Abstract
This retrospective cohort study aimed to evaluate the association between acetylcholinesterase inhibitors (AChEI) usage and the risk of lung cancer. Data from 116,106 new users of AChEI and 348,318, at a ratio of 1:3, matched by age, sex, and index-year, between 2000 and 2015 controls were obtained from the Taiwan Longitudinal Health Insurance Database in this cohort study. The Cox regression model was used to compare the risk of lung cancer. The adjusted hazard ratio (aHR) of lung cancer for AChEI users was 1.198 (95% confidence interval [CI] = 0.765–1.774, p = 0.167). However, the adjusted HR for patients aged ≥ 65 was adjusted to HR: 1.498 (95% CI = 1.124–1.798, p < 0.001), in contrast to the comparison groups. In addition, patients with comorbidities such as pneumonia, bronchiectasis, pneumoconiosis, pulmonary alveolar pneumonopathy, hypertension, stroke, coronary artery disease, diabetes mellitus, chronic kidney disease, depression, anxiety, smoking-related diseases, dementia, and seeking medical help from medical centers and regional hospitals, were associated with a higher risk in lung cancer. Furthermore, longer-term usage of rivastigmine (366–730 days, ≥ 731 days) and galantamine (≥ 731 days) was associated with the risk of lung cancer. AChEI increased the risk of lung cancer in the older aged patients, several comorbidities, and a longer-term usage of rivastigmine and galantamine. Therefore, physicians should estimate the risks and benefits of AChEI usage and avoid prescribing antidepressants concurrently.
Subject terms: Neurology, Oncology, Risk factors
Introduction
Dementia could very well be a heavy burden for the patients and their caregivers, community, and society1–4, and the most common type is Alzheimer dementia (AD). Acetylcholinesterase inhibitors (AChEI), such as donepezil, rivastigmine, and galantamine, are efficacious and safe for the treatment of mild to moderate AD5,6, and donepezil is also effective in treating moderate-severe to severe AD7. In addition, previous studies have shown that AChEI usage was associated with a decreased risk of injury8, mortality9, myocardial infarction10, and ischemic stroke11.
Lung cancer is one of the leading causes of death worldwide including Taiwan. Despite all the innovations in radiology imaging diagnostic testing, surgical techniques, chemotherapy treatments, the 5-year survival rates for the lung cancer patients were just 6–18%12. Lacking specific biomarkers and tools for early detection, most of the patients were diagnosed at advance stages. The etiology and pathogenesis of the lung cancer are very complex. Cigarette smoking bears a strong association with lung carcinogenesis. Other contributing risk factors include the occupational or environmental exposure to the crystalline silica dust, asbestos, arsenic, residential radon, polycyclic aromatic hydrocarbons, passive smoking, and ambient air pollution. Prompt investigations for risk factors from other chemicals of lung cancer are now ongoing for improving the patients’ diagnosis and treatment survival rates13. However, several studies have found that the neurotransmitter acetylcholine (ACh) acts as an autocrine growth factor for human lung cancer14 and acetylcholinesterase (AChE) is also a promising tumor suppressor15. The inhibition of AChEI could increase the levels of Ach, and this finding raised a possibility of the association between AChEI and lung cancer.
Thus, there is no study, as yet, for the relationship between AChEI and the risk of lung cancer. Furthermore, no large, population-based study has comprehensively analyzed and evaluated the potential risk of lung cancer according to the age of the patients and the duration of AChEI usage. To this end, we conducted a population-based cohort study so as to determine the risk of lung cancer over 15 years.
Results
Baseline and end-point characteristics of the study population
Table 1 depicts the baseline characteristics of the study population. There were 116,106 subjects in the AChEI usage group and 348,318 in the non-AChEI controls, with a similar distribution of sex and age. The AChEI usage group tended to be higher in all the comorbidities, with monthly insured premiums of 18,000–34,999 and ≥ 35,000 New Taiwan Dollars (NT$), married, and education levels ≥ 12 years, geographical area of residence in the North, East, and outlying islands of Taiwan, urbanization levels 1 and 4, and levels of medical care in the medical center. Characteristics of the study at the endpoint are as illustrated in Table S2.
Table 1.
