Abstract
BACKGROUND
Oocyte activation deficiency (OAD) is attributed to the majority of cases underlying failure of ICSI cycles, the standard treatment for male factor infertility. Oocyte activation encompasses a series of concerted events, triggered by sperm-specific phospholipase C zeta (PLCζ), which elicits increases in free cytoplasmic calcium (Ca2+) in spatially and temporally specific oscillations. Defects in this specific pattern of Ca2+ release are directly attributable to most cases of OAD. Ca2+ release can be clinically mediated via assisted oocyte activation (AOA), a combination of mechanical, electrical and/or chemical stimuli which artificially promote an increase in the levels of intra-cytoplasmic Ca2+. However, concerns regarding safety and efficacy underlie potential risks that must be addressed before such methods can be safely widely used.
OBJECTIVE AND RATIONALE
Recent advances in current AOA techniques warrant a review of the safety and efficacy of these practices, to determine the extent to which AOA may be implemented in the clinic. Importantly, the primary challenges to obtaining data on the safety and efficacy of AOA must be determined. Such questions require urgent attention before widespread clinical utilization of such protocols can be advocated.
SEARCH METHODS
A literature review was performed using databases including PubMed, Web of Science, Medline, etc. using AOA, OAD, calcium ionophores, ICSI, PLCζ, oocyte activation, failed fertilization and fertilization failure as keywords. Relevant articles published until June 2019 were analysed and included in the review, with an emphasis on studies assessing large-scale efficacy and safety.
OUTCOMES
Contradictory studies on the safety and efficacy of AOA do not yet allow for the establishment of AOA as standard practice in the clinic. Heterogeneity in study methodology, inconsistent sample inclusion criteria, non-standardized outcome assessments, restricted sample size and animal model limitations render AOA strictly experimental. The main scientific concern impeding AOA utilization in the clinic is the non-physiological method of Ca2+ release mediated by most AOA agents, coupled with a lack of holistic understanding regarding the physiological mechanism(s) underlying Ca2+ release at oocyte activation.
LIMITATIONS, REASONS FOR CAUTION
The number of studies with clinical relevance using AOA remains significantly low. A much wider range of studies examining outcomes using multiple AOA agents are required.
WIDER IMPLICATIONS
In addition to addressing the five main challenges of studies assessing AOA safety and efficacy, more standardized, large-scale, multi-centre studies of AOA, as well as long-term follow-up studies of children born from AOA, would provide evidence for establishing AOA as a treatment for infertility. The delivery of an activating agent that can more accurately recapitulate physiological fertilization, such as recombinant PLCζ, is a promising prospect for the future of AOA. Further to PLCζ, many other avenues of physiological oocyte activation also require urgent investigation to assess other potential physiological avenues of AOA.
STUDY FUNDING/COMPETING INTERESTS
D.G. was supported by Stanford University’s Bing Overseas Study Program. J.K. was supported by a Healthcare Research Fellowship Award (HF-14-16) made by Health and Care Research Wales (HCRW), alongside a National Science, Technology, and Innovation plan (NSTIP) project grant (15-MED4186-20) awarded by the King Abdulaziz City for Science and Technology (KACST). The authors have no competing interests to declare.
Keywords: assisted oocyte activation (AOA), oocyte activation, calcium ionophores, ICSI, phospholipase C zeta (PLCζ), oocyte activation deficiency (OAD), calcium, male infertility, sperm, oocyte
WHAT DOES THIS MEAN FOR PATIENTS?
At fertilization, oocyte activation is triggered by sperm-specific phospholipase C zeta (PLCζ) by releasing calcium in specific patterns within the oocyte. A deficiency in this process underlies most cases of fertilization failure in mammals. This process of calcium release can be clinically mimicked via assisted oocyte activation (AOA), involving a combination of mechanical, electrical and/or chemical stimuli.
Recent advances in AOA techniques warrant a review of the safety and efficacy of these practices and of how AOA may be clinically implemented in the clinic. Herein, following a detailed literature review examining studies assessing large-scale efficacy and safety, the main concern impeding clinical AOA implementation is its non-physiological nature, coupled with a lack of holistic understanding of physiological mechanism(s) underlying calcium release at fertilization.
We find that numerous questions require urgent attention before widespread clinical utilization of such protocols can be advocated. We hope that this article will be able to aid the burgeoning number of researchers investigating the clinical efficacy of such methodology in further refining the practice until large-scale utilization can be achieved and accepted.
Introduction
Of all cases of infertility, 30–50% can be attributed to a male causative factor (Kumar and Singh, 2015; Leaver, 2016), while about 30% of all cases of infertility cannot currently be explained (Ray et al., 2012). In both instances, ICSI (whereby a single sperm is injected directly into the oocyte) represents a standard mode of treatment (Palermo et al., 2015; Rubino et al., 2016; Simopoulou et al., 2016; Palermo et al., 2017; Borges et al., 2020b). ICSI itself is just one of a suite of laboratory techniques designed to treat various forms of infertility, collectively termed ART. However, ICSI usage increased from 15.4% to 66.9% between 1996 and 2012, even though ICSI is not universally recommended for normospermic men (Boulet et al., 2015; Sustar et al., 2019; Ten et al., 2019). ICSI yields fertilization rates of 70–80%, but failed fertilization following ICSI, sometimes recurrent over repeated cycles and miscarriage remain a difficult reality for many couples (Montag et al., 2012; Yoon et al., 2013; D’Haeseleer et al., 2014; Nikiforaki et al., 2016; Karabulut et al., 2018; Koot et al., 2019; Rumbold et al., 2019; Duran‐Retamal et al., 2020). Worryingly, numerous indications now suggest an increased incidence of birth defects in ART babies compared to naturally conceived counterparts (Zhao et al., 2020).
Total fertilization failure (TFF) occurs in 1–5% of ICSI cases (Kyono et al., 2012; Montag et al., 2012; Vanden Meerschaut et al., 2014b; Kashir et al., 2018; Basirat et al., 2019; Bassiri et al., 2020). Moreover, associations have been identified between embryo grading and congenital malformations, endocrine profile changes and deficient hearing in ICSI-conceived babies (Abel et al., 2019; Belva et al., 2019; Yasemin Sert et al., 2019), while age, antimullerian hormone levels and antral follicle counts are associated with live birth rates following ICSI, with other hormones currently under investigation (Metello et al., 2019; Zheng et al., 2019; Song et al., 2020; Tarín et al., 2020; Tiegs et al., 2020; Wang et al., 2020; Ye et al., 2020; Zanetti et al., 2020). The use of ART however does not seem linked to an increase in the risk of autism spectrum disorder or preeclampsia (Diop et al., 2019; Kennedy et al., 2019). Only frozen embryo transfer was associated with a small yet statistically significant increased risk for childhood cancer (Hargreave et al., 2019), and the cognitive, behavioural and school performance of children born from IVF versus ICSI remain comparable (Heineman et al., 2019; Norrman et al., 2020).
Various factors account for ICSI failure, including unexplained non-male factor infertility (Gennarelli et al., 2019). However, a failure or defect in a series of concerted events at fertilization, collectively termed oocyte activation deficiency (OAD), whether sperm- or oocyte-borne, is the main cause for TFF (Tesarik et al., 2002; Vanden Meerschaut et al., 2014b; Deemeh et al., 2015; Capalbo et al., 2016; Tosti and Ménézo, 2016; Karabulut et al., 2018; Kashir et al., 2018), resulting in the inability of mature oocytes to undergo activation and complete fertilization by sperm. This is thought to be directly responsible for 40% of ICSI failures (Heindryckx et al., 2008; Yeste et al., 2016a), with perhaps a higher level attributable in an indirect manner (Sang et al., 2018; Yang et al., 2019; Kashir, 2020; Kashir et al., 2020b).
Perhaps due to the increasing proportion of ICSI cycles, the increasing rates of TFF and OAD are to be expected (Montag et al., 2012; Santella and Dale, 2015; Aydinuraz et al., 2016; Ebner and Montag, 2016; Bonte et al., 2019). Significant efforts have focussed on elucidating the molecular mechanisms underlying both oocyte activation and fertilization failure, and the clinical methodologies used to rectify cases of OAD, termed assisted oocyte activation (AOA). However, the safety and efficacy of such methods remains controversial, with no clear indication as to whether such protocols should be applied or not within the clinic. Herein, we review recent findings regarding AOA efficacy and safety, exploring the major obstacles preventing widespread use of AOA in clinical practice, in line with recent clinical evaluation on the utilization of AOA. We also briefly examine the molecular mechanisms underlying oocyte activation and posit potential alternatives to current strategies for AOA to improve the efficacy of such treatments, with perhaps an improvement in ART success rates.
Methods
This article is based on a critical review of literature on peer-reviewed article indexing databases including PubMed, Scopus and Medline, using AOA, OAD, calcium ionophores, ICSI, phospholipase c zeta (PLCζ), oocyte activation, failed fertilization and fertilization failure as keywords.
Physiological mechanism of oocyte activation
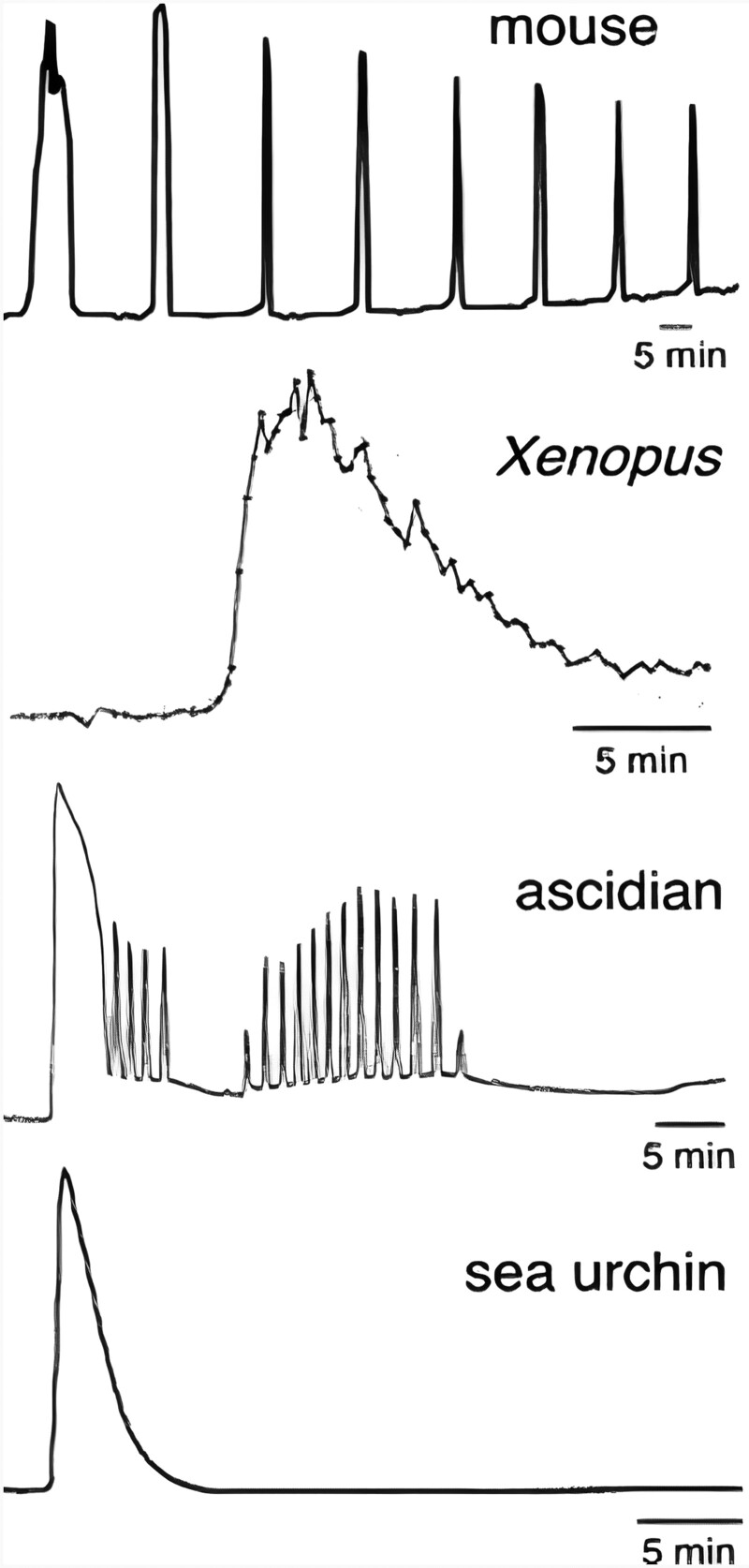
Oocyte activation is a spatially and temporally orchestrated process (Xu and Yang, 2017), resulting in established endpoints including resumption of meiosis II (MII), second polar body (2PB) extrusion, cortical granule exocytosis and cytoskeletal rearrangements (Hosseini et al., 2008; Tosti and Ménézo, 2016; Kashir et al., 2018; Sha et al., 2019). In mammals, these events are a collective culmination of temporally-mediated cytoplasmic calcium (Ca2+) levels, initiated in an inositol 1,4,5-trisphosphate receptor-dependent manner from Ca2+ stores such as the endoplasmic reticulum (ER) (Alberio et al., 2000; Yeste et al., 2016a). Observed in all species till date, the spatial and temporal pattern of the Ca2+ release is species-specific in amplitude, frequency and number (Fig. 1) and in molecular modulation (Kashir et al., 2018). Other components at the transcriptional and translational level certainly play a role (Dorfeshan et al., 2019; Rong et al., 2019; Sha et al., 2019; Zhang et al., 2019; Kang et al., 2020; Sacha et al., 2020; Sha et al., 2020; Zhang et al., 2020), but we focus herein on the wave-like Ca2+ diffusion that is integral to oocyte activation.
Figure 1.
Representative Ca2+ responses at fertilization in eggs/oocytes of several species. Figure reproduced from Miyazaki (2006) with permission.
Ca2+ at oocyte activation
Ca2+-sensitive dyes have revealed in non-mammalian species that activation is triggered by a single large transient increase in Ca2+ (Horner and Wolfner, 2008). However, mammalian oocytes undergo a series of these Ca2+ transients, defined as oscillations (Publicover et al., 2007; Swann and Yu, 2008). Importantly, the specific patterns of Ca2+ release in terms of amplitude, duration and frequency over time seem largely species-specific in all species studied to date (Miyazaki et al., 1993; Jones et al., 1998b; Stricker, 1999; Ducibella et al., 2002, 2006; Ducibella and Fissore, 2008). Ca2+ oscillations in mammalian oocytes are a direct consequence of cytosolic inositol trisphosphate (IP3), indicating that this signalling cascade initiates with hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) (Fig. 2) (Parrington, 2001; Swann et al., 2006; Whitaker, 2006; Parrington et al., 2007; Swann and Yu, 2008). Microinjecting Ca2+ ions triggers mouse blastocyst development (Fulton and Whittingham, 1978; Swann and Yu, 2008), while blocking, down-regulating or reducing levels of IP3-Rs inhibits Ca2+ oscillations and oocyte activation in mouse and hamster oocytes (Miyazaki et al., 1993; Brind et al., 2000; Jellerette et al., 2000; Xu et al., 2003). Cytosolic IP3 peaks are also observed during fertilization in mammalian oocytes (Swann and Yu, 2008).
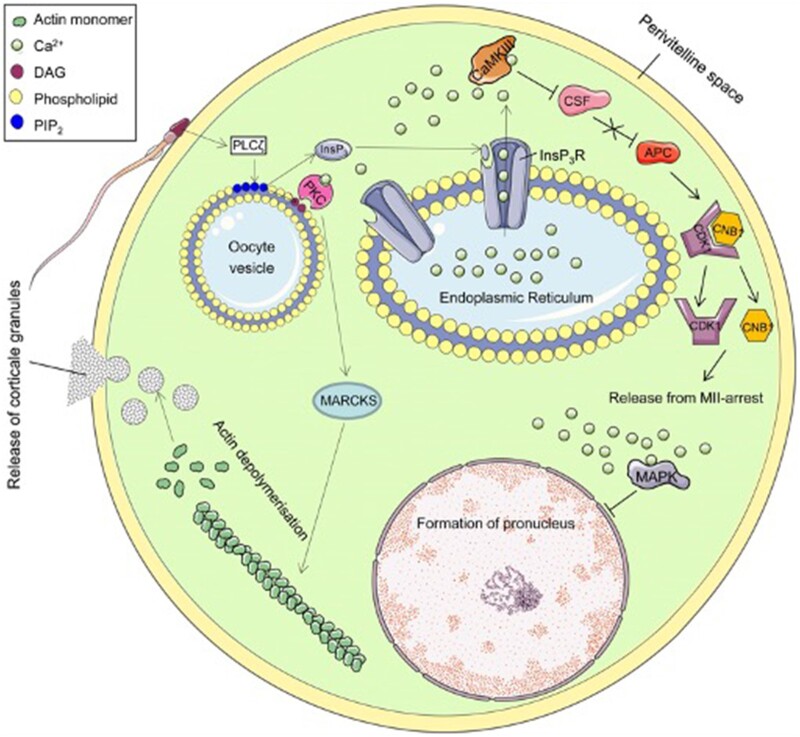
Figure 2.
Schematic summary of the proposed mechanism underlying Ca2+ release at oocyte activation. The fertilizing sperm triggers Ca2+ following delivery of sperm-specific phospholipase C zeta (PLCζ) to the oolemma during or following oocyte-sperm membrane fusion. PLCζ interacts with an as yet unknown oocyte-borne factor(s), facilitating hydrolysis of PIP2 into DAG and InsP3, which subsequently triggers Ca2+ release from intracellular stores, alleviating the MII-arrest. The proposed mechanism mediates cortical granules exocytosis, MAPK deactivation and subsequent pronuclei formation and CaMKII activation, inhibiting CSF (Emi2) and liberating APC. This reduces levels of Cyclin B1 in the maturation-promoting factor (MPF) complex comprising CDK1 and Cyclin B1, which inactivates MPF, releasing the oocyte from MII-arrest. APC, anaphase-promoting complex/cyclosome; CaM/CaMKII, calcium/calmodulin-dependent protein kinase II; CSF, cytostatic factor; CNB1, cyclin B1; CDK1, cyclin-dependent kinase 1; DAG, diacylglycerol; InsP3, inositol 1,4,5-trisphosphate; InsP3R, InsP3 receptor; MAPK, mitogen-activated protein kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C. Schematic reproduced with permission from Yeste et al. (2016b).
