Abstract
Microbial enzymes have gained interest for their widespread use in various industries and medicine due to their stability, ease of production, and optimization. Endophytic fungi in plant tissues produce a wide range of secondary metabolites and enzymes, which exhibit a variety of biological activities. The present review illustrates promising applications of enzymes produced by endophytic fungi and discusses the characteristic features of the enzymes, application of the endophytic fungal enzymes in therapeutics, agriculture, food, and biofuel industries. Endophytic fungi producing ligninolytic enzymes have possible biotechnological applications in lignocellulosic biorefineries. The global market of industrially important enzymes, challenges, and future prospects are illustrated. However, the commercialization of endophytic fungal enzymes for industrial purposes is yet to be explored. The present review suggests that endophytic fungi can produce various enzymes and may become a novel source for upscaling the production of enzymes of industrial use.
Keywords: Endophytic fungi, Enzymes, Cellulase, Xylanase, Co-cultivation, Epigenetic modifiers, l-Asparaginase
Introduction
Enzymes have enormous potential in various industrial sectors such as pharmaceuticals, food, beverages, detergents, leather processing, and paper and pulp. For decades, enzymes are being isolated from different sources such as animals and plants. Presently, microorganisms are more preferred sources for the production of industrial enzymes due to their easy availability and fast growth rate (Shankar et al. 2016). With recombinant DNA technology, genetic changes can easily be made on microbial cells for enhanced enzyme production (Illanes et al. 2014). The production of microbial enzymes is necessary for the industrial sectors because of their superior performances and work under a wide range of varied physical and chemical conditions with innocuous pollution and cost-effectiveness (Singh et al. 2016). Many microorganisms such as bacteria, yeast, and fungi have been explored for the production of enzymes. Production of industrial enzymes by bacteria has been studied for a long time, and among different bacterial species, the genus Bacillus has been extensively prospected (Joo and Choi 2012). Other bacterial genera employed as enzyme production sources are Streptomyces and Pseudomonas. Besides bacterial species, fungal species are also extensively explored for enzyme production (Chambergo and Valencia 2016). In biotechnology, approximately a few dozen fungal species are explored commercially for their enzyme-producing ability (Corrêa et al. 2014). Trichoderma reesei, Aspergillus niger, and Aspergillus oryzae have become preferred enzyme producers because their enzymes are robust and capable of resisting a wide range of conditions such as extreme pH, temperature, osmolarity, and pressure (Corrêa et al. 2014). Moreover, fungal enzyme production benefits from high yields with low cost, rapid development, low-cost growth media, high stability, and easy recovery of the produced enzymes (Wanderley et al. 2017). In addition, fungal enzymes are secreted extracellularly, and different complex substrates like corn-steep liquor of lower cost could be used, and most of the solid fermentation can be performed with fungi compared to bacteria (Gopinath et al. 2017). Microbial proteins can be expressed in recombinant bacteria, yeasts, or filamentous fungal cell cultures. Though bacterial expression systems have an edge on efficiency because of their growing ability and high density on substrates (Terpe 2006), filamentous fungi became attractive hosts due to increased secretion levels of bioactive enzymes (Yang et al. 2017).
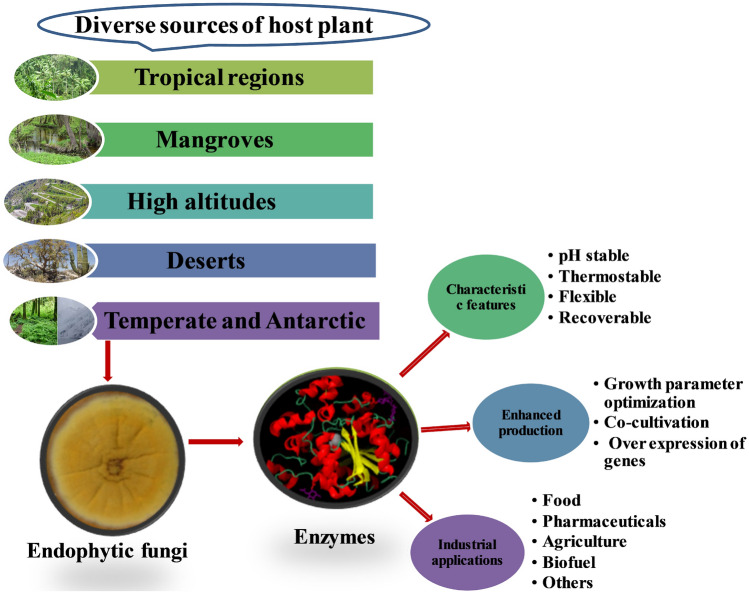
Endophytes live inside the plant tissues without causing any sign of infection during all or part of the plant life cycle (Schulz et al. 2002). They establish unique relationships with the plant species ranging from symbiosis to latent pathogens. The interaction between plant and endophyte is intricately modulated by different factors such as environmental parameters, host genotype, developmental stage, and immune system signaling (Aamir et al. 2020). Unveiling the folds of endophyte–host interaction reveals a hotspot for the exploration of repertoire of hyperdiverse and valuable secondary metabolites (Choudhary et al. 2021). For the last 2 decades, endophytic fungi have been explored for various bioactive compounds for therapeutic purposes. Diverse compounds such as terpenoids, steroids, xanthones, quinones, phenols, cytochalasins, benzopyrones, and isocoumarins are originating from these fungi through different biosynthetic pathways (Schulz and Boyle 2005). Presently, the main focus of endophytic fungal research is linked to the production of secondary metabolites with applications in the environment, agriculture, medicine, and the food industry (Kusari et al. 2014). Though the ability of endophytic fungi to produce bioactive metabolites and novel drugs have been explored in a greater way, little attention has been drawn to use them as sources for industrial enzymes. In association with their host plants, endophytic fungi secrete proteins presumed to aid in growth, nourishment, and defense. The endophytic fungi produce some of the enzymes such as pectinases, cellulases, amylases, laccases, proteases, and lipases as one of the resistant mechanisms against pathogenic organisms and also for gaining nutrients from the host (Vasundhara et al. 2019). The cellulose and lignin production depicts a strategic benefit to decompose the tissues and endure as saprobes after senescence (Oses et al. 2006). Different extracellular enzymes secreted by endophytic fungi have applications in food, textile, leather, confectionery, agriculture, beverage, and human health (Mishra et al. 2017a). Several researchers have acknowledged the potential of endophytic fungal enzymes in industrial applications and secondary metabolites production (Joo and Choi 2012; Yadav 2019; Gupta et al. 2016; Khan et al. 2017). In the present review, various enzymes produced by endophytic fungi, their characteristic features such as thermal stability, pH tolerance, and approaches to increase yield by optimizing growth parameters, co-cultivation, and overexpression of genes were discussed. An attempt has also been made to review the application of endophytic fungal enzymes in therapeutics, agriculture, biofuel, the food industry, and other relevant industries. The global market for industrially important enzymes and challenges and prospects are also discussed.
Enzymes from endophytic fungi
Endophytic fungi help the host plant to combat biotic and abiotic stress by synthesizing metabolites and extracellular enzymes, which can act as resistance mechanisms against pathogens and help to nourish the host plants (Jagannath et al. 2021). Hence, endophytic fungi serve as a promising source for producing a wide range of extracellular enzymes (Table 1). Endophytic fungi such as Pestalotiopsis microspora, A. oryzae, and Penicillium chrysogenum were reported to produce amylases (Joel and Bhimba 2012; Fouda et al. 2015). In addition, the endophytes, Pestalotiopsis sp., Acremonium sp., Microsphaeropsis sp., Sclerocystis sp., Nigrospora sp., Phomopsis sp., Keteleeria davidiana, Cephalosporium sp., Penicillium sp., Fusarium oxysporum, Aspergillus sp., P. chrysogenum, and Xylaria sp., can produce cellulase enzymes (Maria et al. 2005; de Almeida et al. 2011; Peng and Chen 2007; Syed et al. 2013; Onofre et al. 2013; Tasia and Melliawati 2017; Fouda et al. 2015; Bezerra et al. 2012). The β-glucosidase enzymes production from the Periconia sp. and A. versicolor were reported (Harnpicharnchai et al. 2009; Huang et al. 2021). de Almeida et al. (2013) reported that A. zeae and F. verticillioides were capable of producing endoglucanase enzymes. Gelatinase was obtained from the Alternaria alternata (Fouda et al. 2015). The endophytic fungi, Hormonema sp., Neofusicoccum sp., Monotospora sp. were capable of producing laccases (Fillat et al. 2016; Wang et al. 2006). In addition, lipase enzyme was obtained from Rhizopus oryzae, Cercospora kikuchii, and Lasiodiplodia theobromae (Torres et al., 2003; Costa-silva et al. 2011; Venkatesagowda et al. 2012). Pestalotiopsis sp., A. alternata, and Penicillium glandicola have been reported to produce pectinases (Maria et al. 2005; Fouda et al. 2015; Bezerra et al. 2012). Tannase was obtained from the endophytic fungi, A. niger and A. fumigatus (Cavalcanti et al. 2017). The xylanase enzyme was isolated from Pestalotiopsis sp., Acremonium sp., and A. alternata (Maria et al. 2005; Tasia and Melliawati 2017; Fouda et al. 2015). Despite the ability to produce enzymes by endophytic fungi, this group of fungi was less explored (Corrêa et al. 2014). Exploring biodiversity to discover efficient enzymes is an upcoming challenge for researchers (Patel et al. 2016). Among these endophytic fungi, Fusarium, Trichoderma, Aspergillus, and Alternaria species are the most common producers of various industrially important enzymes.
Table 1.
