Abstract
Mycoplasma pneumonia usually causes asymptomatic to mild respiratory tract infection. However, nonrespiratory manifestations are not rare with involvement of various organ including skin, cardiovascular, central nervous system. We are presenting a 43-year-old male who presented with diffuse rash, sever mucositis, confusion, and complicated by ischemic stroke; also, review of mycoplasma related stroke and Stevens-Johnson syndrome.
Keywords: mycoplasma, Stevens-Johnson syndrome, stroke
Introduction
Mycoplasma pneumonia is an atypical bacterium which does not have cell wall. It is one of the common respiratory pathogen usually causes asymptomatic to mild respiratory symptoms. Some people also develop extrarespiratory symptoms along with respiratory symptoms or without respiratory symptoms. Mucocutaneous diseases are common manifestation among nonrespiratory manifestation which include maculopapular or vesicular rash, urticarial, erythema multiforme (EM), and Stevens-Johnson syndrome (SJS). Other nonrespiratory manifestation includes hepatitis, pancreatitis, carditis, encephalitis, aseptic meningitis, myelitis, and glomerulonephritis.
Case Presentation
A 43-year-old previously healthy male presented to the emergency department with a 7-day history of fever, dry cough followed by the development of nonpruritic rash all over the body involving palms, soles, and mucosa associated with bleeding from his nose, mouth, palms, and soles for 2 days. On presentation, he was confused and found to have maculopapular, vesicular, and target like lesions noted over torso and extremities involving palms and soles (Figure 1-4) and severe mucositis involving lips, buccal mucosa, conjunctivae, and urethral meatus. Laboratory investigations revealed normal hemoglobin of 14.2g/dL, leukopenia with white count of 3.4 × 103/µL, thrombocytopenia of 51 × 103/µL, normal creatinine & electrolytes and mildly elevated liver enzyme with alanine aminotransferase (ALT) of 93 U/L and aspartate aminotransferase (AST) of 273 U/L. Computed tomography (CT) of lungs revealed left upper lung tree-in-bud and ground glass opacities. CT head and abdomen-pelvis were negative for an acute intracranial and intra-abdominal pathology, respectively. Cerebrospinal fluid (CSF) analysis and polymerase chain reaction (PCR) for neurotropic pathogens (Bio-Fire) were unremarkable. Skin lesions PCR for herpes simplex and varicella-zoster virus, serological test for HIV, syphilis, and hepatitis viral panel were negative. Blood, sputum, urine, and CSF cultures for bacteria and oropharynx culture for neisseria stayed sterile.
Figure 1.
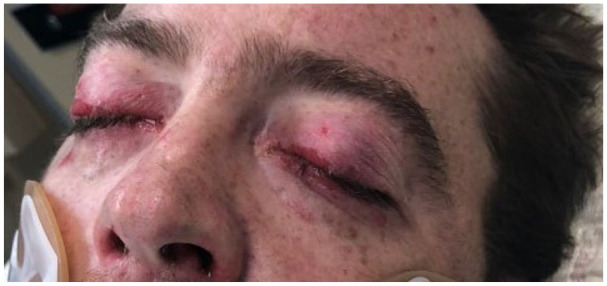
Mucositis involving eyes.
Figure 2.
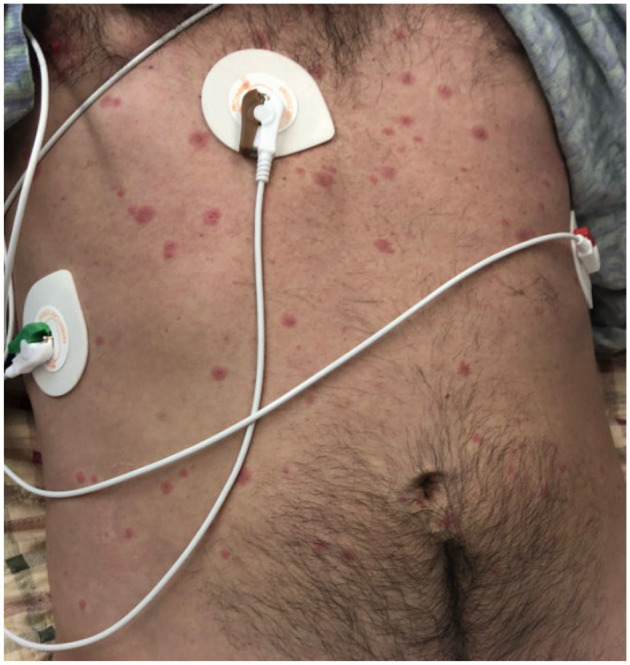
Maculopapular and target like lesion over chest and abdomen.
