Version Changes
Revised. Amendments from Version 1
There are several changes from version 1:
Add reference metal test standards in the Methods (The standard reference used for the analysis of Pb metal is SNI 6989.8:2009, Cd metal SNI 06-6989.16-2004, Cu metal refers to the SNI 6989.6:2009 standard, Fe heavy metal refers to SNI 06-6989.4-2004, and Zn metal refers to measurement standard according to SNI 06-6989.7-2004)
Add PCa and Correlation Test on Statistical analysis (In this study, the PCa test and the correlation between heavy metals contained in water, Caulerpa, and sediment were carried out with water quality parameters)
Additional citations for the acid digestion used in this study (V. van Ginneken and E. de Vries, Seaweeds as Biomonitoring System for Heavy Metal (HM) Accumulation and Contamination of Our Oceans, Am. J. Plant Sci. 09 (2018), pp. 1514–1530)
Changes in data presentation from Table 3-5 to Figure 3-5.
Pca analysis includes the scatterplot (the addition of PCa and Correlation of heavy metal is shown in Figure 6 and Table 4-6).
Addition of the water analysis and sediment analysis.
Addition of the detailed digestion method provided.
Addition of The conclusion water and sediment
Added references (44, 59, and 60).
44 Ginneken VV, de Vries E: Seaweeds as Biomonitoring System for Heavy Metal (HM) Accumulation and Contamination of Our Oceans. Am. J. Plant Sci. 2018;9:1514–1530.44 59 Puspitasari, R. Logam dalam Ekosiste Perairan. Bawal. 2006;1: 1-6. 60 Tampubolon OFR, Ismanto A, Suryo AAD, Muslin, Indrayanti E. Simulasi Pola Sebaran Logam Berat Tembaga (Cu) di Perairan Kota Pekalongan. Indonesian Journal of Oceanography.2021;3:1-15.
Abstract
Background: Heavy metals are materials naturally occurring in nature and increase with a rise in human activity. Ex-mining areas and domestic waste from human settlements are sources of heavy metal contamination that enter and pollute water, which then accumulates in various organisms including the Caulerpa racemosa community. The accumulation of heavy metals in C. racemosa has a wide impact on the food chain in aquatic ecosystems and humans because this alga is a consumptive commodity.
Methods: Sampling of C. racemosa was carried out at seven sites on Bintan Island, Indonesia covering the eastern (Teluk Bakau, Beralas Pasir, Malang Rapat), northern (Berakit and Pengudang), western (Sakera), and southern parts (Tg. Siambang). Sampling was carried out during different monsoons, and heavy metals in water and sediment samples were measured to determine the heavy metal concentration. Heavy metals were analyzed by a spectrophotometric method using Atomic Absorption Spectrophotometry.
Results: The results showed that heavy metal concentrations fluctuate according to changes in the wind season, which carry currents and spread pollutants into the water. The concentration of metal in the water is also from anthropogenic activities. The heavy metal content of cadmium (Cd), lead (Pb), copper (Cu), iron (Fe), and zinc (Zn) in C. racemosa is high in locations close to settlements. Meanwhile, in seawater samples, Fe and Zn metals have the highest concentrations compared to others.
Conclusions: Ex-bauxite mines are a source of Fe and Zn metal contamination in the environment, especially at Tg. Siambang. The levels of these heavy metals in the sediment are also high, as surface particle deposits accumulate at the bottom of the sediment. In general, the levels of heavy metals Cd, Pb, Cu, Fe, and Zn increase in the northern monsoon because the dynamics of the water transport greater heavy metal pollution.
Keywords: Heavy metal, Caulerpa racemosa, Bintan Island, Atomic Absorption Spectrophotometry
Introduction
Heavy metals are materials naturally contained in nature. These metals increase along with a rise in human activity and become a threat to environmental pollution. The increase in heavy metals is caused by mining, 1 agriculture and industry 2 activities, as well as anthropogenic processes from residential and household domestic waste. 3, 4 Therefore, increased coastal activity leads to a rise in the content of these metals up to the quality standard threshold. 5
Heavy metal particles that accumulate in the environment affect aquatic ecosystems. The contamination is distributed in the water column and accumulates in organisms over a prolonged period, affecting the food chain. 5 Briffa et al. (2020) stated that heavy metal contamination in organisms affect biological functions and cause various toxic effects in the long term. 6 According to Liu et al. (2020) and Apiratikul et al. (2004), these metals are generally dominated by cadmium (Cd), lead (Pb), copper (Cu), iron (Fe), and zinc (Zn). 1, 7– 9
Cadmium (Cd) and lead (Pb) are non-essential heavy metals whose function in the human body are unknown. These metals are categorized as carcinogenic (or mixed) agents for humans by the International Agency for Research on Cancer. Exposure to Cd and Pb causes cancer or infection in human organs such as the urinary tract, reproductive tract, central nervous system, respiratory system, 10, 11 they cause kidney problems, and increase blood pressure. 11 The content of these heavy metals are measured in blood, urine, hair, nail, and saliva samples and are characterized by low levels of excretion. 12 Furthermore, excess exposure to Cd and Pb is lethal when entering mammalian cells and they accumulate high concentrations in the cytoplasmic space and nucleus. 13, 14 The maximum limits of Cd and Pb metals in the human body are 0.005 mg/L and 0.015 mg/L, respectively. 15 Therefore, due to the high level of toxicity of heavy metals, serious monitoring of the accumulation of Cd and Pb is needed.
Meanwhile, copper (Cu), zinc (Zn), and iron (Fe) are essential heavy metals, with a certain amount needed in the human body. However, when the amount exceeds the maximum limit, it becomes poisonous and very dangerous to health. Cu is considered a carcinogen metal because it causes damage to DNA. Meanwhile, Cu 2+ interacts with lipid hydroperoxides to form malondialdehyde and 4-hydroxynonenal, which are considered carcinogens and cause DNA and tissue damage. 16, 17 Excessive accumulation of Zn metal causes depletion of Cu 2+ in cells and decreases superoxide dismutase (SOD) and cytochrome C oxidase levels by increasing cholesterol levels. Furthermore, it causes cardiac dysfunction with impaired iron mobilization, inhibits the enzymes SOD, peroxidase, and catalase, thereby rapidly increasing the concentration of superoxide free radicals and oxidative stress. Excess Zn also causes severe damage to the cell wall and DNA while increasing gene mutations. 18 Fe metal is also one of the important elements in the body with a tightly controlled concentration. Excess Fe is caused by several conditions such as frequent transfusions, exploitation of iron consumption (as a supplement), and chronic hepatitis. It triggers the formation of free radicals, thereby causing severe complications such as mental retardation, Alzheimer's, multiple sclerosis, reproductive system dysfunction, heart problems, liver cirrhosis, liver cancer, hepatitis, and metabolic dysfunction. 19 The maximum limits for the essential heavy metals Cu, Zn, and Fe in the human body are 1.3 mg/L, 3 mg/L, 0.2 mg/L, respectively. 15 Therefore, it is necessary to monitor the concentration of these heavy metals to prevent them from passing the accumulated threshold.
Aquatic ecosystems consist of various types of biota including the macroalgae community such as Caulerpa racemosa. Heavy metal pollution in the aquatic environment also impacts the accumulation of these metals in macroalgae. 7 Caulerpa sp. is a type of algae that is able to absorb Cd, Pb, Cu, Fe and Zn metals in water. 20 The absorption ability of Pb, 21 Cd 30 g/L, 22 Zn and Cu 10 mg/L, 23 and Fe 7.63 mg/L 24 in water is 0.35 mg/kg. This proves that C. racemosa has the ability to bioaccumulate heavy metals in marine waters.
On the other hand, C. racemosa has consumptive benefits for coastal communities, especially as a traditional food ingredient. Khandaker et al. (2021) stated that the accumulation of heavy metals in these organisms have the potential to cause various adverse health effects. 25 The content of heavy metals contained in C. racemosa in Bintan Island, Indonesia waters is unknown, therefore, it is important to carry out this research in order to determine the impact of its exposure to surrounding communities.
