Graphical abstract

Keywords: COVID-19, Genomics, Genomic surveillance, Reverse vaccinology, SARS-CoV-2
Abbreviations: ACE2, Angiotensin-Converting Enzyme – 2; COVID-19, Coronavirus Disease; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; CSIR, Council Of Scientific And Industrial Research; GISAID, Global Initiative on Sharing All Influenza Data; ICMR, Indian Council of Medical Research; ICTV, International Committee on Taxonomy of Viruses; MERS-CoV, Middle East respiratory syndrome coronavirus; NGS, Next-Generation Sequencing; NSP, Non-Structural Protein; ORF, Open Reading Frame; PANGOLIN, Phylogenetic Assignment of Named Global Outbreak Lineages; PRF, Programmed -1 Ribosomal Frameshifting; RBD, Receptor-binding domain; RdRp, RNA-dependent RNA polymerase; RTC, Replication-Transcription Complex; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TMEM106B, Transmembrane protein 106B; TMPRSS2, Transmembrane Protease Serine 2; UTR, Untranslated region; VOC, Variants of Concern; VOI, Variants of Interest; WHO, World Health Organization
Abstract
The coronavirus disease 2019 (COVID-19) quickly swept over the world, becoming one of the most devastating outbreaks in human history. Being the first pandemic in the post-genomic era, advancements in genomics contributed significantly to scientific understanding and public health response to COVID-19. Genomic technologies have been employed by researchers all over the world to better understand the biology of SARS-CoV-2 and its origin, genomic diversity, and evolution. Worldwide genomic resources have greatly aided in the investigation of the COVID-19 pandemic. The pandemic has ushered in a new era of genomic surveillance, wherein scientists are tracking the changes of the SARS-CoV-2 genome in real-time at the international and national levels. Availability of genomic and proteomic information enables the rapid development of molecular diagnostics and therapeutics. The advent of high-throughput sequencing and genome editing technologies led to the development of modern vaccines. We briefly discuss the impact of genomics in the ongoing COVID-19 pandemic in this review.
1. Introduction
Coronavirus disease 2019 (COVID-19) has wreaked havoc on the world, costing millions of lives, severely affecting public health systems, and inflicting social and economic crises. It has rapidly spread globally, becoming one of the most devastating outbreaks in the history of mankind. As of December 3, 2021, there have been more than 263 million confirmed cases of COVID-19 and over 5.2 million deaths worldwide (https://covid19.who.int/). Continuous attempts are being made to effectively tackle this deadly disease. Being the first pandemic in the post-genomic era, advancements in genomics contributed a lot to scientific understanding and the public health response to the COVID-19, to a greater degree which was not feasible during the past outbreaks like 2002–2003 severe acute respiratory syndrome (SARS) epidemic. Genomic technologies have been employed by researchers all over the world to better understand the viral origin, outbreak dynamics, transmission, and evolution. Integration of genomics and other omics technologies played a crucial role in the development of new diagnostics, therapeutics, and vaccines.
Genomics is a branch of biology that focuses on the study of structure, function, mapping, and editing of the entire genome of an organism (McKusick and Ruddle, 1987). Genomics has many sub-disciplines such as structural genomics, functional genomics, comparative genomics, epigenomics, metagenomics, pharmacogenomics, and others, which use bioinformatics and computational tools to explore the characteristics of genomes. The advent of next-generation sequencing platforms has transformed genomics from a discipline into a technology that is commonly used in labs around the world to solve scientific problems. Genomics is now widely employed in medicine, research, biotechnology, and agriculture.
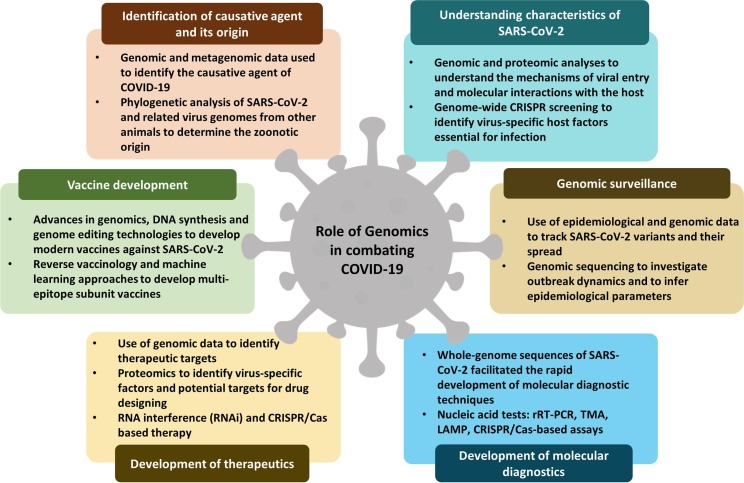
In this review, we provide a brief history of the identification of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) and its origin, outlining how genomics helps in understanding the biology of SARS-CoV-2, and discuss the importance of genomic surveillance in tracking SARS-CoV-2 variants and their spread. Finally, we highlight how genomic data is exploited in the development of molecular diagnostics, therapeutics, and vaccines to combat COVID-19 (Fig. 1 ).
Fig. 1.
A summary of various roles of genomics in the fight against COVID-19 pandemic.
2. Identification of causative agent and its origin
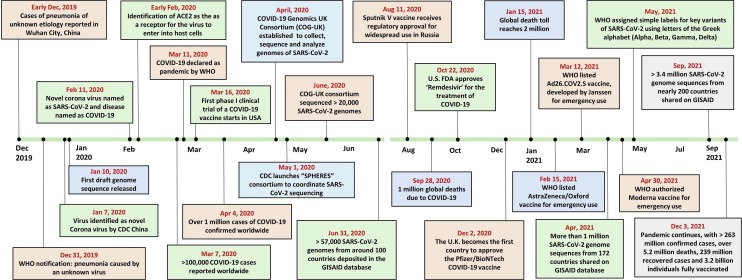
In December 2019, a cluster of cases with atypical pneumonia of unknown etiology was reported in some of the local hospitals in Wuhan city of China (Wu et al., 2020). Initial investigations identified that the pathogen was a novel coronavirus (CoV) and named 2019-nCoV by the World Health Organization (WHO). The disease has spread around the world in a very short period and crossed one hundred thousand COVID-19 cases worldwide within two months. Then, COVID-19 was declared a pandemic by WHO on March 11, 2020 (as shown in Fig. 2 ). During the initial stages of the outbreak, sequencing of samples from patients led to the identification of the causative organism (Wu et al., 2020, Lu et al., 2020).
Fig. 2.
Timeline of key events of COVID-19 and genomics, starting from the first report in December 2019 to the current situation as of December 2021.
Identification of the origin and source of infection is very important to take necessary public health measures to reduce disease spread. The analysis of the viral genomic sequences, from Wuhan and surrounding areas, provided insights into the early transmission dynamics and enabled the determination of the times of origin and diversification (Li et al., 2020, Boni et al., 2020). Lu et al. (2020) reported that the sequences obtained from nine patients were highly similar, with more than 99.98% sequence identity. Analysis of sequence data revealed that the virus belongs to the genus Betacoronavirus and subgenus Sarbecovirus (Wu et al., 2020). Lu et al. (2020) showed that 2019-nCoV had a sequence identity of 79% with SARS-CoV and 50% with MERS-CoV. The virus was subsequently renamed as SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) by the International Committee on Taxonomy of Viruses (ICTV) and the disease was named as COVID-19 (Coronavirus disease 2019) by WHO (Zhou et al., 2020b).
Phylogenetic analysis of genome sequences from SARS-CoV-2 and related viruses from other animals was carried out to determine the zoonotic origin of SARS-CoV-2. These investigations revealed that SARS-CoV2 was more closely related to two bat-derived coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21 with more than 88% sequence identity (Lu et al., 2020). Zhou et al. (2020b) also showed that the genomic sequences were 96% identical to a coronavirus, BatCoV RaTG13 in horseshoe bat (Rhinolophus affinis). So far, the closest known sequence to SARS-CoV-2 was BatCoV RaTG13. Several studies identified SARS-CoV-2-related coronaviruses in Chinese pangolins (Manis pentadactyla) and Malayan pangolins (Manis javanica), but pangolin coronaviruses were less closely related to SARS-CoV-2 (with 85.5–92.4% sequence similarity) than bat coronaviruses (Lam et al., 2020, Liu et al., 2020). Most of the findings suggested that the bats could be the most probable natural reservoir for SARS-CoV-2 lineage (Andersen et al., 2020, Lu et al., 2020, Zhou et al., 2020b, Wacharapluesadee et al., 2021). Pangolins are suspected to be an intermediate host of SARS‑CoV‑2 due to sequence similarity between pangolin coronaviruses and SARS-CoV-2 (Lam et al., 2020, Liu et al., 2020, Xiao et al., 2020). Therefore, the comparative analysis of genomic and metagenomic data from various animal sources will be crucial in unraveling the origin and evolution of SARS-CoV-2.