Characteristics of study at the baseline.
| AChEI | With | Without | P | ||
|---|---|---|---|---|---|
| Variables | n | % | n | % | |
| Total | 116,106 | 25.00 | 348,318 | 75.00 | |
| Gender | 0.999 | ||||
| Male | 59,052 | 50.86 | 177,156 | 50.86 | |
| Female | 57,054 | 49.14 | 171,162 | 49.14 | |
| Age (years) | 60.21 ± 18.25 | 60.31 ± 19.94 | 0.131 | ||
| Age groups (years) | 0.999 | ||||
| 50–64 | 58,712 | 50.57 | 176,136 | 50.57 | |
| ≥ 65 | 57,394 | 49.43 | 172,182 | 49.43 | |
| Monthly insurance premiums | 0.020 | ||||
| < 18,000 | 92,246 | 79.45 | 278,012 | 79.82 | |
| 18,000–34,999 | 13,459 | 11.59 | 39,454 | 11.33 | |
| ≥ 35,000 | 10,401 | 8.96 | 30,852 | 8.86 | |
| Marital status | < 0.001 | ||||
| Without | 50,982 | 43.91 | 158,617 | 45.54 | |
| With | 65,124 | 56.09 | 189,701 | 54.46 | |
| Education levels (years) | < 0.001 | ||||
| < 12 | 60,986 | 52.53 | 191,336 | 54.93 | |
| ≥ 12 | 55,120 | 47.47 | 156,982 | 45.07 | |
| Pneumonia | 8715 | 7.51 | 14,541 | 4.17 | < 0.001 |
| Bronchiectasis | 9011 | 7.76 | 19,701 | 5.66 | < 0.001 |
| Pneumoconiosis | 8875 | 7.64 | 16,701 | 4.79 | < 0.001 |
| PAP | 1597 | 1.38 | 1714 | 0.49 | < 0.001 |
| COPD | 10,875 | 9.37 | 22,472 | 6.45 | < 0.001 |
| Asthma | 5556 | 4.79 | 15,701 | 4.51 | < 0.001 |
| Hypertension | 22,106 | 19.04 | 64,013 | 18.38 | < 0.001 |
| Stroke | 14,512 | 12.50 | 34,012 | 9.76 | < 0.001 |
| Coronary artery disease | 17,541 | 15.11 | 33,451 | 9.60 | < 0.001 |
| Diabetes mellitus | 23,895 | 20.58 | 69,123 | 19.84 | < 0.001 |
| Chronic kidney disease | 15,872 | 13.67 | 44,012 | 12.64 | < 0.001 |
| Osteoporosis | 3340 | 2.88 | 6124 | 1.76 | < 0.001 |
| Depression | 6701 | 5.77 | 3978 | 1.14 | < 0.001 |
| Anxiety | 5512 | 4.75 | 2811 | 0.81 | < 0.001 |
| Hyperlipidemia | 5101 | 4.39 | 11,022 | 3.16 | < 0.001 |
| Smoking-related diseases | 4841 | 4.17 | 12,901 | 3.70 | < 0.001 |
| Dementia | 29,785 | 25.65 | 66,971 | 19.23 | < 0.001 |
| CCI_R | 1.02 ± 1.85 | 0.84 ± 1.72 | < 0.001 | ||
| Location | < 0.001 | ||||
| Northern Taiwan | 44,123 | 38.00 | 131,010 | 37.61 | |
| Middle Taiwan | 28,452 | 24.51 | 91,225 | 26.19 | |
| Southern Taiwan | 32,013 | 27.57 | 98,720 | 28.34 | |
| Eastern Taiwan | 10,712 | 9.23 | 25,451 | 7.31 | |
| Outlying islands | 806 | 0.69 | 1912 | 0.55 | |
| Urbanization level | < 0.001 | ||||
| 1 (the highest) | 40,112 | 34.55 | 118,706 | 34.08 | |
| 2 | 45,124 | 38.86 | 139,126 | 39.94 | |
| 3 | 10,245 | 8.82 | 34,529 | 9.91 | |
| 4 (the lowest) | 20,625 | 17.76 | 55,957 | 16.06 | |
| Level of care | < 0.001 | ||||
| Medical center | 44,802 | 38.59 | 105,601 | 30.32 | |
| Regional hospital | 42,121 | 36.28 | 128,701 | 36.95 | |
| Local hospital | 29,183 | 25.13 | 114,016 | 32.73 | |
P: Chi-square/Fisher exact test on category variables and t test on continue variables.
AChEI acetylcholinesterase inhibitors, CCI_R Charlson Comorbidity Index, dementia removed, COPD chronic obstructive pulmonary disease, NT$ New Taiwan Dollars, PAP pulmonary alveolar pneumonopathy.
The association between AChEI and lung cancer
Of the AChEI usage group, 4713 (371.04 per 100,000 person-years) suffered lung cancer when compared to 14,071 (362.52 per 100,000 person-years) in the control group, after the 15-year follow-up. Figure 1 shows the Kaplan–Meier analysis was used for the cumulative incidence of lung cancer in the AChEI users and non-user controls (log-rank test, p = 0.245).
Figure 1.
