Abstract
Group A streptococci are model extracellular gram-positive pathogens responsible for pharyngitis, impetigo, rheumatic fever, and acute glomerulonephritis. A resurgence of invasive streptococcal diseases and rheumatic fever has appeared in outbreaks over the past 10 years, with a predominant M1 serotype as well as others identified with the outbreaks. emm (M protein) gene sequencing has changed serotyping, and new virulence genes and new virulence regulatory networks have been defined. The emm gene superfamily has expanded to include antiphagocytic molecules and immunoglobulin-binding proteins with common structural features. At least nine superantigens have been characterized, all of which may contribute to toxic streptococcal syndrome. An emerging theme is the dichotomy between skin and throat strains in their epidemiology and genetic makeup. Eleven adhesins have been reported, and surface plasmin-binding proteins have been defined. The strong resistance of the group A streptococcus to phagocytosis is related to factor H and fibrinogen binding by M protein and to disarming complement component C5a by the C5a peptidase. Molecular mimicry appears to play a role in autoimmune mechanisms involved in rheumatic fever, while nephritis strain-associated proteins may lead to immune-mediated acute glomerulonephritis. Vaccine strategies have focused on recombinant M protein and C5a peptidase vaccines, and mucosal vaccine delivery systems are under investigation.
Streptococcus pyogenes (group A streptococcus) is an important species of gram-positive extracellular bacterial pathogens. Group A streptococci colonize the throat or skin and are responsible for a number of suppurative infections and nonsuppurative sequelae. As pathogens they have developed complex virulence mechanisms to avoid host defenses. They are the most common cause of bacterial pharyngitis and are the cause of scarlet fever and impetigo. The concept of distinct throat and skin strains arose from decades of epidemiological studies, in which it became evident that there are serotypes of group A streptococci with a strong tendency to cause throat infection, and similarly, there are other serotypes often associated with impetigo (62, 543). In the past, they were a common cause of puerperal sepsis or childbed fever. Today, the group A streptococcus is responsible for streptococcal toxic shock syndrome, and most recently it has gained notoriety as the “flesh-eating” bacterium which invades skin and soft tissues and in severe cases leaves infected tissues or limbs destroyed.
The group A streptococcus has been investigated for its significant role in the development of post-streptococcal infection sequelae, including acute rheumatic fever, acute glomerulonephritis, and reactive arthritis. Acute rheumatic fever and rheumatic heart disease are the most serious autoimmune sequelae of group A streptococcal infection and have afflicted children worldwide with disability and death. Group A streptococcal infections have recently been associated with Tourette's syndrome, tics, and movement and attention deficit disorders. This review will address the potential pathogenic mechanisms involved in poststreptococcal sequelae.
The Lancefield classification scheme of serologic typing distinguished the beta-hemolytic streptococci based on their group A carbohydrate, composed of N-acetylglucosamine linked to a rhamnose polymer backbone. Streptococci were also serologically separated into M protein serotypes based on a surface protein that could be extracted from the bacteria with boiling hydrochloric acid. Currently, more than 80 M protein serotypes have been identified, and a molecular approach to identification of emm (M protein) genes has been achieved. Vaccines containing the streptococcal M protein as well as other surface components are under investigation for prevention of streptococcal infections and their sequelae. This review will focus on the pathogenic mechanisms in group A streptococcal diseases and on new developments which have an impact on our understanding of group A streptococcal diseases in humans.
RESURGENCE OF SEVERE GROUP A STREPTOCOCCAL INFECTIONS AND SEQUELAE
Although group A streptococci are exquisitely sensitive to penicillin, an unexplained resurgence of group A streptococcal infections has been observed since the mid-1980s (275). The first indication that infections due to S. pyogenes were on the rise was an outbreak of rheumatic fever which affected approximately 200 children during a 5-year period (531). From the mid-1980s to the 1990s, eight rheumatic fever outbreaks were documented in the United States, with the largest in Salt Lake City, Utah (17, 275, 531). Outbreaks were reported in Pennsylvania, Ohio, Tennessee, and West Virginia and at the Naval Training Center in San Diego, Calif. (17). A decline in rheumatic fever with a milder disease pattern had been observed in the previous decade (59). Therefore, the increased severity and the attack on middle-class families deviated from the past epidemiological patterns. Streptococcal M protein serotypes associated with the new outbreaks of rheumatic fever were M types 1, 3, 5, 6, and 18 (280).
In the late 1980s, streptococcal toxic shock syndrome, bacteremia, and severe, invasive group A streptococcal skin and soft tissue infections were reported in the United States and Europe (103, 212, 241, 275, 376, 498). Increased bacteremic infections were reported in Colorado, Sweden, and the United Kingdom (93a, 510). These severe and invasive diseases have high morbidity and mortality and may be linked to the emergence of certain serotypes or clonotypes. Although several different M protein serotypes have been isolated from severe, invasive streptococcal diseases, M protein serotype 1 has dominated (241, 245). Host susceptibility may be an important factor in production of toxic streptococcal syndrome. These aspects will be considered in this review. The resurgence of these serious infections and sequelae is strong evidence for increased awareness and surveillance of group A streptococci in the community.
FEATURES OF GROUP A STREPTOCOCCAL SUPPURATIVE INFECTIONS
Group A streptococci are extracellular bacterial pathogens which produce a variety of pyogenic infections involving the mucous membranes, tonsils, skin, and deeper tissues, including pharyngitis, impetigo/pyoderma, erysipelas, cellulitis, necrotizing fasciitis, toxic streptococcal syndrome, scarlet fever, septicemia, pneumonia, and meningitis. Infections may be mild to extremely severe. Complications such as sepsis, pneumonia, and meningitis can occur, which may lead to a fatal outcome. Several specific clinical syndromes, such as toxic streptococcal syndrome and necrotizing fasciitis, have reemerged and perhaps become more prevalent in the past 10 years due to infections with S. pyogenes.
Pharyngitis and Scarlet Fever
Group A streptococci are the most common bacterial cause of pharyngitis and primarily affect school-age children 5 to 15 years of age (62). All ages are susceptible to spread of the organism under crowded conditions, such as those at schools and military facilities. Pharyngitis and its association with rheumatic fever are seasonal, occurring in the fall and winter (62, 506, 507). This is in contrast to pyoderma or skin infection, which occurs in the summer and can be associated with the production of acute glomerulonephritis (61). Organisms which colonize the skin can also colonize the throat, but streptococcal strains which commonly produce skin infections do not lead to rheumatic fever. Groups C and G can also cause pharyngitis and must be distinguished from group A organisms after throat culture (58, 62). Although they are not considered normal flora, pharyngeal carriage of group A streptococci can occur without clinical symptoms of disease.
Certain M protein serotypes, such as M types 1, 3, 5, 6, 14, 18, 19, and 24 of S. pyogenes, are found associated with throat infection and rheumatic fever. These pharyngitis-associated serotypes do not produce opacity factor as do M serotypes such as 2, 49, 57, 59, 60, and 61, which are associated with pyoderma and acute glomerulonephritis (60, 62, 506, 507; Facklam, personal communication). The skin and throat serotypes have been divided epidemiologically on the basis of (i) opacity factor production, (ii) the presence of the class I C repeat region epitope identified on M proteins by anti-M protein monoclonal antibody (MAb) 10B6, and (iii) emm (M protein) gene patterns A through E (50, 57).
Although usually associated with streptococcal throat infection, scarlet fever may occur due to infections at other sites (62). The group A streptococcal strain producing scarlet fever does so because it carries the genes for one or more of the streptococcal pyrogenic exotoxins A, B, and C. The genes for exotoxins A and C are encoded on a lysogenic temperate bacteriophage (66, 546), while exotoxin B is chromosomal. The pyrogenic exotoxins, currently known as streptococcal superantigens, are responsible for the rash, strawberry tongue, and desquamation of the skin seen in scarlet fever.
Pyoderma and Streptococcal Skin Infections
Group A streptococci which invade the skin and cause impetigo are different M protein serotypes from those that cause pharyngitis (50, 61, 506, 507). In addition, some of the skin strains are associated with production of acute poststreptococcal glomerulonephritis. The skin infections and nephritis are seasonal, usually occurring during the summer months and in temperate climates. The infection is limited to the epidermis, usually on the face or extremities, and is highly contagious (65). Streptococcal strains which cause pyoderma do not cause rheumatic fever. Staphylococci may be mixed with streptococci in impetigo, and thus the treatment of choice is not penicillin for penicillinase-producing staphylococci (65). Group A streptococcal strains may enter the skin through abrasions and other types of lesions to penetrate the epidermis and produce erysipelas or cellulitis. Erysipelas is a distinctive form of cellulitis with characteristically raised and erythematous superficial layers of the skin, while cellulitis affects subcutaneous tissues (65). Cellulitis may occur from infected burns or wounds. Both erysipelas and cellulitis can be caused by streptococcal groups A, B, C, and G.
Invasive Streptococcal Disease: Streptococcal Toxic Shock Syndrome, Necrotizing Fasciitis, and Septicemia
Introduction.
In 1987, Cone and colleagues described a toxic shock-like syndrome due to S. pyogenes (103). Later in 1989, Stevens described an unusually severe form of group A streptococcal disease which was similar to the staphylococcal toxic shock syndrome (498). Streptococcal toxic shock syndrome was characterized by hypotension and multiple organ failure. The Working Group on Severe Streptococcal Infections suggested a case definition of streptococcal toxic shock syndrome and necrotizing fasciitis (569a). Necrotizing fasciitis may accompany the toxic streptococcal syndrome. Group A streptococcal infection producing the toxic syndrome may occur in muscle and fascia and follow mild trauma with entrance of streptococci through the skin. In addition, group A streptococci may infect the vaginal mucosa and uterus, leading to severe disease or septicemia. Several excellent recent reviews on severe invasive streptococcal disease report on its features and pathogenesis (3, 290, 379, 498). The features of invasive streptococcal infections include hypotension and shock, multiple organ failure, systemic toxicity, severe local pain, rapid necrosis of subcutaneous tissues and skin, and gangrene (65). Predisposing factors include skin trauma, surgery, varicella, and burns.
Pyrogenic exotoxins and superantigens in invasive disease.
Several virulence factors of group A streptococci are likely to be involved in the pathogenesis of toxic shock, invasion of soft tissues and skin, and necrotizing fasciitis. These virulence factors are the extracellular pyrogenic exotoxins A, B, and C as well as newly discovered exotoxins and superantigens such as exotoxin F (mitogenic factor) and streptococcal superantigen (SSA) (366, 394, 395). In addition, several new superantigens with strong mitogenic activity have recently been reported as SpeG, SpeH, SpeJ, SmeZ, and SmeZ-2 (274, 434). Details about the mitogenic toxins are described in a separate section under virulence factors. These new data point to the fact that there are a large number of superantigens which may play a role in toxic streptococcal syndrome. All of these toxins act as superantigens which interact with major histocompatibility complex (MHC) class II molecules and a limited number of Vβ regions of the T-lymphocyte receptor to activate massive numbers of T cells nonspecifically. The activation liberates large amounts of interleukins as well as other inflammatory cytokines such as tumor necrosis factor and gamma interferon (171, 221, 395). The pyrogenic exotoxins are potentially responsible for at least some of the manifestations of toxic streptococcal syndrome. Kotb and colleagues demonstrated evidence for selective depletion of T cells expressing Vβ1, Vβ5.1, and Vβ12 in patients with streptococcal toxic shock syndrome, further supporting the hypothesis that the streptococcal superantigens play an important role in disease pathogenesis (544). Further evidence also suggests that streptococcal isolates from toxic streptococcal syndrome induce a Th1 rather than a Th2 cytokine response, which is characteristic of superantigens (393).
Pyrogenic exotoxin B.
Pyrogenic exotoxin B is an extracellular cysteine protease which has been shown to cleave fibronectin and vitronectin (288), extracellular matrix proteins, and human interleukin-1β into the active form of the molecule (287). Therefore, the protease may be important in inflammation, shock, and tissue destruction. Humans with a diverse range of invasive disease (erysipelas, cellulitis, pneumonia, bacteremia, septic arthritis, toxic shock syndrome, and necrotizing fasciitis) all produced elevated levels of antibodies against streptococcal pyrogenic exotoxin B following infection (214).
M protein serotypes.
M protein serotypes are nonrandomly represented among invasive-disease-causing strains (91, 100, 102, 369, 377, 378, 381, 389, 482). In clinical reports and epidemiological studies of invasive and toxic streptococcal diseases, M types 1, 3, 11, 12, and 28 have frequently been reported, with M1 and M3 being the most common. Other serotypes have occasionally been reported. Using restriction fragment length polymorphism (RFLP) as detected in pulsed-field gel electrophoresis, two subclones from invasive disease were identified by RFLP type and multilocus enzyme electrophoretic type (377, 378). The M1T1 genotype was recovered from both invasive disease and uncomplicated pharyngitis, suggesting that host factors as well as the streptococcal strain play a role in the development of severe disease (369, 379). The M1T1 genotype was studied during a recent epidemic in Sweden; the conclusion was that M1T1 did not appear to be clonal, since it had genetic diversity downstream of the emm-1 gene and both genes for erythrogenic toxins A and B exhibited clonal variation (389). This was in contrast to findings reported by Cleary and others that M1 may be clonotypic (100). Recent data reported by Stockbauer and colleagues (500) show that invasive M1 organisms are a heterogeneous array of related organisms that differ by variation in the streptococcal inhibitor of complement.
Animal models of invasive soft-tissue infection.
Wessels and colleagues have developed a murine model of human invasive soft-tissue infection (16). The animals challenged with wild-type M3 streptococci developed a spreading soft-tissue necrosis, with bacteremia and death. Animals challenged with acapsular or M protein-deficient M3 mutants did not develop the spreading, necrotizing disease but developed a localized abscess. In further studies, mutants of the M3 strain from which the cysteine protease gene was deleted caused the same necrosis and spreading disease as the wild type. Therefore, in the murine model of soft-tissue infection described by Ashbaugh et al. (16), the capsule and M protein play a major role in development of the spreading necrotic lesion.
Boyle has utilized an air sac model of skin infection in an attempt to demonstrate an association between expression of immunoglobulin G (IgG)-binding proteins and invasive potential (71, 442, 467). In recent studies, Raeder and Boyle have shown that fresh clinical M1 serotype isolates from blood cultures could be subgrouped based on their invasive potential in the mouse skin air sac model and IgG-binding protein expression (442). The expression of M1 protein and its IgG-binding properties were associated with the invasive potential of the M1 serotype studied. Subtle differences in the M1 serotype have been reported previously which may affect the invasive potential of the M1 strain (225). The virulence of an isolate in the skin model does not necessarily correlate with virulence potential when the streptococcal strain is administered intraperitoneally (71, 442, 467). In the skin air sac model of group A streptococcal infection, phenotypic variation of the same M protein serotype alters virulence. The skin models of infection and invasion suggest that the virulence factors required for skin invasion may be different from those for invasion through the pharyngeal route of infection or by injection intraperitoneally, which bypasses the normal entrance mechanisms of the bacterium. The section on virulence factors in this review will address some of these issues.
Treatment.
Treatment of toxic and severe invasive disease with antibiotics is not always effective, and mortality can exceed 50% (149). The failure of penicillin to treat severe invasive streptococcal infections successfully is attributed to the phenomenon that a large inoculum reaches stationary phase quickly and penicillin is not very effective against slow-growing bacteria (497, 499). Treatment with clindamycin in experimental models of fulminant streptococcal infections appears to be more efficacious than penicillin, but this has not yet been demonstrated in humans. Clindamycin acts on protein synthesis rather than on cell wall synthesis.
A number of studies have suggested that treatment of streptococcal toxic shock syndrome with intravenous immunoglobulin (IVIG) reduces the mortality rate (291, 292, 494). The reason for this may be that IVIG provides neutralizing or protective antibody to the patient. Plasma from patients with severe invasive group A streptococcal infections who were given IVIG inhibited streptococcal superantigen-induced T-cell proliferation and cytokine production (392). All three streptococcal pyrogenic exotoxins, A, B, and C, were inhibited by the IVIG. The data suggested that a deficiency of neutralizing antibodies against the superantigens may increase the risk of developing disease (23, 24). In addition, opsonic antibodies against M1 protein were found in pooled IVIG, and the anti-M1 antibodies combined with superantigen neutralizing antibodies in the IVIG should contribute to decreased mortality by reducing the bacterial load and neutralizing the effects of the toxins in patients with severe disease (24).
IDENTIFICATION OF THE ORGANISM: OLD AND NEW TECHNIQUES
Description and Clinical Microbiology
Throat culture.
The group A streptococci have long been recognized as the Streptococcus sp. associated with acute pharyngitis. In a positive throat culture, group A streptococci appear as beta-hemolytic colonies among other normal throat flora which are usually alpha- or nonhemolytic on 5% sheep blood agar. S. pyogenes may appear as highly mucoid to nonmucoid; colonies are catalase negative. Optimal recovery of group A streptococci may be achieved by use of blood agar plates containing sulfamethoxazole-trimethoprim to inhibit some of the normal flora and growth under anaerobic conditions to enhance streptolysin O activity (303). Throat culture is still recognized as the most reliable method for detecting the presence of group A streptococci in the throat (170). Presumptive identification of the beta-hemolytic group A streptococci relies on susceptibility to bacitracin or a positive pyrrolidonylarylamidase test (170).
Lancefield group.
The Lancefield serological grouping system for identification of streptococci is based on the immunological differences in their cell wall polysaccharides (groups A, B, C, F, and G) or lipoteichoic acids (group D) (303). The group A carbohydrate antigen is composed of N-acetyl-β-d-glucosamine linked to a polymeric rhamnose backbone. Confirmation of S. pyogenes is done by highly accurate serological methods, such as the Lancefield capillary precipitin technique and the slide agglutination procedure, which utilize standardized grouping antisera (170). These methods, including Streptex on primary plates (24 h) or subculture (48 h), would confirm group A streptococci. For this reason, rapid tests which screen for the presence of group A streptococci in the throat have been developed and are popular in the clinical setting (170, 303). Facklam has recently reviewed the currently available group A screening tests and discusses their sensitivity and specificity in comparison with the conventional methods (170). It is beyond the scope of this review to describe the many tests available for identification of group A streptococci from throat swabs. However, the most rapid tests take 5 to 30 min and use some form of nitrous acid or enzymatic extraction of the group A carbohydrate (170). Fluorescent-antibody and genetic probe tests can be performed directly on throat swabs but are not easily adapted to the clinical setting. Once extracted, the group A carbohydrate antigen is detected by one of four methods, including slide agglutination, enzyme-linked immunosorbent assay (ELISA), optical immunoassay, and a modified one-step ELISA procedure (170).
M protein and T typing: development of a molecular biology approach.
Streptococcal M protein, which extends from the cell membrane of group A streptococci, has been used to divide S. pyogenes into serotypes. Quite a number of years ago, Lancefield designed a serotyping system for the identification of the M protein serotypes (317). The method consisted of treating group A streptococci grown in Todd-Hewitt broth with boiling 0.1 N HCl. This method extracted the group A carbohydrate, M protein, and cell wall, and the clarified extract was used in capillary precipitin tests to determine the M protein serotype with standardized typing sera. The N-terminal region of the M protein has been demonstrated to contain the type-specific moiety and is recognized by specific typing sera in the precipitin test (28, 179, 271, 318). There were several difficulties with M serotyping, including ambiguities in the results, discovery of new M types, difficulty in obtaining high-titered antisera against opacity factor-positive strains, and the availability and high cost of preparing high-titered antisera for all known serotypes (170). Currently, more than 80 different serotypes of M protein have been identified (170).
Because of the difficulty in preparation of M-typing antisera, an alternative to the preparation of M-typing antisera has been developed (518, 559, 560). Approximately half of group A streptococci produce opacity factor, a lipoproteinase which causes various types of mammalian serum to increase in opacity. Antibodies against the opacity factor are type specific and correlate with the M type. By using an opacity factor inhibition test, the M type of a group A streptococcus can be determined by determining the type of opacity factor (518, 559, 560).
The T protein antigen is present at the surface of the group A streptococci along with the M and R protein antigens. Although the genes for the M (see section on M protein) and T proteins (272, 470) have been investigated, the R protein sequence has not been elucidated. Although there is homology between tee genes, there is a much greater diversity among them compared with emm genes (272). Unlike the M protein, the most conserved region appeared to reside in the amino-terminal half of the T protein molecule. These observations were made from comparison of 25 different T types (272). In addition, the T protein was not present in streptococcal groups C and G.
In the laboratory, the T typing assay is performed as an agglutination test. The T typing of group A streptococci has been important in the investigation of epidemiology of group A streptococcal infections and has identified strains associated with outbreaks when the M type was not identifiable. Because certain M and emm types are associated with certain T types, the testing for M or emm type can be shortened by knowledge of the T type. Most (>95%) group A streptococci have well-defined T-type antigens, and certain T serotypes are associated with each of the specific M protein serotypes (34, 36). The use of T typing in addition to emm gene sequence analysis allows the identification of strain diversity. This type of characterization of group A streptococcal isolates is extremely important in the current climate of emerging invasive disease and sequelae.
Recently, a molecular biology approach has been developed for the identification of M protein serotypes (35). In this study, the emm types of 95 known M serotypes (reference strains) and 74 of 77 clinical isolates were identified by rapid PCR analysis. A nucleotide primer pair was used for amplification and identification of the emm allele. Of the 95 reference strains analyzed, 81 closely matched sequences in GenBank, 5 were new gene sequences added to GenBank, and the rest had small discrepancies which will be resolved. In general, a good correlation was seen between the known serotype and the identification by emm gene sequencing by the rapid PCR technique. This technique has advantages over hybridization techniques, where problems arise in identification of new genes or hybrid M protein molecules which result from interstrain recombination (553). Recently, rapid hybridization techniques utilizing emm-specific oligonucleotide probes have been shown to be useful in identification of M protein serotypes (289). Information on emm types can be accessed through the internet at http://www.cdc.gov/hcidod/biotech/infotech_hp.html.
Serological Diagnosis of Streptococcal Infection: Anti-Streptolysin O, Anti-DNase B, and Other Diagnostic Antibodies
The host responds immunologically to streptococcal infection with a plethora of antibodies against many streptococcal cellular and extracellular components. Host responses against the M protein serotype protect against reinfection with that particular serotype. Routinely, serotype-specific antibodies are measured only for research purposes and not for diagnosis of streptococcal infection. Responses against other cellular components are observed, including antibodies against the cell wall mucopeptide, the group A streptococcal carbohydrate moieties N-acetylglucosamine and rhamnose, and the other protein cell wall antigens R and T. None of the cell wall antigens are used in the routine diagnosis of group A streptococcal infections.
Serological diagnosis of group A streptococcal infections is based on immune responses against the extracellular products streptolysin O, DNase B, hyaluronidase, NADase, and streptokinase, which induce strong immune responses in the infected host (507). Anti-streptolysin O (ASO) is the antibody response most often examined in serological tests to confirm antecedent streptococcal infection. Todd developed the assay for ASO antibodies by 1932 (520). An increase in the ASO titer of ≥166 Todd units is generally accepted as evidence of a group A streptococcal infection. In previous studies it has been shown that infants are born with maternal levels of antistreptococcal antibodies and that infants develop streptococcal infections after the first year of life. ASO antibodies may not demonstrate a detectable rise in 1- to 3-year-olds, who have had few previous group A streptococcal infections (349). At <2 years of age, >50% of the patients had ASO titers of <50 Todd units, and none of the patients had titers above 166 (349). In the same study, older school-age children developed higher ASO titers. All five of the extracellular streptococcal enzymes may become significantly elevated over normal levels during a streptococcal infection. Although the ASO titer is the standard serological assay for confirmation of a group A streptococcal infection, assay of several of the enzymes enhances the chance for a positive test if the patient did not produce high levels of antibody against one or more of the extracellular enzymes. In general, the titers of antibodies against the extracellular products parallel each other; however, exceptions may be seen in infections with pyoderma or nephritogenic strains, when the anti-DNase B titers have been found to be a reliable indicator of streptococcal infection (507). Infection of the skin does not always elicit a strong ASO response.
Confirmation of a group A streptococcal infection is imperative for the diagnosis of rheumatic fever, since most often streptococci cannot be cultured from the pharynx. In rheumatic fever, approximately 80% of the patients will have an elevated ASO titer (>200 Todd units) at 2 months after onset (61). If an anti-DNase B or antihyaluronidase assay is performed on sera from these patients, the number of patients with at least one positive antistreptococcal enzyme titer rises to 95%. Thus, most acute rheumatic fever cases demonstrate an elevated ASO titer with some exceptions which require more than one antibody test to detect previous group A streptococcal infection. The streptozyme test was developed some years ago as a hemagglutination assay for the detection of multiple antibodies against extracellular products such as anti-streptolysin O, anti-DNase B, antihyaluronidase, antistreptokinase, or anti-NADase, and it is used clinically in some laboratories as an additional diagnostic test (61).
PATHOGENESIS: INTERACTION BETWEEN HOST AND PATHOGEN
Adherence and Colonization
Host-pathogen interactions occur due to binding of surface streptococcal ligands to specific receptors on host cells. Attachment of group A streptococci to pharyngeal or dermal epithelial cells is the most important initial step in colonization of the host. Without strong adherence mechanisms, group A streptococci could not attach to host tissues and would be removed by mucous and salivary fluid flow mechanisms and exfoliation of the epithelium. In skin attachment and colonization by group A streptococci, a site of previous damage may be important in overcoming the dermal barrier. Specific adhesion allows competition between normal flora and group A streptococci for tissue sites where normal flora reside. The investigation of adherence determinants of both streptococcal and host cells is vital to the understanding of pathogenic mechanisms in disease and in the development of antiadhesive therapies or vaccines to prevent colonization. Immunization or exposure of humans or animals to microbial adhesins may induce antibodies which concentrate in the mucosal layer and block adherence and colonization at the mucosal epithelium.
The adhesion process involves multiple group A streptococcal adhesins reported by several investigators as detailed below and described in excellent reviews (19, 108, 226, 227). Adherence has been outlined in these reviews as an initial weak interaction with the mucosa which is followed by a second adherence event which confers tissue specificity and high-avidity adherence (227). In addition, one could speculate that the presence of multiple adhesins in strains could give them the advantage of more avid adherence and potentially enhanced virulence. Although not yet well understood, environmental factors expressed in a particular body site may be important cues for expression of adhesins important for colonization of a tissue-specific site. It is possible that movement of streptococci from the mucosa or skin into deeper tissues may be facilitated by specialized adhesion mechanisms. Recent studies indicate that adherence to particular types of host cells may induce localized cytokine production and inflammatory responses (110, 536).
Multiple adhesins.
In early studies of bacterial adherence, Ellen and Gibbons suggested that an adhesin was associated with the M protein fimbriae on the surface of the group A streptococci (168, 169). Shortly thereafter, Beachey and Ofek published the first paper describing lipoteichoic acid (LTA) as an adhesin (27). Their data suggested that M protein was not the adhesin, but that LTA, an amphipathic molecule, was the adhesin for buccal epithelial cells. In these first studies of LTA, experiments characterizing adhesion were established (27). Antibody against LTA on the group A streptococci blocked adhesion to epithelial cells, and LTA, when reacted with epithelial cells, saturated the epithelial cell adhesin and inhibited adherence. Later, fibronectin was identified as the epithelial cell receptor binding LTA (488). In these studies, fibronectin was found to inhibit adhesion of group A streptococci to epithelial cells, and antifibronectin was found to block the binding of streptococci to epithelial cells. The LTA molecule was found to act as an adhesin by reacting with molecules on the streptococcal surface, such as the M protein, through its negatively charged polyglycerol phosphate backbone and positively charged residues of surface proteins. The lipid moiety of LTA projected outward and interacted with fatty acid-binding sites on fibronectin and epithelial cells (397). Evidence suggested that LTA accounted for approximately 60% of adhesion to epithelial cells, indicating that other adhesins were involved in the adherence of group A streptococci to epithelial cells. In a recent review (226), Hasty and Courtney state that at least 11 adhesins have been described for group A streptococci, including M protein (90, 136, 168, 169, 401, 540), LTA (106, 109, 111, 398, 488, 489), protein F/Sfb (223, 224), a 29-kDa fibronectin-binding protein (105), glyceraldehyde-3-phosphate dehydrogenase (412, 563), a 70-kDa galactose-binding protein (201, 535), a vitronectin-binding protein (526), a collagen-binding protein (533), serum opacity factor (310), a 54-kDa fibronectin binding-protein, FBP54 (109), and the hyaluronate capsule (549). Several extracellular host cell proteins have been implicated in attachment or adherence to group A streptococci, including fibronectin (105, 488), fibrinogen (463), collagen (533), vitronectin (526), a fucosylated glycoprotein (541), and integral membrane proteins including CD46, the membrane cofactor protein on keratinocytes (400), and CD44, the hyaluronate-binding receptor on keratinocytes (471). Table 1 summarizes the streptococcal adhesins and host receptors described previously.
TABLE 1.
Group A streptococcal adhesins and their host cell receptors
| Adhesin | Host cell receptor | Comment | Reference(s) |
|---|---|---|---|
| LTA | Epithelial cell/fibronectin receptor | 27, 488 | |
| M protein | HEp-2 cells | IL-6 production | 110, 418, 540 |
| Keratinocytes/CD46 receptor/factor H | IL-1 and prostaglandin E2 production | 168, 400, 401 | |
| Protein F/SfbI | Epithelial cell/fibronectin/CD46 receptor on keratinocytes | 223, 224, 400 | |
| Fibronectin-binding protein (FBP54) | Fibronectin/fibrinogen | 109 | |
| Serum opacity factor | Fibronectin | 311, 446 | |
| Hyaluronic acid capsule | Keratinocyte/CD44 (hyaluronate receptor) | 471, 549 | |
| Glyceraldehyde-3-phosphate dehydrogenase | Pharyngeal epithelium/fibronectin/cytoskeletal proteins/plasminogen-plasmin | 412, 563 | |
| Fibronectin-binding protein (29 kDa) | Fibronectin | 105 | |
| Vitronectin-binding protein | Vitronectin | 526 | |
| 70-kDa galactose-binding protein | Galactose | 201, 535 | |
| Collagen-binding protein | Collagen | 533 |
As previously discussed, the group A streptococcal M protein has been implicated as an adhesin since the early work of Ellen and Gibbons (168, 169). Studies of isogenic M-positive and M-negative strains indicate that the M-positive strains adhered to HEp-2 cells while the M-negative strains demonstrated greatly reduced adherence (109). Neither M-negative nor M-positive isogenic strains bind to buccal epithelial cells (90). Hollingshead found that M protein was required for persistence of group A streptococci in a rat model of infection (240). Hasty et al. have demonstrated that adherence of M protein to HEp-2 cells stimulates the release of interleukin-6 (IL-6) and potential localized inflammatory responses (Table 1) (110).
The M protein is important for attachment to keratinocytes in skin infections (401). Keratinocytes in the skin bind to the C repeat region of M protein (418). When the C1 and C2 repeats were deleted, the M protein was decreased in its ability to bind keratinocytes (418). Membrane cofactor protein (CD46) has been demonstrated to be a receptor on keratinocytes for the streptococcal M protein. CD46 binds M protein through factor H-like repeats present in CD46 (400). The importance of CD46 as a cellular receptor for group A streptococci is uncertain, as transfection of L cells with cDNA encoding human CD46 failed to increase binding of group A streptococci expressing type 3, 6, or 18 M protein (41). Attachment of group A streptococci to the skin may involve different adhesive mechanisms from those required for colonization of the pharynx. This hypothesis has not been widely investigated but is important in the understanding of the pathogenesis of skin and throat strains. In addition, the role of M protein in adherence appears to depend on the M protein serotype and host cell source, such as the pharynx or skin (471).
Of all the adhesins studied, M protein and LTA were the only adhesins known to prevent colonization in animals, and their antibodies protected against lethal infection by group A streptococci (106, 226). However, recently it was shown that SfbI, also known as protein F, induced a protective response in the serum and lungs of animals vaccinated intranasally with SfbI. SfbI-treated animals were protected against homologous and heterologous serotypes of group A streptococci (219). Both M protein and LTA block binding to HEp-2 cells (111). Studies by Bessen and Fischetti demonstrated that antibodies against the C repeat region of M protein protected animals against mucosal challenge and colonization with group A streptococci of multiple serotypes (49). In fact, in an intact organism these domains are exposed on the cell surface and accessible to antibody.
Other streptococcal adhesins which bind fibronectin are protein F (SfbI) (401), fibronectin-binding protein FBP54 (107, 109), and serum opacity factor (311, 446). Protein F and fibronectin each have two different adhesive domains (480) and appear to be involved in binding to the dermis and Langerhans cells (401). The two fibronectin-binding domains in protein F have sequence homology with fibronectin-binding repeats described in other bacteria. Protein F was expressed in 75 to 80% of the streptococci investigated (382) and is regulated by a superoxide signal (203). Evidence supporting this hypothesis is that protein F was found to be overexpressed in superoxide dismutase deletion mutants (203). Thus, superoxide availability appears to be an environmental cue for protein F expression. The role of protein F in virulence is not known, but it is definitely a streptococcal adhesin and mediates internalization of group A streptococci into nonphagocytic cells (219). Caparon and colleagues have also found that constitutive expression of fibronectin binding in group A streptococci is in response to growth under anaerobic conditions and activation of rofA, a potential regulator of adhesion (188). Adherence of streptococci to keratinocytes upregulates production of the inflammatory mediators IL-1 and prostaglandin E2 (536). Induction of proinflammatory responses in keratinocytes is associated with adherence of streptococci and their production of streptolysin O (458).
FBP54 is expressed on the surface of group A streptococci and binds to fibronectin (107, 109). The fibronectin-binding domain was identified in the first 89 residues of FBP54. Antibodies to FBP54 were present in sera from patients with streptococcal disease, and it was not expressed by some strains of group A streptococci (108).
The mechanisms of adhesion by group A streptococci must be accommodated in a hypothetical model. The model most often described is that of Courtney and Hasty and colleagues (108, 226, 227). They describe adhesion as two steps, one of which is relatively weak and overcomes electrostatic repulsion. They suggest that LTA is the mediator of the first-step adhesion. Second-step adhesion may then involve M protein, FBP54, protein F, opacity factor, and any number of other adhesins which have been described. The model must take into account the fact that the adhesins may differ in the throat and skin due to the presence or absence of receptor ligands and to the cues required to induce expression of an adhesin in a particular environment. In addition, changes in host cells caused by the presence of proinflammatory cytokines may change conditions for host cell adherence.