Figure 3.
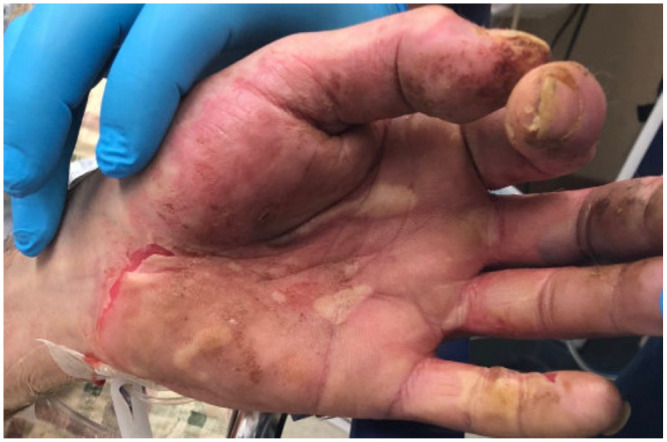
Vesicular rash in hand with some ruptured and bleeding.
Figure 4.
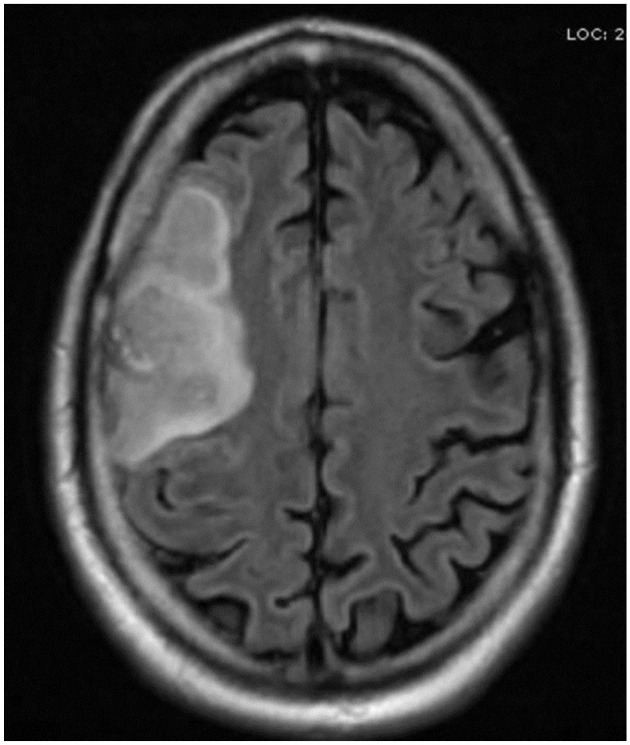
Ischemic stroke involving right MCA.
Abbreviation: MCA, middle cerebral artery.
Respiratory viral panel of nasopharyngeal aspirate and serum IgM antibody resulted positive for M pneumoniae. Given the severity of mucosal involvement and respiratory failure, he was intubated and started on azithromycin. His leukopenia, thrombocytopenia, and liver enzymes improved slowly to normal but was still intubated due to severe desquamation of the oropharyngeal mucosa and copious secretion when left-sided weakness was noted on the ninth day of hospital stay. CT head followed by magnetic resonance imaging (MRI) of the brain revealed acute infarct with hemorrhagic transformation in the right frontal lobe involving the middle cerebral artery (MCA) territory (Figure 4). However, CT angiogram of head and neck were negative for occlusion, stenosis, aneurysm, dissection, or arteriovenous malformation in the major intracranial or neck arteries except re-demonstration of right anterior MCA territory infarct with hemorrhagic transformation. Subsequently, Echo and Doppler of limbs were done and were negative for cardiac abnormality including atrial septal defect, vegetation or deep vein thrombosis. His skin biopsy resulted findings consistent with Steven-Johnson syndrome. The patient was successfully extubated and subsequently discharged home after full recovery.
