Abstract
High mobility group box 1 (HMGB1) is a nonhistone nuclear protein that has multiple functions according to its subcellular location. In the nucleus, HMGB1 is a DNA chaperone that maintains the structure and function of chromosomes. In the cytoplasm, HMGB1 can promote autophagy by binding to BECN1 protein. After its active secretion or passive release, extracellular HMGB1 usually acts as a damage-associated molecular pattern (DAMP) molecule, regulating inflammation and immune responses through different receptors or direct uptake. The secretion and release of HMGB1 is fine-tuned by a variety of factors, including its posttranslational modification (e.g., acetylation, ADP-ribosylation, phosphorylation, and methylation) and the molecular machinery of cell death (e.g., apoptosis, pyroptosis, necroptosis, alkaliptosis, and ferroptosis). In this minireview, we introduce the basic structure and function of HMGB1 and focus on the regulatory mechanism of HMGB1 secretion and release. Understanding these topics may help us develop new HMGB1-targeted drugs for various conditions, especially inflammatory diseases and tissue damage.
Subject terms: Molecular biology, Medical research
Inflammation: Nuclear protein offers target for controlling immune response
A nuclear protein that gets released after cell death or is actively secreted by immune cells offers a promising therapeutic target for treating diseases linked to excessive inflammation. Daolin Tang from the University of Texas Southwestern Medical Center in Dallas, USA, and colleagues review how cellular stresses can trigger the accumulation of HMGB1, a type of alarm signal protein that promotes the recruitment and activation of inflammation-promoting immune cells. The researchers discuss various mechanisms that drive both passive and active release of HMGB1 into the space around cells. These processes, which include enzymatic modifications of the HMGB1 protein, cell–cell interactions and molecular pathways of cell death, could be targeted by drugs to lessen tissue damage and inflammatory disease caused by HMGB1-induced immune responses
Introduction
In 1973, Ernest Johns, Graham Goodwin and colleagues extracted a set of nonhistone proteins from calf thymus chromatin1,2. Subsequently, these proteins were named “high mobility group” (HMG) proteins because of their high mobility in polyacrylamide gel electrophoresis systems with no signs of aggregation. Currently, HMG family proteins include HMGB, HMGN, and HMGA subfamilies, which are highly evolutionarily conserved nuclear proteins3. As the most abundant protein among all the HMG family members and the second-most abundant protein in the nucleus, HMGB1 (also known as HMG14, HMG-15, and amphoterin6) is widely expressed in mammalian cells and tissues7. Normally, HMGB1 is mainly located in the nucleus and binds to chromatin, but it can shuttle from the nucleus to the cytoplasm under various stress conditions and then into the extracellular space, for example, when induced by elevated reactive oxygen species (ROS) production during porcine circovirus type 2 infection8,9. The subcellular location of HMGB1 varies depending on cell type, tissue, and stress signals, and its location is key to its functions10,11. HMG proteins have multiple nuclear functions, and additional extracellular and proinflammatory activities are continuously being discovered for HMGB1 5,12and related proteins, such as HMGB213 and HMGN114. In response to infection and tissue damage, HMGB1 can be actively secreted and passively released outside cells, where they function as damage-associated molecular pattern molecules (DAMPs) to mediate inflammation and immune responses15,16. In this minireview, we briefly introduce basic HMGB1 biology and focus on the mechanisms involved in HMGB1 secretion and release in the context of stressors. Gaining knowledge of HMGB1 may help us develop new HMGB1-targeted drugs for disease treatment.
HMGB1 structure
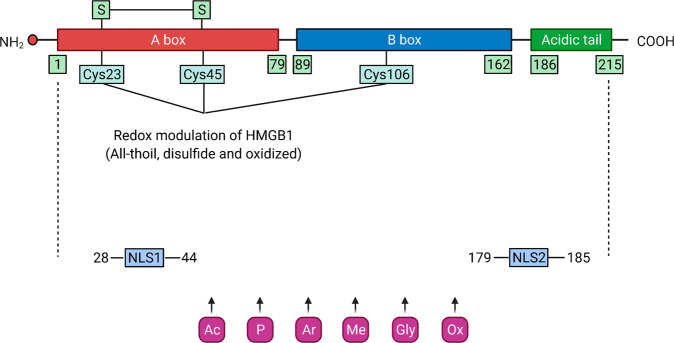
The mRNA of HMGB1 is polyadenylated17, and the protein sequence of HMGB1 shows 100% homology between mice and rats and 99% homology between rodents and humans18. The human HMGB1 protein consists of 215 amino acid residues, which are arranged in two consecutive DNA binding domains (namely, the HMG A box domain [9–79 amino acids] and HMG B box domain [95–163 amino acids]) followed by a C-terminal acidic tail (186–215 amino acids) and a short but functionally significant N-terminal region. The two HMG boxes of HMGB1 are structurally composed of three alpha helices and two loops that are arranged in an “L” shape19. The steady location of HMGB1 in the nucleus is due to two nuclear localization signals (NLSs): NLS1 (28–44 amino acids) and NLS2 (179–185 amino acids)20, while the nuclear export signal (NES) is contained in the DNA binding domains. Thus, an abnormal HMGB1 subcellular location can be induced with changes in the NES and NLS2. Specific residues in the HMGB1 sequence are responsible for the interaction, binding, activity, and function of HMGB119,21,22. The extracellular B box can recapitulate proinflammatory activity, whereas the A box can act as an HMGB1 antagonist due to its ability to bind to the B box23. After fusion with the C-terminal acid tail, the anti-inflammatory activity of the HMGB1 A box is enhanced24. Overall, the structure of HMGB1 is highly conserved across species (Fig. 1).
Fig. 1. The structure and modification of HMGB1.
HMGB1 consists of three domains: two DNA binding domains (Box A and Box B), a C-terminus and an N-terminus. HMGB1 undergoes various posttranslational modifications after cell activation induced by external stimuli, resulting in its translocation from the nucleus to the cytoplasm and finally its release. Ac, acetylation; Ar, ADP-ribosylation; Cys, cysteine; Gly, N-glycosylation; Me, methylation; NLS1/2, nuclear localization signal 1/2; Ox, oxidation; P, phosphorylation.
HMGB1 modification
The position and activity of HMGB1 are affected by posttranslational modifications. HMGB1 can shuttle between the nucleus and the cytoplasm by modifying NLS1 and NLS2 through acetylation and deacetylation mediated by histone acetyltransferase (HAT) family proteins and histone deacetylase (HDAC) family proteins12,20,25. In contrast, acetylation of lysine in the NLS prevents nuclear reentry of HMGB120,26. In addition, HMGB1 can bind with exportin 1 (XPO1, best known as chromosome region maintenance 1 [CRM1]) and be acetylated under oxidative stress and then transferred to the cytoplasm27. Animal studies have confirmed that various oxidative stresses can induce the release of hyperacetylated HMGB1 under various pathological conditions28,29. Various signaling pathways are implicated in HMGB1 acetylation and deacetylation, such as the interferon (IFN)-Janus kinase (JAK) signal transducer and activator of transcription 1 (STAT1) pathway30,31 and sirtuin1 (SIRT1) pathway32. In addition to acetylation33, other modifications, such as methylation, N-glycosylation, phosphorylation, and oxidation, can regulate the translocation and release of HMGB1 to the extracellular space in response to various stresses20,27,34–40. For example, phosphorylation of the serine residues of NLS1 and NLS2, which is mediated by protein kinase C (PKC), regulates the nucleus-cytoplasm shuttling of HMGB138. Lys42 methylation of nuclear HMGB1 can alter HMGB1 protein conformation and reduce HMGB1–DNA binding activity35,41, leading to passive diffusion of HMGB1 out of the nucleus. Secretion of HMGB1 from macrophages can be facilitated when HMGB1 is poly(ADP-ribosyl)ated, a posttranslational modification produced by poly(ADP-ribose) polymerase 1 (PARP1)37,42. Glutamate residues at 40, 47, and 179 have been shown to be possible ADP-ribosylation sites43–45. Liquid chromatography tandem mass spectrometry showed that N-glycosylation of three residues of HMGB1 (N37, N134, and N135) attenuates the binding of HMGB1 with DNA and promotes HMGB1 release39. HMGB1 possesses three cysteine residues at amino acids C23, C45, and C106. Three redox forms of HMGB1 depend on the redox conditions of the environment and are associated with its function26,46. Intracellular fully reduced HMGB1 (fr-HMGB1), with three conserved cysteines containing thiol groups, can form a complex with CXCL12 and promote the migration of immune cells. Partially reduced HMGB1 with a disulfide bond between cysteine 23 and cysteine 45, termed disulfide HMGB1 (ds-HMGB1), triggers inflammatory responses. Fully oxidized HMGB1 (ox-HMGB1) has no chemokine or cytokine activity26,46. According to the redox status of these residues, HMGB1 can induce or suppress the immune response through its subcellular location40. In summary, various modifications mediate the translocation of HMGB1 from the nucleus to the cytoplasm and then to the extracellular space (Fig. 1 and Table 1). However, whether and how these posttranscriptional modifications are competitively, cooperatively, or independently regulated under different conditions remains obscure7.
Table 1.
Sites and functions of posttranslational modification (PTM) of HMGB1.
| PTM | Site | Function |
|---|---|---|
| Acetylation/deacetylation | Lysine in NLS | HMGB1 translocation between the nucleus and cytoplasm12,20,25,34 |
| Methylation | Lys42 | Alters HMGB1 protein conformation and reduces its DNA binding activity35,41 |
| Phosphorylation | Serine residues in NLS1 and NLS2 | Regulates the nucleus-cytoplasm shuttling of HMGB136,38 |
| ADP-ribosylation | C-terminal tail | Promotes HMGB1 secretion in macrophages37,42–45 |
| N-glycosylation | N37, N134, and N135 | Attenuates the binding of HMGB1 with DNA and promotes HMGB1 release39 |
| Redox and oxidation | C23, C45, and C106 | Regulates the inflammatory activity of HMGB140; induces translocation of HMGB1 protein from the nucleus to the cytoplasm53–61 |
Active HMGB1 secretion
Wang and colleagues first reported in 1999 that cultured macrophages treated with the endotoxin lipopolysaccharide (LPS) can secrete HMGB1 in large quantities into the extracellular space5. The release of HMGB1 was further confirmed in a mouse model of lethal endotoxemia5. In addition to LPS, accumulating evidence has documented that exogenous microbial products, various pathogen infections, and endogenous host stimuli can induce active HMGB1 secretion in multiple cells, such as, but not limited to, immune cells, fibroblasts, and epithelial or endothelial cells47. Due to the lack of a leader sequence, HMGB1 cannot be actively secreted through the conventional endoplasmic reticulum-Golgi secretory pathway that most soluble secretory proteins utilize19,48. Nevertheless, two models of HMGB1 active release have been proposed and frequently cited49. One involves activation of target cells with stimuli, resulting in secretion of HMGB1 into the extracellular space20,50. The second is packaging of HMGB1 into intracellular vesicles (such as lysosomes or autophagosomes) and subsequent release of HMGB1 outside the cell after fusion of the vesicles with the cell plasma membrane25,51. The detailed signals and modulators (proteins or organelles) involved in HMGB1 active secretion are described below (Fig. 2).