Mammalian Ca2+ oscillations alleviate MII arrest through cyclin B1 proteolysis, mediated by ubiquitin or proteasome activation (Fig. 2) (Miyazaki and Ito, 2006). Ca2+–calmodulin association activates calmodulin-dependent kinase II (CaMKII) (Miyazaki and Ito, 2006) in a repetitive manner that occurs coincident with each Ca2+ peak in fertilizing mouse oocytes, resulting in Cyclin B1 polyubiquitination by the anaphase promoting complex/cyclosome (APC/C), a E3 ubiquitin ligase (Swann and Lai, 2016). This cascade is prevented in unfertilized oocytes by cytostatic factor (CSF), maintaining MII arrest (Hyslop et al., 2004; Jones, 2004; Miyazaki and Ito, 2006). Upon fertilization, CaMKII inhibits CSF components (Hyslop et al., 2004). Persistent Ca2+ oscillations also contribute to pronuclear formation via reduction of mitogen-associated protein kinase activity (Ducibella et al., 2002; Miyazaki and Ito, 2006).
Such is the importance of Ca2+ profiles at fertilization that the frequency and amplitude of Ca2+ oscillations affected early embryo protein profiles in mice (Ducibella et al., 2002) and embryonic development in rabbits, determining transplantation rates of four-cell embryos (Swann and Ozil, 1994; Miyazaki and Ito, 2006). Oocyte activation events are also temporally and spatially sensitive to specific Ca2+ oscillation profiles in mammals in a chronological manner, with early events such as cortical granule exocytosis requiring fewer oscillations than later events such as the alleviation of MII arrest (Malcuit et al., 2006; Stitzel and Seydoux, 2007). Furthermore, the number of Ca2+ pulses required to complete oocyte activation is greater than the number required to initiate exit from MII arrest (Ducibella et al., 2002; Krauchunas and Wolfner, 2013). Thus, this degree of sensitivity and potential downstream effect on embryogenesis necessitated the elucidation of the causative stimulant that initiates these essential patterns of Ca2+ release.
The precise mechanisms underlying Ca2+ oscillations in mammals have been subject to much debate, particularly regarding the roles played by both gametes. Three predominant models were hypothesized: (i) the Ca2+ conduit model (Jaffe, 1983, 1991), (ii) the membrane receptor model (Jaffe, 1990; Schultz and Kopf, 1995; Evans and Kopf, 1998; Parrington et al., 2007) and (iii) the soluble sperm factor model (Swann et al., 2006; Parrington et al., 2007; Saunders et al., 2007). Data obtained for one or more species can be interpreted as supporting each of these models as being the causative theory behind oocyte activation, which are more thoroughly reviewed elsewhere (Stricker, 1999; Kashir et al., 2013a). However, significant evidence supports the theory of sperm factor mediation within mammals and other taxa (Jones et al., 1998b; Swann et al., 2006; Whitaker, 2006; Parrington et al., 2007; Iwao, 2012).
The ‘sperm-factor’ model suggests that oocyte activation is triggered by a soluble factor released from the sperm into the oocyte during, or immediately following, gamete fusion (Lawrence et al., 1997). Injecting sperm extracts into the eggs of a variety of species resulted in successful Ca2+ release and oocyte activation (Swann, 1990; Kyozuka et al., 1998; Stricker, 1999; Dong et al., 2000; Coward et al., 2003) suggesting that similar sperm-based oocyte activation mechanisms exist throughout a wide spectra of (at least invertebrate) species. The soluble sperm factor responsible for initiating activation in mammalian oocytes seems sperm-specific, as injection of other somatic cells into oocytes does not cause Ca2+ transients (Swann, 1990; Wu et al., 1997; Kashir et al., 2013a), while ICSI also successfully results in oocyte activation and fertilization.
Regulation of calcium stores
While the major source of Ca2+ release at fertilization is the ER, extracellular Ca2+ influx is required to maintain these Ca2+ oscillations. Indeed, depletion of extracellular Ca2+ reduces the frequency of or completely ceases Ca2+ oscillations (Igusa and Miyazaki, 1983; Kline and Kline, 1992a; Takahashi et al., 2013). Indeed, Ca2+ influx also seems to play a crucial role in determining the intervals between Ca2+ transients (Takahashi et al., 2013). A candidate phenomenon underlying Ca2+ store regulation has been a mechanism termed store operated Ca2+ entry (SOCE), which maintains intracellular Ca2+ homeostasis from the extracellular milieu within at least somatic cells (Putney, 1986; Miao and Williams, 2012). Major regulators of SOCE seem to be the STIM proteins (STIM1/STIM2), transmembrane ER proteins that bind Ca2+ (Williams et al., 2001; Roos et al., 2005; Cai, 2007; Hewavitharana et al., 2007; Miao and Williams, 2012).
The sensitivity of STIM2 to even minor decreases in Ca2+ levels within the ER lumen allows it to stabilize basal cytosolic and ER Ca2+ levels under non-stimulated conditions (Brandman et al., 2007). STIM1, however, can only be activated by larger decreases in ER luminal Ca2+ following large-scale Ca2+ release (such as at oocyte activation) (Miao and Williams, 2012). In somatic cells, plummeting ER Ca2+ causes oligomerization and redistribution of STIM1, activating STIM1 (Liou et al., 2005; Smyth et al., 2008), which then signal ORAI proteins, stimulating transport of extracellular Ca2+ into the cytosol (Zhang et al., 2005; Feske et al., 2006; Vig et al., 2006). Sarcoplasmic/ER Ca2+ ATPase pumps can then transport Ca2+ back into the ER, replenishing cellular stores (Miao and Williams, 2012).
Considering that mammalian oocytes express both STIM1 and ORAI1 (Gómez-Fernández et al., 2009; Koh et al., 2009), while mouse oocytes also express STIM2, this would suggest a similar mechanism for Ca2+ store regulation in oocytes (Miao and Williams, 2012). However, recent data have indicated that female mice lacking one or both STIM proteins remained fertile, with oocytes exhibiting normal patterns of Ca2+ release post-fertilization, as well as ER Ca2+ stores or Ca2+ influx following depletion (Bernhardt et al., 2017). Similar observations were also made with oocytes from mice lacking ORAI (Bernhardt et al., 2017). Such data perhaps indicate that the STIM1/STIM2/ORAI mechanisms do not play a major role at least within mouse oocytes.
Indeed, neither known SOCE blockers nor the expression of STIM1/ORAI inhibitory protein fragments affect the Ca2+ oscillation frequency or influx rate (Takahashi et al., 2013). Intriguingly, however, Bernhardt et al. (2017) also indicated that fertilization-associated patterns of Ca2+ release were impaired by NS8593, a TRPM7-specific inhibitor. Oocytes depleted of both TRPM7 and CaV3.2 terminate oscillations prematurely, with a concurrent delay in resumption of oscillations, strongly indicating a collective action of multiple factors in maintaining the majority of Ca2+ influx following fertilization (Bernhardt et al., 2018). Collectively, such findings suggest that CaV3.2 and TRPM7 serve as essential mediators of Ca2+ influx following fertilization, at least within mice (Stein et al., 2020). Double knock out mice depleted of both TRPV3 and CaV3.2 were subfertile, with reduced oocyte Ca2+ stores. Furthermore, the number of double knock-out oocytes exhibiting Ca2+ release was significantly lower that WT at fertilization, and oscillations were also of reduced frequency (Mehregan et al., 2021). Collectively such studies indicate that the collective action of TRPV3 and CaV3.2 is required for both initiation and specific profiles (amplitude, frequency and longevity) of Ca2+ oscillations during fertilization, at least within mammals (Mehregan et al., 2021).
While such suggestions may be true for mice, this may not be the entire picture for all mammals. Indeed, evidence suggests that in the pig, Ca2+ oscillations are indeed supported by SOCE, with Ca2+ release accompanied by repeated interactions between STIM1 and ORAI, while a STIM1 puncta formation inhibitor (ML-9) blocks SOCE, also disrupting Ca2+ oscillations in fertilized oocytes (Zhang et al., 2018). Lysosomes may also exhibit some degree of Ca2+ uptake mechanisms. However, the exact underlying mechanisms remain unknown (Lloyd-Evans and Waller-Evans, 2020). Mitochondria can also uptake Ca2+ through the mitochondrial Ca2+ uniporter, and in the presence of high cytoplasmic Ca2+, through voltage-dependent anion channels (Romero-Garcia and Prado-Garcia, 2019; Stein et al., 2020).
PLCζ, oocyte activation and male infertility
Numerous studies have attempted to identify the most physiologically relevant candidate for the sperm factor in mammals, initially suggesting that a 33 kDa oscillogen initiated Ca2+ oscillations (Parrington et al., 1996), yet recombinant versions were unable to elicit Ca2+ oscillations in mouse oocytes (Wolosker et al., 1998; Parrington et al., 1999; Swann et al., 2004). Subsequently, the truncated form of the c-kit receptor, tr-kit, was proposed to induce parthenogenetic mouse oocyte activation (Sette et al., 1997, 2002) purportedly via activation of phospholipase C gamma-1 (PLCγ1) through phosphorylation by a Src-like Kinase Fyn (Sette et al., 1998). However, independent efforts have thus far failed to replicate such results, or emulate these in humans (Kashir et al., 2014).
Another more recent proposal is the post-acrosomal sheath WW domain-binding protein (PAWP), microinjection of which into porcine, bovine, macaque and Xenopus eggs resulted in pronuclear formation (Wu et al., 2007). PAWP injection also caused a Ca2+ increase when injected into Xenopus eggs but did not appear to mimic the single large Ca2+ wave normally seen at fertilization in such eggs (Aarabi et al., 2009; 2014a,b). However, despite earlier preliminary studies (Aarabi et al., 2014a,b), repeated independent experiments could not demonstrate the ability of recombinant mouse and human PAWP to elicit any detectable Ca2+ release when microinjected into mouse oocytes (Kashir et al., 2014; Nomikos et al., 2015). However, when one considers that sperm-induced Ca2+ oscillations seem to be caused by activation of the inositol 1,4,5-trisphosphate (InsP3) signalling pathway (Miyazaki et al., 1992), this suggests that the sperm factor might itself be a phospholipase C (PLC) isoform (Jones et al., 1998a).
Of the known mammalian phosphoinositide (PI)-specific PLC isozymes at the time (Kelley et al., 2001; Rhee, 2001; Song et al., 2001; Hwang et al., 2005; Nakahara et al., 2005; Zhou et al., 2005), very few were able to successfully and physiologically result in successful oocyte activation (Yeste et al., 2016a; Kashir et al., 2018). Indeed, while several PLCs exert specific roles at fertilization within both gametes (PLC delta 4 in the acrosome reaction, or PLC beta 1 in regulating calcium dynamics in the oocyte) (Fukami et al., 2001, 2003; Igarashi et al., 2007), most PLC isoforms do not seem directly involved in oocyte activation, failing to elicit Ca2+ release upon injection into mouse oocytes (Kashir, 2020).
It was not until mouse express sequence tag (EST) databases were used that a testis-specific PLC was described, termed PLCzeta (PLCζ), a ∼74 kDa protein in mice and ∼70 kDa in humans (Cox et al., 2002; Saunders et al., 2002). Immunodepletion of PLCζ from sperm extracts diminished Ca2+ release following injection into mouse oocytes or sea urchin egg homogenates (Saunders et al., 2002), while recombinant PLCζ injections in mouse oocytes elicited fertilization-like Ca2+ oscillations, supporting blastocyst development (Saunders et al., 2002; Kouchi et al., 2004). Finally, disruption of PLCζ expression in mice testes through RNA interference exhibited sperm that induced prematurely ending Ca2+ oscillations. While these mice were not infertile, mating experiments yielded significantly reduced litter sizes (Knott et al., 2005). Finally, two papers (Hachem et al., 2017; Nozawa et al., 2018) recently reported the creation of transgenic knockout mouse models of PLCζ, both concluding (albeit with caveats as discussed later herein) that PLCζ is the primary physiological stimulus of Ca2+ oscillations at fertilization.
PLCζ is much more potent in mouse oocytes compared to other PLCs (Swann et al., 2006, 2007; Swann and Lai, 2016). Although further details regulating the molecular mechanisms underlying PLCζ mechanistic action is urgently required, PLCζ is thought to target PIP2-containing cytoplasmic vesicular lipids within the oocyte (Yu et al., 2012) to form IP3, leading to subsequent Ca2+ release from stores such as the ER in an IP3R-dependent manner (Fig. 2) (Swann and Lai, 2016; Swann, 2020). PLCs usually produce IP3 through PIP2 that are present almost exclusively in membranes, suggesting that PLCζ should target the oocyte plasma membrane, where cells normally contain the bulk of their PIP2 (Halet et al., 2002; Yu et al., 2012). However, rather than a decrease of PIP2 as would be expected in such a case, an increase in PIP2 levels at the plasma membrane is observed following both normal fertilization and PLCζ microinjection (Halet et al., 2002), while conversely, PLCδ1 injection led to a loss of plasma membrane PIP2 (Yu et al., 2012; Swann and Lai, 2016). Indeed, the first Ca2+ transient during mammalian fertilization initiates from the point of gamete fusion, with subsequent Ca2+ release occurring from multiple regions throughout the fertilizing oocyte, implying that the majority of PIP2 hydrolysis and InsP3 generation occurs within the oolemma (Fig. 2); these assertions supported by numerous experimental and theoretical models (Whitaker and Irvine, 1984; Dupont and Dumollard, 2004; Sanders and Swann, 2016).
PLCζ was observed uniformly distributed throughout the oocyte cytoplasm, not at the plasma membrane (Yoda et al., 2004; Yu et al., 2008), specifically within vesicles no bigger than 1 µm (Yu et al., 2012). This suggests that IP3 is generated from an intracellular source of PIP2 during fertilization. Indeed, mammalian sperm extracts (containing PLCζ) hydrolyze PIP2 in sea urchin egg homogenates, while maximal IP3 was generated in those fractions of homogenates that were richest in yolk, vesicles which demonstrably contain PIP2 (Snow et al., 1996; Rice et al., 2000). However, as mouse oocytes do not contain yolk, the nature of the observed vesicles remains to be determined (Swann and Lai, 2016).
Several conditions of male infertility (accounting for 19–57% of cases of total infertility) currently remain untreatable following application of ART (Kashir, 2020; Kashir et al., 2010; Saleh et al., 2020), even following ICSI. Indeed, up to 5% of ICSI treatment cycles still fail, which is largely attributed to a defect in oocyte activation (Kashir et al., 2010). Sperm from infertile males which consistently fail to fertilize oocytes following ART (IVF or ICSI) either fail to elicit Ca2+ oscillations, or do so abnormally (Yoon et al., 2008; Heytens et al., 2009). Such sperm also exhibited abnormal patterns and levels of PLCζ within the sperm head, suggesting that defects in sperm PLCζ (at both gene and protein levels) may underlie such cases of fertilization failure, particularly considering that such fertilization failure can be ‘rescued’ following concurrent microinjection of infertile human sperm with recombinant PLCζ (Yoon et al., 2008).
However, further to the 1–5% of ICSI cycles expected to experience TFF, even the response from ‘fertile’ males is extremely variable, with ∼20% of human sperm exhibiting only 1 or 2 Ca2+ transients upon injection into oocytes (Ferrer-Buitrago et al., 2018a,b), which is unlikely to activate oocytes (Swann, 2020). Furthermore, 10% of such sperm did not elicit any Ca2+ release at all, suggesting that the ability of normal human sperm to cause Ca2+ signals is likely to be highly variable; such assertions are in line with observations of variable localization patterns and levels of PLCζ in human sperm, and may underlie cases of low fertilization success (a much more common occurrence) in addition to TFF (Kashir et al., 2013b, 2020b; Yelumalai et al., 2015; Yeste et al., 2016b).
Clinically, complete fertilization failure is attributed to defective oocyte activation failure in a sperm-specific manner, more so than any other potential cause (Kashir et al., 2010). Furthermore, an increasing body of evidence is now associating PLCζ defects with not just outright OAD, but also a growing number of male factor conditions affecting sperm DNA integrity, morphology, count and motility, as well as the efficacy of cell cycle resumption rates and resulting embryogenesis (refer to (Kashir, 2020) for details).
Common methods of AOA
While the complete extent of the role of PLCζ in male fertility/infertility is currently the subject of much investigation (Kashir, 2020; Kashir et al., 2020a; Meng et al., 2020; Cheung et al., 2020), it is clear that removal or abrogation of the pattern of Ca2+ release at oocyte activation underlies numerous cases of male infertility and abnormal embryogenesis (Kashir et al., 2010). To this degree, AOA aims to trigger meiotic resumption in the oocyte by artificially elevating intracellular levels of Ca2+ (Ferrer-Buitrago et al., 2018a,b). Mechanical, electrical, chemical or a combination of these stimuli each present different means of AOA (Fig. 3), and are associated with unique risks and benefits (Alberio et al., 2000; Amdani et al., 2013, 2016; Nasr-Esfahani et al., 2010; Vanden Meerschaut et al., 2014b).
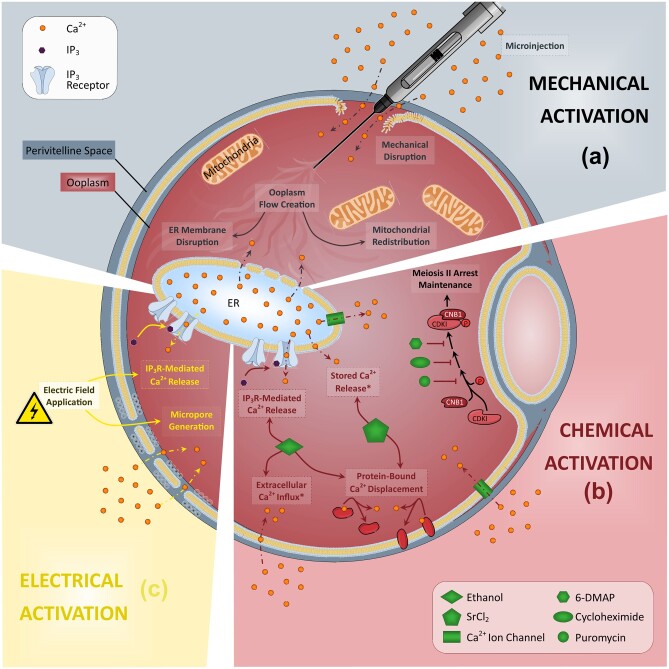
Figure 3.
Schematic representation of the purported mechanisms underlying the three most commonly applied methods of assisted oocyte activation. (a) Mechanical activation usually involves a disruption of the plasma membrane and/or components within the oolemma, leading to an elevation of Ca2+ within the oocyte due to influx of Ca2+ and/or disruption of Ca2+ store membranes such as the endoplasmic reticulum (ER). (b) The mechanisms underlying chemical activation vary on the type of agent utilized, but usually involve the facilitated transport of extracellular Ca2+ into the oocyte either directly or via transport channels. (c) Electrical activation involves generation of pores within the oocyte membrane via application of varying electrical fields, allowing extracellular Ca2+ influx into the oolemma.