3. Understanding the characteristics of SARS-CoV-2
In the early stages of the pandemic, genomic and proteomic analyses have proven helpful in understanding the mechanisms of viral entry and molecular interactions with hosts which are vital to the spread of the disease. SARS-CoV-2 is an enveloped, single-stranded, positive-sense, RNA virus with a genome size of ∼29.9 kb classified under the genus Betacoronavirus in the family Coronaviridae (V’kovski et al., 2020). The genome comprises 5′ UTR, replicase (ORF1a/ORF1b), four structural genes, 3′ UTR, and a poly (A) tail (Hu et al., 2020). SARS-CoV-2 genome has 14 different open reading frames (ORF) which encode 27 proteins including 4 major structural proteins (Spike (S), Envelope (E), Membrane (M), Nucleocapsid (N) proteins) (Lokman et al., 2020). Apart from these, several ORFs encoding non-structural proteins (such as papain-like protease, 3-chymotrypsin-like protease, RNA-dependent RNA polymerase, helicase) and accessory proteins are distributed in the SARS-CoV-2 genome (Chan et al., 2020). These accessory proteins play a significant role in modulating host responses to infection, such as enhancing or inhibiting the production of pro-inflammatory cytokines, and are the determinants of the pathogenicity of the virus (Shang et al., 2020). The virus binds to the host cell using surface spike glycoproteins that comprise 2 functional subunits, S1 and S2. The receptor-binding domain (RBD) of the S1 subunit recognizes and attaches to the host cell receptor, while the S2 subunit is needed for fusion with the host cell membrane (Wrapp et al., 2020). Thus the Spike proteins were mostly used as therapeutic targets to prevent the entry of the virus into the host cells (Letko et al., 2020). SARS-CoV-2 and other related coronaviruses share genetic similarities in the spike protein RBD motif, which facilitated in identifying the cell entry receptor to which SARS-CoV-2 attaches, and hence the type of cells that it may infect. Experiments using reverse genetics methods have shown that the SARS-CoV-2 uses the same receptor angiotensin-converting enzyme 2 (ACE2) as SARS-CoV to enter into the human cells, but with higher affinity than the SARS-CoV virus (Letko et al., 2020). The spike protein is cleaved and activated by the host proteases mainly transmembrane protease serine 2 (TMPRSS2), cathepsin L1 (CTSL), and furin, which make necessary conformational changes for the fusion and entry of the virus into the target cell (Shang et al., 2020).
During the translation of the SARS-CoV-2 RNA genome, programmed −1 ribosomal frameshifting (PRF) is a critical step in which the translational reading frame is switched at the junction of ORF 1a and 1b (Bhatt et al., 2021). PRF is necessary for the synthesis of RNA-dependent RNA polymerase (RdRp) and downstream proteins which are crucial for virus propagation. The replication-transcription complex (RTC) and RNA-dependent RNA polymerase (RdRP) activity facilitates a more complicated replication and transcription process in coronavirus genomes than in other kinds of RNA viruses. RNA polymerase synthesizes complementary negative-strand RNAs from the positive sense template genomic RNA (gRNA). The continuous replication leads to full-length gRNAs, whereas discontinuous jumping of RdRP is called template switching which yields subgenomic RNAs (sgRNAs) with shared 5′ and 3′ ends. Next-generation sequencing (NGS) and nanopore sequencing technologies enabled the researchers to identify hundreds of template switches and to construct the subgenomic landscapes of SARS-CoV-2 (Wang et al., 2021). As a result, the molecular basis for deciphering subgenome synthesis and developing new antiviral drugs will be laid. Understanding the structural biology of the viral proteins is also very essential for improving therapeutic and preventive measures. Proteins are the prime targets in immunological interventions since they are the key factors responsible for viral pathogenicity (Naik et al., 2020). Sequence-based prediction studies generated a vast amount of data on SARS-CoV-2 proteins and their interactions with other molecules. As of December 2021, the PDB repository (https://www.rcsb.org/) contains 1640 files related to SARS-CoV-2. Genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screening has uncovered several key features of SARS-CoV-2 including the virus-specific host factors that are essential for infection (Wei et al., 2021). For example, TMEM106B is a lysosomal protein that acts as a proviral host factor for SARS-CoV-2 infection (Baggen et al., 2021). Availability of genomic and proteomic information along with the in-vitro and in-vivo studies enabled the researchers to better understand the characteristics of SARS-CoV-2.
4. Genomic surveillance
The pandemic has opened a new era of genomic surveillance, wherein scientists are monitoring changes of the viral genome in real-time to understand the evolution of SARS-CoV-2 and to predict the emergence of new variants at the global and national levels (Cyranoski, 2021, Joonlasak et al., 2021). Genomic surveillance involves the use of epidemiological, genomics, and phenomics data to monitor the emergence of new strains and to track pathogen transmission and evolution (Lo and Jamrozy, 2020). Both genomic and epidemiological information should be brought together promptly to guide public health and social measures (PHSMs), diagnosis, treatment, and vaccination. Genomic epidemiology has been widely applied in various countries to track the origin and routes of transmission of COVID-19 (Deng et al., 2020, Fauver et al., 2020, Miller et al., 2020, Rockett et al., 2020, Seemann et al., 2020).
4.1. Sequencing initiatives across the world
Advances in next-generation sequencing have enabled the rapid and efficient production of entire viral genomes at a low cost. Genomic sequencing plays a major role in the continuous monitoring of the evolution of SARS-CoV-2 genome. The WHO recommended the nations speed up genome sequencing and share the genomic data and findings in a coordinated way through a publicly accessible database. To coordinate sequencing operations, several initiatives and consortia have been formed in various countries (Table 1 ). For example, in April 2020, the COVID-19 Genomics UK Consortium (COG-UK) was formed in the United Kingdom to collect, sequence, and analyze SARS-CoV-2 genomes to understand viral transmission and evolution (https://www.cogconsortium.uk/). Other initiatives such as CDC’s “SPHERES” (SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance) (https://www.cdc.gov/coronavirus/2019-ncov/variants/spheres.html), Canadian COVID Genomics Network (CanCOGeN) (https://www.genomecanada.ca/en/cancogen), Germany’s Deutsche COVID-19 OMICS Initiative (DeCOI) (https://decoi.eu/), Switzerland’s Swiss SARS-CoV-2 Sequencing Consortium (S3C) (https://bsse.ethz.ch/cevo/research/sars-cov-2/swiss-sars-cov-2-sequencing-consortium.html), and Irish Coronavirus Sequencing Consortium (https://www.teagasc.ie/). Indian SARS-CoV-2 Genomics Consortium (INSACOG) was formed by the Department of Biotechnology, Ministry of Science & Technology, Government of India along with CSIR and ICMR, including 28 national laboratories to sequence and monitor the SARS-CoV-2 genomic variations (https://dbtindia.gov.in/insacog). Apart from the national initiatives, several global networks are also involved in SARS-CoV2 genome sequencing and surveillance (Table 1).
Table 1.
