Abstract
Objectives:
Intraoperative localization and preservation of parathyroid glands (PGs) are challenging during thyroid surgery. A new non-invasive technique of combined near-infrared PG autofluorescence detection and dye-free imaging angiography that allows intraoperative feedback has recently been introduced. The objective of this study was to evaluate this technique in real-time.
Materials and Methods:
A pilot feasibility study of a portable imaging device in four patients who underwent either thyroid lobectomy or total thyroidectomy is presented. PG autofluorescence and vascularity/tissue perfusion were monitored using a real-time screen display during the surgical procedure.
Results:
Three lobectomies and one total thyroidectomy were performed. Among the nine PGs identified by the operating surgeon, eight PGs were confirmed using the autofluorescence device. Each PG was successfully determined to be either well-perfused or devascularized, and devascularized PGs were autotransplanted.
Conclusions:
The preliminary results suggest that the combination of PG autofluorescence detection and dye-free angiography can potentially be used to assess PG function. With further validation studies, the effectiveness of this technique in clinical practice can be further delineated.
Keywords: Parathyroid gland (PG), Vascularity, Dye-free imaging angiography, Perfusion assessment, Thyroidectomy
1. Introduction
Although thyroid surgery is performed safely due to advances in surgical techniques and the development of new surgical instruments, hypocalcemia is one of the most common complications after thyroid surgery. The incidence of transient hypocalcemia is reported to be 0.3%–49%, and that of permanent hypocalcemia is reported to be 0%–13%. 1 It is difficult even for an experienced surgeon to identify the parathyroid gland (PG) because it is a small, yellow-brown gland that has a shape similar to that of the surrounding lymph nodes, perithyroidal soft tissue, and fat. The causes of post-operative hypocalcemia include direct damage to PGs or their inadvertent removal, damage to the vasculature of PGs, hematoma, stunning after dissection, secretion of calcitonin (parathyroid hormone [PTH] suppression), and dilution of blood due to fluid therapy. 2 Among these causes, damage to PGs and vessels during surgery is the main cause of post-operative hypocalcemia. 3
To preserve PGs safely, they should be carefully identified, and their perfusion should be maintained by avoiding damage to their vasculature. Conventionally, surgeons rely on visual inspection to identify or assess the viability of PGs. 4 However, this method gives inconsistent results because it depends on the individual surgeon’s experience and training. In 2011, an optical method for identifying PGs using near-infrared autofluorescence (NIRAF) was first introduced by Paras et al., 5,6 and subsequent studies have reported that the NIRAF technique is effective for intraoperative real-time localization of PGs. 5–12 This method has several benefits owing to the use of non-invasive technology. However, surgeons cannot evaluate the viability of PGs intraoperatively because autofluorescence signals are emitted by both viable and non-viable PGs. Moreover, presence of metastatic lymph nodes, brown fat, and thyroid tissue may lead to false positive results. 13–15
Viability assessment of PGs based on the surgeon’s judgment is unreliable and subjective because surgeons rely on the color or swelling of the PG observed during inspection. To address this concern, studies have reported the use of exogenous dyes, such as indocyanine green (ICG), to identify and assess PG perfusion. 16–19 Although ICG has been widely adopted in recent randomized controlled trials, 15 assessment of the viability of PGs using fluorescent dyes is limited by the disruption of surgical workflow, delayed onset of fluorescence signals, and temporal limitations in multiple applications. 16–19
Although new surgical platforms combined with non-invasive imaging techniques such as laser speckle contrast imaging (LSCI) are being introduced to assess PG vascularity 20–22, these devices do not display real-time videos. These imaging modalities take still images that require additional post-processing for assessment. Moreover, they typically require bulky and costly equipment, in addition to already complex surgical tools. 20 Furthermore, no standardized or practical equipment is accessible for surgeons to conveniently locate and assess the viability of PGs intraoperatively in real-time. We recently developed and introduced a real-time, portable hand-held imager to localize and assess the viability of PGs intraoperatively, and proved its efficacy in preclinical studies. 23 The fundamental operation utilizes the same laser speckle contrast analysis method. However, our software permits a dye-free imaging angiography that selectively visualizes the vasculature and tissue microcirculation levels with distinct false colors by digitally fine-tuning arbitrary blood flow indices. For example, according to the relative blood flow speed (arbitrary values between 0 and 255) threshold settings, veins (<128) and arteries (>128) are selectively displayed in distinctive false colors of green and red, respectively. Similarly, the PG perfusion levels are displayed in distinct false colors, such as blue (poorly perfused), green (well-perfused), and red (pulsatile blood flow).