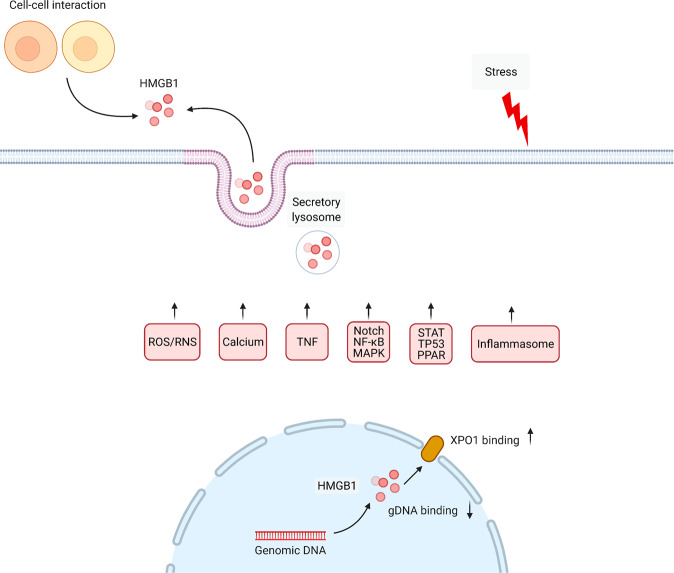
Fig. 2. Active secretion of HMGB1 during stress.
HMGB1 secretion is mediated by secretory lysosomes, which can be triggered by a variety of cytokines, signaling pathways, and cell–cell interactions.
ROS
ROS are oxygen atom-containing free radicals generated during various physiological processes and pathological disorders. The ROS family includes singlet oxygen (1O2•), superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). ROS generation is one of the basic mechanisms that causes the antibacterial activity of immune cells, and it has multiple roles in signal transduction. However, ROS are a main contributor to oxidative stress, and excessive ROS accumulation can lead to cell damage and cell death52. Mounting evidence has demonstrated the important role of ROS in the active secretion and passive release of HMGB1 in immune and nonimmune cells53. For example, paclitaxel increases ROS accumulation and phosphorylation of p38 mitogen-activated protein kinase 1 (MAPK1)-nuclear factor kappa B (NF-κB) signaling molecules, which leads to HMGB1 secretion from macrophages54. Cultured normal human melanocytes can release HMGB1 following treatment with H2O227,28,55. H2O2 induces HMGB1 release in macrophages and monocytes through activation of the MAPK and NF-κB pathways27,41. Antioxidants, such as N-acetylcysteine56, quercetin57, ethyl pyruvate54, edaravone58, pyrrolidine dithiocarbamate59, EUK-860, tert-butylhydroqinone60, and resveratrol61, significantly inhibit HMGB1 release in various infection and injury models in vitro and in vivo.
The key regulator of the response to oxidative stress is NFE2 like BZIP transcription factor 2 (NFE2L2, best known as nuclear factor erythroid 2-related factor [NRF2]), which acts as a transcription factor to trigger the expression of a series of antioxidant genes, such as heme oxygenase-1 (HMOX1, best known as HO-1)62. In addition to its primary function in heme homeostasis, HMOX1 also plays a vital role in antioxidant protection and the anti-inflammatory process of macrophages63. HMOX1 catalyzes the first and rate-limiting step in the oxidative degradation of heme to carbon monoxide, biliverdin, and ferrous iron. Biliverdin and carbon monoxide have anti-inflammatory properties. A dysfunctional NFE2L2-HMOX1-HMGB1 signaling pathway has been implicated in various pathological conditions, especially in hypoxia-induced injury in the heart, intestine, and brain62,64–69. Upregulation of HMOX1 inhibits the translocation of HMGB1 to the cytosol, thereby reducing its active secretion64. Hemin blocks radiation-induced HMGB1 release in an HMOX1-dependent manner in human keratinocyte HaCaT cells70. As expected, the absence of NFE2L2 reduces the expression of HMOX1 during inflammation and increases the release of HMGB170,71. However, downregulation of HMGB1 is necessary for NFE2L2-mediated HMOX1 production during pulmonary fibrosis72. The activation of the NFE2L2-HMOX1-HMGB1 axis is further regulated by β-1-adrenergic receptors, leading to a reduction in cardiomyocyte damage caused by hypoxia/reoxygenation68. Overall, the mutual regulation between HMOX1 and HMGB1 signals is important for elucidating the feedback mechanism of oxidative stress. Further exploration of the collaboration between HMGB1 and NFE2L2-targeted genes in infection and innate immunity is important.
RNS
Reactive nitrogen species (RNS), including nitric oxide (NO) and peroxynitrite (ONOO-), are important regulators of inflammatory diseases73. NO is a powerful vasodilator produced by NO synthase from L-arginine, O2, and nicotinamide adenine dinucleotide phosphate (NADPH). Although NO has an extremely short half-life in the blood, it plays a dual role in regulating cellular signal transduction during physiological and pathological processes. The production of NO is mediated by NO synthases, such as nitric oxide synthase 2 (NOS2, also known as iNOS), in response to endotoxins or cytokines, which contributes to killing or inhibiting the growth of invading microorganisms or neoplastic tissue. Carbon monoxide-releasing molecule 2 (CORM-2) specifically inhibits NOS2 expression and NO production, which blocks LPS-induced HMGB1 release in macrophages through the IFNB-JAK2-STAT1 pathway. In contrast, the presence of an NO donor (NOC-18) reverses this process, indicating a role of NO metabolism in regulating HMGB1 release74. As a feedback mechanism, HMGB1 inhibits NOS2 mRNA in a dose-dependent manner in the presence of IFNG and TNF, thereby antagonizing the immunosuppressive ability and therapeutic effects of mesenchymal stem cells during acute kidney injury75. HMGB1 also binds to toll like receptor 4 (TLR4) to inhibit nitric oxide synthase 3 (NOS3, also known as eNOS) expression and subsequent NO release in the setting of diabetes76,77. These findings highlight the complex relationship between HMGB1 and NO under various stresses.
During ischemia/reperfusion injury, explosive production of ONOO- is rapidly induced by a surge in NO and O2−78–80. ONOO− exhibits more potent toxicity than its precursor81. ONOO− also mediates HMGB1 release and subsequent signaling pathways in different experimental models82,83. Free radicals or ONOO− scavengers, such as edaravone, baicalin, and phenylboronic acid, effectively inhibit the translocation and release of HMGB1 as well as HMGB1-mediated production of proinflammatory cytokines84–88. In addition, HMGB1 promotes ONOO− generation by activating NADPH oxidase via interaction with TLR4 or advanced glycosylation end-product specific receptor (AGER, best known as RAGE)87,89–91. However, there is still a lack of clear evidence to clarify the interaction between HMGB1 and RNS signaling pathways, which requires further in-depth investigations.
Calcium ions
As a universal second messenger, calcium ions are involved in a wide range of cellular processes by exerting allosteric regulatory effects on intracellular enzymes or proteins92. The release of calcium ions and their transport between the cytoplasm and intracellular storage are regulated by numerous proteins, channels, and pumps93–95. Disrupted intracellular calcium signaling leads to severe cell damage and even cell death96–98. Calcium-mediated signal transduction is implicated in HMGB1 translocation and release during infection99, sterile inflammation100,101, and cancer102,103. Of note, the phosphorylation and release of HMGB1 are regulated by the activation of calcium-mediated protein kinases, especially Ca2+/calmodulin-dependent protein kinase kinase (CaMKK)92,102,104,105. In addition, IFNB regulated by PKC activity and calcium signaling pathways is also involved in the release of HMGB1106. Consequently, calcium signaling inhibitors (e.g., STO609, CV159, 2-APB, and U73122), calcium chelators (e.g., BAPTA) or knockdown/knockout of CaMKK inhibit HMGB1 secretion and protect animals in various disease models99,107. Further determining whether different sources of calcium play a similar role in mediating HMGB1 secretion in activated immune cells is necessary.
XPO1
XPO1 is a member of the importin β superfamily of nuclear transport receptors involved in centrosome duplication and spindle assembly during mitosis108. XPO1 recognizes and exports proteins containing a leucine-rich NES, acting as a nuclear transport receptor engaged in extranuclear transportation of proteins or RNA108,109. Redistribution of HMGB1 is also strictly regulated by XPO1 in response to various inflammatory stimuli40. XPO1 inhibitors significantly inhibit HMGB1 translocation from the nucleus to the cytoplasm110,111. In contrast, the molecular chaperone heat shock protein family A (Hsp70) member 1A (HSPA1A, also known as HSP72) inhibits the interaction between HMGB1 and XPO1, thereby blocking HMGB1 secretion in macrophages following treatment with LPS or tumor necrosis factor (TNF)112. In addition, N-linked glycosylation of HMGB1 is crucial for the HMGB1-XPO1 interaction and nucleocytoplasmic transport as well as for extracellular secretion during inflammation39. XPO1-mediated nuclear export of tumor suppressor proteins has been implicated in tumorigenesis and drug resistance in several cancer types113,114. Since the release of HMGB1 plays an important role in shaping the tumor immune microenvironment, it is also expected that XPO1 can promote the translocation and release of HMGB1 in cancer.
TNF
Macrophages and monocytes release TNF and HMGB1 in a time-dependent manner in response to LPS and interferon gamma (IFNG, also known as IFN-γ) stimulation5,115,116. Direct TNF suppression via genetic TNF knockout or TNF-neutralizing antibodies partially inhibits IFNG- and LPS-induced HMGB1 release in macrophages116, suggesting that HMGB1 secretion is partially mediated through a TNF-dependent mechanism. In addition, CD14 and Janus kinase 2 (JAK2) act as upstream regulators in a TNF-mediated HMGB1 release signaling pathway in response to LPS and IFNG, respectively116, highlighting the notion that an integrated signaling pathway controls HMGB1 secretion in activated macrophages.