Discussion
M pneumoniae is an atypical bacterium and usually causes asymptomatic or mild respiratory tract infection. Nonrespiratory manifestations occurs in 25% of all patients with M pneumoniae infection and include encephalitis, aseptic meningitis, acute transverse myelitis, stroke, polyradiculopathy, acute disseminated encephalomyelitis (ADEM), Guillain-Barre syndrome (GBS), cranial nerve palsies, cerebellar ataxia, psychosis, hemolysis, carditis, conduction abnormalities, hepatitis, pancreatitis, glomerulonephritis, arthritis, maculopapular or vesicular rash, urticarial, EM, and SJS and mycoplasma pneumonia–associated mucositis (MPAM, also called atypical SJS).1-7 These nonrespiratory manifestations can occur along with respiratory tract infection or independently. Extrapulmonary manifestations are common in children and young adult ranging from 6 to 21 years of age who are more prone to contract M pneumoniae respiratory infection, but uncommon in older age group. 8
Mucocutaneous and cutaneous diseases are more common among extrapulmonary manifestations and can occur in up to 17% patient with M pneumoniae infection. 9 SJS is an uncommon dermal manifestation following the M pneumoniae infection and it appears to be associated more commonly than other infectious agents. 10 SJS is characterized by an immune-mediated extensive necrosis and detachment of epidermis as this patient has had in his skin biopsy.
Central nervous system (CNS) manifestations occur in approximately 0.1% of all patients with M pneumoniae infection and up to 7% in all patients requiring hospitalization11,12 which includes encephalitis, aseptic meningitis, acute transverse myelitis, stroke, polyradiculopathy, acute disseminated encephalomyelitis (ADEM), Guillain-Barre syndrome (GBS), cranial nerve palsies, cerebellar ataxia, and psychosis. Encephalitis and meningoencephalitis are the most common CNS complication of mycoplasma infection followed by polyradiculitis, aseptic meningitis, and cerebellar ataxia. 13 Acute ischemic stroke (AIS) related to M pneumoniae infection is a rare manifestation, and thus far a total of 28 cases have been reported in English literature in all age group, among them only 9 were adult (Table 1)
Table 1.
Descriptions of Patients With Stroke Related to MP.
| References | Age & sex | Presenting symptoms | Onset of stroke (days) | Modality of MP diag. | CSF analysis | Abnormal laboratory findings (coagulation profile and antibodies) |
|---|---|---|---|---|---|---|
| Dowd et al. 14 | 31/F | PNA | 21 | NA | NA | Cold agglutinin |
| Mulder et al. 15 | 30 /F | PNA | 7 | CF | Normal | ↑fibrinogen, ↑FDP |
| Socan et al. 16 | 28/F | Cough | 6 | I | Normal, culture (+), PCR (+ | Normal |
| Padovan et al. 17 | 36/F | URTI | 8 | CAA | Normal, serology (+) and PCR (+) | Positive IgM aCL,↑aPTT, positive lupus anticoagulant, ↑D-dimer |
| Idbaih et al. 18 | 35/F | PNA | 21 | NA | NA | NA |
| Siclari et al. 19 | 40/F | PNA | 7 | CAA | NA | Normal |
| Senda et al. 20 | 21/M | URTI | 7 | CAA | Normal | IgM (+) of aCL, ↑PT, ↑APPT, ↑fibrinogen, ↑D-dimer, ↑thrombin-antithrombin III-complex, ↓protein C |
| Benghanem et al 21 | 57/F | PNA | 14 | E | NA | Positive aCL and anti beta2 GP1 Ab; Hemolytic anemia |
| Sarathchandran et al 22 | 39/M | Fever and headache | NA | 58 leucocytes/μL, culture (−) | NA |
Abbreviations: MP, Mycoplasma pneumonia; CSF, cerebrospinal fluid; PNA, pneumonia; NA, not available; CF, complement fixation; FDP, fibrin degradation product; PCR, polymerase chain reaction; URTI, upper respiratory tract infection; CAA, cold agglutinin assay; aCL, anticardiolipin antibody; Ab, antibody.
