Abstract
Background
Eculizumab modifies the course of disease in patients with atypical haemolytic uraemic syndrome (aHUS), but data evaluating whether eculizumab discontinuation is safe are limited.
Methods
Patients enrolled in the Global aHUS Registry who received ≥1 month of eculizumab before discontinuing, demonstrated haematologic or renal response prior to discontinuation and had ≥6 months of follow-up were analysed. The primary endpoint was the proportion of patients suffering from thrombotic microangiopathy (TMA) recurrence after eculizumab discontinuation. Additional endpoints included: estimated glomerular filtration rate changes following eculizumab discontinuation to last available follow-up; number of TMA recurrences; time to TMA recurrence; proportion of patients restarting eculizumab; and changes in renal function.
Results
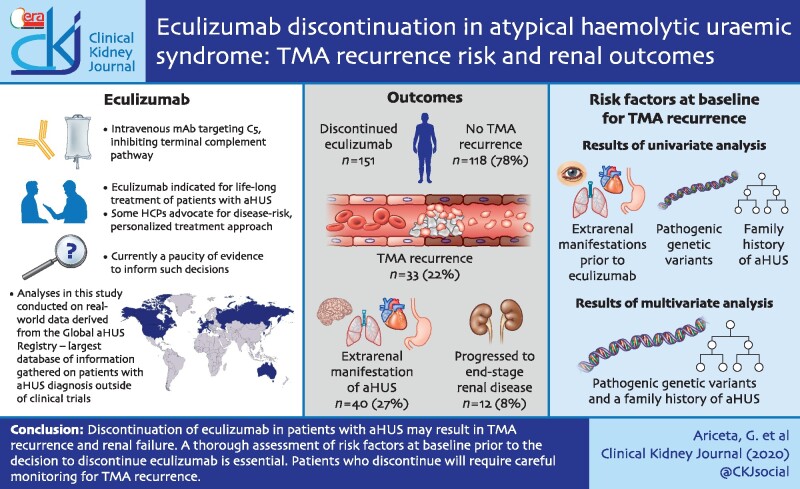
We analysed 151 patients with clinically diagnosed aHUS who had evidence of haematologic or renal response to eculizumab, before discontinuing. Thirty-three (22%) experienced a TMA recurrence. Univariate analysis revealed that patients with an increased risk of TMA recurrence after discontinuing eculizumab were those with a history of extrarenal manifestations prior to initiating eculizumab, pathogenic variants or a family history of aHUS. Multivariate analysis showed an increased risk of TMA recurrence in patients with pathogenic variants and a family history of aHUS. Twelve (8%) patients progressed to end-stage renal disease after eculizumab discontinuation; seven (5%) patients eventually received a kidney transplant. Forty (27%) patients experienced an extrarenal manifestation of aHUS after eculizumab discontinuation.
Conclusions
Eculizumab discontinuation in patients with aHUS is not without risk, potentially leading to TMA recurrence and renal failure. A thorough assessment of risk factors prior to the decision to discontinue eculizumab is essential.
Keywords: anaemia, GFR, haemoglobin, thrombosis, thrombotic microangiopathy
GRAPHICAL ABSTRACT
INTRODUCTION
Atypical haemolytic uraemic syndrome (aHUS) is a rare complement-mediated disease with an estimated annual incidence of 0.23–1.9 per million [1]. Despite plasma therapy, historically aHUS had poor outcomes, with many patients developing end-stage renal disease (ESRD) or dying [2, 3].
Eculizumab (Alexion Pharmaceuticals, Inc., Boston, MA, USA), a humanized monoclonal antibody that blocks terminal complement C5 activation, was approved for the treatment of patients with aHUS in 2011 [4, 5]. Data from four prospective clinical trials [6–10], the Global aHUS Registry [11] and observational studies [12–14] have demonstrated that eculizumab changes the clinical progression of patients with aHUS, substantially improving haematologic, renal, transplant and quality of life outcomes, with a favourable safety profile.
While eculizumab is indicated for life-long treatment [4], some experts advocate for a risk-based, personalized treatment approach in certain patients [15–17]. However, there is a paucity of evidence informing such decisions. Thrombotic microangiopathy (TMA) recurrence has been reported in 20–30% of patients after eculizumab discontinuation. Thus, any decision to discontinue treatment must be carefully considered [13, 15–22]. Data from a long-term, prospective trial showed that patients discontinuing eculizumab had a 13.5-fold higher rate of TMA and trended towards reduced renal function versus those remaining on treatment [13]. Several groups were identified as particularly high risk for TMA recurrence, including patients with: (i) identified complement pathogenic variants or complement factor H (CFH) autoantibodies [2, 3]; (ii) paediatric onset; and (iii) multiple episodes of TMA [13]. These data agree with retrospective and registry analyses [15, 16, 21]. Pregnant patients, renal transplant patients and patients with low estimated glomerular filtration rate (eGFR <20 mL/min/1.73 m2) may also be at increased risk following eculizumab discontinuation [18, 19].
