Abstract
Babesiosis is an emerging, tick-transmitted, zoonotic disease caused by hematotropic parasites of the genus Babesia. Babesial parasites (and those of the closely related genus Theileria) are some of the most ubiquitous and widespread blood parasites in the world, second only to the trypanosomes, and consequently have considerable worldwide economic, medical, and veterinary impact. The parasites are intraerythrocytic and are commonly called piroplasms due to the pear-shaped forms found within infected red blood cells. The piroplasms are transmitted by ixodid ticks and are capable of infecting a wide variety of vertebrate hosts which are competent in maintaining the transmission cycle. Studies involving animal hosts other than humans have contributed significantly to our understanding of the disease process, including possible pathogenic mechanisms of the parasite and immunological responses of the host. To date, there are several species of Babesia that can infect humans, Babesia microti being the most prevalent. Infections with Babesia species generally follow regional distributions; cases in the United States are caused primarily by B. microti, whereas cases in Europe are usually caused by Babesia divergens. The spectrum of disease manifestation is broad, ranging from a silent infection to a fulminant, malaria-like disease, resulting in severe hemolysis and occasionally in death. Recent advances have resulted in the development of several diagnostic tests which have increased the level of sensitivity in detection, thereby facilitating diagnosis, expediting appropriate patient management, and resulting in a more accurate epidemiological description.
Babesiosis, caused by infection with intraerythrocytic parasites of the genus Babesia, is one of the most common infections of free-living animals worldwide and is gaining increasing interest as an emerging zoonosis in humans. Although capable of infecting a wide range of vertebrates, babesial parasites require both a competent vertebrate and nonvertebrate host to maintain transmission cycles. All babesial parasites described to date are transmitted by ixodid ticks to their vertebrate hosts. The parasites replicate in the vertebrate hosts' red blood cells and are called piroplasms due to their pear-shaped appearance when within the infected host cells (99, 226). Most of what is known about the host response to babesial infections comes from observations of and studies on vertebrates other than humans. All mammalian hosts examined have been able to develop immunity to Babesia species, either after an episode of infection and recovery or after prophylactic immunization. Both humoral and cellular factors are involved in immunity to babesiosis.
Human babesiosis is caused by one of several babesial species that have distinct geographic distributions based on the presence of competent hosts. In North America, babesiosis is caused predominantly by Babesia microti (49, 158, 169, 213), a rodent-borne piroplasm, and also occasionally by a newly recognized species, the so-called WA1 piroplasm (161, 176, 231). In Europe, babesiosis is considerably rarer but more lethal; it is caused by the bovine pathogen Babesia divergens. The spectrum of disease is broad, ranging from an apparently silent infection to a fulminant, malaria-like disease resulting occasionally in death. Various determinants are involved in the severity of disease manifestation; among those identified are age, immunocompetence, and coinfection with other pathogenic agents. In this review, we will provide an overview of recent developments in the investigation of this interesting emerging zoonosis. Because most of what is known about babesiosis comes from animal studies, we will focus initially on work in animal models and then draw attention to features in common with the human disease.
CHARACTERIZATION OF THE ORGANISM
Host Specificity and Ecology
The babesias are one of the most ubiquitous and widespread blood parasites in the world based on numbers and distribution of species in animals, second only to the trypanosomes (114, 226). They generally have two classes of hosts, an invertebrate and a vertebrate host. The maintenance of Babesia spp. is dependent on both hosts; the specific tick vector must feed on a vertebrate reservoir that is competent in maintaining the Babesia organisms in an infectious state. Therefore, B. microti presents itself as an emerging zoonosis only in areas where there is a primary competent reservoir.
Invertebrate hosts.
Babesias can be found wherever certain species of ticks flourish. To date, only ixodid ticks have been identified as vectors for Babesia spp. except for one report that identified a nonixodes tick, Ornithodoros erraticus, as a reservoir for Babesia meri (72). Six of the seven main genera of ixodid ticks have been demonstrated as experimental or natural vectors of diverse Babesia spp. (202, 213, 226). Some Babesia species, such as Babesia bigemina and Theileria equi (Babesia equi) can infect more than one genus of ticks (99, 206), whereas B. microti can only infect ticks from the genus Ixodes (226). Several tick vectors can carry more than one Babesia species. For instance, Ixodes dammini can harbor B. microti, usually but not exclusively (165) in its nymphal stage (169, 215), along with Babesia odocoilei (6). It is not known if they can harbor more than one Babesia species at a time or if they can transmit more than one at a time.
The ecology and life cycle of B. microti and its interaction with I. dammini (also known as Ixodes scapularis [214]) is the best understood of the Babesia species (226). The nymphal stage of I. dammini and its interaction with Peromyscus leucopus (white-footed mouse) is essential for the maintenance of B. microti. Field surveys estimate that up to 40% of these mice are infected (83, 166, 215), and in one study as many as 60% were infected (55). The adult stages of I. dammini feed primarily on deer (Odocoileus virginianus), which do not serve as reservoirs for B. microti (170). They feed in the fall and again in the spring, after which they lay eggs (241). The eggs hatch in the summer (late July), and the larvae feed primarily on mice during August and September. This is the point at which the tick can acquire Babesia organisms. These infected larvae overwinter and molt to become nymphs in the spring (166). It is estimated that approximately 40% of the nymphal ticks in some areas (e.g., Nantucket Island) where babesiosis is endemic may be infected (166). The nymphs feed on hosts from May through July. Finally, nymphs that have fed molt into adults in the fall, completing the tick life cycle. In areas where human babesiosis is endemic, the nymphal ticks feed primarily on P. leucopus (i.e., northeastern United States) (77, 215). However, the range of the tick extends to the southeastern United States, where the nymphs primarily feed on lizards (216). It has been suggested that the lizards are poor reservoirs and are not able to maintain B. microti as an infectious agent (216), whereas mice can maintain the organisms. There have been only two reported cases of B. microti infection in Europe (69, 90). This is likely because of limited or no interaction between the tick host for B. microti in Europe and humans (227). The mouse-specific tick Ixodes trianguliceps is the reservoir for B. microti (226) and does not feed on humans.
It is believed that the tick responsible for transmission of B. divergens to humans is Ixodes ricinus (69, 227). The life cycle of I. ricinus requires 3 years, as larva in the first year, nymphs in the second, and adults in the third. A noteworthy observation is that a high incidence of B. divergens infections occur in cattle when ambient air temperatures are elevated, presumably when ticks are more active. In addition, most human cases have occurred in individuals who have frequent contact with cattle (40). Finally, I. ricinus is also the vector for the Lyme disease spirochete in Europe.
The tick host for the more recently discovered species WA1 is not known. There are a few candidates, however. The ticks Dermacentor variabilis, Ornithodoros coriaceus, and Ixodes pacificus are found in areas where cases of WA1 infection have occurred (227). An inability to infect I. dammini in the laboratory suggests that I. pacificus might not be the vector (227). The vertebrate host reservoir for WA1 is also unknown. WA1 is most closely related to the canine pathogen Babesia gibsoni, but WA1 does not seem to infect dogs. It will infect rodents and can be lethal, depending on the mouse strain used for study (142).
Vertebrate hosts.
More than 100 known Babesia species have been identified (113, 226) which infect many types of mammalian hosts, most numerously the order Rodentia, and also several avian species (99, 113, 226). Almost any mammal that serves as a host for a Babesia-infected tick is a potential reservoir (226). The host ranges of B. microti and B. divergens vary from small terrestrial mammals (15, 55, 213) to subhuman primates (139, 199) to humans for B. microti and from cattle to various rodent species and to humans for B. divergens (40, 119, 143). There are several examples of different and often more serious disease manifestations resulting from transmission of a Babesia species (e.g., B. microti) that is common in a wild vertebrate species (e.g., P. leucopus) to a poorly adapted vertebrate host (e.g., humans). As a natural reservoir for B. microti, most white-footed mice (P. leucopus) in babesiosis-enzootic areas are parasitemic; however, it is not unusual for less than 0.1% of the host erythrocytes to be infected (55, 215). In addition, white-footed mice seem to remain parasitemic for life (215). In contrast, hamsters and laboratory mice can develop rather high parasitemias, often as high as 40 to 50% in particularly susceptible hosts (12, 67, 116). B. equi (T. equi), naturally found in horses and transmitted by Hyalomma spp., produces an acute tick-borne hemolytic anemia in susceptible horses. This can be followed by a chronic carrier state that can result in reduced oxygen-carrying capacity, which causes decreased performance of racehorses (75, 150). B. equi infections are also a problem for the importation and exportation of horses (65). Babesia canis is found worldwide, being the most widespread and most pathogenic Babesia species in dogs. It is transmitted primarily by the tick Rhipicephalus sanguineus (via either transovarian or transtadial means) or Dermacentor reticulatus. Clinical infection in dogs can be hyperacute, acute, or chronic (42, 43, 76, 237). Symptoms in the acute form of the disease can include fever, jaundice, hemoglobinuria, and anemia and can result in death (42, 43, 237). B. bigemina, a cattle pathogen, has perhaps the greatest potential for economic impact in the United States. The vertebrate hosts include water buffalo and other wild ruminants, and transmission occurs through Boophilus spp. Although infections are not as virulent as those seen with Babesia bovis (not seen in the United States), there is an acute hemolytic phase which is often fatal.
The observation that so many Babesia species infect so many vertebrates without any apparent disease manifestations begs the question of whether there might be some selective advantage conferred on the carrier. Clearly, B. microti poses no real health threat to the white-footed mouse, so it is possible that as long as the infection does not cause any real problems for the host, there is no selection against it. Alternatively, there might be some benefit conferred on the host, such as protection against infection with the more pathogenic Plasmodium spp. The recent recognition of unusual biochemical pathways resembling those found in blue-green algae suggests that the organisms are capable of producing potentially useful metabolic products such that, for instance, nutritional requirements for certain compounds might be ameliorated.
Life Cycle
Apicomplexans/sporozoans (including the genera Babesia and its close relative Theileria) generally go through at least three stages of reproduction (Fig. 1) (99): (i) gamogony—formation and fusion of gametes inside the tick gut, (ii) sporogony—asexual reproduction in salivary glands, and (iii) merogony—asexual reproduction in the vertebrate host (reviewed in more detail in reference 99).
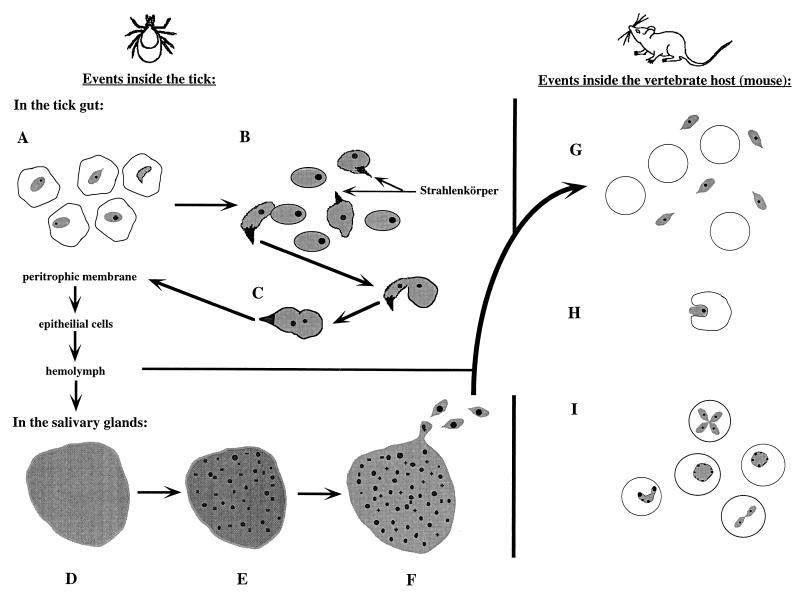
FIG. 1.
Life cycle of Babesia spp. in the tick and vertebrate hosts. Events in the tick begin with the parasites still visible in consumed erythrocytes. Some are beginning to develop Strahlenkörper forms (A). The released gametes begin to fuse (note that only one of the proposed mechanisms is pictured; one gamete has a Strahlenkörper form, whereas the other does not) (B). The formed zygote then goes on to infect and move through other tissues within the tick (C) to the salivary glands. Once a parasite has infected the salivary acini, a multinucleate but undifferentiated sporoblast is formed (D). After the tick begins to feed, the specialized organelles of the future sporozoites form (E). Finally, mature sporozoites bud off of the sporoblast (F). As the tick feeds on a vertebrate host, these sporozoites are inoculated into the host (G). Not shown is the preerythrocytic phase seen in Theileria spp. and T. equi (B. equi). Sporozoites (or merozoites) contact a host erythrocytic and begin the process of infection by invagination (H). The parasites become trophozoites and can divide by binary fission within the host erythrocyte, creating the various ring forms and crosses seen on stained blood smears (I). Illustrations are not to scale.
Events in the tick.
Much of what has been learned about the life cycle of Babesia spp. in the tick has been obtained from studies with B. microti (226). The organisms are first detectable in the tick about 10 h after the tick begins to feed on an infected vertebrate. After about 46 to 60 h of feeding, the parasites are still detectable within the consumed erythrocytes, but some of them (the gametocytes) begin to develop new organelles (Fig. 1A); most notable is the development of an arrowhead-shaped organelle at the anterior end of the organism (Fig. 1) called Strahlenkörper (101), or ray bodies. Organisms containing this arrowhead structure within the tick host have been found in all infections with Babesia and Theileria spp. (99) that have been examined. These arrowhead forms are likely involved in the fusion of the gametes (99, 189) (Fig. 1C). The resulting zygote uses the arrowhead to enter the epithelial cells of the tick gut approximately 80 h after the tick starts feeding. From the epithelial cells, the parasites move to the salivary acini via the hemolymph (188).
Sporozoite development within the salivary gland can be divided into three stages. First, the parasite expands and fills the hypertrophied host cell (Fig. 1D), forming a multinucleate sporoblast which is a relatively undifferentiated, three-dimensional, branching meshwork from which the sporozoites will bud (100). The second step starts only after the tick host begins feeding again; the specialized organelles of the future sporozoites (micronemes, rhoptries, and double membrane segments beneath the plasma membrane) develop within the meshwork (Fig. 1E). Finally, the mature sporozoites form through a budding process (Fig. 1F). Mature sporozoites are approximately 2.2 by 0.8 μm in size and pyriform-shaped and contain a smooth endoplasmic reticulum, free ribosomes, mitochondrion-like organelles, a single anterior rhoptry, and several micronemes (99, 100). Approximately 5,000 to 10,000 sporozoites can be produced within a single sporoblast.
It is estimated that several thousand sporozoites are deposited in the dermis around the tick's mouth during the final hours of attachment and feeding. This is a smaller inoculum than the approximately 10,000 to 25,000 sporozoites needed to syringe-inoculate white-footed mice or hamsters (169). The efficiency of tick transmission is attributed to the tick saliva, which probably facilitates infection with its anti-inflammatory and/or immunosuppressive pharmacological activity (178).
“Large” Babesia species, like B. divergens, can be transmitted transovarially. After the zygotes (also called ookinetes) have entered the hemolymph, they may invade other cells, such as fat body cells or nephrocytes, and undergo a second cycle of division (226). These secondary ookinetes can then invade the ovaries and be transmitted transovarially. The implications, if any, of this mode of transmission in the tick for human infections are unclear, except that transovarial transmission can theoretically result in large numbers of infected ticks in areas where Babesia spp. are endemic.
Events in the vertebrate.
The length of time that the tick is attached to the vertebrate host directly affects the efficacy of sporozoite transmission to hamsters and white-footed mice (167) (i.e., the longer the tick is attached, the more likely it is that transmission of the sporozoites will occur). If the tick is allowed to feed to repletion, infection rates approach 100% (167).
Once in the vertebrate, the transmitted sporozoites seem to infect the erythrocytes, except in the case of Theileria and some Babesia species, which invade lymphocytes first. Sporozoites invade the lymphocytes and then differentiate into multinucleate schizonts (131). These go on and differentiate further into merozoites, which bud off from the schizont and lyse the cell. These merozoites or sporozoites (from Babesia species without a preerythrocytic stage) infect the host erythrocytes (Fig. 1G). The merozoite invades the host erythrocyte through a process of invagination (Fig. 1H) (191), forming a parasitophorous vacuole. The vacuole membrane gradually disintegrates, and the parasite is left with the defining piroplasm feature of a single membrane, in contrast to Plasmodium species, which invade by a similar mechanism but retain the host membrane in addition to its own (191). Within the host erythrocytes, most merozoites become trophozoites and divide by binary fission (Fig. 1I); this asexual reproduction produces more merozoites, which lyse the cell and go on to infect additional erythrocytes. Four parasites can form at the same time, giving rise to a Maltese cross form (Fig. 1I). Rapid reproduction destroys the host cell and leads to hemoglobinuria in the host. Some trophozoites can, however, become potential gametocytes (129, 190). These trophozoites do not reproduce at this point but instead increase in size (187). Later on, when they are in the gut of the tick, these gametocytes will develop into gametes prior to leaving the erythrocytes within the tick gut (190).
