Abstract
Background
Dyspnea is a common and distressing symptom in patients with cancer. To improve its management, multicenter confirmatory studies are necessary. Research policy would be useful in conducting these studies. Here, we propose a new research policy for the management of dyspnea in patients with cancer.
Methods
The first draft was developed by a policy working group of 11 specialists in the field of supportive care or palliative care for dyspnea. Then, a provisional draft was developed after review by a research support group (the Japanese Supportive, Palliative and Psychosocial Care Study Group) and five Japanese scientific societies (Japanese Association of Supportive Care in Cancer, Japanese Society of Medical Oncology, Japanese Society of Palliative Medicine, Japanese Association of Rehabilitation Medicine and Japanese Society of Clinical Oncology), and receipt of public comments.
Results
The policy includes the following components of research policy on dyspnea: (i) definition of dyspnea, (ii) scale for assessment of dyspnea, (iii) reason for dyspnea or factors associated with dyspnea and (iv) treatment effectiveness outcomes/adverse events. The final policy (Ver1.0) was completed on 1 March 2021.
Conclusions
This policy could help researchers plan and conduct studies on the management of cancer dyspnea.
Keywords: dyspnea, cancer, supportive care, palliative care, clinical research, clinical study
We propose a research policy for the management of dyspnea in patients with cancer. This policy could help researchers plan and conduct studies on the management of cancer dyspnea.
Introduction
Dyspnea is a common symptom in advanced cancer patients and is most frequent in lung cancer, with a prevalence of 74% of the cases. Dyspnea is a distressing symptom not only for patients but also for their families. It has a significant impact on the quality of life (1). The aetiology of dyspnea in cancer patients is diverse, including primary and metastatic lung cancer, malignant pleural effusion, major airway obstruction, atelectasis, lymphangitis carcinomatosa and superior vena cava syndrome. Sometimes more than one aetiology can lead to dyspnea development.
Although several treatment interventions such as opioids and oxygen therapy have been commonly used for palliation of cancer dyspnea in daily practice, the evidence of their efficacy has generally been scarce. Thus, to improve the quality of dyspnea management in cancer patients, quality clinical studies are warranted. We recently developed a general research policy for cancer supportive and palliative care in Japan (2). Here, we propose a specific policy for research on the management of cancer dyspnea to standardize its clinical research design.
Methods
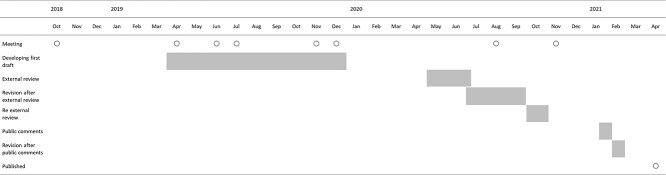
The task group for developing a specific research policy for the study of cancer dyspnea was launched in October 2018. The group consisted of 11 clinical specialists and researchers in this field which comprised nine palliative care physicians, one radiation oncologist and one oncology nurse specialist. Of them, three were additionally specialized in respiratory medicine including thoracic oncology, one in psycho-oncology and one in respiratory therapy. The first draft was developed via e-mail and eight online and in-person meetings. Three pairs of authors contributed each section and then it was revised by the other members. After external review was conducted by a domestic research support group in this field (the Japanese Supportive, Palliative and Psychosocial Care Study Group) and five domestic academic societies (Japanese Association of Supportive Care in Cancer, Japanese Society of Medical Oncology, Japanese Society for Palliative Medicine, Japanese Association of Rehabilitation Medicine and Japanese Society of Clinical Oncology) and receipt of public comments, the final statement was fixed. The date of each step is shown in Fig. 1.
Figure 1.
The date of each step.
Results
Definition of dyspnea
Dyspnea is defined as a subjective experience of breathing discomfort (3). This policy mainly deals with dyspnea caused by cancer (primary/metastatic lung cancer, malignant pleural effusion, superior vena cava syndrome, lymphangitis carcinomatosa, etc.). Thus, dyspnea associated with cancer treatments (drug-induced lung injury, radiation pneumonitis, etc.), and dyspnea primarily caused by non-cancer co-morbidities (COPD, interstitial lung disease, heart failure, etc.) are beyond this policy. Moreover, since this policy focuses on capable patients, those who lack the capacity to rate patient-reported outcomes due to unconsciousness or cognitive impairment are beyond this policy. Episodic breathlessness is not included in this policy because this concept has not been fully recognized internationally.