Currently, no prospective controlled studies defining eculizumab discontinuation criteria have been published, although several European studies are in progress [SETS aHUS (2017-003916-37); CUREiHUS (NTR5988/NL5833); STOPECU (NCT02574403)]. Current recommendations stipulate that if treatment is to be discontinued, it should be on a patient-by-patient basis with close monitoring [15, 17, 18, 21, 23, 24]. Restrictive dosing of eculizumab with gradual tapering of therapy may also be considered [17], but lacks robust evidence [25].
The Global aHUS Registry provides the opportunity to analyse prospective real-world data from a large number of patients. Here, we report outcomes in patients with aHUS whose condition improved on eculizumab, and who subsequently discontinued. The aims of this analysis were to describe the characteristics and outcomes of patients who suffered a TMA recurrence following eculizumab discontinuation compared with those who did not.
MATERIALS AND METHODS
Study design
The Global aHUS Registry (NCT01522183) is an observational, non-interventional, multicentre registry that retrospectively and prospectively collects demographic characteristics, medical and disease history and treatment outcomes in patients with aHUS. Further details of the registry have been described [26].
Patients
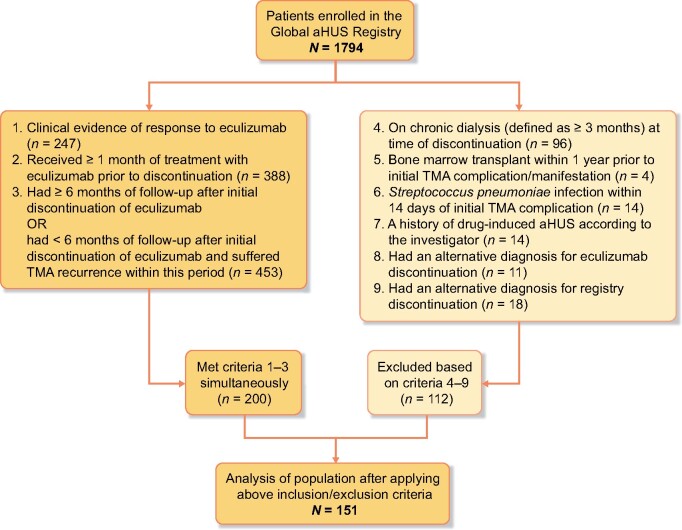
Registry-enrolled patients (all with an investigator-determined clinical diagnosis of aHUS) were included in the current analysis if they: (i) had received treatment with eculizumab and had evidence of therapeutic response, defined as lactate dehydrogenase (LDH) <1.5× upper limit of normal or platelet count ≥150 000/μL or eGFR >30 mL/min/1.73 m2 or a decrease in serum creatinine by 25% compared with baseline (last available value prior to eculizumab) within 6 months prior to discontinuation; (ii) received at least 1 month of eculizumab treatment prior to discontinuation; and (iii) had at least 6 months of follow-up after initial eculizumab discontinuation, unless TMA recurred sooner (Figure 1).
FIGURE 1:
Patient disposition. A total of 1794 patients were enrolled in the Global aHUS Registry, of whom 151 patients were subsequently included in this analysis. These patients had been treated with eculizumab with clinical evidence of a response to therapy, and had received at least 1 month of therapy, AND had at least 6 months of follow-up after discontinuation OR had suffered a TMA recurrence within 6 months of follow-up. Additionally, patients fulfilling these criteria must also not have met any of the exclusion criteria.
Patients were excluded if they were on chronic dialysis (defined as ≥3 months’ duration of dialysis) at time of discontinuation; had temporally related triggers of TMA including bone marrow transplant within 1 year; had a Streptococcus pneumoniae infection within 14 days; or a history of drug-induced TMA according to the investigator. Patients were also excluded if they had an alternative diagnosis as a reason for eculizumab or registry discontinuation.
Endpoints
The primary endpoint was the proportion of patients presenting with a TMA recurrence after eculizumab discontinuation. The definition of TMA recurrence was per treating physician observation, who must have actively indicated that a TMA had occurred in the patient’s clinical report form. A patient was considered to have a TMA relapse if the investigator reported ‘TMA complications related to aHUS’, ‘TMA signs and symptoms’ or any other TMA in the patients’ clinical report form per their own clinical judgement. A patient was considered to have suffered a TMA recurrence if a TMA was reported in any of these forms after the date of eculizumab discontinuation. The secondary endpoint was change in eGFR from time of eculizumab discontinuation to last available follow-up. Exploratory endpoints included: the number of TMA recurrences after discontinuation; time to TMA recurrence after discontinuation; proportion of patients that restarted eculizumab after discontinuation; change in renal function, as evaluated by eGFR, classified as improved (increase ≥15 mL/min/1.73 m2), stable (no change) or worsened (reduction ≥15 mL/min/1.73 m2) compared from time of eculizumab discontinuation to last available follow-up; time to ESRD after discontinuation; and change in anti-hypertensive management after discontinuation. Renal outcomes were also investigated in a subset of patients who showed an initial renal response while on eculizumab, defined as ≥25% decrease in serum creatinine.