Phylogenetic Classification
The taxonomic classification of Babesia spp. places them in the phylum Apicomplexa (also called Sporozoa), class Aconoidasida (Piroplasmea), and the order Piroplasmida (113, 115, 129). Piroplasms are characterized by intraerythrocytic forms which can be pear-shaped (113). They have apical complex organelles (including rhoptries and micronemes), a merogonic stage within the vertebrate host erythrocytes, and sexual development and sporozoite formation within the invertebrate host (which in the case of Babesia spp. has been only described in ticks [99, 227]). Two of the families within the order Piroplasmida are Babesiidae and Theileriidae; the primary distinction between them is usually defined as the absence of a preerythrocytic cycle in Babesia and the absence of transovarial transmission in Theileria (99, 180, 226).
Initially, Babesia species were identified based on morphological parameters of the intraerythrocytic forms (i.e., trophozoites) visible on stained blood smears from infected animals and vertebrates. This analysis, along with host specificity, has provided a means of classifying the various species. More than 100 species of Babesia have been described, infecting a wide variety of vertebrates. It is suspected, however, that many of these descriptions may be of similar or identical species that the traditional methods could not distinguish (160). These traditional methods of classification are gradually being supplanted by more recent molecular biological methods which are useful in differentiating between similar organisms and confirming distinctions based on more subjective characteristics. There are several reasons to justify using molecular analyses to classify Babesia rather than methods based on morphological parameters and host specificity. (i) Different parasites in the same hosts may appear to be morphologically similar (e.g., Plasmodium and some Babesia species). (ii) The same parasite may have different microscopic appearances in different hosts, probably due to host-specific factors, such as splenic function and immunologic predisposition (e.g., B. divergens has its “characteristic” appearance in bovine erythrocytes, but in humans it exhibits extensive pleomorphism, which complicates its diagnosis). (iii) The classification of Babesia species on the basis of host specificity appears to be less useful than once thought, since certain extensively studied species such as B. microti have been shown to have a broad host specificity (15, 55, 139, 198, 215). The newer techniques are arguably more objective than those based on observation of visible characteristics (160). Thus, it is anticipated that classification based on comparison of nucleic acid sequences will likely show that many babesial species (like B. microti) can infect many different host organisms, resulting in synonymy of previously distinct species (160).
Babesia are grouped informally into the small Babesia (trophozoites are 1.0 to 2.5 μm; species include B. gibsoni, B. microti, and Babesia rodhaini) and large Babesia (2.5 to 5.0 μm; species include B. bovis, Babesia caballi, and B. canis). These morphological classifications are generally consistent with the phylogenetic characterization based on nuclear small subunit-ribosomal DNA (nss-rDNA) sequences, which shows that the large and small babesias fall into two phylogenetic clusters (Fig. 2), with the small babesias being more related to Theileria spp. than the large (one exception to this is the human pathogen B. divergens, which appears small on blood smears [0.4 to 1.5 μm] but is genetically related to large babesias [Fig. 2]). Indeed, some sequences in B. microti (nss-rDNA fragment) show greater similarity to Theileria annulata (91%), a bovine pathogen, than to members of its own genus (e.g., B. bigemina is 88% similar to B. microti [162]). This was the first molecular evidence that the small Babesia spp. were in fact evolutionarily linked to Theileria (162). This, coupled with the observation that, like Theileria species and in contrast to large Babesia spp., none of the small Babesia spp. seem to be transmitted transovarially in ticks, has led to the suggestion that the small babesias should be classified with Theileria (132, 226, 227). In fact, descriptions of such a stage in B. equi (132, 202) have led to this species' reclassification as T. equi (130), which further supports the reevaluation of the former classification system. It has also been suggested that a preerythrocytic cycle exists for B. microti as well (132), but further confirmation is needed. Evaluation of other genetic loci should help clarify the relationship of the two genera and lead to a better understanding of the taxonomic position of such species as B. equi and B. microti.
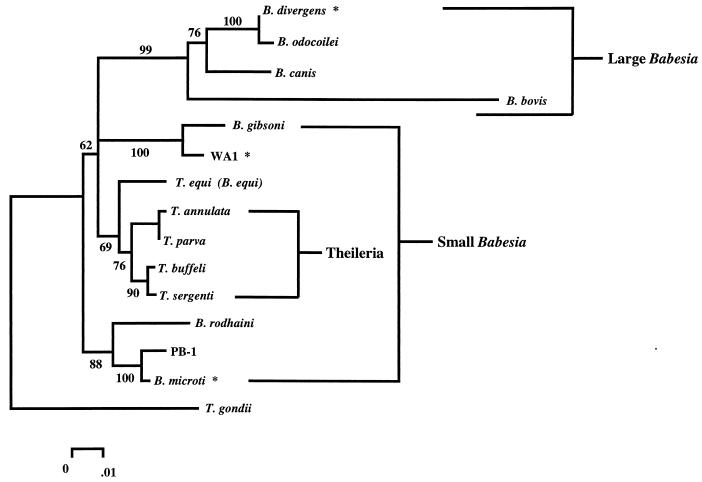
FIG. 2.
Phylogenetic tree representation of a neighbor-joining analysis of several species of piroplasms. Five hundred nucleotides of the nuclear small-subunit rDNA were aligned by using the Pileup program of the Wisconsin Genetics Computer Group package. Phylogenetic analysis of the alignment was performed as described previously (102) with the Molecular Evolutionary Genetics Analysis (MEGA) computer program, version 1.01 (109), to make a Jukes-Cantor distance measurement and perform a neighbor-joining analysis with 500 bootstrap replicates. The phylogenetic analysis using parsimony (PAUP) computer program, version 3.1.1 (222), was used to confirm the order observed by the neighbor-joining analysis (using a branch-and-bound algorithm with 100 bootstrap replicates). The percentage of neighbor-joining bootstrap replications (>50%) is shown above each node. This tree is consistent with previously published analyses (160, 161). Species that are known to infect humans are marked with an asterisk. The groups of large and small babesias are bracketed and labeled.
The two primary Babesia species which have been found to infect humans are B. microti and B. divergens, along with the as yet unnamed species WA1 (176, 231), CA1 (161), and MO1 (81) (Fig. 2, marked with *). There are also reports of human infection with other species such as B. bovis (30, 207) and B. canis (94), but some have not been well documented. It is interesting to note that members of both the “large” (B. divergens) and the “small” (B. microti) babesias are capable of infecting humans. Not surprisingly, they have different host requirements and the disease manifestations are somewhat different. This will be discussed in more detail below.
Molecular phylogenetic analysis has been useful for further defining the phylogenetic relationship between Babesia and Theileria (162, 176). The potential is there for genetic analyses to also aid in the discovery of new and/or previously undetectable pathogens. An example of this was the discovery of WA1 (176, 231). In 1991, an acute malaria-like syndrome in a patient was attributed to a new Babesia-like piroplasm, designated WA1. Although WA1 is morphologically similar to B. microti, several differences were noted, including antigen cross-reactivity (176), virulence in hamsters (100% fatality within 10 days), and Southern restriction fragment length polymorphisms of DNA digests (176, 231). All of these data indicated that WA1 was a new human pathogen, distinct from B. microti. Subsequent studies involving determination of the ribosomal subunit sequences and comparison with other piroplasm-derived sequences showed that WA1 was most closely related to B. gibsoni (a pathogen of dogs that produces a chronic condition with poor susceptibility to antimicrobial treatment). Phylogenetically, WA1 falls within a cluster (Fig. 2) that includes T. equi (B. equi) and the known lymphoproliferative Theileria piroplasms.
Another piroplasm was discovered from several cases of human babesiosis occurring between 1991 and 1993 in California. The patients were all splenectomized and were blood smear positive for piroplasms on admission; two had complicated courses, and one died. To identify the species, broad-range PCR was used (161). Prior to this, broad-range PCR was mostly used for identification of unculturable bacterial pathogens from human clinical specimens. These new cases posed a problem because the presence of human host rDNA sequences complicated the analysis of what was presumably unculturable eukaryotic pathogens; the highly conserved regions of the nss-rRNA gene used to recover the protozoal sequences are shared by the human homolog. To circumvent this problem, DNA sequencing primers were selected to hybridize only to the protozoal DNA, thereby allowing the protozoan-specific DNA to be sequenced out of the pool containing both piroplasm and human DNA (161). The protozoal DNA sequences obtained from the patient samples were nearly identical to each other (99.8%) and 95% similar to the above-mentioned WA1 (161). This analysis correlated with serologic cross-reactivity (161). Phylogenetic analysis showed that although related, this California protozoan (CA1) was distinct from both WA1 and B. gibsoni.
Most recently, a broad-range PCR survey of samples from baboons in various colonies maintained in the United States not only discovered a “new” Babesia species (PB-1; Fig. 2) (18) but demonstrated that up to 40% of the baboons in these colonies were infected (M. J. Homer and D. H. Persing, unpublished data). The newly discovered species is most closely related to B. microti (Fig. 2) and may be the organism previously described as Entopolypoides (18, 114). Recognition of this infection will be important in the prevention of experimental complications as well as potential zoonotic transmission.
Theileria
The identification of a preerythrocytic stage in the vertebrate host differentiates Theileria species from Babesia species. However, such a stage is suspected to exist in B. microti (131) and has been more definitively identified in T. equi (B. equi) (131, 202). Studies of this stage in Theileria have shown it to have some remarkable qualities, and it therefore merits some discussion and may provide valuable insights into piroplasmic infections. The preerythrocytic stages of Theileria parva and T. annulata are intralymphocytic schizonts that are capable of blastogenesis and clonal expansion of predominantly T and B cells, respectively (8, 60, 217). This transformation is reversible; treatment with buparvaquone results in elimination of the schizonts, and subsequent proliferation is inhibited. Theileria species undergo a repeated schizogony in the lymphocytes, resulting in the release of small merozoites that subsequently infect red cells and become trophozoites. It is the lymphocytic stage that causes many of the severe disease manifestations of Theileria infections (lymphadenopathy, pyrexia, thrombocytopenia, and panleukopenia).
It is not surprising that the lymphoproliferative process caused by infection has generated considerable interest—this system provides a unique and potentially powerful tool for examining and possibly elucidating mechanisms of cell cycle control in lymphocytes. The two species most commonly used in these studies are T. parva (the cause of East Coast fever), which preferentially causes T-cell proliferation (in T cells expressing either αβ or γδ T-cell receptors), and the closely related T. annulata (the cause of tropical theileriosis), which infects primarily B cells and macrophages (217). Both species cause severe lymphoproliferative disease, and the infected cells can proliferate indefinitely in cell cultures. These transformed cells have several characteristic traits, including changes in surface epitopes for monoclonal antibodies (146), pleiomorphism, and short generation times (16 to 25 h in vitro) (219). Furthermore, when the infected lymphocytes are injected into athymic (92) or SCID (61) mice, they infiltrate tissues and form tumor-like metastatic masses. Remarkably, the transformation remains reversible upon drug treatment even after many years in culture (85, 126, 181). The exact mechanism through which Theileria induces proliferation is not known, but it is possible that T. annulata and T. parva might employ different mechanisms. The dysregulation of several kinases has been implicated (54, 62, 66, 148, 149) as well as disruption or induction of various transcriptional activators (14, 154).
The importance of these studies and observations when considering Babesia is unclear. Although preerythrocytic stages have been detected in some species, nothing as definitive or remarkable as the Theileria-transformed cell lines have been developed or even observed. Despite the lack of evidence, there could indeed be effects on cell cycle control due to babesial infection that may have serious consequences for the host. Some studies have even implicated Babesia species in a leukemogenic role (87–89), which could involve similar mechanisms to those employed by Theileria, but further studies would be needed to draw such conclusions.
Host Immune Response
All mammalian hosts examined have been able to develop immunity to Babesia species, either after an episode of infection and recovery or after prophylactic immunization (see Vaccine section below). Both humoral and cellular factors are involved in immunity to babesiosis (Fig. 3). It is in the first stages of a babesial infection that the immune system gets effectively “primed.” When the infection from a tick first occurs, the sporozoites are free in the plasma in the bloodstream for a short period of time. At this stage, immunoglobulin G (IgG) antibodies can prevent infection by binding and neutralizing sporozoites before they succeed in invading their target cells (Fig. 3A). A new stage begins when babesial organisms establish their intraerythrocytic infection (Fig. 3B). It is during this progression stage that parasitemia rises and acute disease can occur. Cells of the innate immune system are responsible for controlling the growth rate of the parasite and therefore the extent of parasitemia. In the absence of macrophages and NK cells, a higher parasitemia develops in a shorter period of time. The inhibition is most likely accomplished by the production of soluble factors: gamma interferon (IFN-γ) by NK cells and tumor necrosis factor alpha (TNF-α), nitric oxide (NO), and reactive oxygen species (ROSs) by macrophages. However, it is unclear how these molecules can interfere with the development of the parasite inside the erythrocyte.
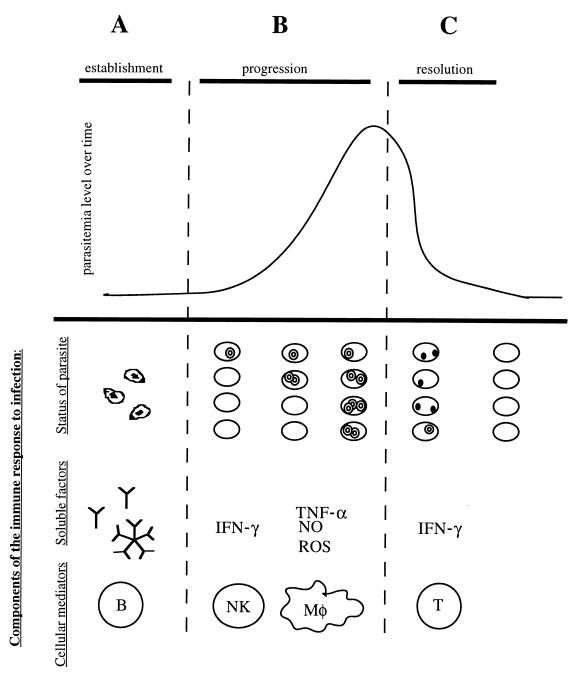
FIG. 3.
Theoretical model of the cells and molecules involved in immunity to Babesia species. Different immune mechanisms contribute to resistance during each stage of babesial infection. During the establishment stage (A), antibodies (IgG) play a role in preventing erythrocyte infection by binding the free sporozoites. During this progression stage (B), the Babesia organisms succeed in invading the erythrocyte, and the resulting merozoites start proliferating and lyse the infected cell. After lysis has occurred, parasites reach the bloodstream again to initiate a new round of invasion. Several rounds of this cycle cause the overall parasitemia level to increase. Cells of the innate immune system are thought to control the growth rate of the merozoites and therefore the rate of increasing parasitemia. Specifically, NK cells and macrophages have been implicated in antibabesial activity. The inhibition seems to rely on the production of soluble factors: IFN-γ by NK cells and TNF-α, nitric oxide (NO), and ROSs by macrophages (Mφ). The specific mechanism of protection, however, remains unclear. In the resolution stage (C), parasitemia levels in babesiosis usually reach a maximum and then decline. The decrease in parasite numbers seems to be due at least in part to intracellular degeneration inside the erythrocyte, as evidenced by the appearance of crisis forms. T-cell lymphocytes seem to be the cells responsible for parasite clearance, specifically the subpopulation of CD4+ IFN-γ producers. The mechanism of parasite eradication and its relation to IFN-γ production remain unknown.