The results of this preliminary study suggest that our dye-free, portable imaging angiography technique combined with PG autofluorescence may permit seamless real-time visualization of tissue perfusion and viability for the first time in thyroid surgery. Herein, we report four surgical cases showcasing the assessment PG perfusion in real-time without any external dye injection using our optical imaging technique.
2. Materials and Methods
This clinical case report is part of a pilot study protocol approved by the Kyungpook National University Chilgok Hospital Institutional Review Board (IRB FILE No. 2019-09-026-001). Four patients were recruited for this study. Among the four patients, three patients underwent thyroid lobectomy and one patient underwent total thyroidectomy. All surgeries were performed at a single institution by an experienced surgeon (Dr. Wan Wook Kim).
2.1. Surgical technique
As reported in our recent paper, 23 our in-house portable hand-held imager is equipped with two cameras for visible and NIR spectral ranges (Fig. 1). The beam splitter/dichroic filter in the imager splits light into red–green–blue (RGB) and NIR images. Furthermore, the on-board software processes the data and displays a dual image consisting of RGB and NIR components on a 4k Ultra-High-Definition Screen intraoperatively. Mode 1 in this system permits label-free, non-invasive localization of PGs using NIRAF, and mode 2 enables continuous visualization of the vasculature and viability assessment of PG tissue using real-time dye-free angiography using an 830 nm laser source. 23 Intraoperatively, an operator held the imager approximately 20–30 cm from the surgical field (FOV: 50 mm diameter, working distance: 30 cm) and imaging was performed with the surgical lights on. During the procedure, the operating surgeon first identified PGs by visual inspection and the device was then used to check if the PG autofluorescence signal was observed. A separate screen was prepared to display the PG autofluorescence and dye-free vascularity/perfusion in real-time. Once autofluorescence signals were confirmed, PG vascularity and perfusion were assessed in the same manner.
Fig. 1.
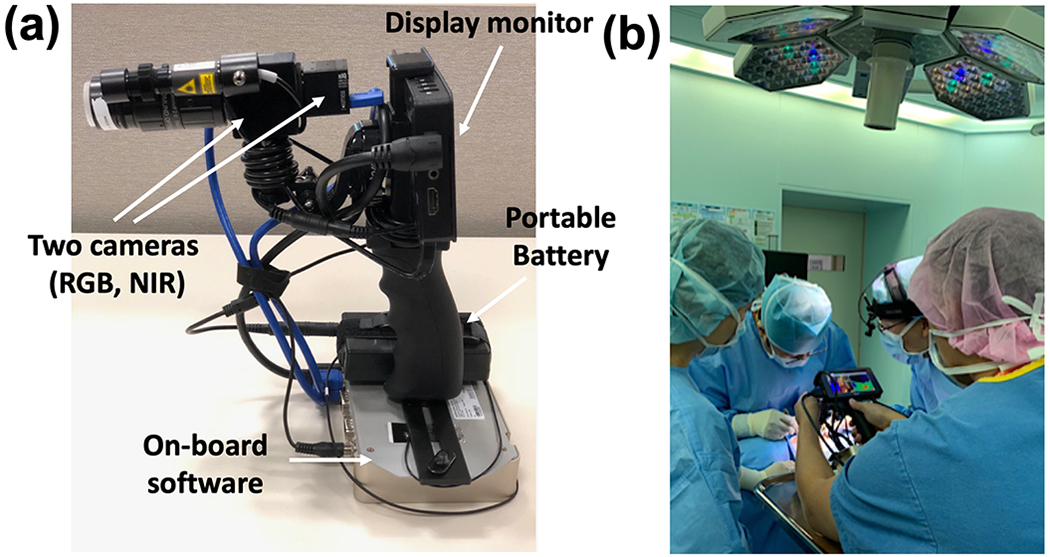
Portable hand-held imager and imaging setup (a) Two cameras of Red-Green-Blue (RGB) and near-infrared (NIR) with an embedded processing board, (b) An operator held the portable imager at 20–30 cm distance.