The analysis also assessed other TMA complications/manifestations related to aHUS, which included the occurrence of any of the following: hypertension (including malignant hypertension), thrombosis, biopsy-proven TMA, seizures, worsening renal function, proteinuria, haematuria and worsening of haemolytic anaemia, as diagnosed by the attending physician. TMA manifestations also included extrarenal symptoms associated with cardiovascular (CV), pulmonary, neurological and gastrointestinal (GI) disease.
Statistical analysis
Continuous data were summarized descriptively in terms of median (range); categorical data were summarized in terms of the number of patients with non-missing data, and frequency counts with percentages were also calculated. This analysis defined eculizumab discontinuation as >30 days between doses of eculizumab or until last available follow-up. There was no imputation of missing data; for the primary endpoint of TMA recurrence it was assumed that missing data were missing at random, i.e. those without their TMA report form completed were assumed to not have a TMA recurrence. Logistic regression was performed to calculate unadjusted odds ratios (ORs) for the risk of TMA recurrence, and a multivariate analysis was performed to calculate adjusted ORs for significant variables identified from the univariate analysis. Additionally, a composite clinical endpoint was used. The composite endpoint was defined if any of the following were satisfied: (i) disease relapse or (ii) eGFR decrease by 10 mL/min/1.73 m2 or (iii) eGFR decrease by 10%, as reported by the investigator. The data-cut for this analysis was 1 April 2019.
RESULTS
Patient disposition
A total of 1794 patients were enrolled in the Global aHUS Registry and 151 patients were included in this analysis (Figure 1). The most common reasons given for eculizumab discontinuation were physician advice (n = 37, 25%), improvement in condition (n = 21, 14%) and ‘other’ (n = 17, 11%; e.g. dose change, completion of treatment series, pharmaceutical benefits/financial reasons). Of the 151 patients, 30 patients (19.9%) restarted eculizumab.
Patient demographics stratified by TMA recurrence status
The median [interquartile range (IQR)] duration of eculizumab therapy was 0.96 (0.4–1.6) years and patients were followed for a median (IQR) of 2.3 (1.4–3.4) years. Thirty-three (21.9%) patients experienced a TMA recurrence at a median (IQR) of 5.3 (2.4–17.0) months after discontinuing eculizumab, 11 of 51 (21.6%) children at 5.1 (2.7–17.0) months and 22 of 100 (22%) adults at 10.2 (1.8–18.3) months. Most patients [21 of 30 (66.7%)] who restarted eculizumab had experienced a TMA recurrence [7 of 10 children (70.0%) and 14 of 20 adults (70%)]. Three patients restarted eculizumab for other reasons (pregnancy, transplantation and prophylaxis for a medical procedure) and for the remaining six patients, no reason was recorded. The median (IQR) number of TMA recurrences in affected patients was 2.0 (1.0–3.0) overall, 2.0 (2.0–3.0) in adults and 2.0 (1.0–2.0) in children.
Characteristics of patients who suffered a TMA recurrence were similar to those who did not, with the following exceptions: a lower median eGFR before discontinuation, a higher incidence of extrarenal manifestations before first commencing eculizumab, and a higher proportion of patients with a family history of aHUS (Table 1).
Table 1.
Characteristics of patients stratified by TMA recurrence status
| Variable | All patients (N = 146)a |
TMA recurrence (n = 33) | No recurrence (n = 113) |
|---|---|---|---|
| Age at aHUS onset, years | 23.1 (5.6–37.7) | 24.8 (3.7–38.9) | 22.9 (6.4–35.6) |
| Female, n (%) | 88 (60.3) | 18 (54.5) | 70 (61.9) |
| Dialysis at any time prior to eculizumab discontinuation, n (%) | 73 (50.0) | 16 (48.5) | 57 (50.4) |
| Family history of aHUS | 16 (11.0) | 9 (27.3) | 7 (6.2) |
| Kidney transplant at any time prior to discontinuation, n (%) | 31 (21.2) | 6 (18.2) | 25 (22.1) |
|
Pregnancy after discontinuation, n (%) |
6 (4.1) | 2 (6.1) | 4 (3.5) |
| eGFR prior to discontinuation, mL/min/1.73 m2 | 57.1 (30.0–94.4) | 50.5 (35.3–91.3) |
64.5 (29.4–95.8) |
| Duration of eculizumab before discontinuation, years | 0.96 (0.4–1.6) | 0.95 (0.3–1.4) | 0.97 (0.5–1.7) |
| Time to TMA recurrence after eculizumab discontinuation, months | – | 5.3 (2.4–17.0) | – |
| Adults | 10.2 (1.8–18.3) | ||
| Children | 5.1 (2.7–8.4) | ||
| Extrarenal manifestations prior to eculizumab, n (%) | |||
| Patients with ≥1 manifestation | 81 (55.5) | 23 (69.7) | 58 (51.3) |
| CV | 30 (20.5) | 9 (27.3) | 21 (18.6) |
| Pulmonary | 19 (13.0) | 9 (27.3) | 10 (8.8) |
| CNS | 36 (24.7) | 10 (30.3) | 26 (23.0) |
| GI | 54 (37.0) | 13 (39.4) | 41 (36.3) |
Five of 151 patients included in the analysis did not have data on TMA recurrence, so were excluded from the analysis. All values median (IQR) unless otherwise stated.