Electrical activation usually involves the direct application of a voltage current, inducing charged lipid bilayer protein migration and pore formation in the membrane, enabling extracellular Ca2+ influx into the oolemma (Yanagida et al., 1999, 2008; Egashira et al., 2009; Vanden Meerschaut et al., 2014b). Mechanical activation usually involves oolemma piercing using micromanipulation, followed by vigorous cytoplasmic aspiration using a modified ICSI procedure (Tesarik et al., 2002; Ebner, 2004), eliciting a Ca2+ influx, usually followed by which ICSI is performed (Tesarik et al., 1994; Neri et al., 2014). Another mechanical method of activation is the microinjection of Ca2+ into the oocyte (Heindryckx et al., 2008; Neri et al., 2014). However, such methods are likely to be difficult to standardize, and as with most other physical methods, they will only induce a single Ca2+ increase (Kashir et al., 2010). In contrast, chemical activation is mediated by Ca2+ ionophores, which are lipid-soluble molecules that can transport Ca2+ across the oolemma, by increasing Ca2+ permeability and causing extracellular Ca2+ influx, and elicit intracellular Ca2+ stores to release stored Ca2+ (Yoshida and Plant, 1992; Vanden Meerschaut et al., 2013).
Mechanical activation
Mechanical AOA involves modified ICSI techniques using a microinjection ICSI needle (Tesarik et al., 2002), relying on oolemma piercing to elicit a calcium influx from the extracellular medium, following which ICSI is performed (Tesarik et al., 1994; Nasr-Esfahani et al., 2010; Neri et al., 2014). A popular mechanical AOA method is to manually disrupt the plasma membrane, followed by vigorous cytoplasmic aspiration, increasing the oocyte Ca2+ load during injection and leading to higher fertilization rates (Tesarik et al., 2002; Ebner, 2004). Such a methodology may also establish closer contact of the injected sperm with oocyte intracellular Ca2+ stores, enabling a more rapid diffusion of the physiological signalling pathway (Tesarik and Sousa, 1995). Less invasive techniques rely upon creating an ooplasm flow by mechanical disruption of Ca2+ stores (Tesarik et al., 2002; Ebner, 2004). Another method for mechanical oocyte activation is the direct microinjection of Ca2+ into the oocyte (Heindryckx et al., 2005, 2008; Neri et al., 2014).
Electrical activation
Electrical AOA protocols involve directly applied and/or alternate voltage currents to induce charged lipid bilayer proteins to move and form pores in the membrane, enabling extracellular Ca2+ flow into the oolemma (Yanagida et al., 2008; Egashira et al., 2009; Baltaci et al., 2010; Vanden Meerschaut et al., 2014b). A single electric pulse produces long-lasting, rapid Ca2+ elevations in the oocyte, which gradually return to baseline levels (Mansour et al., 2009; Vanden Meerschaut et al., 2014b), effectively parthenogenetically activating both human and mouse oocytes, inducing blastocyst formation (Versieren et al., 2010) and significantly improving fertilization rates compared to standard ICSI (Baltaci et al., 2010). Electrical resistance changes can also serve as a marker for confirming oocyte viability and penetration (Mor et al., 2020). Technological advances in nanoscale electrostimulation have also allowed for a preferential targeting of intracellular membranes, without much effect on the plasma membrane (Batista Napotnik et al., 2016), yielding high activation rates and improving parthenogenetic embryogenesis (Stein et al., 2020).
However, such technologies in species other than mouse have yet to be tested (Stein et al., 2020), while the overall efficiency of electrostimulation depends on multiple factors including pore size formed and ionic content of the surrounding medium. While such methods have successfully been applied on bovine and human oocytes (Yanagida et al., 2008), these procedures may induce reactive oxygen species (ROS) within oocytes (Koo et al., 2008). Furthermore, increases in Ca2+ are transient, with Ca2+ levels returning to basal values without the induction of oscillations (Neri et al., 2014).
Chemical activation
Chemical AOA protocols employ compounds that facilitate intracellular Ca2+ transients in the oolemma mediated via extracellular influx. Such agents are usually lipid-soluble compounds able to transport Ca2+ across cell membrane by increasing Ca2+ permeability and extracellular Ca2+ influx. Additionally, some compounds, such as IP3, also act on intracellular Ca2+ stores (Yoshida and Plant, 1992; Vanden Meerschaut et al., 2013). Examples of popular chemical activating agents include ethanol, ionomycin and A23187 (by far the most popular clinical agents). Most agents result in a single prolonged Ca2+ rise but fail to elicit normal Ca2+ patterns. Other activating agents have been shown to cause multiple transients, and include strontium chloride (SrCl2) in mice (Kline and Kline, 1992b; Kishigami and Wakayama, 2007) and phorbol esters (Cuthbertson and Cobbold, 1985) or thimerosal (Fissore et al., 1995). While strontium chloride’s mechanism of action in humans remains unclear (Swann, 2020), this compound is effective in mouse oocyte activation (Versieren et al., 2010; Nikiforaki et al., 2016; Kashir et al., 2018).
Further compounds also used include 6-dimethylaminopurine (6-DMAP) and puromycin (Alberio et al., 2000; Heindryckx et al., 2008; Darwish and Magdi, 2015; Kim et al., 2015; Yeste et al., 2016a; Aydinuraz et al., 2016; Capalbo et al., 2016; Nikiforaki et al., 2016; Yeste et al., 2016b; Shang et al., 2019), while exposure to low concentrations of ethanol also elicits a single rise in Ca2+, which also seems to increase the rate of high-quality embryo and blastocyst formation from fresh and vitrified human oocytes, in vitro (Zhang et al., 2017). The combination of multiple chemical mediators of AOA is able to produce embryos in animal models (Borges et al., 2020a). Ca2+ ionophores, such as ionomycin or A23187, are the most commonly used form of AOA in IVF clinics today (Ebner et al., 2012; D’Haeseleer et al., 2014; Ebner et al., 2015; Aydinuraz et al., 2016; Capalbo et al., 2016; Economou et al., 2017; Karabulut et al., 2018), and can work in conjunction with other compounds to enhance the processes important for fertility, such as acrosomal exocytosis (Akter et al., 2019).
Thimerosal is another compound that induces Ca2+ release in several cell types and is capable of eliciting Ca2+ oscillations in mammalian oocytes (Swann, 1991) by increasing IP3R sensitivity to Ca2+ (Cheek et al., 1993). However, thimerosal causes oxidation of tubulin, preventing polymerization and impairing spindle formation in oocytes (Alexandre et al., 2003), and requires sequential treatment with dithiothreitol to prevent tubulin oxidation. While this does indeed successfully initiates activation that closely resembles fertilization signaling (McDougall et al., 1993; Herbert et al., 1997; Nakada and Mizuno, 1998; Stein et al., 2020), the requirement for the use of a reducing reagent with thimerosal (which also prematurely terminates Ca2+ oscillations) has prevented its widespread clinical use (Stein et al., 2020).
Combined activation
Combinations of mechanical, electrical and/or chemical activation present innovative means of attempting to harness the unique benefits of each mode of activation, although with mixed results. One group (Heindryckx et al., 2005) injected a small amount of CaCl2 with sperm during ICSI followed by exposure to ionomycin, leading to improved fertilization success rates. In another study, after AOA using electrical activation, ionomycin or SrCl2, the media was supplemented with cycloheximide and/or DMAP. However, while no differences were observed in activation and cleavage, higher morulation and blastulation rates were observed for both mouse and human oocytes (Versieren et al., 2010). Another study utilized A23187 for AOA, supplementing the culture medium with granulocyte-macrophage colony stimulating factor (GM-CSF), increasing the number of high-quality embryos undergoing cleavage and blastulation. Analysis via array comparative genomic hybridization further suggested that exposure to GM-CSF after initial AOA could also result in fewer chromosomal abnormalities due to the cytogenic enhancing properties of GM-CSF (Economou et al., 2017). A point to note is that the oocytes used in this study (upon exposure to AOA and GM-CSF) were relatively older (18–20 h post-oocyte retrieval). Although aged oocytes are an unavoidable reality for such studies (as failure to fertilize requires time to confirm), the age here is perhaps another factor to be mindful of.
Clinical use of Ca2+ ionophores
By far, the most commonly used chemical means of AOA in both research and the clinic is A23187 (also known as calcimycin), a carboxylic antibiotic that binds divalent cations such as Ca2+ and Mg2+ and freely transports them across all biological membranes. The second-most common ionophore used in oocyte activation is ionomycin, which is far more specific for Ca2+ compared to A23187 and can activate and stimulate gene expression (Santella and Dale, 2015). A ready-to-use solution (CultActive), similar to A23187, has also been applied for clinical use with better success rates (Ebner et al., 2012). However, ionomycin seems more potent and specific compared to A23187 (Kauffman et al., 1980; Versieren et al., 2010; Nikiforaki et al., 2016).
Thus, while reports have been extensively described, the actual clinical applications of such chemicals remain limited. This is particularly problematic due to human oocytes not being particularly responsive to most of the aforementioned agents, relying on a combination of chemical treatments, coupled with sperm injection (Yamano et al., 2000; Neri et al., 2014). Furthermore, even following successful evocation of Ca2+ release in oocytes, most ionophores do not elicit the characteristic pattern of Ca2+ transients required for competent oocyte activation in humans, with only strontium chloride treatment in mice resulting in Cas+ oscillations, accompanied by oocyte activation and efficient parthenogenesis (Bos-Mikich et al., 1995; Ma et al., 2005). The efficiency of strontium chloride in humans remains debatable, as no Ca2+ oscillations are observed (Rogers et al., 2004). Strontium ions (Sr2+) are thought to gate oocyte IP3R1 receptors via the TRPV3 channel, which is thought to be involved in mediating Sr2+ influx in rodents (Brind et al., 2000; Jellerette et al., 2000; Carvacho et al., 2013). However, considering that Sr2+ is thought to mediate oocyte activation via CaMKIIγ (Backs et al., 2010), this very much remains a non-physiological mechanism of action.
Several studies have now examined the applicability of AOA within the clinic, for various chemical activating agents (Table I), with an equivalent body of research devoted to examining protocols using A23187 (Table II). One of the first reports on Ca2+ ionophore application examined ICSI couples characterized by poor fertilization rates, wherein ionophore treatment post-ICSI resulted in moderate zygote formation (Tesarik and Sousa, 1995). Subsequently, a study involving patients with a history of inconsistent fertilization and severe sperm morphological abnormalities used ionomycin to enhance fertilization but failed to generate good quality embryos (Moaz et al., 2006). Conversely, however, separate studies examining cases of sperm defects and failed fertilization that were treated with CaCl2 injection concurrent with ICSI, followed by sequential Ca2+ ionophore treatments, showed increased fertilization rates and clinical pregnancies and births neonates (Heindryckx et al., 2008; Nasr-Esfahani et al., 2008; Mansour et al., 2009; Neri et al., 2014). Numerous case reports now exist demonstrating that ICSI combined with AOA greatly increases fertilization and subsequent pregnancy rates (Rybouchkin et al., 1997; Kim et al., 2001; Tesarik et al., 2002; Heindryckx et al., 2005; Kyono et al., 2008; Tejera et al., 2008; Taylor et al., 2010), while a recent meta-analysis concluded that ionophore treatment significantly improved clinical pregnancy rates as well as oocyte activation (Murugesu et al., 2017). However, this contradicted an earlier meta-analysis that suggested the opposite (Sfontouris et al., 2015).
Table I.
A comparative overview of study design and outcomes of AOA protocols using various chemical activators.
| Endpoint type | Study type (AOA stimulus) | Fertilized oocytes (Total) | Experimental group (Total) | Control group | Primary findings | References |
|---|---|---|---|---|---|---|
| Efficacy |
|
Undisclosed |
|
Standard ICSI | Rates of fertilization and transferable embryos increased with AOA Blastulation, pregnancy and implantation rates not improved. |
Li et al. (2019b) |
| Safety |
|
|
|
Standard ICSI |
|
Li et al. (2019a) |
| Safety |
|
|
|
Standard ICSI |
|
Deemeh et al. (2015) |
| Efficacy |
|
|
|
Standard ICSI—split by sibling oocytes | Fertilization rates in patients with low fertilization history not always increased, even upon pre-screening for OAD. | Vanden Meerschaut et al. (2012) |
| Safety |
|
|
|
Natural conception | No intellectual or language disabilities identified in AOA children | D’Haeseleer et al. (2014) |
| Safety |
|
|
|
Natural conception | Cognitive, language, motor development and behaviour within general population standards | Vanden Meerschaut et al. (2014) |
| Safety |
|
|
|
None | Congenital malformations detected in 6.3% of children born following ionomycin treatment. | Mateizel et al. (2018) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Heindryckx et al. (2008) |
| Efficacy |
|
|
|
ICSI with activation- capable (control) or activation-deficient sperm |
|
Nikiforaki et al., (2016) |
| Safety and Efficacy |
|
|
|
Standard ICSI |
|
Kyono et al. (2012) |
| Efficacy |
|
|
|
|
|
Norozi-Hafshejani et al. (2018) |
| Efficacy |
|
|
|
Standard ICSI |
|
Zhang et al. (2017) |
| Efficacy |
|
|
|
Standard ICSI | Significantly improved high-quality embryo and blastocyst formation rates from vitrified oocytes to those comparable to fresh oocytes. | Liu et al. (2013) |
AOA, artificial oocyte activation; GM-CSF, granulocyte-macrophage colony stimulating factor; OAD, oocyte activation deficiency.
Table II.
An overview of study design and outcomes of AOA protocols utilizing A23187, CultActive and protocols supplemented with GM-CSF.
| Endpoint type | Study type | Fertilized oocytes (Total) | Experimental group (Population) | Control group | Primary findings | References |
|---|---|---|---|---|---|---|
| Efficacy |
|
|
|
Standard ICSI |
|
Nazarian et al. (2019) |
| Efficacy |
|
|
|
None | Treatment successfully produced live births for males with globozoospermia. | Shang et al. (2018) |
| Safety |
|
|
|
Standard ICSI |
|
Miller et al. (2016) |
| Safety |
|
|
|
IVF |
|
Capalbo et al. (2016) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Sdrigotti et al. (2015) |
| Safety |
|
|
|
None |
|
Lu et al. (2014) |
| Safety and Efficacy |
|
|
|
Standard ICSI |
|
Eftekhar et al. (2013) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Yoon et al. (2013) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Montag et al. (2012) |
| Safety and Efficacy |
|
|
|
Standard ICSI |
|
Economou et al. (2017) |
| Safety and Efficacy |
|
|
|
Standard ICSI | Improved fertilization and pregnancy rates, and comparable embryogenesis. | Karabulut et al. (2018) |
| Safety and Efficacy |
|
|
|
Standard ICSI and split by sibling oocytes | No improvement in fertilization rates and impeded embryogenesis quality. | Aydinuraz et al. (2016) |
| Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Darwish and Magdi (2015) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Ebner et al. (2015) |
| Safety and Efficacy |
|
|
|
Standard ICSI from previous cycles |
|
Ebner et al. (2012) |
AOA, artificial oocyte activation; GM-CSF, granulocyte-macrophage colony stimulating factor; IMSI, intra-cytoplasmic morphologically selected sperm injections; OAT, oligoasthenoteratozoospermia.
There seems to be an overall lack of consensus regarding the efficacy of improvements in fertilization and pregnancy rates following AOA (Neri et al., 2014). Furthermore, Vanden Meerschaut et al. (2012) indicated that AOA may not benefit all patients experiencing OAD, with fertilization history and sperm parameters seemingly playing an important role (Vanden Meerschaut et al., 2012, 2013; Neri et al., 2014). Thus, it is not yet clear which group of AOA patients would be most likely to benefit, apart from severe cases of OAD, without further clinical investigations. Indeed, current opinion with regards to this is split within the literature. A recent prospective multi-centre study concluded that Ca2+ ionophore treatment successfully increases clinical pregnancy and live-birth rates in patients with low or failed fertilization (Ebner et al., 2015).
Fertilization and pregnancy rates following AOA seem highly variable, most likely due to the heterogenic and low number of patients recruited in the vast majority of studies, with differences between patient baseline characteristics and activating agents employed, making it hard to compare different reports (Vanden Meerschaut et al., 2013). AOA protocols used throughout the published literature diverge in the ionophore concentration used, duration of ionophore exposure, the moment of ionophore exposure following ICSI and the number of ionophore exposures (Vanden Meerschaut et al., 2013). Thus, it appears likely that while AOA can be significantly effective to resolve at least cases of extreme OAD, further detailed and focused investigations are required to ascertain specific protocols for all groups of patients. Perhaps ionophore treatment success is related to fertilization rates in previous cycles, with AOA presenting with the best results in patients with a history of < 30% fertilization in a previous ICSI cycle (Vanden Meerschaut et al., 2012; Ebner et al., 2015).
Efficacy and safety of AOA
An obstacle to the widespread use of AOA is that the safety and efficacy of such practice is not yet fully established, with a dearth of randomized control trials and follow-up studies leaving the safety and efficacy of AOA unclear (Vanden Meerschaut et al., 2012; Santella and Dale, 2015; Sfontouris et al., 2015; van Blerkom et al., 2015; Aydinuraz et al., 2016; Ebner and Montag, 2016; Ferrer-Buitrago et al., 2018a,b). A further concern is that the Ca2+ oscillation pattern produced by AOA is distinct from that of physiological oocyte activation, whereby most Ca2+ ionophores release Ca2+ from intracellular stores in a temporally and spatially uncontrolled fashion in a single ‘tidal wave-like’ flow that does not correspond to the physiological activation process (Fig. 4) (Versieren et al., 2010; Ebner et al., 2012; Santella and Dale, 2015; van Blerkom et al., 2015). Perhaps the most reason to be cautious is that most ionophores are toxic to oocytes followed prolonged exposure (Steinhardt et al., 1974; Swann, 2018), and thus care must be taken to ensure the correct dosages and timings are applied.
