994;P=0.008), as well as NRS-2002 (OR, 11.949; 95% CI, 2.232–63.986; P=0.004) The data indicate serum prealbumin and NRS score increased the likelihood of progression into severe status.
Table 2. Single factor regression analysis.
| Variables | P-value | EXP (B) | 95% CI |
|
P-values were calculated by univariate analysis. CI: confidence interval; TSF: triceps skinfold thickness; AMC: arm medium circumference; NRS-2002: nutritional risk screening 2002; CRP: C-reactive protein; IL-6: interleukin-6; PaO2/FiO2: arterial partial pressure of oxygen/fraction of inspired oxygen.
| |||
| Age | 0.126 | 1.029 | 0.992–1.066 |
| Serum albumin | <0.001 | 0.686 | 0.563–0.837 |
| Serum prealbumin | <0.001 | 0.974 | 0.964–0.985 |
| TSF (% of normal value) | 0.816 | 0.998 | 0.986–1.011 |
| AMC (% of normal value) | 0.024 | 0.954 | 0.916–0.994 |
| Lymphocyte count | 0.004 | 0.221 | 0.078–0.622 |
| NRS-2002 | <0.001 | 10.912 | 3.161–37.673 |
| Leukocytes | 0.024 | 1.193 | 1.024–1.391 |
| Hemoglobin | <0.001 | 0.924 | 0.890–0.961 |
| Urea | 0.002 | 1.586 | 1.189–2.115 |
| CRP | 0.009 | 1.070 | 1.017–1.126 |
| IL-6 | 0.184 | 1.019 | 0.991–1.047 |
| PaO2/FiO2 | <0.001 | 0.977 | 0.967–0.987 |
Receiver operating characteristic analysis
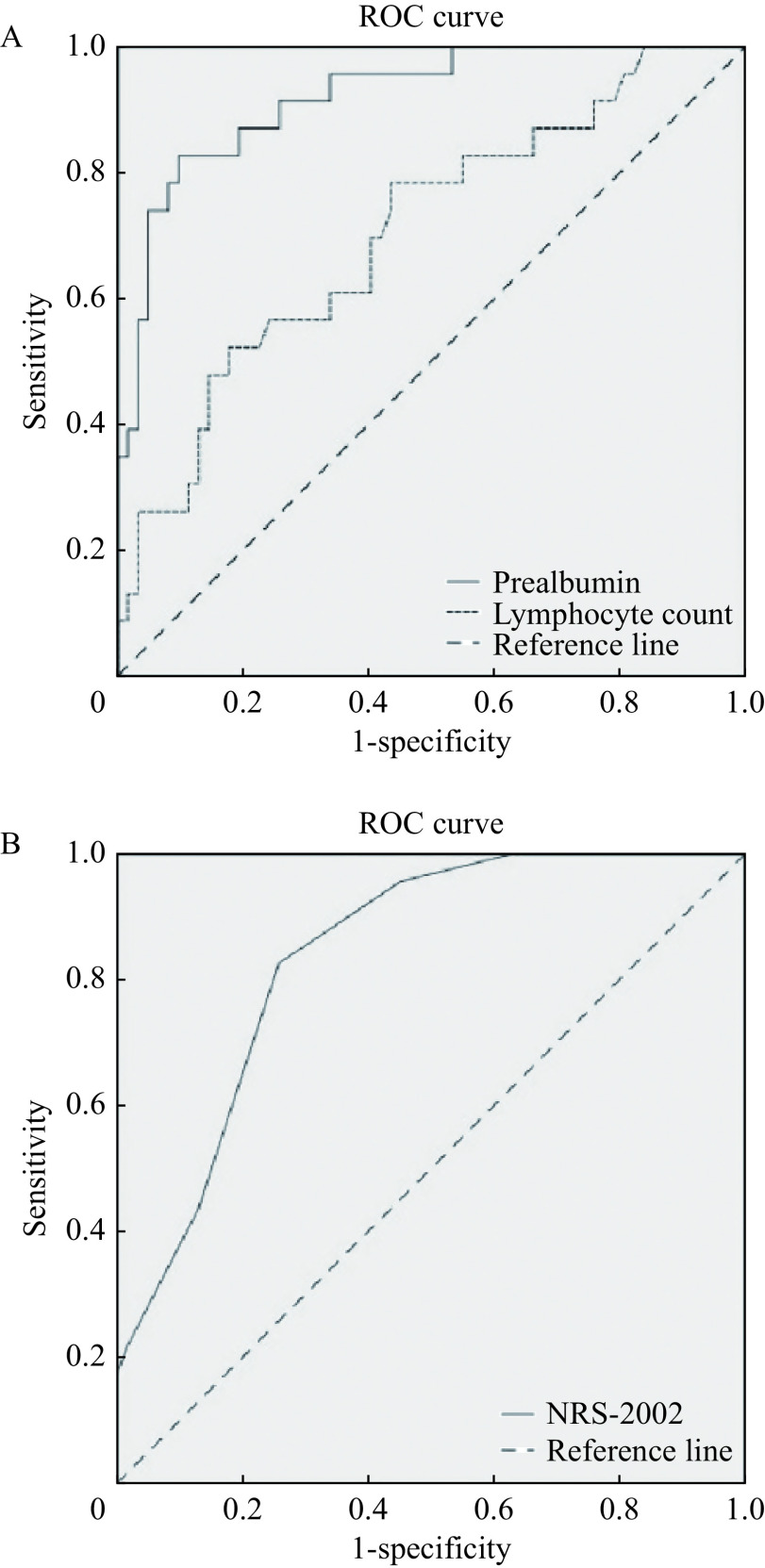
ROC analysis was used for evaluating the predictive value of variables for the severity of illness. Fig. 1 shows the ROC curve analysis of the sensitivities and specificities of prealbumin, lymphocyte count, and NRS-2002 in predicting severely ill status. The comparison among prealbumin, lymphocyte count, and NRS-2002 in these patients revealed the area under the ROC