Methods
Data were collected from seven sites on Bintan Island, Indonesia covering the eastern (Teluk Bakau, Beralas Pasir, Malang Rapat), the northern (Berakit and Pengudang), the western (Sakera), and the southern part (Tg. Siambang). In addition, data was also taken based on seasonal differences to determine the effect of seasonal changes on the existing conditions of C. racemosa on the coast of Bintan Island. The difference in seasons is taken to refer to the difference in the direction of the monsoon, namely the northerly, easterly, southerly and westerly monsoon. Figure 1 shows the distribution of research sites on Bintan Island.
Figure 1.
Map of research locations and field data collection, This figure has been reproduced with permission from Apriadi et al. (2018). 58
Sampling of C. racemosa was carried out using a scuba set (Amscud), 50 × 50 cm 2 transect plot, underwater camera (Canon D30 82 '/25m rated depth), GPS (Garmin GPSMAP 78S), Oven (Memmert UN 55), analytical scales (Kern ABJ 220, accuracy level of 0.001 g), Van Dorn sampler, sediment shovel, mortar, aluminium foil, plastic samples, label paper, and boxes. Heavy metal levels were tested by the wet digestion method using a solution of nitrite acid (HNO 3), sulfuric acid (H 2SO 4), hydrogen peroxide (H 2O 2), and hydrochloric acid (HCl). 20 Samples were analyzed using Atomic Abstraction Spectrophotometry (AAS) using the Shimadzu AA-7000. 26 The standard reference used for the analysis of Pb is SNI 6989.8:2009, Cd SNI 06-6989.16-2004, Cu refers to the SNI 6989.6:2009 standard, Fe heavy metal refers to SNI 06-6989.4-2004, and Zn to measurement standard according to SNI 06-6989.7-2004.
Caulerpa racemosa sampling
The sample preparation process was carried out by weighing 5 grams of the dry weight of the C. racemosa sample, which had previously been cleaned and dried in an oven at 60°C for 24 hours. 27, 28 The sample was then crushed with a mortar until homogenous before adding a HNO 3 solution and 20 ml of HCl. Furthermore, the solution was placed in a water heater until it reacted and was digested. It was then filtered with Whatman 42 filter paper to determine the readings of Cd, Pb, Cu, Fe, Zn on the AAS tool.
Water and sediment sampling
Water and sediment sampling for heavy metal analysis refers to the digestion method. 29 Approximately three liters of seawater sample were taken with a Van Dorn sampler and then concentrated by baking at 80°C until the volume reduced to 50 ml. Furthermore, 4 ml of H 2SO 4, and 10 ml of H 2O 2 were added to the sample and heated until the oxidation was complete. After cooling the sample, it was filtered with 0.45 μm Whatman 42 filter paper and distilled water was added until it reached a volume of 50 ml.
The sediment taken in the field was weighed, and as much as 30 g was dried in an oven at 105°C. In addition, approximately 1 g of the sediment was added to 5 ml of HNO 3 and heated at 80°C until the volume changed (20–30 ml). The process of adding HNO 3 was repeated and it was reheated, after which it was filtered with 0.45 μm Whatman filter paper and distilled water was added until it reached a volume of 50 ml.
Statistical analysis
The results of the metal content readings of Cd, Pb, Cu, Fe, Zn were analyzed using IBM SPSS Statistics 22 software with one-way Anova analysis (p <0.05) to determine the concentration of heavy metals at each site based on the season. Furthermore, the level of correlation between metals was also analyzed to determine the influence of each metal being measured. In addition, in this study, PCA tests were carried out and the correlation between heavy metals contained in water, Caulerpa, and sediments with water quality parameters.
Results and discussion
Heavy metals in Caulerpa racemosa samples
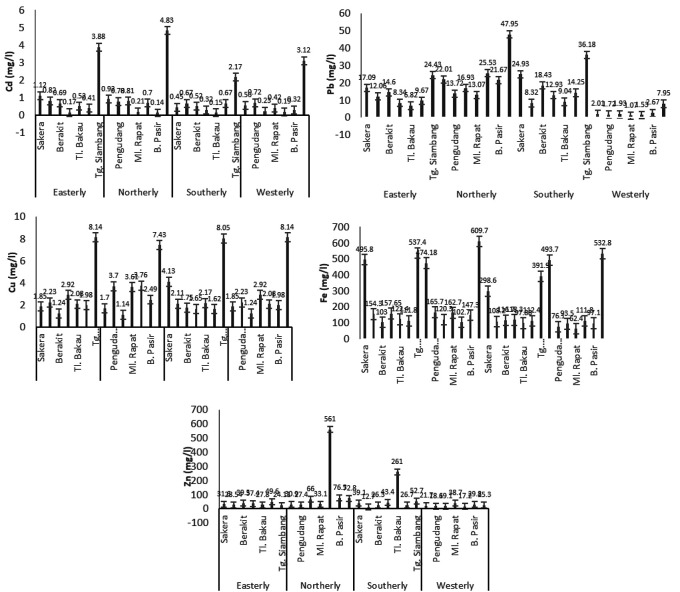
Heavy metal analysis was distinguished by season to determine the content in C. racemosa and to explain the effect of the season on the water. Those heavy metals measured in the C. racemosa sample consisted of Cd, Pb, Cu, Fe, and Zn in micrograms per gram (μg/g), with the analysis results shown in Table 1. The concentration of the metals Cd, Pb, Cu, Fe, and Zn were in the range of 0.14–4.83 μg/g, 1.07–47.95 μg/g, 1.14–8.14 μg/g, 62.4–609.7 μg/g, and 12.7–76.5 μg/g with an average concentration of 0.95 ± 1.15 μg/g, 14.17 ± 10.98 μg/g, 3.07 ± 2.16 μg/g, 220.58 ± 175.16 μg/g, and 35.25 ± 16.54 μg/g, respectively.
Table 1. Average concentration of heavy metal (μg/g) of Cd, Pb, Cu, Fe, and Zn in the Caulerpa racemosa during Northerly–Westerly monsoon in Bintan Island coastal water.