List of SARS-CoV-2 Genomic Consortia in various countries.
| Name of the genomics consortium/sequencing initiative | Country/region | Source link |
|---|---|---|
| Africa CDC Institute for Pathogen Genomics | Africa | https://africacdc.org/institutes/ipg/ |
| Canadian COVID Genomics Network (CanCOGeN) | Canada | https://www.genomecanada.ca/en/cancogen |
| Coronavirus Sequencing in Quebec (CoVSeQ) | Quebec, Canada | https://covseq.ca/ |
| COVID-19 Genomics UK Consortium (COG-UK) | United Kingdom | https://www.cogconsortium.uk/ |
| COVID-19 Network Investigations (CONI) alliance | Thailand | https://coni.team/ |
| Danish Covid-19 Genome Consortium (DCGC) | Denmark | https://www.covid19genomics.dk/home |
| Deutsche COVID-19 OMICS Initiative (DeCOI) | Germany | https://decoi.eu/ |
| Indian SARS-CoV-2 Genomics Consortium (INSACOG) | India | https://dbtindia.gov.in/insacog |
| Irish Coronavirus sequencing consortium | Ireland | https://www.teagasc.ie/ |
| Mutational Dynamics of SARS-CoV-2 in Austria | Austria | https://www.sarscov2-austria.org/cemm/ |
| National Institute of Infectious Diseases | Japan | https://www.niid.go.jp/niid/en/ |
| RIVM – National Institute for Public Health and the Environment | Netherlands | https://www.rivm.nl/en/coronavirus-covid-19 |
| SeqCOVID – genomic epidemiology of SARS-CoV-2 | Spain | http://seqcovid.csic.es/ |
| SPHERES consortium (SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance) | United States of America | https://www.cdc.gov/coronavirus/2019-ncov/variants/spheres.html |
| Switzerland’s Swiss SARS-CoV-2 Sequencing Consortium (S3C) | Switzerland | https://bsse.ethz.ch/cevo/research/sars-cov-2/swiss-sars-cov-2-sequencing-consortium.html |
| ARTIC network’s Real-Time Molecular Epidemiology For Outbreak Response | Global | https://artic.network/ |
| COVID-19 High Performance Computing (HPC) Consortium | Global | https://covid19-hpc-consortium.org/ |
| Public Health Alliance for Genomic Epidemiology (PHA4GE) | Global | https://pha4ge.org/ |
| The COVID-19 host genetics initiative | Global | https://www.covid19hg.org/ |
Sequencing data of SARS-COV-2 genomes from multiple countries across the world are now shared through open access repositories like Global Initiative on Sharing All Influenza Data (GISAID). GISAID was originally developed for rapid international exchange of all influenza viral genomes and related clinical data (Shu and McCauley, 2017), but it has now been expanded to include SARS-CoV-2 genomic data. The first SARS-CoV-2 whole-genome sequences were made publicly available on GISAID's SARS-CoV-2 database on January 10, 2020, allowing for worldwide responses to the pandemic (Lu et al., 2020). Within six months more than 57,000 SARS-CoV-2 genomes from around 100 countries were deposited. GISAID combines sequence data with epidemiological information and provides real-time genomic surveillance to monitor the emergence of SARS-CoV-2 variants in different parts of the world. GISAID is the most commonly used database for the SARS-CoV-2. As of December 2021, more than 5.7 million SARS-CoV-2 genome sequences from nearly 200 countries around the world were shared on the GISAID database (https://www.gisaid.org/). The largest proportion of sequences shared from Europe (58.2%), then North America (31.8%), Asia (5.8%), South America (1.9%), Africa (1.16%), with the fewest from Oceania (0.91%) (https://www.gisaid.org/). In addition to GISAID, other existing genomic and proteomic databases have been updated and used to provide SARS-CoV-2 resources (listed in Table 2 ). Some databases like China National Center for Bioinformation (CNCB) (https://ngdc.cncb.ac.cn/ncov/) combine information from five different sources – National Microbiome Data Collaborative (NMDC), China National Genebank Database (CNGdb), GISAID, Genome warehouse, and NCBI GenBank. However, accurate SARS-CoV-2 genomic surveillance has been hampered by several issues. The sequencing quality of SARS-CoV-2 genomes in public databases varies for a number of reasons like sequencing methods and laboratory-specific implementation, which can lead to significant bias while studying SARS-CoV-2 variants and evolution dynamics. Due to the lack of proper quality control for genomic data, there were many sequences that were significantly shorter or longer than the reference genome (Zelenova et al., 2021). A database update can be highly recommended in order to increase the quality of the genomic data. So many user-friendly web-based tools were created to overcome the problem of data processing and interpretation, for example, Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN) (https://pangolin.cog-uk.io/) for lineage assignment, Nextstrain (https://nextstrain.org/), CoVizu (http://filogeneti.ca/covizu/), and Microreact (https://microreact.org/) for data visualization. All these databases aided the scientists in deciphering SARS-CoV-2 mutations, developing appropriate diagnostic kits, and tracking the outbreaks all around the planet.
Table 2.
List of commonly used SARS-CoV-2 genomic/proteomic databases.
| Database | Data type (No. of entries)1 | References | Source link |
|---|---|---|---|
| GISAID SARS-CoV-2 database | SARS-CoV-2 genome sequences (3,445,483) | Khare et al., 2021 | https://www.gisaid.org/ |
| DNA Databank of Japan (DDBJ) | Sequence data of SARS-CoV-2 (47 entries) | Okido et al., 2021 | https://www.ddbj.nig.ac.jp/ |
| EMBL-EBI COVID-19 Data Portal (CDP) | Sequences (485,396), Raw reads (167,051), Sequenced samples (376,298), Studies (392), Genes (22), Browser (1), Variants (12,691) | De Silva et al., 2021, Harrison et al., 2021 |
https://www.ebi.ac.uk/ena/pathogens/covid-19 https://www.covid19dataportal.org/ |
| NCBI SARS-CoV-2 Resources | SRA runs (1,107,163), Nucleotide records (1,368,700), Clinical studies related to COVID-19 (6,533), PubMed (175,769), PMC (202,051) | Sayers et al., 2022 | https://www.ncbi.nlm.nih.gov/sars-cov-2/ |
| NGDC-CNCB’s Resource for Coronavirus 2019 (RCoV19) | Coronavirus Sequence (7,801,242), New Coronavirus Strain (3,597,465), Novel Coronavirus Sequence (3,617,804). | Gong et al., 2020, Song et al., 2020, Zhao et al., 2020 | https://ngdc.cncb.ac.cn/ncov/ |
| PDBe-KB (Protein Data Bank in Europe) – COVID-19 Data Portal | Entries (1849), Macromolecules (867), ompounds (725), Protein families (171) | Varadi et al., 2022 | https://www.ebi.ac.uk/pdbe/covid-19 |
| CoV3D | SARS-CoV-2 protein structures: spike (467), protease (374), NSP (458) |
Gowthaman et al., 2021 | https://cov3d.ibbr.umd.edu/ |
| RCSB-PDB (Protein Data Bank) | PDB Structures (1449 files) | Burley et al., 2021 | https://rcsb.org/covid19 |
As of 03.12.2021.
4.2. Tracking SARS-CoV-2 variants and their spread
SARS-CoV-2, like other viruses, changes over time to adapt to changing environments. The majority of mutations are neutral that have little effect on the functional properties of the virus. There are certain mutations that may be significant, for example, when they encode essential components like the SARS-CoV-2 spike glycoprotein, which serves as a key for the virus to enter host cells and initiate infection (Zhang et al., 2020a). Genomic analyses indicate that some changes may confer a selective advantage to the virus and lead to increased fitness such as antiviral drug resistance and immune escape (Harvey et al., 2021). Even a single amino acid change may alter the severity of illness it causes, infectivity, transmissibility, host immunity responses, the effectiveness of vaccines, therapeutics, and other public health measures (Van Dorp et al., 2020). Since the beginning of the SARS-CoV-2 pandemic, the World Health Organization (WHO) and its international networks have been tracking the evolution of the SARS-CoV-2 genome and updating the variants of interest (VOI) and variants of concern (VOC) (Konings et al., 2021). As of December 2021, there are five variants of concern (VOC) designated by WHO such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/). Alpha (B.1.1.7) variant, the first VOC exhibiting greater transmissibility, was emerged in the United Kingdom (UK) in September 2020 (Davies et al., 2021). The Delta (B.1.617.2) variant, first identified in December 2020 in India, is now replacing the pre-existing lineages such as Kappa (B.1.617.1) and Alpha (B.1.1.7) and causing re-emergence even in countries with high vaccination coverage (Kannan et al., 2021). Genomic sequencing plays a major role in identifying the viral variants and hotspots of transmission. Korber et al. (2020) designed a bioinformatics pipeline to monitor SARS-CoV-2 variants using the data from the GISAID SARS-CoV-2 sequence database. The pipeline tracks the changes in spike glycoprotein overtime to find the variants that are increasing in different geographic regions at the same time. Their findings showed that during a month, a SARS-CoV-2 variant bearing a specific spike mutation (D614G) became globally dominant.