In the murine experimental model, parasite clearing starts and parasitemia levels begin to decline approximately 10 days after infection (Fig. 3C). The falling parasitemia is due to Babesia degeneration inside the erythrocyte and clearance by the spleen, which is hyperreactive. When this happens, the infection enters the resolution stage and the disease subsides. Intraerythrocytic killing at the resolution stage requires T lymphocytes, specifically the subpopulation of CD4+ IFN-γ producers. It has been proposed that IFN-γ is directly responsible for the intraerythrocytic parasite degradation, but such direct involvement has not been proven (see below). In contrast, immune animals display an already developed antibabesial immune response upon encountering a new infectious challenge; these animals do not show the stage of rising parasitemia, and often no parasites can be detected in circulating blood.
Humoral responses.
The humoral component of the immune system is currently considered of limited importance in protection against babesial infections. Mice immune to B. rodhaini remain protected after an irradiation dose that suppresses B-lymphocyte antibody production (242). Likewise, in cattle infected with B. divergens, it was found that the antibody response was not the factor determining the development of the primary parasitemia (33). Moreover, transferring immune serum to immunodeficient mice infected with B. microti does not confer the ability to resolve the infection (123). However, some degree of immunity to B. microti can be transferred to cattle and mice with serum containing specific antibabesial antibodies (118). Immune serum can also delay the onset of B. rodhaini parasitemia, but it neither prevents the development of infection nor protects the infected mice from death (1). It has been demonstrated that antibodies in the serum neutralize babesial sporozoites or merozoites at the extracellular stage (1, 82, 177, 234). Indeed, antibodies have more effect on free parasites than on infected red blood cells (1). Therefore, the protective role of antibodies seems to be restricted to a short window of time between the moment that the parasite gains access to the bloodstream and the time that it invades the target cells (Fig. 3A).
Some observations suggest that Babesia species can subvert the humoral immune response and manipulate it to its advantage. Recent studies with B. bigemina show that a parasite protein expressed on the surface of the host erythrocyte is involved in binding IgM (52). The authors propose that IgM binding might be somehow useful for parasite growth and survival (52). This hypothesis is supported by the observation that IgM-deficient mice are unexpectedly resistant to B. microti infection (184), which would support a theory that the parasites utilize IgM to facilitate the infection process.
A similar stratagem seems to exist in relation to the complement pathway. No complement-mediated lysis of Babesia parasites has been found. On the contrary, it has been observed that several components of the complement pathway are essential in the invasion of erythrocytes by B. rodhaini (31). Also, in a study in which complement was used to promote macrophage phagocytosis of B. rodhaini merozoites, the unexpected finding was that the presence of complement inhibited phagocytosis of the pathogen (157).
Cell-mediated responses.
Insight into the possible involvement of cellular immune responses in the resistance to babesial infections came from the recognized importance of the spleen in defense of the host against Babesia species (reviewed in reference 245). The spleen is a large lymphoid organ, populated by T cells, B cells, natural killer (NK) cells, and macrophages. Some of these cell populations, then, could be responsible for the protective effects observed. In fact, it is possible to protect mice from Babesia infections by the adoptive transfer of spleen cells from immune animals (127, 128, 182, 242). Moreover, good levels of protection seem to be conferred specifically by splenocytes and not by lymph node cells (194), probably reflecting the fact that the systemic antigens are channeled preferentially to the spleen and not to peripheral lymph nodes.
The specific involvement of T cells has been examined by using thymus-deficient animals. Infecting congenically athymic mice (36, 193) with B. microti results in an elevated persistent parasitemia, which contrasts with the transient parasitemia observed in normal mice. These data indicate that the T cells are critical in resistance to babesiosis and also that the T-cell-mediated mechanisms occur at the resolution stage (193). T cells have also been implicated in the protection against lethal Babesia species; mice immunized against B. rodhaini experience a rising parasitemia and high mortality when treated with antithymocyte serum (244). Further, it has been shown that the transfer of purified T lymphocytes obtained from immune animals is sufficient to confer immunity to B. microti in naive mice (194), and the adoptive transfer of immune thymocytes to immunodeficient mice confers the ability to resolve a B. microti infection (123) (Fig. 3C).
B. microti antigens can trigger specific activation of T cells. Parasite-infected erythrocytes as well as free merozoites are able to sensitize mice for delayed-type hypersensitivity, an immune phenomenon mediated by T lymphocytes, in particular by the subpopulation known as CD4+ Th1 cells (195). Mice depleted of CD4+ T helper cells are more susceptible to B. microti infection than normal mice (91, 205). In contrast, susceptibility to infection is unaffected (91) or even decreased (205) in mice depleted of CD8 cytotoxic T cells. Therefore, CD4+ T helper cells seem to be the subpopulation chiefly responsible for protection against B. microti.
Although the presence of a specific immune subpopulation correlates with resistance to babesiosis, it is still unclear what effector mechanisms could be responsible for clearance of the pathogen in the infected host. In particular, there is no evidence that immune cells actually kill free parasites or infected erythrocytes by direct lysis. In the early studies, it was found that the remains of dead B. microti (crisis forms) appeared inside erythrocytes at the time of a decline in the parasitemia. This suggested that a soluble mediator was responsible for degeneration of the parasite (39). To date, this remains the most plausible mechanism of parasite clearance, since complement lysis and CD8 cytotoxicity have been ruled out. It is interesting to note that the production of IFN-γ by CD4+ T cells was found to be at least partially responsible for the resolution of parasitemia after primary infection (91). There is some evidence suggesting that IFN-γ could be directly toxic to intracellular parasites, including those that are intraerythrocytic (reviewed in reference 240) (Fig. 3B and C).
Although the activation of immune cells in general seems to be protective against babesiosis, the possibility remains that some of the expanded immune cell populations could be taking part in the pathogenesis of the disease. This possibility is supported by the observation that immunosuppressed mice challenged with the lethal B. rodhaini survived better than untreated, immunocompetent mice (242).
Nonspecific responses (innate immunity).
There is evidence to suggest that protection against babesial infections could be mediated through nonspecific components of the immune response, the so-called innate immunity. Several specific molecules involved in innate immunity have been elucidated in the past several years. Specifically, NK cells and macrophages have been implicated in antibabesial activity.
The role of natural killer cells was first proposed based on a highly suggestive relationship between levels of NK cell activity and resistance to B. microti in inbred strains of mice (56). It is possible that NK cells might be mediating protection in the early stages of infection (212) (Fig. 3B). Other studies found high NK cell activity during peak parasitemia and the recovery phase (95). Evidence of NK cell activity has also been obtained from studies on human babesiosis. A cell population with characteristics of NK cells was found to be significantly elevated in patients with acute babesiosis (11). A recent case report also showed a marked increase in NK cells during the acute phase of the infection (203). However, although the authors propose that NK cells might be involved in the host defense against acute babesiosis, such a conclusion cannot be made simply on the basis of a correlation.
The protective role of macrophages has been analyzed in the mouse model of babesiosis. Macrophage depletion with silica eliminates protection against B. microti (145). Also, macrophage inhibition (244) or depletion (201) totally abolished the protection of mice immunized against B. rodhaini, causing high mortality. Conversely, it is possible to protect naive mice against B. microti by the adoptive transfer of macrophages from immune animals, and the protection is even better than that obtained by adoptive transfer of immune T cells (127) (Fig. 3B).
A defining characteristic of macrophages and NK cells is that they are able to produce soluble mediators in response to a variety of nonspecific infectious stimuli. This could be of primary importance for babesiosis, since it is extensively documented that activation of nonspecific immune responses via unrelated stimuli can confer resistance against babesiosis (35, 37, 38, 47, 110, 243). In all these cases, a nonspecific soluble mediator is thought to be responsible for the protection.
Macrophage stimulation has been found to inhibit parasite growth in infected mice through the production of nitric oxide (185). Another macrophage soluble mediator, TNF-α, has also been proposed to mediate parasite death in babesiosis (34). There is also evidence that supports a role for ROSs in the intraerythrocytic killing of B. bovis (97) (Fig. 3B).
Immunological effects of coinfection with other pathogens.
Multiple infections due to distinct pathogens in the same host may have nonspecific effects on each other through host immune responses. There have been studies that report on the antagonistic or synergistic effects of coinfecting agents. The establishment of a particular pattern of immune response (i.e., type 1 versus type 2) early in the course of many infections may radically affect the course of disease progression or resolution. The role of helper T cells and their differentiation into Th1 and Th2 subsets has been the focus of recent studies attempting to elucidate the mechanisms involved in the synergism observed during coinfection. Similar to the immunologic effects seen during infection with unrelated pathogens in the experiments conducted by Clark et al. in the 1970s (35–39), it is possible that immunologic interactions occur within the Lyme disease transmission cycle involving combinations of agents within the mouse reservoir; currently, the list includes Ehrlichia, Babesia, Borrelia, and Bartonella. It is reasonable to hypothesize that an immune response to one organism may have trickle-down effects related to the infection process due to a coinfecting agent, either synergistically or antagonistically.
Immunosuppression is a common characteristic among various parasitic infections (71, 144, 220). There are several lines of evidence that demonstrate the immunosuppressive effects of B. microti infections on the maintenance of coinfecting agents. B. microti infections can impair the ability of host mice to reject Trichuris muris (nematode) infections (163), prolong and enhance Trypanosoma musculi infections in mice (144), result in decreased Trypanosoma-specific antibody production (144), and decrease the ability of mice to mount an immune response to sheep erythrocytes (2, 175).
In the case of coinfection with the agent for Lyme disease, infections with B. microti may elicit an immune response that results in establishment of higher numbers of Lyme disease spirochetes (160). Borrelia burgdorferi establishment and pathogenesis are favored in a Th1-dominant environment, whereas the infection can be effectively controlled by a Th2-dominant CD4+ T-cell response (124, 160). Studies with C3H mice (respond to B. burgdorferi with a Th1-dominant response) and BALB/c mice (Th2-dominant response to B. burgdorferi) have shown that C3H mice maintain higher numbers of spirochetes than BALB/c mice when infected with B. burgdorferi. It is possible that coinfection with B. microti could skew T-cell development towards a Th1 response, thereby facilitating a more established infection of B. burgdorferi. The alternative situation is also possible, in which B. burgdorferi could enhance babesial infection; this could be consistent with recent field survey results in mice, in which B. microti was primarily found in mice that were also infected with B. burgdorferi (5). Other, unexpected pathogen combinations are also found; another survey examined 152 baboons in two colonies (maintained in the United States) for the prevalence and distribution of simian T-lymphotrophic virus (STLV) and babesial infections among the two populations. The data suggest that the baboons become infected with STLV at an earlier age than with Babesia spp. and that infection with STLV predisposes to babesial infection (Homer and Persing, unpublished data).
There have also been several documented cases of antagonism between Babesia spp. and other infectious agents. As mentioned above, some other infections or pathogenic antigens can confer or elicit a protective immune response against infections with Babesia spp. (34, 35, 37, 38). Isospora felis infections (223), inoculation with Mycobacterium bovis BCG or Mycobacterium phlei (37, 230), and killed Corynebacterium parvum (38, 44) can also protect mice against babesial infections.
HISTORY OF CLINICAL APPEARANCE OF BABESIOSIS
Babesial infections have probably been complicating the lives of humans since antiquity, primarily through infections of domestic livestock. Only recently, in the latter half of this century, have these infections become a documented immediate threat to human health, earning the title of an emerging infectious zoonosis. The Biblical book of Exodus may contain the first “historical” reference to babesial infection. The plague of the Egyptians' cattle is described as a “grievous murrain” that could have been red water fever of cattle (caused by B. bovis) and could have included hematuria as a prevalent sign. However, the genus was not formally recognized until the work of Babes (7) in 1888, who studied the cause of febrile hemoglobinuria in cattle. Shortly thereafter, it was discovered that ticks provided the mode of transmission of B. bigemina, the Texas cattle fever pathogen (211).
The first documented case of babesiosis in humans was in 1957 (207). A splenectomized farmer in Yugoslavia was diagnosed with a B. bovis infection (207). Given the subsequent observation that most cases in Europe are due to B. divergens and the difficulty of accurate diagnosis of B. divergens by blood smears, it is more probable that this first case was due to B. divergens. Subsequently, there have been several cases of zoonotic babesiosis in Europe; most cases occurred in splenectomized individuals and often resulted in fatality (69), and the majority of these cases were due to infection with B. divergens.
Human babesiosis in the United States is most often caused by B. microti (226), but other distinct piroplasms are also emerging as causative agents (160). B. microti was described in P. leucopus in the 1930s, but P. leucopus was not identified as the reservoir until 1976 (77). Babesiosis was one of the first zoonoses in the United States to be identified definitively as a tick-transmitted disease. It was considered a common infection in many animals and not a threat to human health until the 1960s, when a series of B. microti infections were identified in residents of Nantucket Island (with Nantucket fever) (77, 168, 196, 198, 213). Since then, babesial infections have become a relatively commonly diagnosed tick-transmitted disease in the northeastern coastal regions and upper midwestern United States.
CLINICAL PRESENTATION
Epidemiology
Most cases of babesial infections in humans have been acquired in temperate regions of the United States and Europe. The actual frequency of B. microti and WA1 infection in the United States is probably much greater than the number of reported cases (136 cases in New York between 1970 and 1991 and 160 cases in Nantucket between 1969 and 1998) because babesiosis is self-limiting and mild in most persons, and it is likely that there are undiagnosed carriers (160). B. microti infections are endemic in the northeastern and Great Lakes regions, but the range is probably expanding. Infections in Europe are caused by B. divergens, mostly in splenectomized individuals; to date, about 30 cases of babesiosis have been reported.
Serosurveys have been the primary technique used to survey populations for babesial infections. Most have been performed in areas where clinically apparent cases have occurred. Surveys of blood donors have shown 3 to 8% prevalence for B. microti. One survey in California showed as high as 16% prevalence of antibodies against the WA1-like organism (161), but high seroprevalence rates in blood donors from areas where babesiosis is not endemic suggest that the WA1 serologic test lacks specificity. The mortality rate for clinically apparent infections of B. microti is about 5% in the United States (133).
With only 29 reported cases, babesiosis is a relatively rare occurrence in Europe. It is, however, very serious, as the infection has a 42% mortality rate (69). Most of the cases have been reported in France and the British Isles, but this is probably not an accurate representation of distribution of the organism itself, since heightened medical and scientific interest in babesiosis will probably result in more reported cases (69). A few cases of babesiosis have been described in other parts of the world, including China (69), Taiwan (204), Egypt (134), South Africa (22), and Mexico (69).
There have been several cases of transfusion-acquired babesiosis in the United States and none reported thus far in Europe or elsewhere. Most of these have involved the transmission of B. microti from an asymptomatic donor (57, 70, 120, 136, 172, 210), and the blood had been stored for from 5 to 35 days, including one case of transmission by frozen-deglycerolized blood (50, 70, 172). The incubation period for appearance of the infection has varied from 17 days to 8 weeks (136, 210). There has also been one case of transfusion-acquired WA1 (80).
Symptoms in Humans
The disease manifestations of human babesiosis are caused by the asexual reproductive stage of the organism in the erythrocytes of the host and the subsequent lysis of host cells. Consequently, there is a very broad clinical spectrum which is probably directly reflective of the level of parasitemia in the blood. The incubation period from the time of tick transmission of the organism to the appearance of symptoms varies from 1 to 6 weeks and may be as long as 3 months (10). Host factors associated with the biological variation in disease presentation are poorly understood.
The extreme end of the spectrum is often described as a fulminating malaria-like infection; symptoms may include malaise, chills, myalgia, anemia, fatigue, and fever (which can be as high as 40°C). Some cases also described nausea, emesis, night sweats, weight loss, and hematuria, which are believed to be associated with higher levels of parasitemia (10, 160). Hepatomegaly and splenomegaly may also be present. Hemolytic anemia that lasts for several days to a few months can occur in clinically severe cases, most commonly in asplenic or elderly hosts. Complications are more likely in immunocompromised patients and can include worsening of an already weakened state or, rarely, adult respiratory distress syndrome.
The cases due to B. divergens infections seen in Europe are usually more severe than those caused by B. microti. Onset of disease symptoms usually occurs within 1 to 3 weeks of the infecting tick bite (69). Most patients had been splenectomized prior to infection. Illness appears suddenly, with hemoglobinuria as the presenting symptom followed by jaundice due to severe hemolysis. In the most severe cases, patients develop a shock-like picture, with renal failure and pulmonary edema (69).
The presence or absence of many laboratory manifestations generally depends on the level of parasitemia (173). Clinically apparent cases may develop high levels of transaminases, alkaline phosphatases, unconjugated bilirubin, and lactic dehydrogenase in serum. Normochromia, normocytic anemia, thrombocytopenia, and, occasionally, leukopenia may also be present. This may be due to TNF-mediated inflammation responses, similar to the pathogenesis of severe malarial infections. However, in light of the recent recognition of coinfection in humans with multiple tick-transmitted agents, it is possible that some of the more variable aspects of the disease could also be associated with coinfection (see below).
