Abstract
Polysaccharide immunomodulators were first discovered over 40 years ago. Although very few have been rigorously studied, recent reports have revealed the mechanism of action and structure-function attributes of some of these molecules. Certain polysaccharide immunomodulators have been identified that have profound effects in the regulation of immune responses during the progression of infectious diseases, and studies have begun to define structural aspects of these molecules that govern their function and interaction with cells of the host immune system. These polymers can influence innate and cell-mediated immunity through interactions with T cells, monocytes, macrophages, and polymorphonuclear lymphocytes. The ability to modulate the immune response in an appropriate way can enhance the host's immune response to certain infections. In addition, this strategy can be utilized to augment current treatment regimens such as antimicrobial therapy that are becoming less efficacious with the advent of antibiotic resistance. This review focuses on recent studies that illustrate the structural and biologic activities of specific polysaccharide immunomodulators and outlines their potential for clinical use.
Recently, many advances have been made toward understanding host immune responses to infectious diseases. Novel cell surface and soluble signaling molecules produced by cells of the immune system have been discovered that regulate host responses to microorganisms. It is now widely appreciated that these molecules interact in a concerted fashion to maintain a balance that governs an appropriate response to infectious organisms. Investigators have focused on discovering compounds that positively or negatively modulate the biologic response of immune cells and enhance the host's ability to resist microbial infection. Several classes of these compounds, such as proteins, peptides, lipopolysaccharides, glycoproteins, and lipid derivatives, have all been characterized as molecules that have potent effects on the host immune system. Peptides such as cytokines and chemokines are well-known examples of such molecules. While polysaccharides have long been believed to have benign biologic properties, certain polymers have recently been shown to act as potent immunomodulating agents. This review will focus on polysaccharides that exhibit this biologic activity and their potential for clinical use.
DEFINITION OF IMMUNOMODULATION
With recent advances in the understanding of how cells communicate with each other to signal effector functions, it has become possible to conceive of strategies to manipulate these signaling pathways in order to influence host responses. Compounds that are capable of interacting with the immune system to upregulate or downregulate specific aspects of the host response can be classified as immunomodulators or biologic response modifiers. Whether certain compounds enhance or suppress immune responses can depend on a number of factors, including dose, route of administration, and timing of administration of the compound in question. The type of activity these compounds exhibit can also depend on their mechanism of action or the site of activity.
The cell types involved in orchestrating specific aspects of innate and acquired immune responses are well known. However, until recently the pathways by which these cells communicate were less well understood. These studies have begun to unravel the network of signaling molecules and cell surface receptors that direct an appropriate host response to infectious agents. The basic strategy underlying immunomodulation is to identify aspects of the host response that can be enhanced or suppressed in such a way as to augment or complement a desired immune response. This approach, which should allow the host to better defend itself against invading microorganisms during the course of infection, is attractive because (i) it allows for enhanced host-derived mechanisms to take part in the immune response and (ii) it does not involve the use of organism-specific therapeutics such as antibiotics. The latter point is especially important due to the increase in the number of bacterial species that have developed resistance to the effects of various antimicrobial agents.
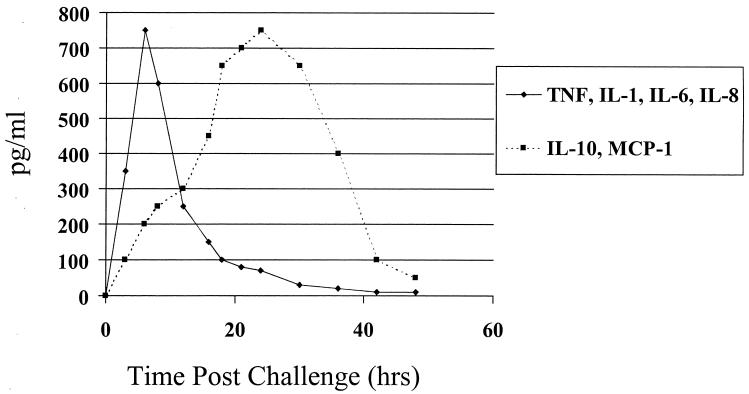
Knowledge of the specific components of cytokine networks and signaling pathways and their role in the regulation of immune responses is important in designing strategies to augment these responses. For example, recent studies have demonstrated the role of anti-inflammatory cytokines such as interleukin 10 (IL-10) and MCP-1 in dampening endotoxic shock associated with bacterial sepsis (26, 80). The influx of bacteria into otherwise sterile spaces such as the peritoneal cavity results in a massive inflammatory response that causes an almost immediate production of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6. In experimental animal models, upregulation of these mediators is associated with increased endotoxic shock and mortality. Following elicitation of proinflammatory cytokines, levels of anti-inflammatory mediators such as IL-10 and MCP-1 are increased and suppress the activity of TNF-α and other proinflammatory molecules such as nitric oxide (NO) (26). The role of IL-10 in the suppression of deleterious effects associated with sepsis has recently been documented (27, 33, 58). MCP-1 complements the role of IL-10 in its ability to increase innate immune defenses associated with bacterial clearance (41). Furthermore, administration of inhibitors of proinflammatory mediators such as NO leads to increased levels of IL-10 and MCP-1 and significantly reduces mortality associated with sepsis in animals (26). These data support the concept that a balance between pro- and anti-inflammatory mediators is maintained during acute infections such as bacterial sepsis and underscore how modulation of this balance of cytokines in favor of anti-inflammatory mediators can be used to ameliorate disease processes. A schematic representation of this process is shown in Fig. 1.
FIG. 1.
Schematic representation of the balance between pro- and anti-inflammatory cytokines elicited during bacterial sepsis. The onset of bacterial sepsis immediately leads to the production of numerous proinflammatory mediators, such as TNF-α, IL-1β, IL-6, IL-8, and NO. To prevent an overwhelming inflammatory response anti-inflammatory mediators such as IL-10 and MCP-1 are produced a few hours later. These cytokines have pleiotropic effects functioning to directly inhibit proinflammatory cytokine synthesis and promote the synthesis of specific cytokine inhibitors, such as IL-1 receptor antagonist and soluble TNF receptors. In addition, they downregulate chemokine and chemokine receptor production and inhibit Th1 cytokines, such as IL-2 and IFN-γ.
POLYSACCHARIDE IMMUNOMODULATORS
Generally, polysaccharides are considered to be classic T-cell-independent antigens that do not elicit cell-mediated immune responses. Rather, most bacterial polysaccharides elicit humoral immunity that results in the induction of low-affinity immunoglobulin M (IgM) and some IgG antibodies. This response is short-lived due to the absence of T-cell help in the development of immunologic memory and the lack of long-lasting antibody response with a switch in class from IgM to IgG. It is believed that the inability of antigen-presenting cells to process polysaccharide antigens leads to the lack of T-cell help, in contrast to the immune response elicited by protein antigens, which stimulate long-lived cell-mediated and humoral responses. Antigen-presenting cells are able to internalize protein antigens, degrade them to peptide subunits, and present these subunits along with major histocompatibility class II molecules on the cell surface. T cells recognize these antigens and are activated to perform effector functions: providing T-cell help in the production of specific IgG antibody by B cells or acting as cytotoxic cells in the lysis of infected host cells.
Recently, certain polysaccharides of microbial origin have been described that act as potent immunomodulators with specific activity for both T cells and antigen-presenting cells such as monocytes and macrophages. While quite a few polysaccharide immunomodulators have been identified, most of the studies have been anecdotal in nature. Relatively few polysaccharides have been examined in detail where both structure-function and mechanism of action studies have been performed. For the purposes of this review, specific examples of polysaccharide immunomodulators will be discussed for cases in which information regarding their biologic activity is available. The review is not meant to provide a complete list of compounds of this nature but rather illustrates their potential for clinical use.