Intracellular Invasion
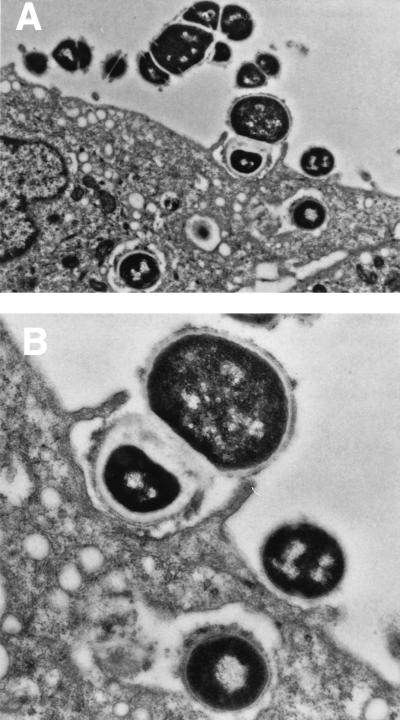
In the past few years, new evidence suggested that group A streptococci not only adhere to epithelial cells but also invade them (324). LaPenta and colleagues demonstrated that group A streptococci have the potential to invade human epithelial cells at frequencies equal to or greater than classical intracellular bacterial pathogens, such as Listeria and Salmonella spp. (324). This initial report generated considerable interest and was confirmed by several laboratories (187, 211, 261, 365). Figure 1 illustrates invasion of epithelial cells by group A streptococci (187). High-frequency invasion requires expression of M protein (118) and/or fibronectin-binding proteins such as SfbI (261, 365). Both the M1 protein and SFbI are considered invasins because latex beads coated with either protein are efficiently internalized by epithelial cells. In addition, mutations in genes that encode these proteins reduce the capacity of streptococci to invade cultured cells. Most recent work has demonstrated high-frequency intracellular invasion of epithelial cells by the M1 serotype of group A streptococci (160). The investigation demonstrated cytoskeletal rearrangements within the cells during the invasion process.
FIG. 1.
Electron micrographs demonstrating the attachment and internalization of streptococci by human cultured pharyngeal cells. Group A streptococci were observed to associate with microvilli upon initial contact with the pharyngeal cells. Membrane extension occurred during the internalization process. Surface interaction can be seen between the pharyngeal cell and streptococcus. Intracellular streptococci were found ingulfed in cytoplasmic vacuoles. Magnifications: A, ×12,700; B, ×24,300. (Reprinted from reference 187 with permission from the publisher.)
Fibronectin (118) and high-affinity fibronectin-binding proteins (406) trigger invasion by engagement of α5β1 integrin receptors on epithelial cells. Group A streptococci can invade human cells by other mechanisms. Laminin has been shown to bind group A streptococci (515) and will induce ingestion of M1 streptococci, independent of serum and fibronectin. Small peptides with the RGD amino acid sequence stimulate uptake in the absence of serum and M protein. The fact that group A streptococci evolved multiple routes to the interior of epithelial cells is a strong indication that intracellular invasion plays an important role in their pathogenesis. The most direct evidence that intracellular invasion is more than a laboratory phenomenon comes from studies of patients with recurrent tonsillitis. Failure to eradicate streptococci from the throat in pharyngotonsillitis occurs in approximately 30% of cases (348, 421). Osterlund and colleagues showed that tonsils excised from such individuals harbored intracellular group A streptococci (403). Others report that strains isolated from carriers are exceptionally able to invade HEp-2 cells in vitro (364). The reason for invasion of host cells is not entirely clear, although the streptococci may find the intracellular environment to be a good place to avoid host defense mechanisms. Therefore, internalization of streptococci may lead to carriage and persistence of streptococcal infection. In further studies, an association of the presence of the fibronectin-binding gene prtF1 with streptococcal strains from antibiotic treatment failures was found (383). In the treatment failures, 92.3% of the strains contained the prtF1 gene, while 29.6% of strains from eradicated infections did not.
Two theories have been proposed for the role of internalization of group A streptococci in disease pathogenesis. It has been suggested to potentially play a role in the carriage and persistence of streptococci, as stated above. Second, studies suggest that internalization may lead to invasion of deeper tissues (324), while other studies have found that low virulence was associated with internalization (472). Perhaps both theories are correct depending on the virulence and properties of the invading bacterium and whether the invasion is of the throat or skin epithelium. It is also possible that internalization of group A streptococci by host epithelial cells represents successful containment of the pathogen by the host. This hypothesis is supported by the observation that poorly encapsulated strains are internalized most efficiently but are relatively avirulent in infection models (472). Future study of these and other questions about intracellular invasion will no doubt yield new and unexpected clues to the role of internalization in the pathogenesis of group A streptococci.
Host Response to Infection: Opsonization and Phagocytosis
It is well established that group A streptococci are antiphagocytic due to surface exposed M protein and hyaluronic acid capsule (368, 551). Two mechanisms have been proposed to explain the antiphagocytic behavior of M-positive streptococci. One mechanism is the binding of factor H, which inhibits the activation of the complement pathway (246). Factor H is a regulatory component of the complement pathway, which inhibits the deposition of soluble C3b. Factor H binds to the C repeat region of the M proteins, and deletion of the C1 and C2 repeat regions reduces factor H binding (418).
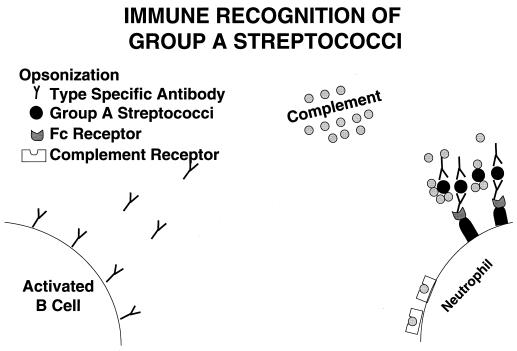
The antiphagocytic behavior of group A streptococci is also mediated by the binding of fibrinogen to the surface of M protein (555–557). Fibrinogen binding to the surface of group A streptococci blocks the activation of complement via the alternate pathway and greatly reduces the amount of C3b bound to streptococci, which therefore reduces phagocytosis by polymorphonuclear leukocytes (247). Type-specific M protein antibodies overcome this effect by binding to the exposed N-terminal M protein epitopes. This results in activation of the classical complement pathway, deposition of C3b, and subsequent phagocytosis. Figure 2 illustrates opsonization of group A streptococci by M type-specific antibody and complement.
FIG. 2.
How the immune system recognizes group A streptococci and uses opsonization by complement and type-specific antibody against M protein or any other surface molecule capable of generating opsonic antibody. Fc receptors shown on macrophages bind to the antibody Fc region, inducing phagocytosis and killing of the streptococci.
In addition to the antiphagocytic properties of the M protein, other surface molecules contribute to resistance to phagocytosis by the group A streptococcus. Recent studies have shown that mutations in Mrp (M-related protein) affect resistance to phagocytosis compared with wild-type strains, and in fact, insertion mutations in any one of the genes emm-49, enn-49, and mrp-49 resulted in reduced resistance to phagocytosis in human blood and purified polymorphonuclear leukocytes (PMNs) (425). From these results Podbielski suggested that resistance to phagocytosis depended on the cooperative effects of all three genes. Further studies by Ji, Cleary, and colleagues confirmed this hypothesis by demonstrating that failure to produce all three M-like proteins, M49, Mrp, and Enn-49, reduced resistance to phagocytosis but did not alter the persistence of streptococci at the oral mucosa (266).
It was found that C5a-activated PMNs were able to kill M-positive streptococci. It has been shown previously that C5a alters the clearance and trafficking of group A streptococci during infection (265). By inactivating C5a, C5a peptidase blocks chemotaxis of PMNs and mononuclear phagocytes to the site of infection (265, 552), while the M protein is limiting the deposition of complement on the surface of the streptococci (260). Therefore, the multiple mechanisms involved in resistance to phagocytosis in a bacterium where antiphagocytic behavior is essential for survival may have only recently been appreciated.
Extracellular Surface Molecules and Virulence Factors
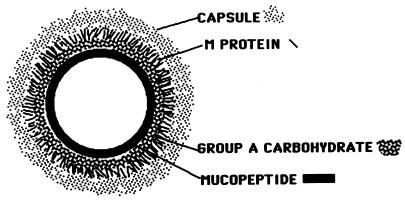
The group A streptococci are covered with an outer hyaluronic acid capsule (298), while the group A carbohydrate antigen and the type-specific M protein are attached to the bacterial cell wall and membrane, as shown in Fig. 3. Both the M protein and the capsule are considered virulence factors conferring antiphagocytic properties upon the streptococcal cell (179, 189, 368).
FIG. 3.
Diagram of the group A streptococcal cell covered with an outer hyaluronic acid capsule and the group A carbohydrate, consisting of a polymer of rhamnose with N-acetylglucosamine side chains. Streptococcal M protein extends from the cell wall and is anchored in the membrane. (Reprinted from reference 119 with permission from the publisher.)
Hyaluronic acid capsule.
The group A streptococcal capsule is composed of a polymer of hyaluronic acid containing repeating units of glucuronic acid and N-acetylglucosamine (509). Synthesis of the polymer involves the products of three genes, hasA, hasB, and hasC, which are all in the same operon (161). The hasA gene encodes hyaluronate synthase (152, 163); hasB encodes UDP-glucose dehydrogenase (162); and hasC encodes UDP-glucose pyrophosphorylase (112). The expression of the has genes is transcriptionally controlled. The three has genes are transcribed as a single message from the promotor upstream of hasA (113). However, only hasA and hasB are required for capsule expression in group A streptococci (15).
The hyaluronic acid capsule is required for resistance to phagocytosis (549). Acapsular mutant strains were altered in their virulence and colonization capacities in animal models (256, 466, 549). Acapsular mutants of serotypes M18 and M24 had drastically reduced virulence in mice after intraperitoneal challenge (549, 551). In a mouse model of skin infection, encapsulated M24 strain Vaughn produced dermal necrosis with purulent inflammation and bacteremia, while the acapsular M 24 strain produced no lesions or minor superficial inflammation with no bacteremia (472). In addition, the capsule may be an important adherence factor in the pharynx, since it binds CD44 on epithelial cells (471). Streptococcal isolates vary in the amount of hyaluronic acid capsule that they produce, which could be related to the has operon promotor. The promoter was more active in a well-encapsulated strain and less active in a poorly encapsulated strain (8). Most recently, Levin and Wessels have demonstrated a negative regulator (CsrR) of hyaluronate synthesis which is part of a two-component regulatory system influencing capsule production and virulence (327). Epidemiologic evidence linking highly mucoid strains with rheumatic fever and severe invasive streptococcal disease suggests that the capsule could play an important role in invasive infections in humans (267). The studies described above also recognize the capsule as a major virulence determinant in conjunction with the streptococcal M protein. Studies of type 18 and type 24 streptococci indicated that the hyaluronate capsule and M proteins were variably important in resistance of different group A streptococci to phagocytosis and that opsonization with C3 did not always lead to phagocytosis and killing (146).
Although hyaluronic acid is identical to the polysaccharide in bovine vitreous humor and human umbilical cord and has been defined as a weak immunogen due to similarity to self, immunization of animals has been shown to elicit antihyaluronate antibodies (175, 176). The fact that the capsule is antiphagocytic and promotes resistance to phagocytosis is supported by data demonstrating that hyaluronidase treatment of encapsulated streptococci increases their susceptibility to phagocytosis (190, 457). However, anticapsule antibody is not opsonic and does not protect against infection by neutralizing the antiphagocytic effects of the capsule. More recent work provides definitive evidence that the capsule is a major virulence determinant involved in resistance to phagocytosis (146, 368, 550). The mechanism of resistance to phagocytosis does not appear to be due to effects on the amount of complement component C3 deposited on the surface of the streptococci (146). The mechanism may be due to the physical barrier of the capsule in preventing access of phagocytes to opsonic complement proteins bound to the bacterial surface.
M protein.
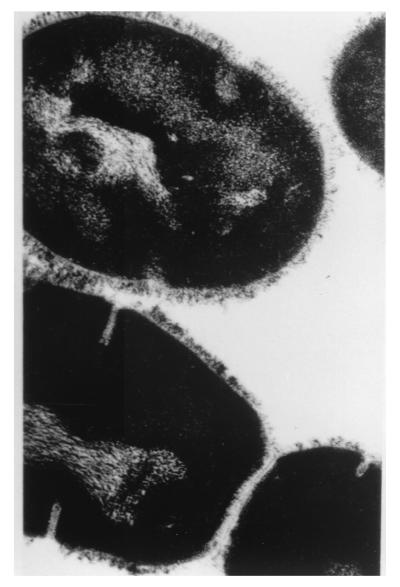
The group A streptococcal M proteins have been studied extensively since their discovery (317–320). Two excellent comprehensive reviews by Fischetti describe the characteristics of the M protein molecule in detail (177, 179). The M protein is a major surface protein and virulence factor of group A streptococci, with more than 80 distinct serotypes identified. The amino-terminal region extends from the surface of the streptococcal wall, while the carboxy-terminal region is within the membrane. The M protein is anchored in the cell membrane by the LPSTGE motif identified by Fischetti and colleagues (183). The M protein extends from the cell surface as an alpha-helical coiled-coil dimer which appears as fibrils on the surface of group A streptococci (419) (Fig. 4).
FIG. 4.
Electron micrograph of group A M type 24 streptococci. The surface fimbriae or hairlike projections indicate the presence of M protein on the surface of the streptococci.
Over the past 60 years, new procedures have been developed to obtain purified M protein. Beachey and Cunningham investigated the treatment of M protein with pepsin at suboptimal pH (125), which led to the development of a pepsin-extracted M protein, PepM protein (25). Pepsin-extracted M proteins have been used to study the M protein molecule due to the ease of the extraction procedure. The method releases the amino-terminal half of the M protein molecule from the surface of the streptococci. Although the PepM protein will appear to be nearly purified on polyacrylamide gel electrophoresis, the preparation contains traces of other streptococcal molecules. Other procedures which have been used to extract M proteins from the streptococcal surface include boiling HCl (318), sonic oscillation (43), alkali treatment (194), isoelectric focusing (124), group C bacteriophage-associated lysin (185), nonionic detergent (181), nitrous acid (222), cyanogen bromide cleavage (534), and guanidine hydrochloride extraction (459). The extraction and purification of M proteins attempted to obtain more homogeneous preparations, but it was not until later that the very heterogeneous banding patterns of the extracted M proteins was overcome through the use of PepM proteins and recombinant M proteins. Even after purification, recombinant M proteins demonstrated multiple banding. It was deduced from the data obtained from PepM protein that the phenomenon of multiple banding of the purified M protein was associated with the C-terminal end.
Structural studies of the streptococcal M protein were begun in the late 1970s and early 1980s by Beachey and colleagues (29, 30, 32) and Manjula, Fischetti, and colleagues (342, 344) when the technology of protein chemistry was used to obtain the amino acid sequence of peptide fragments of the M proteins. M protein types 24, 5, and 6 were investigated first and their sequences were elucidated. From these sequence data and the previously known amino acid sequence data, Fischetti and Manjula defined the alpha-helical coiled-coil structure of the M proteins and their heptad repeating structure, which was quite similar to the alpha-helical coiled-coil structure in host tissue proteins such as tropomyosin and the keratin-desmin-vimentin and keratin-myosin-epidermin-fibrinogen families of molecules (343, 345, 346).
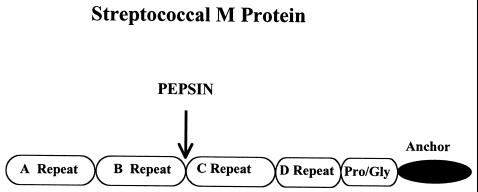
The age of molecular biology brought cloning technology to the study of streptococcal antigens, and the emm genes from M types 24, 5, 6, and 12 and group G were cloned and their nucleotide sequences were deduced (58, 236, 297, 369, 454). The cloning and sequencing of the emm genes (58, 236, 297, 370, 454, 477) revealed repeating sequence motifs within (i) the N-terminal region, (ii) the midmolecule region and pepsin-sensitive region, and (iii) the conserved carboxy-terminal region. The N-terminal region, called the A repeat region, confers serotype specificity on the group A streptococcus and was found to be highly variable among M protein serotypes. The midregion was also variable and was called the B repeat region (179). The carboxy-terminal region also contained amino acid sequence repeats which extend throughout the carboxy-terminal one-third of the molecule. Figure 5 shows a diagram of the M protein molecule, illustrating the repeating regions and pepsin cleavage site. Using molecular probes, Hollingshead, Fischetti, and Scott (237) determined that the highly conserved carboxy-terminal region of M protein contained sequence homology shared among most of the M protein serotypes. Information about the carboxy-terminal region of the molecule was not known until the gene for M protein (emm) was cloned and expressed in Escherichia coli (477). Size variation among M proteins and within a serotype is dependent on the number of repeating units in the A and B repeat regions (179). Variations can occur in the number and size of the repeat blocks within the M protein amino acid sequence. Fischetti reported that streptococcal isolates taken weekly from throats of patients were found in electrophoretic gels to exhibit changes in size of the M protein molecule (179). Such changes in size may provide the bacterium with a selective advantage, as it would change antigenically and not be recognized by host antibody.
FIG. 5.
The A, B, C, and D repeat regions of M protein, with the protein anchor and pepsin cleavage site shown. The A repeat region varies between serotypes and contains the highly variable serotype-specific amino acid sequence of M protein at the N terminus. The B repeat region varies from serotype to serotype, while the C repeat region contains a conserved sequence shared among all of the serotypes. The anchor region contains the LPXTGX motif required to anchor gram-positive proteins in the cell membrane. (Reprinted from reference 119 with permission from the publisher.)
Functionally, the M proteins inhibit phagocytosis, which is a primary virulence mechanism for survival in tissues. Absence of the emm gene allowed rapid phagocytosis of the streptococcus (479, 511). Introduction of the emm gene into an M-negative strain converted it to an M-positive strain and restored resistance to phagocytosis to the M-negative strain (416, 478). The antiphagocytic activity of M protein is due to its binding of complement regulatory protein factor H (246, 418) and fibrinogen (555, 556) as described above. The binding of fibrinogen also leads to the acquisition and activation of plasminogen, which is then converted by streptokinase to active plasmin (97, 334). Human kininogen has also been reported to bind to M protein with the subsequent release of bradykinin, a vasoactive peptide released in plasma (38, 39). In a recent review (108), Courtney and colleagues point out that not all M proteins exhibit the same structures or functions, since M3 protein binds fibronectin (469), while M5 and M24 proteins do not (109).
M proteins have been divided into class I and class II molecules (50, 52). The division of the M proteins into two classes is based on their reaction with antibodies (such as anti-M protein MAb 10B6) against the C repeat region of M protein. Class I M proteins are reported to contain a surface-exposed epitope on whole group A streptococci that reacts with the antibodies against the C repeat region. Streptococcal strains containing the class II M proteins do not react with these antibodies and do not contain the class I epitope (50, 52). In addition, the class I M protein serotypes were opacity factor negative, while the class II serotypes were opacity factor positive. In studies of 130 streptococcal isolates, there was a strong correlation between serotypes known to produce rheumatic fever and the presence of the class I epitope (50, 52). In fact, serologic data suggest that rheumatic fever patients were recently infected with a class I group A streptococcal strain (57). These data correlate with the M-associated protein (MAP) I and II antigen profiles and may be the basis of the MAP reactivity previously studied by Widdowson (558, 561). Antibodies against the heart in rheumatic fever were associated with the MAP I antigen. Bisno has reported that there is a strong correlation between streptococcal serotype and the occurrence of rheumatic fever (60). The epidemiologic data have led to the proposal that group A streptococci associated with acute rheumatic fever outbreaks harbor a unique antigen or epitope that is associated with the development of acute rheumatic fever and carditis in the susceptible host.
Using nearly the entire M6 gene sequence or parts thereof, homology between M6 protein and the M proteins of streptococcal groups C and G was found (479). The M proteins from strains producing human disease appear to be structurally and functionally similar (492). In addition, other streptococcal proteins which were not M proteins shared sequence homologies with the group A streptococcal M protein. One of the first to be recognized was protein G from group G streptococci. Protein G is an IgG-binding protein which binds albumin and all four subclasses of human and animal IgG (5). Likewise, protein A, an IgG-binding protein from staphylococci, shares regions of homology with M proteins in the carboxy-terminal region. In further studies of the C-terminal region of M proteins, Pancholi and Fischetti discovered that gram-positive surface proteins have a similar C-terminal region that is responsible for attachment. This region is composed of a charged C terminus followed by a hydrophobic domain and a highly conserved LPXTGX motif (183). The similarities among these proteins of gram-positive cocci reside in the carboxy-terminal region near the membrane anchor.
Immunity to the M protein is protective against group A streptococcal infection and has led to the study of M protein vaccines (54, 142). The immune response against the alpha-helical M protein is a two-edged sword, leading to production of protective antibody which promotes phagocytosis and killing (179, 318) as well as antibodies which may react with host tissues (122, 132, 137, 140). The M protein has been and will continue to be the subject of intensive investigation due to its role as a major virulence factor and its potential as a vaccine against streptococcal infections.
emm-like genes and the emm gene superfamily.
Genes related to the M protein gene (emm) are called the M gene superfamily and include immunoglobulin-binding proteins, M-related proteins, and M proteins. These proteins may possess functional properties of immunoglobulin binding or antiphagocytic behavior. According to Hollingshead and colleagues (238), more than 20 genes have been identified in the emm gene superfamily, in which the genes shared greater than 70% DNA sequence identity at their 5′ ends (238). The highly conserved identity is found within three distinct domains in the cell-associated region of the M protein molecule. These domains include domain H, which may serve to anchor the protein in the membrane; the peptidoglycan-associated domain; and the cell wall-associated domain. The domains are all highly conserved among emm gene products compared with the other regions of the molecules which are surface exposed (238). These domains do not include the C repeat region of M proteins, which can be divided into class I and class II M proteins depending on the presence of the class I epitope detected by MAb 10B6 and others (50, 52). Phylogenetic analysis of the region has revealed four major lineages, designated subfamilies SF1 through SF4, that contain differences in the peptidoglycan-spanning domain of the M or M-like protein (53, 238, 239). Five major emm chromosomal patterns of the subfamily genes were identified based on the number and arrangement of the emm subfamily genes (56). These subfamily gene arrangement patterns were designated A through E. A given strain has one, two, or three emm or emm-like genes that are tandemly arranged on the chromosome near the positive transcriptional regulator called the multiple gene regulator of group A streptococci (mga). The emm gene patterns display several phenotypes. Table 2 summarizes the patterns of emm genes associated with skin and throat infections.
TABLE 2.
Characteristics of five chromosomal patterns of emm genes associated with skin and throat infectionsa
| Chromosomal patternb | Infection | emm gene subfamily | M classc | Opacity factor reaction | Usual tissue site of isolation | Typical M serotype(s) |
|---|---|---|---|---|---|---|
| A | Pharyngitis | SF1 | I | Negative | Throat | 1, 3, 6, 12, 17, 19, 24 |
| B | Pharyngitis | SF1-SF1 | I | Negative | Throat | 1, 5, 14 |
| C | Pharyngitis | SF1-SF3 | I | Negative | Throat | 18 |
| D | Impetigo | SF4-SF1-SF3 | I | Negative | Skin | 33, 41, 42, 52, 53, 70 |
| E | Pharyngitis or impetigo | SF4-SF2-SF3 | II | Positive | Throat or skin | 2, 4, 11, 22, 28, 49, 75 |
Acute rheumatic fever found in chromosomal patterns A to C (studies in United States); AGN found among all chromosomal patterns.
The immunoglobulin-binding proteins identified for group A streptococci are encoded by emm or emm-related genes which express M proteins or M-like proteins, respectively. Immunoglobulin-binding proteins have structural characteristics similar to those defined above for M-related proteins. They are M-like molecules which interact with immunoglobulins outside their antigen-combining site in the Fc region of the immunoglobulin molecule. Approximately six functional types of IgG-binding proteins expressed by gram-positive bacteria have been reviewed by Boyle (67). Group A streptococci express type II Fc binding receptors on their cell surface, which are further classified by the subclass of IgG with which they react. The type II Fc-binding proteins generally bind to human, rabbit, and pig immunoglobulins most strongly but not to mouse, rat, goat, cat, or dog immunoglobulins. Weak binding to cow and sheep immunoglobulins is seen (67). Boyle classified the streptococcal IgG-binding proteins into types, IIo, II′o, IIa, IIb, and IIc, based on the subclasses bound from different species, including humans (408). In addition, IgA-binding proteins may be expressed by group A streptococci (67). Other streptococcal groups may express immunoglobulin-binding proteins, but in general their binding profiles with immunoglobulins from different species are different from that of group A streptococcal immunoglobulin-binding proteins.
Heath and Cleary cloned and sequenced the first group A streptococcal IgG-binding protein gene, the fcrA gene. The fcrA gene in strain CS110 expressed a protein which bound IgG1, IgG2, and IgG4 (230, 422) and was a type IIa IgG-binding protein based on the Boyle classification scheme described previously (408). Quite a number of IgG-binding proteins have been identified (6, 207, 230) which belong to the M protein gene superfamily (53, 69, 72, 404, 405, 423, 453, 495, 553; T. D. Pack and M. D. P. Boyle, Abstr. 4th Int. Conf. Streptococcal Genet., abstr. M74). Genetic studies have suggested that a common ancestral gene has undergone gene duplication to produce the diversified family of immunoglobulin-binding proteins (197, 220, 231, 238, 262, 296, 424). Table 3 summarizes the emm gene superfamily of related molecules. The genes encoding the immunoglobulin-binding proteins are controlled by mga, a positive transcriptional regulator also controlling expression of M protein.
TABLE 3.
Variable organization of the emm gene superfamily of M-related proteinsa
| Chromosomal emm pattern | Subfamily
|
||
|---|---|---|---|
| First gene: mrp (fcrA), mrp | Central gene: emm, emmL, sir, arp | Last gene: emmL, enn, sph | |
| A | SF1 | ||
| B | SF1 | SF1 | |
| C | SF1 | SF3 | |
| D | SF4 | SF1 | SF3 |
| Eb | SF4 | SF2 | SF3 |
Similarities of genes in the emm gene superfamily include the amino-terminal leader sequence and carboxy-terminal anchor; a peptidoglycan-spanning region rich in proline, glycine, serine, and threonine residues; tandemly arranged amino acid sequence repeats; and a seven-residue periodicity in amino acid placement (238). The fcrA gene encodes a type IIa immunoglobulin-binding protein, mrp encodes an M-related protein that binds to IgG and fibrinogen, emmL encodes a protein that binds IgG, sir and arp encode IgA-binding proteins, and that encoded by sir can also bind to IgG; enn encodes a protein that binds either IgG3 or IgA, and sph encodes protein H, which binds IgG. The central gene contains emm gene sequence at the 5′ end. The class I epitope is found in the C repeat region of central SF1 emm genes (44, 50, 52, 56, 238, 239). Members of the emm gene family in group A streptococci encode antiphagocytic cell surface proteins and/or immunoglobulin-binding proteins (239). IgG binding is observed for almost all of the genes listed above in at least one or more strains, but not all are antiphagocytic. The emm gene immunoglobulin-binding product varies in human subclass specificity. For example, SF4 usually binds human IgG1, -2, and -4 but not IgG3. Some emm gene products only bind IgG3, while others bind all four human subclasses. The nomenclature scheme devised by Boyle includes characterization by immunoglobulin binding to human as well as other immunoglobulin species (408). Boyle classification is types IIo, II′o, IIa, IIb, and IIc based on the subclasses of immunoglobulins bound from different species (408).
IgA binding is restricted to pattern E in either the SF2 or SF3 gene, depending on the strain. The emm gene immunoglobulin-binding product varies in human subclass specificity.
Analysis of the immunoglobulin-binding domains suggests that they are distinct regions of the M and M-related proteins. In addition, the M and M-like proteins may contain unique domains which bind other plasma proteins, such as albumin, factor H, fibrinogen, and plasminogen as well as IgG and IgA. Structural domains that are similar in all M and M-like proteins reside in the carboxy-terminal two-thirds of the molecule or C repeat region, as described above for M proteins. Recent reviews by Boyle (68) and Kehoe (296) are available on the subject. A single protein may contain multiple unique binding functions. Boyle suggests that this variation in the surface M and M-like proteins may confer a particular pathogenic profile and allow variation in the protein as a virulence strategy (68). Likewise, Bessen and Fischetti (45, 55) have suggested that each unique combination of domains imparts a unique virulence profile to a particular streptococcal strain. Boyle suggests that these multifunctional M or M-like proteins enable the organism to sense its environment and then express the appropriate virulence factors accordingly. Such variation may lead to temporary avoidance of the host antibody defense against the extracellular streptococcal pathogen until specific antibody to the variation can be generated.
The role of immunoglobulin-binding proteins in virulence has been studied in the mouse air sac model of skin infection. When strains carrying insertionally inactivated emm or mrp (M-related protein) genes were compared to the wild-type isogenic strain for virulence in skin infection, the loss of emm gene expression resulted in a loss of virulence (71). Loss of the emm gene product resulted in a significant loss of virulence when the isolate was injected into the skin, while no loss of virulence was observed when the isolate was injected intraperitoneally. Similar results were observed for the 64/14 strain and the M2 strain, for which isogenic mutants lacking expression of emm or mrp were created and tested in the skin infection model (71, 467). Expression of the IgG-binding proteins was associated with more resistance to phagocytosis, survival in blood, and more invasiveness in the skin infection model (425, 442, 443).
Studies of throat- and skin-derived streptococcal isolates show that human IgG-binding activity was associated with all impetigo isolates tested whether isolated from the throat or skin (45, 52). Expression of class II M proteins and opacity factor was almost always accompanied by expression of the human IgG-binding receptor irrespective of the site of infection. By contrast, all class I isolates were lacking expression of IgG-binding receptors. Thus, there was a strong correlation between class I or II M proteins, tissue site of isolation, and IgG binding by the streptococcal strain (44, 52).
In studies of invasive M1 isolates, Raeder and Boyle found that there were two immunoglobulin-binding phenotypes among group A streptococcal M1 isolates from invasive disease (441, 444). One group of the M1 isolates bound all four human IgG subclasses (type IIo), while the second group of M1 strains bound only IgG3 (type IIb). In these isolates, the M1 protein is the major IgG-binding protein (468). Differences among the M1 isolates may help explain their pathogenic potential. Evaluation of the M1 phenotypes in the mouse air sac model of skin infection revealed that the M1 phenotype IIo was more invasive than the IIb phenotype (444). The IIb phenotype changed to the IIo phenotype in the blood and spleens of mice injected with the IIb phenotype (444). The IgG-binding phenotype predicted severity of invasive skin infection leading to death of the animals. Conversion of type IIo to type IIb was associated with a posttranslational modification event involving the action of a bacterial cysteine protease, SpeB (445).
Studies of an M-like protein, protein H, from group A streptococci have shown that two adjacent IgG-binding domains were present in protein H which bound two different subclasses of human IgG (55). The study reported that the two coding regions were tightly linked and that strong selective pressures may maintain the two-domain binding motif. The motif was associated with impetigo isolates and not with nasopharyngeal isolates. The strong correlation of the motif with impetigo isolates suggests that it may play a role in virulence and tissue site distinction (55). Protein H also has a separate binding site for albumin (195).
IgA-binding proteins have also been reported in group A streptococci. An IgA-binding protein of group A streptococci designated Arp4 (IgA receptor protein from serotype 4 strain) binds both subclasses of IgA with high affinity, binds IgG weakly, but does not bind to fibrinogen (330, 495). Arp4 was demonstrated to possess antiphagocytic function as well as the seven-residue periodicity associated with M and M-related proteins (255). Arp4 is distinctly different from Mrp4 (M-related protein from serotype 4 strain), which binds IgG as well as fibrinogen (329, 495). The IgA-binding motif of Arp4 and an IgA-binding protein from a serotype 2 strain (ML2.2) was identified by Bessen and localized to a 58-residue peptide containing amino acid residues 14 to 71 of the protein (51, 232). Bessen and Fischetti also reported that a significantly higher number of wound and deep tissue isolates possessed IgA-binding activity (45). The gene similar to Arp4 designated ML2.2 (IgA-binding protein from a serotype 2 strain) has been shown to have extensive sequence homology with Arp4 (53).
Immunoglobulin-binding proteins have been suggested to play a role in streptococcal sequelae. Totolian, Burova, Schalen, and colleagues report in several studies that Fc receptor-positive streptococci induce the production of anti-IgG antibodies, which deposit in heart and kidney tissues and may trigger renal or myocardial damage (85–87). Streptococcal strains which did not contain immunoglobulin-binding proteins on the surface did not produce tissue damage. The studies, however, used whole streptococci, which could have possessed other factors which induce deposition of IgG in tissues.
Plasminogen-binding proteins: glyceraldhyde-3-phosphate dehydrogenase, enolase, and streptokinase.
Bacteria such as group A streptococci may bind plasmin(ogen) to their surface receptor proteins (Table 4). This strategy by the streptococci may enhance bacterial invasion or movement through normal tissue barriers. Table 4 summarizes the plasmin-plasminogen binding of proteins of group A streptococci. An excellent review by Boyle and Lottenberg describes the capture of host plasminogen as a potential common mechanism by which invasive bacteria cross tissue barriers (70). Invasion is thought to be influenced by plasmin bound at the bacterial surface. Surface-bound plasmin would activate extracellular matrix metalloproteases or collagenases to facilitate invasion (334). The majority of studies on plasmin(ogen) receptors have focused on group A streptococci. The studies of plasmin(ogen) binding have identified surface glyceraldehyde-3-phosphate dehydrogenase as a plasmin(ogen)-binding receptor (77, 78, 332, 333, 412). Lottenberg and colleagues identified lysine-binding sites in the region of the plasmin(ogen) molecule which interacted with the streptococcal plasmin(ogen)-binding protein Plr (76, 77). Group A streptococci grown in human plasma acquire surface-associated plasmin activity, suggesting that in vivo the bacteria can bind and generate plasmin (333). The recombinant plasmin receptor gene product Plr was evaluated for binding of plasmin(ogen) and found to react with human Lys-plasmin(ogen), defined as modified zymogen lacking 77 amino acid residues and with a lysine at the amino terminus (151). The vast majority of group A strains bind to Lys-plasmin(ogen), while only a small number of M serotypes bind to Glu-plasmin(ogen). A small number of M protein serotypes express an antiphagocytic M protein which has the property of binding to Glu-plasmin(ogen) directly (40, 566).
TABLE 4.
Group A streptococcal plasminogen-plasmin binding proteins
Further studies by Winram and Lottenberg utilized site-directed mutagenesis of Plr/dehydrogenase to demonstrate that the carboxy-terminal lysine-334 was essential for binding of plasmin(ogen), but mutation of the plr gene did not reduce plasmin binding to intact group A streptococci (565). An IgM MAb which inhibited plasmin(ogen) binding to the recombinant Plr protein did not inhibit binding of plasmin(ogen) to whole intact group A streptococci (151). Collectively, these data suggested that Plr did not account for all of the plasmin binding to group A streptococci. In addition to the direct binding of plasmin, group A streptococci were demonstrated to have plasmin(ogen) activity due to another pathway that involved fibrinogen, plasmin(ogen), streptokinase, and surface-expressed M or M-like fibrinogen-binding protein (98, 150, 538, 539).
Subsequently, the recombinant protein Plr was identified as a glyceraldehyde-3-phosphate dehydrogenase (563). Studies by Pancholi and Fischetti reported the presence of the glyceraldehyde-3-phosphate dehydrogenase or streptococcal surface dehydrogenase of multiple M protein serotypes and other streptococcal groups (412). Following identification and purification of the glyceraldehyde-3-phosphate dehydrogenase (streptococcal surface dehydrogenase), it was demonstrated to have multiple binding activity (412). The streptococcal surface dehydrogenase was found to bind fibronectin, lysozyme, and the cytoskeletal proteins myosin and actin (412). Due to its binding of fibronectin, the dehydrogenase was proposed to play a role in adherence and colonization of the pharyngeal epithelium. As described above, the surface dehydrogenase was demonstrated to bind to plasmin, but very weakly (563). The surface dehydrogenase was also discovered to be an ADP-ribosylating enzyme which was enhanced in the presence of nitric oxide (411). It was also found that whole streptococci or streptococcal surface dehydrogenase activated tyrosine kinase and protein kinase C in human pharyngeal cells (413). The signaling of host pharyngeal cells may be important in the infectious process. Other studies have shown that under conditions of iron starvation, the plasmin receptor Plr/surface glyceraldehyde-3-phosphate dehydrogenase is released from the surface into the growth medium (165). The significance of this mechanism in pathogenesis is yet to be determined, but it could play a role in bacterium-host cell communication.