Host Susceptibility
There are probably many host characteristics that affect the severity of babesiosis; among those identified are age and immunocompetence. The most severe infections occur predominantly in the elderly and in splenectomized or immunocompromised hosts. There appears to be a correlation between the severity of the infection and the age of the patient (10, 197). In patients infected with B. microti, the ages have ranged from 3 weeks to 86 years, with the majority of clinically apparent cases falling in the range from 50 to 60 years (108). This finding was most striking in a recent study of the persistence of parasitemia after acute babesiosis; the mean age of mild or asymptomatic subjects was approximately 30 years less than that of severe cases (104). It has been observed that adult P. leucopus are more frequently parasitemic than juveniles (215). Another study showed that older laboratory (BALB/c) mice had reduced and delayed peak parasitemias compared with more juvenile mice but that the older mice could not clear the parasites and experienced periodic parasitemias until death (74). Also, resistance to B. divergens is seen in young cattle (17).
Additional factors determining the severity of babesiosis are asplenia and coinfection with other infectious agents (58, 152, 210, 227, 229). Almost all the cases of babesiosis in Europe (∼83%) have been attributed to B. divergens; these infections have reportedly been more severe and almost always occurred in patients who had been splenectomized prior to infection (69). These cases have often been fatal. In contrast, most of the cases in North America have been caused by B. microti and occurred in normosplenic patients. The exceptions to this are the cases in the western United States, which were caused by piroplasms other than B. microti, such as WA1 (176, 231), CA1 (96, 161), and MO1 (81). Coinfection with other tick-transmitted infectious agents can result in more severe manifestations (108). This could be due to an overall immunosuppressive effect that facilitates establishment of infection, or perhaps there is a more specific synergy between organisms that occupy the same transmission cycle. As will be discussed in more detail below, several other infectious agents transmitted by I. dammini can affect the course of infection as well. For instance, patients coinfected with B. burgdorferi (the causative agent in Lyme disease) and B. microti experienced increased disease severity (108). Finally, human immunodeficiency virus (HIV) infection may also exacerbate the symptoms of babesial infection; several relatively treatment-resistant cases have been described (13, 58, 152).
Inbred mice have allowed various genetic susceptibility questions to be examined. A study examining the susceptibility of various mouse strains to B. microti found profound differences in peak parasitemia levels between strains, with the C3H and A strains being highly susceptible and C57BL/6 notably resistant (192). The data also suggested that the resistance was a dominant trait and that it was not due to the presence of a specific major histocompatibility complex (MHC) haplotype (56). A more recent study reexamined this question using WA1 as the infectious agent on several inbred and congenic strains encompassing five different haplotypes. Differences in susceptibility within each haplotype were observed, demonstrating that the susceptible phenotype was independent of MHC haplotype and attributable instead to the genetic background (142). An interesting characteristic of the WA1 model is that differences in susceptibility are manifested not only in parasitemia levels, but also in the dramatically polarized outcome of the infection: full recovery or death. Additional data from our laboratory indicate that the resistance is conferred by a small number of autosomal dominant genes (M. Moro and I. Aguilar-Delfin, unpublished data).
DIAGNOSIS
The diagnosis of babesiosis should begin with a descriptive history, which might include appropriate clinical manifestations, history of travel to an area where it is endemic, tick bite or exposure to a tick-infested area, recent blood transfusion, and splenectomy. Subsequent analysis should include examination of stained blood smears (described below) as well as serologic evaluation with indirect (immuno)fluorescent antibody tests (IFATs) (32) and possibly PCR (162). Morphologic changes in the spleen may be identified with magnetic resonance imaging or computerized axial tomography scan in severe cases. The presence of other definitive laboratory findings (described in the Symptoms in Humans section) usually depends directly on the level of parasitemia in the patient (and will likely be within normal ranges for clinically mild or silent cases). Identification of B. divergens is currently performed by direct-smear evaluation, IFATs, and animal subinoculation; PCR assays (151) are not used routinely in diagnosis, since they are only performed in reference laboratories.
Before the development of a PCR-based assay for B. microti, inoculation of hamsters with patient blood was the most sensitive method for detection of B. microti (160). The organisms require several weeks or longer to establish a detectable infection, and the results may be uninterpretable due to factors such as host adaptation, isolate variation, and dose of inoculum (15, 55). There have also been cases of novel emerging Babesia species (96, 176) that could not be isolated via hamster inoculation and were eventually identified by broad-range PCR (160, 161). Hamster inoculation has been very useful for monitoring persistent infection (for up to 7 months) in asplenic hosts, but improved sensitivity is a necessity for detection of such a state in normosplenic persons (104). Thus, PCR is rapidly becoming the test of choice for confirmation of actual infection in antibody-reactive persons and for monitoring therapeutic responses. However, great care must be taken to avoid contamination with the PCR method, which can lead to false-positive results (162). Thus, PCR data should always be corroborated by immunologic testing whenever possible.
Hematology
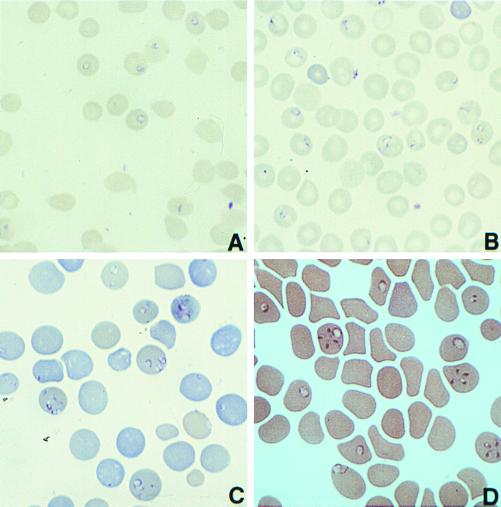
Examination of thin blood smears for the presence of parasites within erythrocytes is the most frequently used technique for diagnosing both infections with B. microti in the United States and infections with B. divergens in Europe (20, 55, 69, 197). Peripheral blood smears are stained with Wright's or Giemsa stain. The organisms are apparent within the red blood cells as darkly staining ring forms with light blue cytoplasm (Fig. 4). B. microti merozoites are approximately 1.5 to 2 μm (Fig. 4A and C) (99), and B. divergens merozoites are variable (1 to 3 μm), depending on which host they have infected (69). Morphologically, there is great variation in the forms seen (Fig. 4); simple rings (annular), paired or single pear-shaped trophozoites (pyriform), and the rarely seen but often described Maltese cross (Fig. 4D, tetrad form). B. microti infections can have parasitemias that are detectable to levels as high as 85% (on peripheral blood smears). The duration of detectable parasitemia on blood smears varies from 3 weeks (3) to 12 weeks (197), with the longest duration of smear positivity being 7 months for a splenectomized patient (221).
FIG. 4.
Giemsa-stained thin blood smears from a nonobese diabetic (NOD)-SCID mouse infected with (A) B. microti strain MN1, (B) a hamster infected with B. microti MN1, (C) a NOD-SCID mouse infected with the related piroplasm WA1, and (D) a hamster infected with WA1.
In general, the analysis of blood smears is a fairly subjective process which consequently depends on the experience of the observer and the time spent examining the smear. The need to discriminate the subtleties of babesial morphology and possible low parasitemias may result in inaccurate diagnoses which might necessitate further analysis. In most instances, however, an accurate patient history, clinical presentation, and observation of characteristic morphologic features are sufficient to establish the appropriate diagnosis; otherwise molecular techniques may be used.
There are some points of caution with respect to blood smear analyses. The ring forms visible within erythrocytes can vary greatly and can be confused with Plasmodium falciparum, but the absence of the pigment hemozoin should distinguish Babesia spp. (173). Note that early stages of P. falciparum might also lack pigment (227). In all cases due to infections with Babesia spp. (both B. microti and B. divergens), blood autoanalyzers might not differentiate between infected and uninfected erythrocytes (21). There have been several cases in which the patient has been initially diagnosed with malaria, which resulted in delayed appropriate treatment, which for serious cases (e.g., B. divergens infections) might prove fatal.
Serology and Immunology
Serological testing with IFATs is useful in diagnosing B. microti infections, particularly chronic infections (227). This test uses hamster-derived B. microti antigen. The IFAT is both specific and sensitive and is the current recommended serologic method (32). The cutoff titer for determination of a positive result varies from laboratory to laboratory; some report titers above 1:64 to be diagnostic (107). In general, higher cutoff titers (1:128 to 1:256) are associated with greater diagnostic specificity. In our experience, titers of 1:128 to 1:256 are rarely associated with false positivity, but screening of blood donor populations at a 1:64 titer may result in occasional false-positive results. In the acute phase of infection, the antibody titers might be 10 to 20 times higher than the cutoff, with a steady decline afterwards over a variable time period (weeks to months) (173).
Antibody is usually detectable when patients are first diagnosed with infections of B. microti (227). Antibody titers can remain elevated for as long as 13 months to 6 years after infection (160). Although persistence of antibody does not necessarily reflect a measurable infection (160, 198), levels of IgG antibody decline less rapidly in persistently infected patients (>3 months, as measured by B. microti DNA detectable in the blood) than in patients whose infections cleared in less than 3 months (104). Persistence of infection does correlate, however, with persistent elevated antibody levels in B. gibsoni infections in dogs (43). In smear-negative or smear-inconclusive cases, the IFAT is still sensitive and specific (and parasitemia is usually apparent in 2 to 4 weeks) (15).
One theoretical drawback to serologic testing is that other protozoal parasites might elicit cross-reactivity, generating false-positive results in B. microti or WA1 IFAT procedures, especially when IgM is the antibody class being detected. Patients with connective tissue disorders such as systemic lupus erythematosus and rheumatoid arthritis (160) may also generate false-positive results by other mechanisms. Conversely, immunosuppressed patients and patients from whom samples are collected early in the course of the infection could generate false-negative results (13, 152); HIV-infected and splenectomized patients generally have very low titers (S. R. Telford III, unpublished data).
B. divergens infections are usually too severe or serious to allow serological diagnosis, as B. divergens antibodies do not become detectable in serum until 7 to 10 days after the onset of hemoglobinuria (69). IFATs can be used, however, to distinguish infections due to different Babesia species, since B. microti, WA1, and B. divergens have limited serologic cross-reactivity.
Molecular Diagnostic Approaches
Although clinically apparent cases are usually diagnosed, patients with mild infection often remain undiagnosed and therefore untreated. Detection of these mild cases of babesiosis requires more sensitive techniques than the ones described thus far. With the evolution of more sensitive PCR-based techniques, the molecular diagnosis and monitoring of even mild cases of babesial infections has become possible.
Development of PCR-based detection assays for both B. microti (162) and B. divergens (151) have been described. Studies have shown these assays to be more sensitive than and equally specific for the detection of acute cases as smear evaluation and hamster inoculation (161, 162, 231). Briefly, these assays usually rely on the amplification of highly conserved sequences (with species-informative regions within the conserved sequence) such as nss-rDNA. Subsequent sequence analysis of the amplified fragments and comparison with a database of known sequences allow definitive identification of the infecting agent.
Patients with detectable babesial DNA in their blood are likely to be parasitemic; various studies have shown that microbial DNA is rapidly cleared from the blood in the absence of microbial replication, so that the detectable presence of DNA is probably reflective of an active infection (98, 104, 147). In studies of other infectious agents, DNA clearance was directly related to a decline in the number of these organisms (98, 147). The exception to this rule appears to be mycobacteria (78, 79, 138).
TREATMENT
Most cases of B. microti infection are mild and usually resolve on their own, without treatment. In more severe cases, however, a combination of clindamycin and quinine is administered as the standard treatment. This particular therapeutic regimen was discovered during the management of a case of presumed transfusion-acquired malarial infection (236). Initially, chloroquine was used to treat the patient, which proved to be unsuccessful in resolving the infection. The patient was then treated with quinine and clindamycin, which successfully eradicated the organisms. Subsequent studies in animals have supported the usefulness of this combination of antimicrobial agents (186). Comparisons between the duration of B. microti DNA (parasitemia) in babesiosis patients who were treated with quinine and clindamycin and babesiosis patients who were untreated showed that treatment reduces the duration of parasitemia (104). However, the potential for drug-related toxicity with this regimen is significant (41) and includes hearing loss, tinnitus, syncope, hypotension, and gastrointestinal distress.
In very serious cases, anti-infective therapy might not be sufficient, and procedures such as erythrocyte exchange transfusion can be beneficial or even life-saving (23, 57, 68, 93). Patients who are iatrogenically immunosuppressed (23), HIV infected (117), or severely infected with Babesia sometimes do not respond to antimicrobial therapy and require extra treatment. Alternative combinations for treatment are being investigated because of the occasional failure and frequent toxicity of quinine and clindamycin. Studies with hamster models have shown that antimalarial agents are ineffective for B. microti infections in vivo (135).
Patients with B. divergens infections, regarded as medical emergencies, require prompt treatment that includes erythrocyte exchange transfusion along with intravenous clindamycin and oral quinine to arrest hemolysis and prevent renal failure (69, 227). In vitro evaluations of B. divergens and its susceptibility to various antimicrobial agents also demonstrated that Imidocarb and the combination of oxomemazine and phenamidine were most effective in vitro (16). Imidocarb has not been approved for use in humans. There have been reports of success with other agents, such as pentamidine and cotrimoxazole, but the side effects of pentamidine make this course of treatment less desirable (173). Furthermore, one study in dogs showed that pentamidine was effective in arresting or reversing the progression of the disease but not in clearing the organisms (in this case, B. gibsoni) from the blood (59).
Theileria infections in cattle are often treated with a regimen including hydroxynaphthoquinone derivatives (such as atovaquone). Given the phylogenetic relatedness of the small babesias with members of the genus Theileria, similar regimens might eventually prove to be useful for managing refractory cases of B. microti infection. There have been studies that have shown the effectiveness of atovaquone in treating B. microti infections (73, 86, 235), and apparently, atovaquone might be even more effective than Imidocarb in treating B. divergens infections (174). Various other pharmacologic interventions have been tried for the treatment of babesiosis, including chloroquine, tetracycline, primaquine, sulfadiazine, and pyrimethamine, with variable results.
HUMAN COINFECTION
A phenomenon that has caused growing concern is coinfection with B. microti and other tick-borne pathogens, particularly B. burgdorferi (the causative agent of Lyme disease). It is estimated from serosurveys that as many as 13% of Lyme disease patients in babesia-endemic areas are coinfected with B. microti (10, 105, 108). Furthermore, it has been suggested that the increase in B. microti seropositivity seen during the past 30 years is consistent with the increased incidence of Lyme disease (108). There are some reports of potential coinfection with B. divergens, as determined by seroreactivity (asymptomatic infection), and B. burgdorferi sensu lato in Europe (69).
In eastern North America, B. microti is transmitted by the same Ixodes tick that perpetuates the agents of Lyme disease and human granulocytic ehrlichiosis and possibly by a novel Bartonella species (83, 121, 156, 213). P. leucopus is also the vertebrate reservoir for at least three of the known pathogens (77, 112, 225) and probably a recently described fourth agent (Bartonella spp. [83]) and is itself commonly coinfected (4, 5, 83, 225). A field surveillance study of P. leucopus populations (in Lyme disease-endemic areas) found that B. burgdorferi-infected mice often had coinfections of B. microti or a Bartonella sp. or both but that mice were not often infected with B. microti or Bartonella in the absence of B. burgdorferi (5, 83). Humans are apparently susceptible to infection with one or a combination of these agents, the disease manifestations being perhaps dependent on the particular combination of infectious agents along with host susceptibility factors (108). Human infection with a Bartonella sp. closely related to the species found in P. leucopus has now been described (233). The diagnosis of coinfections presents a serious challenge to clinicians and public health professionals, who should rely on epidemiologic information about case distribution in exposure areas and be aware of the potential for coinfection.