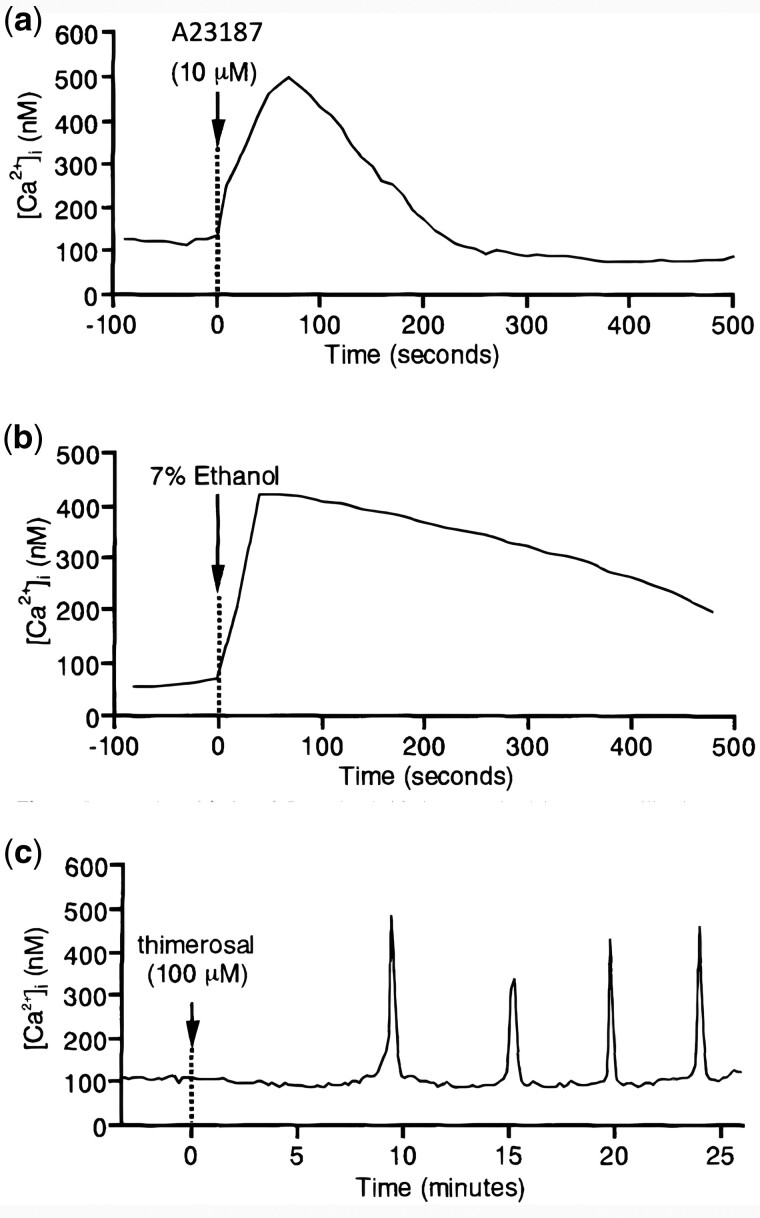
Figure 4.
Representative Ca2+ responses in mammalian oocytes following treatment. Treatment with (a) A23187, (b) 7% ethanol and (c) thimerosal. Figure reproduced from Nakada and Mizuno (1998) with permission.
Given the complexity of cellular mechanisms regulated by intra-cytoplasmic Ca2+ levels and oscillations (Alberio et al., 2000), Ca2+ ionophores have raised concerns of molecular downstream consequences, issues with long-term gene expression and epigenetic alterations (i.e. DNA methylation), with possible implications affecting embryonic development post-implantation (Ebner et al., 2012; Vanden Meerschaut et al., 2014b; Santella and Dale, 2015; Capalbo et al., 2016; Nikiforaki et al., 2016; Anifandis et al., 2019). For example, protein synthesis and degradation changes in the first cell cycle may occur as a result of AOA, based on data with parthenogenetically activated mammalian oocytes (Ebner and Montag, 2016). Furthermore, the effects of ionophores upon oocyte mitochondrial metabolism and cellular homeostasis remain unknown (Santella and Dale, 2015; van Blerkom et al., 2015; Kashir et al., 2018).
Mammalian oocyte activation involves a concerted profile of Ca2+ oscillations, with characteristic frequencies and amplitude of each transient (Kashir et al., 2013a, 2014), released in an IP3-dependant manner. Several animal studies have demonstrated that the number and amplitude of Ca2+ transients not only affects activation efficiency, but also profoundly influences subsequent embryonic development (Ducibella et al., 2002; Ozil et al., 2006; Kim et al., 2011b), blastocyst quality (Bos-Mikich et al., 1997) and the implantation potential of rabbit parthenogenotes (Ozil and Huneau, 2001) and mouse zygotes, resulting in altered embryonic gene expression (Ozil et al., 2006). Indeed, a higher activation rate does not necessarily correlate to a higher birth rate, which is potentially regulated by mechanisms within the oocyte activation process that affects later developmental stages (Yamamoto et al., 2020).
Although embryo quality does not seem to differ between the use of AOA or standard ICSI (Ebner et al., 2012, 2015; Ebner and Montag, 2016; Karabulut et al., 2018), there were only limited advantages for the application of AOA regarding fertilization rates (Karabulut et al., 2018). For example, although a recent study comparing the spindle-chromosome normalcy and IP3R1 distribution among embryos that failed either traditional ICSI or ICSI followed by AOA was unable to conclude a significant difference in Ca2+ releasing deficiencies between both groups, it identified a downstream activation deficiency that could not be overcome by Ca2+ ionophores alone (Ferrer-Buitrago et al., 2019). Both the efficacy and safety of AOA procedures have come under scrutiny, warranting further studies on the reproductive technique before widespread application in patients (Vanden Meerschaut et al., 2012; Santella and Dale, 2015; Sfontouris et al., 2015; van Blerkom et al., 2015; Aydinuraz et al., 2016; Capalbo et al., 2016; Ferrer-Buitrago et al., 2018b).
Recent studies have also indicated, at least on the face of it, that utilizing AOA does not significantly alter the morphokinetic parameters of embryos resulting from either ionomycin (Martínez et al., 2021) or calcimycin (A23187) (Shebl et al., 2021), with AOA embryos developing normally for most milestones. However, a key difference when utilizing ionomycin was that the time taken for second polar body extrusion (tPB2) and the third cell cycle (t3) were both significantly faster compared to the normal ICSI groups (Martínez et al., 2021), which perhaps is a reflection of the rapid and non-physiologic release of Ca2+ associated with use of ionophores (Kashir et al., 2020b; Martínez et al., 2021). Similarly, utilization of ready-to-use A23187 (calcimycin) solution did not result in major differences between groups, with most morphokinetic parameters exhibiting convergence, with the exception of the time taken for pronuclear formation (which was faster in the ionophore group) and third cell cycle (s3 in this study) (Shebl et al., 2021). It should be noted that pregnancy rates between ionophore and control groups remained non-significant, requiring more studies before such morphokinetics can be linked to pregnancy success.
The use of A23187 for AOA has raised mixed concerns over safety and the need for varying degrees of risk management. A23187 exposure has been shown to degrade embryos, and could underlie the risk of failed second polar body extrusion by lack of coordination with telophase in MII, although there has been no increase in chromosome segregation errors (Aydinuraz et al., 2016; Capalbo et al., 2016). When A23187 use was supplemented with GM-CSF, 62.5% of embryos were free of chromosomal abnormalities (Economou et al., 2017). Other studies have failed to link chromosomal malformations with AOA and A23187, by focusing on later stages of development, gestational outcomes, neonatal health (Miller et al., 2016), rates of medical abortions, birth defects, congenital malformations and multiple pregnancy risk, all indicating no significant change between cases without AOA (Sato et al., 2011; Ebner et al., 2012; Yoon et al., 2013; Deemeh et al., 2015; Ebner et al., 2015; Matsukawa et al., 2015; Sdrigotti et al., 2015; Miller et al., 2016).
Chromosomal alterations in children born from AOA were similar to those of their parents (Lu et al., 2014), while such children exhibited normal physical and mental development, as well as comparable language, cognitive and behavioural abilities to the general population (D’Haeseleer et al., 2014; Vanden Meerschaut et al., 2014b). In a decade-long study of children born from either A23187 or ionomycin use, only 6.3% of children had congenital malformations, all from treatment of ionomycin (Mateizel et al., 2018). Mouse zygotes treated with ionomycin exhibited normal embryogenesis and development of fertile pups (Heytens et al., 2008). However, application of high concentrations of ionomycin following mouse sperm ICSI increased the frequency and amplitude of Ca2+ release, influencing mitochondrial metabolism, increasing reactive oxygen species (ROS) and decreasing ATP, and impairing blastocyst formation (Chen et al., 2020).
Other reports showed no adverse effects following the application of ionomycin, electrical pulses or strontium chloride in an activation-deficient mouse model, resulting in normal development and fertile pups (Vanden Meerschaut et al., 2013). However, ionomycin-induced Ca2+ transients in starfish eggs were followed by rapid alterations of the actin cytoskeleton, while cortical granules were disrupted or fused with other vesicles. This collectively prevented egg cortical maturation, despite normal fertilization progression, with monospermic zygotes failing to proceed past the first cell division or displaying problematic subsequent cell cleavage (Vasilev et al., 2012; Santella and Dale, 2015). Considering that actin cytoskeletal dynamics have been linked intricately with profiles of Ca2+ oscillations in mammalian fertilization (Ajduk et al., 2011; Swann et al., 2012), similar investigations are required in the mammalian scenario to ensure embryogenic competency is maintained following use of similar protocols in humans.
Examining the safety and efficacy of ionomycin, ethanol, electrical activation and combinations of activators reveals the experimental nature of AOA procedures. Notably, oocyte Ca2+ analysis may be a valid tool, together with the heterologous mouse oocyte activation test (MOAT), to pre-screen patients with ICSI fertilization failure before proceeding to AOA (Ferrer-Buitrago et al., 2018a,b). Most ionophores are capable of uncontrollably releasing Ca2+ from all intracellular stores, including those not normally involved in activation, potentially affecting factors downstream of the spatial and temporal regulation of Ca2+ transients (Ducibella and Fissore, 2008; Santella and Dale, 2015). Finally, ionophores exert multiple effects on cellular homeostasis, the effects of which require investigation in oocytes, which may exert long-term genetic/epigenetic, biochemical and physiological effects (Santella and Dale, 2015). It is thus essential that such downstream effects upon progeny are examined, which is admittedly difficult to do for numerous reasons, the major one being that AOA protocols are a relatively recent development in ART.
While some limited population studies have been performed on humans, the sizes of populations were quite small, ranging from 10 to 25 children being examined. Furthermore, such studies examine gross developmental and behavioural parameters, with potential differences in gene expression, epigenetic modifications and molecular alterations still requiring investigation. One group (Isom et al., 2013) examined the transcriptional profile of porcine embryos generated via a series of ART methodologies, finding significant alterations compared to normal embryos, including downregulation of the transforming growth factor β signalling in IVF embryos, including aberrant regulation of ubiquitin-mediated proteolysis and ErbB signalling. Significantly, however, expression of genes involved in chromatin modification, RNA-mediated gene silencing and apoptosis were significantly disrupted in embryos created via somatic cell nuclear transfer, which involved the use of AOA. Until further assessment of the efficacy and safety of AOA is conducted, AOA will remain limited and confined to select clinical applications.
Assessment of AOA efficacy and safety
Although numerous studies have attempted to assess the safety and efficacy of AOA, major challenges must be overcome before broad application within clinics. These include heterogeneity in varied experimental methodologies and inconsistent patient inclusion criteria between research groups, as well as nonstandard endpoint choices in assessing safety outcomes. Combined with the small sample size of many studies and the limited applicability of animal models for explaining biological processes in humans, such challenges must be understood and addressed before the efficacy and safety of AOA can be improved.
Heterogeneity in study methodology
The lack of homogeneity in AOA study design is evident in the range of protocol variants in AOA studies, even when subdivided by activation type. No industry standard is present for study methodology, further compounding this issue. This stems from a lack of homogeneity in protocols for other aspects of ART. In the case of chemical activation, there is a significant variety among chemicals used, chemical concentration, timing and duration of exposure, and number of times the chemical is applied (Heindryckx et al., 2008; Liu et al., 2011; Ebner et al., 2012, 2015; Kyono et al., 2012; Montag et al., 2012; Vanden Meerschaut et al., 2012; Yoon et al., 2013; Darwish and Magdi, 2015; Aydinuraz et al., 2016; Capalbo et al., 2016; Miller et al., 2016; Nikiforaki et al., 2016; Economou et al., 2017; Zhang et al., 2017; Karabulut et al., 2018).
Such variation not only exists between studies but also within the same study among different patients recruited. In addition, the choice of using either laboratory-generated chemical activators or commercially available options, such as CultActive (whose exact concentration remains undisclosed by the manufacturer, although Nikiforaki et al. (2016) estimated this to be at least >15 μmol/l), is another source of variability. The rise in amplitude of Ca2+ is more standardized and reproducible under laboratory conditions, but commercial chemical activators would be used in the clinical setting and may produce differing results (Ebner et al., 2012; Aydinuraz et al., 2016; Goksan Pabuccu et al., 2016; Nikiforaki et al., 2016).
Electrical AOA is also constrained by non-standardized activation methodologies, although the techniques themselves differ from those of chemical activation. Variance in the types of electrical pulses, time duration of pulses and repetition rates of pulses all contribute to the heterogeneity of studies (Mansour et al., 2009; Baltaci et al., 2010; Versieren et al., 2010). Additionally, although studies that combine different activation techniques present novel means of overcoming OAD, it becomes increasingly difficult to compare the safety and efficacy of these studies with others that rely on only one activation mechanism for AOA. Studies experimenting with various combinations of chemical and mechanical activation, administration of different chemical activators and different orders of administration of chemical activators all suffer from a lack of standardized means for evaluating safety (Tesarik et al., 2002; Kyono et al., 2008). Nonetheless, the importance of investigating combinations of stimuli for AOA necessitates such studies.
Inconsistent sample inclusion criteria
Diverse inclusion criteria for patient samples across studies further complicate matters, particularly as gametes used in ICSI and AOA are subject to a variety of treatments and procedures. There are a multitude of mechanisms for sperm selection without homogeneity in methodology (Oseguera-López et al., 2019; Vaughan and Sakkas, 2019). For example, immature testicular sperm require exposure to higher levels of the activating compound than ejaculated or epididymal sperm, as well as lower sperm retrieval rates, suggesting the need for careful protocol adjustment (Boeri et al., 2020). Immature oocyte incidence may also pose a similar issue (Braga et al., 2020). Furthermore, testicular spermatozoa are expected to be in better condition as they have not yet been exposed to post-testicular DNA fragmentation (Agarwal et al., 2020). Thus, this variation in sperm quality between samples makes it difficult to have a standard comparison for verifying safety and efficacy across studies.
Female gametes also suffer from such variation among study samples, although standard protocols require use of freshly collected oocytes arrested at MII. However, the impact of AOA on oocytes matured in vitro (IVM) after vitrification and cells cryopreserved with ethylene glycol and sucrose or vitrified with sucrose or trehalose as cryoprotectants have also been studied (Liu et al., 2011; Capalbo et al., 2016; Zhang et al., 2017). Moreover, a dearth of gametes for experimentation has led to the use of IVM oocytes derived from stimulated cycles or aged oocytes that remain unfertilized post-ICSI for experiments; these samples are used to evaluate chemical activator efficacy or AOA safety and efficiency (Nikiforaki et al., 2016; Economou et al., 2017).
Additionally, gametes are routinely pre-screened prior to ICSI to improve outcomes, adding bias to cross-study evaluations of AOA efficacy. For instance, intra-cytoplasmic morphologically selected sperm injections can select sperm based on fine morphological features under high magnification and the activation capacity of sperm can be selected for via MOAT (Heindryckx et al., 2008; Versieren et al., 2010; Vanden Meerschaut et al., 2012; Aydinuraz et al., 2016). This is further complicated by the fact that sperm with specific morphologies tend to exhibit different capacities for oocyte activation, even within the same sample (Kashir et al., 2010, 2012; Kashir, 2020; Sermondade et al., 2011; Vanden Meerschaut et al., 2013). When such techniques are employed as a screen for patient samples before the start of a study, it is improper to compare these results to those of other studies that do not have any such screen in place.
Varying grades of male factor infertility are accepted as inclusion criteria, with most studies not further subdividing patient groups according to infertility aetiology and/or fertilization failure rate. Each patient’s history includes information on the incidence of teratozoospermia, asthenozoospermia, oligozoospermia, cryptozoosperia, azoospermia, globozoospermia, female factor infertility and/or any other unknown factor infertility (Esteves et al., 2018; Esteves and Roque, 2019; Lin et al., 2019; Moretti et al., 2019; Stimpfel et al., 2019; Fesahat et al., 2020; Morin et al., 2020). Many studies include all couples with a history of ICSI fertilization failure, regardless of aetiology, and some do not even specify the specific inclusion criteria besides a general history of ICSI failure. In fact, a few studies have recommended AOA treatment for patients even when the couple has no history of previous ICSI failure (Eftekhar et al., 2013; Karabulut et al., 2018).
Finally, inconsistencies in patient group inclusion can arise due to different criteria for control groups between studies. Often, due to a dearth of embryos and the ethical considerations of producing many embryos for research, data from previously failed ICSI cycles are employed as controls in many efficacy and safety studies. Alternatively, some studies use sibling oocyte split randomizations, in which oocytes from the same woman are divided and assorted into experimental or control groups, with the latter undergoing standard ICSI. Standard ICSI performed on different patients and the babies born from this procedure are used as controls for both prospective and retrospective studies assessing safety. Assessing the long-term safety of AOA using children born through standard ICSI or children from the general population as controls can vary significantly in terms of the baseline outcome for comparison, introducing yet another source of variability.
Non-standardized outcome assessments
While efficacy endpoints classically include fertilization, cleavage, embryo transfer, implantation and clinical pregnancy rates, as well as embryo and blastocyst quality assessments, the criteria for evaluating safety outcomes are much more variable. Commonly cited outcome measures for safety include miscarriage rates, congenital and neonatal malformation occurrence, as well as chromosomal analysis and cytogenetic analysis, although endpoint reporting is not carried out in a consistent manner. As for the long-term follow-up of children born through AOA, studies are scarce; still, those conducted till date include a variety of analyses, such as physical, mental, cognitive, language, behavioural and motor development. Variations in AOA methods and patient inclusion criteria prohibit the pooling of results from different studies. In a study using couples with a history of TFF, only 10 of 690 electronically identified records were eligible for direct comparison between ICSI and ICSI-AOA. Hence, no robust conclusions could be drawn for both AOA efficacy and safety (Sfontouris et al., 2015). This undoubtedly constitutes one of the largest obstacles to the establishment of a standard clinical protocol for measuring the safety of AOA.