Complement pathogenic variants
A higher proportion of patients who suffered a TMA recurrence had an identified pathogenic variant compared with patients without a TMA recurrence (30.3% versus 15.0%, respectively; Table 2). CFH and membrane cofactor protein (MCP) pathogenic variants were the most common, occurring in 12 (12.1%) and 10 (10.8%) patients across the cohort, respectively. Pathogenic variants in both genes occurred in a higher proportion of patients who suffered a TMA recurrence (CFH, 18.2% versus 10.4%; and MCP, 15.0% versus 9.6%, respectively; Table 3). CFH autoantibodies occurred in a higher proportion of those who did not have a TMA recurrence (23.6% versus 8.7% of tested patients, respectively).
Table 2.
Summary of pathogenic variants
| Variable | All patients (N = 146) |
TMA recurrence (n = 33) | No recurrence (n = 113) |
|---|---|---|---|
| Tested for at ≥5 pathogenic variants with no mutation identified | 62 (42.5) | 11 (33.3) | 51 (45.1) |
| Tested for ˂5 pathogenic variants and no mutation identified | 16 (11.0) | 2 (6.1) | 14 (12.4) |
| Any pathogenic variant found | 27 (18.5) | 10 (30.3) | 17 (15.0) |
| Anti-CFH antibody tested and positive | 19 (13.0) | 2 (6.1) | 17 (15.0) |
| Any pathogenic variant found or anti-CFH antibody positive | 42 (28.8) | 12 (36.4) | 30 (26.5) |
All values n (%).
Table 3.
Genotype data stratified by TMA recurrence status
| All patients (N = 146) |
TMA recurrence (n = 33) |
No recurrence (n = 113) |
||||
|---|---|---|---|---|---|---|
| Pathogenic variant | Tested, n | Pathogenic variant identified, n (%) | Tested, n | Pathogenic variant identified, n (%) | Tested, n | Pathogenic variant identified, n (%) |
| CFH | 99 | 12 (12.1) | 22 | 4 (18.2) | 77 | 8 (10.4) |
| C3 | 89 | 3 (3.4) | 17 | 1 (5.9) | 72 | 2 (2.8) |
| CFI | 94 | 2 (2.1) | 20 | 1 (5.0) | 74 | 1 (1.4) |
| CFB | 83 | 3 (3.6) | 17 | 1 (5.9) | 66 | 2 (3.0) |
| MCP | 93 | 10 (10.8) | 20 | 3 (15.0) | 73 | 7 (9.6) |
| THBD | 46 | 2 (4.3) | 9 | 2 (22.2) | 37 | 0 |
| DGKE | 36 | 1 (2.8) | 5 | 1 (20.0) | 31 | 0 |
C3, complement component 3; CFB, complement factor B; CFI, complement factor I; DGKE, the gene encoding diacylglycerol kinase epsilon; THBD, the gene encoding thrombomodulin.
Risk of TMA recurrence
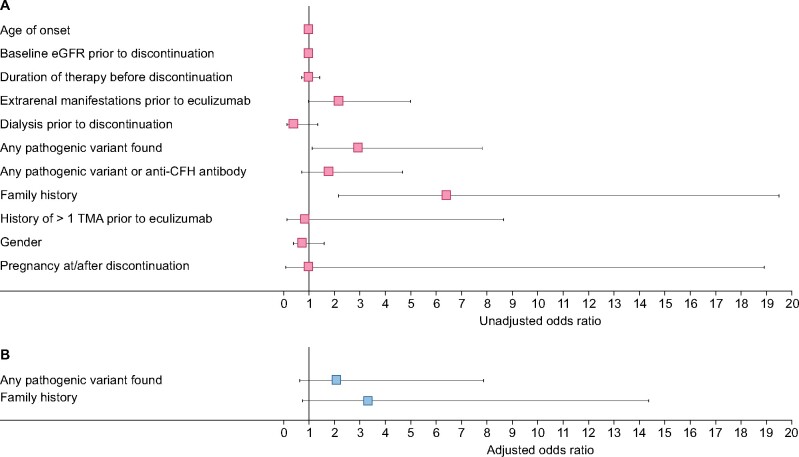
Univariate logistic regression revealed that patients with an increased risk of TMA recurrence after discontinuing eculizumab were those with extrarenal manifestations prior to eculizumab [unadjusted OR = 2.2, 95% confidence interval (CI) 0.95–5.0], any pathogenic variant found (unadjusted OR = 2.9, 95% CI 1.1–7.9) or family history of aHUS (unadjusted OR = 6.4, 95% CI 2.1–19.5; Figure 2A). In the multivariate analysis, an increased risk was shown for any pathogenic variant found (adjusted OR = 2.1, 95% CI 0.6–7.9) and family history of aHUS (adjusted OR = 3.3, 95% CI 0.7–14.4; Figure 2B).
FIGURE 2:
(A) Univariate logistic regression analysis and (B) multivariate logistic regression analysis to determine risk of TMA recurrence after eculizumab discontinuation. Data are shown as unadjusted (A) and adjusted (B) ORs with 95% CI error bars.