curve values for severely ill status (0.92, prealbumin; 0.705, lymphocyte count; 0.838, NRS-2002). In addition, the difference among the three variables was statistically significant (P<0.05) (Table 3). The result shows prealbumin and NRS score could well predict severe status for COVID-19 patients.
Figure 1.
Comparison of ROCs of the prealbumin, lymphocyte count and NRS-2002 for predicting severely ill status.
A: ROC of the prealbumin and lymphocyte count. B: ROC of NRS-2002. ROC: receiver operating characteristic.
Table 3. ROC analysis.
| Variable | AUC | Standard error | P-value | 95% CI | Cut-off | Sensitivity | Specificity |
| P-values were calculated by ROC analysis. AUC: area under the curve. | |||||||
| Prealbumin | 0.920 | 0.032 | <0.001 | 0.857–0.983 | 183.35 | 0.826 | 0.903 |
| Lymphocyte count | 0.705 | 0.064 | 0.004 | 0.579–0.830 | 0.945 | 0.522 | 0.823 |
| NRS-2002 | 0.838 | 0.043 | <0.001 | 0.754–0.923 | 2.5 | 0.826 | 0.742 |
Effects of nutritional status on the prognosis
Student's t-test or χ2 test were used to compare the in-hospital outcomes between the malnutrition group and the normal nutrition group. The morbidity of patients who finally developed into severe status in the malnutrition group (NRS score ≥3) was 54.3%, which was higher than that in the normal nutrition group (NRS score <3). There was a statistical difference in the length of in-hospital stay ([37.06±8.98] days vs. [29.74±8.31] days, P<0.001) between the two groups. Death was observed in 6/35 patients (17.14%) with NRS score ≥3, but none with NRS score <3 (P<0.001) (Table 4), which shows the malnutrition group have worse prognosis.