4.3. Inferring epidemiological parameters and transmission dynamics
Various studies used whole-genome sequences (WGS) as a surveillance tool to investigate outbreak dynamics (Bandoy and Weimer, 2021, Oude Munnink et al., 2020, Bukin et al., 2021) and to infer epidemiological parameters like reproductive number (Geidelberg et al., 2021). For instance, Banu et al. (2020) analyzed the phylogenetic clusters of SARS-CoV-2 genomes to rule out the emergence of COVID-19 in India and suggested that the common ancestor might have emerged at the end of January 2020 and resulted in an outbreak followed by the nationwide spread. Bousali et al. (2021) performed phylogenetic and phylodynamics analysis using SARS-CoV-2 genome sequences derived from ten European regions to investigate the Molecular Transmission Clusters (MTCs). Pan et al. (2021) conducted a phylogenetic analysis using a large number of SARS-CoV-2 genomic sequences from GenBank and GISAID databases to identify the epidemiological traits of COVID-19 and observed the diverse sources of transmission and transmission routes of SARS-CoV-2 in different countries. Geidelberg et al. (2021) estimated the growth rate and reproduction number of SARS-CoV-2 by phylogenetic analysis of genetic sequences obtained from confirmed COVID-19 cases in China. Similarly, Romero et al. (2021) estimated effective reproductive number (Rt) using genomic data of SARS-CoV-2 in Peru. Based on these studies, researchers were able to combine genomic data with epidemiological data to understand the transmission dynamics of SARS-CoV-2 and to take timely public health measures, including regional lockdowns and travel restriction.
5. Role of genomics in diagnosis and therapy of COVID-19
Genomic medicine is an advanced discipline that focuses on how genomic information is used in clinical diagnosis, therapy, and predicting outcomes (Oishi et al., 2015).
5.1. Development of molecular diagnostics
Management of COVID-19 requires prompt diagnosis, effective therapy, and future prevention. The availability of the first whole-genome sequences of SARS-CoV-2 facilitated the rapid development of molecular diagnostic techniques, particularly nucleic acid-based diagnostic assays such as real-time reverse transcription-polymerase chain reaction (rRT‐PCR), Transcription-Mediated Amplification (TMA), loop‐mediated isothermal amplification (LAMP), and CRISPR/Cas-based assays (Broughton et al., 2020, Carter et al., 2020, Caruana et al., 2020, Corman et al., 2020, Shen et al., 2020, Wang et al., 2020). These approaches were further improved and refined to make them more specific to the viral variants in different geographical regions. Because of its sensitivity and specificity, RT-PCR is considered the 'gold standard' among nucleic acid tests for detection and screening of COVID-19 (Corman et al., 2020). Viral genomic sequences are needed for designing the primers and probes that would efficiently bind to SARS-CoV-2 nucleic acid. Several SARS-CoV-2 genomic areas, including the RdRP gene in the ORF1ab sequence, the S gene, N gene, and E gene, are used in RT-PCR assays to diagnose COVID-19 (Wang et al., 2020). Mutations in the primer and probe-target areas of the SARS-CoV-2 genome can lead to false-negative results (Khan and Cheung, 2020). Therefore to improve the accuracy of detection and to reduce the risk of false negatives, the virus is detected with several targets, such as multiplex real-time RT-PCR methods targeting two or more sections of the viral genome (Ishige et al., 2020, Tahamtan and Ardebili, 2020). The risk of diminished diagnostic efficiency is also avoided by developing diagnostics based on relatively stable conserved regions of the genome (Ascoli, 2021). As the virus continues to evolve, genome sequencing is necessary for monitoring the mutations that would hinder the ability of diagnostic assays to detect SARS-CoV-2 (Jain et al., 2021). Advancements in genomics and proteomics enabled the cloning and expression of SARS-CoV-2 viral proteins, which aided the development of inexpensive rapid diagnostic tests for detection of SARS-CoV-2 at the point of care such as antigen and serological tests (Toptan et al., 2021, Mercer and Salit, 2021).
5.2. Development of therapeutics
Having access to the genome of the SARS-CoV-2 virus allows researchers to identify therapeutic targets and to build models of epitopes and immune responses, allowing the development of new therapeutics and vaccines (Chellapandi and Saranya, 2020, Li et al., 2020, Zhou et al., 2020a, Peng et al., 2021). Both genomics and proteomics enabled the rapid understanding of viral protein function and pathogenesis, as well as the identification of virus-specific factors and potential targets for drug design. COVID-19 might be treated using drugs that target any of the key proteins involved in viral replication (Table 3 ). For example, the drug Remdesivir inhibits RdRp (RNA-dependent RNA polymerase) and has been approved for the treatment of COVID-19 in various countries after showing improvement in clinical studies (Beigel et al., 2020, Buckland et al., 2020, Riva et al., 2020, Rubin et al., 2020). Apart from these antiviral drugs, several other potential therapeutic approaches have been developed by various researchers to combat COVID-19 such as RNA interference (RNAi) and CRISPR/Cas based therapy that target viral RNAs to functionally disrupt the virus (Berber et al., 2020). Chen et al. (2020) developed an RNAi-based approach using small interfering RNA (siRNA) molecules that inhibit gene expression and block SARS-CoV-2 replication. Genomic knowledge serves as the basis for theoretical predictions of the potential siRNA targets in the SARS-CoV-2 genome. On the other hand, the CRISPR/Cas technique employs guide RNAs (gRNAs) that simultaneously target and degrade different regions of the virus-like replicase-transcriptase (ORF1ab) and spike (S) genes (Nguyen et al., 2020). RdRp gene of the SARS-CoV-2 can also be targeted by the CRISPR-Cas system due to its highly conserved amino acid sequence and chemical structure (Kumar et al., 2020). Abbott et al. (2020) suggested a CRISPR-Cas13-based COVID-19 treatment called Prophylactic Antiviral CRISPR in huMAN cells (PAC-MAN) to suppress SARS-CoV-2. The CRISPR-Cas13 system allows for the rapid development of guide RNAs that specifically target highly conserved regions of the viral genome, allowing it to combat rapidly mutating SARS-CoV-2 strains (Kumar et al., 2020). Apart from the viral targeting agents, the host factors that are necessary for viral replication and transcription could be used as therapeutic targets (Li and De Clercq, 2020). For example, soluble ACE2, TMPRSS2, and CTSL inhibitors have been demonstrated to have significant antiviral activity and might be used to treat COVID-19 (Ghanbari et al., 2020, Gordon et al., 2020, Hoffmann et al., 2020). Therefore, understanding the genomics of both virus and host is critical for developing and delivering therapeutics for COVID-19.
Table 3.
List of potential antiviral drugs for SARS-CoV-2 infection.
| Drug | Mechanism | References |
|---|---|---|
| Favipiravir | RdRp (RNA-dependent RNA polymerase) inhibitor | Driouich et al., 2021 |
| Lopinavir and Ritonavir | Mpro (main protease) inhibitors | Rut et al., 2020 |
| Mizoribine | IMPDH (Inosine-5′-monophosphate dehydrogenase) inhibitor | Borbone et al., 2021 |
| Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir | RdRp inhibitor | Beigel et al., 2020, Buckland et al., 2020, Elfiky, 2020 |
| Tocilizumab | IL-6 receptor inhibitor | Gupta and Leaf, 2021, Salama et al., 2021 |
6. Modern vaccine technologies
In order to combat the ongoing COVID-19 pandemic, an effective and safe vaccine is very important. The availability of genomic information together with advances in DNA synthesis and genome editing technologies enabled the researchers to develop modern vaccines such as RNA-based, DNA-based, protein subunit, and recombinant viral vector vaccines. According to the recent report by WHO, around 135 vaccine candidates were undergoing clinical trials, with 194 in preclinical development, as of December 3, 2021 (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines). Of the 135 candidates, 39 are undergoing phase 3 or phase 4 clinical trials (Table 4 ). At present, eight vaccines including three viral vector-based, two RNA-based, and three inactivated virus vaccines are approved for emergency use by WHO (Table 4). Supplementary Fig. S1 and Table S1 depict the relative progress of vaccine candidates in various phases of clinical development. The majority of the SARS-CoV-2 vaccine candidates (>70%) were developed through genomic methodologies.
Table 4.