Group A streptococci also express a surface enolase with strong plasminogen-binding activity (410). This is particularly important since the Plr/dehydrogenase described above does not function as the only plasmin(ogen) receptor on group A streptococci. In further studies, it was determined that lysines in the carboxy-terminal region of the surface enolase are important in plasmin(ogen) binding (410). The plasmin-binding activity of the enolase may be important in streptococcal invasion of tissues. The surface enolase was found to make a greater contribution to plasmin binding than the surface dehydrogenase (410). The enolase appeared to be more exposed and was reported to serve as the primary receptor for plasminogen binding (410). The streptococcal enolase was shown to be on the surface of the streptococci by immunoelectron microscopy using specific antienolase MAb (410). Using a photoactivatable cross-linker, it was shown that the streptococcal enolase and not the streptococcal dehydrogenase contributes to the direct binding of plasminogen by group A streptococci. In addition, antibody to the surface enolase was opsonophagocytic. Antibodies against the enolase have been reported in systemic rheumatic diseases and could play a role in poststreptococcal sequelae (410). Since the enolase is exposed on the surface of human tissues, it is possible that antibodies to the enolase could result in autoimmune tissue damage (410).
Streptokinase, a secreted plasminogen-binding protein, is a 46,000-molecular-weight protein with four compact domains (148, 249, 341). As early as 1933, group A streptococci were shown to dissolve fibrin clots (519). Eventually the fibrinolytic plasminogen activator was identified as streptokinase, which forms a 1:1 complex with plasminogen and converts other plasminogen molecules to plasmin (334). Antistreptokinase antibody will neutralize the activity of streptokinase, while host protease inhibitors will not (70). The streptokinases are a family of secreted streptococcal proteins with the common function of converting plasminogen to plasmin (334). The streptokinase gene is found in most group A streptococcal isolates as one of nine polymorphic genotypes (248, 269, 341). The polymorphic regions of streptokinase span amino acid residues 174 to 244 and 270 to 290 (269, 341). Despite the polymorphism in the variable regions of the molecule, the overall structure (hydrophilicity, hydrophobicity, antigenic sites, amphipathic regions, etc.) is maintained (285). Streptokinase activity found in different streptococci reflects the host range and is not active in hosts which it does not normally infect (70). Streptokinase has been associated with the pathogenesis of acute poststreptococcal glomerulonephritis (244, 341, 399). The major variable region of streptokinase is the V1 region, which contains 70 amino acids (269, 341). In group A streptococcal isolates from acute poststreptococcal glomerulonephritis, the V1 region is reported to contain more ordered secondary structures (341). It is possible that this region of streptokinase can bind to renal glomeruli and activate plasminogen, resulting in nephritis (341). The plasminogen-activating activity of streptokinase may also directly contribute to streptococcal virulence and invasion of tissues (314, 333).
Streptococcal pyrogenic exotoxins and the novel mitogen SMEZ: role as superantigens.
The streptococcal pyrogenic exotoxins, or erythrogenic toxins, are well known for their pyrogenicity, enhancement of endotoxic shock, and superantigenic effects on the immune system (10, 11). Schlievert has written a review on the role of superantigens in disease (464), and Kotb has reviewed the characteristics and effects of bacterial pyrogenic exotoxins as superantigens (304). Superantigens are molecules which are mitogenic for certain T-cell subsets but do not require processing by antigen-presenting cells. They activate a much larger number of T cells than conventional antigens (284, 306). The superantigen is capable of binding to the beta chain (Vβ) of a characteristic set of T-cell receptors and also to the MHC class II molecule expressed on B cells, monocytes, and dendritic cells. Binding to the T-cell receptor and/or to MHC class II molecules causes the T cells to proliferate and release excessive amounts of inflammatory cytokines (171, 233, 304). The streptococcal pyrogenic exotoxins have been defined in four distinct antigenic groups, A to D. Types A, B, and C are well defined, while type D is not well characterized (10, 11). The pyrogenic exotoxins appear to be responsible for many of the manifestations of scarlet fever and toxic streptococcal syndrome described in this review. Administration of streptococcal pyrogenic exotoxin A to animals resulted in fever, hypotension, and other symptoms associated with streptococcal toxic shock syndrome (326). It is now evident, as will be described below, that there are a large number of mitogenic exotoxins which function as superantigens and may be important in the manifestations of toxic streptococcal syndrome.
The pyrogenic exotoxins have homology with other pyrogenic exotoxins, such as staphylococcal enterotoxins A and C (210). The speA and speC genes are encoded by a bacteriophage (209, 546, 547, 574), while the gene for SpeB is chromosomal (210, 228, 268, 573). SpeB is a cysteine proteinase which degrades vitronectin, fibronectin, and IL-1 precursor (287, 288). The proteinase will be discussed below. The Vβ T-cell subsets expanded by SpeA are Vβ 2, 12, 14, and 15, while SpeB expands Vβ 8 and SpeC expands Vβ 1, 2, 5.1, and 10 (523).
Other pyrogenic exotoxins include mitogenic factor (SpeF), streptococcal superantigen (SSA), streptococcal mitogenic exotoxin Z (SMEZ) (259, 274, 367), and three most recently identified, novel streptococcal superantigen genes speG, speH, and speJ from the S. pyogenes M1 genomic database at the University of Oklahoma, have been investigated (434). A fourth novel group A streptococcal superantigen, SMEZ-2, was identified from strain 2035 due to its homology with SMEZ. Recombinant molecules of SMEZ, SMEZ-2, SpeG, and SpeH were mitogenic for human peripheral blood lymphocytes, with SMEZ-2 the most potent of all of the superantigens reported thus far (434). SMEZ-2 was also shown to be mitogenic for rabbit T cells (274). Table 5 summarizes the human Vβ specificities of the group A streptococcal superantigens. Stimulation of the Vβ 2, 4, 7.4, and 8 T-cell subsets was observed for SMEZ; Vβ 4 and 8 for SMEZ-2; Vβ 2, 4, 6.9, and 12.3 for SpeG; and Vβ 2, 7.3, and 9.1 for SpeH (434). SpeF was shown to have cytokine induction patterns similar to those of SpeA and SpeB (395), and it showed expansion of the Vβ 2, 4, 8, 15, and 19 T-cell subsets (390). SSA caused expansion of the Vβ 1, 3, 5.2, and 15 T-cell subsets (367). SMEZ was distinct from any known mitogenic exotoxins, and anti-SMEZ antisera did not cross-react with SpeA, SpeB, or SpeC in neutralization tests of mitogenic activity (274, 434). However, it was reported that SMEZ-2, SpeG, and SpeJ were the most closely related to SMEZ and SpeC. SpeA, SpeC, and SSA have sequence identities of 20 to 90% with the staphylococcal enterotoxins (527). SpeH was found to be more closely related to the staphylococcal enterotoxins (434).
TABLE 5.
Vβ specificity of group A streptococcal superantigens
| Superantigen | Vβ specificitya | Reference |
|---|---|---|
| Streptococcal pyrogenic exotoxins | ||
| SpeA | 2, 12, 14, 15 | 523 |
| SpeB | 8 | 523 |
| SpeC | 1, 2, 5.1, 10 | 523 |
| SpeD | Not characterized | |
| SpeF | 2, 4, 8, 15, 19 | 390 |
| SpeG | 2, 4, 6.9, 12.3 | 434 |
| SpeH | 2, 7.3, 9.1 | 434 |
| SpeJ | ? | 434 |
| SSA | 1, 3, 5.2, 15 | 366 |
| SMEZ | 2, 4, 7.4, 8 | 434 |
| SMEZ-2 | 4, 8 | 434 |
Human Vβ T-cell subsets stimulated by superantigen.
The clinical manifestations in streptococcal toxic shock syndrome may result in part from the massive superantigen-induced cytokine production (304). SpeA, SpeB, and SpeF were shown to induce massive amounts of gamma interferon and tumor necrosis factor-beta, with weak Th2 cytokine responses (395). Superantigens characteristically induce cytokines IL-1, IL-6, gamma interferon, and tumor necrosis factor beta (305, 464). A study by Norrby-Teglund and colleagues reported that the cytokine profiles and proliferative responses to SpeF differed among individuals (395). Individual variance in response to superantigens may result in varying clinical severity.
The nucleotide sequence of the streptococcal pyrogenic exotoxin A gene was reported by Weeks and Ferretti (547) and the gene has been found to be expressed in most streptococcal strains isolated from cases of streptococcal toxic shock syndrome (229). In this study, 85% of the patients who manifested toxic streptococcal syndrome had strains which produced pyrogenic exotoxin A. In studies by Yu and Ferretti, 45% of scarlet fever isolates and 15% of non-disease-associated isolates contained the gene for pyrogenic exotoxin A (573). Xu and Collins have also found that the gene is expressed at fourfold-higher levels when the strains are grown at 37°C than at 26°C (571). Nucleotide sequencing of the streptococcal pyrogenic exotoxin A (speA) gene in strains associated with various outbreaks has revealed that there are four naturally occurring alleles of speA (384). The speA1, speA2, and speA3 alleles encode toxins which differ in a single amino acid, while speA4 encodes a toxin which is 9% divergent from the other three and has 26 amino acid substitutions (384). The speA2 and speA3 alleles are expressed in most of the recent isolates from toxic streptococcal syndrome. Twenty mutant speA toxins were produced by Kline and Collins, and their data suggest that speA3 is especially more active mitogenically and had higher affinity for the HLA DQ molecule than the SpeA1 form of the toxin (302). Recent research by Kotb suggested that the class II haplotype of the individual is a risk factor for clinically severe toxic streptococcal syndrome (Kotb, personal communication). The data therefore suggest that there may be a genetic predisposition toward development of superantigen-related toxic streptococcal syndrome and invasive disease.
Streptococcal superantigens may play a role in autoimmune responses in streptococcal sequelae. Studies by Kotb and colleagues suggested that pepsin-extracted M protein (PepM protein) acted as a superantigen and expanded the Vβ 2, 4, and 8 T-cell subsets (521, 522, 537). Studies in other laboratories suggested that the M protein is not a superantigen (154, 186, 465). Nevertheless, a superantigenic site in streptococcal M5 protein has been reported within the amino acid sequence (M5 residues 157 to 197, KEQENKETIGTLKKILDETVKDKLAKEQKSKQNIGALKQEL) (537). The superantigenic site is the B3 repeat in the B repeat region of the M5 protein and shares significant amino acid sequence homology with other superantigens (537). The site contains the QKSKQ sequence previously shown to react with antimyosin antibodies in acute rheumatic fever (128).
Inflammatory responses triggered by the streptococcal pyrogenic exotoxins or superantigens may lead to autoimmune responses and manifestations. The alteration of the cytokine network toward production of inflammatory cytokines may be responsible in part for autoimmune manifestations in streptococcal sequelae.
Streptococcal proteinase (streptococcal exotoxin B).
Streptococcal proteinase or streptococcal exotoxin B is an extracellular cysteine protease produced by all group A streptococci (288, 380). However, some strains produce very large amounts of the protease (150 mg/liter) (200). The cysteine protease is secreted as a 40-kDa zymogen which is cleaved to a proteolytically active 28-kDa mature enzyme under reducing conditions (200, 228, 380). In group A streptococcal diseases such as pharyngitis, rheumatic fever, and invasive disease, patients produce antibodies against the proteinase. In fatal invasive disease, patients have lower levels of antibody to exotoxin B than do those with milder group A streptococcal infections (245). Neutralization of exotoxin B activity may protect humans against severe disease manifestations. Immunization of mice with the protease or streptococcal pyrogenic exotoxin B prolonged the survival of mice after challenge with heterologous strains of group A streptococci (286, 313). Passive protection or enhanced survival time was achieved when mice were administered antibodies against the protease. Therefore, the streptococcal protease elicits antibodies with protective effects in animals. These studies have led to the investigation of pyrogenic exotoxin B as a potential vaccine candidate which would enhance immunity against severe invasive disease as well as other streptococcal diseases.
Musser and colleagues have shown that the cysteine protease converts IL-1β precursor to active IL-1β, an inflammatory cytokine (287). Studies have also shown that the protease degrades vitronectin and fibronectin (288) and activates a 66-kDa human endothelial cell matrix metalloprotease (84). In addition, it has been shown that the protease cleaves and releases urokinase plasminogen activator receptor from the surface of mononuclear leukocytes (568) and releases biologically active kinins from their precursors (234). Activation of enzymes which can degrade the extracellular matrix in tissues could result in the tissue damage observed in invasive streptococcal infections. The protease produced damage to human umbilical vein endothelial cells in culture, and endothelial cell damage is observed in invasive streptococcal disease (263). Evidence that the streptococcal protease activates an extracellular matrix metalloprotease supports the hypothesis that the streptococcal protease is important in the pathogenesis of invasive disease.
Sequence analysis of the streptococcal pyrogenic exotoxin B gene from 200 group A streptococcal isolates revealed that there were three variants (501). Investigation of the variants identified one, mSpeB2, with an arginine-glycine-aspartic acid (RGD) motif which preferentially binds human integrins αvβ3 and αIIbβ3 (501). The mSpeB2 variant of the protease is expressed by all unusually virulent M1 strains and several other clones that cause invasive disease worldwide (501). This unique allelic variation may influence host-pathogen interactions in invasive disease.
In studies from one laboratory, inactivation of exotoxin B significantly decreased mouse lethality caused by M serotypes 3 and 49 (336, 337), while Ashbaugh and colleagues found that inactivation of exotoxin B had no effect on virulence in a mouse model of invasive tissue infection (16). Exotoxin B enhanced in vitro internalization of group A streptococci by human epithelial and endothelial cells (83). Genetic inactivation of the cysteine protease resulted in more rapid clearance from the peritoneal cavity of the streptococcal pyrogenic exotoxin B mutant and reduced its dissemination to organs (335). The evidence suggested that the streptococcal protease promoted resistance to phagocytosis and dissemination to organs. The studies also suggested that expression of the cysteine protease contributed to extracellular survival of group A streptococci in the host (83). The exact mechanisms by which the protease functions to prevent phagocytosis and enhance virulence are unknown. However, proteolytic activity toward M proteins, C5a peptidase, or host molecules may have an effect on virulence and phagocytosis.
C5a peptidase.
The C5a peptidase is a proteolytic enzyme (endopeptidase) found on the surface of group A streptococci. It is a highly specific 130-kDa serine peptidase that is anchored to the streptococcal cell wall. The peptidase cleaves the complement-derived chemotaxin C5a at its PMN-binding site (99). This event then inhibits the recruitment of phagocytic cells to the site of infection (265, 552). Therefore, the surface of group A streptococci presents a double barrier to the complement defenses of the host. First, the M protein, discussed above, reduces activation of the alternative pathway, and second, the streptococcal C5a peptidase inactivates C5a and chemotaxis. Most recently, C5a was shown to activate PMNs to kill M-positive streptococci (266). Although resistant to phagocytosis in fresh human blood, M-positive streptococci were found to be engulfed by activated phagocytic cells.
The C5a peptidase is encoded by a gene which is regulated by mga in concert with M protein (325). The enzyme is produced by group A, B, and G streptococci that have been isolated from human disease. The amino acid sequence is greater than 95% identical among class I and class II M protein serotypes of group A and B streptococci (96). Three lines of evidence suggest that the C5a peptidase is important in virulence of group A streptococci. Mutations in the scpA gene increase clearance of streptococci from subdermal sites of infection and from the nasopharyngeal mucosa of intranasally infected mice (264, 265). However, these mutations did not affect the virulence of the streptococci in a mouse air sac model of infection (265). Moreover, intranasal immunization of mice produced a vigorous serum and secretory antibody response that enhanced clearance of the bacteria from the oral mucosa of mice (264). Although fewer than 15% of children under 10 years of age exhibit measurable antibody against C5a peptidase, most adults have evidence of a strong immune response to this streptococcal protein (396). These findings prompted investigators to suggest that the immune response to the C5a peptidase may account for the relative resistance of adults to infection.
In recent studies, group A streptococci containing an inactivated scpA gene for the C5a peptidase were compared with the wild-type strain containing the intact scpA gene in a mouse model of long-term throat colonization and pneumonia (256). The group A streptococcus strain B514-Sm, a natural mouse pathogen, was the strain used for the genetic and mouse model studies with C57BL/10SnJ mice. Deletion of the scpA gene resulted in a small but significant difference from the wild type in the incidence of pneumonia in the C3HeB/FeJ mouse model after intratracheal inoculation but not in throat colonization in the C57BL/10SnJ mouse model following intranasal inoculation (256). A vaccine containing the C5a peptidase may protect against both group A and B streptococcal infections.
Streptococcal inhibitor of complement-mediated lysis.
The sic gene, which encodes the streptococcal inhibitor of complement-mediated lysis (SIC), has been investigated by Akesson and colleagues and Musser and colleagues (7, 235, 415, 500). sic was found to be a uniquely variable gene among M1 serotypes. SIC inhibited the complement membrane attack complex and may possibly contribute to group A streptococcal virulence, but the role of SIC in virulence in vivo has not been proven yet.
Recent molecular population genetic analysis of the gene encoding SIC in serotype M1 strains has discovered a uniquely high level of polymorphism (415, 500). The SIC protein was found to be remarkably hypervariable, with the amount of polymorphism far exceeding that present in any bacterial protein except those directly associated with antibiotic resistance. Importantly, SIC variants were rapidly selected on mucosal surfaces, and selection of these variants may perpetuate and increase the magnitude of M1 epidemic waves (James Musser, personal communication).
Analysis of the Group A Streptococcal Genome
Ferretti and colleagues are in the process of completing the entire sequence of the group A streptococcal genome from S. pyogenes SF 370, an M1 serotype classified as a T1/19/8 isolate from a wound infection (University of Oklahoma strain collection; originally obtained from the Communicable Disease Center in Atlanta, Ga.). The SF 370 strain has an RFLP profile identical to that of other severe invasive group A streptococcal strains. In addition, the strain contains a bacteriophage which encodes streptococcal erythrogenic toxin C and three other novel superantigens (J. J. Ferretti, personal communication). Analysis of the genomic sequence has revealed no evidence of pathogenicity islands. However, the gene for the cyclic AMP (cAMP) factor, regarded as a group B streptococcal extracellular cohemolysin in the presence of Staphylococcus aureus sphingomyelinase C, was found to be present in the genome of group A streptococci. The gene for the cAMP factor, cfa, was expressed by group A streptococci and found to be widely spread among group A streptococcal isolates (82 of 100) (199). In addition, the presence of multiple phage and insertion sequence elements suggests horizontal transfer of new virulence properties, and 11 two-component signal transduction systems with histidine kinases were identified (J. J. Ferretti, personal communication). The Oklahoma University S. pyogenes database can be accessed by internet at http://www.genome.ou.edu/strep.html.
Global Regulators of Virulence Genes
The group A streptococci must adapt to the various niches which they encounter in the human host. Therefore, regulatory elements must respond to environmental signals and control the expression of virulence genes in group A streptococci. Because the M protein gene was central to the virulence of group A streptococci, studies began with investigation of control of its expression. In 1987, both Cleary and colleagues and Scott and Caparon independently defined a gene which was a positive transcriptional regulator of expression of the M protein (89, 454, 490). The locus defined by Cleary was virR (491), and the locus defined by Caparon and Scott was mry (417). The mry/virR gene was renamed mga for multiple gene regulator in group A streptococci. The nucleotide sequence of mga contained sequence homology with phosphorylation acceptor motifs, consistent with the second component of a two-component regulatory signal transduction system (417). Deletion of sequences in mga resulted in the loss of M protein expression (493), and these deletion mutants were complemented by a complete mga gene in trans. It is now established that Mga activates the transcription of several genes, including those for M protein (emm), C5a peptidase (scpA), M-like proteins (mrp, enn, and fcR), serum opacity factor (sof), and secreted inhibitor of complement (sic) (95, 101, 355, 358, 422). Mga feeds back to positively regulate itself and functions as a 62-kDa protein to bind to the promoter region of the genes that it regulates (417).
The genes involved in the mga regulon are shown in Table 3, and an overview of regulation is shown in Fig. 6. The genes regulated by mga were found to be expressed in the late exponential growth phase but not in the stationary growth phase (357). Caparon and colleagues demonstrated that transcription of the M protein gene was regulated by the concentration of carbon dioxide (88). It was proposed that the level of carbon dioxide in tissues was one of the factors which regulated the expression of M protein during infection in vivo. Scott and colleagues have also found that transcription of the emm gene was downregulated by increased osmolarity (high salt), low temperature (25°C), growth in free exchange of gases (shaking), and restricted availability of iron (356). Their data suggested that group A streptococci can sense multiple signals in the environment which control expression of virulence determinants. Thus, Mga activates expression of several virulence genes in response to different environmental conditions. Hollingshead and colleagues reported that of 32 streptococcal M protein serotypes studied, many hybridized with the mga-1 gene (238, 422). Genes such as slo (streptolysin O) and many other virulence genes in group A streptococci were not affected by mga but appeared to be controlled by growth phase-dependent regulation, suggesting that expression of virulence genes in group A streptococci was under complex global regulation (357). In recent studies, a global negative regulator of mga was identified in a few strains and called nra. In addition to repressing Mga synthesis 4- to 16-fold, nra was a negative regulator of prtF2, the gene for fibronectin-binding protein F2, and the collagen-binding protein gene (cpa) in group A streptococci. The Nra protein sequence was 62% homologous to RofA, a positive transcriptional regulator of the fibronectin-binding protein (prtF) and itself in response to increased oxygen levels (426). RofA and Mga may influence the expression of genes involved in adhesion in different environments (426).
FIG. 6.
Overview of the regulatory networks in expression of group A streptococcal virulence genes. +, positive regulation; —, negative regulation. Short dashed lines indicate CovR regulation, and long dashed lines represent regulation by Mga. Solid lines show the correlation between growth phase (exponential [light lines] or stationary [darker lines]). As shown in the figure, Mga activates transcription of several genes, including those for M protein (emm), C5a peptidase (scpA), M-like proteins (mrp, enn, and fcR), serum opacity factor (sof), and secreted inhibitor of complement (sic) (95, 101, 355, 358, 422). Mga feeds back to positively regulate itself and functions as a 62-kDa protein to bind to the promotor region of genes that it regulates (417). The genes involved in the mga regulon are shown in Table 3, and an overview of regulation is shown in the diagram above. A global negative regulator of mga was identified in a few strains and called nra. In addition to repressing Mga synthesis 4- to 16-fold, nra was a negative regulator of prtF2, the gene for the fibronectin-binding protein F2, and the collagen-binding protein gene (cpa) in group A streptococci. The Nra protein sequence was 62% homologous to RofA, a positive transcriptional regulator of the fibronectin-binding protein (prtF) and itself in response to increased oxygen levels (426). RofA and Mga may influence the expression of genes involved in adhesion in different environments (426). csrR and csrS or covR and covS are a pair of genetic loci in group A streptococci that encode a two-component regulatory system (327). Inactivation of csrR or covR resulted in a striking increase in transcription of the capsule synthesis genes of the has operon and a corresponding increase in hyaluronic acid capsule production (327). Subsequent work confirmed these observations and demonstrated binding of the CsrR protein to the promoter region upstream of the has operon (42). The CsrR or CovR protein acts as a transcriptional regulator. Production of a nonpolar mutation in csrR or covR increased transcription of several other virulence genes, including ska (streptokinase), sagA (streptolysin O), and speF (mitogenic factor) but had no effect on mga, emm, scpA, speB, or speC. Thus, the CovR or CsrR response regulator repressed transcription of several virulence operons in group A streptococci (172). Since multiple unrelated genes were controlled by csrR, an alternative nomenclature was given to the csrR-csrS locus, covR-covS for control of virulence genes. The csrR-csrS or covR-covS sensor-regulator gene pair represent a new regulatory pathway affecting expression of several group A streptococcal virulence genes which are not regulated by mga and has been found in all group A streptococcal strains tested (172). (Reprinted from reference 172 with permission from the publisher.)
Levin and Wessels identified csrR-csrS, a pair of genes in group A streptococci that encode a two-component regulatory system (327). Inactivation of csrR resulted in a striking increase in transcription of the capsule synthesis genes of the has operon and a corresponding increase in hyaluronic acid capsule production (327). Subsequent work by Bernish and van de Rijn confirmed these observations and demonstrated binding of the CsrR protein to the promoter region upstream of the has operon (42). Their results provided further evidence that the CsrR protein acts as a transcriptional regulator. Federle, Scott, and colleagues found that a nonpolar mutation in csrR increased transcription of several other virulence genes, including ska (streptokinase), sagA (streptolysin O), and speMF (mitogenic factor) but had no effect on mga, emm, scpA, speB, or speC. Thus, the CsrR response regulator repressed transcription of several virulence operons in group A streptococci (172) (Fig. 6). Since multiple unrelated genes were controlled by csrR, Scott and colleagues (172) proposed an alternative designation for the csrR-csrS loci, covR-covS for control of virulence genes. CovR repressed itself and acted independently of growth phase (172). The csrR/csrS or covR-covS sensor-regulator gene pair represents a new regulatory pathway affecting expression of several group A streptococcal virulence genes which are not regulated by mga, and it has been found in all group A streptococcal strains tested (172). Figure 6 is a diagram illustrating the regulatory networks in group A streptococci. Bernish and van de Rijn discovered that csrR or covR acts directly on the hasA promoter or through another regulatory circuit on other promoters (42). Although a global regulator has not yet been identified that affects both mga and covR or csrR, growth phase affects both sets of genes (Fig. 6). Even in a strain lacking csrR or covR, the expression of genes was growth phase dependent. CsrR or CovR repressed some genes that are maximally expressed in either the exponential or stationary growth phase (172). Thus, the CsrR or CovR pathway appears to be independent of growth phase. According to Federle, Scott, and colleagues, genes encoding streptolysin s, capsule synthesis, streptokinase, and mitogenic factor were regulated by at least two distinct signals. One signal acts on CsrR or CovR and the other signal is the growth phase, about which little is known. In the mga regulon, the genes are shut off as growth enters the stationary phase. While a report by Steiner and Malke suggests that amino acid starvation affects the CovRS-mediated suppression of virulence genes (K. Steiner and H. Malke, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. B-261, 2000), future studies of global regulation in group A streptococci should uncover more about the mechanisms of these three distinct pathways and explain the regulation of crucial virulence factors associated with the many different types of streptococcal diseases.
Determinants of Protective Immunity
Protective opsonic and mucosal antibody against M protein.
Protection against group A streptococci correlates with the presence of opsonizing antibody against type-specific M protein (318). Type-specific opsonic antibodies against the M protein recognize epitopes in the amino-terminal region of the M protein molecule (271). Type-specific antibody is essential for effective clearance of the group A streptococci by polymorphonuclear leukocytes or neutrophils (Fig. 2). Although immune responses appear in humans to other parts of the M protein molecule, these antibodies are not opsonic and protective. It has been suggested that opsonic antibodies are produced late in infection (178, 317). Antibodies against nonopsonic epitopes of the M protein appeared to be produced prior to the opsonic response (178). This may be due to the fact that nonopsonic, non-type-specific epitopes are shared among group A streptococci and a secondary response would occur faster to the epitopes to which the host had been previously exposed. Once the host is exposed to the type-specific epitopes, a primary response occurs and long-term immunity to the infecting serotype is acquired (319). Antibodies against the B repeat region appear first in rabbits immunized with M protein (184) and are not opsonic (271). Human opsonic antisera contain antibodies against the N-terminal A repeat region, while nonopsonic sera contain antibodies to other regions but not to the amino-terminal A repeat region (179). All human sera were found to contain antibodies against the highly conserved C repeat region, which has the highest homology among the M protein serotypes (237). It makes sense that humans should have high levels of antibody against this region. Although antibodies produced against conserved-region peptides may not opsonize group A organisms, opsonic human antibodies specific for a conserved epitope on the M protein of group A streptococci have been reported (73). Additional studies suggest that human antibodies to the conserved region of the M protein opsonize heterologous strains of group A streptococci (74). In contrast, other reports suggest that epitopes shared among group A streptococcal M proteins in the C repeat region generate an immune response which is not opsonic but in animal models confers protective mucosal immunity against colonization with heterologous serotypes (46, 49, 81, 271). In these studies, peptides from the conserved C repeat region of the M protein molecule were administered intranasally to mice. Upon challenge with group A streptococci intranasally, the mice were protected against colonization and infection. Figure 7 illustrates the importance of mucosal immunity in protection against pharyngeal colonization by group A streptococci.
FIG. 7.
Streptococcal adherence and inhibition of adherence to the mucosa by specific antibody. Mucosal antibody against surface adhesins or epitopes in the C repeat region of M proteins protects against colonization with group A streptococci.
Studies with humans show that adults have significantly fewer group A streptococcal infections than children (75). This finding most likely reflects multiple streptococcal infections in childhood with the development of antibodies that afford protection against infection. Since it is unlikely that an individual would encounter a plethora of M serotypes during childhood, the protection seen in adults may in part be due to a mechanism other than opsonization. Studies have investigated the role of IgA at mucosal surfaces in protection against group A streptococcal infection. In mice, passively administered M protein-specific IgA provided protection against mucosal infection and also delayed disseminated infection and death (47). IgA blocks adherence of bacteria to mucosal surfaces and plays a key role in host protection at mucous membranes (301). IgA could also cause the streptococci to become trapped in mucus and cleared by host fluid flow mechanisms. Secretory anti-M protein-specific IgA prevented attachment of group A streptococci to pharyngeal epithelial cells in vitro (187). M protein-specific IgG did not diminish adherence but reduced invasion and internalization of the group A streptococci into the pharyngeal epithelial cells. The data suggest that IgG blocks invasion and IgA prevents adherence and colonization (187). M protein-specific opsonic IgG binds to the surface of the streptococci, where complement is then bound, and serves to facilitate opsonization and clearance of the bacteria. M protein-specific IgG is important in protection against growth of streptococci in the blood and tissues and follows an established infection.
Therefore, protective immunity has two major mechanisms. First, organisms entering the host can be blocked from attachment to mucosal surfaces by IgA specific for the C repeat region of M proteins (Fig. 7). Second, the group A streptococcus, once it has entered the host tissues, is effectively eliminated by opsonization with type-specific antibody and complement, with subsequent phagocytosis and killing (Fig. 2). One mechanism prevents colonization, while the other mechanism prevents multiplication in the host and elimination of the bacterium in host tissues or blood. Immunization of animals with M protein or passively administered serum antibody against M protein will protect against challenge with live streptococcal organisms (158). M protein vaccine strategies have taken advantage of the epitopes conferring these two modes of protection from infection.
Other streptococcal surface components potentially involved in protection against infection.
Various components of the group A streptococci may result in production of opsonizing antibodies or IgA which protects against colonization at the mucosal surface. Although the group A streptococcal M protein has been recognized as the streptococcal antigen capable of inducing protective, opsonic antibody, it is becoming increasingly clear that other surface molecules may participate in inducing protection and opsonization against infection. Dale and colleagues have reported a new antigen isolated from M-negative type 18 streptococci which elicited opsonizing protective antibody against serotypes 3, 18, and 28 (143). Likewise, M-like genes which contain antiphagocytic domains may potentially induce opsonizing antibody and in part contribute to protection (143).
Surface molecules such as the C5a peptidase induce antibodies which protect against intranasal challenge of group A streptococci of homologous and heterologous M serotypes (264). This evidence indicating mucosal anti-C5a peptidase antibody protection against challenge makes the C5a peptidase a candidate for streptococcal vaccines. Evidence has also demonstrated that the group A streptococcal carbohydrate could induce protective antibodies which opsonized multiple M serotypes of S. pyogenes (461). Antibodies against the streptococcal pyrogenic exotoxins A (392), B (245), and C (391) may play a protective role in humans by neutralizing the toxicity and mitogenicity of the toxins. Mice immunized with streptococcal erythrogenic toxin B (streptococcal proteinase) had prolonged survival following challenge with multiple group A streptococcal strains (286). Protection afforded by these various antigens may be important in overall protective immunity in vivo.
T-cell immunity to infection with group A streptococci.
Although a strong antibody response against the M protein is required for protection against homologous streptococcal infection, T-cell responses are important in B-helper T-cell function. T-cell epitopes have been reported for M5 protein, and a summary of our current knowledge of T-cell epitopes has been reported (123). T-cell responses to M protein have been investigated in a number of studies (123, 138, 154, 167, 216, 250, 435, 436, 455, 456, 521, 522). Although many of the studies related to T-cell responses in acute rheumatic fever and autoimmunity, studies of T-cell epitopes involved in protective immune responses against infection are particularly important in vaccine development.
Several studies have investigated T-cell epitopes potentially involved in M protein-specific protective immune responses (436, 455, 456). Robinson and colleagues have mapped multiple T-cell epitopes of streptococcal M proteins. In particular, two immunodominant epitopes were identified and characterized by using T-cell clones generated from mice immunized with recombinant M5 protein (156, 157). The two epitopes included an Ed-restricted epitope (M5 residues 17 to 31) and an Ad-restricted epitope (M5 residues 308 to 319). Their results suggested that the two epitopes were processed by two different antigen-processing pathways (156, 157). The study suggested that the following factors may be important in the processing of protective antigens such as streptococcal M proteins: (i) type and stage of antigen-presenting cell, (ii) receptors used to capture soluble and particulate antigens, (iii) enzyme cleavage sites flanking MHC class II binding peptides, and (iv) the presence of cytokines and other pharmacological agents which modify antigen processing by the antigen-presenting cell (156, 157). It is possible that the method of antigen processing will influence CD4+ T-cell specificity and functional differentiation.
NONSUPPURATIVE SEQUELAE
Acute Poststreptococcal Glomerulonephritis
Introduction.
Scarlet fever was first associated with the occurrence of acute glomerulonephritis in the late 1800s and early 1900s, and shortly thereafter acute glomerulonephritis was commonly associated with a previous streptococcal infection (402). Most cases of acute glomerulonephritis seen today are associated with a group A streptococcal infection and are not usually associated with scarlet fever (487). Acute poststreptococcal glomerulonephritis occurs primarily in children and young adults, with males affected twice as often as females, and individuals over 40 can also be subjected to the disease (487). Many investigators have noted a relationship between group A streptococcal infection and development of acute glomerulonephritis (159, 431, 449, 543). The epidemiology of acute poststreptococcal glomerulonephritis is related to its presence in southern and temperate climates, where pyoderma-associated glomerulonephritis demonstrated peak occurrence in the summer, while rheumatic fever peaked in the autumn and winter months of the year (64). In northern climates, acute glomerulonephritis is associated with throat infection (487). However, frequently the same organism infecting the skin in impetigo will also infect the throat. In general, skin infection precedes that of the throat. Past epidemics in the United States have been community associated, with the most notable outbreaks in the Red Lake Indian Reservation in Minnesota in 1953 and 1966 (13, 487). Other factors such as crowding, poor hygiene, and poverty are also associated with acute glomerulonephritis outbreaks. Recurrent epidemics in certain communities have also been reported (430), although it is rare that an individual has a second occurrence (487). The general absence of individual recurrences may be due to the presence of type-specific antibodies or antibodies against the nephritogenic factors. Long term prophylaxis with penicillin in patients following a poststreptococcal acute glomerulonephritis attack is not recommended (487). Unlike rheumatic fever, the outbreaks of acute glomerulonephritis have continued to decline and may be due to changes in the streptococci or the host (487). Regions of the world which still exhibit a high incidence of poststreptococcal acute glomerulonephritis include Africa, the Caribbean, South America, New Zealand, and Kuwait.