Some fungal endophytes that have been reported to produce various enzymes and their hosts
| Enzyme | Endophytic fungi | Source | References |
|---|---|---|---|
| Amylase | Pestalotiopsis microspore, Aspergillus oryzae, Penicillium chrysogenum | Rhizophora mucronata, Avicennia officinalis, A. marina, Asclepias sinaica | Joel and Bhimba (2012), Fouda et al. (2015) |
| Cellulase | Pestalotiopsis sp., Acremonium sp., Microsphaeropsis sp., Sclerocystis sp., Nigrospora sp., Phomopsis sp., Keteleeria davidiana, Cephalosporium sp., Penicillium sp., Fusarium oxysporum, Aspergillus sp., Penicillium chrysogenum, Xylaria sp. | Acanthus ilicifolius, Zea mays, Sabina chinensis, Taxus chinensis, Keteleeria evelyniana, Pinus massoniana, Cupressus torulosa, Chlorophytum comosum, Baccharis dracunculifolia, Glochidion Borneese, Asclepias sinaica, Opuntia ficus-indica | Maria et al. (2005), de Almeida et al. (2011), Peng and Chen (2007), Syed et al. (2013), Onofre et al. (2013), Tasia and Melliawati (2017), Fouda et al. (2015), Bezerra et al. (2012) |
| β-glucosidase | Periconia sp., Aspergillus versicolor | Cola acuminata | Harnpicharnchai et al. (2009), Huang et al. (2021) |
| Endoglucanase | Acremonium zeae, Fusarium verticillioides | Zea mays | de Almeida et al. (2013) |
| Gelatinase | Alternaria alternata | Asclepias sinaica | Fouda et al. (2015) |
| Laccase | Hormonema sp., Neofusicoccum sp., Monotospora sp. | Eucalyptus globulus, Cynodon dactylon | Fillat et al. (2016), Wang et al. (2006) |
| Lipase | R. oryzae, Cercospora kikuchii, Lasiodiplodia theobromae | F. Vulgare, Tithonia diversifolia, Cocos nucifera | Torres et al. (2003), Costa-silva et al. (2011), Venkatesagowda et al. (2012) |
| Pectinase | Pestalotiopsis sp., Alternaria alternata, Penicillium glandicola | Acanthus ilicifolius, Asclepias sinaica, Opuntia ficus-indica | Maria et al. (2005), Fouda et al. (2015), Bezerra et al. (2012) |
| Tannase | A. niger, A. fumigatus | Anadenanthera colubrina, Anacardium occidentale | Cavalcanti et al. (2017) |
| Xylanase | Pestalotiopsis sp., Acremonium sp., Alternaria alternata | Acanthus ilicifolius, Memecylon excelsum, Asclepias sinaica | Maria et al. (2005), Tasia and Melliawati (2017), Fouda et al. (2015) |
Characteristic features of enzymes
The properties of enzymes, such as thermostability, pH stability, pressure, and chemical inertness, are essentially required for industrial and medical processes. Higher yield with the cost-effective fermentation procedure is the priority for the enzyme industries. Basic knowledge of microbial diversity can help to characterize new enzymes discovered in various environmental conditions (Thapa et al. 2019). Different environmental conditions such as low to high pH, extreme temperature, elevated pressure, and oxidative stress will help endophytic fungi to produce enzymes for better industrial performance (Alkalde et al. 2006). For obtaining such resistant and stable enzymes, molecular engineering and other biotechnological techniques are used. The enzymes with such variability are required for efficient and cost-effective bioconversion processes (Rigoldi et al. 2018). The key features of the enzymes produced by various endophytic fungi from the diverse environments were depicted in Fig. 1.
Fig. 1.
Some of the key features of enzymes produced by fungal endophytes from diverse regions
Thermostable enzymes
In many studies, it has been proven that microbial enzymes offer higher thermostability. Internal and external properties are equally crucial for the thermostability of the enzymes.
The molecular alterations at cellular and subcellular levels aid them in adapting to harsh environmental habitats. Thermostability and kinetic stability are the two crucial features of thermostable enzymes. Similar to the native enzyme, β-glucosidase produced by the endophytic fungus Periconia sp. (BCC2871) exhibited its optimal activity at 70 °C with pH of 5 and 6 and hydrolyzed the rice straw into simple sugars. Few enzymes such as heat-tolerant xylanase and cold-tolerant lipase have been isolated from endophytic fungi in different thermal regions (Bradner et al. 1999). Such enzymes are good candidates for converting the lignocellulosic biomass to biofuels and chemicals. Thermostable glucoamylase produced by Streptosporangium sp., isolated from leaves of maize exhibited the maximum enzyme activity (158 U mg−1 protein) at pH 4.5 and temperature 70 °C (Stamford et al. 2002). Two endophytic fungal strains Botryosphaeria sp. AM01 and Saccharicola sp. EJC04 produced three thermostable enzymes that are endoglucanase, β-glucosidases, and xylanases. Among these, β-glucosidases exhibited maximum activity at a wide range of pH (3.5–8.5), followed by xylanases, with its optimum activity at 60–75 °C (Marques et al. 2018). The tannase enzyme produced by the endophytic fungus Pestalotiopsis guepini exhibited thermal stability and retained its activity of 91.4% at 90 °C (de Sena et al. 2014). Xylanase produced by the endophytic fungus A. terreus isolated from Memora peregrine showed thermostability at 55 °C (Sorgatto et al. 2012). Wipusaree et al. (2011) reported that the xylanase produced by A. alternata isolated from Croton oblongifolius was thermostable up to 40 °C. Phytase enzyme produced by F. verticillioides exhibited its thermal stability up to 60 °C (Mehdipour-Moghaddam et al. 2010). External factors that affect thermostability are the concentration of protein and biochemical properties of the substrate (Bendourou et al. 2021). Some of the co-factors such as Mg2+, Ca2+, Sr2+, and Ba2+ help to stabilize protein structures by interacting specifically with the unfolded polypeptide and accelerates the formation of the functional protein by priorly coordinating with polypeptide folding (Wittung 2000).
pH stability
The pH plays a vital role in nature as it significantly impact all biological functions. The interactions among protein–protein, protein–membrane, and protein–ligand seem to be highly affected by the pH variations (Dittrich et al. 2008). Enzymes used for various biotechnological applications must possess pH stability with optimum activity to execute their functions efficiently. Different endophytic fungi were reported to produce stable enzymes with different pH, ranging from alkaline to acidic. Torres et al. (2003) isolated endophytic fungus R. oryzae from the Mediterranean that produced stable lipase enzyme, with maximum activity at pH 4 and 7. In another study, out of 34 isolates of endophytic fungi isolated from Glycine max (L), two isolates, Rhizoctonia sp. and F. verticillioides exhibited pH stability between 2.5 and 6.0 (Marlida et al. 2010). The pH stability was assessed for extracellular enzymes (amylase, cellulase, chitinase, laccase, lipase, protease, and tyrosinase) produced by Pestalotiopsis sp., Acremonium sp., Aspergillus chlamydosporus, Aspergillus sp., and Fusarium sp. The highest cellulase activity was recorded at pH 7, while xylanase and protease at pH 9 for these isolates (Maria et al. 2005). Thiol-dependent protease produced by the endophytic fungus Aspergillus ochraceus BT21 isolated from Ruprechita saicifolia exhibited its stability at alkaline pH of 8.0 (Mohamed et al. 2021). The cellulase produced by endophytic fungus F. oxysporum isolated from Baccharis dracunculifolia exhibited its maximum activity at pH 5.96 (Onofre et al. 2013). The tannase enzyme produced by A. fumigatus and A. niger was stable at 30 °C and pH 4.0 (Cavalcanti et al. 2017). Renuka and Ramanujam (2016) reported the phytase production in F. verticillioides and Rhizoctonia sp. showed stability at optimal pH of 5.0 and 4.0, respectively. Panuthai et al. (2012) reported the production of extracellular lipases from F. oxysporum, isolated from the leaves of Croton oblongifolius with optimal activity at pH 8.0 and moderate activity between 8 and 12. The cellulase produced by Penicillium sp. CPF2 has exhibited its maximal activity at pH 5.5 (Syed et al. 2013).
Approaches to enhance the yield of enzymes
Endophytic fungi represent an inexhaustible source of important metabolites with varied biological activities. Most of the biosynthetic gene clusters (BCGs) responsible for secondary metabolites production remain silent in fungi or are only weakly expressed under standard laboratory growth conditions. Therefore, there is a need to come up with techniques that can be used to induce the activation of cryptic biosynthetic pathways to enhance secondary metabolites biosynthesis in fungal endophytes. Below, we highlight some of the approaches that have been employed to activate silent gene clusters in fungal endophytes.
Optimization of growth parameters
Endophytic fungi produce enzymes under controlled physiochemical conditions. When these conditions alter, then, it affects the production of enzymes. Therefore, enzyme production with a high yield and more stability can be carried out at optimized growth parameters. The one factor at a time approach can enhance the production of industrially important enzymes (Mishra et al. 2017b). Response surface methodology is associated with the interaction and modeling of operational growth parameters. This technique involves three steps: (a) to perform the experiments which are statistically designed, (b) coefficient estimation in a mathematical model and (c) to predict and analyze the model adequacy. The regression equation obtained after the interaction helps to optimize the parameters for enhancing the production of enzymes from the fungi. Several factors such as temperature, pH, and carbon to nitrogen ratio, inducers, medium composition, and aeration rate may influence the production of enzymes. Therefore, supplementation of suitable inducers with appropriate growth conditions would be an excellent strategy to enhance the production of enzymes. It was reported that copper is mainly used as an inducer for the production and catalytic activity of laccase enzyme obtained from the endophytic fungi. The results showed an 85% increase in lignocellulosic activity for endophytic fungi Neofusicoccum luteum and Hormonema sp. while a 95% increase for N. australe (Fillat et al. 2016). The endophytic fungus Caribena versicolor is known to have the lignin degradation ability. In the presence of syringic acid, gallic acid, and copper sulfate, this fungus degraded the lignin 2.43 times higher than without supplementation (Mishra et al. 2017a, b). By optimizing the incubation period, temperature, pH, and carbon source, the production of l-asparaginase was enhanced (El-Gendy et al. 2021). The enhanced production of β-glucosidase and amylase in endophytic fungi was achieved (Hegde et al. 2011; Huang et al. 2021). After optimizing different parameters, the amylase enzyme obtained from the Discosia sp. showed maximum production at pH 7.0 and temperature of 30 °C. The parameters optimized by response surface methodology showed 14.4-fold increased production of β-glucosidase with 812.86 U/mL activity and 25.98% recovery (Huang et al. 2021).
Co-cultivation and epigenetic modifications
Co-cultivation is the growth of two or more microorganisms together to mimic the myriad of interactions, which occur when they coexist naturally (Bertrand et al. 2014). In fungi, genes that encode the enzymes needed for the biosynthesis and transport of secondary metabolites and pathway-specific regulatory genes are clustered on a single gene locus, forming biosynthetic gene clusters (BGCs) (Keller et al. 2005). In several instances, it has been shown that the activation of cryptic BGCs among certain microbes requires an ecological context like interaction with other microbes and host plants for the plant-associated microorganisms. Such interactions between microbes alter the biosynthetic profile of the involved strains and produce compounds that are not produced in monoculture (Stroe et al. 2020). Cultivation of fungus–bacterium and fungus–fungus are the two main strategies employed to induce cryptic BGCs in fungal endophytes. An endophytic fungus Fusarium tricinctum, when grown in the presence of Bacillus subtilis increased (78-fold increase) the secondary metabolite production (Ola et al. 2013). Co-cultivation of endophytic fungus Chaetomium sp., isolated from Sapium ellipticum with B. subtilis, resulted in an eightfold increase in secondary metabolites production (Akone et al. 2016). When two endophytic fungi, Paraconiothyrium sp., and Alternaria sp., were cultured together, a threefold increase in the production of paclitaxel was recorded (Soliman and Raizada 2013). A mixed fermentation of Phoma sp., an endophyte, and Armillaria sp., a fungal symbiont, resulted in the biosynthesis of novel compounds (Li et al. 2019). Two fungi T. reesei (LM-UC4) and Aspergillus phoenicis (QM329) were reported to produce a complete set of cellulase enzymes through co-culture using bagasse as substrate in solid-state fermentation conditions. A study showed a twofold increase in the yield and a sixfold increase in β-glucosidase activity compared to the monoculture. Another study showed a 30–50% increase in the production of extracellular enzymes by mixed culture of Trichoderma viride and A. niger compared to monoculture. The co-culture of Aspergillus carbonarius and T. reesei showed enhanced cellulase enzyme production. When T. reesei and Monascus purpureus were cultured together, enhanced production of hydrolytic enzymes was observed (Singh et al. 2021). These studies reveal that co-cultivation is becoming a powerful tool for unlocking the chemical diversity of fungal endophytes by increasing the expression of cryptic or weakly expressed BGCs.
