Abstract
We aimed to evaluate the association between the prevalence of diabetic retinopathy (DR) and coffee consumption in a Korean population. This cross-sectional study was based on data from the 2008–2011 Korean National Health and Nutrition Survey. Among 37,753 survey participants, the data of 1350 subjects with type 2 diabetes who underwent DR examination were analyzed. DR was graded using the modified Airlie House classification system. Coffee consumption data were obtained through food frequency questionnaires and categorized into four groups: almost none, < 1 cup/day, 1 cup/day, and ≥ 2 cups/day. The relationship between DR and coffee consumption was evaluated using multivariable logistic regression models adjusted for age, sex, education, occupation, income, smoking, alcohol intake, body mass index, physical activity, hypertension, dyslipidemia, diabetes duration, and glycated hemoglobin. The prevalence of DR was 20.0%. Non-proliferative DR was observed in 87.8% of all DR patients, and proliferative DR in 12.2%. The prevalence of DR and vision-threatening DR showed a significantly decreasing tendency according to daily coffee consumption (P for trend 0.025 and 0.005, respectively) after adjustment for possible confounders. This tendency was more prominent in those aged < 65 years (P for trend 0.005 and 0.003, respectively). Our findings suggest coffee consumption might be associated with DR reduction especially in Koreans with diabetes mellitus aged < 65 years.
Subject terms: Retinal diseases, Diabetes, Nutrition
Introduction
Diabetic retinopathy (DR) is an important complication of diabetes mellitus (DM) and is a major cause of vision impairment and blindness. DR can significantly affect an individual’s quality of life1. The prevalence of DR among diabetic patients varies across countries, ranging from 18% in India to 40% in the United State2. Given the global burden of diabetes, there is a great deal of interest in developing nutritional and dietary approaches to reduce or prevent diabetic complications3–5.
Coffee is one of the most consumed beverages worldwide6. Over the past few decades, many studies have investigated the associations between coffee consumption and various diseases, including cancer7, Alzheimer's disease8, cardiovascular disease9, and type 2 diabetes10. Numerous studies including several meta-analyses have indicated that coffee consumption lowers the risk of type 2 diabetes5,11,12.
Despite the great interest in the relationship between coffee consumption and diabetes, few studies have evaluated the association between DR and coffee consumption. Some animal studies have suggested that ingestion of coffee might be effective in preventing DR13–15; however, the only study conducted in humans showed no significant association between intake of coffee and DR16. Therefore, the purpose of this study was to assess the relationship between the prevalence of DR and coffee consumption in a large population.
Results
Among 37,753 survey participants, the data of 1350 participants diagnosed with type 2 diabetes who underwent DR examination were analyzed (Fig. 1). The basic characteristics of the participants are presented in Table 1. The prevalence of any DR and vision-threatening DR (VTDR) in this population was 20.0% and 5.3%, respectively. Among the participants with DR, 87.8% had NPDR and 12.2% had PDR. Compared to participants without DR, the DR group had a significantly higher HbA1c level, a longer duration of diabetes, and a lower BMI; however, the effects of BMI were no longer significant once they were adjusted for demographic and socioeconomic factors (Supplementary Table S1). The VTDR group exhibited a lower rate of high school or college education, lower BMI, higher HbA1c level, and longer diabetes duration compared with the no VTDR group. Additionally, the baseline characteristics of the participants according to inclusion and exclusion participants are presented in Supplementary Table S2.
Figure 1.
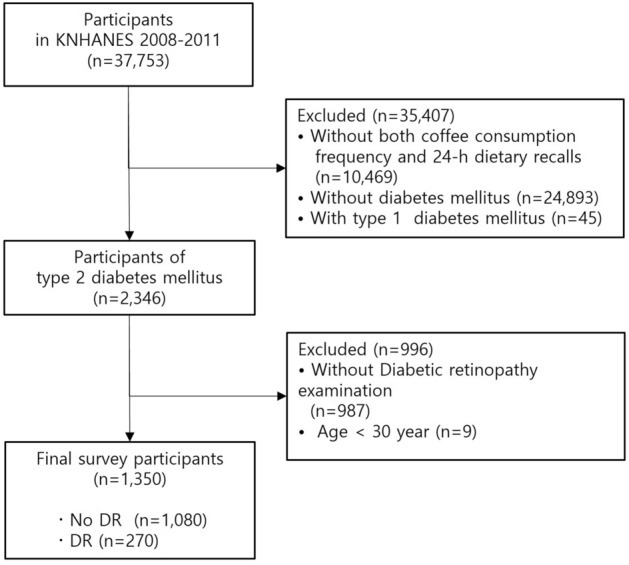
Flowchart of the study participants diagnosed with type 2 diabetes who underwent diabetic retinopathy examination. (KNHANES: Korea National Health and Nutrition Examination Survey).
Table 1.