Restricted sample size
As AOA is only recommended for only select patients, this severely restricts the availability of embryos for efficacy and safety assessments. Thus, although a large number of studies have been conducted, each has a small sample size (Versieren et al., 2010; Ebner et al., 2012; Kyono et al., 2012; Eftekhar et al., 2013; Vanden Meerschaut et al., 2014a,b; Darwish and Magdi, 2015; Aydinuraz et al., 2016; Economou et al., 2017; Karabulut et al., 2018). In conjunction with ethical restrictions on mass producing embryos for research, the small sample size of studies explains the difficulty establishing optimal control groups with randomization (Sfontouris et al., 2015). After all, embryos fertilized via standard ICSI are not abundant nor readily available for research purposes. It follows that the clinical follow-up of children born from AOA is even more limited than analyses of embryo or neonatal safety. The rarity of children born via AOA compared to the general population renders large-scale studies comprehensibly scarce, complicates the establishment of sound controls and limits the ability to draw conclusions of statistical significance across studies (Heindryckx et al., 2008; Yoon et al., 2013; D’Haeseleer et al., 2014; Vanden Meerschaut et al., 2014a,b; Deemeh et al., 2015; Sfontouris et al., 2015; Capalbo et al., 2016).
Animal model limitations
Animal models are a routinely used alternative to the use of human gametes for AOA studies (Tesarik et al., 2002; Versieren et al., 2010; van Blerkom et al., 2015; Capalbo et al., 2016; Nikiforaki et al., 2016; Ogura, 2020). However, numerous differences exist between oocyte activation mechanisms of humans and commonly employed animal models, such as mice (Vanden Meerschaut et al., 2013, 2014b; van Blerkom et al., 2015; Nikiforaki et al., 2016; Kashir et al., 2018). Above a certain Ca2+ threshold, mouse and rabbit oocyte activation efficiency are independent of amplitude, pattern and the duration of a rise in Ca2+ levels, although embryo/blastocyst quality, long-term gene expression and development to term are affected (Heindryckx et al., 2008; Nikiforaki et al., 2014, 2016). Such variation among species suggests the need for the use of utmost caution in extrapolating results to the human condition from animal models. Indeed some ionophores, such as strontium chloride (Sr2+), may be effective in mouse models, but may not yield consistent/successful results in humans (Lu et al., 2018b).
Alternative AOA avenues
Apprehension towards AOA protocols and the need for more robust and reliable studies to support the technique are consequences of the artificial mechanism of action of AOA, which creates non-physiological patterns of Ca2+ release. To this degree, it is of utmost importance that studies focus not only on further elucidating the mechanistic modalities underlying Ca2+ release and regulation, but also establish more physiologically relevant modalities of eliciting the required pattern of Ca2+ release at fertilization.
PLCζ-based AOA strategies
A goal of ART is to mimic as closely as possible the physiological processes that occur throughout mammalian fertilization, making PLCζ a potential replacement for most current agents of AOA as a physiologically relevant mechanism within the clinic. Several reports have linked defects in human PLCζ with cases of OAD. Indeed, sperm from men who routinely fail IVF and/or ICSI either fail to elicit Ca2+ oscillations in oocytes or do so in an uncharacteristic or abnormal profile (Yoon et al., 2008; Heytens et al., 2009). Importantly, sperm from such patients exhibit reduced or absent levels of PLCζ within the sperm head (Yoon et al., 2008; Heytens et al., 2009; Kashir et al., 2011), while mutations in PLCζ may be contributing not only to male infertility but also perhaps to cases of male sub-fertility (for a detailed review, see Kashir (2020)). Furthermore, levels of PLCζ in sperm and the proportion of sperm exhibiting detectable PLCζ positively correlate with ICSI success rates (Yelumalai et al., 2015). Thus, the clinical potential of PLCζ is apparent, both as a therapeutic intervention and as a prognostic indicator of OAD. One study (Meng et al., 2020) recently further indicated the importance of examining PLCζ in the context of OAD by showing that 80% of patients exhibiting a significant ‘PLCζ deficiency’ who opted for AOA exhibited a significantly improved fertilization rate (∼40% higher) and improved pregnancy and live birth rates (both increased by 40% per initiated cycle).
Yoon et al. (2008) countered abnormalities in sperm PLCζ by co-injection with mouse PLCζ mRNA, while Rogers et al. (2004) demonstrated successfully generated parthenogenetic blastocysts following PLCζ cRNA injection into human oocytes. However, such therapeutic employment of PLCζ cRNA is not viable, as PLCζ transcription would be uncontrollable and extremely variable within oocytes, likely proving detrimental to pre-implantation development (Ducibella et al., 2002; Rogers et al., 2004; Ozil et al., 2006). Consequently, the synthesis of a pure and active recombinant form of PLCζ has been a key goal (Kashir et al., 2011; Nomikos et al., 2013b), resulting in the generation of stable, purified recombinant human PLCζ protein, able to induce Ca2+ oscillations within a physiological range and able to counter the deleterious effects of mutant PLCζ (Nomikos et al., 2013b). Furthermore, a mouse model of ICSI failure showed that success rates following PLCζ injection co-incident with such sperm were comparable to control injections (Sanusi et al., 2015).
Importantly, the potential use of recombinant PLCζ as an AOA agent has one striking advantage; estimates of the quantity of PLCζ present in a single, healthy human sperm have been determined (50–100 fg/sperm), identifying a potential dosage range to examine (Kashir et al., 2010; Nomikos et al., 2013a; Kashir et al., 2014; Saleh et al., 2020). Furthermore, differences in PLCζ potency and Ca2+ oscillation patterns between humans and mouse models have been described (Heindryckx et al., 2008; Nomikos et al., 2013a; Swann and Lai, 2016; Swann, 2018, 2020), further facilitating a more accurate extrapolation of the effects of PLCζ on humans from animal models. Collectively, such studies underscore the potential for standardization of the dosage of recombinant PLCζ as an AOA activation agent and for overcoming the major obstacle of AOA activation agents.
However, despite PLCζ representing perhaps the most encouraging current physiological alternative to AOA with recombinant PLCζ still representing the most physiologically relevant AOA strategy, recent studies have necessitated a rethinking of what is currently accepted in terms of the physiological mechanism underlying mammalian oocyte activation. While both Hachem et al. (2017) and Nozawa et al. (2018) concluded that PLCζ was indeed the key driver of Ca2+ oscillations at least in mammals, indicating that sperm lacking PLCζ could not induce Ca2+ release following microinjection into mouse oocytes, IVF experiments with such sperm led to observations of Ca2+ oscillations, albeit lower in number and frequency, alongside a high degree of polyspermy and OAD (Nozawa et al., 2018; Satouh and Ikawa, 2018). This abnormal pattern of Ca2+ release alongside low numbers of embryos and offspring, has been suggestively attributed towards spontaneous activation, unrelated to Ca2+ release, which is common in some strains of mice (Cheng et al., 2012; Jones, 2018).
Another (perhaps more controversial) suggestion is that sperm potentially possess a secondary factor capable of Ca2+ releasing activity, albeit weaker than PLCζ (Jones, 2018; Swann, 2020). Proposed as an alternative ‘primitive’ or ‘cryptic’ sperm factor, some studies have suggested that this (yet unidentified) factor may also be involved in or contribute to events leading to oocyte activation (Nozawa et al., 2018; Satouh and Ikawa, 2018; Swann, 2020). Alternatively, this cryptic factor could be one of the other potential candidates for the sperm factor apart from PLCζ as discussed previously herein, or perhaps PLCζ RNA may also be involved as the abnormal profile of Ca2+ release is very similar to injections of low RNA concentrations in mouse oocytes (Jones, 2018; Swann, 2020). However, as previously discussed, no other proposed factor has been independently and consistently confirmed to elicit physiological Ca2+ release, while the total amount of PLCζ RNA present within sperm may not be enough to elicit any Ca2+ at all (Jones, 2018; Swann, 2020). Collectively, this is an exciting and emerging area of investigation, and highlights just how much we do not know regarding the molecular intricacies involved in regulating Ca2+ release and oscillation patterns at fertilization.
Further investigative avenues
Numerous further factors may contribute towards the efficacy of oocyte activation that could be utilized to replace current non-physiological mechanisms of oocyte activation, and thus require urgent further investigations. The mammalian oocyte machinery involved in Ca2+ release may also exert significant effects upon the efficacy of oocyte activation (Miyara et al., 2003; Kilani and Chapman, 2014), with potential connections proposed between fertilization failure and the expression profiles of genes involved in oocyte maturation (Gasca et al., 2008; Grøndahl et al., 2013; Yeste et al., 2016a, 2017). Such factors could also be playing at least a secondary role in the process of oocyte activation. Indeed, oocyte PLCs have often been suggested to play minor roles at least in the regulation of Ca2+ maintenance (Coward et al., 2003; Runft et al., 2004; Yeste et al., 2016a).
Disruption of starfish egg cytoskeletal dynamics with heparin prevents the rapid Ca2+ wave upon interaction with sperm, instead delaying and reducing Ca2+ release and amplitude, failing to prevent polyspermy. This perhaps suggests that disruption of actin dynamics at fertilization influenced Ca2+ release (Puppo et al., 2008; Santella et al., 2015; Limatola et al., 2019a), potentially also impacting upon subsequent events in egg activation such as cortical granule exocytosis (Santella et al., 2015; Limatola et al., 2019b). Thus, perhaps it is prudent to examine further preventative precautions to preserve actin cytoskeletal dynamics at fertilization as a part of AOA protocols. Furthermore, both direct PLCζ- and ICSI-induced mammalian oocyte activation generate zinc sparks, which coordinate with Ca2+ oscillations for embryos activated by PLCζ. These recent developments reveal the importance of fluctuations in zinc levels during activation (Tokuhiro and Dean, 2018; Que et al., 2019). Accumulation of zinc throughout maturation seems essential for meiotic progression and meiotic arrest at metaphase II, and is then expelled from the oocyte at fertilization, immediately following the characteristic series of Ca2+ oscillations (Kim et al., 2011a; Bernhardt et al., 2012; Duncan et al., 2016). Such dynamics of zinc appear to function by modulating CSF activity, in turn affecting maintenance of metaphase II arrest via modulating EMI2, a zinc-binding component of the CSF (Bernhardt et al., 2012).
Indeed, zinc chelators in and of themselves prompted oocyte activation and blastocyst formation, lending themselves as possible additions to novel AOA protocols (Kerns et al., 2018; Uh et al., 2019). One such prospect is TPEN (Swann, 2018), a zinc chelator that enables meiotic resumption and embryogenesis in mice following ICSI using sperm in a mouse model of ICSI-failure (Suzuki et al., 2010; Lee et al., 2015). However, studies in humans have not yielded as promising results (Duncan et al., 2016). In pig oocytes, TPEN is effective at causing activation, but only at lower concentrations and in combination with currently used Ca2+ ionophores (Lee et al., 2015).
Further to direct processes, Ca2+-influx mechanisms are increasingly being revealed as indispensable aspects of oocyte activation, not only replenishing Ca2+ stores, but also underlying specific events such as polar body emission and cortical granule exocytosis. Defects in mediators of Ca2+ influx, including the TRPM7 and CaV3.2 channels, potentially alter the developmental potential of offspring by inducing a premature cessation of Ca2+ oscillations (Miao and Williams, 2012; Saleh et al., 2020). Thus, perhaps mediation of such mechanisms represents an alternative method of treatment for cases of OAD and associated conditions of PLCζ-deficiency. Both the TRPM7 and CaV3.2 channels almost completely account for Ca2+ influx in at least mammalian oocytes, while TRPM7 acts as a membrane sensor of extracellular magnesium (Mg2+) and Ca2+ concentrations, modulating the dynamics of the Ca2+ oscillatory response at fertilization (Miao and Williams, 2012; Miao et al., 2012).
Indeed, culture conditions may also be another important contributory factor underlying successful utilization of AOA in the clinic. Patterns of Ca2+ release and subsequent embryogenesis were significantly affected by the concentration of Ca2+ in the culture media during AOA, indicating that the type of culture conditions used significantly exerts effects upon embryogenic competency in relation to Ca2+ release and embryogenesis (Lu et al., 2018a). Mouse AOA with ionomycin in Ca2+-free medium for the 10 min duration of AOA yielded no blastocyst formation, despite subsequent culture of the embryos in media containing Ca2+ (Lu et al., 2018a).
Altering the extracellular Mg2+ to Ca2+ ratio in culture media alters Ca2+ release dynamics in mouse oocytes at fertilization, as well as the developmental capacity of resultant embryos (Ozil et al., 2017), suggesting that limiting Mg2+ availability in culture media may represent a potential intervention to increase Ca2+ release in cases where PLCζ-induced Ca2+ release may be defective via influx mechanisms including TRPM7 (Ozil et al., 2017). However, focused clinical studies have not yet been undertaken to examine the overall effects of such alterations in culture media. It is essential to further examine whether modulation of Ca2+ influx mechanisms through alteration of culture media composition would improve pregnancy and delivery rates in the clinic, particularly in relation to PLCζ-associated conditions (Kashir, 2020).
Future prospects
Future of AOA on patients in the clinic
Despite various outstanding challenges to establishing the safety and efficacy of AOA, existing studies do not necessarily implicitly indicate that such methods are not promising or are unsafe. Importantly, most existing studies do not refer to the health of children born from AOA, but rather are endpoints during embryonic development that could potentially be remedied, depending of course on early detection and knowledge of potential problems in line with scientific advances to provide remedial solutions. For example, GM-CSF supplementation after AOA could reduce the occurrence of chromosomal abnormalities at in vitro embryonic developmental during the fifth day (Economou et al., 2017). Perhaps the main risk after AOA lies in a failure to coordinate the telophasic extrusion of the 2PB during MII, suggesting the adoption of chromosome screening to examine chromosomal ploidy (Capalbo et al., 2016). Furthermore, embryo selection via the embryo grading system has yielded no further differences in pregnancy or implantation rates between AOA and standard ICSI groups (Aydinuraz et al., 2016).
Importantly, as AOA is usually the final option for couples experiencing OAD, researchers have learned to utilize gametes in sub-optimal conditions, such as vitrified gametes or unfertilized oocytes post-ICSI (Liu et al., 2011; Capalbo et al., 2016; Economou et al., 2017; Zhang et al., 2017). Given that most ART protocols, including standard ICSI, prolonged in vitro culture and cryopreservation, have been linked to altered gene expression (Ebner and Montag, 2016), hopes for the final safety and efficacy of AOA should be anticipated accordingly. The final clinical outcomes of AOA will likely be the result of the cumulative effect of all these procedures on the embryo, rather than just AOA alone. Therefore, adequate step-by-step screening for anomalies must be conducted to pinpoint exactly where and when AOA has the potential to cause harm. A thorough dissection of each technique’s molecular mechanism and affected pathways becomes all the more relevant. Still, it must be recognized that AOA remains the end of the line treatment option for an increasing number of patients, rendering this technique relevant and promising.
Redesigning further studies
Further research on the efficacy and safety of AOA is required, particularly through larger scale studies. The establishment of an internationally recognized set of guidelines for AOA study design is of utmost importance. These include standardized protocols, clear criteria for patient inclusion and sub-classification, as well as a list of mandatory endpoints that can be universally used for efficacy measurement. As for safety assessments, the selection of a preferred set of tests, be it readily accessible DNA screening protocols, standardized tests or questionnaires, is recommended. Past studies indicate heterogeneity in clinical prediction models for ART (Ratna et al., 2020). Additionally, multi-centre studies and international cooperation between different fertility centres would be preferred over single-centre studies to increase sample sizes and further promote protocol homogeneity. Finally, the establishment of a single, generalized database for AOA results could further orient and promote a standardized study design while facilitating direct comparison between studies. This would allow for quick protocol improvement, wide-spread acceptance of the technique and clinical establishment of AOA.
In contrast, combinations and variations of experimental techniques should only be explored if and when standardized protocols prove insufficient or unsafe. Furthermore, clear and direct technique efficacy comparisons should be made across studies, as has been done by precedent studies (Tesarik et al., 2002; Kyono et al., 2012; Nikiforaki et al., 2016; Economou et al., 2017). Nonetheless, the exact molecular mechanisms of each ART are still unclear in humans, further complicating the choice of the best protocol for each situation.
Finally, it is vital that current research on the safety, efficacy and efficiency of ARTs, including AOA, is clearly conveyed to patients, who might otherwise take it upon themselves to seek information online about infertility treatment options. Many freely available resources do not predominantly rely upon academic studies, yet they may sway public opinion on AOA. Hence, the public availability of clear and informative studies about AOA could crucially influence public awareness, create a greater sense clinician-patient trust and help patients make informed, autonomous decisions about their own treatment plans. On balance, AOA appears an immensely promising avenue of treatment for not just OAD and TFF, but perhaps even to improve the efficacy of ART overall. However, the identification of more physiological agents and protocols that better mimic physiological Ca2+ release is essential, coupled with further studies to evaluate the safety and efficacy of such treatments, before such procedures can be widely applied within clinics.
Data availability
No new data were generated or analysed in support of this research.
Authors’ roles
J.K. and D.G. contributed towards most of the literature search, quality assessment, data extraction and interpretation of results, alongside C.J. and K.C. J.K., D.G., C.J. and K.C. were all involved in drafting of the manuscript, and all authors approved of the final version of the article.
Funding
D.G. was supported by Stanford University’s Bing Overseas Study Program. J.K. was supported by a Healthcare Research Fellowship Award (HF-14-16) from Health and Care Research Wales (HCRW), alongside a National Science, Technology, and Innovation plan (NSTIP) project grant (15-MED4186-20) awarded by the King Abdulaziz City for Science and Technology (KACST).
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Junaid Kashir, College of Medicine, Alfaisal University, Riyadh, Kingdom of Saudi Arabia; Department of Comparative Medicine, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia.
Durga Ganesh, Nuffield Department of Women’s & Reproductive Health, University of Oxford, Level 3, Women’s Centre, John Radcliffe Hospital, Oxford, UK; David Geffen School of Medicine, University of California—Los Angeles, Los Angeles, CA, USA.
Celine Jones, Nuffield Department of Women’s & Reproductive Health, University of Oxford, Level 3, Women’s Centre, John Radcliffe Hospital, Oxford, UK.
Kevin Coward, Nuffield Department of Women’s & Reproductive Health, University of Oxford, Level 3, Women’s Centre, John Radcliffe Hospital, Oxford, UK.