The exact mechanism of extrapulmonary manifestations of M pneumoniae is poorly understood. These manifestations can be result of dissemination of infection resulting from direct tissue invasion by the pathogen, especially in patients with humoral immunodeficiency (eg, early onset encephalitis, myelitis, aseptic meningitis, and septic arthritis); postinfectious autoimmune phenomena (eg, late onset encephalitis, myelitis, peripheral neuropathy, cerebellar dysfunction, GBS, and SJS); vascular occlusion secondary to vasculitis or hypercoagulable state (eg, stroke); or possibly ADP ribosylating toxin.9,23,24 Isolation of M pneumoniae in synovial fluid, pericardial fluid, and CSF has been reported,16,25,26 which support the direct tissue invasion hypothesis; however, their numbers are limited. Moreover, only a single report has demonstrated the presence of M pneumoniae within the parenchymal brain tissue 27 and not at all in synovial membrane and pericardium. The low reporting to isolate the organism from the infected sites could be due to difficulty in culturing the organism and lack of facility in diagnostic laboratories. The presence of autoantibodies to brain (anti-neuronal and anti-galactocerebroside antibodies) and other human tissues including well-known IgM class cold agglutinins 28 and delayed onset of extrapulmonary manifestation like hemolysis, late onset encephalitis, GBS, ADEM after several days to a few weeks from the onset of disease support autoimmune mechanism, however, their role is not well defined. Hypercoagulability induced by M pneumoniae is another mechanism that may lead to stroke. Elevated markers of hypercoagulability (eg, fibrinogen, D-dimer), endothelial dysfunction and evolution of IgM anticardiolipin antibodies, lupus anticoagulant after M pneumoniae infection did support the hypothesis.20,17,29 However, their numbers are limited and the presence of anticardiolipin antibodies, lupus anticoagulant were transient in contrary to antiphospholipid antibody syndrome and generally not thought to increase risk of thrombosis. 30 The underlying mechanism of MCA occlusion in our patient remained unreveal, however, thought to be from direct CSF invasion by M pneumoniae and subsequent meningeal reaction causing vascular occlusion given presentation but arguable given normal CSF picture.
PCR-based assays are the test of choice for diagnosis of M pneumoniae infection which include direct PCR for M pneumoniae (when very high suspicion) and multiplex PCR-based assays (which detect multiple respiratory tract pathogens on a single sample). Samples used for these molecular tests in the setting of respiratory infection are nasopharyngeal swab, sputum, and bronchial alveolar lavage. Serological test with 4-fold rise in IgG titer during convalescent phase can be used as an alternative molecular test. Detection of single high IgM titer may be used as presumptive diagnosis although the specificity of the diagnostic approach is very low. 31 Culture of the M pneumoniae is extremely difficult being a fastidious and slow-growing organism. Etiological diagnosis of extrapulmonary manifestations of the organism is difficult and made by detection of M pneumoniae by PCR or culture from the site of infection if the manifestations result from direct tissue invasion (eg, detection of mycoplasma in CSF, synovial fluid, and pericardia fluid in cases of meningitis/encephalitis, pericarditis, and septic arthritis, respectively). However, if the direct detection is unrevealing or clinical manifestations presumed to immune mediated then 4-fold rise of IgG titer during convalescent period is the primary way to make microbiological diagnosis. Sometimes presumptive diagnosis is made if extrapulmonary manifestation is concurrently present with respiratory infection and the organism is detected in respiratory tract.