| Season | Location | Cd | Pb | Cu | Fe | Zn |
|---|---|---|---|---|---|---|
| Northerly monsoon | Malang Rapat | 0.21±0.31 | 13.07±6.38 | 3.61±0.69 | 162.7±31.16 | 33.1±30.69 |
| Teluk Bakau | 0.70±0.36 | 25.53±4.31 | 3.76±1.31 | 102.7±22.46 | 56.1±7.42 | |
| Beralas Pasir | 0.14±0.38 | 21.67±3.99 | 2.49±1.28 | 147.3±22.84 | 76.5±25.85 | |
| Berakit | 0.81±0.073 | 16.93±4.18 | 1.14±1.35 | 120.3±19.54 | 66.0±21.35 | |
| Pengudang | 0.78±2.29 | 13.72±17.86 | 3.7±2.9 | 165.7±22.54 | 27.4±25.26 | |
| Sakera | 0.92±2.18 | 22.01±14.7 | 1.7±2.94 | 474.18±19.17 | 30.9±20.95 | |
| Tg. Siambang | 4.83±2.45 | 47.95±20.03 | 7.43±2.47 | 609.7±27.98 | 72.8±17.84 | |
| Average | 1.20±1.63 | 22.98±11.92 | 3.40±2.05 | 254.65±201.36 | 51.12±22.94 | |
| Easterly monsoon | Malang Rapat | 0.17±0.18 | 8.34±1.42 | 2.92±0.51 | 157.65±24.0 | 37.4±10.92 |
| Teluk Bakau | 0.52±0.14 | 6.82±3.93 | 2.08±0.45 | 122.4±9.7 | 27.8±10.9 | |
| Beralas Pasir | 0.41±0.21 | 9.67±2.46 | 1.98±0.51 | 111.8±27.42 | 49.6±10.53 | |
| Berakit | 0.69±0.22 | 14.60±2.51 | 1.24±0.49 | 103.0±23.51 | 39.5±5.65 | |
| Pengudang | 0.82±1.68 | 12.06±6.22 | 2.23±3.52 | 154.3±10.2 | 28.54±3.76 | |
| Sakera | 1.12±1.6 | 17.09±5.67 | 1.85±3.36 | 495.8±16.85 | 31.6±5.18 | |
| Tg. Siambang | 3.88±1.87 | 24.43±6.53 | 8.14±3.43 | 537.4±25.5 | 24.11±9.64 | |
| Average | 1.09±1.27 | 13.29±6.07 | 2.92±2.35 | 240.34±190.19 | 34.08±8.73 | |
| Southerly monsoon | Malang Rapat | 0.32±0.26 | 12.93±2.71 | 1.65±0.31 | 118.3±10.5 | 43.4±11.8 |
| Teluk Bakau | 0.15±0.27 | 9.04±4.7 | 2.17±0.28 | 97.88±9.86 | 26.1±0.28 | |
| Beralas Pasir | 0.67±0.08 | 14.25±5.08 | 1.62±0.25 | 112.4±4.25 | 26.7±7.96 | |
| Berakit | 0.52±0.11 | 18.43±8.37 | 1.75±1.28 | 116.7±17.5 | 26.3±13.2 | |
| Pengudang | 0.67±0.93 | 8.32±14.01 | 2.11±3.02 | 108.2±14.59 | 12.7±20.33 | |
| Sakera | 0.45±0.92 | 24.93±9.3 | 4.13±2.62 | 298.6±17.39 | 39.1±9.88 | |
| Tg. Siambang | 2.17±0.93 | 36.18±17.56 | 8.05±2.91 | 391.9±17.2 | 52.7±9.93 | |
| Average | 0.71±0.67 | 17.73±9.93 | 3.07±2.36 | 177.71±117.76 | 33.48±14.34 | |
| Westerly monsoon | Malang Rapat | 0.42±0.11 | 1.07±0.82 | 2.92±0.51 | 62.4±25.36 | 38.7±10.8 |
| Teluk Bakau | 0.19±0.07 | 1.53±0.57 | 2.08±0.45 | 111.8±9.69 | 17.2±6.79 | |
| Beralas Pasir | 0.32±0.26 | 2.67±0.49 | 1.98±0.51 | 97.1±11.23 | 29.8±6.32 | |
| Berakit | 0.23±0.24 | 1.93±0.15 | 1.24±0.49 | 93.5±26.23 | 19.1±1.66 | |
| Pengudang | 0.72±1.43 | 1.72±3.51 | 2.23±3.52 | 76.1±25.14 | 18.6±3.35 | |
| Sakera | 0.56±1.41 | 2.01±3.24 | 1.85±3.36 | 493.7±17.6 | 21.7±1.86 | |
| Tg. Siambang | 3.12±1.3 | 7.95±5.74 | 8.14±2.96 | 532.8±18.35 | 25.3±6.04 | |
| Average | 0.79±1.04 | 2.70±2.37 | 2.92±2.3 | 209.63±208.31 | 24.34±7.69 |
The residential areas: Ml. Rapat, Tl. Bakau, B. Pasir, Pengudang, Sakera.
The ex-bauxsite mine: Tg. Siambang.
Ginneken and Vries (2018) research that conducted heavy metal tests on several Caulepa species ( C. Sertlatloides, and C. branchypus) described different metal content. The metal content of Cd is in the range of 4.43-5.72 g/g, Pb is in the range of 1.33-2.45 g/g, Cu is in the range of 117.85-204.75 g/g, Fe is in the range of 0.90-1.08 g/g, and Zn is 1136-1169.5 g/g. 30 Yulianto et al. (2018) showed that the concentration of Cd metal in seaweed ranged from 0.018 to 0.87 μg/g. 31 Meanwhile, research by Llanos and Dalawampu (2017) on a C. racemosa sample stated that the concentration of heavy metals such as Cd, Pb, and Zn was in the range of 0.21–0.29 μg/g, 2.61–7.23 μg/g and 6.03–7.23 μg/g, respectively. 32 Intawongse et al. (2018) research in C. racemosa obtained metal concentrations of Pb (0.43 μg/g), Cd (0.79 μg/g), Cu (1.55 μg/g), Fe (311 μg/g), and Zn (13.4 μg/g). 33 The results of the above studies indicate that metal levels in sea water fluctuate according to the conditions, with the concentrations influenced by other effects, especially anthropogenic activities.
Table 1 shows an increasing trend of heavy metal concentrations in residential areas and as a result of former bauxite mining activities. As is shown from seven observations, the waters of Malang Rapat, Teluk Bakau, Berakit, Pengudang, and Sakera. The observation station in Tg Siambang waters is a former bauxite mining area whose effects are now affecting the surrounding aquatic environment.
The metal concentrations of Cd, Pb, Cu, and Fe in the ex-mining area have a higher value than in other areas. For example, the concentration of Cd in ex-mining areas is between 2.17–4.83 μg/g, while in other areas, it is lower between 0.14–1.12 μg/g. Similarly, the levels of Pb, Cu, and Fe increased along with a rise in the intensity of community activities.
According to Sarie (2019) this system is known as the open pit mining system by removing the top soil. The effect that occurs is the release or erosion of soil chemicals, then erosion and sedimentation occur into the waters. This triggers an increase in heavy metal levels in the waters and affects biota, including Caulerpa.
Rehman et al. (2013) compared the levels of several metals in residential areas and found that the Zn, Fe, Cu and Pb content reached 199.94 μg/g, 195.62 μg/g, 9.18 μg/g, and 13.09 μg/g, respectively. 34 According to Vongdala et al. (2019) and Agbeshie et al., the concentrations of Fe, Pb, Zn, and Cd at domestic waste disposal sites tend to experience a significant increase. 35, 36 Kinuthia et al. (2020) stated that an increase in heavy metal concentration positively correlates with the intensity of residential spots. 37 This is in line with the results of the study, which identified an increase in the levels of heavy metals Cd, Pb, Cu, Fe, and Zn. Furthermore, this activity causes metal accumulation in C. racemosa to be higher with a greater effect.
Heavy metal concentrations in C. racemosa samples were also differentiated according to the wind season, as shown in Figure 2 and Figure 3. The aim was to determine the effect of the season on changes in the concentration of heavy metals that accumulated in C. racemosa. The concentration of Cd, Pb, Cu, and Fe metals increased in seasons with high wave dynamics and currents (Northerly monsoon). The heavy metals that accumulate in C. racemosa are lower in the east-west monsoon (Easterly–Westerly monsoon) than in the north (Northerly monsoon). The Cd metal content in the east-west monsoon, namely 0.71 ± 0.67 and 1.09 ± 1.27 μg/g increased to 1.20 ± 1.63 μg/g in the northern monsoon. Furthermore, the concentration of Pb in the east-west monsoon, namely 2.70 ± 2.37 and 13.29 ± 6.07 μg/g increased in the northern monsoon to 22.98 ± 11.92 μg/g. Additionally, the concentration of Cu in the east-west monsoon was 2.92 ± 2.3 μg/g compared to 3.40 ± 2.05 μg/g in the northern monsoon. Fe content in the east-west monsoon, namely 209.63 ±208.31 and 240.34 ± 190.19 μg/g increased to 254.65 ± 201.36 μg/g in the north monsoon. Zn metal in the east-west monsoon increased from 24.34 ± 7.69 and 34.08 ± 8.73 μg/g to 51.12 ± 22.94 μg/g in the north monsoon. These results indicate that all the tested metals increased in the northern season.
Figure 2. Comparative of heavy metal concentration on Northerly–Westerly monsoon in Bintan coastal water.
Figure 3. Average concentration of heavy metal (µg/g) of Cd, Pb, Cu, Fe, and Zn in the Caulerva racemosa during Northerly–Westerly monsoon in Bintan Island coastal water.