The regulation of fungal secondary metabolism is a complex process that depends on an intricate network of cellular, chemical, and genetic determinants, which occurs via the cluster-specific and globally acting regulators (Rojas-Aedo et al. 2018). Chromatin-level control of gene silencing or activation is one of the mechanisms involved in the regulation of fungal secondary metabolites biosynthesis (Brakhage 2013). The use of small chemical molecules known as epigenetic modifiers is one such approach in regulating secondary metabolites production. Remodeling the chromatin landscape by chemically targeting the histone and DNA post-translation modifications has been found to enhance the quantity of constitutive fungal secondary metabolites by activating or suppressing secondary metabolite encoding gene clusters (Cichewicz, 2010). Epigenetic modification can be achieved by cultivating the target strains in the presence of histone deacetylases (HDAC) inhibitors or DNA methyltransferases (DNMT) inhibitors. The addition of these compounds at micromolar or even nanomolar concentrations has been found to suppress or activate the associated enzymes, resulting in the reengineering of secondary metabolites biosynthesis pathways in fungi (Yang et al. 2014). The endophytic fungus Pestalotiopsis crassiuscula isolated from Fragaria chiloensis when grown in the presence of 500 µM 5-azacytidine resulted in drastic chemical differences in the extracts than the fungus grown in the absence of DMT inhibitor (Cichewicz 2010). A study exploring the influence of epigenetic modification and co-cultivation of the endophytic fungus Chaetomium sp., and B. subtilis resulted in the production of isosulochrin, when the fungus was grown in rice media in the presence of either suberoylanilide hydroxamic acid (SAHA) or 5-azacytidine (Akone et al. 2016).
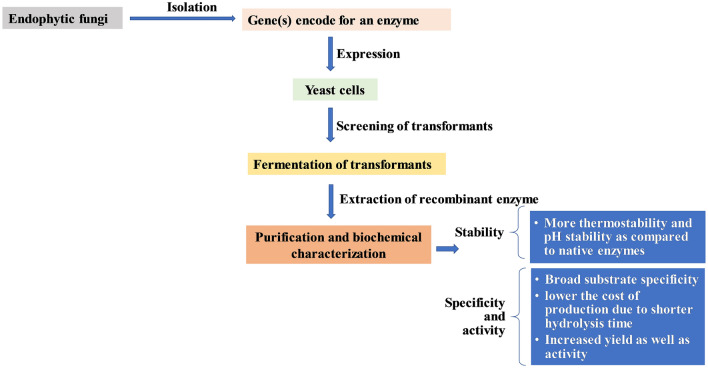
Overexpression of genes
Genetic transcription and protein secretion are the two mainly used strategies that can help in the gene manipulation of fungi, which further helps in producing enzymes with better quality and higher yield (El-Gendy et al. 2021). The general view of the overexpression of genes encoding for various enzymes from endophytic fungi to enhance the yield and stability was depicted in Fig. 2. Endophytic fungi can be manipulated to produce enzymes employing over-expressing the genes coding for the respective enzymes (Yadav et al. 2009). The novel gene (TdAmyA) encoding for α-amylase isolated from Thermomyces dupontii (L18) over-expressed in Pichia pastoris showed the highest activity of 38,314 U/mL with a protein content of 28.7 mg/mL (Wang et al. 2019). The two new genes PsLPMOA and PsLPMOB obtained from Pestalotiopsis sp., encode for the lytic polysaccharide monooxygenases were over-expressed in yeast P. pastoris showed better quality even in the presence of sea salt compared to the native enzyme (initial fermentation at 30 °C for 20 h with oxygen flow rate of 0.1–0.2 vvm, pH 5 and agitated at 600 rpm) (Patel et al. 2016). The β-glucosidase gene from Periconia sp. BCC2871 cloned into the P. pastoris KM71 showed optimal activities at a temperature of 70 ˚C and pH of 5 and 6. The recombinant enzyme retained 60% of its activity when incubated at 70 °C for 1.5 h (Harnpicharnchai et al. 2009). The gene encoding for the pectin lyase of Penicillium griseoroseum over-expressed in Aspergillus nidulans showed an increased yield than the non-cloned fungi (Yadav et al. 2009).
Fig. 2.
General view of the overexpression of genes encoding for various enzymes from endophytic fungi to enhance the yield and stability
Applications
Endophytic fungi have been recognized as valuable sources of natural products for agronomy, industry, and biomedical development and also produce enzymes with varied applications. The wide applications of enzymes produced by endophytic fungi can meet the growing demand in different sectors. Below, we summarize some of the applications of endophytic fungal enzymes in various industries.
Therapeutics
Enzymes play an essential role in pharmaceutics and healthcare, as these are used as tools in the manufacturing of active pharmaceutical ingredients and sometimes directly as drugs for therapeutic purposes. The high accuracy, strong affinity, and specificity for target location with minimum side effects make the enzymes more favorable candidates to use them as preventive and therapeutic medicine. Biofilms are often considered as spreaders of contaminants, which cause various health issues due to their persistent nature. Lipases and proteases isolated from an endophytic fungus Myrcia guianensis hydrolyzed the biofilm formed by Staphylococcus aureus within 10 and 30 min, respectively (Matias et al. 2021). Another naturally occurring enzyme is chitinase that acts as an antibacterial and antifungal agent. Many studies showed enormous pharmaceutical potential in human medicines because of the antitumor activity (indicated by chitohexaose and chitoheptaose), wound healing property, and anti-hypertensive activity (Rathore and Gupta 2015). Chitin is the element of the cell wall of various lethal microbes, is an easy target for antimicrobials. Thirty-one isolated endophytic fungi out of 162 were capable of producing chitinase that belongs to genera Phomopsis, Colletotrichum, and Fusarium isolated from leaves of tree species of the forests of Western Ghats, Southern India (Rajulu et al. 2011). l-Asparaginase is an enzyme that is used for the treatment of acute lymphocytic leukemia. It catalyzes the hydrolysis of l-asparagine to l-aspartic acid and ammonia (Sanghvi et al. 2016). Fungal asparaginases have gained importance as they are produced extracellularly (Batool et al. 2016). Various l-asparaginase-producing endophytic fungi, Plectosphaerella, Fusarium, Stemphylium, Septoria, Alternaria, Didymella, Phoma, Chaetosphaeronema, Sarocladium, Nemania, Epicoccum, Ulocladium, and Cladosporium were reported (Hatamzadeh et al. 2020). Different approaches have been used to produce L-asparaginase from the endophytic fungus Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. The enzyme production was carried out by both submerged fermentation (SmF) followed by solid-state fermentation (SSF). SSF was carried out using polyurethane foam (PUF) as a novel approach to enhance the enzyme yield. The production was enhanced from 80.8 U/mL in unoptimized medium to 94.4 U/mL in the optimized medium under SmF. It has been further increased to 120.3 U/mL under SSF using PUF soaked in the optimized liquid medium (Krishnapura et al. 2020). In another study, l-asparaginase production was enhanced using SmF of F. equiseti AHMF4 (El-Gendy et al. 2021). Microbial l-Asparaginase is preferred over chemical drugs because of its biodegradability and non-toxicity (Mhatre et al. 2017).
l-Glutaminase is an emerging anticancer agent with efficient anti-retroviral activity (Roberts and McGregor 1991) that makes it active against HIV. Glutaminase lowers glutamine levels in serum and tissues for prolonged periods and can cause a reduction in reverse transcriptase activity of HIV in serum. Glutaminase helps in the lysis of glutamine-dependent tumor cells by depriving these cells of glutamine (Kumer et al. 2013). The antitumour properties of l-glutaminase isolated from A. oryzae under submerged fermentation have been well studied (Dutt et al. 2014). Endophytic fungus Aspergillus sp. ALAA-2000 recovered from the soft marine sponge showed the highest l-glutaminase production with an activity of 91.92 U/ml (El-Gendy et al. 2016). In another study, two endophytic fungi, Cladosporium sp. and Trichoderma sp., were reported to produce l-glutaminase (El-Gendy et al. 2017). Xylarinase is an fibrinolytic metalloprotease, isolated from endophytic fungus Xylaria curta. The enzyme displayed both plasmin and tissue plasminogen activity by hydrolyzing the α and β chains of the fibrinogen (Meshram et al. 2016). Lipases have therapeutic potential as they can be used as antifungal agents. Lipases produced by endophytic fungi, Emericella nidulans, Dichotomophtora portulacae and D. boerhaaviae eliminated promastigote forms of Leishmania amazonensis at 5 mg/mL concentration with antifungal activity of 14.65 μg/mL against Malassezia sp and Microsporum canis (Alves et al. 2018). β-Glucuronidase is a potent enzyme with many applications in the medical industry. A novel β-glucuronidase (cg-GUS) by endophytic fungus C. globosum DX-THS3 displayed excellent thermostability and pH stability (Zhang et al. 2020). Apart from l-Asparaginase, xylarinase, lipase, β-glucuronidase, other enzymes such as amylase, protease, and l-tyrosinase have medical importance and the same has been listed in Table 2 along with their sources and applications.
Table 2.