General characteristics of DR in patients with type 2 diabetes (n = 1,350).
| All | No DR | Any DR | P | No PDR | PDR | P | No VTDR | VTDR | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1350) | (n = 1080) | (n = 270) | (n = 1317) | (n = 33) | (n = 1278) | (n = 72) | ||||
| Age (years) | 58.8 ± 0.4 | 58.6 ± 0.4 | 59.9 ± 0.8 | 0.143 | 58.8 ± 0.4 | 59.2 ± 1.6 | 0.648 | 58.8 ± 0.4 | 60.2 ± 1.4 | 0.332 |
| 30–49 | 180 (22.4) | 152 (23.7) | 28 (17.1) | 0.169 | 176 (22.6) | 4 (12.2) | 0.153 | 173 (23.0) | 7 (10.7) | 0.051 |
| 50–64 | 529 (43.8) | 409 (42.7) | 120 (48.2) | 511 (43.4) | 18 (61.9) | 495 (43.1) | 34 (57.0) | |||
| ≥ 65 | 641 (33.8) | 519 (33.6) | 122 (34.7) | 630 (34.0) | 11 (25.9) | 610 (33.9) | 31 (32.3) | |||
| Sex, male | 657 (55.5) | 527 (55.5) | 130 (55.4) | 0.980 | 644 (55.7) | 13 (46.0) | 0.350 | 623 (55.4) | 34 (57.2) | 0.789 |
| Education | ||||||||||
| ≤ Elementary school | 644 (41.7) | 516 (41.1) | 128 (44.0) | 0.267 | 626 (41.4) | 18 (58.0) | 0.372 | 607 (41.3) | 37 (49.4) | 0.005 |
| Middle school | 218 (16.3) | 168 (15.4) | 50 (20.0) | 212 (16.3) | 6 (16.2) | 202 (15.6) | 16 (29.5) | |||
| High school | 320 (27.1) | 256 (27.7) | 64 (24.5) | 314 (27.3) | 6 (16.4) | 306 (27.6) | 14 (15.8) | |||
| ≥ College | 159 (15.0) | 135 (15.8) | 24 (11.5) | 157 (15.1) | 2 (9.4) | 156 (15.4) | 3 (5.3) | |||
| Household income | ||||||||||
| Quartile 1 (low) | 463 (29.1) | 378 (29.0) | 85 (29.2) | 0.981 | 454 (29.2) | 9 (19.9) | 0.543 | 442 (29.0) | 21 (30.1) | 0.266 |
| Quartile 2 | 340 (26.3) | 260 (26.1) | 80 (27.5) | 328 (26.2) | 12 (34.0) | 314 (25.8) | 26 (37.7) | |||
| Quartile 3 | 283 (23.1) | 229 (23.3) | 54 (22.2) | 278 (23.2) | 5 (17.2) | 271 (23.5) | 12 (15.7) | |||
| Quartile 4 (high) | 242 (21.5) | 196 (21.5) | 46 (21.1) | 235 (21.3) | 7 (28.9) | 230 (21.7) | 12 (16.5) | |||
| Occupation | ||||||||||
| White-collar | 105 (11.2) | 88 (11.9) | 17 (8.2) | 0.409 | 101 (11.2) | 4 (14.5) | 0.814 | 101 (11.5) | 4 (5.4) | 0.263 |
| Blue-collar | 532 (44.4) | 433 (44.4) | 99 (44.5) | 520 (44.4) | 12 (46.8) | 504 (44.0) | 28 (52.2) | |||
| Others | 702 (44.4) | 552 (43.7) | 150 (47.2) | 686 (44.5) | 16 (38.7) | 663 (44.5) | 39 (42.4) | |||
| Current smoking status | 350 (31.3) | 276 (31.0) | 74 (32.5) | 0.718 | 342 (31.3) | 8 (34.0) | 0.804 | 325 (30.7) | 25 (44.0) | 0.089 |
| Alcohol | ||||||||||
| Non-drinker | 559 (35.3) | 439 (34.4) | 120 (39.3) | 0.443 | 541 (35.2) | 18 (44.1) | 0.166 | 522 (35.0) | 37 (41.0) | 0.638 |
| Social drinker | 623 (48.0) | 505 (48.4) | 118 (46.2) | 610 (47.9) | 13 (51.3) | 595 (48.1) | 28 (46.2) | |||
| Heavy drinker | 161 (16.7) | 130 (17.2) | 31 (14.5) | 159 (16.9) | 2 (4.6) | 154 (16.9) | 7 (12.8) | |||
| Walking physical activity | 573 (40.0) | 458 (40.6) | 115 (37.7) | 0.505 | 556 (39.8) | 17 (53.1) | 0.164 | 537 (39.7) | 36 (45.6) | 0.393 |
| Moderate physical activity | 149 (10.0) | 128 (11.0) | 21 (5.8) | 0.033 | 148 (9.9) | 1 (11.9) | 0.839 | 145 (10.1) | 4 (8.3) | 0.734 |
| Aerobic physical activity | 202 (15.2) | 164 (15.3) | 38 (14.7) | 0.843 | 199 (15.3) | 3 (8.9) | 0.326 | 194 (15.4) | 8 (11.9) | 0.542 |
| BMI (kg/m2) | 25.1 ± 0.1 | 25.2 ± 0.1 | 24.3 ± 0.2 | < 0.001 | 25.1 ± 0.1 | 23.2 ± 0.6 | 0.064 | 25.1 ± 0.1 | 23.4 ± 0.3 | < 0.001 |
| BMI < 18.5, underweight | 24 (1.5) | 17 (1.3) | 7 (2.4) | 0.036 | 22(1.5) | 2(6.6) | 0.150 | 22 (1.5) | 2 (2.5) | 0.126 |
| 18.5 ≤ BMI < 23.0, normal | 374 (26.4) | 278 (24.7) | 96 (33.8) | 362(26.4) | 12(29.9) | 343 (25.9) | 31 (37.8) | |||
| 23.0 ≤ BMI < 25.0, overweight | 313 (23.2) | 248 (23.3) | 65 (22.6) | 305(23.1) | 8(27.6) | 298 (23.0) | 15 (26.4) | |||