NF-κB
As a classical inflammatory signaling pathway, NF-κB plays a crucial role in the complex regulatory network of inflammation and immunity through the production and secretion of multiple proinflammatory cytokines and chemokines117. In the classical NF-κB activation pathway, subunit p65/p50 dimers are activated in response to various stress signals (such as cytokines, LPS, growth factors and antigen receptors). After inhibitor of NF-κB beta (IKBKB) protein is phosphorylated, its subsequent degradation by the proteasome leads to translocation of NF-κB p65/p50 to the nucleus, thereby inducing target gene expression alone or in combination with other transcription factor families. The NF-κB pathway is involved in HMGB1 release, whereas inhibition of the canonical NF-κB pathway limits HMGB1 secretion in activated immune cells118–120, although the NF-κB target gene directly responsible for this process is still u nclear. One possibility is that, as mentioned above, TNF (the classic NF-κB target gene) may be involved in the NF-κB-dependent release of HMGB1.
Notch
As an evolutionarily conserved pathway, the Notch signaling cascade plays a vital role in various developmental and physiological processes, regulating cell fate, proliferation, survival, and homeostasis121. There are four Notch receptors (Notch 1 to 4) and five Notch ligands (Delta-like 1, 3, and 4 and Jagged canonical Notch ligand 1/2 [Jagged 1 and 2]) in mammalian cells. Dysregulation of the Notch-mediated signaling pathway is also correlated with human diseases and pathological conditions121. For example, impaired Notch signaling is involved in inflammatory disorders and inflammatory cytokine release122–125. Notch signaling is activated after LPS stimulation, and Jagged 1 expression is increased in a JNK-dependent manner. In addition, in vitro and in vivo, Notch signaling can be inhibited by DAPT (a γ-secretase inhibitor), which was found to significantly attenuate the release of HMGB1 in response to LPS. These findings indicate that Notch signaling activation favors LPS-induced HMGB1 release124–126.
MAPK
MAPKs are signal transduction systems in eukaryotic cells that mediate the response of extracellular signals to intracellular signals. MAPKs are mainly composed of three subfamilies of highly conserved serine/threonine protein kinases, namely, extracellular regulated protein kinase (ERK), Jun N-terminal kinase (JNK) and MAPK14 (best known as p38). They conduct extracellular signals through a tertiary kinase cascade, specifically, extracellular signal → MAPK kinase (MKKK) → MAPK kinase (MKK) → MAPK127. MAPK family members divergently contribute to HMGB1 release in different inflammatory and injury models128–131. For example, the expression and release of HMGB1 is mediated through the p38 MAPK signaling pathway induced by rhesus rotavirus, while inhibition of p38 prevents HMGB1 release in cholangiocytes131. MAPK-mediated HMGB1 secretion is also implicated in endothelial inflammation, thus providing a potential therapeutic strategy for vascular diseases132. In vascular endothelial cells under high homocysteine conditions, the secretion of HMGB1 is positively regulated by the neuropilin 1 (NRP1)-MAPK14-MAPK pathway. Consequently, the knockdown of NRP1 by siRNA or administration of the NRP1 inhibitor ATWLPPR inhibits HMGB1 secretion in vivo and in vitro132. Together, these findings establish a functional role of MAPK in mediating HMGB1 secretion, although the detailed signal transduction mechanism remains unclear.
STAT
STATs are a family of transcription factors (containing STAT1-6) that were initially identified by gene expression analysis triggered by IFN133. The STAT family plays a central role in signal transduction derived from different extracellular stimuli. STAT protein is usually located in the cytoplasm in an inactive form and is activated by tyrosine phosphorylation mediated by JAK1 and JAK2134. Phosphorylated STATs form homodimers or heterodimers, leading them to translocate to the nucleus and ultimately regulate the transcription of many target genes135. A large number of studies have shown that JAK-regulated activation of STAT1 and STAT3 plays a positive role in the expression, modification, and/or release of HMGB1 in different inflammation and injury models136–139. Disruption of the JAK-STAT signaling pathway inhibits HMGB1 release, thereby playing a protective role in sepsis and ischemia/reperfusion injury models139,140. Reciprocally, extracellular HMGB1 is able to trigger activation of the STAT1 and STAT3 pathways139–143. These findings reveal that an interplay exists between HMGB1 and STAT signaling in inflammation.
TP53
HMGB1 and tumor protein P53 (TP53, best known as p53) can mutually regulate the signaling pathways they mediate and participate in many physiological and pathological processes144,145. For example, in cancer cells, HMGB1 and TP53 form a complex to regulate DNA repair and the balance between autophagy and apoptosis146. Knockout of TP53 increases the expression of cytosolic HMGB1 and induces autophagy, while depletion of HMGB1 in mouse embryonic fibroblasts promotes TP53 cytoplasmic localization and reduces autophagy146,147. Interestingly, after carcinogen administration, there is a cytoplasmic shift of HMGB1 in wild-type rat hepatocytes, and circulating HMGB1 levels are increased compared with levels in TP53+/− rats, indicating that TP53 activation can control neoplastic inflammation by inducing HMGB1 release148. Moreover, HMGB1 also transports its nuclear binding target TP53 from the nucleus to the cytoplasm and participates in the resistance to sunitinib, indicating a direct interaction and cross-regulation between HMGB1 and TP53 in the presence of disease149.
PPAR
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear hormone receptor family and contain three isotypes, namely, PPAR-α, PPAR-βδ, and PPAR-γ150. PPAR binds to specific ligands and activates the transcription of PPAR target genes, which plays a vital role in cell differentiation, tissue development, cell metabolism, inflammation, and tumor progression151. After administration of poly(I:C) or LPS, the secretion of HMGB1 in activated macrophages is negatively regulated by PPAR. Accordingly, treatment with rosiglitazone, a PPAR-γ ligand, decreases HMGB1 release and protects against sepsis in vivo and in vitro152. These findings provide new insights into the pleiotropic role of PPAR ligands in inhibiting lethal inflammation by targeting the release and activity of HMGB1153.
Inflammasomes
Inflammasomes are polyprotein oligomers assembled in cells after the cells recognize DAMPs and pathogen-associated molecular patterns (PAMPs) and act as a platform for activation of canonical caspase-1 or noncanonical caspase-11 and the subsequent secretion of proinflammatory cytokines154. In addition to mediating maturation and release of the interleukin 1 (IL1) family, activated inflammasomes also promote HMGB1 release in immune cells155–157 or cancer cells158 through different signaling pathways. The phosphorylation and activation of eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2, also termed double-stranded RNA-dependent protein kinase [PKR]) is required for inflammasome-dependent interleukin 1 beta (IL1B) and HMGB1 release in macrophages159. In contrast, complement component 5a receptor 2 (C5aR2) deficiency restricts activation of the NLRP3 inflammasome and the release of HMGB1 in vitro and in vivo160. Moreover, M2 isoform of pyruvate kinase M2 (PKM2)-dependent glycolysis is involved in HMGB1 release through selective activation of NLR family pyrin domain containing 3 (NLRP3) and is absent in melanoma 2 (AIM2) inflammasomes in macrophages161. Therefore, pharmacological and genetic inhibition of inflammasome signaling pathways can attenuate the release of HMGB1 and protect mice from sepsis or ischemia/reperfusion damage162–165. Of note, NLRP3 can regulate HMGB1 release via an inflammasome-independent pathway166. Recent data demonstrated that in vitro HMGB1 release after inflammasome activation occurs after cellular rupture, which is likely inflammasome-independent in vivo49. Nevertheless, it is still difficult to distinguish between the active or passive release of HMGB1 mediated during inflammasome activation and subsequent pyroptotic cell death.
Secretory lysosomes
In addition to mediating degradation, some lysosomes also act as secretory compartments, namely, secretory lysosomes. Secretory lysosomes are found in different types of immune cells and are Ca2+-regulated secretory organelles responsible for lysosome exocytosis167. It has been proposed that cytokine secretion can be regulated without leaders, such as IL1B and HMGB1, through lysosomal exocytosis19. Double immunofluorescence staining showed that HMGB1 colocalized with the lysosomal marker lysosomal-associated membrane protein 1 (LAMP1) but not the early endosomal marker early endosome antigen 1 (EEA1) in LPS-treated monocytes168. IFI30 lysosomal thiol reductase (IFI30, best known as gamma-interferon inducible lysosomal thiol reductase [GILT]) attenuates the formation of disulfide bonds in proteins, which assists in the complete unfolding of proteins for lysosomal degradation. NF-κB and STAT1, respectively, directly mediate the regulation of GILT expression induced by LPS and IFNG169,170. Depletion of IFI30 in immune cells (e.g., T cells and monocytes) can inhibit cytokine production (e.g., IL1B and TNF) and HMGB1 release in response to LPS, antigen exposure169, or mitochondrial oxidative damage1,171,172. Nuclear HMGB1 protein cytosolic translocation is then activated and associated with increased autophagy in IFI30−/− fibroblasts172. These findings establish a role of IFI30 in regulating secretory lysosome-mediated HMGB1 release.
Cell–cell interaction
The release of HMGB1 mediated by cell-cell interactions has been demonstrated in several studies. For example, the crosstalk between natural killer (NK) cells and dendritic cells (DCs) can cause HMGB1 release173. During vascular endothelial cell injury, platelets release a large amount of extracellular HMGB1 in the form of a disulfide isoform174, which plays a key role in neutrophil activation and thrombosis174–176. Clearance of apoptotic cells by phagocytic cells plays a role in the resolution of inflammation. Interestingly, the release of HMGB1 in macrophages induced by apoptotic cells has been considered one of the lethal mechanisms of apoptosis-mediated sepsis177. Impaired clearance of apoptotic cells also leads to the release of HMGB1, which in turn promotes TLR4-mediated cytokine production178. Thus, there is a dynamic relationship between the production and clearance of apoptotic cells in regulating HMGB1 release in macrophages. Moreover, the interaction between infiltrating uveitogenic T cells and retinal cells can induce rapid release of HMGB1 through the Fas cell surface death receptor (Fas)/Fas ligand inflammatory signaling pathway179. These findings further indicate that HMGB1 is a signaling mediator that coordinates the communication between different cells during an immune response.