Some of the fungal endophytes that have been reported to produce various enzymes with their therapeutic applications
| Enzyme | Endophytic fungi | Applications | References |
|---|---|---|---|
| Xylarinase | Xylaria sp. | Thrombolytic | Meshram et al. (2016) |
| Lipases | Emericella nidulans, Dichotomophtora portulacae and D. boerhaaviae | Leishmanicidal, fungicidal and anti-inflammatory | Alves et al. (2018) |
| l-Asparaginase | Plectosphaerella, Fusarium, Stemphylium, Septoria, Alternaria, Didymella, Phoma, Chaetosphaeronema, Sarocladium, Nemania, Epicoccum, Ulocladium and Cladosporium | Anticancer, act as biosensor in Leukemia | Hatamzadeh et al. (2020), Batool et al. (2016) |
| Protease | Aspergillus sp., Pestalotiopsis microspore, Umbelopsis isabellia, Xylaria psidii KT30 | Helps in healing of burns, wounds, carbuncles, act as clot buster | Budiarto et al. (2015), Mayerhofer et al. (2015), Russell et al. (2011), Ahmad et al. (2014), Razzaq et al. (2019) |
| β-Glucuronidase | Chaetomium globosum DX-THS3 | Liver detoxification, estrogen metabolism, and carbohydrate digestion | Zhang et al. (2020) |
| Amylase |
Colletotrichum sp., Macrophomina phaseolina, Nigrospora sphaerica and Fusarium solani Cylindrocephalum sp. (Ac-7) |
Used in diagnosis of cystic fibrosis, diabetes mellitus, and pancreatic infection | Ayob and Simarani (2016), Azzopardi et al (2016), Sunitha et al (2012), Zhang et al. (2020) |
| l-Tyrosinase | Aspergillus terreus and Penicillium copticola, Fusarium sp., Alternaria sp. | Melanin biosynthesis, l- DOPA manufacturing, antitumour | Kamal et al. (2013), Zaidi et al. (2014), Maamoun et al. (2021) |
Agriculture
Lignocellulose is a recalcitrant solid complex that originated from the combination of lignin and hemicellulose. Microorganisms play a significant role in the degradation of agricultural waste without polluting the environment. Some important enzymes, including glucanases, xylanases, glucosidases, and xylosidases are essential for the complete degradation of lignocellulosic biomass and oxidative enzymes such as laccase, manganese peroxidase, and lignin peroxidase (Maciel et al. 2012). Lignocellulolytic enzymes produced by endophytic fungi are potential alternative sources for organic matter degradation (Moretti et al. 2014). Production of endoglucanase activity by endophytic fungi C. cladosporioides PAJ03 (88.51 ± 1.0 U/g), Phomopsis stipata SC04 (83.44 ± 7.7 U/g), T. viridae PAJ01 (64.56 ± 4.0 U/g) and Botryosphaeria sp. AM 01 (42.79 ± 1.6 U/g) were reported. The β-glucosidase activity of endophytic fungi Saccharicola sp. EJC04 (51.56 ± 2.7 U/g), Paecilomyces sp. SF021 (33.19 ± 9.2 U/g), Ustilaginoidea sp. CV04 (29.75 ± 0.8 U/g) and Ustilaginoidea sp. XYA04 (21.72 ± 3.05 U/g) have been reported. The endophytic fungus P. stipata SC04 produced xylanase, and Botryosphaeria AM01 xylanase, and β-xylosidase (Moretti et al. 2014).
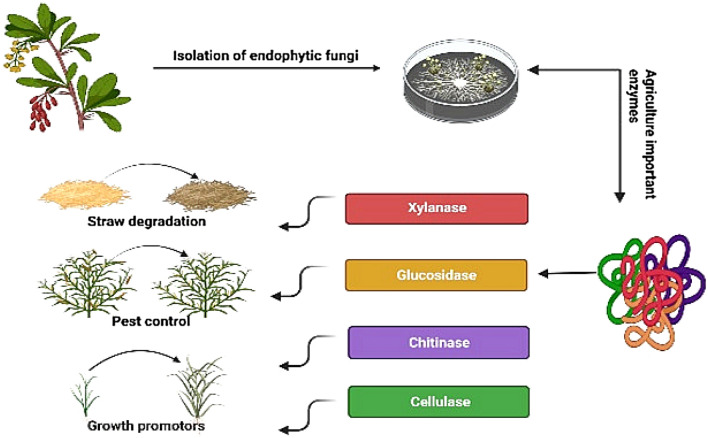
Two endophytic fungi, Botryosphaeria sp., AM01, and Saccharicola sp., EJC04, served as potential sources for the production of cellulases and xylanases, which can be applied in pretreated sugarcane bagasse saccharification processes (Marques et al. 2018). Beauveria bassiana SAN01, an endophytic fungus isolated from onion leaves, enhanced xylanase and endoglucanase production in wheat bran compared to other substrates (Amobonye et al. 2021a). Amobonye et al. (2021b) isolated xylanase from B. bassiana with a specific activity of 324.2 U/mg and capable of deink the wastepaper. In another study, six strains, namely A. niger DR02, Trichoderma atroviride DR17, and DR19, Alternaria sp. DR45, Annulohypoxylon stigyum DR47, and Talaromyces wortmannii DR49 were identified with the highest hemicellulose activity suitable for lignocellulosic biomass decomposition (Robl et al. 2013). Goldbeck et al. (2013) reported the cellulase production using variable substrates by an endophytic fungus, A. strictum. Two endophytic fungi, Colletotrichum sp. and Alternaria sp. produced cellulase along with substantial amounts of lipids in rice straw and wheat bran under solid-state fermentation (Devi et al. 2012). Furthermore, endophytic fungus Pistacia vera has shown prospects for the production of agriculture-relevant enzyme, i.e., chitinase (Falade et al. 2021). Endophytic fungi usually produce it as a defense mechanism against pests and pathogens. Endophytic fungal enzymes have the potential to degrade biomass, pest control and plant growth promotion activities (Fig. 3). For treating lignin-rich biomass waste, it is essential to give the pre-treatment, which remove or modify lignin into lignocellulosic fiber to get hydrolase polysaccharides. Rao et al. (2019) isolated an endophytic fungus Irpex lacteus from Euphorbia milii and studied the production of laccase with enzyme activity of 122 U/L. Five endophytes, Hormonema sp., Pringsheim smilacis, Ulocladium sp., and N. luteum, and N. australe have shown the highest laccase activity with pH stability pH in the range of 4–5 (Fillat et al. 2016; Torre et al. 2017). The endophytic fungus Phomopsis liquidambari used phenolic 4-hydroxybenzoic acid as the sole carbon and energy source, and produces the ligninolytic enzymes laccase and lignin peroxidase when cultured in submerged fermentation (Chen et al. 2013). Production of plant health and growth-promoting enzymes by endophytic fungi would enhance agricultural productivity and help in agro-industrial sustainability. In a study, consortium of five fungal endophytes, Beauveria bassiana, T. asperellum, Metarhizium anisopliae, Purpureocillium lilacinum and Pochonia chlamydosporia increased in the arylsulfatase activity indicating the increase in the uptake from the soil. Such findings can benefit sustainable agricultural management systems in the treatments to reduce the environmental impacts of mineral fertilizers and pesticides (Alves et al. 2021). Some of the enzymes applied in agriculture sector are presented in Table 3.
Fig. 3.
Graphical representation of the application of the endophytic fungal enzymes in agriculture sector
Table 3.
Some of the fungal endophytes that have been reported to produce different enzymes with potential agricultural applications
| Enzyme | Endophytic fungi | Applications | References |
|---|---|---|---|
| Chitinase | Aureobasidium pullulans, Lasiodiplodia theobromae, Neotyphodium sp., Plodia theobromae | Biopesticide, bio-fungicide | Li et al. (2004); Nagpure et al. (2014) |
| Arylsulfatase | B. bassiana, T. asperellum, M. anisopliae, P. chlamydosporia | Helps in the uptake of sulfur from the soil | Alves et al. (2021) |
| Cellulase | Pestalotiopsis microspore TKBRR, Coelomycetes sp., Acremonium sp., Aspergillus niger | Helps in soil fertility, manufacturing of compost, acts as a biocontrol agent | Phitsuwan et al. (2012); Goukanapalle and Kanderi (2020) |
| Pectinase | Aspergillus sp., C. gloeosporioides, Trichoderma sp. | Saccharification of agricultural substrates | Shubha and Srinivas (2017), Garg et al. (2016) |
| Xylanase | Botryosphaeria sp. AM01 and Saccharicola sp. EJC 04 | Pretreatment of lignocellulosic waste to simple sugars, Production of biobutanol | Shankar et al. (2016), Bendourou et al. (2021) |
| Phosphatase | Penicillium sp., Aspergillus sp., Piriformospora, Curvularia, Trichoderma | Convert organic phosphate esters to orthophosphate ions and helps in the plant nutrition | Mehta et al. (2019), Antonious et al. (2020), Sujatha et al. (2020) |
Biofuel
Plant biomass is a goldmine of energy-rich carbohydrates, and microbes can efficiently convert it into biofuels. Endophytic fungus Ascocoryne sarcoides has been studied genomically and for the production of myco-diesel and bioactive volatiles with fuel potential (Gianoulis et al. 2012). The combined action of xylanase with other enzymes such as mannanase, ligninase, glucanase, and glucosidase, can be applied to generate biofuels from lignocellulosic biomass (Parajó et al. 1998). The production of bioethanol requires the delignification of lignocellulose to liberate cellulose and hemicellulose. The next step includes the depolymerization of the carbohydrate polymers to produce free sugars (hexose and pentose) that are further used in fermentation to produce bioethanol (Adegboye et al. 2021). Endophytic fungi, Penicillium, Phoma, Lasiodiplodia, Paraconiothyrium, Fusicoccum, Xylaria, Diaporte, Cercospora, Colletotrichum, Nigrospora, Pestalotiopsis and Guignardia isolated from Terminalia catappa, Terminalia mantaly, and Canangao dorata reported to produce amylase, lipase, and cellulase (Toghueo et al. 2016). These three enzymes play an essential role in the production of biofuels such as ethanol. To produce ethanol, Almeida et al. (2013) studied two corn endophytic fungi, F. verticillioides and A. zeae. The ethanol yield from glucose, xylose, and a mixture of both sugars were 0.47, 0.46, and 0.50 g/g ethanol/sugar for F. verticillioides and 0.37, 0.39, and 0.48 g/g ethanol/sugar for A. zeae. Both fungi could co-ferment glucose and xylose with a higher ethanol production yield. Sugarcane bagasse and lignocellulosic biomass have been used as substrates for ethanol production. Lipases have been used to produce biodiesel produced by endophytic fungi, Stemphylium lycopersici, Sordaria sp, and C. kikuchii (Costa-Silva et al. 2011; Francisco et al. 2015). Dey et al. (2011) evaluated two endophytic fungal isolates, Colletotrichum sp. and Alternaria sp., for their potential as biodiesel feedstock. The results showed that these two endophytes grew successfully in solid-state fermentation with the use of two substrates; rice straw and wheat bran that led to the high production of cellulase (1.21–2.51 U/g dry substrate (gds)) and a substantial amount of lipid (60.32–84.30 mg/gds).
Food industry
The application of enzymes in the food industry has added more advancements in the brewing process. Enzymes enhance animal and vegetable proteins’ functional and nutritional properties via hydrolysis of proteins. Many of these enzymes, e.g., amylolytic enzymes, are used for the production of glucose syrups, crystalline glucose, high fructose corn syrups, and maltose syrups (Sindhu et al. 2016, 2021). The application of enzymes has been divided into different food sectors, such as baking, dairy, juice production, and brewing. The baking industry is the top on the list in utilizing enzymes to improve the dough stability, crumb softness, and shelf life, followed by the dairy industry and brewery industry.
Production of amylase by endophytic fungi, Pseudopestalotiopsis theae, Clindrocephalum sp., Discosia sp., and Phyllosticta sp. has been reported (Sopalun and Iamtham 2020; Hegde et al. 2011; Amirita et al. 2012; Sunitha et al. 2012). They improve the quality of products with much safety and enhance the texture, volume, stability, color, uniformity, softness, and prolonged freshness. Endophytic fungi, A. terreus, A. fumigatus, F. oxysporum, and T. harzianum have been isolated from soybean, which produces lipases and xylanase (Farouk et al. 2020). Blends of these two enzymes are used for dough stability and conditioning, while glucose oxidase and lipoxygenase are added to improve dough strengthening and whiteness (Singh et al. 2016). Endo and exo-proteases from A. oryzae (BakeZyme B500BG) have been utilized for degrading proteins in flour dough for preparing biscuits, cakes, crackers, and cookies (Sawant and Nagendran 2014).