When conducting clinical research on dyspnea, it is necessary to clearly define the circumstance under which dyspnea is studied.
(i) Resting dyspnea: We defined resting dyspnea as ‘persistent dyspnea after the patient had been lying on the bed or sitting for at least 5 min.’
(ii) Exertional dyspnea: We defined exertional dyspnea as ‘dyspnea induced by exercise load from activities of daily life.’ One specific example of exertional dyspnea is the modified Medical Research Council (MRC) Dyspnea Scale (4) of Grade 2 or higher.
Scales for assessment of dyspnea in cancer patients
Because dyspnea is a subjective symptom, the intensity of dyspnea should be measured using a patient-reported outcome scale, which should be adopted as the primary outcome in clinical research on cancer dyspnea treatment.
Numerical rating scale
The numerical rating scale (NRS) is a subjective scale used for the quantitative assessment of dyspnea intensity. The NRS is an 11-point numerical scale ranging from 0 (no dyspnea) to 10 (worst possible dyspnea). Several studies reported that the minimally clinically important difference (MCID) of the NRS within the group for cancer dyspnea was ~1 (5,6). Thus, we propose a change of 1 or more as MCID in the NRS. Although MCID between groups has not been fully investigated, some clinical research on dyspnea has adopted an MCID of 1 (7,8). Permission to use the NRS in clinical research is not required.
Visual analogue scale
The visual analogue scale (VAS) is another major subjective scale for the quantitative assessment of dyspnea intensity. Although it has several variations, VAS is typically a 100 mm straight horizontal or vertical line, where one end of the line defined ‘no dyspnea’ and the other end ‘worst possible dyspnea.’ The patient is asked to mark on the line where their perception of dyspnea best fits. Although its reliability and validity for assessing cancer patients have not been verified, it is an assessment scale that is often used in clinical studies in the field of palliative treatment. Some clinical studies suggested MCID of VAS within the group for cancer dyspnea as ~10–20 mm (6) (9). Permission for the use of VAS in clinical research is not required.
Modified Borg scale
The modified Borg scale is also a subjective scale for quantitative assessment of dyspnea intensity. The modified Borg scale is vertically lined with a 12-point numerical scale from 0 to 10 (including 0.5) anchored corresponding verbal expression of intensity. Each point is set to have equal intervals. Its validity has been verified mainly in exertional dyspnea in healthy subjects and in non-cancer chronic respiratory diseases. Although it has not been formally validated in cancer dyspnea, several clinical studies of cancer dyspnea have used the modified Borg scale (10,11). Permission to use the modified Borg scale in clinical research is not required.
Cancer dyspnoea scale
The cancer dyspnoea scale (CDS) is a multidimensional scale for the assessment of subjective perception of dyspnea in cancer patients, which was developed in Japanese and has been validated in other languages. The CDS consists of 12 items, including five for sense of effort, three for sense of discomfort and four for sense of anxiety (12). Each item was scored on a 5-point scale from ‘not at all (= 1)’ to ‘very much (= 5).’ Its content validity and reliability have been verified in patients with advanced cancers. Permission to use the CDS in clinical research is not required.
Multidimensional dyspnea profile
The multidimensional dyspnea profile (MDP) is a comprehensive scale for assessing the sensory and affecting dimensions of dyspnea. The MDP consists of several parts: the A1 scale assesses the ‘discomfort’ and ‘difficulty’ of the respiratory sensation on an 11-point scale from 0 (neutral) to 10 (unbearable), and patients can fill in and identify exactly what actions this difficulty level refers to; the SQ selection includes five items addressing the sensory quality of dyspnea. Each item is evaluated on an 11-point scale from 0 (none) to 10 (as intense as I can imagine); the A2 scale includes five items addressing emotional responses related to dyspnea. Each item is evaluated on an 11-point scale from 0 (none) to 10 (most I can imagine). Although its reliability and validity have been verified in emergency room patients (13,14), it has rarely been used in clinical research on cancer dyspnea. The validity of the Japanese version of the MDP has been confirmed in patients with COPD (15). When it is used in clinical research, it is necessary to obtain permission from the Mapi Research Trust.