| BMI ≥ 25.0, obese | 634 (48.8) | 533 (50.7) | 101 (41.1) | 623(49.1) | 11(35.8) | 610 (49.6) | 24 (33.2) | |||
| HbA1c | 7.4 ± 0.1 | 7.3 ± 0.1 | 8.2 ± 0.1 | < 0.0001 | 7.4 ± 0.1 | 8.6 ± 0.4 | 0.199 | 7.4 ± 0.1 | 8.8 ± 0.2 | < 0.001 |
| Hypertension | 832 (58.3) | 670 (58.5) | 162 (57.2) | 0.754 | 811 (58.2) | 21 (60.8) | 0.814 | 793 (58.9) | 33 (54.1) | 0.069 |
| Hypercholesterolemia | 359 (29.4) | 279 (28.3) | 80 (33.9) | 0.152 | 350 (29.4) | 9 (28.6) | 0.938 | 340 (29.3) | 19 (31.1) | 0.828 |
| Diabetes duration, years | 7.7 ± 0.2 | 6.9 ± 0.3 | 10.2 ± 0.5 | < 0.001 | 7.5 ± 0.2 | 13.9 ± 2.0 | 0.057 | 7.3 ± 0.2 | 13.7 ± 1.3 | < 0.001 |
| Energy intake (kcal/day) | 1915.9 ± 31.3 | 1913.4 ± 33.5 | 1926.3 ± 87.0 | 0.891 | 1919.3 ± 31.5 | 1730.6 ± 197.1 | 0.339 | 1925.0 ± 32.3 | 1735.0 ± 98.3 | 0.065 |
| Coffee consumption | ||||||||||
| Almost none | 231 (15.2) | 178 (14.9) | 53 (16.5) | 0.376 | 223 (15.1) | 8 (19.5) | 0.226 | 217 (15.3) | 14 (13.6) | 0.252 |
| < 1 time/day | 310 (22.1) | 249 (21.6) | 61 (24.6) | 301 (22.1) | 9 (24.5) | 292 (21.8) | 18 (29.5) | |||
| 1 time/day | 365 (27.7) | 293 (27.2) | 72 (29.6) | 356 (27.4) | 9 (39.4) | 342 (27.4) | 23 (33.8) | |||
| ≥ 2 times/day | 444 (35.0) | 360 (36.3) | 84 (29.3) | 437 (35.3) | 7 (16.6) | 427 (35.6) | 17 (23.1) | |||
Data are expressed as means ± standard errors for continuous variables or numbers (proportions) for categorical variables. P values were based on the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables.
DR, diabetic retinopathy; BMI, body mass index; HbA1c, glycated hemoglobin; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy.
Patient characteristics compared in terms of the amount of coffee consumption are presented in Supplementary Table S3. The variables with significant differences between different coffee consumption groups included age (P < 0.001), sex (P < 0.001), education level (P < 0.001), household income (P < 0.001), occupation (P < 0.001), current smoking status (P < 0.001), alcohol drinking (P < 0.001), walking physical activity (P < 0.001), prevalence of hypertension (P = 0.002), Energy intake (P < 0.001), and BMI (P = 0.012). The average age of the group who rarely drank coffee was 61.5 ± 1.1, and that of the group who drank ≥ 2 cups/day was 56.3 ± 0.6. The proportion of men in the group who rarely drank coffee was 41.0%, and that in the group who drank ≥ 2 cups/day was 71.4%. In the group who rarely drink coffee, 9.9% had a college education or higher compared to the 23.3% in the group that drank ≥ 2 cups/day. In the group that rarely drink coffee, 10.7% had a household income above the 4th quartile, and 27.9% in the group who drank ≥ 2 cups/day. The current smoking rate was 19.7% in the group that rarely drank coffee, and 44.9% in the group that drank ≥ 2 cups/day. The prevalence of hypertension was 70.9% in the group that rarely drank coffee, and 51.8% in the group that drank ≥ 2 cups/day. Finally, the average BMI was 24.7 ± 0.2 kg/m2 for those who rarely drank coffee, and 25.5 ± 0.2 kg/m2 for those who drank ≥ 2 cups/day.
After adjusting for potential confounders, coffee consumption was found to be inversely correlated with the prevalence of any DR and VTDR (P for trend = 0.025 for any DR, P for trend = 0.005 for VTDR; Table 2). Participants who consumed ≥ 2 cups of coffee per day had lower odds of having any DR (odds ratio [OR] 0.53, 95% confidence interval [CI] 0.28–0.99) and VTDR (OR: 0.30, 95% CI 0.10–0.91) than those who drank almost none after adjustment. The OR for the 1 cup/day group was not statistically significant in the any DR or the VTDR groups. There were no significant correlations between the prevalence of PDR and coffee consumption.
Table 2.