The initial symptoms of both babesiosis and Lyme disease overlap significantly (Table 1). Like babesiosis, Lyme disease presents with nonspecific symptoms of fever, fatigue, and other flu-like symptoms (108). Patients coinfected with B. microti and B. burgdorferi experience more severe symptoms, resulting in fatality in rare cases (108) and the persistence of postinfectious fatigue. B. burgdorferi DNA persisted in the blood for prolonged periods in coinfected patients, which was correlated with the persistence of fatigue in the small number of patients studied (108). Studies have shown that coinfection with babesiosis and Lyme disease did not have any significant effect on the duration of parasitemia with B. microti, as measured by the duration of detectable B. microti DNA (108), but it may well affect the frequency of recognition of infections due to B. microti. It is important to note that the antibiotic therapy used for the treatment of Lyme disease is not likely to eradicate the underlying infection with B. microti. A coinfected patient treated only for (early) Lyme disease could therefore still have a persistent babesial infection after therapy. Management of patients with persistent symptoms after appropriate therapy for proven Lyme disease might therefore include an evaluation for other tick-transmitted agents.
TABLE 1.
Symptoms associated with Lyme disease and babesiosisa
| Symptom | % of patients surveyed exhibiting the indicated symptoms
|
||
|---|---|---|---|
| Lyme disease (n = 214) | Babesiosis (n = 10) | Both (n = 26) | |
| Fatigue | 49 | 60 | 81 |
| Headache | 42 | 60 | 77 |
| Erythema migrans | 85 | 0 | 62 |
| Fever | 42 | 80 | 58 |
| Sweats | 11 | 20 | 46 |
| Chills | 23 | 50 | 42 |
| Myalgia | 31 | 20 | 38 |
| Anorexia | 14 | 10 | 31 |
| Arthralgia | 36 | 50 | 27 |
| Emotional lability | 7 | 0 | 23 |
| Nausea | 5 | 10 | 23 |
| Neck stiffness | 21 | 30 | 23 |
| Multiple EM | 14 | 0 | 19 |
| Cough | 10 | 20 | 15 |
| Sore throat | 9 | 20 | 15 |
| Conjunctivitis | 3 | 0 | 12 |
| Splenomegaly | 0 | 10 | 8 |
| Vomiting | 4 | 0 | 8 |
| Joint swelling | 3 | 0 | 4 |
Adapted from reference 108.
PERSISTENT INFECTION
The existence of the chronic asymptomatic carrier state in babesial infections of domestic and wild animals has been recognized for many years (43, 63, 116, 198, 226), and consequently, most information about the chronic carrier state of babesial infections is from animal models. Dogs infected with B. gibsoni, for instance, can remain chronic carriers after the clinical symptoms have resolved (43). Chronically infected animals maintain elevated antibody titers, and some can develop signs of other chronic diseases, such as pathologic evidence of liver disease, chronic membranoproliferative glomerulonephritis, or both (43). Hamsters display an initial parasitemia that can resolve to a carrier state in which the parasites can only be detected infrequently. The carrier state can last 2 or more years, but in the last month of life, the animals show signs of relapse characterized by a rise in parasitemia, increasing ascites, anorexia, and lethargy (116). There have also been studies that have demonstrated chronic infections in primates as well (199). From these studies, it seems that the chronic carrier state of babesial infections in experimental animal models is more common than realized. The B. microti reservoir P. leucopus seems to maintain a chronic carrier state, generally harboring low parasitemias, while large numbers of the P. leucopus population seem to be infected (55, 83, 166, 215). The carrier state is probably an essential component of the B. microti transmission cycle that may contribute significantly to the overall prevalence of infections.
Relatively little is known about the chronic carrier state in humans. Until recently, the actual duration of infection with Babesia and Babesia-like organisms in humans has been difficult to determine because the tests used to determine the chronic carrier state lacked sensitivity. With the advent of PCR, however, serial measurements of patient blood samples have shown that the chronic carrier state can last for months to years (104). The best evidence that an asymptomatic carrier state exists is provided by the demonstration on several occasions of transfusion-acquired or -transmitted babesiosis (57, 70, 120, 136, 172, 210). One study screened subjects with the passive hemagglutination test for B. canis antigen and found that 38 of 101 subjects reacted positively. Although babesial organisms have been recovered by hamster inoculation from 3 of the 38 positive subjects (153), these findings still await confirmation after more than 20 years. Several serosurveys have suggested that exposure to babesial antigens occurs much more frequently than expected (64, 106, 196). However, all of these studies await confirmation of babesial infection by subinoculation of animals or PCR. Our own studies in California showed a 16% prevalence of antibodies to WA1 (161); however, these findings must be interpreted in light of a known lack of specificity of the WA1 IFATs (D. H. Persing, unpublished data). There is some evidence of a chronic carrier state in Europe as well. A serosurvey of 798 foresters showed that only two individuals had been exposed to B. microti, and no parasites could be visualized on thin blood smears (103). A serosurvey of 190 blood donors in France showed that serum samples from only two were reactive with B. divergens antigen (69). Clearly much work must be done to improve the specificity of the methods used to detect babesial antibody in humans and to correlate antibody results with the persistence of infection. The apparent existence and surprising prevalence of these chronic infections may become an increasingly important practical concern for blood transfusion recipients in areas where babesiosis is endemic, since infections can be transmitted by transfusion. Unfortunately, behavioral screening (inquiries about history of tick bites and travel to babesiosis-endemic areas) may not be sufficient to eliminate true carriers from becoming blood donors; some sort of screening test may eventually become necessary in highly babesiosis-endemic areas.
PREVENTION
Preventive measures range from simple avoidance to habitat modification. Simple measures include use of tick repellents before entering a tick-infested area, avoidance of or minimization of exposure to tick-infested areas, and thorough examination of skin after exposure. Ticks found before attachment should obviously be removed, and ticks found after attachment can also be removed to limit the possibility of transmission if they can be removed within 24 h after attachment (164, 168).
Various public health measures have been put into effect which depend on reducing the density of the tick population. The use of acaricides is the most common method; the application of this pesticide to host nests and on the coats of reservoir hosts can interrupt transmission of B. microti (122, 218). Likewise, the application of acaricides to cattle could reduce the possible transmission of B. divergens to humans (227). The major drawback is that ticks may rapidly develop resistance to acaricides, so that this pesticide is not suitable for prevention by itself but must be used in conjunction with other methods.
Vaccines
At the present time, there are no vaccines for humans against babesial organisms, nor is infection frequent enough at present to warrant a large-scale vaccine effort. Significant effort has been invested in the development of vaccines for cattle and other animals, however, which might eventually prove useful for developing a human vaccine. Much of the work done to develop vaccines has focused on the large babesias, such as B. bovis, B. divergens, and B. bigemina (reviewed in references 9, 28, and 155). Vaccine development has resulted in attenuated vaccines (24–27), vaccines that use soluble antigens from in vitro cultures (137, 140, 209), and recombinant vaccines (reviewed in references 155 and 239). Comparisons of various aspects of attenuated and virulent strains have furthered our understanding of the disease process and helped us to identify potential key components that are involved, such as the genes specific to virulent (45, 228) and attenuated or avirulent (45, 46) strains. Studies aimed at the development of vaccines against various species of Babesia have also helped elucidate some of the immune mechanisms that occur in response to the infection process. Should vaccines eventually provide effective protection, they might become useful for splenectomized or immunocompromised patients living in high-risk areas (227).
Live vaccines.
The use of living parasites to immunize cattle against the spread of babesiosis has been employed for a long time in livestock management (24–27) with varying success. In 1964, an attenuated strain of B. bovis was produced by adaptation in splenectomized calves (24). This strain has proved to be a very useful vaccine (27, 29). Since then, numerous attenuated vaccines have been developed for several Babesia species (51, 53, 111, 171).
Recombinant vaccines.
Although attenuated vaccines have been effective, they have several problems associated with them; among the most important is the cotransmission of other enzootic agents, such as bovine leukosis virus, and a short shelf life (238). Therefore, there are studies targeted at developing other preventive techniques. Irradiated B. bovis-, B. bigemina-, and Babesia major-infected erythrocytes have been used to prevent parasitemia but are not as effective as other vaccines (99, 155).
Research for recombinant vaccines has focused on developing vaccines from the major surface antigens of the sporozoite form. In particular, the apical complex proteins are of special interest because of their putative role in host cell invasion. Antigens from these proteins have been shown to elicit an immune response that could be sufficient for protection. Several candidate proteins have been identified in Babesia species, including the rhoptry-associated proteins (RAP), which are encoded by a multigene family (48, 155, 179, 208). RAP-1 from both B. bovis and B. bigemina is highly immunogenic for both B and T cells (19, 183), elicits a Th1-type response from T helper cells, and can induce partial protective immunity against challenge (125, 200, 232, 239). However, the presence of specific RAP-1 antibodies does not consistently correlate with the degree of protective immunity (232), implying a more elaborate role for both the effector and helper functions of CD4+ T cells. Additionally, the immune effector mechanisms responsible for protection require more elucidation to select an appropriate antigen delivery system. It is clear that many facets of the babesial life cycle and infectious process still need to be clarified to aid in vaccine development.
CONCLUSION
Human babesiosis was first described in 1957 but is now known to have worldwide distribution. The increase in reported cases is likely due to increases in actual incidence as well as increased awareness of the disease. Despite the diagnostic and preventive advances resulting from extensive research and a greater understanding of the disease, babesiosis continues to have significant medical impact as a confounding variable in the diagnosis and treatment of Lyme disease and as a potential threat to the blood supply, especially in the United States. Diagnostic advances, like the development of PCR assays, have resulted in increased sensitivity for detection as well as the discovery and characterization of new babesial species. Further studies using the molecular tools now available and those to be developed will lead to a better understanding of the natural history of these organisms, including the transmission cycle and the potential role of Babesia parasites themselves as immunomodulators.
ACKNOWLEDGMENT
Irma Aguilar-Delfin is supported by a fellowship from the Consejo Nacional de Ciencia y Techologia (CONACyT), Mexico.
REFERENCES
- 1.Abdalla H S, Hussein H S, Kreier J P. Babesia rodhaini: passive protection of mice with immune serum. Tropenmed Parasitol. 1978;29:295–306. [PubMed] [Google Scholar]
- 2.Adachi K, Kawano H, Makimura S. Suppressed antibody response to sheep erythrocytes in experimentally Babesia rodhaini-infected mice. J Vet Med Sci. 1993;55:189–190. doi: 10.1292/jvms.55.189. [DOI] [PubMed] [Google Scholar]
- 3.Anderson A E, Cassaday P B, Healy G R. Babesiosis in man: sixth documented case. Am J Clin Pathol. 1974;62:612–618. doi: 10.1093/ajcp/62.5.612. [DOI] [PubMed] [Google Scholar]
- 4.Anderson J F, Johnson R C, Magnarelli L A, Hyde F W, Myers J E. Peromyscus leucopus and Microtus pennsylvanicus simultaneously infected with Borrelia burgdorferi and Babesia microti. J Clin Microbiol. 1986;23:135–137. doi: 10.1128/jcm.23.1.135-137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J F, Mintz E D, Gadbaw J J, Magnarelli L A. Babesia microti, human babesiosis, and Borrelia burgdorferi in Connecticut. J Clin Microbiol. 1991;29:2779–2783. doi: 10.1128/jcm.29.12.2779-2783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong P M, Katavolos P, Caporale D A, Smith R P, Spielman A, Telford S R., III Diversity of Babesia infecting deer ticks (Ixodes dammini) Am J Trop Med Hyg. 1998;58:739–742. doi: 10.4269/ajtmh.1998.58.739. [DOI] [PubMed] [Google Scholar]
- 7.Babes V. Sur l'hemoglobinurie bactérienne du boeuf. C R Acad Sci Ser III Sci Vie. 1888;107:692–694. [Google Scholar]
- 8.Baldwin C L, Black S J, Brown W C, Conrad P A, Goddeeris B M, Kinuthia S W, Lalor P A, MacHugh N D, Morrison W I, Morzaria S P, et al. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988;56:462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barriga O O. A review on vaccination against protozoa and arthropods of veterinary importance. Vet Parasitol. 1994;55:29–55. doi: 10.1016/0304-4017(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 10.Benach J L, Habicht G S. Clinical characteristics of human babesiosis. J Infect Dis. 1981;144:481. doi: 10.1093/infdis/144.5.481. [DOI] [PubMed] [Google Scholar]
- 11.Benach J L, Habicht G S, Hamburger M I. Immunoresponsiveness in acute babesiosis in humans. J Infect Dis. 1982;146:369–380. doi: 10.1093/infdis/146.3.369. [DOI] [PubMed] [Google Scholar]
- 12.Benach J L, White D J, McGovern J P. Babesiosis in Long Island: host-parasite relationships of rodent- and human-derived Babesia microti isolates in hamsters. Am J Trop Med Hyg. 1978;27:1073–1078. [PubMed] [Google Scholar]
- 13.Benezra D, Brown A E, Polsky B, Gold J W, Armstrong D. Babesiosis and infection with human immunodeficiency virus (HIV) Ann Intern Med. 1987;107:944. doi: 10.7326/0003-4819-107-6-944_1. [DOI] [PubMed] [Google Scholar]
- 14.Botteron C, Dobbelaere D. AP-1 and ATF-2 are constitutively activated via the JNK pathway in Theileria parva-transformed T-cells. Biochem Biophys Res Commun. 1998;246:418–421. doi: 10.1006/bbrc.1998.8635. [DOI] [PubMed] [Google Scholar]