Other mechanisms
Targeted temperature management inhibits the extracellular release of HMGB1 in myocardial ischemic/reperfusion injury, indicating a role of temperature-sensitive mechanisms in HMGB1 secretion180. In addition, α7 nicotinic acetylcholine receptors (α7nAChRs) and α7nAChR-dependent cholinergic signaling are implicated in suppressing the release of HMGB1 and reducing the inflammatory response in acute lung injury181, highlighting the idea that HMGB1 is a mediator and target of neuroimmunity during critical illness. Interestingly, as a commonly used anti-inflammatory drug, corticosterone induces HMGB1 release via pannexin 1-dependent mechanisms in primary cultured rat cortical astrocytes182. The use of a large number of genetically defective mice (e.g., activating transcription factor 3 [ATF3]−/−183, C-C motif chemokine receptor 7 [CCR7]−/−184, IL17A−/−185, secretory leukocyte peptidase inhibitor [SLPI]−/−186, complement C3 [C3]−/−187, and ADAM metallopeptidase with thrombospondin type 1 motif 13 [ADAMTS13]−/− mice188) in infection models further confirms the complexity and diversity of the HMGB1 release mechanism.
Passive release of HMGB1
Cell death is generally divided into accidental cell death and regulated cell death. HMGB1 can be passively released after various types of cell death (such as necrosis, necroptosis, apoptosis, NETosis, lysosome-mediated cell death, pyroptosis, autophagy-dependent cell death, and ferroptosis) in response to various stimuli or damage. In this section, we discuss some key regulators of HMGB1 passive release (Fig. 3).
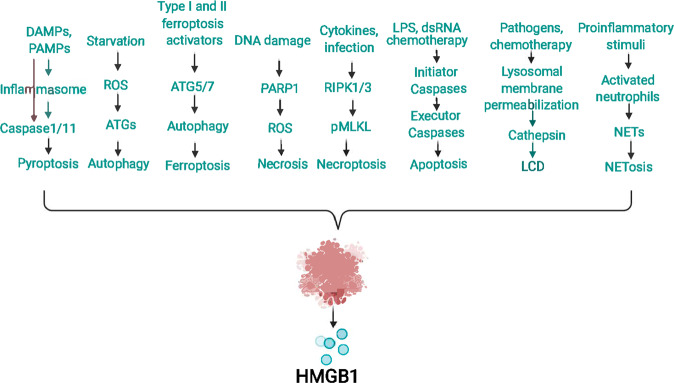
Fig. 3. Passive release of HMGB1 during cell death.
HMGB1 is a typical damage-associated molecular pattern (DAMP) that can be passively released during various types of cell death, such as pyroptosis, necrosis, apoptosis, necroptosis, ferroptosis, NETosis, and lysosome-mediated and autophagy-dependent cell death.
PARP1
The PARP family comprises many enzymes responsible for catalyzing ADP-ribose transfer from nicotinamide adenine dinucleotide (NAD)+ to proteins during several types of stress189. PARP1 is the most abundant and characteristic PARP member in mammalian cell nuclei190,191. Extensive DNA damage has been identified as the most potent promoter of PARP overactivation, resulting in an extravagant demand for NAD+, ATP depletion, and subsequent necrosis192. Several groups of proteins (such as histones and HMG proteins) and certain transcription factors (such as TP53 and NF-κB) are substrates of PARP193,194. PARP1 activation is responsible for the active secretion of HMGB1 in response to LPS-challenged macrophages and mouse embryonic fibroblasts37. In addition, DNA-damaging drugs can induce HMGB1 release during necrosis through PARP1 activation. This PARP1-dependent translocation and release of HMGB1 has been observed in inflammation, fibrosis, and cell death193,195. In contrast, PARP inhibitors or genetic depletion of PARP1 in mouse embryo fibroblasts significantly attenuate alkylating DNA injury-induced HMGB1 translocation and release. Loss of HMGB1 in the cell will lead to excessive activation of PARP, thereby providing a positive feedback mechanism that promotes tissue damage.
RIPK3
It has been well demonstrated that HMGB1 can be passively released by necrotic cells and causes sterile inflammation2. Previously, it was thought that necrosis was not a type of programmed cell death. However, recent progress has shown that necrosis that occurs in response to cytokines (such as TNF or type 1 or type 2 IFN), viral infection or chemotherapy may lead to highly programmed cell death, called necroptosis196–198. Receptor-interacting serine-threonine kinase (RIPK) family members (RIPK1 and RIPK3) and mixed-lineage kinase domain-like pseudokinase (MLKL) are key promoters of necroptosis due to their roles in the formation of necrosomes2,199. As a transition between TNF-induced apoptosis and necrosis, RIPK3 regulates necroptosis more specifically than RIPK1. RIPK3 knockout mice showed a reduction in sepsis and donor kidney inflammatory damage, which was found to be related to a decrease in DAMP release (including HMGB1)200. Pretreatment with the RIPK3 inhibitor necrostatin-1 can also reduce HMGB1 levels and protect animals from jejunal morphological injury201. In addition, RIPK3-regulated necroptosis is involved in dsRNA/poly (I:C)-induced HMGB1 release in a mouse model, which is the cause of inflammation during retinal degeneration202. In RIPK3−/− mice, HMGB1 release was attenuated, and subsequent necrosis and inflammation were prevented, providing substantial protection against poly(I:C)-induced retinal degeneration200.
Cathepsin
The concept of lysosome-dependent cell death (LCD) was first proposed by Christian de Duve, who famously identified cellular lysosomes as “recycle bins”203. This form of cell death is mainly mediated by lysosomal cathepsin; depends on leaked contents and the cell environment; and shows mixed characteristics of necrosis, apoptosis, and ferroptosis204. In response to a variety of stimuli, such as pathogen invasion and chemotherapy, the permeability of the lysosomal membrane increases, leading to cytoplasmic release of cathepsins and other hydrolytic enzymes204. Several members of the cathepsin family are involved in the release of HMGB1. For example, cathepsin B participates in L. pneumophila-induced LCD and HMGB1 release205. Cathepsin B-dependent NLRP3 inflammasome activation also contributes to nicotine-induced HMGB1 release and endothelial barrier dysfunction206. When cells are pretreated with specific cathepsin B inhibitors, cathepsin B may interact with nuclear proteins (such as core histone H3207 and HMGB1), thereby inhibiting cell death and subsequent HMGB1 release. In addition, transport of cathepsin B from the lysosome to the nucleus can cause DNA damage and subsequent release of HMGB1 for ferroptosis induction. Similarly, cathepsin D is involved in necroptosis-mediated HMGB1 release in immune cells7. In turn, HMGB1 can regulate cathepsin release and subsequent cell death208. Altogether, these studies indicate that cathepsin plays a significant role in regulating the release of HMGB1 during various types of cell death.
Antioxidant enzymes
ROS are a double-edged sword in cellular processes, and their role changes depending on the threshold of oxidative stress. Excessive ROS accumulation can trigger cell damage and death209. In addition to inducing the active secretion of HMGB1, ROS can also promote the passive release of HMGB1 during various cell death modalities (such as necrosis, apoptosis, necroptosis, and ferroptosis)210. Accordingly, a group of antioxidant enzymes regulate the release of HMGB1 during ROS-related cell death. For example, dysfunction in superoxide dismutase 1 (SOD1) and superoxide dismutase 2 (SOD2) is implicated in promoting HMGB1 release during apoptosis211–215. Other antioxidant enzymes, such as peroxiredoxins216, glutathione reductase172, and thioredoxin217, also inhibit HMGB1 passive release in a context-dependent manner. Therefore, targeting HMGB1 with antioxidant compounds may be an attractive treatment strategy for tissue damage-related diseases.
DNase
During cell death, DNA is degraded into fragments by different deoxyribonucleases (DNases), which participate in autoimmune diseases and other diseases. In apoptotic cells, DNA is degraded into nucleosomal units by DNA endonuclease (DNase-γ), while in necrotic cells, DNA is randomly degraded by extracellular DNase I or II218. NETosis is a form of regulated cell death first identified in neutrophils. In response to proinflammatory stimuli, neutrophils release their DNA and DNA-binding proteins (including HMGB1 and histones), which form neutrophil extracellular traps (NETs)219. The degradation of NETs caused by DNase significantly increases the release of HMGB1 during neutrophil activation220. NET-mediated activation of TLR4 and TLR9 signals can further enhance the release of HMGB1221,222. In addition to NETosis, DNase is also involved in regulating the release of HMGB1 in necrosis and apoptosis223,224. DNase inhibitors, such as DR396, can limit HMGB1 release during cell death223,224. Interestingly, bacterial-derived DNase can interfere with DNA stability through HMGB1, thereby impairing the type 1 IFN response in infection225. It is expected that the DNA sensor pathway may link cell death, HMGB1 release, and the immune response.
Caspase
Caspases are a family of intracellular cysteine-aspartic proteases and are mainly involved in apoptosis and pyroptosis226. Two types of caspases, namely, initiators and executors, are involved in the apoptosis process. In response to stress signals, the initiator caspase is activated and then cleaves the executor caspase, which mediates apoptosis by hydrolyzing target proteins227. For example, caspase-8 is involved in cytokine processing and death receptor-mediated apoptosis signaling pathways228. PPAR-α activator fenofibrate-induced rat myocardial I/R damage is related to caspase-9-dependent mitochondrial apoptosis229. Caspase-9 can prevent accessibility of cytochrome c to complex III in the mitochondria, resulting in increased ROS production230. Moreover, apoptosis signals cause mitochondria to release cytochrome c and activate APAF1 (apoptosome), which then cleaves the proenzyme of caspase-9 into an active dimer form to mediate caspase-3 activation. Caspase-3 and caspase-7 mediate cleavage of the mitochondrial complex 1 protein p75, which promotes subsequent HMGB1 release during apoptosis. Whether other caspase cleavage substrates are related to the release of HMGB1 in apoptosis remains unknown. In addition, pretreatment with caspase inhibitors can significantly reduce the release of HMGB1 in experimental sepsis177. Unlike apoptosis, pyroptosis is typically triggered by activation of caspase-1 or caspase-11231. HMGB1 release is decreased by inhibiting caspase-1 activity during pyroptosis165. Compared with wild-type mice, the serum HMGB1 level in caspase-1/caspase-11 double-knockout mice is significantly reduced during lethal endotoxemia156. In addition, amino acids 67, 158, and 169 of HMGB1 can be cleaved directly by caspase-1 but not other caspases (-2, -3, -5, -7, -9, or -11)232. The caspase-1-modified A box (especially residues 23–50) is different from full-length HMGB1 in activity, and its binding to receptor AGER can remedy apoptosis-induced immune tolerance in sepsis232. All these results indicate that the processing and release of HMGB1 are regulated by caspases during inflammation and the immune response.