PS A
Structural attributes and role in biologic function.
Polysaccharide A (PS A) is the prototype of a group of polysaccharides termed zwitterionic polysaccharides (Zps) that have both positively and negatively charged moieties and share the same biologic function. PS A is one of two capsular polysaccharides isolated from the gram-negative anaerobic bacterium Bacteroides fragilis that share this dual-charge motif (47, 66). They are present on the surface of this organism and exist as an ionically linked aggregate that can be separated on the basis of electrophoretic mobility (66). These molecules make up the capsular polysaccharide complex (CPC) of B. fragilis and exhibit distinct biologic properties. Early studies by Onderdonk et al. and Kasper et al. showed that the CPC modulates abscess formation associated with experimental intra-abdominal sepsis (29, 44). Intraperitoneal challenge with the polysaccharide complex along with sterile cecal contents induced abscess formation in animals. Conversely, subcutaneous immunization with the CPC prior to intraperitoneal challenge with B. fragilis prevented subsequent abscess formation. The ability to both induce abscesses and prevent their formation could be transferred to naïve animals by T cells, while B cells failed to transfer this activity (45, 53, 54). These studies were the first to indicate that the B. fragilis polysaccharides behaved as immunomodulators with activity specific for T cells.
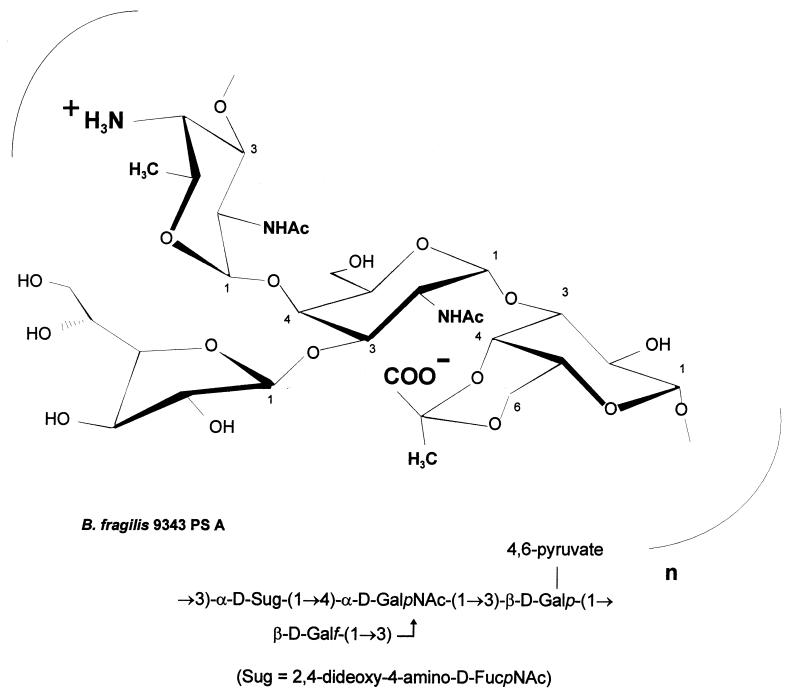
The CPC of B. fragilis NCTC 9343 comprises two structurally distinct high-molecular-weight polysaccharides, termed PS A and PS B. The component polysaccharides are coexpressed on the surface of B. fragilis (66) and have repeating-unit structures that possess positively and negatively charged groups (6). PS A is a tetrasaccharide repeating unit with a balanced positively charged amino group and negatively charged carboxyl group, while PS B is a hexasaccharide repeating unit with a 2-aminoethylphosphonate substituent containing a free amino group and a negatively charged phosphonate group. An additional negative charge conferred by a galacturonic acid residue gives this repeating unit one positive and two negative charges. Of the hundreds of capsular polysaccharides whose structures have been determined, very few possess this zwitterionic charge motif. The capsule of Streptococcus pneumoniae type 1 and the group polysaccharide from this organism, C substance, are two such polymers. The structure of PS A is shown in Fig. 2.
FIG. 2.
Structure of B. fragilis PS A. This repeating unit comprises a trisaccharide backbone with a galactofuranose side chain. PS A has a positively charged free amino group on the 2,4-dideoxy-4-amino-d-FucNAc and a 4,6-pyruvate ring on the galactose moiety.
B. fragilis Zps are potent inducers of intraabdominal abscesses in a rat model of sepsis. Intraperitoneal challenge with a dose of less than 1 μg of PS A results in abscess formation in more than 50% of animals (AD50) (Table 1). PS B (AD50 = 25 μg) and CPC (AD50 = 22 μg) also exhibit this activity (64). Structurally distinct Zps induce abscesses in a similar manner. The type 1 capsular polysaccharide of S. pneumoniae and C substance have potent abscess-inducing ability. Polysaccharides with only one negatively charged group per repeat (such as the Vi antigen of Salmonella enterica serovar Typhi) can be converted into abscess-inducing polymers by the chemical addition of positively charged groups. Finally, chemical modification of PS A or PS B to neutralize or eliminate positively or negatively charged groups on each repeating unit significantly reduces potency by 2 orders of magnitude. It is important to point out that bacterial polysaccharides with repeating-unit structures that lack charged groups or have one negatively charged group per repeating unit do not induce abscesses in this model. Taken together, these studies delineate a structure-activity relationship that explains the ability of certain polysaccharides to interact with the host immune system to induce abscess formation.
TABLE 1.
Abscess induction by Zpsa
| Polysaccharide | AD50b (μg) |
|---|---|
| PS A | 0.67 |
| PS B | 25 |
| CPC | 22 |
| C substance | 5 |
| S. pneumoniae type 1 CP | 31 |
| Group B meningococcal CP | >200 |
| Group B streptococcal type Ia CP | >200 |
Animals were challenged intraperitoneally with 10-fold dilutions of each polysaccharide mixed 1:1 with sterile cecal-content adjuvant (64). Animals were examined for intra-abdominal abscesses 6 days later, and the AD50 was calculated as described (64). Zps were potent abscess-inducing agents, while polysaccharides that did not have this charge motif had AD50s greater than 200 μg.
The dose of polysaccharide calculated to yield abscesses in 50% of animals as previously described (64).
Model of intra-abdominal abscess formation by Zps.