- 15.Brandt F, Healy G R, Welch M. Human babesiosis: the isolation of Babesia microti in golden hamsters. J Parasitol. 1977;63:934–937. [PubMed] [Google Scholar]
- 16.Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet J J. In vitro evaluation of drug susceptibilities of Babesia divergens isolates. Antimicrob Agents Chemother. 1998;42:818–820. doi: 10.1128/aac.42.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brocklesby D W, Harness E, Sellwood S A. The effect of age on the natural immunity of cattle to Babesia divergens. Res Vet Sci. 1971;12:15–17. [PubMed] [Google Scholar]
- 18.Bronsdon M A, Homer M J, Magera J M H, Harrison C, Andrews R G, Bielitzki J T, Emerson C L, Persing D H, Fritsche T R. Detection of enzootic babesiosis in baboons (Papio cynocephalus) and phylogenetic evidence supporting synonymy of the genera Entopolypoides and Babesia. J Clin Microbiol. 1998;37:1548–1553. doi: 10.1128/jcm.37.5.1548-1553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown W C, McElwain T F, Ruef B J, Suarez C E, Shkap V, Chitko-McKown C G, Tuo W, Rice-Ficht A C, Palmer G H. Babesia bovis rhoptry-associated protein 1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains. Infect Immun. 1996;64:3341–3350. doi: 10.1128/iai.64.8.3341-3350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruce-Chwatt L J. Essential malariology. New York, N.Y: John Wiley & Sons, Inc.; 1985. [Google Scholar]
- 21.Bruckner D A, Garcia L S, Shimizu R Y, Goldstein E J, Murray P M, Lazar G S. Babesiosis: problems in diagnosis using autoanalyzers. Am J Clin Pathol. 1985;83:520–521. doi: 10.1093/ajcp/83.4.520. [DOI] [PubMed] [Google Scholar]
- 22.Bush J B, Isaacson M, Mohamed A S, Potgieter F T, de Waal D T. Human babesiosis—a preliminary report of 2 suspected cases in South Africa. S Afr Med J. 1990;78:699. [PubMed] [Google Scholar]
- 23.Cahill K M, Benach J L, Reich L M, Bilmes E, Zins J H, Siegel F P, Hochweis S. Red cell exchange: treatment of babesiosis in a splenectomized patient. Transfusion. 1981;21:193–198. doi: 10.1046/j.1537-2995.1981.21281178156.x. [DOI] [PubMed] [Google Scholar]
- 24.Callow L L. Proceedings of the 19th World Veterinary Congress. 1971. The control of babesiosis with a highly effective attenuated vaccine; pp. 357–360. Lioate, Mexico. [Google Scholar]
- 25.Callow L L. Tick-borne livestock diseases and their vectors. III. The Australian methods of vaccination against anaplasmosis and babesiosis. World Anim Rev. 1976;18:9–15. [Google Scholar]
- 26.Callow L L. Vaccination against bovine babesiosis. Adv Exp Med Biol. 1977;93:121–149. doi: 10.1007/978-1-4615-8855-9_9. [DOI] [PubMed] [Google Scholar]
- 27.Callow L L. Some aspects of the epidemiology and control of bovine babesiosis in Australia. J S Afr Vet Assoc. 1979;50:353–356. [PubMed] [Google Scholar]
- 28.Callow L L, Dalgliesh R J, de Vos A J. Development of effective living vaccines against bovine babesiosis—the longest field trial? Int J Parasitol. 1997;27:747–767. doi: 10.1016/s0020-7519(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 29.Callow L L, Mellors L T, McGregor W. Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int J Parasitol. 1979;9:333–338. doi: 10.1016/0020-7519(79)90083-3. [DOI] [PubMed] [Google Scholar]
- 30.Calvo de Mora A, Garcia Castellano J M, Herrera C, Jimenez-Alonso J. Human babesiosis: report of a case with fatal outcome. Med Clin. 1985;85:515–516. . (In Spanish.) [PubMed] [Google Scholar]
- 31.Chapman W E, Ward P A. Babesia rodhaini: requirement of complement for penetration of human erythrocytes. Science. 1977;196:67–70. doi: 10.1126/science.841340. [DOI] [PubMed] [Google Scholar]
- 32.Chisholm E S, Ruebush II T K, Sulzer A J, Healy G R. Babesia microti infection in man: evaluation of an indirect immunofluorescent antibody test. Am J Trop Med Hyg. 1978;27:14–19. doi: 10.4269/ajtmh.1978.27.14. [DOI] [PubMed] [Google Scholar]
- 33.Christensson D A. Inverse age resistance to experimental Babesia divergens infection in cattle. Acta Vet Scand. 1989;30:453–464. doi: 10.1186/BF03548023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark I A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978;ii:75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- 35.Clark I A. Resistance to Babesia spp. and Plasmodium sp. in mice pretreated with an extract of Coxiella burnetii. Infect Immun. 1979;24:319–325. doi: 10.1128/iai.24.2.319-325.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark I A, Allison A C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974;252:328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- 37.Clark I A, Allison A C, Cox F E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976;259:309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- 38.Clark I A, Cox F E, Allison A C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977;74:9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- 39.Clark I A, Richmond J E, Wills E J, Allison A C. Immunity to intra-erythrocytic protoza. Lancet. 1975;ii:1128–1129. doi: 10.1016/s0140-6736(75)91010-7. [DOI] [PubMed] [Google Scholar]
- 40.Clarke C S, Rogers E T, Egan E L. Babesiosis: underreporting or case-clustering? Postgrad Med J. 1989;65:591–593. doi: 10.1136/pgmj.65.766.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clyde D F, Gilman R H, McCarthy V C. Antimalarial effects of clindamycin in man. Am J Trop Med Hyg. 1975;24:369–370. doi: 10.4269/ajtmh.1975.24.369. [DOI] [PubMed] [Google Scholar]
- 42.Colly L P, Nesbit J W. Fatal acute babesiosis in a juvenile wild dog (Lycaon pictus) J S Afr Vet Assoc. 1992;63:36–38. [PubMed] [Google Scholar]
- 43.Conrad P A, Thomford J W, Yamane I, Whiting J, Bosma L, Uno T, Holshuh H J, Shelly S. Hemolytic anemia caused by Babesia gibsoni infection in dogs. J Am Vet Med Assoc. 1991;199:601–605. [PubMed] [Google Scholar]
- 44.Corrier D E, Wagner G G. The protective effect of pretreatment with killed Corynebacterium parvum against acute babesiosis in calves. Vet Parasitol. 1984;15:165–168. doi: 10.1016/0304-4017(84)90032-3. [DOI] [PubMed] [Google Scholar]
- 45.Cowman A F, Bernard O, Stewart N, Kemp D J. Genes of the protozoan parasite Babesia bovis that rearrange to produce RNA species with different sequences. Cell. 1984;37:653–660. doi: 10.1016/0092-8674(84)90397-0. [DOI] [PubMed] [Google Scholar]
- 46.Cowman A F, Timms P, Kemp D J. DNA polymorphisms and subpopulations in Babesia bovis. Mol Biochem Parasitol. 1984;11:91–103. doi: 10.1016/0166-6851(84)90057-4. [DOI] [PubMed] [Google Scholar]
- 47.Cox F E. Heterologous immunity between piroplasms and malaria parasites: the simultaneous elimination of Plasmodium vinckei and Babesia microti from the blood of doubly infected mice. Parasitology. 1978;76:55–60. doi: 10.1017/s0031182000047387. [DOI] [PubMed] [Google Scholar]
- 48.Dalrymple B P, Casu R E, Peters J M, Dimmock C M, Gale K R, Boese R, Wright I G. Characterisation of a family of multi-copy genes encoding rhoptry protein homologues in Babesia bovis, Babesia ovis and Babesia canis. Mol Biochem Parasitol. 1993;57:181–192. doi: 10.1016/0166-6851(93)90194-3. [DOI] [PubMed] [Google Scholar]
- 49.Dammin G J, Spielman A, Benach J L, Piesman J. The rising incidence of clinical Babesia microti infection. Hum Pathol. 1981;12:398–400. doi: 10.1016/s0046-8177(81)80020-2. [DOI] [PubMed] [Google Scholar]
- 50.Eberhard M L, Walker E M, Steurer F J. Survival and infectivity of Babesia in blood maintained at 25 C and 2–4 C. J Parasitol. 1995;81:790–792. [PubMed] [Google Scholar]
- 51.Echaide I E, de Echaide S T, Guglielmone A A. Live and soluble antigens for cattle protection to Babesia bigemina. Vet Parasitol. 1993;51:35–40. doi: 10.1016/0304-4017(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 52.Echaide I E, Hines S A, McElwain T F, Suarez C E, McGuire T C, Palmer G H. In vivo binding of immunoglobulin M to the surfaces of Babesia bigemina-infected erythrocytes. Infect Immun. 1998;66:2922–2927. doi: 10.1128/iai.66.6.2922-2927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edelhofer R, Kanout A, Schuh M, Kutzer E. Improved disease resistance after Babesia divergens vaccination. Parasitol Res. 1998;84:181–187. doi: 10.1007/s004360050380. [DOI] [PubMed] [Google Scholar]
- 54.Eichhorn M, Dobbelaere D A E. Induction of signal transduction pathways in lymphocytes infected by Theileria parva. Parasitol Today. 1994;10:469–472. doi: 10.1016/0169-4758(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 55.Etkind P, Piesman J, Ruebush II T, Spielman A, Juranek D D. Methods for detecting Babesia microti infection in wild rodents. J Parasitol. 1980;66:107–110. [PubMed] [Google Scholar]
- 56.Eugui E M, Allison A C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasitol Immunol. 1980;2:277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 57.Evenson D A, Perry E, Kloster B, Hurley R, Stroncek D F. Therapeutic apheresis for babesiosis. J Clin Apheresis. 1998;13:32–36. doi: 10.1002/(sici)1098-1101(1998)13:1<32::aid-jca7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 58.Falagas M E, Klempner M S. Babesiosis in patients with AIDS: a chronic infection presenting as fever of unknown origin. Clin Infect Dis. 1996;22:809–812. doi: 10.1093/clinids/22.5.809. [DOI] [PubMed] [Google Scholar]
- 59.Farwell G E, Legrand E K, Cobb C C. Clinical observations on Babesia gibsoni and B. canis infections in dogs. J Am Vet Med Assoc. 1982;180:507–511. [PubMed] [Google Scholar]
- 60.Fawcett D W, Doxsey S, Stagg D A, Young A S. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro: electron microscopic observations. Eur J Cell Biol. 1982;27:10–21. [PubMed] [Google Scholar]
- 61.Fell A H, Preston P M, Ansell J D. Establishment of Theileria-infected bovine cell lines in scid mice. Parasitol Immunol. 1990;12:335–339. doi: 10.1111/j.1365-3024.1990.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 62.Fich C, Klauenberg U, Fleischer B, Broker B M. Modulation of enzymatic activity of src-family kinases in bovine T cells transformed by Theileria parva. Parasitology. 1998;117:107–115. doi: 10.1017/s0031182098002832. [DOI] [PubMed] [Google Scholar]
- 63.Figueroa J V, Chieves L P, Johnson G S, Buening G M. Detection of Babesia bigemina-infected carriers by polymerase chain reaction amplification. J Clin Microbiol. 1992;30:2576–2582. doi: 10.1128/jcm.30.10.2576-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filstein M R, Benach J L, White D J, Brody B A, Goldman W D, Bakal C W, Schwartz R S. Serosurvey for human babesiosis in New York. J Infect Dis. 1980;141:518–521. doi: 10.1093/infdis/141.4.518. [DOI] [PubMed] [Google Scholar]
- 65.Friedhoff K T, Tenter A M, Muller I. Haemoparasites of equines: impact on international trade of horses. Rev Sci Tech. 1990;9:1187–1194. [PubMed] [Google Scholar]
- 66.Galley Y, Hagens G, Glaser I, Davis W, Eichhorn M, Dobbelaere D. Jun NH2-terminal kinase is constitutively activated in T cells transformed by the intracellular parasite Theileria parva. Proc Natl Acad Sci USA. 1997;94:5119–5124. doi: 10.1073/pnas.94.10.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gleason N N, Healy G R, Western K A, Benson G D, Schultz M G. The “Gray” strain of Babesia microti from a human case established in laboratory animals. J Parasitol. 1970;56:1256–1257. [PubMed] [Google Scholar]
- 68.Gorenflot A, Brasseur P, Bonmarchand G, Laneele D, Simonin D. Two cases of severe human babesiosis treated successfully. Presse Med. 1990;19:335. . (In French.) [PubMed] [Google Scholar]
- 69.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters T P. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 70.Grabowski E F, Giardina P J, Goldberg D, Masur H, Read S E, Hirsch R L, Benach J L. Babesiosis transmitted by a transfusion of frozen-thawed blood. Ann Intern Med. 1982;96:466–467. doi: 10.7326/0003-4819-96-4-446. [DOI] [PubMed] [Google Scholar]
- 71.Greenwood B M, Playfair J H L, Torrigiani G. Immunosuppression in murine malaria. 1. General characteristics. Clin Exp Immunol. 1971;8:467–478. [PMC free article] [PubMed] [Google Scholar]
- 72.Gunders A E. Piroplasmal sporozoites in the argasid Ornithodoros erraticus (Lucas) Experientia. 1977;33:892–893. doi: 10.1007/BF01951265. [DOI] [PubMed] [Google Scholar]
- 73.Gupta P, Hurley R W, Helseth P H, Goodman J L, Hammerschmidt D E. Pancytopenia due to hemophagocytic syndrome as the presenting manifestation of babesiosis. Am J Hematol. 1995;50:60–62. doi: 10.1002/ajh.2830500113. [DOI] [PubMed] [Google Scholar]
- 74.Habicht G S, Benach J L, Leichtling K D, Gocinski B L, Coleman J L. The effect of age on the infection and immunoresponsiveness of mice to Babesia microti. Mech Ageing Dev. 1983;23:357–369. doi: 10.1016/0047-6374(83)90036-2. [DOI] [PubMed] [Google Scholar]
- 75.Hailat N Q, Lafi S Q, al-Darraji A M, al-Ani F K. Equine babesiosis associated with strenuous exercise: clinical and pathological studies in Jordan. Vet Parasitol. 1997;69:1–8. doi: 10.1016/s0304-4017(96)01100-4. [DOI] [PubMed] [Google Scholar]
- 76.Harvey J W, Taboada J, Lewis J C. Babesiosis in a litter of pups. J Am Vet Med Assoc. 1988;192:1751–1752. [PubMed] [Google Scholar]
- 77.Healy G R, Spielman A, Gleason N. Human babesiosis: reservoir of infection on Nantucket Island. Science. 1976;192:479–480. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- 78.Hellyer T J, DesJardin L E, Hehman G L, Cave M D, Eisenach K D. Quantitative analysis of mRNA as a marker for viability of Mycobacterium tuberculosis. J Clin Microbiol. 1999;37:290–295. doi: 10.1128/jcm.37.2.290-295.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellyer T J, Fletcher T W, Bates J H, Stead W W, Templeton G L, Cave M D, Eisenach K D. Strand displacement amplification and the polymerase chain reaction for monitoring response to treatment in patients with pulmonary tuberculosis. J Infect Dis. 1996;173:934–941. doi: 10.1093/infdis/173.4.934. [DOI] [PubMed] [Google Scholar]
- 80.Herwaldt B L, Kjemtrup A M, Conrad P A, Barnes R C, Wilson M, McCarthy M G, Sayers M H, Eberhard M L. Transfusion-transmitted babesiosis in Washington State: first reported case caused by a WA1-type parasite. J Infect Dis. 1997;175:1259–1262. doi: 10.1086/593812. [DOI] [PubMed] [Google Scholar]
- 81.Herwaldt B L, Persing D H, Precigout E A, Goff W L, Mathiesen D A, Taylor P W, Eberhard M L, Gorenflot A F. A fatal case of babesiosis in Missouri—identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 82.Hines S A, McElwain T F, Buening G M, Palmer G H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989;37:1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- 83.Hofmeister E K, Kolbert C P, Abdulkarim A S, Magera J M, Hopkins M K, Uhl J R, Ambyaye A, Telford III S R, Cockerill III F R, Persing D H. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177:409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 84.Reference deleted.