The nephritogenicity of group A streptococci appears to be related to specific M protein serotypes of S. pyogenes which cause acute glomerulonephritis, and certain strains within the serotype are nephritogenic. Thus, not all strains of the same M protein serotype are nephritogenic. Both pharyngeal and skin infection can lead to glomerulonephritis. However, the predominant M protein serotypes associated with pyoderma or skin infections and glomerulonephritis are M types 2, 49, 42, 56, 57, and 60, while M types 1, 4, 12, and 25 are associated with throat infections and glomerulonephritis (61, 487, 507). It is well documented that not all M type 12 strains are nephritogenic (486). The pyoderma or skin strains characteristically produce opacity factor or lipoproteinase, while in general the throat strains do not (559, 560). Clearly, there are differences between the throat and skin strains. Class I M protein serotypes, as defined by their reactivity with MAb 10B6, are the strains of S. pyogenes associated with pharyngitis and rheumatic fever, while the skin-pyoderma-nephritogenic strains do not react with MAb 10B6 (50). Furthermore, skin strains can be differentiated from pharyngitis strains by their chromosomal pattern (56).
Diagnosis.
The characteristics of poststreptococcal acute glomerulonephritis include edema, hypertension, hematuria, urinary sediment abnormalities, and decreased serum complement levels, with little fever (61). There is a latent period of 1 to 4 weeks (average, 10 days) between the streptococcal infection and development of acute glomerulonephritis, and antistreptococcal antibody titers for anti-DNase B or antihyaluronidase are elevated (63, 487). In glomerulonephritis following pyoderma or skin infection, the latency period may be 3 to 6 weeks and the ASO titers are generally low. After throat infection, the latency period may be 1 to 2 weeks and the ASO titers may be higher. Clinical manifestations include discolored or coffee-colored urine due to hematuria, edema of the face and extremities which has a sudden onset, and circulatory congestion due to renal impairment (487). Recurrent attacks of glomerulonephritis do not cause more severe disease, and in general there is no permanent damage to the kidney in children following the disease attack. However, according to Bisno, 1% or fewer children develop severe or irreversible renal failure, but in the adult population this may be a different scenario, with more acute glomerulonephritis patients developing chronic glomerulonephritis or hypertension (61). Silva describes pathological and clinical outcomes of postinfectious glomerulonephritis in an excellent review (487).
Potential mechanisms of pathogenesis.
The pathogenic events that lead to the development of poststreptococcal acute glomerulonephritis are related to an immunologic phenomenon involving immune complexes, nephritogenic streptococcal proteins, or both. A latency period, decrease in serum complement, and observed effects on the glomeruli suggest an immune-mediated event. Several mechanisms have been proposed, including immune complex deposition, reaction of antibodies cross-reactive with streptococcal and glomerular antigens, alteration of glomerular tissues by streptococcal products such as a proteinase or streptokinase, and direct complement activation by streptococcal components deposited in the glomeruli (487).
Circulating immune complexes.
Circulating immune complexes may play a role in development of disease. Elevated serum levels of IgG and IgM have been observed in 90% of patients with acute postinfectious glomerulonephritis, and 58% demonstrated circulating immune complexes, compared to 4% of normal individuals (487). In poststreptococcal acute glomerulonephritis, 66% have cryoglobulins (487), and the immune complexes appeared to contain streptococcal antigens (196). Immune complexes were also found in sera of patients with uncomplicated pharyngitis; therefore, it is possible that immune complexes reflect systemic inflammatory responses rather than glomerular damage (572).
Molecular mimicry.
Kefalides and colleagues demonstrated that sera from patients with poststreptococcal acute glomerulonephritis contained antibodies against laminin, collagen, and other macromolecules found in the glomerular basement membrane (295). In further studies, they found that the epitope recognized in collagen resides in the 7-S domain of type IV collagen (294). Previous evidence showed that streptococcal antigens, immunoglobulins, and complement are present in the kidney glomeruli in poststreptococcal glomerulonephritis (360). Early studies by Lange and Markowitz suggested that the glomerular basement membrane shared antigens with streptococcal M12 protein (347). Additional studies have suggested that antigens are shared between the group A streptococcus and glomeruli (61, 208, 309, 321). Kraus and Beachey identified a renal autoimmune epitope (Ile-Arg-Leu-Arg) in M protein (308). The association of nephritis with certain M protein serotypes suggested that the M protein could play a role in the disease. In animal models of nephritis induced by nephritogenic streptococci (M type 12), antiglomerular antibodies eluted from kidney glomeruli reacted with the type 12 streptococcal M protein (331). In addition, a monoclonal antibody against glomeruli reacted with the M12 protein (208). These studies support immune-mediated mechanisms in the pathogenesis of acute glomerulonephritis. Molecular mimicry between streptococcal and renal antigens may be important in the development of glomerulonephritis.
Streptococcal antigens and nephritis strain-associated proteins.
Evidence has suggested that streptococcal antigens other than M protein may be deposited in the kidney and result in deposits of immunoglobulin and/or complement. Serum IgG from patients with poststreptococcal acute glomerulonephritis reacted with glomeruli and mesangium taken from patients with early-stage glomerulonephritis (524). In addition, nephritogenic group A streptococci or membranes could absorb the glomerular reactivity from the serum. Lange (322, 323) and others (12) have suggested that free streptococcal antigen may deposit in glomeruli and attract circulating antistreptococcal antibody or complement components or both.
A component of the streptococcus, endostreptosin, was found to absorb antiglomerular antibody from sera of patients following poststreptococcal acute glomerulonephritis (487). In late stages of nephritis, endostreptosin is not detectable, but increased serum antiendostreptosin titers are diagnostic of poststreptococcal acute glomerulonephritis (481, 487). Endostreptosin is a protein of 40 to 50 kDa derived from the streptococcal cell cytoplasm. Endostreptosin was observed to deposit on glomerular basement membranes in rats (116). It also activated complement component C3 by the alternate pathway and was not related immunologically to other streptococcal exoenzymes or cell wall components (116).
Investigations by Zabriskie and coworkers have focused on the nephritogenic streptococci isolated from cases of acute glomerulonephritis. Streptococcal isolates from cases of nephritis were first noted to produce a protein not found in strains from patients without acute glomerulonephritis. At first the protein was called nephritis strain-associated protein (532). A number of years later, the nephritis strain-associated protein was found to be a plasmin-binding protein (429). The molecule had antigenic, biochemical, and structural similarity to group C streptokinase, but it was not related to the group A streptokinase (429). Upon amino acid sequence analysis, the 46,000-Da protein was identified as streptococcal pyrogenic exotoxin B precursor, also known as the streptococcal proteinase zymogen (429). It is possible that the protease activity or the superantigenic properties of this exotoxin/protease/plasmin-binding protein may play a role in the inflammatory changes seen in nephritis. Antibody titers to streptococcal pyrogenic exotoxin B were significantly elevated in acute poststreptococcal glomerulonephritis in comparison with acute rheumatic fever, scarlet fever, and normal individuals (117). Streptococcal pyrogenic exotoxin B (SpeB) was found in the glomeruli of 67% of glomerulonephritis biopsy specimens examined, while only 16% of normal biopsies were positive (117). If pyrogenic exotoxin B deposited in the glomeruli and activated the alternate complement pathway, this might explain the complement deposits in the kidney in poststreptococcal acute glomerulonephritis. Alternatively, the plasmin-binding activity could play a role by penetration of the pyrogenic exotoxin B-bound plasmin into the glomerular tissues, where excess plasmin could activate complement and produce inflammation in the glomeruli (117).
Although streptokinase has not been found in the glomeruli of poststreptococcal acute glomerulonephritis patients, there is evidence that it may play a role in this disease. Evidence indicated that nephritogenic streptokinases have unique domains which are attracted to renal tissues (387, 388). Molecular comparison analysis of the variable streptokinase domains demonstrated that streptokinases from nephritogenic strains had >95% homology, while <60% homology was observed for streptokinases from nonnephritogenic strains (269). Nine different polymorphic genotypes of streptokinase have been identified, and ska-1, ska-2, ska-6, and ska-9 have been associated with clinical and experimental acute poststreptococcal nephritis. Streptokinase was proposed to bind and activate plasminogen to plasmin, a potent protease, which then could activate the complement system and lead to glomerulonephritis (387).
Studies have been directed toward the determination of the role of streptokinase in acute glomerulonephritis. One study of glomerulonephritis strains in Ethiopian children did not associate certain genotypes with specific diseases such as acute glomerulonephritis (517). However, in other studies, deletion of the streptokinase gene from a type 49 strain eliminated the nephritogenic properties of the strain (244). Studies to investigate allelic variants of streptokinase demonstrated that a change from a nephritis-associated to a non-nephritis-associated allele abolished the capacity of the strain to produce nephritis in a mouse model of acute glomerulonephritis (386). Although streptokinase has not been found in biopsies of nephritis patients (117), it is possible that nephritis strains have more than one mechanism or complementary mechanisms not yet understood by which complement can be fixed in the glomerulus, leading to nephritis. It is also possible that undetectable amounts of streptokinase in glomeruli can have profound effects on complement activation and production of disease. In this regard, Holm and colleagues reported that a more sensitive method than immunofluorescence was required to detect streptokinase in glomeruli (387).
Stinson and colleagues also found that a number of surface proteins from nephritogenic streptococci, M12 serotype, were capable of binding to glomerular capillary walls in experiments in vitro (204). These data further support the possibility that multiple streptococcal antigens may react with sites in the glomeruli and bind antibody or activate complement in the pathogenesis of acute poststreptococcal glomerulonephritis.
Animal models of poststreptococcal acute glomerulonephritis.
In both humans and animal models of experimental glomerulonephritis, similar types of electron-dense deposits have been observed (487). Several studies have tested live streptococci, culture supernatants, or streptococcal proteins for induction of glomerulonephritis (37, 242, 270, 363). Glomerular inflammatory changes were reported in rabbits immunized with Fc receptor-positive group A streptococci (86), which induced high levels of anti-IgG antibodies and IgG deposits in the glomerulus, with glomerular changes. It was proposed that the anti-IgG deposits lead to activation of C3 (86).
One successful animal model is the tissue cage model of poststreptococcal acute glomerulonephritis, in which live organisms were grown in a tissue cage and the extracellular products from the bacteria were allowed to naturally diffuse into the body of the animal (242). The rabbit model appeared to be similar to poststreptococcal acute glomerulonephritis histologically and clinically. Strains which did not contain the nephritis-associated protein did not cause glomerulonephritis or glomerular deposits. Further studies by Holm and colleagues investigated the osmotic pump model, in which small amounts of the nephritis strain-associated protein were released slowly into the animal, with development of acute glomerulonephritis (243). A most recent report describes deposition of streptokinase and the C3 component of complement in the glomeruli of BALB/c mice implanted with tissue cages infected with the nephritogenic NZ131 and EF514 strains (387). However, no nephritis or deposition was observed when mice were infected with the NZ131 streptokinase deletion mutant or nonnephritogenic strain S84. These data continue to support a potential role for streptokinase in glomerulonephritis. It is possible that streptokinase may lead to early deposition of complement in the glomeruli.
Rheumatic Fever
Introduction.
Rheumatic fever is a delayed sequel to group A streptococcal pharyngitis. The disease manifests as an inflammation of the joints (arthritis), heart (carditis), central nervous system (chorea), skin (erythema marginatum), and/or subcutaneous nodules (505). These five major clinical manifestations, any of which may be seen in rheumatic fever, were established by the Jones criteria and revised by the American Heart Association (134, 273, 529). The disease is autoimmune in nature and most likely results in part from the production of autoreactive antibodies and T cells shown to cross-react with components of the group A streptococcus and host tissues. The medical importance of rheumatic fever is serious cardiac involvement, with myocarditis or valvulitis leading to death or valve replacement (505, 529). At one time in the United States, rheumatic fever was considered the most common cause of acquired heart disease in school-age children (351). Massell has published a most extraordinary historical account of rheumatic fever, from the earliest records of the disease to the most current research investigations (351).
Rheumatic fever is a major cause of acquired heart disease in children worldwide, with the disease occurring most frequently in underdeveloped countries where access to medical care is limited and children live in poverty and unsanitary, crowded conditions (506). In a recent review, Stollerman points out that inadequate prevention of streptococcal infection and deprivation of children in communities near Johannesburg, South Africa, and the aborigines of northern Australia have led to very high rates of rheumatic fever (≥20 cases per 1,000) compared to communities in the developed world with adequate access to medical care (0.2 to 0.5 case per 1,000) (506). Other regions cited for increased incidence of rheumatic fever were Hawaii, Sri Lanka, and Auckland, New Zealand (506). Ganguly, Kaplan, and colleagues have cited that the incidence of rheumatic heart disease worldwide ranges from 0.55 to 11 per 1,000 (293). A recent epidemiological survey in rural north India cited 210 cases of rheumatic heart disease per 100,000 school children aged 5 to 15 years (293). The epidemiological surveys leave little doubt that rheumatic fever is a world health problem. Outbreaks of rheumatic fever have also been observed in the United States since 1983, when Utah reported an outbreak associated with children from middle- to upper-income families with good access to medical care, but crowding was a factor associated with disease (530, 531). Mucoid M18 strains have been associated with the outbreak.
Although progress has been made in our understanding of rheumatic fever and its pathogenesis as an autoimmune disease, there is still much to be elucidated about the disease process. Disease susceptibility factors, including the major histocompatibility antigens and potential tissue-specific antigens, are under investigation as potential risk determinants in the disease. Autoantibodies which develop during streptococcal infection and in rheumatic fever are being investigated for their potential role in the disease, while it is evident that T lymphocytes play a key role in the pathogenesis of rheumatic carditis. Pathogenic epitopes of streptococcal and host antigens which cause autoimmune disease in animal models have been defined. This review will put these developments in perspective with the disease manifestations. Several other recent reviews on rheumatic fever have been written and describe past and present investigations of the clinical manifestations and pathogenesis of the disease (61, 119, 202, 351, 353, 502, 503, 506).
Association of group A streptococci with rheumatic fever.
S. pyogenes is the primary initiating factor in the development of acute rheumatic fever. Although a susceptible host and autoimmune responses are involved in development of the disease, the group A streptococcus plays a central role. The strongest evidence supporting the hypothesis that rheumatic fever is a result of a group A streptococcal throat infection is that rheumatic fever parallels the occurrence of streptococcal infections (351, 506, 507). In addition, elevated antistreptococcal antibodies accompany rheumatic fever, such as rises in ASO and anti-DNase B antibody titers (507, 520, 542). Antibodies against streptolysin O are now used to document antecedent streptococcal infection in cases of rheumatic fever. The risk of developing rheumatic fever has been related to the overall magnitude of the immune response against group A streptococcal infection (447, 448, 496).
Clinical features of rheumatic fever.
Symptoms of rheumatic fever can vary depending on the severity of the disease and the body systems involved. There is a latency period between the onset of streptococcal pharyngitis and the development of rheumatic fever. The latency period can range from 1 to 5 weeks after pharyngitis (62, 506, 507). This presumably allows the host time to develop an immune response, which subsequently attacks the tissues involved in the disease. The Jones criteria (134, 273) are a group of major and minor manifestations used in the diagnosis of rheumatic fever. The diagnosis requires the presence of either two major manifestations or one major and two minor manifestations. Major manifestations include carditis, arthritis, chorea, subcutaneous nodules, and erythema marginatum. Minor manifestations include fever, arthralgia, elevated sedimentation rate, elevated C-reactive protein, and elevated P-R interval. An elevated ASO titer, elevated anti-DNase B titer, or a positive throat culture for group A streptococci is considered supportive evidence of a group A streptococcal infection. Although carditis is the most serious major manifestation, migratory, polyarticular arthritis is the most common. The arthritis does not produce permanent damage, while injury to the heart valves can be serious enough for replacement of the damaged valve(s). The carditis presents as mitral or aortic regurgitation with a heart murmur upon auscultation or evidence by Doppler echocardiography (362). Myocarditis and pericarditis may be present. Permanent heart damage is related to the extent of the valvular involvement, but heart failure due to rheumatic fever is seen less frequently in the developed parts of the world than in the past, when it was a major cause of heart disease.
Other manifestations observed in rheumatic fever are seen less frequently and include subcutaneous nodules, Sydenham's chorea, and erythema marginatum (134). Sydenham's chorea is a neurological disorder causing involuntary movements, muscle weakness, and emotional disturbances. After puberty, chorea is seen only in females (507). Chorea can be the only major manifestation present in rheumatic fever, but in the presence of an elevated ASO or anti-DNase B titer, a diagnosis of rheumatic fever can be ascertained. Antibrain antibodies reactive with neurons of basal ganglia and other brain tissues have been reported in the sera of patients with Sydenham's chorea (82, 254). Experimental therapy by plasma exchange has been reported to be rapidly curative in patients with chorea (512).
Erythema marginatum is a distinct red circinate rash which is characteristic of rheumatic fever. However, it is rarely seen in the disease. Subcutaneous nodules occur over the surfaces of the joints and spine, and they are not unique to rheumatic fever, since they are seen in cases of rheumatoid arthritis and systemic lupus erythematosus (507). The minor manifestations are more general and not as unique as the major manifestations. The minor characteristics of rheumatic fever include fever, elevated erythrocyte sedimentation rate, elevated C-reactive protein, leukocytosis, prolonged P-R interval on electrocardiogram, and arthralgia. Supporting evidence of a recent streptococcal infection, either by serology or by culture, is required to make the diagnosis of acute rheumatic fever (134).
Host susceptibility.
Although there is a high prevalence of streptococcal pharyngitis in populations, only a small percentage of individuals develop acute rheumatic fever (508). Studies of families suggest that the disease is familial but that the genetic factor, characterized as autosomal recessive, has limited penetrance (94, 205, 516, 562). Therefore, it is believed that there is genetic susceptibility to rheumatic fever. However, twins do not usually both develop rheumatic fever, suggesting that environmental factors play a role in susceptibility to disease (516). Repeated exposure to streptococcal infections plays a central role in the development of rheumatic fever. Genes and environmental factors other than a group A streptococcal infection, which may play a role in the disease, remain virtually unknown.
The hypothesis that rheumatic fever requires a genetic predisposition is not surprising, since autoimmune and rheumatic diseases have long been associated with genetic susceptibility related to expression of a particular MHC antigen phenotype. Ayoub described a higher frequency of DR4 in Caucasian patients with rheumatic fever and a higher frequency of DR2 in African-American populations with rheumatic fever (18). However, the DR4-DR2 associations were not found when HLA typing was performed with molecular techniques (4). In South African populations with rheumatic fever, HLA DR1 and DRw6 were frequently associated with the disease (340). The association of different HLA phenotypes with different ethnic groups suggested that the MHC class I and MHC class II associations in rheumatic fever were complex and conflicting. This confusion may be due in part to the fact that in the majority of these studies analysis was performed by serological methods and not by more current molecular methods for detection of the MHC class II type. In addition, previous studies of HLA associations included a heterogeneous group of patients with and without heart disease, which may have led to inconsistent or inconclusive results. Guedez and Kotb have recently reevaluated MHC class II associations with rheumatic fever and rheumatic heart disease (215). They grouped the patients into a more defined group which had only mitral valve disease, such as mitral regurgitation and mitral stenosis. A group of Egyptian patients with established mitral valve disease were evaluated by molecular analysis of their HLA class II haplotype. Significant increases in the frequency of the DRB1*0701 and DQA1*0201 alleles and the DRB1*0701-DQA1*0201 and DRB1*13-DQA1*0501-3-DQB1*0301 haplotypes were found in the affected patients compared to ethnic controls. The data indicated that certain class II alleles and haplotypes were associated with rheumatic heart disease and that this association appeared to be stronger and more consistent when analyzed in patients with relatively more homogeneous clinical manifestations. The results from the study by Guedez, Kotb, and colleagues (215) suggested that DRB1*0701, DR6, and DQB1*0201 confer susceptibility to rheumatic fever and are in agreement with those reported for Turkish (407), Mexican (153), South African (340), and Japanese (307) rheumatic fever patients, in which the majority of cases (>50%) evaluated involved mitral valve disease.
Studies have also focused on identifying individuals who may be predisposed to acute rheumatic fever. Zabriskie and colleagues identified a non-HLA B-cell marker, known as 883 or D8/17, which identified 100% of rheumatic fever patients evaluated in the United States (New York and New Mexico) (299, 414). MAb D8/17 reacted with 33 to 40% of the B cells from patients with a history of acute rheumatic fever, whereas only low-level staining was observed in control groups (5 to 7% of B cells stained). The B-cell marker was elevated in patients from all ethnic groups, but only 66% of a north Indian study group were positive (293). Kaur and colleagues developed a new MAb against the B cells of the north Indian population with rheumatic fever. The MAb, PGI/MNII, reacted with samples from a larger number of north Indian rheumatic fever patients, suggesting that the B-cell marker D8/17 did not identify all of the north Indians with a history of rheumatic fever and that some differences in the B-cell marker may exist among ethnic populations. Individuals whose B cells reacted >10% with the MAb were considered positive. In these studies, 58 to 68% of individuals with acute rheumatic fever and chronic rheumatic heart disease, respectively, were positive with MAb D8/17. Fourteen percent of normal individuals were positive (293). In the north Indian population study, 86 and 94% with rheumatic fever and heart disease, respectively, were positive with MAb PGI/MN SII. Of the normal controls, only 8% were positive. Study of siblings in families with rheumatic fever or rheumatic heart disease revealed that 60% were positive (>10% staining positive) when tested with the PGI/MN SII MAb (293). It is possible that MAbs for different ethnic populations will be needed to detect B-cell markers or alloantigens associated with rheumatic fever in the majority of the population. Identification of individuals and populations at risk will be an important part of a program for control and elimination of rheumatic fever. Studies are currently in progress to identify candidate B-cell alloantigens associated with the disease.
Associated with antimyosin antibodies in acute rheumatic fever, the My1 idiotype was found to be elevated in acute rheumatic fever, acute glomerulonephritis, systemic lupus erythematosus, and Sjögren's syndrome (354) (Fig. 8). It was not elevated above normal levels in uncomplicated pharyngitis, IgA nephropathy, myocarditis, Chagas' disease, or rheumatoid arthritis. The data showed that the My1 idiotype was not present in heart failure and supported the findings of Zabriskie and colleagues (575), who showed that the antiheart antibodies in acute rheumatic fever were different from those in sera from postcardiotomy and heart failure patients. Idiotypes which may image the host-bacterial determinant hypothetically could act together with other genetic and predisposing factors in the host to initiate or exacerbate the disease. A description of human monoclonal antimyosin antibodies in rheumatic fever is found below in the section on autoantibodies that cross-react with streptococcal antigens.
FIG. 8.
Identification of an idiotype (My1) present on antibodies in sera from patients with acute rheumatic fever (ARF) and acute glomerulonephritis (AGN) as well as systemic lupus erythematosus (SLE) and Sjögren's syndrome (not shown). Sera from normal individuals (N) and patients with uncomplicated streptococcal disease (UC), heart failure (HF), Chagas' disease (not shown), and IgA nephropathy (not shown) are compared in the figure, and all have normal levels of the My1 idiotype. (Reprinted from reference 354 with permission from the publisher.)
Although little is known about target organ sensitivity in rheumatic fever, it is possible that rheumatic fever patients express unique determinants in the target tissue which predispose them to react with antibodies which may target inflammation to the particular sites affected in the disease, mainly the heart, joints, brain, and skin. In studies of mouse antimyosin antibodies, Diamond and colleagues present data in support of the hypothesis that genetic differences in mouse strains affected the deposition of mouse antimyosin antibodies in mice (328). Antimyosin antibodies deposited in DBA/2 mice but not in SCID or BALB/c mouse strains and resulted in myocarditis in the DBA/2 mice. Although host susceptibility has been investigated for many years, the exact genes which may predispose an individual to development of rheumatic fever remain unidentified. Like many autoimmune diseases, acute rheumatic fever is a multifactorial disease due to a combination of factors, including the susceptible host, group A streptococcal infection, and other environmental and host factors affecting the immune response.
Autoimmunity and Molecular Mimicry in Rheumatic Fever
Autoantibodies cross-reactive with streptococcal antigens.
Autoantibodies against the heart were associated with acute rheumatic fever in 1945 by Cavelti (93). In 1969 Kaplan and Frengley demonstrated antiheart antibodies in acute rheumatic fever sera using immunofluorescence techniques (278). These findings were supported and confirmed by Zabriskie and colleagues in 1970 (577). Antibody and complement were reported to be deposited in the hearts of patients with acute rheumatic heart disease (277). Antiheart antibodies persisted in patients with rheumatic recurrences but declined by 5 years after the initial rheumatic episode. Zabriskie suggested that repeated episodes of streptococcal infections were important in development of acute rheumatic fever (575). These previous data supported the hypothesis that acute rheumatic fever has an autoimmune origin.
The first indications that antiheart antibodies reacted with group A streptococci were found by Kaplan and colleagues, who demonstrated that the antiheart antibodies could be absorbed from human sera by group A streptococci or their cell walls or membranes (276, 277, 281–283, 576). Sera from rheumatic fever patients and rabbit anti-group A streptococcal sera reacted with heart and skeletal muscle (575). The streptococcal cross-reactive antigen was reported by Kaplan and colleagues to be in the cell wall, while Zabriskie and colleagues reported the cross-reactive antigen in streptococcal membranes (575, 576). Beachey and Stollerman reported the non-type-specific M antigen, and Widdowson and Maxted reported the M-associated protein (MAP) as candidates for antigens associated with antibody cross-reactivity (31, 558, 561). Additional studies by van de Rijn and colleagues of the group A streptococcal membrane indicated that four peptides were purified from the membranes which reacted with antiheart antibody from acute rheumatic fever sera (528). However, the origins of the peptides and their sequences were not determined. Studies by Goldstein and colleagues suggested that the group A polysaccharide and the glycoproteins in heart valves containing N-acetylglucosamine were responsible for the antibody cross-reactivity between streptococci and heart tissues (206). Lyampert and colleagues in Russia also suggested that the group A streptococcal polysaccharide induced responses against host tissues (338, 339). Sandson suggested that the hyaluronic acid capsule might also play a role in antibody cross-reactivity with joint tissues (462). Although a wide array of studies in the 1960s and 1970s left little doubt that group A streptococci were associated with autoantibody responses against heart and other tissues, cross-reactivity was not understood due to the large number of antibodies present in human and animal sera.
In the 1980s, human and mouse MAbs against group A streptococci and heart tissue were produced (127, 131, 312). Strong immunofluorescent staining of heart tissue sections by the MAbs was reported to be similar to that seen for human and animal sera studied previously (Fig. 9). The cross-reactive MAbs identified myosin as the autoantigen in the heart (126, 312), and the MAbs have been shown to recognize streptococcal M protein as well as streptococcal membranes (22, 132, 137, 138, 139). In addition, the MAb reactivities have indicated that the streptococcal cross-reactive antigens were present in both the cell wall and cell membrane (21, 22, 127, 131, 132). The specificities of mouse cross-reactive MAbs are shown in Table 6, and the human cross-reactive antibodies are shown in Table 7. The important difference between the mouse and the human MAbs is that the human antimyosin-antistreptococcal MAbs all react with N-acetylglucosamine, the immunodominant epitope of the group A carbohydrate (1, 483). This is an important feature of the human antimyosin antibodies, since elevated and persistent levels of anti-group A carbohydrate antibodies were previously reported in cases of chronic rheumatic valvulitis (164). A few of the mouse antimyosin-antistreptococcal MAbs reacted with DNA and were antinuclear, which is not seen in the human MAbs (133, 173). Human sera from rheumatic fever in general do not contain antinuclear antibody. Mouse and human cross-reactive MAbs have been categorized into three major groups. Group 1 includes antibodies which recognize only α-helical coiled-coil molecules such as myosin, tropomyosin, and keratin; group 2 antibodies recognize myosin and DNA and are antinuclear; and group 3 antibodies recognize myosin and N-acetylglucosamine. Studies using affinity-purified human antimyosin antibodies from acute rheumatic fever sera have been important in verifying that the reactivities observed with the MAbs are observed in the rheumatic fever sera (129, 130, 140). Antimyosin antibodies affinity purified from acute rheumatic fever sera identified a cross-reactive epitope near the pepsin cleavage site in M5 and M6 proteins (128). The amino acid sequence of the epitope was identified as Gln-Lys-Ser-Lys-Gln. Proteolytic fragments and synthetic peptides of human cardiac myosin were used to identify sites of cross-reactivity in the myosin molecule (155). MAbs reacted with the heavy chain of either skeletal or cardiac myosins and were mapped to sites in the α-helical rod region of the heavy chain. Cross-reactivity with sites in cardiac myosin is particularly important, since cardiac myosin has been shown to induce myocarditis when administered to susceptible animals, whereas skeletal myosin does not (155, 438, 440).
FIG. 9.
Reactivity of antistreptococcal-antimyosin MAb with human myocardium in an immunofluorescence assay. (Reprinted from reference 173 with permission from the publisher. Copyright 1989. The American Association of Immunologists.)
TABLE 6.
Cross-reactivity of antistreptococcal mouse MAbsa
| MAb | Antigen specificityb |
|---|---|
| 6.5.1 | Actin, vimentin, keratin, GlcNac |
| 8.5.1 | Keratin, vimentin, GlcNac |
| 27.4.1 | Myosin, tropomyosin, keratin, M5, M6 |
| 36.2.2d | Myosin, tropomyosin, actin, keratin, laminin, PepM1, PepM5, PepM6 |
| 49.8.9 | Vimentin, actin, keratin, M5, M6, GlcNac |
| 54.2.8 | Myosin, tropomyosin, vimentin, DNAc, keratin, M5, M6 |
| 101.4.1 | Myosin, vimentin, actin, keratin, M5, M6, GlcNac |
| 112.2.2 | Myosin, tropomyosin, actin |
| 654.1.1 | Myosin, tropomyosin, DNA, M5, M6 |
Adapted from reference 119 with permission from the publisher.
GlcNac, N-acetyl-β-d-glucosamine.
DNA, antinuclear antibody
Cytotoxic for heart cells in 51Cr release assay
TABLE 7.
Human antistreptococcal MAb specificitiesa
| MAb | Antigen specificity |
|---|---|
| 10.2.3 | Myosin, keratin, vimentin, actin, M5, M6, GlcNacb |
| 1.C8 | Myosin, vimentin, keratin, GlcNac |
| 1.H9c | Myosin, keratin, GlcNac |
| 4.F2 | Myosin, keratin, GlcNac |
| 5.G7d | Heat-aggregated IgG, myosin, keratin, GlcNac |
| 5.G3 | Keratin, GlcNac |
| 1.C3 | Laminin, keratin, GlcNac |
| 9.B12 | Keratin, GlcNac |
| 2.H11 | Keratin, GlcNac |
| 3.B6c | Myosin, tropomyosin, vimentin, actin |
Adapted from reference 119 with permission from the publisher.
GlcNac, N-acetylglucosamine.
Cytotoxic MAbs.
Rheumatoid factor.
Polyspecific autoantibodies which recognize more than one antigen have emerged as a theme in autoimmunity, cross-reactivity, and molecular mimicry (2, 92, 132, 316). Tables 6 and 7 illustrate the polyspecificity of the cross-reactive antistreptococcal-antimyosin MAbs. Since their discovery, the V-D-J region genes have been sequenced, but there is no consensus sequence to explain the molecular basis of the polyspecificity and cross-reactivity. However, as described in our reports, the V genes of cross-reactive MAbs from rheumatic fever sera were encoded by a heterogeneous group of VH3 family genes (VH-3, VH-8, VH-23, and VH-30) and a VH4-59 gene segment (1). Young and colleagues have also sequenced a similar group of V genes (570). Many sequences were either in germ line configuration or were highly homologous with a germ line sequence. In studies of cross-reactive antibody specificity, aromatic and hydrophobic interactions appeared to play an important role in reactivity with peptides which mimicked N-acetylglucosamine (484). It has been proposed that a germ line antibody may be polyreactive due to conformational rearrangement and configurational change, permitting binding of diverse molecules such as carbohydrates and peptides (545).
Molecular mimicry and M protein.
Molecular mimicry is defined as the sharing of epitopes between antigens, which in this case are the host and streptococcal bacteria. Three types of mimicry have been defined between antigens, including the sharing of identical amino acid sequences, homologous but nonidentical amino acid sequences, and epitopes on dissimilar molecules such as peptides and carbohydrates (483–485) or between DNA and peptides (133, 437). Shikhman has recently shown that some of the antistreptococcal-antimyosin mouse and human MAbs recognized cytoskeletal proteins and the epitope of the group A carbohydrate N-acetylglucosamine. Antibodies which recognized N-acetylglucosamine also recognized peptides which could bind lectins and induce an immune response against N-acetylglucosamine (460, 483–485).
Of the streptococcal antigens which are involved in molecular mimicry between the host and streptococcus, the M protein antigen has been the best studied and characterized. The M protein sequence was identified by Manjula and Fischetti as an alpha-helical heptad repeating structure which resembled other α-helical proteins, such as tropomyosin and the desmin-keratin family of molecules (343, 346). At the same time, the crossreactive MAbs described in Tables 6 and 7 identified the α-helical coiled-coil proteins cardiac and skeletal myosin, tropomyosin, vimentin, laminin, and keratin as host antigens that cross-react with M protein, as well as retinal S antigen and DNA (119, 121, 122, 126, 128, 130, 132, 173). Microbial antigens immunologically similar to M protein include coxsackievirus capsid proteins, mycobacterial heat shock protein HSP-65, and streptococcal group A carbohydrate N-acetylglucosamine (119).
In addition to the myosin cross-reactive sequence (Gln-Lys-Ser-Lys-Gln) near the pepsin cleavage site in the M5 and M6 proteins (128), other myosin cross-reactive sites were identified by Dale and Beachey in M5 (139, 140) and M19 (80). Using overlapping synthetic peptides of M5 protein, myosin cross-reactive B-cell epitopes were identified in peptides from the A, B, and C repeat regions of M5 protein (123). Investigation of MAb 10B6, an anti-M protein MAb which recognized the class I epitope of M proteins, revealed that it reacted with M5 peptides containing the class I epitope and with cardiac and skeletal myosins (440). The class I epitope shared homology with both skeletal and cardiac myosins, but peptides containing the class I epitope did not cause any tissue inflammation in animal models, as described below (440).