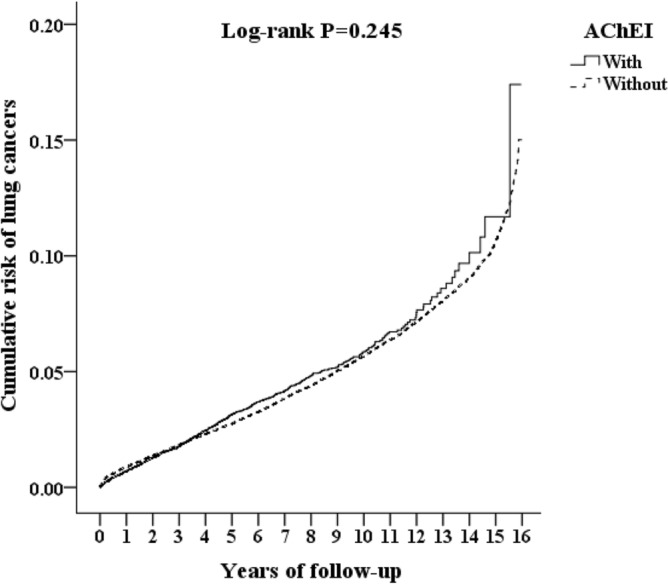
Kaplan–Meier for cumulative risk of lung cancers aged 50 and over stratified by AChEI with log-rank test.
Table 2 discloses that the Cox regression model revealed that the adjusted HR for dementia was 1.198 (95% CI = 0.765–1.774, p = 0.167), when compared with the controls, after adjusting for age, sex, comorbidities, CCI scores, and all the covariates. However, the adjusted HR for patients aged ≥ 65 was 1.498 (95% CI = 1.124–1.798, p < 0.001), in contrast to the comparison groups. The adjusted HR’s for patients with comorbidities such as pneumonia, bronchiectasis, pneumoconiosis, pulmonary alveolar pneumonopathy, hypertension, stroke, coronary artery disease, diabetes mellitus, chronic kidney disease, depression, anxiety, smoking-related diseases, dementia, and seeking medical help from medical centers and regional hospitals, were associated with a higher risk in lung cancer.
Table 2.
Factors of lung cancers by using Cox regression model.
| Variables | Crude HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| AChEI (reference: without) | 1.240 | 0.792 | 1.798 | 0.198 | 1.198 | 0.765 | 1.774 | 0.167 |
| Age ≥ 65 (reference: age of 50–64) | 1.524 | 1.165 | 1.823 | < 0.001 | 1.498 | 1.124 | 1.798 | < 0.001 |
| Pneumonia (reference: without) | 2.501 | 2.134 | 2.801 | < 0.001 | 2.495 | 2.101 | 2.786 | < 0.001 |
| Bronchiectasis (reference: without) | 1.776 | 1.254 | 2.148 | < 0.001 | 1.754 | 1.241 | 2.131 | < 0.001 |
| Pneumoconiosis (reference: without) | 2.345 | 1.955 | 2.794 | < 0.001 | 2.284 | 1.925 | 2.765 | < 0.001 |
| PAP (reference: without) | 1.731 | 1.213 | 2.124 | < 0.001 | 1.724 | 1.201 | 2.111 | < 0.001 |
| Hypertension (reference: without) | 1.848 | 1.364 | 2.124 | < 0.001 | 1.842 | 1.345 | 2.097 | < 0.001 |
| Stroke (reference: without) | 1.767 | 1.303 | 2.048 | < 0.001 | 1.752 | 1.289 | 2.035 | < 0.001 |
| Coronary artery disease (reference: without) | 1.772 | 1.296 | 2.010 | < 0.001 | 1.722 | 1.264 | 1.999 | < 0.001 |
| Diabetes mellitus (reference: without) | 1.598 | 1.134 | 1.995 | < 0.001 | 1.579 | 1.124 | 1.986 | < 0.001 |
| Chronic kidney disease (reference: without) | 2.341 | 1.598 | 2.685 | < 0.001 | 2.297 | 1.583 | 2.674 | < 0.001 |
| Depression (reference: without) | 1.682 | 1.168 | 1.989 | < 0.001 | 1.591 | 1.154 | 1.984 | < 0.001 |
| Anxiety (reference: without) | 1.703 | 1.203 | 2.024 | < 0.001 | 1.687 | 1.197 | 2.015 | < 0.001 |
| Smoking-related diseases (reference: without) | 1.099 | 1.037 | 1.134 | 0.017 | 1.095 | 1.033 | 1.129 | 0.014 |
| Dementia (reference: without) | 1.089 | 1.026 | 1.129 | 0.024 | 1.087 | 1.024 | 1.124 | 0.026 |
| CCI_R | 1.127 | 1.103 | 1.149 | < 0.001 | 1.124 | 1.101 | 1.145 | < 0.001 |
| Medical center (reference: local hospital) | 1.602 | 1.131 | 2.005 | < 0.001 | 1.598 | 1.128 | 1.986 | < 0.001 |
| Regional hospital (reference: local hospital) | 1.467 | 1.108 | 1.996 | < 0.001 | 1.451 | 1.105 | 1.973 | < 0.001 |
HR hazard ratio, CI confidence interval, Adjusted HR Adjusted variables listed in the table 1, AChEI acetylcholinesterase inhibitors, CCI_R Charlson Comorbidity Index, dementia removed, PAP pulmonary alveolar pneumonopathy.