Table 4. Comparison of in-hospital outcomes between the malnutrition group and the normal nutrition group.
| Outcomes | NRS-2002<3 (n=50) | NRS-2002≥3 (n=35) | P-value |
| aData are presented as mean±SD. NRS-2002: nutritional risk screening 2002. P-values were calculated by *chi-square test or #Student's t-test or Mann-Whitney U test. | |||
| Critical status, n (%) | 4 (8) | 19 (54.3) | <0.001 * |
| Length of in-hospital day (days)a | 29.7±8.3 | 37.1±9.0 | 0.032# |
| Deaths, n (%) | 0 (0) | 6 (17.14) | <0.001 * |
Discussion
Malnutrition affects 20% to 50% of hospitalized patients. It has a direct effect on the patients' prognosis and length of hospitalization[8], especially in those with higher nutritional risk, such as diabetic patients[9]. To determine whether diabetic patients being at risk of malnutrition is an essential part of clinical assessment. But current knowledge of nutritional risk in diabetic patients with COVID-19 is limited. Previous studies have shown that COVID-19 patients with chronic diseases such as diabetes have more severe status and worsen prognosis. Our study investigated the characteristics of nutritional indicators in hospitalized COVID-19 patients in severe and non-severe status.
Increased nutritional risk was found in 35 patients (41.8%), confirmed that malnutrition is a common health problem among diabetic patients with COVID-19. In our study, it seemed that BMI and TSF are not sufficient as a tool to assess the nutritional status of the patients. Both single factor regression analysis and multivariate analysis determined that serum prealbumin and NRS-2002 were independent predictors for developing severely ill status. Furthermore, ROC curve showed that prealbumin and NRS-2002 could well predict severely ill status for diabetic patients with COVID-19. Additionally, patients with higher risk of malnutrition would be more likely to have worse outcomes, including more possibly getting to be severely ill, having higher mortality and longer in-hospital stay. A recent report showed that a low prealbumin level predicts progression to acute respiratory distress syndrome (ARDS), suggesting that poor nutritional status is associated with bad outcomes[10]. Similar results were obtained by previous studies on other viral infections, which indicated that nutritional status plays a significant role in patient outcomes[6,11]. Healthy nutritional status may support immune function to prevent the onset of severe infection[12]. So maintaining a healthy nutritional status is warranted at this time to prime the immune systems of individuals infected or not infected with COVID-19. Some researchers have proposed nutritional interventions to increase the likelihood of beneficial clinical outcomes for non-critically ill and severely ill hospitalized COVID-19 patients[13].
One of the major causes for malnutrition in the course of COVID-19 is gastrointestinal disorder. Besides lungs, SARS-CoV-2 can affect many other organs through an intense inflammatory reaction, including the gastrointestinal tract. It has been observed that many COVID-19 patients have diarrhea, vomiting, and loss of appetite which led to less food intake. Delay of enteral nutrition often occurs in critically ill patients for life-threatening hypoxemia. In our study, nutritional indicators of severely ill patients were statistically worse than those of generally ill patients. In fact, diabetes itself is a risk factor for developing COVID-19 and increases the vulnerability to malnourishment[14]. Previous studies have implicated the close relationship of malnutrition with diabetes in patients[15]. Elderly patients with COVID-19 and diabetes mellitus had higher rate of malnutrition[2]. This was consistent with the results of our study. Obviously, it has to be underlined that the patients receive one additional point for being with diabetes in the NRS-2002, introducing bias to the above-mentioned results. It is mainly ascribable to the diet control, disorders of metabolism, and other factors.
The limitation of our study is the relatively small group of diabetic COVID-19 patients. Despite its limitation, the results of the present study are valuable because they indicate a problematic presence of malnutrition in diabetic patients with COVID-19. Further studies in these patients are needed to confirm our results. Therefore, nutritional assessment should be performed to screen for the risk of malnutrition, especially in diabetic COVID-19 patients.