3. Results
Four patients with ages ranging from 27 to 61 years were enrolled in the study. Patient information, including type of surgery, result summary, and pre- and post-operative serum calcium and PTH levels is presented in Table 1. There were no intraoperative complications associated with the imaging procedure.
Table 1.
Details of case series of four patients including patients, operation, summary of results, and comments (AF – 1: Found, 0: Not found, perfusion – 1: Well-perfused PG, 0: Devascularized PG, Units for calcium and PTH: mg/dl and pg/ml).
| Patient | Sex | Age | Operation | Rt sup PG | Rt inf PG | Lt sup PG | Lt inf PG | Comments | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | perfusion | AF | perfusion | AF | perfusion | AF | perfusion | ||||||
| 1 | F | 61 | Lt lobectomy | N/A | N/A | N/A | N/A | 1 | 0 | N/A | N/A | Devascularized Lt sup PG confirmed Autotransplanted | |
| 2 | F | 50 | Total thyroidectomy | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | Devascularized Rt sup & inf PGs confirmed | |
| 3 | M | 31 | Lt lobectomy | N/A | N/A | N/A | N/A | 1 | 1 | 1 | 0 | Lt sup PG perfusion confirmed | |
| 4 | M | 27 | Lt lobectomy | 1 | 0 | 1 | 0 | N/A | N/A | N/A | N/A | Devascularized Lt sup PG confirmed Autotransplanted | |
| Patient | Sex | Age | Operation | Preop Calcium | Preop PTH | POD1 Calcium | POD1 PTH | POD2 Calcium | POD2 PTH | ||||
|
| |||||||||||||
| 1 | F | 61 | Lt lobectomy | 8.6 | 17.7 | 8.5 | 35 | 9 | |||||
| 2 | F | 50 | Total thyroidectomy | 8.8 | 31.2 | 8.9 | 14.6 | 8.2 | 20.9 | ||||
| 3 | M | 31 | Lt lobectomy | 9.3 | 28.5 | 8.9 | 42.6 | 8.9 | |||||
| 4 | M | 27 | Lt lobectomy | 8.8 | 70.3 | 9.5 | 36.7 | 9.3 | |||||
As summarized in Table 1, a 61-year-old woman underwent a left thyroid lobectomy. The left superior PG was first identified by the operating surgeon through visual inspection and confirmed with autofluorescence using the hand-held imager. In the NIRAF image (Fig. 2), the left superior PG showed bright autofluorescence when illuminated by an NIR laser (100 mW, 785 nm laser diode), which confirmed the identification. Real-time dye-free imaging angiography showed no perfusion or blood flow in the PG.
Fig. 2.

Left superior parathyroid gland (PG), indicated in red circles, are identified using (a) near-infrared autofluorescence (NIRAF), (b) RGB image, and (c) dye-free angiography, which shows no tissue perfusion. (Note that the ambient light is on and simultaneously PG autofluorescence and perfusion map are observed in NIR mode); Colorbar indicates blood flow.
We further investigated vascular perfusion in the inferior thyroid artery, as shown in Fig. 3. The inferior thyroid artery is highlighted in red in the dye-free angiography mode. After careful dissection around the left superior PG, we confirmed that feeding vessels from the superior and inferior thyroid arteries were damaged. After resection of pathological tissue, we reconfirmed the devascularization of the left superior PG and the operating surgeon then autotransplanted the PG. The left inferior PG was not identified within the operative field in this patient.