ATG
Autophagy is a conserved degradation process that can remove unused proteins or damaged organelles by forming specific membrane structures (phagophores, autophagosomes, and autolysosomes). This process is mainly driven by a series of autophagy-related (ATG) proteins and usually plays a role in promoting cell survival233. Recently, HMGB1 secretion was reported to be regulated by heat shock protein 90 alpha family class A member 1 (HSP90AA1), Golgi reassembly stacking protein 2 (GORASP2)-mediated autophagy-based secretion machinery and the formation of multivesicular bodies234. However, excessive activation of the autophagic molecular machinery may cause cell death233. Iron-dependent ferroptosis is recognized as a form of autophagy-dependent cell death by degrading various anti-ferroptosis regulators210. In addition, autophagy-dependent secretion affects a number of extracellular factors, ranging from granule contents to inflammatory mediators. Knockdown of crucial autophagy proteins, such as ATG5, ATG7, and ATG12, significantly decreases extracellular HMGB1 release during necrosis233 or ferroptosis235. In addition, the secretion of HMGB1 by macrophages and fibroblasts also depends on ATG5 in response to LPS and starvation, which requires the participation of ROS signals236. Interestingly, HMGB1 release was observed in autophagy-deficient (ATG7−/−) hepatocytes and was found to be dependent on NFE2L2-regulated inflammasome activation. It is also worth noting that intracellular or extracellular HMGB1 is a promoter of autophagy through different mechanisms (e.g., by activating the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha [PI3K] complex or by inducing HSP27 expression), which may exacerbate autophagy-dependent ferroptosis237–243. The interaction between autophagy and HMGB1 provides an example of the complexity of the stress response during cell death.
pH
Alkaliptosis is a pH-dependent cell death in cancer cells244. The drug JTC801 can induce alkaliptosis by activating the NF-κB pathway and inhibiting the expression of carbonic anhydrase 9 (CA9), a transmembrane metalloenzyme responsible for maintenance of intracellular pH245,246. Alkaliptosis is associated with the translocation and release of HMGB1, which is inhibited by the DNA repair pathway mediated by FA complementation group D2 (FANCD2)247. Once released by alkaliptotic cells, extracellular HMGB1 binds to the AGER receptor in macrophages, and then activates the stimulator of interferon response CGAMP interactor 1 (STING1, also known as TMEM173) pathway to produce pro-inflammatory cytokines (such as TNF and IL6)247. Thus, HMGB1 is an immune mediator of DNA sensor pathway activation induced by abnormal pH-mediated cell death.
Conclusions and perspectives
HMGB1 is a position-dependent multifunctional protein. Inside the cell, nuclear HMGB1 regulates the structure and function of chromosomes, while cytoplasmic HMGB1 sustains autophagy. Outside the cell, HMGB1 is a mediator of inflammation, immunity, and the metabolic response. The release of HMGB1 is related to active or passive processes and can be adjusted at various levels. In particular, ROS and redox signals play a central role in driving the secretion and release of HMGB1 by coupling various posttranslational modifications. Targeting the release and activity of HMGB1 provides a strategy for the treatment of various diseases, especially infection and tissue damage. The following questions are worthy of our continued pursuit: How can we distinguish between the active secretion and passive release mechanisms of HMGB1? What is the main mechanism of HMGB1 secretion? Is there any difference in the activity of HMGB1 released by immune cells and nonimmune cells? What is the structural basis of HMGB1 lysosomal localization? Are the immune consequences of strategies that interfere with the translocation and release of HMGB1 different? How can we evaluate the acute and chronic effects of HMGB1 release on cell death-related immune responses? Compared with other DAMPs, what immune uniqueness does HMGB1 have? How can we develop an HMGB1-dependent combination drug strategy for disease treatment?
Acknowledgements
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript.
Author contributions
Conceptualization, R.C. and D.T.; writing—original draft, R.C.; writing—review and editing, D.T. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript was supported by grants from the National Natural Sciences Foundation of Hunan Province (#2019JJ30041), National Natural Sciences Foundation of China (#82070613), and Innovation-Driven Project of Central South University (#2020CX044).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruochan Chen, Email: 84172332@qq.com.
Daolin Tang, Email: daolin.tang@utsouthwestern.edu.
References
- 1.Goodwin GH, Johns EW. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur. J. Biochem. 1973;40:215–219. doi: 10.1111/j.1432-1033.1973.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 2.Xue J, et al. HMGB1 as a therapeutic target in disease. J. Cell. Physiol. 2021;236:3406–3419. doi: 10.1002/jcp.30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi ME, Beltrame M. Flexing DNA: HMG-box proteins and their partners. Am. J. Hum. Genet. 1998;63:1573–1577. doi: 10.1086/302170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas JOHMG. I and 2 architectural DNA-binding proteins. Biochem. Soc. Trans. 2001;4:395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- 5.Wang HC, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 6.Huttunen HJ, Rauvala H. Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J. Intern. Med. 2004;255:351–366. doi: 10.1111/j.1365-2796.2003.01301.x. [DOI] [PubMed] [Google Scholar]
- 7.Kang R, et al. HMGB1 in health and disease. Mol. Asp. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun R, et al. PCV2 induces reactive oxygen species to promote nucleocytoplasmic translocation of the viral DNA binding protein HMGB1 to enhance its replication. J. Virol. 2020;94:e00238–20. doi: 10.1128/JVI.00238-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, et al. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain. Behav. Immun. 2020;88:132–143. doi: 10.1016/j.bbi.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Gao SQ, et al. Neuronal HMGB1 in nucleus accumbens regulates cocaine reward memory. Addict. Biol. 2020;25:e12739. doi: 10.1111/adb.12739. [DOI] [PubMed] [Google Scholar]
- 11.Deng M, Scott MJ, Fan J, Billiar TR. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J. Leukoc. Biol. 2019;106:161–169. doi: 10.1002/JLB.3MIR1218-497R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Pusterla T, de Marchis F, Palumbo R, Bianchi ME. High mobility group B2 is secreted by myeloid cells and has mitogenic and chemoattractant activities similar to high mobility group B1. Autoimmunity. 2009;42:308-310. doi: 10.1080/08916930902831845. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J. Exp. Med. 2012;209:157–171. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini L, et al. HMGB1 and repair: focus on the heart. Pharmacol. Ther. 2019;196:160–182. doi: 10.1016/j.pharmthera.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen R, et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6:e05672. doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustin M, Neihart NK, Fagan JB. mRNA of chromosomal proteins HMG-1 and HMG-2 are polyadenylated. Biochem. Biophys. Res. Commun. 1981;101:893–897. doi: 10.1016/0006-291x(81)91833-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari S, Ronfani L, Calogero S, Bianchi ME. The mouse gene coding for high mobility group 1 protein (HMG1) J. Biol. Chem. 1994;269:28803–28808. [PubMed] [Google Scholar]
- 19.Kwak MS, et al. Immunological significance of HMGB1 post-translational modification and redox biology. Front. Immunol. 2020;11:1189. doi: 10.3389/fimmu.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonaldi T, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo. J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 22.Gong W, Li Y, Chao F, Huang G, He F. Amino acid residues 201–205 in C-terminal acidic tail region plays a crucial role in antibacterial activity of HMGB1. J. Biomed. Sci. 2009;16:83. doi: 10.1186/1423-0127-16-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Gong W, et al. The anti-inflammatory activity of HMGB1 A box is enhanced when fused with C-terminal acidic tail. J. Biomed. Biotechnol. 2010;2010:915234. doi: 10.1155/2010/915234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu B, et al. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev. Clin. Immunol. 2014;10:713–727. doi: 10.1586/1744666X.2014.909730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, et al. Regulation of posttranslational modifications of HMGB1 during immune responses. Antioxid. Redox Signal. 2016;24:620–634. doi: 10.1089/ars.2015.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dave SH, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J. Leukoc. Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sitapara RA, et al. The alpha7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol. Med. 2020;26:98. doi: 10.1186/s10020-020-00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhupar R, et al. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl Acad. Sci. USA. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabadi MM, et al. High-mobility group box 1 is a novel deacetylation target of Sirtuin1. Kidney Int. 2015;87:95–108. doi: 10.1038/ki.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W, Ku SK, Bae JS. Zingerone reduces HMGB1-mediated septic responses and improves survival in septic mice. Toxicol. Appl. Pharmacol. 2017;329:202–211. doi: 10.1016/j.taap.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, et al. Evidence for SIRT1 mediated HMGB1 release from kidney cells in the early stages of hemorrhagic shock. Front. Physiol. 2019;10:854. doi: 10.3389/fphys.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito N, et al. Cytolytic cells induce HMGB1 release from melanoma cell lines. J. Leukoc. Biol. 2007;81:75–83. doi: 10.1189/jlb.0306169. [DOI] [PubMed] [Google Scholar]
- 36.Oh YJ, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J. Immunol. 2009;182:5800–5809. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- 37.Davis K, et al. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol. Med. 2012;18:359–369. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge X, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD) J. Biol. Chem. 2014;289:22672–22691. doi: 10.1074/jbc.M114.552141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YH, et al. N-linked glycosylation plays a crucial role in the secretion of HMGB1. J. Cell Sci. 2016;129:29–38. doi: 10.1242/jcs.176412. [DOI] [PubMed] [Google Scholar]