Risk of malnutrition is highly prevalent among diabetic COVID-19 patients, which is associated with severely ill status and prognosis. Evaluation of nutritional status, especially the indicators of NRS-2002 and the level of serum prealbumin, should be applied in routine clinical assessment and prognostic stratification.
This work was supported by grants from the National Natural Science Foundation of China (No. 81970217 to WG); Science and Technology Development Foundation, Nanjing Medical University, China (No. NMUB2019074 to CZ); Natural Science Foundation for Colleges and Universities in Jiangsu Province (No. 20KJB320010 to CZ); Science and Technology Development Foundation of Geriatric, Geriatrics Society of Jiangsu (No. JGS2019ZXYY06 to XL); National Key R&D Program of China (No. 2020YFC2008505 to XL)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81970217 to WG); Science and Technology Development Foundation, Nanjing Medical University, China (No. NMUB2019074 to CZ); Natural Science Foundation for Colleges and Universities in Jiangsu Province (No. 20KJB320010 to CZ); Science and Technology Development Foundation of Geriatric, Geriatrics Society of Jiangsu (No. JGS2019ZXYY06 to XL); National Key R&D Program of China (No. 2020YFC2008505 to XL)
Footnotes
CLC number: R153, Ducument code: A
The authors reported no conflict of interests.
Contributor Information
Wei Zhao, Email: zhaowei@njmu.edu.cn.
Wei Gao, Email: gaowei84@njmu.edu.cn.
References
- 1.Zabetakis I, Lordan R, Norton C, et al COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecora F, Persico F, Argentiero A, et al The role of micronutrients in support of the immune response against viral infections. Nutrients. 2020;12(10):3198. doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuong C, Bates TA, Akter S, et al Nutritional status impacts dengue virus infection in mice. BMC Biol. 2020;18(1):106. doi: 10.1186/s12915-020-00828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck MA, Handy J, Levander OA Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12(9):417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arkin N, Krishnan K, Chang MG, et al Nutrition in critically ill patients with COVID-19: challenges and special considerations. Clin Nutr. 2020;39(7):2327–2328. doi: 10.1016/j.clnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrup J, Rasmussen HH, Hamberg O, et al Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi: 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 7.Singh AK, Gupta R, Ghosh A, et al Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr:Clin Res Rev. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano-Torres EA, Simental-Mendía LE, Morales-Garza LA, et al Impact of nutritional intervention on length of hospital stay and mortality among hospitalized patients with malnutrition: a clinical randomized controlled trial. J Am Coll Nutr. 2017;36(4):235–239. doi: 10.1080/07315724.2016.1259595. [DOI] [PubMed] [Google Scholar]
- 9.Reber E, Gomes F, Vasiloglou MF, et al Nutritional risk screening and assessment. J Clin Med. 2019;8(7):1065. doi: 10.3390/jcm8071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Chen X, Cai Y, et al Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haley KP, Gaddy JA Nutrition and Helicobacter pylori: host diet and nutritional immunity influence bacterial virulence and disease outcome . Gastroenterol Res Pract. 2016;2016:3019362. doi: 10.1155/2016/3019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsoupras A, Zabetakis I Comment on "Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181" . Nutrients. 2020;12(8):2321. doi: 10.3390/nu12082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caccialanza R, Laviano A, Lobascio F, et al Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Zhong W, Tian Y, et al The effect of diabetes on mortality of COVID-19: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99(27):e20913. doi: 10.1097/MD.0000000000020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibovitz E, Adler H, Giryes S, et al Malnutrition risk is associated with hypoglycemia among general population admitted to internal medicine units. Results from the MENU study. Eur J Clin Nutr. 2018;72(6):888–893. doi: 10.1038/s41430-018-0143-9. [DOI] [PubMed] [Google Scholar]