Enzymes play a vital role in the dairy industry as they are used to develop and enhance organoleptic characteristics (aroma, flavor, and color). Few endophytic fungi have been studied to produce proteases, such as A. japonicus, C. cladosporioides, P. guepinnii, T. flavus (Bezerra et al. 2012). Endophytic fungi such as Penicillium, Alternaria, Aspergillus sp., and Fusarium were reported to produce catalase with a significant yield that can replace the prevailing resources (Bind and Nema 2019). Microbial enzymes are employed in the brewing industry to digest plant cell walls during extraction to produce enhanced yield, color, aroma, and more transparent products. Endophytic fungi Aspergillus sp., Trichoderma sp., and Orpinomyces sp. produce beneficial enzymes such as β-glucanase and amylo-glucosidase that has an important place in the beverage industry. These enzymes help in the malting and mashing fermentation process that aid in the clarification of wort (Gomaa 2018).
Other applications
In the pulp and paper industry, xylanases, cellulases, lipases, and laccases are used as an alternative to conventional chlorine and chlorine-based bleaching compounds (Singh et al. 2016). Phytate (myo-inositol-hexaphosphate) is one of the phosphorylated anti-nutrients in cereals, pollens, legumes, and oilseeds, which is monogastric, and animals do not metabolize due to meager amount or even the absence of the phytase enzyme in their digestive tracts. Phytate is consequently discharged in the feces of these animals in waterways that lead to eutrophication of surface waters, particularly in areas of livestock production (Takizawa 1998). In one of the reported studies, endophytic fungi Penicillium daleae and Aspergillus sp. isolated from Taxus wallichiana produced maximum calcium phytase at 25 and 15 °C, 10.33 ± 0.13 and 10.37 ± 0.37 µM/ml (Adhikari and Pandey 2019).
Global market of industrially important enzymes
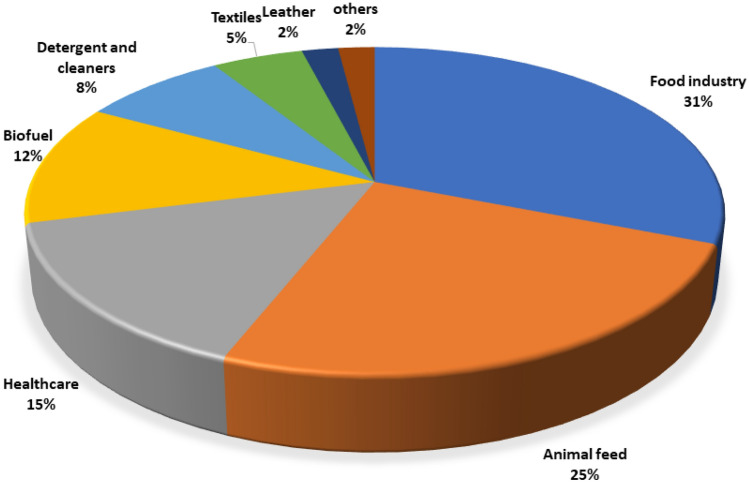
From the last few years, enzymes have covered the major area in the industrial market such as food, pharmaceutical, and leather, directly or indirectly utilized for human consumption. In bulk, enzymes are being processed from microorganisms rather than plants and animals, as microbes are the most convenient source, giving the easy scale-up, faster production with good yield by purification, strain manipulation for overexpression, etc. About 200 types of microbial enzymes from 4000 known enzymes are used commercially (Liu and Kokare 2017). Based on the Allied Research Market, majority of the global industrial enzymes share is on food and animal feed industry followed by healthcare and biofuel (Fig. 4) (www.alliedresearchmarket.com).
Fig. 4.
Global industrial enzymes market share based on their applications between the year 2020 and 2024 based on the data provided by Allied Research Market (www.alliedresearchmarket.com)
Globally, the industrial enzymes market is expected to grow exponentially, with an annual growth rate of 6.8% from 2019 to 2024, as reported in the article by Mordor intelligence (Globe Newswire 2019). A few key players in the enzyme market are Novozymes A/S, Koninklijke DSM N.V., DuPont de Nemours, Inc., BASF SE, Advanced Enzyme Technologies Ltd., AB Enzymes GmbH, Codexis, Inc., Amano Enzyme, Inc., F. Hoffmann-La Roche Ltd., and Thermo Fisher Scientific Inc. Other players in the value chain include BBI Enzymes Ltd., Procter & Gamble Co., Puratos Group, Novus International, Inc., and Chr. Hansen A/S. (Allied research market).
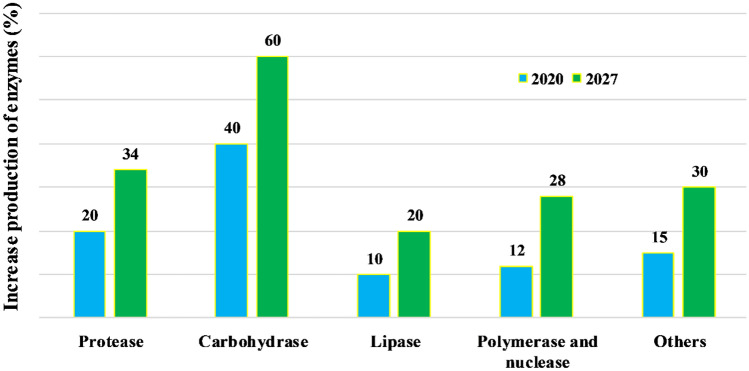
The analysis showed North America holds the highest share in the enzyme market in 2019, followed by Europe and Asia–Pacific. It is predicted that Asia–Pacific will grow at the highest CAGR of 8.2% from 2020 to 2027, with the launch of new therapy enzymes required to treat different diseases (Allied research market 2021). Only a few enzymes such as protease, carbohydrases, and lipases are the three enzymes that are most widely used in every sector (Fig. 5).
Fig. 5.
Predicted increase production of various industrial enzymes from the year 2020 to 2027 based on the data provided by Allied Research Market (www.alliedresearchmarket.com)
The global market of proteases is predicted to be increased by 2 billion USD by 2024. The health industry will see the maximum development in the proteases market due to its numerous benefits such as immunomodulating, anti-inflammatory properties, and application in healing skin burns and stomach ulcers. However, protease is also employed to improve fodder’s nutritional or digestive characteristics and indicate its significant share in the animal feed market. Carbohydrases have crossed USD 2.5 billion in 2016 and are expected to increase by 1/3 over the existing figure by 2024. The prominent application sectors shall be in the food, beverage, and pharmaceutical industry. Lipases are expected to achieve a 6.8% increase by 2024. The major area of the lipase market shall be in the field of healthcare and food industry.
Challenges and prospects
The bioactive potential of endophytic fungi has been known for many years, but the production of enzymes with good quantity and quality is the new eye-opener for many research areas. The current article upgrades the information about various endophytic fungi used as a bioresource for the production of industrially important enzymes.
There is a need for basic and applied multidisciplinary research on endophytic fungi producing enzymes, which will aid in different biotechnological applications and fulfill industry demand. The enzyme-producing ability of endophytic fungi varies with the host, geographical, and physiological conditions. Therefore, the research on endophytic fungi from different sources under different climatic conditions with enzyme-producing ability are needed to be explored.
Furthermore, optimizing a fungal strain to produce enzymes with superior qualities in an economical manner is more challenging for scientists. This can be attained by optimizing the growth parameters of endophytic fungi by providing all the similar physiological conditions as of their host plant. Another technique includes the dual culture that helps to enhance enzyme production with a good yield. During the optimization, enzymes’ intact activity and stability with higher yield and less production cost is a matter of concern, but that can be solved by utilizing endophytic fungi as enzymatic sources as it is more manageable and less time-consuming.
Moreover, detailed knowledge about molecular biology and the mechanism of enzyme production by endophytic fungi may provide a platform for engineering the potent strains. For this, various strategies can be used such as genetic engineering, metabolomics, and proteomics. The stability and reusability of enzymes during operational processes is another challenge for industrialists and researchers. Immobilization of enzymes that can be used without any loss in their activities, but the recovery of enzymes after the immobilization is somehow difficult. Hence, this area needed more attention so that immobilization of the enzymes becomes easy and beneficial for the industrial processes (stable pH, thermostability, easily recoverable, and reusable). Therefore, the exploration of endophytic fungi for producing enzymes, their optimized growth parameters, and suitable immobilization techniques will surely help the industrialists to use cost-effective and stable industrially important enzymes in the biotechnological fields.
Conclusions
Endophytic fungi are studied for many years; still, they remain an underexplored group of endophytes. They are majorly known as the bioactive compound producers, but the area as enzyme producers is barely explored. The question of microbes being used as potent industrial enzyme sources in the near future is not known with the present knowledge. New and more extensive studies can be used such as optimization of cultivation of cultures, expression of regulatory mechanisms of genes coding for enzymes, followed by the characterization of the physicochemical properties of these enzymes to evaluate the real potential of endophytic fungi as sources of industrially important enzymes.
Production cost is another factor that can be minimized by making enzyme mixtures. Many strains are genetically engineered to produce different enzymes, but identifying the target genes is still needed. From all of this, one can say there is a need for integrated approaches such as—(a) by developing methods for isolating the genes coding for the enzymes, (b) by designing advanced bioreactors for the production of enzymes, (c) by studying the molecular interaction between substrate and enzymes, (d) by optimizing combined biophysical, mathematical and enzymological approaches to enhance the production of the stable enzyme cost-effectively.
Industrially important enzymes are produced worldwide by solid-state fermentation and submerged fermentation techniques. The demand for enzymes is still on the top for various industrial processes, but there is vast scope for advancement in enzyme properties. There is a need to develop the pH and temperature stable enzyme production for various industrial applications. Enzymes independent of metal ions for their activities and do not affect the inhibitory agents are mostly desired for industrial applications. While optimizing the qualities of the enzyme, one must keep the consistency or improvement in the catalytic efficiency in mind. However, various enzyme immobilization techniques are there to develop reusability quality, but still, this is the major challenge. There is another big problem of enzyme specificity towards natural substrates.
Acknowledgements
The authors are thankful to Thapar Institute of Engineering and Technology, Patiala for providing the facilities
Author contributions
FB collected the literature, compiled the findings related to industrial enzymes by endophytic fungi and prepared a draft review. AG collected the literature, compiled the findings of endophytic enzymes in therapeutics and prepared the draft. MV is involved in conceptualization and draft checking. MSR conceptualized the idea and prepared the final review.
Declarations
Conflict of interest
The authors declare they have no competing interests.
Footnotes
Fatima Bhadra and Anu Gupta have equal contribution.