Patient Outcomes
Long-term renal outcomes
Median (IQR) eGFR values of 57.8 (30.1–95.8; n = 143), 67.0 (35.2–96.4; n = 97), 46.8 (32.5–88.5; n = 52) and 62.0 (33.1–93.0; n = 35) mL/min/1.73 m2 were observed at the nearest timepoint before discontinuation, and at 1-, 2- and 3-years of follow-up, respectively. Twelve (8.0%) patients progressed to ESRD in a median (IQR) of 5.4 (0.8–16.5) months after eculizumab discontinuation, five of whom had a prior kidney transplant. Eleven of these 12 patients had kidney failure prior to eculizumab treatment and 6 of these patients had eGFR ˂25 mL/min/1.73 m2. Two of the 12 patients progressed to ESRD after multiple eculizumab discontinuations, restarts and TMA recurrences and 7 patients eventually received a transplant.
All patients had a renal or haematologic response while on eculizumab. Among the subgroup of 64 patients (43.8%) who had a renal response (≥25% decrease in serum creatinine), 55 (85.9%) did not suffer a TMA recurrence, while 9 (14.1%) did. In patients who did not suffer a TMA recurrence, eGFR at the last follow-up remained stable (Table 4). Of the nine patients who suffered a TMA recurrence (Table 5), eGFR decrease was noted in three (33.3%) patients and remained stable in six (66.7%) at last follow-up (which ranged from 4.4 to 52.1 months where data were available). All nine patients who had an initial renal response and suffered a TMA recurrence restarted eculizumab after the recurrence. However, those with a decline in eGFR at the last follow-up restarted eculizumab 87, 192 and 310 days after TMA recurrence, comparatively later compared with those with a stable eGFR who restarted eculizumab 0, 5, 5, 7, 22 and 95 days after TMA recurrence.
Table 4.
Long-term renal outcomes by age and kidney status, in patients who did not suffer a TMA recurrence
| Paediatric, native kidney (n = 17) | Paediatric, transplant (n = 1) | Adult, native kidney (n = 28) | Adult, transplant (n = 9) |
|
|---|---|---|---|---|
| Improved | 3 (18) | 0 (0) | 6 (21.5) | 2 (22) |
| Stable | 11 (65) | 0 (0) | 20 (71.5) | 7 (78) |
| Declined | 3 (17) | 1 (100) | 2 (7) | 0 (0) |
Data depict only those patients with an initial renal response (≥25% decrease in serum creatinine while on eculizumab). For all patients, eGFR was compared using the most recent value prior to eculizumab discontinuation, and at last follow-up following discontinuation; Improved = increase in eGFR of ≥15 mL/min/1.73 m2, Declined = decrease in eGFR of ≥15 mL/min/1.73 m2. All values n (%).
Table 5.
Long-term renal outcomes by age and kidney status, in patients who suffered a TMA recurrence
| Paediatric, native kidney (n = 1) | Paediatric, transplant (n = 1) | Adult, native kidney (n = 7) | Adult, transplant (n = 0) |
|
|---|---|---|---|---|
| Improved | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stable | 1 (100) | 1 (100) | 4 (57) | 0 (0) |
| Declined | 0 (0) | 0 (0) | 3 (43) | 0 (0) |
Data depict only those patients with an initial renal response (≥25% decrease in serum creatinine while on eculizumab). For all patients, eGFR was compared using the most recent value prior to eculizumab discontinuation, and at last follow-up following discontinuation; Improved = increase in eGFR of ≥15 mL/min/1.73 m2, Declined = decrease in eGFR of ≥15 mL/min/1.73 m2. All values n (%).
Of the 151 patients included in this analysis, 75 (49.3%) patients reached the composite endpoint after eculizumab discontinuation in a median (IQR) time of 4.5 (2.4–11.5) months.
Extra-renal manifestations
After discontinuing eculizumab, 40 (27.4%) patients experienced an extrarenal manifestation, 21 (63.6%) patients who suffered a TMA recurrence and 19 (16.8%) patients who did not suffer a TMA recurrence. GI manifestations were the most common, occurring in 23 (15.8%) patients, followed by central nervous system (CNS) [17 (11.6%) patients] and CV [15 (10.3%) patients] manifestations. Patients with TMA recurrence after discontinuing eculizumab were at increased risk of extra-renal manifestations (adjusted OR = 6.32, 95% CI 2.06–19.43).
Antihypertensive medications
At the last available follow-up, a higher proportion of patients were receiving antihypertensive medications compared with at the time of eculizumab discontinuation [107 (71.3%) versus 51 (54.3%) patients, respectively]. This increase was observed for both paediatric [36 (70.6%) versus 15 (50.0%) patients, respectively] and adult patients [71 (71.7%) versus 36 (56.3%) patients, respectively] and across all types of antihypertensive therapies analysed (which included agents acting on the renin–angiotensin system, antihypertensives, beta-blocking agents, calcium channel blockers, diuretics and other cardiac therapies).