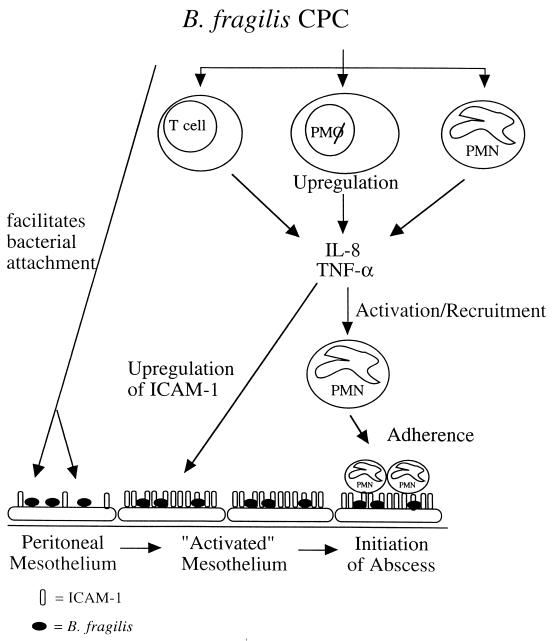
The recruitment, infiltration, and sequestration of polymorphonuclear lymphocytes (PMNs) within the peritoneal cavity form the basis for the development of intra-abdominal abscesses. Cell adhesion molecules (CAMs) expressed by eukaryotic cells are critical in the trafficking of white blood cells from circulating blood to sites of inflammation. The expression of CAMs on the surfaces of cells increases in response to proinflammatory cytokines and chemokines and mediates cellular extravasation and localization of PMNs to host cell surfaces. The role of the B. fragilis CPC in regulating the production of cytokines in the peritoneal cavity and the expression of CAMs such as ICAM-1 on mesothelial cells has been studied in vitro. The CPC of B. fragilis stimulates both TNF-α and IL-1α from murine peritoneal macrophages, which interact with peritoneal mesothelial cells to induce the expression of ICAM-1. Surface-expressed ICAM-1 on mesothelial cells serves as a functional ligand for infiltrating PMNs and increases the binding of this cell type in vitro (25). The CPC also stimulates IL-8 production from human peripheral blood monocytes and PMNs, which suggests that it also serves to recruit PMNs to sites of infection. It has been proposed that the Zps of B. fragilis have many roles in coordinating events that could lead to abscess formation in the peritoneal cavity: 1) they allow for the adherence of bacteria to mesothelial surfaces lining the peritoneal cavity; and 2) stimulate proinflammatory cytokines and chemokines that induce the expression of CAMs on host cells and recruit PMNs to the abdominal cavity. CAMs such as intracellular adhesion molecule 1 (ICAM-1) facilitate the adherence of infiltrating PMNs to mesothelial tissue. The recruitment of PMNs into the peritoneal cavity and subsequent adherence of these cells to inflamed mesothelial tissue form the first stages of intra-abdominal abscess formation in the infected host. The proposed mechanism by which Zps coordinate these events is shown in Fig. 3.
FIG. 3.
Proposed model of intra-abdominal abscess formation. The CPC of B. fragilis interacts with cells of the host immune system within the peritoneal cavity. This interaction (i) allows for the localization of B. fragilis within the abdominal cavity, thus resulting in enhanced adherence to the mesothelial surface and the ability to resist clearance from the peritoneum, and (ii) stimulates proinflammatory cytokines and chemokines, the production of which stimulates the expression of CAMs (such as ICAM-1) on host cells and the recruitment of PMNs to the abdominal cavity. Infiltration and sequestration of PMNs within the peritoneal cavity are the hallmark of intra-abdominal abscess formation. The CPC of B. fragilis interacts with T cells, peritoneal macrophages, and PMNs. In response to this interaction, these cells produce TNF-α and IL-8, which serve to recruit activated PMNs to the peritoneal cavity and upregulate ICAM-1 expression on mesothelial cells. The production of ICAM-1 by mesothelial cells serves as a functional ligand for infiltrating PMNs. The recruitment of PMNs into the peritoneal cavity and subsequent adherence of these cells to activated mesothelial tissue form the first stages of intra-abdominal abscess formation in the infected host.
Prevention of abscess formation by Zps.
While intraperitoneal challenge with Zps and sterile cecal contents induces intra-abdominal abscess formation, subcutaneous or intramuscular treatment of animals with these polymers prevents the development of this host response following bacterial challenge. Subcutaneous administration of PS A in the absence of any adjuvant mixtures protects against abscess formation following challenge with B. fragilis or different intestinal organisms capable of causing intra-abdominal abscesses. Protection by PS A can also be conferred by administration 24 h following bacterial challenge.
The ability of Zps to prevent abscess formation is dependent on the presence of positively and negatively charged groups on their repeating-unit structures. Naturally occurring polysaccharides or polysaccharides chemically modified to possess these charged groups protect animals against challenge with B. fragilis or heterologous abscess-inducing bacteria. Protection from abscess formation by antigenically distinct, heterologous bacterial species that induce this pathobiologic host response demonstrates that PS A does not behave as a classic immunogen that confers specific immunity. Rather, it appears this polymer modulates the immune system to suppress a generalized host response that leads to abscess formation. This hypothesis is further supported by the fact that PS A can be administered shortly before or even after challenge with bacteria and still protect against abscess formation.
T-cell immunomodulation by Zps.
Despite the conventional view regarding humoral immune mechanisms governing the host response to bacterial polysaccharides, a role for T cells in mediating protection against abscess formation has been demonstrated (45, 53, 63, 65). T cells taken from animals previously treated with PS A protect naïve recipient animals against abscess formation induced by viable B. fragilis or a combination of Fusobacterium varium and Enterococcus faecium.
CD4+ T cells transfer the immunomodulatory activity in this system (67). A role for soluble mediators produced by CD4+ T cells was demonstrated when cell lysates could also transfer this protective activity. Analysis of the cytokine profile of splenic T cells taken from animals treated with PS A showed that mRNA transcripts were produced for IL-2, gamma interferon (IFN-γ), and IL-10, but not for IL-4. This pattern did not follow a classic Th1 or Th2 profile but rather seemed to represent a combination of the two patterns. This cytokine profile is characteristic of the response to the superantigen staphylococcal enterotoxin B (3), in which IL-2 and IFN-γ are seen early in the response, while IL-10 is produced 48 h later. Since IL-10 has been shown to inhibit Th1 responses, these investigators hypothesized that cells stimulated with staphylococcal enterotoxin B likely produced this cytokine in order to help downregulate the stimulatory response.
IL-2 produced by T cells appears to be central to the ability of PS A to modulate the prevention of abscesses, since neutralization of this cytokine with specific antibody abrogates the ability of T-cell lysates to transfer protection to naïve animals (67). In contrast, mixing of IFN-γ- or IL-10-specific antibody did not abrogate the protective activity. Furthermore, administration of recombinant IL-2 to animals at the time of challenge with B. fragilis also prevented abscess formation. Parallel studies have shown that PS A stimulates CD4+ T-cell proliferation in vitro (11).
On the basis of this work, the mechanism of protection by Zps is thought to occur through the modulation of CD4+ T-cell activity and the production of IL-2. It has been reported that certain activated CD4+ T cells can induce suppression in naïve T cells in a manner that obviates unwanted host responses to specific antigens or alloantigens (22, 51, 60). The mechanism by which tolerance is induced in these cells is unclear, but some investigators have suggested that induction is mediated through control of IL-2 secretion and the upregulation of the IL-2 receptor CD25 on T cells (37, 60). It is possible that the transfer of PS A-activated CD4+ T cells from rats confers protection to naïve animals in a similar manner. The ability of Zps to both upregulate and downregulate the host immune system to induce or prevent abscess formation clearly demonstrates their immunomodulatory properties. The interaction of Zps with T cells and other host cells to regulate the production of cytokines exemplifies how these molecules can influence the immune system to prevent deleterious host responses.
β-(1-3)-Glucans
Structure-function attributes.
β-(1-3)-Glucans are glucose homopolymers purified from fungi and yeasts. These polymers are generally 1-3 linked throughout the repeating unit but can be isolated with 1-6 branches of the 1-3 polymer. Glucans can be obtained in crude form by boiling and enzyme treatment of yeast strains to yield insoluble and soluble material. Insoluble glucans have been derivatized by phosphorylation, sulfation, and amination to improve solubility. The soluble glucans exist mainly as linear triple-helical structures in aqueous solution (8).
The biologic activity of particulate yeast glucans that differed in molecular weight and the number of 1-6 linkages has been determined. Mice injected with high-, medium-, or low-molecular-weight glucans all induced peritoneal cell activation. Treatment with these polymers resulted in an increase in the proportion of neutrophils and eosinophils and an alteration in macrophage morphology. Macrophages also showed an increase in phosphatase activity and lipopolysaccharide (LPS)-stimulated NO production (15).