References
- Aamir M, Rai KK, et al. Fungal endophytes: classification, diversity, ecological role, and their relevance in sustainable agriculture. Microb Endophytes. 2020 doi: 10.1016/b978-0-12-818734-0.00012-7. [DOI] [Google Scholar]
- Adegboye MF, Ojuederie OB, Talia PM. Bioprospecting of microbial strains for biofuel production: metabolic engineering, applications, and challenges. Biotechnol Biofuels. 2021;14:1–21. doi: 10.1186/s13068-020-01853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari P, Pandey A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere. 2019;9:2–9. doi: 10.1016/j.rhisph.2018.11.002. [DOI] [Google Scholar]
- Ahmad MS, Noor ZM, Ariffin ZZ. Isolation and identification fibrinolytic protease endophytic fungi from Hibiscus leaves in Shah Alam. Int J Biol Vet Agric Food Eng. 2014;8:1027–1030. [Google Scholar]
- Akone SH, Mándi A, et al. Inducing secondary metabolite production by the endophytic fungus Chaetomium sp. through fungal–bacterial co-culture and epigenetic modification. Tetrahedron. 2016;72:6340–6347. doi: 10.1016/j.tet.2016.08.022. [DOI] [Google Scholar]
- Alkalde M, Ferrer M, Palou FJ, Ballesteros A. Environmental biocatalysts: from remediation with enzymes to novel green processes. Trends Biotechnol. 2006;24:281–287. doi: 10.1016/j.tibtech.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Alves DR, de Morais SM, Tomiotto-Pellissier F, et al. Leishmanicidal and fungicidal activity of lipases obtained from endophytic fungi extracts. PLoS ONE. 2018;13:e0196796. doi: 10.1371/journal.pone.0196796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GS, Bertini SCB, et al. Fungal endophytes inoculation improves soil nutrient availability, arbuscular mycorrhizal colonization and common bean growth. Rhizosphere. 2021;18:100330. doi: 10.1016/j.rhisph.2021.100330. [DOI] [Google Scholar]
- Amirita A, Sindhu P et al (2012) Enumeration of endophytic fungi from medicinal plants and screening of extracellular enzymes. W J Sci Technol 2:13–19. https://www.academia.edu/4674128
- Amobonye A, Bhagwat P, Singh S, Pillai S. Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol. 2021;125:39–48. doi: 10.1016/j.funbio.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Amobonye A, Bhagwat P, Singh S, Pillai S. Beauveria bassiana Xylanase: characterization and wastepaper deinking potential of a novel glycosyl hydrolase from an endophytic fungal entomopathogen. J Fungi. 2021;27(8):668. doi: 10.3390/jof7080668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonious GF, Turley ET, Dawood MH. Monitoring soil enzymes activity before and after animal manure application. Agriculture. 2020;10:166. doi: 10.3390/agriculture10050166. [DOI] [Google Scholar]
- Ayob FW, Simarani K. Endophytic filamentous fungi from a Catharanthus roseus: identification and its hydrolytic enzymes. Saudi Pharm J. 2016;24:273–278. doi: 10.1016/j.jsps.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi E, Lloyd C, Teixeira SR, Conlan RS, Whitaker IS. Clinical applications of amylase: novel perspectives. Surgery. 2016;160:26–37. doi: 10.1016/j.surg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Batool T, Makky EA, Jalal M, Yusoff MM. A comprehensive review on l-asparaginase and its applications. Appl Biochem Biotechnol. 2016;178(900):923. doi: 10.1007/s12010-015-1917-3. [DOI] [PubMed] [Google Scholar]
- Bendourou FE, Suresh G, Laadila MA, et al. Feasibility of the use of different types of enzymatically treated cellulosic fibres for polylactic acid recycling. J Waste Manag. 2021;21:237–247. doi: 10.1016/j.wasman.2020.11.058. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Bohni N, et al. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv. 2014;32:1180–1204. doi: 10.1016/j.biotechadv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Bezerra JDP, Santos MGS, Svedese VM. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol. 2012;28:1989–1995. doi: 10.1007/s11274-011-1001-2. [DOI] [PubMed] [Google Scholar]
- Bind M, Nema S. Isolation and molecular characterization of endophytic bacteria from Pigeon Pea along with antimicrobial evaluation against Fusarium udum. Appl Microbiol. 2019;5:1–12. doi: 10.35248/2471-9315.19.5.163. [DOI] [Google Scholar]
- Bradner JR, Gillings M, Nevalainen KMH. Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. W J Microbiol Biotechnol. 1999;15:131–132. doi: 10.1023/A:1008855406319. [DOI] [Google Scholar]
- Brakhage AA. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- Budiarto BR, Mustopa AZ, Tarman K. Isolation, purification and characterization of extracellular protease produced by marine-derived endophytic fungus Xylaria psidii KT30. J Coast Life Med. 2015;3:56–63. [Google Scholar]
- Cavalcanti RMF, Ornela P, Jorge JA, Guimarães L. Screening, selection and optimization of the culture conditions for tannase production by endophytic fungi isolated from caatinga. J Appl Biol Biotechnol. 2017;5:1–9. doi: 10.7324/JABB.2017.50101. [DOI] [Google Scholar]
- Chambergo FS, Valencia EY. Fungal biodiversity to biotechnology. Appl Microbiol Biotechnol. 2016;100:2567–2577. doi: 10.1007/s00253-016-7305-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xie XG, Ren CG, Dai CC. Degradation of N-heterocyclic indole by a novel endophytic fungus Phomopsis liquidambari. Bioresour Tech. 2013;129:568–574. doi: 10.1016/j.apsoil.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Choudhary M, Gupta S, Dhar MK, Kaul S. Endophytic fungi-mediated biocatalysis and biotransformations paving the way toward green chemistry. Front Bioeng Biotechnol. 2021;9:664705. doi: 10.3389/fbioe.2021.664705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz RH. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Rep. 2010;27:11–22. doi: 10.1039/B920860G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa RCG, Rhoden SA, Mota TR, et al. Endophytic fungi: expanding the arsenal of industrial enzyme producers. J Ind Microbiol Biotechnol. 2014;41:1467–1478. doi: 10.1007/s10295-014-1496-2. [DOI] [PubMed] [Google Scholar]
- Costa-Silva TA, Nogueira MA, et al. Lipase production by endophytic fungus Cercospora kikuchii: stability of enzymatic activity after spray drying in the presence of carbohydrates. Drying Technol. 2011;29:1112–1119. doi: 10.1080/07373937.2011.573153. [DOI] [Google Scholar]
- de Almeida MN, Guimaraes VM, Bischoff KM. Cellulases and hemicellulases from endophytic Acremonium sp. and its application on sugarcane bagasse hydrolysis. Appl Biochem Biotechnol. 2011;165(2):594–610. doi: 10.1007/s12010-011-9278-z. [DOI] [PubMed] [Google Scholar]
- de Almeida MN, Guimarães VM, Falkoski DL. Direct ethanol production from glucose, xylose and sugarcane bagasse by the corn endophytic fungi Fusarium verticillioides and Acremonium zeae. J of Biotechnol. 2013;168:7–71. doi: 10.1016/j.jbiotec.2013.07.032. [DOI] [PubMed] [Google Scholar]
- de Sena AR, dos Santos ACB, Gouveia MJ, et al. Production, characterization and application of a thermostable tannase from Pestalotiopsis guepinii URM 7114. Food Technol Biotechnol. 2014;52:459–467. doi: 10.17113/ftb.52.04.14.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi NN, Prabakaran JJ, Wahab F. Phytochemical analysis and enzyme analysis of endophytic fungi from Centella asiatica. Asian Pac J Tropical Biomed. 2012;2:S1280–S1284. doi: 10.1016/S2221-1691(12)60400-6. [DOI] [Google Scholar]
- Dey P, Banerjee J, Maiti MK. Comparative lipid profiling of two endophytic fungal isolates-Colletotrichum sp. and Alternaria sp. having potential utilities as biodiesel feedstock. Bioresour Technol. 2011;102(10):5815–5823. doi: 10.1016/j.biortech.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Dittrich MT, Klau GW, Rosenwald A, Dandekar T, Müller T. Identifying functional modules in protein–protein interaction networks: an integrated exact approach. Bioinformatics. 2008;2:i223–i231. doi: 10.1093/bioinformatics/btn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Naveen M, Saroj Y, Siddalingeshwara KG. Role of carbon source for the production of l-glutaminase from Aspergillus oryzae. J Drug Deliv Ther. 2014;4:193–196. doi: 10.22270/jddt.v4i5.983. [DOI] [Google Scholar]
- El-Gendy MMA, Taha TM, Abo-Dahab NF, Hassan FSM. Process optimization of l-glutaminase production; a tumour inhibitor from marine endophytic isolate Aspergillus sp. ALAA-2000. JMBT. 2016;8:256–267. doi: 10.4172/1948-5948.1000313. [DOI] [Google Scholar]
- El-Gendy MMA, Al-Zahrani SHM, El-Bondkly AMA. Construction of potent recombinant strain through intergeneric protoplast fusion in endophytic fungi for anticancerous enzymes production using rice straw. Appl Biochem Biotechnol. 2017;183:30–50. doi: 10.1007/s12010-017-2429-0. [DOI] [PubMed] [Google Scholar]
- El-Gendy MMA, Awad MF, El-Shenawy FS, El-Bondkly AMA. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular l-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci. 2021;28:2540–2548. doi: 10.1016/j.sjbs.2021.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade AO, Adewole KE, Ekundayo TC. Aptitude of endophytic microbes for production of novel biocontrol agents and industrial enzymes towards agro-industrial sustainability. Beni-Suef Univ J Basic Appl Sci. 2021 doi: 10.1186/s43088-021-00146-3. [DOI] [Google Scholar]
- Farouk HM, Attia EZ, El-Katatny MH. Hydrolytic enzyme production of endophytic fungi isolated from soybean (Glycine max) J of Mod Res. 2020;2:1–7. doi: 10.21608/jmr.2019.15748.1008. [DOI] [Google Scholar]
- Fillat U, Martín-Sampedro R, Macaya-Sanz D, et al. Screening of eucalyptus wood endophytes for laccase activity. Process Biochem. 2016;51(5):589–598. doi: 10.1016/j.procbio.2016.02.006. [DOI] [Google Scholar]
- Fouda AH, Hassan SE-D, Eid AM, Ewais EE-D. Biotechnological applications of fun-gal endophytes associated with medicinal plant Asclepias sinaica (Bioss.) Ann Agric Sci. 2015;60:95–104. doi: 10.1016/j.aoas.2015.04.001. [DOI] [Google Scholar]