The prevalence of DR by frequency of coffee consumption from the food frequency questionnaire among participants with type 2 diabetes (n = 1350).
| Coffee consumption | |||||
|---|---|---|---|---|---|
| Almost none (n = 231) |
< 1 cup/day (n = 310) |
1 cup/day (n = 365) |
≥ 2 cups/day (n = 444) |
P for trend | |
| Any DR | |||||
| Case | 53 | 61 | 72 | 84 | |
| Crude OR (95% CI) | 1.00 (ref.) | 1.03 (0.63–1.67) | 0.98 (0.61–1.59) | 0.73 (0.45–1.18) | 0.133 |
| Age and sex adjusted OR (95% CI) | 1.00 (ref.) | 1.04 (0.64–1.68) | 0.99 (0.61–1.60) | 0.74 (0.45–1.21) | 0.165 |
| Multivariable adjusted OR (95% CI) | 1.00 (ref.) | 0.95 (0.54–1.68) | 0.67 (0.36–1.24) | 0.53 (0.28–0.99) | 0.025 |
| VTDR | |||||
| Case | 14 | 18 | 23 | 17 | |
| Crude OR (95% CI) | 1.00 (ref.) | 1.52 (0.68–3.40) | 1.39 (0.64–2.99) | 0.73 (0.30–1.75) | 0.238 |
| Age and sex adjusted OR (95% CI) | 1.00 (ref.) | 1.53 (0.69–3.38) | 1.38 (0.64–2.98) | 0.71 (0.30–1.69) | 0.235 |
| Multivariable adjusted OR (95% CI) | 1.00 (ref.) | 1.44 (0.60–3.43) | 0.57 (0.21–1.53) | 0.30 (0.10–0.91) | 0.005 |
| PDR | |||||
| Case | 8 | 9 | 9 | 7 | |
| Crude OR (95% CI) | 1.00 (ref.) | 0.86 (0.30–2.48) | 1.11 (0.38–3.31) | 0.37 (0.12–1.13) | 0.086 |
| Age and sex adjusted OR (95% CI) | 1.00 (ref.) | 0.86 (0.30–2.48) | 1.13 (0.40–3.23) | 0.38 (0.12–1.20) | 0.103 |
| Multivariable adjusted OR (95% CI) | 1.00 (ref.) | 0.73 (0.20–2.60) | 0.41 (0.10–1.67) | 0.28 (0.06–1.42) | 0.071 |
Multivariable adjustments included age, gender, education, occupation, income, body mass index, energy intake, hypertension, dyslipidemia, duration of diabetes, glycated hemoglobin (%), smoking, drinking, and physical activity (aerobic, moderate, walking level).
DR, diabetic retinopathy; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy; OR, odds ratio; CI, confidence interval.
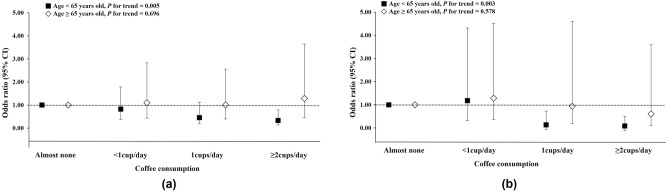
When the population was divided into two groups according to age, this decreasing tendency of DR according to coffee consumption became more pronounced among participants aged under 65 years (Fig. 2). The overall trend of the inverse relationship between coffee consumption and the prevalence of any DR and VTDR became more significant after adjustment (P for trend = 0.005 and 0.003, respectively). However, these trends were not seen in participants aged ≥ 65 years.
Figure 2.
Prevalence of diabetic retinopathy (DR) in terms of coffee consumed among patients with type 2 diabetes by age. Odds ratio of (a) any DR and (b) vision-threatening diabetic retinopathy (VTDR). The prevalence of any DR and VTDR significantly lowered with higher coffee consumption in all participants (P for trend = 0.025 and 0.005, respectively). When the population was divided into two groups according to age, the trend was significant in participants aged < 65 years (P for trend = 0.005 and 0.003, respectively) but not in those aged ≥ 65 years.
We performed a comparison of demographics between the two groups (< 65 vs. ≥ 65 years). The ≥ 65-years-old group had a longer duration of diabetes, a higher rate of hypertension, and a higher proportion of participants who drank < 1 cup of coffee per day. This group had a lower proportion of men, a lower education level, a lower income level, a lower consumption of alcohol and cigarettes, a lower BMI, and a lower HbA1c (Supplementary Table S4).
Next, we analyzed the correlation between the prevalence of DR and the type of coffee consumed (Table 3). Individuals who drank black coffee had lower odds of DR (P for trend = 0.040). This tendency was the same in the group who consumed coffee with sugar or cream (P for trend = 0.031).
Table 3.
DR according to the tertiles of coffee intake consumed from one-day 24-h dietary recall among patients with type 2 diabetes (n = 1350).
| Tertile for black coffee intake | Tertile for coffee with sugar or cream intake | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 (low) | T2 | T3 (high) | P for trend | T1(low) | T2 | T3(high) | P for trend | |
| Intake (cup/day) | None | ≤ 1cup/day | > 1cup/day | None | ≤ 1cup/day | > 1cup/day | ||
| Any DR | ||||||||
| Case/at risk | 193/933 | 27/137 | 50/280 | 190/921 | 46/251 | 34/178 | ||
| Crude OR (95% CI) | 1.00 (ref.) | 0.93 (0.53–1.73) | 0.77 (0.51–1.65) | 0.233 | 1.00 (ref.) | 0.99 (0.62–1.59) | 0.85 (0.50–1.42) | 0.559 |
| Multivariable adjusted OR (95% CI) | 1.00 (ref.) | 0.90 (0.45–1.79) | 0.57 (0.34–0.95) | 0.040 | 1.00 (ref.) | 0.63 (0.35–1.13) | 0.55 (0.29–1.06) | 0.031 |
Multivariable adjustments included age, gender, education, occupation, income, body mass index, energy intake, hypertension, dyslipidemia, duration of diabetes, glycated hemoglobin (%), smoking, drinking, and physical activity (aerobic, moderate, walking level).
DR, diabetic retinopathy; the black coffee intake group and coffee with sugar or cream intake group were mutually adjusted; OR, odds ratio; CI, confidence interval.