The challenge in fully assessing the structure-function properties of the β-(1-3)-glucans has been in obtaining purified soluble preparations of this material. Many investigators have demonstrated immunomodulatory properties with cell wall-derived insoluble material. However, more recent efforts have been directed toward identifying the structural and biologic characteristics of the soluble form of the molecule in order to evaluate its potential for clinical use.
Conversion of particulate glucan to a soluble material can be achieved with treatment in methyl sulfoxide and urea (8 M) at 100°C. This preparation is endotoxin free and composed of 34.06% C, 6.15% H, 50.30% O, 5.69% S, and 3.23% N. Following this treatment, two polymer peaks were resolved by size exclusion chromatography: peak 1 was approximately 1 × 106 Da and represented 1% of the total polymer mass, and peak 2 was about 1.5 × 104 Da and represented 99% of the total polymer mass (76). These polymers formed a triple helix in solution and were biologically active.
Further work simplified the extraction of water-insoluble β-(1-3)-d-glucan from Saccharomyces cerevisiae by employing hydrochloric acid. However, other protic acids tested for the extraction of water-soluble glucan showed promising results. Purified glucan could be obtained from acetic, formic, and phosphoric acids and varied in molecular mass and viscosity; however, the basic structure and side chain branching were not altered (39).
Anti-infective activity.
The biologic properties of crude preparations of β-(1-3)-glucans have been studied since the 1960s. Although these molecules exhibit a wide range of biologic functions, including antitumor activity, their ability to prevent a range of experimental infectious diseases has been studied in the greatest detail (8). Numerous reports have documented the ability of β-(1-3)-glucans to nonspecifically activate cellular and humoral components of the host immune system. Primarily, these molecules increase antimicrobial activity of mononuclear cells and neutrophils (36, 49, 69) and enhance the functional activity of macrophages (12). β-(1-3)-Glucans also stimulate the proliferation of monocytes and macrophages and have potent hematopoietic activities (48, 49).
The anti-infective activity of β-(1-3)-glucans has been tested in animal models of bacterial, fungal, parasitic, and viral disease but has been most extensively tested in experimental bacterial diseases. β-(1-3)-Glucans have been evaluated in experimental models of Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Mycobacterium leprae infection (19, 30, 73–75, 77, 79). In each case, treatment with β-(1-3)-glucan had a beneficial effect in reducing mortality or decreasing bacterial counts in infected animals.
Insoluble and derivatized β-(1-3)-glucans stimulate the production of proinflammatory mediators such as complement components, IL-1, TNF-α, IL-2, and eicosanoids (9, 16, 17, 21, 32). Due to their potential for adverse reactions in animals and humans, the insoluble compounds have not been evaluated in detail for clinical use.
The soluble β-(1-3)-glucans have been examined extensively for their ability to prevent bacterial sepsis. Studies by Williams and DiLuzio and coworkers were among the first to identify the anti-infective properties of these compounds and their ability to prevent bacterial sepsis in experimental models (20, 73–75). Subsequent studies focused on the nature and mechanism of this protective activity. Williams and coworkers showed that administration of soluble β-(1-3)-glucan has multiple effects. It significantly increased the number of circulating neutrophils in animals and enhanced bone marrow proliferation. Neutrophils from glucan-treated mice showed increased phagocytosis of E. coli in vitro (77). Subsequent work with PGG-glucan, a highly purified proprietary β-(1-3)-glucan with β-1,6 branches demonstrated similar activity. Administration of PGG-glucan to mice or rats prior to bacterial challenge protected against lethal peritonitis (43) and yielded a dose-dependent protective effect against lethal E. coli and S. aureus challenge. Prophylactic treatment also resulted in a decrease in mortality associated with intra-abdominal sepsis. This model of sepsis simulates fecal contamination of the peritoneal cavity following disruption of the bowel and results in severe peritonitis with associated mortality and abscess formation. Fewer organisms were present in blood cultures of PGG-glucan-treated animals, and studies of leukocytes from rats and mice following a single injection of PGG-glucan showed a transient increase in the total leukocyte count (43). Treatment with PGG-glucan could be administered up to 4 h postchallenge and still effect a significant reduction in mortality (62). This activity can be transferred by spleen cells taken from glucan-treated animals and is thought to be mediated, at least in part, by an increase in the production of prostaglandins by these cells (14).
Recent evidence suggests that PGG-glucan modulates the production of pro-inflammatory cytokines from lymphocytes and monocytes during sepsis. Lymphocytes and monocytes taken from mice treated with PGG-glucan produced significantly less TNF-α and other proinflammatory cytokines when stimulated in vitro with LPS or a superantigen (56). In addition, treatment with glucan increases the level of cellular apoptosis, suggesting that inflammatory cells induced by the septic process are deleted from circulation. The ability to dampen the proinflammatory response associated with bacterial sepsis and to remove cells producing these cytokines from sites of inflammation furthers our understanding of the anti-infective properties of PGG-glucan.
In work done to determine the effect of β-(1-3)-glucan on transcriptional activation, cytokine expression, and mortality in a mouse model of sepsis, decreased NF-κB and NF-IL-6 nuclear binding activity and TNF-α and IL-6 mRNA levels were observed compared to control animals (72). The downregulation of transcription factor activation and cytokine message expression was associated with an increase in survival in animals with sepsis. Animals treated with the glucan in a prophylactic or therapeutic mode showed increased survival rates compared to controls following the induction of sepsis by cecal ligation puncture.
The immunomodulatory properties of PGG-glucan have been studied in many in vitro models. Incubation of phagocytic cells such as PMNs with this compound increases their bactericidal capabilities (57, 69). Incubation of human whole blood with PGG-glucan does not directly stimulate oxidative burst, phagocyte degranulation, or the release of proinflammatory cytokines but does prime PMNs and monocytes for subsequent activation following exposure to opsonized bacteria or particulate matter (69).
The basis for the biologic activity of the β-(1-3)-glucans lies in their ability to interact directly with macrophages and PMNs. Muller et al. showed that glucan phosphate, a water-soluble, chemically defined (1-3)-β-d-glucan, binds to human and murine monocyte/macrophage cell lines (40). This work suggests that the binding is specific and leads to internalization of bacteria and increased cytoplasmic vacuolization. Complement receptor 3 (CR3) has been identified as a receptor for certain glucans (78). CR3-mediated phagocytosis and degranulation are dependent upon simultaneous ligation of an iC3b binding site and a glucan binding site, both within the domain of CR3 (50). However, PGG-glucan appears to bind to a receptor that is distinct from CR3 (1). The concentration-dependent and high-affinity binding of PGG-glucan resulted in the activation of rat macrophages. Recent studies showing that monoclonal antibodies specific for a lactosylceramide glycosphingolipid inhibited specific binding of PGG-glucan suggest that this molecule is the receptor (69). Although this binding was specific, lactosylceramides with different fatty acid chain lengths and varying degrees of saturation could act as PGG-glucan receptors.
β-(1-3)-Glucan binding to eukaryotic cells results in the activation of signal transduction pathways. PGG-glucan elicits NF-κB-like and NF-IL-6-like transcription factors in a time- and concentration-dependent manner (1). The signal transduction pathways used are distinct from those used by LPS. Treatment of neutrophils with the monoclonal antibody specific for the PGG-glucan glycosphingolipid receptor inhibited the activation of the NF-κB-like factor in these studies (69).