Integrated palliative care outcome scale
The integrated palliative care outcome scale (IPOS) consists of 17 items that represent the most important patient-reported concerns: 10 physical, two emotional, one spiritual; two related to communication, one to family anxiety and one to a practical issue. Dyspnea (shortness of breath) is included as one of ‘physical symptoms.’ The dyspnea item of the IPOS is evaluated on a 5-point scale from ‘not at all’ (= 1) to ‘overwhelmingly’ (= 4). IPOS has two versions: self-reported by patient and proxy-reported by staff. The validity and reliability of the IPOS was confirmed in patients receiving palliative care (16). The Japanese version of the IPOS was also validated (17,18). Permission to use the IPOS in clinical research is not required.
Support team assessment schedule symptom version
The support team assessment schedule (STAS) symptom version is a proxy rating scale used by staff in palliative care. The STAS symptom version includes dyspnea, and it can assess the impact that breathlessness/dyspnea has on the patient on a 5-point scale from none (= 0) to ‘severe and continuous overwhelming breathlessness, unable to think of other matters’ (= 4). The STAS symptom version was developed in Japan. Its inter-rater reliability between physicians and nurses has been confirmed (19). Permission to use the STAS in clinical research is not required.
Causes/factors related to dyspnea
Known factors related to dyspnea should be evaluated in clinical research on cancer dyspnea to set eligibility criteria, stratification factors and allocation adjustment factors. It is necessary to define individual causes/factors before starting the study. Table 1 lists the causes and factors related to dyspnea (20–22).
Table 1.
Causes and factors related to dyspnea
| Direct cause | Lung tumour, mediastinal mass, airway obstruction (neoplastic, vocal cord paralysis, etc.), superior vena cava syndrome, pulmonary embolism, lymphangitis carcinomatosa, pericardial effusion, pleural effusion, pneumonia (infectious, non-infectious) |
| Indirect cause | Anaemia, ascites, diaphragm elevation due to hepatomegaly, respiratory muscle loss due to cachexia (fatigue) |
| Physical factors | Age, sex, ECOG PS [20], pulse rate, respiratory rate, percutaneous oxygen saturation [21], intensity of dyspnea [21], pain, cough and malaise [20, 21] |
| Disease factors (condition of primary disease) | Primary lesion (oesophageal cancer or mediastinal tumour [22]), disease staging [20], organic lung lesions (primary lung cancer, metastatic lung cancer) [20], airway obstruction (neoplastic, vocal cord paralysis etc.) [22], pericardial fluid retention [22], ascites |
| Mental/psychological factors | Anxiety, depression [20–22] |
| Other factors | History of smoking Cancer treatment, thoracentesis drainage, oxygen inhalation, drugs (opioids, benzodiazepines, antidepressants, steroids, diuretics, bronchodilators, etc.) that patients are receiving concurrently with the study intervention. COPD, interstitial lung disease and chronic heart failure as comorbidities |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; COPD, chronic obstructive pulmonary disease.
Treatment effectiveness outcomes/adverse events
Clinical research evaluating the efficacy for resting dyspnea
Primary outcome
The primary outcome should be based on the intensity of dyspnea as evaluated by patient-reported outcomes.
Although the NRS is the preferred scale for measuring primary outcome, alternatively, the VAS or the modified Borg scale may be used. We propose using the NRS for evaluating dyspnea as the primary outcome, because it is simple and widely used in previous research on cancer dyspnea. Regarding the assessment time-point for the primary outcome, it should be individually set based on the characteristics of the study intervention, such as onset of action, half-time of action and time until steady state.
For example, in a study evaluating the efficacy of a single dose of a short-acting medication (i.e. Tmax 1 h, T1/2 3 h), the time point for assessing the primary outcome should be 1 to 2 h after administration. Usually, NRS ‘now’ is chosen as the time frame for assessment.