Discussion
In this study, we found that participants who reported drinking ≥ 2 cups of coffee per day had a lower prevalence of any DR and VTDR compared with those who reported drinking less than 1 cup of coffee per day. Moreover, we found a negative correlation between the degree of coffee consumption and the prevalence of any DR and VTDR by trend analysis. We also observed that, regardless of the type of coffee, the prevalence of DR tended to decrease with increases in coffee intake of both black coffee and coffee with sugar or cream, adjusted for confounders such as energy intake.
Many studies have shown the inverse association between coffee drinking and the risk of type 2 diabetes4–6,17. Carlström et al. reviewed 30 articles about the relationship between coffee and type 2 diabetes and performed a systematic review and meta-analysis17. Based on available evidence, they concluded that coffee consumption is inversely associated with the risk of type 2 diabetes. The thermogenic, antioxidative, and anti-inflammatory effects of coffee consumption were all suggested as possible mechanisms behind this association.
However, very few studies have analyzed the relationship between coffee intake and DR. Shin et al. showed that chlorogenic acid (CGA) in coffee sufficiently preserved the expression of occludin and decreased vascular endothelial growth factor (VEGF) levels, leading to decreased blood–retinal barrier breakdown and vascular leakage in a diabetic rat model13. Jang et al. demonstrated the protective effects of CGA and coffee on retinal degeneration in mice14,15. Under hypoxic conditions, pretreatment with CGA prevented cell death in a concentration-dependent manner14. Further, coffee metabolites significantly decreased injury to retinal ganglion cells after induced optic nerve crush15. These studies showed that coffee consumption may provide health benefits by preventing retinal degeneration13–15. A previous report suggested that CGA inhibits retinal neoangiogenesis during DR by impeding high glucose-induced hypoxia-inducible factor 1-alpha-mediated paracrine VEGF expression in microglia cells and by preventing VEGF-induced angiogenesis in retinal endothelial cells18. Collectively, these in vivo animal studies suggest that the action of ingredients present in coffee, such as CGA, may mediate the preventative effect of coffee consumption on DR. To the best of our knowledge, only one large population study by Neelam et al.16 has evaluated the association between coffee intake and DR and found no significant association. However, this previous study had a smaller sample size (113 DR cases, 240 controls, total sample size of 353) than ours (270 DR cases, 1080 controls, total sample size of 1350), and this lack of adequate power could be the reason for the lack of statistical significance in the previous study. The authors acknowledged this limitation in their paper. In this study, we divided the participants into four groups based on the amount of coffee consumption. As daily coffee intake increased, the prevalence of DR decreased. However, our study is a cross-sectional study, and therefore it cannot clearly explain the causal relationship between the lower prevalence of DR seen with increased coffee consumption. Thus, cohort studies with a prospective design will be required to fully elucidate the causal relationship.
In this study, participants who drank more coffee tended to have a higher BMI, and BMI was significantly lower in participants with DR than in those without DR. Some studies have reported an association between a higher BMI and a lower prevalence of DR19,20. However, other studies reported no association or a positive association with DR21,22. Most results showing an inverse association between obesity and DR were obtained in Asian study participants23; therefore, ethnic differences are a possible explanation for inconsistency between obesity and DR. Unlike the relatively clear association between higher BMI and increased risk of diabetes, the association between BMI and DR is disputed, due to the conflicting results of previous studies20,22. In this study, participants who drank more coffee had a higher BMI and a lower prevalence of DR; however, the association between BMI and DR was not statistically significant (Supplementary Table S1) and it should not be overlooked that BMI remains an important risk factor for the development of DM. Further longitudinal cohort studies are required to clarify whether higher BMI lowers the incidence of DR.
In Korea, the middle aged and older populations drink more coffee with sugar or creamer than any other age group24, but the daily total sugar intake was 61.4 g25, which is considerably lower than that in the United States (116.4 g)26. Although no clear results have been reported with creamers or sugars, it should not be overlooked that excessive intake of saturated fat or simple sugars in cream or sugar can lead to weight gain and insulin resistance27.
In this study, the association between coffee consumption and DR was only significant among participants under 65 years of age. There are a few possible reasons for this. First, the duration of diabetes was longer in the ≥ 65-years-old group, and the effect of the duration of diabetes on DR may have been too strong to allow other factors, such as consumption of coffee, to have an impact on DR. Second, it is more likely that the diet pattern, including coffee intake, changed after being diagnosed with diabetes among participants ≥ 65 years old. This assumption is supported by the fact that the ≥ 65-years-old group consumed less alcohol and cigarettes, had a lower BMI and HbA1c, and had a higher proportion of participants who consumed less than one cup of coffee per day than the group aged < 65 years. In particular, the lower HbA1c may be a result of more thorough glycemic control than in the < 65-years-old group and supports our theory of behavioral and dietary changes. However, because this was a cross-sectional study, it was not possible to confirm the change in coffee consumption patterns in patients with our data set. Finally, in the ≥ 65-years-old group, potential people with severe disease may have been excluded due to death, resulting in a selective survival bias.
Our study has several limitations. First, due to the cross-sectional nature of the analysis performed using a predesigned survey, we were not able to define the causal relationship between DR and coffee consumption. Additionally, although we found a negative correlation between the degree of coffee consumption and the prevalence of DR, our results might be affected by “collider bias” because coffee consumption would be associated with both DR and DM28. Second, the exact mechanism by which coffee components affect the progression of DR remains unknown. Further studies should be conducted to elucidate this mechanism. Third, the number of participants with DR was small. There were no significant correlations between the prevalence of PDR and coffee consumption, despite coffee intake being inversely correlated with the prevalence of any DR or VTDR. This may be because there were too few patients with PDR to produce statistically significant results. Nevertheless, this study used a nationwide, stratified, multistage, clustered sampling, and standardized assessment methods of seven standardized photographs. To our knowledge, this is the first study to demonstrate the relationship between coffee intake and DR in a large survey analysis.