Most mycoplasma infections are self-limiting; however, respiratory infection usually treated with antibiotics. Treatment with antibiotic is useful if the extrapulmonary manifestation is due to direct invasion of the organism; however, if related to autoimmune phenomenon, role of antibiotic is questionable. Macrolide, tetracycline, or fluoroquinolone classes of antibiotics are preferred, considering age of the patient and local antibiotic resistance patterns. The duration of antibiotic in pulmonary infection is usually 5 to 7 days, but in extrapulmonary infection undetermined. Use of steroid is controversial but studies have shown benefit in the setting of immune-mediated manifestations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iDs: Sanjay K. Yadava  https://orcid.org/0000-0003-0097-8802
https://orcid.org/0000-0003-0097-8802
Jain Hanish  https://orcid.org/0000-0002-2144-9056
https://orcid.org/0000-0002-2144-9056
References
- 1. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luby JP. Pneumonia caused by Mycoplasma pneumoniae infection. Clin Chest Med. 1991;12(2):237-244. [PubMed] [Google Scholar]
- 3. Tsiodras S, Kelesidis I, Kelesidis T, et al. Central nervous system manifestations of Mycoplasma pneumoniae infections. J Infect. 2005; Dec51(5):343-354. [DOI] [PubMed] [Google Scholar]
- 4. Ali NJ, Sillis M, Andrews BE, Jenkins PF, Harrison BD. The clinical spectrum and diagnosis of Mycoplasma pneumoniae infection. Q J Med. 1986;58(227):241-251. [PubMed] [Google Scholar]
- 5. Agarwala BN, Ruschhaupt DG. Complete heart block from Mycoplasma pneumoniae infection. Pediatr Cardiol. 1991;12(4):233-236. [DOI] [PubMed] [Google Scholar]
- 6. Vujic I, Shroff A, Grzelka M, et al. Mycoplasma pneumoniae-associated mucositis—case report and systematic review of literature. J Eur Acad Dermatol Venereol. 2015;29(3):595-598. [DOI] [PubMed] [Google Scholar]
- 7. Daxboeck F. Mycoplasma pneumoniae central nervous system infections. Curr Opin Neurol. 2006;19(4):374-378. [DOI] [PubMed] [Google Scholar]
- 8. Pönkä A. The occurrence and clinical picture of serologically verified Mycoplasma pneumoniae infections with emphasis on central nervous system, cardiac and joint manifestations. Ann Clin Res. 1979;11(suppl 24):1-60. [PubMed] [Google Scholar]
- 9. de Groot RCA, Meyer Sauteur PM, Unger WWJ, et al. Things that could be Mycoplasma pneumoniae. J Infect. 2017;74(suppl 1):S95-S100. [DOI] [PubMed] [Google Scholar]
- 10. Tay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol. 1996;35(5 pt. 1):757-760. [DOI] [PubMed] [Google Scholar]
- 11. Mansel JK, Rosenow EC, III, Smith TF, et al. Mycoplasma pneumoniae pneumonia. Chest. 1989;95(3):639-646. [DOI] [PubMed] [Google Scholar]
- 12. Abramovitz P, Schvartzman P, Harel D, Lis I, Naot Y. Direct invasion of the central nervous system by Mycoplasma pneumoniae: a report of two cases. J Infect Dis. 1987;155(3):482-487. [DOI] [PubMed] [Google Scholar]
- 13. Guleria R, Nisar N, Chawla TC, Biswas NR. Mycoplasma pneumoniae and central nervous system complications: a review. J Lab Clin Med. 2005;146(2):55-63. [DOI] [PubMed] [Google Scholar]
- 14. Dowd AB, Grace R, Rees WD. Cerebral infarction associated with Mycoplasma pneumoniae infection. Lancet. 1987;2(8558):567. [DOI] [PubMed] [Google Scholar]