Longer term of usage and comorbidities of AChEI and the risk of lung cancer
Table 3 shows that longer-term usage of rivastigmine (366–730 days, ≥ 731 days) and galantamine (≥ 731 days) was associated with the risk of lung cancer. Table S3 depicts that the AChEI usage patients with pneumonia and pneumoconiosis were associated with a higher risk of lung cancer in comparison with the controls without the usage of AChEI.
Table 3.
Factors of lung cancers among AChEI subgroups by using Cox regression and Fine and Gray's competing risk model.
| AChEI subgroups | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|
| Without AChEI | 362.52 | Reference | |||
| With AChEI | 371.04 | 1.198 | 0.765 | 1.774 | 0.167 |
| With Donepezil | 367.91 | 1.188 | 0.759 | 1.759 | 0.189 |
| With Donepezil, 1–30 days | 363.38 | 1.173 | 0.749 | 1.737 | 0.204 |
| With Donepezil, 31–365 days | 369.39 | 1.193 | 0.762 | 1.766 | 0.187 |
| With Donepezil, 366–730 days | 365.10 | 1.179 | 0.756 | 1.747 | 0.201 |
| With Donepezil, ≥ 731 days | 372.94 | 1.204 | 0.769 | 1.783 | 0.158 |
| With Rivastigmine | 369.48 | 1.194 | 0.862 | 1.685 | 0.225 |
| With Rivastigmine, 1–30 days | 362.63 | 1.173 | 0.743 | 1.642 | 0.313 |
| With Rivastigmine, 31–365 days | 364.59 | 1.178 | 0.751 | 1.659 | 0.286 |
| With Rivastigmine, 366–730 days | 373.47 | 1.245 | 1.041 | 1.884 | 0.003 |
| With Rivastigmine, ≥ 731 days | 375.29 | 1.297 | 1.043 | 1.889 | 0.001 |
| With Galantamine | 367.70 | 1.187 | 0.905 | 1.825 | 0.165 |
| With Galantamine, 1–30 days | 363.11 | 1.172 | 0.849 | 1.734 | 0.297 |
| With Galantamine, 31–365 days | 365.14 | 1.179 | 0.853 | 1.749 | 0.183 |
| With Galantamine, 366–730 days | 366.48 | 1.183 | 0.884 | 1.755 | 0.145 |
| With Galantamine, ≥ 731 days | 375.01 | 1.211 | 1.070 | 1.978 | < 0.001 |
PYs person-years, Adjusted HR adjusted hazard ratio: adjusted for the variables listed in Table 3, CI confidence interval.
Discussion
After adjusting for the covariates, the overall adjusted HR was 1.198 (95% CI = 0.765–1.774, p = 0.167), when compared with the controls. The Kaplan–Meier analysis revealed that the 15-year cumulative incidence rate between the AChEI-cohort and the controls was not significant (p = 0.245). Nonetheless, the adjusted HR for patients aged ≥ 65 was 1.498 (95% CI = 1.124–1.798, p < 0.001), in contrast to the comparison groups. In other words, the patients in the AChEI-cohort aged ≥ 65 had a nearly 1.5-fold increased risk of the development of lung cancer.
In addition, the patients with comorbidities, such as pneumonia, bronchiectasis, pneumoconiosis, pulmonary alveolar pneumonopathy, hypertension, stroke, coronary artery disease, diabetes mellitus, chronic kidney disease, depression, anxiety, smoking-related diseases, dementia, and seeking medical help from medical centers and regional hospitals, were associated with a higher risk of lung cancer. To the best of our knowledge, this is the first study on the topic of the association between the usage of ACEI and the risk of lung cancer.
Several reports have shown that AChEI was associated with a higher risk of bradycardia16, syncope17, and other adverse cardiovascular events18,19. There are no studies about the potential disadvantageous effects of AChEI except the cardiovascular events. The present study has pointed out that the future pharmacological and clinical studies are important when referring to the potential effects of carcinogenesis of the AChEI.