In conclusion, this study showed that coffee intake inversely correlated with the prevalence of DR in Koreans with DM under 65 years of age, suggesting that coffee consumption might be associated with a reduction in DR. Cohort studies are warranted to fully elucidate the cross-sectional association between coffee consumption and DR.
Materials and methods
Study population
This study was based on data acquired from the Korean National Health and Nutritional Examination Survey (KNHANES), which has been conducted by the Division of Health and Nutritional Survey, Korean Centers for Disease Control and Prevention (KCDC) since 199829. It is a cross-sectional, population-based, nationally representative ongoing survey. A multi-stage, stratified, and clustered probability design was used to choose a sample of civilian, non-institutionalized Korean adults. The KNHANES contains three components: a health interview, nutrition survey, and health examination. Data were assembled via household interviews and standardized physical examinations conducted at mobile examination centers (Supplementary Table S5) 29.
Data from the Fourth and Fifth KNHANES conducted from 2008 to 2011 (KNHANES IV-2,3 and V-1) were used. A 5-year ophthalmic survey designed by the Korean Ophthalmological Society was conducted between July 2008 and December 2011. Detailed survey information has been previously published29,30.
A total of 37,753 patients were surveyed between 2008 and 2011. Participants aged 30–79 years with type 2 diabetes were eligible for inclusion in the present study. Participants aged 30–79 years with type 2 diabetes were eligible for inclusion in the present study. We excluded participants with missing data of both FFQs and 24-h dietary recalls (n = 10,469), without type 2 diabetes (n = 24,893), and with type 1 diabetes (n = 45). Furthermore, we excluded participants who did not have accessible data regarding fundus photographs in the diabetic retinopathy examination (n = 987) or were younger than 30 years of age (n = 9). A total of 1,350 participants were enrolled and included in the final analysis (Fig. 1). This survey was reviewed and approved by the Institutional Review Boards (IRBs) of the Korean Centers for Disease Control and Prevention (IRB numbers: 2008-04EXP-01-c, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C) and written informed consent was provided by all participants. All experiments and examinations were performed in accordance with relevant guidelines and regulations.
Evaluation of diabetes
Diabetes was defined as a diagnosis of diabetes, the use of oral hypoglycemic medications or insulin, and/or a fasting blood glucose level ≥ 126 mg/dL. Participants aged younger than 30 years at the time of diagnosis were considered to have type 1 diabetes and were excluded.
Evaluation of DR
In cooperation with the KCDC, eye examinations were conducted by ophthalmologists from the Korean Ophthalmologic Society. Non-mydriatic 45° digital fundus photography (TRC-NW6S; Topcon, Tokyo, Japan) was performed in all participants ≥ 19 years old. For each participant, one 45° nonmydriatic digital retinal image centered on the fovea was taken per eye (two images per person). In participants who had a history of diabetes, random blood glucose level ≥ 200 mg/dL, or doubtful DR findings in the non-mydriatic fundus photographs, seven standard photographs from the Early Treatment for Diabetic Retinopathy Study (ETDRS) were obtained from both eyes after pharmacologic pupil dilatation with the same Non-mydriatic 45° digital fundus photography (TRC-NW6S; Topcon, Tokyo, Japan)31. All 1350 final participants with type 2 diabetes received seven standard fundus photographs from the ETDRS.
DR was defined if any of the following characteristic lesions were present, based on the Early Treatment for Diabetic Retinopathy Study severity scale: microaneurysms, hemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading, or new vessels. Each eye of participants was assigned a DR severity score according to the modified Airlie House Classification system31; detailed grading information has been previously published19. The level of DR was measured depending on the worse eye. Eyes were graded as either no DR (levels 10–13) or any DR (levels 14–80). DR was divided further into minimal non-proliferative DR (NPDR, levels 14–20), mild NPDR (level 31), moderate NPDR (levels 41–47), severe NPDR (level 51), and proliferative DR (level > 60). Any DR was further subdivided into non-proliferative DR (NPDR, levels 14–51), and proliferative DR (PDR, level > 60).
Macular edema (ME) was defined by hard exudates within one disk diameter from the foveal center in the presence of blot hemorrhage and microaneurysms or by the existence of focal photocoagulation scars in the macular area. Vision-threatening DR (VTDR) was determined by the presence of severe NPDR, PDR, or clinically-significant ME31.
Coffee consumption
Dietary information was collected by trained dietitians through a 63-item non-quantitative food frequency questionnaire (FFQ) over the past year and through a one-day 24-h dietary recall. The overall frequency of coffee consumption in the past year was obtained from a non-quantitative food frequency questionnaire (FFQ). Participants were asked to report how frequently they consumed coffee over the previous year based on 10 categories: 3 cups a day, 2 cups a day, 1 cup a day, 4–6 cups a week, 2–3 cups a week, 1 cup a week, 2–3 cups a month, 1 cup a month, 6–11 cups a year, and almost none. The frequency of coffee consumption was categorized into the following four groups: almost none (6–11 cups a year, and almost none), < 1 cup a day (4–6 cups a week, 2–3 cups a week, 1 cup a week, 2–3 cups a month, 1 cup a month), 1 cup a day, and ≥ 2 cups a day32. Information on types and amount of coffee calculated from the 24-h dietary recalls was collected from the same participants who completed the FFQs. We quantified the intake (serving or gram) of specific coffee types such as black coffee or coffee with sugar and powder creamer from a single 24-h dietary recalls, because the FFQ did not include the consumption by type of coffee. The quantitative 24-h dietary recall questionnaire collected detailed quantitative information on all foods and beverages consumed in the past 24 h (time, location, type of food, amount, cooking method) using open-ended dietary assessment methods. Using data from 24-h dietary recalls, we categorized types of coffee as “black coffee” and “coffee with sugar or powder creamer,” and the intake of coffee type was divided into three groups as follows: none, ≤ 1 cup a day, and > 1 cup a day.