- Francisco B, Luis M, Mathew C, Karla M. Lipase catalyzed biodiesel production and quality with Jatropha curcas oil: exploring its potential for Central America. J Biol Eng. 2015 doi: 10.1186/s13036-015-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R. Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech. 2016;6:1–13. doi: 10.1007/s13205-016-0371-4.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulis TA, Griffin MA, Spakowicz DJ, et al. Genomic analysis of the hydrocarbon-producing, cellulolytic, endophytic fungus Ascocorynesarcoides. PLoS Genet. 2012;8:e1002558. doi: 10.1371/journal.pgen.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globe Newswire (2019) Global Industrial Enzymes Market Growth Trends and Forecast 2019-2024: Competition for raw materials with other industries and price volatility restraining market growth. https://www.globenewswire.com/news-release/2019/03/29/1788385/0/en
- Goldbeck R, Ramos MM, Pereira GAG, Maugeri-Filho F. Cellulase production from a new strain Acremonium strictum isolated from the Brazilian biome using different substrates. Bioresour Tech. 2013;128:797–803. doi: 10.1016/j.biortech.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Gomaa AM. Application of enzymes in brewing. J Nutr Food Sci Forecast. 2018;1:1002. [Google Scholar]
- Gopinath SCB, Anbu P, Md Arshad MK, et al. Biotechnological processes in microbial amylase production. BioMed Res Int. 2017 doi: 10.1155/201S7/1272193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goukanapalle PKR, Kanderi DK, et al. Optimization of cellulase production by a novel endophytic fungus Pestalotiopsis microspora TKBRR isolated from Thalakona forest. Cellulose. 2020;27:6299–6316. doi: 10.1007/s10570-020-03220-8. [DOI] [Google Scholar]
- Gupta VK, Kubicek CP, Berrin JG, et al. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. 2016;41:633–645. doi: 10.1016/j.tibs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Harnpicharnchai P, Champreda V, Sornlake W, Eurwilaichitr L. A thermotolerant β-glucosidase isolated from an endophytic fungi-Periconia sp., with a possible use for biomass conversion to sugars. Protein Expr Purif. 2009;67(2):61–69. doi: 10.1016/j.pep.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Hatamzadeh S, Rahnama K, Nasrollahnejad S, et al. Isolation and identification of l-asparaginase-producing endophytic fungi from the Asteraceae family plant species of Iran. PeerJ. 2020;8:e8309. doi: 10.7717/peerj.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SV, Ramesha A, Srinvas C. Optimization of amylase production from an endophytic fungi Discosia sp. isolated from Calophyllum inophyllum. J Agric Tech. 2011;7:805–813. [Google Scholar]
- Huang C, Feng Y, Patel G, et al. Production, immobilization and characterization of beta-glucosidase for application in cellulose degradation from a novel Aspergillus versicolor. Int J Biological Macromol. 2021;177:437–446. doi: 10.1016/j.ijbiomac.2021.02.154. [DOI] [PubMed] [Google Scholar]
- Illanes A, Cauerhff A, Wilson L, Castro GR. Recent trends in biocatalysis engineering. Bioresour Technol. 2014;115:48–57. doi: 10.1016/j.biortech.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Konappa N, Lokesh A, et al. Bioactive compounds guided diversity of endophytic fungi from Baliospermum montanum and their potential extracellular enzymes. Anal Biochem. 2021;614:114024. doi: 10.1016/j.ab.2020.114024. [DOI] [PubMed] [Google Scholar]
- Joel EL, Bhimba V. Production of alpha amylase by mangroves associated fungi microspora strain VB5 and Aspergillus oryzae strain VB6. Indian J Geo-Mar Sci. 2012;41:279–283. [Google Scholar]
- Joo HS, Choi JW. Purification and characterization of a novel alkaline protease from Bacillus horikoshii. J Microbiol Biotechnol. 2012;22:58–68. doi: 10.4014/jmb.1109.09006. [DOI] [PubMed] [Google Scholar]
- Kamal MZ, Yedavalli P, Deshmukh MV, Rao NM. Lipase in aqueous-polar organic solvents: activity, structure, and stability. Protein Sci. 2013;22:904–915. doi: 10.1002/pro.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microb. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- Khan AL, Shahzad R, Al-Harrasi A, Lee IJ. Endophytic microbes: a resource for producing extracellular enzymes in endophytes: crop productivity and protection. Sustain Develop Biodivers. 2017;16:95–110. doi: 10.1007/978-3-319-66544-3_5. [DOI] [Google Scholar]
- Krishnapura PR, Belur PD. L-asparaginase production using solid-state fermentation by an endophytic Talaromyces pinophilus isolated from rhizomes of Curcuma amada. J Pure Appl Microb. 2020;14:307–318. doi: 10.22207/JPAM.14.1.32. [DOI] [Google Scholar]
- Kumer SS, Muthuvelayudham R, Viruthagiri T. Statistical optimization based production of l-glutaminase by Serratia marcescens under submerged fermentation. Res J Chem Sci. 2013;3:43–53. [Google Scholar]
- Kusari S, Singh S, Jayabaskaran C. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol. 2014;32:297–303. doi: 10.1016/j.tibtech.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Li HM, Sullivan R, Moy M, Kobayashi DY, Belanger FC. Expression of a novel chitinase by the fungal endophyte in Poa ampla. Mycologia. 2004;96:526–536. doi: 10.1080/15572536.2005.11832951. [DOI] [PubMed] [Google Scholar]
- Li HT, Zhou H, et al. Inducing secondary metabolite production by co-culture of the endophytic fungus Phoma sp. and the symbiotic fungus Armillaria sp. J Nat Prod. 2019;82:1009–1013. doi: 10.1021/acs.jnatprod.8b00685. [DOI] [PubMed] [Google Scholar]
- Liu X, Kokare C. Microbial enzymes of use in industry, biotechnology of microbial enzymes: production, biocatalysis and industrial applications. Elsevier. 2017;2017:267–298. doi: 10.1016/B978-0-12-803725-6.00011-X. [DOI] [Google Scholar]
- Maamoun HS, Rabie GH, et al. Biochemical properties of tyrosinase from Aspergillus terreus and Penicillium copticola; undecanoic acid from Aspergillus flavus, an endophyte of Moringa oleifera, is a novel potent tyrosinase inhibitor. Molecules. 2021;26:1309. doi: 10.3390/molecules26051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel GM, Bracht A, et al. Fundamentals, diversity and application of white rot fungi. In: Silva AP, Sol M, et al., editors. Fungi: types, environmental impact and role in disease. United States: Nova Science Publishers Inc.; 2012. pp. 409–458. [Google Scholar]
- Maria G, Sridhar K, Raviraja N. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agri Technol. 2005;1:67–80. [Google Scholar]
- Marlida Y, Delfita R, Gusmanizar N, Ciptaan G. Identification characterization and production of phytase from endophytic fungi. WASET. 2010;65:1043–1046. [Google Scholar]
- Marques NP, de Cassia PJ, Gomes E, et al. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind Crops Prod. 2018;122:66–75. doi: 10.1016/j.indcrop.2018.05.022. [DOI] [Google Scholar]
- Matias RR, Sepúlveda AMG, Batista BN, et al. Degradation of Staphylococcus aureus biofilm using hydrolytic enzymes produced by Amazonian endophytic fungi. Appl Biochem Biotechnol. 2021 doi: 10.1007/s12010-021-03542-8. [DOI] [PubMed] [Google Scholar]
- Mayerhofer MS, Fraser E, Kernaghan G. Acid protease production in fungal root endophytes. Mycologia. 2015;107:1–11. doi: 10.3852/14-106. [DOI] [PubMed] [Google Scholar]
- Mehdipour-Moghaddam M, Emtiazi G, et al. Novel phytase and cellulase activities in endophytic Azospirilla. World Appl Sci J. 2010;10:1129–1135. doi: 10.1100/tsw.2010.111. [DOI] [Google Scholar]
- Mehta P, Sharma R, Putatunda C, Walia A. Endophytic fungi: role in phosphate solubilization. In: Singh BP, editor. Advances in endophytic fungal research: present status and future challenges. Cham: Springer; 2019. pp. 183–209. [Google Scholar]
- Mhatre A, Narwankar R, Rawat A, Tembadmani K, Mishra S. Current perspectives in sustainable environment management. 1. Nerul: SIES Indian Institute of Environment Management; 2017. Characterization of endophytic fungi from medicinal plants for application in therapeutic enzyme extraction; pp. 230–239. [Google Scholar]
- Mishra V, Jana AK, Jana MM, Gupta A. Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet Sorghum bagasse. Bioresour Tech. 2017;236:49–59. doi: 10.1016/j.biortech.2017.03.148. [DOI] [PubMed] [Google Scholar]
- Mishra VK, Passari AK, Chandra P, et al. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD GC-MS. PLoS ONE. 2017;12:1–24. doi: 10.1371/journal.pone.0186234. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Meshram V, Saxena S, Paul K. Xylarinase: a novel clot busting enzyme from an endophytic fungus Xylaria curta. J Enz Inhib Med Chem. 2016;31:1502–1511. doi: 10.3109/14756366.2016.1151013. [DOI] [PubMed] [Google Scholar]
- Mohamed I, El-Khonezy MI, et al. Detergent stable thiol-dependant alkaline protease produced from the endophytic fungus Aspergillu sochraceus BT21: purification and kinetics. Biocatal Agric Biotechnol. 2021;35:102046. doi: 10.1016/j.bcab.2021.102046. [DOI] [Google Scholar]
- Moretti MMS, Bocchini-Martins DA, et al. Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl Ener. 2014;122:189–195. doi: 10.1016/j.apenergy.2014.02.020. [DOI] [Google Scholar]
- Nagpure A, Choudhary B, Gupta RK. Chitinases: in agriculture and human healthcare. Crit Rev Biotechnol. 2014;34:215–232. doi: 10.3109/07388551.2013.790874. [DOI] [PubMed] [Google Scholar]
- Ola ARB, Thomy D, et al. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J Nat Prod. 2013;76:2094–2099. doi: 10.1021/np400589h. [DOI] [PubMed] [Google Scholar]
- Onofre SB, Mattiello SP, et al. Production of cellulases by the endophytic fungus Fusarium oxysporum. J Microbiol Res. 2013;3:131–134. doi: 10.5923/j.microbiology.20130304.01. [DOI] [Google Scholar]
- Oses R, Valenzuela S, Freer J, Baeza J, Rodríguez J. Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. Int Biodeter Biodegr. 2006;57:129–135. doi: 10.1016/j.ibiod.2006.01.002. [DOI] [Google Scholar]
- Panuthai T, Sihanonth P, Piapukiew J, Sooksai S, Sangvanich P, Karnchanatat A. An extracellular lipase from the endophytic fungi Fusarium oxysporum isolated from the Thai medicinal plant, Croton oblongifolius Roxb. Afr J Microbiol Res. 2012;6:2622–3263. [Google Scholar]
- Parajó JC, Dominguez H, Domínguez J. Biotechnological production of xylitol. Part 3: operation in culture media made from lignocellulose hydrolysates. Bioresour Tech. 1998;66:25–40. doi: 10.1016/S0960-8524(98)00037-6. [DOI] [Google Scholar]
- Patel I, Kracher D, Ma S, et al. Salt-responsive lytic polysaccharide monooxygenases from the mangrove fungus Pestalotiopsis sp. NCi6. Biotechnol Biofuels. 2016;9(1):1–12. doi: 10.1186/s13068-016-0520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-W, Chen H-Z. Microbial oil accumulation and cellulase secretion of the endophytic fungi from oleaginous plants. Ann Microbiol. 2007 doi: 10.1007/BF03175213. [DOI] [Google Scholar]