Mannans
Candida albicans mannan.
Mannan from C. albicans exhibits certain immunomodulatory properties. In general, these compounds consist mostly of a polysaccharide component but also include proteins (5% by weight). The biologic activity of this molecule was initially noted in patients suffering from candidiasis. Patients infected with C. albicans had suppressed immune system function and elevated levels of circulating mannan. Mannose-binding lectins present on macrophages can bind mannan and activate the host immune system via a non-self-recognition mechanism (68). The mannose receptor recognizes a repeating-unit pattern associated with carbohydrates that surround infectious agents and mediates endocytosis and phagocytosis (2). This recognition leads to signal transduction, cytokine production, and complement activation.
The immunosuppressive activity of C. albicans mannan was documented in studies in which delayed hypersensitivity was inhibited in animals following intravenous injection of this polymer (23). It is interesting that treatment with mannan elicited specific CD8+ T cells that were responsible for this downregulatory activity (23). These cells are not produced in the absence of CD4+ or I-A+ cells following administration of mannan. Subsequently, these investigators found that IL-4 is the key cytokine that mediates the induction of mannan-specific downregulatory cells. It is believed that Th2 CD4+ T cells that secrete IL-4 are involved in this process. Additional studies showed that IL-12p40, IL-10, and IFN-γ may also play a role in the production of CD8+ downregulatory effector cells (70).
The C. albicans mannan also induces the release of proinflammatory cytokines. Animals injected with this compound have increased levels of TNF-α in serum (24). The response is concentration dependent and can be blocked by administration of mannan-specific antibodies. It is hypothesized that, upon saturation of mannan clearance mechanisms via mannan-binding proteins, free mannan stimulates an inflammatory response that is important in the pathogenesis of Candida sepsis.
Cryptococcus neoformans GXM.
C. neoformans infections are characterized by a generalized suppression of the host immune system that is mediated by T-suppressor cells. The capsular polysaccharide of this organism, glucuronoxylomannan (GXM), elicits the production of these cells during the course of infection (7). However, GXM mixed directly with antigen-presenting cells inhibits the induction of T suppressor responses and increases delayed-type hypersensitivity responses in infected mice. Treatment of mice with a mixture of GXM and antigen-presenting cells results in an increase in survival following infection.
PSK and PSP
Protein bound polysaccharides PSK and PSP have been isolated from distinct strains of mushrooms. These compounds are chemically similar and have a molecular mass of about 100 kDa (42). The polysaccharide component is made up of monosaccharides with α-(1-4) and β-(1-3) glucosidic linkages, while aspartic and glutamic acids predominate in the peptide component of these compounds. PSK and PSP differ mainly in the presence of fucose in PSK and rhamnose and arabinose in PSP. PSK and PSP are classified as biological response modifiers that stimulate T-cell activation and induce IFN-γ and IL-2 production. The biologic activity is characterized by their ability to increase white blood cell counts, IL-2 production and delayed-type hypersensitivity reactions. In addition, these polysaccharides have been shown to inhibit the growth of tumor cell lines and have in vivo antitumor activity.
The antitumor activity has been evaluated in Japan for prevention of esophageal, gastric, and lung cancer in humans with promising results (42). In phase II and phase III trials in China, PSP significantly enhanced immune status in 70 to 97% of patients with cancers of the stomach, esophagus, lung, ovary, and cervix. In these studies, PSK and PSP increased the number of immune cells and facilitated dendritic and cytotoxic T-cell infiltration of tumors. The polysaccharides were well-tolerated and compatible with chemotherapy and radiation therapy.
The mechanism of action of these polymers is not clear. Studies have suggested that in treatment of mice with PSK, the PSK binds to and inhibits the immunosuppressive cytokine transforming growth factor beta (38). PSK and PSP were also found to enhance superoxide dismutase (SOD) activity in mice. Tumor-bearing mice that were treated with the polysaccharides showed decreased tumor growth and increased SOD activity in this tissue. Since SOD is believed to protect cells against superoxide radical-mediated damage it is believed that these polysaccharides ameliorated disease by increasing the activity of this enzyme (71).
The effect of PSK treatment on neutrophil activity in vivo has been studied and suggests that the activation of this cell type may be responsible in part for efficacy in the treatment of experimental cancers (28). Animals receiving PSK had increased neutrophil levels with concomitant target cell toxicity and a marked decrease in size and number of lung metastatic foci. This was associated with an increase in chemotactic factors produced by neutrophils.
HA
Hyaluronic acid (HA) is produced by streptococci and is also a major carbohydrate component of the extracellular matrix of mammalian tissue and can be found in skin, joints, eyes, and most other organs and tissues. It is a disaccharide repeating unit that is the simplest example of the class of anionic glycosaminoglycans. The HA capsule of group A streptococci acts as an adhesin for attachment of this organism to CD44 on human keratinocytes. This capsule protects the organism from ingestion by both phagocytes and epithelial cells and enhances virulence in mouse models of lethal systemic infection, upper airway colonization, and pneumonia (52).
Modulation of the immune system is believed to occur via binding of HA to the CD44 receptor on eukaryotic cells. This receptor-ligand interaction is critical in regulating cell-cell communication and leukocyte extravasation. T-cell activation via T-cell receptor signaling induces activated CD44 and CD44-dependent primary adhesion and the extravasation of activated T cells to inflamed sites, thus suggesting that HA-CD44 interactions lead to the development of pathogenic T cells in chronic inflammation (55).
Low-molecular-weight HA can be used to block interactions between T lymphocyte CD44 and eukaryotic cell-derived hyaluronate. Blockade in this manner prevents allograft rejection and preserves organ function (31). In addition, HA has skin wound-healing properties (13) and can be used as a replacement of naturally occurring hyaluronate in eye and joint surgery (59).
POTENTIAL CLINICAL APPLICATIONS
The potential usefulness of immunomodulating polysaccharides in the treatment of infectious diseases has been demonstrated in preclinical and clinical studies on PGG-glucan and B. fragilis PS A. Glucans have long been known to enhance the effect of antimicrobial agents in the treatment of experimental sepsis (10, 34, 35). However, the recent alarming increase in the number of antibiotic-resistant bacterial strains isolated from infected patients has refocused attention on alternative treatment regimens. This has led investigators to study the effectiveness of glucan treatment on infectious diseases caused by antibiotic-resistant bacteria. In a rat model of intra-abdominal sepsis, treatment with PGG-glucan significantly reduced mortality associated with challenge by antibiotic-resistant strains of E. coli or S. aureus. Combination treatment with glucan and the antibiotic to which these organisms were sensitive resulted in greater protection than with antibiotic therapy alone (61). Similar studies with guinea pigs showed that administration of PGG-glucan increased the infective dose of methicillin-resistant strains of S. aureus and Streptococcus epidermidis by as much as 2.5- and 60-fold, respectively (30). Finally, rats treated with PGG-glucan and challenged with a multiple-antibiotic-resistant S. aureus strain had reduced bacterial counts in blood, elevated absolute monocyte and neutrophil counts, and increased neutrophil oxidative burst activity. The enhancement of the innate immune function by PGG-glucan in animals treated with ampicillin resulted in an overall reduction in mortality following challenge with ampicillin-resistant S. aureus (36). In summary, these studies clearly demonstrate the ability of β-(1-3)-glucan to augment standard antibiotic regimens in clinical cases where antibiotic-resistant bacteria can predominate.