- 85.Hudson A T, Randall A W, Fry M, Ginger C D, Hill B, Latter V S, McHardy N, Williams R B. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 86.Hughes W T, Oz H S. Successful prevention and treatment of babesiosis with atovaquone. J Infect Dis. 1995;172:1042–1046. doi: 10.1093/infdis/172.4.1042. [DOI] [PubMed] [Google Scholar]
- 87.Hugoson G. Studies on lymphocytosis in regions with high and low incidences of bovine leukosis and babesiosis. Bibl Haematol. 1970;36:537–543. doi: 10.1159/000391748. [DOI] [PubMed] [Google Scholar]
- 88.Hugoson G, Lagerlof B, Thoreel B. The effect of chronic protozoan infection by Babesia rodhaini on leukemogenesis in mice. Int J Cancer. 1977;20:947–950. doi: 10.1002/ijc.2910200619. [DOI] [PubMed] [Google Scholar]
- 89.Hugoson G, Vennstrom R, Henriksson K. The occurrence of bovine leukosis following the introduction of babesiosis vaccination. Bibl Haematol. 1968;30:157–161. doi: 10.1159/000391244. [DOI] [PubMed] [Google Scholar]
- 90.Humiczewska M, Kuzna-Grygiel W. A case of imported human babesiosis in Poland. Wiad Parazytol. 1997;43:227–229. . (In Polish.) [PubMed] [Google Scholar]
- 91.Igarashi I, Waki S, Ito M, Omata Y, Saito A, Suzuki N. Role of CD4+ T cells in the control of primary infection with Babesia microti in mice. J Protozool Res. 1994;4:164–171. [Google Scholar]
- 92.Irvin A D, Stagg D A, Kanhai G K, Brown C G, Omwoyo P L. Studies on cell fusion between Babesia rodhaini-infected mouse erythrocytes and baby hamster kidney cells. Int J Parasitol. 1975;5:465–470. doi: 10.1016/0020-7519(75)90015-6. [DOI] [PubMed] [Google Scholar]
- 93.Jacoby G A, Hunt J V, Kosinski K S, Demirjian Z N, Huggins C, Etkind P, Marcus L C, Spielman A. Treatment of transfusion-transmitted babesiosis by exchange transfusion. N Engl J Med. 1980;303:1098–1100. doi: 10.1056/NEJM198011063031906. [DOI] [PubMed] [Google Scholar]
- 94.Jacquemin L, Bazin C, Lamy C, Chubilleau C, Barale T, Daoudal P, Duhamel C. Babesiose (ou piroplasmose) humaine: à propos de trois observations récentes en France. Maghreb Inf Med. 1980;2:31–38. [Google Scholar]
- 95.James M A. Immunology of babesiosis. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press; 1988. pp. 119–130. [Google Scholar]
- 96.Jerant A F, Arline A D. Babesiosis in California. West J Med. 1993;158:622–625. [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson W C, Cluff C W, Goff W L, Wyatt C R. Reactive oxygen and nitrogen intermediates and products from polyamine degradation are babesiacidal in vitro. Ann N Y Acad Sci. 1996;791:136–147. doi: 10.1111/j.1749-6632.1996.tb53520.x. [DOI] [PubMed] [Google Scholar]
- 98.Kain K C, Brown A E, Lanar D E, Ballou W R, Webster H K. Response of Plasmodium vivax variants to chloroquine as determined by microscopy and quantitative polymerase chain reaction. Am J Trop Med Hyg. 1993;49:478–484. doi: 10.4269/ajtmh.1993.49.478. [DOI] [PubMed] [Google Scholar]
- 99.Kakoma I, Mehlhorn H. Babesia of domestic animals. In: Kreier J P, editor. Parasitic protozoa. 2nd ed. Vol. 7. San Diego, Calif: Academic Press; 1993. pp. 141–216. [Google Scholar]
- 100.Karakashian S J, Rudzinska M A, Spielman A, Lewengrub S, Piesman J, Shoukrey N. Ultrastructural studies on sporogony of Babesia microti in salivary gland cells of the tick Ixodes dammini. Cell Tissue Res. 1983;231:275–287. doi: 10.1007/BF00222180. [DOI] [PubMed] [Google Scholar]
- 101.Koch R. Kultivierungversuch der Hunde piroplasmen. Z Hyg Infektionskr. 1906;54:1–9. [Google Scholar]
- 102.Kolbert C P, Bruinsma E S, Abdulkarim A S, Hofmeister E K, Tompkins R B, Telford III S R, Mitchell P D, Adams-Stich J, Persing D H. Characterization of an immunoreactive protein from the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1172–1178. doi: 10.1128/jcm.35.5.1172-1178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krampitz H E, Buschmann H, Munchhoff P. Gibt es latente Babesien-infektionen beim Menschen in Süddeutschland? Mitt Österr Ges Tropenmed Parasitol. 1986;8:233–243. [Google Scholar]
- 104.Krause P J, Spielman A, Telford III S R, Sikand V K, McKay K, Christianson D, Pollack R J, Brassard P, Magera J, Ryan R, Persing D H. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 105.Krause P J, Telford III S, Pollack R J, Ryan R, Brassard P, Zemel L, Spielman A. Babesiosis: an underdiagnosed disease of children. Pediatrics. 1992;89:1045–1048. [PubMed] [Google Scholar]
- 106.Krause P J, Telford III S, Ryan R, Hurta A B, Kwasnik I, Luger S, Niederman J, Gerber M, Spielman A. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29:1–4. doi: 10.1128/jcm.29.1.1-4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krause P J, Telford III S R, Ryan R, Conrad P A, Wilson M, Thomford J W, Spielman A. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–926. doi: 10.1093/infdis/169.4.923. [DOI] [PubMed] [Google Scholar]
- 108.Krause P J, Telford III S R, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 109.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetic analysis. ed. 1.01. University Park, Pa: Pennsylvania State University; 1993. [Google Scholar]
- 110.Kurtzhals J, Andersen B J, Christensen N O. Effects on in vitro growth of Babesia microti by cells and serum from B. microti and Schistosoma mansoni infected mice. Acta Vet Scand. 1988;29:357–362. doi: 10.1186/BF03548629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lawrence J A. Conventional vaccines for tick-borne haemoparasitic diseases of sheep and goats. Parasitologia. 1997;39:119–121. [PubMed] [Google Scholar]
- 112.Levine J F, Wilson M L, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 113.Levine N D. Taxonomy of the piroplasms. Trans Am Microsc Soc. 1971;90:2–33. [Google Scholar]
- 114.Levine N D. The protozoan phylum apicomplexa. Vol. 2. Boca Raton, Fla: CRC Press; 1988. [Google Scholar]
- 115.Levine N D, Corliss J O, Cox F E, Deroux G, Grain J, Honigberg B M, Leedale G F, Loeblich A R, Lom J, Lynn D, Merinfeld E G, Page F C, Poljansky G, Sprague V, Vavra J, Wallace F G. A newly revised classification of the protozoa. J Protozool. 1980;27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- 116.Lykins J D, Ristic M, Weisiger R M. Babesia microti: pathogenesis of parasite of human origin in the hamster. Exp Parasitol. 1975;37:388–397. doi: 10.1016/0014-4894(75)90008-9. [DOI] [PubMed] [Google Scholar]
- 117.Machtinger L, Telford III S R, Inducil C, Klapper E, Pepkowitz S H, Goldfinger D. Treatment of babesiosis by red blood cell exchange in an HIV-positive, splenectomized patient. J Clin Apheresis. 1993;8:78–81. doi: 10.1002/jca.2920080205. [DOI] [PubMed] [Google Scholar]
- 118.Mahoney D F. Bovine babesiosis: the passive immunization of calves against Babesia argentina with special reference to the role of complement fixing antibodies. Exp Parasitol. 1967;20:119–124. doi: 10.1016/0014-4894(67)90029-x. [DOI] [PubMed] [Google Scholar]
- 119.Mahoney D F. Babesia of domestic animals. In: Kreier J P, editor. Parasitic protozoa. Vol. 4. San Francisco, Calif: Academic Press; 1977. pp. 1–52. [Google Scholar]
- 120.Marcus L C, Valigorsky J M, Fanning W L, Joseph T, Glick B. A case report of transfusion-induced babesiosis. JAMA. 1982;248:465–467. [PubMed] [Google Scholar]
- 121.Mather T N, Mather M E. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J Med Entomol. 1990;27:646–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- 122.Mather T N, Ribeiro J M, Spielman A. Lyme disease and babesiosis: acaricide focused on potentially infected ticks. Am J Trop Med Hyg. 1987;36:609–614. doi: 10.4269/ajtmh.1987.36.609. [DOI] [PubMed] [Google Scholar]
- 123.Matsubara J, Koura M, Kamiyama T. Infection of immunodeficient mice with a mouse-adapted substrain of the gray strain of Babesia microti. J Parasitol. 1993;79:783–786. [PubMed] [Google Scholar]
- 124.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McElwain T F, Perryman L E, Musoke A J, McGuire T C. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. 1991;47:213–222. doi: 10.1016/0166-6851(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 126.McHardy N. Recent advances in the chemotherapy of theileriosis. Prev Vet Med. 1984;2:179–192. [Google Scholar]
- 127.Meeusen E, Lloyd S, Soulsby E J. Babesia microti in mice: adoptive transfer of immunity with serum and cells. Aust J Exp Biol Med Sci. 1984;62:551–566. doi: 10.1038/icb.1984.53. [DOI] [PubMed] [Google Scholar]
- 128.Meeusen E, Lloyd S, Soulsby E J. Babesia microti in mice: subpopulations of cells involved in the adoptive transfer of immunity with immune spleen cells. Aust J Exp Biol Med Sci. 1984;62:567–575. doi: 10.1038/icb.1984.54. [DOI] [PubMed] [Google Scholar]
- 129.Mehlhorn H, Peters W, Haberkorn A. The formation of kinetes and oocysts in Plasmodium gallinaceum and considerations on phylogenetic relationships between Haemosporidia, Piroplasmida, and other Coccidia. Protistologica. 1980;16:135–154. [Google Scholar]
- 130.Mehlhorn H, Schein E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res. 1998;84:467–475. doi: 10.1007/s004360050431. [DOI] [PubMed] [Google Scholar]
- 131.Mehlhorn H, Schein E, Ahmed J S. Theileria. In: Kreier J P, editor. Parasitic protozoa. Vol. 7. New York, N.Y: Academic Press; 1993. pp. 217–304. [Google Scholar]
- 132.Mehlhorn H, Shein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1984;23:37–103. doi: 10.1016/s0065-308x(08)60285-7. [DOI] [PubMed] [Google Scholar]
- 133.Meldrum S C, Birkhead G S, White D J, Benach J L, Morse D L. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis. 1992;15:1019–1023. doi: 10.1093/clind/15.6.1019. [DOI] [PubMed] [Google Scholar]
- 134.Michael S A, Morsy T A, Montasser M F. A case of human babesiosis (preliminary case report in Egypt) J Egypt Soc Parasitol. 1987;17:409–410. [PubMed] [Google Scholar]
- 135.Miller L H, Neva F A, Gill F. Failure of chloroquine in human babesiosis (Babesia microti): case report and chemotherapeutic trials in hamsters. Ann Intern Med. 1978;88:200–202. doi: 10.7326/0003-4819-88-2-200. [DOI] [PubMed] [Google Scholar]
- 136.Mintz E D, Anderson J F, Cable R G, Hadler J L. Transfusion-transmitted babesiosis: a case report from a new endemic area. Transfusion. 1991;31:365–368. doi: 10.1046/j.1537-2995.1991.31491213305.x. [DOI] [PubMed] [Google Scholar]
- 137.Montenegro-James S, Ristic M, Toro Benitez M, Leon E, Lopez R. Heterologous strain immunity in bovine babesiosis using a culture-derived soluble Babesia bovis immunogen. Vet Parasitol. 1985;18:321–337. doi: 10.1016/0304-4017(85)90067-6. [DOI] [PubMed] [Google Scholar]
- 138.Moore D F, Curry J I, Knott C A, Jonas V. Amplification of rRNA for assessment of treatment response of pulmonary tuberculosis patients during antimicrobial therapy. J Clin Microbiol. 1996;34:1745–1749. doi: 10.1128/jcm.34.7.1745-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moore J A, Kuntz R E. Babesia microti infections in nonhuman primates. J Parasitol. 1981;67:454–456. [PubMed] [Google Scholar]
- 140.Moreau Y, Martinod S, Fayet G. Epidemiologic and immunoprophylactic aspects of canine babesiosis in France. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press; 1988. pp. 191–196. [Google Scholar]
- 141.Reference deleted.
- 142.Moro M H, David C S, Magera J M, Wettstein P J, Barthold S W, Persing D H. Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect Immun. 1998;66:492–498. doi: 10.1128/iai.66.2.492-498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murphy T M, Gray J S, Langley R J. Effects of rapid passage in the gerbil (Meriones unguiculatus) on the course of infection of the bovine piroplasm Babesia divergens in splenectomised calves. Res Vet Sci. 1986;40:285–287. [PubMed] [Google Scholar]
- 144.Murray P K, Jennings F W, Murray M, Urquhart G M. The nature of immunosuppression in Trypanosoma brucei infections in mice. 2. The role of the T and B lymphocytes. Immunology. 1974;27:825–840. [PMC free article] [PubMed] [Google Scholar]
- 145.Mzembe S A, Lloyd S, Soulsby E J. Macrophage mediated resistance to Babesia microti in Nematospiroides dubius-infected mice. Z Parasitenkd. 1984;70:753–761. doi: 10.1007/BF00927128. [DOI] [PubMed] [Google Scholar]
- 146.Naessens J, Newson J, Bensaid A, Teale A J, Magondu J G, Black S J. De novo expression of T cell markers on Theileria parva-transformed lymphoblasts in cattle. J Immunol. 1985;135:4183–4188. [PubMed] [Google Scholar]
- 147.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 148.ole-MoiYoi O K, Brown W C, Iams K P, Nayar A, Tsukamoto T, Macklin M D. Evidence for the induction of casein kinase II in bovine lymphocytes transformed by the intracellular protozoan parasite Theileria parva. EMBO J. 1993;12:1621–1631. doi: 10.1002/j.1460-2075.1993.tb05807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.ole-MoiYoi O K, Nayar A, Iams K, Musoke A J, Yilma T. Molecular aspects of Theileria parva and approaches to vaccine development for animals. Ann N Y Acad Sci. 1989;569:174–182. doi: 10.1111/j.1749-6632.1989.tb27367.x. [DOI] [PubMed] [Google Scholar]
- 150.Olivier A, Nurton J P, Guthrie A J. An epizoological study of wastage in thoroughbred racehorses in Gauteng, South Africa. J S Afr Vet Assoc. 1997;68:125–129. doi: 10.4102/jsava.v68i4.893. [DOI] [PubMed] [Google Scholar]
- 151.Olmeda A S, Armstrong P M, Rosenthal B M, Valladares B, del Castillo A, de Armas F, Miguelez M, Gonzalez A, Rodriguez Rodriguez J A, Spielman A, Telford S R., III A subtropical case of human babesiosis. Acta Trop. 1997;67:229–234. doi: 10.1016/s0001-706x(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 152.Ong K R, Stavropoulos C, Inada Y. Babesiosis, asplenia, and AIDS. Lancet. 1990;336:112. doi: 10.1016/0140-6736(90)91624-j. [DOI] [PubMed] [Google Scholar]
- 153.Osorno B M, Vega C, Ristic M, Robles C, Ibarro S. Isolation of Babesia spp. from asymtomatic human beings. Vet Parasitol. 1976;2:111–120. [Google Scholar]
- 154.Palmer G H, Machado J, Jr, Fernandez P, Heussler V, Perinat T, Dobbelaere D A. Parasite-mediated nuclear factor kappaB regulation in lymphoproliferation caused by Theileria parva infection. Proc Natl Acad Sci USA. 1997;94:12527–12532. doi: 10.1073/pnas.94.23.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palmer G H, McElwain T F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 156.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 157.Parrodi F, Jacobson R H, Wright I G, Fitzgerald C J, Dobson C. The effect of immune serum and complement on the in vitro phagocytosis of Babesia rodhaini. Parasitol Immunol. 1991;13:457–471. doi: 10.1111/j.1365-3024.1991.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 158.Parry M F, Fox M, Burka S A, Richar W J. Babesia microti infection in man. JAMA. 1977;238:1282–1283. [PubMed] [Google Scholar]
- 159.Reference deleted.