List of vaccine candidates in phase 3 or 4 clinical trials.
| Vaccine platform | Vaccine name | Type of candidate vaccine | Developer/manufacturer | Clinical stage | Reference |
|---|---|---|---|---|---|
| DNA based vaccine | ZyCoV-D | Plasmid DNA Covid-19 vaccine | Zydus Cadila | Phase 3 | Momin et al., 2021 |
| INO-4800 COVID-19 Vaccine | Plasmid DNA Covid-19 vaccine | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | Phase 3 | Andrade et al., 2021 | |
| Inactivated virus | CoronaVac* | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Sinovac Biotech | Phase 4 | Tanriover et al., 2021 |
| WIBP-CorV | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products (WIBP) | Phase 3 | Al Kaabi et al., 2021 | |
| BBIBP-CorV* | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products (BIBP) | Phase 4 | Xia et al., 2021 | |
| Covidful or IMBCAMS COVID-19 vaccine | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Institute of Medical Biology (IMB) + Chinese Academy of Medical Sciences (CAMS) | Phase 3 | Huang et al., 2021 | |
| QazVac or QazCovid-in | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Research Institute for Biological Safety Problems, Kazakhstan | Phase 3 | Zakarya et al., 2021 | |
| Covaxin (BBV152)* | Whole-virion Inactivated SARS-CoV-2 Vaccine (Vero Cell) | Bharat Biotech International Limited + Indian Council of Medical Research (ICMR) | Phase 3 | Ganneru et al., 2021 | |
| KCONVAC or Minhai COVID-19 vaccine | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Shenzhen Kangtai Biological Products Co., Ltd. + Beijing Minhai Biotechnology | Phase 3 | https://en.biokangtai.com/ | |
| VLA2001 or Valneva COVID-19 vaccine | Inactivated SARS-CoV-2 vaccine, produced in Vero cells | Valneva, National Institute for Health Research, United Kingdom | Phase 3 | https://valneva.com/research-development/covid-19-vla2001/ | |
| ERUCOV-VAC or TURKOVAC | Inactivated SARS-CoV-2 vaccine (Vero cell) | Health Institutes of Turkey + Erciyes University. | Phase 3 | http://www.erciyes.edu.tr/ | |
| Protein subunit | NVX-CoV2373 | SARS-CoV-2 rS/Matrix M1-Adjuvant (Full-length recombinant SARS-CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M) | Novavax + Coalition for Epidemic Preparedness Innovations (CEPI) | Phase 3 | Heath et al., 2021 |
| ZIFIVAX or ZF2001 | Recombinant SARS-CoV-2 (CHO Cell) – RBD-based protein subunit vaccine | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Phase 3 | Yang et al., 2021 | |
| VAT00002 | SARS-CoV-2 S protein with adjuvant | Sanofi Pasteur + GSK | Phase 3 | https://www.sanofi.com/en/our-covid-19-vaccine-candidates | |
| SCB-2019 | Trimeric subunit Spike Protein vaccine + CpG 1018 adjuvant plus Alum adjuvant | Clover Biopharmaceuticals Inc./GSK/Dynavax | Phase 3 | Richmond et al., 2021 | |
| COVAX-19 (or SpikoGen) | Recombinant spike protein + adjuvant | Vaxine + CinnaGen Co. | Phase 3 | https://vaxine.net/ | |
| MVC-COV1901 | Spike-2P protein + adjuvant CpG 1018 | Medigen Vaccine Biologics Corporation + Dynavax Technologies + National Institute of Health | Phase 4 | Hsieh et al., 2021 | |
| FINLAY-FR-2 or Soberana 2 | RBD chemically conjugated to tetanus toxoid plus adjuvant | Instituto Finlay de Vacunas Cuba | Phase 3 | Chang-Monteagudo et al., 2021 | |
| EpiVacCorona | Based on peptide antigens | Federal Budgetary Research Institution (FBRI) State Research Center of Virology and Biotechnology VECTOR, | Phase 3 | Ryzhikov et al., 2021 | |
| Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | RBD (baculovirus production expressed in Sf9 cells) | West China Hospital + Sichuan University | Phase 3 | Meng et al., 2021 | |
| CIGB-66 (or Abdala) | RBD + aluminium hydroxide | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 3 | http://www.cigb.edu.cu/ | |
| BECOV2A (Corbevax) | RBD + aluminium hydroxide + CpG 1018 | Biological E. Limited | Phase 3 | https://www.biologicale.com/Vaccines_Biologics/products.html | |
| Nanocovax | Recombinant Sars-CoV-2 Spike protein, Aluminum adjuvanted | Nanogen Pharmaceutical Biotechnology JSC | Phase 3 | https://nanogenpharma.com/products/nanocovax-141.html | |
| GBP510 | Recombinant surface protein vaccine with adjuvant AS03 (Aluminium hydroxide) | SK Bioscience Co., Ltd. and Coalition for Epidemic Preparedness Innovations (CEPI) | Phase 3 | https://www.skbioscience.co.kr/ | |
| Razi Cov Pars | Recombinant spike protein | Iranian Razi Vaccine and Serum Research Institute | Phase 3 | http://www.rvsri.ac.ir/ | |
| RNA based vaccine | mRNA-1273 (Spikevax)* | Nucleoside-modified mRNA (modRNA) encoding a spike protein, encapsulated in lipid nanoparticles | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 | Baden et al., 2021 |
| BNT162b2/Comirnaty Tozinameran (INN)* |
Nucleoside-modified mRNA encapsulated in a lipid nanoparticle (LNP) | Pfizer/BioNTech + Fosun Pharma | Phase 4 | Haas et al., 2021 | |
| CVnCoV | Unmodified mRNA that encodes the full-length, pre-fusion stabilized coronavirus spike protein | CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI) | Phase 3 | https://www.curevac.com/en/covid-19/ | |
| ARCoV (Walvax COVID-19 vaccine) | Lipid nanoparticle (LNP)-encapsulated mRNA encoding the Receptor Binding Domain of SARS-CoV-2 | Walvax Biotechnology, Suzhou Abogen Biosciences, and PLA Academy of Military Science. | Phase 3 | Zhang et al., 2020b | |
| mRNA-1273.351 | LNP-encapsulated mRNA- vaccine encoding full-length, prefusion stabilized S protein of the SARS-CoV-2B.1.351 variant | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 | Choi et al., 2021 | |
| ARCT-154 (VBC-COV19-154) | Nucleoside-modified mRNA encapsulated in a lipid nanoparticle (LNP) | Vinbiocare Biotechnology + Arcturus Therapeutics, Inc. | Phase 3 | https://arcturusrx.com/mrna-medicines-pipeline/#pipelineGroup_lunarCovid | |
| Viral vector (Non-replicating) | AZD1222 (Vaxzevria)* Covishield* |
ChAdOx1 replication-deficient simian adenovirus vector, containing the full‐length codon-optimized coding sequence of SARS-CoV-2 spike protein | AstraZeneca + University of Oxford; Serum Institute of India |
Phase 4 | Knoll and Wonodi, 2021 |
| AD5-nCOV (Convidecia) | Recombinant novel coronavirus vaccine (Adenovirus type 5 vector) | CanSino Biological Inc./Beijing Institute of Biotechnology | Phase 4 | Zhu et al., 2020 | |
| Ad5-nCoV-IH (Convidecia) | Recombinant COVID-19 vaccine (adenovirus type 5 vector) for Inhalation (Ad5-nCoV-IH) | CanSino Biological Inc./Beijing Institute of Biotechnology | Phase 3 | Wu et al., 2021 | |
| Gam-COVID-Vac (Sputnik V) | Adenovirus viral vector vaccine – based on rAd26-S + rAd5-S | Gamaleya Research Institute, Health Ministry of the Russian Federation | Phase 3 | Logunov et al., 2021 | |
| Ad26.COV2.S (Janssen COVID-19 Vaccine)* | Recombinant adenovirus type 26 (Ad26) vector expressing SARS-CoV-2 spike (S) protein | Janssen Pharmaceuticals | Phase 4 | Alter et al., 2021 | |
| Viral vector (Replicating) | DelNS1-nCoV-RBD LAIV | Comprises weakened flu viruses, such as H1N1, H3N2, and B, with genetic segments of the S-protein (Intranasal flu-based-RBD) | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | Phase 3 | https://clinicaltrials.gov/ct2/show/NCT05200741 |
| Virus like particle | CoVLP | Plant-produced virus-like particle (VLP) vaccine | Medicago Inc. + GlaxoSmithKline (GSK) | Phase 3 | Ward et al., 2021 |
| Live attenuated virus | COVI-VAC (CDX-005) | Intranasal live attenuated vaccine | Codagenix/Serum Institute of India | Phase 3 | https://codagenix.com/vaccine-programs/covid-19/ |
World Health Organization (WHO) Emergency Use Authorization (EUA) qualified COVID-19 vaccines (as of December 3, 2021). (https://covid19.trackvaccines.org/agency/who/).