Assessment of other variables
Covariates for the statistical models were assessed based on related studies33–36. Potential confounders included age; sex; health-related behaviors such as smoking, alcohol use, and physical activity; socioeconomic status including education, occupation, and household income; and comorbid medical conditions, such as hypertension, hypercholesterolemia, glycated hemoglobin (HbA1c) level, body mass index (BMI), and diabetes duration. Information regarding demographic and social factors was collected using a standardized questionnaire during a health interview. “Current smoker” was defined as currently smoking with a smoking history of 100 or more cigarettes in the participant’s lifetime. The participants were divided into three groups based on their level of alcohol consumption: non-drinker, social drinker, and heavy drinker. The Korean National Nutrition Survey defined the high-risk drinking rate as an average drinking rate of seven or more glasses (five glasses for women) per drinking session, with a frequency of drinking more than twice a week. Physical activity was categorized into the following independent categories: aerobic, moderate, and walking. Aerobic activity was defined as physical activity for least 2 h and 30 min/week, medium- or high-intensity physical activity for 1 h and 15 min, or both medium- and high-intensity physical activity. Moderate physical activity was defined as participation in at least five days of ≥ 30 min/day of less intensive activity. Participants performing at least five days of ≥ 30 min/day of walking were classified into the walking physical activity group. Educational level was categorized into following four groups: elementary school graduate or less, middle school graduate, high school graduate, and college graduate or above. The participants were stratified into four quartiles according to their equivalent household income (quartile 1, lowest; quartile 4, highest). Occupation was categorized into the following three groups: white collar, blue collar, and others.
Blood pressure (BP) was measured using standard methods with the patient in a sitting position. Three measurements were taken in all participants at 5-min intervals, and the average of the second and third measurements was used in the analysis. Hypertension was defined as a systolic BP > 140 mmHg and diastolic BP > 90 mmHg, or if the individual had been prescribed antihypertensive medication. Blood samples were collected in the morning after fasting for at least 8 h. Fasting glucose, HbA1c, and total cholesterol levels were measured at a certified laboratory. Hypercholesterolemia was defined as a total cholesterol concentration > 240 mg/dL or if the individual had been prescribed cholesterol-lowering medication. Diabetes duration was determined as the difference between the current age of the patient and the age at diabetes onset.
Statistical analysis
The demographic and clinical characteristics of the study participants are presented as means (standard error) or number (proportion). Differences between proportions were tested using the chi-square test and differences in continuous variables were tested using the Wilcoxon rank-sum test.
Logistic regression models were used to investigate the association between coffee consumption and the prevalence of any DR, PDR, and VTDR. The first logistic regression model was adjusted for age and sex. A second model was adjusted for other potential confounders, including education, occupation, income, smoking, alcohol intake, BMI, physical activity, energy intake, hypertension, dyslipidemia, duration of diabetes, and HbA1c (%). Moreover, we assessed the association of coffee without sugar or cream and coffee with sugar or cream. If participants drank both types of coffee, the association was assessed after adjusting for the other type of coffee. To test for a linear trend across increasing amounts of coffee consumption, we modeled categories of coffee consumption as a continuous variable. In addition, we created subgroups divided by age group (< 65 and ≥ 65 years) and examined associations between subgroups and diagnosis of any DR, PDR, and VTDR.
Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical analyses accounted for the KNHANES’s complex sample design and weighting. Statistical significance was set at P < 0.05.
Supplementary Information
Acknowledgements
This research was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIT) (No. 120210290) and by 2016 Kangwon National University Hospital Grant. The funding organizations had no role in the design or conduct of this study.
Author contributions
Conceptualization, D.D.H.; software, S.O.K.; validation, D.D.H. and H.J.L. and J.I.P.; formal analysis, S.O.K.; investigation, H.J.L. and D.D.H.; resources, J.I.P. and D.D.H.; data curation, S.O.K.; writing—original draft preparation, H.J.L.; writing—review and editing, J.I.P. and D.D.H.; visualization, S.O.K.; supervision, J.I.P. and D.D.H.; project administration, D.D.H.; All authors have read and agreed to the submitted version of the manuscript.
Funding
National Research Foundation of Korea (Grant No. 120210290) and 2016 Kangwon National University Hospital Grant.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hak Jun Lee and Ji In Park.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07192-6.