M proteins were also found to share homology and immunological cross-reactivity with a number of other strong bacterial antigens. We have postulated that one purpose of antibody cross-reactivity is to protect the host against multiple pathogens (120, 122). An antibody molecule that can neutralize several different infectious agents is an important first line of defense. Supporting evidence is the neutralization of enteroviruses by antistreptococcal-antimyosin antibodies (120, 122). A site in a coxsackievirus VPI protein shared homology and cross-reactivity with M protein. Coxsackieviruses are involved in the induction of myocarditis in a susceptible host (251, 252). Another bacterial antigen which shares epitopes with M proteins is heat shock protein Hsp-65, which plays a role in the development of arthritis and diabetes (439). Shared epitopes among pathogens may be important in triggering autoimmune diseases.
The M protein is not the only group A streptococcal antigen associated with immunological cross-reactivity with the MAbs or affinity-purified antimyosin antibodies from rheumatic fever sera. Other group A streptococcal antigens which cross-reacted with myosin include a 60-kDa actin-like protein (20), a 60-kDa protein associated with M-negative group A streptococci (21, 22), and a 67-kDa protein which was cloned using rheumatic fever sera (300). The 67-kDa protein contained sequence homology with class II HLA antigens such as DR, DP, and DQ in humans (300). Whether rheumatic fever patients produce antibodies to class II HLA antigens and whether the 67-kDa protein plays a role in pathogenesis remain to be determined. Interestingly, the gene for the 67-kDa protein was present only in group A, C, and G streptococci (300).
Potential pathogenic mechanisms of cross-reactive antibodies in rheumatic fever.
The role of cross-reactive and polyspecific antibodies in the pathogenesis of rheumatic fever is not clear. However, I and my colleagues have reported two MAbs, human MAb 1.H9 and mouse MAb 36.2.2, which were cytotoxic for heart cells in culture in the presence of complement (1, 122). The cytotoxic antibodies recognize epitopes on the surface of heart cells (Fig. 10), and MAb 36.2.2 was shown to recognize laminin, an extracellular matrix α-helical coiled-coil protein surrounding heart cells and also present in the valve (217, 218). Another human MAb, 3B.6, from a rheumatic carditis patient, which reacted with valvular endothelium, myocardium, and myosin, was cytotoxic for human umbilical cord endothelial cells in vitro in the presence of complement and reacted with laminin (198). Pathogenic cross-reactive antibody may be limited to a few antibody idiotypes which recognize the extracellular matrix in myocardium or at the valvular surface endothelium. Extracellular matrix may act like a sieve to trap antibodies in tissues and initiate inflammation. Diamond and colleagues reported that antimyosin MAbs deposited in the extracellular matrix of myocardium in susceptible DBA/2 mice which developed myocarditis (328). These studies provide evidence that antistreptococcal-antimyosin antibodies could be potentially damaging to cells or tissues.
FIG. 10.
Reactivity of antistreptococcal-antimyosin MAb 36.2.2 with the surface or extracellular matrix of rat myocardial cells in culture. MAb 36.2.2 exhibits cytotoxicity against rat heart cells in the presence of complement. (Reprinted from reference 14 with permission from the publisher. Copyright 1997. The American Association of Immunologists.)
Autoreactive T cells in rheumatic fever.
Historically, previous studies have indicated that lymphocytes were highly responsive to streptococcal cell wall and membrane antigens (450–452). T-cell responses to M proteins have been investigated in a number of studies (138, 216, 435, 436, 455, 456, 521–523). Cytotoxic T lymphocytes were reported to be stimulated by M protein, and cytotoxic lymphocytes were reported in the blood of patients with acute rheumatic fever (138, 257). Studies have suggested that PepM protein is a superantigen which acts to stimulate Vβ 2, 4, and 8 T-cell subsets (521–523, 537). Researchers in other laboratories report that neither recombinant nor native forms of M proteins are superantigenic (154, 186, 465). A superantigenic site reported for the M5 protein is localized to the amino acid sequence located in M5 residues 157 to 197 (KEQENKETIGTLKKILDETVKDKLAKEQKSKQNIGALKQEL) in the B3 repeat of the M molecule (537). This site has a significant degree of sequence homology with other superantigens.
Most of the studies on T-cell epitopes studied in rheumatic fever and in animal models focus on the streptococcal M5 protein molecule, because for many years M5 has been a serotype associated with acute rheumatic fever outbreaks (61). T- and B-cell epitopes of the M5 protein were defined in previous studies by Robinson and colleagues (455, 456), by Good and colleagues (435, 436), and in my own laboratory (123). The T-cell epitopes defined have been summarized previously (123). Kalil and colleagues recently reported that T cells isolated from the valves of rheumatic fever patients previously infected with M5 group A streptococci were responsive to several M5 peptides and heart-extracted proteins. Although three peptides from the A and B repeat regions of M5 protein stimulated the valvular T cells, peptides from the C repeat region were not tested. B- and T-cell epitopes of M5 protein which cross-reacted with myosin were mapped using 23 overlapping synthetic peptides (18-mers) of the A, B, and C repeat regions of the M5 protein (123). Of note, there were six dominant myosin cross-reactive sites throughout the M5 molecule which consistently stimulated T cells from mice sensitized with human cardiac myosin (123). Table 8 summarizes the dominant myosin cross-reactive T-cell epitopes of M5 protein in BALB/c mice (123), the M5 sequences recognized by T-cell clones from rheumatic heart valves (216), and M5 peptides reported by Good and colleagues to stimulate human T cells from normal and rheumatic fever subjects which were cross-reacted with myosin peptides (435, 436). An important correlation seen in Table 8 is that M5 peptides NT5, B1B2, and B2, dominant cross-reactive T-cell epitopes in the BALB/c mouse, contained sequences that were similar to those recognized by T cells from rheumatic valves (216). Peptide NT5 produced inflammatory infiltrates in the myocardia of BALB/c mice (123). The collective evidence suggested that amino acid sequences in M5 protein which share homology with cardiac myosin may break tolerance and promote T-cell-mediated inflammatory heart disease in animals and humans (123).
TABLE 8.
Summary of myosin and heart cross-reactive T-cell epitopes of streptococcal M5 proteina
| Peptide (amino acids) | Sequenceb | Origin of T-cell clone or response (reference)c |
|---|---|---|
| 1–25 | TVTRGTISDPQRAKEALDKYELENH | ARF/valve (216) |
| 81–96 | DKLKQQRDTLSTQKETLEREVQN | ARF/valve (216) |
| 163–177 | ETIGTLKKILDETVK | ARF/valve (216) |
| 337–356 | LRRDLDASREAKKQVEKAL | Normal/PBL (435) |
| 347–366 | AKKQVEKALEEANSKLAALE | Mice (436)/normal PBL (435) |
| 397–416 | LKEQLAKQAEELAKLRAGKA | ARF/PBL (435) |
| NT4 (40–58) | GLKTENEGLKTENEGLKTE | BALB/c lymph noded |
| NT5 (59–76) | KKEHEAENDKLKQQRDTL | BALB/c lymph noded |
| B1B2 (137–154) | VKDKIAKEQENKETIGTL | BALB/c lymph noded |
| B2 (150–167) | TIGTLKKILDETVKDKIA | BALB/c lymph noded |
| C2A (254–271) | EASRKGLRRDLDASREAK | BALB/c lymph noded |
| C3 (293–308) | KGLRRDLDASREAKKQ | BALB/c lymph noded |
Reprinted from reference 123 with permission from the publisher.
The amino-terminal TVTRGTIS sequence was taken from the M5 amino acid sequence published by Manjula et al. (342) and deviates from the M5 sequence published by Miller et al. (361) at positions 1 and 8. Other sequences (81–96 and 163–177) were taken from the PepM5 sequence reported by Manjula (342). These two sequences are found in the sequence as 67 to 89 and 174 to 188, respectively, as reported by Miller et al. (361). All other sequences shown are from the M5 gene sequence reported by Miller et al. (361). Underlined sequences are those shared among the epitopes or sequences compared in Table 2.
PBL, peripheral blood lymphocytes; ARF, acute rheumatic fever.
BALB/c mice were immunized with purified human cardiac myosin, and the recovered lymph node lymphocytes were stimulated with each of the peptides in tritiated-thymidine uptake assays (123).
Animal models of carditis.
Animal models for the study of rheumatic fever are limited because humans are the host and reservoir of group A streptococci. One of the complications of developing a model of infection which leads to rheumatic fever symptoms is that animals are not easily infected and once infected do not maintain an infection for a lengthy period of time. Therefore, most animal models which have been reported have relied on immunization of rabbits, mice, rats, and monkeys (114, 115, 166, 191, 192, 473–476, 548). A review by Unny and Middlebrook on rheumatic carditis described many early studies of rheumatic fever in animals, noting the streptococcal antigen used, the type of immunization, and the animal tested (525). Most of the preparations used in the animal studies were whole streptococci or crude streptococcal preparations. The outcomes of many of the experiments were equivocal. Murphy and Swift reported focal heart lesions in rabbits (371–374). Schwab and colleagues studied group A streptococcal cell walls and peptidoglycan-polysaccharide complexes in mice and rats which developed carditis, arthritis, or uveiitis (114, 115, 166, 191, 192, 213, 253, 295, 473, 474–476, 548). None of the models previously investigated have been used to adequately study the mechanisms of pathogenesis in rheumatic fever. Antibody, cell-mediated immunity, and histopathology in the tissues have been reported. Schwab and colleagues suggested that the peptidoglycan-polysaccharide persisted in the tissues of animals and acted as a chronic stimulus for continued tissue injury and immune complex deposition.
Deposition of antimyosin antibody in the hearts of DBA/2 mice but not BALB/c or SCID mice may be a model of antibody-mediated heart inflammation which may be applicable to rheumatic heart disease (328). The DBA/2 mice developed immunoglobulin deposits in the myocardium with subsequent histopathological myocarditis. It was postulated that the DBA/2 mice have a target organ sensitivity not seen in the other mouse strains. It is not known if this model is applicable to rheumatic fever or if there is a target organ sensitivity in rheumatic carditis.
Recent studies have focused on identification of animal models and peptides of the streptococcal M protein which may elicit rheumatoid lesions in mice and rats. These studies have been performed in the anticipation of identifying an animal model which through immunization procedures could be used to study rheumatic fever or rheumatic heart disease. The first of these studies identified an amino acid sequence, GLKTENEGLKTENEGLKTE (NT4 peptide), in M5 protein which shared similarities with cardiac myosin and produced myocarditis in MRL+/+ mice (250). The carditis produced by immunization with 30 μg of NT4 peptide was shown to be caused by CD4+ T lymphocytes, since anti-CD4 antibody administered to the animals prevented NT4-induced disease. Anti-IAk antibody also prevented disease, whereas anti-IEk antibody did not, demonstrating an MHC association of the I-Ak class II molecule with disease. Further studies demonstrated that administration of the NT4 peptide conjugated to syngeneic splenocytes made the mice tolerant of coxsackievirus-induced myocarditis (250). Epitopes in the M protein have been shown to share immunological cross-reactivity and similarity with the coxsackievirus proteins VP1, VP2, VP3, and VP4 (122). Coxsackieviruses are one of the causes of autoimmune myocarditis in humans and animals (569). The importance of the finding is that an epitope from a streptococcal protein produced myocarditis which was abrogated with anti-CD4 and anti-MHC class II antibody. The model has implications for rheumatic heart disease in that mimicry of a cryptic self-epitope by a foreign antigen associated with a pathogen may break tolerance and cause autoimmune manifestations in the susceptible host (250). A second important point is that use of the NT4 peptide as immunotherapy was successful in preventing autoimmune heart disease. Immunotherapies or vaccines against autoimmune diseases such as rheumatic fever and myocarditis may be important in future considerations of therapy and prevention.
Myocarditis was observed in BALB/c mice immunized with peptides of M5 protein from the A and B repeat regions. The peptides NT4, NT5, NT6, B1A, and B3A elicited cellular infiltrates in the myocardium, as previously described (123). The repeated regions involved in the A and B repeats contained sequence homology with cardiac myosin, a known autoantigen in myocarditis (Fig. 11). Peptides from the C repeat region shared homology with both skeletal and cardiac myosins and did not elicit an inflammatory reaction in the myocardium (123). The data are consistent with the hypothesis that only cardiac myosins and not skeletal myosin induce inflammatory heart disease (385). The hypothesis in rheumatic heart disease is that unique sequences in M proteins break immune tolerance to pathogenic epitopes in human cardiac myosin and lead to an autoimmune-mediated pathogenesis in rheumatic fever and rheumatic carditis. Although animal models support this hypothesis, multiple factors must be considered in an animal model of a human disease.
FIG. 11.
(A) Homology between human cardiac myosin residues 1313 to 1329 and streptococcal M5 protein peptide B2 (M5 residues 150 to 167). Identity (47%) was observed in a 17-amino-acid overlap. The M5 peptides B2, B1B2, and B3A contain large amounts of overlapping sequence. :, identical residues; ., conserved substitutions. Three of the residues shown in panel A in the myosin sequence are unique to human cardiac myosin. (B) Amino acid sequence identity (LKTEN) between M5 peptide NT4 and human cardiac myosin (LQTEN). NT4 contains residues 40 to 58 of the M5 protein. The cardiac myosin sequence shown is found in residues 1279 to 1286 near the beginning of the light meromyosin tail and the end of the S-2 fragment of myosin. The myosin sequence shown in panel B is conserved among cardiac myosins. (C) Amino acid sequence identity between serotype M6 protein and human cardiac myosin within the same regions. The repeat in the M6 protein is LTTEN, which is repeated five times in the N-terminal region of the M6 protein. In M5 and M6 proteins, the LKTEN and LTTEN sequences, respectively, are conserved and are similar to the LQTEN sequence in cardiac myosins. (Reprinted from reference 123 with permission from the publisher.)
Streptococcal Reactive Arthritis
Streptococcal reactive arthritis is a nonpurulent arthritis which occurs following group A streptococcal infection but does not fit the Jones criteria for diagnosis of rheumatic fever (4). The arthritis is prolonged in streptococcal reactive arthritis for several weeks or months. Treatment of streptococcal reactive arthritis with aspirin or salicylates is not very effective, unlike rheumatic fever, which is usually treated with high-dose aspirin. Carditis due to mitral insufficiency has been reported in 5 to 6% of patients with streptococcal reactive arthritis (4). Penicillin prophylaxis is recommended in cases of carditis to prevent further attacks and further heart injury.
Streptococcal reactive arthritis appeared to be more similar to the reactive arthritides described for enteric pathogens than to rheumatic fever (4). Susceptibility to streptococcal reactive arthritis may be associated with certain major histocompatibility antigens or class II molecules expressed by patients developing this syndrome. Recent studies suggested that there was an increased frequency of the HLA-DRB1*01 allele in streptococcal reactive arthritis compared with normal individuals or patients with rheumatic fever (4). The presence of HLA-B27 alleles in individuals has been shown to be associated with the development of reactive arthritis caused by chlamydiae and enteric pathogens, but the HLA-B27 allele was not found more frequently in streptococcal reactive arthritis than in normal individuals (4). Rheumatic fever patients were shown to have an increased frequency of the HLA-DRB1*16 allele, not the HLA-DRB1*01 allele, which was increased in streptococcal reactive arthritis cases.
Tics and Other Brain Disorders
Recent studies have related group A streptococcal infections to the onset of obsessive-compulsive disorder and Tourette's syndrome (9, 514). A subgroup of tics and obsessive compulsive behavior in children following group A streptococcal infections has been defined as pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Patients with PANDAS expressed the D8/17 marker at the same rate that it was found to be present in rheumatic fever patients on DR+ cells in the peripheral blood (513). In that study, the rheumatic fever patients who were compared with the PANDAS group had Sydenham's chorea as a major manifestation. In both patient groups, 85 to 89% of the individuals were positive for D8/17, a marker of rheumatic fever on B cells, while only 17% of normal subjects were positive (513). Further studies confirmed the D8/17 positivity in a large percentage of PANDAS patients compared with controls (375). It was unclear if antineuronal antibodies play a role in the disease, which has similarities to Sydenham's chorea, a major manifestation in rheumatic fever. Chorea has the longest latency period following streptococcal pharyngitis and may appear with low antistreptococcal antibody titers, emotional disturbances, and involuntary movements. A long latency period before the detection of PANDAS has made the association with streptococcal infection more difficult to assess.
VACCINATION AND PREVENTION
Antibiotic Therapy and Prophylaxis
Since the 1940s, penicillin has been the treatment of choice for group A streptococcal infections (504). Furthermore, penicillin prophylaxis is still used to prevent recurrences of rheumatic fever (61). Early treatment of streptococcal infection with penicillin prevents an immune response against streptococcal antigens and concomitantly prevents the streptococcal sequelae rheumatic fever and glomerulonephritis.
Problems of treatment failures have been reported (279, 421) but were thought to be due to the coexistence of lactamase-producing bacteria in the tonsillopharynx or to streptococci which invade epithelial cells and were protected from the action of penicillin (383). Adjacent bacteria may degrade penicillin in the infected area and allow survival of the group A streptococci. Streptococci were eradicated in these situations by administering amoxicillin and clavulanate together (279). It is amazing that resistance to penicillin has not developed in group A streptococci, and they have remained exquisitely sensitive. The reason for the lack of penicillin resistance is not known. However, some strains have developed penicillin tolerance by inhibiting the bactericidal effects of the antibiotic (421). For penicillin-tolerant strains, the MBC of penicillin will be at least 32-fold higher than the MIC. Other choices for treatment of streptococcal pharyngitis include cephalosporins, erythromycin, and amoxicillin-clavulanate, but penicillin remains the drug of choice.
Vaccine Strategies
M proteins.
Most recent vaccine strategies have targeted either the type-specific N-terminal region of the M proteins (26, 28, 135, 142, 144, 145) or the highly conserved carboxy-terminal region of the M protein molecule (49, 54, 179, 359). Vaccination against the N-terminal type-specific region induced protective bactericidal and opsonic antibody against the specific M protein serotype, while vaccination against the conserved carboxyl-terminal region of the M protein protected against multiple serotypes and prevented colonization at mucosal surfaces. The studies described below suggest that parenteral or mucosal immunization effectively protects against infection. Investigators have worked over the past two decades to develop a safe, efficacious M protein vaccine to be used for immunization, especially against rheumatogenic serotypes. In addition, with the resurgence of serious streptococcal infections, it is prudent to pursue a group A streptococcal vaccine against serotypes that produce invasive disease.
The development of a vaccine has always been met with enthusiasm, but certain problems must be overcome. First, it should not exacerbate the rheumatic disease that the vaccine would be designed to prevent. M protein sites associated with tissue cross-reactivity or tissue infiltrates should be avoided, and selected sites should be thoroughly tested in animals. Second, the immune response should provide lasting protection. Third, because more than 80 different M serotypes cause infections, only a limited number of M protein serotypes are practical for a type-specific vaccine. In addition, it has been observed that M serotypes which cause infection are cyclic in populations and also that different M serotypes are responsible for rheumatic fever in different parts of the world (229, 275). Vaccines which targeted epitopes common to all M proteins also appeared to be effective against colonization. Common group A streptococcal antigens other than M protein have also been under investigation as vaccines for protection against colonization and infection.
In a 1989 review, Fischetti (179) discussed the development of M protein vaccines, and Bessen and Fischetti have most recently reviewed vaccines against group A streptococci (54). Many outstanding scientists have made contributions to our understanding of the M protein antigen since its discovery by Lancefield (315, 317–320). Historically, Massell vaccinated humans with M protein vaccines in 1968 using a partially purified M3 vaccine which had been HCl extracted from whole M3 streptococci (352). Vaccinees produced protective antistreptococcal antibodies. The vaccine was given to siblings in families with rheumatic fever. There were three episodes of rheumatic fever in the vaccinees, but it was not certain if the vaccine was related to the development of rheumatic fever (350). In the study, 3 of 21 vaccinees developed rheumatic fever following group A streptococcal infections occurring during the period of immunization. Only 5 of 447 controls who did not receive the vaccine developed acute rheumatic fever.
In the 1970s, the work on M protein vaccines began with the work of Fox and Wittner, who studied M protein vaccines and the immune response in mice (193, 567) and also in humans (137, 428). Fox and colleagues immunized 200 healthy adults and children with M types 1, 3, 6, 12, and 24. Both mucosal and parenteral vaccines were tested and demonstrated approximately 70% efficacy (137, 428). By 1979 Beachey and colleagues had taken advantage of the pepsin extraction method for M protein and had produced highly purified PepM24 (33). PepM24 contained the N-terminal half of the M protein molecule and was mixed with alum as an adjuvant and used to immunize a small group of 12 human volunteers. The volunteers developed opsonic antibody against the type 24 streptococcus and had no delayed-type hypersensitivity reaction, and no heart cross-reactive antibodies were observed by immunofluorescence tests of heart sections. Further studies by Beachey and colleagues were the first studies in humans with a synthetic M protein vaccine antigen (28).
One of the major problems of immunization with the streptococcal M protein as effective prevention of group A streptococcal infection is the more than 80 M protein serotypes. For effective protection against rheumatic fever, a combination of M protein serotypes from rheumatogenic strains would be required, because different M serotypes prevail in different regions of the world. In 1986, studies by Beachey and colleagues described opsonic antibodies against a hybrid peptide containing copies of the type 5 and type 24 M proteins synthesized in tandem (26). The peptide induced antibodies against both serotypes of M protein, suggesting that effective immunization with multivalent synthetic vaccines was possible. More recently, Dale has examined multivalent hybrid recombinant tetravalent and octavalent M protein vaccines (135, 142, 145). Four M protein serotypes were represented in the peptide, which contained the N terminus of M types 24, 5, 6, and 19 (142). The immune sera from rabbits immunized with the multivalent peptide proved to opsonize all four serotypes of group A streptococci. Recombinant vaccines containing as many as eight M type sequences have been shown to be effective at inducing opsonic, serotype-specific responses in rabbits (145).
To enhance immunogenicity, M protein sequences were conjugated to E. coli labile toxin B subunit (141). Intranasal immunization with a recombinant group A streptococcal M5 protein fragment (M5 residues 1 to 15) protected mice against intraperitoneal challenge with M type 5 streptococci. The emm-5 gene was introduced into Salmonella enterica serovar Typhimurium, which was effectively used to orally immunize BALB/c mice (427). The mice were protected from challenge with M type 5 streptococci but not heterologous serotypes.
Bessen and Fischetti demonstrated that infection and colonization were influenced by passive administration of M protein-specific IgA antibody (47, 48, 180). Affinity-purified salivary anti-M6 IgA was mixed with M6 streptococci prior to intranasal challenge. The type-specific IgA delayed and decreased the mortality rate from infection with M6 streptococcal organisms. When the N-terminal type-specific region was used as a vaccine, it delivered protection against the homologous serotype. The M type-specific IgG was opsonic, while M type-specific IgA was not opsonic (48).
For protection against multiple serotypes and prevention of colonization, mucosal immunity was shown to be effective by immunization with synthetic peptides corresponding to conserved epitopes found in the carboxy-terminal region of M protein (49). Vaccination of mice with conserved region M protein peptides (residues 216 to 235, 248 to 269, and 275 to 284) conjugated to cholera toxin B subunit protected the mice against colonization with group A streptococci (46). Although the peptides from the conserved region given intranasally did not induce an opsonic antibody response or protect against systemic infection, they reduced colonization at the nasopharyngeal mucosal surface (46). Studies by Bronze and colleagues have also demonstrated that mucosal immunity was enhanced by local administration of vaccines (79).
Fischetti and colleagues expressed the carboxy-terminal region of the M protein in vaccinia virus (182) and in Streptococcus gordonii (359, 432). The utilization of S. gordonii as a gram-positive oral commensal bacterium is a novel vaccine delivery system which expressed a portion of the emm-6 gene on its surface (432). The administration of mucosal vaccines containing the common carboxy-terminal region of M protein were shown to induce both IgA and IgG antibodies in the recipient animals and prevented colonization of the mucosa by homologous and heterologous serotypes (54). Exactly how antibody to the C repeat region epitopes prevented adherence is not known. Since protection against both systemic infection and local colonization must be achieved, it remains to be seen if mucosal immunity will be strong enough to prevent systemic disease.
Current potential M protein vaccines for human use have appeared to be free of deleterious epitopes that might generate high levels of heart-reactive antibody, cardiac inflammation, or rheumatic fever-like symptoms in vaccine recipients (54). Over the years, many volunteers have received and tolerated streptococcal vaccines relatively well, suggesting that immunization of humans against streptococcal infection is possible and potentially safe. Since streptococcal sequelae are autoimmune types of disorders, as described in this review, and molecular mimicry may potentially play a role in their pathogenesis, safety issues are a major consideration in the development and use of group A streptococcal vaccines in humans.
C5a peptidase.
The C5a peptidase is another promising vaccine candidate. Intranasal administration of a truncated form of the enzyme induced measurable salivary IgA and serum IgG responses in mice (264). Immunized mice were shown to eradicate streptococci from their throats more rapidly after intranasal challenge. The goal of a vaccine is to prevent the initiation of throat infections before the bacteria have an opportunity to become established on the mucosal epithelium. Since the C5a peptidase is antigenically stable and 95 to 98% identical among different serotypes, a vaccine that contains this protein would presumably produce protection against all serotypes. This prediction was confirmed in mice (264). Immunization with C5a peptidase from an M49 serotype reduced the capacity of serotype M1, M2, and M11 strains to persist on the mouse oral mucosa. The mechanism by which antibody directed against the peptidase increases the rate of clearance of streptococci is not fully understood. The most likely explanation is that antibody neutralizes protease activity, thereby preserving the C5a chemotactic gradient that surrounds the bacteria in human tissue. This in turn could intensify the local inflammatory response and the infiltration of mononuclear phagocytes. Alternatively, antibody directed against the C5a peptidase could activate the classical complement pathway, which will also nonspecifically magnify the inflammatory response and the influx of phagocytic cells. In addition, the peptidase is not likely to induce tissue-reactive antibodies, although experiments have not yet tested this hypothesis. Evidence supporting this prediction is based on the fact that group B streptococci express a C5a peptidase that is 98% identical in amino acid sequence to the C5a peptidase produced by group A streptococci (96), yet the group B streptococcus has not been reported to induce a tissue-cross-reactive immune response or rheumatic fever-like disease.
Other vaccine candidates.
Other candidates for future group A streptococcal vaccines include antigens such as the streptococcal proteinase (pyrogenic exotoxin B) (286) and group A carbohydrate-protein conjugates (461). Surface antigens expressed during infection or environmentally controlled in vivo may eventually become candidates for a multipotential vaccine. Vaccines which protect large numbers of individuals against streptococcal infection may consist of multiple streptococcal surface antigens and exotoxins for protection against invasion, colonization, and sequelae. Long-term immunity should be sought in the vaccines, and it will be a challenge to develop long-lasting immunity against group A streptococci at the mucosal surface. Human trials will be forthcoming in the next few years with each or a combination of these vaccines, which hopefully will lead to effective vaccination against group A streptococcal diseases and their sequelae.
ACKNOWLEDGMENTS
Deepest appreciation is expressed to all of my colleagues who helped make this review possible by sending their reprints and providing comments on portions of the review. Gratitude is also expressed to Debra Bessen, Yale University School of Medicine, for constructing Tables 2 and 3.
This work was supported by grants HL35280 and HL56267 from the National Heart, Lung and Blood Institute.