Advances in bioinformatics, immunogenetics, and molecular simulations led to more rapid, precise, and cost-effective designing of protein subunit/peptide vaccines. A technique called 'reverse vaccinology' uses the genome sequences of pathogens rather than organisms to develop novel antigens, which need to be tested by using experimental biology (Bambini and Rappuoli, 2009). Pan-genomic reverse vaccinology, which involves the comparison of genomic data from different strains of SARS-CoV-2, enhances the opportunity of developing novel vaccines (Enayatkhani et al., 2020). Novel epitopes in proteins encoded in the genomes can be predicted in-silico using bioinformatics/immunoinformatics tools based on sequence similarities to previously reported immunogenic motifs or structural approaches such as molecular docking simulations (Ishack and Lipner, 2021). As the genomic and proteomic information of SARS-CoV-2 is rapidly becoming accessible, numerous studies applied reverse vaccinology and machine learning approaches to develop multi-epitope subunit vaccines against SARS-CoV-2 (Enayatkhani et al., 2020, Ong et al., 2020, Sanami et al., 2020, Tahir ul Qamar, 2020, Almofti et al., 2021, Saha et al., 2021). Recombinant protein vaccines against SARS-CoV-2 include spike-protein-based, RBD-based, and virus-like particle (VLP)-based vaccines (Krammer, 2020) and 53 of them are in the clinical phase. For example, Novavax's NVX-CoV2373 vaccine is made up of full-length recombinant SARS-CoV-2 spike glycoproteins nanoparticles that have been adjuvanted with Matrix-M1 (Keech et al., 2020). Despite the widespread occurrence of the B.1.1.7 (or alpha) variant, preliminary findings of phase 3 clinical trial in the UK showed an efficacy rate of 86.3% against the alpha variant and 96.4% efficacy against non-alpha variants (Heath et al., 2021). The majority of the recombinant protein subunit vaccines against SARS-CoV-2 have entered the phase 3 clinical trials (Table 4).
Nucleic acid vaccines either RNA or DNA deliver the genetic information of antigen (such as spike glycoprotein) rather than the antigen itself. 15 DNA-based and 21 mRNA-based candidate vaccines against SARS-CoV-2 are in clinical trials. Due to the ease of handling, simple manufacture, and stability of plasmid DNA, DNA-based vaccination methods have become a reality. Of the eleven DNA-based vaccines, only two vaccines, ZyCoV-D (developed by Zydus Cadila) and INO-4800 (by Inovio Pharmaceuticals) have undergone phase 3 clinical trials. ZyCoV-D uses plasmid DNA that contains the genetic information to make the ‘spike protein’ (Momin et al., 2021). It is the world’s first plasmid DNA vaccine for COVID-19 to be approved for emergency use (Mallapaty, 2021). Next to protein vaccines, the majority of the vaccine candidates are mRNA-based which accounts for 16% of all vaccines developed across platforms (Supplementary Table S1). Two mRNA vaccines, Pfizer-BioNTech’s BNT162b2 (Comirnaty) and Moderna’s mRNA-1273 (Spikevax) were the first to be authorized for use in many countries (Baden et al., 2021, Haas et al., 2021). These vaccines use nucleoside-modified mRNA (modRNA) encoding SARS-CoV-2 spike protein that is encapsulated in lipid nanoparticles (LNP). Other mRNA vaccines, such as CVnCoV (developed by CureVac and CEPI) and ARCoV (developed by Walvax Biotechnology) are in phase 3 clinical trials (Table 4). The mRNA vaccines provide a variety of advantages over other vaccine platforms, including efficient delivery, flexibility, short development time, use of the host's protein translational machinery, and no risk of genome integration (Momin et al., 2021). The era of synthetic genomics led to the development of viral vector-based vaccines that deliver antigen-coding nucleic acid fragments to host cells through viral vectors. Viruses are altered to lower their virulence and their reproduction capability but retaining their ability to infect human cells (Alter et al., 2021). At present, four non-replicating adenovirus-vector vaccines such as Oxford/AstraZeneca’s AZD1222, Janssen’s Ad26.COV2.S, CanSino’s AD5-nCOV (Convidecia), Gamaleya Research Institute’s Gam-COVID-Vac (Sputnik V) are now in widespread use (Table 4). All these contain DNA that encodes a SARS‑CoV‑2 spike protein.
7. Conclusion
The COVID-19 pandemic startled the globe, pushing science to develop new strategies to combat the virus. The availability of genomic data enables a very rapid, thorough, and precise global follow-up of the progression of the COVID-19. Early detection of SARS-CoV-2 variants, along with a better knowledge of the mutational processes behind shifting patterns of virulence, transmissibility, and antigenicity, have greatly aided in making timely public health decisions. It is critical to emphasize that genomic information must be utilized with caution while taking public health decisions. The use of improper bioinformatics tools, sampling bias, sequencing errors, and misinterpretation of findings may all lead to wrong conclusions. The genomic sequence of SARS-CoV-2 also enabled the cloning and synthesis of specific viral proteins, which aided in the development of rapid diagnostic tests for SARS-CoV-2 screening. In order to understand SARS-CoV-2 variant spread in different countries, it is essential to integrate genomic and epidemiological data. The increased adoption of genomic technologies in various facets of the worldwide response to the COVID-19 pandemic is major evidence of the role of genomics in modern medicine. Furthermore, the tremendous advances in genomics and lessons learnt from the battle against SARS-CoV-2 offer a great potential to reduce the future threats to mankind and bolster preparedness for future outbreaks.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All the authors of the manuscript wish to thank Director and Joint Director (Research), ICAR-Indian Veterinary Research Institute (IVRI), Izatnagar, an institute under the Indian Council of Agricultural Research (ICAR), Department of Agricultural Research and Education (DARE), Government of India for their support and providing infrastructural facility to review this study.
Edited by John Doe
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gene.2022.146387.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abbott T.R., Dhamdhere G., Liu Y., Lin X., Goudy L., Zeng L., et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell. 2020;181:865–876.e12. doi: 10.1016/j.cell.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. JAMA. 2021;326:35. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almofti Y.A., Abd-elrahman K.A., Eltilib E.E.M. Vaccinomic approach for novel multi epitopes vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) BMC Immunol. 2021;22 doi: 10.1186/s12865-021-00412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G., Yu J., Liu J., Chandrashekar A., Borducchi E.N., Tostanoski L.H., et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596:268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade V.M., Christensen-Quick A., Agnes J., Tur J., Reed C., Kalia R., et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell activity against global SARS-CoV-2 variants. npj Vaccines. 2021;6:121. doi: 10.1038/s41541-021-00384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli C.A. Could mutations of SARS-CoV-2 suppress diagnostic detection? Nat. Biotechnol. 2021;39:274–275. doi: 10.1038/s41587-021-00845-3. [DOI] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggen J., Persoons L., Vanstreels E., Jansen S., Van Looveren D., Boeckx B., et al. Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2. Nat. Genet. 2021;53:435–444. doi: 10.1038/s41588-021-00805-2. [DOI] [PubMed] [Google Scholar]
- Bambini S., Rappuoli R. The use of genomics in microbial vaccine development. Drug Discov. 2009;14:252–260. doi: 10.1016/j.drudis.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoy D.J.D.R., Weimer B.C. Analysis of SARS-CoV-2 genomic epidemiology reveals disease transmission coupled to variant emergence and allelic variation. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-86265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu S., Jolly B., Mukherjee P., Singh P., Khan S., Zaveri L., et al. A Distinct Phylogenetic Cluster of Indian Severe Acute Respiratory Syndrome Coronavirus 2 Isolates. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the Treatment of Covid-19 — Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berber B., Aydin C., Kocabas F., Guney-Esken G., Yilancioglu K., Karadag-Alpaslan M., et al. Gene editing and RNAi approaches for COVID-19 diagnostics and therapeutics. Gene Ther. 2020;28:290–305. doi: 10.1038/s41434-020-00209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P.R., Scaiola A., Loughran G., Leibundgut M., Kratzel A., Meurs R., et al. Structural basis of ribosomal frameshifting during translation of the SARS-CoV-2 RNA genome. Science. 2021;372(6548):1306–1313. doi: 10.1126/science.abf3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T.Y., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Borbone N., Piccialli G., Roviello G.N., Oliviero G. Nucleoside Analogs and Nucleoside Precursors as Drugs in the Fight against SARS-CoV-2 and Other Coronaviruses. Molecules. 