- 160.Persing D H, Conrad P A. Babesiosis: new insights from phylogenetic analysis. Infect Agents Dis. 1995;4:182–195. [PubMed] [Google Scholar]
- 161.Persing D H, Herwaldt B L, Glaser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Phillip D F, Conrad P A. Infection with a babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 162.Persing D H, Mathiesen D, Marshall W F, Telford III S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Phillips R S, Wakelin D. Trichuris muris: effect of concurrent infections with rodent piroplasms on immune expulsion from mice. Exp Parasitol. 1976;39:95–100. doi: 10.1016/0014-4894(76)90015-1. [DOI] [PubMed] [Google Scholar]
- 164.Piesman J, Hicks T C, Sinsky R J, Obiri G. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J Clin Microbiol. 1987;25:2012–2013. doi: 10.1128/jcm.25.10.2012-2013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piesman J, Karakashian S J, Lewengrub S, Rudzinska M A, Spielman A. Development of Babesia microti sporozoites in adult Ixodes dammini. Int J Parasitol. 1986;16:381–385. doi: 10.1016/0020-7519(86)90118-9. [DOI] [PubMed] [Google Scholar]
- 166.Piesman J, Mather T N, Dammin G J, Telford III S, Lastavica C C, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–1189. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- 167.Piesman J, Mather T N, Sinsky R J, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Piesman J, Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- 169.Piesman J, Spielman A. Babesia microti: infectivity of parasites from ticks for hamsters and white-footed mice. Exp Parasitol. 1982;53:242–248. doi: 10.1016/0014-4894(82)90065-0. [DOI] [PubMed] [Google Scholar]
- 170.Piesman J, Spielman A, Etkind P, Ruebush II T, Juranek D D. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol. 1979;15:537–540. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- 171.Pipano E. Live vaccines against hemoparasitic diseases in livestock. Vet Parasitol. 1995;57:213–231. doi: 10.1016/0304-4017(94)03122-d. [DOI] [PubMed] [Google Scholar]
- 172.Popovsky M A. Transfusion-transmitted babesiosis. Transfusion. 1991;31:296–298. doi: 10.1046/j.1537-2995.1991.31491213290.x. [DOI] [PubMed] [Google Scholar]
- 173.Pruthi R K, Marshall W F, Wiltsie J C, Persing D H. Human babesiosis. Mayo Clin Proc. 1995;70:853–862. doi: 10.1016/S0025-6196(11)63943-8. [DOI] [PubMed] [Google Scholar]
- 174.Pudney M, Gray J S. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J Parasitol. 1997;83:307–310. [PubMed] [Google Scholar]
- 175.Purvis A C. Immunodepression in Babesia microti infections. Parasitology. 1977;75:59–61. doi: 10.1017/s003118200006234x. [DOI] [PubMed] [Google Scholar]
- 176.Quick R E, Herwaldt B L, Thomford J W, Garnett M E, Eberhard M L, Wilson M, Spach D H, Dickerson J W, Telford III S R, Steingart K R, Pollock R, Persing D H, Kobayashi J M, Juranek D D, Conrad P A. Babesiosis in Washington State: a new species of Babesia? Ann Intern Med. 1993;119:284–290. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 177.Reduker D W, Jasmer D P, Goff W L, Perryman L E, Davis W C, McGuire T C. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol Biochem Parasitol. 1989;35:239–247. doi: 10.1016/0166-6851(89)90210-7. [DOI] [PubMed] [Google Scholar]
- 178.Ribeiro J M. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 179.Ridley R G, Takacs B, Lahm H W, Delves C J, Goman M, Certa U, Matile H, Woollett G R, Scaife J G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990;41:125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- 180.Riek R F. Babesiosis. In: Weinman D, Ristic M, editors. Infectious blood diseases of man and animals. Vol. 2. New York, N.Y: Academic Press; 1968. pp. 219–268. [Google Scholar]
- 181.Rintelen M, Schein E, Ahmed J S. Buparvaquone but not cyclosporin A prevents Theileria annulata-infected bovine lymphoblastoid cells from stimulating uninfected lymphocytes. Trop Med Parasitol. 1990;41:203–207. [PubMed] [Google Scholar]
- 182.Roberts J A. Adoptive transfer of immunity to Babesia rodhaini by spleen cells from immune rats. Aust J Exp Biol Med Sci. 1968;46:807–808. doi: 10.1038/icb.1968.191. [DOI] [PubMed] [Google Scholar]
- 183.Rodriguez S D, Palmer G H, McElwain T F, McGuire T C, Ruef B J, Chitko-McKown M G, Brown W C. CD4+ T-helper lymphocyte responses against Babesia bigemina rhoptry-associated protein I. Infect Immun. 1996;64:2079–2087. doi: 10.1128/iai.64.6.2079-2087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosenberg Y J, Evans C B. Resistance of mice suppressed for IgM production to Babesia microti infection. Nature. 1979;281:302–304. doi: 10.1038/281302a0. [DOI] [PubMed] [Google Scholar]
- 185.Rosenblatt-Bin H, Klein A, Sredni B. Antibabesial effect of the immunomodulator AS101 in mice: role of increased production of nitric oxide. Parasitol Immunol. 1996;18:297–306. doi: 10.1046/j.1365-3024.1996.d01-104.x. [DOI] [PubMed] [Google Scholar]
- 186.Rowin K S, Tanowitz H B, Wittner M. Therapy of experimental babesiosis. Ann Intern Med. 1982;97:556–558. doi: 10.7326/0003-4819-97-4-556. [DOI] [PubMed] [Google Scholar]
- 187.Rudzinska M A. Ultrastructure of intraerythrocytic Babesia microti, with emphasis on the feeding mechanism. J Protozool. 1976;23:224–233. doi: 10.1111/j.1550-7408.1976.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 188.Rudzinska M A, Lewengrub S, Spielman A, Piesman J. Invasion of Babesia microti into epithelial cells of the tick gut. J Protozool. 1983;30:338–346. doi: 10.1111/j.1550-7408.1983.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 189.Rudzinska M A, Spielman A, Lewengrub S, Trager W, Piesman J. Sexuality in piroplasms as revealed by electron microscopy in Babesia microti. Proc Natl Acad Sci USA. 1983;80:2966–2970. doi: 10.1073/pnas.80.10.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rudzinska M A, Spielman A, Riek R F, Lewengrub S J, Piesman J. Intraerythrocytic “gametocytes” of Babesia microti and their maturation in ticks. Can J Zool. 1979;57:424–434. doi: 10.1139/z79-050. [DOI] [PubMed] [Google Scholar]
- 191.Rudzinska M A, Trager W, Lewengrub S J, Gubert E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976;169:323–334. doi: 10.1007/BF00219605. [DOI] [PubMed] [Google Scholar]
- 192.Ruebush M J, Hanson W L. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979;65:430–433. [PubMed] [Google Scholar]
- 193.Ruebush M J, Hanson W L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980;29:507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- 194.Ruebush M J, Hanson W L. Transfer of immunity to Babesia microti of human origin using T lymphocytes in mice. Cell Immunol. 1980;52:255–265. doi: 10.1016/0008-8749(80)90347-0. [DOI] [PubMed] [Google Scholar]
- 195.Ruebush M J, Troutman E H, Kennedy D A. Delayed-type hypersensitivity to Babesia microti-infected erythrocytes in mice. Cell Immunol. 1986;98:289–299. doi: 10.1016/0008-8749(86)90289-3. [DOI] [PubMed] [Google Scholar]
- 196.Ruebush T, II, Juranek D D, Chisholm E S, Snow P C, Healy G R, Sulzer A J. Human babesiosis on Nantucket Island: evidence for self-limited and subclinical infections. N Engl J Med. 1977;297:825–827. doi: 10.1056/NEJM197710132971511. [DOI] [PubMed] [Google Scholar]
- 197.Ruebush T K, Cassaday P B, Marsh H J, Lisker S A, Voorhees D B, Mahoney E B, Healy G R. Human babesiosis on Nantucket Island: clinical features. Ann Intern Med. 1977;86:6–9. doi: 10.7326/0003-4819-86-1-6. [DOI] [PubMed] [Google Scholar]
- 198.Ruebush T K, Juranek D D, Spielman A, Piesman J, Healy G R. Epidemiology of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30:937–941. doi: 10.4269/ajtmh.1981.30.937. [DOI] [PubMed] [Google Scholar]
- 199.Ruebush T K, Piesman J, Collins W E, Spielman A, Warren M C. Tick transmission of Babesia microti to rhesus monkeys (Macaca mulatta) Am J Med Hyg. 1981;30:555–559. doi: 10.4269/ajtmh.1981.30.555. [DOI] [PubMed] [Google Scholar]
- 200.Ruef B J, Tuo W, Rodriguez S D, Roussel A J, Chitko-McKown C G, Palmer G H, McElwain T F, Canals A, Zarlenga D S, Gasbarre L C, Brown W C. Immunization with Babesia bigemina rhoptry-associated protein 1 induces a type 1 cytokine response. J Interferon Cytokine Res. 1997;17:45–54. doi: 10.1089/jir.1997.17.45. [DOI] [PubMed] [Google Scholar]
- 201.Saeki H, Ishii T. Effect of silica treatment on resistance to Babesia rodhaini infection in immunized mice. Vet Parasitol. 1996;61:201–210. doi: 10.1016/0304-4017(95)00823-3. [DOI] [PubMed] [Google Scholar]
- 202.Schein E, Rehbein G, Voigt W P, Zweygarth E. Babesia equi (Laveran 1901) 1. Development in horses and in lymphocyte culture. Tropenmed Parasitol. 1981;32:223–227. [PubMed] [Google Scholar]
- 203.Shaio M F, Lin P R. A case study of cytokine profiles in acute human babesiosis. Am J Trop Med Hyg. 1998;58:335–337. doi: 10.4269/ajtmh.1998.58.335. [DOI] [PubMed] [Google Scholar]
- 204.Shih C M, Liu L P, Chung W C, Ong S J, Wang C C. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shimada T, Shikano S, Hashiguchi R, Matsuki N, Ono K. Effects of depletion of T cell subpopulations on the course of infection and anti-parasite delayed type hypersensitivity response in mice infected with Babesia microti and Babesia rodhaini. J Vet Med Sci. 1996;58:343–347. doi: 10.1292/jvms.58.343. [DOI] [PubMed] [Google Scholar]
- 206.Shortt H E. Ticks and piroplasms, no. 6. In: Arthur D R, editor. Aspects of disease transmission by ticks. London, United Kingdom: Zoological Society of London; 1962. [Google Scholar]
- 207.Skrabalo Z, Deanovic Z. Piroplasmosis in man: report of a case. Doc Med Geogr Trop. 1957;9:11–16. [PubMed] [Google Scholar]
- 208.Skuce P J, Mallon T R, Taylor S M. Molecular cloning of a putative rhoptry associated protein homologue from Babesia divergens. Mol Biochem Parasitol. 1996;77:99–102. doi: 10.1016/0166-6851(96)02570-4. [DOI] [PubMed] [Google Scholar]
- 209.Smith R D, James M A, Ristic M, Aikawa M, Vega y Murguia C A. Bovine babesiosis: protection of cattle with culture-derived soluble Babesia bovis antigen. Science. 1981;212:335–338. doi: 10.1126/science.7209532. [DOI] [PubMed] [Google Scholar]
- 210.Smith R P, Evans A T, Popowsky M, Mills L, Spielman A. Transfusion-acquired babesiosis and failure of antibiotic treatment. JAMA. 1986;256:2726–2727. [PubMed] [Google Scholar]
- 211.Smith T. Investigations into the nature, causation, and prevention of Texas or Southern cattle tick fever. Washington, D.C.: Bureau of Animal Industries, bulletin no. 1. U.S. Department of Agriculture; 1893. pp. 177–304. [Google Scholar]
- 212.Solomon J B, Forbes M G, Solomon G R. A possible role for natural killer cells in providing protection against Plasmodium berghei in early stages of infection. Immunol Lett. 1985;9:349–352. doi: 10.1016/0165-2478(85)90061-6. [DOI] [PubMed] [Google Scholar]
- 213.Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25:784–787. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- 214.Spielman A, Clifford C M, Piesman J, Corwin M D. Human babesiosis on Nantucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n. sp. (Acarina: Ixodidae) J Med Entomol. 1979;15:218–234. doi: 10.1093/jmedent/15.3.218. [DOI] [PubMed] [Google Scholar]
- 215.Spielman A, Etkind P, Piesman J, Ruebush II T K, Juranek D D, Jacobs M S. Reservoir hosts of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30:560–565. doi: 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- 216.Spielman A, Wilson M L, Levine J F, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 217.Spooner R L, Innes E A, Glass E J, Brown C G. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology. 1989;66:284–288. [PMC free article] [PubMed] [Google Scholar]
- 218.Stafford K C., 3rd Effectiveness of carbaryl applications for the control of Ixodes dammini (Acari: Ixodidae) nymphs in an endemic residential area. J Med Entomol. 1991;28:32–36. doi: 10.1093/jmedent/28.1.32. [DOI] [PubMed] [Google Scholar]
- 219.Steuber S, Frevert U, Ahmed J S, Hauschild S, Schein E. In vitro susceptibility of different mammalian lymphocytes to sporozoites of Theileria annulata. Z Parasitenkd. 1986;72:831–834. doi: 10.1007/BF00925104. [DOI] [PubMed] [Google Scholar]
- 220.Strickland G T, Voller R A, Pettit L E, Fleck D G. Immunodepression associated with concomitant Toxoplasma and malarial infections in mice. J Infect Dis. 1972;126:54–60. doi: 10.1093/infdis/126.1.54. [DOI] [PubMed] [Google Scholar]
- 221.Sun T, Tenenbaum M J, Greenspan J, Teichberg S, Wang R T, Degnan T, Kaplan M H. Morphologic and clinical observations in human infection with Babesia microti. J Infect Dis. 1983;148:239–248. doi: 10.1093/infdis/148.2.239. [DOI] [PubMed] [Google Scholar]
- 222.Swofford D L. PAUP: phylogenetic analysis using parsimony. ed. 3.1.1. Champaign: Illinois Natural History Survey; 1993. [Google Scholar]
- 223.Takahashi M, Omata Y, Oikawa H, Claveria F, Igarashi I, Saito A, Suzuki N. Protective immune response of Isospora felis-infected mice against Babesia microti infection. J Vet Med Sci. 1993;55:587–590. doi: 10.1292/jvms.55.587. [DOI] [PubMed] [Google Scholar]
- 224.Reference deleted.
- 225.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Telford S R, III, Gorenflot A, Brasseur P, Spielman A. Babesial infections in humans and wildlife. In: Kreier J P, editor. Parasitic protozoa. 2nd ed. Vol. 5. San Diego, Calif: Academic Press; 1993. pp. 1–47. [Google Scholar]
- 227.Telford S R, III, Spielman A. Babesiosis of humans. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. Vol. 5. London, England: Arnold; 1998. pp. 349–359. [Google Scholar]
- 228.Tetzlaff C L, McMurray D N, Rice-Ficht A C. Isolation and characterization of a gene associated with a virulent strain of Babesia microti. Mol Biochem Parasitol. 1990;40:183–192. doi: 10.1016/0166-6851(90)90040-s. [DOI] [PubMed] [Google Scholar]
- 229.Teutsch S M, Etkind P, Burwell E L, Sato K, Dana M M, Fleishman P R, Juranek D D. Babesiosis in post-splenectomy hosts. Am J Trop Med Hyg. 1980;29:738–741. doi: 10.4269/ajtmh.1980.29.738. [DOI] [PubMed] [Google Scholar]
- 230.Tewari A K, Sharma N N, Rao J R, Mishra A K, Das S K. Effect of Mycobacterium phlei on the development of immunity to Babesia bigemina. Vet Parasitol. 1996;62:223–230. doi: 10.1016/0304-4017(95)00873-x. [DOI] [PubMed] [Google Scholar]
- 231.Thomford J W, Conrad P A, Telford III S R, Mathiesen D, Bowman B H, Spielman A, Eberhard M L, Herwaldt B L, Quick R E, Persing D H. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis. 1994;169:1050–1056. doi: 10.1093/infdis/169.5.1050. [DOI] [PubMed] [Google Scholar]
- 232.Ushe T C, Palmer G H, Sotomayor L, Figueroa J V, Buening G M, Perryman L E, McElwain T F. Antibody response to a Babesia bigemina rhoptry-associated protein 1 surface-exposed and neutralization-sensitive epitope in immune cattle. Infect Immun. 1994;62:5698–5701. doi: 10.1128/iai.62.12.5698-5701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Welch D F, Carroll K C, Hofmeister E K, Persing D H, Robison D A, Steigerwalt A G, Brenner D J. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37:2598–2601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Winger C M, Canning E U, Culverhouse J D. A monoclonal antibody-derived antigen of Babesia divergens: characterization and investigation of its ability to protect gerbils against virulent homologous challenge. Parasitology. 1989;3:341–348. doi: 10.1017/s0031182000059059. [DOI] [PubMed] [Google Scholar]
- 235.Wittner M, Lederman J, Tanowitz H B, Rosenbaum G S, Weiss L M. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg. 1996;55:219–222. doi: 10.4269/ajtmh.1996.55.219. [DOI] [PubMed] [Google Scholar]
- 236.Wittner M, Rowin K S, Tanowitz H B, Hobbs J F, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy G R. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–604. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]
- 237.Wozniak E J, Barr B C, Thomford J W, Yamane I, McDonough S P, Moore P F, Naydan D, Robinson T W, Conrad P A. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris) J Parasitol. 1997;83:692–699. [PubMed] [Google Scholar]
- 238.Wright I G. Towards a synthetic Babesia vaccine. Int J Parasitol. 1991;21:156–159. [PubMed] [Google Scholar]
- 239.Wright I G, Casu R, Commins M A, Dalrymple B P, Gale K R, Goodger B V, Riddles P W, Waltisbuhl D J, Abetz I, Berrie D A, Bowles Y, Dimmock C, Hayes T, Kalnins H, Leatch G, McCrae R, Montague P E, Nesbit I T, Parrodi F, Peters J M, Scheiwe P C, Smith W, Rode-Bramanis K, White M A. The development of a recombinant Babesia vaccine. Vet Parasitol. 1992;44:3–13. doi: 10.1016/0304-4017(92)90138-y. [DOI] [PubMed] [Google Scholar]
- 240.Yabrov A A. Interferon and non-specific resistance. New York, N.Y: Human Sciences Press; 1980. [Google Scholar]
- 241.Yuval B, Spielman A. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1990;27:196–201. doi: 10.1093/jmedent/27.2.196. [DOI] [PubMed] [Google Scholar]
- 242.Zivkovic D, Seinen W, Kuil H, Albers-van Bemmel C M, Speksnijder J E. Immunity to Babesia in mice. I. Adoptive transfer of immunity to Babesia rodhaini with immune spleen cells and the effect of irradiation on the protection of immune mice. Vet Immunol Immunopathol. 1984;5:343–357. doi: 10.1016/0165-2427(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 243.Zivkovic D, Speksnijder J E, Kuil H, Seinen W. Immunity to Babesia in mice. II. Cross protection between various Babesia and Plasmodium species and its relevance to the nature of Babesia immunity. Vet Immunol Immunopathol. 1984;5:359–368. doi: 10.1016/0165-2427(84)90004-7. [DOI] [PubMed] [Google Scholar]
- 244.Zivkovic D, Speksnijder J E, Kuil H, Seinen W. Immunity to Babesia in mice. III. The effects of corticosteroids and anti-thymocyte serum on mice immune to Babesia rodhaini. Vet Immunol Immunopathol. 1985;9:131–142. doi: 10.1016/0165-2427(85)90013-3. [DOI] [PubMed] [Google Scholar]
- 245.Zwart D, Brocklesby D W. Babesiosis: non-specific resistance, immunological factors and pathogenesis. Adv Parasitol. 1979;17:49–113. doi: 10.1016/s0065-308x(08)60549-7. [DOI] [PubMed] [Google Scholar]