REFERENCES
- 1.Adderson E E, Shikhman A R, Ward K E, Cunningham M W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetyl-glucosamine/anti-myosin antibody V region genes. J Immunol. 1998;161:2020–2031. [PubMed] [Google Scholar]
- 2.Adrezejewski C, Jr, Rauch J, Stollar B D, Schwartz R S. Antigen binding diversity and idiotypic cross-reactions among hybridoma autoantibodies to DNA. J Immunol. 1980;126:226–231. [PubMed] [Google Scholar]
- 3.Ahmed S, Ayoub E M. Severe, invasive group A streptococcal disease and toxic shock. Pediatr Ann. 1998;27:287–292. doi: 10.3928/0090-4481-19980501-08. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Ayoub E M, Scornik J C, Wang C-Y, She J-X. Poststreptococcal reactive arthritis: clinical characteristics and association with HLA-DR alleles. Arthritis Rheum. 1998;41:1096–1102. doi: 10.1002/1529-0131(199806)41:6<1096::AID-ART17>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Akerstrom B, Brodin T, Reis K, Bjorck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985;135:2589–2592. [PubMed] [Google Scholar]
- 6.Akesson P, Cooney J, Kishimoto G, Bjorck L. Protein H—a novel IgG binding bacterial protein. Mol Immunol. 1990;27:523–531. doi: 10.1016/0161-5890(90)90071-7. [DOI] [PubMed] [Google Scholar]
- 7.Akesson P, Sjoholm A G, Bjorck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 8.Alberti S, Ashbaugh C D, Wessels M R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen A J, Leonard H L, Swedo S E. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1995;34:307–311. doi: 10.1097/00004583-199503000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Alouf J E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin) Pharmacol Ther. 1980;11:661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 11.Alouf J E, Knoll H, Kohler W. The family of mitogenic, shock-inducing and superantigenic toxins from staphylococci and streptococci. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. [Google Scholar]
- 12.Andres G A, Accinni L, Hsu K C, Zabriskie J B, Seegal B C. Electron microscopic studies of human glomerulonephritis with ferritin conjugated antibody. J Exp Med. 1966;123:399. doi: 10.1084/jem.123.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthony B F, Kaplan E L, Wannamaker L W, Briese F W, Chapman S S. Attack rates of acute nephritis after type-49 streptococcal infection of the skin and of the respiratory tract. J Clin Investig. 1969;48:1697. doi: 10.1172/JCI106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antone S M, Adderson E E, Mertens N M J, Cunningham M W. Molecular analysis of V gene sequences encoding cytotoxic anti-streptococcal/anti-myosin monoclonal antibody 36.2.2 that recognizes the heart cell surface protein laminin. J Immunol. 1997;159:5422–5430. [PubMed] [Google Scholar]
- 15.Ashbaugh C D, Alberti S, Wessels M R. Molecular analysis of the capsule gene region of group A streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol. 1998;180:4955–4959. doi: 10.1128/jb.180.18.4955-4959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayoub E M. Resurgence of rheumatic fever in the United States. Postgrad Med. 1992;92:133–142. doi: 10.1080/00325481.1992.11701445. [DOI] [PubMed] [Google Scholar]
- 18.Ayoub E M, Barrett D J, Maclaren N K, Krischer J P. Association of class II human histocompatibility leukocyte antigens with rheumatic fever. J Clin Investig. 1986;77:2019–2026. doi: 10.1172/JCI112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baddour L M, Christensen G D, Simpson W A, Beachey E H. Microbial adherence. In: Mandel G L, Douglas R G Jr, Bennett J E, editors. The principles and practices of infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 9–25. [Google Scholar]
- 20.Barnett L A, Cunningham M W. Evidence for actinlike proteins in an M protein-negative strain of Streptococcus pyogenes. Infect Immun. 1992;60:3932–3936. doi: 10.1128/iai.60.9.3932-3936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett L A, Cunningham M W. A new heart-cross-reactive antigen in Streptococcus pyogenes is not M protein. J Infect Dis. 1990;162:875–882. doi: 10.1093/infdis/162.4.875. [DOI] [PubMed] [Google Scholar]
- 22.Barnett L A, Ferretti J J, Cunningham M W. A 60-kDa acute rheumatic fever associated antigen of Streptococcus pyogenes. In G. Orefici (ed.), New perspectives on streptococci and streptococcal diseases. Proceedings of the XI Lancefield International Symposium. 1992. pp. 216–218. . Zentralbl. Bakteriol. Suppl. 22. Gustav-Fisher-Verlag, New York, N.Y. [Google Scholar]
- 23.Basma H, Norrby-Teglund A, Low D, McGeer, El-Ahmady O, Kotb M. Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect Immun. 1999;67:1871–1877. doi: 10.1128/iai.67.4.1871-1877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basma H, Norrby-Teglund A, McGeer A, Low D E, El-Ahmedy O, Dale J B, Schwartz B, Kotb M. Opsonic antibodies to the surface M protein of group A streptococci in pooled normal immunoglobulins (IVIG): potential impact on the clinical efficacy of IVIG therapy for severe invasive group A streptococcal infections. Infect Immun. 1998;66:2279–2283. doi: 10.1128/iai.66.5.2279-2283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beachey E H, Campbell G L, Ofek I. Peptide digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974;9:891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beachey E H, Gras-Masse H, Tarter A, Jolivet M, Audibert F, Chedid L, Seyer J M. Opsonic antibodies evoked by hybrid peptide copies of types 5 and 24 streptococcal M proteins synthesized in tandem. J Exp Med. 1986;163:1451–1458. doi: 10.1084/jem.163.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beachey E H, Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976;143:759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beachey E H, Seyer J M, Dale J B, Simpson W A, Kang A H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature. 1981;292:457–459. doi: 10.1038/292457a0. [DOI] [PubMed] [Google Scholar]
- 29.Beachey E H, Seyer J M, Kang A H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980;255:6284–6289. [PubMed] [Google Scholar]
- 30.Beachey E H, Seyer J M, Kang A H. Repeating covalent structure of streptococcal M protein. Proc Natl Acad Sci USA. 1978;75:3163–3167. doi: 10.1073/pnas.75.7.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beachey E H, Stollerman G H. Mediation of cytotoxic effects of streptococcal M protein by non-type-specific antibody in human sera. J Clin Investig. 1973;52:2563–2570. doi: 10.1172/JCI107448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beachey E H, Stollerman G H, Chiang E Y, Chiang T M, Seyer J M, Kang A H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977;145:1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beachey E H, Stollerman G H, Johnson R H, Ofek I, Bisno A L. Human immune response to immunization with a structurally defined polypeptide fragment of streptococcal M protein. J Exp Med. 1979;150:862–877. doi: 10.1084/jem.150.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beall B, Facklam R, Hoenes T, Schwartz B. Survey of emm gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J Clin Microbiol. 1997;35:1231–1235. doi: 10.1128/jcm.35.5.1231-1235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beall B, Facklam R R, Elliott A, Franklin A R, Hoenes T, Jackson D, LaClaire L, Thompson T, Viswanathan R. Streptococcal emm types associated with T-agglutination types and the use of conserved emm gene restriction fragment patterns for subtyping group A streptococci. J Med Microbiol. 1998;47:893–898. doi: 10.1099/00222615-47-10-893. [DOI] [PubMed] [Google Scholar]
- 37.Becker C G, Murphy G E. The experimental induction of glomerulonephritis like that in man by infection with group A streptococci. J Exp Med. 1968;127:1–24. doi: 10.1084/jem.127.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Nasr A, Herwald H, Muller-Esterl W, Bjorck L. Human kininogens interact with M protein, abacterial surface protein and virulence determinant. Biochem J. 1995;305:173–180. doi: 10.1042/bj3050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Nasr A, Herwald H, Sjobring U, Renne T, Muller-Esterl W, Bjorck L. Absorption of kininogen from human plasma by Streptococcus pyogenes is followed by release of bradykinin. Biochem J. 1997;326:657–660. [PMC free article] [PubMed] [Google Scholar]
- 40.Berge A, Sjobring U. PAM, a novel plaminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;34:25417–25424. [PubMed] [Google Scholar]
- 41.Berkower C, Ravins M, Moses A E, Hanski E. Expression of different group A streptococcal M proteins in an isogenic background demonstrates diversity in adherence to and invasion of eukaryotic cells. Mol Microbiol. 1999;31:1463–1475. doi: 10.1046/j.1365-2958.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 42.Bernish B, van de Rijn I. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem. 1999;274:4786–4793. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- 43.Besdine R W, Pine L. Preparation and description of high-molecular-weight soluble surface antigens of a group A streptococcus. J Bacteriol. 1968;96:1953–1958. doi: 10.1128/jb.96.6.1953-1960.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bessen D, Beall B, Hollingshead S. ASM conference on streptococcal genetics: genetics of the streptococci, enterococci, and lactococci. Washington, D.C.: American Society for Microbiology; 1998. emm genes and tissue tropisms of group A streptococci; pp. 19–20. [Google Scholar]
- 45.Bessen D, Fischetti V A. A human IgG receptor of group A streptococci is associated with tissue site of infection and streptococcal class. J Infect Dis. 1990;161:747–754. doi: 10.1093/infdis/161.4.747. [DOI] [PubMed] [Google Scholar]
- 46.Bessen D, Fischetti V A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessen D, Fischetti V A. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988;167:1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bessen D, Fischetti V A. Role of non-opsonic antibody in protection against group A streptococcal infection. In: Lasky L, editor. Technology advances in vaccine development. New York, N.Y: Alan R. Liss; 1988. pp. 493–502. [Google Scholar]
- 49.Bessen D, Fischetti V A. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990;145:1251–1256. [PubMed] [Google Scholar]
- 50.Bessen D, Jones K F, Fischetti V A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989;169:269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bessen D E. Localization of immunoglobulin A-binding sites within M or M-like proteins of group A streptococci. Infect Immun. 1994;62:1968–1974. doi: 10.1128/iai.62.5.1968-1974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bessen D E, Fischetti V A. Differentiation between two biologically distinct classes of group A streptococci by limited substitutions of amino acids within the shared region of M protein-like molecules. J Exp Med. 1990;172:1757–1764. doi: 10.1084/jem.172.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bessen D E, Fischetti V A. Nucleotide sequences of two adjacent M and M-like protein genes of group A streptococci: different RNA transcript levels and identification of a unique immunoglobulin A-binding protein. Infect Immun. 1992;60:124–135. doi: 10.1128/iai.60.1.124-135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bessen D E, Fischetti V A. Vaccines against Streptococcus pyogenes infections. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 783–802. [Google Scholar]
- 55.Bessen D E, Izzo M W, McCabe E J, Sotir C M. Two-domain motif for IgG-binding activity by group A streptococcal emm gene products. Gene. 1997;196:75–82. doi: 10.1016/s0378-1119(97)00201-1. [DOI] [PubMed] [Google Scholar]
- 56.Bessen D E, Sotir C M, Readdy T L, Hollingshead S K. Genetic correlates of throat and skin isolates of group A streptococci. J Infect Dis. 1996;173:896–900. doi: 10.1093/infdis/173.4.896. [DOI] [PubMed] [Google Scholar]
- 57.Bessen D E, Veasy L G, Hill H R, Augustine N H, Fischetti V A. Serologic evidence for a class I group A streptococcal infection among rheumatic fever patients. J Infect Dis. 1995;172:1608–1611. doi: 10.1093/infdis/172.6.1608. [DOI] [PubMed] [Google Scholar]
- 58.Bisno A, Craven D E, McCabe W R. M proteins of group G streptococci isolated from bacteremic human infection. Infect Immun. 1987;55:753–757. doi: 10.1128/iai.55.3.753-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisno A L. Acute rheumatic fever: a present day perspective, classics in medicine. Medicine. 1993;72:278–283. [Google Scholar]
- 60.Bisno A L. The concept of rheumatogenic and non-rheumatogenic group A streptococci. In: Read S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press Inc.; 1980. pp. 789–803. [Google Scholar]
- 61.Bisno A L. Non-suppurative poststreptococcal sequelae: rheumatic fever and glomerulonephritis. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Vol. 2. New York, N.Y: Churchill Livingstone; 1995. pp. 1799–1810. [Google Scholar]
- 62.Bisno A L. Streptococcus pyogenes. In: Mandell G L, Bennett R G, Dolin R, editors. Principles and practice of infectious diseases. Vol. 2. New York, N.Y: Churchill Livingstone; 1995. pp. 1786–1799. [Google Scholar]
- 63.Bisno A L, Ofek I. Serologic diagnosis of streptococcus infection. Comparison of a rapid hemmagglutination technique with conventional antibody tests. Am J Dis Child. 1974;127:676–681. doi: 10.1001/archpedi.1974.02110240062007. [DOI] [PubMed] [Google Scholar]
- 64.Bisno A L, Pearce I A, Wall H P, Stollerman G S. Contrasting epidemiology of acute rheumatic fever and acute glomerulonephritis: nature of the antecedent streptococcal infection. N Engl J Med. 1970;283:561–565. doi: 10.1056/NEJM197009102831103. [DOI] [PubMed] [Google Scholar]
- 65.Bisno A L, Stevens D L. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 66.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and relate illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 67.Boyle M D P. Encyclopedia of immunology. 2nd ed. New York, N.Y: Academic Press, Inc.; 1998. Bacterial immunoglobulin-binding proteins; pp. 323–327. [Google Scholar]
- 68.Boyle M D P. Variation of multifunctional surface proteins-a virulence strategy for group A streptococci? J Theor Biol. 1995;173:415–426. doi: 10.1006/jtbi.1995.0073. [DOI] [PubMed] [Google Scholar]
- 69.Boyle M D P, Hawlitzky J, Raeder R, Podbielski A. Analysis of genes encoding two unique type IIa immunoglobulin G binding proteins expressed by a single group A streptococcal isolate. Infect Immun. 1994;62:1336–1347. doi: 10.1128/iai.62.4.1336-1347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyle M D P, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemostasis. 1997;77:1–10. [PubMed] [Google Scholar]
- 71.Boyle M D P, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. doi: 10.1086/515241. [DOI] [PubMed] [Google Scholar]
- 72.Boyle M D P, Weber-Heyneman J, Raeder R, Podbielski A. Genetic and serological analysis of IgG binding proteins expressed by an opacity factor negative group A streptococcus. Mol Immunol. 1995;32:669–678. doi: 10.1016/0161-5890(95)00022-7. [DOI] [PubMed] [Google Scholar]
- 73.Brandt E R, Hayman W A, Currie B, Carapetis J, Wood Y, Jackson D C, Cooper J, Melrose W D, Saul A J, Good M F. Opsonic human antibodies from an endemic population specific for a conserved epitope on the M protein of group A streptococci. Immunology. 1996;89:331–337. doi: 10.1046/j.1365-2567.1996.d01-754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandt E R, Hayman W A, Currie B, Pruksakorn S, Good M F. Human antibodies to the conserved region of the M protein: opsonization of heterologous strains of group A streptococci. Vaccine. 1997;15:1805–1812. doi: 10.1016/s0264-410x(97)00178-3. [DOI] [PubMed] [Google Scholar]
- 75.Breese B B, Hall C B. Beta hemolytic streptococcal diseases. Boston, Mass: Houghton Mifflin; 1978. [Google Scholar]
- 76.Broder C C, Lottenberg R, Boyle M D P. Mapping of the human plasmin domain recognized by the unique plasmin receptor of group A streptococci. Infect Immun. 1989;57:2597–2605. doi: 10.1128/iai.57.9.2597-2605.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broder C C, Lottenberg R, Mering G O, Johnston K H, Boyle M D P. Isolation of a prokaryotic plasmin receptor. J Biol Chem. 1991;266:4922–4928. [PubMed] [Google Scholar]
- 78.Broeseker T A, Boyle M D P, Lottenberg R. Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb Pathog. 1988;5:19–27. doi: 10.1016/0882-4010(88)90077-0. [DOI] [PubMed] [Google Scholar]
- 79.Bronze M, McKinsey D, Corbett C, Beachey E H, Dale J B. Protective immunity evoked by locally administered group A streptococcal vaccines in mice. J Immunol. 1988;141:2767. [PubMed] [Google Scholar]
- 80.Bronze M S, Beachey E H, Dale J B. Protective and heart-crossreactive epitopes located within the NH2 terminus of type 19 streptococcal M protein. J Exp Med. 1988;167:1849–1859. doi: 10.1084/jem.167.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bronze M S, Courtney H S, Dale J B. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J Immunol. 1992;148:888–893. [PubMed] [Google Scholar]
- 82.Bronze M S, Dale J B. Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J Immunol. 1993;151:2820–2828. [PubMed] [Google Scholar]
- 83.Burns E H, Jr, Lukomski S, Rurangirwa J, Podbielski A, Musser J M. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb Pathog. 1998;24:333–339. doi: 10.1006/mpat.1998.0204. [DOI] [PubMed] [Google Scholar]
- 84.Burns E H, Jr, Marceil A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burova L A, Koroleva I V, Ogurtzov R P, Murashov S V, Svensson M-L, Schalen C. Role of IgG Fc receptor in tissue deposition of IgG in rabbits immunized with Streptococcus pyogenes. APMIS. 1992;100:567–574. doi: 10.1111/j.1699-0463.1992.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 86.Burova L A, Nagornev V A, Pigarevsky P V, Gladilina M M, Seliverstova V G, Schalen C, Totolian A. Triggering of renal tissue damage in the rabbit by IgG Fc-receptor-positive group A streptococci. APMIS. 1998;106:277–287. doi: 10.1111/j.1699-0463.1998.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 87.Burova L A, Schalen C, Koroleva I V, Svensson M-L. Role of group A streptococcal IgG Fc-receptor in induction of anti-IgG by immunization in the rabbit. FEMS Microbiol Immunol. 1989;47:443–448. doi: 10.1111/j.1574-6968.1989.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 88.Caparon M G, Geist R T, Perez-Casal J, Scott J R. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J Bacteriol. 1992;174:5693–5701. doi: 10.1128/jb.174.17.5693-5701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caparon M G, Scott J R. Identification of a gene that regulates expression of M protein: the major virulence determinant of group A streptococci. Proc Natl Acad Sci USA. 1987;84:8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caparon M G, Stevens D S, Olsen A, Scott J R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991;59:1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carapetis J, Robins-Browne R, Martin D, Shelby-James T, Hogg G. Increasing severity of invasive group A streptococcal disease in Australia: clinical and molecular epidemiological features and identification of a new virulent M-nontypeable clone. Clin Infect Dis. 1995;21:1220–1227. doi: 10.1093/clinids/21.5.1220. [DOI] [PubMed] [Google Scholar]
- 92.Carroll P, Stafford D, Schwartz R S, Stollar B D. Murine monoclonal anti-DNA antibodies bind to endogenous bacteria. J Immunol. 1985;135:1086. [PubMed] [Google Scholar]
- 93.Cavelti P A. Autoantibodies in rheumatic fever. Proc Soc Exp Biol Med. 1945;60:379–381. doi: 10.3181/00379727-60-15197p. [DOI] [PubMed] [Google Scholar]
- 93a.Centers for Disease Control and Prevention. Invasive group A streptococcal infections—United Kingdom, 1994. Morb Mortal Wkly Rep. 1994;43:401–402. [PubMed] [Google Scholar]
- 94.Cheadle W B. Harvean lectures on the various manifestations of the rheumatic state as exemplified in childhood and early life. Lancet. 1989;i:821–832. [Google Scholar]
- 95.Chen C, Bormann N, Cleary P P. VirR and Mry are homologous trans-acting regulators of M protein and C5a peptidase expression in group A streptococci. Mol Gen Genet. 1993;241:685–693. doi: 10.1007/BF00279912. [DOI] [PubMed] [Google Scholar]
- 96.Chmouryguina I, Suvorov A, Ferrieri P, Cleary P P. Conservation of the C5a peptidase genes in group A and B streptococci. Infect Immun. 1996;64:2387–2390. doi: 10.1128/iai.64.7.2387-2390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christner R, Li Z, Raeder R, Podbielski A, Boyle M. Identification of key gene products required for acquisition of plasmin-like enzymatic activity by group A streptococci. J Infect Dis. 1997;175:1115–1120. doi: 10.1086/516450. [DOI] [PubMed] [Google Scholar]
- 98.Christner R, Zhuqing L, Raeder R, Podbielski A, Boyle M D P. Identification of key gene products required for acquisition of plasmin-like enzymatic activity by group A streptococci. J Infect Dis. 1997;175:1115–1120. doi: 10.1086/516450. [DOI] [PubMed] [Google Scholar]
- 99.Cleary P, Prabu U, Dale J, Wexler D, Handley J. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect Immun. 1992;60:5219–5223. doi: 10.1128/iai.60.12.5219-5223.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 101.Cleary P P, LaPenta D, Heath D, Haanes E J, Chen C. A virulence regulon in Streptococcus pyogenes. In: Dunny G M, Cleary P P, McKays L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 147–151. [Google Scholar]
- 102.Cockerill F R, III, Thompson R L, Musser J M, Schlievert P M, Talbot J, Holley K E, Harmsen W S, Ilstrup D M, Kohner P C, Kim M H, Frankfort B, Manahan J M, Steckelberg J M, Roberson F, Wilson W R Southeastern Minnesota Streptococcal Working Group. Molecular, serological, and clinical features of 16 consecutive cases of invasive streptococcal disease. Clin Infect Dis. 1998;26:1448–1458. doi: 10.1086/516376. [DOI] [PubMed] [Google Scholar]
- 103.Cone L, Woodard D R, Schlievert P M, Tomory G S. Clinical and bacteriologic observations of a toxic-shock like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 104.Reference deleted.
- 105.Courtney H A, Hasty D L, Dale J B, Poirer T P. A 29-kilodalton fibronectin binding protein of group A streptococci. Curr Microbiol. 1992;25:245–250. doi: 10.1007/BF01575856. [DOI] [PubMed] [Google Scholar]
- 106.Courtney H S, Bronze M S, Dale J B, Hasty D L. Analysis of the role of M24 protein in streptococcal adhesion and colonization by use of omega-interposon mutagenesis. Infect Immun. 1994;62:4868–4873. doi: 10.1128/iai.62.11.4868-4873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Courtney H S, Dale J B, Hasty D L. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect Immun. 1996;64:2415–2419. doi: 10.1128/iai.64.7.2415-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Courtney H S, Dale J B, Hasty D L. Strategies for prevention of group A streptococcal adhesion and infection. In: An Y H, Friedman R J, editors. Handbook of bacterial adhesion: principles, methods, and applications. Totowa, N.J: Humana Press; 1999. pp. 553–579. [Google Scholar]
- 109.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Courtney H S, Ofek I, Hasty D L. M protein mediated adhesion of M type 24 Streptococcus pyogenes stimulates release of interleukin-6 by HEp-2 tissue culture cells. FEMS Microbiol Lett. 1997;151:65–70. doi: 10.1016/s0378-1097(97)00139-0. [DOI] [PubMed] [Google Scholar]
- 111.Courtney H S, von Hunolstein C, Dale J B, Bronze M S, Beachey E H, Hasty D L. Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb Pathog. 1992;12:199–208. doi: 10.1016/0882-4010(92)90054-r. [DOI] [PubMed] [Google Scholar]
- 112.Crater D L, Dougherty B A, van de Rijn I. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1995;270:28676–28680. doi: 10.1074/jbc.270.48.28676. [DOI] [PubMed] [Google Scholar]
- 113.Crater D L, van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. J Biol Chem. 1995;270:18452–18458. doi: 10.1074/jbc.270.31.18452. [DOI] [PubMed] [Google Scholar]
- 114.Cromartie W, Craddock J, Schwab J H, Anderle S, Yang C. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cromartie W J, Craddock J G. Rheumatic like cardiac lesions in mice. Science. 1966;154:285–287. doi: 10.1126/science.154.3746.285. [DOI] [PubMed] [Google Scholar]
- 116.Cronin W J, Lange K. Immunologic evidence for the in situ deposition of a cytoplasmic streptococcal antigen (endostreptosin) on the glomerular basement membrane in rats. Clin Nephrol. 1990;34:143. [PubMed] [Google Scholar]
- 117.Cu G A, Mezzano S, Bannan J D, Zabriskie J B. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 1998;54:819–826. doi: 10.1046/j.1523-1755.1998.00052.x. [DOI] [PubMed] [Google Scholar]
- 118.Cue C, Dombek P E, Lam H, Cleary P P. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cunningham M W. Streptococci and rheumatic fever. In: Rose N R, Friedman H, editors. Microorganisms and autoimmune disease. New York, N.Y: Plenum Publishing Corp.; 1996. pp. 13–66. [Google Scholar]
- 120.Cunningham M W, Antone S M, Gulizia J M, Gauntt C J. Common epitopes shared between streptococcal M protein and viruses may be a link to autoimmunity. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Proceedings of the XI Lancefield International Symposium, Zentralbl. Bakteriol. Suppl. 22. 1992. pp. 534–536. Gustav-Fisher-Verlag, New York, N.Y. [Google Scholar]
- 121.Cunningham M W, Antone S M, Gulizia J M, McManus B A, Gauntt C J. α-Helical coiled-coil molecules: a role in autoimmunity against the heart. Clin Immunol Immunopathol. 1993;68:129–134. doi: 10.1006/clin.1993.1106. [DOI] [PubMed] [Google Scholar]
- 122.Cunningham M W, Antone S M, Gulizia J M, McManus B M, Fischetti V A, Gauntt C J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cunningham M W, Antone S M, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cunningham M W, Beachey E H. Immunochemical properties of streptococcal M protein purified by isoelectric focusing. J Immunol. 1975;115:1002–1006. [PubMed] [Google Scholar]
- 125.Cunningham M W, Beachey E H. Peptic digestion of streptococcal M protein. I. Effect of digestion at suboptimal pH upon the biological and immunochemical properties of purified M protein extracts. Infect Immun. 1974;9:244–248. doi: 10.1128/iai.9.2.244-248.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cunningham M W, Hall N K, Krisher K K, Spanier A M. A study of monoclonal antibodies against streptococci and myosin. J Immunol. 1985;136:293–298. [PubMed] [Google Scholar]
- 127.Cunningham M W, Krisher K, Graves D C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984;46:34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cunningham M W, McCormack J M, Fenderson P G, Ho M K, Beachey E H, Dale J B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989;143:2677–2683. [PubMed] [Google Scholar]
- 129.Cunningham M W, McCormack J M, Fenderson P G, Ho M K, Beachey E H, Dale J B. Human monoclonal antibodies reactive with antigens of the group A streptococcus and human heart. J Immunol. 1989;141:2760. [PubMed] [Google Scholar]
- 130.Cunningham M W, McCormack J M, Talaber L R, Harley J B, Ayoub E M, Muneer R S, Chun L T, Reddy D V. Human monoclonal antibodies reactive with antigens of the group A streptococcus and human heart. J Immunol. 1988;141:2760–2766. [PubMed] [Google Scholar]
- 131.Cunningham M W, Russell S M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A streptococci. Infect Immun. 1983;42:531–538. doi: 10.1128/iai.42.2.531-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cunningham M W, Swerlick R A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986;164:998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cunningham M W, Swerlick R W. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986;164:998. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dajani A S. Guidelines for the diagnosis of rheumatic fever (Jones criteria, 1992 update) JAMA. 1992;268:2069–2073. [PubMed] [Google Scholar]
- 135.Dale J B. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine. 1999;17:193–200. doi: 10.1016/s0264-410x(98)00150-9. [DOI] [PubMed] [Google Scholar]
- 136.Dale J B, Baird R W, Courtney H S. Passive protection of mice against group A streptococcal pharyngeal infection by lipoteichoic acid. J Infect Dis. 1994;169:319–323. doi: 10.1093/infdis/169.2.319. [DOI] [PubMed] [Google Scholar]
- 137.Dale J B, Beachey E H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985;162:583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dale J B, Beachey E H. Human cytotoxic T lymphocytes evoked by group A streptococcal M proteins. J Exp Med. 1987;166:1825–1835. doi: 10.1084/jem.166.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dale J B, Beachey E H. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J Exp Med. 1985;161:113–122. doi: 10.1084/jem.161.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dale J B, Beachey E H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J Exp Med. 1986;164:1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dale J B, Chiang E C. Intranasal immunization with recombinant group A streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J Infect Dis. 1995;171:1038–1041. doi: 10.1093/infdis/171.4.1038. [DOI] [PubMed] [Google Scholar]
- 142.Dale J B, Chiang E Y, Lederer J W. Recombinant tetravalent group A streptococcal M protein vaccine. J Immunol. 1993;151:2188–2194. [PubMed] [Google Scholar]
- 143.Dale J B, Chiang E Y, Liu S, Courtney H S, Hasty D L. New protective antigen of group A streptococci. J Clin Invest. 1999;103:1261–1268. doi: 10.1172/JCI5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dale J B, Seyer J M, Beachey E H. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J Exp Med. 1983;158:1727–1732. doi: 10.1084/jem.158.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dale J B, Simmons M, Chiang E C, Chiang E Y. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine. 1996;14:944–948. doi: 10.1016/0264-410x(96)00050-3. [DOI] [PubMed] [Google Scholar]
- 146.Dale J B, Washburn R G, Marques M B, Wessels M R. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64:1495–1501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.D'Alessandri R, Plotkin G, Kluge R M, et al. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with type 3 and type 12 group A streptococcus. J Infect Dis. 1978;138:712. doi: 10.1093/infdis/138.6.712. [DOI] [PubMed] [Google Scholar]
- 148.Damaschun G, Damaschun H, Gast K, Gerlach D, Misselwitz R, Welfle H, Zirwer D. Streptokinase is a flexible multi-domain protein. Eur Biophys J. 1992;20:355–361. doi: 10.1007/BF00196594. [DOI] [PubMed] [Google Scholar]
- 149.Davies H D, McGeer A, Schwartz B, Green K, Cann D, Simor A E, Low D E Ontario Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 150.D'Costa S S, Boyle M D P. Interaction of a group A streptococcus within human plasma results in assembly of a surface plasminogen activator that contributes to occupancy of surface plasmin-binding structures. Microb Pathog. 1998;24:341–349. doi: 10.1006/mpat.1998.0207. [DOI] [PubMed] [Google Scholar]
- 151.D'Costa S S, Wang H, Metzger D W, Boyle M D P. Group A streptococcal isolate 64/14 expresses surface plasmin-binding structures in addition to Plr. Res Microbiol. 1997;148:559–572. doi: 10.1016/S0923-2508(97)88080-1. [DOI] [PubMed] [Google Scholar]
- 152.DeAngelis P L, Papacontantinou J, Weigel P H. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem. 1993;268:19181–19184. [PubMed] [Google Scholar]
- 153.Debaz H, Olivo A, Perez-Luque E, Vasquez-Garcia M N, Burguete A, Chavez-Negrete A, Velasco C, Arguero R, Gorodeszky C. DNA analysis of HLA class II alleles in rheumatic heart disease in Mexicans. Hum Immunol. 1996;49(Suppl. 1):63. [Google Scholar]
- 154.Degnan B, Taylor J, Goodacre J A. Streptococcus pyogenes type 5 M protein is an antigen, not a superantigen, for human T cells. Hum Immunol. 1997;53:206–215. doi: 10.1016/S0198-8859(97)00028-1. [DOI] [PubMed] [Google Scholar]
- 155.Dell A, Antone S M, Gauntt C J, Crossley C A, Clark W A, Cunningham M W. Autoimmune determinants of rheumatic carditis: localization of epitopes in human cardiac myosin. Eur Heart J. 1991;12:158–162. doi: 10.1093/eurheartj/12.suppl_d.158. [DOI] [PubMed] [Google Scholar]
- 156.Delvig A, Robinson J H. Different endosomal proteolysis requirements for antigen processing of two T cell epitopes of the M5 protein from viable Streptococcus pyogenes. J Biol Chem. 1998;273:3291–3295. doi: 10.1074/jbc.273.6.3291. [DOI] [PubMed] [Google Scholar]
- 157.Delvig A, Robinson J H. Two T cell epitopes from the M5 protein of viable Streptococcus pyogenes engage different pathways of bacterial antigen processing in mouse macrophages. J Immunol. 1998;160:5267–5272. [PubMed] [Google Scholar]
- 158.Dillon H C. Streptococcal infections of the skin and their complications: impetigo and nephritis. In: Wannamaker L W, Matsen J M, editors. Streptococci and streptococcal diseases. New York, N.Y: Academic Press, Inc.; 1972. pp. 571–587. [Google Scholar]
- 159.Dillon H C, Derrick C W, Dillon M S. M-antigens common to pyoderma and acute glomerulonephritis. J Infect Dis. 1974;130:257–267. doi: 10.1093/infdis/130.3.257. [DOI] [PubMed] [Google Scholar]
- 160.Dombek P, Cue D, Sedgewick J, Lam H, Ruschkowski B, Finlay B, Cleary P. High frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 mediated invasion and cytoskeletal rearrangements. Mol Microbiol. 1999;31:859–870. doi: 10.1046/j.1365-2958.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 161.Dougherty B A, van de Rijn I. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. J Exp Med. 1992;175:1291–1299. doi: 10.1084/jem.175.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dougherty B A, van de Rijn I. Molecular characterization of hasB from has operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1993;268:7118–7124. [PubMed] [Google Scholar]
- 163.Dougherty B A, van de Rijn I. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1994;269:169–175. [PubMed] [Google Scholar]
- 164.Dudding B A, Ayoub E M. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968;128:1081. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eichenbaum Z, Green B D, Scott J R. Iron starvation causes release from the group A streptococcus of the ADP-ribosylating protein called plasmin receptor or surface glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 1996;64:1956–1960. doi: 10.1128/iai.64.6.1956-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eisenberg R, Fox A, Greenblatt J, Anderle S, Cromartie W J, Schwab J H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982;38:127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.El-Demellawy M, El-Ridi R, Guirguis N I, Alim M A, Kotby A, Kotb M. Preferential recognition of human myocardial antigens by T lymphocytes from rheumatic heart disease patients. Infect Immun. 1997;65:2197–2205. doi: 10.1128/iai.65.6.2197-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ellen R P, Gibbons R J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972;5:826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ellen R P, Gibbons R J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974;9:85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Facklam R R. Screening for streptococcal pharyngitis: current technology. Infect Med. 1997;14:891–898. [Google Scholar]
- 171.Fast D J, Schlievert P M, Nelson R D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989;57:291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Federle M J, McIver K S, Scott J. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fenderson P G, Fischetti V A, Cunningham M W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989;142:2475–2481. [PubMed] [Google Scholar]
- 174.Ferretti J J, Huang T, Hynes W L. Extracellular product genes of group A streptococci. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 201–205. [Google Scholar]
- 175.Fillit H, Blake M, MacDonald C, McCarty M. Immunogenicity of liposome-bound hyaluronate in mice: at least two different antigenic sites on hyaluronate are identified by mouse monoclonal antibodies. J Exp Med. 1988;168:971–982. doi: 10.1084/jem.168.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fillit H, McCarty M, Blake M. Induction of antibodies to hyaluronic acid by immunization of rabbits with encapsulated streptococci. J Exp Med. 1986;164:762–776. doi: 10.1084/jem.164.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fischetti V A. Streptococcal M protein. Sci Am. 1991;264:58–65. doi: 10.1038/scientificamerican0691-58. [DOI] [PubMed] [Google Scholar]
- 178.Fischetti V A. Streptococcal M protein extracted by nonionic detergent. II. Analysis of the antibody response to the multiple antigenic determinants of the M-protein molecule. J Exp Med. 1977;146:1108–1123. doi: 10.1084/jem.146.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischetti V A, Bessen D. Effect of mucosal antibodies to M protein on colonization by group A streptococci. In: Hook N, editor. Molecular mechanisms of microbial adhesion. New York, N.Y: Springer-Verlag; 1988. pp. 128–142. [Google Scholar]
- 181.Fischetti V A, Gotschlich E C, Siviglia G, Zabriskie J B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976;144:32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fischetti V A, Hodges W M, Hruby D E. Protection against streptococcal pharyngeal colonization with a vaccinia: M protein recombinant. Science. 1989;244:1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- 183.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of the surface proteins of gram positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 184.Fischetti V A, Windels M. Mapping the immuno-determinants of the complete streptococcal M6 protein molecule: identification of an immunodominant region. J Immunol. 1988;141:3592–3599. [PubMed] [Google Scholar]
- 185.Fischetti V A, Zabriskie J B, Gotschlich E C. Physical, chemical, and biological properties of type 6 M protein extracted with purified streptococcal phage-associated lysin. In: Haverkorn M J, editor. Streptococcal disease and the community. Amsterdam, The Netherlands: Excerpta Medica; 1974. pp. 26–36. [Google Scholar]
- 186.Fleischer B, Schmidt K-H, Gerlach D, Kohler W. Separation of T-cell-stimulating activity from streptococcal M protein. Infect Immun. 1992;60:1767–1770. doi: 10.1128/iai.60.5.1767-1770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fluckiger U, Jones K F, Fischetti V A. Immunoglobulins to group A streptococcal surface molecules decrease adherence to and invasion of human pharyngeal cells. Infect Immun. 1998;66:974–979. doi: 10.1128/iai.66.3.974-979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fogg G C, Caparon M G. Constitutive expression of fibronectin binding in Streptococcus pyogenes as a result of anaerobic activation of rofA. J Bacteriol. 1997;179:6172–6180. doi: 10.1128/jb.179.19.6172-6180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Foley M J, Wood W B. Studies of the pathogenicity of group A streptococci. II. The anti-phagocytic effects of the M protein and its capsular gel. J Exp Med. 1959;110:617–628. doi: 10.1084/jem.110.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Foley S M J, Wood W B. Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J Exp Med. 1959;110:617–628. doi: 10.1084/jem.110.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fox A, Brown R, Anderle S, Chetty C, Cromartie W J, Gooder W, Schwab J H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982;35:921–925. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fox A, Schallinger L, Kirkland J. Sedimentation field flow fractionation of bacterial cell wall fragments. J Microbiol Methods. 1985;3:273. [Google Scholar]
- 193.Fox E N. M proteins of group A streptococci. Bacteriol Rev. 1974;38:57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fox E N, Wittner M K. New observations on the structure and antigenicity of the M proteins of the group A streptococcus. Immunochemistry. 1969;6:11–24. doi: 10.1016/0019-2791(69)90174-8. [DOI] [PubMed] [Google Scholar]
- 195.Frick L-M, Akesson P, Cooney J, Sjobring U, Schmidt K-H, Gomi H, Hattoir S, Tagawa C, Kishimoto F, Bjorck L. Protein H—a surface protein of Streptococcus pyogenes with separate binding sites for IgG and albumin. Mol Microbiol. 1994;12:143–151. doi: 10.1111/j.1365-2958.1994.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 196.Friedman J, van de Rijn I, Ohkuni H, Fischetti V, Zabriskie J. Immunological studies of post-streptococcal sequelae: evidence for presence of streptococcal antigens in circulating immune complexes. J Clin Investig. 1984;74:1027–1034. doi: 10.1172/JCI111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Frithz E, Heden L O, Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989;3:1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 198.Galvin, J. E., M. E. Hemric, K. Ward, and M. W. Cunningham. Cytotoxic monoclonal antibody from rheumatic carditis recognizes heart valves and laminin. J. Clin. Invest., in press. [DOI] [PMC free article] [PubMed]
- 199.Gase K, Ferretti J J, Primeaux C, McShan W M. Identification, cloning, and expression of the cyclic AMP factor gene (cfa) of group A streptococci. Infect Immun. 1999;67:4725–4731. doi: 10.1128/iai.67.9.4725-4731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gerlach D, Knoll H, Kohler W, Ozegowski J H, Hribalova V. Isolation and characterization of erythrogenic toxins. V. Communication: identity of erythrogenic exotoxin type B and streptococcal proteinase precursor. Zentralbl Bakteriol Hyg Abt I Orig Reihe A. 1983;255:221–223. [PubMed] [Google Scholar]
- 201.Gerlach D, Schalen C, Tigyi Z, Nilsson B, Forsgren A, Naidu A S. Identification of a novel lectin in Streptococcus pyogenes and its possible role in bacterial adherence to pharyngeal cells. Curr Microbiol. 1994;28:331–338. [Google Scholar]
- 202.Gibofsky A, Kerwar S, Zabriskie J B. Rheumatic fever: the relationships between host, microbe and genetics. Rheum Dis Clin N Am. 1998;24:237–259. doi: 10.1016/s0889-857x(05)70007-7. [DOI] [PubMed] [Google Scholar]
- 203.Gibson C, Caparon M. Insertional inactivation of sod in Streptococcus pyogenes suggests that prtF is regulated via a superoxide signal. J Bacteriol. 1996;178:4688–4695. doi: 10.1128/jb.178.15.4688-4695.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Glurich I, Winters B, Albini B, Stinson M. Identification of Streptococcus pyogenes proteins that bind to rabbit kidney in vitro and in vivo. Microb Pathog. 1991;10:209–220. doi: 10.1016/0882-4010(91)90055-f. [DOI] [PubMed] [Google Scholar]
- 205.Glynn L E, Halborrow E J. Relationship between blood groups, secretion status and susceptibility to rheumatic fever. Arthritis Rheum. 1961;4:203–207. doi: 10.1002/art.1780040210. [DOI] [PubMed] [Google Scholar]
- 206.Goldstein I, Halpern B, Robert L. Immunological relationship between streptococcus A polysaccharide and the structural glycoproteins of heart valve. Nature. 1967;213:44–47. [Google Scholar]
- 207.Gomi H, Hozumi T, Hattori S, Tagawa C, Kishimoto F, Bjorck L. The gene sequence and some properties of protein H: a novel IgG binding protein. J Immunol. 1990;144:4046–4052. [PubMed] [Google Scholar]
- 208.Goroncy-Bermes P, Dale J B, Beachey E H, Opferkuch W. Monoclonal antibody to human renal glomeruli cross-reacts with streptococcal M protein. Infect Immun. 1987;55:2416–2419. doi: 10.1128/iai.55.10.2416-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Goshorn S C, Schlievert P M. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J Bacteriol. 1989;171:3068–3073. doi: 10.1128/jb.171.6.3068-3073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goshorn S C, Schlievert P M. Nucleotide sequence of streptococcal pyrogenic exotoxin type C. Infect Immun. 1980;56:2518–2520. doi: 10.1128/iai.56.9.2518-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Greco R, DeMartino L, Donnarumma G, Conte M P, Seganti L, Valenti P. Invasion of cultured human cells by Streptococcus pyogenes. Res Microbiol. 1995;146:5551–5560. doi: 10.1016/0923-2508(96)80561-4. [DOI] [PubMed] [Google Scholar]
- 212.Greenberg R N, Willoughby B G, Kennedy D J, Otto T J, McMillan R, Bloomster T G. Hypocalcemia and “toxic” syndrome associated with streptococcal fasciitis. South Med J. 1983;76:916–918. doi: 10.1097/00007611-198307000-00026. [DOI] [PubMed] [Google Scholar]
- 213.Greenblatt J, Hunter N, Schwab J H. Antibody response to streptococcal cell wall antigens associated with experimental arthritis in rats. Clin Exp Immunol. 1980;42:450–457. [PMC free article] [PubMed] [Google Scholar]
- 214.Gubba S, Low D E, Musser J M. Expression and characterization of group A streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect Immun. 1998;66:765–770. doi: 10.1128/iai.66.2.765-770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guedez Y, Kotby A, El-Demellawy M, Galal A, Thomson G, Zaher S, Kassen S, Kotb M. HLA class II associations with rheumatic heart disease are more evident and consistent among clinically homogeneous patients. Circulation. 1999;99:2784–2790. doi: 10.1161/01.cir.99.21.2784. [DOI] [PubMed] [Google Scholar]
- 216.Guilherme L, Cunha-Neto E, Coelho V, Snitcowsky R, Pomerantzeff P M A, Assis R V, Pedra F, Neumann J, Goldberg A, Patarroyo M E, Pileggi F, Kalil J. Human heart-filtrating T cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation. 1995;92:415–420. doi: 10.1161/01.cir.92.3.415. [DOI] [PubMed] [Google Scholar]
- 217.Gulizia J M, Cunningham M W, McManus B M. Antistreptococcal monoclonal antibodies recognize multiple epitopes in human heart valves: cardiac myosin, vimentin, and elastin as potential valvular autoantigens. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Proceedings of the XI Lancefield International Symposium, Zentralbl. Bakteriol. Suppl. 22. 1992. pp. 267–269. Gustav-Fischer-Verlag, New York, N.Y. [Google Scholar]
- 218.Gulizia J M, Cunningham M W, McManus B M. Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves: evidence for multiple cross-reactive epitopes. Am J Pathol. 1991;138:285–301. [PMC free article] [PubMed] [Google Scholar]
- 219.Guzman C A, Talay S R, Molinari G, Medina E, Chhatwal G S. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect Dis. 1999;179:901–906. doi: 10.1086/314655. [DOI] [PubMed] [Google Scholar]
- 220.Haanes E J, Cleary P P. Identification of a divergent M protein gene and M protein-related gene family in Streptococcus pyogenes serotype 49. J Bacteriol. 1989;171:6397–6408. doi: 10.1128/jb.171.12.6397-6408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hackett S P, Stevens D L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992;165:879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- 222.Hafez K, El Kholy A M, Facklam R R. Extraction of group A streptococcal M protein with nitrous acid. J Clin Microbiol. 1981;14:530–533. doi: 10.1128/jcm.14.5.530-533.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hanski E, Caparon M G. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hanski E, Horwitz P, Caparon M G. Introduction of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, into heterologous streptococcal and enterococcal strains promotes their adherence to repiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harbaugh M P, Podbielski A, Hugi S, Cleary P P. Nucleotide substitutions and small scale insertion produce size and antigenic variation in group A streptococcal M1 protein. Mol Microbiol. 1993;8:981–991. doi: 10.1111/j.1365-2958.1993.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 226.Hasty D L, Courtney H S. Group A streptococcal adhesion: all of the theories are correct. In: Kahane I, Ofek I, editors. Toward anti-adhesion therapy for microbial diseases. New York, N.Y: Plenum Press; 1996. pp. 81–94. [PubMed] [Google Scholar]
- 227.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hauser A R, Schlievert P M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990;172:4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hauser A R, Stevens D L, Kaplan E L, Schlievert P M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991;29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Heath D G, Cleary P P. Cloning and expression of the gene for an immunoglobulin G Fc receptor protein from a group A streptococcus. Infect Immun. 1987;55:1233–1238. doi: 10.1128/iai.55.5.1233-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Heath D G, Cleary P P. Fc receptor and M protein are products of gene duplication. Proc Natl Acad Sci USA. 1989;86:4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Heden L-O. Identification of the IgA-binding region in streptococcal protein arp. J Immunol. 1994;153:3557–3564. [PubMed] [Google Scholar]
- 233.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 234.Herwald H, Collin M, Muller-Ester W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:1–9. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hoe N, Nakashima K, Grigsby D, Pan X, Dou S-J, Naidich S, Garcia M, Kahn E, Bergmire-Sweat D, Musser J. Rapid molecular genetic subtyping of serotype M1 group A streptococcus strains. Emerg Infect Dis. 1999;5:254–263. doi: 10.3201/eid0502.990210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hollingshead S K, Fischetti V A, Scott J R. Complete nucleotide sequence of type 6 M protein of the group A streptococcus: repetitive structure and membrane anchor. J Biol Chem. 1986;261:1677–1686. [PubMed] [Google Scholar]
- 237.Hollingshead S K, Fischetti V A, Scott J R. A highly conserved region present in transcripts encoding heterologous M proteins of group A streptococci. Infect Immun. 1987;55:3237–3239. doi: 10.1128/iai.55.12.3237-3239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hollingshead S K, Readdy T L, Yung D L, Bessen D E. Structural heterogeneity of the emm gene cluster in group A streptococci. Mol Microbiol. 1993;8:707–717. doi: 10.1111/j.1365-2958.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 239.Hollingshead S K, Ready T, Arnold J, Bessen D E. Molecular evolution of a multi-gene family in group A streptococci. Mol Biol Evol. 1994;11:208–219. doi: 10.1093/oxfordjournals.molbev.a040103. [DOI] [PubMed] [Google Scholar]
- 240.Hollingshead S K, Simecka J W, Michalek S M. Role of M protein in pharyngeal colonization by group A streptococci in rats. Infect Immun. 1993;61:2277–2283. doi: 10.1128/iai.61.6.2277-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Holm S E. Invasive group A streptococcal infections. N Engl J Med. 1996;335:590–591. doi: 10.1056/NEJM199608223350811. [DOI] [PubMed] [Google Scholar]
- 242.Holm S E. The pathogenesis of acute poststreptococcal glomerulohephritis in new lights. APMIS. 1988;96:189–193. doi: 10.1111/j.1699-0463.1988.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 243.Holm S E, Bergholm A-M, Johnston K H. A possible role of NSAP in the pathogenesis of the initial phase of experimental APSGN. Abstr. Xth Lancefield Int. Symp. 1987. Streptococci and Streptococcal Dis., Cologne, Germany. Abstr. L54. [Google Scholar]
- 244.Holm S E, Ferretti J, Simon D, Johnston K. Deletion of a streptokinase gene eliminates the nephritogenic capacity of a type 49 strain. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Proceedings of the XI Lancefield International Symposium. Zentralbl. Bakteriol. Suppl. 22. 1992. pp. 261–263. Gustav-Fischer-Verlag, New York, N.Y. [Google Scholar]
- 245.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 246.Horstmann R D, Sievertsen H J, Knobloch J, Fischetti V A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci USA. 1988;85:1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Horstmann R D, Sievertsen H J, Leippe M, Fischetti V A. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect Immun. 1992;60:5036–5041. doi: 10.1128/iai.60.12.5036-5041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang T T, Malke H, Ferretti J J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989;57:502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huang T T, Malke H, Ferretti J J. The streptokinase gene of group A streptococci: cloning, expression in Escherichia coli, and sequence analysis. Mol Microbiol. 1989;3:197–205. doi: 10.1111/j.1365-2958.1989.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 250.Huber S A, Cunningham M W. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsackieviral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- 251.Huber S A, Job L P, Woodruff J F. Lysis of infected myofibers by coxsackievirus B3 immune lymphocytes. Am J Pathol. 1980;98:681–694. [PMC free article] [PubMed] [Google Scholar]
- 252.Huber S A, Lodge P A. Coxsackievirus B-3 myocarditis: identification of different pathogenic mechanisms in DBA/2 and BALB/c mice. Am J Pathol. 1986;122:284–291. [PMC free article] [PubMed] [Google Scholar]
- 253.Hunter N, Anderle S, Brown R, Dalldorf F, Clark R, Cromartie W J, Schwab J H. Cell mediated response during experimental arthritis induced in rats with streptococcal cell walls. Clin Exp Immunol. 1980;42:441–449. [PMC free article] [PubMed] [Google Scholar]
- 254.Husby G, van de Rijn I, Zabriskie J B, Abdin Z H, Williams R C J. Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Husman L K, Scott J R, Lindahl G, Stenberg L. Expression of the Arp protein, a member of the M protein family, is not sufficient to inhibit phagocytosis of Streptococcus pyogenes. Infect Immun. 1995;63:345–348. doi: 10.1128/iai.63.1.345-348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Husman L K, Yung D-L, Hollingshead S K, Scott J R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect Immun. 1997;65:1422–1430. doi: 10.1128/iai.65.4.1422-1430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hutto J H, Ayoub E M. Cytotoxicity of lymphocytes from patients with rheumatic carditis to cardiac cells in vitro. In: Read S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press; 1980. pp. 733–738. [Google Scholar]
- 258.Reference deleted.