2021;26:986. doi: 10.3390/molecules26040986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousali M., Dimadi A., Kostaki E.G., Tsiodras S., Nikolopoulos G.K., Sgouras D.N., et al. SARS-CoV-2 Molecular Transmission Clusters and Containment Measures in Ten European Regions during the First Pandemic Wave. Life. 2021;11:219. doi: 10.3390/life11030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland M.S., Galloway J.B., Fhogartaigh C.N., Meredith L., Provine N.M., Bloor S., et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukin Y.S., Bondaryuk A.N., Kulakova N.V., Balakhonov S.V., Dzhioev Y.P., Zlobin V.I. Phylogenetic reconstruction of the initial stages of the spread of the SARS-CoV-2 virus in the Eurasian and American continents by analyzing genomic data. Virus Res. 2021;305 doi: 10.1016/j.virusres.2021.198551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S.K., Bhikadiya C., Bi C., Bittrich S., Chen L., Crichlow G.V., et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49(D1):D437–D451. doi: 10.1093/nar/gkaa1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana G., Croxatto A., Coste A.T., Opota O., Lamoth F., Jaton K., Greub G. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin. Microbiol. Infect. 2020;26:1178–1182. doi: 10.1016/j.cmi.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.-F.-W., Kok K.-H., Zhu Z., Chu H., To K.-K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Monteagudo A., Ochoa-Azze R., Climent-Ruiz Y., Macías-Abraham C., Rodríguez-Noda L., Valenzuela-Silva C., et al. A single dose of SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg. Health Am. 2021;4 doi: 10.1016/j.lana.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellapandi P., Saranya S. Genomics insights of SARS-CoV-2 (COVID-19) into target-based drug discovery. Med. Chem. Res. 2020;29:1777–1791. doi: 10.1007/s00044-020-02610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Feng P., Liu K., Wu M., Lin H. Computational Identification of Small Interfering RNA Targets in SARS-CoV-2. Virol. Sin. 2020;35:359–361. doi: 10.1007/s12250-020-00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 2021;27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.es.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Alarming COVID variants show vital role of genomic surveillance. Nature. 2021;589:337–338. doi: 10.1038/d41586-021-00065-4. [DOI] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Gu W., Federman S., du Plessis L., Pybus O.G., Faria N.R., et al. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into Northern California. Science. 2020;369:582–587. doi: 10.1126/science.abb9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva N.H., Bhai J., Chakiachvili M., Contreras-Moreira B., Cummins C., Frankish A., et al. The Ensembl COVID-19 resource: ongoing integration of public SARS-CoV-2 data. Nucleic Acids Res. 2021;50(D1):D765–D770. doi: 10.1093/nar/gkab889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich J.S., Cochin M., Lingas G., Moureau G., Touret F., Petit P.R., et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayatkhani M., Hasaniazad M., Faezi S., Gouklani H., Davoodian P., Ahmadi N., Einakian M.A., Karmostaji A., Ahmadi K. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J. Biomol. Struct. Dyn. 2020;39:2857–2872. doi: 10.1080/07391102.2020.1756411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauver J.R., Petrone M.E., Hodcroft E.B., Shioda K., Ehrlich H.Y., Watts A.G., et al. Coast-to-Coast Spread of SARS-CoV-2 during the Early Epidemic in the United States. Cell. 2020;181:990–996.e5. doi: 10.1016/j.cell.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganneru B., Jogdand H., Daram V.K., Das D., Molugu N.R., Prasad S.D., et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. iScience. 2021;24 doi: 10.1016/j.isci.2021.102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geidelberg L., Boyd O., Jorgensen D., Siveroni I., Nascimento F.F., Johnson R., et al. Genomic epidemiology of a densely sampled COVID-19 outbreak in China. Virus Evol. 2021;7 doi: 10.1093/ve/veaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari R., Teimoori A., Sadeghi A., Mohamadkhani A., Rezasoltani S., Asadi E., Jouyban A., Sumner S.C. Existing antiviral options against SARS-CoV-2 replication in COVID-19 patients. Future Microbiol. 2020;15:1747–1758. doi: 10.2217/fmb-2020-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z., Zhu J.W., Li C.P., Jiang S., Ma L.N., Tang B.X., et al. An online coronavirus analysis platform from the National Genomics Data Center. Zool Res. 2020;41(6):705–708. doi: 10.24272/j.issn.2095-8137.2020.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowthaman R., Guest J.D., Yin R., Adolf-Bryfogle J., Schief W.R., Pierce B.G. CoV3D: a database of high resolution coronavirus protein structures. Nucleic Acids Res. 2021;49(D1):D282–D287. doi: 10.1093/nar/gkaa731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Leaf D.E. Tocilizumab in COVID-19: some clarity amid controversy. Lancet. 2021;397:1599–1601. doi: 10.1016/s0140-6736(21)00712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/s0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P.W., Lopez R., Rahman N., Allen S.G., Aslam R., Buso N., et al. The COVID-19 Data Portal: accelerating SARS-CoV-2 and COVID-19 research through rapid open access data sharing. Nucleic Acids Res. 2021;49(W1):W619–W623. doi: 10.1093/nar/gkab417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021 doi: 10.1056/nejmoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S.M., Liu W.D., Huang Y.S., Lin Y.J., Hsieh E.F., Lian W.C., et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Jiang Q., Wang Y., Yang J., Du T., Yi H., et al. SARS-CoV-2 inactivated vaccine (Vero cells) shows good safety in repeated administration toxicity test of Sprague Dawley rats. Food Chem. Toxicol. 2021;152 doi: 10.1016/j.fct.2021.112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishack S., Lipner S.R. Bioinformatics and immunoinformatics to support COVID-19 vaccine development. J. Med. Virol. 2021;93:5209–5211. doi: 10.1002/jmv.27017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige T., Murata S., Taniguchi T., Miyabe A., Kitamura K., Kawasaki K., Nishimura M., Igari H., Matsushita K. Highly sensitive detection of SARS-CoV-2 RNA by multiplex rRT-PCR for molecular diagnosis of COVID-19 by clinical laboratories. Clin. Chim. Acta. 2020;507:139–142. doi: 10.1016/j.cca.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Rophina M., Mahajan S., Krishnan B.B., Sharma M., Mandal S., et al. Analysis of the potential impact of genomic variants in global SARS-CoV-2 genomes on molecular diagnostic assays. Int. J. Infect. Dis. 2021;102:460–462. doi: 10.1016/j.ijid.2020.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joonlasak K., Batty E.M., Kochakarn T., Panthan B., Kümpornsin K., Jiaranai P., et al. Genomic surveillance of SARS-CoV-2 in Thailand reveals mixed imported populations, a local lineage expansion and a virus with truncated ORF7a. Virus Res. 2021;292 doi: 10.1016/j.virusres.2020.198233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S.R., Spratt A.N., Cohen A.R., Naqvi S.H., Chand H.S., Quinn T.P., Lorson C.L., Byrareddy S.N., Singh K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021;124 doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/nejmoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.A., Cheung P. Presence of mismatches between diagnostic PCR assays and coronavirus SARS-CoV-2 genome. R. Soc. Open Sci. 2020;7 doi: 10.1098/rsos.200636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Gurry C., Freitas L., Schultz M.B., Bach G., Diallo A., et al. GISAID’s Role in Pandemic Response. China CDC Wkly. 2021;3(49):1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/s0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings F., Perkins M.D., Kuhn J.H., Pallen M.J., Alm E.J., Archer B.N., et al. SARS-CoV-2 Variants of Interest and Concern naming scheme conducive for global discourse. Nat. Microbiol. 2021;6:821–823. doi: 10.1038/s41564-021-00932-w. [DOI] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Kumar P., Malik Y.S., Ganesh B., Rahangdale S., Saurabh S., Natesan S., et al. CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.576875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H.H., Jiang J.F., Zhu H.C., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jiang J.-Z., Wan X.-F., Hua Y., Li L., Zhou J., Wang X., Hou F., Chen J., Zou J., Chen J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.W., Jamrozy D. Genomics and epidemiological surveillance. Nat. Rev. Microbiol. 2020;18:478. doi: 10.1038/s41579-020-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/s0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokman S.M., Rasheduzzaman M.d., Salauddin A., Barua R., Tanzina A.Y., Rumi M.H., et al. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein: A computational biology approach. Infect. Genet. Evol. 2020;84 doi: 10.1016/j.meegid.2020.104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. India’s DNA COVID vaccine is a world first – more are coming. Nature. 2021;597:161–162. doi: 10.1038/d41586-021-02385-x. [DOI] [PubMed] [Google Scholar]
- McKusick V.A., Ruddle F.H. A new discipline, a new name, a new journal. Genomics. 1987;1:1–2. doi: 10.1016/0888-7543(87)90098-x. [DOI] [Google Scholar]