One study has shown that a chemical compound, eserine, and an acetylcholinesterase inhibitor, was capable of inducing carcinogenesis in the epithelium of rat mammary glands20. Another study reported that the Ach could act as an autocrine growth factor for human lung cancer cells14, the association between AChEI, which could increase the level of Ach, and the risk of lung cancer. Another study has found that it is also a promising tumor suppressor, therefore, the inhibition of the AChE would not be beneficial for the suppression of carcinogenesis15. The degradation of the acetylcholinesterase and butyrylcholinesterase would lead to the consequent release of acetylcholine that binds back to the nicotinic and muscarinic receptors, could accelerate their proliferation, migration, and invasion of the lung cancer cells15, in both the carcinogenesis and progression of lung cancer. In the present study, it would take a longer duration of rivastigmine and galantamine usage for the development of the risk, therefore, we speculate that the interaction between ACEI and the aging process might well play an important role in the pathogenesis of lung cancer.
In the present study, the association between long-term usage of rivastigmine and galantamine, but not donepezil, has been found. The underlying reasons for this difference are yet to be clarified. Not only the degradation of acetylcholinesterase, but also the butyrylcholinesterase, and the consequent release of acetylcholine that binds back to the nicotinic and muscarinic receptors could accelerate their proliferation, migration, and invasion of the lung cancer cells15. In addition, previous studies have shown that the decreased circulating butyrylcholinesterase predicts a shorter survival for patients with pancreatic cancer21, and non-muscle-invasive bladder cancer22. Therefore, for rivastigmine, the dual inhibition of acetylcholinesterase and butyrylcholinesterase might play an important role for the increased risk of lung cancer, especially in the long-term usage. On the other hand, although galantamine is a selective AChEI23, some other medications, such as escitalopram, could result in the synergistic inhibition on the butyrylcholinesterase24. We speculate that in the long-term usage of galantamine, the concurrent usage of other medications might be a reason for the additional inhibition on butyrylcholinesterase and the subsequent increased risk of lung cancer.
Limitations
First, even though the NHIRD have recorded inpatient care, ambulatory care, dental care, and prescription drugs availed by the insured and their date of birth. However, pursuant to the Personal Information Protection Act, individual identifiers are encrypted before being released for research. Therefore, information such as the severity, laboratory parameters, neurological symptom severity, electrophysiological testing, or rehabilitation potential could not be assessed in the present study for dementia due to the lack of such data in the NHIRD. Besides, we could not include data on the psychosocial, environmental, and genetic factors in our analyses due to the same reason. Second, the NHIRD does not contain the data of initial chest imaging or detailed pulmonary evaluation. Third, the information for the smoking and the pack-years of cigarettes was not included in Taiwan’s NHIRD. However, in this dataset, smoking-related diseases, such as tobacco usage disorder (ICD-9-CM code: 305.1), interstitial emphysema (ICD-9-CM code: 518.1), pulmonary eosinophilia (ICD-9-CM code: 518.3–518.4), nonspecific abnormal results of pulmonary function study (ICD-9-CM code: 794.2), and personal history of tobacco usage (ICD-9-CM code: V15.82), were included, as listed in Table S1. Therefore, the role of health disadvantages for smoking has been analyzed in the present study. However, despite these limitations, our derived data are highly likely to be valid and representative due to the NHIRD containing data covering all hospitals within Taiwan and over 99% of the population for the relevant 15-year period.
Methods
Data sources
Taiwan’s National Health Insurance (NHI) Program was launched in 1995, and had contracts with 97% of the medical providers and enrolled more than 99% of the 23 million population, as of June, 200925. The details of the program have been documented in previous studies26–34. We used the Longitudinal Health Insurance Database (LHID), a subset of two million randomly sampled patients from the NHIRD, during a 15-year period (2000–2015) in this study.
Ethics approval
This study was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). This study was approved by the Institutional Review Board (IRB) of the Tri-Service General Hospital (TSGH). The TSGH IRB waived the need of individual consents since all the identification data were encrypted in the NHIRD (IRB No. B-110-30).
Study design and participants
The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)35 diagnostic codes were used in the NHIRD. Each diagnosis of dementia was made by a board-certified psychiatrist or neurologist according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) and its Text-revised edition (DSM-IV-TR)36,37. Other causes of dementia must likewise be excluded: patients with cerebral vascular disease history were excluded. Patients with old vascular insults, hydrocephalus, brain tumor, or any other potential cause of dementia other than AD noted in these neuro-images were excluded. The Mini-Mental Status Examination (MMSE) score must be between 10 and 26 and clinical dementia rating (CDR) grade either 1 or 2. The requested blood tests include the venereal disease research laboratory, thyroid function, complete blood count, fasting sugar, glutamic–oxaloacetic transaminase, glutamic–pyruvic transaminase, blood urea nitrogen, creatinine, serum B12 and folic acid levels. Aside from the clinical presentation, cognition tests and blood tests, all of the patients must have neuro-image studies, with either a brain computerized tomogram or a magnetic resonance image. The diagnostic work-up must be performed and confirmed by a certificated neurologist or psychiatrist. The AChEI treatment for AD is covered by the NHI program. According to the NHI regulations, the AChEI medications are exclusively used in AD patients38.