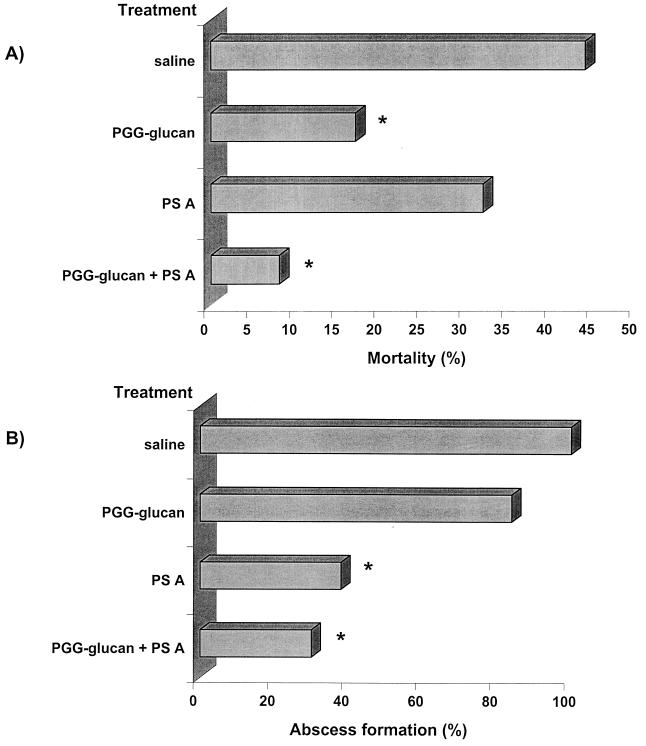
The potential clinical use of polysaccharide immunomodulators can be further illustrated by studies of the effectiveness of PGG-glucan and PS A in preventing intra-abdominal sepsis in the absence of antimicrobial therapy (62). Clinically, this disease process is associated with acute bacterial sepsis followed by the development of intra-abdominal abscess formation. Its pathogenesis has been studied in animals and can be simulated by the implantation of rat cecal contents into the peritoneal cavity of rats. Animals challenged with this inoculum develop acute sepsis associated with mortality and a chronic phase marked by abscess formation in surviving animals (46). This is a severe sepsis model in which the cecal-content inoculum contains several hundred aerobic and anaerobic bacterial species. In these studies, animals were given prophylactic treatment with PGG-glucan and/or PS A in the absence of conventional antibiotic therapy (data are shown in Fig. 4). Administration of the combination of both PGG-glucan and PS A to animals prior to challenge with cecal contents significantly reduced the rate of mortality and abscesses compared with saline-treated controls. Animals treated with PGG-glucan alone had a reduced mortality rate, but there was no effect on the number of animals with abscesses compared with controls. However, treatment with PS A alone significantly reduced the abscess rate. Although there was a reduction in the mortality rate in PS A-treated animals, it was not statistically significant, demonstrating that suppression of the ability of these animals to form abscesses does not lead to increased mortality as some would hypothesize. These data clearly delineate the potential usefulness of polysaccharide immunomodulators for the treatment of infectious diseases. This strategy enhances the host's ability to combat infections and could augment the efficacy of current anti-infective regimens that consist solely of antibiotic therapy. In addition, this approach obviates the problems associated with the development of antibiotic-resistant bacterial strains.
FIG. 4.
Polysaccharide-mediated protection against mortality and abscess formation associated with experimental intra-abdominal sepsis (62). Animals were treated prophylactically with saline, PGG-glucan, PS A, or a combination of these polymers and challenged with rat cecal contents. The challenge inoculum is titrated to yield approximately a 50% mortality rate with 100% abscess formation in surviving animals. Results are taken from two separate experiments. (A) Mortality rates in animals treated with PGG-glucan or with the combination of PGG-glucan and PS A were significantly reduced (∗) compared with the saline-treated control group. (B) Abscess formation was significantly reduced (∗) in animals treated with PS A or the combination of PS A and PGG-glucan compared with the saline-treated controls. These results demonstrate that administration of two polysaccharide immunomodulators can prevent both phases of intra-abdominal sepsis in the absence of antibiotic therapy.
Clinical Testing of PGG-Glucan
PGG-glucan has been evaluated in clinical trials to assess its ability to prevent serious postoperative infections following high-risk gastrointestinal procedures (4, 5, 18). In phase I-II and phase II trials, treatment with PGG-glucan reduced infection rates and the length of hospitalization. In a subsequent phase III trial, there was no overall difference in the number of serious infections and deaths between PGG-glucan-treated groups and placebo groups. In a prospectively defined group of patients who had undergone noncolorectal surgery, PGG-glucan administration resulted in a statistically significant reduction in serious infections and death. However, this study was terminated due to an increased incidence of adverse effects in patients receiving PGG-glucan (18). While initial trials seemed promising, it was unclear why PGG-glucan failed to achieve an overall clinically significant effect in the later stages of these trials. Given the fact that the β-(1-3)-glucans are so structurally diverse, it is possible that further analysis of the structure-function properties of these compounds will eventually yield a compound with clinical efficacy.
CONCLUSIONS
The collection of work reviewed here illustrates the distinct biologic properties associated with polysaccharide immunomodulators. While the activity of some of these polymers has been known for over 30 years, the lack of defined structural and mechanistic information has limited efforts to study their potential for clinical use. Recent investigations have led to a more detailed understanding of structural aspects of polysaccharides that govern biologic function and regulation of cytokine networks that influence host immune responses. Particular structure-function relationships have been identified and polysaccharide-specific receptors have been discovered that should provide a foundation for the development of compounds with novel activities.
The use of immunomodulating agents provides distinct advantages over conventional therapies. Most important, the enhancement of the host immune system's innate ability to combat bacterial infection obviates the problems associated with antibiotic resistance. Because bacteria can rapidly mutate in a manner that blunts the activities of many antimicrobial agents, resistance to these agents will be an ongoing problem. As seen from the numerous preclinical studies presented here, the use of a combination of immunomodulation and antimicrobial therapy may be a valuable new tool in the treatment of infectious diseases.
ACKNOWLEDGMENTS
I thank Andrew B. Onderdonk and Jaylynn Olivo for critically reading the manuscript.