- 259.Iwasaki M, Igarashi H, Hinuma Y, Yutsudo T. Cloning, characterization and overexpression of a Streptococcus pyogenes gene encoding a new type of mitogenic factor. FEBS Lett. 1993;331:187–192. doi: 10.1016/0014-5793(93)80323-m. [DOI] [PubMed] [Google Scholar]
- 260.Jacks-Weis J, Kim Y, Cleary P. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982;128:1897–1902. [PubMed] [Google Scholar]
- 261.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 262.Jeppson H, Frithz E, Heden L O. Duplication of a DNA sequence homologous to genes for immunoglobulin receptors and M proteins in Streptococcus pyogenes. FEMS Microbiol Lett. 1992;92:139–146. doi: 10.1016/0378-1097(92)90502-f. [DOI] [PubMed] [Google Scholar]
- 263.Jevon G P, Dunne W M, Jr, Hawkins H K, Armstrong D L, Musser J M. Fatal group A streptococcal infections in Sweden, 1988–1989. Clin Infect Dis. 1994;18:91–93. doi: 10.1093/clinids/18.1.91. [DOI] [PubMed] [Google Scholar]
- 264.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ji Y, Schnitzler N, DeMaster E, Cleary P. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect Immun. 1998;66:5399–5405. doi: 10.1128/iai.66.11.5399-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Johnson D R, Stevens D L, Kaplan E L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 267a.Johnson D R, Kaplan E, Sramek J, Bicova R, Havlicek J, Havlickova H, Motlova J, Kriz P. Laboratory methods for the diagnosis of group A streptococcal infections. Geneva, Switzerland: World Health Organization; 1996. pp. 1–120. [Google Scholar]
- 268.Johnson L P, Tomai M A, Schlievert P M. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986;166:623–627. doi: 10.1128/jb.166.2.623-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Johnston K H, Chaiban J E, Wheeler R C. Analysis of the variable domain of the streptokinase gene from streptococci associated with poststreptococcal glomerulonephritis. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Proceedings of the XI Lancefield International Symposium. Zentralbl. Bakteriol. Suppl. 22. 1992. p. 339. Gustav-Fischer-Verlag, New York, N.Y. [Google Scholar]
- 270.Johnston K H, Zabriskie J B. Purification and partial characterization of the nephritis strain associated protein from Streptococcus pyogenes. J Exp Med. 1986;163:697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jones K F, Fischetti V A. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci. J Exp Med. 1988;167:1114–1123. doi: 10.1084/jem.167.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jones K F, Schneewind O, Koomey J M, Fischetti V A. Genetic diversity among the T-protein genes of group A streptococci. Mol Microbiol. 1991;5:2947–2952. doi: 10.1111/j.1365-2958.1991.tb01854.x. [DOI] [PubMed] [Google Scholar]
- 273.Jones T D. The diagnosis of rheumatic fever. JAMA. 1944;126:481–484. [Google Scholar]
- 274.Kamezawa Y, Nakahara T, Nakano S, Abe Y, Nozsaki-Renard J, Isono T. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect Immun. 1997;65:3828–3833. doi: 10.1128/iai.65.9.3828-3833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kaplan E L. The resurgence of group A streptococcal infections and their sequelae. Eur J Clin Microbiol Infect Dis. 1991;10:55–57. doi: 10.1007/BF01964407. [DOI] [PubMed] [Google Scholar]
- 276.Kaplan M H. Immunologic relation of streptococcal and tissue antigens. I. Properties of an antigen in certain strains of group A streptococci exhibiting an immunologic cross reaction with human heart tissue. J Immunol. 1963;90:595–606. [PubMed] [Google Scholar]
- 277.Kaplan M H, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever: association with cardiac failure. N Engl J Med. 1964;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 278.Kaplan M H, Frengley J D. Autoimmunity to the heart in cardiac disease: current concepts of the relation of autoimmunity to rheumatic fever, postcardiotomy and post infarction syndromes and cardiomyopathies. Am J Cardiol. 1969;24:459–473. doi: 10.1016/0002-9149(69)90489-5. [DOI] [PubMed] [Google Scholar]
- 279.Kaplan M H, Johnson D R. Eradication of group A streptococci from the upper respiratory tract by amoxicillin with clavulanic acid after oral penicillin V treatment failure. J Pediatr. 1988;113:400–403. doi: 10.1016/s0022-3476(88)80291-9. [DOI] [PubMed] [Google Scholar]
- 280.Kaplan M H, Johnson D R, Cleary P P. Group A streptococcal serotypes isolated from patients and sibling contacts during the resurgence of rheumatic fever in the United states in the mid-1980's. J Infect Dis. 1989;159:101–103. doi: 10.1093/infdis/159.1.101. [DOI] [PubMed] [Google Scholar]
- 281.Kaplan M H, Meyeserian M. An immunological cross reaction between group A streptococcal cells and human heart tissue. Lancet. 1962;i:706–710. doi: 10.1016/s0140-6736(62)91653-7. [DOI] [PubMed] [Google Scholar]
- 282.Kaplan M H, Suchy M L. Immunologic relation of streptococcal and tissue antigens. II. Cross reactions of antisera to mammalian heart tissue with a cell wall constituent of certain strains of group A streptococci. J Exp Med. 1964;119:643–650. doi: 10.1084/jem.119.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kaplan M H, Svec K H. Immunologic relation of streptococcal and tissue antigens. III. Presence in human sera of streptococcal antibody cross reactive with heart tissue: association with streptococcal infection, rheumatic fever, and glomerulonephritis. J Exp Med. 1964;119:651–666. doi: 10.1084/jem.119.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kappler J W, Kotzin B, Herron L, Gelfand E, Bigler R, Boyston A, Carrell S, Posnett D, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 285.Kapur V, Kanjilal S, Hamrick M, Li L-L, Whittam T S, Sawyer S A, Musser J M. Molecular population genetic analysis of the streptokinase gene of Streptococcus pyogenes: mosaic alleles generated by recombination. Mol Microbiol. 1995;16:509–519. doi: 10.1111/j.1365-2958.1995.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 286.Kapur V, Maffei J, Greer G, Adams G, Musser J. Vaccination with streptococcal extracellular cysteine protease (interleukin-1 convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 287.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1b precursor to produce active IL-1b by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kapur V, Topouzis S, Majesky M W, Li L-L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cyteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 289.Kaufold A, Podbielski A, Blockpoel M, Schouls L. Rapid typing of group A streptococci by use of DNA amplification and non-radioactive allele-specific oligonucleotide probes. FEMS Microbiol Lett. 1994;119:19–26. doi: 10.1111/j.1574-6968.1994.tb06861.x. [DOI] [PubMed] [Google Scholar]
- 290.Kaul R, McGeer A, Low D E, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Am J Med. 1997;103:18–24. doi: 10.1016/s0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 291.Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Low D E. Intravenous immunoglobulin (IVIG) therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28:800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 292.Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Low D E. Polyspecific intravenous immunoglobulin therapy in streptococcal toxic shock syndrome: a stratified case-control study and evidence for the in vivo inhibition of T-cell stimulation and cytokine production. Clin Infect Dis. 1998;28:800–807. [Google Scholar]
- 293.Kaur S, Kumar D, Grover A, Khanduja K L, Kaplan E L, Gray E D, Ganguly N K. Ethnic differences in expression of susceptibility marker(s) in rheumatic fever/rheumatic heart disease patients. Int J Cardiol. 1998;64:9–14. doi: 10.1016/s0167-5273(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 294.Kefalides N A, Ohno N, Wilson C B, Fillit H, Zabriskie J, Rosenbloom J. Identification of antigenic epitopes in type IV collagen by use of synthetic peptides. Kidney Int. 1993;43:94–100. doi: 10.1038/ki.1993.16. [DOI] [PubMed] [Google Scholar]
- 295.Kefalides N A, Pegg N T, Ohno N, Poon-King T, Zabriskie J B, Fillit H. Antibodies to basement membrane collagen and to laminin are present in sera from patients with poststreptococcal glomerulonephritis. J Exp Med. 1986;163:588. doi: 10.1084/jem.163.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kehoe M A. Cell wall associated proteins in gram positive bacteria. New Compr Biochem. 1994;27:217–261. [Google Scholar]
- 297.Kehoe M A, Poirier T P, Beachey E H, Timmis K N. Cloning and genetic analysis of serotype 5 M protein determinant of group A streptococci: evidence for multiple copies of the M5 determinant in the Streptococcus pyogenes genome. Infect Immun. 1985;48:190–197. doi: 10.1128/iai.48.1.190-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kendall F E, Heidelberger M, Dawson M N. A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic streptococcus. J Biol Chem. 1941;118:61–69. [Google Scholar]
- 299.Khanna A K, Buskirk D R, Williams R C, Jr, Gibofsky A, Crow M K, Menon A, Fotino M, Reid H M, Poon-King T, Rubinstein P, Zabriskie J B. Presence of a non-HLA B cell antigen in rheumatic fever patients and their families as defined by a monoclonal antibody. J Clin Investig. 1989;83:1710–1716. doi: 10.1172/JCI114071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kil K S, Cunningham M W, Barnett L A. Cloning and sequence analysis of a gene encoding a 67-kilodalton myosin-cross-reactive antigen of Streptococcus pyogenes reveals its similarity with class II major histocompatibility antigens. Infect Immun. 1994;62:2440–2449. doi: 10.1128/iai.62.6.2440-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Killian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A protease. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kline J B, Collins C M. Analysis of the superantigenic activity of mutant and allelic forms of streptococcal pyrogenic exotoxin A. Infect Immun. 1996;64:861–869. doi: 10.1128/iai.64.3.861-869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. [Google Scholar]
- 304.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kotb M. Role of superantigens in the pathogenesis of infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–374. [Google Scholar]
- 306.Kotzin B L, Leung D Y, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 307.Koyanagi T, Koga Y, Nishi H, Toshima H, Sasazuki T, Imaizumi T, Kimura A. DNA typing of HLA class II genes in Japanese patients with rheumatic heart disease. J Mol Cell Cardiol. 1996;28:1349–1353. doi: 10.1006/jmcc.1996.0125. [DOI] [PubMed] [Google Scholar]
- 308.Kraus W, Beachey E H. Renal autoimmune epitope of group A streptococci specified by M protein tetrapeptide: Ile-Arg-Leu-Arg. Proc Natl Acad Sci USA. 1988;85:4516–4520. doi: 10.1073/pnas.85.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kraus W, Dale J B, Beachey E H. Identification of an epitope of type 1 streptococcal M protein that is shared with a 43-kDa protein of human myocardium and renal glomeruli. J Immunol. 1990;145:4089–4093. [PubMed] [Google Scholar]
- 310.Kreikmeyer B, Talay S R, Chhatwal G S. Characterization of a novel fibronectin- binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 311.Kreikmeyer S, Talay R, Chhatwal G. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 312.Krisher K, Cunningham M W. Myosin: a link between streptococci and heart. Science. 1985;227:413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 313.Kuo C-F, Wu J-J, Lin K-Y, Tsai P-J, Lee S C, Jin J-T, Lei H-Y, Lin Y-S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kuusela P, Ullberg M, Sakela O, Kronvall G. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect Immun. 1992;60:196–201. doi: 10.1128/iai.60.1.196-201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 316.Lafer E M, Rauch J, Andrezejewski C, Jr, Mudd D, Furie B, Schwartz R S, Stollar B D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981;153:897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lancefield R C. The antigenic complex of Streptococcus hemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus hemolyticus. J Exp Med. 1928;47:9–10. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 319.Lancefield R C. Persistence of type specific antibodies in man following infection with group A streptococci. J Immunol. 1959;110:271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lancefield R C. Specific relationship of cell composition to biological activity of hemolytic streptococci. Harvey Lect. 1941;36:251. [Google Scholar]
- 321.Lange C F. Chemistry of cross-reactive fragments of streptococcal cell membrane and human glomerular basement membrane. Transplant Proc. 1969;1:959–963. [PubMed] [Google Scholar]
- 322.Lange K, Ahmed U, Kleinberger H, Treser G. A hitherto unknown streptococcal antigen and its probable relation to acute poststreptococcal glomerulonephritis. Clin Nephrol. 1976;5:207–215. [PubMed] [Google Scholar]
- 323.Lange K, Seligson G, Cronin W. Evidence for the in situ origin of poststreptococcal GN: glomerular localization of endostreptosin and the clinical significance of the subsequent antibody response. Clin Nephrol. 1983;19:3–10. [PubMed] [Google Scholar]
- 324.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.LaPenta D, Zhang X P, Cleary P P. Streptococcus pyogenes type IIa IgG Fc receptor expression is co-ordinately regulated with M protein and streptococcal C5a peptidase. Mol Microbiol. 1994;12:873–879. doi: 10.1111/j.1365-2958.1994.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 326.Lee P K, Schlievert P M. Quantification and toxicity of group A streptococcal pyrogenic exotoxins in an animal model of toxic shock syndrome-like illness. J Clin Microbiol. 1989;27:1890–1892. doi: 10.1128/jcm.27.8.1890-1892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Levin J, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 328.Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. 1995;187:1123–1131. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lindahl G. Cell surface proteins of a group A streptococcus type M4: the IgA receptor and a receptor related to M proteins are coded for by closely linked genes. Mol Gen Genet. 1989;216:372–379. doi: 10.1007/BF00334378. [DOI] [PubMed] [Google Scholar]
- 330.Lindahl G, Akerstrom B. Receptor for IgA in group A streptococci: cloning of the gene and characterization of the protein expressed in Escherichia coli. Mol Microbiol. 1989;3:339–347. doi: 10.1111/j.1365-2958.1989.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 331.Lindberg L H, Vosti K L. Elution of glomerular bound antibodies in experimental streptococcal glomerulonephritis. Science. 1969;166:1032–1033. doi: 10.1126/science.166.3908.1032. [DOI] [PubMed] [Google Scholar]
- 332.Lottenberg R, Broder C C, Boyle M D P. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987;55:1914–1928. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lottenberg R, DesJardin L E, Wang H, Boyle M D P. Streptokinase-producing streptococci grown in human plasma acquire unregulated cell-associated plasmin activity. J Infect Dis. 1992;166:436–440. doi: 10.1093/infdis/166.2.436. [DOI] [PubMed] [Google Scholar]
- 334.Lottenberg R, Minning-Wenz D, Boyle M. Capturing host plasmin(ogen): a common mechanism for invasive pathogens. Trends Microbiol. 1994;2:20–24. doi: 10.1016/0966-842x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 335.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lukomski S L, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1798. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lyampert I M, Beletskrya L V, Ugryumova G A. The reactions of heart and other organ extracts with the sera of animals immunized with group A streptococci. Immunology. 1968;15:845–854. [PMC free article] [PubMed] [Google Scholar]
- 339.Lyampert I M, Vvedenskaya O I, Danilova T A. Study on streptococcus group A antigens common with heart tissue elements. Immunology. 1966;11:313–320. [PMC free article] [PubMed] [Google Scholar]
- 340.Maharaj B, Hammond M G, Appadoo B, Leary W P, Pudifin D J. HLA-A, B, DR, and DQ antigens in black patients with severe chronic rheumatic heart disease. Circulation. 1987;76:259–261. doi: 10.1161/01.cir.76.2.259. [DOI] [PubMed] [Google Scholar]
- 341.Malke H. Polymorphism of the SK gene: implications for the pathogenesis of post-streptococcal glomerulonephritis. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;278:246–257. doi: 10.1016/s0934-8840(11)80842-x. [DOI] [PubMed] [Google Scholar]
- 342.Manjula B N, Acharya A S, Mische S M, Fairwell T, Fischetti V A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984;259:3686–3693. [PubMed] [Google Scholar]
- 343.Manjula B N, Fischetti V A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986;140:684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- 344.Manjula B N, Fischetti V A. Studies on group A streptococcal M-proteins: purification of type 5 M-protein and comparison of its amino terminal sequence with two immunologically unrelated M-protein molecules. J Immunol. 1980;124:261–267. [PubMed] [Google Scholar]
- 345.Manjula B N, Fischetti V A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980;151:695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Manjula B N, Trus B L, Fischetti V A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad Sci USA. 1985;82:1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Markowitz A S, Lange C F. Streptococcal related glomerulonephritis. I. Isolation, immunocytochemistry and comparative chemistry of soluble fractions from type 12 nephritogenic streptococci and human glomeruli. J Immunol. 1964;92:565. [PubMed] [Google Scholar]
- 348.Markowitz M, Gerber M A, Kaplan L. Treatment of streptococcal pharyngotonsillitis: reports of penicillin's demise are premature. J Pediatr. 1993;123:679–685. doi: 10.1016/s0022-3476(05)80840-6. [DOI] [PubMed] [Google Scholar]
- 349.Markowitz M, Gordis L. Rheumatic fever. 2nd ed. Philadelphia, Pa: W. B. Saunders; 1972. [Google Scholar]
- 350.Massell B, Honikman L, Amezcua Rheumatic fever following streptococcal immunization. J Am Med Assoc. 1969;207:115–119. [PubMed] [Google Scholar]
- 351.Massell B F. Rheumatic fever and streptococcal infection: unraveling the mysteries of a dread disease. Harvard University Press for the Francis A; 1997. . Countway Library of Medicine, Boston, Mass. [Google Scholar]
- 352.Massell B F, Michael J G, Amezcua J, Siner M. Secondary and apparent primary antibody responses after group A streptococcal vaccination of 21 children. Appl Microbiol. 1968;16:509–518. doi: 10.1128/am.16.3.509-518.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Massell B F, Narula J. Rheumatic fever and rheumatic carditis. In: Braunwald E, editor. Atlas of heart diseases. Vol. 2. Philadelphia, Pa: Current Medicine; 1995. pp. 10.1–10.20. [Google Scholar]
- 354.McCormack J M, Crossley C A, Ayoub E M, Harley J B, Cunningham M W. Poststreptococcal anti-myosin antibody idiotype associated with systemic lupus erythematosus and Sjogren's syndrome. J Infect Dis. 1993;168:915–921. doi: 10.1093/infdis/168.4.915. [DOI] [PubMed] [Google Scholar]
- 355.McIver K S, Heath A S, Green B D, Scott J R. Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.McIver K S, Heath A S, Scott J R. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect Immun. 1995;63:4540–4542. doi: 10.1128/iai.63.11.4540-4542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.McIver K S, Scott J R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.McLandsborough L A, Cleary P P. Insertional inactivation of VirR in Streptococcus pyogenes M49 demonstrates that VirR functions as a positive regulator of streptococcal C5a peptidase and M protein in OF+ strains. Dev Biol Stand. 1995;85:149–152. [PubMed] [Google Scholar]
- 359.Medaglini D, Pozzi G, King T P, Fischetti V A. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium streptococcus gordonii after oral colonization. Proc Natl Acad Sci. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Michael A F, Jr, Drummond K N, Good R A, Vernier R L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Investig. 1966;45:237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Miller L C, Gray E D, Beachey E H, Kehoe M A. Antigenic variation among group A streptococcal M proteins: nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988;263:5668–5673. [PubMed] [Google Scholar]
- 362.Minich L L, Tani L Y, Pagotto L T, Shaddy R E, Veasy L G. Doppler echocardiography distinguishes between physiologic and pathologic “silent” mitral regurgitation in patients with rheumatic fever. Clin Cardiol. 1997;20:924–926. doi: 10.1002/clc.4960201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Miyamoto Y, Kotake I, Ochiai S, Kodama T. Experimental nephritis produced in rabbits by inoculation of group A hemolytic streptococci isolated from epidemic nephritis patients: a focal infection experiment in the paranasal sinuses. Jpn J Microbiol. 1960;4:105. doi: 10.1111/j.1348-0421.1960.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 364.Molinari G, Chhatwal G S. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J Infect Dis. 1998;177:1600–1607. doi: 10.1086/515310. [DOI] [PubMed] [Google Scholar]
- 365.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwasl G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Mollick J A, Miller G G, Musser J M, Cook R G, Grossman D, Rich R R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Investig. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Reference deleted.
- 368.Moses A E, Wessels M R, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Muotiala A, Seppala H, Huovinen P, Vuopio-Varkila J. Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis. 1997;175:392–399. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 370.Mouw A R, Beachey E H, Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988;170:676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Murphy G. The characteristic rheumatic lesions of striated and of nonstriated or smooth muscle cells of the heart. Medicine (Baltimore) 1963;42:73–118. [Google Scholar]
- 372.Murphy G E. Nature of rheumatic heart disease with special reference to myocardial disease and heart failure. Medicine (Baltimore) 1960;39:289–384. [PubMed] [Google Scholar]
- 373.Murphy G E, Swift H F. Induction of cardiac lesions closely resembling those of rheumatic fever in rabbits, following repeated skin infections with group A streptococci. J Exp Med. 1949;89:687–698. doi: 10.1084/jem.89.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Murphy G E, Swift H F. The induction of rheumatic-like cardiac lesions in rabbits by repeated focal injections with group A streptococci: comparison with the cardiac lesions of serum disease. J Exp Med. 1950;91:485–498. doi: 10.1084/jem.91.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Murphy T K, Goodman W K, Fudge M W, Williams R C, Ayoub E M, Dalal M, Lewis M H, Zabriskie J B. B lymphocyte antigen D8/17: a peripheral marker for childhood-onset obsessive-compulsive disorder and Tourette's syndrome. Am J Psychiatry. 1997;154:402–407. doi: 10.1176/ajp.154.3.402. [DOI] [PubMed] [Google Scholar]
- 376.Musher D M. Trends in bacteremic infection due to streptococcus pyogenes (group A streptococcus), 1986–1995. Emerg Infect Dis. 1996;2:54–56. doi: 10.3201/eid0201.960107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock like-syndrome. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Musser J M, Kapur V, Kanjilal S, Shah U, Musher D M, Barg N L, Johnston K H, Schlievert P M, Henrichson J, Gerlach D, Rakita R M, Tanna A, Cookson B D, Huang J C. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (scarlet fever toxin) J Infect Dis. 1993;167:337–346. doi: 10.1093/infdis/167.2.337. [DOI] [PubMed] [Google Scholar]
- 379.Musser J M, Krause R M. The revival of group A streptococcal diseases, with a commentary on staphylococcal toxic shock syndrome. New York, N.Y: Academic Press; 1998. [Google Scholar]
- 380.Musser J M, Stockbauer K, Kapur V, Rudgers G W. Substitution of cysteine 192 in a highly conserved Streptococcus pyogenes extracellular cysteine protease (interleukin 1b convertase) alters proteolytic activity and ablates zymogen processing. Infect Immun. 1996;64:1913–1917. doi: 10.1128/iai.64.6.1913-1917.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mylvaganam H, Bjorvatn B, Hofstad T, Hjetland R, Hoiby E A, Holm S. Small-fragment restriction endonuclease analysis in epidemiological mapping of group A streptococci. J Med Microbiol. 1994;40:256–260. doi: 10.1099/00222615-40-4-256. [DOI] [PubMed] [Google Scholar]
- 382.Natanson S, Sela S, Moses A, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 383.Neeman R, Keller N, Barzilai A, Korenman Z, Sela S. Prevalence of the internalization-associated gene, prtF1, among persisting group A streptococcus strains isolated from asymptomatic carriers. Lancet. 1998;352:1974–1977. doi: 10.1016/S0140-6736(97)12452-7. [DOI] [PubMed] [Google Scholar]
- 384.Nelson K, Schlievert P M, Selander R K, Musser J M. Characterization and clonal distribution of four alleles of the speA gene encoding pyrogenic exotoxin A (scarlet fever toxin) in Streptococcus pyogenes. J Exp Med. 1991;174:1271–1274. doi: 10.1084/jem.174.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Neu N, Rose N R, Beisel K W, Herskowitz A, Gurri-Glass G, Craig S W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 386.Nordstrand A, McShan W M, Ferretti J J, Holm S E, Norgren M. Allele substitution of the streptokinase gene reduces the nephritogenic capacity of group A streptococcal strain N2131. Infect Immun. 2000;68:1019–1025. doi: 10.1128/iai.68.3.1019-1025.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nordstrand A, Norgren M, Ferretti J J, Holm S E. Streptokinase as a mediator of acute post-streptococcal glomerulonephritis in an experimental mouse model. Infect Immun. 1998;66:315–321. doi: 10.1128/iai.66.1.315-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Nordstrand A, Norgren M, Holm S E. An experimental model for acute poststreptococcal glomerulonephritis in mice. APMIS. 1996;104:805–816. doi: 10.1111/j.1699-0463.1996.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 389.Norgren M, Norrby A, Holm S E. Genetic diversity in T1M1 group A streptococci in relation to clinical outcome of infection. J Infect Dis. 1992;166:1014–1020. doi: 10.1093/infdis/166.5.1014. [DOI] [PubMed] [Google Scholar]
- 390.Norrby-Teglund A. Ph.D. dissertation. Umea, Sweden: Umea University; 1994. [Google Scholar]
- 391.Norrby-Teglund A, Kaul R, Low D E, McGeer A, Andersson J, Andersson U, Kotb M. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun. 1996;64:5395–5398. doi: 10.1128/iai.64.12.5395-5398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Norrby-Teglund A, Kaul R, Low D E, McGeer A, Newton D W, Andersson J, Andersson U, Kotb M. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol. 1996;156:3057–3064. [PubMed] [Google Scholar]
- 393.Norrby-Teglund A, Lustig R, Kotb M. Differential induction of Th1 versus Th2 cytokines by group A streptococcal toxic shock syndrome isolates. Infect Immun. 1997;65:5209–5215. doi: 10.1128/iai.65.12.5209-5215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Norrby-Teglund A, Norgren M, Holm S E. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994;62:3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.O'Connor S P, Darip D, Fraley K, Nelson C, Kaplan E L, Cleary P. The human antibody response to streptococcal C5a peptidase. J Infect Dis. 1990;163:109–116. doi: 10.1093/infdis/163.1.109. [DOI] [PubMed] [Google Scholar]
- 397.Ofek I, Simpson W A, Beachey E H. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J Bacteriol. 1982;149:426–433. doi: 10.1128/jb.149.2.426-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Ofek L, Beachey E H, Jefferson W, Campbell G L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975;141:900–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ohkuni H, Todome Y, Yoshimura K, Yamamoto T, Suzuki H, Yokomuro K, Johnston K H, Zabriskie J B. Detection of nephritis strain-associated streptokinase by monoclonal antibodies. J Med Microbiol. 1991;35:60–63. doi: 10.1099/00222615-35-1-60. [DOI] [PubMed] [Google Scholar]
- 400.Okada N, Liszewski M, Atkinson J, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Okada N, Pentland A P, Falk P, Caparon M G. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Investig. 1994;94:965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Osman A A, Close H G, Carter H. Studies in Bright's disease. VIII. Observations on the aitiology of scarlatinal nephritis. Guy's Hosp Rep. 1933;83:360. [Google Scholar]
- 403.Osterlund A, Popa R, Nikkila T, Scheynium A, Engstrand L. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope. 1997;107:640–647. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 404.O'Toole P, Stenberg L, Rissler M, Lindahl G. Two major classes in the M protein family in group A streptococci. Proc Natl Acad Sci USA. 1992;89:8661–8665. doi: 10.1073/pnas.89.18.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Otten R A, Raeder R, Heath D G, Lottenberg R, Cleary P P, Boyle M D P. Identification of two type IIa IgG binding proteins expressed by a single group A streptococcus. J Immunol. 1992;148:3174–3182. [PubMed] [Google Scholar]
- 406.Ozeri V, Rosenshine I, Mosher D F, Fassler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 407.Ozkan M, Carin M, Sonnez G, Senocak M, Ozdemin M, Yokut C. HLA antigens in Turkish race with rheumatic heart disease. Circulation. 1993;87:1974–1978. doi: 10.1161/01.cir.87.6.1974. [DOI] [PubMed] [Google Scholar]
- 408.Pack T D, Boyle M D P. Characterization of a type II′o group A streptococcal immunoglobulin-binding protein. Mol Immunol. 1995;32:1235–1243. doi: 10.1016/0161-5890(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 409.Reference deleted.