- 40.Kwak MS, et al. Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol. 2019;24:101203. doi: 10.1016/j.redox.2019.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu F, Zhao ZH, Ding ST, Wu HH, Lu JJ. High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian Pac. J. Cancer Prev. 2013;14:5789–5795. doi: 10.7314/apjcp.2013.14.10.5789. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Wang H, Chavan SS, Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol. Med. 2015;21(Suppl 1):S6–S12. doi: 10.2119/molmed.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong Q, et al. SIRT6-PARP1 is involved in HMGB1 polyADP-ribosylation and acetylation and promotes chemotherapy-induced autophagy in leukemia. Cancer Biol. Ther. 2020;21:320–331. doi: 10.1080/15384047.2019.1702397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Xie J, Li X, Fang J. Poly (ADP-ribosylation) of HMGB1 facilitates its acetylation and promotes HMGB1 translocation-associated chemotherapy-induced autophagy in leukaemia cells. Oncol. Lett. 2020;19:368–378. doi: 10.3892/ol.2019.11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang M, et al. Poly-ADP-ribosylation of HMGB1 regulates TNFSF10/TRAIL resistance through autophagy. Autophagy. 2015;11:214–224. doi: 10.4161/15548627.2014.994400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raucci A, et al. The Janus face of HMGB1 in heart disease: a necessary update. Cell. Mol. Life Sci. 2019;76:211–229. doi: 10.1007/s00018-018-2930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai X, Biswas I, Panicker SR, Giri H, Rezaie AR. Activated protein C inhibits lipopolysaccharide-mediated acetylation and secretion of high-mobility group box 1 in endothelial cells. J. Thromb. Haemost. 2019;17:803–817. doi: 10.1111/jth.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 49.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat. Commun. 2020;11:4561. doi: 10.1038/s41467-020-18443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WJ, et al. Profibrogenic effect of high-mobility group box protein-1 in human dermal fibroblasts and its excess in keloid tissues. Sci. Rep. 2018;8:8434. doi: 10.1038/s41598-018-26501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisetsky DS. The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol. Med. 2014;20:158–163. doi: 10.2119/molmed.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.min HJ, et al. Th2 cytokines-DUOX2-ROS-HMGB1 translocation axis is important in the pathogenesis of allergic rhinitis. Clin. Sci. 2021;135:483–494. doi: 10.1042/CS20201212. [DOI] [PubMed] [Google Scholar]
- 53.min HJ, et al. ROS-dependent HMGB1 secretion upregulates IL-8 in upper airway epithelial cells under hypoxic condition. Mucosal Immunol. 2017;10:685–694. doi: 10.1038/mi.2016.82. [DOI] [PubMed] [Google Scholar]
- 54.Sekiguchi F, et al. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: Evidence for a neuroimmune crosstalk. Neuropharmacology. 2018;141:201–213. doi: 10.1016/j.neuropharm.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 55.Cui T, et al. Oxidative stress-induced HMGB1 release from melanocytes: a paracrine mechanism underlying the cutaneous inflammation in vitiligo. J. Invest. Dermatol. 2019;139:2174–2184.e2174. doi: 10.1016/j.jid.2019.03.1148. [DOI] [PubMed] [Google Scholar]
- 56.Tsung A, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang D, et al. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am. J. Respir. Cell Mol. Biol. 2009;41:651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kato S, et al. Edaravone, a novel free radical scavenger, reduces high-mobility group box 1 and prolongs survival in a neonatal sepsis model. Shock. 2009;32:586–592. doi: 10.1097/SHK.0b013e3181a2b886. [DOI] [PubMed] [Google Scholar]
- 59.Zhang ZW, et al. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig. Dis. Sci. 2010;55:2529–2536. doi: 10.1007/s10620-009-1073-0. [DOI] [PubMed] [Google Scholar]
- 60.Hosakote YM, Brasier AR, Casola A, Garofalo RP, Kurosky A. Respiratory syncytial virus infection triggers epithelial HMGB1 release as a damage-associated molecular pattern promoting a monocytic inflammatory response. J. Virol. 2016;90:9618–9631. doi: 10.1128/JVI.01279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delucchi F, et al. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS ONE. 2012;7:e39836. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys. Acta Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Vijayan V, Wagener F, Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Hu X, Jiang H. Nrf-2–HO-1–HMGB1 axis: an important therapeutic approach for protection against myocardial ischemia and reperfusion injury. Int. J. Cardiol. 2014;172:223–224. doi: 10.1016/j.ijcard.2013.12.273. [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Zhu C, Wang G, Xu R, Zhu Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm. Res. 2015;64:395–403. doi: 10.1007/s00011-015-0817-x. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Hu X, Xie J, Xu W, Jiang H. Beta-1-adrenergic receptors mediate Nrf2-HO-1-HMGB1 axis regulation to attenuate hypoxia/reoxygenation-induced cardiomyocytes injury in vitro. Cell. Physiol. Biochem. 2015;35:767–777. doi: 10.1159/000369736. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, et al. Hydrogen gas protects against intestinal injury in wild type but not NRF2 knockout mice with severe sepsis by regulating HO-1 and HMGB1 release. Shock. 2017;48:364–370. doi: 10.1097/SHK.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 68.Faridvand Y, et al. Nrf2 activation and down-regulation of HMGB1 and MyD88 expression by amnion membrane extracts in response to the hypoxia-induced injury in cardiac H9c2 cells. Biomed. Pharmacother. 2019;109:360–368. doi: 10.1016/j.biopha.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 69.Rivera-Perez J, et al. Epigallocatechin 3-gallate has a neuroprotective effect in retinas of rabbits with ischemia/reperfusion through the activation of Nrf2/HO-1. Int. J. Mol. Sci. 2020;21:3716. doi: 10.3390/ijms21103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park EJ, Kim YM, Chang KC. Hemin reduces HMGB1 release by UVB in an AMPK/HO-1-dependent PAthway in Human Keratinocytes HaCaT cells. Arch. Med. Res. 2017;48:423–431. doi: 10.1016/j.arcmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Mazur-Bialy AI, Pochec E. The time-course of antioxidant irisin activity: role of the Nrf2/HO-1/HMGB1 axis. Antioxidants. 2021;10:88. doi: 10.3390/antiox10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qu J, et al. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J. Cell. Physiol. 2019;234:8862–8872. doi: 10.1002/jcp.27548. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Chen X, Luo Y, Shen J. Potential molecular targets of peroxynitrite in mediating blood-brain barrier damage and haemorrhagic transformation in acute ischaemic stroke with delayed tissue plasminogen activator treatment. Free Radic. Res. 2018;52:1220–1239. doi: 10.1080/10715762.2018.1521519. [DOI] [PubMed] [Google Scholar]
- 74.Tsoyi K, et al. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-beta/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock. 2010;34:608–614. doi: 10.1097/SHK.0b013e3181e46f15. [DOI] [PubMed] [Google Scholar]
- 75.Wang S, et al. High-mobility group box 1 protein antagonizes the immunosuppressive capacity and therapeutic effect of mesenchymal stem cells in acute kidney injury. J. Transl. Med. 2020;18:175. doi: 10.1186/s12967-020-02334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Z, et al. HMGB1 impairs endothelium-dependent relaxation in diabetes through TLR4/eNOS pathway. FASEB J. 2020;34:8641–8652. doi: 10.1096/fj.202000242R. [DOI] [PubMed] [Google Scholar]
- 77.Gliozzi M, et al. Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. Int. J. Mol. Sci. 2019;20:3294. doi: 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 79.Shen J, et al. Nitric oxide down-regulates caveolin-1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J. Neurochem. 2006;96:1078–1089. doi: 10.1111/j.1471-4159.2005.03589.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen XM, Chen HS, Xu MJ, Shen JG. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 2013;34:67–77. doi: 10.1038/aps.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crow JP, Beckman JS. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr. Top. Microbiol. Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- 82.Loukili N, et al. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc. Res. 2011;89:586–594. doi: 10.1093/cvr/cvq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen H, et al. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl. Stroke Res. 2020;11:967–982. doi: 10.1007/s12975-019-00772-1. [DOI] [PubMed] [Google Scholar]
- 84.Kikuchi K, et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J. Pharmacol. Exp. Ther. 2009;329:865–874. doi: 10.1124/jpet.108.149484. [DOI] [PubMed] [Google Scholar]
- 85.Xu M, et al. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2013;150:116–124. doi: 10.1016/j.jep.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 86.Wang H, Liu D. Baicalin inhibits high-mobility group box 1 release and improves survival in experimental sepsis. Shock. 2014;41:324–330. doi: 10.1097/SHK.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 87.Chandrashekaran V, et al. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017;13:8–19. doi: 10.1016/j.redox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H, et al. Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurological outcome in ischemic stroke rats with delayed t-PA treatment: involvement of ONOO(-)-MMP-9 pathway. Transl. Stroke Res. 2018;9:515–529. doi: 10.1007/s12975-017-0598-3. [DOI] [PubMed] [Google Scholar]
- 89.Park HS, et al. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 90.Fan J, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J. Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 91.Tang D, Kang R, Zeh H, Jr, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao P, et al. HMGB1 release by H2O2-induced hepatocytes is regulated through calcium overload and 58-F interference. Cell Death Disco. 2017;3:17008. doi: 10.1038/cddiscovery.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hwang SM, Lee JY, Park CK, Kim YH. The role of TRP channels and PMCA in brain disorders: intracellular calcium and pH homeostasis. Front Cell Dev. Biol. 2021;9:584388. doi: 10.3389/fcell.2021.584388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Irvine R. 20 years of Ins(1,4,5)P3,and 40 years before. Nat. Rev. Mol. Cell. Biol. 2003;4:586–590. doi: 10.1038/nrm1152. [DOI] [PubMed] [Google Scholar]
- 95.Tu CL, Chang W, Bikle DD. Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J. Invest. Dermatol. 2005;125:187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 96.Carafoli E, Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhosale G, Sharpe JA, Sundier SY, Duchen MR. Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann. N. Y. Acad. Sci. 2015;1350:107–116. doi: 10.1111/nyas.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Criddle DN. Reactive oxygen species, Ca(2+) stores and acute pancreatitis; a step closer to therapy? Cell Calcium. 2016;60:180–189. doi: 10.1016/j.ceca.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, et al. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J. Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng HH, et al. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci. Rep. 2017;7:16628. doi: 10.1038/s41598-017-16778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian T, et al. Sphingosine kinase 1 regulates HMGB1 translocation by directly interacting with calcium/calmodulin protein kinase II-delta in sepsis-associated liver injury. Cell Death Dis. 2020;11:1037. doi: 10.1038/s41419-020-03255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen S, et al. Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett. 2017;394:22–32. doi: 10.1016/j.canlet.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Falk H, et al. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology. 2017;6:e1301332. doi: 10.1080/2162402X.2017.1301332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quan H, et al. Stearoyl lysophosphatidylcholine inhibits LPS-induced extracellular release of HMGB1 through the G2A/calcium/CaMKKbeta/AMPK pathway. Eur. J. Pharmacol. 2019;852:125–133. doi: 10.1016/j.ejphar.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 105.Li W, et al. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 2020;11:229. doi: 10.3389/fimmu.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma L, Kim SJ, Oh KI. Calcium/calmodulin-dependent protein kinase is involved in the release of high mobility group box 1 via the interferon-beta signaling pathway. Immune Netw. 2012;12:148–154. doi: 10.4110/in.2012.12.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin JH, et al. Ethyl pyruvate inhibits HMGB1 phosphorylation and release by chelating calcium. Mol. Med. 2015;20:649–657. doi: 10.2119/molmed.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem. Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr. Med. Chem. 2008;15:2648–2655. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, et al. Translocation of endogenous danger signal HMGB1 from nucleus to membrane microvesicles in macrophages. J. Cell. Physiol. 2016;231:2319–2326. doi: 10.1002/jcp.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu M, et al. KPT-330, a potent and selective CRM1 inhibitor, exhibits anti-inflammation effects and protection against sepsis. Biochem. Biophys. Res. Commun. 2018;503:1773–1779. doi: 10.1016/j.bbrc.2018.07.112. [DOI] [PubMed] [Google Scholar]