REFERENCES
- 1.Adams D S, Pero S C, Petro J B, Nathans R, Mackin W M, Wakshull E. PGG-glucan activates NF-kappaB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J Leukoc Biol. 1997;62:865–873. doi: 10.1002/jlb.62.6.865. [DOI] [PubMed] [Google Scholar]
- 2.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Assenmacher M, Lohning M, Scheffold A, Manz R A, Schmitz J, Radbruch A. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur J Immunol. 1998;28:1534–1543. doi: 10.1002/(SICI)1521-4141(199805)28:05<1534::AID-IMMU1534>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Babineau T J, Hackford A, Kenler A, Bistrian B, Forse R A, Fairchild P G, Heard S, Keroack M, Caushaj P, Benotti P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994;129:1204–1210. doi: 10.1001/archsurg.1994.01420350102014. [DOI] [PubMed] [Google Scholar]
- 5.Babineau T J, Marcello P, Swails W, Kenler A, Bistrian B, Forse R A. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994;220:601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann H, Tzianabos A O, Brisson J R, Kasper D L, Jennings H J. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using resolution NMR spectroscopy. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- 7.Blackstock R, Casadevall A. Presentation of cryptococcal capsular polysaccharide (GXM) on activated antigen-presenting cells inhibits the T-suppressor response and enhances delayed-type hypersensitivity and survival. Immunology. 1997;92:334–339. doi: 10.1046/j.1365-2567.1997.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleicher P, Mackin W. Betafectin PGG-glucan: a novel carbohydrate immunomodulator with anti-infective properties. J Biotechnol Healthcare. 1995;2:207–222. [Google Scholar]
- 9.Browder W, Williams D, Pretus H, Olivero G, Enrichens F, Mao P, Franchello A. Beneficial effect of enhanced macrophage function in the trauma patient. Ann Surg. 1990;211:605–613. [PMC free article] [PubMed] [Google Scholar]
- 10.Browder W, Williams D, Sherwood E, McNamee R, Jones E, DiLuzio N. Synergistic effect of nonspecific immunostimulation and antibiotics in experimental peritonitis. Surgery. 1987;102:206–214. [PubMed] [Google Scholar]
- 11.Brubaker J O, Li Q, Tzianabos A O, Kasper D L, Finberg R W. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 12.Burgaleta C, Territo M C, Quan S G, Golde D W. Glucan-activated macrophages: functional characteristics and surface morphology. J Reticuloendothel Soc. 1978;23:195–204. [PubMed] [Google Scholar]
- 13.Chen W Y, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 14.Cisneros R L, Gibson F C, Tzianabos A O. Passive transfer of poly-(1-6)-β-glucotriosyl-(1-3)-β-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect Immun. 1996;64:2201–2205. doi: 10.1128/iai.64.6.2201-2205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary J A, Kelly G E, Husband A J. The effect of molecular weight and beta-1,6-linkages on priming of macrophage function in mice by (1,3)-beta-d-glucan. Immunol Cell Biol. 1999;77:395–403. doi: 10.1046/j.1440-1711.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 16.Czop J K, Austen K F. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc Natl Acad Sci USA. 1985;82:2751–2755. doi: 10.1073/pnas.82.9.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czop J K, Austen K F. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J Immunol. 1985;135:3388–3393. [PubMed] [Google Scholar]
- 18.Dellinger E P, Babineau T J, Bleicher P, Kaiser A B, Seibert G B, Postier R G, Vogel S B, Norman J, Kaufman D, Galandiuk S, Condon R E. Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Betafectin Gastrointestinal Study Group. Arch Surg. 1999;134:977–983. doi: 10.1001/archsurg.134.9.977. [DOI] [PubMed] [Google Scholar]
- 19.Delville J, Jacques P J. Therapeutic effect of yeast glucan in mice infected with Mycobacterium leprae. Arch Int Physiol Biochim. 1977;85:965–966. doi: 10.3109/13813457709053306. [DOI] [PubMed] [Google Scholar]
- 20.DiLuzio N R, Williams D L. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect Immun. 1978;20:804–810. doi: 10.1128/iai.20.3.804-810.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doita M, Rasmussen L T, Seljelid R, Lipsky P E. Effect of soluble aminated beta-1,3-d-polyglucose on human monocytes: stimulation of cytokine and prostaglandin E2 production but not antigen-presenting function. J Leukoc Biol. 1991;49:342–351. doi: 10.1002/jlb.49.4.342. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Sanz J A, Mikulits W, Livingstone A, Lefkovits I, Mullner E W. Translational control: a general mechanism for gene regulation during T cell activation. FASEB J. 1998;12:299–306. doi: 10.1096/fasebj.12.3.299. [DOI] [PubMed] [Google Scholar]
- 23.Garner R E, Childress A M, Human L G, Domer J E. Characterization of Candida albicans mannan-induced, mannan-specific delayed-hypersensitivity suppressor cells. Infect Immun. 1990;58:2613–2620. doi: 10.1128/iai.58.8.2613-2620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garner R E, Hudson J A. Intravenous injection of Candida-derived mannan results in elevated tumor necrosis factor alpha levels in serum. Infect Immun. 1996;64:4561–4566. doi: 10.1128/iai.64.11.4561-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson F C, 3rd, Onderdonk A B, Kasper D L, Tzianabos A O. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- 26.Hogaboam C M, Steinhauser M L, Schock H, Lukacs N, Strieter R M, Standiford T, Kunkel S L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect Immun. 1998;66:650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss R S, Swanson P E, Knudson C M, Chang K C, Cobb J P, Osborne D F, Zollner K M, Buchman T G, Korsmeyer S J, Karl I E. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 28.Ishihara Y, Fujii T, Iijima H, Saito K, Matsunaga K. The role of neutrophils as cytotoxic cells in lung metastasis: suppression of tumor cell metastasis by a biological response modifier (PSK) In Vivo. 1998;12:175–182. [PubMed] [Google Scholar]
- 29.Kasper D L, Onderdonk A B, Crabb J, Bartlett J G. Protective efficacy of immunization with capsular antigen against experimental infection with Bacteroides fragilis. J Infect Dis. 1979;140:724–731. doi: 10.1093/infdis/140.5.724. [DOI] [PubMed] [Google Scholar]
- 30.Kernodle D, Gates H, Kaiser A B. Prophylactic anti-infective activity of poly-[1-6]-beta-d-glucopyranosyl-[1-3]-beta-d-glucopryanose glucan in a guinea pig model of staphylococcal wound infection. Antimicrob Agents Chemother. 1998;42:545–549. doi: 10.1128/aac.42.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knoflach A, Azuma H, Magee C, Denton M, Murphy B, Iyengar A, Buelow R, Sayegh M H. Immunomodulatory functions of low-molecular weight hyaluronate in an acute rat renal allograft rejection model. J Am Soc Nephrol. 1999;10:1059–1066. doi: 10.1681/ASN.V1051059. [DOI] [PubMed] [Google Scholar]
- 32.Konopski Z, Seljelid R, Eskeland T. Cytokines and PGE2 modulate the phagocytic function of the beta-glucan receptor in macrophages. Scand J Immunol. 1993;37:587–592. doi: 10.1111/j.1365-3083.1993.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 33.Kopydlowski K M, Salkowski C A, Cody M J, van Rooijen N, Major J, Hamilton T A, Vogel S N. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 34.Lahnborg G, Hedstrom K G, Nord C E. The effect of glucan--a host resistance activator--and ampicillin on experimental intraabdominal sepsis. J Reticuloendothel Soc. 1982;32:347–353. [PubMed] [Google Scholar]
- 35.Lahnborg G, Hedstrom K G, Nord C E. Glucan-induced enhancement of host resistance in experimental intraabdominal sepsis. Eur Surg Res. 1982;14:401–408. doi: 10.1159/000128314. [DOI] [PubMed] [Google Scholar]