Fig. 3.

Visualization of blood flow through the inferior thyroid artery (ITA) in (a) RGB mode and (b) Dye-free angiography. Red circle highlights a high flow of the ITA. White arrow indicates the same left superior PG observed in Fig. 2 with no perfusion.
The second case involved a 50-year-old woman who underwent total thyroidectomy. Four PGs were identified by the surgeon after careful dissection. Once the PGs were surgically exposed, three PGs (right superior, right inferior, and left inferior) were identified by autofluorescence; however, the left superior PG was not identified because it was deeply embedded in the tissue. Fig. 4 shows the autofluorescence and vascularity of the left inferior PG before dissection. We visualized the pulsation in the vasculature (Fig. 4c) and confirmed a well-perfused PG.
Fig. 4.

Total thyroidectomy case with PG showing both autofluorescence and feeding vessel flow in (a) NIRAF (b) RGB (c) Dye-free angiography, note that the red circles indicate the same parathyroid gland while the white-dashed circle shows pedicle vessel connected to the parathyroid gland with pulsation.
After dissection and removal of pathological tissue, we assessed the perfusion status of the right superior and inferior PGs using dye-free imaging angiography. Despite meticulous dissection, the blood flow to both PGs disappeared after surgery (Fig. 5). The operating surgeon autotransplanted both PGs. This patient had normal PTH levels without hypocalcemic symptoms and did not require any post-operative calcium medication.
Fig. 5.

Post-operative PGs showing no perfusion flows.(a) Color images show both right superior PG and right inferior PG, (b) Both PGs visualized in dye-free imaging angiography mode. Note that simultaneous assessment of both right superior and inferior glands was possible with the dye-free imaging angiography.
In another patient, the microvasculature in the left superior PG was visualized in bright red color, indicating high blood flow (Fig. 6c). High blood flow was confirmed through clinical assessment by the surgeon and further verified by post-operative assessment (Fig. 7b). Since the left superior PG was saved, autotransplantation of the left inferior PG was not required. Post-operative calcium and PTH levels were normal without any complications.
Fig. 6.

Pre-operative left-inferior PG confirmation using (a) autofluorescence, (b) RGB, and (c) dye-free angiography shows vascularity and well-perfused flows with pulsation.
Fig. 7.

Post-operative assessment of the same left-inferior PG viability in (a) RGB image and (b) dye-free angiography. Note that the PG shows vascularity with pulsation.
4. Discussion
Early detection of PGs during thyroid surgery is essential for their preservation and prevention of permanent hypocalcemia. It is often difficult for surgeons to distinguish whether PGs are healthy through visual inspection. When venous drainage of PGs is poor, surgeons can distinguish PGs without difficulty because the glands typically turn dark red or swell up. However, when the arterial supply is insufficient, identification is often difficult because there is no notable color difference in the appearance of PGs. Currently, fluorescent dyes such as ICG are widely used to identify and assess the perfusion of PGs; however, there are several limitations to this method in surgical settings. First, it is difficult to obtain accurate real-time viability information regarding PGs because of the delayed response to ICG. 18 Moreover, as the fluorescent dyes are washed out of the organ within a few minutes, not all four PGs can be simultaneously evaluated for perfusion and function. 24 This necessitates multiple injections of fluorescent dyes, which may lead to adverse reactions in patients. 18 Most importantly, ICG cannot be properly delivered to blood vessels flowing to devascularized PGs and thus may fail to monitor such devascularized but normal PGs. In our study, the portable imager could detect NIRAF signals emitted from PGs and allowed dye-free imaging angiography to assess PG vascularity and perfusion in real-time.