- 112.Tang D, et al. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and roinflammatory function in macrophages. J. Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun YQ, et al. Expression of CRM1 and CDK5 shows high prognostic accuracy for gastric cancer. World J. Gastroenterol. 2017;23:2012–2022. doi: 10.3748/wjg.v23.i11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang AY, Liu H. The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig. 2019;6:6. doi: 10.21037/sci.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit. Care. Med. 2000;28:2949–2955. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 116.Chen G, et al. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J. Leukoc. Biol. 2004;76:994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- 117.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 118.Lin F, et al. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J. Cell. Physiol. 2018;233:6683–6692. doi: 10.1002/jcp.26468. [DOI] [PubMed] [Google Scholar]
- 119.Hiramoto S, et al. Cystitis-related bladder pain involves ATP-dependent HMGB1 release from macrophages and its downstream H2S/Cav3.2 signaling in mice. Cells. 2020;9:1748. doi: 10.3390/cells9081748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang CM, Jiang M, Wang HJ. Effect of NFkappaB inhibitor on highmobility group protein B1 expression in a COPD rat model. Mol. Med Rep. 2013;7:499–502. doi: 10.3892/mmr.2012.1181. [DOI] [PubMed] [Google Scholar]
- 121.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat. Rev. Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 122.Garis M, Garrett-Sinha LA. Notch signaling in B cell immune responses. Front. Immunol. 2020;11:609324. doi: 10.3389/fimmu.2020.609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castro RC, Goncales RA, Zambuzi FA, Frantz FG. Notch signaling pathway in infectious diseases: role in the regulation of immune response. Inflamm. Res. 2021;70:261–274. doi: 10.1007/s00011-021-01442-5. [DOI] [PubMed] [Google Scholar]
- 124.Niranjan T, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 125.Okamoto M, et al. Essential role of Notch signaling in effector memory CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J. Exp. Med. 2008;205:1087–1097. doi: 10.1084/jem.20072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsao PN, et al. Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J. Biomed. Sci. 2011;18:56. doi: 10.1186/1423-0127-18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou JR, et al. Neuropeptide Y induces secretion of high-mobility group box 1 protein in mouse macrophage via PKC/ERK dependent pathway. J. Neuroimmunol. 2013;260:55–59. doi: 10.1016/j.jneuroim.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 128.Huang W, et al. Heat stress induces RIP1/RIP3-dependent necroptosis through the MAPK, NF-kappaB, and c-Jun signaling pathways in pulmonary vascular endothelial cells. Biochem. Biophys. Res. Commun. 2020;528:206–212. doi: 10.1016/j.bbrc.2020.04.150. [DOI] [PubMed] [Google Scholar]
- 129.Kim HS, et al. Sulfatide inhibits HMGB1 secretion by hindering toll-like receptor 4 localization within lipid rafts. Front. Immunol. 2020;11:1305. doi: 10.3389/fimmu.2020.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishioku T, et al. Dimethyl fumarate prevents osteoclastogenesis by decreasing NFATc1 expression, inhibiting of erk and p38 MAPK phosphorylation, and suppressing of HMGB1 release. Biochem. Biophys. Res. Commun. 2020;530:455–461. doi: 10.1016/j.bbrc.2020.05.088. [DOI] [PubMed] [Google Scholar]
- 131.Mohanty SK, et al. High mobility group box 1 release by cholangiocytes governs biliary atresia pathogenesis and correlates with increases in afflicted infants. Hepatology. 2021;74:864–878. doi: 10.1002/hep.31745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma Y, et al. NRP1 regulates HMGB1 in vascular endothelial cells under high homocysteine condition. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1039–H1046. doi: 10.1152/ajpheart.00746.2018. [DOI] [PubMed] [Google Scholar]
- 133.Darnell JEJ. The JAK-STAT pathway summary of initial studies and recent advances. Recent Prog. Horm. Res. 1996;51:391–403. [PubMed] [Google Scholar]
- 134.Ou A, Ott M, Fang D, Heimberger AB. The role and therapeutic targeting of JAK/STAT signaling in glioblastoma. Cancers. 2021;13:437. doi: 10.3390/cancers13030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 136.Zhou S, et al. Angiotensin II enhances the acetylation and release of HMGB1 in RAW264.7 macrophage. Cell Biol. Int. 2018;42:1160–1169. doi: 10.1002/cbin.10984. [DOI] [PubMed] [Google Scholar]
- 137.Hao J, et al. IFN-gamma induces lipogenesis in mouse mesangial cells via the JAK2/STAT1 pathway. Am. J. Physiol. Cell Physiol. 2013;304:C760–C767. doi: 10.1152/ajpcell.00352.2012. [DOI] [PubMed] [Google Scholar]
- 138.Park EJ, Kim YM, Kim HJ, Chang KC. Degradation of histone deacetylase 4 via the TLR4/JAK/STAT1 signaling pathway promotes the acetylation of high mobility group box 1 (HMGB1) in lipopolysaccharide-activated macrophages. FEBS Open Bio. 2018;8:1119–1126. doi: 10.1002/2211-5463.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Imbaby S, et al. Beneficial effect of STAT3 decoy oligodeoxynucleotide transfection on organ injury and mortality in mice with cecal ligation and puncture-induced sepsis. Sci. Rep. 2020;10:15316. doi: 10.1038/s41598-020-72136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Y, et al. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol. Med. Rep. 2018;17:5007–5012. doi: 10.3892/mmr.2018.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang G, Zhang J, Dui D, Ren H, Liu J. High mobility group box 1 induces the activation of the Janus kinase 2 and signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway in pancreatic acinar cells in rats, while AG490 and rapamycin inhibit their activation. Bosn. J. Basic Med. Sci. 2016;16:307–312. doi: 10.17305/bjbms.2016.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo HF, et al. High mobility group box 1 induces synoviocyte proliferation in rheumatoid arthritis by activating the signal transducer and activator transcription signal pathway. Clin. Exp. Med. 2011;11:65–74. doi: 10.1007/s10238-010-0116-3. [DOI] [PubMed] [Google Scholar]
- 143.Conti L, et al. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013;27:4731–4744. doi: 10.1096/fj.13-230201. [DOI] [PubMed] [Google Scholar]
- 144.Zhang B, Yang N, Mo ZM, Lin SP, Zhang F. IL-17A enhances microglial response to OGD by regulating p53 and PI3K/Akt pathways with involvement of ROS/HMGB1. Front. Mol. Neurosci. 2017;10:271. doi: 10.3389/fnmol.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang X, et al. Silencing of functional p53 attenuates NAFLD by promoting HMGB1-related autophagy induction. Hepatol. Int. 2020;14:828–841. doi: 10.1007/s12072-020-10068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Livesey K, et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72:1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davalos AR, et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yan HX, et al. p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release. J. Hepatol. 2013;59:762–768. doi: 10.1016/j.jhep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luo P, et al. HMGB1 represses the anti-cancer activity of sunitinib by governing TP53 autophagic degradation via its nucleus-to-cytoplasm transport. Autophagy. 2018;14:2155–2170. doi: 10.1080/15548627.2018.1501134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shao XR, et al. Peroxisome proliferator-activated receptor-γ: master regulator of adipogenesis and obesity. Curr. Stem. Cell Res. Ther. 2016;11:282–289. doi: 10.2174/1574888x10666150528144905. [DOI] [PubMed] [Google Scholar]
- 151.Hernandez-Quiles M, Broekema MF, Kalkhoven E. PPARgamma in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front. Endocrinol. 2021;12:624112. doi: 10.3389/fendo.2021.624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hwang JS, et al. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone inhibits lipopolysaccharide-induced release of high mobility group box 1. Mediators Inflamm. 2012;2012:352807. doi: 10.1155/2012/352807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ying S, Xiao X, Chen T, Lou J. PPAR ligands function as suppressors that target biological actions of HMGB1. PPAR Res. 2016;2016:2612743. doi: 10.1155/2016/2612743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Craven RR, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamkanfi M, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller JM, et al. Curcumin: a double hit on malignant mesothelioma. Cancer Prev. Res. 2014;7:330–340. doi: 10.1158/1940-6207.CAPR-13-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu S, et al. The complement receptor C5aR2 promotes protein kinase R expression and contributes to NLRP3 inflammasome activation and HMGB1 release from macrophages. J. Biol. Chem. 2019;294:8384–8394. doi: 10.1074/jbc.RA118.006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie M, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiang M, et al. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J. Immunol. 2011;187:4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu P, et al. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum. Gene Ther. 2011;22:853–864. doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- 164.Kamo N, et al. ASC/caspase-1/IL-1beta signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Khambu B, et al. HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J. Clin. Invest. 2018;128:2419–2435. doi: 10.1172/JCI91814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Willingham SB, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blott EJ, Griffiths GM. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 168.Gardella S, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. Embo. Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rausch MP, Hastings KT. GILT modulates CD4+ T-cell tolerance to the melanocyte differentiation antigen tyrosinase-related protein 1. J. Invest. Dermatol. 2012;132:154–162. doi: 10.1038/jid.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lackman RL, Cresswell P. Exposure of the promonocytic cell line THP-1 to Escherichia coli induces IFN-gamma-inducible lysosomal thiol reductase expression by inflammatory cytokines. J. Immunol. 2006;177:4833–4840. doi: 10.4049/jimmunol.177.7.4833. [DOI] [PubMed] [Google Scholar]