- 36.Liang J, Melican D, Cafro L, Palace G, Fisette L, Armstrong R, Patchen M L. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. Int J Immunopharmacol. 1998;20:595–614. doi: 10.1016/s0192-0561(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 38.Matsunaga K, Hosokawa A, Oohara M, Sugita N, Harada M, Nomoto K. Direct action of a protein-bound polysaccharide, PSK, on transforming growth factor-beta. Immunopharmacology. 1998;40:219–230. doi: 10.1016/s0162-3109(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 39.Muller A, Ensley H, Pretus H, McNamee R, Jones E, McLaughlin E, Chandley W, Browder W, Lowman D, Williams D. The application of various protic acids in the extraction of (1→3)-beta-d-glucan from Saccharomyces cerevisiae. Carbohydr Res. 1997;299:203–208. doi: 10.1016/s0008-6215(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 40.Muller A, Rice P J, Ensley H E, Coogan P S, Kalbfleisch J H, Kelle J L, Love E J, Portera C A, Ha T, Browder I W, Williams D L. Receptor binding and internalization of a water-soluble (1-3)-13-glucan biologic response modifier in two monocyte/macrophage cell lines. J Immunol. 1996;156:3418–3425. [PubMed] [Google Scholar]
- 41.Nakano Y, Kasahara T, Mukaida N, Ko Y C, Nakano M, Matsushima K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect Immun. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng T B. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae) Gen Pharmacol. 1998;30:1–4. doi: 10.1016/s0306-3623(97)00076-1. [DOI] [PubMed] [Google Scholar]
- 43.Onderdonk A B, Cisneros R L, Hinkson P L, Ostroff G R. Anti-infective effect of poly β1-6 glucotriosyl-β1-3-glucopyranose glucan in vivo. Infect Immun. 1992;60:1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onderdonk A B, Kasper D L, Cisneros R L, Bartlett J G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- 45.Onderdonk A B, Markham R B, Zaleznik D F, Cisneros R L, Kasper D L. Evidence for T cell-dependent immunity to Bacteroides fragilis in an intraabdominal abscess model. J Clin Investig. 1982;69:9–16. doi: 10.1172/JCI110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onderdonk A B, Weinstein W M, Sullivan N M, Bartlett J G, Gorbach S L. Experimental intra-abdominal abscesses in rats: quantitative bacteriology of infected animals. Infect Immun. 1974;10:1256–1259. doi: 10.1128/iai.10.6.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantosti A, Tzianabos A O, Onderdonk A B, Kasper D L. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 1991;59:2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patchen M L, Lotzova E. Modulation of murine hemopoiesis by glucan. Exp Hematol. 1980;8:409–422. [PubMed] [Google Scholar]
- 49.Riggi S J, DiLuzio N R. Identification of a reticuloendothelial stimulating agent in zymosan. Am J Physiol. 1961;200:297–300. doi: 10.1152/ajplegacy.1961.200.2.297. [DOI] [PubMed] [Google Scholar]
- 50.Ross G D, Cain J A, Myones B L, Newman S L, Lachmann P J. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 51.Ryan K R, Evavold B D. Persistence of peptide-induced CD4+ T cell anergy in vitro. J Exp Med. 1998;187:89–96. doi: 10.1084/jem.187.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J Clin Investig. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shapiro M E, Kasper D L, Zaleznik D F, Spriggs S, Onderdonk A B, Finberg R W. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J Immunol. 1986;137:341–346. [PubMed] [Google Scholar]
- 54.Shapiro M E, Onderdonk A B, Kasper D L, Finberg R W. Celluar immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982;154:1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegelman M H, DeGrendele H C, Estess P. Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol. 1999;66:315–321. doi: 10.1002/jlb.66.2.315. [DOI] [PubMed] [Google Scholar]
- 56.Soltys J, Quinn M T. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect Immun. 1999;67:244–252. doi: 10.1128/iai.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stashenko P, Wang C Y, Riley E, Wu Y, Ostroff G, Niederman R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dent Res. 1995;74:323–330. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 58.Steinhauser M L, Hogaboam C M, Kunkel S L, Lukacs N W, Strieter R M, Standiford T J. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 59.Sutherland I W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–46. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 60.Thornton A M, Shevach E M. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tzianabos A O, Cisneros R L. Prophylaxis with the immunomodulator PGG glucan enhances antibiotic efficacy in rats infected with antibiotic-resistant bacteria. Ann N Y Acad Sci. 1996;797:285–287. doi: 10.1111/j.1749-6632.1996.tb52980.x. [DOI] [PubMed] [Google Scholar]
- 62.Tzianabos A O, Gibson F C, 3rd, Cisneros R L, Kasper D L. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J Infect Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- 63.Tzianabos A O, Kasper D L, Cisneros R L, Smith R S, Onderdonk A B. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Investig. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 65.Tzianabos A O, Onderdonk A B, Zaleznik D F, Smith R S, Kasper D L. Structural characteristics of polysaccharides that induce protection against intra-abdominal abscess formation. Infect Immun. 1994;62:4881–4886. doi: 10.1128/iai.62.11.4881-4886.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzianabos A O, Pantosti A, Baumann H, Brisson J R, Jennings H J, Kasper D L. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- 67.Tzianabos A O, Russell P R, Onderdonk A B, Gibson F C, 3rd, Cywes C, Chan M, Finberg R W, Kasper D L. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J Immunol. 1999;163:893–897. [PubMed] [Google Scholar]
- 68.Vasta G R, Quesenberry M, Ahmed H, O'Leary N. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol. 1999;23:401–420. doi: 10.1016/s0145-305x(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 69.Wakshull E, Brunke-Reese D, Lindermuth J, Fisette L, Nathans R S, Crowley J J, Tufts J C, Zimmerman J, Mackin W, Adams D S. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology. 1999;41:89–107. doi: 10.1016/s0162-3109(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Li S P, Moser S A, Bost K L, Domer J E. Cytokine involvement in immunomodulatory activity affected by Candida albicans mannan. Infect Immun. 1998;66:1384–1391. doi: 10.1128/iai.66.4.1384-1391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei W S, Tan J Q, Guo F, Ghen H S, Zhou Z Y, Zhang Z H, Gui L. Effects of Coriolus versicolor polysaccharides on superoxide dismutase activities in mice. Chung Kuo Yao Li Hsueh Pao. 1996;17:174–178. [PubMed] [Google Scholar]
- 72.Williams A, Sun X, Fischer J E, Hasselgren P O. The expression of genes in the ubiquitin-proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery. 1999;126:744–750. [PubMed] [Google Scholar]
- 73.Williams D L, Browder I W, DiLuzio N R. Immunotherapeutic modification of Escherichia coli-induced experimental peritonitis and bacteremia by glucan. Surgery. 1983;93:448–454. [PubMed] [Google Scholar]
- 74.Williams D L, Browder W, McNamee R, Di Luzio N R. Glucan immunomodulation in experimental E. coli sepsis. Adv Exp Med Biol. 1982;155:701–706. doi: 10.1007/978-1-4684-4394-3_77. [DOI] [PubMed] [Google Scholar]
- 75.Williams D L, Di Luzio N R. Glucan induced modification of experimental Staphylococcus aureus infection in normal, leukemic and immunosuppressed mice. Adv Exp Med Biol. 1979;121:291–306. doi: 10.1007/978-1-4684-3593-1_25. [DOI] [PubMed] [Google Scholar]
- 76.Williams D L, Pretus H A, McNamee R B, Jones E L, Ensley H E, Browder I W. Development of a water-soluble, sulfated (1→3)-beta-d-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydr Res. 1992;235:247–257. doi: 10.1016/0008-6215(92)80093-g. [DOI] [PubMed] [Google Scholar]
- 77.Williams D L, Sherwood E R, Browder I W, McNamee R B, Jones E L, Rakinic J, DiLuzio N R. Effect of glucan on neutrophil dynamics and immune function in Escherichia coli peritonitis. J Surg Res. 1988;44:54–61. doi: 10.1016/0022-4804(88)90122-9. [DOI] [PubMed] [Google Scholar]
- 78.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll M C, Mayadas T N, Ross G D. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–3052. [PubMed] [Google Scholar]
- 79.Yokota M, Kambayashi J, Tsujinaka T, Sakon M, Mori T, Tsuchiya M, Oishi H, Matsuura S. A new method for the quantification of beta-glucan in plasma and its application in the diagnosis of postoperative infection. Jpn J Surg. 1990;20:559–566. doi: 10.1007/BF02471013. [DOI] [PubMed] [Google Scholar]
- 80.Zisman D A, Kunkel S L, Strieter R M, Tsai W C, Bucknell K, Wilkowski J, Standiford T J. MCP-1 protects mice in lethal endotoxemia. J Clin Investig. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]