- 410.Pancholi V, Fischetti V A. α-Enolase, a novel strong plasmin(ogen)-binding protein on the surface of pathogenic streptococci. J Biol Chem. 1998;273:14503–14515. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- 411.Pancholi V, Fischetti V A. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc Natl Acad Sci USA. 1993;90:8154–8188. doi: 10.1073/pnas.90.17.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Pancholi V, Fischetti V A. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J Exp Med. 1997;186:1633–1643. doi: 10.1084/jem.186.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Patarroyo M E, Winchester R J, Vejerano A, Gibofsky A, Chalem F, Zabriske J B, Kinkel H G. Association of a B-cell alloantigen with susceptibility to rheumatic fever. Nature. 1979;278:173–174. doi: 10.1038/278173a0. [DOI] [PubMed] [Google Scholar]
- 415.Perea Mejia L M, Stockbauer K E, Pan X, Cravioto A, Musser J M. Characterization of group A Streptococcus strains recovered from Mexican children with pharyngitis by automated DNA sequencing of virulence-related genes: unexpectedly large variation in the gene (sic) encoding a complement-inhibiting protein. J Clin Microbiol. 1997;35:3220–3224. doi: 10.1128/jcm.35.12.3220-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Perez-Casal J, Caparon M G, Scott J R. Introduction of the emm6 gene into an emm-deleted strain of Streptococcus pyogenes restores its ability to resist phagocytosis. Res Microbiol. 1992;143:549–558. doi: 10.1016/0923-2508(92)90112-2. [DOI] [PubMed] [Google Scholar]
- 417.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Perez-Casal J, Okada N, Caparon M, Scott J R. Role of the conserved C repeat region of the M protein of Streptococcus pyogenes. Mol Microbiol. 1995;15:907–916. doi: 10.1111/j.1365-2958.1995.tb02360.x. [DOI] [PubMed] [Google Scholar]
- 419.Phillips G N, Flicker P F, Cohen C, et al. Streptococcal M protein: α-helical coiled-coil structure and arrangements on the cell surface. Proc Natl Acad Sci USA. 1981;78:4698. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Reference deleted.
- 421.Pichichero M E. The rising incidence of penicillin treatment failures in group A streptococcal tonsillopharyngitis: an emerging role for the cephalosporins? Pediatr Infect Dis J. 1991;10:S50–S55. doi: 10.1097/00006454-199110001-00011. [DOI] [PubMed] [Google Scholar]
- 422.Podbielski A, Flosdorff A, Weber-Heynemann J. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Podbielski A, Hawlitzky J, PacK T D, Flosdorff A, Boyle M D P. A group A streptococcal enn protein potentially resulting from intergenomic recombination exhibits atypical immunoglobulin characteristics. Mol Microbiol. 1994;12:725–736. doi: 10.1111/j.1365-2958.1994.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 424.Podbielski A, Krebs B, Kaufold A. Genetic variability of emm-related genes of the large vir regulon of group A streptococci: potential intra- and intergenomic recombinational events. Mol Gen Genet. 1994;243:691–698. doi: 10.1007/BF00279579. [DOI] [PubMed] [Google Scholar]
- 425.Podbielski A, Schnitzler N, Beyhs P, Boyle M D P. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 426.Podbielski A, Woischnik M, Leonard B A B, Schmidt K-H. Characterization of nra, a global negative regulator gene in group A streptococci. Mol Microbiol. 1999;31:1051–1064. doi: 10.1046/j.1365-2958.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 427.Poirer T P, Kehoe M A, Beachey E H. Humoral and mucosal immunity against group A streptococci evoked by attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988;168:25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Polly S M, Waldman R H, High P, Wittner M K, Dorfman A, Fox E N. Protective studies with a group A streptococcal M protein vaccine. II. Challenge of volunteers after local immunization in the upper respiratory tract. J Infect Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 429.Poon-King R, Bannan J, Viteri A, Cu G, Zabriskie J B. Identification of an extracellular plasmin binding protein from nephritogenic streptococci. J Exp Med. 1993;178:759–763. doi: 10.1084/jem.178.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Poon-King T, Mohammed I, Cox R, Potter E V, Simon N M, Siegel A C, Earle D P. Recurrent epidemic nephritis in South Trinidad. N Engl J Med. 1967;277:728. doi: 10.1056/NEJM196710052771403. [DOI] [PubMed] [Google Scholar]
- 431.Potter E V, Ortiz J S, Sharrett R, et al. Changing types of nephritogenic streptococci in Trinidad. J Clin Investig. 1971;50:1197–1205. doi: 10.1172/JCI106597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Pozzi G, Oggioni M R, Manganelli R, Fischetti V A. Expression of M6 protein gene of Streptococcus pyogenes in Streptococcus gordonii after chromosomal integration and transcriptional fusion. Res Microbiol. 1992;143:449–457. doi: 10.1016/0923-2508(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 433.Reference deleted.
- 434.Proft T, Moffatt S L, Berkahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–101. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Pruksakorn S, Currie B, Brandt E, Hunsakunachai P C S, Manmontri A, Robinson J H, Kehoe M A, Galbraith A, Good M F. Identification of T cell autoepitopes that cross-react with the C-terminal segment of the M protein of group A streptococci. Int Immunol. 1994;6:1235–1244. doi: 10.1093/intimm/6.8.1235. [DOI] [PubMed] [Google Scholar]
- 436.Pruksakorn S, Galbraith A, Houghten R A, Good M F. Conserved T and B cell epitopes on the M protein of group A streptococci: induction of bactericidal antibodies. J Immunol. 1992;149:2729–2735. [PubMed] [Google Scholar]
- 437.Putterman C, Diamond B. Immunization with a peptide surrogate for double stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Quinn A, Adderson E E, Shackelford P G, Carroll W L, Cunningham M W. Autoantibody germ-line gene segment encodes VH and VL regions of a human anti-streptococcal monoclonal antibody recognizing streptococcal M protein and human cardiac myosin epitopes. J Immunol. 1995;154:4203–4212. [PubMed] [Google Scholar]
- 439.Quinn A, Shinnick T M, Cunningham M W. Anti-Hsp65 antibodies recognize M proteins of group A streptococci. Infect Immun. 1996;64:818–824. doi: 10.1128/iai.64.3.818-824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Quinn A, Ward K, Fischetti V, Hemric M, Cunningham M W. Immunological relationship between the class I epitope of streptococcal M protein and myosin. Infect Immun. 1998;66:4418–4424. doi: 10.1128/iai.66.9.4418-4424.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Raeder R, Boyle M D P. Analysis of IgG-binding expression by invasive isolates of Streptococcus pyogenes. Clin Diagn Lab Immunol. 1995;2:484–486. doi: 10.1128/cdli.2.4.484-486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Raeder R, Boyle M D P. Association between expression of immunoglobulin G-binding proteins by group A streptococci and virulence in a mouse skin infection model. Infect Immun. 1993;61:1378–1384. doi: 10.1128/iai.61.4.1378-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Raeder R, Boyle M D P. Association of type II immunoglobulin G-binding protein expression and survival of group A streptococci in human blood. Infect Immun. 1993;61:3696–3702. doi: 10.1128/iai.61.9.3696-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Raeder R, Boyle M D P. Properties of IgG-binding proteins expressed by Streptococcus pyogenes isolates are predictive of invasive potential. J Infect Dis. 1996;173:888–895. doi: 10.1093/infdis/173.4.888. [DOI] [PubMed] [Google Scholar]
- 445.Raeder R, Woischnik M, Podbielski A, Boyle M D P. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin-binding properties. Res Microbiol. 1998;149:539–548. doi: 10.1016/s0923-2508(99)80001-1. [DOI] [PubMed] [Google Scholar]
- 446.Rakonjac J V, Robbins J C, Fischetti V A. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect Immun. 1993;63:622–631. doi: 10.1128/iai.63.2.622-631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Rammelkamp C H., Jr The Lewis A. Conner Memorial Lecture: rheumatic heart disease—a challenge. Circulation. 1958;17:842–851. doi: 10.1161/01.cir.17.5.842. [DOI] [PubMed] [Google Scholar]
- 448.Rammelkamp C H, Jr, Denny F W, Wannamaker L W. Studies on the epidemiology of rheumatic fever in the armed services. In: Thomas L, editor. Rheumatic fever. Minneapolis, Minn: University of Minnesota Press; 1952. pp. 72–89. [Google Scholar]
- 449.Rammelkamp C H, Jr, Weaver R S. Acute glomerulonephritis: the significance of variations in the incidence of the disease. J Clin Investig. 1953;32:345–358. doi: 10.1172/JCI102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Read S E, Reid H F, Fischetti V A, Poon-King T, Ramkissoon R, McDowell M, Zabriskie J B. Serial studies on the cellular immune response to streptococcal antigens in acute and convalescent rheumatic fever patients in Trinidad. J Clin Immunol. 1986;6:433–441. doi: 10.1007/BF00915249. [DOI] [PubMed] [Google Scholar]
- 451.Read S E, Zabriske J B, Fischetti V A, Utermohlen V, Falk R. Cellular reactivity studies to streptococcal antigens in patients with streptococcal infections and their sequelae. J Clin Investig. 1974;54:439–450. doi: 10.1172/JCI107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Reid H G M, Read S E, Poon-King T, Zabriske J B. Lymphocyte response to streptococcal antigens in rheumatic fever patients in Trinidad. In: Read S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press; 1980. pp. 681–693. [Google Scholar]
- 453.Retnoningrum D S, Podbielski A, Cleary P P. Type M12 protein from Streptococcus pyogenes is a receptor for IgG3. J Immunol. 1993;150:2332–2340. [PubMed] [Google Scholar]
- 454.Robbins J C, Spanier J G, Jones S J, Simpson W J, Cleary P P. Streptococcus pyogenes type 12 M protein regulation by upstream sequences. J Bacteriol. 1987;169:5633–5640. doi: 10.1128/jb.169.12.5633-5640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Robinson J H, Atherton M C, Goodacre J A, Pinkney M, Weightman H, Kehoe M A. Mapping T-cell epitopes in group A streptococcal type 5 M protein. Infect Immun. 1991;59:4324–4331. doi: 10.1128/iai.59.12.4324-4331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Robinson J H, Case M C, Kehoe M A. Characterization of a conserved helper T-cell epitope from group A streptococcal M proteins. Infect Immun. 1993;61:1062–1068. doi: 10.1128/iai.61.3.1062-1068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Rothbard S. Protective effect of hyaluronidase and type specific anti-M serum on experimental group A streptococcus infections in mice. J Exp Med. 1948;88:325–342. doi: 10.1084/jem.88.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Ruiz N, Wang B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol. 1997;27:337–346. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- 459.Russell H, Facklam R R. Guanidine extraction of streptococcal M protein. Infect Immun. 1975;12:679–686. doi: 10.1128/iai.12.3.679-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Sabbaga J, Line S R P, Potocnjak P, Madaio M P. A murine nephritogenic monoclonal anti-DNA autoantibody binds directly to mouse laminin, the major non-collagenous protein component of the glomerular basement membrane. Eur J Immunol. 1989;19:137–143. doi: 10.1002/eji.1830190122. [DOI] [PubMed] [Google Scholar]
- 461.Salvadori L G, Blake M S, McCarty M, Tai J Y, Zabriskie J B. Group A streptococcus-liposome ELISA titers to group A polysaccharide and opsonic capabilities of the antibodies. J Infect Dis. 1995;171:593–600. doi: 10.1093/infdis/171.3.593. [DOI] [PubMed] [Google Scholar]
- 462.Sandson J, Hamerman D, Janis R. Immunologic and chemical similarities between the streptococcus and human connective tissue. Trans Assoc Am Physicians. 1968;81:249–257. [PubMed] [Google Scholar]
- 463.Sanford B A, Davison V E, Ramsay M A. Fibrinogen-mediated adherence of group A streptococci to influenza A virus-infected cell cultures. Infect Immun. 1982;38:513–520. doi: 10.1128/iai.38.2.513-520.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Schlievert P M. Role of superantigens in disease. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 465.Schmidt K-H, Gerlach D, Wollweber L, Reichardt W, Mann K, Ozegowski J-H, Fleischer B. Mitogenicity of M5 protein extracted from Streptococcus pyogenes cells is due to streptococcal pyrogenic exotoxin C and mitogenic factor MF. Infect Immun. 1995;63:4569–4575. doi: 10.1128/iai.63.12.4569-4575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Schmidt K-H, Gunther E, Courtney H S. Expression of both M protein and hyaluronic acid capsule by group A streptococcal strains results in a high virulence for chicken embryos. Med Microbiol Immunol. 1996;184:169–173. doi: 10.1007/BF02456131. [DOI] [PubMed] [Google Scholar]
- 467.Schmidt K-H, Podbielski A, Raeder R, Boyle M D P. Inactivation of single genes within the virulence regulon of an M2 group A streptococcal isolate results in differences in virulence for chicken embryos and for mice. Microb Pathog. 1997;23:347–355. doi: 10.1006/mpat.1997.0166. [DOI] [PubMed] [Google Scholar]
- 468.Schmidt K-H, Wadstrom T. A secreted receptor related to M1 protein of Streptococcus pyogenes binds to fibrinogen, IgG and albumin. Zentralbl Bakteriol Hyg Reihe A. 1990;273:216–228. doi: 10.1016/s0934-8840(11)80252-5. [DOI] [PubMed] [Google Scholar]
- 469.Schmidt K H, Mann K, Cooney J, Kohler W. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol Med Microbiol. 1993;7:135–144. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 470.Schneewind O, Jones K F, Fischetti V A. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990;172:3310–3317. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A streptococcus to CD44 on human keratinocytes. J Clin Investig. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Schwab J H. Analysis of the experimental lesion of connective tissue produced by a complex of C polysaccharide from group A streptococci. II. Influence of age and hypersensitivity. J Exp Med. 1964;119:401–408. doi: 10.1084/jem.119.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Schwab J H. Analysis of the experimental lesion of connective tissue produced by a complex of C polysaccharide from group A streptococci. I. In vivo reaction between tissue and toxin. J Exp Med. 1962;116:17–28. doi: 10.1084/jem.116.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Schwab J H. Biological properties of streptococcal cell wall particles. I. Determinants of the chronic nodular lesion of connective tissue. J Bacteriol. 1965;90:1405–1411. doi: 10.1128/jb.90.5.1405-1411.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Schwab J H, Allen J, Anderle S, Dalldorf F, Eisenberg R, Cromartie W J. Relationship of complement to experimental arthritis induced in rats with streptococcal cell walls. Immunology. 1982;46:83–88. [PMC free article] [PubMed] [Google Scholar]
- 477.Scott J R, Fischetti V A. Expression of streptococcal M protein in Escherichia coli. Science. 1983;221:758–760. doi: 10.1126/science.6192499. [DOI] [PubMed] [Google Scholar]
- 478.Scott J R, Guenther P C, Malone L M, Fischetti V A. Conversion of an M- group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986;164:1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Scott J R, Pulliam W M, Hollingshead S K, Fischetti V A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci USA. 1985;82:1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Sela S, Aviv A, Tovi A, Burstein I, Caparon M G, Hanski E. Protein F—an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains at two different binding sites. Mol Microbiol. 1994;10:1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 481.Seligson G, Lange K, Majeed H A, Deol H L, Cronin W, Boyle R. Significance of endostreptosin antibody titers in poststreptococcal glomerulonephritis. Clin Nephrol. 1985;24:69–75. [PubMed] [Google Scholar]
- 482.Seppala H, He Q, Osterblad M, Huovinen P. Typing of group A streptococci by random amplified polymorphic DNA analysis. J Clin Microbiol. 1994;32:1945–1948. doi: 10.1128/jcm.32.8.1945-1948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Shikhman A R, Cunningham M W. Immunological mimicry between N-acetyl-beta-d-glucosamine and cytokeratin peptides: evidence for a microbially driven anti-keratin antibody response. J Immunol. 1994;152:4375–4387. [PubMed] [Google Scholar]
- 484.Shikhman A R, Greenspan N S, Cunningham M W. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-d-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J Immunol. 1994;153:5593–5606. [PubMed] [Google Scholar]
- 485.Shikhman A R, Greenspan N S, Cunningham M W. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-d-glucosamine. J Immunol. 1993;151:3902–3913. [PubMed] [Google Scholar]
- 486.Siegel A C, Johnson E E, Stollerman G H. Controlled studies of streptococcal pharyngitis in a pediatric population. I. Factors related to the attack rate of rheumatic fever. N Engl J Med. 1961;265:559–566. [Google Scholar]
- 487.Silva F G. Acute postinfectious glomerulonephritis and glomerulonephritis complicating persistent bacterial infection. In: Jennette J C, Olson J L, Schwartz M M, Silva F G, editors. Hepinstall's pathology of the kidney. 5th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1998. pp. 389–453. [Google Scholar]
- 488.Simpson W A, Beachey E H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983;39:275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Simpson W A, Ofek I, Sarasohn C, Morrison J C, Beachey E H. Characteristics of the binding of streptococcal lipoteichoic acid to human oral epithelial cells. J Infect Dis. 1980;141:457–462. doi: 10.1093/infdis/141.4.457. [DOI] [PubMed] [Google Scholar]
- 490.Simpson W J, Cleary P P. Expression of M type 12 protein by a group A streptococcus exhibits phase-like variation: evidence of coregulation of colony opacity determinants and M protein. Infect Immun. 1987;55:2448–2455. doi: 10.1128/iai.55.10.2448-2455.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Simpson W J, LaPenta D, Chen C, Cleary P P. Coregulation of type 12 M protein and streptococcal C5a peptidase genes in group A streptococci: evidence for a virulence regulon controlled by the virR locus. J Bacteriol. 1990;172:696–700. doi: 10.1128/jb.172.2.696-700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Simpson W J, Robbins J C, Cleary P P. Evidence for group A-related M protein genes in human but not animal-associated group G streptococcal pathogens. Microb Pathog. 1987;3:339–350. doi: 10.1016/0882-4010(87)90004-0. [DOI] [PubMed] [Google Scholar]
- 493.Spanier J G, Jones S J C, Cleary P. Small DNA deletions creating avirulence in Streptococcus pyogenes. Science. 1984;225:935–938. doi: 10.1126/science.6089334. [DOI] [PubMed] [Google Scholar]
- 494.Stegmayer B, Bjorck S, Holm S, Nisell J, Rydvall A, Settergren B. Septic shock induced by group A streptococcal infection: clinical and therapeutic aspects. Scand J Infect Dis. 1992;24:589–597. doi: 10.3109/00365549209054644. [DOI] [PubMed] [Google Scholar]
- 495.Stenberg L, O'Toole P, Lindahl G. Many group A streptococcal strains express two immunoglobulin binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol. 1992;6:1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 496.Stetson C A. The relation of antibody response to rheumatic fever. In: McCarty M, editor. Streptococcal infection. New York, N.Y: Columbia University Press; 1954. pp. 208–218. [Google Scholar]
- 497.Stevens D L, Gibbons A E, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–28. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 498.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–8. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 499.Stevens D L, Yan S, Bryant A E. Penicillin binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation of the inoculum effect. J Infect Dis. 1993;167:1401–1405. doi: 10.1093/infdis/167.6.1401. [DOI] [PubMed] [Google Scholar]
- 500.Stockbauer K E, Grigsby D, Pan X, Fu Y-X, Perea Mejia L M, Cravioto A, Musser J. Hypervariability generated by natural selection in an extracellular complement-inhibiting protein of serotype M1 strains of group A Streptococcus. Proc Natl Acad Sci USA. 1998;95:3128–3133. doi: 10.1073/pnas.95.6.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Stockbauer K E, Magoun L, Liu M, Burns E H, Jr, Gubba S, Renish S, Pan X, Bodary S, Baker E, Coburn J, Leong J, Musser J M. A natural variant of the cysteine protease virulence factor of group A streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc Natl Acad Sci USA. 1999;96:242–247. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Stollerman G H. The changing face of rheumatic fever in the 20th century. J Med Microbiol. 1998;47:1–3. doi: 10.1099/00222615-47-8-655. [DOI] [PubMed] [Google Scholar]
- 503.Stollerman G H. Changing streptococci and prospects for the global eradication of rheumatic fever. Perspect Biol Med. 1997;40:165–189. doi: 10.1353/pbm.1997.0044. [DOI] [PubMed] [Google Scholar]
- 504.Stollerman G H. The global impact of penicillin. Mt Sinai J Med. 1993;60:112–119. [PubMed] [Google Scholar]
- 505.Stollerman G H. Rheumatic and heritable connective tissue diseases of the cardiovascular system. In: Braunmald E, editor. Heart disease: a textbook of cardiovascular medicine. Vol. 11. Philadelphia, Pa: W. B. Saunders; 1988. pp. 1706–1734. [Google Scholar]
- 506.Stollerman G H. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 507.Stollerman G H. Rheumatic fever and streptococcal infection. In: Stollerman G H, editor. Clinical cardiology monographs. New York, N.Y: Grune and Stratton; 1975. pp. 1–303. [Google Scholar]
- 508.Stollerman G H. Rheumatogenic streptococci and autoimmunity. Clin Immunol Immunopathol. 1991;61:131–142. doi: 10.1016/s0090-1229(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 509.Stoolmiller A C, Dorfman A. The biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1969;244:236–246. [PubMed] [Google Scholar]
- 510.Stromberg A, Romanus V, Burman L G. Outbreak of group A streptococcal bacteremia in Sweden: an epidemiologic and clinical study. J Infect Dis. 1991;164:595–598. doi: 10.1093/infdis/164.3.595. [DOI] [PubMed] [Google Scholar]
- 511.Swanson J, Hsu K C, Gotschlich E C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969;130:1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Swedo S E. Sydenham's chorea: a model for childhood autoimmune neuropsychiatric disorders. JAMA. 1994;272:1788–1791. doi: 10.1001/jama.272.22.1788. [DOI] [PubMed] [Google Scholar]
- 513.Swedo S E, Leonard H L, Mittleman B B, Allen A J, Rapoport J L, Dow S P, Kanter M E, Chapman F, Zabriskie J. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry. 1997;154:110–112. doi: 10.1176/ajp.154.1.110. [DOI] [PubMed] [Google Scholar]
- 514.Swedo S E, Leonard H L, Schapiro M B, Casey B J, Mannheim G B, Lenane M C, Rettew D C. Sydenham's chorea: physical and psychological symptoms of St. Vitus dance. Pediatrics. 1993;91:706–713. [PubMed] [Google Scholar]
- 515.Switalski L, Speziale P, Hook M, Waldstrom T, Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984;259:3734. [PubMed] [Google Scholar]
- 516.Taranta A, Torosdag S, Metrakos J D. Rheumatic fever in monozygotic and dizygotic twins. Circulation. 1959;20:778–792. [Google Scholar]
- 517.Tewodros W, Nordstrand A, Kronvall G, Holm S E, Norgren M. Streptokinase gene polymorphism in group A streptococci isolated from Ethiopian children with various disease manifestations. Microb Pathog. 1993;15:303–311. doi: 10.1006/mpat.1993.1080. [DOI] [PubMed] [Google Scholar]
- 518.Reference deleted.
- 519.Tillett W S, Garner R L. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933;58:485–502. doi: 10.1084/jem.58.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Todd E W. Antigenic streptococcal hemolysin. J Exp Med. 1932;55:267–280. doi: 10.1084/jem.55.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Tomai M, Aileon J A, Dockter M E, Majumbar G, Spinella D G, Kotb M. T cell receptor V gene usage by human T cells stimulated with the superantigen streptococcal M protein. J Exp Med. 1991;174:285–288. doi: 10.1084/jem.174.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Tomai M, Kotb M, Majumdar G, Beachey E H. Superantigenicity of streptococcal M protein. J Exp Med. 1990;172:359–62. doi: 10.1084/jem.172.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Tomai M, Schlievert P M, Kotb M. Distinct T cell receptor Vβ gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and M protein. Infect Immun. 1992;60:701–705. doi: 10.1128/iai.60.2.701-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Treser G, Semar M, McVicar M, Franklin M, Ty A, Sagel I, Lange K. Antigenic streptococcal components in acute glomerulonephritis. Science. 1969;163:676. doi: 10.1126/science.163.3868.676. [DOI] [PubMed] [Google Scholar]
- 525.Unny S K, Middlebrooks B L. Streptococcal rheumatic carditis. Microbiol Rev. 1983;47:97–120. doi: 10.1128/mr.47.1.97-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Valentin-Weigand P, Grulich-Henn J, Chhatwal G S, Muller-Berghaus G, Blobel H, Preissner K T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin) Infect Immun. 1988;56:2851–2855. doi: 10.1128/iai.56.11.2851-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Van Den Busche R A, Lyon J D, Bohach G A. Molecular evolution of the staphylococcal and streptococcal pyrogenic toxin gene family. Mol Phylogenet Evol. 1993;2:281–292. doi: 10.1006/mpev.1993.1027. [DOI] [PubMed] [Google Scholar]
- 528.van de Rijn I, Zabriske J B, McCarty M. Group A streptococcal antigens cross-reactive with myocardium: purification of heart reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977;146:579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Veasy L G. Rheumatic fever—T. Duckett Jones and the rest of the story. Cardiol Young. 1995;5:293–301. [Google Scholar]
- 530.Veasy L G, Tani L Y, Hill H R. Persistence of acute rheumatic fever in the intermountain area of the United States. J Pediatr. 1994;124:9–16. doi: 10.1016/s0022-3476(94)70247-0. [DOI] [PubMed] [Google Scholar]
- 531.Veasy L G, Wiedmeier S E, Orsmond G S. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316:421–427. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]
- 532.Villarreal H, Fischetti V A, van de Rijn I, Zabriskie J B. The occurrence of a protein in the extracellular products of streptococci isolated from patients with acute glomerulonephritis. J Exp Med. 1979;149:459–472. doi: 10.1084/jem.149.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Visai L, Bozzini S, Raucci G, Toniolo A, Speziale P. Isolation and characterization of a novel collagen-binding protein from Streptococcus pyogenes strain 6414. J Biol Chem. 1995;270:347–353. doi: 10.1074/jbc.270.1.347. [DOI] [PubMed] [Google Scholar]
- 534.Vosti K L, Williams W K. Extraction of streptococcal type 12 M protein by cyanogen bromide. Infect Immun. 1978;21:546–555. doi: 10.1128/iai.21.2.546-555.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Wadstrom T, Tylewska S. Glycoconjugates as possible receptors for Streptococcus pyogenes. Curr Microbiol. 1982;7:343–346. [Google Scholar]
- 536.Wang B, Ruiz N, Pentland A, Caparon M. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect Immun. 1997;65:2119–2126. doi: 10.1128/iai.65.6.2119-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Wang B, Schlievert P M, Gaber A O, Kotb M. Localization of an immunologically functional region of the streptococcal superantigen pepsin-extracted fragment of type 5 M protein. J Immunol. 1993;151:1419–1429. [PubMed] [Google Scholar]
- 538.Wang H, Lottenberg R, Boyle M D P. Analysis of the interaction of group A streptococci with fibrinogen, streptokinase and plasminogen. Microb Pathog. 1995;18:153–166. doi: 10.1016/s0882-4010(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 539.Wang H, Lottenberg R, Boyle M D P. A role for fibrinogen in the streptokinase-dependent acquisition of plasmin(ogen) by group A streptococci. J Infect Dis. 1995;171:85–92. doi: 10.1093/infdis/171.1.85. [DOI] [PubMed] [Google Scholar]
- 540.Wang J, Stinson M. M protein mediates streptococcal adhesion to HEp-2 cells. Infect Immun. 1994;62:442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Wang J, Stinson M. Streptococcal M6 protein binds to fucose-containing glycoproteins on cultured human epithelial cells. Infect Immun. 1994;62:1268–1274. doi: 10.1128/iai.62.4.1268-1274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Wannamaker L W. The chain that links the throat to the heart. Circulation. 1973;48:9–18. doi: 10.1161/01.cir.48.1.9. [DOI] [PubMed] [Google Scholar]
- 543.Wannamaker L W. Differences between streptococcal infections of the throat and of the skin. N Engl J Med. 1970;282:23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- 544.Watanabe-Ohnishi R, Low D E, McGeer A, Stevens D L, Schlievert P M, Newton D, Schwartz B, Kreiswirth B, Kotb M Ontario Streptococcal Study Project. Selective depletion of V-beta bearing T cells in patients with severe invasive group A streptococcal infections and streptococcal toxic shock syndrome. J Infect Dis. 1995;171:74–84. doi: 10.1093/infdis/171.1.74. [DOI] [PubMed] [Google Scholar]
- 545.Wedemayer G J, Patten P A, Wang L H, Schultz P G, Stevens R C. Structural insights into the evolution of an antibody combining site. Science. 1997;276:1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 546.Weeks C R, Ferretti J J. The gene for type A streptococcal exotoxin (erythrogenic toxin) is located in the bacteriophage T12. Infect Immun. 1984;46:531–536. doi: 10.1128/iai.46.2.531-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Weeks C R, Ferretti J J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986;52:144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Wells A, Parajasegaram G, Baldwin M, Yang C, Hammer M, Fox A. Uveitis and arthritis induced by systemic injection of streptococcal cell walls. Invest Ophthalmol Vis Sci. 1986;27:921–925. [PubMed] [Google Scholar]
- 549.Wessels M R, Bronze M S. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA. 1994;91:12238–12242. doi: 10.1073/pnas.91.25.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Wessels M R, Goldberg J B, Moses A E, DiCesare T J. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun. 1994;62:433–441. doi: 10.1128/iai.62.2.433-441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wexler D, Chenoweth E, Cleary P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci USA. 1985;82:8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Whatmore A M, Kehoe M A. Horizontal gene transfer in the evolution of group A streptococcal emm-like genes: gene mosaics and variation in vir regulons. Mol Microbiol. 1994;11:363–374. doi: 10.1111/j.1365-2958.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 554.Reference deleted.
- 555.Whitnack E, Beachey E H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Investig. 1982;69:1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Whitnack E, Beachey E H. Biochemical and biological properties of the binding of human fibrinogen to M protein in group A streptococci. J Bacteriol. 1985;164:350–358. doi: 10.1128/jb.164.1.350-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Whitnack E, Dale J B, Beachey E H. Common protective antigens of group A streptococcal M proteins masked by fibrinogen. J Exp Med. 1984;159:1201–1202. doi: 10.1084/jem.159.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Widdowson J P. The M-associated protein antigens of group A streptococci. In: Read S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press; 1980. pp. 125–147. [Google Scholar]
- 559.Widdowson J P, Maxted W R, Grant D L. The production of opacity in serum by group A streptococci and its relationship with the presence of M antigen. J Gen Microbiol. 1970;61:343–353. doi: 10.1099/00221287-61-3-343. [DOI] [PubMed] [Google Scholar]
- 560.Widdowson J P, Maxted W R, Grant D L, Pinney A M. The relationship between M-antigen and opacity factor in group A streptococci. J Gen Microbiol. 1971;65:69–80. doi: 10.1099/00221287-65-1-69. [DOI] [PubMed] [Google Scholar]
- 561.Widdowson J P, Maxted W R, Pinney A M. An M-associated protein antigen (MAP) of group A streptococci. J Hyg. 1976;69:553–564. doi: 10.1017/s0022172400021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Wilson M G, Schweitzer M D, Lubschez R. The familial epidemiology of rheumatic fever. J Pediatr. 1943;22:468–492. [Google Scholar]
- 563.Winram S B, Lottenberg R. The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology. 1996;142:2311–2320. doi: 10.1099/13500872-142-8-2311. [DOI] [PubMed] [Google Scholar]
- 564.Reference deleted.
- 565.Winram S B, Lottenberg R. Site-directed mutagenesis of streptococcal plasmin receptor protein (Plr) identifies the C-terminal Lys334 as essential for plasmin binding, but mutation of the plr gene does not reduce plasmin binding to group A streptococci. Microbiology. 1998;144:2025–2035. doi: 10.1099/00221287-144-8-2025. [DOI] [PubMed] [Google Scholar]
- 566.Wistedt A C, Ringdahl U, Muller-Estrel W, Sjobring U. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol Microbiol. 1995;18:569–578. doi: 10.1111/j.1365-2958.1995.mmi_18030569.x. [DOI] [PubMed] [Google Scholar]
- 567.Wittner M K, Fox E N. Homologous and heterologous protection of mice with group A streptococcal M protein vaccines. Infect Immun. 1977;15:104–108. doi: 10.1128/iai.15.1.104-108.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Wolf B B, Gibson C A, Kapur V, Hussaini M, Musser J M, Gonias S L. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J Biol Chem. 1994;269:30682–30687. [PubMed] [Google Scholar]
- 569.Woodruff J F. Viral myocarditis—a review. Am J Pathol. 1980;101:425–466. [PMC free article] [PubMed] [Google Scholar]
- 569a.Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA. 1993;269:390–391. [PubMed] [Google Scholar]
- 570.Wu X, Liu B, Van der Merwe P L, Kalis N N, Berney S M, Young D C. Myosin-reactive autoantibodies in rheumatic carditis and normal fetus. Clin Immunol Immunopathol. 1998;87:184–192. doi: 10.1006/clin.1998.4531. [DOI] [PubMed] [Google Scholar]
- 571.Xu S, Collins C. Temperature regulation of the streptococcal pyrogenic exotoxin A-encoding gene (speA) Infect Immun. 1996;64:5399–5402. doi: 10.1128/iai.64.12.5399-5402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yoshizawa N, Treser G, McClung J A, Sagel I, Takahashi K. Circulating immune complexes in patients with uncomplicated group A streptococcal pharyngitis and patients with acute poststreptococcal glomerulonephritis. Am J Nephrol. 1983;3:23–29. doi: 10.1159/000166682. [DOI] [PubMed] [Google Scholar]
- 573.Yu C, Ferretti J J. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A streptococci. Infect Immun. 1991;59:211–215. doi: 10.1128/iai.59.1.211-215.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Yu C-E, Ferretti J J. Molecular characterization of new group A streptococcal bacteriophages containing the gene for streptococcal erythrogenic toxin A (speA) Mol Gen Genet. 1991;231:161–168. doi: 10.1007/BF00293833. [DOI] [PubMed] [Google Scholar]
- 575.Zabriskie J B. Mimetic relationships between group A streptococci and mammalian tissues. Adv Immunol. 1967;7:147–188. doi: 10.1016/s0065-2776(08)60128-5. [DOI] [PubMed] [Google Scholar]
- 576.Zabriskie J B, Freimer E H. An immunological relationship between the group A streptococcus and mammalian muscle. J Exp Med. 1966;124:661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Zabriskie J B, Hsu K C, Seegal B C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970;7:147–159. [PMC free article] [PubMed] [Google Scholar]