References
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril 2014a;102:440–447. [DOI] [PubMed] [Google Scholar]
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 2014b;28:4434–4440. [DOI] [PubMed] [Google Scholar]
- Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev 2009;77:249–256. [DOI] [PubMed] [Google Scholar]
- Abel K, Healey M, Finch S, Osianlis T, Vollenhoven B. Associations between embryo grading and congenital malformations in IVF/ICSI pregnancies. Reprod Biomed Online 2019;39:981–989. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, Finelli R, Leisegang K, Sengupta P, Barbarosie C et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health 2020;38:412–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajduk A, Ilozue T, Windsor S, Yu Y, Seres KB, Bomphrey RJ, Tom BD, Swann K, Thomas A, Graham C et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun 2011;2:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter QS, Rajabi-Toustani R, Shimizu K, Kuwahara Y, Murase T. Polymyxin B enhances acrosomal exocytosis triggered by calcium and the calcium ionophore A23187 in ejaculated boar spermatozoa. Anim Sci J 2019;90:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberio R, Motlik J, Stojkovic M, Wolf E, Zakhartchenko V. Behavior of M-phase synchronized blastomeres after nuclear transfer in cattle. Mol Reprod Dev 2000;57:37–47. [DOI] [PubMed] [Google Scholar]
- Alexandre H, Delsinne V, Goval JJ. The thiol reagent, thimerosal, irreversibly inhibits meiosis reinitiation in mouse oocyte when applied during a very early and narrow temporal window: a pharmacological analysis. Mol Reprod Dev 2003;65:454–461. [DOI] [PubMed] [Google Scholar]
- Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53:292–308. [DOI] [PubMed] [Google Scholar]
- Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments. Adv Biol Regul 2016;61:58–67. [DOI] [PubMed] [Google Scholar]
- Anifandis G, Michopoulos A, Daponte A, Chatzimeletiou K, Simopoulou M, Messini CI, Polyzos NP, Vassiou K, Dafopoulos K, Goulis DG. Artificial oocyte activation: physiological, pathophysiological and ethical aspects. Syst Biol Reprod Med 2019;65:3–11. [DOI] [PubMed] [Google Scholar]
- Aydinuraz B, Dirican EK, Olgan S, Aksunger O, Erturk OK. Artificial oocyte activation after intracytoplasmic morphologically selected sperm injection: a prospective randomized sibling oocyte study. Human Fertility 2016;19:282–288. [DOI] [PubMed] [Google Scholar]
- Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A 2010;107:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaci V, Ayvaz ÖÜ, Ünsal E, Aktaş Y, Baltacı A, Turhan F, Özcan S, Sönmezer M. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril 2010;94:900–904. [DOI] [PubMed] [Google Scholar]
- Basirat Z, Kashifard M, Golsorkhtabaramiri M, Mirabi P. Factors associated with spontaneous abortion following intracytoplasmic sperm injection (ICSI). JBRA Assist Reprod 2019;23:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri F, Nasr-Esfahani MH, Forozanfar M, Tavalaee M. Relationship between sperm parameters with sperm function tests in infertile men with at least one failed cycle after intracytoplasmic sperm injection cycle. Int J Fertil Steril 2020;13:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista Napotnik T, Reberšek M, Vernier PT, Mali B, Miklavčič D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): a systematic review. Bioelectrochemistry 2016;110:1–12. [DOI] [PubMed] [Google Scholar]
- Belva F, Bonduelle M, Tournaye H. Endocrine and reproductive profile of boys and young adults conceived after ICSI. Curr Opin Obstet Gynecol 2019;31:163–169. [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 2012;86:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Padilla-Banks E, Stein P, Zhang Y, Williams CJ. Store-operated Ca(2+) entry is not required for fertilization-induced Ca(2+) signaling in mouse eggs. Cell Calcium 2017;65:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Stein P, Carvacho I, Krapp C, Ardestani G, Mehregan A, Umbach DM, Bartolomei MS, Fissore RA, Williams CJ. TRPM7 and Ca(V)3.2 channels mediate Ca(2+) influx required for egg activation at fertilization. Proc Natl Acad Sci U S A 2018;115:E10370–E10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri L, Palmisano F, Preto M, Sibona M, Capogrosso P, Franceschelli A, Ruiz‐Castañé E, Sarquella‐Geli J, Bassas‐Arnau L, Scroppo FI et al. Sperm retrieval rates in non‐mosaic Klinefelter patients undergoing testicular sperm extraction: what expectations do we have in the real‐life setting? Andrology 2020;8:680–687. [DOI] [PubMed] [Google Scholar]
- Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, De Gheselle S, Steyaert N, Boel A, Vanden Meerschaut F et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril 2019;112:266–274. [DOI] [PubMed] [Google Scholar]
- Borges AA, Santos MVdO, Nascimento LE, Lira GPdO, Praxedes ÉA, Oliveira MFd, Silva AR, Pereira AF. Production of collared peccary (Pecari tajacu Linnaeus, 1758) parthenogenic embryos following different oocyte chemical activation and in vitro maturation conditions. Theriogenology 2020;142:320–327. [DOI] [PubMed] [Google Scholar]
- Borges E Jr, Souza AS, Braga D, Iaconelli A Jr. Successful twin pregnancy with intracytoplasmic sperm injection using surgical sperm retrieval after 25 years of vasectomy: a case report. JBRA Assist Reprod 2020;24:87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos-Mikich A, Swann K, Whittingham DG. Calcium oscillations and protein synthesis inhibition synergistically activate mouse oocytes. Mol Reprod Dev 1995;41:84–90. [DOI] [PubMed] [Google Scholar]
- Bos-Mikich A, Whittingham DG, Jones KT. Meiotic and mitotic Ca2+ oscillations affect cell composition in resulting blastocysts. Dev Biol 1997;182:172–179. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015;313:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga D, Zanetti BF, Setti AS, Iaconelli A Jr, Borges E Jr. Immature oocyte incidence: contributing factors and effects on mature sibling oocytes in intracytoplasmic sperm injection cycles. JBRA Assist Reprod 2020;24:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007;131:1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind S, Swann K, Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca2+ or egg activation. Dev Biol 2000;223:251–265. [DOI] [PubMed] [Google Scholar]
- Cai X. Molecular evolution and functional divergence of the Ca(2+) sensor protein in store-operated Ca(2+) entry: stromal interaction molecule. PloS One 2007;2:e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo A, Ottolini CS, Griffin DK, Ubaldi FM, Handyside AH, Rienzi L. Artificial oocyte activation with calcium ionophore does not cause a widespread increase in chromosome segregation errors in the second meiotic division of the oocyte. Fertil Steril 2016;105:807–814.e2. [DOI] [PubMed] [Google Scholar]
- Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep 2013;5:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek TR, McGuinness OM, Vincent C, Moreton RB, Berridge MJ, Johnson MH. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development 1993;119:179–189. [DOI] [PubMed] [Google Scholar]
- Chen C, Sun T, Yin M, Yan Z, Yu W, Long H, Wang L, Liao X, Yan Z, Li W et al. Ionomycin induced mouse oocyte activation can disrupt preimplantation embryo development through increased reactive oxygen species reaction and DNA damage. Mol Hum Reprod 2020;26:773–783. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Zhong Z, Latham KE. Strain-specific spontaneous activation during mouse oocyte maturation. Fertil Steril 2012;98:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S, Xie P, Parrella A, Keating D, Rosenwaks Z, Palermo GD. Identification and treatment of men with phospholipase Cζ-defective spermatozoa. Fertil Steril 2020;114:535–544. [DOI] [PubMed] [Google Scholar]
- Coward K, Campos-Mendoza A, Larman M, Hibbitt O, McAndrew B, Bromage N, Parrington J. Teleost fish spermatozoa contain a cytosolic protein factor that induces calcium release in sea urchin egg homogenates and triggers calcium oscillations when injected into mouse oocytes. Biochem Biophys Res Commun 2003;305:299–304. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 2002;124:611–623. [DOI] [PubMed] [Google Scholar]
- Cuthbertson KS, Cobbold PH. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature 1985;316:541–542. [DOI] [PubMed] [Google Scholar]
- D'Haeseleer E, Vanden Meerschaut F, Bettens K, Luyten A, Gysels H, Thienpont Y, De Witte G, Heindryckx B, Oostra A, Roeyers H et al. Language development of children born following intracytoplasmic sperm injection (ICSI) combined with assisted oocyte activation (AOA). Int J Lang Commun Disord 2014;49:702–709. [DOI] [PubMed] [Google Scholar]
- Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod Biomed Online 2015;31:799–804. [DOI] [PubMed] [Google Scholar]
- Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation. Reprod Sci 2015;22:322–328. [DOI] [PubMed] [Google Scholar]
- Diop H, Cabral H, Gopal D, Cui X, Stern JE, Kotelchuck M. Early autism spectrum disorders in children born to fertile, subfertile, and ART-treated women. Matern Child Health J 2019;23:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J-B, Tang T-S, Sun F-Z. Xenopus and chicken sperm contain a cytosolic soluble protein factor which can trigger calcium oscillations in mouse eggs. Biochem Biophys Res Commun 2000;268:947–951. [DOI] [PubMed] [Google Scholar]
- Dorfeshan P, Ghaffari Novin M, Salehi M, Farifteh F. Expression of miR-302 in human embryo derived from in-vitro matured oocyte. Int J Reprod Biomed 2019;17:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 2008;315:257–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 2002;250:280–291. [PubMed] [Google Scholar]
- Ducibella T, Schultz R, Ozil J. Role of calcium signals in early development. Semin Cell Dev Biol 2006;17:324–332. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep 2016;6:24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G, Dumollard R. Simulation of calcium waves in ascidian eggs: insights into the origin of the pacemaker sites and the possible nature of the sperm factor. J Cell Sci 2004;117:4313–4323. [DOI] [PubMed] [Google Scholar]
- Duran‐Retamal M, Morris G, Achilli C, Gaunt M, Theodorou E, Saab W, Serhal P, Seshadri S. Live birth and miscarriage rate following intracytoplasmic morphologically selected sperm injection vs intracytoplasmic sperm injection: an updated systematic review and meta‐analysis. Acta Obstet Gynecol Scand 2020;99:24–33. [DOI] [PubMed] [Google Scholar]
- Ebner T. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod 2004;19:1837–1841. [DOI] [PubMed] [Google Scholar]
- Ebner T, Köster M, Shebl O, Moser M, Van der Ven H, Tews G, Montag M. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril 2012;98:1432–1437. [DOI] [PubMed] [Google Scholar]
- Ebner T, Montag M. Artificial oocyte activation: evidence for clinical readiness. Reprod Biomed Online 2016;32:271–273. [DOI] [PubMed] [Google Scholar]
- Ebner T, Montag M, Montag M, Van der Ven K, Van der Ven H, Ebner T, Shebl O, Oppelt P, Hirchenhain J, Krüssel J et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod BioMed Online 2015;30:359–365. [DOI] [PubMed] [Google Scholar]
- Economou KA, Christopikou D, Tsorva E, Davies S, Mastrominas M, Cazlaris H, Koutsilieris M, Angelogianni P, Loutradis D. The combination of calcium ionophore A23187 and GM-CSF can safely salvage aged human unfertilized oocytes after ICSI. J Assist Reprod Genet 2017;34:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: a randomized clinical trial. Iran J Reprod Med 2013;11:875–882. [PMC free article] [PubMed] [Google Scholar]
- Egashira A, Murakami M, Haigo K, Horiuchi T, Kuramoto T. A successful pregnancy and live birth after intracytoplasmic sperm injection with globozoospermic sperm and electrical oocyte activation. Fertil Steril 2009;92:2037.e5–2039. [DOI] [PubMed] [Google Scholar]
- Esteves SC, Roque M. Extended indications for sperm retrieval: summary of current literature. F1000Res 2019;8:F1000 Faculty Rev-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves SC, Roque M, Bedoschi G, Haahr T, Humaidan P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat Rev Urol 2018;15:535–562. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kopf GS. Molecular mechanisms of sperm-egg interactions and egg activation. Andrologia 1998;30:297–307. [DOI] [PubMed] [Google Scholar]
- Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca2+ transients vs oscillatory Ca2+ signaling for assisted oocyte activation: limitations and benefits. Reproduction 2018a;155:R105–R119. [DOI] [PubMed] [Google Scholar]
- Ferrer-Buitrago M, Bonte D, Dhaenens L, Vermorgen S, Lu Y, De Sutter P, Heindryckx B. Assessment of the calcium releasing machinery in oocytes that failed to fertilize after conventional ICSI and assisted oocyte activation. Reprod Biomed Online 2019;38:497–507. [DOI] [PubMed] [Google Scholar]
- Ferrer-Buitrago M, Dhaenens L, Lu Y, Bonte D, Vanden Meerschaut F, De Sutter P, Leybaert L, Heindryckx B. Human oocyte calcium analysis predicts the response to assisted oocyte activation in patients experiencing fertilization failure after ICSI. Hum Reprod 2018b;33:416–425. [DOI] [PubMed] [Google Scholar]
- Fesahat F, Henkel R, Agarwal A. Globozoospermia syndrome: an update. Andrologia 2020;52:e13459. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006;441:179–185. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Pinto-Correia C, Robl JM. Inositol trisphosphate-induced calcium release in the generation of calcium oscillations in bovine eggs. Biol Reprod 1995;53:766–774. [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N et al. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science 2001;292:920–923. [DOI] [PubMed] [Google Scholar]
- Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J Cell Biol 2003;161:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton BP, Whittingham DG. Activation of mammalian oocytes by intracellular injection of calcium. Nature 1978;273:149–151. [DOI] [PubMed] [Google Scholar]
- Gasca S, Reyftmann L, Pellestor F, Rème T, Assou S, Anahory T, Dechaud H, Klein B, De Vos J, Hamamah S. Total fertilization failure and molecular abnormalities in metaphase II oocytes. Reprod Biomed Online 2008;17:772–781. [DOI] [PubMed] [Google Scholar]
- Gennarelli G, Carosso A, Canosa S, Filippini C, Cesarano S, Scarafia C, Brunod N, Revelli A, Benedetto C. ICSI versus conventional IVF in women aged 40 years or more and unexplained infertility: a retrospective evaluation of 685 cycles with propensity score model. J Clin Med 2019;8:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksan Pabuccu E, Sinem Caglar G, Dogus Demirkiran O, Pabuccu R. Uncommon but devastating event: total fertilisation failure following intracytoplasmic sperm injection. Andrologia 2016;48:164–170. [DOI] [PubMed] [Google Scholar]
- Gómez-Fernández C, Pozo-Guisado E, Gañán-Parra M, Perianes MJ, Alvarez IS, Martín-Romero FJ. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction 2009;138:211–221. [DOI] [PubMed] [Google Scholar]
- Grøndahl ML, Borup R, Vikeså J, Ernst E, Andersen CY, Lykke-Hartmann K. The dormant and the fully competent oocyte: comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. Mol Hum Reprod 2013;19:600–617. [DOI] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Ferrer Buitrago M, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P et al. PLCζ is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017;144:2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P(2) at fertilization of mouse eggs. J Cell Sci 2002;115:2139–2149. [DOI] [PubMed] [Google Scholar]
- Hargreave M, Jensen A, Hansen MK, Dehlendorff C, Winther JF, Schmiegelow K, Kjær SK. Association between fertility treatment and cancer risk in children. JAMA 2019;322:2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online 2008;17:662–668. [DOI] [PubMed] [Google Scholar]
- Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod 2005;20:2237–2241. [DOI] [PubMed] [Google Scholar]
- Heineman KR, Kuiper DB, Bastide-van Gemert S, Heineman MJ, Hadders-Algra M. Cognitive and behavioural outcome of children born after IVF at age 9 years. Hum Reprod 2019;34:2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M, Gillespie JI, Murdoch AP. Development of calcium signalling mechanisms during maturation of human oocytes. Mol Hum Reprod 1997;3:965–973. [DOI] [PubMed] [Google Scholar]
- Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 2007;42:173–182. [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon S-Y, Fissore RA, Hamer R, Deane CM, Ruas M et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLC) in spermatozoa from infertile men. Hum Reprod 2009;24:2417–2428. [DOI] [PubMed] [Google Scholar]
- Heytens E, Soleimani R, Lierman S, De Meester S, Gerris J, Dhont M, Van der Elst J, De Sutter P. Effect of ionomycin on oocyte activation and embryo development in mouse. Reprod Biomed Online 2008;17:764–771. [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn 2008;237:527–544. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Hajian M, Moulavi F, Shahverdi AH, Nasr-Esfahani MH. Optimized combined electrical–chemical parthenogenetic activation for in vitro matured bovine oocytes. Anim Reprod Sci 2008;108:122–133. [DOI] [PubMed] [Google Scholar]
- Hwang JI, Oh YS, Shin KJ, Kim H, Ryu SH, Suh PG. Molecular cloning and characterization of a novel phospholipase C, PLC-eta. Biochem J 2005;389:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop LA, Nixon VL, Levasseur M, Chapman F, Chiba K, McDougall A, Venables JP, Elliott DJ, Jones KT. (2+)-promoted cyclin B1 degradation in mouse oocytes requires the establishment of a metaphase arrest. Dev Biol 2004;269:206–219. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol 2007;312:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol 1983;340:611–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom SC, Stevens JR, Li R, Spollen WG, Cox L, Spate LD, Murphy CN, Prather RS. Transcriptional profiling by RNA-Seq of peri-attachment porcine embryos generated by a variety of assisted reproductive technologies. Physiol Genomics 2013;45:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao Y. Egg activation in physiological polyspermy. Reproduction 2012;144:11–22. [DOI] [PubMed] [Google Scholar]
- Jaffe LA. First messengers at fertilization. J Reprod Fertil Suppl 1990;42:107–116. [PubMed] [Google Scholar]
- Jaffe LF. Sources of calcium in egg activation: a review and hypothesis. Dev Biol 1983;99:265–276. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A 1991;88:9883–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 2000;223:238–250. [DOI] [PubMed] [Google Scholar]
- Jones KT. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol Hum Reprod 2004;10:1–5. [DOI] [PubMed] [Google Scholar]
- Jones KT. Mammalian sperm contain two factors for calcium release and egg activation: Phospholipase C zeta and a cryptic activating factor. Mol Hum Reprod 2018;24:465–468. [DOI] [PubMed] [Google Scholar]
- Jones KT, Cruttwell C, Parrington J, Swann K. A mammalian sperm cytosolic phospholipase C activity generates inositol trisphosphate and causes Ca2+ release in sea urchin egg homogenates. FEBS Lett 1998a;437:297–300. [DOI] [PubMed] [Google Scholar]
- Jones KT, Soeller C, Cannell MB. The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development 1998b;125:4627–4635. [DOI] [PubMed] [Google Scholar]
- Kang W, Harada Y, Yamatoya K, Kawano N, Kanai S, Miyamoto Y, Nakamura A, Miyado M, Hayashi Y, Kuroki Y et al. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab Invest 2020;100:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabulut S, Aksünger Ö, Ata C, Sağıroglu Y, Keskin İ. İ. Artificial oocyte activation with calcium ionophore for frozen sperm cycles. Syst Biol Reprod Med 2018;64:381–388. [DOI] [PubMed] [Google Scholar]