In a previously published article, Thomas et al. reported that thyroid, brown fat, and other tissues showed autofluorescence. They reported a false positive rate of 8.1% out of the 386 PGs examined. 14 However, in our study of four patients, we found that the surrounding tissues did not exhibit autofluorescence in NIR light, as shown in Fig. 2. We expected to see brighter signals in the PGs because of their intrinsic properties that are responsible for autofluorescence when illuminated by the NIR excitation light (785 nm). Based on our image analysis using ImageJ, the target-to-background ratio of the PG compared with its surroundings was 3.05. Thus, within our limited sample size, we found that only PGs exhibited autofluorescence, and no autofluorescence was detected in the surrounding tissues. Although we did not encounter any false positives in this study, we recognize that potential false positives can arise from brown fat, thyroid nodules, lymph nodes, or fibrofatty tissues. 14,15 By applying machine learning to future clinical data, the issue of false positives may be mitigated. Moreover, we also recognize the potential for false negatives, which may occur when PGs are deeply situated, covered by blood or water in the surgical field, or diseased. 25 In a future study, we will examine the nature of autofluorescence in surrounding tissue types with a larger sample size to compare our findings with those reported in a published paper. 14 Previously, it has also been reported that diseased PGs, such as PGs with adenomas, show heterogeneous autofluorescence patterns. 26 We aim to identify the characteristics of different types of PGs by collecting more clinical data and utilizing machine learning to distinguish between normal and diseased PGs by training both shape, colors and their NIRAF patterns.
Meanwhile, Mannoh et al. reported that LSCI successfully distinguished between well- and poorly vascularized PGs with an accuracy of 91.5%. 22 Recently, they proposed a combined imaging system integrating both NIRAF and LSCI techniques to simultaneously localize and assess the vascularity of PGs. 20 However, both the technique and device had limitations with regard to pre-identifying PGs and post-processing for analysis. The surgical light had to be turned off during the measurements, and due to quasi-real-time data acquisition and processing, the device took several minutes to display perfusion results, requiring compensation by averaging the signals from motion artifacts. On the other hand, our portable hand-held imaging device allowed us to monitor tissue perfusion continuously in real-time, without disrupting the surgical workflow, as none of the ambient lights (operating room lights, surgical lights, and surgeon’s headlamp) needed to be turned off during its operation. We chose dye-free imaging angiography to highlight PGs without labeling and emphasize the blood flow visualization, which shows the perfusion levels and vasculature of the PGs in false colors according to the relative blood flow indices. Although there is no fundamental difference between LSCI used by Mannoh et al. and dye-free angiography, 20,21 our system includes a co-registered RGB portion and displays the video-streams in real-time with selective false-color imaging and threshold values. The key features of our device are 1) wireless connectivity, 2) portability, and 3) a compact hand-held design. Our device has a built-in display, as well as an extendable screen display, a dual RGB-NIR camera, and different modes that allow a quick change from NIRAF to dye-free angiography, which makes it easier for surgeons to navigate the target areas and maneuver the device as necessary within the surgical field. More clinical studies with the device improvement would further validate the usefulness of this prototype, in cases of total parathyroidectomy with autotransplantation or subtotal thyroidectomy in secondary or tertiary hyperparathyroidism. 27 Also, our technology will be of great help in measuring the blood flow of the PG left in this scenario and evaluating viability in subtotal thyroidectomy. 27 As we make successive iterations of the device, we also hope to deliver a more user-friendly version of the device and output by incorporating quantifiable numbers and thresholds to indicate the PGs and the percentage of viability and blood flow in the glands or vasculature. We will continue improving upon the usability of the screen and the imager by adding control buttons and features that can help surgeons easily navigate through the different modes of our system.