DISCUSSION
This prospective, registry-based analysis is the largest reported data set exploring patient outcomes, including TMA recurrence and renal function changes, in patients with aHUS who discontinue eculizumab therapy. Importantly, it is illustrative of the real-world setting, where physicians navigate complex decisions related to treatment discontinuation and its consequences. Patients included in this analysis were treated with eculizumab for at least 1 month and demonstrated an initial response (haematologic, renal or both) before discontinuing treatment due to treating physician judgement, patient choice or other factors (such as local access restrictions).
Current literature reports that the risk of TMA recurrence after eculizumab discontinuation may be as high as 31%, making identification of at-risk patients essential [19]. In alignment with this, 21.9% of patients in this analysis suffered a TMA recurrence after discontinuing eculizumab; however, the time to recurrence was longer in this study (median of 5 months) than in previous studies (<6 weeks [15], and median of 3 months [19]). We also observed a shorter time to TMA recurrence in children than in adults (5.1 and 10.2 months, respectively), suggesting that children, particularly, should be closely monitored for TMA recurrence following discontinuation. Further, these analyses showed a trend towards increased TMA recurrence in patients with complement pathogenic variants; this was particularly notable in patients with CFH and MCP variants (18.2% and 15.0% of patients with these respective variants suffered a TMA recurrence), consistent with previous reports [15, 19–21]. In contrast, the analysis also showed that anti-CFH antibodies occurred more frequently in the group without TMA recurrence. The data also highlighted an increasing trend towards TMA recurrence in patients with a family history of aHUS.
Contrary to other studies, however, where patients experiencing a TMA recurrence had a shorter duration of treatment, the duration of eculizumab therapy in our study was similar between patients who did and did not suffer a TMA recurrence [19, 21]. This disparity with other studies may be partially explained by methodological differences; the current analysis only included patients who demonstrated an initial response to treatment, with a median treatment duration of ∼1 year. Optimal dosing and treatment duration have been an area of ongoing debate, particularly due to the high costs of the treatment [27]. Restrictive dosing of eculizumab with gradual tapering of treatment while monitoring disease activity has been attempted [28], but there is no validated method or reliable predictors of early relapse to date. It should be noted that, as shown in the logistic regression analyses presented in this manuscript, disease stability following long-term eculizumab treatment does not guarantee a reduced risk of TMA recurrence following treatment discontinuation. Further, we observed that eGFR prior to eculizumab discontinuation was numerically lower in patients with subsequent TMA recurrence (50.5 versus 64.5 mL/min/1.73 m2, respectively), but this was not a statistically confirmed risk factor for TMA recurrence. Additionally, while studies have suggested that patients with paediatric disease onset [13] and/or with multiple TMAs have a higher risk of TMA recurrence [16], our analysis could not confirm this, nor could we identify any increased risk based on eculizumab duration, dialysis, gender or pregnancy.
Ours is the first study comparing the proportion of extrarenal manifestations between patients who did and did not suffer a TMA recurrence after discontinuing eculizumab. Current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that, once on treatment, patients do not discontinue complement inhibitors while extrarenal manifestations persist [24]. In this study, a higher proportion of patients presenting with extrarenal manifestations prior to eculizumab initiation suffered a subsequent TMA recurrence (69.7% versus 51.3%), suggesting that those with multi-organ involvement prior to eculizumab treatment are at a greater risk of TMA recurrence the following discontinuation. Furthermore, more patients with extrarenal manifestations of aHUS after eculizumab discontinuation subsequently suffered a TMA recurrence versus those who did not present with extrarenal manifestations (63.6 versus 16.8%, respectively). The most common manifestations were GI, followed by CNS and CV, consistent with previous studies [29, 30]. However, 16.8% of patients who did not suffer a TMA recurrence also reported extrarenal manifestations after eculizumab discontinuation; it is unclear whether these resulted directly from aHUS disease activity.
We also investigated a subgroup of 64 patients with an initial renal response to eculizumab (≥25% decrease in serum creatinine). In these patients, eGFR was generally stable in those without TMA recurrence, but was worse in those who suffered a recurrence, with six patients remaining stable and three with reduced eGFR at last follow-up (one progressed to ESRD and transplant); no patients with an initial renal response who suffered a TMA recurrence had improved renal function at last follow-up. Our data suggest that those restarting eculizumab earlier had a better renal function at last follow-up, in agreement with previous reports [13, 31]. Given the established relationship between acute kidney injury and chronic kidney disease [32], only patients at least risk of TMA recurrence should be considered for eculizumab discontinuation. Indeed, 12 (8.0%) patients overall progressed to ESRD after discontinuing eculizumab, 5 of whom previously underwent kidney transplantation; KDIGO advises that eculizumab should not be discontinued in patients that have received a renal transplant [24].
Lastly, we found a higher proportion of patients receiving antihypertensive medications at last available follow-up compared with at the time of eculizumab discontinuation (71.3% versus 54.3% patients, respectively). Finding more frequent and severe hypertension in the group of patients who discontinued eculizumab may indicate increased CV involvement and chronic kidney disease progression risk, and in some cases subclinical disease activity.