- Kashir J. Increasing associations between defects in phospholipase C zeta and conditions of male infertility: not just ICSI failure? J Assist Reprod Genet 2020;37:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. Comparative biology of sperm factors and fertilization-induced calcium signals across the animal kingdom. Mol Reprod Dev 2013a;80:787–815. [DOI] [PubMed] [Google Scholar]
- Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update 2010;16:690–703. [DOI] [PubMed] [Google Scholar]
- Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, Ruas M, Tee ST, Heindryckx B, Galione A et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod 2011;26:3372–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, de Sutter P, Parrington J, Turner K, Child T et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril 2013b;99:107–117.e3. [DOI] [PubMed] [Google Scholar]
- Kashir J, Mistry BV, BuSaleh L, Abu‐Dawas R, Nomikos M, Ajlan A, Abu‐Dawud R, AlYacoub N, AlHassan S, Anthony Lai F et al. Phospholipase C zeta profiles are indicative of optimal sperm parameters and fertilization success in patients undergoing fertility treatment. Andrology 2020a;8:1143–1159. [DOI] [PubMed] [Google Scholar]
- Kashir J, Mistry BV, BuSaleh L, Abu‐Dawas R, Nomikos M, Ajlan A, Abu‐Dawud R, AlYacoub N, AlHassan S, Lai FA et al. Phospholipase C zeta profiles are indicative of optimal sperm parameters and fertilisation success in patients undergoing fertility treatment. Andrology 2020b;8:1143–1159. [DOI] [PubMed] [Google Scholar]
- Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv Biol Regul 2018;67:148–162. [DOI] [PubMed] [Google Scholar]
- Kashir J, Nomikos M, Lai FA, Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem Biophys Res Commun 2014;450:1204–1211. [DOI] [PubMed] [Google Scholar]
- Kashir J, Sermondade N, Sifer C, Oo SL, Jones C, Mounce G, Turner K, Child T, McVeigh E, Coward K. Motile sperm organelle morphology evaluation-selected globozoospermic human sperm with an acrosomal bud exhibits novel patterns and higher levels of phospholipase C zeta. Hum Reprod 2012;27:3150–3160. [DOI] [PubMed] [Google Scholar]
- Kauffman RF, Taylor RW, Pfeiffer DR. Cation transport and specificity of ionomycin. Comparison with ionophore A23187 in rat liver mitochondria. J Biol Chem 1980;255:2735–2739. [PubMed] [Google Scholar]
- Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. Embo J 2001;20:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AL, Stern CJ, Tong S, Hastie R, Agresta F, Walker SP, Brownfoot FC, MacLachlan V, Vollenhoven BJ, Lindquist AC. The incidence of hypertensive disorders of pregnancy following sperm donation in IVF: an Australian state-wide retrospective cohort study. Hum Reprod 2019;34:2541–2548. [DOI] [PubMed] [Google Scholar]
- Kerns K, Zigo M, Drobnis EZ, Sutovsky M, Sutovsky P. Zinc ion flux during mammalian sperm capacitation. Nat Commun 2018;9:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilani S, Chapman MG. Meiotic spindle normality predicts live birth in patients with recurrent in vitro fertilization failure. Fertil Steril 2014;101:403–406. [DOI] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O’Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 2011a;6:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BY, Yoon S-Y, Cha SK, Kwak KH, Fissore RA, Parys JB, Yoon TK, Lee DR. Alterations in calcium oscillatory activity in vitrified mouse eggs impact on egg quality and subsequent embryonic development. Pflugers Arch 2011b;461:515–526. [DOI] [PubMed] [Google Scholar]
- Kim J-W, Yang S-H, Yoon S-H, Kim S-D, Jung J-H, Lim J-H. Successful pregnancy and delivery after ICSI with artificial oocyte activation by calcium ionophore in in-vitro matured oocytes: a case report. Reprod Biomed Online 2015;30:373–377. [DOI] [PubMed] [Google Scholar]
- Kim ST, Cha YB, Park JM, Gye MC. Successful pregnancy and delivery from frozen-thawed embryos after intracytoplasmic sperm injection using round-headed spermatozoa and assisted oocyte activation in a globozoospermic patient with mosaic Down syndrome. Fertil Steril 2001;75:445–447. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Wakayama T. Efficient strontium-induced activation of mouse oocytes in standard culture media by chelating calcium. J Reprod Dev 2007;53:1207–1215. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol 1992a;149:80–89. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem 1992b;267:17624–17630. [PubMed] [Google Scholar]
- Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol Reprod 2005;72:992–996. [DOI] [PubMed] [Google Scholar]
- Koh S, Lee K, Wang C, Cabot RA, Machaty Z. STIM1 regulates store-operated Ca2+ entry in oocytes. Dev Biol 2009;330:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo OJ, Jang G, Kwon DK, Kang JT, Kwon OS, Park HJ, Kang SK, Lee BC. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology 2008;70:1111–1118. [DOI] [PubMed] [Google Scholar]
- Koot YEM, Hviid Saxtorph M, Goddijn M, de Bever S, Eijkemans MJC, Wely M, van der Veen F, Fauser BCJM, Macklon NS. What is the prognosis for a live birth after unexplained recurrent implantation failure following IVF/ICSI? Hum Reprod 2019;34:2044–2052. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 2004;279:10408–10412. [DOI] [PubMed] [Google Scholar]
- Krauchunas AR, Wolfner MF. Molecular changes during egg activation. Curr Top Dev Biol 2013;102:267–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 2015;8:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyono K, Kumagai S, Nishinaka C, Nakajo Y, Uto H, Toya M, Sugawara J, Araki Y. Birth and follow-up of babies born following ICSI using SrCl2 oocyte activation. Reprod Biomed Online 2008;17:53–58. [DOI] [PubMed] [Google Scholar]
- Kyono K, Takisawa T, Nakajo Y, Doshida M, Toya M. Birth and follow-up of babies born following ICSI with oocyte activation using strontium chloride or calcium ionophore A23187. J Mamm Ova Res 2012;29:35–40. [Google Scholar]
- Kyozuka K, Deguchi R, Mohri T, Miyazaki S. Injection of sperm extract mimics spatiotemporal dynamics of Ca2+ responses and progression of meiosis at fertilization of ascidian oocytes. Development 1998;125:4099–4105. [DOI] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development 1997;124:233–241. [DOI] [PubMed] [Google Scholar]
- Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs 2016;25:S35–S40. [DOI] [PubMed] [Google Scholar]
- Lee K, Davis A, Zhang L, Ryu J, Spate LD, Park K-W, Samuel MS, Walters EM, Murphy CN, Machaty Z et al. Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology 2015;84:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zheng X, Lian Y, Li M, Lin S, Zhuang X, Chen L, Liu P, Qiao J. Artificial oocyte activation improves cycles with prospects of ICSI fertilization failure: a sibling oocyte control study. Reprod Biomed Online 2019a;39:199–204. [DOI] [PubMed] [Google Scholar]
- Li B, Zhou Y, Yan Z, Li M, Xue S, Cai R, Fu Y, Hong Q, Long H, Yin M, Du T, Wang Y, Kuang Y, Yan Z, Lyu Q. Pregnancy and neonatal outcomes of artificial oocyte activation in patients undergoing frozen-thawed embryo transfer: a 6-year population-based retrospective study. Arch Gynecol Obstet 2019b;300:1083–1092. [DOI] [PubMed] [Google Scholar]
- Limatola N, Vasilev F, Chun JT, Santella L. Altered actin cytoskeleton in ageing eggs of starfish affects fertilization process. Exp Cell Res 2019a;381:179–190. [DOI] [PubMed] [Google Scholar]
- Limatola N, Vasilev F, Chun JT, Santella L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019b;27:241–249. [DOI] [PubMed] [Google Scholar]
- Lin P-Y, Huang C-C, Chen H-H, Huang B-X, Lee M-S. Failed sperm retrieval from severely oligospermic or non-obstructive azoospermic patients on oocyte retrieval day: Emergent oocyte cryopreservation is a feasible strategy. PloS One 2019;14:e0224919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005;15:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao YX, Zhang ZG, Xing Q. Artificial oocyte activation and human failed-matured oocyte vitrification followed by in vitro maturation. Zygote 2011;21:71–76. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Waller-Evans H. Lysosomal Ca(2+) homeostasis and signaling in health and disease. Cold Spring Harb Perspect Biol 2020;12:a035311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Chen X, Shen H, Zhang X, Li Y, Liang R, Li S, Wei L. No genetic alterations in infants from intracytoplasmic sperm injection in combination with artificial oocyte activation: a pilot study. Chin Med J (Engl) 2014;127:383–385. [PubMed] [Google Scholar]
- Lu Y, Bonte D, Ferrer-Buitrago M, Popovic M, Neupane J, Van der Jeught M, Leybaert L, De Sutter P, Heindryckx B. B. Culture conditions affect Ca(2+) release in artificially activated mouse and human oocytes. Reprod Fertil Dev 2018a;30:991–1001. [DOI] [PubMed] [Google Scholar]
- Lu Y, Reddy R, Ferrer Buitrago M, Vander Jeught M, Neupane J, D, Vos WH, Van den Abbeel E, Lierman S, De Sutter P, Heindryckx B. Strontium fails to induce Ca(2+) release and activation in human oocytes despite the presence of functional TRPV3 channels. Hum Reprod Open 2018;2018:hoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S-F, Liu X-Y, Miao D-Q, Han Z-B, Zhang X, Miao Y-L, Yanagimachi R, Tan J-H. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology 2005;64:1142–1157. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol 2006;206:565–573. [DOI] [PubMed] [Google Scholar]
- Mansour R, Fahmy I, Tawab NA, Kamal A, El-Demery Y, Aboulghar M, Serour G. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril 2009;91:133–139. [DOI] [PubMed] [Google Scholar]
- Martínez M, Durban M, Santaló J, Rodríguez A, Vassena R. Assisted oocyte activation effects on the morphokinetic pattern of derived embryos. J Assist Reprod Genet 2021;38:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateizel I, Verheyen G, Van de Velde H, Tournaye H, Belva F. Obstetric and neonatal outcome following ICSI with assisted oocyte activation by calcium ionophore treatment. J Assist Reprod Genet 2018;35:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Shibasaki S, Takahashi M, Sasaki C, Nakamura Y, Sato Y, Hattori H, Nakajo Y, Aono N, Okuyama N et al. Follow-up of child growth regarding new technologies: testicular sperm extraction (TESE), in vitro maturation (IVM), and assisted oocyte activation (AOA). Fertil Steril 2015;104:e290. [Google Scholar]
- McDougall A, Gillot I, Whitaker M. Thimerosal reveals calcium-induced calcium release in unfertilised sea urchin eggs. Zygote 1993;1:35–42. [DOI] [PubMed] [Google Scholar]
- Mehregan A, Ardestani G, Akizawa H, Carvacho I, Fissore R. Deletion of TRPV3 and CaV3.2 T-type channels in mice undermines fertility and Ca2+ homeostasis in oocytes and eggs. J Cell Sci 2021;134:jcs257956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Melo P, Jones C, Ross C, Mounce G, Turner K, Child T, Coward K. Use of phospholipase C zeta analysis to identify candidates for artificial oocyte activation: a case series of clinical pregnancies and a proposed algorithm for patient management. Fertil Steril 2020;114:163–174. [DOI] [PubMed] [Google Scholar]
- Metello JL, Tomás C, Ferreira P. Can we predict the IVF/ICSI live birth rate? JBRA Assist Reprod 2019;23:402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A 2012;109:4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev 2012;79:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril 2016;106:590–596.e2. [DOI] [PubMed] [Google Scholar]
- Miyara F, Migne C, Dumont-Hassan M, Meur AL, Cohen-Bacrie P, Aubriot F-X, Glissant A, Nathan C, Douard S, Stanovici A et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev 2003;64:458–470. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Thirty years of calcium signals at fertilization. Semin Cell Dev Biol 2006;17:233–243. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci 2006;100:545–552. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 1993;158:62–78. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 1992;257:251–255. [DOI] [PubMed] [Google Scholar]
- Moaz MN, Khattab S, Foutouh IA, Mohsen EA. Chemical activation of oocytes in different types of sperm abnormalities in cases of low or failed fertilization after ICSI: a prospective pilot study. Reprod Biomed Online 2006;13:791–794. [DOI] [PubMed] [Google Scholar]
- Montag M, Köster M, van der Ven K, Bohlen U, van der Ven H. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reproductive Biomed Online 2012;24:521–526. [DOI] [PubMed] [Google Scholar]
- Mor A, Zhang M, Esencan E, Simsek B, Nichols-Burns SM, Liu Y, Lo J, Kelk DA, Flores V, Gao X-B et al. A step towards the automation of intracytoplasmic sperm injection: real time confirmation of mouse and human oocyte penetration and viability by electrical resistance measurement. Fertil Steril 2020;113:234–236. [DOI] [PubMed] [Google Scholar]
- Moretti E, Collodel G, Salvatici MC, Belmonte G, Signorini C. New insights into sperm with total globozoospermia: increased fatty acid oxidation and centrin1 alteration. Syst Biol Reprod Med 2019;65:390–399. [DOI] [PubMed] [Google Scholar]
- Morin SJ, Hanson BM, Juneau CR, Neal SA, Landis JN, Scott RT, Hotaling JM. A comparison of the relative efficiency of ICSI and extended culture with epididymal sperm versus testicular sperm in patients with obstructive azoospermia. Asian J Androl 2020;22:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, Serhal P, Ben-Nagi J. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril 2017;108:468–482.e3. [DOI] [PubMed] [Google Scholar]
- Nakada K, Mizuno J. Intracellular calcium responses in bovine oocytes induced by spermatozoa and by reagents. Theriogenology 1998;50:269–282. [DOI] [PubMed] [Google Scholar]
- Nakahara M, Shimozawa M, Nakamura Y, Irino Y, Morita M, Kudo Y, Fukami K. A novel phospholipase C, PLC(eta)2, is a neuron-specific isozyme. J Biol Chem 2005;280:29128–29134. [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril 2010;94:520–526. [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Razavi S, Javdan Z, Tavalaee M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril 2008;90:2231–2237. [DOI] [PubMed] [Google Scholar]
- Nazarian H, Azad N, Nazari L, Piryaei A, Heidari MH, Masteri-Farahani R, Karimi M, Ghaffari-Novin M. Effect of Artificial Oocyte Activation on Intra-Cytoplasmic Sperm Injection Outcomes in Patients with Lower Percentage of Sperm Containing Phospholipase Cζ: A Randomized Clinical Trial. J Reprod Infertil 2019;20:3–9. [PMC free article] [PubMed] [Google Scholar]
- Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014;55:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforaki D, Vanden Meerschaut F, De Gheselle S, Qian C, Van den Abbeel E, De Vos WH, Deroo T, De Sutter P, Heindryckx B. Sperm involved in recurrent partial hydatidiform moles cannot induce the normal pattern of calcium oscillations. Fertil Steril 2014;102:581–588.e1. [DOI] [PubMed] [Google Scholar]
- Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, Heindryckx B. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril 2016;105:798–806.e2. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Kashir J, Swann K, Lai FA. Sperm PLCζ: from structure to Ca2+oscillations, egg activation and therapeutic potential. FEBS Lett 2013a;587:3609–3616. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Sanders JR, Kashir J, Sanusi R, Buntwal L, Love D, Ashley P, Sanders D, Knaggs P, Bunkheila A et al. Functional disparity between human PAWP and PLCζ in the generation of Ca2+oscillations for oocyte activation. Mol Hum Reprod 2015;21:702–710. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, Zikos C, Livaniou E, Amso N, Nounesis G et al. Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril 2013b;99:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norozi-Hafshejani M, Tavalaee M, Azadi L, Bahadorani M, Nasr-Esfahani MH. Effects of assisted oocyte activation with calcium- ionophore and strontium chloride on in vitro ICSI outcomes. Iran J Basic Med Sci. 2018;21:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrman E, Petzold M, Bergh C, Wennerholm U-B. School performance in children born after ICSI. Hum Reprod 2020;35:340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep 2018;8:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A. How to improve mouse cloning. Theriogenology 2020;150:215–220. [DOI] [PubMed] [Google Scholar]
- Oseguera-López I, Ruiz-Díaz S, Ramos-Ibeas P, Pérez-Cerezales S. Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front Cell Dev Biol 2019;7:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil J-P, Sainte-Beuve T, Banrezes B. [Mg2+]o/[Ca2+]o determines Ca2+ response at fertilization: tuning of adult phenotype? Reproduction 2017;154:675–693. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 2006;300:534–544. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development 2001;128:917–928. [DOI] [PubMed] [Google Scholar]
- Palermo GD, Neri QV, Rosenwaks Z. To ICSI or not to ICSI. Semin Reprod Med 2015;33:92–102. [DOI] [PubMed] [Google Scholar]
- Palermo GD, O’Neill CL, Chow S, Cheung S, Parrella A, Pereira N, Rosenwaks Z. Intracytoplasmic sperm injection: state of the art in humans. Reproduction 2017;154:F93–F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J. Does a soluble sperm factor trigger calcium release in the egg at fertilization? J Androl 2001;22:1–11. [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G. Flipping the switch: How a sperm activates the egg at fertilization. Dev Dyn 2007;236:2027–2038. [DOI] [PubMed] [Google Scholar]
- Parrington J, Jones KT, Lai FA, Swann K. Soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem J 1999;341: 1–4. [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 1996;379:364–368. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm–making the most of what you've got. Nat Cell Biol 2007;9:235–242. [DOI] [PubMed] [Google Scholar]
- Puppo A, Chun JT, Gragnaniello G, Garante E, Santella L. Alteration of the cortical actin cytoskeleton deregulates Ca2+ signaling, monospermic fertilization, and sperm entry. PloS One 2008;3:e3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986;7:1–12. [DOI] [PubMed] [Google Scholar]
- Que EL, Duncan FE, Lee HC, Hornick JE, Vogt S, Fissore RA, O’Halloran TV, Woodruff TK. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology 2019;127:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratna MB, Bhattacharya S, Abdulrahim B, McLernon DJ. A systematic review of the quality of clinical prediction models in in vitro fertilisation. Hum Reprod 2020;35:100–116. [DOI] [PubMed] [Google Scholar]
- Ray A, Shah A, Gudi A, Homburg R. Unexplained infertility: an update and review of practice. Reprod Biomed Online 2012;24:591–602. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 2001;70:281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K. Mammalian sperm contain a Ca(2+)-sensitive phospholipase C activity that can generate InsP(3) from PIP(2) associated with intracellular organelles. Dev Biol 2000;228:125–135. [DOI] [PubMed] [Google Scholar]
- Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K. Phospholipase Cζ causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 2004;128:697–702. [DOI] [PubMed] [Google Scholar]
- Romero-Garcia S, Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review). Int J Oncol 2019;54:1155–1167. [DOI] [PubMed] [Google Scholar]