Talwar et al. (2014) carried out research related to differences in heavy metal concentrations by season. The results showed that Pb, Cr, and Cu tend to increase during the rainy season by 0.03–0.8 ppm, 0.01–0.4 ppm, and 0.02–0.7 ppm, respectively. 38 According to research by Mondol et al. (2011), the highest concentration of heavy metals in marine plants is during the wet season, where Pb, Cr, and Cu metals range from 6.18 to 14.91 μg/g, 19.65–19.83 μg/g, and 23.88–33.16 μg/g. 30 Zhang et al. (2017) stated that the rainy season has the highest effect on the increase in heavy metals. 39 This condition is similar to the results found at this research location, where the concentration of heavy metals tends to increase in the northern monsoon due to the effect of current and wave dynamics, which are the driving force for marine dynamics that carry and spread pollutants in waters. 40
Koropitan and Cordova (2017) stated that flow is a component of heavy metal transport from a river transported into open water, thereby increasing its value in the aquatic ecosystem. 41 Budiyanto and Lestari (2017) examined the difference between metals in the eastern (March) and the southern seasons (June). 42 The results showed an increase in Cd, Cu, Pb and Zn metals from 1.18 μg/g to 1.74 μg/g, 94.8 μg/g to 157.0 μg/g, 41.7 μg/g to 89.4 μg/g, and 503.0 μg/g to 1270 μg/g, respectively. These results clearly show that changes in environmental conditions, especially wind direction, dynamics of currents and waves, as well as season, were factors that affected heavy metal concentrations in nature.
The one-way Anova test ( Table 2) showed that the concentration of Pb in C. racemosa in the northern monsoon was significantly different compared to other seasons (f = 0.051). Meanwhile, the Cu, Fe, Zn and Cd results were not significantly different between seasons with f values of 0.722, 0.481, 0.121, and 0.493, respectively. Therefore, it can be concluded that the fluctuating season conditions do not significantly affect changes in metal concentrations. The levels of correlation between Cd, Pb, Cu, Fe, and Zn metals are shown in Table 3.
Table 2. Anova test of heavy metal concentration on Caulerpa racemosa in different monsoon.
| Metals | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Cd | Between Groups | 1.146 | 3 | 0.382 | 0.263 | 0.851 |
| Within Groups | 34.811 | 24 | 1.450 | |||
| Total | 35.958 | 27 | ||||
| Pb | Between Groups | 1559.008 | 3 | 519.669 | 7.336 | 0.001 |
| Within Groups | 1700.020 | 24 | 70.834 | |||
| Total | 3259.028 | 27 | ||||
| Cu | Between Groups | 1.095 | 3 | 0.365 | 0.070 | 0.975 |
| Within Groups | 125.409 | 24 | 5.225 | |||
| Total | 126.504 | 27 | ||||
| Fe | Between Groups | 24562.954 | 3 | 8187.651 | 0.244 | 0.864 |
| Within Groups | 803898.015 | 24 | 33495.751 | |||
| Total | 828460.969 | 27 | ||||
| Zn | Between Groups | 42365.448 | 3 | 14121.816 | 1.247 | 0.315 |
| Within Groups | 271682.633 | 24 | 11320.110 | |||
| Total | 314048.081 | 27 | ||||
Table 3. Correlation of heavy metal in Caulerpa racemosa Northerly–Westerly monsoon in Bintan Island coastal water.
| Heavy Metal | Cd | Pb | Cu | Fe | Zn |
|---|---|---|---|---|---|
| Cd | 1 | - | - | - | - |
| Pb | 0.631 ** | 1 | - | - | - |
| Cu | 0.835 ** | 0.571 ** | 1 | - | - |
| Fe | 0.77 ** | 0.526 ** | 0.651 ** | 1 | - |
| Zn | −0.059 | 0.241 | 0.052 | −0.158 | 1 |
Correlation is significant at the 0.01 level (2-tailed).
The results of metal analysis in C. racemosa showed a strong correlation level in Pb with Cd, Cu with Cd, Fe with Cd, Cu with Pb, Fe with Pb, Zn with Pb, Fe with Cu, and Zn with Cu. Research by Wang and Liu (2004) found a correlation between Pb and Cd, Cu with Pb, and Zn with Pb metals. 43 Furthermore, research by Khaled et al. (2014) showed a positive correlation value between metals Cu and Cd, Fe with Cd, metal Cd with Pb, metal Cu with Fe, Cu with Pb, Cu with Zn, and Fe with Pb. 44 Harikumar et al. (2010) also obtained a positive correlation value between heavy metals Cu and Pb. 45 Mourad and El-Azim's research (2019) on macroalgae showed a positive correlation between Cu and Cd, Cu with Pb, Fe with Pb, Zn with Pb, Fe with Cu, and Zn with Cu. 46 The concentrations of metals in C. racemosa correlate with each other, which indicates that an increase in the levels of one metal affects others.
Heavy metals in water samples
The concentration of heavy metals in the tested water samples fluctuated according to seasonal conditions. It was found that the highest average concentrations of Cd, Pb, Cu and Fe were in the eastern monsoon, namely 0.0013 ± 0.00056 mg/l, 0.0010 ± 0.00048 mg/l, 0.0010 ± 0.00074 mg/l, and 0.0013 ± 0.00092 mg/l, respectively. Meanwhile, Zn metal was the highest in the western monsoon with an average of 0.0240 ± 0.0054 mg/l, as shown in Figure 4.
Figure 4. Average concentration of heavy metal (mg/l) of Cd, Pb, Cu, Fe, and Zn in the water during Northerly-Westerly monsoon in Bintan Island coastal water.
Several studies stated that in marine waters, Pb metal concentrations ranged from 0.0059–1.07 mg/l, 47 Cadmium (Cd) ranged from 0.01–0.03 mg/l, 48 Cu ranged from 0.0472–0.0725 mg/l, Zn ranged from 32.59–45.79 mg/l, 49 and Fe ranged from 0.17–0.45 mg/l. 50 In general, the concentrations of heavy metals in the water samples at the research locations were similar to previous studies, though the levels of Fe and Zn were categorized as high in this research.
Bazzi (2014) stated that anthropogenic activity is a determining factor for high Zn and Fe metal contamination in waters. 51 Furthermore, Sun et al. (2020) emphasized that heavy metal levels are influenced by changes in salinity, pH, and biological activity, they tend to increase at the location of bays and estuaries that are close to residential activities. 52 Zn and Fe metal contamination in the research location is influenced by community activities, especially settlements that produce various types of waste. In addition, the open land of former bauxite mining on Bintan Island is a source of contamination of these metals in the waters. According to Rezaei et al. (2019) and Ismail et al. (2019), bauxite waste is a source of Fe and Zn metal contamination in the environment. 53, 54 It is one of the causes of the high concentration of Zn and Fe metals in the research location.
Heavy metals in sediment samples
Heavy metals that enter the water accumulate at the bottom and affect the metal composition of the sediments. According to preliminary studies, the highest content of heavy metal Cd in sediments occurred in the westerly monsoon with an average of 0.0289 ± 0.037 μg/g, while the northerly monsoon produced strong current dynamics of 0.0045 ± 0.001 μg/g. The highest Pb content was in the easterly monsoon with an average of 0.0449 ± 0.013 μg/g, the highest Cu and Fe was in the west season with an average of 0.0321 ± 0.024 μg/g and 18.43 ± 5.25 μg/g, respectively. However, the heavy metal Zn actually increased during the northerly monsoon with an average value of 11.63 ± 14.041 μg/g, as shown in Figure 5.
Figure 5. Average concentration of heavy metal (µg/g) of Cd, Pb, Cu, Fe, and Zn in the sediment during Northerly-Westerly monsoon in Bintan Island coastal water.
The concentration of heavy metals in sediments decreases and increases in the northern monsoon and east to west monsoons, respectively. The stirring of sediment that occurs in the northern monsoon is a factor in the difference in metal concentrations between seasons. The water currents stir the sediment in seasons with hydrodynamic conditions, therefore, the heavy metals contained are dispersed more quickly. Meanwhile, in other seasons, the dynamics are smaller and support the deposition of metal particles that enter the waters. This affects the accumulation of heavy metals in the water to the bottom sediments. Wardani et al. (2020) stated that heavy metals are transported by currents where the flow conditions differ in strength in accordance with the season. 55 Wisha et al. (2018) and Hamuna and Tanjung (2021) stated that the distribution of heavy metals in the sea is influenced by several water inputs and dynamics, topography, wind patterns, movement and surface current circulation. 56, 57
PCa and correlation of heavy metal
The principal component analysis in Figure 6a and the correlation analysis in Table 4. show a correlation between the metal content of the C. racemosa sample. The heavy metal content of Cd in Caulerpa has a relationship with water temperature although it is not significant. Pb metal has a relationship with current, depth, salinity, pH, DO, BOD, COD. TSS and TDS although also not significant. Meanwhile, Cu has a positive correlation with depth, salinity, pH, DO, BOD, COD, and TDS but also not significant. The Fe metal in Caulerpa only has a positive correlation with current and depth.