- Meng F.Y., Gao F., Jia S.Y., Wu X.H., Li J.X., Guo X.L., et al. Safety and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials. Signal Transduct. Target. Ther. 2021;6 doi: 10.1038/s41392-021-00692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T.R., Salit M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021;22:415–426. doi: 10.1038/s41576-021-00360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D., Martin M.A., Harel N., Tirosh O., Kustin T., Meir M., et al. Full genome viral sequences inform patterns of SARS-CoV-2 spread into and within Israel. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin T., Kansagra K., Patel H., Sharma S., Sharma B., Patel J., et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik B., Gupta N., Ojha R., Singh S., Prajapati V.K., Prusty D. High throughput virtual screening reveals SARS-CoV-2 multi-target binding natural compounds to lead instant therapy for COVID-19 treatment. Int. J. Biol. Macromol. 2020;160:1–17. doi: 10.1016/j.ijbiomac.2020.05.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.M., Zhang Y., Pandolfi P.P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020;30:189–190. doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S.M., Marshall N., Hamilton A.B., Yano E.M., Lerner B., Scheuner M.T. Assessing multilevel determinants of adoption and implementation of genomic medicine: an organizational mixed-methods approach. Genet. Med. 2015;17:919–926. doi: 10.1038/gim.2015.7. [DOI] [PubMed] [Google Scholar]
- Okido T., Kodama Y., Mashima J., Kosuge T., Fujisawa T., Ogasawara O. DNA Data Bank of Japan (DDBJ) update report 2021. Nucleic Acids Res. 2021;50(D1):D102–D105. doi: 10.1093/nar/gkab995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong E., Wong M.U., Huffman A., He Y. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Nieuwenhuijse D.F., Stein M., O’Toole Á., Haverkate M., Mollers M., et al. Rapid SARS-CoV-2 whole-genome sequencing and analysis for informed public health decision-making in the Netherlands. Nat. Med. 2020;26:1405–1410. doi: 10.1038/s41591-020-0997-y. [DOI] [PubMed] [Google Scholar]
- Pan B., Ji Z., Sakkiah S., Guo W., Liu J., Patterson T.A., Hong H. Identification of Epidemiological Traits by Analysis of SARS−CoV−2 Sequences. Viruses. 2021;13:764. doi: 10.3390/v13050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Shen L., Xu J., Tian X., Liu F., Wang J., Tian G., Yang J., Zhou L. Prioritizing antiviral drugs against SARS-CoV-2 by integrating viral complete genome sequences and drug chemical structures. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-83737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond P., Hatchuel L., Dong M., Ma B., Hu B., Smolenov I., Li P., Liang P., Han H.H., Liang J., Clemens R. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/s0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett R.J., Arnott A., Lam C., Sadsad R., Timms V., Gray K.A., et al. Revealing COVID-19 transmission in Australia by SARS-CoV-2 genome sequencing and agent-based modeling. Nat. Med. 2020;26:1398–1404. doi: 10.1038/s41591-020-1000-7. [DOI] [PubMed] [Google Scholar]
- Romero P.E., Sánchez-Yupari M., Montero S., Tsukayama P. Uso de genomas de SARS-CoV-2 para la estimación del número reproductivo efectivo (Rt) en Perú durante marzo y abril del 2020. Rev. Peru. Med. Exp. Salud Publica. 2021;38:267–271. doi: 10.17843/rpmesp.2021.382.6417. [DOI] [PubMed] [Google Scholar]
- Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA Approval of Remdesivir—A Step in the Right Direction. N. Engl. J. Med. 2020;383:2598–2600. doi: 10.1056/nejmp2032369. [DOI] [PubMed] [Google Scholar]
- Rut W., Groborz K., Zhang L., Sun X., Zmudzinski M., Pawlik B., et al. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2020;17:222–228. doi: 10.1038/s41589-020-00689-z. [DOI] [PubMed] [Google Scholar]
- Ryzhikov, A.B., Ryzhikov, E.A., Bogryantseva, M.P., Usova, S.V., Danilenko, E.D., Nechaeva, E.A., et al., 2021. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the “EpiVacCorona” Vaccine for the prevention of COVID-19, in volunteers aged 18–60 years (phase I–II). iimm 11, 283–296. 10.15789/2220-7619-asb-1699.
- Saha, R., Ghosh, P., Burra, V.L.S.P., 2021. Designing a next generation multi-epitope based peptide vaccine candidate against SARS-CoV-2 using computational approaches. 3 Biotech 11. 10.1007/s13205-020-02574-x. [DOI] [PMC free article] [PubMed]
- Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:20–30. doi: 10.1056/nejmoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanami S., Zandi M., Pourhossein B., Mobini G.-R., Safaei M., Abed A., Arvejeh P.M., Chermahini F.A., Alizadeh M. Design of a multi-epitope vaccine against SARS-CoV-2 using immunoinformatics approach. Int. J. Biol. Macromol. 2020;164:871–883. doi: 10.1016/j.ijbiomac.2020.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers E.W., Cavanaugh M., Clark K., Pruitt K.D., Schoch C.L., Sherry S.T., Karsch-Mizrachi I. GenBank. Nucleic Acids Res. 2022;50(D1):D161–D164. doi: 10.1093/nar/gkab1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T., Lane C.R., Sherry N.L., Duchene S., Gonçalves da Silva A., Caly L., et al. Tracking the COVID-19 pandemic in Australia using genomics. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, M., Zhou, Y., Ye, J., Abdullah AL-maskri, A.A., Kang, Y., Zeng, S., Cai, S., 2020. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 10, 97–101. 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed]
- Shu Y., McCauley J. GISAID: Global initiative on sharing all influenza data – from vision to reality. Eurosurveillance. 2017;22 doi: 10.2807/1560-7917.es.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Ma L., Zou D., Tian D., Li C., Zhu J., et al. The Global Landscape of SARS-CoV-2 Genomes, Variants, and Haplotypes in 2019nCoVR. Genom. Proteom. Bioinf. 2020;18(6):749–759. doi: 10.1016/j.gpb.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir ul Qamar, M., Shahid, F., Aslam, Sadia, Ashfaq, U.A., Aslam, Sidra, Fatima, I., Fareed, M.M., Zohaib, A., Chen, L.-L., 2020. Reverse vaccinology assisted designing of multiepitope-based subunit vaccine against SARS-CoV-2. Infect. Dis. Poverty 9. 10.1186/s40249-020-00752-w. [DOI] [PMC free article] [PubMed]
- Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/s0140-6736(21)01429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., Corman V.M. Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V’kovski, P., Kratzel, A., Steiner, S., Stalder, H., Thiel, V., 2020. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170. 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed]
- Varadi M., Anyango S., Armstrong D., Berrisford J., Choudhary P., Deshpande M., et al. PDBe-KB: collaboratively defining the biological context of structural data. Nucleic Acids Res. 2022;50(D1):D534–D542. doi: 10.1093/nar/gkab988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L., et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharapluesadee S., Tan C.W., Maneeorn P., Duengkae P., Zhu F., Joyjinda Y., et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Jiang A., Feng J., Li G., Guo D., Sajid M., et al. The SARS-CoV-2 subgenome landscape and its novel regulatory features. Mol. Cell. 2021;81:2135–2147.e5. doi: 10.1016/j.molcel.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li X., Li T., Zhang S., Wang L., Wu X., Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1629–1635. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.Y., et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Alfajaro M.M., DeWeirdt P.C., Hanna R.E., Lu-Culligan W.J., Cai W.L., et al. Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell. 2021;184:76–91.e13. doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021;1(12):1654–1664. doi: 10.1016/s1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/s1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.J., et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- Yang S., Li Y., Dai L., Wang J., He P., Li C., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021;21:1107–1119. doi: 10.1016/s1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarya K., Kutumbetov L., Orynbayev M., Abduraimov Y., Sultankulova K., Kassenov M., et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine. 2021:101078. doi: 10.1016/j.eclinm.2021.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenova M., Ivanova A., Semyonov S., Gankin Y. Analysis of 329,942 SARS-CoV-2 records retrieved from GISAID database. Comput. Biol. Med. 2021;139 doi: 10.1016/j.compbiomed.2021.104981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang L., Jackson C.B., Mou H., Ojha A., Peng H., Quinlan B.D., et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., Yang G., et al. A Thermostable mRNA Vaccine against COVID-19. Cell. 2020;182:1271–1283.e16. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.M., Song S.H., Chen M.L., Zou D., Ma L.N., Ma Y.K., et al. The 2019 novel coronavirus resource. Yi Chuan. 2020;42(2):212–221. doi: 10.16288/j.yczz.20-030. [DOI] [PubMed] [Google Scholar]
- Zhou L., Wang J., Liu G., Lu Q., Dong R., Tian G., Yang J., Peng L. Probing antiviral drugs against SARS-CoV-2 through virus-drug association prediction based on the KATZ method. Genomics. 2020;112:4427–4434. doi: 10.1016/j.ygeno.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/s0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.