- 171.Lackman RL, Jamieson AM, Griffith JM, Geuze H, Cresswell P. Innate immune recognition triggers secretion of lysosomal enzymes by macrophages. Traffic. 2007;8:1179–1189. doi: 10.1111/j.1600-0854.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 172.Chiang HS, Maric M. Lysosomal thiol reductase negatively regulates autophagy by altering glutathione synthesis and oxidation. Free Radic. Biol. Med. 2011;51:688–699. doi: 10.1016/j.freeradbiomed.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 173.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 174.Stark K, et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood. 2016;128:2435–2449. doi: 10.1182/blood-2016-04-710632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maugeri N, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 176.Vogel S, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 2015;125:4638–4654. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qin S, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Velegraki M, et al. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica. 2013;98:1206–1215. doi: 10.3324/haematol.2012.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang G, et al. HMGB1 release triggered by the interaction of live retinal cells and uveitogenic T cells is Fas/FasL activation-dependent. J. Neuroinflammation. 2015;12:179. doi: 10.1186/s12974-015-0389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Beom JH, et al. Targeted temperature management at 33 degrees C or 36 induces equivalent myocardial protection by inhibiting HMGB1 release in myocardial ischemia/reperfusion injury. PLoS ONE. 2021;16:e0246066. doi: 10.1371/journal.pone.0246066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang ZK, et al. Electroacupuncture pretreatment attenuates acute lung injury through α7 nicotinic acetylcholine receptor-mediated inhibition of HMGB1 release in rats after cardiopulmonary bypass. Shock. 2018;50:351–359. doi: 10.1097/SHK.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hisaoka-Nakashima K, et al. Corticosterone induces HMGB1 release in primary cultured rat cortical astrocytes: involvement of pannexin-1 and P2X7 receptor-dependent mechanisms. Cells. 2020;9:1068. doi: 10.3390/cells9051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lai PF, et al. ATF3 Protects against LPS-induced inflammation in mice via inhibiting HMGB1 expression. Evid. Based Complement. Altern. Med. 2013;2013:716481. doi: 10.1155/2013/716481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawakami M, et al. The role of CCR7 in allergic airway inflammation induced by house dust mite exposure. Cell. Immunol. 2012;275:24–32. doi: 10.1016/j.cellimm.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 185.Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J. Pharmacol. Exp. Ther. 2011;339:93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 186.Nakamura A, et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J. Exp. Med. 2003;197:669–674. doi: 10.1084/jem.20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cai C, et al. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1175–R1182. doi: 10.1152/ajpregu.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fujioka M, et al. ADAMTS13 gene deletion enhances plasma high-mobility group box1 elevation and neuroinflammation in brain ischemia-reperfusion injury. Neurol. Sci. 2012;33:1107–1115. doi: 10.1007/s10072-011-0913-9. [DOI] [PubMed] [Google Scholar]
- 189.Noguchi T, et al. Gefitinib initiates sterile inflammation by promoting IL-1beta and HMGB1 release via two distinct mechanisms. Cell Death Dis. 2021;12:49. doi: 10.1038/s41419-020-03335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nguewa PA, Fuertes MA, Valladares B, Alonso C, Perez JM. Poly(ADP-ribose) polymerases: homology, structural domains and functions. Novel therapeutical applications. Prog. Biophys. Mol. Biol. 2005;88:143–172. doi: 10.1016/j.pbiomolbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 191.Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair (Amst.) 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 192.Pandey N, Black BE. Rapid detection and signaling of DNA damage by PARP-1. Trends Biochem. Sci. 2021;46:744–757. doi: 10.1016/j.tibs.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ni SY, et al. Puerarin alleviates lipopolysaccharide-induced myocardial fibrosis by inhibiting PARP-1 to prevent HMGB1-mediated TLR4-NF-kappaB signaling pathway. Cardiovasc. Toxicol. 2020;20:482–491. doi: 10.1007/s12012-020-09571-9. [DOI] [PubMed] [Google Scholar]
- 194.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Disco. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 195.Qin WD, et al. Low shear stress induced HMGB1 translocation and release via PECAM-1/PARP-1 pathway to induce inflammation response. PLoS ONE. 2015;10:e0120586. doi: 10.1371/journal.pone.0120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 198.Thapa RJ, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl Acad. Sci. USA. 2013;110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019;20:19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Murakami Y, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu Y, et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021;12:62. doi: 10.1038/s41419-020-03365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Allocca M, Corrigan JJ, Mazumder A, Fake KR, Samson LD. Inflammation, necrosis, and the kinase RIP3 are key mediators of AAG-dependent alkylation-induced retinal degeneration. Sci. Signal. 2019;12:eaau9216. doi: 10.1126/scisignal.aau9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aits S, Jaattela M. Lysosomal cell death at a glance. J. Cell Sci. 2013;126:1905–1912. doi: 10.1242/jcs.091181. [DOI] [PubMed] [Google Scholar]
- 204.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morinaga Y, et al. Legionella pneumophila induces cathepsin B-dependent necrotic cell death with releasing high mobility group box1 in macrophages. Respir. Res. 2010;11:158. doi: 10.1186/1465-9921-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Y, Chen Y, Zhang Y, Li PL, Li X. Contribution of cathepsin B-dependent Nlrp3 inflammasome activation to nicotine-induced endothelial barrier dysfunction. Eur. J. Pharmacol. 2019;865:172795. doi: 10.1016/j.ejphar.2019.172795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamalisto S, et al. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat. Commun. 2020;11:229. doi: 10.1038/s41467-019-14009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen L, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9:597. doi: 10.1038/s41419-018-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 211.Brambilla L, Martorana F, Guidotti G, Rossi D. Dysregulation of astrocytic HMGB1 signaling in amyotrophic lateral sclerosis. Front. Neurosci. 2018;12:622. doi: 10.3389/fnins.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yon JM, Kim YB, Park D. The ethanol fraction of white rose petal extract abrogates excitotoxicity-induced neuronal damage in vivo and in vitro through inhibition of oxidative stress and proinflammation. Nutrients. 2018;10:1375. doi: 10.3390/nu10101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lo Coco D, Veglianese P, Allievi E, Bendotti C. Distribution and cellular localization of high mobility group box protein 1 (HMGB1) in the spinal cord of a transgenic mouse model of ALS. Neurosci. Lett. 2007;412:73–77. doi: 10.1016/j.neulet.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 214.Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 as an autophagy sensor in oxidative stress. Autophagy. 2011;7:904–906. doi: 10.4161/auto.7.8.15704. [DOI] [PubMed] [Google Scholar]
- 215.Tang D, Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid. Redox Signal. 2011;15:2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shichita T, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012;18:911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- 217.Vezzoli M, et al. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid. Redox Signal. 2011;15:2161–2174. doi: 10.1089/ars.2010.3341. [DOI] [PubMed] [Google Scholar]
- 218.Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10:108–116. doi: 10.1038/sj.cdd.4401161. [DOI] [PubMed] [Google Scholar]
- 219.Kim SW, Lee JK. Role of HMGB1 in the interplay between NETosis and thrombosis in ischemic stroke: a review. Cells. 2020;9:1794. doi: 10.3390/cells9081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tohme S, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang H, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yamada Y, et al. DR396, an apoptotic DNase gamma inhibitor, attenuates high mobility group box 1 release from apoptotic cells. Bioorg. Med. Chem. 2011;19:168–171. doi: 10.1016/j.bmc.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 224.Yamada Y, et al. The release of high mobility group box 1 in apoptosis is triggered by nucleosomal DNA fragmentation. Arch. Biochem. Biophys. 2011;506:188–193. doi: 10.1016/j.abb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 225.Keller N, et al. Group A streptococcal DNase Sda1 impairs plasmacytoid dendritic cells’ type 1 interferon response. J. Invest. Dermatol. 2019;139:1284–1293. doi: 10.1016/j.jid.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 226.Liu B, et al. Inflammatory caspases drive pyroptosis in acute lung injury. Front. Pharmacol. 2021;12:631256. doi: 10.3389/fphar.2021.631256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kopeina GS, Prokhorova EA, Lavrik IN, Zhivotovsky B. Alterations in the nucleocytoplasmic transport in apoptosis: caspases lead the way. Cell Prolif. 2018;51:e12467. doi: 10.1111/cpr.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mandal R, Barron JC, Kostova I, Becker S, Strebhardt K. Caspase-8: the double-edged sword. Biochim Biophys. Acta Rev. Cancer. 2020;1873:188357. doi: 10.1016/j.bbcan.2020.188357. [DOI] [PubMed] [Google Scholar]
- 229.Zhao Q, et al. Fenofibrate protects against acute myocardial I/R injury in rat by suppressing mitochondrial apoptosis as decreasing cleaved caspase-9 activation. Cancer Biomark. 2017;19:455–463. doi: 10.3233/CBM-170572. [DOI] [PubMed] [Google Scholar]
- 230.Makazan Z, Saini HK, Dhalla NS. Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1986–H1994. doi: 10.1152/ajpheart.01214.2006. [DOI] [PubMed] [Google Scholar]
- 231.Briard B, Malireddi RKS, Kanneganti TD. Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 2021;17:e1009358. doi: 10.1371/journal.ppat.1009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.LeBlanc PM, et al. An immunogenic peptide in the A-box of HMGB1 protein reverses apoptosis-induced tolerance through RAGE receptor. J. Biol. Chem. 2014;289:7777–7786. doi: 10.1074/jbc.M113.541474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.New J, Thomas SM. Autophagy-dependent secretion: mechanism, factors secreted, and disease implications. Autophagy. 2019;15:1682–1693. doi: 10.1080/15548627.2019.1596479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kim YH, et al. Secretory autophagy machinery and vesicular trafficking are involved in HMGB1 secretion. Autophagy. 2021;17:2345–2362. doi: 10.1080/15548627.2020.1826690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 236.Tang D, et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256–1258. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 238.Liu X, et al. Novel dihydroartemisinin derivative DHA-37 induces autophagic cell death through upregulation of HMGB1 in A549 cells. Cell Death Dis. 2018;9:1048. doi: 10.1038/s41419-018-1006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xu T, Jiang L, Wang Z. The progression of HMGB1-induced autophagy in cancer biology. Onco Targets Ther. 2019;12:365–377. doi: 10.2147/OTT.S185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen X, Yu C, Kang R, Kroemer G, Tang D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021;28:1135–1148. doi: 10.1038/s41418-020-00728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu J, et al. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem. Biol. 2020;27:420–435. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2020;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hou W, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu J, Kuang F, Kang R, Tang D. Alkaliptosis: a new weapon for cancer therapy. Cancer Gene Ther. 2020;27:267–269. doi: 10.1038/s41417-019-0134-6. [DOI] [PubMed] [Google Scholar]
- 245.Song X, et al. JTC801 induces pH-dependent death specifically in cancer cells and slows growth of tumors in mice. Gastroenterology. 2018;154:1480–1493. doi: 10.1053/j.gastro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhu S, Liu J, Kang R, Yang M, Tang D. Targeting NF-kappaB-dependent alkaliptosis for the treatment of venetoclax-resistant acute myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2021;562:55–61. doi: 10.1016/j.bbrc.2021.05.049. [DOI] [PubMed] [Google Scholar]
- 247.Fang X, et al. The HMGB1-AGER-STING1 pathway mediates the sterile inflammatory response to alkaliptosis. Biochem. Biophys. Res. Commun. 2021;560:165–171. doi: 10.1016/j.bbrc.2021.05.003. [DOI] [PubMed] [Google Scholar]