Compared with perfusion assessment using ICG fluorescence, our dye-free imaging angiography method permits seamless visualization of diminutive blood supply to PGs (Figs. 4, 6, 7) and can be iteratively used intraoperatively to determine whether the blood flow is disrupted due to a transient vasoconstriction of vessels or devascularization of normal PGs. All PGs assessed using the imager were validated by clinical assessment during surgery and were verified post-operatively by monitoring the patients’ PTH and calcium levels. After the patients fully recovered from surgery, the serum levels of PTH (8–76 pg/ml) and calcium (8.6–10.1 mg/dl) were found to be within the normal range, with no complications overall. Our preliminary data suggested that the imager was highly effective in evaluating real-time perfusion in three cases: viable PG, non-viable PG, and seemingly viable but non-viable PG. In the first case, the PG showed red-colored microvasculature (feeding vessels) and pulsation, indicating a highly perfused and well-vascularized PG. However, in the second case, a dark blue color was noted in the dye-free angiography data, suggesting poorly perfused or devascularized PGs. Most importantly, the imager was able to detect devascularized PGs when they appeared to be viable with visual inspection. For this qualitative study, we interpreted the color of the vasculature or PGs according to a color map with quantification of blue (0–64), yellow to green (65–128), and green to red (129–255); red and green colors were deemed as well perfused vasculature and tissue respectively, and blue was deemed as poorly perfused or devascularized. In a previous study, 23 we validated the false colors in a preclinical ischemic kidney model by ligating renal artery and vein. Although a detection threshold was not established in this study involving four patients due to the assessment of relative flow rates, we hope to further calibrate the absolute flow rates with known microfluidic channel systems described in our previous paper. 28
Despite the key features and advantages of our device, our study had some limitations. Given that our feasibility study was limited to a small number of cases (N=4), further studies are needed to validate the effectiveness of the technique. For example, during parathyroidectomy, dye-free imaging angiography could be used to monitor the viable, hyperplasic PG perfusion before and after clamping the pedicle vasculature to determine if there is a change in blood flow. In addition, the performance of the imager needs to be improved to clearly visualize the microvasculature attached to the PGs. Moreover, as the imager was hand-held, motion artifacts were inevitable when collecting data. To mitigate this issue, we hope to further improve upon the design and make the output less susceptible to motion artifacts by mounting a more compact, lightweight system in the surgeon’s headlamp or on surgical lights. Other potential mitigation strategies to address this issue include the use of a hand-stabilizer or articulated arm to hold the device. Lertsakdadet et al. recently introduced an approach to address both motion artifact and image co-registration for their hand-held laser speckle imaging system that involves adding a fiducial marker into the imaging protocol and post-processing the data. 29 They showed that it was possible to reduce motion artifacts by using a gimbal stabilizer. 30 Laser speckle imaging is directly comparable to dye-free angiography, and we believe that there are various methods to enhance our hand-held system and mitigate motion artifacts.
5. Conclusion
In this study, we demonstrated the early feasibility of a portable hand-held imager that allows both PG autofluorescence detection and dye-free perfusion imaging. The preliminary results show that our imager has the potential to identify and assess PGs intraoperatively during thyroid and parathyroid surgery. With further clinical studies, the effectiveness of this technique can be further delineated.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R43EB030874. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Karadeniz E, Akcay MN. Risk Factors of Incidental Parathyroidectomy and its Relationship with Hypocalcemia after Thyroidectomy: A Retrospective Study. Cureus. 2019;11(10):e5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steen S, Rabeler B, Fisher T, Arnold D. Predictive factors for early postoperative hypocalcemia after surgery for primary hyperparathyroidism. Proc (Bayl Univ Med Cent). 2009;22(2):124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine. 2014;47(2):537–542. [DOI] [PubMed] [Google Scholar]
- 4.Mittendorf EA, McHenry CR. Complications and sequelae of thyroidectomy and an analysis of surgeon experience and outcome. Surg Technol Int. 2004;12:152–157. [PubMed] [Google Scholar]