- 15. Mulder LJ, Spierings EL. Stroke in a young adult with Mycoplasma pneumoniae infection complicated by intravascular coagulation. Neurology. 1987;37(8):1430-1431. [DOI] [PubMed] [Google Scholar]
- 16. Socan M, Ravnik I, Bencina D, Dovc P, Zakotnik B, Jazbec J. Neurological symptoms in patients whose cerebrospinal fluid is culture- and/or polymerase chain reaction-positive for Mycoplasma pneumoniae. Clin Infect Dis. 2001;32(2):E31-E35. [DOI] [PubMed] [Google Scholar]
- 17. Padovan CS, Pfister HW, Bense S, et al. Detection of Mycoplasma pneumoniae DNA in cerebrospinal fluid of a patient with M. Pneumoniae infection-“associated” stroke. Clin Infect Dis. 2001;33(10):E119-E121. [DOI] [PubMed] [Google Scholar]
- 18. Idbaih A, Crassard I, Vahedi K, Guichard JP, Woimant W. Thrombotic cocktail in stroke. Neurology. 2005;64:334. [DOI] [PubMed] [Google Scholar]
- 19. Siclari F, Hirt L, Meuli R, Fellmann F, Gailloud P, Michel P. Bilateral carotid thrombus formation after strenuous coughing. Eur J Neurol. 2009;16(7):e122-e123. [DOI] [PubMed] [Google Scholar]
- 20. Senda J, Ito M, Atsuta N, et al. Paradoxical brain embolism induced by Mycoplasma pneumoniae infection with deep venous thrombus. Intern Med. 2010;49(18):2003-2005. [DOI] [PubMed] [Google Scholar]
- 21. Benghanem S, Rosso C, Leger A, et al. Association between mycoplasma pneumonia and increased risk of ischemic stroke: a case report. Eur J Neurol. 2016;23:260. [Google Scholar]
- 22. Sarathchandran P, Al Madani A, Alboudi AM, Inshasi J. Mycoplasma pneumoniae infection presenting as stroke and meningoencephalitis with aortic and subclavian aneurysms without pulmonary involvement. BMJ Case Reports. 2018;2018:bcr2017221831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagashima M, Higaki T, Satoh H, Nakano T. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interact Cardiovasc Thorac Surg. 2010;11(6):849-851. [DOI] [PubMed] [Google Scholar]
- 24. Narita M. Pathogenesis of neurologic manifestations of Mycoplasma pneumonia infection. Pediatr Neurol. 2009;41(3):159-166. [DOI] [PubMed] [Google Scholar]
- 25. Davis CP, Cochran S, Lisse J, et al. Isolation of Mycoplasma pneumoniae from synovial fluid samples in a patient with pneumonia and polyarthritis. Arch Intern Med. 1988;148(4):969-970. [PubMed] [Google Scholar]
- 26. Szymanski M, Petric M, Saunders FE, Tellier R. Mycoplasma pneumoniae pericarditis demonstrated by polymerase chain reaction and electron microscopy. Clin Infect Dis. 2002; Jan134(1):E16-E17. [DOI] [PubMed] [Google Scholar]
- 27. Launes J, Paetau A, Linnavuori K, Iivanaineu M. Direct invasion of the brain parenchyma by Mycoplasma pneumoniae. Acta Neurol Scand. 1997;95(6):374. [DOI] [PubMed] [Google Scholar]
- 28. Biberfeld G. Antibodies to brain and other tissues in cases of Mycoplasma pneumoniae infection. Clin Exp Immunol. 1971;8(2):319-333. [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka H, Narita M, Teramoto S, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121(5):1493-1497. [DOI] [PubMed] [Google Scholar]
- 30. Mélé N, Turc G. Stroke associated with recent Mycoplasma pneumoniae infection: a systematic review of clinical features and presumed pathophysiological mechanisms. Front Neurol. 2018;9:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nir-Paz R, Michael-Gayego A, Ron M, Block C. Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin Microbiol Infect. 2006;12(7):685-688. [DOI] [PubMed] [Google Scholar]