On the other hand, it should be several days or 1 week after starting intervention in the study evaluating the regular dose of a long-acting medication (i.e. time to steady state will be 24–72 h). In such cases, time frame for assessing the NRS should be unified such as ‘over past 24 h’ or ‘now,’ and it should be clarified whether to assess the ‘average’ or ‘maximum’ intensity of dyspnea. In any case, when setting the assessment time frame, the extent to which deviation is tolerated should be specified.
Secondary outcome
Appendix 1 shows our proposal for the secondary outcomes of dyspnea at rest (23–27).
Clinical research evaluating the efficacy for exertional dyspnea
Methods of inducing exertion
Six-minute walk test (28)
Patients are asked to walk between two cones 30 m apart. The 6-min walk test measures the distance that a patient can quickly walk on a flat, hard surface in 6 min.
This was used in multiple randomized controlled trials related to exertional dyspnea. Moreover, it is possible to apply a load similar to activities of daily living, such as walking. On the other hand, since it is a self-paced test involving patient’s own effort, there is a drawback that the load applied is not constant, such as the possibility that the walking speed and walking distance may change depending on encouragement received and the patient’s mood. In addition, the test may be difficult to adapt to patients who already have walking difficulties.
Two-minute walk test (29)
The method is essentially the same as the 6-min walk test. The walking time is 2 min.
Shuttle walking test (30)
Patients are asked to walk back and forth between two cones 10 m apart. The walking speed is determined by an audio signal, starting at 30 m/min and increasing by 10 m/min every minute until the patient either cannot reach the cone before the next audio signal or becomes too dyspneic. Because the walking speed is determined by the protocol, there is an advantage in that the influence of encouragement received and patient’s mood is small and the load on the patient is constant. On the other hand, many patients with advanced cancer may withdraw from the test because they are unable to tolerate a certain load. In addition, since patients increase the walking speed according to the audio signal, this test cannot be performed in patients with hearing impairment. Similar to the 6-min and 2-min walk tests, this test is difficult to adapt for patients who already have walking difficulties.
Arm exercise (31)
Patients are asked to move an outstretched arm between 20 cm above and 20 cm below the shoulder height. Unlike walking tests, this can be performed by patients with walking difficulties.
Primary outcome
Similar to resting dyspnea, the primary outcome should be based on the intensity of dyspnea evaluated by patient-reported outcomes, such as the NRS, VAS and modified Borg scale. In studies evaluating the efficacy of intervention for exertional dyspnea, the intervention is initiated before inducing exertion, and prophylactic effects are examined, because dyspnea will improve spontaneously after the exertion stops. The intensity of dyspnea immediately after the completion of exertion may alternate (32–34), however, the intensity of dyspnea before inducing exertion can be a significant confounding factor. Thus, we think evaluation of the latter is not appropriate for primary outcome. We propose that primary outcome should be assessing the change in dyspnea intensity immediately after the completion of exertion from baseline (before inducing exertion), and the appropriate time frame of assessment would be ‘now’ (35).
In addition, if the exertion induction is not completed, the insufficient load of exertion or resting during exertion may significantly influence dyspnea intensity. Thus, such cases should be handled as incomplete in the analysis of primary outcomes, and the study protocol should specify how to deal with such incomplete cases in advance. In this policy, we propose that ‘discontinued cases shall be treated as having maximum dyspnea (NRS = 10).’
Secondary outcome
Appendix 2 shows our proposal for the secondary outcomes of dyspnea on exertion.
Discussion
This is the first research policy on the management of cancer dyspnea. Some systematic reviews of interventions for cancer dyspnea have stated the methodological limitations of existing studies (36,37). This research policy may lead to the implementation of uniform studies based on a correct methodological approach.
Compared with the general research policy we published (2), this policy is more detailed, including assessment tools, outcomes and the methods of exertional loading specific to cancer dyspnea research. In the field of palliative and end-of-life care research, methods of researching end of life care (MORCare) have reported some recommendations for general palliative and end-of-life care research (38–44). In addition, in the field of pain research, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has reported some recommendations for pain clinical trials (45–51). However, no recommendations have been reported in the field of cancer dyspnea research specifically. Therefore, this policy is worth noting.