- 5.McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab. 2014;99(12):4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt. 2011;16(6):067012. [DOI] [PubMed] [Google Scholar]
- 7.Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc. 2017;31(7):3020–3027. [DOI] [PubMed] [Google Scholar]
- 8.Abbaci M, De Leeuw F, Breuskin I, et al. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral Oncol. 2018;87:186–196. [DOI] [PubMed] [Google Scholar]
- 9.Squires MH, Jarvis R, Shirley LA, Phay JE. Intraoperative Parathyroid Autofluorescence Detection in Patients with Primary Hyperparathyroidism. Ann Surg Oncol. 2019;26(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 10.Kose E, Rudin AV, Kahramangil B, et al. Autofluorescence imaging of parathyroid glands: An assessment of potential indications. Surgery. 2020;167(1):173–179. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G, McWade MA, Nguyen JQ, et al. Innovative surgical guidance for label-free real-time parathyroid identification. Surgery. 2019;165(1):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SW, Lee HS, Ahn YC, et al. Near-Infrared Autofluorescence Image-Guided Parathyroid Gland Mapping in Thyroidectomy. J Am Coll Surg. 2018;226(2):165–172. [DOI] [PubMed] [Google Scholar]
- 13.Shinden Y, Nakajo A, Arima H, et al. Intraoperative Identification of the Parathyroid Gland with a Fluorescence Detection System. World J Surg. 2017;41(6):1506–1512. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G, Solorzano CC, Baregamian N, et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection - A surgeon-blinded multi-centric study. Am J Surg. 2021. S0002-9610 (21): 00280-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solorzano CC, Thomas G, Berber E, et al. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery. 2021;169(4):868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidal Fortuny J, Sadowski SM, Belfontali V, et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg. 2018;105(4):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ, Ladurner R. Intraoperative Near-Infrared Autofluorescence and Indocyanine Green Imaging to Identify Parathyroid Glands: A Comparison. Int J Endocrinol. 2019;2019:4687951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanaropoulou NM, Chorti A, Markakis M, Papaioannou M, Michalopoulos A, Papavramidis T. The use of Indocyanine green in endocrine surgery of the neck: A systematic review. Medicine (Baltimore). 2019;98(10):e14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spartalis E, Ntokos G, Georgiou K, et al. Intraoperative Indocyanine Green (ICG) Angiography for the Identification of the Parathyroid Glands: Current Evidence and Future Perspectives. In Vivo. 2020;34(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannoh E, Luo M, Thomas G, Solórzano C, Mahadevan-Jansen A. A combined autofluorescence and laser speckle contrast imaging system for parathyroid surgical guidance (Conference Presentation). Vol 10868: SPIE; 2019. [Google Scholar]
- 21.Mannoh EA, Parker LB, Thomas G, Solorzano CC, Mahadevan-Jansen A. Development of an imaging device for label-free parathyroid gland identification and vascularity assessment. J Biophotonics. 2021;14(6):e202100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannoh EA, Thomas G, Solorzano CC, Mahadevan-Jansen A. Intraoperative Assessment of Parathyroid Viability using Laser Speckle Contrast Imaging. Sci Rep. 2017;7(1):14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh E, Kim WW, Nam S-H, Cheon GW, Ning B, Cha J. Development of a portable imager for intraoperative real-time localization of parathyroid glands. Vol 11229: SPIE; 2020. [Google Scholar]
- 24.Suh YJ, Choi JY, Chai YJ, et al. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc. 2015;29(9):2811–2817. [DOI] [PubMed] [Google Scholar]
- 25.Hartl DO Raïs; Guerlain Joanne ; Breuskin Ingrid ; Abbaci Muriel ; Laplace-Builhé Corinne. Intraoperative Parathyroid Gland Identification Using Autofluorescence: Pearls and Pitfalls. World Journal of Surgery and Surgical Research. 2019;2(1). [Google Scholar]
- 26.Demarchi MS, Karenovics W, Bedat B, De Vito C, Triponez F. Autofluorescence pattern of parathyroid adenomas. BJS Open. 2021;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Lau LW, Cha J. Dual-display laparoscopic laser speckle contrast imaging for real-time surgical assistance. Biomed Opt Express. 2018;9(12):5962–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lertsakdadet B, Yang BY, Dunn CE, et al. Correcting for motion artifact in handheld laser speckle images. J Biomed Opt. 2018;23(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lertsakdadet B, Dunn C, Bahani A, Crouzet C, Choi B. Handheld motion stabilized laser speckle imaging. Biomed Opt Express. 2019;10(10):5149–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