The records of ambulatory care visits and inpatient claims by the NHI Administration are randomly reviewed, to verify the accuracy of the diagnoses39. Therefore, using the NHIRD is considered as being suitable to study the association between HMCAA and the risk of developing dementia. The records of the AChEI were also retrieved from the NHIRD. We also calculated the estimated cumulative dosage of AChEI for each subject using the defined daily dose (DDD) that were obtained from the WHO Collaborating Centre for Drug Statistics Methodology (https://www.whocc.no/), and the duration of the usage of AChEI was calculated by dividing the cumulative doses by the DDD of the AChEI. A total of 116,106 new users of AChEI were enrolled, along with 348,318 controls without the usage of AChEI, at a ratio of 1:3, matched by age, sex, and index-year, between 2000 and 2015, from a two million LHID, a subset of Taiwan’s National Health Research Institutes Database, in this retrospective cohort study (Fig. S1).
Covariates
Covariates included sex, age (50–64, ≥ 65), geographical area of residence (northern, central, southern, and eastern Taiwan), urbanization level (levels 1 to 4, as described below), monthly insured premiums (in New Taiwan dollars (NT$): < 18,000, 18,000–34,999, ≥ 35,000), and levels of medical care (medical center, regional hospital, and local hospital). The Charlson comorbidity index (CCI, scores of 0, 1, 2, 3, ≥ 4) is the most widely used comorbidity index40,41. Other comorbidities included pneumonia, bronchiectasis, pneumoconiosis, pulmonary alveolar pneumonopathy, chronic obstructive pulmonary disease, asthma, hypertension, stroke, coronary artery disease, diabetes mellitus, chronic kidney disease, osteoporosis, depression, anxiety, hyperlipidemia, smoking-related diseases, and dementia (Table S1).
Major outcome
All of the study participants were followed from the index date until the onset of lung cancer, death, withdrawal from the NHI program, or the end of 2015. Patients with dementia were identified by the ICD-9-CM codes of lung cancer (ICD-9-CM code: 162).
Statistical analysis
All analyses were performed using the SPSS software version 22.0 for Windows (IBM Corp., Armonk, NY). χ2 and t tests were used to evaluate the distribution of the categorical and continuous variables between the patients who did and did not use AChEI. The Fisher exact test for categorical variables was used to statistically examine the differences between the two cohorts. The Multivariate Cox regression model and regression analysis were used so as to determine the risk of lung cancer, and the results are presented as a hazard ratio (HR) with a 95% confidence interval (CI). Differences in the risk of lung cancer between the two groups were estimated using the Kaplan–Meier method with the log-rank test. A 2-tailed p value < 0.05 was considered to be statistically significant.
Conclusion
In conclusion, this study has shown that the antidepressant users might have a nearly 1.5-fold risk of lung cancer than the non-users in the patients with age ≥ 65. It was also noteworthy for the patients who took a longer duration of rivastigmine and galantamine usage. Therefore, clinicians should be cautious in balancing the benefit and risk of the usage of AChEI in patients with AD.
Supplementary Information
Acknowledgements
The authors thank the provision of the National Health Insurance Research Database by the Taiwan’s Health and Welfare Data Science Center and Ministry of Health and Welfare (HWDC, MOW).
Author contributions
C.T.L., C.C.Y., W.C.C., and N.S.T. conceived, designed, and conducted the study, performed the statistical analyses, analyzed and interpreted the data, and drafted the manuscript. C.H.C., C.S.T., Y.T.T., C.Y.L., Y.C.L., and Y.S.C. participated in its conception, design, assisted with the data collection, analyzed and interpreted the data, and were involved in drafting the manuscript and revised the manuscript critically for important intellectual content. C.T.L. and C.C.Y. wrote the first draft. W.C.C. and N.S.T. conducted the critical revision of the manuscript. All authors read and approved this manuscript.
Funding
Project financial support was provided by the Medical Affairs Bureau, the Ministry of Defense of Taiwan (MND-MAB-110-087), the Tri-Service General Hospital Research Foundation (TSGH-C108-003, TSGH-C108-151, TSGH-B-109-010, TSGH-E-110240, and TSGH-B-110-012), and the Taoyuan Armed Forces General Hospital (TYAFGH-A-110020). The sponsor had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chien-Ting Liu, Chuan-Chi Yang, Wu-Chien Chien and Nian-Sheng Tzeng.