We only included IPOS and STAS symptom version as objective measures for assessing dyspnea for patient who cannot rate self-evaluation reports. Although the Respiratory Distress Observation Scale (RDOS) was another assessment option (52), it was developed for use in the ICU setting and has not yet been validated for cancer patients. Moreover, a previous study that assessed advanced cancer patients showed a weak correlation between self-reported dyspnea intensity and RDOS (53). Therefore, we decided not to include RDOS in this policy. Overall, objective measures for assessing dyspnea in cancer patients are lacking, and this needs to be addressed in future studies.
As a limitation, this policy was developed exclusively by Japanese palliative care specialists, and we only included assessment tools available in Japanese. Therefore, recommended assessment tools may be different in other countries. We are looking forward to comments and criticism from researchers in other countries to improve our policy because our ultimate goal is to develop a consensus global research policy for cancer dyspnea.
Conclusions
This policy may help researchers plan and conduct studies on the management of cancer dyspnea.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED), grant number 20ck0106499h0002.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Supplementary Material
Contributor Information
Yoshinobu Matsuda, Department of Psychosomatic Internal Medicine, National Hospital Organization Kinki-Chuo Chest Medical Center, Sakai, Japan.
Takashi Yamaguchi, Division of Palliative Care, Department of Medicine, Konan Medical Center, Kobe, Japan; Department of Palliative Medicine, Kobe University Graduate School of Medicine, Kobe, Japan.
Yoshihisa Matsumoto, Department of Palliative Medicine, National Cancer Center Hospital East, Kashiwa, Japan.
Hiroto Ishiki, Department of Palliative Medicine, National Cancer Center Hospital, Tokyo, Japan.
Yuko Usui, Division of Palliative Therapy, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan.
Jun Kako, College of Nursing Art and Science, University of Hyogo, Akashi, Japan.
Kozue Suzuki, Department of Palliative Care, Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital, Tokyo, Japan.
Ryo Matsunuma, Division of Palliative Care, Department of Medicine, Konan Medical Center, Kobe, Japan; Department of Palliative Medicine, Kobe University Graduate School of Medicine, Kobe, Japan.
Masanori Mori, Palliative and Supportive Care Division, Seirei Mikatahara General Hospital, Hamamatsu, Japan.
Hiroaki Watanabe, Home Palliative Care Asunaro Clinic, Komaki, Japan.
Sadamoto Zenda, Department of Radiation Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
References
- 1. Kloke M, Cherny N, Committee EG. Treatment of dyspnoea in advanced cancer patients: ESMO clinical practice guidelines. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26 Suppl 5:v169–73. [DOI] [PubMed] [Google Scholar]
- 2. Zenda S, Uchitomi Y, Morita T, Yamaguchi T, Inoue A. Establishment of a research policy for supportive and palliative care in Japan. Jpn J Clin Oncol. 2021;51(4):538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parshall MB, Schwartzstein RM, Adams L et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The global strategy for the diagnosis, management and prevention of COPD. Global initiative for chronic obstructive lung disease (gold). 2022. https://goldcopd.org/2022-gold-reports/
- 5. Hui D, Shamieh O, Paiva CE et al. Minimal clinically important differences in the Edmonton symptom assessment scale in cancer patients: a prospective, multicenter study. Cancer. 2015;121(17):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Clinically important differences in the intensity of chronic refractory breathlessness. Journal of pain and symptom management. 2013;46(6):957–63. [DOI] [PubMed] [Google Scholar]
- 7. Kako J, Morita T, Yamaguchi T et al. Fan therapy is effective in relieving Dyspnea in patients with terminally ill cancer: a parallel-arm, randomized controlled trial. Journal of pain and symptom management. 2018;56(4):493–500. [DOI] [PubMed] [Google Scholar]
- 8. Currow D, Louw S, McCloud P et al. Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75(1):50–6. [DOI] [PubMed] [Google Scholar]