This study has several limitations. Patients must have had an initial response to eculizumab, which likely biased the results towards favourable outcomes; further, patients discontinuing eculizumab due to their treating physician’s opinion likely had more stable disease. Secondly, this was a registry analysis and, as such, data collection was not as extensive as a clinical trial. For example, in patients with anti-CFH antibodies, data on antibody titres were not collected, meaning that further conclusions could not be drawn about a potentially reduced risk of recurrence in patients with low titres. Additional limitations include the potential for underreporting of outcomes and varied interpretation of disease characteristics at baseline.
Overall, based on this analysis, published data and our clinical experience, we recommend that any consideration of eculizumab discontinuation should be discussed in-depth with the patient, alongside a thorough risk assessment. We strongly advise that patients with poor renal function, a family history of aHUS, pathogenic variants, previous extrarenal manifestations or prior transplantations should not discontinue treatment. It is also important to consider whether the patient had an initial renal response to eculizumab, as these patients were at a lower risk of eGFR loss after relapse in this analysis. In patients discontinuing eculizumab, we would suggest patient education on early TMA symptoms, regular urine dipsticks to monitor proteinuria and, if possible, regular blood tests to monitor haemoglobin, platelets, serum creatinine and LDH [15, 16, 18, 21]. If a TMA recurrence is suspected, eculizumab should be rapidly reinitiated.
CONCLUSION
This is the first analysis to investigate the outcomes of eculizumab discontinuation in stable aHUS patients who showed a positive response to treatment, the population likely to be considered for discontinuation in a real-world setting. We found that patients at risk of TMA recurrence were more likely to have extrarenal manifestations prior to discontinuation, test positive for complement pathogenic variants and have a family history of aHUS. Physicians should conduct a thorough risk assessment and ensure patients are regularly monitored after discontinuation, with the rapid recommencement of eculizumab if TMA recurs.
ACKNOWLEDGEMENTS
The sponsor and investigators thank the patients and their families for their participation in and support for this clinical study. The authors would also like to thank all the Registry investigators who have contributed data, and the members of the Global aHUS Registry. The authors would like to acknowledge Ciaran Wright, PhD, Leia Silvagnoli-Compston, MSc and Alexander T. Hardy, PhD of Bioscript Science, Macclesfield, UK, for providing editorial and medical writing support with funding from Alexion Pharmaceuticals, Inc. The authors would like to acknowledge Åsa Lommelé, PhD, and Jonathan Mathias, PhD of Alexion Pharmaceuticals, Inc., for their critical review of the manuscript. The authors would like to thank Veronique Fremeaux-Bacchi for validating the genetic analysis.
FUNDING
This study was funded wholly by Alexion Pharmaceuticals, Inc. The Global aHUS Registry is an observational, non-interventional, multicentre registry that is funded by Alexion Pharmaceuticals, Inc. Medical writing support provided by Bioscript Science was funded by Alexion Pharmaceuticals, Inc.
AUTHORS’ CONTRIBUTIONS
All authors (G. Ariceta F.F., L.S., B.M., V.N., D.C., A.M.S. and G. Ardissino) were involved in the conception, analysis and interpretation and critical review of this manuscript. Medical writing support was provided by Bioscript Science.
CONFLICT OF INTEREST STATEMENT
G. Ariceta has received honoraria for lectures from Alexion Pharmaceuticals and is a member of the Scientific Advisory Board of the Global aHUS Registry, sponsored by Alexion Pharmaceuticals. F.F. has received honoraria for participation in advisory boards, expert meetings and teaching courses from Alexion Pharmaceuticals. L.S. is a member of the Scientific Advisory Board of the Global aHUS Registry, sponsored by Alexion Pharmaceuticals. B.M. is an employee of Alexion Pharmaceuticals. V.N. is an employee of Paraexcel International, a Contract Research Organization engaged by Alexion Pharmaceuticals. D.C. has received honoraria for educational lectures from Alexion Pharmaceuticals, and honoraria for advisory board participation from Natera and ITB Pharmaceuticals. A.M.S. has nothing to disclose. G. Ardissino has received honoraria for participation in expert meetings from Alexion Pharmaceuticals and is a member of the scientific advisory board of the Atypical Hemolytic Uremic Syndrome (aHUS) Global Registry, supported by Alexion Pharmaceuticals.
The results presented within this article have not been published previously in whole or in part, except in abstract or presentation formats.
DATA AVAILABILITY STATEMENT
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development.
Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
CLINICALTRIALS.GOV IDENTIFIER: NCT01522183
Contributor Information
Gema Ariceta, Department of Pediatric Nephrology, Vall d'Hebron Hospital, and the Autonomous University of Barcelona, Barcelona, Spain.
Fadi Fakhouri, Department of Nephrology and Immunology, CHU de Nantes, Nantes, France.
Lisa Sartz, Department of Pediatrics, Skane University Hospital, Lund University, Lund, Sweden.
Benjamin Miller, Alexion Pharmaceuticals, Inc., Boston, MA, USA*.
Vasilis Nikolaou, Parexel International Ltd, Uxbridge, UK.
David Cohen, Department of Medicine, Division of Nephrology, Columbia University Medical Center, New York, NY, USA.