References
- 1.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Elosta A, Ghous T, Ahmed N. Natural products as anti-glycation agents: Possible therapeutic potential for diabetic complications. Curr. Diabetes Rev. 2012;8:92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- 4.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 6.Akash MS, Rehman K, Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30:755–763. doi: 10.1016/j.nut.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Ma D, Zhang Y, Zheng W, Wang P. Coffee consumption and risk of colorectal cancer: A meta-analysis of observational studies. Public Health Nutr. 2013;16:346–357. doi: 10.1017/S1368980012002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barranco Quintana, J. L., Allam, M. F., Serrano Del Castillo, A. & Fernandez-Crehuet Navajas, R. Alzheimer's disease and coffee: A quantitative review. Neurol Res29, 91–95. 10.1179/174313206X152546 (2007). [DOI] [PubMed]
- 9.Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: A dose–response meta-analysis. Circ. Heart Fail. 2012;5:401–405. doi: 10.1161/CIRCHEARTFAILURE.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doo T, Morimoto Y, Steinbrecher A, Kolonel LN, Maskarinec G. Coffee intake and risk of type 2 diabetes: The Multiethnic Cohort. Public Health Nutr. 2014;17:1328–1336. doi: 10.1017/S1368980013000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014;53:25–38. doi: 10.1007/s00394-013-0603-x. [DOI] [PubMed] [Google Scholar]
- 13.Shin JY, Sohn J, Park KH. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013;28:608–613. doi: 10.3346/jkms.2013.28.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang H, et al. Chlorogenic acid and coffee prevent hypoxia-induced retinal degeneration. J. Agric. Food Chem. 2014;62:182–191. doi: 10.1021/jf404285v. [DOI] [PubMed] [Google Scholar]
- 15.Jang H, Choi Y, Ahn HR, Jung SH, Lee CY. Effects of phenolic acid metabolites formed after chlorogenic acid consumption on retinal degeneration in vivo. Mol. Nutr. Food Res. 2015;59:1918–1929. doi: 10.1002/mnfr.201400897. [DOI] [PubMed] [Google Scholar]
- 16.Kumari N. Is coffee consumption associated with age-related macular degeneration and diabetic retinopathy? All Res. J. Biol. 2014;5:7–13. [Google Scholar]
- 17.Carlstrom M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018;76:395–417. doi: 10.1093/nutrit/nuy014. [DOI] [PubMed] [Google Scholar]
- 18.Mei X, et al. Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vascul. Pharmacol. 2018;101:29–37. doi: 10.1016/j.vph.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008–2011. Invest. Ophthalmol. Vis. Sci. 2013;54:6827–6833. doi: 10.1167/iovs.13-12654. [DOI] [PubMed] [Google Scholar]
- 20.Man RE, et al. Differential association of generalized and abdominal obesity with diabetic retinopathy in asian patients with type 2 diabetes. JAMA Ophthalmol. 2016;134:251–257. doi: 10.1001/jamaophthalmol.2015.5103. [DOI] [PubMed] [Google Scholar]
- 21.Lim LS, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2010;51:4458–4463. doi: 10.1167/iovs.09-4939. [DOI] [PubMed] [Google Scholar]
- 22.Wong TY, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am. J. Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song SJ. Obesity and diabetic retinopathy: New perspectives. JAMA Ophthalmol. 2016;134:258. doi: 10.1001/jamaophthalmol.2015.5178. [DOI] [PubMed] [Google Scholar]
- 24.Je Y, Jeong S, Park T. Coffee consumption patterns in Korean adults: The Korean National Health and Nutrition Examination Survey (2001–2011) Asia Pac. J. Clin. Nutr. 2014;23:691–702. doi: 10.6133/apjcn.2014.23.4.11. [DOI] [PubMed] [Google Scholar]
- 25.Lee H-S, et al. Dietary total sugar intake of Koreans: Based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2011. J. Nutr. Health. 2014;47:268–276. doi: 10.4163/jnh.2014.47.4.268. [DOI] [Google Scholar]
- 26.Marriott, B. P., Hunt, K. J., Malek, A. M. & Newman, J. C. Trends in intake of energy and total sugar from sugar-sweetened beverages in the united states among children and adults. NHANES 2003–2016. Nutrients. 10.3390/nu11092004 (2019). [DOI] [PMC free article] [PubMed]
- 27.Wang J, et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J. Nutr. 2014;144:81–86. doi: 10.3945/jn.113.182519. [DOI] [PubMed] [Google Scholar]
- 28.Munafo MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. Collider scope: When selection bias can substantially influence observed associations. Int. J. Epidemiol. 2018;47:226–235. doi: 10.1093/ije/dyx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kweon S, et al. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES) Int. J. Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon KC, et al. Prevalence of eye diseases in South Korea: Data from the Korea National Health and Nutrition Examination Survey 2008–2009. Korean J. Ophthalmol. 2011;25:421–433. doi: 10.3341/kjo.2011.25.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grading diabetic retinopathy from stereoscopic color fundus photographs: An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology98, 786–806 (1991). [PubMed]
- 32.Kim K, Kim K, Park SM. Association between the prevalence of metabolic syndrome and the level of coffee consumption among Korean women. PLoS ONE. 2016;11:e0167007. doi: 10.1371/journal.pone.0167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frith E, Loprinzi PD. The association between bouted and non-bouted physical activity on retinopathy prevalence. Eur. J. Intern. Med. 2018;47:32–35. doi: 10.1016/j.ejim.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Lim Y, et al. Association of bone mineral density and diabetic retinopathy in diabetic subjects: The 2008–2011 Korea National Health and Nutrition Examination Survey. Osteoporos Int. 2016;27:2249–2257. doi: 10.1007/s00198-016-3527-5. [DOI] [PubMed] [Google Scholar]
- 35.Long M, Wang C, Liu D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes. 2017;7:e281. doi: 10.1038/nutd.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao C, et al. Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: Data from a meta-analysis of seven cohort studies. Diagn. Pathol. 2014;9:130. doi: 10.1186/1746-1596-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.