Figure 6. PCa of heavy metal (µg/g) of Cd, Pb, Cu, Fe, and Zn and Water Parameters during Northerly Westerly monsoon in Bintan Island (a: in Caulerpa), (b: in the water), and (c: in sediment).
Table 4. Correlation of heavy metal (μg/g) of Cd, Pb, Cu, Fe, and Zn in the Caulerpa during Northerly-Westerly monsoon in Bintan Island coastal water.
| Correlations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd_C | Pb_C | Cu_C | Fe_C | Zn_C | T ( oC) | Current | depth | Salinity | pH | DO | N | BOD | COD | TSS | TDS | |
| Cd_C | 1 | .631 ** | .835 ** | .770 ** | -0.059 | 0.018 | -0.171 | -0.008 | -0.096 | -0.097 | -0.059 | -0.017 | -0.104 | -0.064 | -0.118 | -0.091 |
| Pb_C | .631 ** | 1 | .571 ** | .526 ** | 0.241 | -0.317 | 0.077 | 0.101 | 0.044 | 0.072 | 0.087 | 0.07 | 0.035 | 0.1 | 0.071 | 0.069 |
| Cu_C | .835 ** | .571 ** | 1 | .651 ** | 0.052 | -0.11 | -0.056 | 0.099 | 0.005 | 0.009 | 0.049 | 0.111 | 0.01 | 0.049 | -0.055 | 0.012 |
| Fe_C | .770 ** | .526 ** | .651 ** | 1 | -0.158 | -0.156 | 0.114 | 0.083 | -0.032 | -0.022 | 0.021 | 0.114 | -0.027 | 0 | -0.076 | -0.019 |
| Zn_C | -0.059 | 0.241 | 0.052 | -0.158 | 1 | -0.139 | -0.108 | -0.169 | -0.107 | -0.081 | -0.091 | -0.192 | -0.039 | -0.027 | -0.129 | -0.098 |
| T ( oC) | 0.018 | -0.317 | -0.11 | -0.156 | -0.139 | 1 | -.416 * | -0.225 | -0.216 | -0.245 | -0.204 | -0.221 | -0.231 | -0.252 | -0.184 | -0.239 |
| Current | -0.171 | 0.077 | -0.056 | 0.114 | -0.108 | -.416 * | 1 | .852 ** | .832 ** | .850 ** | .837 ** | .854 ** | .842 ** | .837 ** | .786 ** | .854 ** |
| Depth | -0.008 | 0.101 | 0.099 | 0.083 | -0.169 | -0.225 | .852 ** | 1 | .960 ** | .961 ** | .947 ** | .931 ** | .952 ** | .953 ** | .889 ** | .966 ** |
| Salinity | -0.096 | 0.044 | 0.005 | -0.032 | -0.107 | -0.216 | .832 ** | .960 ** | 1 | .998 ** | .988 ** | .947 ** | .991 ** | .989 ** | .947 ** | .998 ** |
| pH | -0.097 | 0.072 | 0.009 | -0.022 | -0.081 | -0.245 | .850 ** | .961 ** | .998 ** | 1 | .988 ** | .941 ** | .991 ** | .993 ** | .948 ** | .999 ** |
| DO | -0.059 | 0.087 | 0.049 | 0.021 | -0.091 | -0.204 | .837 ** | .947 ** | .988 ** | .988 ** | 1 | .956 ** | .974 ** | .980 ** | .920 ** | .987 ** |
| BOD | -0.104 | 0.035 | 0.01 | -0.027 | -0.039 | -0.231 | .842 ** | .952 ** | .991 ** | .991 ** | .974 ** | .933 ** | 1 | .988 ** | .927 ** | .990 ** |
| COD | -0.064 | 0.1 | 0.049 | 0 | -0.027 | -0.252 | .837 ** | .953 ** | .989 ** | .993 ** | .980 ** | .927 ** | .988 ** | 1 | .942 ** | .992 ** |
| TSS | -0.118 | 0.071 | -0.055 | -0.076 | -0.129 | -0.184 | .786 ** | .889 ** | .947 ** | .948 ** | .920 ** | .860 ** | .927 ** | .942 ** | 1 | .949 ** |
| TDS | -0.091 | 0.069 | 0.012 | -0.019 | -0.098 | -0.239 | .854 ** | .966 ** | .998 ** | .999 ** | .987 ** | .944 ** | .990 ** | .992 ** | .949 ** | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
The relationship between metal content in seawater ( Figure 6b and Table 5) shows that Cu has a significant correlation with currents, and has a relationship with depth, salinity, pH, DO, Nitrate, BOD, COD, TSS and TDS. Fe has a correlation with current and TSS while Zn has a correlation with current parameters, depth, salinity, pH, DO, nitrate, BOD, COD, TSS, and TDS.
Table 5. Correlation of heavy metal (μg/g) of Cd, Pb, Cu, Fe, and Zn in Water during Northerly-Westerly monsoon in Bintan Island coastal water.
| Correlations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd_w | Pb_w | Cu_w | Fe_w | Zn_w | T ( oC) | Current | depth | Salinity | pH | DO | N | BOD | COD | TSS | TDS | |
| Cd_w | 1 | .509 ** | 0.103 | 0.26 | -0.314 | -0.244 | -0.273 | -0.24 | -0.246 | -0.255 | -0.224 | -0.13 | -0.294 | -0.234 | -0.306 | -0.265 |
| Pb_w | .509 ** | 1 | -0.087 | -0.035 | -.580 ** | -0.148 | -0.314 | -0.208 | -0.216 | -0.226 | -0.241 | -0.228 | -0.275 | -0.236 | -0.17 | -0.223 |
| Cu_w | 0.103 | -0.087 | 1 | .715 ** | -0.236 | -0.277 | .451 * | 0.242 | 0.234 | 0.246 | 0.241 | 0.252 | 0.202 | 0.241 | 0.297 | 0.247 |
| Fe_w | 0.26 | -0.035 | .715 ** | 1 | -0.103 | -0.16 | 0.102 | -0.086 | -0.068 | -0.059 | -0.049 | -0.011 | -0.073 | -0.033 | 0.013 | -0.066 |
| Zn_w | -0.314 | -.580 ** | -0.236 | -0.103 | 1 | -0.053 | 0.246 | 0.108 | 0.125 | 0.141 | 0.1 | 0.069 | 0.214 | 0.191 | 0.084 | 0.136 |
| T ( oC) | -0.244 | -0.148 | -0.277 | -0.16 | -0.053 | 1 | -.416 * | -0.225 | -0.216 | -0.245 | -0.204 | -0.221 | -0.231 | -0.252 | -0.184 | -0.239 |
| Current | -0.273 | -0.314 | .451 * | 0.102 | 0.246 | -.416 * | 1 | .852 ** | .832 ** | .850 ** | .837 ** | .854 ** | .842 ** | .837 ** | .786 ** | .854 ** |
| Depth | -0.24 | -0.208 | 0.242 | -0.086 | 0.108 | -0.225 | .852 ** | 1 | .960 ** | .961 ** | .947 ** | .931 ** | .952 ** | .953 ** | .889 ** | .966 ** |
| Salinity | -0.246 | -0.216 | 0.234 | -0.068 | 0.125 | -0.216 | .832 ** | .960 ** | 1 | .998 ** | .988 ** | .947 ** | .991 ** | .989 ** | .947 ** | .998 ** |
| pH | -0.255 | -0.226 | 0.246 | -0.059 | 0.141 | -0.245 | .850 ** | .961 ** | .998 ** | 1 | .988 ** | .941 ** | .991 ** | .993 ** | .948 ** | .999 ** |
| DO | -0.224 | -0.241 | 0.241 | -0.049 | 0.1 | -0.204 | .837 ** | .947 ** | .988 ** | .988 ** | 1 | .956 ** | .974 ** | .980 ** | .920 ** | .987 ** |
| N | -0.13 | -0.228 | 0.252 | -0.011 | 0.069 | -0.221 | .854 ** | .931 ** | .947 ** | .941 ** | .956 ** | 1 | .933 ** | .927 ** | .860 ** | .944 ** |
| BOD | -0.294 | -0.275 | 0.202 | -0.073 | 0.214 | -0.231 | .842 ** | .952 ** | .991 ** | .991 ** | .974 ** | .933 ** | 1 | .988 ** | .927 ** | .990 ** |
| COD | -0.234 | -0.236 | 0.241 | -0.033 | 0.191 | -0.252 | .837 ** | .953 ** | .989 ** | .993 ** | .980 ** | .927 ** | .988 ** | 1 | .942 ** | .992 ** |
| TSS | -0.306 | -0.17 | 0.297 | 0.013 | 0.084 | -0.184 | .786 ** | .889 ** | .947 ** | .948 ** | .920 ** | .860 ** | .927 ** | .942 ** | 1 | .949 ** |
| TDS | -0.265 | -0.223 | 0.247 | -0.066 | 0.136 | -0.239 | .854 ** | .966 ** | .998 ** | .999 ** | .987 ** | .944 ** | .990 ** | .992 ** | .949 ** | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
The heavy metals contained in the sediment ( Figure 6c and Table 6) show that Cd and Pb only have a correlation with temperature. Cu and Fe in sediments have a correlation with current, depth, salinity, pH, DO, Nitrate, BOD, COD. TSS and TDS. Zn only correlated with temperature, depth, salinity, pH, BOD, and TDS.