Andrew M Siedlecki, Department of Internal Medicine, Renal Division, Brigham and Women's Hospital, Boston, MA, USA.
Gianluigi Ardissino, Centro per la Cura e lo Studio della Sindrome Emolitico-Uremica, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
REFERENCES
- 1. Yan K, Desai K, Gullapalli L et al. Epidemiology of atypical hemolytic uremic syndrome: a systematic literature review. Clin Epidemiol 2020; 12: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noris M, Caprioli J, Bresin E et al. Relative role of genetic complement abnormalities in sporadic and familial ahus and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010; 5: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fremeaux-Bacchi V, Fakhouri F, Garnier A et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EMA. EU/3/09/653. 2011. https://www.Ema.Europa.Eu/en/medicines/human/orphan-designations/eu309653 (22 January 2021, date last accessed)
- 5.FDA. Eculizumab (soliris). 2011. http://wayback.Archive-it.Org/7993/20170113081126/ (22 January 2021, date last accessed)
- 6. Fakhouri F, Hourmant M, Campistol JM et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis 2016; 68: 84–93 [DOI] [PubMed] [Google Scholar]
- 7. Greenbaum LA, Fila M, Ardissino G et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 2016; 89: 701–711 [DOI] [PubMed] [Google Scholar]
- 8. Legendre CM, Licht C, Muus P et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013; 368: 2169–2181 [DOI] [PubMed] [Google Scholar]
- 9. Licht C, Greenbaum LA, Muus P et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 2015; 87: 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vilalta R, Al-Akash S, Davin J et al. Eculizumab therapy for pediatric patients with atypical hemolytic uremic syndrome: efficacy and safety outcomes of a retrospective study (Abstract 1155). Haematologica 2012; 97: 47922492290 [Google Scholar]
- 11. Siedlecki AM, Isbel N, Vande Walle J et al. Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep 2019; 4: 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menne J, Delmas Y, Fakhouri F et al. Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clin Kidney J 2019; 12: 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menne J, Delmas Y, Fakhouri F et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol 2019; 20: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuber J, Frimat M, Caillard S et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol 2019; 30: 2449–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ardissino G, Testa S, Possenti I et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 2014; 64: 633–637 [DOI] [PubMed] [Google Scholar]
- 16. Fakhouri F, Fila M, Provot F et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 2017; 12: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wijnsma KL, Duineveld C, Volokhina EB et al. Safety and effectiveness of restrictive eculizumab treatment in atypical haemolytic uremic syndrome. Nephrol Dial Transplant 2018; 33: 635–645 [DOI] [PubMed] [Google Scholar]
- 18. Olson SR, Lu E, Sulpizio E et al. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol 2018; 48: 96–107 [DOI] [PubMed] [Google Scholar]
- 19. Macia M, de A, Moreno F, Dutt T et al. Current evidence on the discontinuation of eculizumab in patients with atypical haemolytic uraemic syndrome. Clin Kidney J 2017; 10: 310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheerin NS, Kavanagh D, Goodship TH et al. A national specialized service in england for atypical haemolytic uraemic syndrome-the first year’s experience. QJM 2016; 109: 27–33 [DOI] [PubMed] [Google Scholar]
- 21. Neave L, Gale DP, Cheesman S et al. Atypical haemolytic uraemic syndrome in the eculizumab era: presentation, response to treatment and evaluation of an eculizumab withdrawal strategy. Br J Haematol 2019; 186: 113–124 [DOI] [PubMed] [Google Scholar]
- 22. Merrill SA, Brittingham ZD, Yuan X et al. Eculizumab cessation in atypical hemolytic uremic syndrome. Blood 2017; 130: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loirat C, Fakhouri F, Ariceta G et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2016; 31: 15–39 [DOI] [PubMed] [Google Scholar]
- 24. Goodship TH, Cook HT, Fakhouri F et al. Atypical hemolytic uremic syndrome and c3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017; 91: 539–551 [DOI] [PubMed] [Google Scholar]
- 25. Ariceta G. Optimal duration of treatment with eculizumab in atypical hemolytic uremic syndrome (aHUS)-a question to be addressed in a scientific way. Pediatr Nephrol 2019; 34: 943–949 [DOI] [PubMed] [Google Scholar]
- 26. Licht C, Ardissino G, Ariceta G et al. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol 2015; 16: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wijnsma KL, Duineveld C, Wetzels JFM et al. Eculizumab in atypical hemolytic uremic syndrome: strategies toward restrictive use. Pediatr Nephrol 2019; 34: 2261–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galbusera M, Noris M, Gastoldi S et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis 2019; 74: 56–72 [DOI] [PubMed] [Google Scholar]
- 29. Schönermarck U, Ries W, Schröppel B et al. Relative incidence of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome in clinically suspected cases of thrombotic microangiopathy. Clin Kidney J 2020; 13: 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaefer F, Ardissino G, Ariceta G et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 31. Vande Walle J, Delmas Y, Ardissino G et al. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J Nephrol 2017; 30: 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chawla LS, Eggers PW, Star RA et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371: 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development.
Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
CLINICALTRIALS.GOV IDENTIFIER: NCT01522183