- Phitsuwan P, Laohakunjit N, et al. Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia Microbiol. 2012;58:163–176. doi: 10.1007/s12223-012-0184-8. [DOI] [PubMed] [Google Scholar]
- Rajulu MBG, Thirunavukkarasu N, Suryanarayanan TS. Chitinolytic enzymes from endophytic fungi. Fungal Divers. 2011;47:43–53. doi: 10.1007/s13225-010-0071-z. [DOI] [Google Scholar]
- Rao A, Ramakrishna N, Arunachalam S, Sathiavelu M. Isolation, screening and optimization of laccase-producing endophytic fungi from Euphorbia milii. Arabian J Sci Eng. 2019;44:51–64. doi: 10.1007/s13369-018-3431-8. [DOI] [Google Scholar]
- Rathore AS, Gupta RD. Chitinases from bacteria to human: properties, applications, and future perspectives. Enzyme Res. 2015;2015:1–8. doi: 10.1155/2015/791907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A, Shamsi S, et al. Microbial proteases applications. Front Bioeng Biotechnol. 2019;7:110. doi: 10.3389/fbioe.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renuka S, Ramanujam B. Fungal endophytes from maize (Zea mays L.): isolation, identification and screening against maize stem borer, Chilo partellus (Swinhoe) J Pure Appl Microbiol. 2016;10:523–529. [Google Scholar]
- Rigoldi F, Donini S, Redaelli A, Parisini E, Gautieri A. Review: engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018;2:011501. doi: 10.1063/1.4997367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robl D, Delabona PS, et al. The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnol. 2013;13:1–12. doi: 10.1186/1472-6750-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, McGregor WG. Inhibition of mouse retroviral disease by bioactive glutaminase- asparaginase. J Gen Virol. 1991;72:299–305. doi: 10.1099/0022-1317-72-2-299. [DOI] [PubMed] [Google Scholar]
- Rojas-Aedo JF, Gil-Durán C, et al. The developmental regulator Pcz1 affects the production of secondary metabolites in the filamentous fungus Penicillium roqueforti. Microbiol Res. 2018;67(74):212–213. doi: 10.1016/j.micres.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Russell JR, Huang J, Anand P, et al. Biodegradation of polyester polyurethane by endophytic fungi. Appl Env Microbiol. 2011;77:6076–6084. doi: 10.1128/AEM.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi G, Bhimani K, Vaishnav D, et al. Mitigation of acrylamide by l-asparaginase from Bacillus subtilis KDPS1 and analysis of degradation products by HPLC and HPTLC. Springer plus. 2016;5:1–11. doi: 10.1186/s40064-016-2159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant R, Nagendran S. Protease: an enzyme with multiple industrial applications. World J Pharm Pharma Sci. 2014;3:568–579. [Google Scholar]
- Schulz B, Boyle C. The endophytic continuum. Mycol Res. 2005;109:661–686. doi: 10.1017/S095375620500273X. [DOI] [PubMed] [Google Scholar]
- Schulz B, Boyle C, Draeger S, Römmert A, Krohn K. Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res. 2002;106:996–1004. doi: 10.1017/S0953756202006342. [DOI] [Google Scholar]
- Shankar S, Shikha, Gupta M (2016) Microbial xylanases: production, applications and challenges. In: Gupta VK, Sharma GD, Tuohy MG, Gaur R (eds) The handbook of microbial bioresources, CABI, pp 313–330
- Shubha J, Srinivas CA. Diversity and extracellular enzymes of endophytic fungi associated with Cymbidium aloifolium L. Afr J Biotechnol. 2017;16:2248–2258. doi: 10.5897/AJB2017.16261. [DOI] [Google Scholar]
- Sindhu R, Binod P, Pandey A. Biological pretreatment of lignocellulosic biomass-an overview. Bioresour Technol. 2016;199:76–82. doi: 10.1016/j.biorTechnol.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Sindhu R, Shiburaj S, Sabu A. Enzyme technology in food processing: recent developments and future prospects. Innov Food Processing Technol. 2021;3:191–215. doi: 10.1016/B978-0-12-815781-7.00016-0. [DOI] [Google Scholar]
- Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Bajar S, Devi A, Pant D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour Tech Rep. 2021;14:100652. doi: 10.1016/j.biteb.2021.100652. [DOI] [Google Scholar]
- Soliman SSM, Raizada MN. Interactions between co-habitating fungi elicit synthesis of Taxol from an endophytic fungus in host Taxus plants. Front Microbiol. 2013;4:1–14. doi: 10.3389/fmicb.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopalun K, Iamtham S. Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. South Afr J Bot. 2020;134:273–279. doi: 10.1016/j.sajb.2020.02.005. [DOI] [Google Scholar]
- Sorgatto M, Guimarães NCdA, et al. Purification and characterization of an extracellular xylanase produced by the endophytic fungus, Aspergillus terreus, grown in submerged fermentation. Afr J Biotechnol. 2012;11:8076–8084. doi: 10.5897/AJB11.2686. [DOI] [Google Scholar]
- Stamford TLM, Stamford NP, Coelho LCBB, Araujo JM. Production and characterization of a thermostable glucoamylase from Streptosporangium sp. endophyte of maize leaves. Bioresour Technol. 2002;83:105–109. doi: 10.1016/S0960-8524(01)00206-1. [DOI] [PubMed] [Google Scholar]
- Stroe M, Netzker T, Scherlach K, et al. Targeted induction of a silent fungal gene cluster encoding the bacteria-specific germination inhibitor fumigermin. Elife. 2020;9:e52541. doi: 10.7554/eLife.52541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujatha E, Gunaswetha K, Bramhachari PV. Current perspectives on phosphate-solubilizing endophytic. Plant Microbiomes Sustain Agric. 2020;25:79. doi: 10.1007/978-3-030-38453-1_3. [DOI] [Google Scholar]
- Sunitha VH, Ramesha A, Savitha J, Srinivas C. Amylase production by endophytic fungi Cylindrocephalum sp. isolated from medicinal plant Alpinia calcarata (Haw.) Roscoe. Brazilian J Microbiol. 2012;43(3):1213–1221. doi: 10.1590/S1517-83822012000300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S, Syed RUH, Johri S. A novel cellulase from an endophyte—Penicillium sp. NFCCI2862. Am J Microbiol Res. 2013;1:84–89. doi: 10.12691/ajmr-1-4-4. [DOI] [Google Scholar]
- Takizawa N. Utilization of recombinant phytase on the avoidance of water pollution by phosphorus of excretory organ of livestock. Seibutsu-Kogaku. 1998;76:82. [Google Scholar]
- Tasia W, Melliawati R. Cellulase and xylanase production from three isolates of indigenous endophytic fungi. IOP Conf Ser. 2017;101:012035. doi: 10.1088/1755-1315/101/1/012035. [DOI] [Google Scholar]
- Terpe K. Overview of bacterial expression systems for heterologous protein production from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- Thapa S, Li H, Ohair J, et al. Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol Biotechnol. 2019;61(579):601. doi: 10.1007/s12033-019-00187-1. [DOI] [PubMed] [Google Scholar]
- Toghueo RMK, Zabalgogeazcoa I, et al. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. South Afr J Bot. 2016;109:146–153. doi: 10.1016/j.sajb.2016.12.021. [DOI] [Google Scholar]
- Torre MDL, Martín-Sampedro R, et al. Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J Ind Microbiol Biotechnol. 2017;44:1561–1573. doi: 10.1007/s10295-017-1977-1. [DOI] [PubMed] [Google Scholar]
- Torres M, Dolcet MM, Sala N, Canela R. Endophytic fungi associated with Mediterranean plants as a source of mycelium-bound lipases. J Agric Food Chem. 2003;51:3328–3333. doi: 10.1021/jf025786u. [DOI] [PubMed] [Google Scholar]
- Vasundhara M, Reddy MS, Kumar A (2019) Secondary metabolites from endophytic fungi and their biological activities, In: Gupta VK, Pandey A (eds) New and future developments in microbial biotechnology and bioengineering, Elsevier, pp 237–258. 10.1016/b978-0-444-63504-4.00018-9
- Venkatesagowda B, Ponugupaty E, Barbosa AM, Dekker RF. Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World J Microbiol Biotechnol. 2012;28:71–80. doi: 10.1007/s11274-011-0793-4. [DOI] [PubMed] [Google Scholar]
- Wanderley MC, Neto JM, Filho JL, et al. Collagenolytic enzymes produced by fungi: a systematic review. Braz J Microbiol. 2017;48:13–24. doi: 10.1016/j.bjm.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu J, Huang W, Tan R. Laccase production by Monotospora sp., an endophytic fungus in Cynodon dactylon. Bioresour Technol. 2006;97:786–789. doi: 10.1016/j.biortech.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Wang YC, Zhao N, Ma JW, et al. High-level expression of a novel α-amylase from Thermomyces dupontii in Pichia pastoris and its application in maltose syrup production. Int J Biol Macromol. 2019;127:683–692. doi: 10.1016/j.ijbiomac.2019.01.162. [DOI] [PubMed] [Google Scholar]
- Wipusaree N, Sihanonth P, et al. Purification and characterization of a xylanase from the endophytic fungus Alternaria alternata isolated from the Thai medicinal plant, Croton oblongifolius Roxb. African J Microbiol Res. 2011;5:5697–5712. [Google Scholar]
- Wittung-Stafshede P. Role of cofactors in protein folding. Acc Chem Res. 2000;35(4):201–218. doi: 10.1021/ar010106e. [DOI] [PubMed] [Google Scholar]
- Yadav AN (2019) Fungal white biotechnology: conclusion and future prospects in recent advancement, In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi, volume 3: Perspective for Sustainable Environments, Springer, pp 491–498. 10.1007/978-3-030-25506-0_20
- Yadav S, Yadav PK, Yadav D, Yadav KDS. Pectin lyase: a review. Process Biochem. 2009;44(1–10):2009. doi: 10.1016/j.procbio.2008.09.012. [DOI] [Google Scholar]
- Yang XL, Huang L, Ruan XL. Epigenetic modifiers alter the secondary metabolite composition of a plant endophytic fungus, Pestalotiopsis crassiuscula obtained from the leaves of Fragaria chiloensis. J Asian Nat Prod Res. 2014;16:412–417. doi: 10.1080/10286020.2014.881356. [DOI] [PubMed] [Google Scholar]
- Yang H, Li J, et al. Microbial production and molecular engineering of industrial enzymes: challenges and strategies. In: Brahmachari G, Demain A, Adrio J, et al., editors. Biotechnology of microbial enzymes. 1. Elsevier; 2017. pp. 151–165. [Google Scholar]
- Zaidi KU, Ali AS, Ali SA, Naaz I. Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem Res Int. 2014 doi: 10.1155/2014/854687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Gao B, et al. Purification and characterization of a novel β-glucuronidase precisely converts glycyrrhizin to glycyrrhetinic acid 3-O-mono-β-D-glucuronide from plant endophytic Chaetomium globosum DX-THS3. Int J Biol Macromol. 2020;159:782–792. doi: 10.1016/j.ijbiomac.2020.05.047. [DOI] [PubMed] [Google Scholar]