Table 6. Correlation of heavy metal (μg/g) of Cd, Pb, Cu, Fe, and Zn in the Sediment during Northerly-Westerly monsoon in Bintan Island coastal water.
| Correlations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd_Sed | Pb_Sed | Cu_Sed | Fe_Sed | Zn_Sed | T ( oC) | Current | Depth | Salinity | pH | DO | N | BOD | COD | TSS | TDS | |
| Cd_Sed | 1 | -0.23 | -0.203 | -0.037 | -0.08 | 0.075 | -0.073 | -0.154 | -0.106 | -0.113 | -0.169 | -0.07 | -0.037 | -0.106 | -0.143 | -0.108 |
| Pb_Sed | -0.23 | 1 | -.478 * | -0.112 | -0.032 | 0.155 | -0.16 | -0.141 | -0.118 | -0.127 | -0.097 | -0.045 | -0.205 | -0.135 | 0.009 | -0.122 |
| Cu_Sed | -0.203 | -.478 * | 1 | 0.111 | 0.352 | 0.154 | 0.291 | 0.354 | 0.3 | 0.304 | 0.279 | 0.171 | 0.326 | 0.287 | 0.245 | 0.308 |
| Fe_Sed | -0.037 | -0.112 | 0.111 | 1 | -0.122 | -0.369 | .576 ** | 0.343 | 0.224 | 0.252 | 0.244 | 0.305 | 0.25 | 0.252 | 0.152 | 0.256 |
| Zn_Sed | -0.08 | -0.032 | 0.352 | -0.122 | 1 | 0.184 | -0.126 | 0.064 | 0.011 | 0.006 | -0.02 | -0.096 | 0.022 | -0.005 | -0.064 | 0.007 |
| T ( oC) | 0.075 | 0.155 | 0.154 | -0.369 | 0.184 | 1 | -.416 * | -0.225 | -0.216 | -0.245 | -0.204 | -0.221 | -0.231 | -0.252 | -0.184 | -0.239 |
| Current | -0.073 | -0.16 | 0.291 | .576 ** | -0.126 | -.416 * | 1 | .852 ** | .832 ** | .850 ** | .837 ** | .854 ** | .842 ** | .837 ** | .786 ** | .854 ** |
| Depth | -0.154 | -0.141 | 0.354 | 0.343 | 0.064 | -0.225 | .852 ** | 1 | .960 ** | .961 ** | .947 ** | .931 ** | .952 ** | .953 ** | .889 ** | .966 ** |
| Salinity | -0.106 | -0.118 | 0.3 | 0.224 | 0.011 | -0.216 | .832 ** | .960 ** | 1 | .998 ** | .988 ** | .947 ** | .991 ** | .989 ** | .947 ** | .998 ** |
| pH | -0.113 | -0.127 | 0.304 | 0.252 | 0.006 | -0.245 | .850 ** | .961 ** | .998 ** | 1 | .988 ** | .941 ** | .991 ** | .993 ** | .948 ** | .999 ** |
| DO | -0.169 | -0.097 | 0.279 | 0.244 | -0.02 | -0.204 | .837 ** | .947 ** | .988 ** | .988 ** | 1 | .956 ** | .974 ** | .980 ** | .920 ** | .987 ** |
| N | -0.07 | -0.045 | 0.171 | 0.305 | -0.096 | -0.221 | .854 ** | .931 ** | .947 ** | .941 ** | .956 ** | 1 | .933 ** | .927 ** | .860 ** | .944 ** |
| BOD | -0.037 | -0.205 | 0.326 | 0.25 | 0.022 | -0.231 | .842 ** | .952 ** | .991 ** | .991 ** | .974 ** | .933 ** | 1 | .988 ** | .927 ** | .990 ** |
| COD | -0.106 | -0.135 | 0.287 | 0.252 | -0.005 | -0.252 | .837 ** | .953 ** | .989 ** | .993 ** | .980 ** | .927 ** | .988 ** | 1 | .942 ** | .992 ** |
| TSS | -0.143 | 0.009 | 0.245 | 0.152 | -0.064 | -0.184 | .786 ** | .889 ** | .947 ** | .948 ** | .920 ** | .860 ** | .927 ** | .942 ** | 1 | .949 ** |
| TDS | -0.108 | -0.122 | 0.308 | 0.256 | 0.007 | -0.239 | .854 ** | .966 ** | .998 ** | .999 ** | .987 ** | .944 ** | .990 ** | .992 ** | .949 ** | 1 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Overall from the results of PCa analysis and correlation, water parameters have an influence on the level of metal absorption in Caulerpa, water, and sediment. However, current is one of the water parameters that has a significant correlation to the accumulation of several metals in Caulerpa, water, and sediment. This indicates that the current has the ability to transport chemical particles in the waters so that they can be spread over a wider area. Tampubolon et al. (2021) examined the effect of currents on the concentration of Cu, the conclusion obtained is that longshore currents have a considerable influence on the direction of distribution of heavy metal Cu. 59 Puspitasari (2006) also stated that current plays a role in the distribution of Pb, Cd, Cu, and Zn in nature. 60
Conclusions
In conclusion, the concentration of Cd, Pb, Cu, Fe and Zn on C. racemosa ranged from 0.14–4.83 μg/g, 1.07–47.95 μg/g, 1.14–8.14 μg/g, 62.4–609.7 μg/g, and 12.7–76.5 μg/g with averages of 0.95 ± 1.15 μg/g, 14.17 ± 10.98 μg/g, 3.07 ± 2.16 μg/g, 220.58 ± 175.16 μg/g, and 35.25 ± 16.54 μg/g, respectively. Heavy metal fluctuations are closely related to seasonal changes, where in the east-west monsoon (Easterly–Westerly monsoon), those that accumulate in C. racemosa are lower than in the north monsoon (Northerly monsoon). However, based on the one-way Anova analysis, only Pb was significantly different between seasons, while Cd, Cu, Fe, and Zn were insignificant. The correlation between metals showed a positive relationship, which indicates that the increase in one metal affect others. In water samples the highest average concentrations of Cd, Pb, Cu and Fe were in the eastern monsoon, namely 0.0013 ± 0.00056 mg/l, 0.0010 ± 0.00048 mg/l, 0.0010 ± 0.00074 mg/l, and 0.0013 ± 0.00092 mg/l, respectively. Meanwhile, Zn metal was the highest in the western monsoon with an average of 0.0240 ± 0.0054 mg/l. In sediment samples the highest content of heavy metal Cd in sediments occurred in the westerly monsoon with an average of 0.0289 ± 0.037 μg/g, while the northerly monsoon produced strong current dynamics of 0.0045 ± 0.001 μg/g. The highest Pb content was in the easterly monsoon with an average of 0.0449 ± 0.013 μg/g, the highest Cu and Fe was in the west season with an average of 0.0321 ± 0.024 μg/g and 18.43 ± 5.25 μg/g, respectively. However, the heavy metal Zn actually increased during the northerly monsoon with an average value of 11.63 ± 14.041 μg/g.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Acknowledgements
The authors are grateful to the government of Teluk Bakau Village, Malang Meeting, Berakit, Beralas Pasir, Pengudang, Sakera, and Dompak, as well as the survey team for providing access to data collection in the field on Caulerpa racemosa.