- 9. Mishra EK, Corcoran JP, Hallifax RJ, Stradling J, Maskell NA, Rahman NM. Defining the minimal important difference for the visual analogue scale assessing dyspnea in patients with malignant pleural effusions. PLoS One. 2015;10(4):e0123798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hui D, Hernandez F, Larsson L et al. Prophylactic fentanyl sublingual spray for episodic exertional Dyspnea in cancer patients: a pilot double-blind randomized controlled trial. Journal of pain and symptom management. 2019;58(4):605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navigante AH, Cerchietti LC, Castro MA, Lutteral MA, Cabalar ME. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. Journal of pain and symptom management. 2006;31(1):38–47. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the cancer dyspnoea scale: a multidimensional, brief, self-rating scale. British journal of cancer. 2000;82(4):800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meek PM, Banzett R, Parsall MB, Gracely RH, Schwartzstein RM, Lansing R. Reliability and validity of the multidimensional dyspnea profile. Chest. 2012;141(6):1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masashi Kanezaki AT, Kunihiko Terada and Satoru Ebihara. Development of the Japanese version of the Multidimensional Dyspnea Profile The 29th Annual Meeting of the Japan Society for Rrespiratory Care and Rehabilitation. Nagoya; 2019. [Google Scholar]
- 16. Murtagh FE, Ramsenthaler C, Firth A et al. A brief, patient- and proxy-reported outcome measure in advanced illness: validity, reliability and responsiveness of the integrated palliative care outcome scale (IPOS). Palliative medicine. 2019;33(8):1045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakurai H, Miyashita M, Morita T, et al. Comparison between patient-reported and clinician-reported outcomes: validation of the Japanese version of the integrated palliative care outcome scale for staff. Palliative & supportive care. 2021;5:1–7. doi: 10.1017/S1478951521000018. [DOI] [PubMed] [Google Scholar]
- 18. Sakurai H, Miyashita M, Imai K et al. Validation of the integrated palliative care outcome scale (IPOS) - Japanese version. Jpn J Clin Oncol. 2019;49(3):257–62. [DOI] [PubMed] [Google Scholar]
- 19. Miyashita M, Yasuda M, Baba R et al. Inter-rater reliability of proxy simple symptom assessment scale between physician and nurse: a hospital-based palliative care team setting. Eur J Cancer Care (Engl). 2010;19(1):124–30. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Factors correlated with dyspnea in advanced lung cancer patients: organic causes and what else? Journal of pain and symptom management. 2002;23(6):490–500. [DOI] [PubMed] [Google Scholar]
- 21. Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. Journal of pain and symptom management. 2000;19(5):357–62. [DOI] [PubMed] [Google Scholar]
- 22. Chiu TY, Hu WY, Lue BH, Yao CA, Chen CY, Wakai S. Dyspnea and its correlates in taiwanese patients with terminal cancer. Journal of pain and symptom management. 2004;28(2):123–32. [DOI] [PubMed] [Google Scholar]
- 23. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. [DOI] [PubMed] [Google Scholar]
- 24. Groenvold M, Petersen MA, Aaronson NK et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42(1):55–64. [DOI] [PubMed] [Google Scholar]
- 25. Miyashita M, Wada M, Morita T et al. Independent validation of the Japanese version of the EORTC QLQ-C15-PAL for patients with advanced cancer. Journal of pain and symptom management. 2015;49(5):953–9. [DOI] [PubMed] [Google Scholar]
- 26. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–43. [DOI] [PubMed] [Google Scholar]
- 27. Tsuchiya A, Ikeda S, Ikegami N et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11(4):341–53. [DOI] [PubMed] [Google Scholar]
- 28. ATS Comittee on Proficiency Standards for Clinical Pumonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. [DOI] [PubMed] [Google Scholar]
- 29. White KM, Agar MR, Currow DC. Assessing the exertion required to induce breathlessness in a population with advanced cancer: matching measures to the level of physical function. BMC Palliat Care. 2019;18(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilcock A, Walker G, Manderson C et al. Use of upper limb exercise to assess breathlessness in patients with cancer: tolerability, repeatability, and sensitivity. Journal of pain and symptom management. 2005;29(6):559–64. [DOI] [PubMed] [Google Scholar]
- 32. Bruera E, Sweeney C, Willey J et al. A randomized controlled trial of supplemental oxygen versus air in cancer patients with dyspnea. Palliative medicine. 2003;17(8):659–63. [DOI] [PubMed] [Google Scholar]