Contributor Information
Wu-Chien Chien, Email: chienwu@ndmctsgh.edu.tw.
Nian-Sheng Tzeng, Email: pierrens@mail.ndmctsgh.edu.tw.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06377-3.
References
- 1.Tzeng NS, et al. Caregiver burden for patients with dementia with or without hiring foreign health aides: A cross-sectional study in a northern Taiwan Memory Clinic. J. Med. Sci. 2015;35:239–247. doi: 10.4103/1011-4564.172999. [DOI] [Google Scholar]
- 2.Tzeng NS, et al. The impact of pharmacological treatments on cognitive function and severity of behavioral symptoms in geriatric elder patients with dementia. Taiwan. J. Psychiatry. 2017;31:69–79. [Google Scholar]
- 3.Wang HY, et al. Forensic evaluations for offenders with dementia in Taiwan's criminal courts. J. Am. Acad. Psychiatry Law. 2018;46:45–51. [PubMed] [Google Scholar]
- 4.Yeh TC, et al. Detection of malingering in the memory of patients with dementia: A pilot study on coin-in-the-hand test in a Northern Taiwan memory clinic. J. Med. Sci. 2018;39:81–89. doi: 10.4103/jmedsci.jmedsci_100_18. [DOI] [Google Scholar]
- 5.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst. Rev. 2006;1:CD005593. doi: 10.1002/14651858.cd005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammad D, Chan P, Bradley J, Lanctot K, Herrmann N. Acetylcholinesterase inhibitors for treating dementia symptoms—A safety evaluation. Expert Opin. Drug Saf. 2017;16:1009–1019. doi: 10.1080/14740338.2017.1351540. [DOI] [PubMed] [Google Scholar]
- 7.Molino I, Colucci L, Fasanaro AM, Traini E, Amenta F. Efficacy of memantine, donepezil, or their association in moderate-severe Alzheimer's disease: A review of clinical trials. TheScientificWorldJournal. 2013;2013:925702. doi: 10.1155/2013/925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao PC, et al. Cognitive enhancers associated with decreased risk of injury in patients with dementia: A nationwide cohort study in Taiwan. J. Investig. Med. 2018;66:684–692. doi: 10.1136/jim-2017-000595. [DOI] [PubMed] [Google Scholar]
- 9.Ku LE, Li CY, Sun Y. Can persistence with cholinesterase inhibitor treatment lower mortality and health-care costs among patients with Alzheimer's disease? A population-based study in Taiwan. Am. J. Alzheimers Dis. Other Dement. 2018;33:86–92. doi: 10.1177/1533317517734639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordstrom P, Religa D, Wimo A, Winblad B, Eriksdotter M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: A nationwide cohort study in subjects with Alzheimer's disease. Eur. Heart J. 2013;34:2585–2591. doi: 10.1093/eurheartj/eht182. [DOI] [PubMed] [Google Scholar]
- 11.Lin YT, Wu PH, Chen CS, Yang YH, Yang YH. Association between acetylcholinesterase inhibitors and risk of stroke in patients with dementia. Sci. Rep. 2016;6:29266. doi: 10.1038/srep29266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang BY, et al. Lung cancer and prognosis in Taiwan: A population-based cancer registry. J. Thorac. Oncol. 2013;8:1128–1135. doi: 10.1097/JTO.0b013e31829ceba4. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CN, et al. Cancers in Taiwan: Practical insight from epidemiology, treatments, biomarkers, and cost. J. Formos. Med. Assoc. 2019 doi: 10.1016/j.jfma.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Xi HJ, Wu RP, Liu JJ, Zhang LJ, Li ZS. Role of acetylcholinesterase in lung cancer. Thorac. Cancer. 2015;6:390–398. doi: 10.1111/1759-7714.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman JR, et al. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019;194:222–254. doi: 10.1016/j.pharmthera.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs New England healthcare system. J. Am. Geriatr. Soc. 2009;57:1997–2003. doi: 10.1111/j.1532-5415.2009.02488.x. [DOI] [PubMed] [Google Scholar]
- 17.Gill SS, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: A population-based cohort study. Arch. Intern. Med. 2009;169:867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 18.Isik AT, et al. Cardiovascular outcomes of cholinesterase inhibitors in individuals with dementia: A meta-analysis and systematic review. J. Am. Geriatr. Soc. 2018;66:1805–1811. doi: 10.1111/jgs.15415. [DOI] [PubMed] [Google Scholar]
- 19.Wan FJ, et al. Anti-dementia drugs and risk of cardiovascular events—A nationwide cohort study in Taiwan. Neuropsychiatry. 2018;8:739–744. doi: 10.4172/Neuropsychiatry.1000606. [DOI] [Google Scholar]