Funding Statement
The authors are grateful to the Ministry of Education and Culture and the Raja Ali Haji Maritime University for funding this research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Liu R, Xu Y, Zhang J, et al. : Effects of heavy metal pollution on farmland soils and crops: A case study of the Xiaoqinling Gold Belt, China. China Geol. 2020;3:402–410. 10.31035/cg2020024 [DOI] [Google Scholar]
- 2. Armah FA, Quansah R, Luginaah I: A Systematic Review of Heavy Metals of Anthropogenic Origin in Environmental Media and Biota in the Context of Gold Mining in Ghana. Int. Sch. Res. Not. 2014;2014:1–37. 10.1155/2014/252148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vareda JP, Valente AJM, Durães L: Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manage. 2019;246:101–118. 10.1016/j.jenvman.2019.05.126 [DOI] [PubMed] [Google Scholar]
- 4. Jaruga P, Coskun E, Kimbrough K, et al. : Cover Image. Environ. Toxicol. 2017;32:i–i. 10.1002/tox.22462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiwan S, Ajay K: Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011;1:15–21. [Google Scholar]
- 6. Briffa J, Sinagra E, Blundell R: Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6:e04691. 10.1016/j.heliyon.2020.e04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q, Pan XD, Huang BF, et al. : Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018;8:1–7. 10.1038/s41598-018-21732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deswati H, Pardi H, Suyani, et al. : Adsorptive stripping voltammetry for the simultaneous determination of Cd, Cu, Cr, and Pb in water samples using Fluorexon: An optimization single factor. Anal. Bioanal. Electrochem. 2018;10:1491–1505. [Google Scholar]
- 9. Deswati H, Suyani AK, Muchtar EF, et al. : Copper, iron and zinc contents in water, pakcoy (Brassica rapa L.) and tilapia (oreochromis niloticus) in the presence of aquaponics. Rasayan J. Chem. 2019;12, pp.40–49. 10.31788/RJC.2019.1215062 [DOI] [Google Scholar]
- 10. Wang T, Wang S, Tang X, et al. : Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ. Sci. Pollut. Res. 2020;27:8707–8718. 10.1007/s11356-019-06957-3 [DOI] [PubMed] [Google Scholar]
- 11. Paper F: Archive of S Archive o. 2018;10:541–553. [Google Scholar]
- 12. Fernández E, Vidal L, Silvestre-Albero J, et al. : Magnetic dispersive solid-phase extraction using a zeolite-based composite for direct electrochemical determination of lead (II) in urine using screen-printed electrodes. Microchim. Acta. 2020;187:1–10. 10.1007/s00604-019-4062-9 [DOI] [PubMed] [Google Scholar]
- 13. Andreani G, Ferlizza E, Cabbri R, et al. : Essential (Mg, fe, zn and cu) and non-essential (cd and pb) elements in predatory insects (vespa crabro and vespa velutina): A molecular perspective. Int. J. Mol. Sci. 2021;22:1–16. 10.3390/ijms22010228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pardi H, Deswati H, Suyani, et al. : Cathodic stripping voltammetric determination of essential element (Copper and zinc) in drinking water. Anal. Bioanal. Electrochem. 2019;11:691–702. [Google Scholar]
- 15. Latif A, Bilal M, Asghar W, et al. : Heavy Metal Accumulation in Vegetables and Assessment of their Potential Health Risk. J. Environ. Anal. Chem. 2018;05. 10.4172/2380-2391.1000234 [DOI] [Google Scholar]
- 16. Deswati H, Suyani I, Rahmi, et al. : Application of central composite design optimization technique for determination of copper in fruit and vegetable samples with adsorptive stripping voltammetry in the presence of calcein. Rasayan J. Chem. 2017;10:1359–1367. 10.7324/rjc.2017.1041810 [DOI] [Google Scholar]
- 17. Costa FS, Leal RVP, Pacheco CSV, et al. : Multivariate optimization of an ultrasound-assisted extraction procedure for the determination of Cu, Fe, Mn, and Zn in plant samples by flame atomic absorption spectrometry. Anal. Methods. 2020;12:2509–2516. 10.1039/d0ay00554a [DOI] [PubMed] [Google Scholar]
- 18. Nie Y, Zhang X, Zhang Q, et al. : A novel high efficient electrochemiluminescence sensor based on reductive Cu(I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosens. Bioelectron. 2020;160:112217. 10.1016/j.bios.2020.112217 [DOI] [PubMed] [Google Scholar]
- 19. Singh P, Prasad S: Spectroscopic review of chelating agents and their influence on the bioavailability of Fe, Zn and Ca in Fijian foods. Appl. Spectrosc. Rev. 2020;55:574–592. 10.1080/05704928.2019.1695133 [DOI] [Google Scholar]
- 20. van Ginneken V, de Vries E: Seaweeds as Biomonitoring System for Heavy Metal (HM) Accumulation and Contamination of Our Oceans. Am. J. Plant Sci. 2018;9:1514–1530. 10.4236/ajps.2018.97111 [DOI] [Google Scholar]
- 21. Perryman SE, Lapong I, Mustafa A, et al. : Potential of metal contamination to affect the food safety of seaweed (Caulerpa spp.) cultured in coastal ponds in Sulawesi, Indonesia. Aquac. Reports. 2017;5:27–33. 10.1016/j.aqrep.2016.12.002 [DOI] [Google Scholar]
- 22. Tamilselvan N, Saurav K, Kannabiran K: Biosorption of Cr (VI), Cr (III), Pb (II) and Cd (II) from aqueous solutions by Sargassum wightii and Caulerpa racemosa algal biomass. J. Ocean Univ. China. 2012;11:52–58. 10.1007/s11802-012-1843-8 [DOI] [Google Scholar]
- 23. Pavasant P, Apiratikul R, Sungkhum V, et al. : Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006;97:2321–2329. 10.1016/j.biortech.2005.10.032 [DOI] [PubMed] [Google Scholar]
- 24. Selvan T, Krishnan K, David E: Biosorption of heavy metals from aqueous solution by Gracilaria corticata varcartecala and Grateloupia lithophila. J. Coast. Life Med. 2013. 10.12980/JCLM.1.2013J8 [DOI]
- 25. Khandaker MU, Chijioke NO, Heffny NAB, et al. : Elevated concentrations of metal (Loids) in seaweed and the concomitant exposure to humans. Foods. 2021;10:1–18. 10.3390/foods10020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dadolahi-Sohrab A, Seyed AN, Nabavi MB, et al. : Environmental Monitoring of Heavy Metals in Seaweed and Associated Sediment from the Strait of Hormuz, I.R. Iran. World J. Fish Mar. Sci. 2011;3:576–589. [Google Scholar]
- 27. Akta J, Indonesia K, Teheni MT: INDONESIA CHIMICA ACTA Region of Baubau Coastal. 2020;13:65–67. [Google Scholar]
- 28. Yong WTL, Chin JYY, Thien VY, et al. : Heavy metal accumulation in field cultured and tissue cultured Kappaphycus alvarezii and Gracilaria changii. Int. Food Res. J. 2017;24:970–975. [Google Scholar]
- 29. Shanbehzadeh S, Vahid Dastjerdi M, Hassanzadeh A, et al. : Heavy metals in water and sediment: A case study of Tembi River. J. Environ. Public Health. 2014;2014. 10.1155/2014/858720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mondol MN, Bfasfe, Chamon AS: Water and Plant Samples Around Tejgaon Industrial Area of Bangladesh. Bangladesh Acad. Sci. 2011;35:19–41. 10.3329/jbas.v35i1.7968 [DOI] [Google Scholar]