- 33. Ahmedzai SH, Laude E, Robertson A, Troy G, Vora V. A double-blind, randomised, controlled phase II trial of Heliox28 gas mixture in lung cancer patients with dyspnoea on exertion. British journal of cancer. 2004;90(2):366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinna MA, Bruera E, Moralo MJ, Correas MA, Vargas RM. A randomized crossover clinical trial to evaluate the efficacy of oral transmucosal fentanyl citrate in the treatment of dyspnea on exertion in patients with advanced cancer. The American journal of hospice & palliative care. 2015;32(3):298–304. [DOI] [PubMed] [Google Scholar]
- 35. Hui D, Kilgore K, Frisbee-Hume S et al. Effect of prophylactic fentanyl buccal tablet on episodic exertional Dyspnea: a pilot double-blind randomized controlled trial. Journal of pain and symptom management. 2017;54(6):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hui D, Maddocks M, Johnson MJ et al. Management of breathlessness in patients with cancer: ESMO clinical practice guidelines(dagger). ESMO Open. 2020;5(6):e001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feliciano JL, Waldfogel JM, Sharma R et al. Pharmacologic interventions for breathlessness in patients with advanced cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(2):e2037632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farquhar M, Preston N, Evans CJ et al. Mixed methods research in the development and evaluation of complex interventions in palliative and end-of-life care: report on the MORECare consensus exercise. Journal of palliative medicine. 2013;16(12):1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gysels M, Evans CJ, Lewis P et al. MORECare research methods guidance development: recommendations for ethical issues in palliative and end-of-life care research. Palliative medicine. 2013;27(10):908–17. [DOI] [PubMed] [Google Scholar]
- 40. Preston NJ, Fayers P, Walters SJ et al. Recommendations for managing missing data, attrition and response shift in palliative and end-of-life care research: part of the MORECare research method guidance on statistical issues. Palliative medicine. 2013;27(10):899–907. [DOI] [PubMed] [Google Scholar]
- 41. Evans CJ, Benalia H, Preston NJ et al. The selection and use of outcome measures in palliative and end-of-life care research: the MORECare international consensus workshop. Journal of pain and symptom management. 2013;46(6):925–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higginson IJ, Evans CJ, Grande G, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med. 2013;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans CJ, Harding R, Higginson IJ, Morecare. 'Best practice' in developing and evaluating palliative and end-of-life care services: a meta-synthesis of research methods for the MORECare project. Palliative medicine. 2013;27(10):885–98. [DOI] [PubMed] [Google Scholar]
- 44. Evans CJ, Yorganci E, Lewis P et al. Processes of consent in research for adults with impaired mental capacity nearing the end of life: systematic review and transparent expert consultation (MORECare_Capacity statement). BMC Med. 2020;18(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turk DC, Dworkin RH, Allen RR et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–45. [DOI] [PubMed] [Google Scholar]
- 46. Turk DC, Dworkin RH, Burke LB et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125(3):208–15. [DOI] [PubMed] [Google Scholar]
- 47. Dworkin RH, Turk DC, Wyrwich KW et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
- 48. Turk DC, Dworkin RH, McDermott MP et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on methods, measurement, and pain assessment in clinical trials. Pain. 2008;139(3):485–93. [DOI] [PubMed] [Google Scholar]
- 49. Dworkin RH, Turk DC, McDermott MP et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–44. [DOI] [PubMed] [Google Scholar]
- 50. Dworkin RH, Turk DC, Peirce-Sandner S et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149(2):177–93. [DOI] [PubMed] [Google Scholar]
- 51. Smith SM, Dworkin RH, Turk DC et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell ML, Templin T, Walch J. A respiratory distress observation scale for patients unable to self-report dyspnea. Journal of palliative medicine. 2010;13(3):285–90. [DOI] [PubMed] [Google Scholar]
- 53. Hui D, Morgado M, Vidal M et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. Journal of palliative medicine. 2013;16(3):274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.