- 20.Calaf GM, Parra E, Garrido F. Cell proliferation and tumor formation induced by eserine, an acetylcholinesterase inhibitor, in rat mammary gland. Oncol. Rep. 2007;17:25–33. doi: 10.3892/or.17.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Klocker EV, et al. Decreased activity of circulating butyrylcholinesterase in blood is an independent prognostic marker in pancreatic cancer patients. Cancers (Basel) 2020;12:1154. doi: 10.3390/cancers12051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura S, et al. Prognostic value of serum cholinesterase in non-muscle-invasive bladder cancer. Clin. Genitourin. Cancer. 2018;16:e1123–e1132. doi: 10.1016/j.clgc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Scott LJ, Goa KL. Galantamine: A review of its use in Alzheimer's disease. Drugs. 2000;60:1095–1122. doi: 10.2165/00003495-200060050-00008. [DOI] [PubMed] [Google Scholar]
- 24.Walsh R, Rockwood K, Martin E, Darvesh S. Synergistic inhibition of butyrylcholinesterase by galantamine and citalopram. Biochim. Biophys. Acta. 2011;1810:1230–1235. doi: 10.1016/j.bbagen.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho Chan WS. Taiwan's healthcare report 2010. EPMA J. 2010;1:563–585. doi: 10.1007/s13167-010-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao LC, et al. The newly diagnosed amnestic disorders and dementia: A nationwide, cohort study in Taiwan. Taiwan. J. Psychiatry. 2018;32:18–28. [Google Scholar]
- 27.Yang CC, et al. No association between human immunodeficiency virus infections and dementia: A nationwide cohort study in Taiwan. Neuropsychiatr. Dis. Treat. 2019;15:3155–3166. doi: 10.2147/ndt.S225584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TY, et al. Sex and age differences in the association between anxiety disorders and narcolepsy: A nationwide population-based case control study. J. Affect. Disord. 2020;264:130–137. doi: 10.1016/j.jad.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Lin YC, et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea-a nationwide cohort study. BMC Pulmon. Med. 2020;20:28. doi: 10.1186/s12890-019-1041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YP, et al. Are anticholinergic medications associated with increased risk of dementia and behavioral and psychological symptoms of dementia? A nationwide 15-year follow-up cohort study in Taiwan. Front. Pharmacol. 2020;11:30. doi: 10.3389/fphar.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan FJ, Chien WC, Chung CH, Yang YJ, Tzeng NS. Association between traumatic spinal cord injury and affective and other psychiatric disorders—A nationwide cohort study and effects of rehabilitation therapies. J. Affect. Disord. 2020;265:381–388. doi: 10.1016/j.jad.2020.01.063. [DOI] [PubMed] [Google Scholar]
- 32.Wang DS, et al. Association between child abuse exposure and the risk of psychiatric disorders: A nationwide cohort study in Taiwan. Child Abuse Negl. 2020;101:104362. doi: 10.1016/j.chiabu.2020.104362. [DOI] [PubMed] [Google Scholar]
- 33.Lin CH, et al. Increased risk of dementia in patients with genital warts: A nationwide cohort study in Taiwan. J. Dermatol. 2020 doi: 10.1111/1346-8138.15277. [DOI] [PubMed] [Google Scholar]
- 34.Yeh TC, et al. Psychiatric disorders after traumatic brain injury: A nationwide population-based cohort study and the effects of rehabilitation therapies. Arch. Phys. Med. Rehabil. 2020 doi: 10.1016/j.apmr.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Chinese Hospital Association . ICD-9-CM English-Chinese Dictionary. Chinese Hospital Association Press; 2000. [Google Scholar]
- 36.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) American Psychiatric Association; 1994. [Google Scholar]
- 37.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Txt-Revised (DSM-IV-TR) American Psychiatric Association; 2000. [Google Scholar]
- 38.Sun Y, Lai MS, Lu CJ, Chen RC. How long can patients with mild or moderate Alzheimer's dementia maintain both the cognition and the therapy of cholinesterase inhibitors: A national population-based study. Eur. J. Neurol. 2008;15:278–283. doi: 10.1111/j.1468-1331.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 39.National Health Insurance Administration. National Health Insurance Reimbursement Audit Guidelines and Notes.http://www.nhi.gov.tw/webdata/webdata.aspx?menu=20&menu_id=710&webdata_id=2889 (2015).
- 40.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J. Clin. Epidemiol. 2003;56:221–229. doi: 10.1016/S0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