- 31. Nurjanah A, Supriharyono, Yulianto B: The Environmentally Sound Aquaculture Strategies Based on Bioaccumulation of Heavy Metal of Lead (Pb) on Seaweed of Gracilaria verrucosa on Aquaculture Areas of MuararejaVillage, Tegal City. IOP Conf. Ser. Earth Environ. Sci. 2018;116. 10.1088/1755-1315/116/1/012042 [DOI] [Google Scholar]
- 32. Llanos N, Dalawampu S: Heavy Metals in Edible Seaweeds From Coastal Areas of Manila Bay and Roxas City, Philippines. Int. J. Adv. Res. 2017;5:1429–1434. 10.21474/IJAR01/4264 [DOI] [Google Scholar]
- 33. Management IM, Url E: Northumbria Research Link. 2018;46:25–35. [Google Scholar]
- 34. Rehman HU, Rehman A, Ullah F, et al. : Comparative study of heavy metals in different parts of domestic and broiler chickens. Int. J. Pharm. Sci. Rev. Res. 2013;23:151–154. [Google Scholar]
- 35. Vongdala N, Tran HD, Xuan TD, et al. : Heavy metal accumulation in water, soil, and plants of municipal solid waste landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health. 2019;16:1–13. 10.3390/ijerph16010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agbeshie AA, Adjei R, Anokye J, et al. : Municipal waste dumpsite: Impact on soil properties and heavy metal concentrations, Sunyani, Ghana. Sci. African. 2020;8:e00390. 10.1016/j.sciaf.2020.e00390 [DOI] [Google Scholar]
- 37. Kinuthia GK, Ngure V, Beti D, et al. : Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci. Rep. 2020;10:1–13. 10.1038/s41598-020-65359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Talwar R, Agrawal S, Bajpai A, et al. : Assessment of Concentration and Variations due to Seasonal Effect on the Presence of Heavy Metals in the Water of Upper Lake, Bhopal. Curr. World Environ. 2014;9:421–425. 10.12944/CWE.9.2.24 [DOI] [Google Scholar]
- 39. Zhang T, Bai Y, Hong X, et al. : Particulate matter and heavy metal deposition on the leaves of Euonymus japonicus during the East Asian monsoon in Beijing, China. PLoS One. 2017;12:1–16. 10.1371/journal.pone.0179840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerpen NB, Schlurmann T, Schendel A, et al. : Wave-Induced Distribution of Microplastic in the Surf Zone. Front. Mar. Sci. 2020;7. 10.3389/fmars.2020.590565 [DOI] [Google Scholar]
- 41. Koropitan AF, Cordova MR: Study of Heavy Metal Distribution and Hydrodynamic Simulation in Green Mussel Culture Net, Cilincing Water - Jakarta Bay. Makara J. Sci. 2017;21:89–96. 10.7454/mss.v21i2.4406 [DOI] [Google Scholar]
- 42. Budiyanto F, Lestari L: Temporal and Spatial Distribution of Heavy Metal in Sediment of Urban Coastal Waters: A Case Study in Jakarta Bay, Indonesia. Bull. Mar. Geol. 2017;32. 10.32693/bomg.32.1.2017.364 [DOI] [Google Scholar]
- 43. Wang B, Liu C: Factors controlling the distribution of trace metals in macroalgae. Chinese J. Geochemistry. 2004;23:366–372. 10.1007/BF02871309 [DOI] [Google Scholar]
- 44. Ginneken VV, de Vries E: Seaweeds as Biomonitoring System for Heavy Metal (HM) Accumulation and Contamination of Our Oceans. Am. J. Plant Sci. 2018;9:1514–1530. [Google Scholar]
- 45. Harikumar APS, Prajitha BK, Silpa CS: Laboratory Research in Biology Assessment of Heavy Metal Contamination in the Sediments of a River Draining Into a Ramsar Site in the Indian Sub. Analysis I. 2010:157–169. [Google Scholar]
- 46. Mourad FA, El-Azim HA: Use of green alga ulva lactuca (L.) as an indicator to heavy metal pollution at intertidal waters in suez gulf, aqaba gulf and suez canal, Egypt. Egypt. J. Aquat. Biol. Fish. 2019;23:437–449. 10.21608/EJABF.2019.57913 [DOI] [Google Scholar]
- 47. Gafur NA, Sakakibara M, Sano S, et al. : A case study of heavy metal pollution in water of Bone River by Artisanal Small-Scale Gold Mine Activities in Eastern Part of Gorontalo, Indonesia. Water (Switzerland). 2018;10:1–10. 10.3390/w10111507 [DOI] [Google Scholar]
- 48. Rumahlatu D: Konsentrasi Logam Berat Kadmium Pada Air, Sedimen dan Deadema setosum (Echinodermata, Echinoidea) di Perairan Pulau Ambon. ILMU Kelaut. Indones. J. Mar. Sci. 2012;16:78–85. [Google Scholar]
- 49. Lee S, Chung J, Lee YW: Cu and Zn Concentrations in Seawater and Marine Sediments Along Korean Coasts from the Perspective of Antifouling Agents. Bull. Environ. Contam. Toxicol. 2018;101:185–190. 10.1007/s00128-018-2380-y [DOI] [PubMed] [Google Scholar]
- 50. Nindyapuspa A, Ni’Am AC: Distribution of heavy metals (Cu and Fe) in sea water of Gresik coastal area. E3S Web Conf. 2018,2017–2019;31. 10.1051/e3sconf/20183104012 [DOI] [Google Scholar]
- 51. Bazzi AO: Heavy metals in seawater, sediments and marine organisms in the Gulf of Chabahar, Oman Sea. J. Oceanogr. Mar. Sci. 2014;5:20–29. 10.5897/JOMS2014.0110 [DOI] [Google Scholar]
- 52. Sun X, Li BS, Liu XL, et al. : Spatial Variations and Potential Risks of Heavy Metals in Seawater, Sediments, and Living Organisms in Jiuzhen Bay, China. J. Chem. 2020; (2020). 10.1155/2020/7971294 [DOI] [Google Scholar]
- 53. Rezaei A, Hassani H, Fard Mousavi SB, et al. : Evaluation of Heavy Metals Concentration in Jajarm Bauxite Deposit in Northeast of Iran Using Environmental Pollution Indices. Malaysian J. Geosci. 2019;3:12–20. 10.26480/mjg.01.2019.12.20 [DOI] [Google Scholar]
- 54. Ismail SNS, Abidin EZ, Praveena SM, et al. : Heavy metals in soil of the tropical climate bauxite mining area in Malaysia. J. Phys. Sci. 2018;29:7–14. 10.21315/jps2018.29.s3.2 [DOI] [Google Scholar]
- 55. Wardani NK, Prartono T, Sulistiono: Sediments quality based on geo-accumulation index in heavy metals (Pb, cu, and cd) of cengkok coastal waters, banten bay. J. Pendidik. IPA Indones. 2020;9:574–582. 10.15294/jpii.v9i4.24908 [DOI] [Google Scholar]
- 56. Wisha UJ, Heriati A, Ramdhan M, et al. : Spatial Distribution of Dissolved Heavy Metals (Hg, Cd, Cu, Pb, Zn) on the Surface Waters of Pare Bay, South Sulawesi. ILMU Kelaut. Indones. J. Mar. Sci. 2019;23:199. 10.14710/ik.ijms.23.4.199-206 [DOI] [Google Scholar]
- 57. Hamuna B, Tanjung RHR: Heavy metal content and spatial distribution to determine the water pollution index in depapre waters, Papua, Indonesia. Curr. Appl. Sci. Technol. 2021;21:1–11. [Google Scholar]
- 58. Apriadi T, Pratama G, Putra RD, et al. : Comparative study on the fish diversity from natural and bauxite postmining in wetland system of Bintan Island, Indonesia. Biodiversitas. 2018;19:967–973. 10.13057/biodiv/d190327 [DOI] [Google Scholar]
- 59. Puspitasari R. Logam dalam Ekosiste Perairan. Bawal. 2006;1: 1–6. [Google Scholar]
- 60. Tampubolon OFR, Ismanto A, Suryo AAD, et al. : Simulasi Pola Sebaran Logam Berat Tembaga (Cu) di Perairan Kota Pekalongan. Indonesian Journal of Oceanography .2021;3: 1–15. [Google Scholar]