- Rong Y, Ji S-Y, Zhu Y-Z, Wu Y-W, Shen L, Fan H-Y. ZAR1 and ZAR2 are required for oocyte meiotic maturation by regulating the maternal transcriptome and mRNA translational activation. Nucleic Acids Res 2019;47:11387–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005;169:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino P, Viganò P, Luddi A, Piomboni P. The ICSI procedure from past to future: a systematic review of the more controversial aspects. Hum Reprod Update 2016;22:194–227. [DOI] [PubMed] [Google Scholar]
- Rumbold AR, Sevoyan A, Oswald TK, Fernandez RC, Davies MJ, Moore VM. Impact of male factor infertility on offspring health and development. Fertil Steril 2019;111:1047–1053. [DOI] [PubMed] [Google Scholar]
- Runft LL, Carroll DJ, Gillett J, Giusti AF, O'Neill FJ, Foltz KR. Identification of a starfish egg PLC-γ that regulates Ca2+ release at fertilization. Dev Biol 2004;269:220–236. [DOI] [PubMed] [Google Scholar]
- Rybouchkin AV, Van der Straeten F, Quatacker J, De Sutter P, Dhont M. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil Steril 1997;68:1144–1147. [DOI] [PubMed] [Google Scholar]
- Sacha CR, Dimitriadis I, Christou G, James K, Brock ML, Rice ST, Bhowmick P, Bormann CL, Souter I. The impact of male factor infertility on early and late morphokinetic parameters: a retrospective analysis of 4126 time-lapse monitored embryos. Hum Reprod 2020;35:24–31. [DOI] [PubMed] [Google Scholar]
- Saleh A, Kashir J, Thanassoulas A, Safieh-Garabedian B, Lai FA, Nomikos M. Essential role of sperm-specific PLC-zeta in egg activation and male factor infertility: an update. Front Cell Dev Biol 2020;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JR, Swann K. Molecular triggers of egg activation at fertilization in mammals. Reproduction 2016;152:R41–R50. [DOI] [PubMed] [Google Scholar]
- Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z et al. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet 2018;102:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella L, Dale B. Assisted yes, but where do we draw the line? Reprod Biomed Online 2015;31:476–478. [DOI] [PubMed] [Google Scholar]
- Santella L, Limatola N, Chun JT. Calcium and actin in the saga of awakening oocytes. Biochem Biophys Res Commun 2015;460:104–113. [DOI] [PubMed] [Google Scholar]
- Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol Hum Reprod 2015;21:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Nakajo Y, Shibuya Y, Takisawa T, Sakamoto E, Kyono K. Follow up of children following assisted reproductive technology (ART). Fertil Steril 2011;96:S152–S153. [Google Scholar]
- Satouh Y, Ikawa M. New insights into the molecular events of mammalian fertilization. Trends Biochem Sci 2018;43:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 2002;129:3533–3544. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Swann K, Lai FA. PLCzeta, a sperm-specific PLC and its potential role in fertilization. Biochem Soc Symp 2007;74:23–36. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol 1995;30:21–62. [DOI] [PubMed] [Google Scholar]
- Sdrigotti A, Rey Valzacchi GJ, Leocata Nieto FA, Canada VE. Artificial oocyte activation with CALCIUM IONOPHORE (A+23187) following ICSI fertilization failure. Fertil Steril 2015;104:e302. [Google Scholar]
- Sermondade N, Hafhouf E, Dupont C, Bechoua S, Palacios C, Eustache F, Poncelet C, Benzacken B, Levy R, Sifer C. Successful childbirth after intracytoplasmic morphologically selected sperm injection without assisted oocyte activation in a patient with globozoospermia. Hum Reprod 2011;26:2944–2949. [DOI] [PubMed] [Google Scholar]
- Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, Rossi P. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development 1997;124:2267–2274. [DOI] [PubMed] [Google Scholar]
- Sette C, Bevilacqua A, Geremia R, Rossi P. Involvement of phospholipase Cgamma1 in mouse egg activation induced by a truncated form of the C-kit tyrosine kinase present in spermatozoa. J Cell Biol 1998;142:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. Embo J 2002;21:5386–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfontouris IA, Nastri CO, Lima MLS, Tahmasbpourmarzouni E, Raine-Fenning N, Martins WP. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Hum Reprod 2015;30:1831–1841. [DOI] [PubMed] [Google Scholar]
- Sha Q-Q, Zhang J, Fan H-Y. A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals. Biol Reprod 2019;101:579–590. [DOI] [PubMed] [Google Scholar]
- Sha Q-Q, Zhu Y-Z, Li S, Jiang Y, Chen L, Sun X-H, Shen L, Ou X-H, Fan H-Y. Characterization of zygotic genome activation-dependent maternal mRNA clearance in mouse. Nucleic Acids Res 2020;48:879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y-L, Zhu F-X, Yan J, Chen L, Tang W-H, Xiao S, Mo W-K, Zhang Z-G, He X-J, Qiao J et al. Novel DPY19L2 variants in globozoospermic patients and the overcoming this male infertility. Asian J Androl 2019;21:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebl O, Trautner PS, Enengl S, Reiter E, Allerstorfer C, Rechberger T, Oppelt P, Ebner T. Ionophore application for artificial oocyte activation and its potential effect on morphokinetics: a sibling oocyte study. J Assist Reprod Genet 2021;38:3125–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulou M, Gkoles L, Bakas P, Giannelou P, Kalampokas T, Pantos K, Koutsilieris M. Improving ICSI: a review from the spermatozoon perspective. Syst Biol Reprod Med 2016;62:359–371. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Bird GS, Putney JW. Jr., Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci 2008;121:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow P, Yim DL, Leibow JD, Saini S, Nuccitelli R. Fertilization stimulates an increase in inositol trisphosphate and inositol lipid levels in Xenopus eggs. Dev Biol 1996;180:108–118. [DOI] [PubMed] [Google Scholar]
- Song C, Hu CD, Masago M, Kariyai K, Yamawaki-Kataoka Y, Shibatohge M, Wu D, Satoh T, Kataoka T. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J Biol Chem 2001;276:2752–2757. [DOI] [PubMed] [Google Scholar]
- Song M, Liu C, Hu R, Wang F, Huo Z. Administration effects of single-dose GnRH agonist for luteal support in females undertaking IVF/ICSI cycles: A meta-analysis of randomized controlled trials. Exp Ther Med 2020;19:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P, Savy V, Williams AM, Williams CJ. Modulators of calcium signalling at fertilization. Open Biol 2020;10:200118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Epel D, Carroll EJ Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature 1974;252:41–43. [DOI] [PubMed] [Google Scholar]
- Stimpfel M, Jancar N, Vrtacnik-Bokal E, Virant-Klun I. Conventional IVF improves blastocyst rate and quality compared to ICSI when used in patients with mild or moderate teratozoospermia. Syst Biol Reprod Med 2019;65:458–464. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science 2007;316:407–408. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 1999;211:157–176. [DOI] [PubMed] [Google Scholar]
- Sustar K, Rozen G, Agresta F, Polyakov A. Use of intracytoplasmic sperm injection (ICSI) in normospermic men may result in lower clinical pregnancy and live birth rates. Aust N Z J Obstet Gynaecol 2019;59:706–711. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development 2010;137:2659–2669. [DOI] [PubMed] [Google Scholar]
- Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990;110:1295–1302. [DOI] [PubMed] [Google Scholar]
- Swann K. Thimerosal causes calcium oscillations and sensitizes calcium-induced calcium release in unfertilized hamster eggs. FEBS Lett 1991;278:175–178. [DOI] [PubMed] [Google Scholar]
- Swann K. The role of Ca(2+) in oocyte activation during in vitro fertilization: insights into potential therapies for rescuing failed fertilization. Biochim Biophys Acta Mol Cell Res 2018;1865:1830–1837. [DOI] [PubMed] [Google Scholar]
- Swann K. The soluble sperm factor that activates the egg: PLCzeta and beyond. Reproduction 2020;160:V9–V11. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. The sperm phospholipase C-ζ and Ca2+ signalling at fertilization in mammals. Biochem Soc Trans 2016;44:267–272. [DOI] [PubMed] [Google Scholar]
- Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction 2004;127:431–439. [DOI] [PubMed] [Google Scholar]
- Swann K, Ozil J-P. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 1994;152:183–222. [DOI] [PubMed] [Google Scholar]
- Swann K, Saunders C, Rogers N, Lai F. PLCζ(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol 2006;17:264–273. [DOI] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Lai FA. PLCζ, a sperm-specific PLC and its potential role in fertilization. Biochem Soc Symp 2007;74:23. [DOI] [PubMed] [Google Scholar]
- Swann K, Windsor S, Campbell K, Elgmati K, Nomikos M, Zernicka-Goetz M, Amso N, Lai FA, Thomas A, Graham C. Phospholipase C-ζ-induced Ca2+ oscillations cause coincident cytoplasmic movements in human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril 2012;97:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol 2008;52:585–594. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun 2013;430:60–65. [DOI] [PubMed] [Google Scholar]
- Tarín JJ, Pascual E, Pérez-Hoyos S, Gómez R, García-Pérez MA, Cano A. Cumulative probabilities of live birth across multiple complete IVF/ICSI cycles: a call for attention. J Assist Reprod Genet 2020;37:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SL, Yoon SY, Morshedi MS, Lacey DR, Jellerette T, Fissore RA, Oehninger S. Complete globozoospermia associated with PLCζ deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online 2010;20:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera A, Mollá M, Muriel L, Remohí J, Pellicer A, De Pablo JL. Successful pregnancy and childbirth after intracytoplasmic sperm injection with calcium ionophore oocyte activation in a globozoospermic patient. Fertil Steril 2008;90:1202.e1–5. [DOI] [PubMed] [Google Scholar]
- Ten J, Peinado P, Guerrero J, Bernabeu A, Llácer J, Orozco-Beltran D, Carratala-Munuera C, Bernabeu R. Comparison of the assisted reproductive technology outcomes between conventional IVF and ICSI with donor oocytes in normozoospermic patients. Hum Fertil (Camb) 2019;1–7. doi: 10.1080/14647273.2019.1686775. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril 2002;78:619–624. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Sousa M. Key elements of a highly efficient intracytoplasmic sperm injection technique: Ca2+ fluxes and oocyte cytoplasmic dislocation. Fertil Steril 1995;64:770–776. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod 1994;9:511–518. [DOI] [PubMed] [Google Scholar]
- Tiegs AW, Tao X, Landis J, Zhan Y, Franasiak JM, Seli E, Wells D, Fragouli E, Scott RT. Sperm mitochondrial DNA copy number is not a predictor of intracytoplasmic sperm injection (ICSI) cycle outcomes. Reprod Sci 2020;27:1350–1356. [DOI] [PubMed] [Google Scholar]
- Tokuhiro K, Dean J. Glycan-independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev Cell 2018;46:627–640.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti E, Ménézo Y. Gamete activation: basic knowledge and clinical applications. Hum Reprod Update 2016;22:420–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uh K, Ryu J, Zhang L, Errington J, Machaty Z, Lee K. Development of novel oocyte activation approaches using Zn2+ chelators in pigs. Theriogenology 2019;125:259–267. [DOI] [PubMed] [Google Scholar]
- van Blerkom J, Cohen J, Johnson M. A plea for caution and more research in the ‘experimental’ use of ionophores in ICSI. Reprod Biomed Online 2015;30:323–324. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, D'Haeseleer E, Gysels H, Thienpont Y, Dewitte G, Heindryckx B, Oostra A, Roeyers H, Van Lierde K, De Sutter P. Neonatal and neurodevelopmental outcome of children aged 3–10years born following assisted oocyte activation. Reprod Biomed Online 2014a;28:54–63. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, De Gheselle S, Dullaerts V, Van den Abbeel E, Gerris J, Heindryckx B, De Sutter P. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod 2012;27:1977–1984. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, De Roo C, Lierman S, Qian C, Schmitt-John T, De Sutter P, Heindryckx B. Comparison of pre- and post-implantation development following the application of three artificial activating stimuli in a mouse model with round-headed sperm cells deficient for oocyte activation. Hum Reprod 2013;28:1190–1198. [DOI] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online 2014b;28:560–571. [DOI] [PubMed] [Google Scholar]
- Vasilev F, Chun JT, Gragnaniello G, Garante E, Santella L. Effects of ionomycin on egg activation and early development in starfish. PloS One 2012;7:e39231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod 2019;101:1076–1082. [DOI] [PubMed] [Google Scholar]
- Versieren K, Heindryckx B, Lierman S, Gerris J, De Sutter P. Developmental competence of parthenogenetic mouse and human embryos after chemical or electrical activation. Reprod Biomed Online 2010;21:769–775. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006;312:1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Wang Z, Dang Y, Shi Y, Zhao P, Zhang K. The nucleolar protein NOP2 is required for nucleolar maturation and ribosome biogenesis during preimplantation development in mammals. FASEB J 2020;34:2715–2729. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Calcium at fertilization and in early development. Physiol Rev 2006;86:25–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M, Irvine RF. Inositol 1,4,5-trisphosphate microinjection activates sea urchin eggs. Nature 1984;312:636–639. [Google Scholar]
- Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J 2001;357:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Kline D, Bian Y, Blackshaw S, Cameron AM, Fralich TJ, Schnaar RL, Snyder SH. Molecularly cloned mammalian glucosamine-6-phosphate deaminase localizes to transporting epithelium and lacks oscillin activity. FASEB J 1998;12:91–99. [DOI] [PubMed] [Google Scholar]
- Wu ATH, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park K-W, Yi Y-J, Xi YW, Prather RS et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem 2007;282:12164–12175. [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev 1997;46:176–189. [DOI] [PubMed] [Google Scholar]
- Xu Y-R, Yang W-X. Calcium influx and sperm-evoked calcium responses during oocyte maturation and egg activation. Oncotarget 2017;8:89375–89390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-associated increase in IP3 receptor type 1: role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev Biol 2003;254:163–171. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hirose N, Kamimura S, Wakayama S, Ito J, Ooga M, Wakayama T. Production of mouse offspring from inactivated spermatozoa using horse PLCζ mRNA. J Reprod Dev 2020;66:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S, Nakagawa K, Nakasaka H, Aono T. Fertilization failure and oocyte activation. J Med Invest 2000;47:1–8. [PubMed] [Google Scholar]
- Yanagida K, Fujikura Y, Katayose H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol 2008;7:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Katayose H, Yazawa H, Kimura Y, Sato A, Yanagimachi H, Yanagimachi R. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod 1999;14:1307–1311. [DOI] [PubMed] [Google Scholar]
- Yang X, Shu L, Cai L, Sun X, Cui Y, Liu J. Homozygous missense mutation Arg207Cys in the WEE2 gene causes female infertility and fertilization failure. J Assist Reprod Genet 2019;36:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasemin Sert U, Kansu Celik H, Canpolat FE, Sımsek GK, Engin Ustun Y. Hearing screening test results of newborns conceived by intracytoplasmic sperm injection: a retrospective study of tertiary referral center. Fetal Pediatr Pathol 2020;39:288–289. [DOI] [PubMed] [Google Scholar]
- Ye Y, Li N, Yan X, Wu R, Zhou W, Cheng L, Li Y. Genetic analysis of embryo in a human case of spontaneous oocyte activation: a case report. Gynecol Endocrinol 2020;36:294–296. [DOI] [PubMed] [Google Scholar]
- Yelumalai S, Yeste M, Jones C, Amdani SN, Kashir J, Mounce G, Da Silva SJM, Barratt CL, McVeigh E, Coward K. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil Steril 2015;104:561–568.e4. [DOI] [PubMed] [Google Scholar]
- Yeste M, Jones C, Amdani SN, Coward K. Oocyte Activation and Fertilisation: Crucial Contributors from the Sperm and Oocyte. Results Probl Cell Differ 2017;59:213–239. [DOI] [PubMed] [Google Scholar]
- Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update 2016a. Jan-Feb;22:23–47. [DOI] [PubMed] [Google Scholar]
- Yeste M, Jones C, Amdani SN, Yelumalai S, Mounce G, da Silva SJM, Child T, Coward K. Does advancing male age influence the expression levels and localisation patterns of phospholipase C zeta (PLCζ) in human sperm? Sci Rep 2016b;6:27543–27543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S. Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol 2004;268:245–257. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Bae IH, Kim HJ, Jang JM, Hur YS, Kim HK, Yoon SH, Lee WD, Lim JH. Analysis of clinical outcomes with respect to spermatozoan origin after artificial oocyte activation with a calcium ionophore. J Assist Reprod Genet 2013;30:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S-Y, Jellerette T, Salicioni AM, Lee HC, Yoo M-S, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest 2008;118:3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Plant S. Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J Physiol 1992;458:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCζ causes Ca(2+) oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2). Mol Biol Cell 2012;23:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Saunders CM, Lai FA, Swann K. Preimplantation development of mouse oocytes activated by different levels of human phospholipase C zeta. Hum Reprod 2008;23:365–373. [DOI] [PubMed] [Google Scholar]
- Zanetti BF, Braga D, Setti AS, Iaconelli A Jr., Borges E. Jr., Effect of GnRH analogues for pituitary suppression on oocyte morphology in repeated ovarian stimulation cycles. JBRA Assist Reprod 2020;24:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y-L, Zhao L-W, Pi S-B, Zhang S-Y, Tong C, Fan H-Y. The CRL4-DCAF13 ubiquitin E3 ligase supports oocyte meiotic resumption by targeting PTEN degradation. Cell Mol Life Sci 2020;77:2181–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chao C-H, Jaeger LA, Papp AB, Machaty Z. Calcium oscillations in fertilized pig oocytes are associated with repetitive interactions between STIM1 and ORAI1†. Biol Reprod 2018;98:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005;437:902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Du X, Zhao L, He M, Lin L, Guo C, Zhang X, Han J, Yan H, Huang K et al. SIRT1 facilitates primordial follicle recruitment independent of deacetylase activity through directly modulating Akt1 and mTOR transcription. Faseb J 2019;33:14703–14716. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang T, Hao Y, Panhwar F, Chen Z, Zou W, Ji D, Chen B, Zhou P, Zhao G et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology 2017;74:43–49. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yan Y, Huang X, Li Y. Do the children born after assisted reproductive technology have an increased risk of birth defects? A systematic review and meta-analysis. J Matern Fetal Neonatal Med 2020;33:322–333. [DOI] [PubMed] [Google Scholar]
- Zheng D, Zeng L, Yang R, Lian Y, Zhu Y-M, Liang X, Tang L, Wang H, Cao Y, Hao G et al. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): protocol for a multicentre randomised controlled trial. BMJ Open 2019;9:e030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wing MR, Sondek J, Harden TK. Molecular cloning and characterization of PLC-eta2. Biochem J 2005;391:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.